Abstract
Objectives
To determine the health status, exercise capacity, and health related quality of life (HRQoL) of COVID-19 associated acute respiratory distress syndrome (ARDS) survivors, 8 months after diagnosis.
Methods
All eligible patients were interviewed and underwent a physical examination, chest X-ray, and 6 min walk test (6MWT). Scales to evaluate post-traumatic stress disorder, depression, anxiety, and HRQoL were applied.
Results
Of 1295 patients, 365 suffered ARDS and 166 survived to hospital discharge. Five died after discharge and 48 were lost to follow-up. Of the 113 remaining patients, 81% had persistent symptoms. More than 50% of patients completed less than 80% of the theoretical distance on the 6MWT, 50% had an abnormal X-ray and 93% of patients developed psychiatric disorders. Mean SF-36 scores were worse than in the general population. After multivariate regression analysis, female sex, non-Caucasian race, and Charlson index>2 were independent risk factors for a worse mental health component summary score on the SF-36, and age was associated with a better prognosis. Female sex and chronic obstructive pulmonary disease were independently associated with a worse physical component summary score.
Conclusion
COVID-19 associated ARDS survivors have long-term consequences in health status, exercise capacity, and HRQoL. Strategies addressed to prevent these sequelae are needed.
Keywords: Long-term outcomes, Sequelae, COVID-19, SARS-CoV2, ARDS
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the etiological agent of coronavirus disease 2019 (COVID-19) that emerged in Wuhan, China, and spread rapidly across the world, causing a pandemic in just a few weeks.1 Although most patients with COVID-19 will have a mild, self-limiting illness, up to 20% develop severe pneumonia and acute respiratory distress syndrome (ARDS) that can necessitate hospitalization, intensive care, and mechanical ventilation.2
In recent months, much progress has been made in understanding the epidemiology, transmission mechanisms, clinical feature, in-hospital complications, and groups most at risk of a poor evolution and death due to COVID-19.2, 3, 4 Furthermore, multiple studies have been carried out to identify antiviral and immune modulating treatment options.2 , 3 , 5, 6, 7 However, the long-term consequences of COVID-19 are less clear, partly due to its short history, and partly due to the difficulty in monitoring patients after hospital discharge in a severely strained health care system.
Some authors have speculated that the consequences of COVID-19 could be similar to those produced by the severe acute respiratory syndrome coronavirus (SARS-CoV), and the Middle East respiratory syndrome coronavirus (MERS-CoV). Both viruses have a close phylogenetic relationship with SARS-CoV-2 and are probably also similar in terms of clinical sequelae, respiratory function, psychiatric disorders, and health related quality of life (HRQoL)8. In addition, previous studies among ARDS survivors have detected a high prevalence of cognitive impairment and reduced functional status and HRQoL.9 , 10 The few published studies that address the long-term outcomes of COVID-19 have included patients with a broad clinical spectrum of COVID-19 disease and have not focused on the most seriously ill.11, 12, 13
In this prospective study, we aimed to determine the clinical outcomes, exercise capacity, psychiatric disorders, and long-term HRQoL of patients with COVID-19 who developed ARDS and survived to hospital discharge.
Methods
Study design
We performed a follow-up prospective single-center study nested in a large multicentre prospective cohort of hospitalized adults with COVID-19 (COVID-MetroSud). The study was conducted at the Complex Hospitalari Moisés Broggi, a 350-bed public hospital that serves an area of 425,000 inhabitants in Barcelona, Spain.
All patients were adults (>18 years old) admitted with reverse-transcription-polymerase chain reaction (RT-PCR) proven SARS-CoV-2 infection and severe COVID-19 pneumonia between 28 February and 15 April 2020. We included only those patients who had suffered ARDS during hospital admission and survived to hospital discharge, and we excluded those who were institutionalized or admitted to hospital at the time of follow-up, refused to participate, could not be contacted after three calls, and lived outside the area covered by the hospital. All patients were informed of the study and signed the informed consent.
The study conformed to the STROBE checklist and was approved by the ethics committee of the coordinating center (Bellvitge University Hospital) in accordance with Spanish legislation. All procedures complied with the ethical standards of the Helsinki Declaration (PR140/20).
Data collection, clinical evaluation, and follow-up
The acute phase of the SARS-CoV-2 infection was considered the first day of symptom onset to hospital discharge. We recorded demographic and epidemiological data, sociofunctional status, comorbidities, clinical data, radiological findings, laboratory tests, and details of intensive care unit (ICU) admission, including orotracheal intubation, duration admitted (days), complications, and treatment. Data were recorded on a secure web-based software platform for online databases (REDCap).14
Patients were assessed at a follow-up visit between November 2020 and December 2020. This visit included a comprehensive medical evaluation, clinical data collection focused on persistent symptoms, the degree of dyspnea according to the modified British Medical Research Council (mMRC) dyspnea scale,15 and a complete physical examination. Patients were also asked about returning to their usual physical and work activity, as well as whether a close family member had died from COVID-19.
