Abstract
Without cystic fibrosis transmembrane conductance regulator–mediated (CFTR-mediated) HCO3– secretion, airway epithelia of newborns with cystic fibrosis (CF) produce an abnormally acidic airway surface liquid (ASL), and the decreased pH impairs respiratory host defenses. However, within a few months of birth, ASL pH increases to match that in non-CF airways. Although the physiological basis for the increase is unknown, this time course matches the development of inflammation in CF airways. To learn whether inflammation alters CF ASL pH, we treated CF epithelia with TNF-α and IL-17 (TNF-α+IL-17), 2 inflammatory cytokines that are elevated in CF airways. TNF-α+IL-17 markedly increased ASL pH by upregulating pendrin, an apical Cl–/HCO3– exchanger. Moreover, when CF epithelia were exposed to TNF-α+IL-17, clinically approved CFTR modulators further alkalinized ASL pH. As predicted by these results, in vivo data revealed a positive correlation between airway inflammation and CFTR modulator–induced improvement in lung function. These findings suggest that inflammation is a key regulator of HCO3– secretion in CF airways. Thus, they explain earlier observations that ASL pH increases after birth and indicate that, for similar levels of inflammation, the pH of CF ASL is abnormally acidic. These results also suggest that a non-cell-autonomous mechanism, airway inflammation, is an important determinant of the response to CFTR modulators.
Keywords: Pulmonology
Keywords: Epithelial transport of ions and water, Genetic diseases, Ion channels
Introduction
Cystic fibrosis (CF) is the most common life-shortening inherited disorder among White individuals (1). CF is caused by mutations in the gene encoding the CF transmembrane conductance regulator (CFTR), an apical membrane anion channel in human airway epithelia (2). The physiologically relevant CFTR-permeable anions are Cl– and HCO3– (3, 4). Cl– secretion drives transepithelial water movement and regulates the volume of the airway surface liquid (ASL). HCO3– secretion, on the other hand, controls the acid-base balance of the ASL. Loss of CFTR-mediated HCO3– secretion leaves H+ secretion unbalanced and lowers the pH of the ASL (pHASL), impairing at least two respiratory host defenses (5–9). The abnormally decreased pHASL alters the biophysical properties of mucus and thus disrupts mucociliary transport. The acidic pHASL also impairs antimicrobial factor activity against inhaled pathogens. These host defense defects manifest clinically as chronic progressive airway disease with frequent exacerbations and reduced survival (10). The abnormal acidification of the ASL thus links the molecular defect in CF with the clinical disease phenotype (11–13).
In vivo studies in humans and pigs have shown that CF pHASL is abnormally acidic soon after birth (5, 14, 15). This abnormality is expected given the loss of CFTR-mediated HCO3– secretion. However, a few months after birth, pHASL in individuals with CF increases to match the pHASL in non-CF individuals (14–16). This latter observation is puzzling because CFTR activity does not return with time. We considered that inflammation might increase CF pHASL with time. CF airways lack inflammation in the newborn period but quickly develop inflammation within weeks to months after birth (17–19). Previous reports suggest that inflammatory cytokines increase HCO3– secretion (20, 21) and that raising pHASL may enhance host defense (22).
To assess the relevance of inflammation to CF pHASL regulation, we considered two main hypotheses. The first of these is that inflammatory cytokines will increase pHASL in CF epithelia. To test this hypothesis, we treated primary cultures of differentiated human CF airway epithelia with a combination of TNF-α and IL-17 (TNF-α+IL-17). These cytokines are key drivers of neutrophilic inflammation (23–25), a hallmark of CF airway disease, and their levels are elevated in vivo (26–29). Studies in non-CF epithelia indicate that TNF-α+IL-17 alkalinize pHASL, and increases in both CFTR and pendrin were responsible (30). We predicted that, even in the absence of CFTR, an increase in pendrin would increase CF pHASL.
The second considered hypothesis is that restoring CFTR function in inflamed CF epithelia will further increase pHASL. This hypothesis is in part based on the notion that directly comparing pHASL in inflamed CF epithelia to pHASL in noninflamed, non-CF epithelia may not be appropriate; that is, two variables are involved: the presence or absence of CFTR and the presence or absence of inflammation. Finding that pHASL further alkalinizes when CFTR activity increases would be consistent with the observation that both CFTR and non-CFTR HCO3– secretion mechanisms, perhaps pendrin, contribute to the increased pHASL. The availability of FDA-approved drugs that modify CFTR function (CFTR modulators) allowed us to test this hypothesis. CFTR modulators include ivacaftor, which increases CFTR open-state probability and can be used to increase function of CFTR-G551D and CFTR-R117H, and a triple drug combination that includes elexacaftor and tezacaftor, which enhance CFTR-ΔF508 processing, and ivacaftor to increase the open-state probability of the channels that reach the cell surface (31, 32).
Results
TNF-α+IL-17 alkalinize CF ASL.
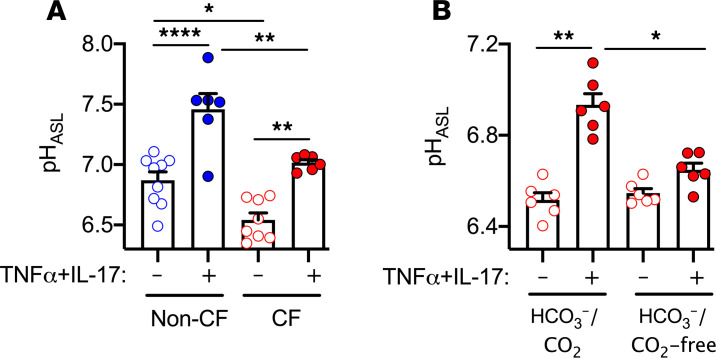
Because TNF-α+IL-17 altered ion transport in non-CF epithelia (30), we investigated their effect on pHASL in CF. We used the pH-sensitive indicator SNARF-1 conjugated to 70 kD dextran and measured pHASL under physiologic conditions (25 mM HCO3–/ 5% CO2 and 37°C). Consistent with our earlier observations (30), applying TNF-α (10 ng/ml) and IL-17 (20 ng/ml) for 48 hours increased pHASL of non-CF epithelia (Figure 1A). As previously reported (11), pHASL was lower in CF epithelia. However, TNF-α+IL-17 increased CF pHASL, albeit to a lower level than in non-CF epithelia. Substituting HEPES for HCO3–/CO2 prevented CF ASL alkalinization (Figure 1B). These results indicate that CF pHASL is relatively acidic under basal conditions but alkalinizes with TNF-α+IL-17 by increasing HCO3– secretion. They also suggest that for similar exposures to TNF-α+IL-17, CF ASL remains abnormally acidic.
Figure 1. CF ASL is abnormally acidic at baseline but alkalinizes with TNF-α+IL-17.
Primary cultures of differentiated human airway epithelia were derived from multiple donors. pHASL was measured using SNARF-1-conjugated dextran on an inverted confocal microscope. (A) Non-CF (blue) versus CF (red) pHASL at baseline and after treatment with TNF-α (10 ng/ml) and IL-17 (20 ng/ml) for 48 hours (n = 6–9). (B) CF epithelia treated with TNF-α+IL-17 for 24 hours. pHASL was measured in the presence of HCO3–/CO2 as well as after removing CO2 from the environment and replacing HCO3– with HEPES (n = 6). Each data point represents an epithelium from a different donor. Data are shown as the mean ± SEM. Statistical significance was tested using ANOVA with post test Tukey’s. *P < 0.05, **P < 0.01, ****P < 0.0001.
Combined TNF-α+IL-17 increase CF pHASL in a time-dependent, durable, and reversible manner.
