Abstract
Bcl2-associated athanogene-3 (BAG3) is expressed ubiquitously in humans, but its levels are highest in the heart, the skeletal muscle, and the central nervous system; it is also elevated in many cancers. BAG3’s diverse functions are supported by its multiple protein-protein binding domains, which couple with small and large heat shock proteins, members of the Bcl2 family, other antiapoptotic proteins, and various sarcomere proteins. In the heart, BAG3 inhibits apoptosis, promotes autophagy, couples the β-adrenergic receptor with the L-type Ca2+ channel, and maintains the structure of the sarcomere. In cancer cells, BAG3 binds to and supports an identical array of prosurvival proteins, and it may represent a therapeutic target. However, the development of strategies to block BAG3 function in cancer cells may be challenging, as they are likely to interfere with the essential roles of BAG3 in the heart. In this Review, we present the current knowledge regarding the biology of this complex protein in the heart and in cancer and suggest several therapeutic options.
Introduction
An important challenge in modern medicine has been that new drugs, biologics, and even vaccines may be precise in modifying the function of a particular target yet still have off-target effects that can hinder their therapeutic development. When the conflict involves proteins that play critical roles in the physiology of the heart but at the same time facilitate the advance of cancer, both caregivers and patients can be challenged. An excellent example can be found in the ongoing development of Bcl2-associated athanogene-3 (BAG3) agonists for the treatment of heart failure (HF) and the development of BAG3 inhibitors for the treatment of cancer.
Cancer cells are highly stressed by metabolic dysfunction, aberrant RNA splicing, accelerated protein synthesis, metabolic reprogramming, and the accumulation of misfolded proteins and other cellular debris. To survive, cancer cells have developed the ability to enhance the activity of the proteasome and the autophagy pathways while simultaneously blocking apoptosis (1). Thus, a logical strategy for ridding the organism of malignancy would be to inhibit protein quality control (PQC) and activate targeted apoptosis (2). Under this strategy, the cellular debris that accumulates in cancer cells would be cleared by BAG3-responsive prosurvival signaling pathways: autophagy, mitophagy, and the ubiquitin-associated proteasomal pathway (3, 4). Unfortunately, efforts to develop BAG3-targeted anticancer therapeutics have been limited in part by the fact that the heart also requires a robust system of BAG3-dependent PQC. Addressing this type of question served as the foundation for the recent emergence of the field of cardio-oncology and for the recent development of strategies that take advantage of innovations in molecular biochemistry, including gene therapy (5–7).
This therapeutic challenge is best illustrated by the recent development of proteasome inhibitors for the treatment of cancer. One of the first proteasome inhibitors to reach clinical trials was bortezomib, which showed limited ability to modify autophagy flux but blocked the proteasome and upregulated levels of BAG3 (8, 9). By contrast, BAG3 knockdown in ex vivo human leukemic cells potentiated bortezomib’s induction of apoptosis (9). Carfilzomib, a proteasome inhibitor that does not upregulate BAG3, proved to be significantly better than bortezomib in treating cancer in a large head-to-head comparison (10, 11). While the overall incidence of HF was low in both groups, nearly 3-fold more events were attributed to HF in the carfilzomib group (8.2%) compared with the bortezomib group (2.8%).
While BAG3 remains an interesting and important target for new therapies in both cancer and heart disease, it has not been tested in either context. However, recent discoveries regarding both the molecular and the cellular biology of BAG3 raise questions regarding its potential usefulness in cancer because of concerns regarding cardiotoxicity. This discussion will focus attention on two specific diseases that illustrate the therapeutic conundrum in which substantive research relevant to the biology of cancer and heart disease has been carried out: pancreatic ductal adenocarcinoma (PDAC) and hereditary dilated cardiomyopathy with severe heart failure with reduced ejection fraction (HFrEF).
BAG3
BAG3, its name derived from “athanatos,” Greek for “against death,” is the third member of a family of six proteins that bind to the ATPase motif of heat shock cognate 70 (heat shock protein 70; hsp70) through a sequence in the BAG domain (12, 13). That BAG3 plays a critical role in living organisms is supported by the fact that a homolog of BAG3 is expressed in all domains of life, including yeast, invertebrates, amphibians, mammals, and even plants (14). BAG3 is a constitutively expressed, multifunctional, and ubiquitous protein that is most abundant in the heart, skeletal muscle, and central nervous system and in many cancers (3, 15). It limits the intrinsic (mitochondria-dependent) pathway of apoptosis by coupling with Bcl2 and activates macroautophagy by cochaperoning both large and small heat shock proteins. Its expression is induced by stress, proteasome inhibitors, 50 Hz electromagnetic fields (14), and aging (12, 16–20).
BAG3 translocates to the nucleus, where it can bind to its own promoter and autoregulate its transcription (21). Heat shock transcription factor 1 (HSF1) interacts with two elements in BAG3’s promoter to increase its expression in an NF-κB–dependent manner (22). It also appears to be sensitive to less well-known transcription factors including the androgen-regulated protein androgen-induced bZIP (AIbZIP) (23), fibroblast growth factor 2 (FGF-2) (24), and early growth response proteins. BAG3 is also expressed in endothelial cells, where its absence leads to decreased angiogenesis (25).