All patients who were able to walk unaided completed a 6-minute walk test (6MWT), following the criteria of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR).16 Median peripheral oxygen saturation (SpO2) was recorded at baseline and after completing the 6MWT, as was the distance walked, in meters. The formula reported by Enright et al.17 was used to calculate the percentage of meters completed based on the theoretical maximum. Any reasons for being unable to complete the test were recorded. Dyspnea on completion of the 6MWT was assessed using the Borg Rating Scale of Perceived Exertion.18
We then verified whether patients had undergone a chest X-ray after hospital discharge. If not, one was requested to ensure that all patients had at least one follow-up chest X-ray. Information from chest computed tomography (CT) and pulmonary function tests (PFTs) was also collected when available.
Patients completed the Beck Depression Inventory second edition (BDI-II),19 Impact of Event Scale Revised (IES-R),20 and State-Trait Anxiety Inventory (STAI)21 questionnaires to assess depression, post-traumatic stress disorder (PTSD), and anxiety, respectively. Finally, all patients completed Version 2 of the Short-Form 36 (SF-36) to assess HRQoL. This consists of 36 questions with answers grouped into eight dimensions across two large groups: the physical component summary (PCS) and the mental health component summary (MCS).22
Definitions
SARS-CoV-2 infection was confirmed by RT-PCR from a nasopharyngeal swab. ARDS was defined according to the Berlin criteria as the appearance or worsening of respiratory failure associated with a known clinical event.23 This required an oxygen arterial pressure (PaO2) / inspired fraction of oxygen (FiO2) ratio ≤300 mmHg, associated with radiology with the appearance of bilateral pulmonary opacities not explained by effusions, atelectasis, or masses. Length of hospital stay was described as the total number of days from admission to hospital discharge. Length of ICU stay was defined as the total number of days spent in critical care.
Statistical methods
Patient characteristics are presented as the number of cases and percentages for categorical variables and as means and standard deviation (SD) or median and interquartile range (IQR) for continuous variables. Fisher's exact test or Pearson's chi-squared test was used to assess the relationship between categorical variables. The student t-test or Mann–Whitney U test was used to compare continuous variables. We then used simple linear regression models to identify factors associated with the MCS and PCS scores of the SF36 questionnaire. Variables were included in the multivariate models if there were non-modifiable (i.e., age, sex, and race) and showed an association (p < 0.2) in the univariate analysis. Results are reported as beta values and 95% confidence intervals. All analyses were performed with a two-sided significance level of 0.05 and conducted using R, version 3.6.3 (cran.r-project.org).24
Results
In total, 1295 patients were admitted for COVID-19, of whom 365 suffered ARDS. Among the ARDS group, 199 died before discharge leaving 166 survivors of COVID-19-associated ARDS. During follow-up, 5 died and 48 did not attend visits, giving a final cohort of 113 patients. Most were male (70%) and had a median age of 64 years (IQR 54–72). Their demographic and clinical data are shown in Table 1 .
Table 1.
Demographic and clinical characteristics, laboratory findings, treatments, and complications of patients during the acute COVID-19 episode.
