Abstract
Motor deficits can significantly affect the completion of daily life activities and have a negative impact on quality of life. Consequently, motor function is an important behavioral endpoint to measure for in-vivo pathophysiological studies in a variety of research areas, such as toxicant exposure, drug development, disease characterization, and transgenic phenotyping. Evaluation of motor function is also critical to the interpretation of cognitive behavioral assays, as many rely on intact motor abilities to derive meaningful data. As such, gait analysis is an important component of behavioral research and can be achieved by manual or video-assisted methods. Manual gait analysis methods, however, are prone to observer bias and are unable to capture many critical parameters. In contrast, automated video-assisted gait analysis can quickly and reliably assess gait and locomotor abnormalities that were previously difficult to collect manually. Here, we describe the evaluation of gait and locomotion in rodents using the automated Noldus CatWalk XT system. We include a step-by-step guide for running an experiment using the CatWalk XT system, and discuss theory and considerations when evaluating rodent gait. The protocol and discussion provided here act as a supplemental resource to the manual for this commercially available system, and can assist CatWalk users in their experimental design and implementation.
Keywords: CatWalk, Rodents, Gait, Locomotion, Motor deficits, Phenotyping
Basic Protocol:
Measuring Gait and Locomotor Parameters in Rodents with the CatWalk XT (Version 9.1) System
INTRODUCTION
The act of walking is a complex behavior, requiring the coordination of limb movement, balance, postural control, proper speed adjustment, and cadence, among other factors. Motor deficits can arise from dysfunction in a variety of systems, such as the cardiovascular (Hattori et al., 2014, Clark et al., 2019), musculoskeletal (Sayed-Zahid et al., 2019), and peripheral and central nervous systems (PNS/CNS) (Carter et al., 1999, Pan et al., 2005, Neumann et al., 2009, Yin et al., 2017). Arthritis and physical injury can cause limping and protective posturing of the limb (Lakes & Allen, 2016), while gait disturbances due to specific CNS injury or affected circuitry can be recognized by their characteristic deficit, such as ataxia (in cerebellar disorders) (Cendelin, 2014) and shuffling gait (in Parkinson’s disease, PD) (Broom et al., 2017). For toxicological studies, evaluating motor function impacts is critical when investigating drug toxicity and potential side effects, as drug-induced movement disorders (DIMD) significantly affect the quality of life of patients and can reduce drug adherence while increasing the risk for adverse outcomes by inhibiting daily life activities (Demler, 2014).
Rodents are a common model used for behavioral studies, but they present unique challenges when evaluating gait. As prey animals, it has been proposed that they are evolutionarily conditioned to hide disability and pain (Mogil, 2015), and can compensate for injury with their quadrupedal stature in ways bipedal humans cannot. For example, quadrupeds can shift their weight to their forelimbs in the case of a hindlimb injury, where bipeds are unable to do so (Saunders et al., 2017). As such, some motor deficits may not be as easily detected in rodents as they are in humans. To assess these subtle deficits, the ability to sensitively collect gait parameters in an environment suited to the animal is crucial. A variety of gait evaluation methods for rodents have been developed over the years, many relying on manual scoring by eye and thus unable to capture subtle effects or measure multiple parameters at once, owing to the combinatory limitations of the human eye and the speed of rodent locomotion.
High-speed video gait analysis solves many of the problems of manual scoring by eye and allows researchers to collect a variety of gait parameters with high sensitivity, providing quantitative measurement for spatiotemporal and kinetic parameters which were previously difficult to collect. Furthermore, these parameters can be measured in a passive environment, such that the animal is free to walk within the analysis arena with minimal handling or intervention of the researcher, limiting the stress of the animal during study. Both open-source systems (Mendes et al., 2015; Jacobs et al., 2018; Zhang et al., 2020) and commercial products, such as the CatWalk XT system (Noldus Information Technology) and MotoRater (TSE Systems), are available for camera-assisted gait tracking with immobile walkways. Active environment analysis with the utilization of a treadmill can also be employed using both open-source (Williams et al., 2020) or commercial products (such as the DigiGait (Mouse Specifics, Inc.) and TreadScan (CleverSys, Inc.)).
In this article, we describe details of the CatWalk XT system for rodents, with many of the core concepts discussed being applicable to other open-source and commercial systems. The CatWalk utilizes a glass walkway placed above a high-resolution camera, where light is internally reflected within the walkway and only released when the animal’s paw contacts the glass, illuminating the paw upon contact. As the rodent travels across the platform, the camera records a video and the CatWalk XT software measures the light intensity of the illuminated paw, providing a host of paw statistics and gait metrics for researchers to analyze. A schematic of the system is shown in Figure 1. Unlike treadmill systems, where researchers set the speed of the walkway belt and force the animal to meet a specified speed, the non-moving walkway of the CatWalk is better suited to analyze natural gait and walk speed. This system can capture numerous independent variables for gait analysis, such as walk and swing speed, cadence, stride, among others, and a variety of paw metrics. These parameters can be used to identify a motor phenotype in transgenic animals or evaluate motor toxicity of chemicals and drugs.
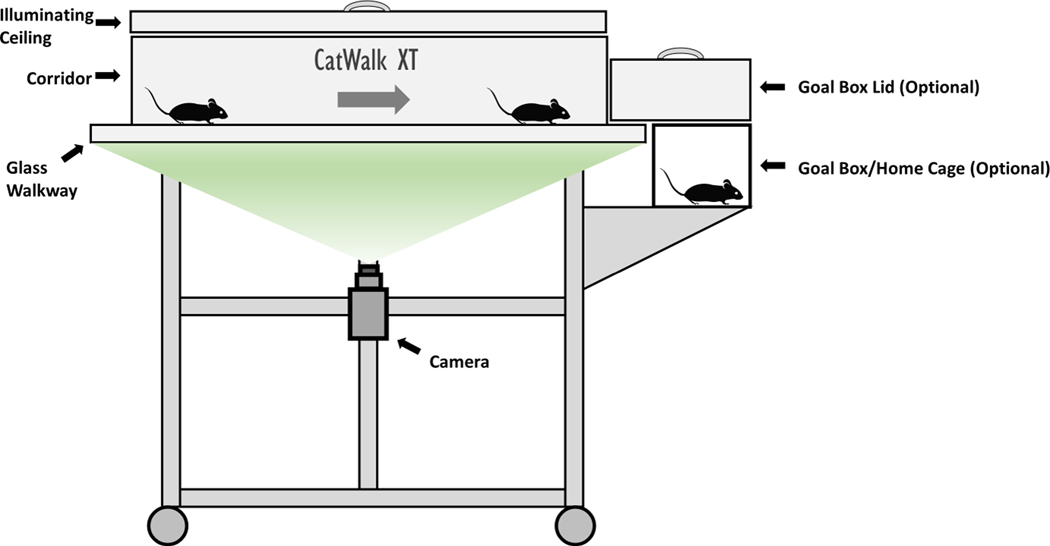
Figure 1 – CatWalk XT System.