Mechanism of action of BAG3
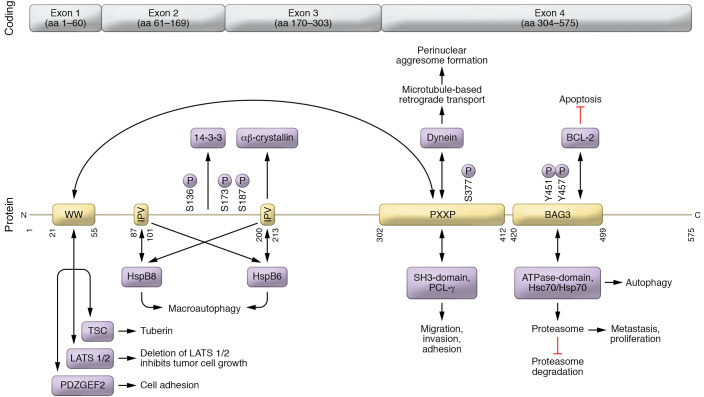
BAG3 interacts with a plethora of signaling pathways that would seem unmanageable were it not for the fact that the protein has numerous protein-protein binding domains that support these different activities. Moving from the carboxy terminus to the amino terminus of the peptide (Figure 1), the first binding site is the BAG domain, which includes a motif that binds the ATPase site of the cochaperone hsp70. The BAG domain also includes the binding site for Bcl2, which initiates the canonical signaling cascade that inhibits the intrinsic limb of apoptosis (26). Next, BAG3’s PXXP domain has two important partners. First, it binds with proteins that have Src domains, as found in phospholipase C-γ or Src, which enhances its ability to regulate cancer cells’ adhesion, migration, and invasion of other cells and tissues (27). Second, it serves as the docking station for the motor protein dynein, a microtubule-based minus-end-directed transport system that carries cargo to perinuclear aggresomes for eventual digestion (16, 28). There are then two Ile-Pro-Val (IPV) domains that bind the small heat shock proteins hspB6 and hspB8 and provide cellular protection during hypoxia/reoxygenation by binding to the heat shock cognate hsc70 (29, 30). Located between the two IPV domains is a binding site for the 14-3-3 protein that targets misfolded proteins to the dynein motor complex (16). The intermediate domain of BAG3 is also a binding site for αβ-crystallin (31). At the amino terminus, the WW domain may interact with proline-rich repeats such as the guanine nucleotide exchange, the pentose base protein, synaptopodin-2, the YAP/TAZ inhibitors LATS1/2 and AMOTL1/2, or the tuberous sclerosis 1 protein (32, 33). The WW domain is required for the induction of autophagy in glioma cells (34).
Figure 1. Structure and function of BAG3.
Binding sites on BAG3 are shown in yellow, and their binding partners are shown in purple. Posttranslational modifications (specifically phosphorylation) are indicated vertically.
BAG3 and the proteasome.
Eukaryotic cells have two primary means of insuring homeostasis and proteostasis: the ubiquitin-proteasome system (UPS) and the autophagy-lysosome system. In the UPS, degradation-prone proteins are first ubiquitinated by the ATP-consuming ubiquitin conjugation machinery. The polyubiquitinated proteins are then degraded by the proteasome, a barrel-shaped and highly conserved multiprotein complex (35). When cells are exposed to stress, they switch from the UPS degradation pathway to the aggresome-lysosome pathways (autophagy) to clear client proteins from the cell. This proteasome-to-autophagy switch is mediated by BAG3 and two proteins that it chaperones: hspB8 and hspA1A.
BAG3 and autophagy.
Autophagy is a complex evolutionarily conserved process that clears the cell of debris that arises from cell division, metabolism, and other cell processes (36). A full description of autophagy is outside the scope of this Review; however, in view of the important role that BAG3 plays in autophagy, a brief introduction is provided. The reader is referred to several excellent reviews for additional information (4, 15, 37, 38).
Autophagy is generally divided into three mechanistic groupings: microautophagy, chaperone-mediated autophagy (CMA), and macroautophagy (Figure 2 and ref. 39). Microautophagy is the process in which cytoplasmic debris directly enters a lysosome through an outpouching of the lysosomal membrane. CMA specifically targets substrates with a biochemical motif related to the sequence KFERQ (40, 41). Misfolded proteins are unfolded through the action of cytosolic chaperones and then translocated across the lysosomal membrane, where they are digested (42). CMA requires lysosomal-associated membrane protein 2A (LAMP2A), hspA8, and hsp90. Macroautophagy is a complex and multistep process in which selective substrates are packaged outside of a lysosome and then transported to the lysosome for degradation (Figure 2). In selective macroautophagy or chaperone-assisted selective autophagy (CASA), the membrane appears to wrap around the cargo in order to fit the specific target (43, 44). The CASA machinery, which impacts the processes of adhesion, migration, and proliferation (44, 45), consists of synaptopodin-2, the WW domain of BAG3, an autophagosome membrane fusion complex, and YAP/TAZ signaling (16, 46–48). Macroautophagy PQC can be impaired by sustained inflammation (49).
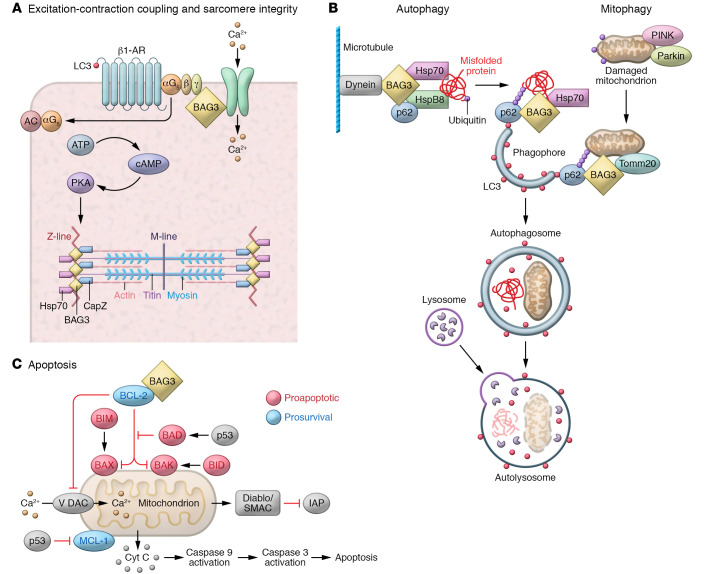
Figure 2. Diverse cellular roles of BAG3.
(A) BAG3 regulates contractility in adult ventricular myocytes, colocalizing with Na+-K+/ATPase and the L-type Ca2+ channel in the sarcolemma and the transverse tubules (t-tubules). Figure 3 shows BAG3’s function in the sarcomere in more detail. (B) BAG3 also plays a central role in autophagy and mitophagy, creating a complex including hsp70 and hspB8 to deliver ubiquitinated, misfolded proteins to the phagophore. This process is both specific and selective. BAG3 is also involved in the maturation of the phagophore into the autophagosome, through its interaction with synaptopodin-2 (SYNPO2) and its associated proteins. BAG3 also serves as an anchoring point for the dynein motor pathway, an intracellular transport system that moves cargo toward the minus ends of microtubules, where they are packaged into perinuclear aggresomes for eventual removal from the cell. (C) Finally, BAG3 couples to Bcl2 to limit the mitochondria-dependent apoptosis pathway.
