Abstract
Aims:
Evaluate the contribution of blast pressure severity to cognitive functioning beyond posttraumatic stress disorder (PTSD) severity and traumatic brain injury (TBI).
Method:
Post-9/11 veterans (N = 254, 86.22% male) completed the Wechsler Adult Intelligence Scale (WAIS-IV) and Trail Making Test (TMT). The Clinician Administered PTSD Scale (CAPS-5), Mid-Atlantic MIRECC Assessment of TBI, and the Salisbury Blast Interview, evaluated PTSD diagnosis/severity, deployment TBI history/severity, and blast exposure history/severity, respectively.
Results:
Veterans with mild deployment TBI had overall significantly lower T-scores on WAIS-IV verbal comprehension index, d = .13, working memory index, d = .30, processing speed index, d = .25, TMT-A, d = .50, and TMT-B, d = .37. Mild deployment TBI was significantly associated with TMT-A, ΔR2 = .05, p < .001, and TMT-B, ΔR2 = .03, p = .001, performance. Blast pressure severity moderated the association between mild deployment TBI and TMT-A, ΔR2 = .02, p = .039, B = −2.01.
Conclusions:
Blast pressure severity exacerbated the effects of mild TBI on a simple attention task, such that participants with TBI had gradual decrements in attention as blast severity increased. Veterans who incur a TBI and are exposed to blasts during deployment may experience persisting difficulties with cognitive functioning due to alterations in basic attention abilities.
Keywords: blast, veteran, cognition, posttraumatic stress disorder, traumatic brain injury
Problems with cognitive function have been associated with psychological conditions related to combat, including posttraumatic stress disorder (PTSD) and traumatic brain injury (TBI), among others (Brewin et al., 2007; Kontos et al., 2013; Scott et al., 2015; Vasterling et al., 2018). Though PTSD and TBI are commonly diagnosed (Cifu et al., 2013), many combat veterans also have the often-unique experience of exposure to blasts and explosions that may be important to consider when evaluating long-term outcomes in this population. Illustratively, between 2000 through early 2018, nearly 384,400 Operations Enduring Freedom/Iraqi Freedom/New Dawn (OEF/OIF/OND) veterans were diagnosed with TBI, an estimated 19–23% of which were due to blast forces (Defense and Veterans Brain Injury Center (DVBIC), 2018; Nelson et al., 2015). Explosions are estimated to be involved in 79% of all combat injuries, and 88% of head and neck injuries of OEF/OIF/OND combatants (Owens et al., 2008). The number of service members with significant blast exposure is unknown, as many exposures are not recorded because they do not result in a TBI. Despite resulting TBI status, blast exposure may have effects on the brain and behavior of veterans beyond PTSD and TBI.
TBI (both blast and non-blast) and PTSD have been associated with poorer cognitive functioning in veterans (Kontos et al., 2013; Vasterling et al., 2018). In valid veteran samples (i.e., studies excluding based on performance validity testing), PTSD has been associated with poorer processing speed and executive functioning compared to counterparts without PTSD diagnosis (Scott et al., 2015; Wrocklage et al., 2016). Most TBI is mild in both military and civilian contexts, and cognitive changes are not expected to endure more than 3–12 months (Carroll et al., 2014; Carroll et al., 2004; Dikmen et al., 2009). Some studies have suggested subtle effects of mild TBI on cognition beyond the expected timeframe for recovery (Vanderploeg et al., 2005). Other studies suggest that apparent effects of TBI on cognition may be better accounted for by other factors, such as PTSD symptoms (Nelson et al., 2012), or multiple injuries (Belanger et al., 2010).
Recent evidence suggests that exposure to subconcussive blast forces (i.e., exposure to blasts or explosions that do not result in symptoms congruent with a TBI) may affect the brain and behavior (Grande et al., 2018; Martindale, Rowland, et al., 2018; Taber et al., 2015). Effects appear to be less severe than outcomes associated with TBI but are worse compared to unexposed controls. This represents a possible range of severity based on forces acting upon the brain, whether blast or blunt force. Recent research on cognitive outcomes almost exclusively evaluates effects of blast exposure in the context of TBI (i.e., TBI caused by blast; Davenport et al., 2012; Davenport et al., 2015; Mac Donald, Barber, Andre, et al., 2017; Mac Donald, Barber, Jordan, et al., 2017). Evidence suggests that cognitive outcomes do not differ by TBI acquisition mechanism (i.e., blast, blunt force), indicating that effects are better explained by TBI status rather than mechanism of injury (Belanger et al., 2009; Greer et al., 2018; Luethcke et al., 2011; Storzbach et al., 2015). What little is known about the effects of blast exposure on cognitive functioning indicate poorer immediate and delayed recall performance in veterans who were in close proximity (< 10m) to a blast or explosion (Grande et al., 2018; Storzbach et al., 2015), though distance doesn’t account for the extreme variability in blast mechanism. Incongruence between TBI, blast TBI, and blast exposure outcomes is likely in part due to differences in characterization and evaluation of blast exposure. As described in this paragraph, studies almost exclusively evaluate effects of blast within the context of a TBI (Belanger et al., 2009), and others operationalize blast severity by distance from the blast, regardless of the variable characteristics of the blast itself (Grande et al., 2018; Storzbach et al., 2015).
Though TBI and PTSD are associated with cognitive complaints (i.e., not necessarily objective cognitive performance deficits) in veteran samples, it is unclear if blast severity should be considered separately in the etiology of lower cognitive functioning or could be identified as an exacerbating factor in the effects of TBI and/or PTSD on cognitive function. The purpose of the present study was to evaluate how blast severity affects cognitive outcomes in combat veterans returning from the wars in Iraq and Afghanistan. The present study represents an initial investigation into how severity of a blast experience, regardless of distance from a blast, affects cognitive outcomes. We hypothesized that experience of increasing blast pressure would: 1) be associated with poorer cognitive outcomes; 2) be associated with poorer cognitive outcomes beyond the presence of PTSD and mild TBI, and; 3) moderate the effects of PTSD and mild TBI on cognitive outcomes.
Methods
Participants
Data for this analysis was collected as part of a study investigating the effects of blast exposure on the brain and behavior of combat veterans. Eligibility criteria were: at least one OEF/OIF/OND deployment with combat exposure (defined as a score > 17 [scale floor, indicating no exposure] on Section D of the Deployment Risk and Resiliency Inventory-2; Vogt et al., 2012), English speaking, 18 years of age or older, able to comply with instructions to complete study tasks, and able to provide informed consent. Exclusion criteria were: a history of moderate or severe TBI; any penetrating head injury; non-deployment TBI with any loss of consciousness; presence of neurologic disorder (e.g., stroke, seizure), severe mental illness, dementia, current substance use disorder, or psychotic symptoms. Psychiatric diagnosis for exclusion criteria were evaluated using the Structured Clinical Interview for DSM-IV Disorders (SCID; First et al., 1996).
