Abstract
Background:
We previously introduced the concept of “two-stage” (or “just-in-time”) informed consent for randomized trials with usual care control. We argued that conducting consent in two stages - splitting information about research procedures from information about the experimental intervention - would reduce the decisional anxiety, confusion and information overload commonly associated with informed consent. We implemented two-stage consent in a low-stakes randomized trial of a mindfulness meditation intervention for procedural distress in patients undergoing prostate biopsy. Here we report accrual rates and patient understanding of the consent process.
Methods:
Patients approached for consent for the biopsy trial were asked to complete the standard “Quality of Informed Consent” (QuIC) questionnaire to assess their knowledge and understanding of the trial.
Results:
Accrual was excellent with 108 of 110 (98%) of patients approached for consent signing first-stage consent. All 51 patients randomized to the experimental arm and who later presented for biopsy signed second stage consent and received the mindfulness intervention. QuIC data were available for 48 patients assigned to the mindfulness treatment arm and 44 controls. The mean QuIC score was similar in the meditation and control arms with and overall mean of 75 (95% C.I. 74, 76) for the knowledge section and 86 (95% C.I. 81, 90) for understanding, comparable to the normative scores of 80 and 88. On further analysis and patient interview, two of the QuIC questions were found to be misleading in the context of a two-stage consent study for a mindfulness intervention. Excluding these questions increased knowledge scores to 88 (95% C.I. 87, 90)).
Conclusions:
We found promising data that two-stage consent facilitated accrual without compromising patient understanding of randomized trials or compliance with allocated treatment. Further research is needed incorporating randomized comparison of two-stage consent to standard consent approaches, patient anxiety and distress as outcomes, suitable modifications to the QuIC questionnaire and trials with higher stakes.
Keywords: Randomized controlled trials, informed consent, anxiety, decisional burden, information overload
Introduction
In a prior paper in Clinical Trials,1 we introduced the concept of “just-in-time” informed consent for randomized trials with usual care control (figure 1). Now more typically described as “two-stage” consent, patients are first informed that they are being invited to join a study and told about research procedures, such as questionnaires. They are then told that they might later be randomly selected to hear about an experimental treatment and, if so, they can decide at that time whether to try it or opt for standard care. Patients who sign the first stage of consent and are randomized to control receive standard of care treatment. Those randomized to the experimental arm are informed about the investigational treatment and are asked to sign a second consent. Following the intent-to-treat principle, patients are analyzed in the experimental arm irrespective of their decision at that second stage.
Figure 1.
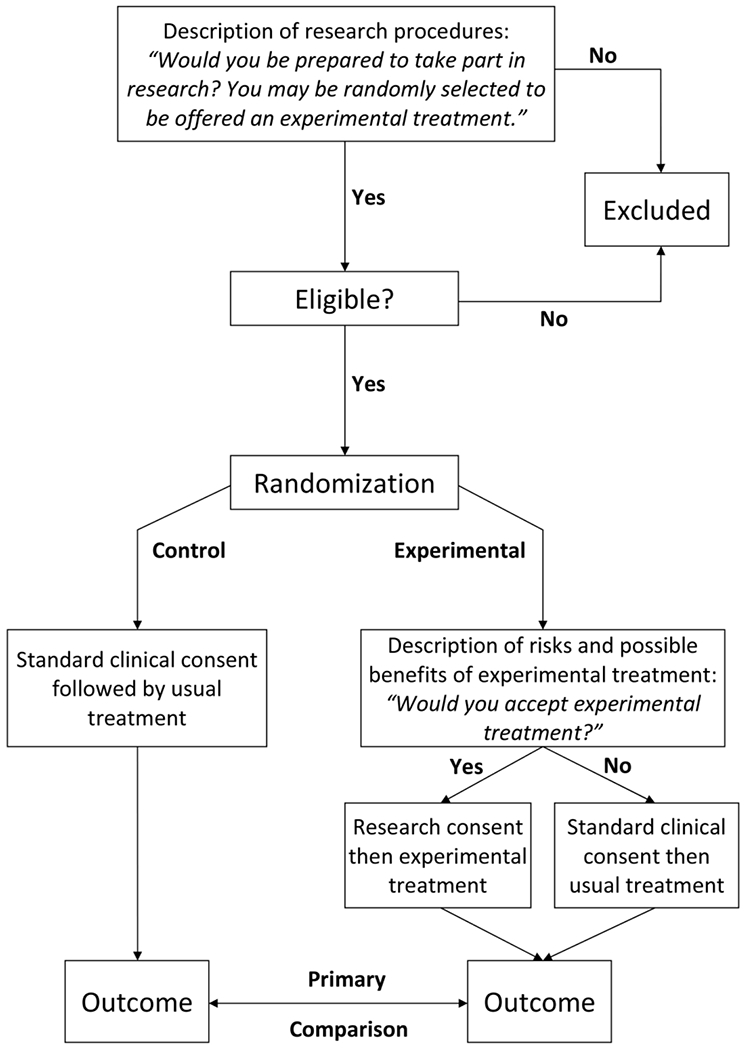
Flow chart of two-stage consent design
Splitting the consent process in two to deal with each type of information separately has several advantages, notably that only patients randomized to the experimental treatment are subject to a discussion of that intervention. We hypothesized that this would reduce much of the burden associated with the consent process, such as decisional anxiety, confusion and information overload. We might also hypothesize that lowering patient distress would improve accrual, on the grounds that investigators are less willing to approach patients about trials when consent discussions are difficult.
