Abstract
The Asian ginseng root (Panax ginseng C.A. Meyer) is a very commonly used herbal medicine worldwide. Ginseng fruit, including the berry (or pulp) and seed, is also valuable for several health conditions including immunostimulation and cancer chemoprevention. In this study, the anticancer and anti-proliferative effects of the extracts of ginseng berry and seed were evaluated. The ginsenosides in the ginseng berry concentrate (GBC) and ginseng seed extract (GSE) were analyzed. We then evaluated their anti-colorectal cancer potentials, including antiproliferation, cell cycle arrest, and apoptotic induction. Further investigation consisted of the berry’s adaptive immune responses, such as the actions on the differentiation of T helper cells Treg, Th1, and Th17. The major constituents in GBC were ginsenosides Re and Rd, which can be compared to those in the root. The GBC significantly inhibited colon cancer cell growth, and its anti-proliferative effect involved mechanisms including G2/M cell cycle arrest via upregulation of cyclin A and induction of apoptosis via regulation of apoptotic related gene expressions. GBC also downregulated the expressions of pro-inflammatory cytokine genes. For the adaptive immune responses, GBC did not influence Th1 and Treg cell differentiation but significantly inhibited Th17 cell differentiation and thus regulated the balance of Th17/Treg for adaptive immunity. Although no ginsenoside was detected in the GSE, interestingly, it obviously enhanced colon cancer cell proliferation with the underlined details to be determined. Our results suggested that GBC is a promising dietary supplement for cancer chemoprevention and immunomodulation.
Keywords: Panax ginseng berry and seed, anticancer, cell cycle, apoptosis, anti-inflammation, adaptive immunity, T helper 17 cells (Th17)
INTRODUCTION
Asian ginseng is a very frequently used herbal medicine in the East and beyond. The root of the ginseng has been used for centuries in Oriental medicine for maintaining general well-being and dealing with various medical conditions (1). It is generally considered that ginsenosides are responsible for beneficial effects of the roots (2–4). Using Asian ginseng, a previous report in Korean subjects disclosed that regular ginseng root consumers had an obviously decreased risk for different cancers compared with the non-consumers (5, 6). Our group previously reported chemopreventive effects of ginseng root on colorectal cancer (CRC) in different animal models (7, 8). In addition to the traditionally used ginseng root, modern phytochemical and biomedical studies exhibited that ginseng fruit, containing both berry and seed, also possessed therapeutic activities (9–11).
Ginseng fruit is precious to the survival of the Panax species and is also valuable for several health conditions. A study published in 2002 discovered that ginseng berry normalized blood glucose levels by improving sensitivity to insulin, lowering cholesterol levels, and decreasing weight in obese diabetic mice (12). A human clinical trial tested the effects of ginseng berry in 119 males with mild to moderate erectile dysfunction, and the data indicated that the berry improved men’s sexual function as an alternative treatment for such cases (13). Recently, immunostimulating and antimetastatic activities of ginseng berry polysaccharide have been reported (14). Using the berry polysaccharide extract, we assessed its antiproliferation and anti-inflammation effects on human CRC cells (11). Alternatively, several ginseng seed studies analyzed chemical constituents and observed its anti-inflammation activity (10, 15, 16). However, compared with the abundant ginseng root investigations, research on ginseng fruit is still fairly new, and further studies will not only help us to better understand its full potential but also elucidate the mechanisms of action.
In previous studies, organic solvents were used to prepare ginseng berry extract, and the extract was subsequently administered to the experimental animals via parenteral routes (11, 12). For the chemically processed extract, the preparation requires a number of steps to accomplish this, and some chemical residues could still exist, especially when the extract was not carefully made. If ginseng berry can be useful as a human dietary supplement, a simple preparation for its oral intake would be a convenient, safe, and practical means to the consumers. Additionally, the effects of ginseng seed, as another portion of ginseng fruit should be evaluated in comparison to those observed from the berry.
In this study, we simplified ginseng berry preparation to yield ginseng berry concentrate. The ginsenosides in the berry concentrate were subsequently analyzed, and its anti-CRC effects were tested, including the actions on cell proliferation, cell cycle arrest, and apoptosis induction. Furthermore, previous observations showed that chronic bowel inflammation is recognized as a risk factor for tumor development, including the CRC (17, 18), and data suggested that anti-inflammatory botanicals suppressed colitis and blocked the cancer through chemoprevention (8, 19). Thus, in this study, we also evaluated the inhibitory effects on inflammation-linked Th17 cell differentiation to elucidate the berry’s mechanisms of action. Further, to compare the anti-CRC effects of ginseng berry concentrate, we prepared and analyzed ginseng seed that had undergone stratification during its sprouting, to observe the effects of other nutrients in the seed on human CRC cells with no ginsenosides from this preparation.
MATERIALS AND METHODS
Chemicals
The chemicals used were all for high performance liquid chromatography or HPLC grade. Plasticware was purchased from Falcon Labware (Franklin Lakes, NJ, USA). Test media, insulin trypsin, glutamine, and buffered saline were purchased from Mediatech (Herndon, VA, USA). Antibiotics, such as streptomycin and penicillin, were purchased from Sigma (St. Louis, MO, USA). For cell proliferation study, the MTS kit was purchased from Promega (Madison, WI, USA). Cell cycle staining buffer was purchased from Biosciences Pharmingen (San Diego, CA, USA), and cell apoptosis detection kit was purchased from Biosciences (Rockville, MD, USA).
Botanical materials and plant extraction
AmorePacific Co. (Seoul, South Korea) provided us the ginseng berry concentrate (GBC) and ginseng seed extract (GSE). Asian ginseng (Panax ginseng) berries were harvested and washed. Ginseng seeds were separated from the berry. Ginseng berry pulp was homogenized, and the berry juice was separated with centrifuge and then concentrated to 20 brix. After filtration, the berry juice was further concentrated to 70 brix and sterilized at 90–95°C. Finally, the berry juice was freeze-dried to obtain GBC. For the GSE, ginseng seeds were washed and maintained in moisture for stratification. The seeds were grounded and extracted with 50% ethanol at 85°C. After filtration, the extraction solution was collected and concentrated to 15 brix. The condensed solution was sterilized at 90–95°C. At last, the solution was freeze-dried to obtain GSE. The extraction process for GBC and GSE was shown in Fig. 1A.
Fig. 1.