We focused on the response to TNF-α+IL-17 because both cytokines are elevated in CF airways (26, 28). Individually, TNF-α and IL-17 applied for 24 hours, increased CF pHASL, albeit modestly compared with the combination TNF-α+IL-17 (Figure 2A). This result is consistent with previous reports of TNF-α and IL-17 synergy (26). We also tested the effect of acute versus chronic TNF-α+IL-17. With acute treatment, CF pHASL did not change at 4 hours, but markedly increased at 24 hours (Figure 2B). With chronic TNF-α+IL-17, pHASL peaked by day 2 and persisted up to day 7 (Figure 2C). Because cytokines can produce lasting changes, for example, epigenetic modifications or changes in cell types, we asked whether TNF-α+IL-17–induced alkalinization was reversible. We exposed CF epithelia to TNF-α+IL-17 for 7 days, stopped treatment, and measured pHASL 7 days later. Withdrawal of cytokines allowed pHASL to return to control levels, whereas continued exposure maintained alkalinization up to 14 days. These results indicate that TNF-α and IL-17 synergistically increase pHASL, and this response is time-dependent, long-lasting, and reversible.
Figure 2. TNF-α+IL-17 combined increase CF pHASL in a time-dependent, durable, and reversible manner.
(A) Human CF airway epithelia were treated with TNF-α (10 ng/ml), IL-17 (20 ng/ml), or both for 24 hours, and pHASL measured using SNARF-1-dextran (n = 6). (B and C) Time-dependent changes in CF pHASL after exposure to combined TNF-α+IL-17 (n = 5). Each data point represents epithelium from a different donor. Data are shown as the mean ± SEM. Statistical significance was tested using repeated-measures ANOVA with post test Tukey’s for A and B and paired Student’s t test for C. *P < 0.05, **P < 0.01, ***P < 0.001.
TNF-α+IL-17 induce profound yet highly similar transcriptomic changes in CF and non-CF epithelia.
Some previous reports have proposed that CFTR loss may alter the responses evoked by inflammatory stimuli (33, 34). To further evaluate this possibility, we compared the TNF-α+IL-17–induced response between CF and non-CF epithelia. We performed RNA-Seq and studied differential gene expression, defined as log2 fold change equal to or greater than 2 and a false discovery rate of less than 0.05. The results are displayed as a volcano plot (Figure 3A). At baseline, few transcripts differed between the CF and non-CF epithelia (Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/JCI150398DS1). Of note, airway-relevant acid-base transporters (e.g., CFTR, ATP12A) were not differentially expressed genes.
Figure 3. TNF-α+IL-17 induce highly similar transcriptomic changes in CF and non-CF epithelia.
Human airway epithelia were treated with vehicle or TNF-α+IL-17 for 48 hours, and RNA-Seq was performed (n = 6 CF or non-CF donors). Differential gene expression was plotted as volcano plots. (A) Baseline CF versus non-CF epithelia; (B) response to TNF-α+IL-17 in non-CF epithelia; (C) response to TNF-α+IL-17 in CF epithelia; and (D) response to TNF-α+IL-17 in non-CF versus CF epithelia. Each data point corresponds to a gene. Statistically different transcripts are identified and marked in red. Analysis for differential expression was performed using DESeq2 and P values derived using Wald test.
To assess CFTR dependence of inflammatory pathways, we compared responses to TNF-α+IL-17. In both CF and non-CF epithelia, TNF-α+IL-17 had a robust effect and altered the expression of hundreds of genes (Figure 3, B and C). After testing for the interaction between genotypes and treatment effects, the CF response was highly similar to the non-CF response (Figure 3D). The vast majority of transcripts clustered close to the line of identity, indicating relatively small or no differences attributable to genotypes. Three transcripts met criteria for differential response in CF versus non-CF: CYP1A1, LINC00330, and MMP1. However, previous literature does not specify a role for these transcripts in acid-base homeostasis. Overall, these results suggest that CF and non-CF responses to TNF-α+IL-17 are highly similar when measured at transcriptional level.
Calcium-activated Cl– channels are not involved in CF ASL alkalinization.
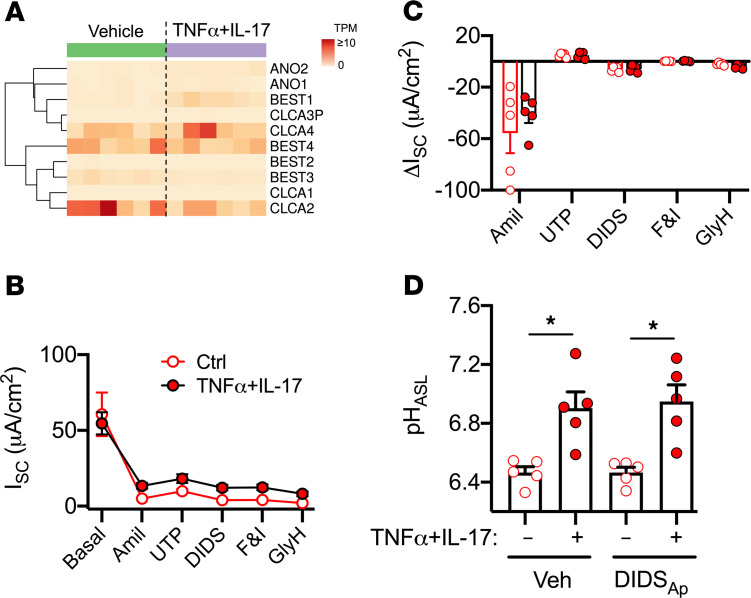
The TNF-α+IL-17–induced response required HCO3–, and CF epithelia lack a functional CFTR. Previous reports indicate that calcium-activated Cl– channels (CaCCs) also conduct HCO3– (35, 36). CaCCs include anoctamins (ANO1 and ANO2), bestrophins (BEST1–BEST4), and chloride channel accessory proteins (CLCA1–CLCA4; ref. 37). In RNA-Seq studies, CaCC-related genes showed no clear change with TNF-α+IL-17 (Figure 4A). To test for electrogenic anion secretion mediated by CaCCs, we mounted epithelia in Ussing chambers with symmetric Krebs-HCO3– solution and recorded short-circuit current (ISC; Figure 4, B and C). We inhibited ENaC with amiloride and noted similar ΔISC in vehicle and TNF-α+IL-17–treated epithelia. Next, we added uridine triphosphate to activate CaCCs, followed by 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS) to inhibit CaCCs. We observed modest responses to these agents; however, ΔISC was not altered in epithelia treated with TNF-α+IL-17. To further assess the role of CaCCs in the pHASL response, we applied DIDS to the apical side and measured pHASL 2 hours later. Apical DIDS did not alter pHASL in either control or TNF-α+IL-17–treated epithelia (Figure 4D). We concluded that CaCCs were not the source of TNF-α+IL-17–mediated CF ASL alkalinization.
Figure 4. CaCCs are not involved in CF ASL alkalinization.
(A) Differential expression of 10 CaCC-related genes in CF airway epithelia measured by RNA-Seq and displayed as a heatmap of raw transcripts per million (TPM). Columns represent epithelia from different CF donors (n = 6). The columns to the left are from 6 separate cultures under baseline conditions, and the columns to the right are from the same 6 donors treated with TNF-α+IL-17 for 48 hours and are displayed in the same sequence as that for the baseline results. Rows represent individual CaCC-related genes. (B and C) CF epithelia were treated with TNF-α+IL-17 for 24 hours and mounted in Ussing chambers with symmetric Krebs-HCO3– solution gassed with 5% CO2. Epithelia were voltage clamped, followed by continuous recording of short-circuit current (ISC), as pharmacologic agents were sequentially added to the apical chamber (n = 5). (D) 100 μM DIDS was suspended in a volatile solvent perfluorocarbon, Fluorinert FC-72 (3M), and applied apically. pHASL was measured 2 hours later using SNARF-1-dextran (n = 5 different donors). Data are shown as the mean ± SEM. *P < 0.05 by repeated-measures ANOVA and Tukey’s multiple comparison test.