BAG3 and apoptosis.
BAG3 inhibits apoptosis in the heart and in some cancers through binding to Bcl2 with subsequent inhibition of the canonical intrinsic pathway of apoptosis (50). However, Bcl2 is but one member of the 25-member Bcl2 family. Therefore, it is not surprising that noncanonical BAG3-mediated pathways might also play a role. This is supported by studies of Bcl2 family members in the hematopoietic system, including Bcl-xL and Bax, which have very similar structures and form ion channels in synthetic membranes (51–53) but, as far as we know, are not strongly regulated by Bcl2 (54–57). The reader is referred to the references for additional details.
BAG3 in cancer
A decade ago, heat shock proteins were thought to be ideal targets for cancer drug therapy, because their expression was increased in cancer, (58); overexpression could induce tumor-specific apoptosis in vitro (59); and a small-molecule inhibitor of hsp70 potentiated hsp90 inhibitor–induced apoptosis in colon carcinoma cells (60). However, the overall effect of early pharmaceuticals on the treatment of cancer was modest, causing investigators to pivot to the cochaperone of hsp70, BAG3 (61). BAG3 is an ideal target: (a) it can promote survival through multiple cell pathways; (b) its expression is induced by stress and growth factors found in cancer cells; (c) high levels of expression correlate directly with chemoresistance; and (d) high levels of BAG3 predict a poor outcome in a variety of cancers, including thyroid, breast, liver, leukemias, and metastatic melanoma (62–65). BAG3 protects estrogen receptor-α–positive neuroblastoma and breast cancer cells through an estrogen response element–independent noncanonical autophagy pathway (66), and also protects non–small cell lung cancer cells from apoptosis (67). However, cancer cells do not follow a script, and in epithelial thyroid cancer cells, knockdown of BAG3 induces epithelial-mesenchymal transition and increased migration and invasion (62, 68).
Oncologists, seeing great potential in BAG3 as a therapeutic target, began studies of its mechanism of action in both animal models of cancer and patients with disease. Colvin et al. showed that BAG3 interacts with the SH3 domain of Src, a non-protein tyrosine kinase (27, 69). This interaction resulted in enhanced mediation of Src signaling with hsp70 by modulating the activity of transcription factors including NF-κB, FoxM1, hypoxia-inducible factor-1α, the translation regulator HuR, and the cell cycle regulators p21 and survivin (27). These studies clearly demonstrated that enhanced coupling of BAG3 and hsp70 supported tumor growth whereas an absence of BAG3-hsp70 had the opposite effect (27). These investigations also identified a small-molecule inhibitor of autophagy, YM-1, which disrupted the hsp70-BAG3 interaction, leading to a loss of Src signaling and to a failure of autophagy to regulate the amount of cellular debris and misfolded proteins (27). These results were confirmed by Li and colleagues, who demonstrated that an allosteric inhibitor of the hsp70-BAG3 protein-protein interaction had antiproliferative activity across all cancer cell lines tested (27, 61).
Another BAG3-related signaling mechanism that has been shown to promote survival of cancer cells is the antiapoptotic Bcl2 family member Mcl-1, which prevents programmed cell death by binding to proapoptotic members of the family, including Bax and Bak (70). By contrast, BAG3 knockdown reduced the level of the antiapoptotic Bcl2 protein Bcl-xL (71–76).
Pancreatic cancer.
Studies of BAG3 in pancreatic ductal adenocarcinoma (PDAC) provide the best examples of the complexity of using BAG3 as a target for anticancer therapy. Two decades ago, cancer biologists reported for the first time that BAG3 was overexpressed in human pancreatic cancer and in cell lines derived from these tumors (77). By contrast, BAG3 mRNA levels were not elevated in other gastrointestinal cancers, including hepatocellular carcinoma, esophageal, stomach, and colon cancers. The elevation in BAG3 levels in pancreatic cancer was inversely related to survival (78). Pancreatic cancer was also associated with an increase in anti-BAG3 antibodies, which proved useful in discriminating between neoplastic lesions and normal pancreas and between neoplastic lesions and other causes of pancreatic disease such as pancreatitis (77, 78). The finding that BAG3 was also detectable in the sera of PDAC patients suggested a role for the secreted form of BAG3 in tumor development (79).
In an important study, Rosati et al. found that not only was BAG3 released from human PDAC cells in culture, but BAG3 could be detected in both the exosome and soluble fractions of PDAC cell lines and colocalized with a cytosolic marker for endosomes (80). These results suggested that BAG3 might be secreted through exosomal pathways. The investigators also found that BAG3 attached to receptors on the surface of macrophages, resulting in their activation and the secretion of PDAC supporting factors. The receptor, identified as interferon-induced transmembrane protein 2 (IFITM-2), signals through the PI3K and the p38 MAP kinase pathways. Notably, BAG3/IFITM-2 binding and the subsequent activation of macrophages were independent of hsp70. The extracellular BAG3 activation of the IFITM-2 receptor on macrophages caused the macrophages to secrete factors that stimulate PDAC cell proliferation. Interruption of macrophage activation leads to decreased tumor growth and metastasis. Importantly, IFITM-2 was required for BAG3 binding and signaling, as its deletion mitigated the untoward effects of BAG3 on macrophage activation. The BAG3 antibody did, however, inhibit tumor growth and metastasis. In aggregate, these studies suggested that the BAG3/IFITM-2 pathway plays an important role in cellular homeostasis and could be another novel therapeutic target.
Although BAG3 fulfills its canonical role of enhancing autophagy and inhibiting apoptosis in the pancreas, it also has a noncanonical effect in that it supports the reprograming of the malignant cells in the pancreas to aerobic glycolysis so that energy levels can be achieved that will support the rapid growth and reproduction that characterize pancreatic cancer (81). Investigators have also identified another noncanonical pathway in which BAG3 promotes cell invasion by stabilizing IL-8 transcription via HuR recruitment and subsequently suppressing the loading of microRNA-4312 containing the miRNA-induced silencing complex in PDAC. This novel pathway links BAG3 expression to enhanced PDAC metastasis (82). In aggregate, these studies support the use of BAG3 as a target for the treatment of patients with PDAC; however, that enthusiasm must be tempered by the recognition that the regulation and function of autophagy in pancreatic cancer are complex and likely require precise targeting (83). Finally, it should be noted that BAG3 controls angiogenesis through regulation of ERK phosphorylation, providing another reason to consider BAG3 as a therapeutic target in cancer (25).
