Abstract
Objective:
There have been significant successes in the fight against HIV/AIDS due to the access to rapid HIV testing, interventions to reduce the mother-to-child transmission (MTCT) risk, potent and effective antiviral medications, and other biomedical prevention strategies. The purpose of this work is to demonstrate that Puerto Rico eliminated Mother-to-Child Transmission of HIV (MTCT) following the 2017 World Health Organization (WHO) criteria for validating the elimination of MTCT and Syphilis.
Methods:
Existing epidemiological data from Puerto Rico was used to document the elimination of MTCT and Syphilis. Data to calculate the indicators was obtained from the various divisions of the Puerto Rico Department of Health, including vital statistics, surveillance data, and programmatic outcomes.
Results:
Puerto Rico eliminated MTCT and syphilis, according to the WHO indicators, earlier than other countries. We can trace the outcomes to 1994 using the incidence rate of perinatally-acquired HIV of <50/100,000; to 2007 using HIV perinatal transmission rates for non-breastfeeding countries (<2%), to 2008 using 90% of women receiving ART at delivery, and to 2005 using the incidence rate of congenital syphilis of <50/100,000.
Conclusion:
Not only have we eliminated the MTCT of HIV and syphilis, but the efforts have been sustained since 2000. The elimination of transmission of infectious diseases requires the intersection of scientific feasibility, coordinated interventions, and political will, successfully attained in Puerto Rico.
Keywords: Perinatal HIV transmission, HIV and Syphilis, HIV and pregnancy, Women-centered HIV Care, Elimination of Mother to Child HIV Transmission
Resumen
Objetivo:
Ha habido avances significativos en la lucha contra el VIH/SIDA: el acceso rápido al diagnóstico de VIH, intervenciones para reducir el riesgo de transmisión maternoinfantil y medicamentos efectivos que disminuyen el riesgo de transmisión sexual y otras estrategias preventivas. El propósito de esta investigación epidemiológica es demostrar cÓmo Puerto Rico ha eliminado la transmisión perinatal del VIH y sífilis, usando las guías publicadas por la Organización Mundial de la Salud (OMS) en el 2017.
Métodos:
Se utilizaron datos epidemiológicos para calcular los indicadores propuestos por la OMS para la validación de la eliminación de la transmisión perinatal del VIH y sífilis. Los datos fueron obtenidos del Departamento de Salud de Puerto Rico, incluyendo estadísticas vitales, datos de vigilancia y logros programáticos.
Resultados:
Puerto Rico eliminó la transmisión perinatal de VIH y sífilis antes que otros países. Podemos confirmar la eliminación en el 1994 usando la tasa de incidencia de VIH perinatal de <50/100,00; en el 2007 usando la tasa de transmisión de VIH perinatal en países sin lactancia (<2%); en el 2008 considerando que el 90% de las mujeres reciben medicamento al momento del parto; y en el 2005 usando la tasa de incidencia de sífilis congénita de <50/100,000.
Conclusión:
No sólo hemos eliminado la transmisión perinatal del VIH y de la sífilis en Puerto Rico, sino que se ha sostenido desde el año 2000. La eliminación de la transmisión de enfermedades infecciosas no es fácilmente alcanzable; requiere la intersección de la ciencia con intervenciones coordinadas y voluntad política.
The HIV/AIDS epidemic has affected millions of individuals worldwide. Puerto Rico was impacted early with the epidemic, exhibiting a different epidemiologic pattern than the one in the US. Initially, injection drug use (IDU) was the most common risk behavior among males (now it is sex with men), while heterosexual transmission has been the most common HIV risk for women. There have been significant successes in the fight against HIV/AIDS. Among them, access to rapid HIV testing, interventions to reduce the mother-to-child transmission (MTCT) risks, and effective antiviral therapies that have demonstrated elimination of sexual transmission risks. Science has demonstrated that treatment saves lives, and that testing and access to therapy reduces the mortality and morbidity and reduces infectivity. Puerto Rico (PR) has contributed to the science and swiftly acted on evidence to provide treatment and care as new developments emerged, eliminating perinatal HIV transmission.
Historically, more than 70 million people have been infected with HIV and about 35 million people have died of HIV complications (1). Globally, 37.9 million [32.7–44 million] people were living with HIV at the end of 2018. An estimated 0.8% [0.6–0.9%] of adults aged 15–49 years worldwide are living with HIV. The African region remains the most affected, with 1 in every 25 adults (3.9%) living with HIV and accounting for nearly two-thirds of the people living with HIV worldwide (1). At the end of 2018, 1.7 million children ages 15 and younger were living with the virus, only about one-third were getting treatment (2). On 2018, UNAIDS estimated that there were 160,000 new HIV infections in children 0–14 years and that 210,000 infections were averted by maternal treatment in pregnancy (3). Without treatment, the HIV MTCT transmission rates range from 15 to 45%.
Approximately 5,000 women living with HIV give birth annually in the US (4). The HIV MTCT has been reduced to less than 1% in the United States due to universal HIV prenatal screening, antiviral treatment (ARV) for pregnant women, elective Cesarean delivery for women with HIV RNA>1,000 copies/ml, infant ART, and infant formula (5, 6). The interventions included in the landmark PACTG 076 study included: treatment during pregnancy (equivalent to Pre-Exposure Prophylaxis/PrEP), treatment during labor and delivery (equivalent to PrEP), and infant therapy for 6 weeks (equivalent to Post-Exposure Prophylaxis/PEP). When women are identified during pregnancy due to universal HIV rapid testing, in most settings (with resources) they are placed in antiviral therapy, what providers now refer as Rapid ART or same-day therapy (7, 8).
In 2014 and 2017, the World Health Organization (WHO) released the Global Guidance on Criteria and Processes for Validation: Elimination of Mother-to-Child Transmission of HIV and Syphilis (EMTCT) (9). Achieving validation of EMTCT is a tremendous accomplishment, but maintaining this status is equally important and requires sustained programmatic efforts. In 2015, the WHO announced that Cuba became the first country to be validated for the elimination of MTCT and syphilis (10, 11).
We had previously reported the reduction of the perinatal HIV transmission in Puerto Rico with data from 1990 to 2006 (12). This paper aims to: a) present additional data confirming that in fact we have eliminated the MTCT using the World Health Elimination Criteria; b) assert the claim that, using the WHO criteria, Puerto Rico eliminated MTCT much earlier than the reported timeframe for Cuba and other countries; c) provide a historical perspective of the convergence of science, practice, and policy that contributed to this achievement. We have eliminated the HIV perinatal transmission and sustained success ever since 2000.
