Abstract
It has become evident that coronavirus disease 2019 (COVID-19) has a multi-organ pathology that includes the brain and nervous system. Several studies have also reported acute psychiatric symptoms in COVID-19 patients. An increasing number of studies are suggesting that psychiatric deficits may persist after recovery from the primary infection. In the current systematic review, we provide an overview of the available evidence and supply information on potential risk factors and underlying biological mechanisms behind such psychiatric sequelae.
We performed a systematic search for psychiatric sequelae in COVID-19 patients using the databases PubMed and Embase. Included primary studies all contained information on the follow-up period and provided quantitative measures of mental health. The search was performed on June 4th 2021.
1725 unique studies were identified. Of these, 66 met the inclusion criteria and were included. Time to follow-up ranged from immediately after hospital discharge up to 7 months after discharge, and the number of participants spanned 3 to 266,586 participants. Forty studies reported anxiety and/or depression, 20 studies reported symptoms- or diagnoses of post-traumatic stress disorder (PTSD), 27 studies reported cognitive deficits, 32 articles found fatigue at follow-up, and sleep disturbances were found in 23 studies. Highlighted risk factors were disease severity, duration of symptoms, and female sex. One study showed brain abnormalities correlating with cognitive deficits, and several studies reported inflammatory markers to correlate with symptoms. Overall, the results from this review suggest that survivors of COVID-19 are at risk of psychiatric sequelae but that symptoms generally improve over time.
Keywords: COVID-19, SARS-CoV-2, Psychiatric sequelae, long-COVID, Systematic review, PTSD, Anxiety, Depression, Cognition, Fatigue, Inflammation
1. Introduction
The typical presentation of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) includes fever and respiratory difficulties. However, studies have shown that COVID-19 has a multi-organ pathology (Gavriatopoulou et al., 2020). Recent studies have reported that more than one-third of the infected patients develop neurological symptoms in the acute phase of the disease, and that 34% show brain abnormalities such as white matter hyperintensities and hypodensities as well as microhemorrhages, hemorrhages, and infarcts (Egbert et al., 2020, Helms et al., 2020a).
Intriguingly, several studies have reported a high incidence of acute psychiatric symptoms in COVID-19 patients. It has been suggested that at least 35% of the patients display symptoms of anxiety and depression (Hu et al., 2020, Kong et al., 2020). Studies on psychiatric sequelae have been performed for the related corona-viruses SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV). A systematic review and meta-analysis on SARS-CoV and MERS-CoV patients concluded that both during the illness and post-illness, an increased incidence of cognitive disabilities, as well as depressed mood and anxiety, was found (Rogers et al., 2020). Data on long term effects of SARS-CoV-2 infection is currently being published at a high rate and include a multitude of symptoms including breathlessness, cough, muscle pains, chest pains, headache, and fatigue. Further, more and more studies demonstrate that psychiatric symptoms might also persist after recovery from the initial infection. These long-term symptoms are collectively referred to as ‘long COVID’ (Long COVID, 2020, Yelin et al., 2020) and it is evident that such sequelae will have severe personal and socioeconomic consequences. It is therefore of utmost importance to characterize and disseminate evidence of long-COVID.
One possible way SARS-CoV-2 could affect the brain is indirect, through the host's immune response to the infection. It has been suggested that COVID-19 patients experience a cytokine storm syndrome and that this is one of the main factors in the pathogenesis of the disease (Kempuraj et al., 2020, Mehta et al., 2020). Furthermore, evidence is beginning to emerge that neuroinflammation is present in some COVID-19 sufferers (Divani et al., 2020, Muccioli et al., 2020, Pilotto et al., 2020, Tang et al., 2021). Increased levels of cytokines, peripherally as well as centrally, can lead not only to lung inflammation and dysfunction (Kempuraj et al., 2020, Wu and Yang, 2020) but also to the development of psychiatric disease (see Dantzer et al., 2008, Drzyzga et al., 2006, Young et al., 2014 for reviews). This link has already been suggested in patients with acute COVID-19, where a tendency for higher levels of the cytokine interleukin (IL)-1β was found in subjects with depressive and/or anxiety symptoms compared to COVID-19 patients who did not display such symptoms (Hu et al., 2020, Kong et al., 2020).
As mentioned above, a systematic review with meta-analysis examining psychiatric sequelae after SARS-CoV and MERS-CoV has already been published, providing valuable information on these related corona-viruses (Rogers et al., 2020). Also, a systematic review focussing primarily on the indirect effects of the COVID-19 pandemic on mental health has been performed (Vindegaard and Benros, 2020). In the current systematic review, the aim was to provide an overview of the current evidence of psychiatric complications in long-COVID after primary symptoms of acute COVID-19 have ceased. Furthermore, we aimed to identify risk factors and molecular mechanisms which could give rise to psychiatric symptoms.
2. Materials and methods
2.1. Search strategy and selection criteria
We searched the databases PubMed and Embase for studies on psychiatric sequelae following SARS-CoV-2 infection. The search was performed on June 4th, 2021 and included all relevant articles published since January 1st 2020. Only primary articles published in peer-reviewed journals in English with a quantitative outcome related to mental health were included, and time since acute COVID-19 had to be defined. Studies were included as sequelae when they were conducted after cessation of acute symptoms. As a minimum two negative qPCR tests had to be provided for the study to be included. Opinion articles, commentaries, reviews, and other articles without original data were excluded. Pre-prints without peer-review and case studies were not included.
2.2. Search string
We used a combined set of keywords, which for PubMed were (((((((((((((“obsessive compulsive disorder”[tiab]) OR (“post traumatic stress”[tiab])) OR (fatigue[tiab])) OR (schizophrenia[tiab])) OR (“neurocognitive disorders”[tiab])) OR (“mood disorder”[tiab])) OR (bipolar[tiab])) OR (depres*[tiab])) OR (anxi*[tiab])) OR (“Mental Disorders”[Mesh])) OR (psych*[tiab])) OR (mental[tiab])) AND (((((((“sars-cov-2”[tiab]) OR (“sars-cov2”[tiab])) OR (“Coronavirus”[Mesh])) OR (“covid-19”[tiab])) OR (“corona virus” [tiab])) OR (“coronavirus” [tiab])) OR (covid [tiab]))) AND ((((“complications” [Subheading]) OR (sequela[tiab])) OR (sequelae[tiab])) OR (“long term”[tiab])) AND (2020:3000/12/12[pdat])) NOT (((review[tiab]) OR (“Systematic Review” [Publication Type])) OR (“Review” [Publication Type])) and for Embase were: ((('covid':ti,ab OR 'coronavirus':ti,ab OR 'corona virus':ti,ab OR 'covid-19′:ti,ab OR 'coronavirinae'/exp OR 'sars-cov2':ti,ab OR 'sars-cov-2':ti,ab) AND ('mental':ti,ab OR 'psych*':ti,ab OR 'mental disease'/exp OR 'anxi*':ti,ab OR 'depres*':ti,ab OR 'bipolar':ti,ab OR 'mood disorder':ti,ab OR 'neurodegenerative disorder':ti,ab OR 'fatigue':ti,ab OR 'schizophrenia':ti,ab OR 'post traumatic stress':ti,ab OR 'obsessive compulsive disorder':ti,ab)) AND ('long term':ti,ab OR 'sequelae':ti,ab OR 'sequela':ti,ab OR 'complication'/exp)) NOT ('review':it OR 'review':ti,ab).
2.3. Study selection and data extraction
Study selection and data extraction were performed using Covidence systematic review software (Veritas Health Innovation, Australia). Duplicate references were removed electronically and manually. Title/abstract screening and full-text screenings were performed independently by two reviewers (CBR and TMS/GW). In cases of disagreements on the inclusion of an article at the title/abstract screening level, it was retained for the next screening stage. Upon disagreement on the inclusion or reason to exclude, the study was forwarded to a third reviewer (SJ), who made the final decision. We included studies containing primary data on psychiatric symptoms in adult patients with prior SARS-CoV-2 infection. We excluded studies without specified psychiatric presentations but included neuropsychiatric manifestations such as cognitive impairments and dyssomnia. Following descriptive variables were extracted for each study and presented in Table 1 : Reference ID and country in which the research was conducted; Primary aim of the study; Study design; Study instruments related to the psychiatric assessment; Numbers of participants; Number of males; Mean age; Time since acute COVID-19 or since negative SARS-CoV-2 test; Inclusion criteria or population description; Exclusion criteria, when reported; Main findings related to psychiatric sequelae and other relevant results.
Table 1.
Studies reporting psychiatric and neuropsychiatric sequelae following SARS-CoV-2 infection.
| Reference (setting) | Primary aim of study | Study design | Study instruments | Number of participants | Males (%) | Mean age | Time since acute COVID-19 | Inclusion criteria | Exclusion criteria | Main findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Al-Aly et al., 2021 (USA) | To identify 6-month incident sequelae in COVID-19 patients who survived > 30 days | Cohort study with controls | ICD-10 diagnoses | 98,661 | 64,593 (87.96) | 59 | 30 days − 6 months | Patients with COVID-19 who survived at least 30 days after diagnosis | Not reported | Non-hospitalized patients showed increased incidence of neurocognitive disorders (HR 3.2), sleep disorders (HR 14.5), anxiety (HR 5.4), trauma- and stress-related disorders (HR 8.9). Further, patients exhibited an excess burden of malaise and fatigue (HR 12.6). Severity of sequelae correlated with severity of the acute COVID-19 |
| Albu et al., 2021 (Spain) | To characterize post COVID-19 sequelae in patients in an outpatient rehabilitation program | Cohort study | MFIS, PSQI, WHOQOL-BREF | 30 (16 post-ICU, 14 non-ICU) | 19 (63%) | 54 | >3 months | PCR confirmed adult COVID-19 patients suffering from sequelae | previous neurological, psychiatric or severe medical condition | The main reason for referral to rehabilitation was fatigue (87%), dyspnea (67%) and cognitive impairment (47%) |
| Alemanno et al., 2021 (Italy) | To examine the impact of COVID-19 on cognitive functions of the disease | Cohort study | MMSE, MoCA, HAM-D, FIM | 56 | N/A | N/A | 1 month after discharge and 2 neg PCR tests | COVID-19 patients discharged from COVID-19 rehabilitation unit | Cognitive dysfunction, psychotropic drug use, COVID-19 encephalitis | >55% of the patients had cognitive deficits at follow up. This correlated with disease severity but had improved compared to results from admission. > 18% had mild/moderate depression at follow up. This correlated with disease severity and had not improved compared to admission. 43% showed signs of PTSD |
| Augustin et al., 2021 (Germany) | To examine post-COVID syndrome in COVID-19 patients following a mild acute COVID-19 disease course | Cohort study | 10 item systematic questionnaire | 353 | 151 (43%) | 43 | 4 and 7 months | Adult non-hospitalised COVID-19 survivors | Not reported | 14% reported fatigue at 7 month follow up. Female sex was a risk factor |
| Bellan et al., 2021 (Italy) | To evaluate lung function, exercise function and psychological seuelae in COVID-19 patients | Cohort study | IES-R (self-rating) | 238 | 142 (59.7%) | 61 | 3–4 months after discharge | Age >=18 years, discharged from hospital after admission for COVID-19 | Not reported | 42.8% of the patients showed PTS symptoms. Male sex was a risk factor for moderate to severe PTS |
| Boari et al., 2021 (Italy) | To assess short-term consequences of COVID-19 | Prospective follow-up cohort study | A structured questionnaire for fatigue, insomnia and anxiety | 94 | Not reported | Not reported | 4 months | COVID-19 survivors with bilateral pulmonary interstitial opacities and ARDS | Not reported | At follow up 52% experienced fatigue, 31% insomnia and 21% anxiety |
| Chen et al., 2020 (China) | To survey health-related quality of life among COVID-19 patients 1 month after discharge compared to the general Chinese population | Cohort study | The Chinese version of the IQOLA SF-36 | 361 | 186 (51.5%) | 47.2 | 1 month after discharge | Cases: COVID-19 patients discharged from 1 of 12 hospitals in Wenzhou City. Controls: A random sample of Chinese adults | Not reported | The severity of the COVID-19 course was negatively associated with the physical functioning, general health, role limitation due to emotional problems, and mental health (p < 0.05). Also, a negative association between lung function and mental health was found and female sex was associated with decreased mental health in the patients |
| Chevinsky et al., 2021 (USA) | To examine post-COVID conditions 1–4 months after COVID diagnosis | Case-control | ICD-10 | 74,446 cases | 31,521 (42%) | Not reported | 31–120 days | Previous in- or outpatients with COVID-19 | Not reported | Post-COVID conditions were most predominant 31–60 days after infection. Patients were more likely to experience anxiety and fatigue than controls up to 60 days after the infection. Depression was also increased in cases vs controls, but improved after 30 days |
| D'Cruz et al., 2021 (UK) | To investigate sequelae of severe COVID-19 | Cohort study | TSQ, GAD-7, PHQ-9 | 119 | 74 (62%) | 59 | 61 days post-discharge | Adult hospital-discharged COVID-19 survivors attending a post-discharge clinical service | Mild or moderate COVID | 68% reported fatigue, 57% sleep disturbances, 25% PTSD, 22% anxiety, 18% depression |
