Abstract
Background
Coronavirus disease 2019 (COVID-19) may cause myocardial injury and myocarditis, and reports of persistent cardiac pathology after COVID-19 have raised concerns of long-term cardiac consequences. We aimed to assess the presence of abnormal cardiovascular resonance imaging (CMR) findings in patients recovered from moderate-to-severe COVID-19, and its association with markers of disease severity in the acute phase.
Methods
Fifty-eight (49%) survivors from the prospective COVID MECH study, underwent CMR median 175 [IQR 105-217] days after COVID-19 hospitalization. Abnormal CMR was defined as left ventricular ejection fraction (LVEF) <50% or myocardial scar by late gadolinium enhancement. CMR indices were compared to healthy controls (n = 32), and to circulating biomarkers measured during the index hospitalization.
Results
Abnormal CMR was present in 12 (21%) patients, of whom 3 were classified with major pathology (scar and LVEF <50% or LVEF <40%). There was no difference in the need of mechanical ventilation, length of hospital stay, and vital signs between patients with vs without abnormal CMR after 6 months. Severe acute respiratory syndrome coronavirus 2 viremia and concentrations of inflammatory biomarkers during the index hospitalization were not associated with persistent CMR pathology. Cardiac troponin T and N-terminal pro-B-type natriuretic peptide concentrations on admission, were higher in patients with CMR pathology, but these associations were not significant after adjusting for demographics and established cardiovascular disease.
Conclusions
CMR pathology 6 months after moderate-to-severe COVID-19 was present in 21% of patients and did not correlate with severity of the disease. Cardiovascular biomarkers during COVID-19 were higher in patients with CMR pathology, but with no significant association after adjusting for confounders.
Trial Registration
COVID MECH Study ClinicalTrials.gov Identifier: NCT04314232
Keywords: COVID-19, cardiac magnetic resonance imaging, CMR, biomarkers, troponin, NT-proBNP
Abbreviations: COVID-19, Coronavirus disease 2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; LGE, Late Gadolinium Enhancement; IL-6, Interleukin-6; ICU, Intensive care unit; NEWS, National Early Warning Score; CRP, C-reactive protein; PCT, Procalcitonin; cTnT, Cardiac troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide; eGFR, Estimated glomerular filtration rate
Cardiac involvement in coronavirus disease 2019 (COVID-19) is common. Patients with underlying cardiovascular disease (CVD) are at increased risk of severe disease and cardiac complications. Arrhythmias, acute coronary syndrome and heart failure related events are known to occur in patients hospitalized with COVID-19,1., 2., 3. in addition to the less frequent fulminant severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) myocarditis.4 Myocardial injury, reflected by elevated concentrations of cardiac troponins, is frequent but the prevalence depends on the baseline risk of the population and clinical setting.5 A large proportion of hospitalized COVID-19 patients with elevated cardiac troponins has been reported to have elevated cardiovascular magnetic imaging (CMR) measurements of T1, extracellular volume or late gadolinium enhancement (LGE) with a non-ischemic pattern in the acute phase.6 Retrospective studies have suggested that cardiac troponin and natriuretic peptides are markers of risk in COVID-19.2 , 7 This is however less clear in prospective studies with unselected patients.8
Persistent symptoms, particularly fatigue and dyspnea, are common after COVID-19.9 Myocardial inflammation and ventricular dysfunction determined by CMR were reported in 78% of patients who recovered from predominantly mild-moderate COVID-19 infection, irrespective of cardiac symptoms.10 In studies of college athletes with COVID-19, the presence of CMR findings consistent with myocarditis varies significantly from 1.5% to 15%. % 11, 12., 13 The presence of abnormal CMR findings in patients recovered from moderate-to-severe COVID-19, and the association with cardiac biomarker concentrations and SARS-CoV-2 viremia in the acute phase is unknown.
Methods
Study design and participants
COVID MECH (NCT04314232) was a prospective, observational study consecutively enrolling unselected adult patients hospitalized with laboratory-confirmed COVID-19. The study was conducted at Akershus University Hospital in Norway between March 18 and May 4, 2020.14 Participants were classified as ICU patients if they were admitted to the ICU and received intensive care treatment (mechanical ventilation) for >24 hours. History of CVD, pulmonary disease, hypertension and diabetes was recorded from the electronic medical records and after interviewing the patients at discretion of the treating physicians. National Early Warning Score is a validated tool used for detection and response to clinical deterioration in adult patients. It is calculated from 6 vital signs, with low risk measurements yielding 0 points, and abnormal values giving up to 3 points per item, with a maximum score of 20.15
After discharge, patients were invited by mail or telephone to a follow-up study. Of 128 participants included in the COVID MECH biobank study, there were 118 survivors at time of the follow-up study, and 102 were eligible for participation. Sixteen patients were excluded due to cognitive impairment, major language barriers, being still hospitalized at time of initiation of follow-up study, residing outside the hospital catchment area or with unavailable contact information. Of the eligible patients who were invited to the follow-up study, 63 (62%) were scheduled for CMR examination, while 28 did not want to participate and 11 did not reply to the invitation. Four patients aborted the CMR examination due to claustrophobia and one did not attend the scheduled CMR, leaving 58 patients with available CMR who make up the population in the current study (Suppl. Figure 1 ).