In our original proposal, we pointed out that two-stage consent was an “empirically testable model” and that “research should be conducted on the patient-experience”. Moreover, in response to a published critique making the point that refusal at second stage consent would lead to a dramatic loss in statistical power,2 we argued3 that pilot studies could provide data on the statistical properties of the design. Here we report on such a pilot study of two-stage consent, giving data on accrual rates and patient understanding of consent.
Methods
Two-stage consent was implemented in a randomized trial of a mindfulness meditation intervention for procedural distress in patients undergoing prostate biopsy (‘mindfulness study’). Despite the use of periprostatic nerve block, prostate biopsy can be uncomfortable, with approximately 1 in 3 men reporting moderate or severe discomfort.4, 5 Guided meditation has been shown to significantly reduce anxiety, pain, and fatigue during imaging-guided breast biopsy.6 We therefore considered whether this technique would be of benefit for prostate biopsy.
Patients being managed by active surveillance for low-risk prostate cancer undergo regular biopsies to determine if the cancer has progressed. We approached patients for consent in this randomized trial typically six months before a scheduled biopsy. At our institution, patients are given questionnaires as to their experience of the biopsy as a routine part of clinical care: 0 – 10 numerical rating scales for pain, anxiety, discomfort, and tolerability. These data are used clinically to assess the need for postprocedural medication. Hence, in the first stage of consent, potential trial participants were told that the trial did not require additional tests, procedures or questionnaires and were simply giving permission to investigators to use their routinely collected data for research purposes. Patients were also told that if they agree to take part, they may be selected at random to be offered “an experimental approach aimed at improving the experience of biopsy”. If so, they would receive further information and could decide at that time of whether to accept the experimental option or receive usual care. Randomization took place shortly after this first stage consent. Patients randomized to control had no further conversations about research. Those randomized to the experimental arm were told of their allocation when presenting for biopsy. The experimental intervention involved headphones connected to an audio player in the clinic area to listen to a 10-minute pre-biopsy mindfulness exercise and then a second 10-minute exercise that guides the patient through the mindfulness intervention during the biopsy procedure. Patients in the experimental arm were asked whether they wanted the mindfulness intervention or to receive biopsy without the listening exercises.
All patients received the “Quality of Informed Consent” (QuIC) survey within 48 hours of their decision whether or not to take part or, for patients who accepted and were randomized to two-stage consent, 48 hours of their second consent, which took place shortly before biopsy. The QuIC is a validated instrument7 with two sections. Part A contained 12 knowledge questions with the answer options of “agree”, “disagree” or “unsure” to items such as “I will have to remain in the clinical trial even if I decide someday that I want to withdraw” (correct answer “disagree”) or “There may not be direct medical benefit to me from my participation in this clinical trial” (correct answer “agree”). Part B consisted of 7 subjective understanding questions scored on a Likert scale from 1 (“Didn’t understand this at all”) to 5 (“Understood this very well”) in response to statements such as: “When you signed the consent form, how well did you understand … why the researchers are doing the clinical trial?” Both section A and section B are scored on a scale of 0 – 100, with higher scores indicating a better knowledge or greater subjective understanding of the consent process.
Results
A total of 110 patients in the pilot study were approached for the first stage of consent, with 107 agreeing and signing consent straight away. One patient wanted to consider it further and signed consent after a week’s consideration and two patients refused immediately: one did not want to participate in research and the other was concerned with privacy. This gives a 98% (108/110) consent rate. Four patients in each arm did not present for biopsy before this study was closed for analysis. All 51 presenting for biopsy in the experimental arm signed second stage consent and received the experimental intervention.