Illustration of the CatWalk XT system. Rodents are placed on one end of the apparatus and walk across a walkway while a camera, from below, records paws to calculate various gait parameters.
Basic Protocol 1 provides a step-by-step guide for setting up and conducting an optimal experiment using the CatWalk XT system. This protocol is designed and intended to be used as a supplemental resource to the CatWalk XT system manual, providing users with tips throughout the setup process and a discussion of common practices when conducting CatWalk experiments.
STRATEGIC PLANNING
Before proceeding, all protocols must be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC), and must conform to governmental regulations regarding the care and use of laboratory animals.
Determining variables of interest
Upwards of 100 variables are produced by the CatWalk XT software and are available for researchers to evaluate. Not all variables are independent, and researchers run the risk of type-1 error by analyzing every variable provided by the software without forethought as to what variables are of interest for a given experiment. Care should be taken to determine, a priori, what parameters are important for the experiment at hand and how they contribute to the description of a motor phenotype in the context of other collected data, such as any mechanistic studies or additional behavioral endpoints. For example, when studying traumatic brain injury (TBI), the location of the induced TBI may be factored in when considering what parameters to evaluate.
Acclimating animals to the walkway and testing room
Acclimating the animals to the walkway for 1 – 2 minutes per day for one week prior to testing until they are consistently walking across the test area will provide a more even baseline between the experimental groups. Variation can also be minimized by running the test at the same time of day for each experimental group and acclimating the mice to the testing room 30 minutes prior to testing. Further discussion on minimizing variability can be found in the “Critical Parameters” section.
Room settings
Utilization of the CatWalk should take place in a dark room (1 lux or less) or under red light conditions. If desired, the walkway and all components can be sequestered in a sealable enclosure that blocks out ambient light, allowing the room to be illuminated while testing.
Configuration of the detection settings
Detection settings for paw prints should be set according to the manufacturer’s instructions. A representative control animal is used to determine the optimal paw detection settings to help the software distinguish between paw prints and background noise; this can be an animal planned for experimentation. When the control animal is placed in the walkway, the camera gain and intensity threshold can be adjusted so the paw print is clearly visualized. An ideal print should have the paw pad and toes clearly visible and separated. If the toes are not visible, or if there is no definition between the toes and/or paw pad, adjust the camera gain and intensity threshold. These settings should be revisited between experiments if there are significant changes in the animals to be used, such as species, strain, age, weight, or if other physical parameters of the animals change which may influence paw impact. Sex may also impact these parameters, and representative male and female animals should be compared to determine if different settings are required. If physical parameters vary greatly within an experiment, e.g., one experimental group is obese and the other is underweight, take a representative rodent from each experimental group and determine if they require different threshold settings for optimal print visualization. If so, two different files should be created, each with different detection settings optimized to the representative rodents. Some parameters rely on clearly visualized paws with separated toes to be calculated, such as print length (measured as the distance from the third toe to the heel of the paw), toe spread (measured as the distance between the first and fifth toes), and intermediate toe spread (measured as the distance between the second and fourth toes). These measurements can be particularly important for the study of specific deficits, such as the recovery from sciatic nerve damage (Baptista et al., 2007). The creation, in this example, of two files with optimized settings ensures that these parameters can be measured appropriately in both groups.
BASIC PROTOCOL 1: Measuring Gait and Locomotor Parameters in Rodents with the CatWalk XT (Version 9.1) System
The CatWalk XT system has been widely used to evaluate rodent gait and locomotion. Here, users will follow the steps provided to set up an experiment within the software and record their test animals as they traverse the walkway. Each traverse of the walkway is termed a “run”, while all runs for a given animal are termed a “trial”. Once all test animals have been measured, gait parameters are exported as an Excel file for further analysis. As the protocol expands on points made in the manual, it serves to assist first time and relatively new users in understanding how the CatWalk works and important considerations of specific settings.
Here, we describe implementation of the system using CatWalk XT version 9.1. While users may have access to a different version, which may exhibit some minor software differences, the theory of gait analysis and other basic concerns discussed in this protocol are applicable to all versions.
Materials
Mice (at least 6 weeks of age) or rats (at least 8 weeks of age)
Computer (System requirements: Windows XP Professional or higher, 2.60GHz CPU, 6GB RAM)
- Noldus CatWalk XT walkway and components
- Adjustable plastic corridor
- Removable metal grills for each end of corridor
- Camera
- Lightbox
- Calibration sheet (15 × 10 cm)
- Goal box for end of corridor (optional)
Noldus CatWalk XT software (Version 9.1 is described in this article)
70% ethanol, standard glass cleaning solution, and low-lint wipes for cleaning walkway
Attractant for goal box (optional)
Protocol Steps
Preparing the walkway
-
1.Clean the walkway and interior corridor walls with 70% ethanol, following up with glass cleaning solution on the walkway surface to minimize streaking.The walkway should be cleaned thoroughly between each animal, as insufficient cleaning may leave residual odors from the previous animal and cause pauses in the run for sniffing or other investigatory behavior in the test area, ultimately leading to an invalid run. Additionally, urine and feces on the walkway can obscure paw prints and cause unnecessary background noise and software read issues. Ensure that the walkway is dry and free of any residual glass cleaning solution before placing an animal inside.
-
2.Place the corridor in the middle of the walkway (Fig. 1) and put the grills in place on each end, ensuring that the corridor is properly seated on its poles and contacts the walkway.The width of the corridor will depend on the size of the animals and should generally be narrow enough for the rodent to walk forward freely and limit turning around; a corridor that is too wide may promote excessive turning behavior. If the rodent turns around during a run within the acquisition window, the run is invalid and will need to be repeated.
-
3.(Optional) Set up goal box at the end of the walkway with preferred attractant (Fig. 1).The goal box may be used at the end of the corridor to create incentive to cross the field, where entrance to the home cage or other attractant (e.g., food pellet or rodent candy) may be used.
Defining the walkway
Defining and calibrating the walkway should be done before each experiment but is not necessary to repeat between animals unless changes are made to the walkway during the experiment. If adjustments are made to the corridor width, camera height, or any other components mid-experiment, then the walkway will need to be re-defined to accommodate these changes. Please refer to the manual for screenshots of the software.
-
4.
Define the layout of the walkway by selecting the “Application Startup Tasks” window in the software, clicking on “Setup the walkway”, and then clicking on “Define the layout of the walkway”. From “Setup”, click on “Define the walkway”. An adjustable white rectangular box should be visible on the runway area.From this window, you should now see the image of the walkway.
-
5.