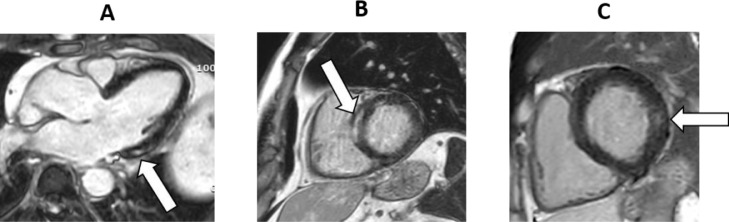
Figure 1.
Title: Non-ischemic left ventricular (LV) scar by late gadolinium enhancement imaging, Caption: Late gadolinium images demonstrating non-ischemic scars in A, the basal inferolateral LV of a 54-year-old male 209 days after hospitalization for COVID-19; B, the basoseptal LV of a 60 year old male 175 days after hospitalization for COVID-19; and C, the basolateral LV of a 50 year old male 202 days after hospitalization for COVID-19.
Patients were compared to 32 healthy participants from the prospective, population-based, age-cohort Akershus Cardiac Examination 1950 Study.16 The controls were normotensive, non-obese non-smokers without known diabetes or cardiovascular disease.
The COVID MECH, COVID CMR and Akershus Cardiac Examination 1950 studies were approved by the Regional Ethics Committee (#20/02873; #20/05884; #2011/1475) and by the institutional Data Protection Officer (#117589; #148701; #12_093). No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
CMR protocol
The CMRs were conducted at Akershus University Hospital between June 24 and November 18 2020 on a 1.5 MRI scanner (Achieva; Philips Medical Systems, Best, The Netherlands). Short-axis, steady-state-free precession sequences were acquired in contiguous 8 mm short axis slices for assessing ventricular volumes and ejection fraction. T2 STIR images were acquired in 10 mm slices in a single midventricular short axis view and 1 four and 1 left ventricular 2 chamber views. Two-dimensional, phase sensitive inversion recovery LGE imaging in contiguous 10 mm short-axis slices covering the ventricles and 3 long-axis views for assessing myocardial scarring was performed starting 10 minutes after injection of 0.15 mmol/kg gadoterate meglumine (Clariscan Gé, GE Healthcare). Myocardial T1 and T2 mapping sequences for the assessment of diffuse myocardial fibrosis and edema were acquired in single 10 mm midventricular short-axis slices. T1 maps were acquired before and 15 minutes after contrast administration using MOLLI sequences with 5s (3s) 3s and 4s (1s) 3s (1s) 2s mapping schemes, respectively. A gradient-spin echo sequence was used for T2 mapping. T1 and T2 maps were generated on dedicated software (cvi42, v5.11.4, Circle Cardiovascular Inc, Calgary, Canada). Blood hematocrit for calculation of the extracellular volume fraction was measured at the time of CMR examination.
CMR assessment
Assessment of ventricular volumes and EF and mass was performed on cvi42 according to SCMR guidelines.17 Trabeculations and papillary muscles were included in the LV volumes. The presence of scar was assessed on LGE sequences by semiautomatic signal intensity thresholding 5 standard deviations above remote myocardium and categorized as ischemic or non-ischemic. Presence of LGE (myocardial scar) or left ventricular EF (LVEF) <50% were defined as abnormal CMR. These were further classified into major pathology (scar and LVEF <50% or LVEF <40%) and minor pathology (scar and LVEF ≥50% or LVEF 40%-49%).
T2 STIR images were visually assessed for focal myocardial and pericardial edema. Myocardial T1 and T2 relaxation times were measured by conservatively placing regions of interest in the midventricular septum. Areas of LGE and significant artifacts were excluded from the measurements. ECV fraction was calculated as previously described.18 Myocardial feature tracking strain analyzes was performed on cvi42. Left ventricular longitudinal strain was assessed in 3 long axis views, and circumferential strain in 3 short axis slices (basal, midventricular, and apical). Examinations with persisting inadequate tracking after up to 2 times contour correction were excluded from analysis.
Laboratory analysis
Blood samples were collected at admission and on target day 3 (day 2-5 accepted) during hospitalization and stored at -80 °C in a study-specific biobank pending analysis. Measurements of interleukin-6 (IL-6), procalcitonin, ferritin, cardiac troponin T (cTnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were performed by the Elecsys immunoassay on the Cobas e801 platform (Roche Diagnostics, Rotkreuz, Switzerland). C-reactive protein was measured as part of clinical routine. Five patients had missing biobank samples, and for these cTnT, NT-proBNP and ferritin were recorded from the clinical routine measurements, while IL-6 and procalcitonin are reported as missing. SARS-CoV-2 RNA in plasma (viremia) was detected by reverse transcription real-time polymerase chain reaction on a QuantStudio 7 system (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Details of the laboratory analysis have been reported previously.14
Statistical analyses
Values are reported as N (%), median (quartile 1 to quartile 3) or mean ± SD, as appropriate. Categorical and continuous variables were compared using the chi-square test for binary variables, ANOVA for parametric continuous variables, and the Kruskal-Wallis test for non-parametric continuous variables. Change in biomarker concentrations from hospital admission to day 3 were calculated by subtraction and compared by non-parametric tests. To account for possible confounders between biomarker concentrations and CMR pathology, we performed multivariable logistic and linear regression models that were adjusted for age, sex, race and established CVD (selected a priori), using log-transformed biomarker concentrations. We also adjusted for time from index hospitalization to CMR examination in additional models. All statistical analyses were performed using Stata Software (version 16, Stata Corp., College Station, TX, USA). A 2-sided P-value of <.05 was considered statistically significant.