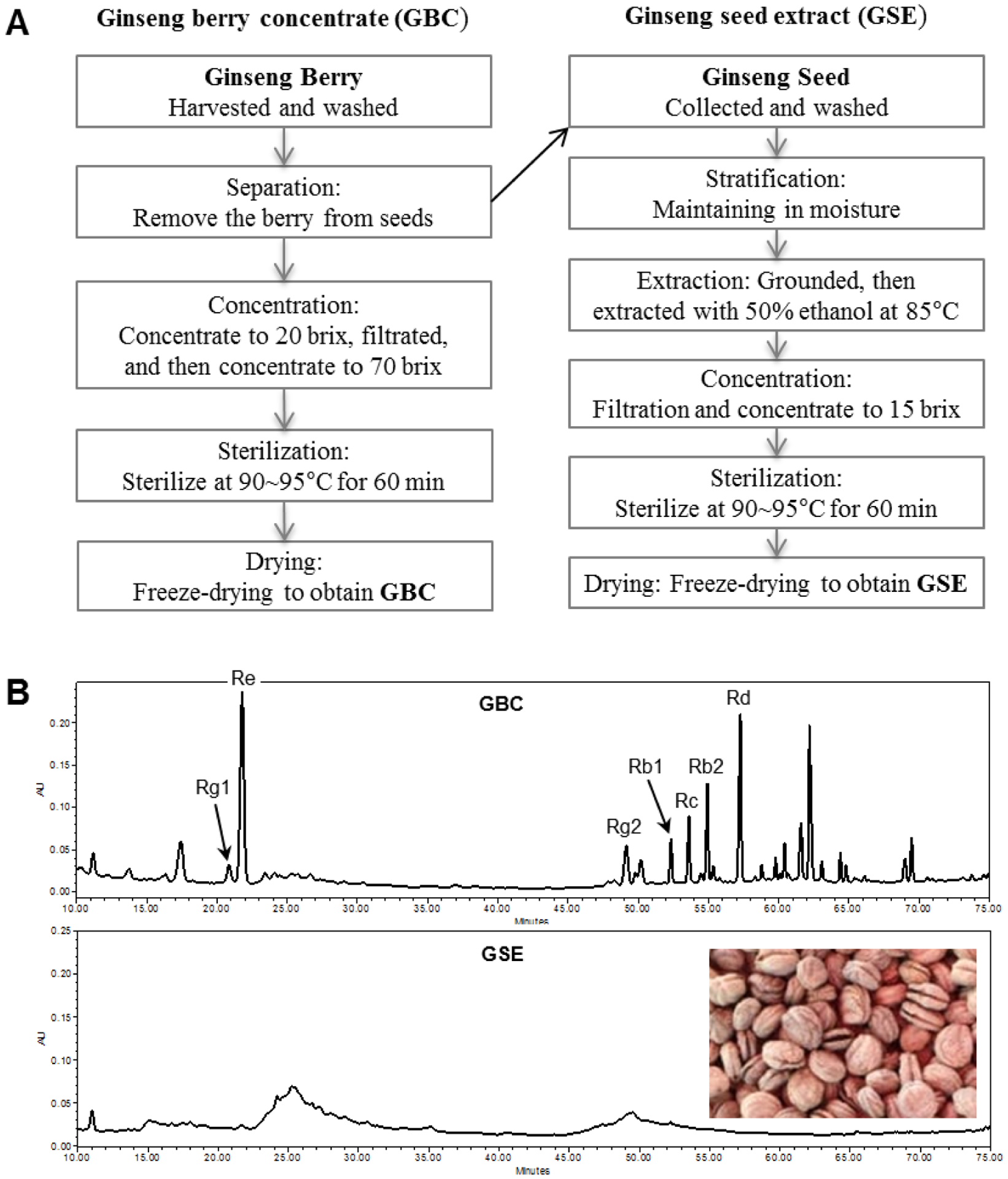
Preparation and HPLC ginsenoside analysis of ginseng berry concentrate (GBC) and ginseng seed extract (GSE). [A]: Flow charts for preparation procedures of GBC and GSE. [B]: HPLC chromatograms of GBC and GSE detected in 202 nm. In the GBC panel, peaks for representative ginsenosides Rg1, Re, Rg2, Rb1, Rc, Rb2, and Rd have been indicated. The peak areas, consistent with responsible compound contents, for Re and Rd are very high, while for Rb1 is relatively low. In the GSE panel, an image for the stratification treated seeds is displayed, and no ginsenoside was detected.
HPLC analysis
Our laboratory uses a Waters instrument 2965 (Milford, MA, USA) for HPLC analysis. This system (Model 996) was used for constituent peak identification and integration, supported by a Waters Empower software. Detailed procedures were reported in our previous publications (8, 20).
Cancer cell lines and cell culture
HCT-116 and HT-29 human colon cancer cells were purchased from American Type Culture Collection (Manassas, VA, USA). HCT-116 is a p53 wild type, whereas HT-29 cells contain a p53 mutation. These cells were grown in McCoy’s 5A medium with 10% FBS and 50 IU streptomycin-penicillin in the humidified air at 37°C with 5% CO2.
Cell proliferation analysis
The solvent used to dissolve GBE and GSE were 50% DMSO. The 96-well plates were used to seed cells and test response to drug treatment. Drug treatment durations were 24, 48, or 72 h. MTS assay was employed to determine cell proliferation. As our previous publications, absorbance was recorded at 490 nm (11, 21).
Cell cycle/cyclin A assays
HCT-116 cells were seeded on 24-well plates. On day 2, the medium was changed, during which different GBC and GSE concentrations were used to treat the cells. After 48 h, we harvested the cells and fixed them with 80% ethanol. After 0.25% Triton X-100 treatment, we resuspended the cells in PBS containing PI, RNase, and cyclin A-FITC. We used FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA) and FlowJo 10.7.1 software (Tree Star, Ashland, OR, USA) to assay cell cycle and cyclin A. At least 10,000 cells were recorded for each assay.
Apoptosis analysis
We seeded HCT-116 cells on 24-well plates. On day 2, we changed the medium, during which different GBC and GSE concentrations were used to treat the cells. After 48 h, we harvested both floating and adherent cells. We stained the cells with PI and annexin V. We used FACScan flow cytometer to assay apoptotic cells. At least 20,000 cells were recorded for each assay.
RNA extraction and qPCR analysis
Using HCT-116 cells and qPCR, the expression of apoptotic genes was analyzed after treatment with GBC/GSE for 24 h. For inflammatory cytokine analysis, HT-29 cells were cultured for 48 h. Fresh medium containing lipopolysaccharide (LPS, 10 ng/ml) was added, which served as a control. GBC or GSE and LPS were added in the medium and treatment time was set for 6 h. The RNAeasy kit (Qiagen, Hilden, Germany) and SuperScript II First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA) were employed to extract total RNA and synthesis of first strand cDNA. Then, the expressions of selected genes were determined by qPCR. We used glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal reference gene. Primers for qPCR are listed in Table 1. The comparative threshold cycle (Ct) method was used to determine relative fold changes of apoptosis- and inflammation-related genes.
Table 1.
Primers used for quantitative real-time polymerase chain reaction analysis of apoptosis and inflammatory cytokine related genes.
| Gene | Primer | Sequence (5’−3’) |
|---|---|---|
| p53 | Forward | GTATTTCACCCTCAAGATCC |
| Reverse | TGGGCATCCTTTAACTCTA | |
| p21 | Forward | GCGGAACAAGGAGTCAGACA |
| Reverse | GAACCAGGACACATGGGGAG | |
| Bad | Forward | AGAGTTTGAGCCGAGTGAGC |
| Reverse | CATCCCTTCGTCGTCCTCC | |
| Bax | Forward | AGTAACATGGAGCTGCAGAGG |
| Reverse | ATGGTTCTGATCAGTTCCGG | |
| Bcl-2 | Forward | GGAGCGTCAACAGGGAGATG |
| Reverse | GATGCCGGTTCAGGTACTCAG | |
| Caspase 3 | Forward | TGGACTGTGGCATTGAGACA |
| Reverse | CAGGTGCTGTGGAGTATGCA | |
| Caspase 8 | Forward | TATCCCGGATGGCTGACT |
| Reverse | GACATCGCTCTCAGGCTC | |
| Caspase 9 | Forward | GAGGGAAGCCCAAGCTGTTC |
| Reverse | GCCACCTCAAAGCCATGG | |
| IL-1β | Forward | CTGATGGCCCTAAACAGATGAAG |
| Reverse | GGTCGGAGATTCGTAGCAGCTGGA | |
| IL-2 | Forward | GAATCCCAAACTCACCAGGATGCTC |
| Reverse | TAGCACTTCCTCCAGAGGTTTGAGT | |
| IL-10 | Forward | GGGAGAACCTGAAGACCCTCA |
| Reverse | TGCTCTTGTTTTCACAGGGAAG | |
| TNF-α | Forward | ATCTTCTCGAACCCCGAGTGA |
| Reverse | CGGTTCAGCCACTGGAGCT | |
| GAPDH | Forward | GAGAAACCTGCCAAGTATG |
| Reverse | GGAGTTGCTGTTGAAGTC |
Naive CD4 cell isolation and differentiation of T-helper cells
Single cell suspensions were prepared by using 4–6 wk old naive C57BL/6 (B6) mice’s spleens. Pooled splenocytes (after erythrocyte lysis) were depleted of CD11b+, CD8α+, and CD19+ cells using biotinylated primary antibodies (BioLegend, San Diego, CA, USA) and streptavidin-coated secondary magnetic particles (Stem Cell Technologies, Vancouver, BC, Canada). T cell differentiation was based on our previous publications (11, 22). Test compounds were initially added into the culture medium. After 3 days, we harvested the differentiated cells, then in the presence of Brefeldin A, the cells were restimulated for 4 h with PMA (50 ng/ml) and ionomycin (750 ng/ml) (Sigma-Aldrich). Harvested cells were then fixed, permeabilized, and stained intracellularly with fluorochrome-conjugated anti-mCD4 (GK1.5), anti-mIFN-γ (XMG1.2), anti–mIL-17 (TC11–18H10), and anti-FoxP3 (FJK-16s) (all from Biolegend) using FoxP3 fixation/permeabilization reagents and protocols from eBiosciences (San Diego, CA, USA). Data were acquired on a Canto II cytometer (BD Biosciences, San Jose, CA, USA) and analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Statistical analysis
Data were expressed as mean ± SE. In general, we used a one-way ANOVA to define the data significant level. In some instances, Student’s t-test was employed for two-group comparison. In all cases, P < 0.05 was considered as statistically significant.