| Patients (N = 113) | |
|---|---|
| Sex (N;%) | |
| Man | 79 (69.9) |
| Woman | 34 (30.1) |
| Age, years (median; IQR) | 64 (54–72) |
| Race (N;%) | |
| Asiatic | 1 (0.9) |
| Caucasian | 80 (70.8) |
| Latin | 26 (23.0) |
| Black | 1 (0.9) |
| Other | 5 (4.4) |
| BMI, Kg/m2(median; IQR) | 29.3 (26.2–32.3) |
| Smoking (N;%) | |
| Smoker | 3 (2.7) |
| Former smoker | 35 (31.0) |
| Nonsmoker | 75 (66.4) |
| Barthel Index score (mean; SD) | 99 (4.1) |
| Comorbidities (N;%) | |
| Hypertension | 57 (50.4) |
| Diabetes mellitus | 30 (26.5) |
| Dyslipidemia | 60 (53.1) |
| Atrial fibrillation | 8 (7.1) |
| Heart failure | 3 (2.7) |
| Moderate-severe chronic kidney disease (CKD) 1 | 3 (2.7) |
| Chronic respiratory disease | 29 (25.7) |
| COPD | 7/29 (24.1) |
| Asthma | 10/29 (34.5) |
| OSAS | 16/29 (55.2) |
| Interstitial lung disease | 1/29 (3.5) |
| Peripheral vascular disease | 8 (7.1) |
| Stroke | 3 (2.7) |
| Solid malignancy | 10 (8.9) |
| Non metastatic neoplasia | 10/10 (100) |
| Metastatic neoplasia | 0 (0) |
| HIV infection | 2 (1.8) |
| Other immunosuppression's 2 | 2 (2.7) |
| Charlson index score (mean; SD) | 1.05 (1.29) |
| Charlson index score ≤2 points (N;%) | 100 (88.5) |
| Charlson index score >2 points (N;%) | 13 (11.5) |
| Symptoms of COVID-19 (N;%) | |
| Days of symptoms upon admission (median; IQR) | 7(5-10) |
| Dyspnea | 113 (100) |
| Cough | 91 (80.5) |
| Rhinorrhea | 8 (7.1) |
| Anosmia | 16 (14.2) |
| Ageusia | 19 (16.8) |
| Odynophagia | 8 (7.1) |
| Fever | 98 (86.7) |
| Diarrhea | 27 (23.9) |
| Sickness | 20 (17.7) |
| Vomiting | 11 (9.7) |
| Asthenia | 60 (53.1) |
| Anorexia | 35 (31.0) |
| Headache | 20 (17.7) |
| Arthromyalgia | 48 (42.5) |
| Chest pain | 18 (15.9) |
| Abdominal pain | 9 (8.0) |
| Delirium | 4 (3.5) |
| Laboratory findings (median, IQR) | |
| Maximum CRP, mg/L (median, IQR) | 198 (149–295) |
| Maximum LDH, UI/L (median, IQR) | 435 (345–548) |
| Maximum d-dimer, ng/ml (median, IQR) | 2.900 (1.780 −7.220) |
| Minimal lymphocyte count, /mm3 | 680 (465–855) |
| Treatments (N;%) | |
| Lopinavir/Ritonavir | 9 (8.0) |
| Beta interferon | 3 (2.7) |
| Hydroxychloroquine | 108 (95.6) |
| Tocilizumab | 47 (41.6) |
| Immunoglobulins | 1 (0.9) |
| Corticosteroids | 76 (67.3) |
| Inhaled corticosteroids | 13 (11.5) |
| LMWH | 109 (96.5) |
| Antibiotics | 113 (100) |
| Azithromycin | 96 (85.0) |
| Ampicillin | 6 (5.3) |
| Amoxicillin-Clavulanic | 11 (9.7) |
| Piperacillin-Tazobactam | 18 (15.9) |
| Ceftriaxone | 65 (57.5) |
| Carbapenems | 15 (13.3) |
| Quinolones | 24 (21.3) |
| Daptomycin | 8 (7.1) |
| Linezolid | 15 (13.3) |
| LOS, days (median; IQR) | 23 (13.0–39.0) |
| ICU admission (N;%) | 42 (37.2) |
| Length of ICU stay, days (median; IQR) | 18 (12.8–34.5) |
| Orotracheal intubation and mechanic ventilation | 36/42 (85.7) |
| Non-invasive mechanic ventilation | 17/42 (40.5) |
| Complications (N;%) | |
| Ventilated-associated pneumonia | 13/36 (36.1) |
| Nosocomial tracheobronchitis | 13 (11.5) |
| Heart failure | 7 (6.2) |
| Arrhythmia | 12 (10.6) |
| Stroke | 1 (0.9) |
| Acute coronary syndrome | 3 (2.7) |
| PE | 16 (14.2) |
| Sepsis | 10 (8.9) |
| Mental status abnormalities | 17 (15.0) |
| Catheter related bacteriemia | 30 (26.5) |
Abbreviations: BMI: Body mass index; COPD: Chronic obstructive pulmonary disease; CRP: Chain polymerase reaction; HIV: Human immunodeficiency virus; ICU: Intensive Care Unit; IQR: Interquartile range; LDH: Lactate dehydrogenase; LMWH: Low molecular weight heparin; LOS: length of hospital stay; OSAS: Obstructive sleep apnea syndrome; PE: pulmonary embolism; SD: Standard deviation.
Creatinine ≥265 μmol/L.
Solid organ transplantation, Hematopoietic stem cell transplantation, Chemotherapy, corticosteroid treatment (prednisone> 10 mg / day or equivalent) or neutropenia.
Follow-up visits occurred a median of 240 days (IQR 230–246) from the first positive RT-PCR for SARS-CoV-2. Table 2 shows that 80% of patients reported at least one persistent symptom, predominantly dyspnea (55%), arthromyalgia (50%), moderate to severe asthenia (45.5%), subjective memory loss (42.5%), and subjective lack of concentration (40.7%). Moreover, 57% of patients had not recovered their physical health prior to admission. Only 9.8% required further hospital admission after discharge.