Puerto Rico is considered an unincorporated territory of the US; it is an associate member of the WHO (13) and of the Pan-American Health Organization (PAHO) (14). Its achievements should be included in the list of countries that have eliminated the MTCT of HIV and Syphilis.
Methods
Epidemiological data was used to validate compliance with impact and process indicators as stated in the 2017 Global Guidance on Criteria and Processes for Validation: Elimination of Mother-to-Child Transmission of HIV and Syphilis (EMTCT). Specifically, we used two impact indicators for validation of EMTCT of HIV (according to section 3.1 of the plan): 1) a population case rate of new pediatric HIV infections due to MTCT of ≤50 per 100,000 live births and 2) an HIV MTCT rate of <2% (non-breastfeeding countries). The impact indicators for validation of EMTCT of syphilis (according to section 3.2 of the plan) was a case rate of congenital syphilis of ≤50 cases per 100,000 live births.
We included the data for three process indicators for validation (according to section 3.5 of the plan): 1) population-level antenatal care (ANC) coverage (at least one visit) of ≥95%, 2) coverage of HIV and/or syphilis testing of pregnant women of ≥95%, and 3) antiretroviral therapy (ART) coverage of HIV-positive pregnant women of ≥95%.
Puerto Rico Department of Health (PR DoH) data was used to document the indicators. Data from the Vital Statistics Office included: total number of live births per year (1980–2016), congenital syphilis cases from 2005 to 2016, and number of women attending prenatal care, trimester of first visit, and total number of visits from 2005 up to 2016. Recent data on births and prenatal care was obtained from the Division of Mothers, Children, and Adolescents. The HIV/AIDS Surveillance Program provided the number of perinatally-acquired HIV infections, number of women who tested positive in pregnancy who gave birth; number of HIV positive women who received antiviral therapy during pregnancy, labor, or delivery; and number of infants who received antiviral therapy. The number of congenital syphilis was obtained from the Sexually-Transmitted Diseases Surveillance Office.
The incidence rate of perinatally-acquired HIV Infections (per 100,000 live births) was computed from 1980 to 2016. The incidence rate for congenital syphilis was computed from 2005 to 2016. The rate of HIV Mother-to-Child Transmission (MTCT), the proportion of pregnant women with at least one prenatal visit, and the proportion of HIV-positive pregnant women who received antiretroviral therapy during pregnancy or birth were computed from 2005 to 2015. Figures for the impact and process indicators were prepared including a line marking of the WHO minimum targets for the elimination of MTCT. Policy documentation was used to demonstrate the synergy of efforts required to eliminate perinatal transmission.
This study did not require Institutional Review Board (IRB) approval since it is not a human or animal study. It used epidemiological data publicly available in declassified databases.
Results
Validation of Elimination of Mother-to-Child Transmission of HIV and Syphilis
The following figures depict the sustained decrease in perinatal HIV transmission as evidenced by various outcomes. Using the WHO outcome indicator of a rate of 50 per 100,000 births, we can observe the peak of perinatal HIV infections starting in the mid-80s and having a peak and a sharp decline after 1991. This coincided with the enrollment of women in the PACTG 076 protocol (15). As illustrated in Figure 1, the rate of perinatal HIV infections has been lower than 50 per 100,000 births since 1994. This is how far back the success in elimination of MTCT can be traced in PR.
Figure 1.
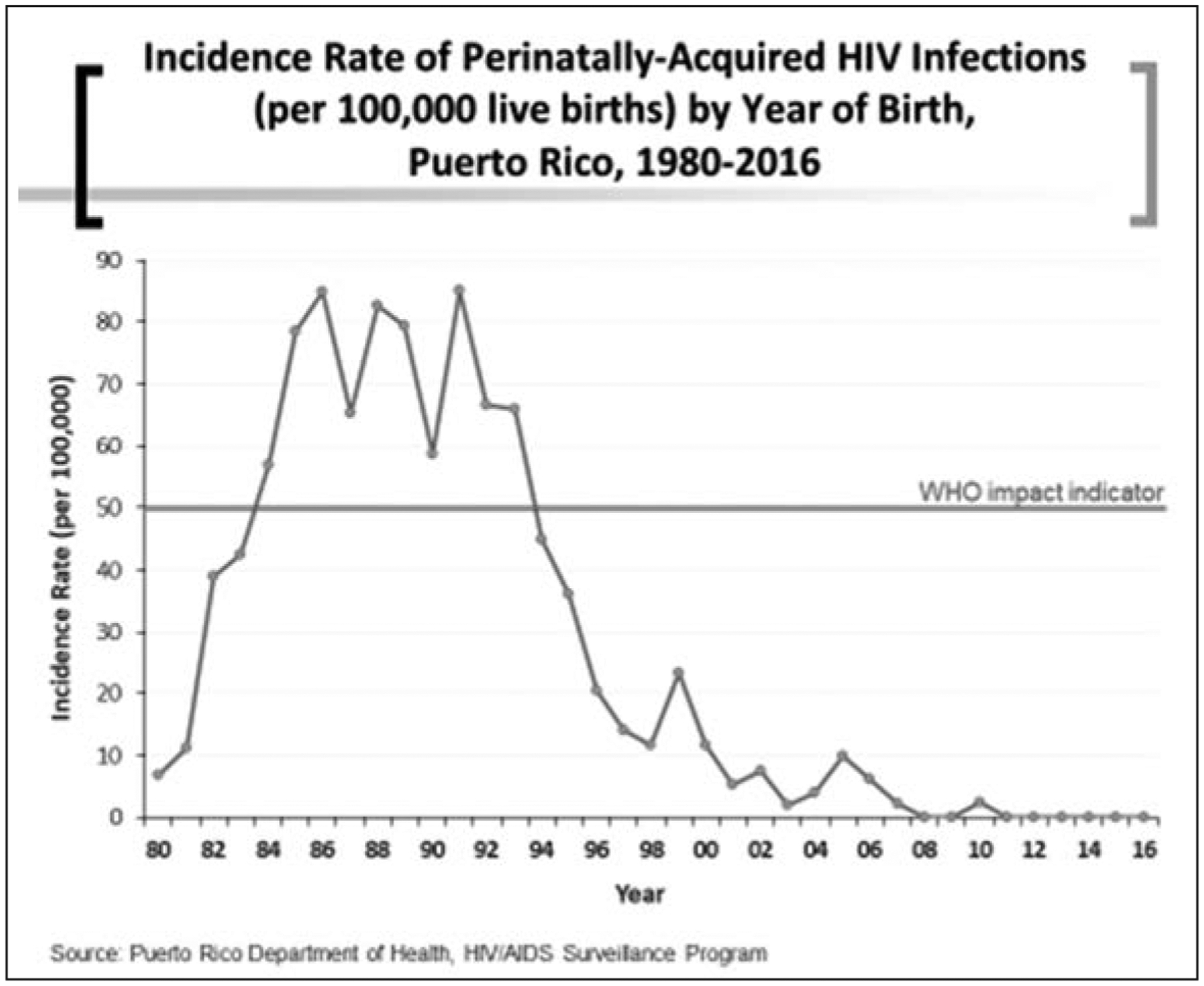
Incidence rate of perinatally-acquired HIV infections (per 100,000 live births) by year of birth, Puerto Rico, 1980–2016
Throughout the years, women have transmitted the HIV infection to their infants, but the rate of MTCT per year in PR had a sharp decline since 2005 (Figure 2). The WHO impact indicator is transmission rate of 2% for non-breastfeeding countries. We have sustained a lower rate since 2007. The PR DoH investigates every case of perinatal transmission in the island to address any gaps in care. The HIV/AIDS Surveillance Program monitors the medical charts of infants and pregnant women where MTCT has been documented (personal communication from Mrs. Eileen Pérez, PR DoH Perinatal HIV Program).