| Daher et al., 2020 (Germany) | To investigate pulmonary impairments, and psychological disorders in patients with COVID-19 six weeks after discharge from hospital | Cohort study | PHQ-9, GAD-7, EQ-5D-5L | 33 | 0.66 | 64 +/-3 | 6 weeks after discharge | Hospitalised COVID-19 patients in isolation ward. Only symptomatic patients with severe disease needing hospitalization | Patients with ARDS who needed mechanical ventilation in the ICU | At follow up 33% had dyspnea, 45% suffered from fatigue. Patients suffered from reduced QoL. No indicators for depression or anxiety |
| Darley et al., 2021 (Australia) | To characterise the effects of COVID-19 during the first year after diagnosis | Prospective cohort study | CogState Cognitive Test Battery; DMI-10 | 78 | 51 (65%) | 47 | 69 days | Adult hospitalised or non-hospitalised COVID-19 survivors. | Not reported | 22% suffered from fatigue, 10% displayed mild/moderate cognitive impairment and 21% had depression at follow up. Follow up symptoms were most frequent following severe illness |
| Daugherty et al., 2021 (USA) | To evaluate sequelae following COVID-19 | Retrospective cohort study | ICD-10 | 266,586 | 126,980 (47.6%) | 42 | 21–120 days | 18–65 year old COVID-19 survivors or matched comparators | Only positive antibody test | COVID-19 survivors showed an increased risk of memory deficits, anxiety, depression, PTSD and fatigue compared to matched comparator groups |
| de Graaf et al., 2021 (Netherlands) | To evaluate cardiopulmonary function and psychological impairment after hospitalization for COVID-19 | Cohort study | GAD-7, PHQ-9, PCL-5, CFQ-25, IQ-CODE-N | 81 | 51 (63%) | 60.8 | 6 weeks after discharge | Adult patients discharged from hospital after COVID-19 | Not reported | 5% showed symptoms of anxiety, 17% depression, 10% PTSS and 27% cognitive failures. No differences were found between ICU or no ICU admission. Higher PCFS scores were associated with depression |
| De Lorenzo et al., 2020 (Italy) | To investigate whether COVID-19 leaves behind residual dysfunction, and identify patients who might benefit from post-discharge monitoring. | Cohort study | Unstructured clinical interviews + self-report questionnaires IES-R, STAI-Y, WHIIRS, WHOQOL-BREF, MoCA | 185 | 123 (66.5%) | 57 | 20–29 days since hospital discharge | All symptomatic adult COVID-19 patients admitted to San Raffaeale University hospital. | Patients admitted for reasons other than COVID-19 who subsequently tested positive for SARS-CoV-2 at routine screenings. | 25.4% patients had new-onset cognitive impairment. PTSD was observed in 22.2% of the patients. PTSD was independently predicted by female sex and hospitalisation, the latter being protective. A previous psychiatric history increased the risk of developing PTSD. BMI was not a predictor for PTSD. Cognitive impairment was found in 25% of the patients who had no history of a cognitive disorder. Anxiety was reported in 30% of the patients and insomnia in 28% |
| Frontera et al., 2021 (USA) | To assess long-term outcomes of patients hospitalized with COVID-19 with and without neurological complications at index | Prospective cohort study | MoCA; Neuro-QoL | 395 | 128 (65%) | 68 | 6 months | Adult, hospital admitted COVID-19 survivors | Outpatients | 47% suffered from anxiety, 29% from depression, 35% from fatigue and 43% from sleep disturbances at follow up. No significant differences were found between neurological COVID-19 patients and non-neurological patients |
| Gautam et al., 2021 (UK) | To study sequelae in severe-to-critical COVID-19 survivors 4–7 months post-illness | Case series | EQ-5D-5L, MoCA | 200 | 125 (62.5%) | 57 | 4–7 months | Adult COVID-19 patients with min 3 days hospital admission | Patients with mild to moderate disease | 53% experienced fatigue at follow up, 13% cognitive difficulties, 15% sleep disturbances. CRP was no longer elevated at follow-up |
| Gennaro et al., 2021 (Italy) | To examine psychiatric and cognitive sequelae following SARS-CoV-2 infection | Cohort study | IES-R, PCL-5, ZSDS, BDI-13, STAI-Y, WHIIRS, OCI | 226 | 149 (66%) | 58 | 90 days post-discharge | COVID-19 pneumonia | Patients < 18 years | 9% suffered from MDD, 9% of anxiety and 3% of insomnia at 3 months follow up. Duration of hospitalisation correlated inversely with ZSDS, OCI and WHIIRS. PTSD symptoms, anxiety and insomnia decreased from 1 to 3 months follow up. Female sex and previous psychiatric history was a predictor of depression at 3 months follow up. 65% showed impaired neurocognitive functioning. Sex, previous psychiatric diagnoses and duration of hospitalisation were not predictors. Systemic inflammation at hospital admission and at follow up predicted severity of depressive psychopathology at 3 months follow up. Systemic inflammation at hospital admission also predicted neurocogntive performance |
| Gonzalez et al., 2021 (Spain) | To explore the long-term pulmonary sequelae in critical COVID-19 survivors | Cohort study | SF-12, HADS | 62 | 46 (74%) | 60 | 3 months after hospital discharge | Adult previously ICU admitted and ARDS suffering COVID-19 survivors | Mental disability and palliative care | 15% showed symptoms of depression, 22% symptoms of anxiety |
| Graham et al., 2021 (USA) | To characterize neurologic manifestations in non-hospitalized COVID-19 'long haulers' | Cohort study | PROMIS | 100 | 30% | 43 | 4–6 months | COVID-19 survivors with neurologic symptoms lasting > 6 weeks | Hospitalisation for pneumonia or hypoxemia | 85% suffered from fatigue, 33% from insomnia |
| Halpin et al., 2021 (UK) | To examine post-discharge symptoms and rehabilitation needs in COVID-19 survivors | Cohort study | A COVID-19 rehabilitation telephone screening tool + EQ-5D-5L | 100 | 54 (54%) | Ward patients 70.5, ICU patients 58.5 | 4–8 weeks after hospital discharge | Adult COVID-19 patients from Leeds | Severe dementia, missing contact details | Fatigue was present in 64% of the patients and was most pronounced in the ICU group. PTSD was found in 31% and was most pronounced in the ICU group. Concentration problems was found in 22% and was most pronounced in the ICU group. Short-term memory deficits was found in 18%. Worsened anxiety/depression was found in 23% and was most pronounced in the ICU group |
| Horn et al., 2020 (France) | To assess the prevalence and predictive factors of PTSD in discharged COVID-19 patients | Cohort study | IES-6 (self-rating); PCL-5 | 138 | 101 (56.1%) | 53 | 7 weeks after after onset of symptoms | Adult COVID-19 patients from Lille University Hospital Center | Communication problems affecting the ability to respond to questionnaires | 6.5% of the patients had PTSD at follow up. Preexisting psychiatric disorder, a high IES-6 score and ICU stay were associated with higher PCL-5 scores |
| Huang et al., 2021a (China) | To descibe the long-term consequences of COVID-19 and associated risk factors in previously hospitalised patients | Cohort study | EQ-5D-5L + self-reported symptom questionnaire | 1733 | 897 (52%) | 57 | 175–199 days | COVID-19 patients discharged from Jin Yin-tan hospital Jan-May 2020 | Psychotic disorders, dementia, readmission to hospital, stroke or similar, living outside Wuhan or in nursing homes | Fatigue or muscle weakness was found in 63% of the patients and sleep difficulties 26%. Anxiety or depression in 23%. Anxiety/depression was highest in patients recovering from severe COVID-19 |
| Huang et al., 2021b (China) | To examine somatic symptom burden and sleep quality over time in COVID-19 survivors | Cohort study | PSQI | 74 | 44 (60%) | 52 | 1 month | Adult COVID-19 survivors | Incomplete medical records | Fatigue had improved by follow up, but sleep disturbances were still present. Severity of acute COVID-19 was a predictor of sequelae |
| Iqbal et al., 2021 (Pakistan) | To assess the prevalence and characteristics of COVID 19 sequelae | Cross sectional study | Self-designed questionnaire | 158 | 71 (45%) | 40 | 20–90 days since recovery | Adult recovered COVID-19 patients | Psychiatric history | 83% suffered from fatigue, 56% from sleep disturbances, 53% anxiety, 42% depression. Fatigue and sleep disturbances correlated negatively with time since recovery. Depression and anxiety did not |
| Liang et al., 2020 (China) | To evaluate COVID-19 sequelae 3 months after hospital discharge | Cohort study | Clinical interview | 76 | 21 (28%) | 41 | 3 months | Adult COVID-19 survivors | History of pulmonary resectioning, neurological or psychiatric disease | 60% experienced fatigue at follow up. Acute levels of serum troponin-I correlated with fatigue at follow up |
| Lorenzo et al., 2021 (Italy) | To evaluate the clinical status of COVID-19 survivors 3 months after hospital discharge | Prospective cohort study | EQ5D, EuroQol | 251 | 179 (71%) | 62 | 80–101 days | Hospitalized COVID-19 patients evaluated 1 and 3 months after hospital discharge | Not reported | Anxiety and insomnia were present in 25% of patients, PTSD in 22%. No difference was found between month 1 and month 3 in anxiety or PTSD. Insomnia decreased at month 3. Current psychiatric disorder as well as anxiety, insomnia and PTSD at month 1 predicted PTSD at month 3 |
| Lu et al., 2020 (China) | To explore the micro-structural changes in the central nervous system after SARS-CoV-2 infection | Cohort study | Self-report questionnaire | 60 | 34 (56.7%) | 44.1 | Follow up was performed 3 month after hospital discharge | Previous hospitalisation with COVID-19 at Fuyang No.2 People's Hospital | Not reported | Memory loss was present in 13.3% of the patients during the acute phase of the disease. At follow up, this affected 28.3% of the patients. 41.7% reported mood changes during the acute stage of the disease, whereas this was present in 16.7% at follow up. 15% reported myalgia acutely and 25% at follow up. The patients also displayed higher bilateral GMV in hippocampus. GMV was negatively correlated with LDH. Global MD of WM was found to correlate with memory loss |
| Matalon et al., 2021 (Israel) | To examine anxiety, depression and stress in COVID-19 patients 1 months after hospitalization | Prospective cohort study | PROMIS | 64 | 35 (55%) | 47 | 1 month | Adult hospitalized COVID-19 survivors | Cognitive or language barriers | Depressive and anxiety symptoms decreased from acute disease to follow up, but was a predictor of PTSS |
| Mattioli et al., 2021 (Italy) | To investigate neurological and cognitive impairments 4 months after COVID-19 | Cohort study | MMSE | 120 | 30 (25%) | 48 | 4 months | Health care workers with COVID-19 | Not reported | No cognitive impairment was found. Anxiety, stress and depression was higher than in the matched comparison group |
| Mazza et al., 2020 (Italy) | To investigate the psychopathological impact of COVID-19 in survivors at one month follow up | Cohort study | Unstructured clinical interview + IES-R, PCL-5, ZSDS, BDI-13, STAI-Y, MOS-SS, WHIIRS, and OCI | 402 | 264 (65.7%) | 57.8 | 31 days after discharge or 28 days after visit to ED | Patients surviving COVID-19 which had either been hospitalised at or evaluated at the ED at San Raffaele Hospital in Milan. | Patients below 18 years | 28% for displayed PTSD, 31% depression, 42% anxiety, 20% OCD symptoms and 40% insomnia. Overall, 56% scored in the pathological range in at least one clinical dimension. Women had lower baseline inflammatory markers, but suffered more for both anxiety and depression. Patients with a previous psychiatric diagnosis showed increased scores on most psychopathological measures, with similar baseline inflammation. Baseline SII was positively associated with depression and anxiety at follow up. No information on follow-up levels of inflammatory markers were reported. |
| Mazza et al., 2021 (Italy) | To study psychopathological and neuro-cognitive impact of COVID-19 in survivors 3-month after clinical recovery | Prospective cohort study | Unstructured clinical interview; IES-R; PCL-5; ZSDS; BDI-13; STAI-Y; WHIIRS; OCI | 226 | 149 (66%) | 59 | 3 months | hospitalized or non-hospitalised COVID-19 survivors | Patients < 18 years old | Persistent depressive symptomatology but not PTSD, anxiety and insomnia at follow up. Sex, previous psychiatric history, and the presence of depression at one month affected the depressive symptomatology at three months. Regardless of clinical physical severity, 78% of the sample showed impaired cognition. Baseline SSI predicted depressive symptomatology and cognitive impairment at follow up |
| McLoughlin et al., 2020 (UK) | To investigate functional and cognitive outcomes in patients with COVID-19 delirium | Cohort study | TICS-m, NEADL | 71 | 51 (72%) | 61 | Follow up was performed 4 weeks after assessment of delirium | SARS-COV-2 positive adult inpatients from University College Hospital, London. | Discharged or diseased prior to assessment patients | Functional but not cognitive impairments found at follow up. |
| Méndez et al., 2021 (Spain) | To evaluate neurocognitive function, psychiatric symptoms and QoL in COVID-19 survivers 2 months after hospital discharge | Cross sectional study | QoL-SF-12, VLT-I, VLT-D, ANT, Digit Span backwards subtest, GAD-7, PHQ-2, DTS | 179 | 102 (58.7%) | 57 | 2 months after discharge | Adult COVID-19 patients discharged from a hospital in Valencia | Age > 84, non-spanish speakers, nursing-home residents, pre-existing dementia, substance abuse, previous major psychiatric disorder | 58.7% of the patients suffered from moderate neurocognitive impairment and 18.4% from severe neurocognitive impairment. Anxiety was found in 29.6% of the patients, depression in 26.8% and PTSD in 25.1%. Stress-related symptoms were associated with neurocognitive impairment. |