There were missing QuIC data from 16 patients, leaving 92 patients in the analysis, 48 assigned to the mindfulness treatment arm and 44 controls. Table 1 shows the QuIC scores separately by group. Scores were similar among both the treatment and control groups with an overall mean was 75 (95% C.I. 74, 76) for section A and 86 (95% C.I. 81, 90) for section B. These are comparable to the normative scores reported for the QuIC7: 80 for section A and 88 for section B.
Table 1.
The mean and 95% CI for the total scores in section A and section B, for the entire cohort and separately by treatment group. The sensitivity analysis excludes questions 3 and 5 from section A.
| Group | Mean (95% CI) |
|---|---|
| QuIC Section A | |
| All Patients | 75 (74, 76) |
| Mindfulness Group | 75 (73, 77) |
| Control Group | 75 (73, 77) |
| QuIC Section A (Sensitivity Analysis) | |
| All Patients | 88 (87, 90) |
| Mindfulness Group | 88 (86, 90) |
| Control Group | 89 (86, 92) |
| QuIC Section B | |
| All Patients | 86 (81, 90) |
| Mindfulness Group | 88 (83, 92) |
| Control Group | 84 (76, 91) |
On further analysis of the responses to individual items (see table 2 and 3) we noted an unusual pattern of responses to two questions, with 70 – 90% of patients reporting “agree” to two questions where the correct answer was “disagree”: question 3 “all the interventions and procedures in my clinical trial are standard for my type of prostate biopsy procedure” and question 5 “in the trial, the researchers will look at treatments that have been proven to be the best treatment for pain and discomfort during prostate biopsy”. Given these unexpected findings, we conducted some informal interviews with a small number of patients. Patients in the control group provided feedback that they never heard about any experimental treatments and had looked at what happened to them during the biopsy, such as getting local anesthesia, and thought that it was standard. Patients in the experimental group said that they thought “interventions and procedures” and “treatment for pain and discomfort” referred to the local anesthesia and other aspects of the biopsy rather than the meditation intervention. Accordingly, we conducted a sensitivity analysis excluding the answers to questions 3 and 5 from the total score calculation. Scores were higher than when including all questions (88, 95% C.I. 87, 90) and similar between the mindfulness intervention group and the control group.
Table 2.
Section A responses in the full cohort.
| Question | Agree | Unsure | Disagree | Unknown |
|---|---|---|---|---|
| A1. When I signed the consent form to take part in research, I knew that I was agreeing to participate in a clinical trial. | 89 (98%) | 1 (1.1%) | 1 (1.1%) | 1 |
| A2. The main reason clinical trials are done is to improve well-being of future patients. | 91 (99%) | 1 (1.1%) | 0 (0%) | 0 |
| A3. All the interventions and procedures in my clinical trial are standard for my type of prostate biopsy procedure. | 70 (77%) | 21 (23%) | 0 (0%) | 1 |
| A4. In my clinical trial, one of the researchers’ major purposes is to find out ways to improve prostate biopsy. | 82 (92%) | 7 (7.9%) | 0 (0%) | 3 |
| A5. In the trial, the researchers will look at treatments that have been proven to be the best treatment for pain and discomfort during prostate biopsy. | 82 (89%) | 10 (11%) | 0 (0%) | 0 |
| A6. After I agreed to participate in my clinical trial, I had a chance of being randomly selected to find out about a new treatment. | 65 (71%) | 19 (21%) | 7 (7.7%) | 1 |
| A7. There may not be direct medical benefit to me from my participation in this clinical trial. | 76 (84%) | 13 (14%) | 2 (2.2%) | 1 |
| A8. By participating in this clinical trial, I am helping the researchers learn information that may benefit future patients undergoing prostate biopsy. | 90 (98%) | 2 (2.2%) | 0 (0%) | 0 |
| A9. Because I am participating in a clinical trial, it is possible that the study sponsor, various government agencies, or others who are not directly involved in my care could review my medical records. | 67 (74%) | 16 (18%) | 8 (8.8%) | 1 |
| A10. The consent form I signed lists the name of the person (or persons) whom I should contact if I have any questions or concerns about the clinical trial. | 65 (71%) | 27 (29%) | 0 (0%) | 0 |
| A11. If I had not wanted to participate in this clinical trial, I could have declined to sign the consent form. | 89 (97%) | 3 (3.3%) | 0 (0%) | 0 |
| A12. I will have to remain in the clinical trial even if I decide someday that I want to withdraw. | 32 (35%) | 23 (25%) | 36 (40%) | 1 |
Table 3.