Participants were recruited through targeted mailings, brochures and flyers posted in the medical center, and by telephone calls if they had participated in previous studies and agreed to be contacted about future research opportunities. Participants were initially screened by telephone and then completed an in-person assessment visit. All participants who completed the in-person assessment visit were reimbursed for their time and effort. Participants were excluded from the present analyses if they failed two or more performance validity measure/s according to indicated manual or published cut-off scores for the b Test, (Boone et al., 2002) Trail Making Test B (TMT-B; Busse & Whiteside, 2012), TMT A+B (Busse & Whiteside, 2012), and the Medical Symptom Validity Test (MSVT; Green, 2004). Participants were additionally excluded from present analyses if they sustained a deployment TBI more than 16 years prior to the assessment visit to reduce the potential for recall bias and memory extinction (n = 7). The final sample size for analyses was N = 254.
All participants provided informed consent prior to participation. All study procedures were approved by the W. G. (Bill) Hefner VA Healthcare System Institutional Review Board.
Measures
Cognitive Battery.
Participants completed the Wechsler Adult Intelligence Scale, fourth edition (WAIS-IV; Wechsler, 2008), Trail Making Test (TMT; Reitan & Wolfson, 1985) forms A and B, Medical Symptom Validity Test (MSVT; Green, 2004), and the b Test (Boone et al., 2002). All cognitive outcome T-scores are corrected for sex, age, race, and education, M = 50, SD = 10. WAIS-IV T-scores are derived from the WAIS-IV Advanced Clinical Systems (ACS) demographically adjusted norms, and TMT T-scores were derived from Heaton norms (Heaton et al., 2004). WAIS-IV index T-scores were used in analyses: Perceptual Reasoning Index (PRI), Verbal Comprehension Index (VCI), Working Memory Index (WMI), and Processing Speed Index (PSI).
Interviews.
The Clinician Administered PTSD Scale for DSM-5 (CAPS-5) determined the presence of current PTSD diagnosis and current severity. Lifetime TBI history was evaluated using the Mid-Atlantic MIRECC Assessment of TBI (MMA-TBI); Rowland, Martindale, Shura et al., 2020]. Deployment mild TBI was chosen as the independent variable due to previous literature that indicates deployment TBI is more likely to affect psychological outcomes in Veterans than mild TBI acquired outside of a deployment environment (Martindale, Epstein, et al., 2018).
Lifetime blast events were evaluated using pressure ratings from the Salisbury Blast Interview (SBI; Rowland, Martindale, Spengler et al., 2020). On the SBI, pressure ratings are provided on a behaviorally-anchored scale of 0 (no pressure/no blast exposure) to 5 (resulted in greater than minor injury) and have been shown to be a good indicator of severity, based on analyses predicting an outcome of TBI (psychometric manuscript in submission). All interviews were conducted by masters-level psychology technicians or doctoral-level psychologists who were trained to VA standards and additionally observed and approved to administer the clinical interviews by the Principal Investigators.
Data Analysis
Analyses were conducted in SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC). Independent samples t-tests were used to evaluate mean differences within condition groups (PTSD and deployment mild TBI, present or absent). Hierarchical linear regression evaluated influence of maximum blast pressure on cognitive outcomes beyond current PTSD severity and deployment TBI status. PTSD severity was represented by the CAPS-5 current severity score. Deployment mild TBI was coded dichotomously, and maximum experienced blast pressure was represented continuously. Confidence intervals are 95%. Moderation analyses were conducted using the PROCESS 3.1 macro for SAS to evaluate interaction effects on cognitive outcomes (Hayes, 2018). Johnson-Neyman analysis was used to probe significant interactions. False discovery rate (FDR; Benjamini & Hochberg, 1995) at α = .05 was used to correct for multiple comparisons across 6 hierarchical regression analyses of cognitive outcomes.
No results were affected by replacing the CAPS-5 severity score with dichotomous CAPS-5 PTSD diagnosis, replacing deployment mild TBI (dichotomous) with the number of deployment mild TBIs (continuous), or adjusting for non-deployment TBI in the models. Psychiatric conditions assessed using the SCID were not correlated with cognitive outcomes and were therefore not included as covariates in analyses. Other potentially confounding variables had negligible correlations with outcomes including years of combat and number of lifetime mild TBI. A time covariate was created and used across all analyses. For individuals with a history of deployment TBI, this variable reflected time since injury, and for those with no history of deployment TBI, this variable reflected time since deployment. Analyses indicated no significant differences between time since injury and time since deployment for the deployment TBI group, t(109) = 1.60, p = .113, therefore time since deployment was determined to be an appropriate proxy for individuals with no deployment TBI history to allow for adjustment of time effects across groups.
Results
Sample characteristics are presented in Table 1. Eligible participants were 254 (86.22% male) veterans between the ages of 23–71, M = 41.16, SD = 10.13, with 12–22 years of education, M = 15.13, SD = 2.17. Deployment mild TBI history was present in 45.67% of the sample. Over one-third (34.25%) had a current PTSD diagnosis with total sample CAPS-5 severity scores ranging from 0–60, M = 17.76, SD = 15.00. Blast event pressure ratings ranged from 0–5, M = 1.85, SD = 1.53. Cognitive T-scores were largely within the normal range, with the highest rate of impairment for the overall sample on TMT-A (4.72%) (see Table 2). There were no significant differences on demographic variables by condition.
Table 1.
Sample Characteristics (N = 254)
| Demographic | M (SD), range or n (%) |
|---|---|
| Age | 41.16 (10.13), 23–71 |
| Education (years) | 15.13 (2.17), 12–22 |
| Number of Deployments | 2.53 (2.15), 1–20 |
| Years since Deployment | 9.71 (3.53), 0.91–15.87 |
| Sex | |
| Male | 219 (86.22%) |
| Female | 35 (13.78%) |
| Race* | |
| White/Caucasian | 148 (58.27%) |
| Black/African American | 96 (37.80%) |
| Other | 16 (6.30%) |
| Branch of Service | |
| Air Force | 26 (10.24%) |
| Army | 182 (71.65%) |
| Marine Corps | 32 (12.60%) |
| Navy | 14 (5.51%) |
| Current PTSD Diagnosis | 87 (34.25%) |
| Current PTSD Symptom Severity | 17.56 (15.00), 0–60 |
| Deployment TBI | 116 (45.67%) |
| Years since Deployment TBI | 10.28 (3.23), 1.06–15.28 |
| Non-Deployment TBI | 78 (30.71%) |
| Blast Pressure Rating | 1.85 (1.53), 0–5 |
Note.