Adjust the camera if you are unable to visualize the walkway. For rodents, 5 – 6 step cycles are ideal for a run. To achieve this, the camera should be set 45 cm below the walkway for mice and 70 cm for rats. Verify that the camera height is appropriate for your experiment and that the walkway is in focus.
-
6.
Adjust the white rectangular box (Step 4) to select the area of interest for the walkway (the walkable space within the corridor).
Calibrating the walkway
-
7.
Select the “Application Startup Tasks” window, click on “Setup the walkway”, and then click on “Calibrate the walkway”. From “Setup”, click on “Calibrate walkway”.
-
8.
Place the 15 × 10 cm calibration sheet onto the walkway in the previously defined area.
-
9.
Resize the white rectangle on the screen to fit the rectangle on the calibration sheet.
Creating an experiment
-
10.From the “File” menu, select “New Experiment”.You may select a previously used experiment profile under “Setup Profile” if you have any saved at this step.
-
11.
Name and select the desired save location for the experiment.
-
12.
To set the experiment settings, navigate to the “New Experiment Tasks” window and select “Experiment Settings”.
-
13.Under the “Time Points” section, add a time point if desired for the experiment.Time points are useful for repeated testing over time if gait effects by treatment are to be evaluated at multiple time points. These may be omitted if they are not of experimental interest.
-
14.Select animal type (rat or mouse).Proper animal selection is critical, as various software components calculate parameters differently for rats and mice.
-
15.Define your experimental groups in the “Treatment Groups” section and click “Add Group” to add a treatment group. Adjust the names of the experimental groups accordingly.The experimental group names will carry through to the exported data files, so it is useful to change the name of the treatment groups. Additionally, you can input further information in the “Description” window, which is exported with the data output and can be analyzed as an independent variable.
Experiment settings
-
16.Set the desired “Run Criteria” by adjusting the “Minimum run duration”, “Maximum run duration”, and “Minimum number of compliant runs to acquire”, and by checking/setting the “Use maximum allowed speed variation”.Note that once runs have been acquired, the run settings cannot be changed for a given experiment. If you find that the run settings were not appropriately set for the experiment after acquiring a few trials, you will need to start a new experiment and repeat the trials with the adjusted settings.
Setting the trial list
-
17.
Navigate to the “Experiment Tasks” window and select “Open Trial List”.
-
18.Select “Add Trial(s)” to add as many trials (animals) as your experiment requires and assign them to the appropriate treatment group. Edit the trial name to your choosing, keeping in mind that all trial names must be unique. You may also adjust the animal ID, time point (if selected; see “Creating an Experiment” section), as well as an optional description.Too many trials within an experiment may cause slow-downs and crash the software. If you have more than 500 trials for an experiment, you should break the experiment up into multiple files.
Acquisition settings
-
19.
Under the “Setup” menu, select “Acquisition Settings”.
-
20.Select criteria for recorded runs under “Run Settings”.These settings are not what decide if a trial is compliant or not; that, instead, is done by editing the “Experiment Settings” (step 16). Under “Run Settings”, you may select a time threshold for which the run will be aborted, and the acquisition will stop. Further, you may select to have the aborted runs discarded and not recorded in the acquisition window. These settings may be useful to save program memory and disc space in situations where the animals routinely make non-compliant runs, and you have many trials in an experiment.
Acquiring a trial
-
21.
Navigate to the acquisition window by selecting “Open Acquisition” in the “Experiment Tasks” window.
-
22.
Select the trial to be acquired in the drop-down menu.
-
23.With an empty and clean walkway, retrieve a background image by clicking “Snap Background”.A background image should be collected prior to each trial, as the cleaning between animals can lead to different background noise and minor positional changes of the corridor.
-
24.
Place the animal at the end of the corridor.
-
25.
Lower the illuminated ceiling by grabbing the handle and gently lowering it onto the walkway.
-
26.On the “Acquisition” window, click “Start Acquisition”.Start acquisition as quickly as possible upon placing the animal inside corridor to ensure you do not miss the first crossing of the test window.
-
27.Allow the animal to cross the test field for as many compliant runs desired for your experiment and visualize each run to verify compliance.A minimum of 3 compliant runs per trial per animal are recommended for data analysis. The software will automatically cease recording once all compliant trials have been acquired. If one or more trials were recorded as complaint based on time but cannot be used for analysis purposes (e.g., the mouse turned around mid-run, stopped mid-run, etc.), you can restart the acquisition by clicking “Start Acquisition” to collect additional runs. For this reason, you should visualize each run on the screen as they occur to verify compliancy based on the chosen experimental parameters and should not rely on the software’s determination based on the time gate alone.
-
28.
Remove the animal from the walkway and return to home cage.
-
29.
Clean the walkway with 70% ethanol and glass cleaner and proceed with the next animal.
-
30.
Repeat steps 22 – 29 until all animals have been tested.
Reviewing and classifying runs
-
31.
Navigate to the “Video” section and select a run from the “View” list.
-
32.
Play the run, adjusting the playback speed as desired.
-
33.If a run is non-compliant and you would like to delete it, right click the run and select “Delete”.Runs which are deleted are permanently deleted and cannot be restored.
-
34.
To classify the runs, navigate to the “Experiment Tasks” window and select “Classify Runs”.
-
35.Utilize the Automatic Footprint Classification (AFC) module to automatically detect paw prints, or manually select prints if this module is not available or manual selection is preferred.The AFC module functions well to label paw prints in situations of normal gait. In situations where the tail, nose, or other parts of the animal body are repeatedly contacting the glass, the AFC does not identify these contacts well, and manual selection will be necessary. For all runs, you should first automatically classify the paw prints (if the module is available), and then go through the run and manually assign any non-paw print contact as well as verify the assignment made by the AFC for each paw print, re-assigning any erroneously labeled paw prints. Users can manually assign prints by drawing a box with their cursor over the print and assigning the appropriate label to it from the pop-up menu. In cases where the glass has significant background, or paw prints are close together, the AFC will have a higher rate of mislabeled or unlabeled paw prints. Refer to the manual for additional usage recommendations of the AFC module as well as detailed manual labeling instructions.
Exporting data
-
36.
Under the “Analyze” menu, select “View Run Statistics”.
-
37.Under the “File” menu, select “Export”. Three export options will be available in the drop-down menu, “Run Data”, “Run Statistics”, and “Trial Statistics”. Select the preferred exportation.Run data will export the frame-by-frame data of a single run. Run statistics compiles the run data into a single value per parameter per run. Trial statistics compiles the run statistics into a single value per parameter per trial/animal. See the Understanding Results section.