BAG3 and the heart
BAG3 genetics and genomics.
The first evidence that BAG3 plays an important part in cardiac homeostasis and proteostasis came, in retrospect, from a genome-wide association study (GWAS) in 2006 by Patrick Ellinor et al. that identified a novel locus for the phenotype of dilated cardiomyopathy, diffuse myocardial fibrosis, and sudden death on chromosome 10q25-26 (84). In 2011, Norton et al. identified a deletion mutation in BAG3 that segregated with family members with a heritable form of dilated cardiomyopathy (DCM) (85). Subsequent studies of probands from multiple countries with hereditary DCM identified disease-causing coding region mutations, the majority of which were either truncations or deletions (86–88). Interestingly, BAG3 levels were also reduced in HF patients with nonheritable forms of DCM (88, 89).
Further confirmation that BAG3 deficiency plays an important role in the pathobiology of HFrEF has come from a group of large GWAS and large proband studies that have identified BAG3 variants as one of 2 to 14 genes that can cause heritable DCM — BAG3 being the second most common after titin in the majority of studies (90–97).
Important information regarding the clinical presentation of BAG3-related disease came from the European Genetic Cardiomyopathies Initiative, describing the clinical epidemiology in over 100 subjects with defined BAG3 genotypes. Presumptive BAG3 mutations were associated with an 80% penetrance and an earlier onset of DCM and a more severe disease in men. The authors also noted a prevalence of truncations or deletions that encompassed the BAG domain and a course of the disease that was more aggressive than seen with other heritable forms of DCM (98).
In 2009, Selcen et al. described the cases of three unrelated children with myofibrillar myopathy who harbored a heterozygous single-nucleotide polymorphism in exon 3 of BAG3 that resulted in a substitution of a leucine for a proline at amino acid position 209 (Pro209Leu) (99). Although this disease does not have a name, it is distinguished from other forms of BAG3-related DCM by its unique phenotype: severe and progressive muscle weakness ultimately resulting in respiratory failure, a restrictive/hypertrophic cardiomyopathy, elevated levels of serum creatine kinase, and neurologic abnormalities including giant axon disease. Recently, investigators reported three missense mutations targeting the same proline 209 codon — each causing a unique phenotype: (a) early cardiomyopathy with distal myopathy (Pro209Leu); (b) late Charcot-Marie-Tooth type 2 neuropathy (Pro209Ser); and (c) late distal myopathy (Pro209Gln) (100). All three Pro209 mutants were found to have a toxic gain-of-function mutation that caused the mutant to aggregate hsp70, and interfere with autophagy (100). However, there is ongoing controversy because a mouse model of the variant did not result in a cardiomyopathy phenotype (101). The reader is therefore referred to several excellent reviews of this disease that is seen predominantly in children (102, 103).
In the more common BAG3 mutations, coding region insertions, or deletions, patients present with a DCM and a positive family history. We now know that BAG3 variants do not necessarily have to cause DCM but can instead influence the phenotype if disease occurs (104). For example, the presence of any one of four variants in patients of African ancestry was associated with a nearly 2-fold increase in either death or worsening HF if the individual developed the disease (104).
BAG3 mechanism of action in the heart.
The first evidence of BAG3’s role in cardiac pathobiology came in 2006 when Homma et al. reported that homozygous deletion of BAG3 in mice led to profound myofibrillar disorganization and death by 4 weeks of age (105). Homma et al. also reported that heterozygotes were normal, but that determination was made at just 4 weeks of age. In 2010, Hishiya et al., using neonatal myocytes in which BAG3 had been knocked down and small cardiac muscle strips from Bag3+/– and WT mice, showed that BAG3 and its cochaperone hsp70 stabilized myofibril structure by forming a complex with the F-actin capping protein CAPZβ1 (106). In the absence of BAG3, even modest stress led to disruption of the myofibril structures (107). Later studies using mice in which one allele of Bag3 was ablated showed that haploinsufficiency was associated with diminished autophagy and increased apoptosis as well as modest left ventricle (LV) dysfunction (108). By contrast with neonatal cardiac myocytes, in adult myocytes BAG3 was predominantly expressed in the sarcolemma and the t-tubules (102, 108). Genetic variants in BAG3 have a similar role in modulating the susceptibility to ischemia in the skeletal musculature, an effect that is strain specific (109).
BAG3 is also cardioprotective during the stress of hypoxia followed by reperfusion (110). When hearts infected with an AAV2/9 vector and the CMV promoter driving the expression of BAG3 underwent exposure to ischemia/reperfusion injury, AAV2/9-BAG3 pretreatment significantly protected the heart. These salutary effects of BAG3 were attributable in part to decreased apoptosis, increased autophagy, and increased autophagic flux (110). BAG3 gene therapy also ameliorated the decrease in LV function seen in hearts after left anterior descending coronary artery occlusion when AAV9-BAG3 was administered two months after the infarction (111). These beneficial effects of BAG3 were accompanied by improved cell shortening, enhanced systolic [Ca2+]i, increased transients in [Ca2+]i amplitudes, and increased maximal L-type Ca2+ current amplitude (112).
To model BAG3 cardiomyopathy in a human system, Judge et al. generated an isogenic series of human induced pluripotent stem cells (iPSCs) with loss-of-function mutations in BAG3 (113). These heterozygous mutations demonstrated reduced protein expression, disrupted myofibril structure, and compromised contractile function. The studies also found that hspB8 is a particularly important partner for BAG3 in cardiomyocytes. Mass spectroscopy suggested that the primary interactions occurred with proteins associated with PQC, ribosomal/RNA binding, nuclear proteins, and secreted proteins (113). However, Judge et al. also reported that the accumulation of ubiquitinated protein after bortezomib treatment was the same in WT and BAG3-deficient cardiomyocytes, suggesting that BAG3 is not required for the regulation of bulk autophagy flux.