| Miskowiak et al., 2021 (Denmark) | To investigate frequency, pattern and severity of cognitive impairments 3–4 months after COVID-19 | Cohort study | SCIP-D; TMT; CFQ | 29 | 17 (59%) | 56 | 3–4 months | Hospitalized COVID-19 survivors | Language barriers; preexisting neurological comorbidity | 59–65% of the patients suffered from clinically significant cognitive impairments. This was associated with d-dimer levels during acute illness and residual pulmonary dysfunction indicating an association with severity of lung function and potentially restricted cerebral oxygen delivery |
| Monti et al., 2021 (Italy) | To assess QoL of invasively ventilated COVID-19 ARDS survivors | Cohort study | EQ-5D-3L, HADS, | 39 | 35 (90%) | 56 | 61 days after ICU discharge | Adult previously ICU admitted COVID-19 ARDS patients | Not reported | 1 patient (2.6%) experienced cognitive decline. 21% reported moderate anxiety or depression |
| Morin et al., 2021 (France) | To describe COVID-19 sequelae 4 months after hospitalization | Prospective uncontrolled cohort study | Q3PC | 478 | 201 (42%) | 61 | 4 months | Adult previously hospitalized COVID-19 survivors | end-stage cancer; dementia; nosocomial COVID-19 | 31% experienced fatigue at follow up, 21% cognitive symptoms, 18% depression 23% anxiety and 7% PTSD |
| Negrini et al., 2021 (Italy) | To examine whether COVID-19 could result in long-term cognitive deficits | Case series | MMSE, STAI, BDI | 9 | 6 (66.7%) | 60 | >= 30 days | Patients previously admitted to a rehabilitation hospital with acute respiratory distress syndrome due to COVID-19. | Cognitive deficits prior to hospitalization. Stroke during acute phase of COVID-19. | A general cognitive decay was observed in 3 patients, with a specific decline in attention, memory, language, and praxis abilities. The cognitive malfunctioning correlated with the length of stay in the ICU. 6 patients displayed anxiety symptoms, 2 of whom also had mild depressive symptoms |
| Noviello et al., 2021 (Italy) | To assess the frequency and risk of gastrointestinal and somatoform symptoms 5 months after COVID-19 compared to a control cohort | Controlled cohort study | SAGIS | 164 cases; 183 controls | 98 (39.8%) | 44 | 5 months | Adult COVID-19 survivors | Previous gastrointestinal comorbidities | Fatigue was significantly more frequent in COVID-19 survivors than controls (32% vs 14%), whereas no sig differences between groups were found for sleep disturbances, depression or anxiety |
| Ortelli et al., 2021 (Italy) | To provide a comprehensive profile of fatigue in COVID-19 survivors compared to healthy controls | Case control study | FRS, FSS, BDI, MoCA, VT, SIT, NT, AES | Cases: 12; controls: 12 | Cases: 83%; controls: 67% | Cases: 67; controls 64 | 12 weeks after disease onset | Prevously hospitalized COVID-19 survivors with FRS score >= 6 | pre-COVID-19 neurological disorder, psychiatric, endocrine, metabolic or cardiopulonary conditions | AES and BDI was higher in patients than controls. AES scores correlated with BDI scores. Cognition was poorer in cases compared to controls, but was only slightly worse than in the backgrund population. Cases displayed a hyperinflammatory state with increased serum levels of CRP and IL-6 during the acute phase |
| Petersen et al., 2020 (Faroe Islands) | To describe symptoms of acute and long COVID in mainly nonhospitalized patients from Faroe Islands | Cohort study | Phone interview | 179 (8 hospitalized) | 82 (46%) | 40 | 125 days | Previous COVID-19 patients | Not reported | At last follow up 47% were asymptomatic compared to 4% during the acute phase. Fatigue was the most prevalent finding at follow up; 29% suffered from fatigue at follow up compared to 74% in the acute phase. Severity of fatigue improved from primarily severe in the acute phase to mild-moderate at follow up |
| Poyraz et al., 2021 (Turkey) | To examine psychiatric symptomatology in recovered COVID-19 patients | Cross sectional study | IES-R, HADS, PSQI, MINI Suicidality Scale | 284 | 140 (50.2%) | 39.7 | average 50 days | COVID-19 survivors from a hospital in Istanbul | Not reported | 34.5% displayed PTSD, anxiety and/or depression. PTSD was most commonly reported. Predictors of PTSD were female gender, past traumatic events, protracted symptoms and stigmatisation |
| Qu et al., 2021 (China) | To determine health-related QoL of COVID-19 patients after discharge | Cohort study | Electronic survey | 540 | 270 (50%) | 48 | 3 months after discharge | Previously hospitalized COVID-19 patients | Other viral infections than SARS-CoV-2, pregnancy | 29% suffered from fatigue at follow-up |
| Romero-Duarte et al., 2021 (Spain) | To quantify COVID-19 sequelae 6 months after discharge | Retrospective cohort study | Structured clinical interviews | 789 | 425 (54%) | 63 | 6 months | Previously hospitalized COVID-19 survivors | Non-hospitalized COVID-19 patients | 22% suffered from fatigue, 4% from depression, 7% from anxiety and 5% from sleep disturbances at follow up. Women were more at risk of developing post-COVID fatigue, depression and anxiety |
| Sami et al., 2020 (Iran) | To examine the incidence of psychological disorders in previously hospitalized COVID-19 survivors | Cohort study | PHQ-9, DASS-21 | 490 | 299 (61%) | 57 | 4 weeks after discharge | Previously hospitalized COVID-19 patients | Not reported | 10–13% suffered from fatigue and 8% from sleep disorders at follow up. These factors were both independent of severity of the acute COVID-19 |
| Shang et al., 2021 (China) | To understand COVID-19 sequelae | Cohort study | Questionnaire | 796 | 404 (51%) | 62 | 6 months | COVID-19 survivors | Not reported | Fatigue was found in 25%, sleep disorder in 23% hypomnesis in 15%. Women were more likely to suffer from fatigue and sleep disturbances. Critical illness was a risk factor for hypomnesis |
| Sudre et al., 2021 (UK/USA/Sweden) | To examine prevalence and risk factors or long-COVID | Prospective controlled cohort study | App questionnaire | 4182 | 29% | 42 | >28 days | Adult COVID-19 survivors with BMI 15–55 | Individuals using the app already feeling unwell; app users without any symptoms throughout the study period | 13% reported symptoms lasting > 28 days. long-COVID was characterised by fatigue, headache and anosmia and was associated with severity of the acute disease |
| Sun et al., 2021 (China) | To evaluate severity and prognosis of COVID-19 | Retrospective cohort study | Standardized electronic questionnaire | 932 | 375 (40%) | 58 | 3 months | Previously hospitalized COVID-19 survivors | Dementia and communication problems; lack of reliable medical history | Median duration of fatigue was 14 days in patients with mild disease and 32 days in severe cases. At follow up 2% suffered from fatigue |
| Sykes et al., 2021 (UK) | To report the long-COVID symptom burden of hospitalized COVID-19 patients | Cohort study | EQ-5D-5L; clinical interview | 134 | 88 (66%) | 60 | 46–167 days | Discharged COVID-19 survivors | Mild symptoms and normal chest x-ray at admission | Females were more likely to report anxiety and fatigue. After 100 days follow up 43% still suffered from anxiety, 33% from extreme fatigue, 35% from sleep disturbances, 31% from memory impairments. These symptoms had improved from previous follow up timepoints. |
| Tanriverdi et al., 2021 (Turkey) | To investigate extrapulmonary COVID-19 sequelae in patients with mild-moderate disease | Cross-sectional study | HADS; PSQI | 48 | 22 (47%) | 39 | >12 weeks | Adult survivors of mild-moderate COVID-19 | Severe COVID-19, neurological, cognitive or orthopedic impairments | Of patients with COVID-19 sequelae, 33% suffered from anxiety, 29% from depression and 50% from sleep disturbances |
| Taquet et al., 2021a (USA) | To examine adverse mental health consequences of COVID-19 by use of data from the TriNetX Analytics Network | Cohort study | ICD-10 | 57,476 compared to several matched control groups | 27,525 (45.1%) | 49.3 | 14–90 days after positive SARS-CoV-2 test | Subjects from the TriNetX Analytics Network who had previously tested positive for COVID-19. Occurance of a first psychiatric diagnosis 14–90 days after positive SARS-CoV-2 test. | Not reported | A diagnosis of COVID-19 was associated with increased incidence of a first psychiatric diagnosis in the following 14–90 days. HR was highest for anxiety disorders, insomnia and dementia. |
| Taquet et al., 2021b (USA) | To provide incidence rates of neurological and psychiatric diagnoses 6 months after COVID-19 | Retrospective cohort study | ICD-10 | 236,379 compared to several comparison groups | 104,015 (44%9 | 46 | 6 months | Subjects from the TriNetX Analytics Network who had previously tested positive for COVID-19 | Not reported | 14% were diagnosed with a mood disorder within the first 6 months after COVID-19, 17% with anxiety, 1.4% with psychosis, 5% with insomnia. Anxiety and insomnia were most pronounced in previously hospitalized survivors. These diagnoses were all higher than in the control groups. HR for anxiety and mood disorders were still elevated but decreasing compared to after 3-months. Mood and anxiety disorders had a weaker relationship with COVID-19 severity and might indicate indirect manifestations of the illness |
| Tenforde et al., 2020 (US) | To evaluate symptom duration and risk factors for milder COVID-19 disease | Cohort study | Telephone interview | 270 | 130 (48%) | 43 | 2–3 weeks | Adult previous COVID-19 outpatients | Asymptomatic COVID-19 | 35% experienced fatigue at follow up compared to 70% in the acute phase. Older age and chronic medical conditions were associated with sequelae. No association between ethnicity and sequelae |
| Tomasoni et al., 2021 (Italy) | To investigate prevalence and possible predictors of anxiety and depression following recovery from COVID-19 | Cross sectional study | HADS: MMSE | 105 | 77 (73%) | 55 | 1–3 months | Discharged COVID-19 patients from a hospital in Milan | Not reported | 29% of the patients displayed anxiety symptoms while depression was found for 11%. These symptoms were not predicted by clinical parameters or disease severity, but patients with anxiety/depression reported a higher degree of persistence of physical symptoms including asthenia |
| Townsend et al., 2020 (Ireland) | To assess patients recovering from COVID-19 for symptoms of severe fatigue, irrespective of severity of initial illness | Cohort study | CFQ-11 | 128 | 59 (54%) | 50 | 56–84 days | COVID-19 survivors | Not reported | 52% reported persistent fatigue at follow up (median time 10 weeks). No association between COVID-19 severity and post-COVID fatigue was found. Neither did markers of inflammation or cell turnover correlate with post-COVID fatigue. Female sex and pre-existing diagnosis of depression/anxiety were risk factors of fatigue |
| Townsend et al., 2021 (Ireland) | To asses whether endothelial function is associated with post-COVID fatigue | Case control study | CFQ-11, GAD-7 | 20 + 20 | 10 | 45 | Median time to follow up was 166.5 days | Adult COVID-19 survivors | Medication affecting heart rate or blood pressure | No pathological differences between fatigued and non-fatigued patients on autonomic testing or on 24-hour blood pressure monitoring. Fatigue was strongly associated with increased anxiety (p < 0.001), with no patients having a pre-existing diagnosis of anxiety |
| van den Borst et al., 2020 (Netherlands) | To assess anxiety, depression, cognitive deficits, PTSD, and fatigue, in patients 3 months after recovery from COVID-19 | Cohort study | HADS; CFQ; PTSS; IES-R; SF-36; TICS; PCL-5 | 124 | 74 (60%) | 59 | 3 months | Discharged COVID-19 patients from the Radboud university medical centre or non-admitted patients with mild disease referred by GP | Not reported | Abnormal HADS-anxiety, HADS-depression, TICS, CFQ, PCL-5 and IES scores were observed in 10%, 12%, 15%, 17%, 7%, and 10% of patients, respectively. Fatigue (extracted from SF-36) was decribed in 69% of the patients |
| Venturelli et al., 2021 (Italy) | To address the main clinical problems of survivors and to set public health priorities, in the wake of possible epidemic resurgences, through evaluation of COVID-19 survivors | Cohort study | IES-R, HADS, RSA, MoCA | 767 | 50 | 63 | 81 days after discharge (median) | Patients admitted with conditions possibly related to previous SARS-CoV-2 infection | Asymptomatic pregnant women admitted for delivery and asymptomatic patients admitted for planned procedures for other conditions | 11% suffered from anxiety, 5% from depression, and < 1% from cognitive deficits at follow up |
| Wang et al., 2020c (China) | To evaluate the long-term impact of Covid-19 in pregnancy on mother's psychological status and infant's development | Cohort study | PCL-C, EPDS, ASQ-3, ASQ:SE-2. This survey was performed 3 months after delivery or abortion | 72 cases (57 delivery and 15 abortion) participated in the follow-up survey | 0 | 31 | > 3 months | Pregnant women with confirmed COVID-19. Onset of COVID-19 was in the pregnancy period. | Onset of COVID-19 before or after pregnancy and those who were lost to follow-up. | 22.2% of the women suffered from PTSD or depression at 3 months after delivery or induced abortion |
| Wang et al., 2020b (USA) | To evaluate prevalence of psychiatric morbidity following discharge after COVID-19 hospitalization | Cross sectional study | PTSD-5; GAD-7; CBS-D 10 | 215 | 95 (44%) | 56 | After hospital discharge | Previous COVID-19 patients discharged home or to a nursing facility | Not reported | 34%, 24%, and 42% of patients screened positive for PTSD, anxiety, and depression respectively |
| Weerahandi et al., 2021 (USA) | To characterize physical health and mental health of patients 1 month after discharge for severe COVID-19 | Cohort study | PROMIS survey instruments | 152 | 92 (62.7%) | 62 | Min 1 month | Adult previous O2 requiring COVID-19 patients discharged to home or a facility | Communication impairment or baseline dementia, discharged to hospise, residing in long-term care, and rehospitalization | One month after COVID-19 infection, both scores in mental health and physical health were significantly lower . Patients also reported worsened ability to carry out social activities after COVID-19 |