= indicates categories are not mutually exclusive. Number of deployments includes contract deployments to warzones in support of OEF/OIF/OND. Race was collapsed due to small numbers in categories; “Other” includes Native America/Alaska Native, Asian, Other, and Refused. Branch of Service indicates most recent branch of service. Branches of service have been collapsed to include Reserve and Guard units. PTSD = posttraumatic stress disorder. CAPS-5 = Clinician Administered PTSD Scale, 5th Edition. TBI = traumatic brain injury. Blast Pressure Rating is measured with the Salisbury Blast Interview (0–5).
Table 2.
Sample Characteristics of Cognitive Measures (N = 254)
| Cognitive Measure | M | SD | range | n (%) Impaired |
|---|---|---|---|---|
| WAIS-IV | ||||
| Perceptual Reasoning Index (PRI) | 49.58 | 10.12 | 25–82 | 6 (2.36%) |
| Verbal Comprehension Index (VCI) | 48.50 | 10.00 | 23–86 | 7 (2.76%) |
| Working Memory Index (WMI) | 45.94 | 9.75 | 26–76 | 3 (1.18%) |
| Processing Speed Index (PSI) | 49.76 | 9.59 | 22–73 | 4 (1.57%) |
| Trail Making Test A (TMT-A) | 48.24 | 10.80 | 19–86 | 12 (4.72%) |
| Trail Making Test B (TMT-B) | 49.52 | 9.67 | 23–81 | 2 (0.79%) |
Note. All reported numbers are T-scores. Impairment is defined as a T score of < 30.
Descriptive information about cognitive tests and impairment rates in the sample are reported in Table 2. The only variable to have significant differences on cognitive variables was deployment mild TBI for WAIS-IV VCI, t(252) = 2.11, p = .036, d = .13; WAIS-IV WMI, t(252) = 2.43, p = .016, d = .30; WAIS-IV PSI, t(252) = 2.02, p = .044, d = .25; TMT-A t(252) = 3.95, p < .001, d = .50, and; TMT-B, t(252) = 2.88, p = .004, d = .37.
Hierarchical Regression
Correlations between independent variables and cognitive outcomes are reported in Table 3. Hierarchical regression models predicting cognitive outcomes are reported in Table 4. Variance inflation factor (VIF) for all models was between 1.01 and 1.33, indicating low collinearity. Correlations between independent variables were as follows: PTSD symptom severity and deployment mild TBI: r = .17, p = .010; PTSD symptom severity and maximum blast pressure: r = .29, p < .001, and; deployment mild TBI and maximum blast pressure: r = .45, p < .001.
Table 3.
Correlations Between Independent Variables and Cognitive Outcomes (N = 254)
| PTSD Severity |
Deployment TBI |
Blast Pressure |
||||
|---|---|---|---|---|---|---|
| Cognitive Measure | r | p | r | p | r | p |
| WAIS-IV | ||||||
| Perceptual Reasoning Index (PRI) | −.01 | .936 | −.06 | .321 | −.06 | .321 |
| Verbal Comprehension Index (VCI) | −.03 | .596 | −.13 | .036 | .02 | .765 |
| Working Memory Index (WMI) | −.09 | .161 | −.15 | .016 | −.08 | .183 |
| Processing Speed Index (PSI) | −.11 | .096 | −.13 | .044 | −.06 | .385 |
| Trail Making Test A (TMT-A) | −.16 | .014 | −.24 | < .001 | −.10 | .129 |
| Trail Making Test B (TMT-B) | −.09 | .145 | −.18 | .004 | −.13 | .041 |
Note. All cognitive measures are represented by demographically corrected T scores. PTSD Severity = CAPS-5 severity score (0–78). TBI = deployment TBI (0 = absent, 1 = present). Blast Pressure = maximum pressure experienced (0–5). r = Pearson’s correlation.
Table 4.
Hierarchical Regression Outcomes (N = 254)
| Omnibus Model |
Parameter Estimates |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | R 2 | p | ΔR2 sig | B | SEB | t | p | LLCI | ULCI | |
| Perceptual Reasoning Index (PRI) | ||||||||||
| Model 1 | PTSD Severity | .005 | .522 | — | 0.00 | 0.04 | −0.01 | .995 | −0.08 | 0.08 |
| Model 2 | PTSD Severity | .009 | .544 | .362 | 0.01 | 0.04 | 0.14 | .887 | −0.08 | 0.09 |
| Deployment TBI | −1.19 | 1.30 | −0.92 | .360 | −3.75 | 1.37 | ||||
| Model 3 | PTSD Severity | .010 | .634 | .776 | 0.01 | 0.04 | 0.29 | .769 | −0.07 | 0.10 |
| Deployment TBI | −0.79 | 1.44 | −0.55 | .583 | −3.62 | 2.04 | ||||
| Blast Pressure | −0.31 | 0.48 | −0.65 | .516 | −1.26 | 0.64 | ||||
| Verbal Comprehension Index (VCI) | ||||||||||
| Model 1 | PTSD Severity | .012 | .231 | — | −0.02 | 0.04 | −0.42 | .671 | −0.10 | 0.06 |
| Model 2 | PTSD Severity | .026 | .085 | .028 | 0.00 | 0.04 | −0.11 | .912 | −0.09 | 0.08 |
| Deployment TBI | −2.45 | 1.27 | −1.93 | .055 | −4.95 | 0.06 | ||||
| Model 3 | PTSD Severity | .035 | .066 | .098 | −0.02 | 0.04 | −0.47 | .642 | −0.11 | 0.07 |
| Deployment TBI | −3.34 | 1.40 | −2.38 | .018 | −6.11 | −0.58 | ||||
| Blast Pressure | 0.70 | 0.47 | 1.49 | .138 | −0.23 | 1.63 | ||||
| Working Memory Index (WMI) | ||||||||||
| Model 1 | PTSD Severity | .013 | .184 | — | −0.05 | 0.04 | −1.33 | .186 | −0.13 | 0.03 |
| Model 2 | PTSD Severity | .031 | .048 | .010 | −0.04 | 0.04 | −0.97 | .331 | −0.12 | 0.04 |
| Deployment TBI | −2.64 | 1.24 | −2.14 | .034 | −5.08 | −0.21 | ||||
| Model 3 | PTSD Severity | .031 | .095 | .999 | −0.04 | 0.04 | −0.93 | .351 | −0.12 | 0.04 |
| Deployment TBI | −2.62 | 1.37 | −1.91 | .057 | −5.32 | 0.08 | ||||
| Blast Pressure | −0.02 | 0.46 | −0.04 | .969 | −0.92 | 0.89 | ||||
| Processing Speed Index (PSI) | ||||||||||