RESULTS
Identification of ginsenosides in GBC and GSE
Ginsenosides, or triterpenoid saponins are recognized as bioactive compounds in ginseng (1, 23, 24). The aforementioned HPLC procedures resulted in the efficiency assay of ginseng saponins. Ginsenosides in GBC and GSE were characterized by comparing UV spectra and retention times of the standards of authentic ginsenosides Rg2, Rg1, Re, Rd, Rd, Rb2, and Rb1. Fig. 1B (upper panel) shows the HPLC chromatogram of GBC. It indicates that the main constituents in GBC were ginsenosides Re and Rd. Several other ginsenosides, including Rb2, Rc, Rb1, Rg2, and Rg1, were identified; however, their contents were less than Re and Rd. On the other hand, there was no ginsenoside detected in GSE. The chromatogram fingerprint of GBC could be used to identify the authentication of ginseng berry extract in future studies.
Antiproliferative effects of GBC and GSE
To investigate the anticancer potential of GBC and GSE, the antiproliferative effects were evaluated through two steps, the first of which investigated one time point with relatively large intervals in extract concentration. Regarding HCT-116 group, Fig. 2A shows GBC completely inhibited cell growth with the concentration of 1000 μg/ml. At 150 and 500 μg/ml, GBC decreased cell proliferation by 16.7% (p < 0.01), 87.7% (p < 0.01), respectively. However, GSE did not show any antiproliferative effects in the treatment concentration range. On the contrary, GSE treatment actually increased cell growth. A similar trend was observed in HT-29 cells (Fig. 2A). Compared to GSE, GBC showed strong antiproliferative effects.
Fig. 2.
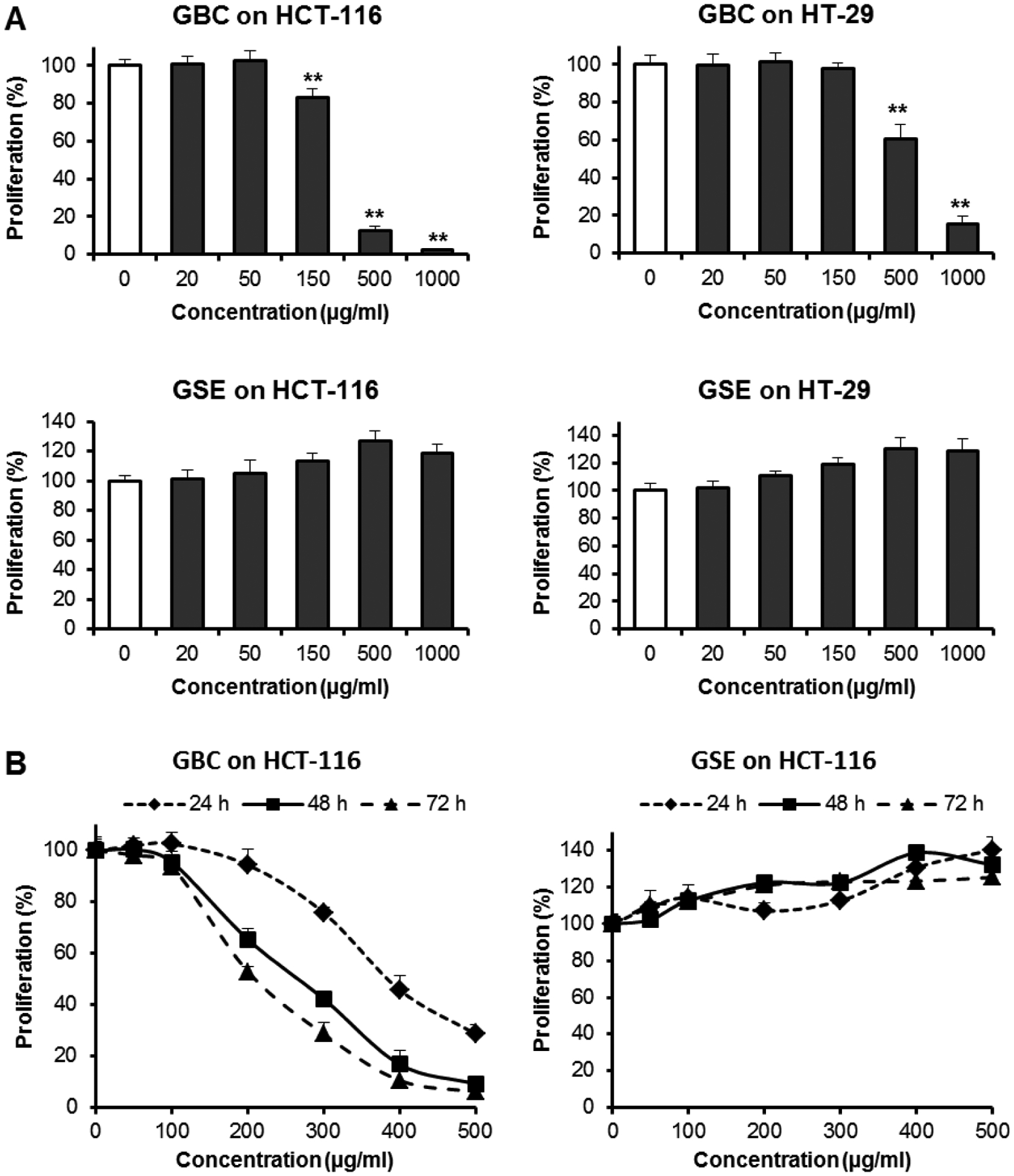
Effects of GBC and GSE on HCT-116 and HT-29 human colorectal cancer cell proliferation. [A]: Concentration-associated effects on HCT-116 and HT-29 cells for 48 h. Cells were treated with 20, 50, 150, 500, and 1000 μg/ml of tested extracts for 48 h. [B]: Time-associated antiproliferative effects on HCT-116 cells. Cells were treated with 50–500 μg/ml of tested extracts for 24, 48, and 72 h. *P<0.05; **P<0.01 vs. control. GBC inhibited cancer cell proliferation in dose- and time-dependent manner. Non-ginsenoside extract GSE increased cell proliferation.
Furthermore, antiproliferative potential was also investigated by measuring time- and dose-dependent effects. Regarding HCT-116 cells, due to GBC showing strong inhibition activities, we employed this cell line for further studies. Cell proliferations were observed at 24, 48, and 72 h. More precise concentration ranges were employed. The treatment concentrations for both extracts were 50–500 μg/ml. As shown in Fig. 2B, after treatment with GBC, by doses over 100 μg/ml, dose- and time-dependent antiproliferative effects were observed. As shown in Fig. 2B, however, GSE did not inhibit cancer cell growth but instead slightly increased cell proliferation.
GBC and GSE on cancer cell cycle
By using HCT-116 cells, MTS results showed that GBC carried out significant antiproliferation with the doses over 100 μg/ml of GBC. Thus, concentrations used in cell cycle tests were of 100–400 μg/ml for 48 h. Fig. 3A shows that there are no changes in cell cycle by the treatment of 100 μg/ml of GBC. However, 200–400 μg/ml of GBC treatment changed cell cycle profiles. At 200 μg/ml, GBC obviously reduced S-phase proportion, and increased G2/M-phase proportion. Upon treatment with 300 μg/ml, more significant changes were observed. After treatment with 400 μg/ml of GBC for 48 h, in comparison to the control (43.1% of G1, 36.4% of M, and 20.4% of G2/M), cells in G1-phase were 28.3% (p < 0.01), M-phase 29.6% (p < 0.05), and G2/M phase 46.8% (p < 0.01). GBC treatment significantly decreased G1-phase cells and increased G2/M-phase cells. However, we did not find cell cycle effects of GSE in HCT-116 cells (Fig. 3B).
Fig. 3.