| Whiteside et al., 2021 (USA) | To investigate neurocognitive sequelae following COVID-19 | Case-series | WAIS IV; RDS; HVLT-R; RBANS; BDAE; TMT; TSAT; ILS; BAI; GDS | 3 | 2 (67%) | 70 | 2 months | English-speaking previous COVID-19 inpatients with severe symptoms, and long-term ICU treatment from a rehabilitation unit | Not reported | The patients demonstrated deficits on formal neuropsychological testing, particularly with encoding and verbal fluency. None of the patients demonstrated rapid forgetting of information. Two patients endorsed new depressive and/or anxiety symptoms |
| Wong et al., 2020 (Canada) | To describe the impact of COVID-19 from the patient's perspective 3 months after symptom onset | Cohort study | EQ-5D-5L; PHQ-9 | 78 | 50 (64%) | 62 | 3 months | Adult COVID-19 patients from Post-COVID-19 respiratory clinics in Vancouver | Not reported | Patients with baseline comorbidities were more likely to suffer from anxiety or depression (22% vs 9% without comorbidities at baseline). 47% of the patients experienced lowered sleep quality. 24% displayed mood impairment |
| Wu et al., 2020 (China) | To examine whether anaesthesiologists experience post-COVID-19 anxiety | Case series | Questionnaire about COVID-19 severity and anxiety | 14 | 7 (50%) | 39 | Min 15 days | Anesthesiologists from Hubei who had survived COVID-19 | Not reported | 93% reported anxiety/fear after recovery. Only 2 subjects reported moderate/severe symptoms. |
| Xiong et al., 2021 (China) | To describe the prevalence, nature and risk factors for the clinical sequelae in COVID-19 survivors | Case control study | Clinical interview | 538 cases + 184 controls | 245 (45.5%) cases + 96 (52,2%) controls | 52 patients; 50 controls | 91–116 days | Cases: adult cured COVID-19 patients discharged from hospital. Controls: Demographically matched volunteers without COVID-19 | Severe and complex underlying diseases or receiving invasive treatment and pregnant/breastfeeding women | Significantly more cases than controls suffered from depression (4%), anxiety (7%) and somnipathy (18%) at the 3 month follow up. The COVID-19 survivors also reported physical decline/fatigue (28%) and respiratory symptoms at this time point (also significantly different from control subjects). Sequealae was more common in female subjects and severity of disease also correlated with subsequent sequelae |
| Yuan et al., 2020 (China) | To evaluate mental health status of 96 convalescent COVID-19 patients. | Cohort study | Online questionnaire; PTSD-SS, SDS, ZSDS. | 96 | 50 (52%) | 45.2 | 6 days after 2 neg SARS-CoV-2 PCR tests | All cured COVID-19 patients discharged from Hospital to quarantine facility | 44% of the cured COVID-19 patients reported depressive symptoms at follow-up. Self-reported depression did not correlate with gender, age, comorbidity, severity of initial infection, or duration of initial illness but did correlate with increased white blood cell and neutrophil counts, and neutrophil-to-lymphocyte ratio | |
| Zhou et al., 2020 (China) | To evaluate the impacts of COVID-19 on cognitive functions and inflammatory profiles in recovered patients | Case control study | GAD-7, PHQ-9, TMT, SCT, CPT, DST | 29 cases + 29 controls | 18 (62%) | 47 | After min 2 neg SARS-CoV-2 PCR tests | Recovered COVID-19 patients; education level > 9 years; Han ethnicity; right-handedness Healthy controls were matched on age, gender and education levels | A history of mental disorders; severe physical illnesses; drug abuse; suicidal thoughts; pregnancy or lactation | Patients with COVID-19 showed a slight cognitive dysfunction compared to controls. This cognitive dysfunction correlated positively with CRP levels. No indication of anxiety or depression in the patient group. |
Abbreviations: Young Manic Rating Scale (YMRS), Impact of Events Scale-Revised (IES-R), post-traumatic stress disorder (PTSD), PTSD Checklist for DSM-5 (PCL-5), Medical Outcomes Study Sleep Scale (MOS-SS), and Obsessive-Compulsive Inventory (OCI), International Quality of Life Assessment Short-Form 36-item questionnaire SF-36 (IQOLA SF-36), Patient Health Questionnaire 9 (PHQ-9), Generalized Anxiety Disorder 7 (GAD-7), Euro Quality of life - five dimensions - five levels (EQ-5D-5L), post-traumatic stress disorder self-rating scale (PTSD-SS), Zung anxiety self-rating scale (SAS), and Zung self-rating depression scale (ZSDS), Trail making test (TMT), Sign Coding Test (SCT), Continuous Performance Test (CPT), and Digital Span Test (DST), Quality of life assessment (WHOQOL-BREF), Montreal Cognitive Assesment (MoCA), Edinburgh Postnatal Depression Scale (EPDS), Ages and Stages Questionnaires, third edition (ASQ-3), Ages and Stages Questionnaire: Social-Emotional, second edition (ASQ:SE-2), Telephone Instrument for Cognitive Status (TICS-m) and Barthel Index and Nottingham Extended Activities of Daily Living (NEADL), Mini-Mental State Examination test (MMSE), State- Trait Anxiety Inventory (STAI) and the Beck Depression Inventory (BDI), intensive care unit (ICU), 13-item Beck's Depression Inventory (BDI-13), State-Trait Anxiety Inventory form Y (STAI-Y), Women's Health Initiative Insomnia Rating Scale (WHIIRS), PTSD Checklist-Civilian Version (PCL-C), Gray matter volumes (GMV), lactate dehydrogenase (LDH), mean diffusion (MD), white matter (WM), Cognitive Failures Questionnaire (CFQ), Informant Questionnaire on Cognitive Functioning in the Elderly (IQ-CODE-N), Impact of Event Scale-6 items (IES-6), QoL Short Form Health Survey 12-item (QoL-SF-12), Verbal Learning Test-Immediate (VLT-I), Verbal Leaning Test - Delayed (VLT-D), Animal Naming Test (ANT), Digit Span backward subtest, 2-item Patient Health Questionnaire (PHQ-2), 17-item Davidson Trauma Scale (DTS), Hospital Anxiety and Depression Scale (HADS), Pittsburgh Sleep Quality Index (PSQI), The Post Traumatic Stress Syndrome Checklist (PTSS), College Breakthrough Series - Depression (CBS-D 10), Patient-Reported Outcomes Measurement Information System (PROMIS), Reliable Digit Span (RDS), Hopkins Verbal Learning Test (HVLT-R), Repeatable Battery for the Assesment of Neuropsychological Status Update (RBANS), Test of Sustained Attention and Tracking (TSAT), Independent Living Scales (ILS), Back Anxiety Inventory (BAI), Geriatric Depression Scale (GDS), Post-COVID-19 functional status (PCFS), Systemic immune-inflammation index (SII), Hamilton Rating Scale for Depression (HAM-D), Functional Independence Measure (FIM), Trauma Screening Questionnaire (TSQ), Short Form Health Survery (SF-12), Euro Quality of life - five dimensions − 3 levels (EQ-5D-3L), Fatigue rating scale (FRS), Fatigue severity scale (FSS), Vigilance Task (VT), Stroop Interference Task (SIT), Navon TAsk (NT), Apathy Evaluation Scale (EAS), Depression Anxiety Stress Scales (DASS-21), Chalder Fatigue Scale (CFQ-11), Resilience Scale for Adults (RSA), Olfactory Dysfunction (OD), Gustatory Dysfunction (GD), Acute Respiratory Distress Syndrome (ARDS), Apathy Evaluation Scale (AES), Screen for Cognitive Impairment in Psychiatry Danish Version (SCIP-D), Cognitive Screening Questionnaire (Q3PC), Depression in the Medically Ill questionnaire (DMI-10), Structured Assessment of Gastrointestinal Symptoms (SAGIS), Hazard ratio (HR), Modified Fatigue Impact Scale (MFIS).
3. Results
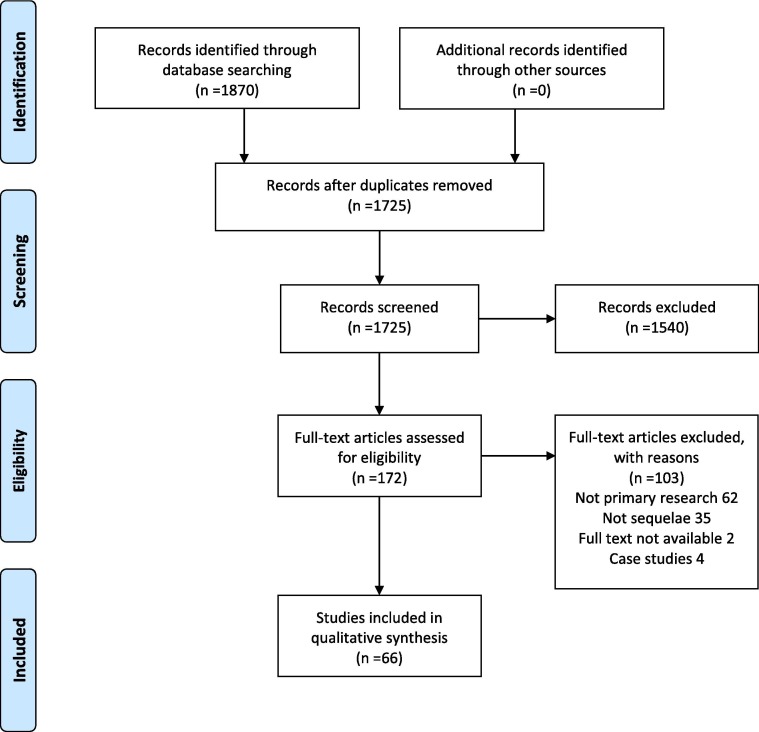
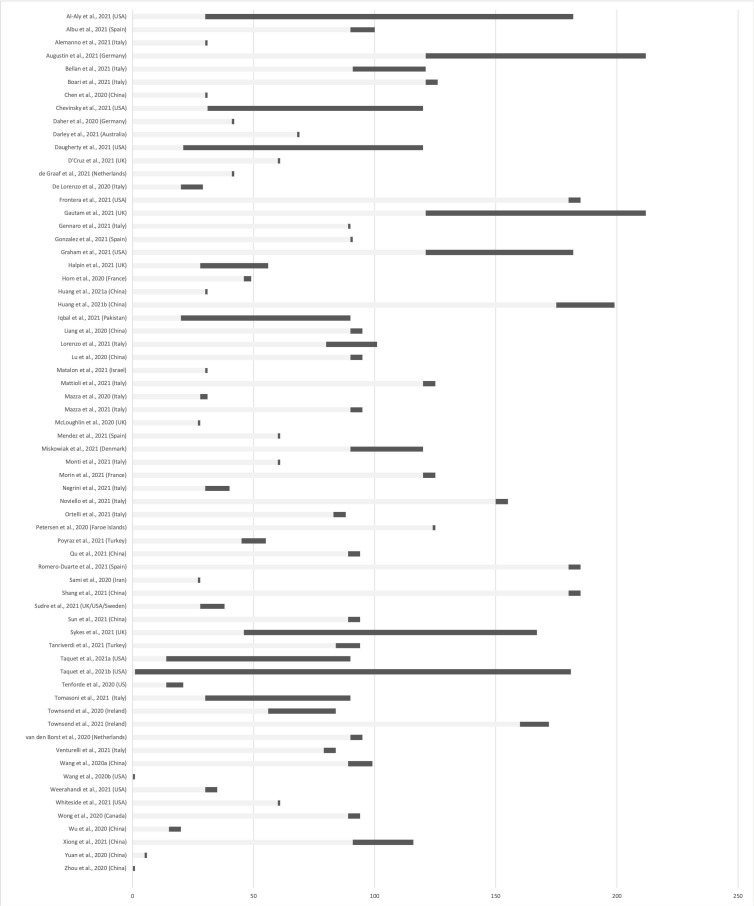
The search resulted in 1725 unique references. Of these, 1540 were excluded as irrelevant during the title-abstract screening. Mostly these references were related to indirect effects of the COVID-19 pandemic on mental health. 172 references were full-text screened. Of these, 116 were excluded, primarily because they 1) did not contain primary data or 2) examined acute effects of SARS-CoV-2 infection rather than sequelae. After this thorough selection process, 66 articles were included in this review (Table 1). The study selection process is illustrated in Fig. 1 . Included studies were from countries in Asia, Europe and North America. The patient material in the included articles was from subjects who were outpatients or had previously been hospitalized with COVID-19 either at the intensive care unit (ICU), in a ward, or the Emergency Department. Study designs include cohort studies, case-control studies, and case series. Time to follow-up ranged from day 1 after recovery until 7 months post-COVID-19 (see Fig. 2 for an overview of follow-up periods).
Fig. 1.
Study selection.
Fig. 2.
Overview of follow-up periods (. ).
).
3.1. Depression and anxiety
The most frequently reported psychiatric deficits were depression and/or anxiety. In total, this was examined in 47 studies (Al-Aly et al., 2021, Alemanno et al., 2021, Boari et al., 2021, Chen et al., 2020, Chevinsky et al., 2021, Daher et al., 2020, Darley et al., 2021, Daugherty et al., 2021, De Lorenzo et al., 2020, Frontera et al., 2021, Gennaro et al., 2021, González et al., 2021, Halpin et al., 2021, Huang et al., 2021a, Iqbal et al., 2021, Lorenzo et al., 2021, Matalon et al., 2021, Mattioli et al., 2021, Mazza et al., 2020, Mazza et al., 2021, Méndez et al., 2021, Monti et al., 2021, Morin et al., 2021, Negrini et al., 2021, Noviello et al., 2021, Ortelli et al., 2021, Poyraz et al., 2021, Romero-Duarte et al., 2021, Sykes et al., 2021, Tanriverdi et al., 2021, Taquet et al., 2021a, Taquet et al., 2021b, Tomasoni et al., 2021, Townsend et al., 2021, van den Borst et al., 2020, Venturelli et al., 2021, Wang et al., 2020b, Wang et al., 2020c, Whiteside et al., 2021, Wong et al., 2020, Wu et al., 2020, Xiong et al., 2021, Yuan et al., 2020, Zhou et al., 2020, D'Cruz et al., 2021, de Graaf et al., 2021, Weerahandi et al., 2021). The results range from no indication of depression or anxiety (Daher et al., 2020, Zhou et al., 2020) to >30% at follow-up (De Lorenzo et al., 2020, Frontera et al., 2021, Iqbal et al., 2021, Mazza et al., 2020, Negrini et al., 2021, Sykes et al., 2021, Wang et al., 2020b, Whiteside et al., 2021, Wu et al., 2020, Yuan et al., 2020). These studies were performed immediately after recovery (measured by min 2 negative SARS-CoV-2 PCR tests) (Wang et al., 2020b) up to 199 days after discharge from the hospital (Huang et al., 2021a).
Disease severity was in several studies suggested to be a risk factor. Al-Aly et al., 2021, Darley et al., 2021, Huang et al., 2021a, Taquet et al., 2021a and (Halpin et al., 2021) but not (de Graaf et al., 2021) reported that anxiety and/or depression was highest in patients recovering from severe COVID-19, Gennaro et al. (2021) showed that duration of hospitalization correlated with depressive symptoms, Alemanno et al. (2021) reported a correlation between depressive symptoms and severity of the initial disease and de Graaf et al. (2021) showed that worse post-COVID functional status was associated with depression. Alemanno et al. (2021) also reported that the depressive symptomatology did not improve compared to depressive status at admission. In contrast Gennaro et al. (2021) reported anxiety to decrease from 1 to 3 months follow-up.
Chevinsky et al. (2021) reported that anxiety was significantly higher in COVID-19 survivors than controls up to 60 days after recovery, but symptoms improved 90 days post-recovery. Depression is significantly higher than controls until 30 days after recovery whereafter the symptoms improve and no significant differences between cases and controls were found. Matalon et al. (2021) reported that depression and anxiety had normalised at the one month follow-up, but were predictors of longer lasting PTSD. Mazza et al. (2021) found persistent depressive symptomatology at 3 month follow-up, whereas anxiety had improved at this timepoint. Taquet et al. (2021a) found HR for anxiety and mood disorders to be elevated at 6 months follow-up but lower than at the 3 months follow-up. Iqbal et al., 2021, Lorenzo et al., 2021 found that depression and anxiety did not correlate with time since recovery. Tomasoni et al. (2021) reported that neither anxiety nor depression was predicted by clinical parameters or disease severity but that the patients reported a higher degree of persistence of physical symptoms. Three studies examined baseline inflammatory markers concerning depression and anxiety at follow-up. Mazza et al. (2020) showed that women displayed lower baseline inflammatory markers but suffered more from both anxiety and depression. Patients with a previous psychiatric diagnosis showed high scores on most psychopathological measures, with similar baseline inflammation. Baseline systemic immune-inflammation index (SII) was positively associated with scores of depression and anxiety at follow-up (Mazza et al., 2020, Mazza et al., 2021). Gennaro et al. (2021) showed that systemic inflammation at admission predicted severity of depressive psychopathology at the 3 months follow-up. Baseline comorbidities have also been suggested to be essential for the development of depression or anxiety. Wong et al. (2020) reported that 22% percent of the patients with comorbidities at baseline suffered from anxiety or depression, whereas this was only 9% without baseline comorbidities. Gennaro et al. (2021), (Mazza et al., 2021), Romero-Duarte et al. (2021) and (Sykes et al., 2021) reported that previous psychiatric history and female sex were predicters of depression and anxiety. Yuan et al. (2020) on the contrary reported depression not to correlate with sex, age, comorbidity, severity of initial infection, or initial illness duration. Instead, they found that these patients exhibited an elevated immune response as measured by increased white blood cell and neutrophil counts.