| Model 1 | PTSD Severity | .019 | .086 | — | −0.06 | 0.04 | −1.57 | .117 | −0.14 | 0.02 |
| Model 2 | PTSD Severity | .030 | .053 | .059 | −0.05 | 0.04 | −1.29 | .198 | −0.13 | 0.03 |
| Deployment TBI | −2.03 | 1.22 | −1.67 | .096 | −4.43 | 0.37 | ||||
| Model 3 | PTSD Severity | .031 | .100 | .772 | −0.06 | 0.04 | −1.34 | .182 | −0.14 | 0.03 |
| Deployment TBI | −2.25 | 1.35 | −1.67 | .097 | −4.90 | 0.41 | ||||
| Blast Pressure | 0.17 | 0.45 | 0.37 | .710 | −0.72 | 1.06 | ||||
| Trail Making Test A (TMT-A) | ||||||||||
| Model 1 | PTSD Severity | .024 | .047 | — | −0.11 | 0.04 | −2.49 | .014 | −0.20 | −0.02 |
| Model 2 | PTSD Severity | .073 | < .001 | < .001 | −0.09 | 0.04 | −1.93 | .055 | −0.17 | 0.00 |
| Deployment TBI | −4.84 | 1.34 | −3.61 | .000 | −7.48 | −2.20 | ||||
| Model 3 | PTSD Severity | .075 | .001 | .582 | −0.09 | 0.05 | −2.05 | .042 | −0.18 | 0.00 |
| Deployment TBI | −5.31 | 1.48 | −3.58 | .000 | −8.23 | −2.39 | ||||
| Blast Pressure | 0.36 | 0.50 | 0.73 | .463 | −0.61 | 1.34 | ||||
| Trail Making Test B (TMT-B) | ||||||||||
| Model 1 | PTSD Severity | .009 | .334 | — | −0.06 | 0.04 | −1.44 | .152 | −0.14 | 0.02 |
| Model 2 | PTSD Severity | .036 | .028 | .001 | −0.04 | 0.04 | −1.01 | .315 | −0.12 | 0.04 |
| Deployment TBI | −3.24 | 1.22 | −2.65 | .009 | −5.65 | −0.83 | ||||
| Model 3 | PTSD Severity | .037 | .050 | .771 | −0.03 | 0.04 | −0.82 | .410 | −0.12 | 0.05 |
| Deployment TBI | −2.87 | 1.36 | −2.12 | .035 | −5.54 | −0.20 | ||||
| Blast Pressure | −0.29 | 0.45 | −0.64 | .526 | −1.18 | 0.61 | ||||
Two of the six hierarchical regressions tested had significant models predicting cognitive outcomes after FDR correction for multiple comparisons. To determine significance of outcomes, the omnibus model was first evaluated to establish if the relationship between parameter estimates and the cognitive measures were significant. Second, if ΔR2 was not significant after including a variable, the omnibus model was not considered an improvement upon the prior model. Following that, parameter estimates were evaluated for individual variable effects. Results for TMT-A reveal that both PTSD symptom severity and deployment TBI were independently significantly associated with poorer performance; however, blast pressure was not associated with outcomes beyond these variables, as indicated by a non-significant ΔR2. The initial model predicting TMT-B from PTSD symptom severity alone was not significant. The model was significant after including deployment TBI, and deployment TBI was a significant predictor of poorer performance. The model remained significant after including blast pressure; however, no individual variable was significantly associated with outcomes. None of the WAIS-IV indices survived correction for multiple comparisons.
Moderation
Interactions between independent variables were evaluated for all outcomes. Only one, two-way interaction was significant across outcomes adjusting for time since deployment. Maximum blast pressure significantly interacted with deployment TBI to predict TMT-A scores, ΔR2 = .02, F(1, 249) = 4.31, p = .039, B = −2.01, CI[−3.92, −0.10]. When deployment TBI was present, increasing blast pressure was associated with increasingly poorer scores on TMT-A. Johnson-Neyman analysis demonstrated that this effect was significant at pressure ratings ≥ 1 (specifically, max pressure rating of 0.92; see Figure 1). There were no significant interaction effects between PTSD symptom severity and deployment TBI, p = .824, or PTSD symptom severity and blast pressure, p = .679 for TMT-A.
Figure 1.
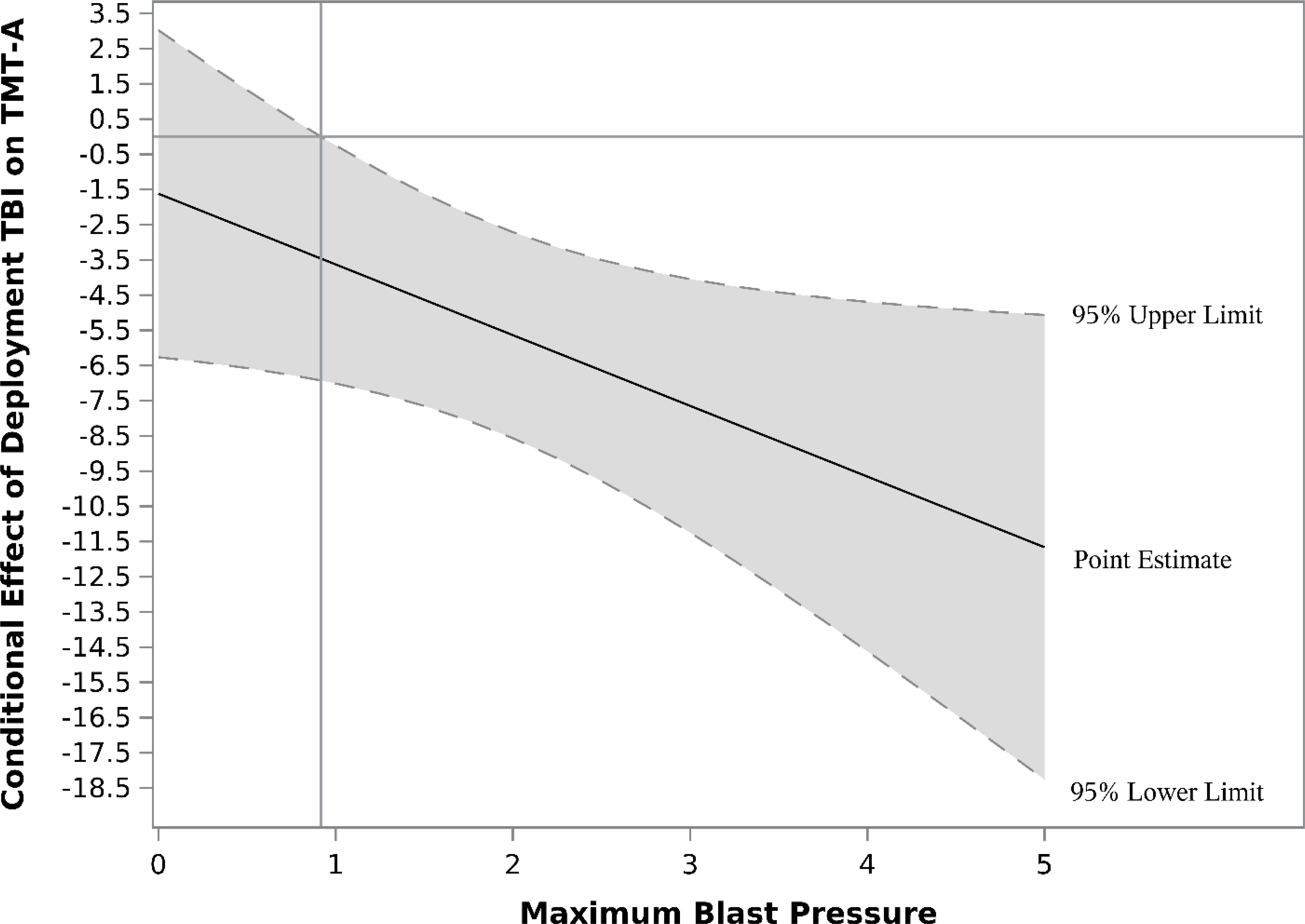
Johnson-Neyman plot illustrating conditional effects of deployment TBI on Trail Making Test A (TMT-A) T-scores. Vertical reference line indicates the point at the moderator, maximum blast pressure, which the conditional effects of deployment TBI is significant (SBI pressure rating of ≥ 1). Horizontal line references an effect of zero (i.e., no conditional effect). Shaded areas represent the 95% confidence interval for effects.
Discussion
The current results demonstrate an influencing effect of deployment TBI on cognitive performance, even beyond the effects of PTSD. Though we did not find an independent effect of blast pressure beyond PTSD and TBI, there was evidence of increasing experience of blast pressure exacerbating outcomes on an isolated cognitive weakness in individuals with TBI.
Research evaluating the effects of blast exposure is relatively new, and most studies to date have evaluated blast exposure in the context of TBI (Nelson et al., 2015). The present study aimed to address this by evaluating the effect of blast exposure on neurocognitive functioning beyond effects of PTSD and mild TBI. Though blast injuries are qualitatively distinct from non-blast injury research examining neurocognitive outcomes of blast-related mild TBI has produced mixed findings (Taber et al., 2006). The present study evaluated blast exposure differently from previous studies. We use an interview specifically designed to evaluate the severity of a blast exposure which allows for a finer evaluation beyond distance from a blast.