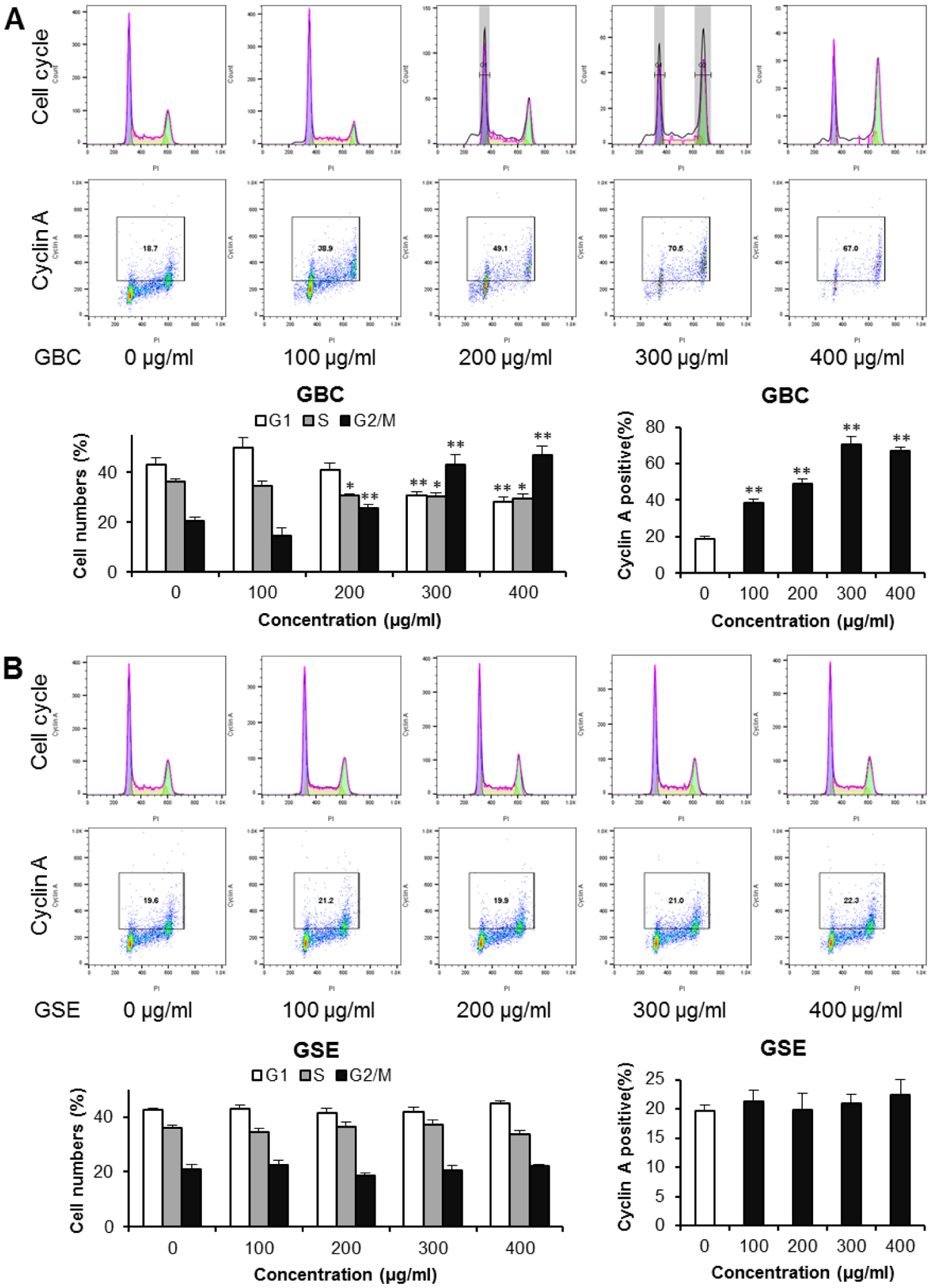
Effects of GBC and GSE on cell cycle arrest and the expression of cyclin A. HCT-116 cells were treated with 100, 200, 300, and 400 μg/ml of GBC and GSE for 48 h. The cell cycle and cyclin A were assessed with PI/RNase and cyclin A-FITC staining using flow cytometry. [A]: The representative histograms of DNA content in different experimental groups (upper panel), and percentage of each cell cycle phase in different groups (lower panel). [B]: The expression of cyclin A. The numbers in the gates are the percentage of cyclin A positive cells (upper panel), and percentage of cyclin A-positive cells in different groups (lower panel). Data obtained from triplicate experiments. * P < 0.05; ** P < 0.01 compared to control.
Effects GBC and GSE on expression of cyclin A
Cell cycle progression is regulated by cyclins, particularly cyclin A for the S-phase and passage through the G2/M-phase. The observation of G2/M phase cancer cell cycle arrest through GBC led to further investigation of cell cycle regulation through evaluating the expression of cyclin A. Using HCT-116 cells, for the control group, the cell proportion for cyclin A positive cells was 18.6%. After 48 h treatment with 100, 200, 300, and 400 μg/ml of GBC, the cyclin A positive proportions increased to 38.3%, 49.2%, 70.1%, and 67.1%, respectively (all p < 0.01) (Fig. 3A). On the other hand, with GSE treatment at same concentrations, the expression of cyclin A was not changed (Fig. 3B). Flow cytometry data for cyclin A analysis supported the observed cell cycle arrest effects by the GBC.
Apoptotic induction of GBC and GSE on colorectal cancer cells
To further investigate mechanisms of GBC’s cell growth inhibition, an apoptotic assay was conducted within the staining of PI and annexin V. For PI and annexin V staining, negative for both are viable cells, while positive for both are late apoptotic or necrotic cells. PI-positive and annexin V-negative are cells that underwent necrosis, while annexin V-positive and PI-negative are early apoptotic cells. The cytograms of bivariate annexin V/PI analysis of HCT-116 cells after GBC and GSE treatment are shown in Fig. 4A. The early and late apoptotic cells for the control were 3.8% and 2.0%, respectively. Treatments with GBC at 300–500 μg/ml increased apoptotic cells obviously. For example, 400 μg/ml of GBC treatment for 48 h resulted in an increasing of apoptotic cells to 41.2% (p < 0.01) (Fig. 4B). Thus, GBC treatment, but not GSE, induced cancer cell apoptosis (Figs. 4A and 4B).
Fig. 4.
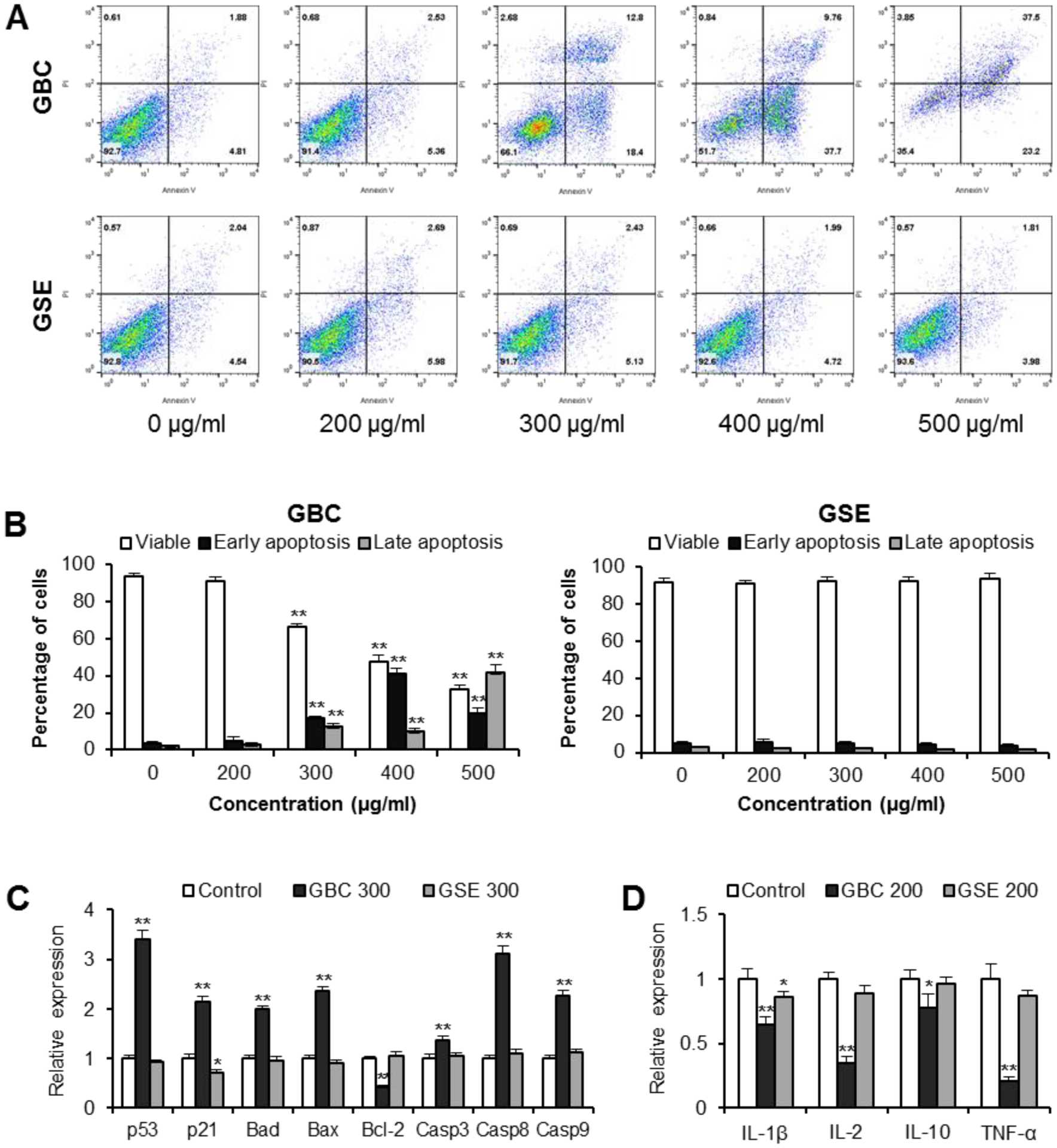
Apoptosis and inflammatory cytokine analysis of cells treated with GBC and GSE. [A]: Representative scatter plots of PI (y-axis) versus annexin V (x-axis). HCT-116 cells were treated with 200–500 μg/ml of GBC and GSE for 48 h. Apoptosis was quantified using annexin V-FITC/propidium iodide (PI) staining followed by flow cytometric analysis. [B]: Percentage of viable, early apoptotic, and late apoptotic cells. [C]: HCT-116 cells were treated with 300 μg/ml of GBC and GSE for 24 h. Expression of apoptotic related genes assayed by real-time PCR. [D]: HT-29 cells were treated with 10 ng/ml of LPS and 200 μg/ml of GBC and GSE for 6 h. Expression of inflammatory cytokine genes assayed by real-time PCR. Data are presented as the mean ± standard error of triplicate experiments. * P < 0.05, ** P < 0.01 vs. control.