Figure 2.
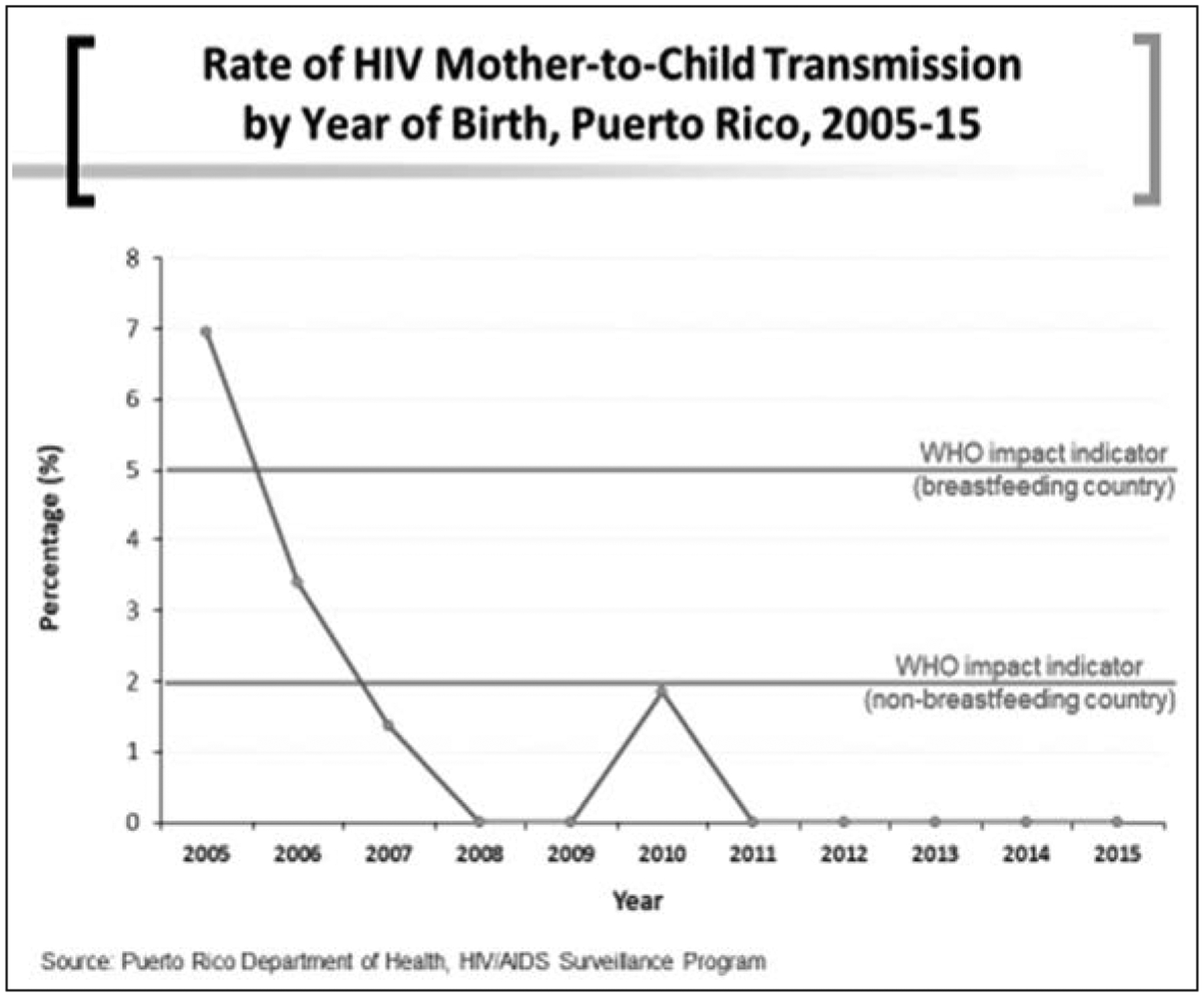
Rate of HIV Mother-to-Child Transmission (MTCT) by year of birth, Puerto Rico, 2005–15
Puerto Rico offers accessible prenatal care to women, irrespective of their economic capacity. Historically, pregnant women attend prenatal care mostly during the first 2 trimesters. Figure 3 shows that over 99% of women have at least one prenatal care visit, higher than the 95% required by the WHO process indicator.
Figure 3.