Results
Baseline characteristics
The time from hospital admission to the CMR examination was median 175 (IQR 105-217) days (range 75-246). The 58 patients with available CMR were aged median 56 (Q1-Q3 50-70) years, 30 (56%) were male, 30 (57%) were Caucasian and median BMI was 27.2 (24.2-29.4) kg/m2. Established CVD was present in 5 (9%), hypertension in 12 (21%), diabetes mellitus in 6 (11%) and chronic kidney disease in 2 (4%) patients. The median length of stay at the index hospitalization was 7 (4-11) days and 11 (19%) were treated with mechanical ventilation in the ICU. Hydroxychloroquine was given to 23 (40%) patients, while no patients were treated with high dose corticosteroids or convalescent plasma. On admission for acute COVID-19, 69% reported dyspnea, 25% chest pain and 56% fatigue. After the acute COVID-19 infection 55% reported persistent dyspnea, 4% chest pain and 64% fatigue. Cardiac arrhythmia was reported in 2 patients during the acute COVID-19. Screening with 24-hour ECG monitoring post-COVID-19 revealed 1 patient with paroxysmal atrial fibrillation and 4 patients with short episodes of self-limiting non-sustained ventricular tachycardia. There was no significant difference with respect to demographics, comorbidities, vital signs on admission, length of stay or ICU treatment between the study population and other participants in the total COVID MECH cohort (Suppl. Table 1).
Cardiac pathology on CMR
By protocol, the 2 patients with chronic kidney disease did not receive contrast and were accordingly not evaluated for myocardial scar by LGE. Both these patients had no other pathology on CMR (comparable LV and RV structure and function, T1, T2 and strain to those with available LGE), and were accordingly classified with normal CMR. In addition, 1 patient had unevaluable LGE sequences due to severe motion artifacts. This patient had reduced LVEF, and was accordingly classified with abnormal CMR.
In total, 12 (21%) patients were classified with abnormal CMR. Among these, 3 patients had major myocardial pathology: 1 with both myocardial scar (a combined ischemic/non-ischemic scar of 6.5% scar volume) and reduced LVEF (38%): 1 with LVEF 37% and no scar; and 1 with LVEF 39% and unavailable LGE-measurements due to unevaluable LGE. The remaining 9 patients with abnormal CMR were classified with minor pathology on CMR. One patient had a combined ischemic/non-ischemic scar of 3.0 % scar volume, the other 8 had non-ischemic scars (mean scar volume 2.0 ± 1.1%; range 0.7%-4.2%) and LVEF ≥50% (mean 57 ± 6%, range 50%-69%) (Figure 1). There were no differences in native T1 or T2 values between patients with and without myocardial pathology by conventional CMR findings. Pericardial enhancement was not identified in any of the patients.
The CMR measurements were compared to 32 healthy controls using the same CMR equipment, method for analysis and analyst. In addition to being free of cardiovascular comorbidities and established risk factors, the healthy controls were older and more frequently female and of white race compared to the COVID CMR patients (Suppl. Table 2). There were no significant differences in LVEF, RVEF, native T1, native T2, extracellular volume, LV strain and myocardial scar between the COVID patients and healthy controls, although there was a borderline significant higher T1 in COVID patients (mean 1006 ± 31 ms vs 993 ± 29 ms, P = .05; Suppl. Table 3).
CMR pathology by clinical characteristics and disease severity at the index hospitalization
Patients with abnormal vs normal CMR were older, with more prevalent CVD and chronic pulmonary disease (Table I ). Vital signs at admission of the index hospitalization were comparable in patients with and without abnormal CMR, apart from lower temperature in those with abnormal CMR. Disease severity scoring, length of hospital stay and the proportion of patients requiring mechanical ventilation in the ICU were also comparable in patients with and without abnormal CMR (Figure 2 ). Clinical characteristics, comorbidities, vital signs during the index hospitalization and hospital outcome stratified by the presence of minor and major pathology on CMR is presented in Suppl. Table 4. There were no differences in patient reported symptoms during acute COVID-19 and post-COVID-19 by presence of CMR pathology (Suppl. Table 5). Cardiac arrhythmias during acute-COVID-19 were more common in patients with CMR pathology after 6 months, while there were no differences in prevalence of arrhythmias from screening post-COVID-19. There was no difference in treatment with hydroxychloroquine between patients with and without abnormal CMR (50% vs 37%, P= .41, respectively).
