Abstract
Neurons broadcast electrical signals to distal brain regions through extensive axonal arbors. Genetically encoded calcium sensors permit the direct observation of action potential activity at axonal terminals, providing unique insights on the organization and function of neural projections. Here, we consider what information can be gleaned from axonal recordings made at scales ranging from the summed activity extracted from multi-cell axon projections to single boutons. In particular, we discuss the application of different recently developed multi photon and fiber photometry methods for recording neural activity in axons of rodents. We define experimental difficulties associated with imaging approaches in the axonal compartment and highlight the latest methodological advances for addressing these issues. Finally, we reflect on ways in which new technologies can be used in conjunction with axon calcium imaging to address current questions in neurobiology.
Keywords: Calcium imaging, Axons, Two-photon imaging, Fiber photometry, Rodents, Neural projections, Protein engineering, Genetically-encoded calcium indicators, Neural circuits
1. Introduction
It is becoming increasingly clear that even simple behaviors engage distributed activity over large number of brain areas (Steinmetz et al., 2019). Understanding how neural circuits give rise to behavior will require identifying the signals relayed across areas. Neurons communicate with distal brain regions through extensive axonal arbors and a great diversity of projection types can be present within individual areas (Chen et al., 2019; Han et al., 2018; Winnubst et al., 2019). Thus, understanding how different brain areas work in concert to give rise to behavior will require projection-type specific recordings (Luo et al., 2018).
Neural recordings in awake animals are usually obtained measuring extracellular action potentials that reflect activity of somata in the vicinity of the electrodes (Gold et al., 2006). Modern multielectrode technology and spike sorting algorithms allow recording from hundreds of neurons simultaneously with exquisite temporal resolution (Steinmetz et al., 2018). While some broad classes of neuronal types can be distinguished using features of the action potential waveform, and optogenetic tagging methods allow the identification of specific projection types in some cases (Li et al., 2015; Lima et al., 2009), imaging methods are better suited for recording neurons projecting to specific areas. Multi-photon imaging, in combination with genetically encoded calcium indicators (GECIs) allows recordings from hundreds to thousands of neurons of defined specific types. When combined with retrograde tracers, multi-photon imaging can be used to record from neurons with defined projections (Chen et al., 2016; Kim et al., 2018; Kwon et al., 2016; Li et al., 2015; Sato and Svoboda, 2010). As with extracellular electrodes, recordings of neural activity using multi-photon imaging using GECIs are usually made from somata. An alternative is to use record neural activity directly from axonal projections. Recording of neural activity from axons allows directly measuring neural signals that are exchanged across areas, providing insights on the function and organization of circuits engaged in interareal brain communication that would not be possible using more traditional recordings. Given the small size of axonal boutons (< 2 μm), electrophysiological recordings of presynaptic structures have been limited to a few exceptional cases. However, presynaptic activity can be optically recorded by filling axons with synthetic calcium indicators (Cox et al., 2000; Koester and Sakmann, 2000; Llinás et al., 1992; Regehr et al., 1994; Sabatini and Regehr, 1998; Tan and Llano, 1999) or genetically-encoded ones. Initial studies were limited to measuring average activity from populations of presynaptic structures. With the advent of multi-photon imaging methods, it became possible to record axonal activity while resolving single axons and boutons (Cox et al., 2000; Koester and Sakmann, 2000) in acute experiments. The development of improved GECIs (Tian et al., 2009), opened up the possibility to record axonal activity with single bouton resolution chronically in vivo, including in behaving animals (Petreanu et al., 2012). Here we will review the methods for measuring activity in axons using calcium imaging and its applications. Due to space constraints, we will focus on applications of functional imaging in the axons of rodents. However, a similar approach can be applied to projections of other species, like zebrafish (Dreosti et al., 2009), tree shrews (Zhang et al., 2018), and marmosets (Sadakane et al., 2015), and most of the issues discussed here can be generalized to the application of the method in non-rodent species. We refer the reader to other review articles for more general discussions on recordings of neural activity using calcium imaging, multiphoton microscopy, genetically encoded indicators, fiber photometry and recordings from other neuronal compartments (Ali and Kwan, 2019; Broussard et al., 2014; Grienberger and Konnerth, 2012; Lin and Schnitzer, 2016; Siciliano and Tye, 2019; Yang and Yuste, 2017).
2. The relation between neural activity and calcium signals in axons
Action potentials (APs) propagate along the complex axonal arbors of neurons until reaching the different target areas. Axons make synaptic contacts in swellings called boutons, where presynaptic structures are located (Kincaid et al., 1998; Shepherd and Harris, 1998). Upon reaching synaptic terminals, APs activate voltage-dependent calcium channels (VDCC), causing a transient rise of intracellular calcium concentration that triggers neurotransmitter release (Katz, 1969). Thus, as in somata, neural activity can be detected in axons by an increase in fluorescence using calcium indicators. While, APs are known to fail at branching points in some invertebrate axons (Debanne et al., 2011), in vertebrate ones they appear to reliably propagate through branches (Cox et al., 2000; Koester and Sakmann, 2000). As a consequence, APs elicits highly correlated calcium activity in boutons from the same axon (Koester and Sakmann, 2000; Petreanu et al., 2012). APs can evoke calcium increases in both axonal shafts and varicosities, but the latter are of larger amplitude because VDCC are concentrated at presynaptic structures (Broussard et al., 2018; Cox et al., 2000; Koester and Sakmann, 2000). As a result, measurements of afferent signals benefits from extracting fluorescent traces selectively in axonal boutons, as these results in higher signal to noise (SNR) compared to measurements done in shafts. Single APs result in highly variable relative changes in fluorescence across boutons of the same axons, presumably because of the variable number of VDCC available at each synapse (Koester and Sakmann, 2000). However, within single boutons, AP-evoked calcium transients show little trial to trial variability. As in the soma, the amplitude of the changes in fluorescence increases as a function of number of APs (Ali et al., 2020a; Marvin et al., 2018; Petreanu et al., 2012; Tanaka et al., 2018). Importantly, many neurotransmitters can modulate VDCC presynaptically (Chalifoux and Carter, 2011; Miller, 1998). Thus, even though APs reliably propagate across axonal branches, calcium activity can be locally modulated in subsets of boutons through presynaptic mechanisms (Pardi et al., 2020). Thus, differences in calcium activity in boutons at two time points can be due to both changes in the firing frequency or local presynaptic actions of neuromodulators. Distinguishing between these two possibilities is not always possible without extra experiments using complementary methods (Schröder et al., 2020).
3. Applications of axonal recordings
APs are initiated in axon initial segments which are adjacent to somata and propagate reliably down the axon. As a consequence AP-evoked calcium transients are highly correlated in axonal and somatic compartments (Koester and Sakmann, 2000). Thus, except for the presynaptic mechanisms that can modulate calcium signaling in individual boutons described above, neural activity in axonal compartments is redundant with the somatic one but more difficult to obtain due to their much smaller size. However, recordings from axons allow specific measurements that would be more difficult, or in many cases, impossible to obtain by recording somatic activity. There are several cases where recordings of calcium signals in axons allow addressing unique questions of neural circuit organization and function. Applications can be divided in 3 major categories of increasing level of detail: 1) recordings of aggregate population activity from specific projections, 2) recordings with single axon resolution to measure the nature and diversity of signals relayed by specific projections and 3), applications that require single bouton measurements to relate the signals single axons carry with the precise location where the synapses are present.