GBC induces gene expressions involved in apoptosis by real time-PCR
Effects of GBC and GSE on selected apoptosis related genes were tested. For those selected targets, pro-apoptotic genes include tumor protein p53 (p53), cyclin-dependent kinase inhibitor 1A (p21), Bcl-2 associated agonist of cell death (Bad), Bcl-2 associated X (Bax), and caspases 3, 8 and 9. The anti-apoptotic gene B-cell lymphoma 2 (Bcl-2) was also included. As shown in Fig. 4C, GSE did not change apoptotic gene expression obviously, except for a slight downregulation of p21. After GBC, p53, p21, Bad, and Bax expressions were significantly increased. Furthermore, GBC induced up-regulation of caspases 3, 8, and 9. In contrast, GBC reduced Bcl-2 expression, which is an anti-apoptotic gene. Our results suggested that activation of the apoptotic pathway plays a role in GBC inhibited cancer cell. In addition, due to p53-wild-type (HCT-116), cells were more sensitive to GBC than p53-mutated (HT-29) cells (Fig. 2). Our data also suggested that GBC induces apoptosis partly through the upregulation of p53.
Inflammatory related gene expression induced by GBC treatment
Chronic gut inflammation is a risk factor for CRC. Some ginseng compounds were previously observed to have antiproliferative potential in relatively high doses, while also possessing anti-inflammatory effects in relatively low doses. To further investigate the contribution of inflammation to cancer cell growth inhibition, an inflammatory cytokine gene expression assay was carried out by real-time PCR. As shown in Fig. 4D, treatment with 200 μg/ml of GBC significantly downregulated the expression for several pro-inflammatory cytokines, including IL-1β, IL-2, and TNF-α (all p < 0.01). GBC treatment also downregulated the expression of IL-10 (p < 0.05). GSE treatment downregulated the expression of IL-1β (p < 0.05) but did not influence the expression of other inflammatory cytokines. Whether the GBC and GSE possess anti-inflammatory potential is confirmed in the observations presented below.
GBC decreased CD4+IL-17+ cell (Th17) differentiation
To investigate GBC on adaptive immunity, GBC on T cell differentiation was evaluated. It has been shown that suppression of Th17-cell differentiation from naive CD4+ T cells has beneficial effects on inflammatory disease management. To test GBC on the differentiation of Treg, Th1 and Th17, CD4+ T cells were maintained under Treg, Th1, and Th17-polarizing conditions. At the treatment concentrations of 50–200 μg/ml, GBC did not influence Treg and Th1 cell differentiation obviously. However, for the Th17 cell differentiation, approximately 30.4% of CD4+ T cells were IL-17+ in the control group. 100 μg/mL of GBC treatment inhibited Th17 differentiation to 21.5% (p < 0.05). Further, 200 μg/mL of GBC treatment inhibited Th17 differentiation down to 12.0% (p < 0.01) (Fig. 5). We observed concentration-dependent negative regulation effects in Th17 differentiation by GBC treatment.
Fig. 5.
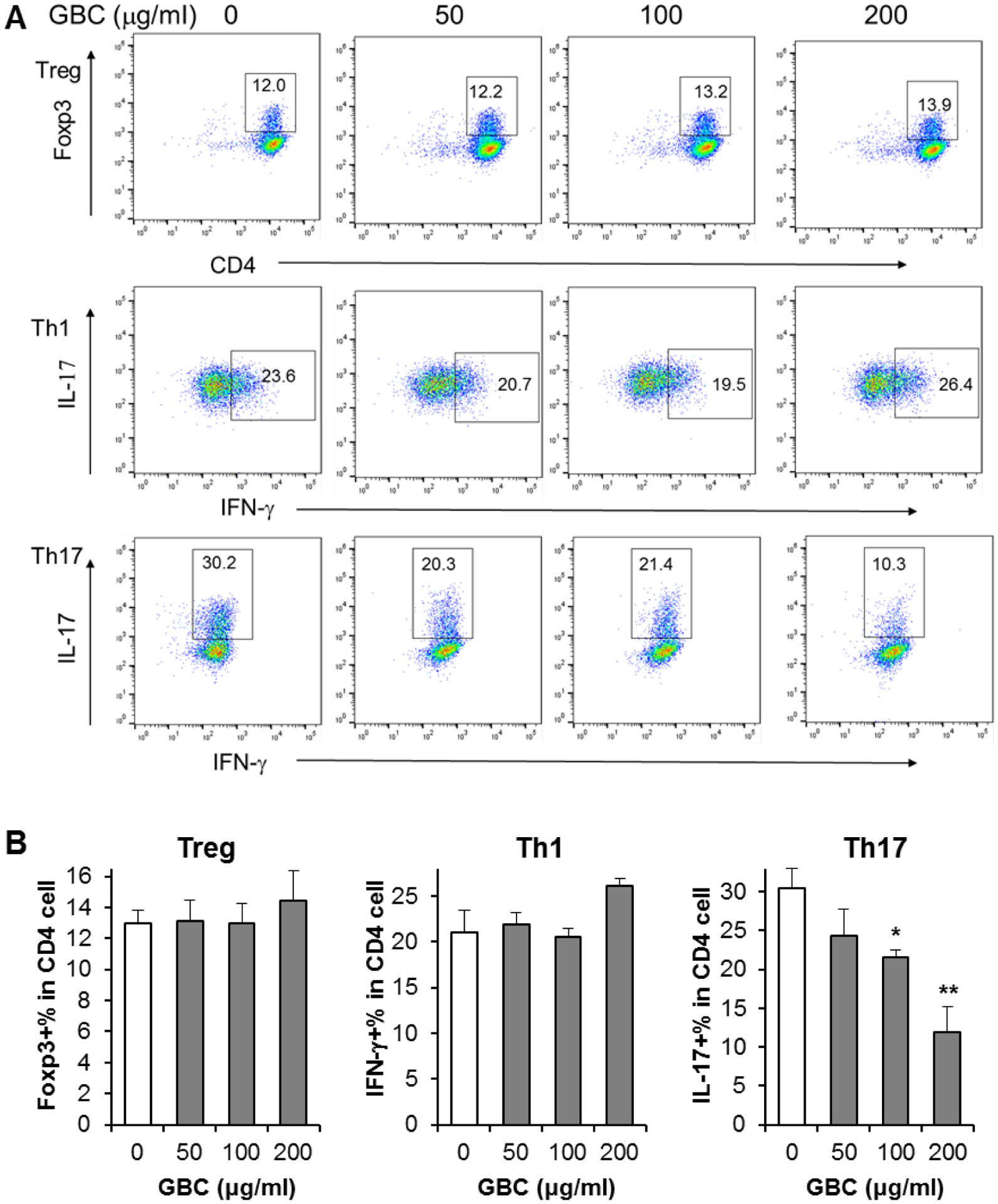
Effect of GBC on Treg, Th1, and Th17 cell differentiation. [A]: Representative scatter plots assayed by flow cytometry. The numbers in the gates are the percentage of each type of T cells. [B]: Data are presented as the mean ± standard error of triplicate experiments. * P < 0.05, ** P < 0.01 vs. control.