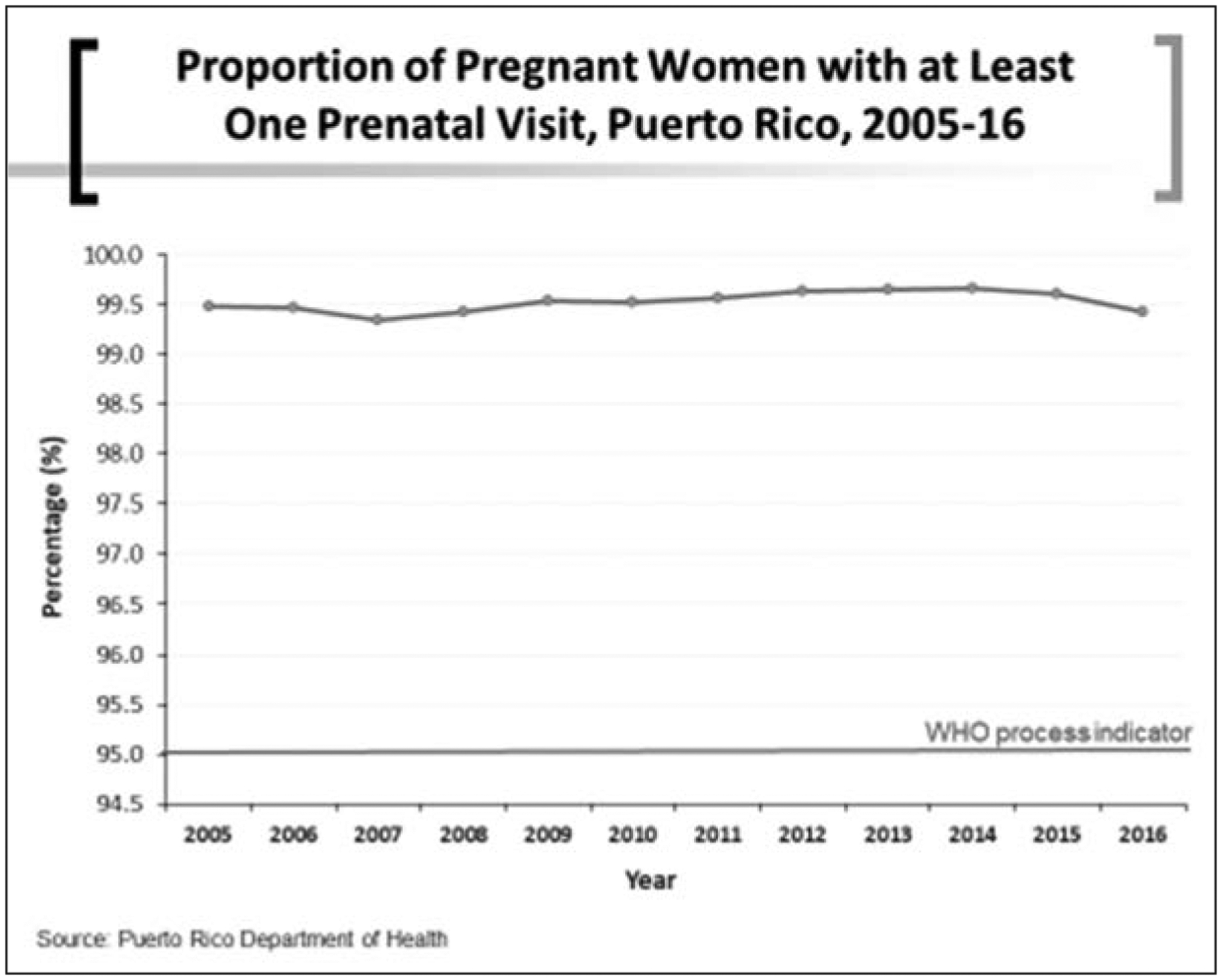
Proportion of pregnant women with at least one prenatal visit, Puerto Rico, 2005–16
According to the WHO indicator of antiviral treatment during pregnancy or labor, Puerto Rico has increased the rate of therapy consistently, but attained the 90% in 2008 and the 95% rate around 2009 (Figure 4). Puerto Rico provides Highly Active Antiretroviral Therapy (HAART) to pregnant women living with HIV. The population of Puerto Rico have had access to HIV treatment and care mostly through the Health Resources and Services Administration’s (HRSA) Ryan White HIV/AIDS Program since 1990 (16). The program provides funding to states, cities, and community organizations for comprehensive clinical care, essential support services, and medications to people living with HIV who are underserved, including pregnant women living with HIV.
Figure 4.
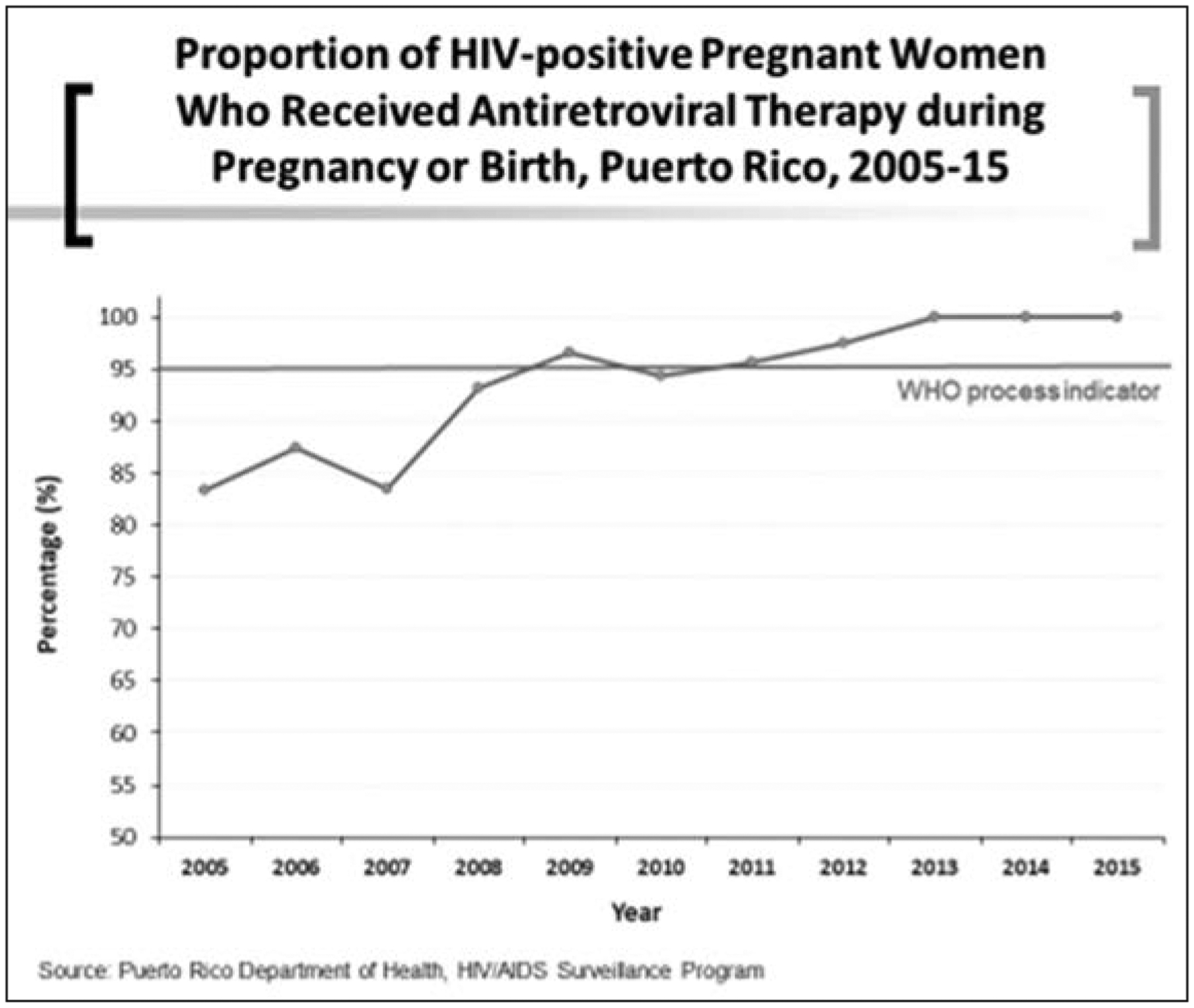
Proportion of HIV-positive pregnant women who received antiretroviral therapy during pregnancy or birth, Puerto Rico, 2005–15
For the elimination of congenial syphilis, the WHO indicator was an incidence rate of congenital syphilis of ≤50 per 100,000 live births. Puerto Rico’s data shows elimination of syphilis since at least 2005. Figure 5 shows the incidence rate of congenital syphilis from 2005 until 2016.