COMMENTARY
Background Information
Locomotion and gait have been studied for decades, with recent technological advancements providing wider access to gait analysis. One of the earliest and simplest methods for evaluating locomotion is observation in an open field environment. While this method is simple, with researchers recording gait measurements by eye or with the assistance of video tracking software for later manual evaluation, observational methods are limited by the capabilities of the human eye and can generally only detect gross locomotor and gait abnormalities (Lakes & Allen, 2016). Treadmills can also be used for observational gait studies, which can be useful in models where motivation to cross an open field is a challenge or when researchers desire to control the speed of the animal (Pereira et al., 2006). Results from observational studies can vary widely between observers, and protocols are difficult to standardize between labs. Further, lack of detection of subtle changes poses significant challenges to such observational methods, highlighting that these methods alone provide only an incomplete picture of locomotion and gait.
A more complex and quantitative test, footprint analysis, can be conducted by dipping a rodent’s paw into ink or photo developer solution and having them walk across a sheet of paper or film, respectively (van der Zee et al., 1987; Carter et al., 1999). From this, calculations based on footfall, print size, and various other parameters can be measured. While more quantitative in nature than strict observational methods, this test has its own limitations, such as the ink itself altering the gait of the animals due to the feel on the paw, smearing of the ink obscuring paw measurements, and the inability to measure desired parameters like velocity (Jacobs et al., 2014).
The CatWalk gait analysis method addresses many of the limitations of observational and ink-based footprint analysis. Developed by Frank Hamers, this method was originally designed to assess front limb–hind limb (FL-HL) coordination in spinal cord injury, a metric historically difficult to assess in open field observation (Hamers et al., 2006). Utilizing a special glass walkway developed by Betts and Duckworth (Betts et al., 1980) that illuminates paw prints, and a camera from below to record, a wealth of parameters were found to be measurable with this method, beyond FL–HL coordination (Hamers et al., 2001). By employing a computer program to analyze the paw prints, intra-observer variability is removed and detailed metrics unable to be caught by the human eye are now able to be recorded. Passive, automated systems such as the Noldus CatWalk XT based on this method have greatly simplified gait analysis in rodents and allowed for collection of dynamic parameters that were previously difficult to collect, such as swing duration, stance duration, and interlimb coordination (Kappos et al., 2017). These parameters are important for the characterization of nerve injuries to both the PNS (Deumens et al., 2007; Chiang et al., 2014) and CNS (Koopmans et al., 2005; Hamers et al., 2006; Zheng et al., 2021). Further, the CatWalk system has been utilized to evaluate motor phenotypes of transgenic animals (Robinson et al., 2012; de Haas et al., 2016) and rodent models of disease, such as multiple sclerosis (Bernardes & Oliveira 2017), Huntington disease (Abada et al. 2013), PD (Wang et al., 2012; Zhou et al., 2015), and stroke (Hetze et al., 2012). Modeling of the impacts of mechanical injury on gait have also been extensively studied with the CatWalk, such as those that occur with TBI (Chen et al., 2012; Walter et al., 2020) and limb injury (Gabriel et al., 2007).
Despite its undeniable importance, it should be mentioned that motor phenotyping is only one piece of the picture when considering a behavioral phenotype and should be part of a larger assessment battery when evaluating, for instance, the effects of toxicants or characterizing a transgenic line. Cognition and motor function are deeply intertwined and overlap in the frontal lobes, cerebellum, and basal ganglia, where the executive function for movement intent and prediction of the movement of others are controlled (Leisman et al., 2016). Given the intertwining of these functions, motor and cognitive deficits often co-exist in specific CNS injuries and both should be investigated for a robust phenotypic analysis. In addition to including cognitive tests when conducting behavioral phenotype analysis, the translational application of the rodent model for gait abnormalities should be considered. There are fundamental differences between quadruped and biped gait that can complicate direct translation of rodent gait abnormalities to humans. For example, rodents and other quadrupeds show an increased frequency of gait patterns at higher speeds, such as trotting and galloping, which are generally not seen in bipedal humans (Broom et al., 2017). These differences will have varying impacts on data interpretation depending on the experimental model and questions being addressed, but should be considered, nonetheless.
Critical Parameters and Troubleshooting
For the CatWalk, there are no concerns with maintaining a novel environment as it is designed to test inherent locomotor ability and gait function and there is no learning component being assessed. Therefore, repeated testing of the same animal is acceptable with no waiting period required between tests. In cases where the mice are tested for long periods or appear to be fatigued by the testing regime, researchers should use their best judgement in applying a wait period between testing to ensure mice are not fatigued when tested, as this could influence their performance.
Reducing stress as much as possible is important for most behavioral assessments. With locomotion and gait evaluation, stress can influence the way in which the animals move and can make subtle treatment effects more difficult to detect due to increased variability. A stressful or fearful rodent in the CatWalk may be less inclined to cross the testing field, may alter their speeds erratically during the test, or may exhibit other undesired behaviors that can impact data analysis. Be mindful to minimize stress for the animals when placing them inside and removing them from the apparatus, as to cause as little stress as possible and to not trigger negative associations with the apparatus itself. Training the animals to travel the walkway beforehand, for example, can familiarize them with the environment, lessening fear and anxiety, and ultimately reduce variability due to run differences by providing more consistently compliant runs. Frequent handling of the animals, in general, can acclimate them to being picked up and moved by researchers, alleviating stress when being placed into and removed from the walkway.
In certain experimental circumstances, there may be justification for studying gait while the animal is under stress. While there may be no spontaneous motor phenotype at baseline, a motor deficit may arise under stress conditions and provide further information for the behavioral phenotype (Sugimoto & Kawakami, 2019). Stress as a modulating factor should be performed in a controlled manner, first following the abovementioned guidelines to allow for each animal to be at a similar baseline, after which stress loading may be applied by the desired technique.
Disruptions to cognitive ability may also present with a motor deficit, depending on the affected brain region. For example, mechanical injury to the cortex by controlled cortical impact (CCI) causes both cognitive (Hamm et al., 1992; Thompson et al., 2006) and motor impairment (Neumann et al., 2009; Chen et al., 2012). Furthermore, phenotypic evaluation of cognition and memory, utilizing classic tests such as the Morris water maze, T-maze, and novel object test, require the animal to move through a testing field to appropriately conduct the test (Rodriguiz and Wetsel 2006). Motor deficits, even in the absence of cognitive deficit, may impact the output of these tests and erroneously display as cognitive deficits if the animal cannot move through the test field. For this reason, motor testing and gait evaluation with the CatWalk or other tests, should be conducted before testing cognitive ability, and any determined motor deficit should be considered when interpreting cognitive tests that require locomotion.