3.1. Population activity in specific projections
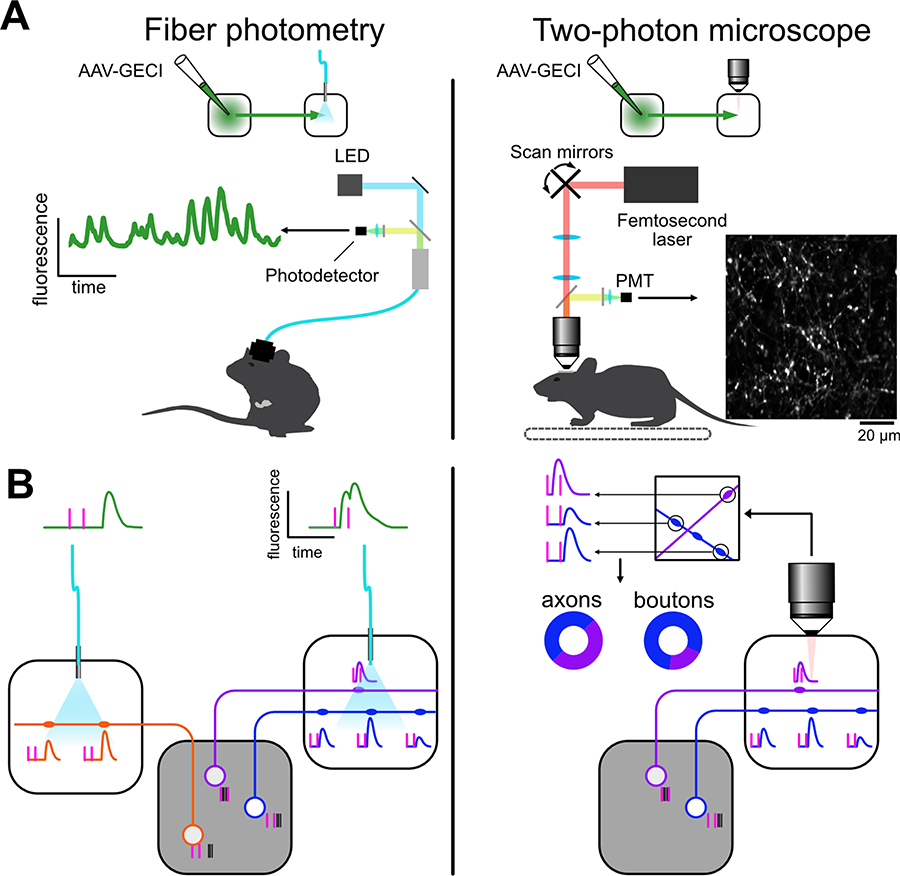
Individual brain regions most often relay signals to multiple downstream targets. Individual neurons often innervate only a subset of these downstream areas (Winnubst et al., 2019), defining projection-specific cell types. Neurons with common projection patterns usually also differ in their gene expression patterns, local connectivity rules, and can be differentially innervated by afferent circuits and might play specialized roles during behavior (Luo et al., 2018). The combination of GECIs with fiber photometry (Cui et al., 2013) allows recording population activity from specific projections. The projection specific neuronal population is usually defined by an injection of an adeno-associated virus (AAV) to transfect a localized population of neurons with GECI. By placing the optical fiber in the one of the targets of this population, projection specific recordings can be obtained (Fig. 1). However, using cell-type specific driver lines, e.g. mouse lines expressing recombinases in subsets of neurons (Daigle et al., 2018), recording can be further targeted to projection- and line-specific population of neurons. For example, this approach revealed that the dopamine neurons in the midbrain relay different signals to their different targets (Gunaydin et al., 2014; Parker et al., 2016; Tsutsui-Kimura et al., 2020).
Fig. 1.
Differences between recordings of calcium signals in axons using fiber photometry and two-photon microscopy. A, Both methods allow recording activity in specific projections. Fiber photometry (left) in not image forming and is amenable to recordings during freely moving behaviors and from regions at any depth in the brain. Two-photon microscopy (right) results in an image of the recorded axons but it is limited to head-fixed animals and, unless tissue is removed, to superficial areas. B, Neurons in a given area can project to different targets. Their activity (black ticks) is locked to different behavioral events (magenta ticks). Calcium activity in axons in the target area reflects somatic activity and is correlated across boutons of the same axons, albeit with different amplitude. Left, fiber photometry-based recordings reflect the average population activity in the target region. Right, using two-photon microscopy, calcium activity can be extracted from individual boutons. Given the high correlation across boutons of the same axon, activity be quantified both in axons and boutons.
Importantly, while it can distinguish activity patterns in different projections, fiber photometry is not an image forming method but provides a population average of the GECI-expressing axons in the vicinity of the fiber tip. As a consequence, the method cannot resolve activity from individual axons, and it is not suited for describing the diversity of signals that might exist within a given projection. Meaningful photometry activity can only be observed if a a large fraction of axons show correlated activity to a given behavioral or sensory event. It is also not possible to distinguish if activity transients locked to different task or sensory events reflects two population of axons responding separately to each of the events, or one population with broader multi-event activity (Fig. 1B). Similarly, axons with sparsely represented activity patterns can remain undetected in fiber photometry recordings regardless of how intense their firing rate is, as their activity might not contribute significantly to the recorded average fluorescent signal. An advantage of fiber photometry is that, as it relies on one-photon excitation and implantable optic fibers, recordings are not limited to superficial structures of the brain. The method is also amenable to freely moving animals. In addition, by implanting multiple fibers, somatic and axonal activity can be recorded simultaneously from several areas (Kim et al., 2016).
3.2. Recordings of single axons
When combined with two-photon microscopy, calcium imaging can resolve single axons and bouton in vivo (Fig. 1). This allows recording from neurons with specific projections like when using fiber photometry but, in addition, allows characterizing the diversity of signals within individual projections. Because it can resolve individual axons, two-photon imaging allows recording signals originating from axons with activity patterns that are sparse and different from the mean population that would be undetectable using fiber photometry. By doing so, this approach can reveal and quantify the diversity of signals within an individual projection (Howe and Dombeck, 2016; Petreanu et al., 2012). The signals relayed to different targets can be similar and largely overlapping, yet show specialization in their relative proportion, providing functional insights on interareal communication (Glickfeld et al., 2013). The method is not very invasive when imaging axons that are close to the surface of the brain, such as in cortical areas (Eggermann et al., 2014; Gambino et al., 2014; Glickfeld et al., 2013; Kwon et al., 2016; Leinweber et al., 2017; Petreanu et al., 2012; Roth et al., 2016), the olfactory bulb (Otazu et al., 2015), and the cerebellum (Shuster et al., 2021; Wilms and Häusser, 2015). In many cases, activity in superficial axons can also be used as a proxy for somatic activity, allowing recordings of neural populations that would not be optically accessible otherwise because their somata are located at depths beyond the limits of two-photon microscopy such as subcortical and neuromodulatory afferents to cortical regions (Burgess et al., 2016; Gambino et al., 2014; Mridha et al., 2021; Roth et al., 2016). Axons terminating in deeper regions can also be recorded, albeit more invasively, by removing overlying tissue (Howe and Dombeck, 2016; Kaifosh et al., 2013; Liang et al., 2020; Schröder et al., 2020) or by combining GRIN lenses with adaptative optics (Meng et al., 2019). A limitation of the method when compared with fiber photometry is that two-photon microscopy requires mice to be head-fixed, but improvements in the miniaturization of two-photon microscopes (Zong et al., 2017) should allow recordings with single axon resolution in freely moving animals in the near future.