Table 2.
Clinical characteristics and functional status at follow-up.
| Patients (N = 113) | |
|---|---|
| Days between COVID-19 diagnosis and follow-up visit (median; IQR) | 240 (230–246) |
| Barthel Index score (mean; SD) | 99 (4.1) |
| Persistent symptoms (N;%) | |
| ≥1 persistent symptom | 91 (80.5) |
| Dyspnea, 0 to 4 points of mMRC | |
| mMRC = 0 | 51 (45.1) |
| mMRC = 1 | 37 (32.7) |
| mMRC = 2 | 17 (15.0) |
| mMRC = 3 | 3 (2.7) |
| mMRC = 4 | 5 (4.4) |
| Cough | 20 (17.7) |
| Chest pain | 28 (24.8) |
| Anosmia | 16 (14.2) |
| Ageusia | 15 (13.3) |
| Odynophagia | 15 (13.3) |
| Asthenia, 0 to 10 points | |
| <5 points | 60 (54.5) |
| ≥5 points | 50 (45.5) |
| Arthromyalgia | 56 (49.6) |
| Headache | 36 (31.9) |
| Subjective memory loss | 48 (42.5) |
| Subjective lack of concentration | 46 (40.7) |
| Insomnia | 33 (36.7) |
| Paresthesia | 27 (24.1) |
| Functional status and family involvement (N;%) | |
| Returned to usual life | 65 (57.5) |
| Returned to work | 39/70 (55.7) |
| Recovery from physical exercise | 48 (42.9) |
| Relatives affected by COVID-19 | 53 (47.3) |
| Relatives deceased by COVID-19 | 11 (9.8) |
| Hospital readmission after hospital discharge | 11 (9.8) |
| Emergency admission after hospital discharge | 24 (21.4) |
Abbreviations: IQR: Interquartile range; SD: Standard deviation; mMRC: modified British Medical Research Council.
Table 3 shows the results of clinical investigations. Concerning the 6MWT results, the baseline SpO2 was 97% (IQR 96–98%) and 30% experienced a saturation drop of ≥4 points after finishing the test. The SpO2 fell below 88% in 5% of patients during or at completion of the 6MWT. Fifty-four of patients completed less than 80% of the theoretical reference meters, adjusted by age. Concerning the radiological findings, 49% of cases had pathological findings on their chest X-ray, mainly showing bilateral interstitial infiltrates (87.5%). Among the 31 chest CTs that were performed, ground-glass opacity and fibrosis were present in 55% and 19%, respectively. Regarding the PFT results, 40% of patients were assessed at a median of 190 days (IQR 144–210) from the diagnosis of COVID-19. The diffusing capacity of the lung for carbon monoxide (DLCO) was <80% in up to three-quarters of PFT results.
Table 3.
6MWT, radiological, and PFT results at follow-up.
| Patients (N = 113) | |
|---|---|
| 6MWT (N;%) | 104 (92.0) |
| Initial SpO2,% (median; IQR) | 97 (96.0–98.8) |
| Final SpO2,% (median; IQR) | 95 (93–96) |
| Decrease on SpO2 ≥4% (N;%) | 31 (30.1) |
| Initial or final SpO2 <88% (N;%) | 5 (4.9) |
| Meters completed (mean; SD) | 377 (117) |
| Completed <80% of theoretical meters (N;%) | 54 (53.5) |
| 6MWT outage (N;%) | 7 (6.8) |
| BORG scale at the end of 6MWT, points (N;%) | |
| 0–2 | 80 (77.7) |
| 3–7 | 23 (22.3) |
| Radiological findings | |
| Chest X-ray, (N;%) | 98 (86.7) |
| Days between COVID-19 diagnosis and | |
| Chest X-ray (median; IQR) | 143 (99–247) |
| Normal | 50 (51.0) |
| Bilateral interstitial infiltrate | 42/48 (87.5) |
| Bilateral alveolar-interstitial infiltrate | 3/48 (6.3) |
| Unilateral alveolar infiltrate | 2/48 (4.2) |
| Unilateral interstitial infiltrate | 1/48 (2.1) |
| Chest CT (N;%) | 31 (36.3) |
| Ground-glass opacification | 17 (54.8) |
| Consolidation areas | 1 (3.2) |
| Ground-glass and consolidation | 9 (29.0) |
| Fibrosis | 6 (19.4) |
| PE | 1 (3.2) |
| PFT (N;%) | 46 (40.7) |
| Days between COVID-19 diagnosis and | |
| PFT (median; IQR) | 190 (144–210) |
| FEV1 <80% | 12/46 (26.1) |
| FVC <80% | 13/46 (28.3) |
| FEV1/FVC <70% | 7/46 (15.2) |
| DLCO <80% | 28/36 (77.8) |
Abbreviations: 6MWT: 6 min walking test; Chest CT: Chest computed tomography; IQR: Interquartile range; mMRC: modified British Medical Research Council; PE: Pulmonary embolism; PFT: Pulmonary function tests; SD: Standard deviation; SpO2: Peripheral oxygen saturation.