A minimum of three compliant runs per trial are recommended for analytic purposes; more may be included, particularly if you suspect that there may be variation in the runs. Runs that fall below the minimum and above the maximum set run duration (Basic Protocol 1, Step 16) will be labeled as non-compliant. All non-compliant runs will still be acquired and recorded in the acquisition window unless otherwise specified in “Acquisition Settings” (discussed in the “Acquisition Settings” section in Basic Protocol 1). The minimum and maximum run settings will vary based on experimental conditions. When deciding on an appropriate time window, you may use the acclimation days to narrow down this window or refer to previously described literature for a given model. For an ideal compliant run, the rodent should freely and calmly cross the test window without interruption. Some treatments may cause fear or anxiety that modify the animal’s run behavior (e.g., the rodents may dart across the test window), and the minimum time will thus need to be adjusted to reflect faster run speeds. Some of this behavior may be mitigated by acclimation to the testing apparatus, but the outcome of acclimation may vary by duration/frequency of the acclimation and experimental treatment conditions. Conversely, some treatments may significantly impact locomotion and cause the treatment groups to move slower compared to the control. In this case, the maximum will need to be adjusted to include longer run times so that all runs by treated animals are not erroneously labeled as non-compliant.
The selection of “Use maximum allowed speed variation” is another layer of compliancy based on speed consistency. Some rodents may start the run fast and slow down, or start slower and speed up mid-run, causing significant variation in their run speed. Speed variations can be due to a variety of factors, such as a startle mid-run or a legitimate effect of treatment. If this option is unselected, only the minimum and maximum times will be used to determine if a run is compliant. If you desire to analyze speed variation as a variable, you should leave this unselected (as is the program default), since determining compliant runs based on speed variation will artificially gate the runs within a specific variation window and may mask differences in this parameter.
For software troubleshooting, please refer to the manual provided with the system.
Understanding Results
The parameters measured by the CatWalk fall into eight general categories: (1) paw statistics, (2) step sequence, (3) base of support (BOS), (4) print positions, (5) support, (6) phase dispersions, (7) couplings, and (8) other. These parameters are automatically calculated by the CatWalk XT software and can be exported on a per-run basis (“Run Statistics”), or alternatively, on a trial basis (“Trial Statistics”) in which all the collected runs are averaged together to output a single value for each parameter per animal. In general, exporting the trial data to obtain one value per parameter per animal is the preferred exportation method. In cases where you are interested in the inter-run variation of a single animal, exporting on a run-basis would be beneficial to examine this variation before averaging the runs to obtain a single trial value. You may refer to the user manual for detailed information on how the parameters in each category are calculated within the software and what total parameters are available. Parameters of interest will vary depending on experimental goals and should be determined a priori for appropriate data analysis (see Figure 2 for graphical representations of select parameters). Some parameters for basic gait and locomotor evaluation include:
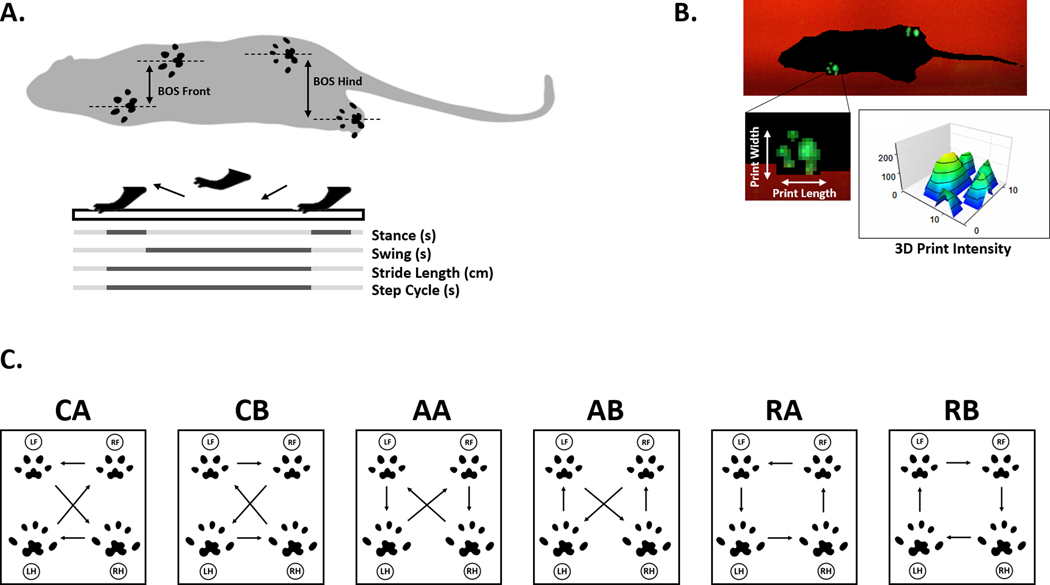
Figure 2 – Selected CatWalk Parameters.
A. Illustration showing base of support (BOS) measurement in front and hind limbs, as well as a graphical representation of stance (s), swing (s), stride length (cm), and step cycle (s). B. Screenshot from a CatWalk XT video demonstrating paw print visualization and intensity output from 3D Footprint Intensities provided in software, along with a graphical representation of paw print statistics for print length and width. C. Common step patterns in rodents, with arrows indicating step directions of left front (LF), left hind (LH), right front (RF), and right hind (RH) paws. Patterns fall into three categories: cruciate (CA, CB), alternate (AA, AB), and rotate (RA, RB).
Average speed: Walk speed measured as distance over time, expressed in centimeter/second (cm/s).
Cadence: Frequency of steps during the trial, expressed as number of steps/second.
Swing: Time of paw spent in the air between two consecutive steps, expressed in seconds (s) (Figure 2A).
Swing speed: Parameter calculated using swing and stride length, expressed in centimeter/second (cm/s).
Stride length: Distance between paw placement in two consecutive steps of the same paw, expressed in centimeters (cm) (Figure 2A).
Stance: Duration of paw contact on glass, expressed in seconds (s) (Figure 2A)
Step cycle: Duration of the stride, expressed in seconds (s) (Figure 2A).
Print intensity: The average pressure exerted by a single paw on floor contact, expressed in arbitrary units (a.u.) (Figure 2B).
Print area: Measurement of the complete paw print including all frames that makes up a stance, expressed in cm2.
Print length: Measured length of the print area, expressed in centimeters (cm) (Figure 2B).
Print width: Measured width of the print area, expressed in centimeters (cm) (Figure 2B).
Base of support: Describes the distance between either two front paws or two hind paws, expressed in centimeters (cm) (Figure 2A).
Sequence Regularity Index: Percentage index measuring interlimb coordination, calculates based on the number of normal step sequence patterns (NSSP), number of paws and paw placements, SRI = 100% * (NSSP * # paws) / # of paw placements.
- Step sequence: Measures percentage spent in the six normal step sequence patterns (NSSP) noted in rodents (Figure 2C):
- Cruciate
- CA : RF – LF – RH – LH
- CB : LF – RF – LH – RH
- Alternate
- AA : RF – RH – LF – LH
- AB : LF – RH – RF – LH
- Rotate
- RA : RF – LF – LH – RH
- RB : LF – RF – RH – LH
Support time: Relative duration spent supporting weight on all combinations of paws. Combinations include zero paw, single paw, girdle two paws (LF & RF or LH & RH), lateral two paws (LF & LH or RF & RH), diagonal two paws (LF & RH or RF & LH), three paws or four.