3.2. Post-traumatic stress disorder
Post-traumatic stress disorder (PTSD) in the patient groups was reported in 20 articles (Al-Aly et al., 2021, Alemanno et al., 2021, Bellan et al., 2021, D'Cruz et al., 2021, Daugherty et al., 2021, de Graaf et al., 2021, De Lorenzo et al., 2020, Gennaro et al., 2021, Halpin et al., 2021, Horn et al., 2020, Lorenzo et al., 2021, Matalon et al., 2021, Mazza et al., 2021, Méndez et al., 2021, Morin et al., 2021, Poyraz et al., 2021, Taquet et al., 2021b, van den Borst et al., 2020, Wang et al., 2020b, Wang et al., 2020c), with results ranging from 6.5% (Horn et al., 2020) to 42.8% of the included patients (Bellan et al., 2021). The studies were performed shortly after discharge from the hospital (Wang et al., 2020b) until 6 months after discharge (Bellan et al., 2021, van den Borst et al., 2020, Wang et al., 2020b). Gennaro et al., 2021, Mazza et al., 2021 reported that PTSD symptoms improved from 1 to 3 months follow-up. On the contrary, Lorenzo et al. (2021) found no improvement from 1 to 3 months follow-up.
Of potential risk factors, several elements were highlighted. One study found no difference between ICU or no ICU admission (de Graaf et al., 2021), whereas both Halpin et al., 2021, Horn et al., 2020 showed that PTSD was most pronounced after ICU admission. (Al-Aly et al., 2021) Another study found that being hospitalized had a preventive effect on PTSD (De Lorenzo et al., 2020). Sex has also been suggested as a risk factor. Bellan et al. (2021) indicated that being male increased the risk of developing PTSD, whereas De Lorenzo et al., 2020, Poyraz et al., 2021 showed that female sex was a predictor of the disorder. De Lorenzo et al., 2020, Horn et al., 2020, Poyraz et al., 2021 all showed that a previous psychiatric history or past traumatic events increased the risk of PTSD and Matalon et al. (2021) found that depressive and anxiety symptoms during acute COVID-19 were predictors of PTSD. Gennaro et al., 2021, Mazza et al., 2020 examined whether baseline SII correlated with PTSD at follow-up, but did not find any such relationship.
3.3. Obsessive-compulsive disorder and psychotic disorders
Symptoms of obsessive–compulsive disorder (OCD) was examined in two studies (Gennaro et al., 2021, Mazza et al., 2020). Mazza et al. (2020) screened patients for OCD and found that 20% suffered from OCD symptoms at follow-up. Gennaro et al. (2021) reported that any signs of OCD improved from 1 to 3 months follow-up. (Taquet et al., 2021a) found an increased incidence of psychotic diagnoses following COVID-19 compared to control cohorts.
3.4. Cognitive deficits
Different aspects of cognitive decline were examined in 27 studies (Al-Aly et al., 2021, Albu et al., 2021, Alemanno et al., 2021, Darley et al., 2021, Daugherty et al., 2021, de Graaf et al., 2021, De Lorenzo et al., 2020, Gautam et al., 2021, Gennaro et al., 2021, Halpin et al., 2021, Lu et al., 2020, Mattioli et al., 2021, Mazza et al., 2021, Mcloughlin et al., 2020, Méndez et al., 2021, Miskowiak et al., 2021, Monti et al., 2021, Morin et al., 2021, Negrini et al., 2021, Ortelli et al., 2021, Shang et al., 2021, Sykes et al., 2021, Taquet et al., 2021b, van den Borst et al., 2020, Venturelli et al., 2021, Whiteside et al., 2021, Zhou et al., 2020). The results range from no cognitive impairments at a 4 months follow-up (Mattioli et al., 2021) to a report of 78% of the patients experiencing impaired performance on at least one cognitive domain 3 months after clinical recovery (Mazza et al., 2021). In total, 11 studies reported cognitive deficits in>25% of their patient populations (Alemanno et al., 2021, de Graaf et al., 2021, De Lorenzo et al., 2020, Gennaro et al., 2021, Lu et al., 2020, Mazza et al., 2021, Méndez et al., 2021, Miskowiak et al., 2021, Negrini et al., 2021, Sykes et al., 2021, Whiteside et al., 2021). The reported deficits were concentration problems (Halpin et al., 2021), short-term memory deficits (Halpin et al., 2021), general memory loss (Daugherty et al., 2021, Lu et al., 2020, Shang et al., 2021, Sykes et al., 2021), a specific decline in attention, memory, language, and praxis abilities (Negrini et al., 2021), encoding and verbal fluency (Whiteside et al., 2021) and an ICD-10 diagnosis of dementia (Taquet et al., 2021b). The studies were performed from immediately after recovery (defined as a minimum of 2 negative SARS-CoV-2 PCR tests) (Zhou et al., 2020) until 7 months after the disease (Gautam et al., 2021).
One study showed that memory loss was present in 13% of the patients in the acute phase, whereas at the follow-up, 3 months later, 28% of the patients were affected by memory loss (Lu et al., 2020). In contrast, Alemanno et al. (2021) showed that cognitive deficits correlated with disease severity and had improved at follow-up 1 month after discharge compared to at admission. Al-Aly et al., 2021, Darley et al., 2021, Halpin et al., 2021, Negrini et al., 2021, and Shang et al. (2021) also reported follow-up symptoms to be most pronounced after severe acute disease. In contrast, de Graaf et al. (2021) did not find differences in cognitive deficits between ICU and non-ICU patients. McLoughlin et al. (2020) did not find delirium to be a predictor of cognitive impairments. Sykes et al. (2021) described that at 100 day follow-up, memory impairments had improved, but 31% were still affected.
Gennaro et al. (2021) reported that cognitive dysfunction was not predicted by sex, previous psychiatric diagnoses, or hospitalization duration, but by severity of depressive symptoms. Lu et al. (2020) performed MRI scans on patients at the 3-month follow-up and showed that patients displayed higher bilateral grey matter volumes (GMV) in the hippocampus. This correlated negatively with lactate dehydrogenase (LDH). Global mean diffusion (MD) of white matter (WM) correlated with memory loss. Furthermore, Zhou et al. (2020) but not Gautam et al. (2021) reported that cognitive dysfunction was associated with increased C-reactive protein (CRP) levels. Mazza et al. (2021) found baseline SSI levels to predict cognitive impairments at follow-up and Gennaro et al. (2021) found that systemic inflammation at hospital admission predicted neurocognitive performance in a multivariate analysis of variance whereas oxygen saturation or duration of hospitalization did not. Miskowiak et al. (2021) reported that cognitive impairments were associated with d-dimer levels during acute illness and residual pulmonary dysfunction (Ortelli et al., 2021).
3.5. Fatigue
Fatigue following recovery from acute COVID-19 was reported in 32 studies (Al-Aly et al., 2021, Albu et al., 2021, Augustin et al., 2021, Boari et al., 2021, Chevinsky et al., 2021, D'Cruz et al., 2021, Daher et al., 2020, Darley et al., 2021, Daugherty et al., 2021, Frontera et al., 2021, Gautam et al., 2021, Graham et al., 2021, Halpin et al., 2021, Huang et al., 2021a, Huang et al., 2021b, Iqbal et al., 2021, Liang et al., 2020, Morin et al., 2021, Noviello et al., 2021, Petersen et al., 2020, Qu et al., 2021, Romero-Duarte et al., 2021, Sami et al., 2020, Shang et al., 2021, Sudre et al., 2021, Sun et al., 2021, Sykes et al., 2021, Tenforde et al., 2020, Townsend et al., 2020, Townsend et al., 2021, van den Borst et al., 2020, Xiong et al., 2021). The results ranged from no fatigue at 1 month follow-up (Huang et al., 2021b) to 87% suffering from fatigue with 12 studies reporting >45% of their populations to be affected (Albu et al., 2021, Boari et al., 2021, D'Cruz et al., 2021, Daher et al., 2020, Gautam et al., 2021, Graham et al., 2021, Halpin et al., 2021, Huang et al., 2021a, Iqbal et al., 2021, Liang et al., 2020, Townsend et al., 2020, van den Borst et al., 2020).
Two studies reported fatigue to be independent of severity of acute COVID-19 (Sami et al., 2020, Townsend et al., 2020) whereas Halpin et al., 2021, Sudre et al., 2021 found fatigue to be most pronounced in patients recovering from severe acute disease. The severity of fatigue improved from the acute phase to follow-up. In support of this, Chevinsky et al., 2021, Iqbal et al., 2021 found that fatigue was still elevated in COVID-19 survivors at follow-up, but improved with time since recovery.
Female sex and pre-existing diagnosis of depression/anxiety were risk factors for fatigue (Augustin et al., 2021, Romero-Duarte et al., 2021, Shang et al., 2021, Sykes et al., 2021, Townsend et al., 2020, Townsend et al., 2021).
Acute levels of serum troponin-I correlated with fatigue at follow-up (Liang et al., 2020). In contrast, no association was found between markers of inflammation and cell turnover (leukocyte, neutrophil or lymphocyte counts, neutrophil-to-lymphocyte ratio, lactate dehydrogenase, CRP) or pro-inflammatory molecules (IL-6 or sCD25) and fatigue post COVID-19 (Townsend et al., 2020).
3.6. Sleep disturbances
Sleep disturbances following COVID-19 was reported in 24 studies (Al-Aly et al., 2021, Boari et al., 2021, D'Cruz et al., 2021, De Lorenzo et al., 2020, Frontera et al., 2021, Gautam et al., 2021, Gennaro et al., 2021, Graham et al., 2021, Huang et al., 2021a, Huang et al., 2021b, Iqbal et al., 2021, Lorenzo et al., 2021, Mazza et al., 2020, Mazza et al., 2021, Negrini et al., 2021, Noviello et al., 2021, Romero-Duarte et al., 2021, Sami et al., 2020, Shang et al., 2021, Sykes et al., 2021, Tanriverdi et al., 2021, Taquet et al., 2021a, Taquet et al., 2021b, Wong et al., 2020). The results range from Noviello et al. (2021) reporting no differences between COVID-19 survivors and controls at a 5 months follow-up to Graham et al. (2021), showing that 85% of the patients experienced sleep disturbances at 4–6 months follow-up. Both Iqbal et al., 2021, Gennaro et al., 2021 found the extent of the sleep disturbances to decrease with time since recovery. Sami et al. (2020) found sleep disturbances to be independent of severity of acute COVID-19. Augustin et al., 2021, Huang et al., 2021b, Sudre et al., 2021, Taquet et al., 2021a on the contrary found that severity of acute COVID-19 was a predictor for sleep disturbances at follow-up. Mazza et al., 2021, Lorenzo et al., 2021, Sykes et al., 2021 reported that insomnia had improved after 3 months. Female sex was reported as a risk factor for sleep disturbances (Shang et al., 2021).
4. Discussion
We identified 5 major areas of deficits, namely depression/anxiety, PTSD, cognition, fatigue, and sleep disturbances. Additionally, OCD was reported in two studies and one article described an increased incidence of psychotic disorders following COVID-19. The results suggest that survivors of COVID-19 are at risk of psychiatric sequelae but that symptoms generally improve over time.
In summary, we identified and included 66 studies that provided information on psychiatric and neuropsychiatric sequelae of COVID-19. The studies were performed in Asia (16), Europe (37), North America (12), and Oceania (1) and follow-up periods ranged from immediately after recovery to 7 months post-recovery (Fig. 2). Thirteen studies compared results to one or more comparison group. The majority of the included studies were based on patients who had been hospitalized. This should be considered when evaluating the results and before extrapolating to, e.g., patients with mild symptomatology.
4.1. Depression and anxiety
Of the 46 studies screening for anxiety and depression, 10 found >30% of the patients affected. These results correspond with SARS-CoV and MERS-CoV sequelae, where an increased incidence of anxiety and depression has also been reported (Rogers et al., 2020). Intriguingly, a recent meta-analysis concluded that the prevalence of anxiety and depression in the background population (with unknown COVID-19 status) during the pandemic was >30% (Salari et al., 2020) suggesting that the increased incidence of depression/anxiety is caused by indirect effects of the pandemic. Speaking against this, is several of the included studies in the current review where large cohorts of COVID-19 survivors are compared to matched comparison groups (e.g. patients having survived other respiratory diseases during the pandemic). In these studies, COVID-19 survivors were in significantly increased risk of developing depression/anxiety at follow up (Al-Aly et al., 2021, Chevinsky et al., 2021, Daugherty et al., 2021, Mattioli et al., 2021, Taquet et al., 2021a, Taquet et al., 2021b). Only Noviello et al. (2021) who included 164 COVID-19 cases failed to find an increased risk compared to a control cohort 5 months after acute disease.
The variation in results between studies is likely to be affected by the use of very different study instruments, and that time to follow-up examinations differs substantially between studies. Disease severity and duration of symptoms also vary between reports and are even highlighted as risks factor in several of the included studies. Generally, anxiety and depressive symptomatology was reported to improve with time from acute disease. (Alemanno et al., 2021, de Graaf et al., 2021, Halpin et al., 2021, Huang et al., 2021a, Tomasoni et al., 2021). Further, only 7 studies contrast their results to comparison groups making it difficult to differentiate between direct and indirect effects of the COVID-19 pandemic.
Inflammatory markers were examined in some articles; one study showing elevated immune response at the time of the follow-up in patients with self-reported depression (Yuan et al., 2020) and two studies showing a correlation between baseline SII and anxiety/depression (Gennaro et al., 2021, Mazza et al., 2020, Mazza et al., 2021). Also, Gennaro et al. (2021) reported that changes in SII predicted changes of depression during follow-up. Wong et al. (2020) reported that patients with baseline comorbidities were more likely to suffer from depression and/or anxiety. It therefore appears that an elevated inflammatory response is likely involved in the development of these symptoms. This is supported by a vast array of work on the relationship between neuroinflammation and depression (Dantzer et al., 2008, Raison et al., 2006). Interestingly, in the two studies which did not find increased incidence of anxiety or depression (Daher et al., 2020, Zhou et al., 2020) blood levels of IL-6 were not elevated. This is particularly interesting as IL-6 has been linked to depressive symptomatology in several studies (Achtyes et al., 2020, Dahl et al., 2014, Lindqvist et al., 2009). It should also be noted that the two studies which failed to report depression or anxiety were of a smaller sample size (n < 34).