Effects of GSE on Treg, Th1 and Th17 cell differentiation
In our previous anti-inflammatory gene expression investigation, we observed that GSE did not show obvious effects on inflammatory cytokines. To compare the effects with GBC, we assayed the effects of GSE on T cell differentiation. As shown in Fig. 6, in most conditions, GSE did not influence T cell differentiation. Only two exceptions include the upregulation of Treg differentiation at 200 μg/ml and upregulation of Th17 differentiation at 50 μg/ml (both p < 0.05). However, we did not observe dose-dependent effects of GSE on T cell differentiation. Therefore, our results did not supply solid evidence that GSE has the potential to regulate T cell differentiation. Further studies with broad treatment concentrations are required.
Fig. 6.
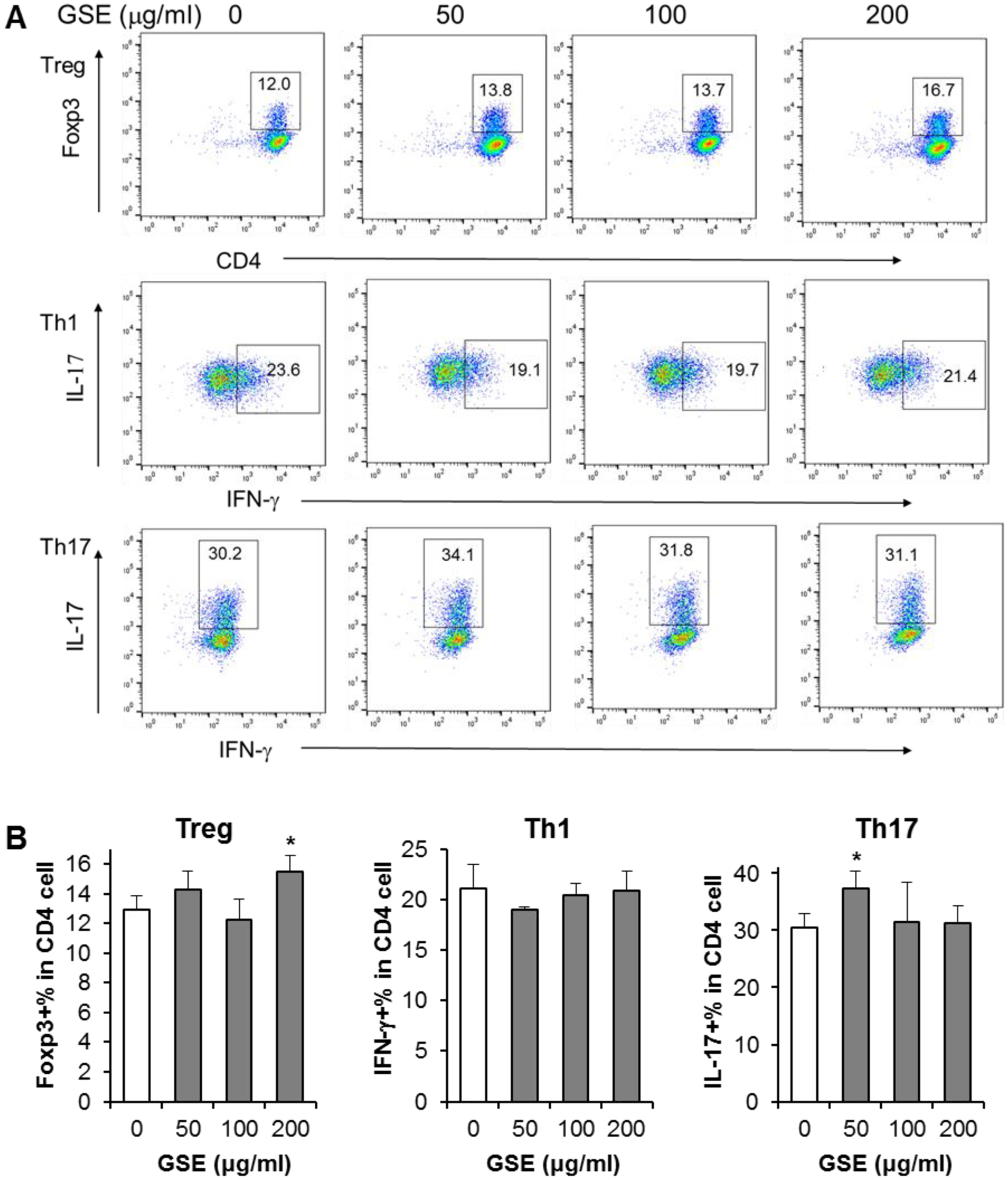
Effect of GSE on Treg, Th1, and Th17 cell differentiation. [A]: Representative scatter plots assayed by flow cytometry. The numbers in the gates are the percentage of each type of T cells. [B]: Data are presented as the mean ± standard error of triplicate experiments. * P < 0.05 vs. control.
DISCUSSION
Herbal medicine is a newly popular field looking to expand the prevention and treatment of diseases, and its wide spectrum encompasses both old and newfound approaches. Among such herbal therapies is ginseng, not only one of the most commonly used herbs at present, but also an acclaimed herbal medicine in Oriental Asian countries widely used for its various beneficial effects. Ginseng also possesses a wide range of pharmacological actions, including angiogenesis-modulating, anti-carcinogenic, anti-inflammatory, and antioxidant activities (25–28).
CRC remains a leading cause of morbidity and mortality worldwide. In spite of the continual advances in developing treatment strategies for CRC patients, dose-limiting toxicity and other severe side effects remain as limitations for chemotherapies (29). The consequences of the drugs can end in refusal to continue with chemotherapy; Cancer patients often try to treat its side effects through complementary and alternative medicinal means. Herbal medicine has recently gained more attentions for the CRC management (30), and prevention of this malignancy by botanical sourced natural compounds can be used as an alternative for potential clinical application (31). Ginseng berry extract was previously demonstrated to have the activities to reduce chemotherapy induced side effects (32). Furthermore, ginseng berry extract even enhances chemopreventive effects of anticancer agents (33). However, solid evidence for ginseng berry extract on the anticancer and involved mechanisms of actions is still limited.
Botanical extracts are composed of complicated bioactive compounds, which noticeably vary in composition based on many factors. Therefore, to supply the phytochemical background of a tested extract for other researchers, botanical constituent analysis is important before pharmacological investigation. It is believed that the major active components of ginseng are ginsenosides, a diverse group of steroid saponins. Ginsenosides are distributed unevenly in different parts of the ginseng plant, including the root, leaf, and berry. As a byproduct of ginseng plant, ginseng berry can also be collected during harvesting the root. Previous studies have demonstrated that in addition to polysaccharides (11), the ginseng berry also contains high amount of ginsenosides with a distinct profile compared to the root (23, 34).
In this study, we use HPLC to determine the composition of ginsenosides in GBC and GSE. There were 7 ginsenosides identified in GBC, of which the major constituents are ginsenosides Re and Rd. With an obvious peak, ginsenoside Rg2, a rare ginsenoside in ginseng root, has also been characterized in GBC. However, there was no ginsenoside detected in GSE. The composition of ginsenosides in GBC was found to be much different with that in ginseng root (23), indicating that its biological potential is different with ginseng root, which is the most commonly used plant part of this herb.
Effects of GBC and GSE on cancer cell growth were determined by using MTS assay. GSE did not show any antiproliferative effects. In contrast, GSE increased cancer cell growth a little, possibly due to our special processed GSE containing some nutrients but not anticancer constituents. GBC, which contains ginsenosides, showed significant anti-CRC cell growth activities. Moreover, GBC showed more potent antiproliferative effects in HCT-116 cells than that in the HT-29 cell line. Thus, further studies should be focused on the HCT-116 cell line.