Rodents typically have a symmetrical gait pattern, with their left and right limb foot-strikes being spaced equidistant, timed at around 50% of the gait cycle, and the front and hind foot-strikes for a given side overlapping in space. An example of this typical gait pattern in a control mouse, obtained by following Basic Protocol 1, can be found in Figure 3. An example video of a control mouse in the walkway can be viewed in the included video (Video 1). Additionally, rodents tend to be balanced and spend an equal amount of time on their left and right limbs (Jacobs et al., 2014). While symmetry in the gait pattern is generally expected across all control animals, size, weight, and other factors may influence other parameters of interest, such as swing and stride length. Comparing the treatment group to a proper control group on an identical background, with as many physical variables held constant as possible, will provide the best interpretation of what parameters are deviating from normal within a given experiment. In cases where physical parameters cannot be held constant (e.g., treated animals have a smaller stature compared controls), these factors should be considered when interpreting the data.
Figure 3 – Basic Rodent Gait Pattern.
Representative run from CatWalk demonstrating a normal gait pattern, viewed from below, with the animal walking from left to right. Left front (LF), left hind (LH), right front (RF), and right hind (RH) paw prints are labeled. Animal is a 4-month-old male wildtype C57BL/6 mouse. The footstrikes are equally spaced between the left and right sides, with the front and hind limbs overlapping in their step.
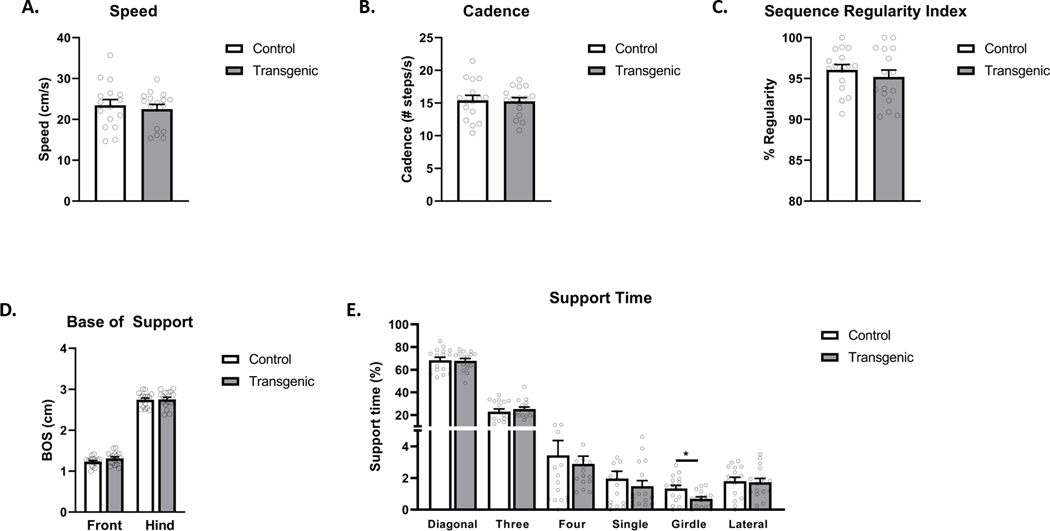
A selection of example parameters, generated by following Basic Protocol 1, can be viewed in Figure 4. Here, control and transgenic mice on a C57BL/6 background (N = 16 per group) were compared to determine baseline differences in their gait and locomotor function. No differences in male and female mice were found for any parameters (not shown), and both sexes were combined for the analysis. Metrics measured include speed of the mice (Figure 4A), cadence (Figure 4B), regularity index of their gait (Figure 4C), base of support (Figure 4D), and the percentage of time the mice spent bearing weight in specific limb configurations (Figure 4E). In this experiment, the transgenic mice did not vary from the controls on most metrics measured. However, the transgenic mice were found to spend significantly less time bearing weight in the “girdle” support position, which is the placement of weight on the two front limbs or two hind limbs simultaneously. These mice have also shown deficits when tested on the rotarod, where the transgenic animals had a shorter latency to fall off a spinning rod than controls. We anticipate that the reduction in girdle support observed in the CatWalk is related to their decreased rotarod success, as bearing weight on the front limbs simultaneously is advantageous for staying on the rod longer. While further analysis of this transgenic line is ongoing to fully understand the associated motor deficits, these data demonstrate that the CatWalk can be an important tool in motor phenotyping to pinpoint deficits, as well as provide plausible explanation for observed deficits in other tests. Data from the CatWalk can be presented in bar graphs as shown in Figure 4, as well as line graphs for displaying data collected over time. Statistical analysis will depend on the experiment and the number of groups being analyzed by the user.
Figure 4 – Example CatWalk Data.
A. Average speed in cm/s, N = 16 per group. B. Cadence ( # steps/s), N = 16 per group. C. Sequence Regularity Index, N = 16 per group. D. Base of Support (cm), N = 16 per group. E. Percent Support Time, N = 16 per group, * p < 0.05 as tested by one-way ANOVA.
Time Considerations
As previously discussed, a 1–2 minute per day per animal acclimation period for one week prior to testing is recommended to familiarize the animals with the testing apparatus. For testing days, if the animals are freely walking across the test area and providing compliant runs, each animal can be tested in under 10 minutes, with the inclusion of clean-up time. This test can also be repeated, as many times as desired, to measure the effect of treatment over time and to conduct a pre- and post-exposure analyses. Manual print assignment can be completed in 1–2 minutes per run, depending on how many prints must be categorized.
Supplementary Material
A compliant run with a 4-month-old C57BL/6 male mouse played at 1/5 normal speed, visualized from below, with the animal walking from left to right. Paw print results displayed on lower half of the video.
