Abstract
Background:
Among children with pharyngitis who test positive for group A Streptococcus (GAS), 10–25% are GAS carriers. Current laboratory methods cannot distinguish acute infection from colonization.
Methods:
We examined two separate longitudinal studies of children with symptomatic pharyngitis associated with a positive GAS throat culture (illness culture). In cohort 1, children presented with pharyngitis symptoms to a clinician, then had follow-up cultures at regular intervals. In cohort 2, throat cultures were performed at regular intervals and with pharyngitis symptoms. Illness cultures were categorized as acute infection or carrier based on follow-up culture results. In cohort 2, carriers were further categorized as a GAS carrier with a new emm-type or a GAS carrier with a previous emm-type based on typing data from prior culture results. For each cohort, we then compared symptoms at the time of illness culture between carriers and those with acute infection.
Results:
Cohort 1 (N=75 illness cultures): 87% of the children were classified as acutely infected versus 13% carriers. Carriers were more likely to have upper respiratory (URI) symptoms (OR: 5.5; 95%CI:1.4–22.1), headache (OR: 6.0; 95%CI:1.2–40.5) or vomiting (OR:5.5; 95%CI:1.2–24.5). Cohort 2 (N=122 illness cultures): 79% were acutely infected and 21% were carriers. Children determined to be carriers with a previous emm-type were more likely to have URI symptoms compared to those with acquisition of a new emm-type.
Conclusion:
Children with symptomatic pharyngitis and GAS on throat culture who were identified as carriers were more likely to present with URI and atypical symptoms than children who were identified as acutely infected.
Keywords: Streptococcus pyogenes, streptococcal pharyngitis, streptococcal colonization, carriage, pharyngitis
Introduction
Between 10–25% of school-aged children with pharyngitis who test positive for group A Streptococcus (GAS) are actually colonized with GAS in their oropharynx and have a viral etiology for their symptoms.(1–3) Both the Infectious Disease Society of America (IDSA) and the American Academy of Pediatrics (AAP) do not support the routine use of GAS testing or antibiotics for children who are suspected GAS carriers as they are less likely to spread the bacteria, to have suppurative complications or to develop acute rheumatic fever compared to children with acute GAS infection(4–11). Yet, children colonized with GAS are more likely to experience unnecessary antibiotic exposure, school absences and healthcare utilization than other children.(12, 13) Thus, efforts to more accurately distinguish children with GAS colonization from those with acute infection are increasingly important.
The challenge in the clinical setting is that clinicians are unlikely to know whether a child presenting with symptomatic pharyngitis is colonized with GAS.(4) This is because GAS colonization is typically identified after the occurrence of multiple, sequential, symptomatic episodes with repetitive, positive GAS testing even after appropriate antibiotic treatment.(2, 4, 14, 15) Thus, to limit GAS testing overall and the overtreatment of GAS colonized children, previous studies and guidelines have focused on clinical symptoms and physical exam signs to determine which children with pharyngitis have a high pre-test probability for a positive GAS throat culture and thus warrant GAS testing.(4, 16, 17) For example, current IDSA guidelines recommend against GAS testing in children with pharyngitis who have upper respiratory infection (URI) symptoms.(10) Despite this recommendation, however, as many as 63% of children who receive GAS testing have at least one URI symptom.(18) Thus, accurate interpretation of a positive GAS test and subsequential management may also be important to limiting the overtreatment of children with GAS colonization.
To this end, it is unknown whether a child’s clinical symptoms and signs can be used to distinguish those with GAS acute infection from colonization in the context of a positive GAS test. Such findings could potentially be used to inform the decision to treat a child with pharyngitis. Therefore, with this study, we aimed to use data from two prospective, longitudinal cohorts of school-aged children with symptomatic pharyngitis and positive GAS throat cultures to identify associations between symptoms at the time of testing and GAS colonization or acute infection. We hypothesized that children who are colonized with GAS would present with more URI symptoms, which could be used to distinguish them from children with acute GAS pharyngitis.
Methods
This was a retrospective study examining previously collected data from two prospective, longitudinal cohorts of school-aged children (ages 5–15) for whom serial throat cultures and symptom assessments were obtained.