Table I.
Baseline characteristics, vital signs on admission and hospital outcome for the acute COVID-19 hospitalization, stratified by the presence of pathology on cardiac magnetic resonance imaging (CMR) 6 months later
| Normal CMR | CMR pathology | P-value | |
|---|---|---|---|
| n = 46 | n = 12 | ||
| Age, years | 54 [46, 70] | 68 [57, 78] | .030 |
| Male sex | 26 (56.5%) | 8 (66.7%) | .53 |
| White race | 23 (50.0%) | 8 (66.7%) | .30 |
| Body mass index, kg/m2 | 27.7 [24.2, 29.4] | 25.8 [24.0, 29.4] | .33 |
| Obesity | 11 (23.9%) | 3 (25.0%) | .94 |
| Diabetes Mellitus | 6 (13.0%) | 0 (0.0%) | .19 |
| Hypertension | 9 (20.0%) | 3 (25.0%) | .71 |
| Cardiovascular disease | 2 (4.3%) | 3 (25.0%) | .023 |
| Chronic pulmonary disease | 1 (2.2%) | 3 (25.0%) | .005 |
| Chronic kidney disease | 2 (4.3%) | 0 (0.0%) | .46 |
| Current smoking | 0 (0.0%) | 1 (8.3%) | .05 |
| Temperature, °C | 38.2 [37.5, 39.0] | 37.3 [36.8, 38.3] | .033 |
| Heart rate, /min | 90 [77, 98] | 86 [73, 89] | .24 |
| Systolic blood pressure, mm Hg | 129 [120, 140] | 131 [125, 138] | .76 |
| Oxygen saturation, % | 95 [93, 96] | 94 [93, 95] | .43 |
| NEWS-score | 5 [3, 7] | 4 [3, 5] | .24 |
| Length of stay (d) | 8 [4, 12] | 6 [4, 9] | .42 |
| ICU admission | 10 (21.7%) | 1 (8.3%) | .29 |
ICU, intensive care unit; NEWS, National Early Warning Score.
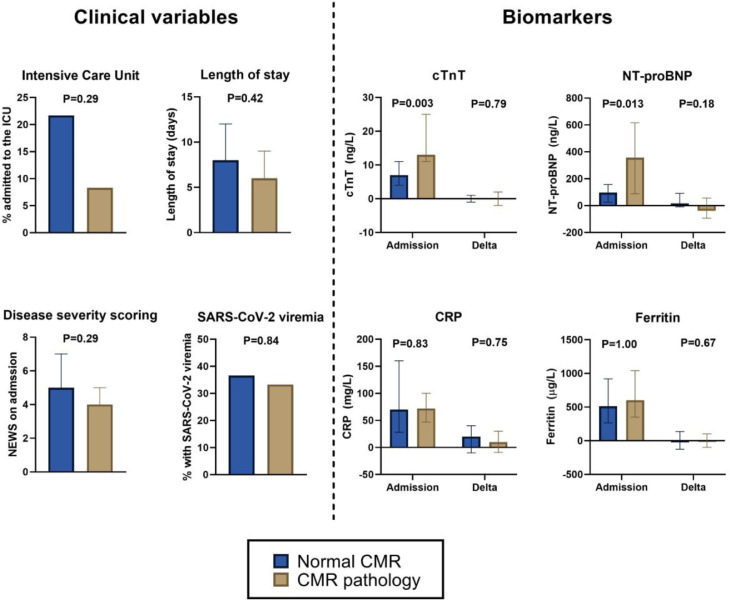
Figure 2.
Title: Clinical variables and biomarker concentrations during hospitalization for COVID-19 in patients with and without pathology on CMR after 6 months, Caption: Proportion of patients with and without pathology on CMR with need for intensive care unit (ICU) treatment, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viremia, National Early Warning Score (NEWS) and length of hospital stay during admission for COVID-19, and concentrations of cardiac troponin T (cTnT), N-terminal pro-B-type natriuretic peptide (NT-proBNP), C-reactive protein (CRP) and ferritin measured at admission and change to day 3 in patients. CMR pathology was defined as myocardial scar or reduced left ventricular ejection fraction. The whiskers represent quartile 1 to quartile 3 for continuous variables.
Eleven (19%) of patients had severe COVID-19 requiring mechanical ventilation in the ICU. Only 1 of these had abnormal CMR (non-ischemic scar and LVEF ≥50%). Table II displays measurements of LV, RV and LA structure and function, myocardial scar, T1, T2 and strain measurements in patients with severe COVID-19 requiring mechanical ventilation in the ICU and in patients with moderate COVID-19 treated in medical wards. Overall, there were no significant differences in CMR measurements between the 2 groups 6 months after hospitalization (Table II).
Table II.