ACKNOWLEDGEMENTS
Work by the authors is supported by the National Institutes of Health (R01ES028273, P42ES04696).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
LITERATURE CITED
- Abada YK, Nguyen HP, Schreiber R, & Ellenbroek B. (2013). Assessment of Motor Function, Sensory Motor Gating and Recognition Memory in a Novel BACHD Transgenic Rat Model for Huntington Disease. PLOS ONE, 8(7), e68584. 10.1371/journal.pone.0068584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AU - Sugimoto H, & AU - Kawakami K. (2019). Low-cost Protocol of Footprint Analysis and Hanging Box Test for Mice Applied the Chronic Restraint Stress. JoVE, 143, e59027. 10.3791/59027 [DOI] [PubMed] [Google Scholar]
- Baptista AF, Gomes JR de S, Oliveira JT, Santos SMG, Vannier-Santos MA, & Martinez AMB (2007). A new approach to assess function after sciatic nerve lesion in the mouse—Adaptation of the sciatic static index. Journal of Neuroscience Methods, 161(2), 259–264. 10.1016/j.jneumeth.2006.11.016 [DOI] [PubMed] [Google Scholar]
- Bernardes D, & Oliveira ALR (2017). Comprehensive catwalk gait analysis in a chronic model of multiple sclerosis subjected to treadmill exercise training. BMC Neurology, 17(1), 160. 10.1186/s12883-017-0941-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts RP, Duckworth T, Austin IG, Crocker SP, & Moore S. (1980). Critical light reflection at a plastic/glass interface and its application to foot pressure measurements. Journal of Medical Engineering & Technology, 4(3), 136–142. 10.3109/03091908009161107 [DOI] [PubMed] [Google Scholar]
- Broom L, Ellison BA, Worley A, Wagenaar L, Sörberg E, Ashton C, Bennett DA, Buchman AS, Saper CB, Shih LC, Hausdorff JM, & VanderHorst VG (2017). A translational approach to capture gait signatures of neurological disorders in mice and humans. Scientific Reports, 7(1), 3225. 10.1038/s41598-017-03336-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, & Morton AJ (1999a). Characterization of Progressive Motor Deficits in Mice Transgenic for the Human Huntington’s Disease Mutation. The Journal of Neuroscience, 19(8), 3248–3257. 10.1523/JNEUROSCI.19-08-03248.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cendelin J. (2014). From mice to men: Lessons from mutant ataxic mice. Cerebellum & Ataxias, 1. 10.1186/2053-8871-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Leung LY, Mountney A, Liao Z, Yang W, Lu X-CM, Dave J, Deng-Bryant Y, Wei G, Schmid K, Shear DA, & Tortella FC (2012). A Novel Animal Model of Closed-Head Concussive-Induced Mild Traumatic Brain Injury: Development, Implementation, and Characterization. Journal of Neurotrauma, 29(2), 268–280. 10.1089/neu.2011.2057 [DOI] [PubMed] [Google Scholar]
- Chiang C-Y, Sheu M-L, Cheng F-C, Chen C-J, Su H-L, Sheehan J, & Pan H-C (2014). Comprehensive analysis of neurobehavior associated with histomorphological alterations in a chronic constrictive nerve injury model through use of the CatWalk XT system: Laboratory investigation. Journal of Neurosurgery, 120(1), 250–262. 10.3171/2013.9.JNS13353 [DOI] [PubMed] [Google Scholar]
- Clark TA, Sullender C, Kazmi SM, Speetles BL, Williamson MR, Palmberg DM, Dunn AK, & Jones TA (2019). Artery targeted photothrombosis widens the vascular penumbra, instigates peri-infarct neovascularization and models forelimb impairments. Scientific Reports, 9(1), 2323. 10.1038/s41598-019-39092-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haas R, Russel FG, & Smeitink JA (2016). Gait analysis in a mouse model resembling Leigh disease. Behavioural Brain Research, 296, 191–198. 10.1016/j.bbr.2015.09.006 [DOI] [PubMed] [Google Scholar]
- Demler TL (2014). Drug-Induced Neurologic Conditions. US Pharmacist, 9(1), 47–51. [Google Scholar]
- Deumens R, Jaken RJP, Marcus MAE, & Joosten EAJ (2007). The CatWalk gait analysis in assessment of both dynamic and static gait changes after adult rat sciatic nerve resection. Journal of Neuroscience Methods, 164(1), 120–130. 10.1016/j.jneumeth.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Gabriel AF, Marcus MAE, Honig WMM, Walenkamp GHIM, & Joosten EAJ (2007). The CatWalk method: A detailed analysis of behavioral changes after acute inflammatory pain in the rat. Journal of Neuroscience Methods, 163(1), 9–16. 10.1016/j.jneumeth.2007.02.003 [DOI] [PubMed] [Google Scholar]
- Hamers FP, Lankhorst AJ, van Laar TJ, Veldhuis WB, & Gispen WH (2001). Automated quantitative gait analysis during overground locomotion in the rat: Its application to spinal cord contusion and transection injuries. Journal of Neurotrauma, 18(2), 187–201. 10.1089/08977150150502613 [DOI] [PubMed] [Google Scholar]
- Hamers FPT, Koopmans GC, & Joosten EAJ (2006). CatWalk-Assisted Gait Analysis in the Assessment of Spinal Cord Injury. Journal of Neurotrauma, 23(3–4), 537–548. 10.1089/neu.2006.23.537 [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, & Hayes RL (1992). Cognitive deficits following traumatic brain injury produced by controlled cortical impact. Journal of Neurotrauma, 9(1), 11–20. 10.1089/neu.1992.9.11 [DOI] [PubMed] [Google Scholar]
- Hattori Y, Kitamura A, Tsuji M, Nagatsuka K, & Ihara M. (2014). Motor and cognitive impairment in a mouse model of ischemic carotid artery disease. Neuroscience Letters, 581, 1–6. 10.1016/j.neulet.2014.08.009 [DOI] [PubMed] [Google Scholar]
- Hetze S, Römer C, Teufelhart C, Meisel A, & Engel O. (2012). Gait analysis as a method for assessing neurological outcome in a mouse model of stroke. Journal of Neuroscience Methods, 206(1), 7–14. 10.1016/j.jneumeth.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Jacobs BY, Kloefkorn HE, & Allen KD (2014). Gait analysis methods for rodent models of osteoarthritis. Current Pain and Headache Reports, 18(10), 456–456. PubMed. 10.1007/s11916-014-0456-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BY, Lakes EH, Reiter AJ, Lake SP, Ham TR, Leipzig ND, Porvasnik SL, Schmidt CE, Wachs RA, & Allen KD (2018). The Open Source GAITOR Suite for Rodent Gait Analysis. Scientific Reports, 8(1), 9797. 10.1038/s41598-018-28134-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos EA, Sieber PK, Engels PE, Mariolo AV, D’Arpa S, Schaefer DJ, & Kalbermatten DF (2017). Validity and reliability of the CatWalk system as a static and dynamic gait analysis tool for the assessment of functional nerve recovery in small animal models. Brain and Behavior, 7(7). 10.1002/brb3.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans GC, Deumens R, Honig WMM, Hamers FPT, Steinbusch HWM, & Joosten EAJ (2005). The Assessment of Locomotor Function in Spinal Cord Injured Rats: The Importance of Objective Analysis of Coordination. Journal of Neurotrauma, 22(2), 214–225. 10.1089/neu.2005.22.214 [DOI] [PubMed] [Google Scholar]