Cohort 1: Acute Illness Study
A longitudinal pediatric office-based study was conducted from May 2005 to May 2008 to examine the natural history of GAS infections following a clinician diagnosis of streptococcal pharyngitis. Study participants were recruited from a pediatric private practice associated with University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh (CHP) who presented with acute symptoms of pharyngitis and received clinician-driven testing for GAS with a Rapid Streptococcal Antigen test (RST). Children between five and fifteen years of age with a positive RST test were enrolled and clinical symptoms, physical exam findings and confirmatory throat cultures were obtained. Each participant was followed approximately every two weeks for up to five visits. Clinical symptoms, physical exam findings, and throat cultures were obtained at each visit using standardized data collection forms. Children unable to tolerate repeated specimen collection were withdrawn from the study.
Cohort 2: Surveillance Study
A longitudinal school-based study of the epidemiology of GAS in school-aged children was conducted each September to May from 1998 to 2003. Detailed study methods have previously been published. (3) Study participants were recruited from a private, tuition-supported, elementary school (kindergarten through grade 8) in Pittsburgh, Pennsylvania that served 285 children. Children between five and fifteen years of age who enrolled in the study engaged in on-site study visits every two weeks during the school-year. In addition, by parental request, acute illness visits were conducted if at least one clinical symptom was present. At each visit, positive and negative clinical symptoms and physical exam findings were recorded on a standardized data form similar to Cohort 1 and throat cultures were obtained.
Symptom and Sign Characterization
All symptoms were documented based on patient/parent report. Sore throat was reported as mild, moderate or severe. Symptoms including fever, headache, vomiting, abdominal pain, congestion/rhinorrhea, and cough were reported as present or absent. Activity was reported as normal, moderately reduced, or severely reduced. Duration of symptoms was reported in days. In Cohort 1, abnormal pharyngeal exam was documented as present if the clinician identified any pharyngeal erythema and/or exudates on physical exam. For Cohort 2, a more detailed pharyngeal exam was recorded including specific documentation of present or absent erythema, enanthem, edema, petechiae or exudates present on the uvula, palate, tonsils or tongue. For this study, presence of any one sign was considered as an abnormal pharyngeal exam.
Specimen Processing
For both cohorts, throat specimens were obtained using a sterile, flexible throat swab in the standard fashion using a Dacron swab and an Aimes Transport Media. Specimens were processed with standard methods. (3) Emm-typing was performed on all GAS isolates from children who participated in Cohort 2 as detailed in the parent study. (3) All children in Cohort 1 were prescribed antibiotics at study entry. In Cohort 2, the majority of children with clinical symptoms and a positive illness cultures were prescribed antibiotics. A subset of suspected GAS carriers did not receive antibiotics at the time of a positive throat culture as they were presumed to be colonized with these bacteria. Treatment for follow-up positive throat cultures was determined based on the presence or absence of symptoms, parent preference and provider assessment.
Throat Culture Inclusion and Exclusion Criteria
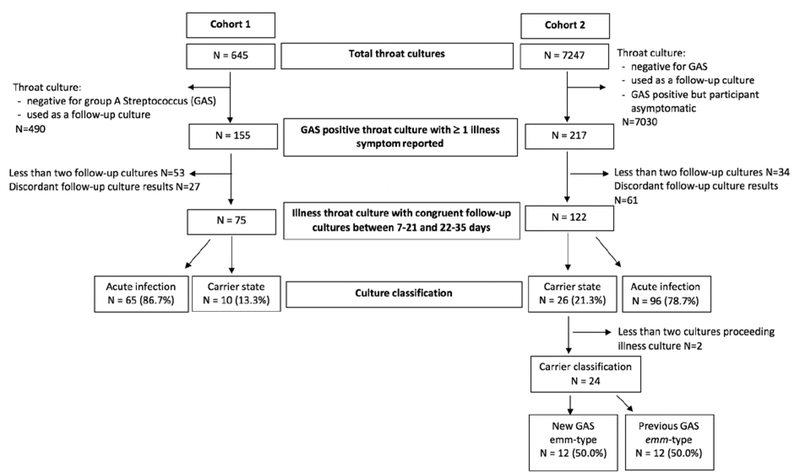
Because of the differences in the design of the two studies it was determined a priori that the cohorts could not be combined. However, inclusion and exclusion criteria for this report were created to identify children who had throat cultures obtained at the time of an illness who also had a sufficient number of longitudinal throat cultures to allow differentiation of those with acute infection versus colonization. Throat cultures were included as “illness cultures” if all of the following were present: 1) a child reported sore throat, 2) the throat culture was positive for GAS, and 3) there were at least two follow-up throat cultures performed between 7–21 days and 22–35 days after the first throat culture. Illness encounters were excluded if the throat culture did not demonstrate GAS, if the child had no signs/symptoms at time of collection, if there were inadequate follow-up study visits, or if the two follow-up throat cultures had discordant results (positive/negative or negative/positive). Since children in Cohort 2 were followed for at least nine months, multiple illness episodes from the same child were included and treated as independent events as long as the culture was not included as a follow-up culture for another illness episode (i.e. minimum timer interval of 36 days between illness episodes).