Table 4 summarizes the psychological evaluation results, revealing that most patients (>90%) had developed a mental disorder. Specifically, 36% presented some degree of depression, almost 50% suffered moderate to severe PTSD, and 34% had state anxiety at or above the 75 percentile.
Table 4.
Results of testing for mental health disorders.
| Patients (N = 113) | |
|---|---|
| BDI-2 inventory of depression (N;%) | 93 (82.3) |
| Minimal or no depression | 59 (63.4) |
| Mild | 10 (10.8) |
| Moderate | 18 (19.4) |
| Severe | 6 (6.5) |
| IES-R of PTSD (N;%) | 109 (96.5) |
| Minimal or no PTSD | 20 (18.3) |
| Mild | 35 (32.1) |
| Moderate | 6 (5.5) |
| Severe | 48 (44.0) |
| STAI anxiety state, percentile (N;%) | 104 (92.0) |
| <75 | 49 (47.1) |
| ≥75 | 55 (52.9) |
| STAI anxiety trait, percentile (N;%) | 103 (91.2) |
| <75 | 68 (66.0) |
| ≥75 | 35 (34.0) |
Abbreviations and definitions: BDI-2: Beck's index, a depression detection inventory; IES-R: Impact of Event Scale Revised, a post-traumatic stress assessment scale; PTSD: post-traumatic stress disorder; STAI: state-trait anxiety index.
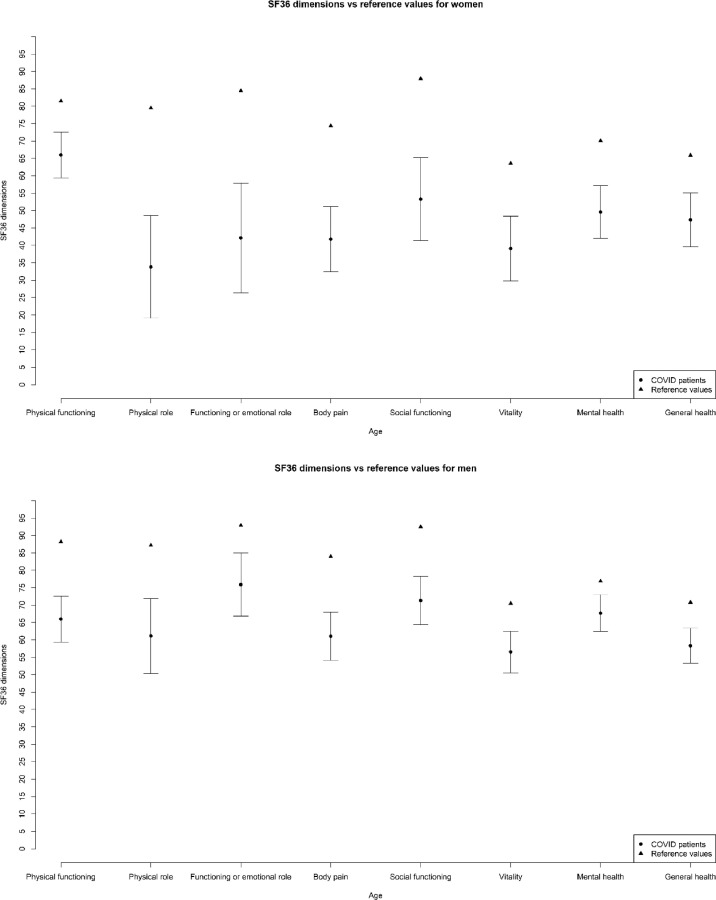
Concerning HRQoL, as shown in Fig. 1 , the mean SF36 scores were worse following admission to hospital with COVID-19 and ARDS than in the general population for each of the eight dimensions, irrespective of sex. Supplementary Fig. 1 then presents the results of the simple linear regression models for the clinical and demographic variables associated with MCS and PCS scores. The MCS score, multivariate regression revealed that female sex (β −9.8; 95%CI −15.12 to −4.49), race other than Caucasian (β −6.51; 95%CI −12.67 to −0.35), and a Charlson index >2 (β −10.52; 95%CI −18.68 to −2.35) were independently associated with a worse score, while age (β 3.56; 95%CI 0.61 to 6.52) was associated with a better score. Concerning the PCS score, a history of chronic obstructive pulmonary disease (COPD) (β −5.78; 95%CI −10.44 to −1.11) and female sex (β −4.79; 95%CI −9.15 to −0.42) were independently associated with a worse score.