Some studies show that neurocognitive outcomes do not differ between blast and non-blast TBI groups (Belanger et al., 2009; Cooper et al., 2012; Luethcke et al., 2011; Nelson et al., 2015; Verfaellie et al., 2013), suggesting that blast may not be categorically different from other TBI mechanisms with regard to cognitive sequelae. Other studies point to subtle neurocognitive differences associated with blast exposure in terms of memory (Grande et al., 2018; Storzbach et al., 2015), reaction time (Haran et al., 2019), executive function (Storzbach et al., 2015), and selective auditory attention (Bressler et al., 2017). Notably, these studies differ as to whether they covary for PTSD and/or TBI. Blast exposure has also been linked to an increased risk of developing PTSD (Nelson et al., 2012), and it has been suggested that neurocognitive effects noted in blast-exposed veterans may be better explained by PTSD symptom severity (Storzbach et al., 2015; Verfaellie et al., 2014). Our results partially support this finding, as blast exposure in our sample was not reliably associated with lower cognitive functioning after adjusting for effects of PTSD and deployment TBI history. Despite an absence of main effects on cognitive outcomes, blast pressure severity significantly moderated the relationship between deployment TBI and a speeded test of attention, such that if the experience of a pressure gradient was reported (SBI pressure rating of ≥ 1), the negative effect of deployment TBI on performance was exacerbated. This relationship amplified linearly as reported blast severity increased. These results further illustrate the complexity in the relationships between PTSD, deployment TBI, and blast exposure, which warrants further exploration in future research.
Our results are congruent with research that suggests limited or no lasting effects after 3 to 12 months following injury (Carroll et al., 2014; Cassidy et al., 2014), because no significant effects of deployment TBI were found on most tasks including WAIS-IV verbal comprehension, working memory, processing speed, and perceptual reasoning indices. However, significant effects of deployment TBI were found on some measures of simple and divided attention, as well as processing speed (TMT parts A & B), which is also consistent with published studies indicating that isolated, subtle cognitive deficits following mild TBI may persist over time, including reaction time (Haran et al., 2013), processing speed (Kontos et al., 2013), and attention (Barker-Collo et al., 2015; Bigler et al., 2013; Rohling et al., 2011; Vanderploeg et al., 2005). These findings are notable because the most recent TBI reported was typically over 10 years prior to testing. Recent work has begun to identify a potential link between mild TBI and problems developing later in life, including neurodegenerative conditions and neurocognitive disorders (Gardner & Yaffe, 2015). It is possible that the current results represent early evidence of the development of these conditions rather than effects associated with the acute experience of concussion. However, it is important to reiterate that effect sizes for significant findings were small, and most participants performed within the average (not impaired) range. Results should be interpreted within this context. Though deployment TBI may be associated with some subtle lasting effects on measures of attention and processing speed, these are not likely to present as clinically significant impairments.
The current findings also demonstrate an effect of deployment TBI on cognitive function independent from, and beyond the effects of PTSD. Previous studies have suggested that potential effects of TBI on cognitive function are better accounted for by psychiatric diagnoses or other symptoms (Martindale, Morissette, et al., 2017; Nelson et al., 2012). One possible explanation for these differences is that PTSD severity is often represented by nonspecific instruments in research studies, such as the PTSD checklist (PCL; Bovin et al., 2016). These instruments likely capture complaints beyond those directly related to a specific traumatic event and may be better conceptualized as reflecting the general distress level of a participant (Miskey & Shura, 2017; Porter et al., 2018). The CAPS-5 is administered by a trained interviewer and may be a more objective measure of PTSD symptom severity. Additionally, previous findings have examined participants much closer to their injury and traumatic events, many within five years. The rate of current PTSD diagnosis in the current study was 34.3%, which is congruent with estimates from prior studies in this population by our group, 28.8–43.8%, that have assessed diagnoses and symptoms long after exposure (Martindale, Epstein, et al., 2018; Martindale, Farrell-Carnahan, et al., 2017). In the current sample, the rate of lifetime PTSD diagnosis was 62.6%, suggesting many participants have recovered from PTSD.