Acute Infection and Carrier Case Definitions
After identifying illness cultures for Cohort 1 and Cohort 2, each encounter was categorized as reflecting a child with acute infection or colonization. As there is no universally accepted, non-serologic-based definition to distinguish acute infection versus colonization with GAS, the following case definitions were used.(4) For Cohort 1 and 2, acute infection was defined as a positive throat culture for GAS obtained while symptomatic with two negative follow-up cultures suggesting clearance. Carrier was defined as a child with a positive throat culture for GAS obtained while symptomatic with two positive follow-up throat cultures suggesting GAS colonization. For Cohort 1, it was not possible to distinguish children with acute infection who then developed colonization from children with colonization that persisted before, during and after illness culture as throat cultures prior to the illness culture were not available. However, for Cohort 2, throat cultures proceeding the illness culture were available, thus carriers were sub-classified based on emm-type data for positive cultures. A GAS carrier who had acute infection followed by colonization was identified if they acquired a new GAS emm-type on illness throat culture, which then persisted on two follow-up cultures. A GAS carrier with a previous emm-type was identified if they had a persistent GAS emm-type identified on throat cultures preceding the illness, during illness and the two follow-up cultures after the illness.
Statistical Analyses
Descriptive statistics were used to characterize demographics and symptoms for both cohorts. Fisher’s exact tests were used to obtain odds ratios estimating the magnitude and significance of associations for clinical symptoms between those with acute infection and carriers (Cohort 1), GAS carrier with a new emm-type and GAS carrier with a previous emm-type (Cohort 2), GAS carrier with a new emm-type and acute infection (Cohort 2) and GAS carrier with a previous emm-type and acute infection (Cohort 2). Finally, we estimated sensitivity, specificity and area under the receiver operating curve (AUC) for URI symptoms in discriminating carrier from acute infection (Cohort 1). An alpha less than 0.05 was considered significant for all statistical tests.
The Institutional Review Board of the University of Pittsburgh approved both studies at the time of data collection and subsequently approved use of this data for this analysis. Legal guardians of study participants provided written informed consent for the original studies.
Results
Cohort 1:
A total of 163 children contributed 645 throat cultures to this longitudinal study. Demographics of this group are presented in Table 1. Seventy-five cultures met our inclusion criteria as an illness culture (Figure 1). Sixty-five (87%) illness episodes were characterized as acute infection, while 10 (13%) were characterized as carriers. Demographics including age, race, and gender were similar across acute and carrier states and all children were prescribed antibiotics (Table 1). Children who reported clinical symptoms of headache, abdominal pain, vomiting, nasal congestion and one or two URI symptoms were more likely to be carriers than those with acute infection (Table 2). There were no differences in symptom reporting for fever, sore throat, cough, or symptom duration or in identification of abnormal pharyngeal exam.
Table 1.
Demographic and treatment characteristics of children enrolled in Cohort 1: Acute Illness Study and Cohort 2: Surveillance Study who presented with clinical symptoms and had a positive group A Streptococcus throat culture.
| Characteristic | Cohort 11 N=75 |
Cohort 2 N=122 |
|---|---|---|
|
| ||
| Age, years, mean (SD), [range] | 9.1 (2.8) [5–15] | 9.2 (2.3) [5–15] |
| Gender Male Female |
37 (49) 38 (51) |
63 (52) 59 (48) |
| Race Caucasian Black Other |
66 (88) 4 (5) 5 (7) |
90 (74) 10 (8) 22 (18) |
| Antibiotic Type Amoxicillin Penicillin/Penicillin VK Other None |
70 (93) 3 (4) 2 (3) 0 (0) |
76 (62) 18 (15) 13 (11) 15 (12) |
Columns N(%) except where otherwise specified.