Investigators have taken advantage of transgenic technology to evaluate the mechanism by which a point mutation or the loss of a single allele of BAG3 results in DCM (114). Knockin of the human mutation BAG3E455K disrupted the interaction between BAG3 and hsp70 and resulted in a DCM phenotype (114). In both the BAG3-knockout and E455K-knockin mouse, the levels of small heat shock proteins were diminished whereas a subset of proteins that maintain cardiac homeostasis were elevated (114, 115). Similarly, cardiac-specific knockouts of one allele of BAG3 also led to a substantial decrease in LV function and cardiac dilatation that were associated with a decrease in small heat shock proteins (114). Other investigators using a similar cardiac-restricted knockout model reported that the decrease in LV function was associated with a decrease in autophagy and autophagy flux as well as a significant increase in myocardial apoptosis (112). Mice in which the ascending aorta had been banded with eventual development of cardiac dilatation and LV dysfunction and mice harboring muscle LIM protein (MLP) knockout showed a 50% reduction in BAG3 levels, consistent with studies in the human heart (88). Thus, diminished levels of BAG3 appear to be a ubiquitous response to stress in the heart.
Another interesting response to stress was reported by De Marco and colleagues. Using mass spectroscopy to measure BAG3 levels in sera, they found for the first time that BAG3 was released from the heart in patients with DCM and stress (116). BAG3 release was associated with an increase in immune response, as anti-BAG3 antibodies were also measurable in the serum of these patients. However, the release of BAG3 was seen only in patients with class IV symptoms, indicative of severe disease.
BAG3 regulates contractility in the adult heart.
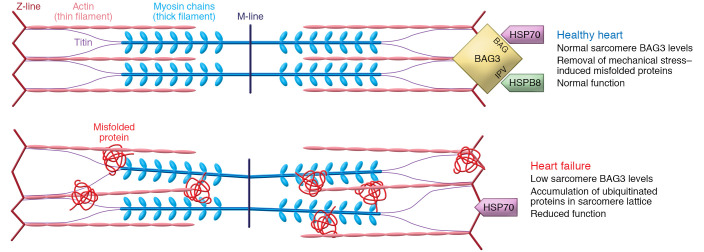
In adult ventricular myocytes, BAG3 is colocalized with Na+/K+-ATPase and the L-type Ca2+ channel in the sarcolemma and the t-tubules (108). In fact, BAG3 coimmunoprecipitated with the β1-adrenergic receptor, the L-type Ca2+ channels, and phospholemman (108). A reduction in maximal calcium-activated force (Fmax) generated by the myofilament is a hallmark of HFrEF in both humans and animal models (117, 118). While the cause of this decline was not known, it was suspected that it arose from structural disorganization and reduced sarcomeric PQC, which is a poorly understood process in the adult heart (119). However, recent work has shown that the BAG3-hsp70-hspB8 complex localizes to the sarcomere to mediate the turnover of a number of clients, such as tropomyosin, myosin light chain, and filamin C (120). In human HF, the decrease in Fmax directly correlated with reduced myofilament and localized BAG3 levels. Heterozygous deletion of Bag3 in mice resulted in a similar phenotype, and AAV-driven restoration of BAG3 in a mouse HF model rescued myofilament function and PQC (Figure 3).
Figure 3. Sarcomere protein quality control requires BAG3.
In the normal heart, the BAG3-hsp70-hspB8 complex localizes to the Z-disc, where it provides chaperone-assisted selective autophagy for sarcomere proteins, which helps to maintain normal contractile function. However, in heart failure (specifically heart failure with reduced ejection fraction, HFrEF), sarcomere-localized BAG3 levels drop, and thus ubiquitinated proteins are not removed and instead stay imbedded in the sarcomere lattice structure. These nuclear, stress-misfolded proteins contribute to the cellular contractile dysfunction observed in heart failure.
The levels of BAG3 in the human heart during HF are not influenced by either age or sex. However, myofilament localization of BAG3 was significantly decreased in cardiac tissue from males with DCM but not in heart tissue from females (121). This difference was independent of estrogen but was closely correlated with changes in LV heat shock transcription factor 1 (HSF1). Specifically, the myofilament-associated HSF1 protein decreases in HF.
BAG3 also plays an important role in modulating the homeostasis of the mitochondria (122). Mitochondrial disruption can result in failure to generate enough ATP and/or the unfettered release of reactive oxygen species, leading to increased apoptosis (123). BAG3 and parkin are recruited to depolarized mitochondria, where they promote mitophagy, whereas suppression of BAG3 significantly reduced autophagy flux and eliminates clearance of TOM20, an essential import receptor for maintaining appropriate levels of mitochondrial proteins (124).
To investigate how a DCM-causing mutation (R477H) could impact myofibrillar organization and the chaperone network in cardiomyocytes, McDermott-Roe et al. generated isogenic genome-edited human iPSC–derived cardiomyocytes (iPSC-CMs) (125). Although normal at baseline, fiber length and alignment declined markedly in R477H and knockout iPSC-CMs following proteasome inhibition with MG132. Proteasome inhibition increased BAG3 and P62 expression in healthy cells, but in mutant cells it caused fiber redistribution, fiber disarray, accumulation of ubiquitinated proteins, and enhanced expression of the autophagy pathway proteins BAG3, P62, and LC3II.
Connexin 43 (Cx43) is a critical protein in the heart that can rapidly change its function by altering the gap junction and slowing cell-cell communication. Early studies suggested that impairment of the autophagy-lysosome pathway degraded Cx43 by either inhibiting lysosomal activity or diminishing the level of BAG3 (126). Inhibition of lysosomal activity enhanced accumulation of Cx43 aggregates and suppressed BAG3 turnover. Knockdown of BAG3 dysregulated Cx43 protein degradation and decreased the levels of Cx43 by changing the stability of the protein. BAG3 can also signal the accumulation of Cx43, suggesting that BAG3 plays a role in Cx43 turnover under normal circumstances and during stress. In fact, when cells were starved, there was a reduction in levels of Cx43-P1 and Cx43-P2, forms of Cx43 that may reflect posttranslational (phosphorylation) modifications. Knockdown of BAG3 reduced levels of Cx43 by dysregulating Cx43 protein stability, and it impaired lysosomal turnover of Cx43.