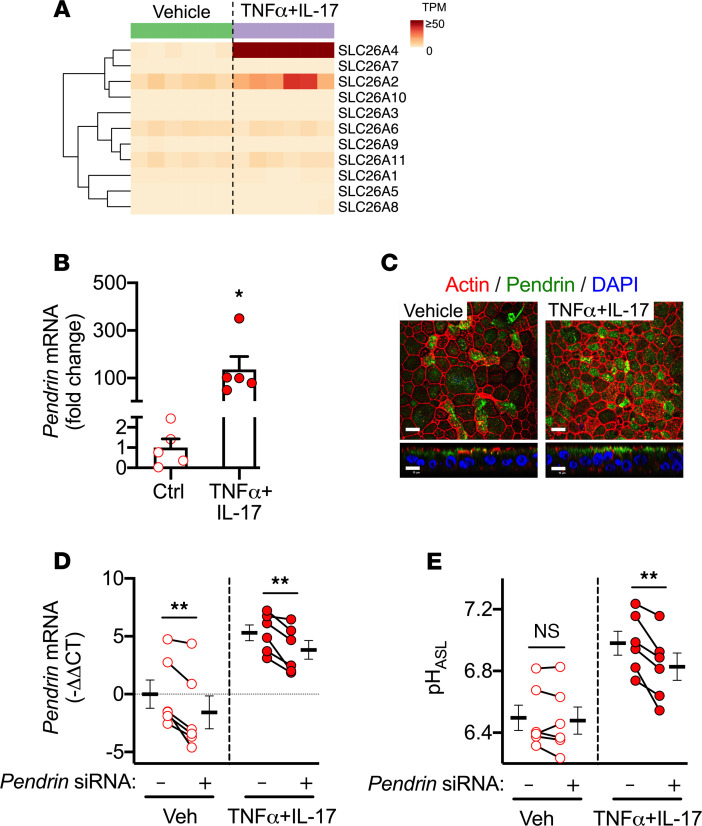
TNF-α+IL-17 alkalinize CF ASL by upregulating pendrin.
In addition to CFTR and CaCC anion channels, SLC26 transporters may also contribute to epithelial HCO3– secretion (38). We used RNA-Seq data to study SLC26 transporters in CF epithelia and displayed results as a heatmap (Figure 5A). The isoform SLC26A4 showed low level expression at baseline, but this expression increased markedly with TNF-α+IL-17. We validated this result with qRT-PCR and noted a >100-fold increase in expression (Figure 5B). SLC26A4 encodes pendrin, an electroneutral, DIDS-insensitive, Cl–/ HCO3– exchanger (39, 40). Using immunocytochemistry, we localized pendrin at the apical pole (Figure 5C). To study the role of pendrin, we performed siRNA-mediated knockdown (Figure 5, D and E). Though no effect was observed under control conditions, pendrin knockdown reduced the already elevated pHASL in TNF-α+IL-17–treated epithelia. These data suggest that pendrin alkalinizes CF ASL and might be particularly relevant to inflamed CF airways.
Figure 5. TNF-α+IL-17 alkalinize CF ASL by upregulating pendrin.
(A) Differential expression of SLC26 family genes in CF airway epithelia by RNA-Seq displayed as a heatmap of raw transcripts per million (TPM). Columns represent epithelia from different CF donors (n = 6). The columns to the left are from 6 separate cultures under baseline conditions, and the columns to the right are from the same 6 donors treated with TNF-α+IL-17 for 48 hours and are displayed in the same sequence as for the baseline results. Rows represent individual SLC26 family genes. (B) SLC26A4 (also known as pendrin) expression, as measured in qRT-PCR (n = 5). (C) Pendrin immunolocalization in CF airway epithelia. Scale bar: 10 μm. (D and E) siRNA directed against pendrin was used to knockdown expression in CF epithelia. TNF-α+IL-17 were applied for 24 hours. pHASL was measured using SNARF-1-dextran (n = 6). Each data point represents epithelium from a different CF donor. Data are shown as the mean ± SEM. *P < 0.05, **P < 0.01 by paired Student’s t test.
A triple combination of CFTR modulators further increases pHASL in TNF-α+IL-17–treated CF epithelia.
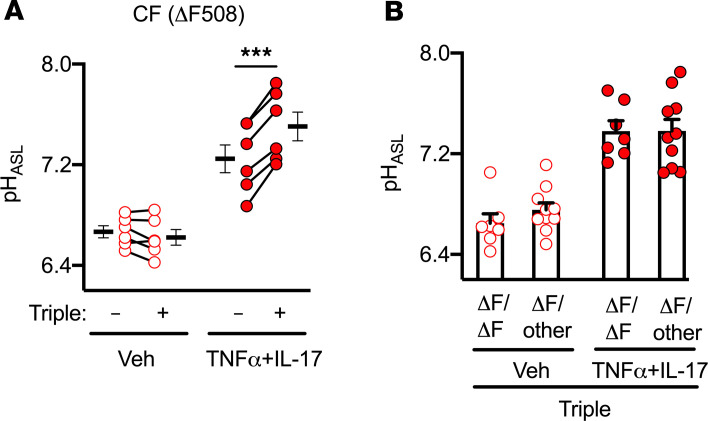
CFTR-ΔF508 is the most common disease-causing CFTR mutation (1). It results in deletion of a single phenylalanine at position 508 and produces a misfolded protein that is prematurely degraded. This defect can be modulated with a triple combination of drugs (elexacaftor, tezacaftor, and ivacaftor; ref. 41). Because airway inflammation is ubiquitous in individuals with CF who take modulators, we studied pHASL in CFTR-ΔF508 epithelia exposed to the triple combination and TNF-α+IL-17. In TNF-α+IL-17–treated epithelia with an already elevated pHASL, the triple combination further increased pHASL (Figure 6A).
Figure 6. A triple combination of CFTR modulators further increases pHASL in TNF-α+IL-17–treated CF epithelia.
(A) Human airway epithelia from CFTR-ΔF508 donors were treated for 48 hours with a combination of elexacaftor (3 μM), tezacaftor (18 μM), and ivacaftor (1 μM), either alone or in the presence of TNF-α+IL-17. pHASL was measured using SNARF-1-dextran (n = 6). (B) pHASL from CF donors homozygous (ΔF508/other [ΔF/other]) or heterozygous (ΔF508/other [ΔF/ΔF]) for ΔF508 allele treated with the triple combination and TNF-α+IL-17 (n = 7-10). Each data point represents epithelium from a different CF donor. Data are shown as the mean ± SEM. Statistical significance was tested using paired (A) or unpaired (B) Student’s t test. ***P < 0.001.
The CFTR-ΔF508 allele is detected in individuals with CF as either homozygous (ΔF508/ΔF508) or compound heterozygous (ΔF508/other) genotypes. In clinical studies, both groups derived similar improvements (increased lung function, reduced pulmonary exacerbations, and reduced sweat [Cl–]) with the triple combination (41). We predicted that this might be reflected in pHASL responses. With the triple combination, pHASL did not vary between ΔF508/ΔF508 and ΔF508/other epithelia (Figure 6B).