Figure 1.
Flow diagram of throat cultures: Cohort 1 (Acute Illness) and Cohort 2 (Surveillance). Acute infection or carrier classification based on two negative or positive follow-up cultures, respectively. Cohort 2 carriers sub-classified as having colonization with new or previous group A Streptococcus (GAS) emm-types based on throat cultures proceeding illness.
Table 2:
Comparison of Cohort 1 sign and symptom characteristics at time of illness presentation for children with group A Streptococcus acute infection and colonization.
| Symptom Characteristics | Acute Infection1 N=65 |
Carrier N=10 |
OR2 (95% CI3) | |
|---|---|---|---|---|
|
| ||||
| Sore Throat4 | Mild Moderate Severe |
26 (40) 31 (48) 8 (12) |
3 (30) 5 (50) 2 (20) |
ref 1.4 (0.3–6.4) 2.2 (0.3–15.3) |
| Abnormal Pharyngeal Exam5 | 56 (86) | 10 (100) | No estimate | |
| Fever4 | 28 (43) | 6 (60) | 2.0 (0.5–7.7) | |
| Headache4 | 26 (40) | 8 (80) | 6.0 (1.2–40.5) | |
| Abdominal Pain4 | 16 (25) | 6 (60) | 4.6 (1.1–18.4) | |
| Vomiting4 | 7 (11) | 4 (40) | 5.5 (1.2–24.5) | |
| Activity4 | Normal Moderate Severe |
24 (37) 28 (43) 13 (20) |
2 (20) 5 (50) 3 (30) |
ref 2.1 (0.4–12.1) 2.8 (0.4–18.7) |
| Cough4 | 11 (17) | 3 (30) | 2.1 (0.5–9.4) | |
| Nasal Congestion4 | 6 (9) | 6 (60) | 14.8 (3.2–67.3) | |
| One URI6 Symptom7 | 14 (21) | 6 (60) | 5.5 (1.4–22.1) | |
| Two URI Symptoms7 | 3 (5) | 3 (30) | 8.9 (1.5–52.6) | |
| Symptom Duration4≤72 hours | 59/63 (94) | 10 (100) | ref | |
| >72 hours | 4/63 (6) | 0 (0) | No estimate | |
Columns N (%) except where otherwise specified.
OR: odds ratio; estimated using Fisher’s exact test
CI: confidence interval
Patient/parent reported
Presence of either pharyngeal erythema or exudates as noted by trained research staff.
URI: upper respiratory infection
Symptoms include cough or nasal congestion.
Cohort 2:
A total of 145 children contributed 7247 throat cultures from 1998 to 2003. We identified 120 illness throat cultures that met our inclusion criteria (Figure 1) from 75 children who contributed an average of 1.6 illness study visits [range 1–5]. Ninety-six (79%) encounters were characterized as acute infection, while 26 (21%) cultures were characterized as carriers. Demographics were similar across acute and carrier states and compared to Cohort 1 (Table 1). 107 (88%) of these episodes were prescribed antibiotics; some children were not treated with an antibiotic if they were a suspected GAS carrier.
In Cohort 2, two carriers could not be sub-classified as a GAS carrier with a new or previous emm-type as they were newly enrolled in the study at time of illness culture. Of the remaining carriers, twelve (50%) children had acquired new GAS emm-types at time of illness that then persisted on follow-up cultures. The other twelve (50%) children had a positive illness culture with a GAS emm-type already identified on previous cultures and persisted on follow-up cultures. Symptom comparison of GAS carriers with new and previous emm-types identified that previous emm-type carriers were more likely to have mild sore throat as well as cough and/or nasal congestion compared to new emm-type carriers (Table 3). Similar differences in URI symptoms were found when comparing GAS carriers with a previous emm-type to children with acute infection (Supplementary Table 1). Finally, no symptom differences were identified when comparing GAS carriers with a new emm-type to children with acute infection (Supplementary Table 2).