4.2. Post-traumatic stress disorder
Symptoms- or diagnoses of PTSD was screened for and reported in 20 studies. Two of these studies reported that as many as 43% of the patients suffered from post-traumatic stress symptoms (Bellan et al., 2021). It is important to note that PTSD has been reported continuously throughout the pandemic as an indirect consequence of living under stress, uncertainty, and altered daily life rather than due to the disease itself (Tan et al., 2020, Wang et al., 2020a). Also, surviving a critical illness has been shown to induce PTS symptoms (Sparks, 2018). Nonetheless, the reported levels of PTS are higher than what has been outlined in the background population, where affected individuals are reported to be 7–10% (Liu et al., 2020, Tan et al., 2020).
The severity of COVID-19 has been highlighted as a risk factor for PTSD. Two studies reported that patients discharged from ICU were more likely to develop PTSD than non-ICU patients (Halpin et al., 2021, Horn et al., 2020), whereas one study found no difference between ICU and non-ICU patients (de Graaf et al., 2021). This difference could be caused by the fact that non-ICU patients can be a heterogeneous group, including severely ill patients who do not qualify for ICU treatment. Male sex was in one study shown to be a risk factor for developing moderate-severe PTS (Bellan et al., 2021) whereas three studies described female sex as the predictor for PTSD symptoms in general (De Lorenzo et al., 2020, Gennaro et al., 2021, Poyraz et al., 2021). Supporting the notion of a role of the female sex is a previous study examining PTSD in SARS survivors 30 months after recovery where female sex was also found to be an independent predictor of the disorder (Mak et al., 2010). A diagnosis of depression/anxiety is frequently reported as risk factors for the development of PTSD (Brady et al., 2000). In the current study, depression and anxiety during acute disease and early follow-up time-points were also reported to be predictors of subsequent development of PTSD (Lorenzo et al., 2021, Matalon et al., 2021). Mazza et al. (2021) but not Lorenzo et al. (2021) found PTSD symptoms to improve over time. Interestingly, Mak et al. (2009) reported PTSD as the most prevalent long-term psychiatric morbidity in SARS survivors.
Inflammation has previously been reported to be a pathophysiological mechanism in the development of PTSD (Lindqvist et al., 2014, Passos et al., 2015). Of the included studies Gennaro et al., 2021, Mazza et al., 2020 examined whether a relationship between baseline SII and PTSD existed, but did not find such relationship. de Graaf et al., 2021, van den Borst et al., 2020 showed that CRP and leukocyte count was elevated at admission, but normalised at follow-up. None of the included articles specifically examined cytokine levels in relation to PTSD, which will be highly relevant to explore in future studies.
4.3. Cognition
Delirium is a recognised complication of, e.g., respiratory illnesses in older adults. For acute COVID-19, the number of patients experiencing this complication is very high, with reports of up to 84% in severe illness cases at all ages (Helms et al., 2020b). The acute symptoms of delirium include disturbances of attention, awareness, and cognition. Not only do acute reports of cognitive deficits exist, but in the current review we have also identified 27 studies that all report cognitive decline at follow-up. The studies use different study instruments and measure various aspects of cognition. The deficits range from concentration problems (Halpin et al., 2021) to memory deficits (Halpin et al., 2021, Lu et al., 2020, Negrini et al., 2021) and praxis disabilities (Negrini et al., 2021) as well as formal diagnoses of dementia (Taquet et al., 2021a, Taquet et al., 2021b). It should be noted that McLoughlin et al. (2020) examined whether delirium was a predictor of cognitive impairments and did not find an association. It is, therefore, unclear whether initial cognitive deficits are related to the long-term effects. Interestingly, Lu et al. (2020) reported a higher degree of memory deficits at the 3 months follow-up compared to during the acute phase whereas Sykes et al. (2021) found memory impairments to improve with time. It should also be noted that Taquet et al. (2021a) found 0.67% of the COVID-19 survivors to suffer from dementia 6 months after the diagnosis and that patients with more severe acute disease were more likely to receive a dementia diagnosis than patients with milder COVID-19.
Lu et al. (2020) showed that COVID-19 patients displayed brain abnormalities at a 3-month follow-up and that this correlated with memory loss and lactate dehydrogenase (LDH). LDH levels have previously been highlighted as markers for the severity of COVID-19 (Henry et al., 2020). The maintenance of normal cognition in older adults is highly correlated with low levels of LDH (Waters et al., 2020). No correlation was found between oxygen saturation and cognitive dysfunction, suggesting that the deficits were not caused by brain hypoxia. This is in contrast to Miskowiak et al. (2021) who suggested that restricted cerebral oxygen delivery could be involved in the development of cognitive sequelae. Zhou et al. (2020) reported that cognitive dysfunction correlated positively with CRP levels at follow-up and Mazza et al. (2021) showed that SSI predicted cognitive impairments at follow-up. This could indicate that neuroimmune alterations are involved.
4.4. Fatigue
COVID-19 induced fatigue can be defined as ‘a decrease in physical and/or mental performance that results from changes in central, psychological, and or/peripheral factors due to the COVID-19 disease’ (Rudroff et al., 2020). Fatigue was reported during acute COVID-19 but is also found to persist following recovery with debilitating effects for the individuals affected. In the present review we identified 32 studies that all found fatigue at follow-up. Fatigue was reported in both more severe COVID-19 cases (requiring hospitalization) as well as in milder cases. For SARS and MERS it was reported that fatigue was one of the most persistent long-term symptoms with accounts up to 39 months after the initial infection (Rogers et al., 2020). For COVID-19 several studies found fatigue to improve from acute disease to follow-up (Chevinsky et al., 2021, Iqbal et al., 2021, Petersen et al., 2020, Tenforde et al., 2020). Sun et al. (2021) reported that the median duration of fatigue in patients with mild COVID-19 was 14 days and 32 days in patients with severe disease. This could suggest that fatigue is not as severe as in the cases of SARS and MERS. It should though be noted that Albu et al. (2021) found that fatigue was the most debilitating long-COVID symptom, and the main reason patients contacted a COVID rehabilitation program.
Risk factors were female sex and pre-existing psychiatric diagnoses. This is consistent with a review identifying risk factors for persistent fatigue following acute infections (Hulme et al., 2017).
Of biological measures, acute levels of serum troponin-I was in one study reported to correlate with fatigue at follow-up suggesting that fatigue is possibly related to myocardial injury (Liang et al., 2020). Several investigators have noted similarities between post-COVID fatigue and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CSF). A recent systematic review found a strong overlap between the post-COVID symptomatology and the clinical presentation of ME/CFS (Wong and Weitzer, 2021). The pathogenesis of ME/CFS is not yet fully understood and likely multifactorial, but interestingly ME/CSF has previously been linked to infection with Epstein-Barr virus (EBV) and the EBV-induced gene-2 leading to neurological and immune-related symptoms (Kerr, 2019). Also enterovirus, cytomegavirus, human herpesvirus-6, human parvovirus B19 and Chlamydophila pneumoniae have been associated with ME/CSF (Chia and Chia, 2008) and it is therefore plausible that a SARS-CoV-2 infection may also be involved in the pathogenesis of ME/CSF. Future studies will be able to shed light on whether the pathophysiology of post-COVID fatigue and ME/CSF is comparable.
Townsend et al. (2020) explored whether markers of inflammation and cell turnover could be related to fatigue but did not find any significant associations. Sample size for the study was n = 20/group.
4.5. Sleep disturbances
Sleep disturbances of various degrees were reported in 24 studies. Such results are similar to reports of SARS and MERS’ long term effects >6 months after acute infection (Rogers et al., 2020). Sleep disturbances were by some authors found to be independent of severity of acute COVID-19, but to decrease with time since recovery (Gennaro et al., 2021, Iqbal et al., 2021, Sami et al., 2020). Others report severity of acute disease to be a predictor of sleep disturbances at follow up (Huang et al., 2021a, Sudre et al., 2021, Taquet et al., 2021a) It should be noted that indirect effects of the pandemic have also led to an increase in reports of sleep disturbances. One study found that up to 20% of the included subjects suffered from insomnia (Lin et al., 2021). Such numbers are still lower than what is presented in the included articles and can therefore not account for the full extent of dyssomnia reported herein. Further, Taquet et al. (2021a) reported that significantly more COVID-19 survivors than comparison subjects suffered from insomnia 6 months after COVID diagnosis and that patients with a more severe acute disease course were at higher risk of insomnia than patients with milder COVID-19 symptoms.
4.6. Limitations
The main limitation of this review is the lack of studies comparing results to appropriate comparison groups. Without such comparisons it is not possible to completely differentiate between direct and indirect effects of COVID-19. In this review 13 out of 66 references compared results to comparison groups.
In the present review the goal was to include all available data at this specific time of the pandemic. By doing so, we have created a full overview of primary studies on psychiatric and neuropsychiatric sequelae, but it should also be stressed that the quality of the studies was very varied. Sample sizes ranged from 3 subjects to 266,586 and study instruments were not always standardized or the most appropriate for a specific outcome. Additionally, to be able to fully evaluate the indirect effects of the pandemic a more thorough evaluation of the temporal aspects will be needed. Further, the very fast time to publication of COVID-19 related studies is a possible concern that is highlighted in the number of retracted articles on the topic (Soltani and Patini, 2020). When more studies of high data quality have been published, meta analyses of the data should also be performed.
5. Conclusion
In summary, the results from the included studies suggest that survivors of COVID-19 are at risk of psychiatric sequelae and that psychiatric and neuropsychiatric sequelae are indeed an essential part of the long-COVID syndrome. The severity and time-frame of such sequelae are, at present, difficult to evaluate. Overall, a tendency towards symptom improvement over time exist. Likewise, severity of the acute infection also appears to be of importance for subsequent sequelae.
Only a few of the included studies contained comparison groups. It is therefore difficult to fully differentiate between the effects of the disease itself and indirect factors, such as living in a pandemic or having survived a severe disease. As highlighted previously, most of the included studies examined patients discharged from hospital, such that patients with mild or asymptomatic COVID-19 are not well represented. Essential for these aspects are the included reports of large cohorts of both hospitalized and non-hospitalized COVID-19 survivors compared to several appropriate comparison groups (Al-Aly et al., 2021, Chevinsky et al., 2021, Daugherty et al., 2021, Taquet et al., 2021a, Taquet et al., 2021b).
Highlighted risk factors were disease severity, duration of symptoms as well as female sex. Increased inflammatory markers were also reported, suggesting that neuroimmune alterations could be at least some of the disease's underlying course. Supporting this notion is the fact that the receptor for SARS-CoV-2 (ACE2) is expressed on neurons and glial cells (Gowrisankar and Clark, 2016, Nemoto et al., 2020), that SARS-CoV-2 can be detected in the brain, and that astrocytes and microglia cells are activated during COVID-19 (Matschke et al., 2020).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Long COVID: let patients help define long-lasting COVID symptoms. Nature 586, 170. [DOI] [PubMed]
- Achtyes E., Keaton S.A., Smart LeAnn, Burmeister A.R., Heilman P.L., Krzyzanowski S., Nagalla M., Guillemin G.J., Escobar Galvis M.L., Lim C.K., Muzik M., Postolache T.T., Leach R., Brundin L. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav. Immun. 2020;83:239–247. doi: 10.1016/j.bbi.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- Albu S., Zozaya N.R., Murillo N., García-Molina A., Chacón C.A.F., Kumru H. What's going on following acute covid-19? Clinical characteristics of patients in an out-patient rehabilitation program. NeuroRehabilitation. 2021;48(4):469–480. doi: 10.3233/NRE-210025. [DOI] [PubMed] [Google Scholar]
- Alemanno F., Houdayer E., Parma A., Spina A., Del Forno A., Scatolini A., Angelone S., Brugliera L., Tettamanti A., Beretta L., Iannaccone S., Di Gennaro F. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: a COVID-rehabilitation unit experience. PLoS One. 2021;16(2):e0246590. doi: 10.1371/journal.pone.0246590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin, M., Schommers, P., Stecher, M., Dewald, F., Gieselmann, L., Gruell, H., Horn, C., Vanshylla, K., Cristanziano, V.D., Osebold, L., Roventa, M., Riaz, T., Tschernoster, N., Altmueller, J., Rose, L., Salomon, S., Priesner, V., Luers, J.C., Albus, C., Rosenkranz, S., Gathof, B., Fätkenheuer, G., Hallek, M., Klein, F., Suárez, I., Lehmann, C., 2021. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg. Health Eur. 6, 100122. [DOI] [PMC free article] [PubMed]
- Bellan, M., Soddu, D., Balbo, P.E., Baricich, A., Zeppegno, P., Avanzi, G.C., Baldon, G., Bartolomei, G., Battaglia, M., Battistini, S., Binda, V., Borg, M., Cantaluppi, V., Castello, L.M., Clivati, E., Cisari, C., Costanzo, M., Croce, A., Cuneo, D., De Benedittis, C., De Vecchi, S., Feggi, A., Gai, M., Gambaro, E., Gattoni, E., Gramaglia, C., Grisafi, L., Guerriero, C., Hayden, E., Jona, A., Invernizzi, M., Lorenzini, L., Loreti, L., Martelli, M., Marzullo, P., Matino, E., Panero, A., Parachini, E., Patrucco, F., Patti, G., Pirovano, A., Prosperini, P., Quaglino, R., Rigamonti, C., Sainaghi, P.P., Vecchi, C., Zecca, E., Pirisi, M., 2021. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw. Open 4, e2036142. [DOI] [PMC free article] [PubMed]