This combination of recovery from PTSD and increased time since TBI raises the possibility of a potential shift in trajectories in the factors influencing cognitive function in veterans and service members of the wars in Iraq and Afghanistan. It is possible that through treatment and the passage of time, the prevalence of PTSD in this population is decreasing, and the individuals who still suffer from PTSD have adopted effective coping strategies to address the effects on cognitive function. It is also possible that the initial stages of long-term consequences of TBI may also be manifesting. This question requires longitudinal data for a true evaluation, and the current results are applicable only within combat-exposed veterans and service members of the wars in Iraq and Afghanistan. Future work will be required to understand the potentially evolving roles of PTSD and TBI in cognitive functioning for this cohort.
This study has limitations that should be considered. Because this is cross-sectional data, it is not possible to make causal inferences. Duration of time since injury and traumatic events (i.e., DSM-5 Criterion A for PTSD) can also affect recall, as recall bias has been shown to play a role in the accuracy of self-reported functioning before and after a TBI (Lange et al., 2010). However, this is a weakness across evaluations of TBI, PTSD, and psychiatric research more generally. The present sample was comprised of veterans who were exposed to combat, and therefore these results may not generalize to the greater military population or civilian populations. We did not collect physical health information about this sample, so evaluating medical effects on outcomes was not possible. Finally, T-scores of significant outcomes were largely within the normal range, and effects were small. Though there may be decrements associated with TBI and/or blast exposure, outcomes should be interpreted within this context.
Conclusions
The current results identified an interaction between blast severity and deployment TBI for a single outcome. Though not robust across tests, this interaction occurs for a simple test of attention and processing speed. Growing literature suggests that blast exposure is associated with changes in brain structure, particularly in white matter tracts (Davenport et al., 2012; Martindale, Rowland, et al., 2018; Robinson et al., 2017; Taber et al., 2015). Such changes would be most likely to manifest on tests requiring speeded responses like TMT-A. However, the same interaction was not seen for a more difficult version of the same test (TMT-B), or the more complex tests making up the WAIS-IV PSI. This finding requires replication and further examination before implications can be fully understood.
Key Points.
Question:
Does experiencing a blast/explosion during deployment affect cognitive functioning beyond other mental health factors?
Findings:
Exposure to a blast/explosion was not a primary factor in cognitive outcomes, but exacerbated negative effects of other conditions on cognitive outcomes.
Importance:
Exposure to blasts/explosions during deployment may contribute to lower cognitive functioning when other conditions are present.
Next Steps:
Identifying specific characteristics of blast exposure (e.g., number of exposures, distance) that are predictive of functional outcomes will be important in elucidating how exposure to blasts affects veterans long-term.
Acknowledgements
This work was supported by grant funding from Department of Defense, Chronic Effects of Neurotrauma Consortium (CENC) Award W81XWH-13-2-0095 and Department of Veterans Affairs CENC Award I01 CX001135. This work was also supported by resources of the W. G. (Bill) Hefner VA Healthcare System, Mid-Atlantic Mental Illness Research Education and Clinical Center (MIRECC), and Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness, Research, and Treatment (MIRT). The authors report no conflicts of interest.
The authors would like to thank the veterans and service members who contributed their time and effort to this research. We would also like to thank Mary Peoples, David J. Curry, MSW, Christine Sortino, MS, and Alana M. Higgins, MA, for their contributions to this project.
Footnotes
Disclaimer
The views, opinions and/or findings contained in this article are those of the authors and should not be construed as an official Veterans Affairs or Department of Defense position, policy or decision, unless so designated by other official documentation.
References
- Barker-Collo S, Jones K, Theadom A, Starkey N, Dowell A, McPherson K, Ameratunga S, Dudley M, Te Ao B, & Feigin V (2015). Neuropsychological outcome and its correlates in the first year after adult mild traumatic brain injury: A population-based New Zealand study. Brain Injury, 29(13–14), 1604–1616. 10.3109/02699052.2015.1075143 [DOI] [PubMed] [Google Scholar]
- Belanger HG, Kretzmer T, Yoash-Gantz R, Pickett T, & Tupler LA (2009). Cognitive sequelae of blast-related versus other mechanisms of brain trauma. Journal of the International Neuropsychological Society, 15(1), 1–8. 10.1017/s1355617708090036 [DOI] [PubMed] [Google Scholar]
- Belanger HG, Spiegel E, & Vanderploeg RD (2010). Neuropsychological performance following a history of multiple self-reported concussions: A meta-analysis. Journal of the International Neuropsychological Society, 16(2), 262–267. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B, 57(1), 289–300. [Google Scholar]
- Bigler ED, Farrer TJ, Pertab JL, James K, Petrie JA, & Hedges DW (2013). Reaffirmed limitations of meta-analytic methods in the study of mild traumatic brain injury: a response to Rohling et al. Clin Neuropsychol, 27(2), 176–214. 10.1080/13854046.2012.693950 [DOI] [PubMed] [Google Scholar]
- Boone KB, Lu P, & Herzberg D (2002). The b Test. Western Psychological Services. [Google Scholar]
- Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, & Keane TM (2016). Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in veterans. Psychological Assessment, 28(11), 1379–1391. 10.1037/pas0000254 [DOI] [PubMed] [Google Scholar]
- Bressler S, Goldberg H, & Shinn-Cunningham B (2017). Sensory coding and cognitive processing of sound in Veterans with blast exposure. Hear Res, 349, 98–110. 10.1016/j.heares.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR, Kleiner JS, Vasterling JJ, & Field AP (2007). Memory for emotionally neutral information in posttraumatic stress disorder: A meta-analytic investigation. Journal of Abnormal Psychology, 116(3), 448–463. 10.1037/0021-843x.116.3.448 [DOI] [PubMed] [Google Scholar]