Fig. 1.
Mean SF36 scores for survivors of COVID-19 with ARDS by dimensions, shown by sex compared with the general population.
Abbreviations: ARDS: Acute severe Respiratory Distress Syndrome; SF36: version 2 of Short-Form 36 quality of life questionnaire.
A sub-analysis comparing Latino with Caucasian patients revealed that the former tended to be younger (52 vs 67 years, p < 0.001) and have less hypertension (30.5% vs 56.2%, p = 0.042) and dyslipidemia (30.5% vs 61.3%, p = 0.013). However, at 8 months, 81% of Latinos had not regained their previous exercise capacity whereas this only applied to 49.4% of Caucasians (p < 0.015). Additionally, significantly more Latino patients completed <80% of the theoretical 6MWT reference distance compared with Caucasians (84% vs 41%, p < 0.001). Latino patients also performed worse on the mMRC scale, the IES-R, and the MCS, but not the PCS (Supplementary Table 1).
Discussion
In this prospective and comprehensive follow-up study of patients who developed ARDS during admission for COVID-19, we found that 80% had at least one persistent symptom 8 months after diagnosis. The most common of these were dyspnea and arthralgia.
The few published articles on the follow-up of patients with COVID-19 have relied on shorter follow-up periods and have reported varying prevalence rates for symptoms.11 , 12 , 25 Our results match those from a Chinese cohort,11 in which 74–86% of patients had at least one persistent symptom, with 26–36%of patients in the most severe group having dyspnea with an mMRC score >1 at 6 months. Similar results have been published by Meije et al. at 45-days’ follow-up, although at 7 months, they report 25% fewer persistent symptoms than in our study.25 However, it is striking that Bellal et al. reported that fewer than 5% of patients had sequelae at 3–4 months. These wide differences may be because the entire population with COVID-19 was included in their study, while we only included the most ill patients. This would be consistent with the fact that our results are similar to those of previous studies published in patients with ARDS of different etiologies, in which 56–84% of patients are reported to have persistent symptoms at 12 months.26, 27, 28, 29
Regarding exercise capacity measured by the 6MWT, more than half of the patients completed <80% of the theoretical distance, adjusted for age. Surprisingly, Huang et al. reported that only 29% of patients in the most severe group had values below the normal range.11 Previous studies in survivors of SARS and MERS are consistent with ours.30 Herridge et al. also suggested that the inability to exercise was mainly due to extrapulmonary disease, such as impaired muscle function.29
Pathological radiological findings were present in a half of the cases that had imaging after hospital discharge. Similarly, Huang et al. describe radiological abnormalities in approximately 50% of patients at 6 months.11 By contrast, pathological findings were identified in only 20% of ARDS survivors, probably because these latter results were analysed 12 months after hospital discharge(29). In our cohort, chest CT was performed in less than a third of patients; had it been performed in all patients, we may have been better placed to define the radiological abnormalities.
In our study, 77% of patients undergoing a PFT had a DLCO <80%. The DLCO is the parameter most affected in Chinese11 and Italian12 cohorts of COVID-19 survivors, but both have fewer patients with a DLCO <80% (22% and 67%, respectively). This difference could be explained by the lower disease severity in the Chinese11 and Italian12 cohorts. Yet, surprisingly, only 56% of patients with the most severe disease in the study by Huang et al. had low DLCOs.11 Our result is still consistent with previous studies of ARDS survivors with different aetiologies and of SARS/MERS survivors affected by severe pneumonia and/or requiring ICU admission. This suggests that pulmonary sequelae may be more related to the appearance of ARDS than to the disease itself.26 , 28, 29, 30, 31, 32, 33
A total of 93% of patients had developed a mental health disorder by 8-months’ follow-up main disorders assessed were PTSD, depression, and anxiety, and all were present at levels considerably higher than published elsewhere.11 , 25 , 34 The differences are also difficult to compare because we analyzed different psychiatric disorders and did not consider a subgroup of who suffered encephalopathy during hospital admission. Nevertheless, the trend seems to indicate that the most severe cases of COVID-19 present a higher risk of mental disorders in the medium term. Previous studies have focused on neurological and psychiatric disorders in patients with COVID-19 (mild to severe) and have found a similar prevalence of PTSD and depression just before hospital discharge.35 , 36 Some authors have hypothesized that this may be related to brain involvement of COVID-19 itself.37 However, psychiatric disorders must be analysed in the global context of the pandemic, which has led to significant changes in lifestyle, economy, work, and social relationships. In fact, the high percentage of PTSD is comparable to that described in studies conducted in survivors of wars and catastrophes.38 , 39
Similar to the mental health problems, the HRQoL of survivors was worse than in the general population. This is also consistent with previous studies among SARS and MERS survivors, as well as among patients affected by ARDS of different aetiologies.9 , 29 , 40 Moreover, high comorbidity and COPD were independently associated with worse scores in the MCS and PCS, respectively.