Figure 5.
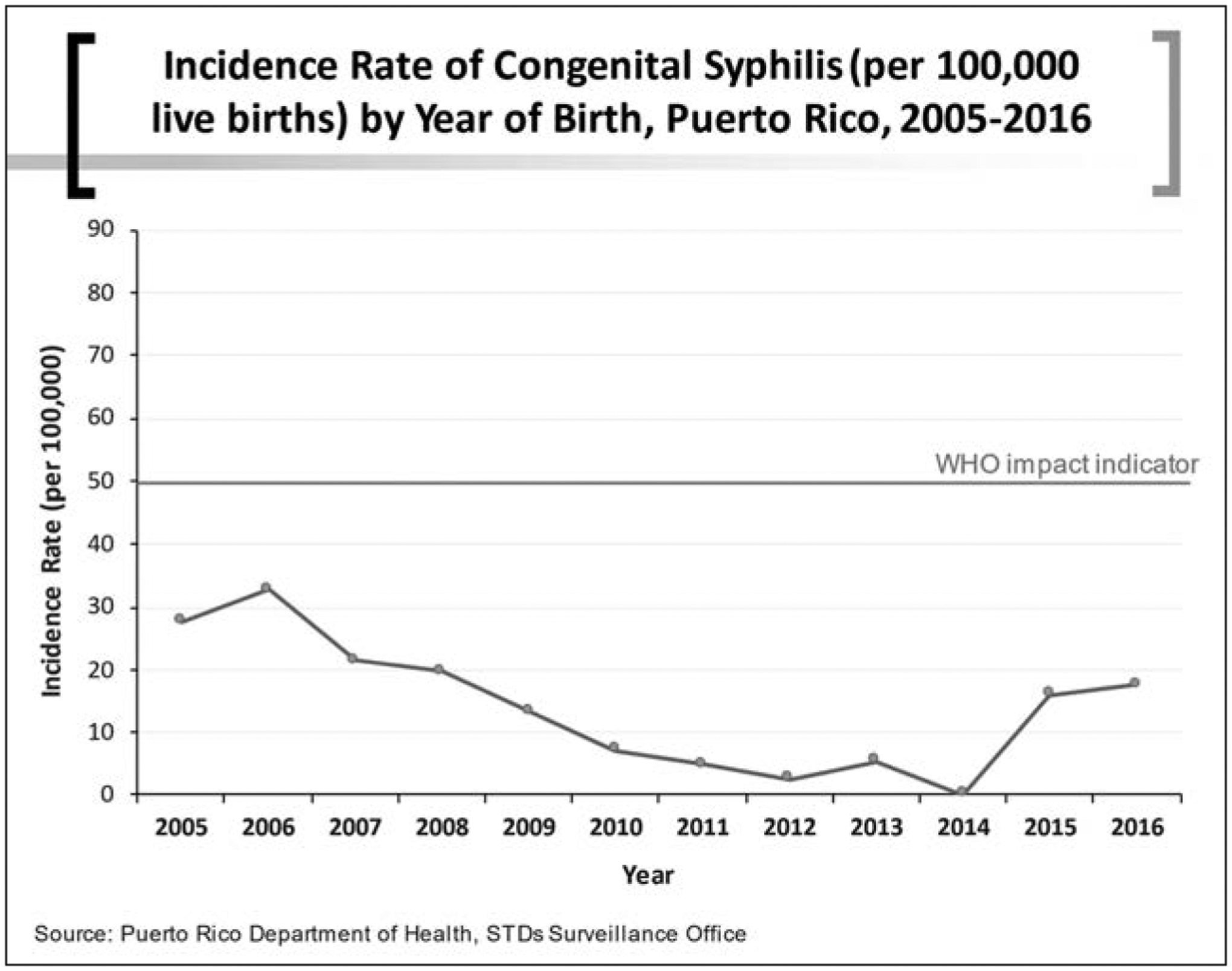
Incidence rate of congenital syphilis (per 100,000 live births) by year of birth, Puerto Rico, 2005–2016
Convergence of Research, Clinical Practices and Policies
Puerto Rico reported the first case of AIDS in 1981. Shortly thereafter, PR DoH established a surveillance unit, which to this date provides the official data on incidence, mortality, and risk behaviors. By 1984, there were 185 AIDS cases reported, 270 in 1985, up to 50,017 cases of HIV/AIDS as of December 31, 2019 (17, 18). Synergy among research, public policy, and availability of treatment and care during pregnancy contributed to the sustained success of PR in the elimination of HIV MTCT since 2007.
Prenatal HIV screening
Puerto Rico established a prenatal universal HIV screening program in 1987 (12). This program targeted women receiving care at the University Hospital catchment area. A previous anonymous survey of pregnant women had raised concerns due to what we then believed was a high prevalence (1.8%) of HIV infection. Immediate responses to the survey were the expansion into universal HIV screening and referrals of women to a centralized clinic at the Puerto Rico Medical Center. The screening program included a written consent and samples processed at one of the Medical Sciences Campus (MSC), University of Puerto Rico (UPR), research laboratories. The Maternal Infant Studies Center (CEMI, for its Spanish acronym) was established to follow the women and their infants throughout pregnancy and longitudinally.