Based on recent work by Xi Fang, we also know that small heat shock proteins closely monitor protein folding for a variety of tissues, including the heart (127). Specifically, they mediate chaperone functions by interacting with BAG3 at the two IPV motifs independent of the actions of the BAG, WW, and PXXP domains. However, the presence of hsp70 may be necessary for these interactions to occur (114). Elevated levels of hspB8 have been found in both animal models and patients with both acute and chronic cardiovascular disease, including acute and chronic myocardial infarction, chronic myocardial ischemia, and pressure overload–induced cardiac hypertrophy.
Targeting BAG3: protecting the heart while treating cancer.
To illustrate the complexity that may arise in treating life-threatening illnesses with agents that lack tissue specificity, we sought examples of a pair of diseases where the therapeutic goal was to inhibit a specific protein to treat one of the diseases but the therapeutic goal for the second disease was to stimulate that same pathway. We also looked for diseases that affected large numbers of patients, that had substantial morbidity and/or mortality, and for which the pharmacologic effects of the therapy were not organ specific. HF and lung cancer were ideal for illustrating this challenge. Both affect large numbers of patients: HF is the most common diagnosis at the time of discharge in individuals over the age of 65 who are hospitalized in the United States, and its incidence is increasing. Lung cancer is the second most common form of cancer, following prostate cancer in men and breast cancer in women. Although the rate of lung cancer has decreased and survival improved for men, the opposite is true for women (128).
From a teleological perspective, BAG3 is unique in that it serves multiple purposes that vary depending on cell type, as opposed to other proteins that do the same thing in multiple cells. In fact, BAG3’s multiple roles in cell survival appear to make its therapeutic reduction more complicated. In fact, in the heart, BAG3 maintains tissue homeostasis and proteostasis through the very same pathways by which it supports the growth and survival of hematopoietic cancers and solid tumors — pro-autophagy and antiapoptosis. However, the studies with bortezomib and carfilzomib provide an important clue: cardiac toxicity was seen in only a very small percentage of subjects receiving either of these two agents.
The results of the bortezomib-carfilzomib studies described above led us to hypothesize that under normal situations, systemic pharmacologic inhibition of BAG3 is not robust enough to alter cardiac function because of inherent redundancy in the autophagy and apoptosis signaling pathways. This hypothesis is supported by the finding that when the proteasome is inhibited, BAG3 and the heat shock proteins can pivot and transport cargo to aggresomes and lysosomal autophagic pathways. Similarly, if the endogenous pathway of apoptosis is inhibited, the cell can pivot and utilize the exogenous (mitochondria-independent) pathway or ship misfolded proteins as appropriate to the proteasome. The theory that loss-of-function genetic mutations in cardioprotective pathways can be associated with a higher incidence of side effects of targeted anticancer therapy is supported by the demonstration of a carfilzomib-sensitive PDAC subgroup with a specific transcriptomic phenotype that could explain the group’s increased sensitivity to the agent (129). This means of interpreting the effectiveness of anticancer therapies and their risk to noncancerous cells and tissues based on genotype is a novel concept, but one that that has gained recent attention (130).
Significant advances in gene therapy and innovations in delivery systems including viral vectors with enhanced tropism, vectors with diminished immunogenicity, and gene therapy that can be delivered on a chip or by other novel delivery systems may also be helpful in preventing new chemotherapeutic agents from causing cardiotoxicity in patients who are at increased risk. We believe that we could learn a great deal more about cardiotoxicity in anticancer therapy and whether there are potential opportunities to mitigate off-target effects if oncologists begin to genotype both the tumor and the person who harbors the tumor (130). Similarly, cardiologists need to begin to genotype patients with new-onset DCM as we continue to recognize new genetic causes of this common disease. A recent report suggests that important information regarding target identification may also be gleaned from new and novel serum biomarkers (131). One potential biomarker is TNF-α, which activates the death receptor pathway, which in turn may lead to cell survival, apoptosis, or a regulated form of necrosis (108). The signaling pathway can then travel alternative paths, activating cell survival, apoptosis, or necrosis. A major decision point is the choice between apoptosis or necrosis — a choice predicated on the activity of caspases (132).
Several clinical points regarding the potential of gene therapy also merit mention. First, because the expression of the transfected gene is often driven by a viral promoter, there is a theoretical concern that “overexpression” could have undue consequences. However, in the case of BAG3, the gene self-regulates, and therefore, endogenous expression should turn off if levels get too high (21). Second, BAG3, when driven by an AAV9 vector, does not integrate into the genome and thus is not passed on to daughter cells during cell division. The corollary of this observation is that therapeutic BAG3 does not serve as an oncogene. Third, in patients with single-nucleotide polymorphisms that are not amenable to traditional gene replacement therapy because of potential dominant-negative effects, CRISPR/Cas9 may soon be used to correct functional single-nucleotide variants. And finally, while this Review has focused on the heart and cancer, we would be disingenuous if we did not report that an increasing number of studies suggest that targeting BAG3 may also be effective in the treatment of diseases attributable to abnormalities in PQC in the central nervous system, including Huntington’s disease, amyotrophic lateral sclerosis, Parkinson’s disease, and Alzheimer’s disease (133–135). The development of a BAG3-targeted gene therapy for neurodegenerative diseases is an exciting new area in the field of PQC and regulation of apoptosis. By contrast with cancer, neurodegenerative disease requires adequate levels of BAG3 consistent with the myocardium. Research is also under way in a hunt for small molecules that might target BAG3 and increase its expression. Regardless of approach, BAG3 remains an enticing target for therapeutic gene and drug discovery.
Acknowledgments
This work was supported by NIH grants HL136737 to JAK and HL91799 and HL12309 to AMF and a grant from Renovacor Inc. to AMF.
Version 1. 08/16/2021
Electronic publication
Footnotes
Conflict of interest: AMF is the founder of Renovacor Inc. and was a director of the company until it became public in 2021. His conflicts include ownership of equity, income, and research support. He is the inventor on US patent US20170016066A1 and European Union patent WO2019237002A1 (Optimization d’une thérapie génique de bag3). All patents have been licensed by Temple University to Renovacor Inc. JYC is an equity holder in Renovacor Inc.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(16):e149415.https://doi.org/10.1172/JCI149415.
Contributor Information
Jonathan A. Kirk, Email: kirk.jonathan@gmail.com.
Joseph Y. Cheung, Email: jcheung6@bwh.harvard.edu.