- Busse M, & Whiteside D (2012). Detecting suboptimal cognitive effort: classification accuracy of the Conner’s Continuous Performance Test-II, Brief Test Of Attention, and Trail Making Test. The Clinical Neuropsychologist, 26(4), 675–687. 10.1080/13854046.2012.679623 [DOI] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, Cancelliere C, Cote P, Hincapie CA, Kristman VL, Holm LW, Borg J, Nygren-de Boussard C, & Hartvigsen J (2014). Systematic review of the prognosis after mild traumatic brain injury in adults: cognitive, psychiatric, and mortality outcomes: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Archives of Physical Medicine and Rehabiliation, 95(3 Suppl), S152–173. 10.1016/j.apmr.2013.08.300 [DOI] [PubMed] [Google Scholar]
- Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, Paniak C, & Pepin M (2004). Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med(43 Suppl), 84–105. [DOI] [PubMed] [Google Scholar]
- Cassidy JD, Cancelliere C, Carroll LJ, Cote P, Hincapie CA, Holm LW, Hartvigsen J, Donovan J, Nygren-de Boussard C, Kristman VL, & Borg J (2014). Systematic review of self-reported prognosis in adults after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Archives of Physical Medicine and Rehabiliation, 95(3 Suppl), S132–151. 10.1016/j.apmr.2013.08.299 [DOI] [PubMed] [Google Scholar]
- Cifu DX, Taylor BC, Carne WF, Bidelspach D, Sayer NA, Scholten J, & Campbell EH (2013). Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND Veterans. J Rehabil Res Dev, 50(9), 1169–1176. 10.1682/jrrd.2013.01.0006 [DOI] [PubMed] [Google Scholar]
- Cooper DB, Chau PM, Armistead-Jehle P, Vanderploeg RD, & Bowles AO (2012). Relationship between mechanism of injury and neurocognitive functioning in OEF/OIF service members with mild traumatic brain injuries. Mil Med, 177(10), 1157–1160. [DOI] [PubMed] [Google Scholar]
- Davenport ND, Lim KO, Armstrong M, & Sponheim S (2012). Diffuse and spatially variable white matter disruptions are associated with blast-related mild traumatic brain injury. NeuroImage, 59, 2017–2024. [DOI] [PubMed] [Google Scholar]
- Davenport ND, Lim KO, & Sponheim SR (2015). White matter abnormalities associated with military PTSD in the context of blast TBI. Human Brain Mapping, 36(3), 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defense and Veterans Brain Injury Center (DVBIC). (2018). Total DoD TBI Worldwide Numbers. http://dvbic.dcoe.mil/files/tbi-numbers/worldwide-totals-2000-2017_feb-14-2018_v1.0_2018-03-08.pdf
- Dikmen SS, Corrigan JD, Levin HS, Machamer J, Stiers W, & Weisskopf MG (2009). Cognitive outcome following traumatic brain injury. Journal of Head Trauma Rehabilitation, 24(6), 430–438. 10.1097/HTR.0b013e3181c133e9 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (1996). Structured Clinical Interview for the DSM-IV-TR Axis I Disorders. American Psychiatric Press, Inc. [Google Scholar]
- Gardner RC, & Yaffe K (2015). Epidemiology of mild traumatic brain injury and neurodegenerative disease. Molecular and Cellular Neuroscience, 66, 75–80. 10.1016/j.mcn.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande LJ, Robinson ME, Radigan LJ, Levin LK, Fortier CB, Milberg WP, & McGlinchey RE (2018). Verbal Memory Deficits in OEF/OIF/OND Veterans Exposed to Blasts at Close Range. Journal of the International Neuropsychological Society, 24(5), 466–475. 10.1017/s1355617717001242 [DOI] [PubMed] [Google Scholar]
- Green P (2004). Test manual for the Medical Symptom Validity Test. Green’s Publishing. [Google Scholar]
- Greer N, Sayer N, Koeller E, Velasquez T, & Wilt TJ (2018). Outcomes Associated With Blast Versus Nonblast-Related Traumatic Brain Injury in US Military Service Members and Veterans: A Systematic Review. Journal of Head Trauma Rehabilitation, 33(2), E16–E29. 10.1097/HTR.0000000000000304 [DOI] [PubMed] [Google Scholar]
- Haran FJ, Alphonso AL, Creason A, Campbell JS, Johnson D, Young E, & Tsao JW (2013). Analysis of post-deployment cognitive performance and symptom recovery in U.S. Marines. PLoS One, 8(11), 79595. 10.1371/journal.pone.0079595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haran FJ, Handy JD, Servatius RJ, Rhea CK, & Tsao JW (2019). Acute neurocognitive deficits in active duty service members following subconcussive blast exposure. Appl Neuropsychol Adult, 1–13. 10.1080/23279095.2019.1630627 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2018). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach (2nd ed.). Guilford Press. [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, & Grant I (2004). Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Psychological Assessment Resources. [Google Scholar]
- Kontos AP, Kotwal RS, Elbin RJ, Lutz RH, Forsten RD, Benson PJ, & Guskiewicz KM (2013). Residual effects of combat-related mild traumatic brain injury. Journal of Neurotrauma, 30(8), 680–686. 10.1089/neu.2012.2506 [DOI] [PubMed] [Google Scholar]
- Lange RT, Iverson GL, & Rose A (2010). Post-concussion symptom reporting and the “good-old-days” bias following mild traumatic brain injury. Archives of Clinical Neuropsychology, 25(5), 442–450. [DOI] [PubMed] [Google Scholar]
- Luethcke CA, Bryan CJ, Morrow CE, & Isler WC (2011). Comparison of Concussive Symptoms, Cognitive Performance, and Psychological Symptoms Between Acute Blast-Versus Nonblast-Induced Mild Traumatic Brain Injury. Journal of the International Neuropsychological Society, 17(1), 36–45. 10.1017/S1355617710001207 [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Barber J, Andre J, Evans N, Panks C, Sun S, Zalewski K, Elizabeth Sanders R, & Temkin N (2017). 5-Year imaging sequelae of concussive blast injury and relation to early clinical outcome. Neuroimage Clinical, 14, 371–378. 10.1016/j.nicl.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Barber J, Jordan M, Johnson AM, Dikmen S, Fann JR, & Temkin N (2017). Early Clinical Predictors of 5-Year Outcome After Concussive Blast Traumatic Brain Injury. JAMA Neurol, 74(7), 821–829. 10.1001/jamaneurol.2017.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale SL, Epstein EL, Taber KH, & Rowland JA (2018). Behavioral and Health Outcomes Associated With Deployment and Nondeployment Acquisition of Traumatic Brain Injury in Iraq and Afghanistan Veterans. Arch Phys Med Rehabil, 99(12), 2485–2495. 10.1016/j.apmr.2018.04.029 [DOI] [PubMed] [Google Scholar]