In collaboration with the CDC and HRSA, the PR DoH established the infrastructure for HIV testing, surveillance, and care. With the accumulated experience of the MSC screening program, universal HIV screening in pregnancy became a public policy on October 1990 (Office of AIDS Affairs and Communicable Diseases/Personal Communication from Dr. Johnny Rullán, Director). This early response allowed the expansion of the prenatal testing throughout the island. Women were identified early and referred to the High-Risk Prenatal Clinic which later became CEMI. Throughout the years, other clinics were available to follow pregnant women living with HIV; however, CEMI remains the largest program. Around 60% of pregnant women living with HIV receive care at CEMI. The initial CDC guidelines for prenatal testing were published on July 1995. Dr. Zorrilla (author of this paper) and Dr. Clemente Diaz (Pediatrics Department, MSC, UPR) participated in initial CDC expert meetings. These guidelines were modified on November 9, 2001, to support universal screening. Puerto Rico had this in place since 1990 (19).
Research programs and early public policy
Early screening policy and researchers’ involvement was instrumental to secure participation in national studies about HIV in pregnancy. Three main strategies facilitated the participation of women living with HIV in research, mostly funded by the National Institutes of Health (NIH). First, a longitudinal clinic for women with HIV was supported through the Research Centers for Minority Institutions (RCMI) as early as 1987. This clinic became CEMI, which has produced the most research, training, and care activities related to pregnancy and HIV.
Second, participation on a large cohort study, the Women and Infant Transmission Study (WITS), which was funded by the National Institute of Child Health and Development (NICHD) and the National Institute of Allergy and Infectious Diseases (NIAID). Enrollment of women started on December 1989 and continued for more than 12 years.
Third, we participated in 1991 as one of the first 10 pilot sites for the PACTG 076 protocol which studied the impact of the drug Zidovudine on the MTCT. The study was conducted at 59 sites of the AIDS Clinical Trials Group (ACTG) funded by NIAID and in France. Puerto Rico had 3 participating sites on this study (15). The PACTG 076 was a Phase III, randomized, double-blind, placebo-controlled clinical trial, designed to evaluate whether zidovudine (AZT) administered to HIV-infected pregnant women and their infants could reduce the rate of transmission from mother to infant. An interim analysis showed that of the 364 evaluated infants, 53 had HIV infection. Of those 53, 13 received zidovudine and 40 received the placebo. The estimated rate of transmission in the treatment group (ZDV) was lower than in the placebo group (8.3% vs. 25.5%). These results were statistically significant (p=0.00006) (20). Due to intervention efficacy, the study was halted and notification of the results were released to the press on February 1994. The placebo arm was eliminated for ethical reasons and women who were on placebo were switched to open-label drug. On March 1994, the PR DoH established as a public policy the availability of free ZDV to any pregnant women with HIV in the island. This was the quickest policy response for treatment of pregnant women living with HIV, one month after a study was halted due to the efficacy of the drug. This policy reinforced prenatal testing, allowed for the availability of treatment during pregnancy, and entailed training of health professionals in obstetrical and pediatric hospitals.
The CDC recommended AZT use in August 1994 after an expert meeting was convened on June 6, 1994 (21). Dr. Zorrilla participated in this meeting. On April 1994, the CDC published an editorial advising providers to discuss the results of the study with their pregnant patients and encouraged treatment with zidovudine. PR had already established the policy of prenatal ZDV treatment on March 1994 (22).
Rapid testing in the labor and delivery rooms
Another step toward the elimination of MTCT was the pilot-testing and implementation of rapid HIV testing in labor rooms. A pilot program was carried out at a Community Hospital in PR (Carolina Area Hospital) in collaboration with the CDC, the PR DoH, and the MSC. This study was implemented on 2002 and the experience from 2002–2008 demonstrated that 6 of 279 women tested in labor were found positive (2.1%). Treatment was made available and no infants were infected (Pilot study 2002–2008 at Carolina Hospital, personal communication from Mrs. Eileen Pérez, PR DoH Perinatal HIV Program).
A public policy for rapid HIV testing in the labor room was established on February 20, 2008. The PR DoH and the CDC provided rapid testing kits to all hospitals in PR. From 2008 to 2016, a total of 19,698 tests were performed (on women without prior testing). This constituted 9% of the total 221,475 deliveries for that period. Although the prevalence was low, because most HIV positive women had been tested during prenatal care, this policy helped to identify twelve additional women and further reduce perinatal HIV transmission risk (Mrs. Eileen Pérez, PR DoH Perinatal HIV Program Monitor, personal communication). An additional HIV test in the 3rd trimester was added to prenatal care, since most women are tested early in the first trimester. This administrative ordinance was signed on August 14, 2013 (23). A second test during pregnancy was also included in the CDC recommendations published on 2001 as well as emphasizing HIV rapid test in labor for women without prior testing (19).
Figure 6 depicts three decades of milestone research and policy, highlighting the response in PR.
Figure 6.