In this study, the analysis on cell cycle distribution demonstrated that GBC induced G2/M arrest, suggesting that GBC targeted cell cycle checkpoint for G2/M arrest. As cyclins are a protein family involved in cell cycle progression, cyclin A plays an important role in controlling the G2/M transition of the cell cycle by binding to CDK2, a required step for cell progression through the S phase (35). Our results showed that GBC up-regulated cyclin A, indicating that GBC could facilitate the cell’s progression through the S phase into the G2/M phase and lead to the accumulation of cell distribution in the G2/M phase.
Apoptosis is considered an important pathway in the inhibition of cancer cells by many anticancer agents (36). Previous studies investigated interactions between natural products and proteins, revealing that some botanical compounds and their molecular pathways were able to modulate the development and metastasis of colorectal tumors (37). To validate the flow cytometric apoptotic data, real time-PCR analysis was preformed to investigate the expression of apoptotic related genes. It was observed that GBC upregulated several pro-apoptotic genes, including p53, p21, Bad, and Bax and downregulated the anti-apoptotic gene Bcl-2. Further, GBC upregulated the expression of caspases 3, 8, and 9. Caspases are a family of cysteine proteases that play essential roles in cell apoptosis (38). These results suggest that GBC exerts its chemopreventive effect by multiple molecular mechanisms of action on the apoptotic pathway in human colorectal cancer cells.
Since IBD and chronic inflammation is recognized as a risk factor for CRC development, an effective prevention method of malignant colon tumor formation and progression is targeting inflammatory pathways, as shown in both animal and human studies (39). While another method to reduce CRC initiation and development is using non-steroidal anti-inflammatory drugs (NSAIDS), this form of prevention is generally not recommended or considered suitable due to the concerns and long-term risks associated with NSAIDS (40). The limitations of present-day standards of practice strongly motivate the exploration of alternative strategies preventing CRC and its ensuing inflammation. The anti-inflammatory effect of ginseng berry has been reported (41, 42). Recently, ginseng berry polysaccharides was observed to show active effects on cancer cell growth inhibition and anti-inflammation (11). However, effects of non-polysaccharide constituents in ginseng berry on inflammation-associated colon cancer chemoprevention has not been investigated.
Previous clinical studies found that high levels of IL-2 and IL-10 in colorectal cancer patents were associated with increased total mortality (43, 44). Further, significantly higher levels of IL-1β and TNF-α were detected in colorectal cancer patients compared to healthy controls. Interestingly, in colorectal cancer patients, circulating levels for these four cytokines decreased progressively after tumor removal (43). In addition to a colon cancer cell model, the HT-29 cells also represent a well characterized model to study the intestinal epithelial response to inflammatory stimulation (45, 46). In this study, GBC inhibited the expressions of pro-inflammatory cytokines, suggesting that GBC may have anti-inflammatory potential.
The adaptive immune response has been considered to play a critical role in the pathogenesis of IBD. T lymphocytes represent the key cell population of the adaptive immunity arm (47). To explore ginseng extracts on adaptive immune responses, the effects of GBC and GSE on the differentiation of Th1, Th17, and Treg cells have been investigated. Based on our results, there is no solid evidence that GSE regulates T cell differentiation. Although GBC treatment did not change Treg and Th1 cell differentiation, dose-dependent inhibition of Th17 cell differentiation has been observed after treatment with GBC. Our data suggests that GBC suppressed differentiation of Th17 cells, which is an important Th subset connected to gut inflammation.
Th17 cells and Treg cells are in dynamic equilibrium under normal circumstances. The balance is broken with the over-proliferation of Th17 cells, excessive enhancement of immunogenicity, and decrease or abnormal function of Treg cells causing intestinal mucosal damage. Based on our data, GBC did not obviously influence Treg but significantly inhibited Th17 differentiation. The ratio of Th17/Treg in IBD patients’ blood is significantly higher. Therefore, regulation of balance between Th17/Treg becomes a new approach for IBD management (48). Our data suggested that GBC may have the potential to regulate the balance of Th17/Treg, to inhibit gut inflammation and prevent colon cancer initiation and development.
In summary, using different processing and extraction methods, GBC and GSE was obtained. HPLC chromatogram fingerprint of both extracts were carried out, and ginsenosides in GBC have been characterized. There was no ginsenoside detected in GSE, and no significant biological effects of GSE were observed. GBC significantly inhibited colon cancer cell growth. The involved anti-proliferative mechanisms of GBC include G2/M cell cycle arrest via upregulation of cyclin A and induction of apoptosis via regulation of apoptotic related gene expressions. GBC also downregulated the expressions of pro-inflammatory cytokine genes. Adaptive immune responses of GBC treatment were investigated. GBC did not influence Th1 and Treg cell differentiation but significantly inhibited Th17 cell differentiation. Our results suggest that GBC is an active anticancer dietary supplement. The mechanisms of actions include inhibition of cancer cell growth via cell cycle arrest and induction of apoptosis, and anti-inflammation via regulation of adaptive immunity.
Acknowledgements:
This work was supported in part by the NIH/NCCAM grants P01 AT004418, K01 AT005362, and 5P30DK042086.
Abbreviations:
- GBC
ginseng berry concentrate
- GSE
ginseng seed extract
- CRC
colorectal cancer
- HPLC
high performance liquid chromatography
- UV
ultraviolet
- FBS
fetal bovine serum
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- FITC
fluorescein isothiocyanate
- ANOVA
analysis of variance
- Treg cells
regulatory T cells
- Th1 cells
type 1 T helper cells
- Th17 cells
type 17 T helper cells
- p53
transformation-related protein 53
- p21
cyclin-dependent kinase inhibitor 1
- Bad
Bcl-2-associated death promoter
- Casp3
caspase 3
- Casp8
caspase 8
- Casp9
caspase 9
- IL-1β
interleukin 1 beta
- IL-2
interleukin 2
- IL-10
interleukin 10
- TNF-α
tumor necrosis factor alpha
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
Footnotes
Conflict of interest: None declared.