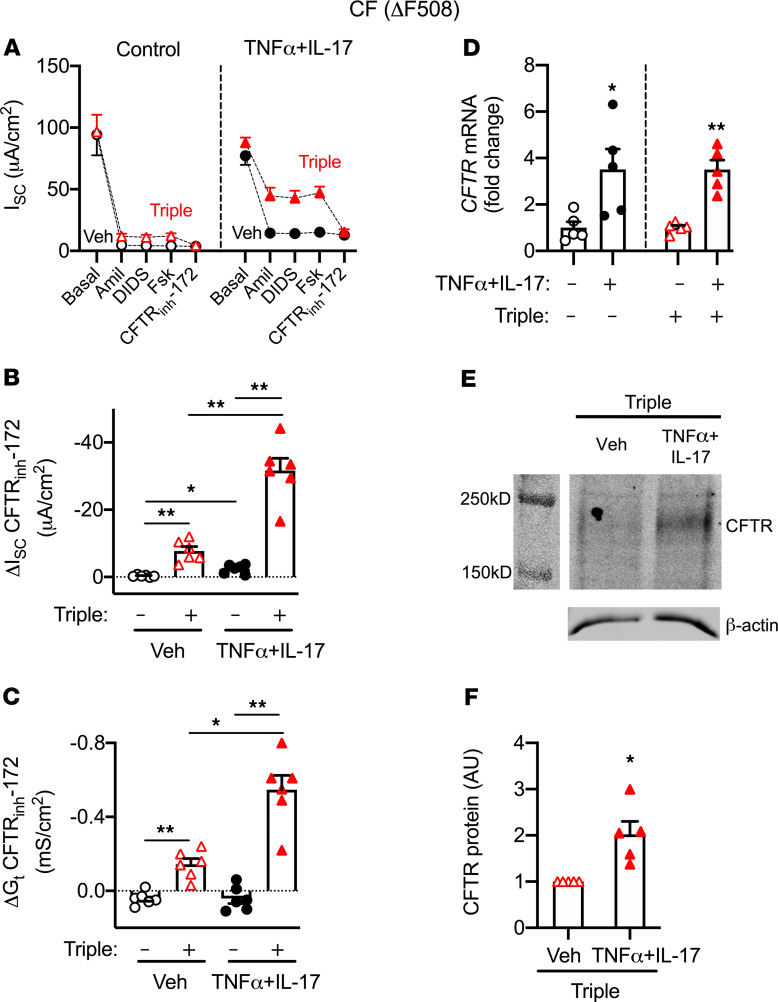
We performed several studies to understand how TNF-α+IL-17 increase the pHASL response to CFTR modulators. CFTR mediates transepithelial electrogenic anion secretion, which can be assayed in Ussing chambers by measuring responses to interventions that increase or inhibit CFTR activity. We mounted epithelia in Ussing chambers with symmetric Krebs-HCO3– solution and recorded ISC and transepithelial conductance (Gt). We inhibited ENaCs with amiloride and CaCCs with DIDS, thereby primarily isolating changes in ISC and Gt to CFTR. We added forskolin to activate CFTR, followed by CFTRinh-172 to inhibit CFTR, and analyzed ΔISC and ΔGt as measures of CFTR activity (Figure 7, A–C). Without modulators, TNF-α+IL-17 modestly increased ΔISC-CFTR but not ΔGt-CFTR. The triple combination significantly increased ΔISC- and ΔGt-CFTR in vehicle-treated controls as well as TNF-α+IL-17–treated epithelia. Remarkably, both measures of CFTR activity were 4 times larger in the presence of TNF-α+IL-17. These results suggested that TNF-α+IL-17 increased the amount of CFTR or its activity. Consistent with an increase in CFTR expression, TNF-α+IL-17 increased CFTR mRNA by 3.5-fold (Figure 7D) and CFTR protein by 2-fold (Figure 7, E and F). Overall, these results indicated that TNF-α+IL-17 increased the CF epithelial response to CFTR modulators by increasing CFTR amount, biosynthesis, and function.
Figure 7. TNF-α+IL-17 increase the response of CFTR-ΔF508 epithelia to the triple combination of CFTR modulators.
Human airway epithelia from CFTR-ΔF508 donors were treated for 48 hours with a combination of elexacaftor (3 μM), tezacaftor (18 μM), and ivacaftor (1 μM), either alone or in the presence of TNF-α+IL-17. (A–C) Epithelia were mounted in Ussing chambers with symmetric Krebs-HCO3– solution gassed with 5% CO2. Epithelia were voltage clamped, followed by recording of ISC and Gt, as pharmacologic agents were sequentially added to the apical chamber (n = 6 different donors). CFTR channel activity was assayed as the response to CFTRinh-172, shown in B as ΔISC and in C as ΔGt. (D) CFTR gene expression measured using qRT-PCR (n = 5). (E) Example of Western blot of CFTR and β-actin in CF airway epithelia treated with the triple combination and either vehicle or TNF-α+IL-17 for 48 hours. (F) Quantification of CFTR protein expression normalized to β-actin (n = 5). Each data point represents epithelium from a different CF donor. Data are shown as the mean ± SEM. Statistical significance was tested using repeated-measures ANOVA and post test Tukey’s test for B and C, and paired Student’s t test for D and F. *P < 0.05, **P < 0.01.
TNF-α+IL-17 increase CFTR activity in G551D CF epithelia.
ΔF508 is a class II mutation that produces a trafficking defect. As a result, very little to no functional CFTR reaches the apical membrane (1). In contrast, a class III mutation (e.g., G551D) produces a gating defect and results in a channel with reduced open state probability. We asked whether TNF-α+IL-17 enhanced CFTR-G551D activity. This is relevant because (a) G551D is the third-most common disease-causing mutation and it produces severe airway disease (42); (b) ivacaftor restores anion secretion and has been in clinical use for several years (43, 44); (c) patients with G551D have substantial airway inflammation, even after long-term ivacaftor use (45–47); (d) as opposed to correctors (elexacaftor, tezacaftor, etc.), ivacaftor works acutely and permits experimental designs that sidestep possible interactions between correctors and cytokines; and (e) chronic ivacaftor may limit corrector efficacy in vitro (48, 49). In G551D epithelia, assessments can be made independent of correctors.
We assayed G551D CF epithelia in Ussing chambers with symmetric Krebs-HCO3– solution, and measured ISC and Gt. We minimized non-CFTR channel activity by applying amiloride and DIDS apically. To assess CFTR, we sequentially added forskolin and ivacaftor, followed by the inhibitor GlyH-101. TNF-α+IL-17 increased CFTR-G551D activity, as evidenced by greater ΔISC and ΔGt in response to CFTR activators as well as the inhibitor (Figure 8, A–C). We also tested pHASL responses under open-circuit conditions at the air-liquid interface. Applied for 1 hour, the combination of forskolin and ivacaftor further alkalinized ASL in TNF-α+IL-17–treated epithelia (Figure 8D). These results indicate that TNF-α+IL-17 enhancement of modulator efficacy is not specific to a particular class of mutations or modulators.
Figure 8. TNF-α+IL-17 increase CFTR activity in CF epithelia with a CFTR-G551D mutation.
Human airway epithelia from G551D CF donors were treated with either vehicle or TNF-α+IL-17 for 48 hours. (A–C) Epithelia were assayed in Ussing chambers containing symmetric Krebs-HCO3– solution, with continuous recording of ISC and Gt (n = 4). (D) pHASL in G551D CF epithelia, stimulated for 2 hours with forskolin 10 μM and ivacaftor 10 μM (n = 3-4). Each data point represents epithelium from a different CF donor. Data are shown as the mean ± SEM. **P < 0.01, ***P < 0.001 by paired Student’s t test.
However, there were some differences in the response to TNF-α+IL-17 in CFTR-G551D and CFTR-ΔF508 epithelia. In the CFTR-G551D epithelia, TNF-α+IL-17 did not alter basal Cl– and HCO3– current after amiloride, but the response to forskolin, the response to ivacaftor, and the total Cl– and HCO3– current were increased consistent with increased CFTR production and acute activation by forskolin and ivacaftor. In CFTR-ΔF508 epithelia, TNF-α+IL-17 increased basal Cl– and HCO3– current after amiloride and total Cl– and HCO3– current, but the response to forskolin was small. These results suggest that, in addition to increasing CFTR production, these inflammatory cytokines may have complex effects on CFTR biology. This is consistent with previous studies reporting that airway inflammation increased CFTR-ΔF508 biosynthesis and function (50, 51). Enhancing CFTR-ΔF508 biosynthesis requires time, and it is possible that the 48-hour treatment with the triple combination might also have contributed to differences between CFTR-G551D and CFTR-ΔF508 epithelia studied in Ussing chambers.
Airway inflammatory markers correlate with ivacaftor-induced lung function improvements.