Section B responses in the full cohort.
| Question | Didn’t understand this at all | Didn’t understand this somewhat | Neither understand/did not understand | Understood this somewhat | Understood this very well | Unknown |
|---|---|---|---|---|---|---|
| B1. The fact that you may be offered a new treatment to your prostate biopsy | 12 (14%) | 0 (0%) | 12 (14%) | 10 (12%) | 51 (60%) | 7 |
| B1. Control group (n=44) | 7 (17%) | 0 (0%) | 7 (17%) | 7 (17%) | 20 (49%) | 3 |
| B2. Mindfulness group (n=48) | 5 (11%) | 0 (0%) | 5 (11%) | 3 (6.8%) | 31 (70%) | 4 |
| B2. Why the researchers are doing the clinical trial | 3 (3.4%) | 0 (0%) | 3 (3.4%) | 12 (14%) | 70 (80%) | 4 |
| B3. How your participation in this clinical trial may benefit future patients | 2 (2.2%) | 0 (0%) | 6 (6.7%) | 7 (7.9%) | 74 (83%) | 3 |
| B4. The effect of the clinical trial on the confidentiality of your medical records | 4 (4.7%) | 0 (0%) | 14 (16%) | 11 (13%) | 56 (66%) | 7 |
| B5. Whom you should contact if you have questions or concerns about the clinical trial | 8 (9.2%) | 0 (0%) | 11 (13%) | 9 (10%) | 59 (68%) | 5 |
| B6. The fact that participation in the clinical trial is voluntary | 2 (2.2%) | 0 (0%) | 0 (0%) | 2 (2.2%) | 85 (96%) | 3 |
| B7. Overall, how well did you understand your clinical trial when you signed the consent form? | 3 (3.4%) | 0 (0%) | 10 (11%) | 13 (15%) | 62 (70%) | 4 |
In section B, scores were lowest for question 1 (“The fact that you may be offered a new treatment to your prostate biopsy”), with only 61 patients (66%) reporting that they understood this “somewhat” or “very well”. There is, as expected, poorer understanding in the control group for item B1, which concerns the experimental treatment.
The investigator (BE) who approached patients for consent, reported that patients readily understood the design and were not confused or annoyed by any aspect. In particular, one concern was that patients might insist on knowing what the experimental intervention was before signing the first consent. But this was not found to be the case. Only a few patients enquired about the experimental intervention and were readily reassured by being given a restatement of the design. The consenting investigator also reported that the consent discussion was without the confusion and anxiety often associated with clinical trial consent and that this increased his motivation to approach patients about the trial on busy clinic days.
Discussion
We have shown good properties of two-stage consent in a first implementation of the design. Accrual and compliance rates were excellent with 98% of patients agreeing to randomization and no patient refusing second-stage consent. Patient knowledge and subjective understanding of research, as evaluated using a standardized questionnaire, were comparable to normative data in both arms, suggesting that two-stage consent maintains patient understanding of research comparing to traditional one-stage consent.
In the light of our findings, we suggest some modifications of the QuIC questionnaire when applied to a study of a behavioral intervention with two-stage consent. First, control group patients will rationally agree that “interventions and procedures are standard” and that treatments “have been proven” because they are not told about an experimental intervention. Second, patients may not see mindfulness as a “treatment”, and therefore be confused by a question asking whether they understood that the trial involved a new treatment. We have therefore modified the QuIC to remove questions A3 and A5 and modified B1 to make reference to “new way to make prostate biopsy less uncomfortable”.
Although we are the first group to assess patient understanding of two-stage consent, supportive data on accrual come from what is known as the “Trials Within Cohorts” (TWiCs) design, where randomized trials are conducted on an existing cohort. TWiCs studies by necessity use what is effectively a two-stage consent approach.8 In the ReFOrM trial, ~2300 patients first agreed to join a cohort of community-dwelling older adults who had experienced falls: 1010 met eligibility criteria for a trial and were randomized; fewer than 10% of those allocated to a multifaceted podiatry intervention refused.9 A second TWiCs trial in a cohort of depressed patients had much higher refusal at second-stage consent (50%), perhaps attributable to the unusual and controversial nature of the experimental intervention (homeopathy) and the attempt to consent by telephone rather than in person. Interestingly, compliance with outcome assessment was much higher than expected, attributed to the lack of a “disappointment effect”, where patients randomized to the control group lose interest in the trial.10, 11
There are three principal limitations of the study. First, we deliberately chose to pilot the two-stage design in a low-stakes setting, on the grounds that it would mitigate harms if the approach turned out not to be effective at informing patients adequately. However, the low-stakes setting also entails that our results might not fully translate to a trial where the stakes are higher. For instance, we had 100% acceptance at the second stage of consent because the intervention is safe, convenient and pleasant. This might not be the case if the experimental intervention was not as benign as a listening exercise, if it was toxic or inconvenient or was given instead of standard care. The second limitation is that the consenting investigator (BE) was an author on the original proposal for two-stage consent and is a strong supporter of the approach. Our very high accrual rates might therefore not be replicated by less motivated investigators. The third limitation was that the QuIC questionnaire included items that were problematic in the context of two-stage consent.