3.3. Relating the position of axonal boutons with the signals they relay
Imaging activity in axons allows the recoding of the diversity of signals in specific projections. Fluorescence signals are usually extracted by placing regions of interest (ROIs) over boutons, as these structures are brighter than shafts because of their larger volume, and because calcium transients are of larger amplitude there as discussed above. As most axonal swellings correspond to presynaptic structures (Shepherd and Harris, 1998), two-photon imaging in axons allows relating the precise position where synapses are made with the signal they relay. Relating recorded signals to the fine position of individual synapses, can provide unique insights into the organization of neural pathways. For example, projections in many instances relay different signals in axons terminating in different layers of the visual cortex (Cruz-Martín et al., 2014; Kondo and Ohki, 2016; Sun et al., 2016). More subtle relations besides laminar position can also be revealed by analyzing the precise position of a presynaptic structure and their functional properties. For example, boutons from different retinal ganglion cells innervating the mouse thalamus tend to cluster together, a few microns from each other, when they share functional properties (Liang et al., 2018). A similar approach was used to measure how the receptive fields of boutons from cortico-cortical afferents relates to their position in the cortical space of the visual cortex. This allowed measuring and comparing the convergence of visual information from defined projections terminating in primary visual cortex (Keller et al., 2020; Marques et al., 2018; Murgas et al., 2020). It also revealed that the position in the retinotopic map of mouse visual cortex where axons make synapses depends both on their receptive field and their tuning to moving oriented gratings (Marques et al., 2018). Simultaneous recording of pre- and postsynaptic signals using dual color calcium imaging (Ali et al., 2020b; Inoue et al., 2019; Mohr et al., 2020) can potentially allow relating the signals that boutons relay, not only with their position in the brain, but with the postsynaptic neurons that they target, inferred from correlated activity.
Like during somatic recordings, calcium imaging using GECIs allows recording chronically from the same boutons across several days (Makino and Komiyama, 2015; Pardi et al., 2020; Petreanu et al., 2012). However, unlike somata, axonal boutons are dynamic and can potentially disappear across recording sessions (De Paola et al., 2006; Stettler et al., 2006), limiting the applicability of longitudinal recordings in boutons.
3.4. Quantifications of axons and boutons in axonal recordings
Unlike somatic recordings, as axons can have many boutons in the same recorded regions, in many cases the activity from the same neuron is repeatedly recorded in multiple ROIs. As activity in ROIs belonging to the same axon is highly correlated, boutons that belong to the same axons can be distinguished from those that do not, even when a physical link is not observed in the images (Petreanu et al., 2012). Thus, both the number of axons carrying a specific signal and the number of synapses they make in the imaged regions can in principle be quantified (Fig. 1B). For some studies it might be more relevant to quantify the number of axons carrying a given signal, e.g., when estimating the relative proportion of neurons relaying different signals to the imaged region. For others, quantifications of boutons might be more relevant, as they reflect the relative abundance of actual inputs received by the neurons in the imaged region. When quantifying signals across boutons it is nevertheless usually necessary to identify those belonging to the same axons, as these are not statistically independent, and this should be taken into account when analyzing the data.
The selection of the ROIs to extract fluorescence traces from boutons can be done by manually or semiautomatically identifying axonal varicosities in registered averaged projections (Gambino et al., 2014; Petreanu et al., 2012). Alternatively, ROIs can be segmented on “hotspots” of neighboring pixels with correlated activity of similar shape and size as boutons (Glickfeld et al., 2013; Liang et al., 2020; Roth et al., 2016). Unsupervised algorithms that segments ROIs using spatiotemporal profile of the recorded image to segment contiguous correlated pixels, such as those of calcium imaging analysis pipelines such as suite2P (Pachitariu et al., 2017) and CalmAm (Giovannucci et al., 2019) can also be applied to axonal recordings by adjusting parameters of the expected size and shape of the ROIs. However, automatic segmentations based on spatiotemporal correlations can in many cases define ROIs that encompass several boutons and the axonal shaft that connects them, as calcium activity will be correlated and spatially contiguous within that region. While this is not an issue when describing activity of axons, if the experimental questions require reporting boutons, further processing might be required to enforce single bouton ROIs.
4. Technical challenges and solutions
Our discussion has highlighted the potential of axon imaging techniques as a systems neuroscience approach. However, it should be noted that this technique also entails considerable technical hurdles. Axon imaging is directed against a structure that is typically <1 μm in diameter, much smaller than the 10 μm diameter of typical neuronal somata. Axons are also distal to the somatic site of the bulk of protein manufacturing. As a result, most of the fluorescent indicator will normally express predominantly within cellular compartments near the soma, limiting the signal that can be extracted at axonal sites (Broussard et al., 2018; Lewis et al., 2011; Padmanabhan et al., 2014). Recordings from distal axons therefore suffer a reduction in SNR. Relatively poor sensor expression in axons also impedes multi-photon imaging of single units at depths typically achievable with somatic imaging (Broussard et al., 2018). Finally, size and distance from the soma make axons refractory for direct electrical recordings. Therefore, unlike in the soma (Sato et al., 2007; Theis et al., 2016; Wei et al., 2020), direct comparison of electrical and fluorescence recordings of activity as a means of establishing ground truth of axon activity patterns is typically not possible. Collectively, these effects make multi-photon recordings of axonal activity more prone to uncorrectable motion artifact contamination, reduced signal-to-noise ratio, and poorly resolved signals in deep tissue.
4.1. Specialized axon-targeted calcium sensors
Specialized calcium sensors that are specifically enriched in the axonal compartment partially address these issues. These methods work by direct fusion of a GCaMP to an axonal subcellular localization peptide derived from growth-associated protein 43 (GAP43) (Broussard et al., 2018) or a full-length synaptophysin protein, which localizes specifically to the presynaptic compartment (Dreosti et al., 2009; Zhang et al., 2018). The resultant probes are called axon-GCaMPs and syGCaMPs, respectively. Both methods result in increased sensor expression in axons, increasing the labeling and effective basal brightness of the structure. In agreement with theoretical models (Broussard et al., 2018; Theer and Denk, 2006; Yasuda et al., 2004) increasing resting-state brightness leads to better motion correction, higher SNR, and better depth resolution. When comparing axon-GCaMPs and syGCaMPs, axon-GCaMPs smoothly labels the entire axonal arbor including varicosities. In contrast, syGCaMPs are tightly localized to the presynaptic compartment and transient transport packets. axon-GCaMPs are thus more diffusible and less prone to photobleaching while syGCaMPs more readily reveal calcium dynamics within putative synaptic boutons (Broussard et al., 2018). Use cases with high structure irradiance and long imaging epochs may therefore benefit from axon-GCaMPs while studies focusing on presynaptic calcium dynamics may be better served by syGCaMPs.
Axon-enriched calcium sensors are an aid, but not a panacea for the technical challenges involved in axon imaging techniques. We now consider motion correction, SNR, and depth resolution individually for best practices for maximizing experimental success.
4.2. Motion artifacts during axonal recordings
Motion artifact is a problem across all in vivo imaging modalities but is particularly pronounced in axon imaging. Small compartment size coupled with low levels of sensor expression in these distal structures limit the total photon flux, which complicates efforts to use standard cross-correlation based methods for image registration (Broussard et al., 2018; Giovannucci et al., 2019; Guizar-Sicairos et al., 2008; Pachitariu et al., 2017). The basal state fluorescence of axonal structures can be increased by the aforementioned trafficking approaches (Broussard et al., 2018; Dreosti et al., 2009) as well as by enhancing the fluorescence output of the sensor in low calcium (Dana et al., 2019) to combat low basal signal. Note, however, that this latter approach comes at the expense of dynamic range of the calcium sensor. Because axons are typically smaller than the size of behavior-related motion artifact (order of 10 μm (Chen et al., 2013a; Dombeck et al., 2007)), they can appear to become brighter and dimmer due to z-axis motion rather than calcium-dependent fluorescence changes. One approach to address this issue is to compare the variance in signal from a calcium-insensitive fluorophore signal with that derived from a calcium sensor introduced in a similar imaging preparation. A cutoff for calcium-derived signals changes can then be enforced at, for example, the 99 percentile of the distribution of changes observed in the static fluorophore (Petreanu et al., 2012; Sekiguchi et al., 2016).