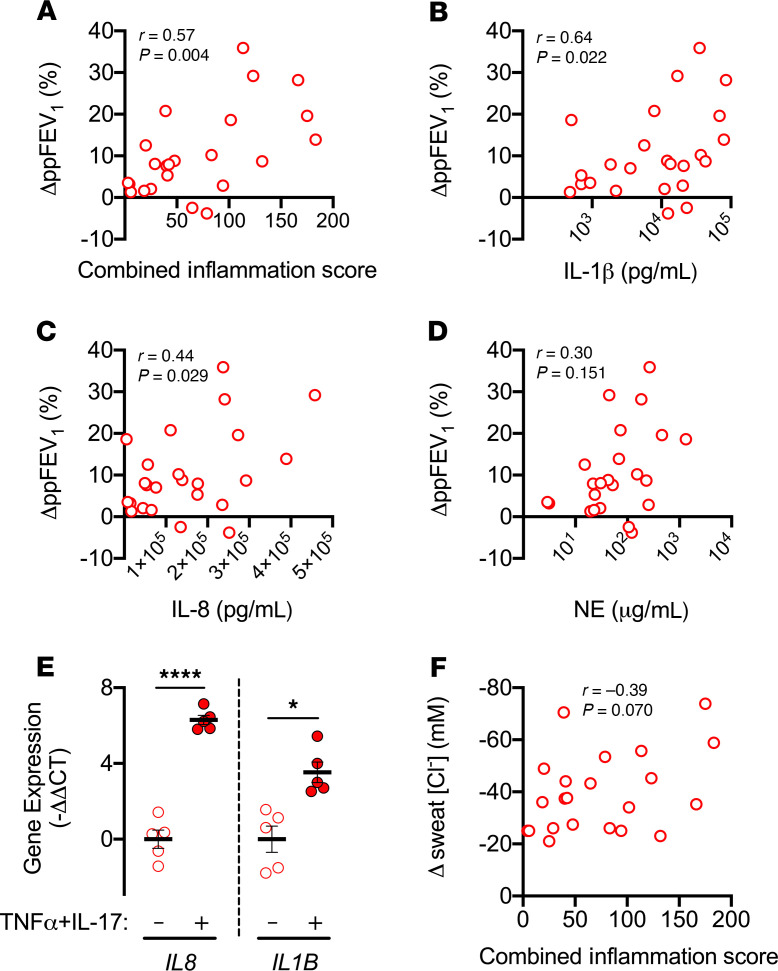
Our in vitro studies indicated that CFTR modulators may have enhanced efficacy in inflamed CF airways. However, in vivo response may differ due to additional factors, including infection, airway remodeling, and drug biodistribution. As an indirect test of the effect of modulators in inflamed airways, we asked if markers of airway inflammation would correlate with modulator-induced early lung function improvements. We analyzed data from an earlier clinical trial of a cohort of individuals with at least 1 G551D or R117H allele, i.e., mutations amenable to ivacaftor potentiation (15, 45, 52, 53). Just before beginning ivacaftor, all individuals had inflammatory markers, IL-1β, IL-8, and neutrophil elastase, measured in sputum. Lung function was assessed at baseline and at day 2 after starting ivacaftor, a time when the effect of any modulator-induced changes in infection and inflammation would be minimal. Supplemental Table 2 shows baseline clinical characteristics of the study participants. We found a positive correlation between baseline inflammation, measured as a combined score of the 3 inflammatory markers, and lung function improvement, measured as % (ΔppFEV1/ ppFEV1) where ppFEV1 is the percentage predicted forced expiratory volume in 1 second (Figure 9A). As for individual markers, we noted significant positive correlations for IL-1β and IL-8 but not neutrophil elastase (Figure 9, B–D). Importantly, TNF-α+IL-17, which we studied in vitro, directly induce IL8 and IL1B expression in CF airway epithelia (Figure 9E).
Figure 9. Airway inflammation correlates with ivacaftor-induced lung function improvements.
Induced sputum samples were obtained from individuals with CF with a CFTR-G551D or a CFTR-R117H mutation immediately before starting ivacaftor. Sputum inflammatory markers IL-1β, IL-8, and neutrophil elastase (NE) were measured using ELISA. Lung function was evaluated immediately before and 2 days after starting ivacaftor. Data are from previously reported studies (15, 45, 53). (A–D) Relationship between baseline airway inflammation and changes in lung function, measured as %(ΔppFEV1/ppFEV1) where ppFEV1 is the percentage predicted forced expiratory volume in 1 second (n = 23). (E) Changes in IL8 and IL1B gene expression in human CF airway epithelia treated with TNF-α+IL-17 for 48 hours (n = 5 different donors). (F) Relationship between baseline airway inflammation and changes in sweat Cl– concentration 2 days after starting ivacaftor (n = 22). Statistical significance was tested using Pearson’s r test for A–D and F and paired Student’s t test for E. Bars in E indicate mean ± SEM. *P < 0.05, ****P < 0.0001.
As a negative control, we also tested for a correlation between airway inflammatory markers and the change in sweat [Cl–], a standard biomarker of CFTR activity in vivo. The sweat gland environment is markedly different from the airway epithelium, in that CF sweat glands do not develop local neutrophil-predominant inflammation. All individuals had decreased sweat [Cl–] at day 2 after starting ivacaftor, indicating CFTR potentiation. However, unlike the improvement in lung function, we observed no correlation between the inflammation index and ivacaftor-induced change in sweat [Cl–] (Figure 9F).
Discussion
CF epithelia lack CFTR-mediated HCO3– secretion, and baseline CF pHASL is abnormally acidic. Here, we found that the inflammatory cytokines TNF-α+IL-17 have a profound impact on CF pHASL. These cytokines induce HCO3– secretion by upregulating pendrin, an apical Cl–/HCO3– exchanger. Strikingly, TNF-α+IL-17 increased the response to CFTR modulators and further alkalinized CF pHASL. Consistent with these findings, analysis of in vivo data from individuals with CF revealed a positive correlation between airway inflammation and CFTR modulator–induced lung function improvements. Collectively, these results suggest that inflammation is a key regulator of HCO3– secretion in CF airways and that it enhances the efficacy of CFTR modulators.
Previous studies have shown that the loss of CFTR function decreases HCO3– secretion in CF airway epithelia. Nonetheless, whether loss of CFTR decreases pHASL in vivo has been debated (16, 54). Our results, together with those of previous studies (11, 30), identify at least two important factors that change CF pHASL: loss of CFTR-mediated HCO3– secretion and inflammation. In the absence of inflammatory cytokines, loss of CFTR-mediated HCO3– secretion decreases pHASL in cultured airway epithelia. Likewise, in neonates tested before the results of genetic tests were known, and, thus, presumably before substantial airway inflammation, pHASL was abnormally acidic in CF (14). Studies in newborn CF pigs also revealed an abnormally acidic pHASL (5). However, inflammatory cytokines modified pHASL in cultured CF airway epithelia: TNF-α+IL-17 increased pHASL by increasing pendrin-mediated HCO3– secretion. Concordantly, in vivo studies of CF in 3-month-old babies, older children, and adults showed that pHASL had increased so that it no longer differed from that in non-CF controls. These results predicted that if CFTR activity were introduced to inflamed CF epithelia, their pHASL would further increase. The use of CFTR modulators allowed us to test that prediction, and CFTR modulators increased pHASL even further in CF epithelia treated with TNF-α+IL-17. These results help explain earlier observations and suggest that for similar levels of inflammation CF pHASL is abnormally low.
Enhancement of CFTR modulator efficacy by 2 cytokines known to be elevated in CF airways suggests that inflammation might have a profound effect in vivo. This prediction is supported by results that showed a significant positive correlation between baseline inflammatory markers and ivacaftor-induced improvements in lung function. Previous work has sought to uncover predictors of response to modulators and therapeutic outcomes (31, 55). In several studies, population-level effects are obvious but are difficult to reconcile with individual-level variability (55–58). Specifically, why some patients derive greater benefit from modulators than others is not known. We speculate that airway inflammation might be a key determinant. To advance personalized CFTR modulation, it may be relevant to study the airway inflammatory milieu and its interactions with approved modulators.
This study has limitations. First, we studied the response to 2 cytokines relevant to CF-like inflammation. However, inflammation is a complex, heterogeneous process (59), and other mediators may modify HCO3– secretion and modulator responses differently. For instance, TGF-β may reduce CFTR expression (60). Second, we did not study the impact of infection, but it remains highly relevant. Some reports have suggested that bacterial factors (e.g., Pseudomonas toxins) may suppress CFTR expression (61, 62). Both inflammation and infection persist despite long-term modulator therapy, and both represent suitable targets to improve CF outcomes. Third, we studied acute improvements in lung function with ivacaftor, as most of the improvement is early and is followed by stabilization of lung function. With chronic treatment, airway inflammatory markers either remain stable (46, 47) or decline but remain abnormally elevated (45). Future studies may define long-term relationships between airway inflammation and the response to modulators.