- Boari G.E.M., Bonetti S., Braglia-Orlandini F., Chiarini G., Faustini C., Bianco G., Santagiuliana M., Guarinoni V., Saottini M., Viola S., Ferrari-Toninelli G., Pasini G., Bonzi B., Desenzani P., Tusi C., Malerba P., Zanotti E., Turini D., Rizzoni D. Short-term consequences of SARS-CoV-2-related pneumonia: a follow up study. High Blood Press Cardiovasc. Prev. 2021;28(4):373–381. doi: 10.1007/s40292-021-00454-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady K.T., Killeen T.K., Brewerton T., Lucerini S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J. Clin. Psychiatry. 2000;61(Suppl 7):22–32. [PubMed] [Google Scholar]
- Chen K.Y., Li T., Gong F.H., Zhang J.S., Li X.K. Predictors of Health-Related Quality of Life and Influencing Factors for COVID-19 Patients, a Follow-Up at One Month. Front Psychiatry. 2020;11:668. doi: 10.3389/fpsyt.2020.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevinsky J.R., Tao G., Lavery A.M., Kukielka E.A., Click E.S., Malec D., Kompaniyets L., Bruce B.B., Yusuf H., Goodman A.B., Dixon M.G., Nakao J.H., Datta S.D., Mac Kenzie W.R., Kadri S., Saydah S., Giovanni J.E., Gundlapalli A.V. Late conditions diagnosed 1–4 months following an initial COVID-19 encounter: a matched cohort study using inpatient and outpatient administrative data - United States, March 1-June 30, 2020. Clin. Infect Dis. 2021 doi: 10.1093/cid/ciab338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia J.K.S., Chia A.Y. Chronic fatigue syndrome is associated with chronic enterovirus infection of the stomach. J. Clin. Pathol. 2008;61(1):43–48. doi: 10.1136/jcp.2007.050054. [DOI] [PubMed] [Google Scholar]
- D'Cruz, R.F., Waller, M.D., Perrin, F., Periselneris, J., Norton, S., Smith, L.J., Patrick, T., Walder, D., Heitmann, A., Lee, K., Madula, R., McNulty, W., Macedo, P., Lyall, R., Warwick, G., Galloway, J.B., Birring, S.S., Patel, A., Patel, I., Jolley, C.J., 2021. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res. 7. [DOI] [PMC free article] [PubMed]
- Daher A., Balfanz P., Cornelissen C., Müller A., Bergs I., Marx N., Müller-Wieland D., Hartmann B., Dreher M., Müller T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020;174:106197. doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J., Ormstad H., Aass H.C.D., Malt U.F., Bendz L.T., Sandvik L., Brundin L., Andreassen O.A. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. 2014;45:77–86. doi: 10.1016/j.psyneuen.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley D.R., Dore G.J., Cysique L., Wilhelm K.A., Andresen D., Tonga K., Stone E., Byrne A., Plit M., Masters J., Tang H., Brew B., Cunningham P., Kelleher A., Matthews G. Persistent symptoms up to four months after community and hospital-managed SARS-CoV-2 infection. Med. J. Aust. 2021;214(6):279–280. doi: 10.5694/mja2.50963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty S.E., Guo Y., Heath K., Dasmariñas M.C., Jubilo K.G., Samranvedhya J., Lipsitch M., Cohen K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2021;373 doi: 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf, M.A., Antoni, M.L., Ter Kuile, M.M., Arbous, M.S., Duinisveld, A.J.F., Feltkamp, M.C.W., Groeneveld, G.H., Hinnen, S.C.H., Janssen, V.R., Lijfering, W.M., Omara, S., Postmus, P.E., Ramai, S.R.S., Rius-Ottenheim, N., Schalij, M.J., Schiemanck, S.K., Smid, L., Stöger, J.L., Visser, L.G., de Vries, J.J.C., Wijngaarden, M.A., Geelhoed, J.J.M., Roukens, A.H.E., 2021. Short-term outpatient follow-up of COVID-19 patients: a multidisciplinary approach. EClinicalMedicine, 100731. [DOI] [PMC free article] [PubMed]
- De Lorenzo R., Conte C., Lanzani C., Benedetti F., Roveri L., Mazza M.G., Brioni E., Giacalone G., Canti V., Sofia V., D’Amico M., Di Napoli D., Ambrosio A., Scarpellini P., Castagna A., Landoni G., Zangrillo A., Bosi E., Tresoldi M., Ciceri F., Rovere-Querini P., Adrish M. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS One. 2020;15(10):e0239570. doi: 10.1371/journal.pone.0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divani A.A., Andalib S., Di Napoli M., Lattanzi S., Hussain M.S., Biller J., McCullough L.D., Azarpazhooh M.R., Seletska A., Mayer S.A., Torbey M. Coronavirus disease 2019 and stroke: clinical manifestations and pathophysiological insights. J. Stroke Cerebrovasc. Dis. 2020;29(8):104941. doi: 10.1016/j.jstrokecerebrovasdis.2020.104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzyzga Ł., Obuchowicz E., Marcinowska A., Herman Z.S. Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain Behav. Immun. 2006;20(6):532–545. doi: 10.1016/j.bbi.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Egbert, A.R., Cankurtaran, S., Karpiak, S., 2020. Brain abnormalities in COVID-19 acute/subacute phase: a rapid systematic review. Brain Behav Immun. [DOI] [PMC free article] [PubMed]
- Frontera J.A., Yang D., Lewis A., Patel P., Medicherla C., Arena V., Fang T., Andino A., Snyder T., Madhavan M., Gratch D., Fuchs B., Dessy A., Canizares M., Jauregui R., Thomas B., Bauman K., Olivera A., Bhagat D., Sonson M., Park G., Stainman R., Sunwoo B., Talmasov D., Tamimi M., Zhu Y., Rosenthal J., Dygert L., Ristic M., Ishii H., Valdes E., Omari M., Gurin L., Huang J., Czeisler B.M., Kahn D.E., Zhou T., Lin J., Lord A.S., Melmed K., Meropol S., Troxel A.B., Petkova E., Wisniewski T., Balcer L., Morrison C., Yaghi S., Galetta S. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J. Neurol. Sci. 2021;426:117486. doi: 10.1016/j.jns.2021.117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam N., Madathil S., Tahani N., Bolton S., Parekh D., Stockley J., Goyal S., Qureshi H., Yasmin S., Cooper B.G., Short J., Geberhiwot T. Medium-term outcome of severe to critically ill patients with SARS-CoV-2 infection. Clin. Infect Dis. 2021 doi: 10.1093/cid/ciab341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavriatopoulou M., Korompoki E., Fotiou D., Ntanasis-Stathopoulos I., Psaltopoulou T., Kastritis E., Terpos E., Dimopoulos M.A. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 2020;20(4):493–506. doi: 10.1007/s10238-020-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro M.M., Mariagrazia P., De Lorenzo R., Cristiano M., Sara P., Roberto F., Fabio C., Patrizia R.Q., Francesco B. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021 doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González J., Benítez I.D., Carmona P., Santisteve S., Monge A., Moncusí-Moix A., Gort-Paniello C., Pinilla L., Carratalá A., Zuil M., Ferrer R., Ceccato A., Fernández L., Motos A., Riera J., Menéndez R., Garcia-Gasulla D., Peñuelas O., Bermejo-Martin J.F., Labarca G., Caballero J., Torres G., de Gonzalo-Calvo D., Torres A., Barbé F. PULMONARY FUNCTION AND RADIOLOGICAL FEATURES IN SURVIVORS OF CRITICAL COVID-19: A 3-MONTH PROSPECTIVE COHORT. Chest. 2021 doi: 10.1016/j.chest.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrisankar Y.V., Clark M.A. Angiotensin II regulation of angiotensin-converting enzymes in spontaneously hypertensive rat primary astrocyte cultures. J. Neurochem. 2016;138(1):74–85. doi: 10.1111/jnc.13641. [DOI] [PubMed] [Google Scholar]
- Graham E.L., Clark J.R., Orban Z.S., Lim P.H., Szymanski A.L., Taylor C., DiBiase R.M., Jia D.T., Balabanov R., Ho S.U., Batra A., Liotta E.M., Koralnik I.J. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021;8(5):1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin S.J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., Walshaw C., Kemp S., Corrado J., Singh R., Collins T., O'Connor R.J., Sivan M. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J. Med. Virol. 2021;93(2):1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Schenck M., Severac F., Clere-Jehl R., Studer A., Radosavljevic M., Kummerlen C., Monnier A., Boulay C., Fafi-Kremer S., Castelain V., Ohana M., Anheim M., Schneider F., Meziani F. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit. Care. 2020;24:491. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., Aggarwal G., Wong J., Benoit S., Vikse J., Plebani M., Lippi G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am. J. Emer. Med. 2020;38(9):1722–1726. doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M., Wathelet M., Fovet T., Amad A., Vuotto F., Faure K., Astier T., Noël H., Henry M., Duhem S., Vaiva G., D’Hondt F. Is COVID-19 associated with posttraumatic stress disorder? J. Clin. Psychiatry. 2020;82(1) doi: 10.4088/JCP.20m13641. [DOI] [PubMed] [Google Scholar]
- Hu, Y., Chen, Y., Zheng, Y., You, C., Tan, J., Hu, L., Zhang, Z., Ding, L., 2020. Factors related to mental health of inpatients with COVID-19 in Wuhan, China. Brain Behav Immun. [DOI] [PMC free article] [PubMed]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L.i., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L.i., Xu D., Li Y., Li C., Peng L.u., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Zhuang W., Wang D., Zha L., Xu X., Li X., Shi Q., Wang X.S., Qiao G. Persistent somatic symptom burden and sleep disturbance in patients with COVID-19 during hospitalization and after discharge: a prospective cohort study. Med. Sci. Monit. 2021;27 doi: 10.12659/MSM.930447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme K., Hudson J.L., Rojczyk P., Little P., Moss-Morris R. Biopsychosocial risk factors of persistent fatigue after acute infection: a systematic review to inform interventions. J. Psychosom. Res. 2017;99:120–129. doi: 10.1016/j.jpsychores.2017.06.013. [DOI] [PubMed] [Google Scholar]
- Iqbal A., Iqbal K., Arshad Ali S., Azim D., Farid E., Baig M.D., Bin Arif T., Raza M. The COVID-19 sequelae: a cross-sectional evaluation of post-recovery symptoms and the need for rehabilitation of COVID-19 survivors. Cureus. 2021;13 doi: 10.7759/cureus.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempuraj, D., Selvakumar, G.P., Ahmed, M.E., Raikwar, S.P., Thangavel, R., Khan, A., Zaheer, S.A., Iyer, S.S., Burton, C., James, D., Zaheer, A., 2020. COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neuroscientist, 1073858420941476. [DOI] [PubMed]
- Kerr J.R. Epstein-barr virus induced gene-2 upregulation identifies a particular subtype of chronic fatigue syndrome/myalgic encephalomyelitis. Front. Pediatr. 2019;7:59. doi: 10.3389/fped.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, X., Zheng, K., Tang, M., Kong, F., Zhou, J., Diao, L., Wu, S., Jiao, P., Su, T., Dong, Y., 2020. Prevalence and factors associated with depression and anxiety of hospitalized patients with COVID-19. medRxiv, 2020.2003.2024.20043075.
- Liang L., Yang B., Jiang N., Fu W., He X., Zhou Y., Ma W.L., Wang X. Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. J. Korean Med. Sci. 2020;35 doi: 10.3346/jkms.2020.35.e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.-y., Wang J., Ou-yang X.-Y., Miao Q., Chen R., Liang F.-X., Zhang Y.-p., Tang Q., Wang T. The immediate impact of the 2019 novel coronavirus (COVID-19) outbreak on subjective sleep status. Sleep Med. 2021;77:348–354. doi: 10.1016/j.sleep.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D., Janelidze S., Hagell P., Erhardt S., Samuelsson M., Minthon L., Hansson O., Björkqvist M., Träskman-Bendz L., Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol. Psychiatry. 2009;66(3):287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Lindqvist D., Wolkowitz O.M., Mellon S., Yehuda R., Flory J.D., Henn-Haase C., Bierer L.M., Abu-Amara D., Coy M., Neylan T.C., Makotkine I., Reus V.I., Yan X., Taylor N.M., Marmar C.R., Dhabhar F.S. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav. Immun. 2014;42:81–88. doi: 10.1016/j.bbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Liu N., Zhang F., Wei C., Jia Y., Shang Z., Sun L., Wu L., Sun Z., Zhou Y., Wang Y., Liu W. Prevalence and predictors of PTSS during COVID-19 outbreak in China hardest-hit areas: gender differences matter. Psychiatry Res. 2020;287:112921. doi: 10.1016/j.psychres.2020.112921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo R., Cinel E., Cilla M., Compagnone N., Ferrante M., Falbo E., Patrizi A., Castellani J., Magnaghi C., Calvisi S.L., Arcidiacono T., Lanzani C.L., Canti V., Mazza M.G., Martinenghi S., Vitali G., Benedetti F., Ciceri F., Conte C., Rovere Querini P. Physical and psychological sequelae at three months after acute illness in COVID-19 survivors. Panminerva Med. 2021 doi: 10.23736/S0031-0808.21.04399-8. [DOI] [PubMed] [Google Scholar]
- Lu Y., Li X., Geng D., Mei N., Wu P.-Y., Huang C.-C., Jia T., Zhao Y., Wang D., Xiao A., Yin B. Cerebral micro-structural changes in COVID-19 patients - An MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25:100484. doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak I.W.C., Chu C.M., Pan P.C., Yiu M.G.C., Chan V.L. Long-term psychiatric morbidities among SARS survivors. Gen. Hosp. Psychiatry. 2009;31(4):318–326. doi: 10.1016/j.genhosppsych.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak I.W.C., Chu C.M., Pan P.C., Yiu M.G.C., Ho S.C., Chan V.L. Risk factors for chronic post-traumatic stress disorder (PTSD) in SARS survivors. Gen. Hosp. Psychiatry. 2010;32(6):590–598. doi: 10.1016/j.genhosppsych.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon N., Dorman-Ilan S., Hasson-Ohayon I., Hertz-Palmor N., Shani S., Basel D., Gross R., Chen W., Abramovich A., Afek A., Ziv A., Kreiss Y., Pessach I.M., Gothelf D. Trajectories of post-traumatic stress symptoms, anxiety, and depression in hospitalized COVID-19 patients: a one-month follow-up. J. Psychosom. Res. 2021;143:110399. doi: 10.1016/j.jpsychores.2021.110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., Dottermusch M., Heinemann A., Pfefferle S., Schwabenland M., Sumner Magruder D., Bonn S., Prinz M., Gerloff C., Püschel K., Krasemann S., Aepfelbacher M., Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli F., Stampatori C., Righetti F., Sala E., Tomasi C., De Palma G. Neurological and cognitive sequelae of Covid-19: a four month follow-up. J. Neurol. 2021:1–7. doi: 10.1007/s00415-021-10579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., Melloni E.M.T., Furlan R., Ciceri F., Rovere-Querini P., group C.-B.O.C.S., Benedetti F. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcloughlin B.C., Miles A., Webb T.E., Knopp P., Eyres C., Fabbri A., Humphries F., Davis D. Functional and cognitive outcomes after COVID-19 delirium. Eur. Geriatr. Med. 2020;11(5):857–862. doi: 10.1007/s41999-020-00353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., Hlh Across Speciality Collaboration U.K. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez R., Balanzá-Martínez V., Luperdi S.C., Estrada I., Latorre A., González-Jiménez P., Feced L., Bouzas L., Yépez K., Ferrando A., Hervás D., Zaldívar E., Reyes S., Berk M., Menéndez R. Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J. Intern. Med. 2021 doi: 10.1111/joim.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K.W., Johnsen S., Sattler S.M., Nielsen S., Kunalan K., Rungby J., Lapperre T., Porsberg C.M. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti G., Leggieri C., Fominskiy E., Scandroglio A.M., Colombo S., Tozzi M., Moizo E., Mucci M., Crivellari M., Pieri M., Guzzo F., Piemontese S., De Lorenzo R., Da Prat V., Fedrizzi M., Faustini C., Di Piazza M., Conte F., Lembo R., Esposito A., Dagna L., Landoni G., Zangrillo A. Two months quality of life of COVID-19 invasively ventilated survivors; an Italian single-center study. Acta Anaesthesiol. Scand. 2021 doi: 10.1111/aas.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin L., Savale L., Pham T., Colle R., Figueiredo S., Harrois A., Gasnier M., Lecoq A.L., Meyrignac O., Noel N., Baudry E., Bellin M.F., Beurnier A., Choucha W., Corruble E., Dortet L., Hardy-Leger I., Radiguer F., Sportouch S., Verny C., Wyplosz B., Zaidan M., Becquemont L., Montani D., Monnet X. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325(15):1525. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccioli L., Pensato U., Cani I., Guarino M., Cortelli P., Bisulli F. Covid-19-associated encephalopathy and cytokine-mediated neuroinflammation. Ann. Neurol. 2020;88(4):860–861. doi: 10.1002/ana.25855. [DOI] [PubMed] [Google Scholar]