Computational and engineering approaches have also been devised for addressing z-axis motion in two-photon imaging. For example, using piezo-based actuators (Göbel et al., 2007) to rapidly move the imaging plane allows recording frames from a short volume along the z-axis (Jaepel et al., 2017; Ryan et al., 2020; Schröder et al., 2020; Tanaka et al., 2018). This approach allows for reconstruction of imaged axonal structures. A particular point in time and space of the 4 dimensional image dataset can then be aligned through time by comparison of adjacent frames within a stack (Schröder et al., 2020), across image stacks (Tanaka et al., 2018), or by correcting fluorescence by comparing ROI position to a reference volume (Ryan et al., 2020). Because of the relative dimness of axonal structures, it has also proven useful to use a secondary marker of the imaged volume which is spectrally resolvable from the GECI. For example, the green GECI GCaMP can be used in axons together with a static red fluorophore expressed in somata in the imaged volume (Schröder et al., 2020; Tanaka et al., 2018), or a red dye injected systemically to allow visualization of vascular structures (Ryan et al., 2020). In this approach, images can be aligned using the more abundant, static fluorophore. As a final note, though it has not yet become common practice for in vivo axon imaging applications, a suite of GCaMPs tethered to static red fluorophores have been developed (Al-Osta et al., 2018; Cho et al., 2017). Using these or GECIs tethered to long stoke shift red fluorophores that allow excitation at the optimal GECI wavelength (Kim et al., 2020; Laviv et al., 2020) may obviate the need for a separately introduced static fluorophore.
Several methods to counteract two-photon motion artifacts rely on adjustments to the excitation beam itself. The first such approach was simple underfilling of the back aperture to create an elongated point spread function for the excitation volume (Zipfel et al., 2003). A more extreme version of this concept is so-called Bessel beam implemented by use of concentric fill of microscope back aperture (Garcés-Chavéz et al., 2002; Lu et al., 2017). In each case, the imaged structures can be situated in a highly elongated excitation volume such that axial motion of the sample results in displacement results in negligible changes to fluorescent output. However, it should be noted that these techniques will sample multiple structures along the axially elongated excitation volume in tissue densely innervated by labeled axons. As a consequence, these methods are suited only in cases when a low density of labelled axons is present in the imaging area. Another approach is to monitor motion in real time (Chen et al., 2013a) and adjust the excitation beam path using electrically tunable acousto-optical devices (Szalay et al., 2016) to counteract the effects of this motion. Indeed, this approach has been recently shown to allow significant improvements to the deblurring of processed image stacks in dendritic compartments in running mice (Griffiths et al., 2020). As such, sample motion-based beam steering can counteract motion artifact in structures with size comparable to axons, though to our awareness this technique has not yet been demonstrated in axon imaging applications.
For fiber photometry-based imaging methods, non-activity related artifact comes predominantly from behavior-related bending of the imaging fiber, intrinsic signal sources and excitation-induced photobleaching (Kim et al., 2016; Martianova et al., 2019; Sych et al., 2019). All of these effects can be corrected for by using a channel for imaging at the isosbestic wavelength (Kim et al., 2016; Lerner et al., 2015). At the isosbestic wavelength, emission of the calcium bound and unbound GECI are equivalent, providing a calcium-insensitive channel that can be used for motion detection and correction. In the isosbestic approach, the GECI is alternatingly excited by an optimal activity wavelength and the isosbestic wavelength. Any changes to fluorescence output on the isosbestic channel can then be regressed out of the signal detected in the activity channel. In addition, similar to two-photon approaches, a static fluor can be used as a reference to correct for non-activity-related fluorescence changes (Akam and Walton, 2019).
4.3. The signal-to-noise ratio of axonal recordings
The small volume, low GECI expression, and relatively fast calcium transients of axonal structures limit available photon budget and resultant SNR. Specifically, fewer available photons increase the difficulty of determining when an activity-related calcium change has occurred by increasing the noise level of the basal state and decreasing the contrast to autofluorescent signal sources (Yasuda et al., 2004). Correspondingly, in fiber photometry experiments, direct comparisons between signal detected in somatic and axonal recordings indicate lower amplitude fluorescent transients emitted from axonal sites (Kim et al., 2016). This effect cannot be ameliorated by increased sensor expression as excessive GECI expression in the soma results in cytotoxicity and eventual morbidity (Tian et al., 2009; Zariwala et al., 2012). Nor can excitation light power be increased indefinitely to increase sensor photon emission as irreversible photobleaching and tissue-damaging heating (Podgorski and Ranganathan, 2016; Wang et al., 2020) occur at excessive laser intensities and dwell times.
Several established and nascent methods exist to combat low SNR in axonal recordings. Preferential enrichment of the GECI within the axonal compartment (Broussard et al., 2018; Dreosti et al., 2009) can improve the sensor expression profile. For detection of fast calcium transients in axons, sensors with faster responses to calcium, including the XCaMP (Inoue et al., 2019), jGCaMP7 (Dana et al., 2019), and particularly the jGCaMP8 series (Zhang et al., 2020) can overcome potential signal loss due to mismatch in sensor kinetic properties and detected calcium events. As discussed above, the detectability of axonal signals using fiber photometry depends not only on the amplitude of the calcium transients in axons, but also on the diversity of signals in the vicinity of the fiber tip. Most of the effective signal in typical photometry systems arises from a volume of around 105 – 106 μm3, extending up to around 200 μm from the optical fiber facet (Aravanis et al., 2007; Pisanello et al., 2019; Yona et al., 2016). Generally, larger core diameter and higher numerical aperture systems provide higher photon collection capabilities (Pisanello et al., 2019). But the impact of collecting more photons on SNR must be balanced with the fact that collecting over larger volumes will entail collecting photons from more spatially distributed structures, increasing the chance of recording functionally heterogenous axonal populations.
4.4. Recording from axons in deep structures
Low sensor enrichment in axons also complicates imaging these structures deep to the tissue surface. Indeed, even under optimal imaging conditions, two-photon imaging is only able to resolve structures a few scattering lengths of the excitation light into tissue. Excitation power can be increased to overcome light lost to scattering, but once the irradiance becomes sufficiently high, fluorescence is produced from outside of the microscope focal plane. This out-of-focus fluorescence obscures emission from the target image region (Theer and Denk, 2006). Therefore, GECIs with green emission spectra such as the GCaMP sensors, produce effective imaging with acceptable SNR only to approximately 500 μm deep to the tissue surface (Takasaki et al., 2020) when not correcting for light aberration due to the tissue. This effect is even more severe for axon imaging as higher excitation light is required to visualize activity due to low sensor enrichment. Multiple methods have been deployed to overcome the depth limit, some of which have now been attempted for imaging axons. The first imaging of axons at depths greater than 300 μm was achieved using adaptive optics (Sun et al., 2016), a process by which the excitation wavefront is shaped by an array of movable mirrors to cancel sample-induced aberrations in the light path to sharply focus the excitation light on its target (Ji, 2014). The effective depth limit using the adaptive optics approach was pushed to nearly 1 mm by correcting for tissue aberrations using a red fluorophore introduced to the vascular lumen of imaged animals (Liu et al., 2019). Sensor enrichment in the axonal compartment has proven to be another useful approach for increasing effective imaging depths (Broussard et al., 2018). Non-invasive imaging of axons at even subcortical depths may be possible by using three-photon microscopy, which uses longer wavelength light for reduced scattering and suppression of out-of-focus emission due to the higher order non-linearity of three-photon emission (Ouzounov et al., 2017). As previously mentioned, invasive methods including fiber photometery and microendoscopy require implantation of fibers or lenses typically hundreds of microns in diameter or removal of overlying tissue can also allow for detection of axonal activity patterns at essentially limitless depths.