- Lakes EH, & Allen KD (2016). Gait analysis methods for rodent models of arthritic disorders: Reviews and recommendations. Osteoarthritis and Cartilage, 24(11), 1837–1849. 10.1016/j.joca.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisman G, Moustafa AA, & Shafir T. (2016). Thinking, Walking, Talking: Integratory Motor and Cognitive Brain Function. Frontiers in Public Health, 4. 10.3389/fpubh.2016.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricelli JW, Lu QL, Lin DC, & Rodgers BD (2016). Trendelenburg-Like Gait, Instability and Altered Step Patterns in a Mouse Model for Limb Girdle Muscular Dystrophy 2i. PLOS ONE, 11(9), e0161984. 10.1371/journal.pone.0161984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CS, Bartos I, Márka Z, Akay T, Márka S, & Mann RS (2015). Quantification of gait parameters in freely walking rodents. BMC Biology, 13(1), 50. 10.1186/s12915-015-0154-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS (2015). Social modulation of and by pain in humans and rodents. PAIN, 156. https://journals.lww.com/pain/Fulltext/2015/04001/Social_modulation_of_and_by_pain_in_humans_and.7.aspx [DOI] [PubMed] [Google Scholar]
- Neumann M, Wang Y, Kim S, Hong SM, Bilgen M, & Liu J. (2009). Assessing gait impairment following experimental traumatic brain injury in mice. Journal of Neuroscience Methods, 176(1), 34–44. 10.1016/j.jneumeth.2008.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Fromholt SE, Hess EJ, Crawford TO, Griffin JW, Sheikh KA, & Schnaar RL (2005). Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate axon stability in the CNS and PNS: Neuropathology and behavioral deficits in single- and double-null mice. Experimental Neurology, 195(1), 208–217. 10.1016/j.expneurol.2005.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen S, Ortega FJ, Kuptsova K, Huttunen J, Tarkka I, & Jolkkonen J. (2013). Gait Impairment in a Rat Model of Focal Cerebral Ischemia. Stroke Research and Treatment, 2013, 410972. 10.1155/2013/410972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JE, Cabrita AM, Filipe VM, Bulas-Cruz J, Couto PA, Melo-Pinto P, Costa LM, Geuna S, Maurício AC, & Varejão ASP (2006). A comparison analysis of hindlimb kinematics during overground and treadmill locomotion in rats. Behavioural Brain Research, 172(2), 212–218. 10.1016/j.bbr.2006.04.027 [DOI] [PubMed] [Google Scholar]
- Robinson L, Guy J, McKay L, Brockett E, Spike RC, Selfridge J, De Sousa D, Merusi C, Riedel G, Bird A, & Cobb SR (2012). Morphological and functional reversal of phenotypes in a mouse model of Rett syndrome. Brain, 135(9), 2699–2710. 10.1093/brain/aws096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguiz RM, & Wetsel WC (2006). Assessments of Cognitive Deficits in Mutant Mice. In Levin ED & Buccafusco JJ (Eds.), Animal Models of Cognitive Impairment. CRC Press; /Taylor & Francis. http://www.ncbi.nlm.nih.gov/books/NBK2527/ [PubMed] [Google Scholar]
- Saunders NR, Dziegielewska KM, Whish SC, Hinds LA, Wheaton BJ, Huang Y, Henry S, & Habgood MD (2017). A bipedal mammalian model for spinal cord injury research: The tammar wallaby. F1000Research, 6. 10.12688/f1000research.11712.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed-Zahid AA, Sher RB, Sukoff Rizzo SJ, Anderson LC, Patenaude KE, & Cox GA (2019). Functional rescue in a mouse model of congenital muscular dystrophy with megaconial myopathy. Human Molecular Genetics, 28(16), 2635–2647. 10.1093/hmg/ddz068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HJ, LeBold DG, Marklund N, Morales DM, Hagner AP, & McIntosh TK (2006). Cognitive evaluation of traumatically brain-injured rats using serial testing in the Morris water maze. Restorative Neurology and Neuroscience, 24(2), 109–114. [PMC free article] [PubMed] [Google Scholar]
- van der Zee CEEM, Schuurman T, Traber J, & Gispen WH (1987). Oral administration of nimodipine accelerates functional recovery following peripheral nerve damage in the rat. Neuroscience Letters, 83(1), 143–148. 10.1016/0304-3940(87)90231-X [DOI] [PubMed] [Google Scholar]
- Walter J, Kovalenko O, Younsi A, Grutza M, Unterberg A, & Zweckberger K. (2020). The CatWalk XT® is a valid tool for objective assessment of motor function in the acute phase after controlled cortical impact in mice. Behavioural Brain Research, 392, 112680. 10.1016/j.bbr.2020.112680 [DOI] [PubMed] [Google Scholar]
- Wang XH, Lu G, Hu X, Tsang KS, Kwong WH, Wu FX, Meng HW, Jiang S, Liu SW, Ng HK, & Poon WS (2012). Quantitative assessment of gait and neurochemical correlation in a classical murine model of Parkinson’s disease. BMC Neuroscience, 13(1), 142. 10.1186/1471-2202-13-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Sater S, Burkhalter C, Schoonen S, Miller J, Shrestha D, Brumley MR, & Schiele NR (2020). Low-cost, open-source, variable speed and incline treadmill for studying impacts of neonatal locomotion. HardwareX, 7, e00097. 10.1016/j.ohx.2020.e00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Valkenburg F, Hornix BE, Mantingh-Otter I, Zhou X, Mari M, Reggiori F, Van Dam D, Eggen BJL, De Deyn PP, & Boddeke E. (2017). Progressive Motor Deficit is Mediated by the Denervation of Neuromuscular Junctions and Axonal Degeneration in Transgenic Mice Expressing Mutant (P301S) Tau Protein. Journal of Alzheimer’s Disease: JAD, 60(s1), S41–S57. 10.3233/JAD-161206 [DOI] [PubMed] [Google Scholar]
- Zhang C, Li H, & Han R. (2020). An open-source video tracking system for mouse locomotor activity analysis. BMC Research Notes, 13(1), 48. 10.1186/s13104-020-4916-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Younsi A, Scherer M, Riemann L, Walter J, Skutella T, Unterberg A, & Zweckberger K. (2021). The CatWalk XT® Gait Analysis Is Closely Correlated with Tissue Damage after Cervical Spinal Cord Injury in Rats. Applied Sciences, 11(9), 4097. 10.3390/app11094097 [DOI] [Google Scholar]
- Zhou M, Zhang W, Chang J, Wang J, Zheng W, Yang Y, Wen P, Li M, & Xiao H. (2015). Gait analysis in three different 6-hydroxydopamine rat models of Parkinson’s disease. Neuroscience Letters, 584, 184–189. 10.1016/j.neulet.2014.10.032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A compliant run with a 4-month-old C57BL/6 male mouse played at 1/5 normal speed, visualized from below, with the animal walking from left to right. Paw print results displayed on lower half of the video.