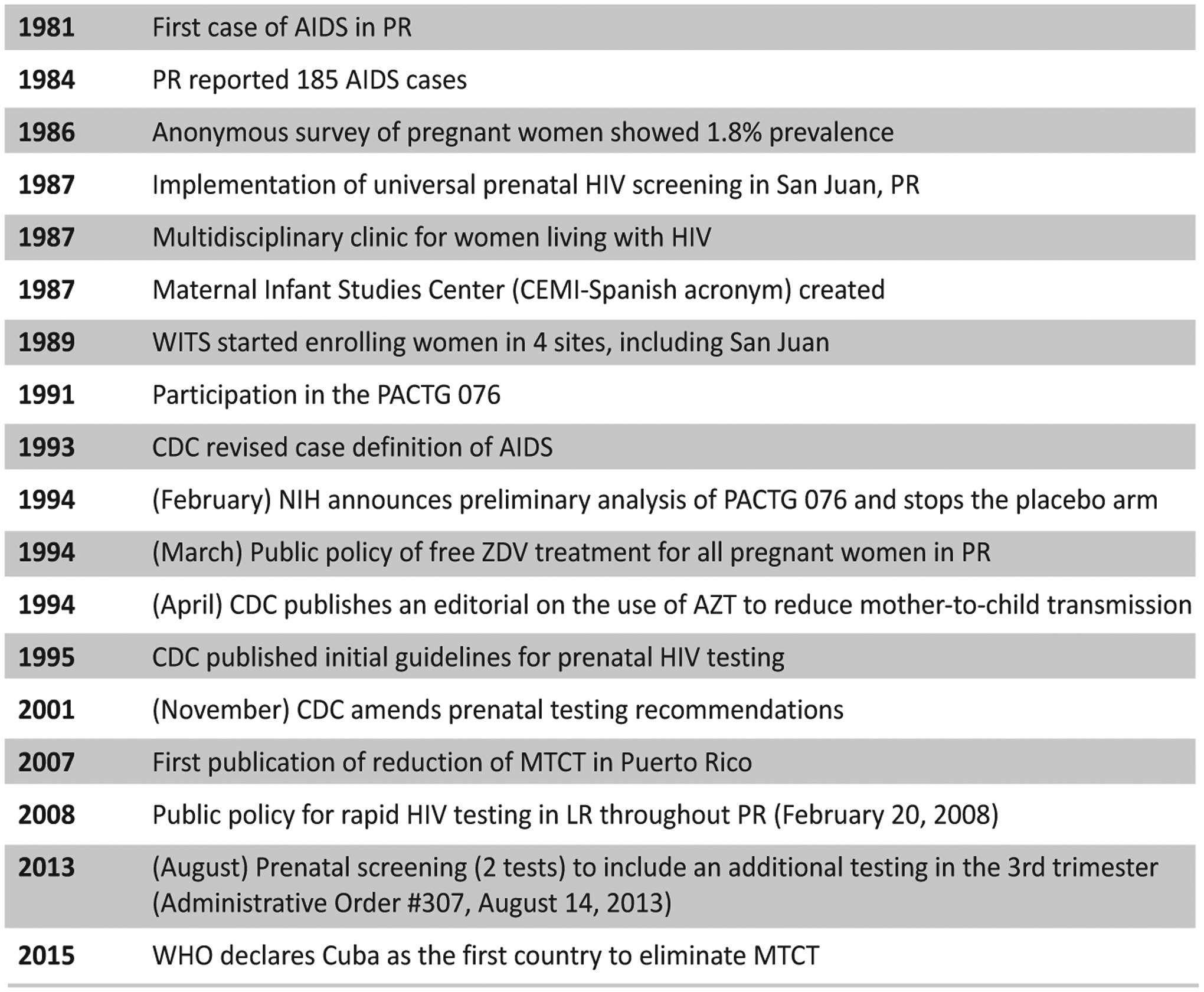
Timeline of advances, recommendations, and public policies regarding perinatal HIV transmission prevention in Puerto Rico and the US.
Discussion
The advances in the prevention of MTCT and the availability of potent lifesaving drugs have changed the course of the epidemic. The availability of antiviral therapy (ART) to women living with HIV during pregnancy and labor has averted 1.6 million pediatric infections since 1995. Efforts were more successful between 2010 and 2016, due to expanded and improved access. Still in 2016, an estimated 120,000 children died due to AIDS (24).
Pregnancy and antenatal screening, at the time when many women learn about their diagnosis and when engaging in longitudinal care, contributed to the elimination of perinatal HIV transmission. Comprehensive care, with access to testing, treatments, counseling case management, and social support in a non-judgmental way are critical to this population.
We presented data that evidences the elimination of HIV and syphilis MTCT in PR long before that of the first country declared by the WHO as doing so. In fact, the sustained elimination of perinatal HIV transmission can be dated according to the outcomes: to 1994 using the incidence rate of <50/100,000; to 2007 using perinatal transmission rates for non-breastfeeding countries (<2%), to 2008 using 90% of women receiving ART at delivery, and to 2005 using the incidence rate of congenital syphilis of <50/100,000.
Puerto Rico had a cadre of investigators, providers, public health officials, and policy makers that assumed a leadership role in the implementation of policies that were pioneer at the time. We implemented universal HIV screening in pregnant women (with written consent) at a time when there were no treatments for HIV. We believed that women needed to know their HIV status and should have access to preventive and risk reduction strategies. When the scientific evidence demonstrated the effectiveness of ZDV in reducing the risk for MTCT, we were ready to change public policy and make free ZDV available to pregnant women identified through the Universal HIV prenatal screening. It took commitment and coordination to implement this policy and distribute the ZDV to all hospitals. Infant formula was available in the island and given for free to women as needed. Elective cesarean deliveries were offered to women living with HIV as soon as the results of the scientific retrospective analyses were published. The Ryan White HIV/AIDS Program provided funding for care and medications free of charge to low-income individuals whose health insurance did not cover such services.
Elimination of transmission of infectious diseases is not easily achieved; it requires the intersection of science, coordinated interventions, and political will. The success and achievement experienced by Puerto Rico regarding MTCT of HIV and syphilis can only be sustained by safeguarding the commitment and investment in research, training, outreach, and women-centered care.
Acknowledgments
The authors want to recognize the following individuals who had a role in the elimination of MTCT in Puerto Rico throughout the years: Dr. Johnny Rullán (deceased), State Epidemiologist - Puerto Rico Department of Health 1987–94, Office of AIDS Affairs and Communicable Diseases (OCASET) Director, and PR Department of Health Secretary 2005–2007- developed the infrastructure for care of people living with HIV and led the early public policy efforts for prenatal HIV testing. Dr. Carmen Feliciano (deceased), PR Department of Health Secretary 2001–2004 - established the public policy for ZDV treatment of pregnant women one month after the results of the PACTG study were made public. Dr. Clemente Diaz, UPR School of Medicine, Pediatrics Department Faculty established the first pediatric clinic for children with AIDS in PR and was a PI for the WITS and other perinatal intervention studies including the PACTG076. Dr. Irma Febo, UPR School of Medicine Pediatrics Department Faculty - currently leads the pediatrics clinic and many clinical, behavioral and intervention studies. Dr. Alberto Carrera, PR Department of Health Ob-Gyn Consultant - was in charge of the rapid HIV testing pilot program at the Carolina Hospital and helped train professionals on perinatal HIV. Dr. Eleonor Jimenez, San Juan City Hospital Pediatrics Department Faculty - established the pediatric program for HIV/AIDS at the San Juan City Hospital and was also part of the PACTG 076. Dr. José M. García-Castro (deceased), UPR School of Medicine, Pediatrics Department Faculty - established the laboratory infrastructure for the first prenatal screening program and for the universal prenatal screening program at the MSC. Dr. George Hillyer (deceased), UPR School of Medicine, Pathology Department Faculty - established the laboratory infrastructure for the WITS and other research programs at the MSC. Prof. Daisy Gely, the PI of the AIDS Education and Training Center (AETC) at the University of Puerto Rico, Medical Sciences Campus, which was responsible for most of the training of professionals on the diverse public policies in PR including antenatal testing, ZDV treatment, Rapid HIV testing, and management of people living with HIV.