Table 3.
Comparison of Cohort 2 sign and symptom characteristics at time of illness presentation for children with GAS1 colonization with a new GAS emm-type and those with a previous GAS emm-type.
| Symptom Characteristics | New GAS emm-type Carrier2
N=113 |
Previous GAS emm-type Carrier N=103 |
OR4 (95% CI5) |
|---|---|---|---|
|
| |||
| Sore Throat6 Mild Moderate/Severe |
0 (0) 11 (100) |
5 (50) 5 (50) |
ref No estimate |
| Abnormal Pharyngeal Exam7 | 8 (73) | 8 (80) | 1.5 (0.2–11.5) |
| Fever6 | 2 (18) | 1 (10) | 0.5 (0.0–6.5) |
| Headache6 | 3 (27) | 0 (0) | No estimate |
| Abdominal Pain6 | 2 (18) | 1 (10) | 0.5 (0.0–6.5) |
| Vomiting6 | 2 (18) | 0 (0) | No estimate |
| Activity6 Normal Decreased |
7 (64) 4 (36) |
9 (90) 1 (10) |
ref 0.2 (0.0–2.2) |
| Cough6,8 | 3 (27) | 7 (78) | 9.3 (1.2–73.0) |
| Nasal Congestion6 | 4 (36) | 8 (80) | 7.0 (1.0–50.6) |
| One URI9 Symptom10 | 5 (45) | 9 (90) | 10.8 (1.0–117.0) |
| Two URI Symptoms10 | 2 (18) | 6 (67) | 9 (1.1–71.0) |
GAS: group A Streptococcus
Columns N (%) except where otherwise specified.
One child with new GAS emm-type carriage and two children with established GAS emm-type carriage had missing symptom data and were excluded from this comparison.
OR: odds ratio; estimated using Fisher’s exact test
CI: confidence interval
Patient/parent reported
Presence of erythema, enanthem, edema, petechiae or exudates present on the uvula, palate, tonsils or tongue as noted by trained research staff.
Missing cough data on one child
URI: upper respiratory infection
Symptoms include cough or nasal congestion
Sensitivity and Specificity
Presence of nasal congestion had the highest AUC (0.75) with a sensitivity of 60% and specificity of 91% for identification of carriers among symptomatic children with positive GAS cultures (Table 4). Presence of two URI symptoms improved specificity to greater than 95%, but decreased sensitivity to only 30%.
Table 4.
Sensitivity and specificity with 95% CI of upper respiratory symptoms in Cohort 1 for the identification of symptomatic children with group A Streptococcus colonization.
| Symptom Characteristics | Sensitivity | Specificity | AUC1 |
|---|---|---|---|
|
| |||
| Cough | 30.0 (6.6–65.2) | 83.1 (71.7–91.2) | 0.57 |
| Nasal congestion | 60.0 (26.2–87.8) | 90.8 (81.0–96.5) | 0.75 |
| Cough or nasal congestion | 60.0 (26.2–87.8) | 78.5 (66.5–87.7) | 0.69 |
| Cough and nasal congestion | 30.0 (6.6–65.2) | 95.4 (87.1–99.0) | 0.63 |
AUC: area under the receiver operating curve
Discussion
Using two independent longitudinal cohorts of school-aged children, we demonstrate that among children with symptomatic pharyngitis and positive GAS cultures, presence of one or more URI symptoms is associated with children who are classified as GAS carriers compared to those with acute infection. In Cohort 1, which most closely mimics clinical practice as provider discretion drove GAS testing, we identified that presence of URI symptoms had modest predictive value in distinguishing children with colonization from those with acute infection. Furthermore, we identified that colonized children were more likely to have other constitutional symptoms including abdominal pain, vomiting, and headache. Similar findings with even more nuanced insight were identified in the analysis of children in Cohort 2. In this cohort, we were able to differentiate the presenting symptoms of children who had established colonization with the same GAS emm-type prior to, during and after illness and those with acute infection with a new GAS emm-type who developed post-treatment colonization. While the former was more likely to present with URI symptoms at time of illness compared to the latter or compared to children with acute infection, the latter had indistinguishable symptoms compared to children with acute infection. This adds nuance to our findings that a child with a positive GAS test who presents with pharyngitis and URI symptoms is more likely to be a child with established GAS colonization with the same emm-type compared a child with acute GAS infection regardless of whether or not the child later develops colonization.