4.5. Ground truth recordings
Finally, unlike somatic recordings, where paired imaging and electrophysiological recordings can be readily performed (e.g.,(Chen et al., 2013b)), axons are relatively refractory to direct electrophysiological interrogation. As a result, the ground truth electrical activity that gives rise to calcium-induced fluorescence observables is much harder to establish in axon imaging techniques. This difficulty has complicated interpretation of axon recordings. Despite this fact, several indirect techniques have been developed to attempt ground truth establishment for axonal recordings. The first and most prevalent of these was direct electrical stimulation of nerve bundles with simultaneous optical recordings of the resultant fluorescence either in acute brain slice preparations (Marvin et al., 2018; Petreanu et al., 2012; Tanaka et al., 2018) or in vivo (Ali et al., 2020a). However, it should be noted that direct stimulation can only approximate average response as this approach does not recover all complexities encountered in behaving animals. Particularly, direct stimulation of afferents is unlikely to engage a similar ensemble of presynaptic modulatory influences that can affect spike-to-spike variability in individual boutons (Pardi et al., 2020). Another approach is to relate the fluorescence signal in axons to a measure of presynaptic neurotransmitter release. This has been achieved by direct electrical detection of neurotransmitter release (see, e.g., (Kupferschmidt et al., 2017)) or by using recently developed genetically encoded sensors of neuromodulators (Patriarchi et al., 2020) to monitor neurotransmitter release during presynaptic stimulation. Relatedly, multicolor calcium imaging allows direct recordings of correlations between pre- and postsynaptic structures that share close spatial appositions (Ali et al., 2020b; Inoue et al., 2019; Mohr et al., 2020). Conceptually, these last two approaches are important advance in that they clarify what postsynaptic effect a particular pattern of fluorescence in the imaged presynaptic compartment will have on the postsynaptic response.
5. Future directions
As discussed here, axon-specific activity imaging techniques give a picture of projection-specific, genetically defined neural activity at multiple scales. These approaches can be leveraged to interrogate the bulk activity of an entire projection or to eavesdrop on the information passed between a particular bouton and its postsynaptic target. We have presented a snapshot of what has been learned through axonal imaging and state of the art methods for dealing with the technical challenges associated with imaging in a small neuronal compartment. We will now consider additional ways that imaging of axons can be used in conjunction with nascent technologies to answer fundamental questions in neurobiology.
An expanded color palette of activity sensors, including GECIs of multiple colors, has opened the door to experiments in which pre- and postsynaptic patterns of activity are simultaneously recorded. Early examples of this concept have involved use of spectrally separable GECIs with one color expresses exclusively in the pre- or postsynaptic compartment (Ali et al., 2020b; Dana et al., 2016; Inoue et al., 2019; Mohr et al., 2020). In these cases, however, synaptic connectivity between cellular elements imaged in the separate channels had to be inferred by patterns of activity. This is because two-photon imaging lacks the spatial resolution to directly determine whether an adjacent axon and dendrite are synaptically coupled. A potential solution to this issue is the use of transsynaptic virus to label both cellular compartments. For example, adeno-associated virus driving expression of a recombinase transduces cells at the site of injection as well as a subset of the postsynaptic targets (Zingg et al., 2017, 2020). By co-injecting a GECI at this first site as well as a spectrally separate, recombinase-dependent GECI in an afferent site, one could in principle image synaptically coupled cell populations simultaneously at the afferent site.
Axon imaging used in conjunction with tools that allow direct visualization of neurotransmitter (Marvin et al., 2013, 2018, 2019) and neuromodulator release (Patriarchi et al., 2018; Sun et al., 2018; Unger et al., 2020) will also help to clarify the functional role of axons that release more than one neurotransmitter (Granger et al., 2017). Using axonal calcium alone to track activity of neuromodulatory afferents does not permit detection of co-release of different neurotransmitters. In contrast, sensors of neuromodulators respond to any changes of local concentrations of their target ligand regardless of the source. Projections with complex neurochemistry such as those from the locus coeruleus (LC), could be effectively interrogated by the combination of these methods. LC is the predominant source of noradrenergic neuromodulation in the brain, but a subpopulation of LC cells co-release dopamine as well as norepinephrine at their terminal fields (Devoto and Flore, 2006; Kempadoo et al., 2016). Imaging of dopamine release using recently developed red dopamine sensors (Patriarchi et al., 2020; Sun et al., 2020) simultaneously with axon-targeted GCaMPs in LC axons should allow direct determination of where and how the brain utilizes dopamine release from this cell population. Relatedly, genetically encoded sensors of neurotransmitter release can in theory allow for direct interrogation of the coupling between calcium influx and neurotransmitter release in vivo, as occurs for example during synaptic facilitation (Helassa et al., 2018). However, there are currently no pairs of red and green sensors of axonal calcium and neurotransmitter release that have been demonstrated to possess sufficient sensitivity for in vivo assays of this type. This limitation can be addressed by the development of either red sensors optimized for axonal calcium or extracellular neurotransmitter release.
Imaging axons will continue to be a fruitful way to isolate the information passed from specified cell populations in one brain region to another. Addressing the technical challenges inherent to this technique will allow wider adoption across the neuroscience community.
Acknowledgments
This work was supported by the Champalimaud Foundation (L.P), Fundaçaõ para a Ciência e a Tecnologia (L.P.) LISBOA-01–0145-FEDER and PTDC/MED-NEU/6645/2020, La “Caixa” Foundation (L.P.) LCF/PR/HR17/52150005, and NIH (G.B) F32 MH120887.
Footnotes
Declaration of Competing Interest
The authors report no declarations of interest.
References
- Akam T, Walton ME, 2019. pyPhotometry: open source Python based hardware and software for fiber photometry data acquisition. Sci. Rep. 9, 3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali F, Kwan AC, 2019. Interpreting in vivo calcium signals from neuronal cell bodies, axons, and dendrites: a review. Neurophotonics 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali F, Gerhard DM, Sweasy K, Pothula S, Pittenger C, Duman RS, Kwan AC, 2020a. Ketamine disinhibits dendrites and enhances calcium signals in prefrontal dendritic spines. Nat. Commun. 11, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali F, Shao L-X, Gerhard DM, Sweasy K, Pothula S, Pittenger C, Duman RS, Kwan AC, 2020b. Inhibitory regulation of calcium transients in prefrontal dendritic spines is compromised by a nonsense Shank3 mutation. Mol. Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Osta I, Mucha M, Pereda D, Piqué-Gili M, Okorocha AE, Thomas R, Hartell NA, 2018. Imaging calcium in hippocampal presynaptic terminals with a ratiometric calcium sensor in a novel transgenic mouse. Front. Cell. Neurosci. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravanis AM, Wang L-P, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K, 2007. An optical neural interface:in vivocontrol of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 4, S143–S156. [DOI] [PubMed] [Google Scholar]