A new and striking finding of our study was that Latino patients presented with worse outcomes (e.g., dyspnea, the 6MWT, MCS, and IES-R) at 8 months after COVID-19. These results should be explored in more detail by adding socioeconomic data or conducting multicentre studies to account for the specific characteristics of the Latino population in our geographic area.
The main strength of this study lies in the prospective and comprehensive data collection for a large cohort of patients with severe COVID-19 who survived ARDS. We are also unaware of any other study that has assessed the survivors of COVID19-associated ARDS globally in physical and mental domains beyond 6 months’ follow-up. However, the study has some equally limitations that should be noted. First, this was a single-center study, and as such, our findings may lack generalizability by failing to reflect the epidemiology of different centres and/or geographical areas. Second, socioeconomic variables were not collected from patients, which may have influenced the outcomes. Finally, we were unable to perform PFT and CT studies in most patients.
In summary, survivors of COVID-19 associated ARDS had poor exercise capacity, health status, and lower HRQoL scores after 8 months compared with the general population. More than 90% presented with mental health disorders, including PTSD, depression, and anxiety. A multidisciplinary approach should be promoted to improve the care of patients with the sequelae of COVID-19.
Declaration of Competing Interest
There are no funders to declare.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.08.018.
Appendix. Supplementary materials
References
- 1.Statement on the second meeting of the International Health Regulations (2005). Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). [Internet]. [cited 2021 May 2]. Available from: https://www.who.int/.
- 2.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutiérrez-Gutiérrez B., del Toro M.D., Borobia A.M., Carcas A., Jarrín I., Yllescas M., et al. Identification and validation of clinical phenotypes with prognostic implications in patients admitted to hospital with COVID-19: a multicenter cohort study. Lancet Infect Dis. 2021;21(3099) doi: 10.1016/S1473-3099(21)00019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M.Y., Zhao R., Gao L.J., Gao X.F., Wang D.P., Cao J.M. SARS-CoV-2: structure, biology, and structure-based therapeutics development. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jan H., Faisal S., Khan A., Khan S., Usman H., Liaqat R., et al. COVID-19: review of epidemiology and potential treatments against 2019 novel coronavirus. Discoveries. 2020;8(2):e108. doi: 10.15190/d.2020.5. (Craiova) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy supplemental content. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tansey C.M., Louie M., Loeb M., Gold W.L., Muller M.P., de Jager J., et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167:1312–1320. doi: 10.1001/archinte.167.12.1312. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed H., Patel K., Greenwood D.C., Halpin S., Lewthwaite P., Salawu A., et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS) coronavirus outbreaks after hospitalization or ICU admission: a systematic review and meta-analysis. J Rehabil Med. 2020;52:63. doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 11.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellan M., Soddu D., Piero B.E., Baricich A., Zeppegno P., et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge + supplemental content. JAMA Netw. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosn J., Piroth L., Epaulard O., le T.P., Mentr F., Covid F., et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect. 2021:10–13. doi: 10.1016/j.cmi.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.REDCap [Internet]. [cited 2021 May 2]. Available from: https://www.projectredcap.org/
- 15.Mahler D.A., Wells C.K. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 16.Manual de procedimientos SEPAR, 4 by SEPAR - Issuu [Internet]. [cited 2021 Mar 14]. Available from: https://issuu.com/separ/docs/procedimientos4?mode=window&backgroundColor=%23222222.