These data support the IDSA guidelines that children presenting with URI symptoms should not be tested for GAS.(10) Not only do these symptoms suggest a viral etiology, but our data confirms that a positive diagnostic test among children with these symptoms is more likely to reflect established colonization with GAS. Yet, there are numerous reasons why a child with URI symptoms will be tested for GAS including testing that is driven by chief-complaint, provider experience or parent preference.(18) We identify that presence of both cough and nasal congestion among children with a positive GAS test was 95.4% (95%CI: 87.1–99.0%) specific for being a GAS carrier. Clinicians could use these data to engage families in shared decision-making about the risks and benefits of testing and of antibiotic treatment. By choosing to not test or treat children with URI symptoms, providers can help to reduce antibiotic use with relatively low risk of encountering GAS complications such as suppurative infections or rheumatic fever.(4, 19)
It is important to emphasize that antimicrobial therapy for children who acquire a new GAS emm-type is warranted. While treatment may not prevent development of GAS colonization, it will reduce the risk of complications from GAS such as rheumatic fever, which are increased when infection with a new emm-type occurs.(10, 11) Our data from Cohort 2 suggests that all children who acquired an infection with a new GAS emm-type, regardless of subsequent clearance or colonization, presented with similar symptoms and were less likely to have URI symptoms. Thus, providers following IDSA guidelines will likely identify and treat such children as part of their regular practice. (10)
Previous decision-rules for children with pharyngitis have focused on symptom presentation to predict positive GAS throat culture and to guide the decision for testing.(20) Absence of URI symptoms is frequently identified as predictive of a positive GAS throat culture. (16, 17) For example, Wald et al found that presence of pharyngitis and fever and absence of viral features (conjunctivitis, rhinorrhea or cough) predicted positive throat cultures in 72% of children.(16) Similarly, the Centor Score, which assigns points for patients who present with fever, tonsillar exudates and swollen/tender anterior cervical lymph nodes, and absence of cough, accurately predicts a positive GAS throat culture in 56% of people with a maximum score of four(17). Similar to other GAS decision-rules, we identified that URI symptoms are also important for distinguishing carriers from children with acute infection and may useful for the decision to treat.
There are several other important limitations of this study. First, despite two moderately sized cohorts, our strict case definitions limited our ability to effectively examine combinations of symptoms in multivariate prediction models. Despite this, we still identified clear symptom differences between children colonized with GAS and those acutely infected with modest test characteristics. Although our data is derived from work originally completed in the 1990s and early 2000s, this does not limit their utility to provide clinical insights into this otherwise challenging study population. In the absence of serologic data to measure immune response to define the carrier state, we used rigorous culture case definitions including emm-typing data to help to differentiate children with acute infection and colonization. Additionally, the two included cohorts had fundamental differences in study design, thus limiting our ability to combine datasets. However, by examining these cohorts separately, we leveraged the strengths of each study to provide more nuanced insight. Cohort 1, which is more representative of the population encountered by clinicians, provides more generalizable findings for clinical practice. Cohort 2, which provides in-depth characterization of carriers that would otherwise be unobtainable in general practice, provides nuanced insight into clinical symptoms revealing the important distinction between children who are GAS carriers with a new versus a previous emm-type.
Conclusion
We identify that children who are GAS carriers are more likely to have URI symptoms at illness presentation than those with acute infection, thus supporting the IDSA guidelines that children with prominent URI symptoms should not be tested for GAS. (4, 10) Novel strategies at the point of care that distinguish children with acute infection from those with colonization or who will become colonized are needed. As sore throat remains one of the most common pediatric complaints, improving diagnostic and decision support for clinicians for the distinction of GAS infection and colonization is important to optimize the benefits and minimize risks of antimicrobial therapy.
Supplementary Material
Acknowledgements:
Enrollment in these clinical studies could not have been done without the assistance of the parents and children who participated.