Cardiac magnetic resonance imaging measurements of left ventricular (LV) and right ventricular (RV) structure and function, myocardial scar by late gadolinium enhancement, T1 and T2 in COVID-19 patients requiring mechanical ventilation at the ICU and in patients treated at the medical wards
| Medical ward n = 47 | ICU n = 11 | P-value | |
|---|---|---|---|
| Myocardial scar | 9 (20.5%) | 1 (9.1%) | .38 |
| Scar volume, % | 2.7 ± 1.8 | 1.9 | .69 |
| LV end diastolic volume indexed, ml/m² | 74.6 ± 13.7 | 79.9 ± 14.3 | .26 |
| LV end systolic volume indexed, ml/m² | 30.8 ± 9.3 | 33.8 ± 6.9 | .32 |
| LV stroke volume, ml | 87.4 ± 20.4 | 95.1 ± 30.0 | .31 |
| LV ejection fraction, % | 59.0 ± 7.8 | 57.6 ± 5.4 | .58 |
| LV mass indexed, g/m² | 48.6 ± 10.6 | 48.9 ± 9.1 | .93 |
| LV circumferential strain short axis, % | -18.7 ± 3.4 | -19.1 ± 1.8 | .69 |
| LV longitudinal strain long axis, % | -16.3 ± 2.2 | -16.4 ± 1.9 | .89 |
| LA volume indexed, ml/m² | 34.1 ± 13.3 | 32.1 ± 9.8 | .67 |
| RV end diastolic volume indexed, ml/m² | 72.9 ± 13.1 | 79.6 ± 15.7 | .15 |
| RV end systolic volume indexed, ml/m² | 31.1 ± 7.9 | 34.8 ± 7.1 | .16 |
| RV stroke volume, ml | 83.3 ± 18.3 | 92.6 ± 29.7 | .19 |
| RV ejection fraction, % | 57.5 ± 6.7 | 56.2 ± 4.0 | .52 |
| Extra cellular volume, % | 25.0 ± 3.0 | 23.8 ± 1.1 | .49 |
| Native T1, ms | 1010 ± 31 | 989 ± 25 | .05 |
| Native T2, ms | 51.5 ± 2.9 | 52.1 ± 2.3 | .32 |
LA, left atrial; LV, left ventricular; RV, right ventricular.
CMR pathology by cardiovascular and inflammatory biomarkers measured during the index hospitalization
cTnT and NT-proBNP concentrations on admission for the index hospitalization were median 8 (IQR 4-13) ng/L and 97 (IQR 35-195) ng/L, respectively. Elevated concentrations of cTnT (≥14 ng/L) was present in 16 (28%) and NT-proBNP (≥250 ng/L) in 20 (35%) at any time point during the hospital stay. SARS-CoV-2 viremia was detectable in 19 (36%) of patients during the index hospitalization. Compared to patients with normal CMR after 6 months, patients with CMR pathology had higher admission concentrations of cTnT (median [IQR] 13 [11-25] ng/L vs 7 [4-11] ng/L, P= .003) and NT-proBNP (357 [88-616] ng/L vs 97 [26-156] ng/L, P= .013) (Figure 2). For cTnT, these differences were attenuated when adjusting for demographics (age, sex and race) and CVD (P= .12), while the association persisted in multivariable models for NT-proBNP (P= .03). For NT-proBNP this difference was driven by patients with major CMR pathology who had particularly high concentrations (median 665 (IQR 487-15461) ng/L) while patients with minor and no CMR pathology had comparable concentrations: median 109 (IQR 86-449) vs 92 (IQR 26-156) ng/L, P= .11 (Suppl. Table 6). Patients with elevated NT-proBNP during hospitalization also had significantly higher CMR markers of myocardial edema (T2) and lower LV ejection fraction, but these associations were attenuated when adjusting for demographics and CVD (Suppl. Table 7), also when analyzing admission NT-proBNP as a continuous variable (P= .16). Elevated cTnT was associated with higher T2 values and larger LV and LA volumes, and the association with T2 persisted also in adjusted models (P= .039; Suppl. Table 8), but not when analyzing cTnT as a continuous variable (P= .62). CMR markers reflecting fibrosis (native T1 and ECV fraction), LV mass, LV function (including strain measurements) and RV function were overall comparable in patients with and without elevation of either cardiovascular biomarker during the index hospitalization (Suppl. Tables 7 and 8). The associations between cardiovascular biomarkers and CMR measurements did not change when additionally adjusting for time from hospitalization to CMR.
Admission concentrations of inflammatory biomarkers (ie, C-reactive protein, IL-6, PCT and ferritin) were comparable in patients with and without pathology on CMR. SARS-CoV-2 viremia was also present in an equivalent proportion of patients with normal CMR (n = 15, 37%) and patients with CMR pathology (n = 4, 33%, P= .84) (Table III , Figure 2).
Table III.