References
- 1.Nandi D, et al. The ubiquitin-proteasome system. J Biosci. 2006;31(1):137–155. doi: 10.1007/BF02705243. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, et al. Proteasome dysregulation in human cancer: implications for clinical therapies. Cancer Metastasis Rev. 2017;36(4):703–716. doi: 10.1007/s10555-017-9704-y. [DOI] [PubMed] [Google Scholar]
- 3.Behl C. Breaking BAG: the co-chaperone BAG3 in health and disease. Trends Pharmacol Sci. 2016;37(8):672–688. doi: 10.1016/j.tips.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Kogel D, et al. At the crossroads of apoptosis and autophagy: multiple roles of the co-chaperone BAG3 in stress and therapy resistance of cancer. Cells. 2020;9(3):574. doi: 10.3390/cells9030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narayan V, Ky B. Common cardiovascular complications of cancer therapy: epidemiology, risk prediction, and prevention. Annu Rev Med. 2018;69:97–111. doi: 10.1146/annurev-med-041316-090622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamo CE, et al. Cancer therapy–related cardiac dysfunction and heart failure. Part 2: Prevention, treatment, guidelines, and future directions. Circ Heart Fail. 2016;9(2):e002843. doi: 10.1161/CIRCHEARTFAILURE.115.002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganatra S, et al. Management of cardiovascular disease during coronavirus disease (COVID-19) pandemic. Trends Cardiovasc Med. 2020;30(6):315–325. doi: 10.1016/j.tcm.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du ZX, et al. Caspase-dependent cleavage of BAG3 in proteasome inhibitors-induced apoptosis in thyroid cancer cells. Biochem Biophys Res Commun. 2008;369(3):894–898. doi: 10.1016/j.bbrc.2008.02.112. [DOI] [PubMed] [Google Scholar]
- 9.Liu P, et al. BAG3 gene silencing sensitizes leukemic cells to Bortezomib-induced apoptosis. FEBS Lett. 2009;583(2):401–406. doi: 10.1016/j.febslet.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 10.Dimopoulos MA, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos MA, et al. Carfilozomib versus bortezomib for relapsed or refractory myeloma — authors’ reply. Lancet Oncol. 2016;17:e126. doi: 10.1016/S1470-2045(16)00145-5. [DOI] [PubMed] [Google Scholar]
- 12.Takayama S, et al. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274(2):781–786. doi: 10.1074/jbc.274.2.781. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, et al. Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene. 1999;18(46):6183–6190. doi: 10.1038/sj.onc.1203043. [DOI] [PubMed] [Google Scholar]
- 14.Rosati A, et al. BAG3: a multifaceted protein that regulates major cell pathways. Cell Death Dis. 2011;2:e141. doi: 10.1038/cddis.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzullo L, et al. The multiple activities of BAG3 protein: mechanisms. Biochim Biophys Acta Gen Subj. 2020;1864(8):129628. doi: 10.1016/j.bbagen.2020.129628. [DOI] [PubMed] [Google Scholar]
- 16.Gamerdinger M, et al. BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO Rep. 2011;12(2):149–156. doi: 10.1038/embor.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thress K, et al. Scythe: a novel reaper-binding apoptotic regulator. EMBO J. 1998;17(21):6135–6143. doi: 10.1093/emboj/17.21.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moribe Y, et al. Samui, a novel cold-inducible gene, encoding a protein with a BAG domain similar to silencer of death domains (SODD/BAG-4), isolated from Bombyx diapause eggs. Eur J Biochem. 2001;268(12):3432–3442. doi: 10.1046/j.1432-1327.2001.02244.x. [DOI] [PubMed] [Google Scholar]
- 19.Briknarova K, et al. Structural analysis of BAG1 cochaperone and its interactions with Hsc70 heat shock protein. Nat Struct Biol. 2001;8(4):349–352. doi: 10.1038/86236. [DOI] [PubMed] [Google Scholar]
- 20.Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 2001;3(10):E237–E241. doi: 10.1038/ncb1001-e237. [DOI] [PubMed] [Google Scholar]
- 21.Gentilella A, Khalili K. Autoregulation of co-chaperone BAG3 gene transcription. J Cell Biochem. 2009;108(5):1117–1124. doi: 10.1002/jcb.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franceschelli S, et al. Bag3 gene expression is regulated by heat shock factor 1. J Cell Physiol. 2008;215(3):575–577. doi: 10.1002/jcp.21397. [DOI] [PubMed] [Google Scholar]
- 23.Qi H, et al. AIbZIP, a novel bZIP gene located on chromosome 1q21.3 that is highly expressed in prostate tumors and of which the expression is up-regulated by androgens in LNCaP human prostate cancer cells. Cancer Res. 2002;62(3):721–733. [PubMed] [Google Scholar]
- 24.Gentilella A, Khalili K. BAG3 expression is sustained by FGF2 in neural progenitor cells and impacts cell proliferation. Cell Cycle. 2010;9(20):4245–4247. doi: 10.4161/cc.9.20.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falco A, et al. BAG3 controls angiogenesis through regulation of ERK phosphorylation. Oncogene. 2012;31(50):5153–5161. doi: 10.1038/onc.2012.17. [DOI] [PubMed] [Google Scholar]
- 26.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 27.Colvin TA, et al. Hsp70-Bag3 interactions regulate cancer-related signaling networks. Cancer Res. 2014;74(17):4731–4740. doi: 10.1158/0008-5472.CAN-14-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kassis JN, et al. CAIR-1/BAG-3 modulates cell adhesion and migration by downregulating activity of focal adhesion proteins. Exp Cell Res. 2006;312(15):2962–2971. doi: 10.1016/j.yexcr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Ni E, et al. The PXXP domain is critical for the protective effect of BAG3 in cardiomyocytes. Clin Exp Pharmacol Physiol. 2019;46(5):435–443. doi: 10.1111/1440-1681.13031. [DOI] [PubMed] [Google Scholar]
- 30.Carra S. The stress-inducible HspB8-Bag3 complex induces the eIF2alpha kinase pathway: implications for protein quality control and viral factory degradation? Autophagy. 2009;5(3):428–429. doi: 10.4161/auto.5.3.7894. [DOI] [PubMed] [Google Scholar]
- 31.Hishiya A, et al. BAG3 directly interacts with mutated alphaB-crystallin to suppress its aggregation and toxicity. PLoS One. 2011;6(3):e16828. doi: 10.1371/journal.pone.0016828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gout E, et al. Co-chaperone BAG3 and adenovirus penton base protein partnership. J Cell Biochem. 2010;111(3):699–708. doi: 10.1002/jcb.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulbricht A, et al. Cellular mechanotransduction relies on tension-induced and chaperone-assisted autophagy. Curr Biol. 2013;23(5):430–435. doi: 10.1016/j.cub.2013.01.064. [DOI] [PubMed] [Google Scholar]