In conclusion, we found promising data that two-stage consent facilitated accrual without compromising patient understanding of research or compliance with allocated treatment. Further research is needed incorporating randomized comparison to standard consent, measuring patient anxiety and distress as an outcome, using suitable modifications to the QuIC questionnaire and in trials with higher stakes.
Acknowledgement
We sincerely thank clinical research study coordinators and managers Sabrina Falcigno, Anna Whalen, and Nicole Benfante in the Department of Surgery (Urology Service) at Memorial Sloan Kettering Cancer Center for their assistance with the conduit of the study and data collection. We thank Robin Hardbattle, mind-body therapist in the Integrative Medicine Service at Memorial Sloan Kettering Cancer Center, for assistance with developing the mindfulness intervention used in the study.
Funding
This work was supported in part by the National Institutes of Health/National Cancer Institute (NIH/NCI) with a Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center [P30 CA008748], a SPORE grant in Prostate Cancer to Dr. H. Scher [P50-CA92629], a PCORI grant [ME-2018C2-13253], the Sidney Kimmel Center for Prostate and Urologic Cancers and David H. Koch through the Prostate Cancer Foundation. S.V.C. was further supported by a career development award from the National Institutes of Health/National Cancer Institute [K22-CA234400].
Footnotes
Conflicts of interest
Andrew Vickers is named on a patent for a statistical method to detect prostate cancer that has been commercialized by OPKO Health. Andrew Vickers receives royalties from sales of the test and has stock options in OPKO Health.
References
- 1.Vickers AJ, Young-Afat DA, Ehdaie B, et al. Just-in-time consent: The ethical case for an alternative to traditional informed consent in randomized trials comparing an experimental intervention with usual care. Clin Trials 2018; 15: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chappell R Comment on Vickers et al. Clin Trials 2019; 16: 214–215. [DOI] [PubMed] [Google Scholar]
- 3.Vickers AJ and Ehadaie B. Response to Chappell. Clin Trials 2019; 16: 216. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez LV and Terris MK. Risks and complications of transrectal ultrasound guided prostate needle biopsy: a prospective study and review of the literature. J Urol 1998; 160: 2115–2120. [DOI] [PubMed] [Google Scholar]
- 5.Naughton CK, Miller DC and Yan Y. Impact of transrectal ultrasound guided prostate biopsy on quality of life: a prospective randomized trial comparing 6 versus 12 cores. J Urol 2001; 165: 100–103. [DOI] [PubMed] [Google Scholar]
- 6.Soo MS, Jarosz JA, Wren AA, et al. Imaging-Guided Core-Needle Breast Biopsy: Impact of Meditation and Music Interventions on Patient Anxiety, Pain, and Fatigue. J Am Coll Radiol 2016; 13: 526–534. [DOI] [PubMed] [Google Scholar]
- 7.Joffe S, Cook EF, Cleary PD, et al. Quality of informed consent: a new measure of understanding among research subjects. J Natl Cancer Inst 2001; 93: 139–147. [DOI] [PubMed] [Google Scholar]
- 8.Kim SY, Flory J and Relton C. Ethics and practice of Trials within Cohorts: An emerging pragmatic trial design. Clin Trials 2018; 15: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cockayne S, Rodgers S, Green L, et al. Clinical effectiveness and cost-effectiveness of a multifaceted podiatry intervention for falls prevention in older people: a multicentre cohort randomised controlled trial (the REducing Falls with ORthoses and a Multifaceted podiatry intervention trial). Health Technol Assess 2017; 21: 1–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viksveen P, Relton C and Nicholl J. Benefits and challenges of using the cohort multiple randomised controlled trial design for testing an intervention for depression. Trials 2017; 18: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viksveen P, Relton C and Nicholl J. Depressed patients treated by homeopaths: a randomised controlled trial using the “cohort multiple randomised controlled trial” (cmRCT) design. Trials 2017; 18: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]