Pathology on CMR after 6 months and concentrations of cardiovascular and inflammatory biomarkers during the index hospitalization for COVID-19
| Normal CMR n = 46 | Abnormal CMR n = 12 | ||
|---|---|---|---|
| cTnT admission (ng/L) | 7.0 [4.0 11.0] | 12.5 [10.5, 25.0] | 0.003 |
| cTnT delta to day 3 (ng/L) | 0.0 [-1.0,1.0] | 0.0 [-2.0,2.0] | 0.79 |
| cTnT ≥14 ng/L during hosp. | 10 (22.2%) | 6 (50.0%) | 0.06 |
| NT-proBNP admission (ng/L) | 97.0 [26.0,156.0] | 357.0 [88.0, 615.5] | 0.013 |
| NT-proBNP delta to day 3 (ng/L) | 15.5 [-9.0,91.0] | -39.0 [-93.0,56.0] | 0.18 |
| NT-proBNP ≥250 ng/L during hosp. | 13 (28.9%) | 7 (58.3%) | 0.06 |
| CRP admission (mg/L) | 70 [28,160] | 72 [47,100] | 0.83 |
| CRP delta to day 3 (mg/L) | 20 [-10,40] | 10 [-9,30] | 0.75 |
| IL-6 admission (pg/mL) | 37.0 [20.8,55.9] | 42.5 [26.1,69.8] | 0.50 |
| IL-6 delta to day 3 (pg/mL) | -8.9 [-36.2,17.1] | -10.5 [-28.8,2.8] | 0.95 |
| PCT admission (g/L) | 0.12 [0.06,0.21] | 0.14 [0.09,0.21] | 0.43 |
| PCT delta to day 3 (g/L) | -0.01 [-0.03,0.06] | -0.01 [-0.04,0.07] | 0.98 |
| Ferritin admission (g/L) | 513 [265, 919] | 602 [351, 1042] | 0.65 |
| Ferritin delta to day 3 (g/L) | 7 [-126, 138] | 13 [-98.0,103] | 0.89 |
| SARS-CoV-2 viremia | 15 (36.6%) | 4 (33.3%) | 0.84 |
Concentrations were measured at admission and changes in concentrations to day 3. Also presented by presence of SARS-CoV-2 viremia, elevated cTnT and NT-proBNP at any time during the index hospitalization for COVID-19 are reported.
CRP, C-reactive protein; cTnT, cardiac troponin T; IL-6, interleukin 6; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCT, procalcitonin; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
There were no differences in delta values of inflammatory and cardiovascular biomarkers from hospital admission to day 3 in patients with and without pathology on CMR (Table III, Figure 2).
Discussion
Among 58 unselected patients hospitalized for COVID-19, cardiac pathology on CMR after 6 months was present in 12 (21%) patients. There were no associations between CMR findings and the need for mechanical ventilation, length-of-stay, severity scorings, inflammatory biomarkers or SARS-CoV-2 viremia during the acute COVID-19 hospitalization. Higher concentrations of cTnT and NT-proBNP during the index hospitalization were associated with a higher prevalence of CMR pathology after 6 months, but this was largely attenuated after adjusting for demographics and established CVD.
Persistent cardiac pathology after recovery from COVID-19 and severity of the acute infection
Among patients with moderate-to-severe COVID-19, we found a substantially lower prevalence of pathology on CMR than in a Germany study by Puntmann et al.10 They reported abnormal findings 10 weeks after the acute infection in 78 of 100 patients who had predominantly mild-to-moderate COVID-19. That prior study identified focal scarring in 32% and pericardial enhancement in 22%. They also reported elevated T1 and T2 values, sensitive measures of myocardial fibrosis and edema, compared to healthy and risk-matched controls. A Chinese study retrospectively assessed 26 patients referred to CMR for cardiac symptoms after hospitalization for COVID-19, and found pathological conventional CMR findings in 58% of patients, myocardial edema being the predominant finding.19 In a study of unselected patients 2-3 months after COVID-19 cardiac abnormalities were found in 26%, while 60%, 29% and 10% had abnormalities in the lungs, kidneys and liver on MRI, and these findings correlated with acute disease severity.20 In a study of patients who all had troponin elevation during the acute COVID-19, CMR after median 68 days demonstrated reduced LVEF in 11% and myocardial scar in 54%, of which approximately half was myocarditis-like scar and half ischemic scar.21 This study found no evidence of diffuse fibrosis (T1) or edema (T2) in the remote myocardium compared to matched controls. In our study, we found a lower proportion of focal myocardial scarring than these studies, and no patients with focal myocardial edema or pericardial enhancement. Also, we found no significant differences in T1 or T2 values, or measures of LV and RV structure and function, between patients with severe COVID-19 (requiring mechanical ventilation) and moderate COVID-19 (requiring hospitalization, but not mechanical ventilation). There were also no clinically meaningful differences in CMR measurements between post-COVID-19 patients and healthy controls. Native T1 was slightly higher (mean 15 ms) compared to healthy controls, and this association reached borderline statistical significance. Elevated T1 has been demonstrated in the subacute phase of COVID-19, and this modest difference may reflect residual changes, but may also be related to a higher prevalence of CVD and risk factors among the COVID-19.
Possible explanations for the lower prevalence of CMR pathology in our COVID-19 patients may relate to differences in time since the acute infection. Myocardial edema decreases in the weeks and months after myocardial injury.22 In our study, median time from diagnosis to CMR was 175 days, which is longer than the aforementioned studies. This may suggest regression of post-COVID-19 cardiac pathology with time. Secondly, differences in baseline risk factors may play a role. Although the patients in the German study were younger and free of heart failure and cardiomyopathy, other risk factors for subclinical cardiac remodeling such as smoking, chronic obstructive pulmonary disease and coronary disease were more common. Also, the proportion of pathological CMR findings in the risk-matched control group was of the German study was high. The cohort in the Chinese study was also young, but with moderate to severe COVID-19 infection, and all patients had cardiac symptoms. Finally, technical CMR differences such as MRI field strength and mapping sequences may potentially explain some of the differences.