REFERENCES
- 1.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol 1999; 58: 1685–1693. [DOI] [PubMed] [Google Scholar]
- 2.Xu W, Choi HK, Huang L. State of Panax ginseng research: A global analysis. Molecules 2017; 22: 1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qi LW, Wang CZ, Yuan CS. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry 2011; 72: 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao H, Xue Q, Zhang Q, et al. How ginsenosides trigger apoptosis in human lung adenocarcinoma cells. Am J Chin Med 2019; 47: 1737–1754. [DOI] [PubMed] [Google Scholar]
- 5.Yun TK, Choi SY. Non-organ specific cancer prevention of ginseng: a prospective study in Korea. Int J Epidemiol 1998; 27: 359–364. [DOI] [PubMed] [Google Scholar]
- 6.Yun TK. Panax ginseng--a non-organ-specific cancer preventive? Lancet Oncol 2001; 2: 49–55. [DOI] [PubMed] [Google Scholar]
- 7.Yu C, Wen XD, Zhang Z, et al. American ginseng attenuates azoxymethane/dextran sodium sulfate-induced colon carcinogenesis in mice. J Ginseng Res 2015; 39: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang CZ, Yu C, Wen XD, et al. American ginseng attenuates colitis-associated colon carcinogenesis in mice: impact on gut microbiota and metabolomics. Cancer Prev Res (Phila) 2016; 9: 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan JY, Huang WH, Zheng W, et al. Multiple effects of ginseng berry polysaccharides: Plasma cholesterol level reduction and enteric neoplasm prevention. Am J Chin Med 2017; 45: 1293–1307. [DOI] [PubMed] [Google Scholar]
- 10.Rho T, Jeong HW, Hong YD, Yoon K, Cho JY, Yoon KD. Identification of a novel triterpene saponin from Panax ginseng seeds, pseudoginsenoside RT8, and its antiinflammatory activity. J Ginseng Res 2020; 44: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CZ, Hou L, Wan JY, et al. Ginseng berry polysaccharides on inflammation-associated colon cancer: inhibiting T-cell differentiation, promoting apoptosis, and enhancing the effects of 5-fluorouracil. J Ginseng Res 2020; 44: 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attele AS, Zhou YP, Xie JT, et al. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 2002; 51: 1851–1858. [DOI] [PubMed] [Google Scholar]
- 13.Choi YD, Park CW, Jang J, et al. Effects of Korean ginseng berry extract on sexual function in men with erectile dysfunction: a multicenter, placebo-controlled, double-blind clinical study. Int J Impot Res 2013; 25: 45–50. [DOI] [PubMed] [Google Scholar]
- 14.Lee DY, Park CW, Lee SJ, et al. Immunostimulating and antimetastatic effects of polysaccharides purified from ginseng berry. Am J Chin Med 2019; 47: 823–839. [DOI] [PubMed] [Google Scholar]
- 15.Hu JN, Lee J-H, Shin JA, Choi JE, Lee K-T. Determination of ginsenosides content in Korean ginseng seeds and roots by high performance liquid chromatography. Food Sci Biotechnol 2008; 17: 430–433. [Google Scholar]
- 16.Sugimoto S, Nakamura S, Matsuda H, Kitagawa N, Yoshikawa M. Chemical constituents from seeds of Panax ginseng: structure of new dammarane-type triterpene ketone, panaxadione, and hplc comparisons of seeds and flesh. Chem Pharm Bull (Tokyo) 2009; 57: 283–287. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy N Tumorigenesis: All together now. Nat Rev Cancer 2013; 13: 148. [DOI] [PubMed] [Google Scholar]
- 18.Swierczynski M, Szymaszkiewicz A, Fichna J, Zielinska M. New insights into molecular pathways in colorectal cancer: Adiponectin, interleukin-6 and opioid signaling. Biochim Biophys Acta Rev Cancer 2020; 1875: 188460. [DOI] [PubMed] [Google Scholar]
- 19.Madka V, Rao CV. Anti-inflammatory phytochemicals for chemoprevention of colon cancer. Curr Cancer Drug Targets 2013; 13: 542–557. [DOI] [PubMed] [Google Scholar]
- 20.Yu C, Wang CZ, Zhou CJ, et al. Adulteration and cultivation region identification of American ginseng using HPLC coupled with multivariate analysis. J Pharm Biomed Anal 2014; 99: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishbein AB, Wang CZ, Li XL, et al. Asian ginseng enhances the anti-proliferative effect of 5-fluorouracil on human colorectal cancer: comparison between white and red ginseng. Arch Pharm Res 2009; 32: 505–513. [DOI] [PubMed] [Google Scholar]
- 22.Hou L, Rao DA, Yuki K, et al. SerpinB1 controls encephalitogenic T helper cells in neuroinflammation. Proc Natl Acad Sci U S A 2019; 116: 20635–20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JW, Choi BR, Kim YC, et al. Comprehensive profiling and quantification of ginsenosides in the root, stem, leaf, and berry of Panax ginseng by UPLC-QTOF/MS. Molecules 2017; 22: 2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu L, Wei F, Liang J, et al. Target molecular-based neuroactivity screening and analysis of Panax ginseng by affinity ultrafiltration, UPLC-QTOF-MS and molecular docking. Am J Chin Med 2019; 47: 1345–1363. [DOI] [PubMed] [Google Scholar]
- 25.Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. J Nutr 2007; 137: 183S–185S. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH. Cardiovascular diseases and Panax ginseng: A review on molecular mechanisms and medical applications. J Ginseng Res 2012; 36: 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adam GO, Kim GB, Lee SJ, Lee H, Kang HS, Kim SJ. Red ginseng reduces inflammatory response via suppression MAPK/P38 signaling and p65 nuclear proteins translocation in rats and Raw 264.7 macrophage. Am J Chin Med 2019; 47: 1589–1609. [DOI] [PubMed] [Google Scholar]
- 28.Jeong D, Ham J, Park S, et al. Ginsenoside Rh2 suppresses breast cancer cell proliferation by epigenetically regulating the long noncoding RNA C3orf67-AS1. Am J Chin Med 2019; 47: 1643–1658. [DOI] [PubMed] [Google Scholar]
- 29.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020; 70: 145–164. [DOI] [PubMed] [Google Scholar]
- 30.Waluga M, Zorniak M, Fichna J, Kukla M, Hartleb M. Pharmacological and dietary factors in prevention of colorectal cancer. J Physiol Pharmacol 2018; 69: 325–336. [DOI] [PubMed] [Google Scholar]
- 31.Chojnacka K, Sosnowska D, Polka D, et al. Comparison of phenolic compounds, antioxidant and cytotoxic activity of extracts prepared from Japanese quince (Chaenomeles japonica L.) leaves. J Physiol Pharmacol 2020; 71: 213–222. [DOI] [PubMed] [Google Scholar]
- 32.Mehendale S, Aung H, Wang A, et al. American ginseng berry extract and ginsenoside Re attenuate cisplatin-induced kaolin intake in rats. Cancer Chemother Pharmacol 2005; 56: 63–69. [DOI] [PubMed] [Google Scholar]
- 33.Li XL, Wang CZ, Sun S, et al. American ginseng berry enhances chemopreventive effect of 5-FU on human colorectal cancer cells. Oncol Rep 2009; 22: 943–952. [PubMed] [Google Scholar]
- 34.Kim YK, Yoo DS, Xu H, et al. Ginsenoside content of berries and roots of three typical Korean ginseng (Panax ginseng) cultivars. Nat Prod Commun 2009; 4: 903–906. [PubMed] [Google Scholar]
- 35.Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science 1989; 246: 629–634. [DOI] [PubMed] [Google Scholar]
- 36.Millimouno FM, Dong J, Yang L, Li J, Li X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev Res (Phila) 2014; 7: 1081–1107. [DOI] [PubMed] [Google Scholar]
- 37.Raschka S, More SK, Devadoss D, Zeng B, Kuhn LA, Basson MD. Identification of potential small-molecule protein-protein inhibitors of cancer metastasis by 3D epitope-based computational screening. J Physiol Pharmacol 2018; 69: 255–263. [DOI] [PubMed] [Google Scholar]
- 38.Medina CB, Mehrotra P, Arandjelovic S, et al. Metabolites released from apoptotic cells act as tissue messengers. Nature 2020; 580: 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis B, Lin J, Wu X, et al. Crohn’s disease-like reaction predicts favorable prognosis in colitis-associated colorectal cancer. Inflamm Bowel Dis 2013; 19: 2190–2198. [DOI] [PubMed] [Google Scholar]
- 40.Garcia Rodriguez LA, Cea-Soriano L, Tacconelli S, Patrignani P. Coxibs: pharmacology, toxicity and efficacy in cancer clinical trials. Recent Results Cancer Res 2013; 191: 67–93. [DOI] [PubMed] [Google Scholar]
- 41.Xu XY, Wang Z, Ren S, et al. Improved protective effects of American ginseng berry against acetaminophen-induced liver toxicity through TNF-alpha-mediated caspase-3/−8/−9 signaling pathways. Phytomedicine 2018; 51: 128–138. [DOI] [PubMed] [Google Scholar]
- 42.Lee DY, Kim MJ, Yoon D, Lee YS, Kim GS, Yoo YC. Ginseng berry prevents alcohol-induced liver damage by improving the anti-inflammatory system damage in mice and quality control of active compounds. Int J Mol Sci 2019; 20: 3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crucitti A, Corbi M, Tomaiuolo PM, et al. Laparoscopic surgery for colorectal cancer is not associated with an increase in the circulating levels of several inflammation-related factors. Cancer Biol Ther 2015; 16: 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen RS, Nijm J, Andersson RE, Dimberg J, Wagsater D. Circulating inflammatory factors associated with worse long-term prognosis in colorectal cancer. World J Gastroenterol 2017; 23: 6212–6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Song ZJ, He X, et al. Antitumor and immunomodulatory activity of genkwanin on colorectal cancer in the APC(Min/+) mice. Int Immunopharmacol 2015; 29: 701–707. [DOI] [PubMed] [Google Scholar]
- 46.Byun EB, Kim WS, Sung NY, Byun EH. Epigallocatechin-3-gallate regulates anti-inflammatory action through 67-kDa laminin receptor-mediated tollip signaling induction in lipopolysaccharide-stimulated human intestinal epithelial cells. Cell Physiol Biochem 2018; 46: 2072–2081. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y, Chen Z. Inflammatory bowel disease related innate immunity and adaptive immunity. Am J Transl Res 2016; 8: 2490–2497. [PMC free article] [PubMed] [Google Scholar]
- 48.Ueno A, Jeffery L, Kobayashi T, Hibi T, Ghosh S, Jijon H. Th17 plasticity and its relevance to inflammatory bowel disease. J Autoimmun 2018; 87: 38–49. [DOI] [PubMed] [Google Scholar]