- Martindale SL, Farrell-Carnahan LV, Ulmer CS, Kimbrel NA, McDonald SD, Workgroup M-AMR, & Rowland JA (2017). Sleep quality in returning veterans: The influence of mild traumatic brain injury. Rehabilitation Psychology, 62(4), 563–570. 10.1037/rep0000159 [DOI] [PubMed] [Google Scholar]
- Martindale SL, Morissette SB, Rowland JA, & Dolan SL (2017). Sleep quality affects cognitive functioning in returning combat veterans beyond combat exposure, PTSD, and mTBI history. Neuropsychology, 31(1), 93–104. [DOI] [PubMed] [Google Scholar]
- Martindale SL, Rowland JA, Shura RD, & Taber KH (2018). Longitudinal changes in neuroimaging and neuropsychiatric status of post-deployment veterans: A CENC pilot study. Brain Injury, 32(10), 1208–1216. 10.1080/02699052.2018.1492741 [DOI] [PubMed] [Google Scholar]
- Miskey HM, & Shura RD (2017). Association of Self-Report Measures with PTSD and Depression in Veterans. Current Treatment Options in Psychiatry, 4(3), 254–261. 10.1007/s40501-017-0120-2 [DOI] [Google Scholar]
- Nelson NW, Davenport ND, Sponheim SR, & Anderson C (2015). Blast-Related Mild Traumatic Brain Injury: Neuropsychological Evaluation and Findings. In Kobeissy F (Ed.), Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- Nelson NW, Hoelzle JB, Doane BM, McGuire KA, & Ferrier-Auerbach AG (2012). Neuropsychological outcomes of U.S. veterans with report of remote blast-related concussion and current psychopathology. Journal of the International Neuropsychological Society, 18, 845–855. [DOI] [PubMed] [Google Scholar]
- Owens BD, Kragh JF Jr., Wenke JC, Macaitis J, Wade CE, & Holcomb JB (2008). Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. J Trauma, 64(2), 295–299. 10.1097/TA.0b013e318163b875 [DOI] [PubMed] [Google Scholar]
- Porter KE, Stein MB, Martis B, Avallone KM, McSweeney LB, Smith ER, Simon NM, Gargan S, Liberzon I, Hoge CW, & Rauch SAM (2018). Postconcussive symptoms (PCS) following combat-related traumatic brain injury (TBI) in Veterans with posttraumatic stress disorder (PTSD): Influence of TBI, PTSD, and depression on symptoms measured by the Neurobehavioral Symptom Inventory (NSI). J Psychiatr Res, 102, 8–13. 10.1016/j.jpsychires.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Reitan RM, & Wolfson D (1985). The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Neuropsychology Press. [Google Scholar]
- Robinson ME, Clark DC, Milberg WP, McGlinchey RE, & Salat DH (2017). Characterization of Differences in Functional Connectivity Associated with Close-Range Blast Exposure. Journal of Neurotrauma, 34(S1), 53–61. 10.1089/neu.2016.4709 [DOI] [PubMed] [Google Scholar]
- Rohling ML, Binder LM, Demakis GJ, Larrabee GJ, Ploetz DM, & Langhinrichsen-Rohling J (2011). A meta-analysis of neuropsychological outcome after mild traumatic brain injury: re-analyses and reconsiderations of Binder et al. (1997), Frencham et al. (2005), and Pertab et al. (2009). The Clinical Neuropsychologist, 25(4), 608–623. 10.1080/13854046.2011.565076 [DOI] [PubMed] [Google Scholar]
- Rowland JA, Martindale SL, Shura RD, Miskey H, Bateman J, Epstein E, Stern M, Hurley R, & Taber KH (2020). Initial Validation of the Mid-Atlantic MIRECC Assessment of Traumatic Brain Injury. Journal of Neurotrauma. 10.1089/neu.2019.6972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland JA, Martindale SL, Spengler KM, Shura RD, & Taber KH (2020). Sequelae of Blast Events in Iraq and Afghanistan War Veterans using the Salisbury Blast Interview: A CENC Study. Brain Injury, 1–11. 10.1080/02699052.2020.1729418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, Krystal JH, & Schweinsburg BC (2015). A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychological Bulletin, 141(1), 105–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storzbach D, O’Neil ME, Roost SM, Kowalski H, Iverson GL, Binder LM, Fann JR, & Huckans M (2015). Comparing the Neuropsychological Test Performance of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) Veterans with and without Blast Exposure, Mild Traumatic Brain Injury, and Posttraumatic Stress Symptoms. Journal of the International Neuropsychological Society, 21(5), 353–363. 10.1017/s1355617715000326 [DOI] [PubMed] [Google Scholar]
- Taber KH, Hurley RA, Haswell CC, Rowland JA, Hurt SD, Lamar CD, & Morey RA (2015). White matter compromise in veterans exposed to primary blast forces. Journal of Head Trauma Rehabilitation, 30(1), E15–E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber KH, Warden DL, & Hurley RA (2006). Blast-related traumatic brain injury: what is known? J Neuropsychiatry Clin Neurosci, 18(2), 141–145. [DOI] [PubMed] [Google Scholar]
- Vanderploeg RD, Curtiss G, & Belanger HG (2005). Long-term neuropsychological outcomes following mild traumatic brain injury. Journal of the International Neuropsychological Society, 11(3), 228–236. 10.1017/s1355617705050289 [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Aslan M, Lee LO, Proctor SP, Ko J, Jacob S, & Concato J (2018). Longitudinal Associations among Posttraumatic Stress Disorder Symptoms, Traumatic Brain Injury, and Neurocognitive Functioning in Army Soldiers Deployed to the Iraq War. Journal of the International Neuropsychological Society, 24(4), 311–323. 10.1017/s1355617717001059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaellie M, Lafleche G, Spiro A, & Bousquet K (2014). Neuropsychological outcomes in OEF/OIF veterans with self-report of blast exposure: associations with mental health, but not MTBI. Neuropsychology, 28(3), 337–346. 10.1037/neu0000027 [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Lafleche G, Spiro AI, Tun C, & Bousquet K (2013). Chronic postconcussion symptoms and functional outcomes in OEF/OIF veterans with self-report of blast exposure. Journal of the International Neuropsychological Society, 19, 1–10. [DOI] [PubMed] [Google Scholar]
- Vogt D, Smith B, King D, & King L (2012). Manual for the Deployment Risk and Resilience Inventory-2 (DRRI-2): A Collection of Measures for Studying Deployment-Related Experiences of Military Veterans. National Center for PTSD. [Google Scholar]
- Wechsler D (2008). Wechseler Adult Intelligence Scale - Fourth Edition. Pearson Education, Inc. [Google Scholar]
- Wrocklage KM, Schweinsburg BC, Krystal JH, Trejo M, Roy A, Weisser V, Moore TM, Southwick SM, & Scott JC (2016). Neuropsychological functioning in veterans with posttraumatic stress disorder: Associations with performance validity, comorbidities, and functional outcomes. Journal of the International Neuropsychological Society, 22, 399–411. [DOI] [PubMed] [Google Scholar]