- Broussard GJ, Liang R, Tian L, 2014. Monitoring activity in neural circuits with genetically encoded indicators. Front. Mol. Neurosci. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard GJ, Liang Y, Fridman M, Unger EK, Meng G, Xiao X, Ji N, Petreanu L, Tian L, 2018. In vivo measurement of afferent activity with axon-specific calcium imaging. Nat. Neurosci. 21, 1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess CR, Ramesh RN, Sugden AU, Levandowski KM, Minnig MA, Fenselau H, Lowell BB, Andermann ML, 2016. Hunger-dependent enhancement of food cue responses in mouse postrhinal cortex and lateral amygdala. Neuron 91, 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG, 2011. Glutamate spillover promotes the generation of NMDA spikes. J. Neurosci. 31, 16435–16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Pfaffli OA, Voigt FF, Margolis DJ, Helmchen F, 2013a. Online correction of licking-induced brain motion during two-photon imaging with a tunable lens. J. Physiol. 591, 4689–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. , 2013b. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Voigt FF, Javadzadeh M, Krueppel R, Helmchen F, 2016. Long-range population dynamics of anatomically defined neocortical networks. ELife 5, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Sun Y-C, Zhan H, Kebschull JM, Fischer S, Matho K, Huang ZJ, Gillis J, Zador AM, 2019. High-throughput mapping of long-range neuronal projection using in situ sequencing. Cell 179, 772–786 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J-H, Swanson CJ, Chen J, Li A, Lippert LG, Boye SE, Rose K, Sivaramakrishnan S, Chuong C-M, Chow RH, 2017. The GCaMP-R family of genetically encoded ratiometric calcium indicators. ACS Chem. Biol. 12, 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Denk W, Tank DW, Svoboda K, 2000. Action potentials reliably invade axonal arbors of rat neocortical neurons. Proc. Natl. Acad. Sci. U. S. A. 97, 9724–9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Martín A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, Nguyen PL, Callaway EM, Ghosh A, Huberman AD, 2014. A dedicated circuit links direction-selective retinal ganglion cells to the primary visual cortex. Nature 507, 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM, 2013. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle TL, Madisen L, Hage TA, Valley MT, Knoblich U, Larsen RS, Takeno MM, Huang L, Gu H, Larsen R, et al. , 2018. A suite of transgenic driver and reporter mouse lines with enhanced brain-cell-type targeting and functionality. Cell 174, 465–480 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana H, Mohar B, Sun Y, Narayan S, Gordus A, Hasseman JP, Tsegaye G, Holt GT, Hu A, Walpita D, et al. , 2016. Sensitive red protein calcium indicators for imaging neural activity. ELife 5, e12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana H, Sun Y, Mohar B, Hulse BK, Kerlin AM, Hasseman JP, Tsegaye G, Tsang A, Wong A, Patel R, et al. , 2019. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 16, 649–657. [DOI] [PubMed] [Google Scholar]
- De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K, 2006. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron 49, 861–875. [DOI] [PubMed] [Google Scholar]
- Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G, 2011. Axon physiology. Physiol. Rev. 91, 555–602. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, 2006. On the origin of cortical dopamine: is it a Co-transmitter in noradrenergic neurons? Curr. Neuropharmacol. 4, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW, 2007. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56, 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreosti E, Odermatt B, Dorostkar MM, Lagnado L, 2009. A genetically-encoded reporter of synaptic activity in vivo. Nat. Methods 6, 883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Kremer Y, Crochet S, Petersen CCH, 2014. Cholinergic signals in mouse barrel cortex during active whisker sensing. Cell Rep. 9, 1654–1660. [DOI] [PubMed] [Google Scholar]
- Gambino F, Pagès S, Kehayas V, Baptista D, Tatti R, Carleton A, Holtmaat A, 2014. Sensory-evoked LTP driven by dendritic plateau potentials in vivo. Nature 515, 116–119. [DOI] [PubMed] [Google Scholar]
- Garcés-Chávez V, McGloin D, Melville H, Sibbett W, Dholakia K, 2002. Simultaneous micromanipulation in multiple planes using a self-reconstructing light beam. Nature 419, 145–147. [DOI] [PubMed] [Google Scholar]
- Giovannucci A, Friedrich J, Gunn P, Kalfon J, Brown BL, Koay SA, Taxidis J, Najafi F, Gauthier JL, Zhou P, et al. , 2019. CaImAn an open source tool for scalable calcium imaging data analysis. ELife 8, 339564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Andermann ML, Bonin V, Reid RC, 2013. Cortico-cortical projections in mouse visual cortex are functionally target specific. Nat. Neurosci. 16, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel W, Kampa BM, Helmchen F, 2007. Imaging cellular network dynamics in three dimensions using fast 3D laser scanning. Nat. Methods 4, 73–79. [DOI] [PubMed] [Google Scholar]
- Gold C, Henze DA, Koch C, Buzsaki G, 2006. On the origin of the extracellular action potential waveform: a modeling study. J. Neurophysiol. 95, 3113–3128. [DOI] [PubMed] [Google Scholar]
- Granger AJ, Wallace ML, Sabatini BL, 2017. Multi-transmitter neurons in the mammalian central nervous system. Curr. Opin. Neurobiol. 45, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienberger C, Konnerth A, 2012. Imaging calcium in neurons. Neuron 73, 862–885. [DOI] [PubMed] [Google Scholar]
- Griffiths VA, Valera AM, Lau JY, Ro?s H, Younts TJ, Marin B, Baragli C, Coyle D, Evans GJ, Konstantinou G, et al. , 2020. Real-time 3D movement correction for two-photon imaging in behaving animals. Nat. Methods 17, 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizar-Sicairos M, Thurman ST, Fienup JR, 2008. Efficient subpixel image registration algorithms. Opt. Lett. 33, 156–158. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, et al. , 2014. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kebschull JM, Campbell RAA, Cowan D, Imhof F, Zador AM, Mrsic-Flogel TD, 2018. The logic of single-cell projections from visual cortex. Nature 556, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helassa N, Dürst CD, Coates C, Kerruth S, Arif U, Schulze C, Wiegert JS, Geeves M, Oertner TG, Torök K, 2018. Ultrafast glutamate sensors resolve high-frequency release at Schaffer collateral synapses. Proc. Natl. Acad. Sci. U. S. A. 115, 5594–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe MW, Dombeck DA, 2016. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535, 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Takeuchi A, Manita S, Horigane S-I, Sakamoto M, Kawakami R, Yamaguchi K, Otomo K, Yokoyama H, Kim R, et al. , 2019. Rational engineering of XCaMPs, a multicolor GECI suite for in vivo imaging of complex brain circuit dynamics. Cell 177, 1346–1360 e24. [DOI] [PubMed] [Google Scholar]
- Jaepel J, Hübener M, Bonhoeffer T, Rose T, 2017. Lateral geniculate neurons projecting to primary visual cortex show ocular dominance plasticity in adult mice. Nat. Neurosci. 20, 1708–1714. [DOI] [PubMed] [Google Scholar]
- Ji N, 2014. The practical and fundamental limits of optical imaging in mammalian brains. Neuron 83, 1242–1245. [DOI] [PubMed] [Google Scholar]
- Kaifosh P, Lovett-Barron M, Turi GF, Reardon TR, Losonczy A, 2013. Septohippocampal GABAergic signaling across multiple modalities in awake mice. Nat. Neurosci. 16, 1182–1184. [DOI] [PubMed] [Google Scholar]
- Katz B, 1969. The Release of Neural Transmitter Substances. Thomas, Springfield, Ill. [Google Scholar]
- Keller AJ, Roth MM, Scanziani M, 2020. Feedback generates a second receptive field in neurons of the visual cortex. Nature 582, 545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER, 2016. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci. U. S. A. 113 (51), 14835–14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CK, Yang SJ, Pichamoorthy N, Young NP, Kauvar I, Jennings JH, Lerner TN, Berndt A, Lee SY, Ramakrishnan C, et al. , 2016. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat. Methods 13, 325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M-H, Znamenskiy P, Iacaruso MF, Mrsic-Flogel TD, 2018. Segregated subnetworks of intracortical projection neurons in primary visual cortex. Neuron 100, 1–9. [DOI] [PubMed] [Google Scholar]