This study has several implications for in vitro and in vivo studies. First, sweat [Cl–] is routinely employed as a CFTR biomarker in translational studies and clinical care. In previous studies, the modulator-induced change in sweat [Cl–] (a biomarker of CFTR activity in vivo) did not correlate consistently with the change in percentage FEV1 (a measure of airway response; refs. 56, 57). This study suggests that non-cell-autonomous mechanisms, such as local inflammation, may control the response to modulators. Further advancement of CF research and care may benefit from the development of airway-specific CFTR biomarkers, and pHASL is an emerging candidate.
Second, the airway inflammation that persists after initiation of CFTR modulators is a potential therapeutic target, and several agents are under evaluation (63). Our results suggest that inflammatory pathways may intersect with CFTR biogenesis pathways. Suppressing inflammation nonspecifically might thus involve a trade-off with modulator efficacy. Some studies have suggested that ivacaftor may reduce the efficacy of correctors when used in combination (48, 49). Whether this is also the case in the presence of inflammation is not known. This study underscores the need to assess interactions between candidate antiinflammatory therapies and CFTR corrector/potentiator combination regimens.
Third, in vitro models are routinely employed to assess CFTR modulators (64). As inflammation is ubiquitous in vivo and modifies the response to modulators, prediction of response based on in vitro assays (theratyping) may be optimized by incorporating assessments of airway inflammatory phenotype and its effect on modulator efficacy.
In summary, the inflammatory milieu in CF airways regulates pHASL and increases the response to CFTR modulators.
Methods
Cell culture.
Primary cultures of differentiated airway epithelia were obtained without passage from multiple human donors as previously reported (65). Briefly, donor tracheae and/or proximal bronchi were enzymatically digested. Epithelial cells were isolated and seeded onto collagen-coated inserts (Costar, 3470 and 3413; Falcon 353180). Epithelia were differentiated at the air-liquid interface for 3 weeks or more prior to assay. To assess cytokine-induced responses, epithelia were treated on the basolateral side with 10 ng/ml TNF-α (R&D Systems), 20 ng/ml IL-17 (R&D Systems), or both.
Pharmacologic reagents.
Elexacaftor was purchased from MedChemExpress, and tezacaftor and ivacaftor were purchased from Selleckchem. GlyH-101 was a gift from the Cystic Fibrosis Foundation Therapeutics Lab and Robert Bridges (Rosalind Franklin University, Chicago, Illinois, USA). Other reagents were purchased from MilliporeSigma.
pHASL measurement.
The protocol for pHASL measurements has previously been reported (11). Briefly, we used a ratiometric pH indicator, SNARF-1, conjugated to 70 kD dextran (Thermo Fisher Scientific). SNARF-1 is a single-excitation (514 nm), dual-emission (580 nm and 640 nm) fluorescence pH indicator, with optimal range near physiologic pH. SNARF-1–conjugated dextran was delivered as a powder to the apical side and allowed to distribute into ASL for 1 hour. Fluorescence ratios were obtained on a laser-scanning confocal microscope (Zeiss LSM 880) and converted to pH values using calibration curves constructed from colorless standard pH solutions. The microscope chamber housing epithelia maintained a humidified environment at 37°C. 5% CO2 was added to the chamber atmosphere whenever the basolateral side was immersed in a HCO3– containing buffer solution but removed when a HCO3–-free (HEPES) buffer solution was used.
Electrophysiologic studies.
Airway epithelia were mounted in modified Ussing chambers (Physiologic Instruments) and bathed in symmetric Krebs-HCO3– buffer solution containing 118.9 mM NaCl, 25 mM NaHCO3, 2.4 mM K2HPO4, 0.6 mM KH2PO4, 1.2 mM MgCl2, 1.2 mM CaCl2, 5 mM dextrose, at 37°C and adjusted to pH 7.4 in the presence of 5% CO2. Epithelia were voltage clamped, followed by recording of ISC and Gt. The following agents were added apically: 100 μM amiloride, 50 μM uridine triphosphate, 100 μM 4,4′-DIDS, 10 μM forskolin and 100 μM 3-isobutyl-2-methylxanthine, and 100 μM GlyH-101 or 10 CFTRinh-172.
Real-time PCR.
Total RNA was isolated from airway epithelia using the RNeasy Lipid Tissue Mini Kit (QIAGEN). Genomic DNA was removed through DNase I (QIAGEN) treatment. Quality of RNA isolation was verified using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific), and samples with a 260:280 ratio ≥ 1.8 were carried forward. RNA was reverse transcribed with SuperScript VILO MasterMix (Invitrogen). cDNA thus obtained was amplified using gene-specific primers and Fast SYBR Green Master Mix (Applied Biosystems) on the QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems). The gene-specific primer pairs used were as follows: SLC26A4 (pendrin), 5′-CTCCCCAAATACCGAGTCAA-3′ and 5′-CCATATCCGACAGGAACTGC-3′; CFTR, 5′- CACCCAGCCATTTTTGGC-3′ and 5′-AGGAGCGATCCACACGAA-3′; β-actin, 5′- AGAGCTACGAGCTGCCTGAC-3′ and 5′-AGCACTGTGTTGGCGTACAG-3′; and SFRS9, 5′-TGCGTAAACTGGATGACACC-3′ and 5′-CCTGCTTTGGTATGGAGAGTC-3′. All reactions were performed in triplicates, and gene expression was quantitated using –ΔΔCT method.
siRNA knockdown.
Gene knockdown in primary airway epithelia was achieved as reported previously (66). siRNAs were obtained from Integrated DNA Technologies (negative control, IDT DS NC 1; pendrin: IDT hs.Ri.SLC26A4.13.2) and transfected into dissociated primary airway epithelial cells using Lipofectamine RNAiMax (Invitrogen). Epithelia were seeded onto collagen-coated inserts (Costar, 3470) and differentiated at the air-liquid interface. pHASL was measured at day 6 or 7 after seeding. The efficiency of gene knockdown was assessed with RT-PCR.
RNA-Seq protocol and analysis.
RNA-Seq was performed in collaboration with the University of Iowa Genomics Division using the manufacturer’s recommended protocols. Briefly, 500 ng DNase I–treated total RNA was enriched for polyA-containing transcripts using beads coated with oligo(dT) primers. The enriched RNA pool was fragmented, converted to cDNA, and ligated to sequencing adaptors using the TruSeq stranded mRNA sample preparation kit (Illumina, RS-122-2101). The molar concentrations of the indexed libraries were measured using the 2100 Bioanalyzer (Agilent) and combined equally into pools for sequencing. The concentrations of the pools were measured with the Illumina Library Quantification Kit (KAPA Biosystems) and sequenced on the Illumina HiSeq 4000 genome sequencer using 75 bp paired-end SBS chemistry.
Pseudoalignment of raw sequencing reads and quantification of transcript-level expression were obtained using Kallisto version 0.45.0 and human transcriptome reference GRCh38. p12 (67). Gene counts were imported into R, and differential expression tests were performed using DESeq2 version 1.22.2 (68). Further, gene expression modeling in DESeq2 accounted for the experimental design, acknowledging and correcting for paired control and treated samples for each donor. Changes in HCO3– transporters were visualized as heatmaps generated using the Clustvis tool (https://biit.cs.ut.ee/clustvis/) (69). RNA-Seq data are available in the NCBI’s GEO database (GEO GSE 176121).
Immunocytochemistry.
Airway epithelia were washed 3 times with PBS, fixed with 4% paraformaldehyde for 15 minutes, and permeabilized with 0.3% Triton-X for 20 minutes. To minimize nonspecific staining, epithelia were treated with SuperBlock (Thermo Fisher Scientific) containing 0.5% normal goat serum for 1 hour at room temperature. Primary antibodies were diluted in SuperBlock and added apically for 3 hours at 37°C. Epithelia were washed and incubated for 45 minutes with appropriate secondary antibodies diluted in PBS. Pendrin was detected using mouse anti-SLC26A4 (1:200; Abnova) and goat anti-mouse secondary antibody conjugated to Alexa Flour 488 (1:1000; Thermo Fisher Scientific). Actin cytoskeleton was stained with Alexa Fluor 633 phalloidin (1:300; Thermo Fisher Scientific) added at the same time as secondary antibodies. Epithelia were mounted on glass slides, and Vectashield with DAPI (Vector Laboratories) was used to secure glass coverslips. Imaging was performed on the Olympus Fluoview FV 3000 confocal microscope. Z-stack images were processed with the Olympus Fluoview program.