Elevated cardiovascular biomarkers during COVID-19 and myocardial pathology after recovery
During acute COVID-19, patients with elevated cardiac troponin have been reported to have elevated T1, extracellular volume and LGE measurements, in addition to enhanced macrophage numbers in myocardial biopsies.6 In our study, higher concentrations of cTnT and NT-proBNP on admission were associated with the presence of scar or reduced LVEF on CMR after 6 months, and the highest concentrations were seen in patients classified with major CMR pathology. However, patients with scar or reduced LVEF did not experience a greater increase in concentrations of these cardiovascular biomarkers during the infection. Moreover, the associations were attenuated when adjusting for demographics and established CVD. Accordingly, we believe this most likely reflects pre-existing subclinical CVD rather than persistent COVID-19-related acute myocardial injury and stress. This is supported by the established link between cTnT and NT-proBNP elevations and subclinical myocardial fibrosis and scar in the general population.23 , 24 There were limited associations between CMR measurements of edema (T2) and elevated cTnT and NT-proBNP during the index hospitalization. Importantly, T2 values has been shown to increase with age,25 and indeed adjusting for this attenuated the association to these cardiovascular biomarkers. However, the association between elevated cTnT concentrations during hospitalization and higher T2 remained significant after adjusting for demographics and established CVD. This finding may imply that patients with myocardial injury during the acute infection may be at risk of persistent myocardial edema after recovery from the acute COVID-19. Still, there were limited correlations between cTnT and other measures of pathology of CMR. Accordingly, the clinical significance of the association between myocardial injury and persistent myocardial edema is uncertain and requires more research with longer follow-up and clinical outcomes such as incident heart failure.
Inflammatory response from COVID-19 and myocardial pathology after recovery
Greater concentrations of inflammatory biomarkers and the presence of SARS-CoV-2 RNA in plasma (viremia) are associated with increased disease severity in COVID-19.26., 27., 28 In our study, we could not identify any association between inflammatory biomarkers or presence of SARS-CoV-2 viremia during acute COVID-19 and pathological findings on CMR after recovery. This supports the notion of limited association between severity of the acute infection, reflected by the inflammatory response, and persistent cardiac pathology. COVID-19 is known to cause an overactive and dysfunctional immune response contributing to disease progression, and our observation suggests that the degree of immune activation does not correlate with persistent cardiac pathology. This is in agreement with an autopsy study that found presence of SARS-CoV-2 in the myocardium to be frequent, but not associated with influx of inflammatory cells into the myocardium or lymphocytic myocarditis.29
Limitations
The study was limited to 49% of survivors from the prospective COVID MECH study. However, patients who agreed to participate in the COVID CMR follow-up study had comparable characteristics and biomarker trajectories compared to patients not willing to participate. Still, we cannot rule out bias that participants were healthier than non-participants. Although the classification of minor and major pathology on CMR is clinically relevant, the application of arbitrary cut-offs has obvious limitations. Importantly, our findings were consistent when the CMR variables were analyzed continuously. Three patients were not assessable for focal myocardial scarring. The multivariable regression models may be overfitted due to the number of covariates relative to the number of outcomes. Moreover, the modest sample size increases the risk of Type 2 errors, particularly in the adjusted analysis. Biomarker measurements from the biobank were not available in all patients (n = 5), however we were able to use measurements of cardiovascular biomarkers obtained in clinical routine for these patients. We did not have cardiac imaging from the patients pre-COVID-19, and can therefore not with certainty determine whether the CMR findings were caused by COVID-19 or were pre-existing. The healthy control group was not age-matched as patients were included from a population study of participants all born in 1950, and we did not include a risk factor matched control group.
Conclusion
Our findings from CMR 6 months after COVID-19 contrast with the previously reported high prevalence of myocardial pathology assessed shorter after the acute infection. Although we do not have serial CMR to confirm this, it may suggest regression of cardiac pathology over time. Moreover, we found no association between markers of disease severity during the index hospitalization and pathology on CMR after 6 months, suggesting that pre-existing subclinical myocardial disease may be more important than COVID-19 for the observed CMR pathology.
Category of submission
Clinical Investigation.
Funding
Dr Myhre is supported by grant number: 2017051 from the South-Eastern Norway Regional Health Authority. The COVID MECH study received assays for measuring interleukin-6, procalcitonin, ferritin, cardiac troponin T and N-terminal pro-B-type natriuretic free of charge from Roche Diagnostics. No extramural funding was directly used to support this work.