- 17.Enright P.L., Sherrill D.L. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 18.González-Mangado N., Rodríguez-Nieto M.J. Prueba de la marcha de los 6 minutos. Respir Med. 2016;9(1):15–22. [Google Scholar]
- 19.Sanz J., Gutiérrez S., Gesteira S., García-Vera M.P. Criterios y baremos para interpretar el “inventario de depresión de Beck-II” (BDI-II) Behav Psychol. 2014;22(1):37–59. [Google Scholar]
- 20.Moncayo F.L.G. Propiedades psicométricas de la escala revisada del impacto del evento estresante (IES-R) en una muestra espanola de pacientes con cancer. Anal Modif Conducta. 2007;33(149):312–331. [Google Scholar]
- 21.Spielberger R.D., Gorsuch R.L., Lushene R.E. STAI Cuestionario de ansiedad estado-rasgo. IEEE Trans Prof Commun. 2015;23(7):3–14. [Google Scholar]
- 22.Madariaga I., Nuñez-Antón V. Aspectos Estadísticos del Cuestionario de calidad de vida relacionada con la salud short form -36 (SF-36) Estad Esp. 2008;50:147–192. [Google Scholar]
- 23.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 24.R Core Team (2020). — European environment agency [Internet]. [cited 2021 May 2]. Available from: https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006.
- 25.Meije Y., Duarte-Borges A., Sanz X., Clemente M., A R., Ortega L., González-Pérez R., Cid R., Pareja J., Cantero I., Ariño M., Sagués T., LLaberia J., Ayestarán A., Fernández-Hidalgo N., Candás-Estébanez B. Hospital de Barcelona COVID19-team, Long-term outcomes of patients following hospitalization for COVID-19: a prospective observational study. Clin Microbiol Infect. 2021;27(8):1151–1157. doi: 10.1016/j.cmi.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hert R., Albert R.K. Sequelae of the adult respiratory distress syndrome. Thorax. 1994;49(1):8–13. doi: 10.1136/thx.49.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghio A.J., Elliott C.G., Crapo R.O., Berlin S.L., Jensen R.L. Impairment after adult respiratory distress syndrome. An evaluation based on American thoracic society recommendations. Am Rev Respir Dis. 1989;139(5):1158–1162. doi: 10.1164/ajrccm/139.5.1158. [DOI] [PubMed] [Google Scholar]
- 28.Luyt C.E., Combes A., Becquemin M.H., Beigelman-Aubry C., Hatem S., Brun A.L., et al. Long-term outcomes of pandemic 2009 influenza A(H1N1)-associated severe ARDS. Chest. 2012;142(3):583–592. doi: 10.1378/chest.11-2196. [DOI] [PubMed] [Google Scholar]
- 29.Herridge M.S., Cheung A.M., Tansey C.M., Matte-Martyn A., Diaz-Granados N., Al-Saidi F., et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Eng J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 30.Hui D.S., Wong K.T., Ko F.W., Tam L.S., Chan D.P., Woo J., et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128(4):2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park W.B., Kang J.I., Kim G., Choi J.P., Rhee J.Y., et al. Brief communication correlation between pneumonia severity and pulmonary complications in middle east respiratory syndrome. J Korean Med Sci. 2018;33(24):169. doi: 10.3346/jkms.2018.33.e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngai J.C., Ko F.W., Ng S.S., To K.W., Tong M., Hui D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and Health status. Respirology. 2010;15(3):543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hui D.S.C., Wong K.T., Antonio G.E., Tong M., Chan D.P., Sung J.J.Y. Long-term sequelae of SARS: physical, neuropsychiatric, and quality-of-life assessment. Hong Kong Med J. 2009;15:21–23. Suppl 8(852) [PubMed] [Google Scholar]
- 34.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236, 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021 doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bo H.X., Li W., Yang Y., Wang Y., Zhang Q., Cheung T., Wu X., Xiang Y.T. Posttraumatic stress symptoms and attitudetoward crisis mental health services amongclinically stable patients with COVID-19 in China. Psychol Med. 2020;51(6):1052–1053. doi: 10.1017/S0033291720000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J., Lu H., Zeng H., Zhang S., Du Q., Jiang T., et al. The differential psychological distress of populations affected by the COVID-19 pandemic. Brain Behav Immun. 2020;87:49–50. doi: 10.1016/j.bbi.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunològic mechanisms. Brain Behav Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fares J., Gebeily S., Saad M., Harati H., Nabha S., Said N., et al. Post-traumatic stress disorder in adult victims of cluster munitions in Lebanon: a 10-year longitudinal study. BMJ Open. 2017;7(8):1–8. doi: 10.1136/bmjopen-2017-017214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamwey M.K., Gargano L.M., Friedman L.G., Leon L.F., Petrsoric L.J., Brackbill R.M. Post-traumatic stress disorder among survivors of the September 11, 2001 world trade center attacks: a review of the literature. Int J Environ Res Public Health. 2020;17(12):1–19. doi: 10.3390/ijerph17124344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batawi S., Tarazan N., Al-Raddadi R., al Qasim E., Sindi A., Johni S., et al. Quality of life reported by survivors after hospitalization for Middle East respiratory syndrome (MERS) BMC. 2019;17:101. doi: 10.1186/s12955-019-1165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.