Western blot.
Airway epithelia were washed 3 times with ice-cold PBS and scraped into 35 μl lysis buffer supplemented with protease inhibitors. The resulting mixture was passed through small bore (27-gauge) needles and centrifuged at 1200g for 5 minutes. The supernatants were collected and ultracentrifuged at 174,000g for 30 minutes. The pellets were resuspended in 1% Triton X-100 in lysis buffer. Samples were run on 7% gels with high-molecular-mass standards (Bio-Rad, 161-0374). Electrophoresed gels were transferred to PVDF membranes (Millipore, IPFL00010) overnight. Membranes were blocked in 0.1% casein in PBS; immunostained with antibodies for CFTR (1:500, no. 596; CFTR Antibody Distribution Program, Cystic Fibrosis Foundation Therapeutics Lab) and β-actin (1:3000, Abcam, ab8227) and secondary antibodies (1:10,000, LI-COR, 926-32212 and 926-32213); and visualized on an Odyssey IR imager (LI-COR). Quantification for CFTR was performed on ImageJ (NIH) after normalizing to β-actin.
Human study participants.
The analysis of data from humans was from previously published studies (15, 45, 52, 53). Adult participants with CF who had at least 1 G551D or R117H allele were enrolled at a single CF center, the National Referral Centre for Adult Cystic Fibrosis, St. Vincent’s University Hospital, and University College Dublin School of Medicine, Dublin, Ireland. Supplemental Table 2 summarizes the clinical characteristics of participants. Inflammatory markers (IL-1β, IL-8, and neutrophil elastase) were measured in induced sputum using ELISA immediately before starting ivacaftor and were used to derive a “combined inflammation score.” For each marker, the highest measurement was assigned a value of 100, and other measurements were expressed as a fraction of 100. In the end, the values for all 3 markers were added to obtain a combined score. Sweat [Cl–] and spirometry were obtained at baseline and 2 days after starting ivacaftor.
Statistics.
Testing for statistical significance was performed on GraphPad Prism 8 Software. Tests included unpaired or paired 2-tailed Student’s t test for comparing 2 groups, repeated-measures 1-way ANOVA with Tukey’s multiple comparison test for comparing more than 2 groups, and Pearson’s r test for assessing correlations. A P value of less than 0.05 was considered significant.
Study approval.
All studies were approved by the University of Iowa Institutional Review Board.
Author contributions
TR, DAS, EFM, PKS, and MJW conceived and designed studies. PHK and PT prepared epithelia for experiments. TR performed experiments, and BJG contributed to Western blots. TR, AAP, ALT, SLD, and MJW analyzed data. IMT and MED provided insightful discussions about study design and results. TR and MJW wrote the manuscript. All authors approved the manuscript.
Supplementary Material
Acknowledgments
We thank the University of Iowa In Vitro Models and Cell Culture Core and the Genomics Division of the Iowa Institute of Human Genetics for technical assistance. This work was supported in part by the NIH (HL140261, HL136813, HL091842, and HL051670), the Cystic Fibrosis Foundation Research Development Program (CFF RDP) (University of Iowa and University of Washington), and an unrestricted grant from the Vertex Investigator-Initiated Studies Program. TR was supported in part by a CFF RDP pilot grant. IMT is supported in part by a Gilead Sciences Research Scholars Program in Cystic Fibrosis. MJW is an investigator of the Howard Hughes Medical Institute.
Version 1. 06/24/2021
In-Press Preview
Version 2. 08/16/2021
Electronic publication
Funding Statement
TR was supported, in part, by a CFF RDP pilot grant
IMT is supported, in part, by a Gilead Sciences Research Scholars Program in Cystic Fibrosis.
MJW is an investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(16):e150398.https://doi.org/10.1172/JCI150398.
Contributor Information
Tayyab Rehman, Email: Tayyab-rehman@uiowa.edu.
Philip H. Karp, Email: philip-karp@uiowa.edu.
Ping Tan, Email: ping-tan@uiowa.edu.
Brian J. Goodell, Email: brian-goodell@uiowa.edu.
Alejandro A. Pezzulo, Email: alejandro-pezzulo@uiowa.edu.
Andrew L. Thurman, Email: andrew-thurman@uiowa.edu.
Ian M. Thornell, Email: Ian-thornell@uiowa.edu.
Samantha L. Durfey, Email: sldurfey@uw.edu.
Michael E. Duffey, Email: duffey@buffalo.edu.
David A. Stoltz, Email: david-stoltz@uiowa.edu.
Edward F. McKone, Email: E.McKone@st-vincents.ie.
Pradeep K. Singh, Email: singhpr@uw.edu.
Michael J. Welsh, Email: michael-welsh@uiowa.edu.
References
- 1.Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015;16(1):45–56. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinton PM. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev. 1999;79(1 Suppl):S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- 3.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79(1 suppl):S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 4.Bridges RJ. Mechanisms of bicarbonate secretion: lessons from the airways. Cold Spring Harb Perspect Med. 2012;2(8):a015016. doi: 10.1101/cshperspect.a015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pezzulo AA, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487(7405):109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang XX, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest. 2016;126(3):879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abou Alaiwa MH, et al. pH modulates the activity and synergism of the airway surface liquid antimicrobials beta-defensin-3 and LL-37. Proc Natl Acad Sci U S A. 2014;111(52):18703–18708. doi: 10.1073/pnas.1422091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birket SE, et al. Development of an airway mucus defect in the cystic fibrosis rat. JCI Insight. 2018;3(1):e97199. doi: 10.1172/jci.insight.97199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clary-Meinesz C, et al. Influence of external pH on ciliary beat frequency in human bronchi and bronchioles. Eur Respir J. 1998;11(2):330–333. doi: 10.1183/09031936.98.11020330. [DOI] [PubMed] [Google Scholar]
- 10.Stoltz DA, et al. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372(16):1574–1575. doi: 10.1056/NEJMc1502191. [DOI] [PubMed] [Google Scholar]
- 11.Shah VS, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351(6272):503–507. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372(9636):415–417. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 13.Simonin J, et al. Airway surface liquid acidification initiates host defense abnormalities in Cystic Fibrosis. Sci Rep. 2019;9(1):6516. doi: 10.1038/s41598-019-42751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abou Alaiwa MH, et al. Neonates with cystic fibrosis have a reduced nasal liquid pH; a small pilot study. J Cyst Fibros. 2014;13(4):373–377. doi: 10.1016/j.jcf.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abou Alaiwa MH, et al. Ivacaftor-induced sweat chloride reductions correlate with increases in airway surface liquid pH in cystic fibrosis. JCI Insight. 2018;3(15):e121468. doi: 10.1172/jci.insight.121468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schultz A, et al. Airway surface liquid pH is not acidic in children with cystic fibrosis. Nat Commun. 2017;8(1):1409. doi: 10.1038/s41467-017-00532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sly PD, et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180(2):146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 18.Sly PD, et al. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368(21):1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 19.Schultz A, Stick S. Early pulmonary inflammation and lung damage in children with cystic fibrosis. Respirology. 2015;20(4):569–578. doi: 10.1111/resp.12521. [DOI] [PubMed] [Google Scholar]
- 20.Kreindler JL, et al. Interleukin-17A induces bicarbonate secretion in normal human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296(2):L257–L266. doi: 10.1152/ajplung.00344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorrieri G, et al. Goblet cell hyperplasia requires high bicarbonate transport to support mucin release. Sci Rep. 2016;6:36016. doi: 10.1038/srep36016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abou Alaiwa MH, et al. Repurposing tromethamine as inhaled therapy to treat CF airway disease. JCI Insight. 2016;1(8):e87535. doi: 10.1172/jci.insight.87535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukacs NW, et al. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. J Immunol. 1995;154(10):5411–5417. [PubMed] [Google Scholar]