Footnotes
The authors have no conflict of interest to disclose.
References
- 1.World Health Organization. Global Health Observatory (GHO) data [WHO Web site]. 2019. Available at: https://www.who.int/gho/hiv/en/. AccessedJanuary 9, 2020.
- 2.WebMD. Children with HIV and AIDS. [WebMD Web site]. January9, 2020. Available at: https://www.webmd.com/hiv-aids/guide/hiv-in-children#1; AccessedJanuary 9, 2020.
- 3.AIDS Info. Epidemics transition metrics. [UNAIDS Web site]. Available at: http://aidsinfo.unaids.org/. AccessedFebruary 21, 2019.
- 4.Nesheim SR, FitzHarris LF, Lampe MA, Gray KM. Reconsidering the number of women with HIV infection who give birth annually in the United States. Public Health Rep. 2018; 133:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nesheim SR, Wiener J, Fitz Harris LF, Lampe MA, Weidle PJ. Brief report: estimated incidence of perinatally acquired HIV infection in the United States, 1978–2013. J Acquir Immune Defic Syndr. 2017;76:461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters H, Francis K, Sconza R, Peckham C, Tookey PA, Thorne C. UK mother-to-child HIV transmission rates continue to decline: 2012–2014. Clin Infect Dis. 2017; 64:527–528. [DOI] [PubMed] [Google Scholar]
- 7.Rosen S, Maskew M, Fox MP, Nyoni C, Mongwenyana C, Malete G, et al. Rapid ART Initiation Reduces Loss Between HIV Testing and Treatment: The RapIT Trial. 2015. Poster session presented at the Conference of Retroviruses and Opportunistic Infections, Seattle, Washington. Available at: http://www.croiconference.org/sessions/rapid-art-initiation-reduces-loss-between-hiv-testing-and-treatment-rapit-trial.AccessedFebruary 21, 2019. [Google Scholar]
- 8.Highleyman L RAPID Program Leads to Faster HIV Suppression [BETA Web site]. July24, 2015. Available at: https://betablog.org/rapid-program-leads-to-faster-hiv-suppression/.AccessedFebruary 21, 2019.
- 9.World Health Organization. Criteria and processes for validation: Elimination of Mother-to-Child transmission of HIV and Syphilis [WHO Web site]. 2014. Available at: https://apps.who.int/iris/bitstream/handle/10665/112858/9789241505888_eng.pdf?sequence=1.AccessedFebruary 21, 2019.
- 10.World Health Organization. WHO validates elimination of mother-to-child-transmission of HIV and syphilis in Cuba. [WHO Web site]. June30, 2015. Available at: https://https://www.who.int/mediacentre/news/releases/2015/mtct-hiv-cuba/en/.AccessedFebruary 21, 2019.
- 11.World Health Organization. Global guidance on criteria and processes for validation: Elimination of Mother-to-Child transmission of HIV and Syphilis [WHO Web site]. 2017. Available at: https://www.who.int/reproductivehealth/publications/emtct-hiv-syphilis/en/.AccessedFebruary 21, 2019.
- 12.Zorrilla C, Tamayo Agrait V, Febo I, Santiago L, Díaz C, Salabarria I, et al. Reduction in the Perinatal HIV transmission at the Maternal Infant Studies Center and Gamma Projects at the UPR School of Medicine. P R Health Sci J. 2007: 26:329–335. [PubMed] [Google Scholar]
- 13.Entrevista al Dr. Raúl Castellanos: Son importantes la continuidad y la visión integral que puede dar el médico de familia. Galenus. Año 11; 68:52–55. [Google Scholar]
- 14.Pan American Health Organization. Member States [PAHO Web site]. Available at: Url: https://www.paho.org/hq/index.php?option=com_content&view=article&id=103:member-states&Itemid=1110&lang=en.AccessedFebruary 21, 2019.
- 15.National Institute of Allergy and Infectious Disease. ACTG 076 Questions and Answers. [AIDS Info Web site]. February20, 1994. Available at: https://aidsinfo.nih.gov/news/101/actg-076-questions-and-answers.AccessedFebruary 21, 2019.
- 16.Health Resource & Services Administration. About the Ryan White HIV/AIDS Program. [HRSA Web site]. February2019. Available at: https://hab.hrsa.gov/about-ryan-white-hivaids-program/about-ryan-white-hivaids-program.AccessedFebruary 21, 2019.
- 17.Puerto Rico HIV/AIDS Surveillance Summary. Cumulative HIV/AIDS Cases Diagnosed as of January 31, 2019. January2019. [Google Scholar]
- 18.Gomez MA, Fernandez DM, Otero JF, Miranda S, Hunter R. The shape of the HIV/AIDS epidemic in Puerto Rico. Rev Panam Salud Pública. 2000; 7:377–383. [DOI] [PubMed] [Google Scholar]
- 19.CDC. Prenatal HIV testing. Morb Mortal Wkly Rep. November9, 2001; 50:1–58. [Google Scholar]
- 20.Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med 1994;331:1173–1180. [DOI] [PubMed] [Google Scholar]
- 21.CDC. US Public Health Service recommendations for human immunodeficiency virus counseling and voluntary testing for pregnant women. Morb Mortal Wkly Rep. July7, 1995; 44:1–14. [PubMed] [Google Scholar]
- 22.CDC. Zidovudine for the Prevention of HIV Transmission from Mother to Infant. Morb Mortal Wkly Rep. April24, 1994; 43:285–287. [PubMed] [Google Scholar]
- 23.Departamento de Salud. Orden Administrativa Núm. 307 para ordenar el ofrecimiento de la prueba de VIH a toda embarazada en el primer y tercer trimestre de embarazo. [Departamento de Salud Gobierno de Puerto Rico Web site]. Available at: http://www.salud.gov.pr/Profesionales-y-Proveedores/Protocolos/Orden%20Adminitrativa%20Num%20307%20-2%20%20Pruebas%20de%20VIH%20Prenatales.pdf.AccessedFebruary 21, 2019.
- 24.UNAIDS. Prevention GAP report [UNAIDS Web site]. 2016. Available at: http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf.AccessedFebruary 21, 2019.