Funding Source: Internal Funding, Department of Pediatrics, Division of General Academic Pediatrics, UPMC Children’s Hospital of Pittsburgh, Research Advisory Committee; Dr. Martin’s NIH K23; University of Pittsburgh HRSA NRSA for Primary Medical Care Research (T32HP22240) for Dr. Rick; and RedCap grant number UL1-TR-001857.
Conflicts of Interest: Martin receives research funding to the institution from the National Institutes of Health, Centers for Disease Control and Prevention, Novus Therapeutics and Medimmune. The other authors have no conflicts of interest to disclose
References
- 1.Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics. 2010;126:e557–564. [DOI] [PubMed] [Google Scholar]
- 2.Pichichero ME, Green JL, Francis AB, et al. Recurrent group A streptococcal tonsillopharyngitis. The Pediatric infectious disease journal. 1998;17:809–815. [DOI] [PubMed] [Google Scholar]
- 3.Martin JM, Green M, Barbadora KA, Wald ER. Group A streptococci among school-aged children: clinical characteristics and the carrier state. Pediatrics. 2004;114:1212–1219. [DOI] [PubMed] [Google Scholar]
- 4.DeMuri GP, Wald ER. The Group A Streptococcal Carrier State Reviewed: Still an Enigma. J Pediatric Infect Dis Soc. 2014;3:336–342. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan EL. The group A streptococcal upper respiratory tract carrier state: an enigma. J Pediatr. 1980;97:337–345. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan EL, Gastanaduy AS, Huwe BB. The role of the carrier in treatment failures after antibiotic for group A streptococci in the upper respiratory tract. J Lab Clin Med. 1981;98:326–335. [PubMed] [Google Scholar]
- 7.Davies HD, McGeer A, Schwartz B, et al. Invasive group A streptococcal infections in Ontario, Canada. Ontario Group A Streptococcal Study Group. N Engl J Med. 1996;335:547–554. [DOI] [PubMed] [Google Scholar]
- 8.Krause RM, Rammelkamp CH Jr. Studies of the carrier state following infection with group A streptococci. II. Infectivity of streptococci isolated during acute pharyngitis and during the carrier state. J Clin Invest. 1962;41:575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker MJ, Barnett TC, McArthur JD, et al. Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clinical microbiology reviews. 2014;27:264–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279–1282. [DOI] [PubMed] [Google Scholar]
- 11.Kimberlin DW BM, Jackson MA, Long SS (Eds), ed. Red Book: Report of the Committee on Infectious Diseases. 31st ed. American Academy of Pediatrics, Elk Grove Village, IL 20182018–2021. [Google Scholar]
- 12.Donnelly JP, Baddley JW, Wang HE. Antibiotic utilization for acute respiratory tract infections in U.S. emergency departments. Antimicrob Agents Chemother. 2014;58:1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfoh E, Wessels MR, Goldmann D, Lee GM. Burden and economic cost of group A streptococcal pharyngitis. Pediatrics. 2008;121:229–234. [DOI] [PubMed] [Google Scholar]
- 14.Martin JM. Pharyngitis and streptococcal throat infections. Pediatr Ann. 2010;39:22–27. [DOI] [PubMed] [Google Scholar]
- 15.Martin JM. The Mysteries of Streptococcal Pharyngitis. Curr Treat Options Pediatr. 2015;1:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wald ER, Green MD, Schwartz B, Barbadora K. A streptococcal score card revisited. Pediatr Emerg Care. 1998;14:109–111. [DOI] [PubMed] [Google Scholar]
- 17.Fine AM, Nizet V, Mandl KD. Large-scale validation of the Centor and McIsaac scores to predict group A streptococcal pharyngitis. Arch Intern Med. 2012;172:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro DJ, Lindgren CE, Neuman MI, Fine AM. Viral Features and Testing for Streptococcal Pharyngitis. Pediatrics. 2017;139. [DOI] [PubMed] [Google Scholar]
- 19.Van Brusselen D, Vlieghe E, Schelstraete P, et al. Streptococcal pharyngitis in children: to treat or not to treat? Eur J Pediatr. 2014;173:1275–1283. [DOI] [PubMed] [Google Scholar]
- 20.Le Marechal F, Martinot A, Duhamel A, Pruvost I, Dubos F. Streptococcal pharyngitis in children: a meta-analysis of clinical decision rules and their clinical variables. BMJ open. 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.