- Negrini F., Ferrario I., Mazziotti D., Berchicci M., Bonazzi M., de Sire A., Negrini S., Zapparoli L. Neuropsychological features of severe hospitalized coronavirus disease 2019 patients at clinical stability and clues for postacute rehabilitation. Arch. Phys. Med. Rehabil. 2021;102(1):155–158. doi: 10.1016/j.apmr.2020.09.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto W., Yamagata R., Nakagawasai O., Nakagawa K., Hung W.Y., Fujita M., Tadano T., Tan-No K. Effect of spinal angiotensin-converting enzyme 2 activation on the formalin-induced nociceptive response in mice. Eur. J. Pharmacol. 2020;872:172950. doi: 10.1016/j.ejphar.2020.172950. [DOI] [PubMed] [Google Scholar]
- Noviello D., Costantino A., Muscatello A., Bandera A., Consonni D., Vecchi M., Basilisco G. Functional gastrointestinal and somatoform symptoms five months after SARS-CoV-2 infection: a controlled cohort study. Neurogastroenterol. Motil. 2021:e14187. doi: 10.1111/nmo.14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortelli P., Ferrazzoli D., Sebastianelli L., Engl M., Romanello R., Nardone R., Bonini I., Koch G., Saltuari L., Quartarone A., Oliviero A., Kofler M., Versace V. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: insights into a challenging symptom. J. Neurol. Sci. 2021;420:117271. doi: 10.1016/j.jns.2020.117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos I.C., Vasconcelos-Moreno M.P., Costa L.G., Kunz M., Brietzke E., Quevedo J., Salum G., Magalhães P.V, Kapczinski F., Kauer-Sant'Anna M. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2(11):1002–1012. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- Petersen, M.S., Kristiansen, M.F., Hanusson, K.D., Danielsen, M.E., B, Á.S., Gaini, S., Strøm, M., Weihe, P., 2020. Long COVID in the Faroe Islands - a longitudinal study among non-hospitalized patients. Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- Pilotto A., Odolini S., Masciocchi S., Comelli A., Volonghi I., Gazzina S., Nocivelli S., Pezzini A., Foca E., Caruso A., Leonardi M., Pasolini M.P., Gasparotti R., Castelli F., Ashton N.J., Blennow K., Zetterberg H., Padovani A. Steroid-responsive encephalitis in coronavirus disease 2019. Ann. Neurol. 2020 doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyraz B., Poyraz C.A., Olgun Y., Gürel Ö., Alkan S., Özdemir Y.E., Balkan İ., Karaali R. Psychiatric morbidity and protracted symptoms after COVID-19. Psychiatry Res. 2021;295:113604. doi: 10.1016/j.psychres.2020.113604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu G., Zhen Q., Wang W., Fan S., Wu Q., Zhang C., Li B., Liu G., Yu Y., Li Y., Yong L., Lu B., Ding Z., Ge H., Mao Y., Chen W., Xu Q., Zhang R., Cao L., Chen S., Li H., Zhang H., Hu X., Zhang Jing, Wang Y., Zhang H., Liang C., Sun L., Sun Y. Health related quality of life of COVID-19 patients after discharge: a multicenter follow up study. J Clin Nurs. 2021;30(11-12):1742–1750. doi: 10.1111/jocn.15733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., Zandi M.S., Lewis G., David A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Duarte Á., Rivera-Izquierdo M., Guerrero-Fernández de Alba I., Pérez-Contreras M., Fernández-Martínez N.F., Ruiz-Montero R., Serrano-Ortiz Á., González-Serna R.O., Salcedo-Leal I., Jiménez-Mejías E., Cárdenas-Cruz A. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: the ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021;19:129. doi: 10.1186/s12916-021-02003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudroff T., Fietsam A.C., Deters J.R., Bryant A.D., Kamholz J. Post-COVID-19 fatigue: potential contributing factors. Brain Sci. 2020;10(12):1012. doi: 10.3390/brainsci10121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari N., Hosseinian-Far A., Jalali R., Vaisi-Raygani A., Rasoulpoor S., Mohammadi M., Rasoulpoor S., Khaledi-Paveh B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16:57. doi: 10.1186/s12992-020-00589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami R., Soltaninejad F., Amra B., Naderi Z., Haghjooy Javanmard S., Iraj B., Haji Ahmadi S., Shayganfar A., Dehghan M., Khademi N., Sadat Hosseini N., Mortazavi M., Mansourian M., Mañanas M.A., Marateb H.R., Adibi P., Di Gennaro F. A one-year hospital-based prospective COVID-19 open-cohort in the Eastern Mediterranean region: the Khorshid COVID Cohort (KCC) study. PLoS One. 2020;15(11):e0241537. doi: 10.1371/journal.pone.0241537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y.F., Liu T., Yu J.N., Xu X.R., Zahid K.R., Wei Y.C., Wang X.H., Zhou F.L. Half-year follow-up of patients recovering from severe COVID-19: analysis of symptoms and their risk factors. J. Intern. Med. 2021;290(2):444–450. doi: 10.1111/joim.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani P., Patini Romeo. Retracted COVID-19 articles: a side-effect of the hot race to publication. Scientometrics. 2020;125(1):819–822. doi: 10.1007/s11192-020-03661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks S.W. Posttraumatic stress syndrome: what is it? J. Trauma Nurs. 2018;25:60–65. doi: 10.1097/JTN.0000000000000343. [DOI] [PubMed] [Google Scholar]
- Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., Pujol J.C., Klaser K., Antonelli M., Canas L.S., Molteni E., Modat M., Jorge Cardoso M., May A., Ganesh S., Davies R., Nguyen L.H., Drew D.A., Astley C.M., Joshi A.D., Merino J., Tsereteli N., Fall T., Gomez M.F., Duncan E.L., Menni C., Williams F.M.K., Franks P.W., Chan A.T., Wolf J., Ourselin S., Spector T., Steves C.J. Attributes and predictors of long COVID. Nat. Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.L., Wang J., Wang Y.S., Hu P.F., Zhao Z.Q., Chen W., Ning B.F., Yin C., Hao Y.S., Wang Q., Wang C., Liu Y.L., Chen C., Yin J.Z., Huang H., Xie W.F. Symptomatic features and prognosis of 932 hospitalized patients with coronavirus disease 2019 in Wuhan. J. Dig. Dis. 2021;22:271–281. doi: 10.1111/1751-2980.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes D.L., Holdsworth L., Jawad N., Gunasekera P., Morice A.H., Crooks M.G. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199(2):113–119. doi: 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W., Hao F., McIntyre R.S., Jiang L., Jiang X., Zhang L., Zhao X., Zou Y., Hu Y., Luo X., Zhang Z., Lai A., Ho R., Tran B., Ho C., Tam W. Is returning to work during the COVID-19 pandemic stressful? A study on immediate mental health status and psychoneuroimmunity prevention measures of Chinese workforce. Brain Behav. Immun. 2020;87:84–92. doi: 10.1016/j.bbi.2020.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S.W., Helmeste D., Leonard B. Inflammatory neuropsychiatric disorders and COVID-19 neuroinflammation. Acta Neuropsychiatr. 2021;33(4):165–177. doi: 10.1017/neu.2021.13. [DOI] [PubMed] [Google Scholar]
- Tanriverdi A., Savci S., Kahraman B.O., Ozpelit E. Extrapulmonary features of post-COVID-19 patients: muscle function, physical activity, mood, and sleep quality. Ir. J. Med. Sci. 2021 doi: 10.1007/s11845-021-02667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8(2):130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenforde M.W., Kim S.S., Lindsell C.J., Billig Rose E., Shapiro N.I., Files D.C., Gibbs K.W., Erickson H.L., Steingrub J.S., Smithline H.A., Gong M.N., Aboodi M.S., Exline M.C., Henning D.J., Wilson J.G., Khan A., Qadir N., Brown S.M., Peltan I.D., Rice T.W., Hager D.N., Ginde A.A., Stubblefield W.B., Patel M.M., Self W.H., Feldstein L.R., Hart K.W., McClellan R., Dorough L., Dzuris N., Griggs E.P., Kassem A.M., Marcet P.L., Ogokeh C.E., Sciarratta C.N., Siddula A., Smith E.R., Wu M.J. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a Multistate Health Care Systems Network - United States, March-June 2020. MMWR Morb. Mortal Wkly Rep. 2020;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasoni D., Bai F., Castoldi R., Barbanotti D., Falcinella C., Mulè G., Mondatore D., Tavelli A., Vegni E., Marchetti G., d'Arminio Monforte A. Anxiety and depression symptoms after virological clearance of COVID-19: a cross-sectional study in Milan, Italy. J. Med. Virol. 2021;93(2):1175–1179. doi: 10.1002/jmv.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L., Dyer A.H., Jones K., Dunne J., Mooney A., Gaffney F., O'Connor L., Leavy D., O'Brien K., Dowds J., Sugrue J.A., Hopkins D., Martin-Loeches I., Ni Cheallaigh C., Nadarajan P., McLaughlin A.M., Bourke N.M., Bergin C., O'Farrelly C., Bannan C., Conlon N., Madeddu G. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):e0240784. doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L., Moloney D., Finucane C., McCarthy K., Bergin C., Bannan C., Kenny R.A. Fatigue following COVID-19 infection is not associated with autonomic dysfunction. PLoS One. 2021;16(2):e0247280. doi: 10.1371/journal.pone.0247280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Borst B., Peters J.B., Brink M., Schoon Y., Bleeker-Rovers C.P., Schers H., van Hees H.W.H., van Helvoort H., van den Boogaard M., van der Hoeven H., Reijers M.H., Prokop M., Vercoulen J., van den Heuvel M. Comprehensive health assessment three months after recovery from acute COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturelli S., Benatti S.V., Casati M., Binda F., Zuglian G., Imeri G., Conti C., Biffi A.M., Spada M.S., Bondi E., Camera G., Severgnini R., Giammarresi A., Marinaro C., Rossini A., Bonaffini P.A., Guerra G., Bellasi A., Cesa S., Rizzi M. Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation. Epidemiol. Infect. 2021;149 doi: 10.1017/S0950268821000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindegaard N., Benros M.E. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav. Immun. 2020;89:531–542. doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Pan R., Wan X., Tan Y., Xu L., McIntyre R.S., Choo F.N., Tran B., Ho R., Sharma V.K., Ho C. A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav. Immun. 2020;87:40–48. doi: 10.1016/j.bbi.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.R., Oyem P.C., Viguera A.C. Prevalence of psychiatric morbidity following discharge after COVID-19 hospitalization. Gen. Hosp. Psychiatry. 2020;69:131–132. doi: 10.1016/j.genhosppsych.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen L., Wu T., Shi H., Li Q., Jiang H., Zheng D., Wang X., Wei Y., Zhao Y., Qiao J. Impact of Covid-19 in pregnancy on mother's psychological status and infant's neurobehavioral development: a longitudinal cohort study in China. BMC Med. 2020;18:347. doi: 10.1186/s12916-020-01825-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters D.L., Vlietstra L., Qualls C., Morley J.E., Vellas B. Sex-specific muscle and metabolic biomarkers associated with gait speed and cognitive transitions in older adults: a 9-year follow-up. Geroscience. 2020;42(2):585–593. doi: 10.1007/s11357-020-00163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerahandi H., Hochman K., Simon E., Blaum C., Chodoch J., Duan E., Garry K., Kahan T., Karmen-Tuohy S., Karpel H., et al. Post-Discharge Health Status and Symptoms in Patients with Severe COVID-19. J. Gen. Internal Med. 2021;36:738–745. doi: 10.1007/s11606-020-06338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside D.M., Oleynick V., Holker E., Waldron E.J., Porter J., Kasprzak M. Neurocognitive deficits in severe COVID-19 infection: case series and proposed model. Clin. Neuropsychol. 2021;35(4):799–818. doi: 10.1080/13854046.2021.1874056. [DOI] [PubMed] [Google Scholar]
- Wong A.W., Shah A.S., Johnston J.C., Carlsten C., Ryerson C.J. Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur. Respir. J. 2020;56(5):2003276. doi: 10.1183/13993003.03276-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, T.L., Weitzer, D.J., 2021. Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)-A Systemic Review and Comparison of Clinical Presentation and Symptomatology. Medicina (Kaunas) 57. [DOI] [PMC free article] [PubMed]
- Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Chen X., Yao S., Liu R. Anxiety persists after recovery from acquired COVID-19 in anaesthesiologists. J. Clin. Anesth. 2020;67:109984. doi: 10.1016/j.jclinane.2020.109984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q., Xu M., Li J., Liu Y., Zhang J., Xu Y., Dong W. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin. Microbiol. Infect. 2021;27(1):89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelin D., Margalit I., Yahav D., Runold M., Bruchfeld J. Long COVID-19-it's not over until? Clin. Microbiol. Infect. 2020;27(4):506–508. doi: 10.1016/j.cmi.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J.J., Bruno D., Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect Disord. 2014;169:15–20. doi: 10.1016/j.jad.2014.07.032. [DOI] [PubMed] [Google Scholar]
- Yuan B., Li W., Liu H., Cai X., Song S., Zhao J., Hu X., Li Z., Chen Y., Zhang K., Liu Z., Peng J., Wang C., Wang J., An Y. Correlation between immune response and self-reported depression during convalescence from COVID-19. Brain Behav. Immun. 2020;88:39–43. doi: 10.1016/j.bbi.2020.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Lu S., Chen J., Wei N., Wang D., Lyu H., Shi C., Hu S. The landscape of cognitive function in recovered COVID-19 patients. J. Psychiatr. Res. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]