- 24.McAleer JP, Kolls JK. Mechanisms controlling Th17 cytokine expression and host defense. J Leukoc Biol. 2011;90(2):263–270. doi: 10.1189/jlb.0211099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoppelenburg AJ, et al. Local IL-17A potentiates early neutrophil recruitment to the respiratory tract during severe RSV infection. PLoS One. 2013;8(10):e78461. doi: 10.1371/journal.pone.0078461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAllister F, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175(1):404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan HL, et al. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2011;184(2):252–258. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karpati F, et al. TNF-alpha and IL-8 in consecutive sputum samples from cystic fibrosis patients during antibiotic treatment. Scand J Infect Dis. 2000;32(1):75–79. doi: 10.1080/00365540050164263. [DOI] [PubMed] [Google Scholar]
- 29.Tiringer K, et al. A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2013;187(6):621–629. doi: 10.1164/rccm.201206-1150OC. [DOI] [PubMed] [Google Scholar]
- 30.Rehman T, et al. TNFα and IL-17 alkalinize airway surface liquid through CFTR and pendrin. Am J Physiol Cell Physiol. 2020;319(2):C331–C344. doi: 10.1152/ajpcell.00112.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guimbellot J, et al. Toward inclusive therapy with CFTR modulators: Progress and challenges. Pediatr Pulmonol. 2017;52(s48):S4–S14. doi: 10.1002/ppul.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clancy JP. Rapid therapeutic advances in CFTR modulator science. Pediatr Pulmonol. 2018;53(s3):S4–S11. doi: 10.1002/ppul.24157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol. 2006;291(2):C218–C230. doi: 10.1152/ajpcell.00605.2005. [DOI] [PubMed] [Google Scholar]
- 34.Murphy SV, Ribeiro CMP. Cystic fibrosis inflammation: hyperinflammatory, hypoinflammatory, or both? Am J Respir Cell Mol Biol. 2019;61(3):273–274. doi: 10.1165/rcmb.2019-0107ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung J, et al. Dynamic modulation of ANO1/TMEM16A HCO3(-) permeability by Ca2+/calmodulin. Proc Natl Acad Sci U S A. 2013;110(1):360–365. doi: 10.1073/pnas.1211594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu K, et al. Bestrophin-2 mediates bicarbonate transport by goblet cells in mouse colon. J Clin Invest. 2010;120(5):1722–1735. doi: 10.1172/JCI41129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang F, et al. International union of basic and clinical pharmacology. LXXXV: calcium-activated chloride channels. Pharmacol Rev. 2012;64(1):1–15. doi: 10.1124/pr.111.005009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alka K, Casey JR. Bicarbonate transport in health and disease. IUBMB Life. 2014;66(9):596–615. doi: 10.1002/iub.1315. [DOI] [PubMed] [Google Scholar]
- 39.Soleimani M, et al. Pendrin: an apical Cl-/OH-/HCO3- exchanger in the kidney cortex. Am J Physiol Renal Physiol. 2001;280(2):F356–F364. doi: 10.1152/ajprenal.2001.280.2.F356. [DOI] [PubMed] [Google Scholar]
- 40.Shcheynikov N, et al. The Slc26a4 transporter functions as an electroneutral Cl-/I-/HCO3- exchanger: role of Slc26a4 and Slc26a6 in I- and HCO3- secretion and in regulation of CFTR in the parotid duct. J Physiol. 2008;586(16):3813–3824. doi: 10.1113/jphysiol.2008.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keating D, et al. VX-445-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379(17):1612–1620. doi: 10.1056/NEJMoa1807120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bobadilla JL, et al. Cystic fibrosis: a worldwide analysis of CFTR mutations — correlation with incidence data and application to screening. Hum Mutat. 2002;19(6):575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 43.Van Goor F, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106(44):18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramsey BW, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hisert KB, et al. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med. 2017;195(12):1617–1628. doi: 10.1164/rccm.201609-1954OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris JK, et al. Changes in airway microbiome and inflammation with ivacaftor treatment in patients with cystic fibrosis and the G551D mutation. Ann Am Thorac Soc. 2020;17(2):212–220. doi: 10.1513/AnnalsATS.201907-493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowe SM, et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190(2):175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cholon DM, et al. Potentiator ivacaftor abrogates pharmacological correction of DeltaF508 CFTR in cystic fibrosis. Sci Transl Med. 2014;6(246):246ra96. doi: 10.1126/scitranslmed.3008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veit G, et al. Some gating potentiators, including VX-770, diminish ΔF508-CFTR functional expression. Sci Transl Med. 2014;6(246):246ra97. doi: 10.1126/scitranslmed.3008889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dupuit F, et al. CFTR and differentiation markers expression in non-CF and delta F 508 homozygous CF nasal epithelium. J Clin Invest. 1995;96(3):1601–1611. doi: 10.1172/JCI118199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bitam S, et al. An unexpected effect of TNF-α on F508del-CFTR maturation and function. F1000Res. 2015;4:218. doi: 10.12688/f1000research.6683.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hisert KB, et al. Ivacaftor-induced proteomic changes suggest monocyte defects may contribute to the pathogenesis of cystic fibrosis. Am J Respir Cell Mol Biol. 2016;54(4):594–597. doi: 10.1165/rcmb.2015-0322LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adam RJ, et al. Acute administration of ivacaftor to people with cystic fibrosis and a G551D-CFTR mutation reveals smooth muscle abnormalities. JCI Insight. 2016;1(4):e86183. doi: 10.1172/jci.insight.86183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McShane D, et al. Airway surface pH in subjects with cystic fibrosis. Eur Respir J. 2003;21(1):37–42. doi: 10.1183/09031936.03.00027603. [DOI] [PubMed] [Google Scholar]
- 55.Seliger VI, et al. The predictive potential of the sweat chloride test in cystic fibrosis patients with the G551D mutation. J Cyst Fibros. 2013;12(6):706–713. doi: 10.1016/j.jcf.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Durmowicz AG, et al. Change in sweat chloride as a clinical end point in cystic fibrosis clinical trials: the ivacaftor experience. Chest. 2013;143(1):14–18. doi: 10.1378/chest.12-1430. [DOI] [PubMed] [Google Scholar]
- 57.Barry PJ, et al. Sweat chloride is not a useful marker of clinical response to Ivacaftor. Thorax. 2014;69(6):586–587. doi: 10.1136/thoraxjnl-2013-204532. [DOI] [PubMed] [Google Scholar]
- 58.Fidler MC, et al. Correlation of sweat chloride and percent predicted FEV1 in cystic fibrosis patients treated with ivacaftor. J Cyst Fibros. 2017;16(1):41–44. doi: 10.1016/j.jcf.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Chen K, et al. Antiinflammatory effects of bromodomain and extraterminal domain inhibition in cystic fibrosis lung inflammation. JCI Insight. 2016;1(11):e87168. doi: 10.1172/jci.insight.87168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snodgrass SM, et al. Tgf-β1 inhibits Cftr biogenesis and prevents functional rescue of ΔF508-Cftr in primary differentiated human bronchial epithelial cells. PLoS One. 2013;8(5):e63167. doi: 10.1371/journal.pone.0063167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bomberger JM, et al. A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog. 2011;7(3):e1001325. doi: 10.1371/journal.ppat.1001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stanton BA. Effects of Pseudomonas aeruginosa on CFTR chloride secretion and the host immune response. Am J Physiol Cell Physiol. 2017;312(4):C357–C366. doi: 10.1152/ajpcell.00373.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roesch EA, et al. Inflammation in cystic fibrosis: an update. Pediatr Pulmonol. 2018;53(s3):S30–S50. doi: 10.1002/ppul.24129. [DOI] [PubMed] [Google Scholar]
- 64.Clancy JP, et al. CFTR modulator theratyping: current status, gaps and future directions. J Cyst Fibros. 2019;18(1):22–34. doi: 10.1016/j.jcf.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karp PH, et al. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol. 2002;188:115–137. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- 66.Ramachandran S, et al. Efficient delivery of RNA interference oligonucleotides to polarized airway epithelia in vitro. Am J Physiol Lung Cell Mol Physiol. 2013;305(1):L23–L32. doi: 10.1152/ajplung.00426.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bray NL, et al. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34(5):525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 68.Love MI, et al. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43(W1):W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.