Conflict of interest
Dr Myhre has served on advisory boards for Novartis and Novo Nordisk, and has received consulting honoraria from Novartis, AmGen and Novo Nordisk. Dr Røsjø has received personal fees from Novartis and Thermo Fischer BRAHMS, CardiNor and SpinChip Diagnostics. Dr Einvik has received research support from Astra Zeneca and Boehringer Ingelheim. Dr Omland has served on advisory boards for Abbott Diagnostics, Roche Diagnostics and Bayer and has received research support from Abbott Diagnostics, Novartis, Roche Diagnostics, Singulex and SomaLogic via Akershus University Hospital, and speaker's or consulting honoraria from Roche Diagnostics, Siemens Healthineers and CardiNor. All other authors report no relevant disclosures.
Acknowledgments
We are grateful for the invaluable contributions by My Svensson MD PhD, Ragnhild Røysland MD PhD, Subaitha Navaruban BSc, Ahmed Meklif MSc, Jannicke Dokken RN, Amyla Abueg RN and Linn Bjørnstad Hagen RN. We also thank Haldor Husby and the Unit of Data Analysis at Akershus University Hospital, Lørenskog, Norway, for help with clinical data acquisition from the data warehouse at Akershus University Hospital.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ahj.2021.08.001.
Appendix. Supplementary materials
References
- 1.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet North Am Ed. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishiga M, Wang DW, Han Y, et al. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawakami R, Sakamoto A, Kawai K, et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis. J Am Coll Cardiol. 2021;77:314–325. doi: 10.1016/j.jacc.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandoval Y, Januzzi JL, Jr, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weckbach LT, Curta A, Bieber S, et al. Myocardial inflammation and dysfunction in COVID-19-associated myocardial injury. Circ Cardiovasc Imaging. 2021;14 doi: 10.1161/CIRCIMAGING.120.011713. [DOI] [PubMed] [Google Scholar]
- 7.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omland T, Prebensen C, Roysland R, et al. Established cardiovascular biomarkers provide limited prognostic information in unselected patients hospitalized With COVID-19. Circulation. 2020;142:1878–1880. doi: 10.1161/CIRCULATIONAHA.120.050089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carfì A, Bernabei R, Landi F, ftGAC-P-ACS Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajpal S, Tong MS, Borchers J, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6(1):116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark DE, Parikh A, Dendy JM, et al. COVID-19 Myocardial Pathology Evaluation in athletes with Cardiac Magnetic Resonance (COMPETE CMR) Circulation. 2021;143:609–612. doi: 10.1161/CIRCULATIONAHA.120.052573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starekova J, Bluemke DA, Bradham WS, et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021;6(8):945–950. doi: 10.1001/jamacardio.2020.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myhre PL, Prebensen C, Strand H, et al. Growth differentiation factor-15 provides prognostic information superior to established cardiovascular and inflammatory biomarkers in unselected patients hospitalized with COVID-19. Circulation;0(0). [DOI] [PMC free article] [PubMed]
- 15.Royal College of Physicians. National early warning score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS. Retrieved from https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2. 6 August 2020
- 16.Berge T, Vigen T, Pervez MO, et al. Heart and brain interactions–the Akershus Cardiac Examination (ACE) 1950 study design. Scand Cardiovasc J. 2015;49:308–315. doi: 10.3109/14017431.2015.1086813. [DOI] [PubMed] [Google Scholar]
- 17.Schulz-Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of trustees task force on standardized post-processing. J Cardiovasc Magn Reson. 2020;22:19. doi: 10.1186/s12968-020-00610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heck SL, Gulati G, Hoffmann P, et al. Effect of candesartan and metoprolol on myocardial tissue composition during anthracycline treatment: the PRADA trial. Eur Heart J Cardiovasc Imaging. 2018;19:544–552. doi: 10.1093/ehjci/jex159. [DOI] [PubMed] [Google Scholar]
- 19.Huang L, Zhao P, Tang D, et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13:2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotecha T, Knight DS, Razvi Y, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. European Heart Journal. 2021;42(19):1866–1878. doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monney PA, Sekhri N, Burchell T, et al. Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart. 2011;97:1312–1318. doi: 10.1136/hrt.2010.204818. [DOI] [PubMed] [Google Scholar]
- 23.Seliger SL, Hong SN, Christenson RH, et al. High-sensitive cardiac troponin T as an early biochemical signature for clinical and subclinical heart failure: MESA (Multi-Ethnic Study of Atherosclerosis) Circulation. 2017;135:1494–1505. doi: 10.1161/CIRCULATIONAHA.116.025505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C-Y, Heckbert SR, Lai S, et al. Association of elevated NT-proBNP with myocardial fibrosis in the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2017;70:3102–3109. doi: 10.1016/j.jacc.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonner F, Janzarik N, Jacoby C, et al. Myocardial T2 mapping reveals age- and sex-related differences in volunteers. J Cardiovasc Magn Reson. 2015;17:9. doi: 10.1186/s12968-015-0118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng F, Huang Y, Guo Y, et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fajnzylber J, Regan J, Coxen K, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11:5493. doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prebensen C, Myhre PL, Jonassen C, et al. SARS-CoV-2 RNA in plasma is associated with ICU admission and mortality in patients hospitalized with COVID-19. Clin Infect Dis. 2021;73(3):e799–e802. doi: 10.1093/cid/ciaa1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindner D, Fitzek A, Bräuninger H, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.