- Kim BB, Wu H, Hao YA, Pan M, Chavarha M, Westberg M, St-Pierre F, Wu JC, Lin MZ, 2020. A red fluorescent protein with improved monomericity enables ratiometric voltage imaging with ASAP3. BioRxiv 2020, 10.09.328922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid AE, Zheng T, Wilson CJ, 1998. Connectivity and convergence of single corticostriatal axons. J. Neurosci. 18, 4722–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester HJ, Sakmann B, 2000. Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex. J. Physiol. 529, 625–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Ohki K, 2016. Laminar differences in the orientation selectivity of geniculate afferents in mouse primary visual cortex. Nat. Neurosci. 19, 316–319. [DOI] [PubMed] [Google Scholar]
- Kupferschmidt DA, Juczewski K, Cui G, Johnson KA, Lovinger DM, 2017. Parallel, but dissociable, processing in discrete corticostriatal inputs encodes skill learning. Neuron 96, 476–489 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SE, Yang H, Minamisawa G, O’Connor DH, 2016. Sensory and decision-related activity propagate in a cortical feedback loop during touch perception. Nat. Neurosci. 19, 1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviv T, Scholl B, Parra-Bueno P, Foote B, Zhang C, Yan L, Hayano Y, Chu J, Yasuda R, 2020. In vivo imaging of the coupling between neuronal and CREB activity in the mouse brain. Neuron 105, 799–812 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinweber M, Ward DR, Sobczak JM, Attinger A, Keller GB, 2017. A sensorimotor circuit in mouse cortex for visual flow predictions. Neuron 95, 1420–1432 e5. [DOI] [PubMed] [Google Scholar]
- Lerner TN, Shilyansky C, Davidson TJ, Evans KE, Beier KT, Zalocusky KA, Crow AK, Malenka RC, Luo L, Tomer R, et al. , 2015. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Mao T, Arnold DB, 2011. A role for myosin VI in the localization of axonal proteins. PLoS Biol. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Chen T, Guo ZV, Gerfen CR, Svoboda K, 2015. A motor cortex circuit for motor planning and movement. Nature 519, 51–56. [DOI] [PubMed] [Google Scholar]
- Liang L, Fratzl A, Goldey G, Ramesh RN, Sugden AU, Morgan JL, Chen C, Andermann ML, 2018. A fine-scale functional logic to convergence from retina to thalamus. Cell 173, 1343–1355 e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Fratzl A, Reggiani JDS, El Mansour O, Chen C, Andermann ML, 2020. Retinal inputs to the thalamus are selectively gated by arousal. Curr. Biol. 30, 3923–3934 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Hromáadka T, Znamenskiy P, Zador AM, 2009. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS One 4, e6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MZ, Schnitzer MJ, 2016. Genetically encoded indicators of neuronal activity. Nat. Neurosci. 19, 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Li Z, Marvin JS, Kleinfeld D, 2019. Direct wavefront sensing enables functional imaging of infragranular axons and spines. Nat. Methods 16, 615–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Sugimori M, Silver RB, 1992. Microdomains of high calcium concentration in a presynaptic terminal. Science 256, 677–679. [DOI] [PubMed] [Google Scholar]
- Lu R, Sun W, Liang Y, Kerlin A, Bierfeld J, Seelig J, Wilson DE, Scholl B, Mohar B, Tanimoto M, et al. , 2017. Video-rate volumetric functional imaging of the brain at synaptic resolution. Nat. Neurosci. 20, 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K, 2018. Genetic dissection of neural circuits: a decade of progress. Neuron 98, 256–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino H, Komiyama T, 2015. Learning enhances the relative impact of top-down processing in the visual cortex. Nat. Neurosci. 18, 1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques T, Nguyen J, Fioreze G, Petreanu L, 2018. The functional organization of cortical feedback inputs to primary visual cortex. Nat. Neurosci. 21, 757–764. [DOI] [PubMed] [Google Scholar]
- Martianova E, Aronson S, Proulx CD, 2019. Multi-fiber photometry to record neural activity in freely-moving animals. JoVE J. Vis. Exp. e60278. [DOI] [PubMed] [Google Scholar]
- Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, Akerboom J, Gordus A, Renninger SL, Chen T-W, Bargmann CI, et al. , 2013. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods 10, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin JS, Scholl B, Wilson DE, Podgorski K, Kazemipour A, Müller JA, Schoch S, Quiroz FJU, Rebola N, Bao H, et al. , 2018. Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR. Nat. Methods 15, 936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin JS, Shimoda Y, Magloire V, Leite M, Kawashima T, Jensen TP, Kolb I, Knott EL, Novak O, Podgorski K, et al. , 2019. A genetically encoded fluorescent sensor for in vivo imaging of GABA. Nat. Methods 16, 763–770. [DOI] [PubMed] [Google Scholar]
- Meng G, Liang Y, Sarsfield S, Jiang W, Lu R, Dudman JT, Aponte Y, Ji N, 2019. High-throughput synapse-resolving two-photon fluorescence microendoscopy for deep-brain volumetric imaging in vivo. ELife 8, e40805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ, 1998. Presynaptic receptors. Annu. Rev. Pharmacol. Toxicol. 38, 201–227. [DOI] [PubMed] [Google Scholar]
- Mohr MA, Bushey D, Aggarwal A, Marvin JS, Kim JJ, Marquez EJ, Liang Y, Patel R, Macklin JJ, Lee C-Y, et al. , 2020. jYCaMP: an optimized calcium indicator for two-photon imaging at fiber laser wavelengths. Nat. Methods 17, 694–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mridha Z, de Gee JW, Shi Y, Alkashgari R, Williams J, Suminski A, Ward MP, Zhang W, McGinley MJ, 2021. Graded recruitment of pupil-linked neuromodulation by parametric stimulation of the vagus nerve. Nat. Commun. 12, 1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgas KA, Wilson AM, Michael V, Glickfeld LL, 2020. Unique spatial integration in mouse primary visual cortex and higher visual areas. J. Neurosci. 40, 1862–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otazu GH, Chae H, Davis MB, Albeanu DF, 2015. Cortical feedback decorrelates olfactory bulb output in awake mice. Neuron 86, 1461–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzounov DG, Wang T, Wang M, Feng DD, Horton NG, Cruz-Hernández JC, Cheng Y-T, Reimer J, Tolias AS, Nishimura N, et al. , 2017. In vivo three-photon imaging of activity of GCaMP6-labeled neurons deep in intact mouse brain. Nat. Methods 14, 388–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachitariu M, Stringer C, Dipoppa M, Schroder S, Rossi LF, Dalgleish H, Carandini M, Harris KD, 2017. Suite2p: beyond 10,000 neurons with standard two-photon microscopy. BioRxiv, 061507. 10.1101/061507. [DOI] [Google Scholar]
- Padmanabhan S, Kareva T, Kholodilov N, Burke RE, 2014. Quantitative morphological comparison of axon-targeting strategies for gene therapies directed to the nigro-striatal projection. Gene Ther. 21, 115–122. [DOI] [PubMed] [Google Scholar]
- Pardi MB, Vogenstahl J, Dalmay T, Spanó T, Pu D-L, Naumann LB, Kretschmer F, Sprekeler H, Letzkus JJ, 2020. A thalamocortical top-down circuit for associative memory. Science 370, 844–848. [DOI] [PubMed] [Google Scholar]
- Parker NF, Cameron CM, Taliaferro JP, Lee J, Choi JY, Davidson TJ, Daw ND, Witten IB, 2016. Reward and choice encoding in terminals of midbrain dopamine neurons depends on striatal target. Nat. Neurosci. 19, 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong W-H, Folk RW, Broussard GJ, Liang R, Jang MJ, et al. , 2018. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarchi T, Mohebi A, Sun J, Marley A, Liang R, Dong C, Puhger K, Mizuno GO, Davis CM, Wiltgen B, et al. , 2020. An expanded palette of dopamine sensors for multiplex imaging in vivo. Nat. Methods 17, 1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Gutnisky DA, Huber D, Xu N, O’Connor DH, Tian L, Looger L, Svoboda K, 2012. Activity in motor-sensory projections reveals distributed coding in somatosensation. Nature 489, 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanello M, Pisano F, Hyun M, Maglie E, Balena A, De Vittorio M, Sabatini BL, Pisanello F, 2019. The three-dimensional signal collection field for Fiber photometry in brain tissue. Front. Neurosci. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgorski K, Ranganathan G, 2016. Brain heating induced by near-infrared lasers during multiphoton microscopy. J. Neurophysiol. 116, 1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Delaney KR, Tank DW, 1994. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J. Neurosci. 14, 523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MM, Dahmen JC, Muir DR, Imhof F, Martini FJ, Hofer SB, 2016. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nat. Neurosci. 19, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TM, Hinojosa AJ, Vroman R, Papasavvas C, Lagnado L, 2020. Correction of z-motion artefacts to allow population imaging of synaptic activity in behaving mice. J. Physiol. 598, 1809–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG, 1998. Optical measurement of presynaptic calcium currents. Biophys. J. 74, 1549–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadakane O, Masamizu Y, Watakabe A, Terada S-I, Ohtsuka M, Takaji M, Mizukami H, Ozawa K, Kawasaki H, Matsuzaki M, et al. , 2015. Long-term two-photon calcium imaging of neuronal populations with subcellular resolution in adult non-human primates. Cell Rep. 13, 1989–1999. [DOI] [PubMed] [Google Scholar]
- Sato TR, Svoboda K, 2010. The functional properties of barrel cortex neurons projecting to the primary motor cortex. J. Neurosci. 30, 4256–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TR, Gray NW, Mainen ZF, Svoboda K, 2007. The functional microarchitecture of the mouse barrel cortex. PLoS Biol. 5, e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder S, Steinmetz NA, Krumin M, Pachitariu M, Rizzi M, Lagnado L, Harris KD, Carandini M, 2020. Arousal modulates retinal output. Neuron 107, 487–495 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi KJ, Shekhtmeyster P, Merten K, Arena A, Cook D, Hoffman E, Ngo A, Nimmerjahn A, 2016. Imaging large-scale cellular activity in spinal cord of freely behaving mice. Nat. Commun. 7, 11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Harris KM, 1998. Three-dimensional structure and composition of CA3–&CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J. Neurosci. Off. J. Soc. Neurosci. 18, 8300–8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster SA, Wagner MJ, Pan-Doh N, Ren J, Grutzner SM, Beier KT, Kim TH, Schnitzer MJ, Luo L, 2021. The relationship between birth timing, circuit wiring, and physiological response properties of cerebellar granule cells. BioRxiv, 2021.02.15.431339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Tye KM, 2019. Leveraging calcium imaging to illuminate circuit dysfunction in addiction. Alcohol 74, 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NA, Koch C, Harris KD, Carandini M, 2018. Challenges and opportunities for large-scale electrophysiology with Neuropixels probes. Curr. Opin. Neurobiol. 50, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NA, Zatka-Haas P, Carandini M, Harris KD, 2019. Distributed coding of choice, action and engagement across the mouse brain. Nature 576, 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler DD, Yamahachi H, Li W, Denk W, Gilbert CD, 2006. Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron 49, 877–887. [DOI] [PubMed] [Google Scholar]
- Sun W, Tan Z, Mensh BD, Ji N, 2016. Thalamus provides layer 4 of primary visual cortex with orientation- and direction-tuned inputs. Nat. Neurosci. 19, 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, et al. , 2018. A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell 174, 481–496 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Zhou J, Dai B, Qian T, Zeng J, Li X, Zhuo Y, Zhang Y, Wang Y, Qian C, et al. , 2020. Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 17, 1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sych Y, Chernysheva M, Sumanovski LT, Helmchen F, 2019. High-density multifiber photometry for studying large-scale brain circuit dynamics. Nat. Methods 16, 553–560. [DOI] [PubMed] [Google Scholar]
- Szalay G, Judak L, Katona G, Ocsai K,Juhász G,Veress M,Szadai Z,Feh ér A, Tompa T, Chiovini B, et al. , 2016. Fast 3D imaging of spine, dendritic, and neuronal assemblies in behaving animals. Neuron 92, 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki K, Abbasi-Asl R, Waters J, 2020. Superficial bound of the depth limit of two-photon imaging in mouse brain. ENeuro 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YP, Llano I, 1999. Modulation by K+ channels of action potential-evoked intracellular Ca2+ concentration rises in rat cerebellar basket cell axons. J. Physiol. 520, 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka YH, Tanaka YR, Kondo M, Terada S-I, Kawaguchi Y, Matsuzaki M, 2018. Thalamocortical axonal activity in motor cortex exhibits layer-specific dynamics during motor learning. Neuron 100, 244–258 e12. [DOI] [PubMed] [Google Scholar]
- Theer P, Denk W, 2006. On the fundamental imaging-depth limit in two-photon microscopy. JOSA A 23, 3139–3149. [DOI] [PubMed] [Google Scholar]
- Theis L, Berens P, Froudarakis E, Reimer J, Romań Roson M, Baden T, Euler T, Tolias AS, Bethge M, 2016. Benchmarking spike rate inference in population calcium imaging. Neuron 90, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. , 2009. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui-Kimura I, Matsumoto H, Akiti K, Yamada MM, Uchida N, Watabe-Uchida M, 2020. Distinct temporal difference error signals in dopamine axons in three regions of the striatum in a decision-making task. ELife 9, e62390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger EK, Keller JP, Altermatt M, Liang R, Matsui A, Dong C, Hon OJ, Yao Z, Sun J, Banala S, et al. , 2020. Directed evolution of a selective and sensitive serotonin sensor via machine learning. Cell 183, 1986–2002 e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wang T, Xu C, 2020. Three-photon neuronal imaging in deep mouse brain. Optica 7, 947–960. [Google Scholar]
- Wei Z, Lin B-J, Chen T-W, Daie K, Svoboda K, Druckmann S, 2020. A comparison of neuronal population dynamics measured with calcium imaging and electrophysiology. PLoS Comput. Biol. 16, e1008198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms CD, Häusser M, 2015. Reading out a spatiotemporal population code by imaging neighbouring parallel fibre axons in vivo. Nat. Commun. 6, 6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnubst J, Bas E, Ferreira TA, Wu Z, Economo MN, Edson P, Arthur BJ, Bruns C, Rokicki K, Schauder D, et al. , 2019. Reconstruction of 1,000 projection neurons reveals new cell types and organization of long-range connectivity in the mouse brain. Cell 179, 268–281 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Yuste R, 2017. In vivo imaging of neural activity. Nat. Methods 14, 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda R, Nimchinsky EA, Scheuss V, Pologruto TA, Oertner TG, Sabatini BL, Svoboda K, 2004. Imaging calcium concentration dynamics in small neuronal compartments. Sci. STKE Signal Transduct. Knowl. Environ. 2004pl5. [DOI] [PubMed] [Google Scholar]
- Yona G, Meitav N, Kahn I, Shoham S, 2016. Realistic numerical and analytical modeling of light scattering in brain tissue for optogenetic applications. ENeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, Zeng H, Looger LL, Svoboda K, Chen T-W, 2012. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J. Neurosci. Off. J. Soc. Neurosci. 32, 3131–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Li H, Chen M, Guo A, Wen Y, Poo M, 2018. Functional organization of intrinsic and feedback presynaptic inputs in the primary visual cortex. Proc. Natl. Acad. Sci. U. S. A. 115, E5174–E5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rózsa M, Bushey D, Zheng J, Broussard GJ, Tsang A, Tsegaye R, Patel S, Narayan JX, Zhang R, et al. , 2020. jGCaMP8 Fast Genetically Encoded Calcium Indicators (Janelia Research Campus). [Google Scholar]
- Zingg B, Chou X-L, Zhang Z-G, Mesik L, Liang F, Tao HW, Zhang LI, 2017. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg B, Peng B, Huang J, Tao HW, Zhang LI, 2020. Synaptic specificity and application of anterograde trans-synaptic AAV for probing neural circuitry. J. Neurosci. 40, 3250–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Webb WW, 2003. Nonlinear magic: multiphoton microscopy in the biosciences. Nat. Biotechnol. 21, 1369–1377. [DOI] [PubMed] [Google Scholar]
- Zong W, Wu R, Li M, Hu Y, Li Y, Li J, Rong H, Wu H, Xu Y, Lu Y, et al. , 2017. Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice. Nat. Methods 14, 713–719. [DOI] [PubMed] [Google Scholar]