- 34.Merabova N, et al. WW domain of BAG3 is required for the induction of autophagy in glioma cells. J Cell Physiol. 2015;230(4):831–841. doi: 10.1002/jcp.24811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saeki Y, Tanaka K. Assembly and function of the proteasome. Methods Mol Biol. 2012;832:315–337. doi: 10.1007/978-1-61779-474-2_22. [DOI] [PubMed] [Google Scholar]
- 36.Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20(3):460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiebel C, et al. BAG3 proteomic signature under proteostasis stress. Cells. 2020;9(11):E2416. doi: 10.3390/cells9112416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giudice A, et al. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol Biol. 2010;647:37–74. doi: 10.1007/978-1-60761-738-9_3. [DOI] [PubMed] [Google Scholar]
- 39.Sturner E, Behl C. The role of the multifunctional BAG3 protein in cellular protein quality control and in disease. Front Mol Neurosci. 2017;10:177. doi: 10.3389/fnmol.2017.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park JS, et al. Regulation of amyloid precursor protein processing by its KFERQ motif. BMB Rep. 2016;49(6):337–342. doi: 10.5483/BMBRep.2016.49.6.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dice JF. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem Sci. 1990;15(8):305–309. doi: 10.1016/0968-0004(90)90019-8. [DOI] [PubMed] [Google Scholar]
- 42.Orenstein SJ, Cuervo AM. Chaperone-mediated autophagy: molecular mechanisms and physiological relevance. Semin Cell Dev Biol. 2010;21(7):719–726. doi: 10.1016/j.semcdb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mijaljica D, et al. The intriguing life of autophagosomes. Int J Mol Sci. 2012;13(3):3618–3635. doi: 10.3390/ijms13033618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(suppl 2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosokawa N, et al. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy. 2009;5(7):973–979. doi: 10.4161/auto.5.7.9296. [DOI] [PubMed] [Google Scholar]
- 46.Gamerdinger M, et al. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 2009;28(7):889–901. doi: 10.1038/emboj.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carra S, et al. HspB8 participates in protein quality control by a non-chaperone-like mechanism that requires eIF2{alpha} phosphorylation. J Biol Chem. 2009;284(9):5523–5532. doi: 10.1074/jbc.M807440200. [DOI] [PubMed] [Google Scholar]
- 48.Gamerdinger M, et al. Emerging roles of molecular chaperones and co-chaperones in selective autophagy: focus on BAG proteins. J Mol Med (Berl) 2011;89(12):1175–1182. doi: 10.1007/s00109-011-0795-6. [DOI] [PubMed] [Google Scholar]
- 49.Hartupee J, et al. Impaired protein quality control during left ventricular remodeling in mice with cardiac restricted overexpression of tumor necrosis factor. Circ Heart Fail. 2017;10(12):e004252. doi: 10.1161/CIRCHEARTFAILURE.117.004252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haudek SB, et al. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J Clin Invest. 2007;117(9):2692–2701. doi: 10.1172/JCI29134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minn AJ, et al. Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature. 1997;385(6614):353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 52.Antonsson B, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277(5324):370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki M, et al. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103(4):645–654. doi: 10.1016/S0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 54.Eskes R, et al. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20(3):929–935. doi: 10.1128/MCB.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei MC, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14(16):2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 56.Sionov RV, et al. Regulation of bim in health and disease. Oncotarget. 2015;6(27):23058–23134. doi: 10.18632/oncotarget.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schenk RL, et al. Characterisation of mice lacking all functional isoforms of the pro-survival BCL-2 family member A1 reveals minor defects in the haematopoietic compartment. Cell Death Differ. 2017;24(3):534–545. doi: 10.1038/cdd.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wisen S, et al. Binding of a small molecule at a protein-protein interface regulates the chaperone activity of hsp70-hsp40. ACS Chem Biol. 2010;5(6):611–622. doi: 10.1021/cb1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Powers MV, et al. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell. 2008;14(3):250–262. doi: 10.1016/j.ccr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Massey AJ, et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother Pharmacol. 2010;66(3):535–545. doi: 10.1007/s00280-009-1194-3. [DOI] [PubMed] [Google Scholar]
- 61.Li X, et al. Validation of the Hsp70-Bag3 protein-protein interaction as a potential therapeutic target in cancer. Mol Cancer Ther. 2015;14(3):642–648. doi: 10.1158/1535-7163.MCT-14-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meng X, et al. Knockdown of BAG3 induces epithelial-mesenchymal transition in thyroid cancer cells through ZEB1 activation. Cell Death Dis. 2014;5:e1092. doi: 10.1038/cddis.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shields S, et al. BAG3 promotes tumour cell proliferation by regulating EGFR signal transduction pathways in triple negative breast cancer. Oncotarget. 2018;9(21):15673–15690. doi: 10.18632/oncotarget.24590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao H, et al. BAG3 and HIF-1 α coexpression detected by immunohistochemistry correlated with prognosis in hepatocellular carcinoma after liver transplantation. Biomed Res Int. 2014;2014:516518. doi: 10.1155/2014/516518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guerriero L, et al. BAG3 protein expression in melanoma metastatic lymph nodes correlates with patients’ survival. Cell Death Dis. 2014;5:e1173. doi: 10.1038/cddis.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Felzen V, et al. Estrogen receptor α regulates non-canonical autophagy that provides stress resistance to neuroblastoma and breast cancer cells and involves BAG3 function. Cell Death Dis. 2015;6:e1812. doi: 10.1038/cddis.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, et al. Bag3 promotes resistance to apoptosis through Bcl-2 family members in non-small cell lung cancer. Oncol Rep. 2012;27(1):109–113. doi: 10.3892/or.2011.1486. [DOI] [PubMed] [Google Scholar]
- 68.Li N, et al. PKCδ-mediated phosphorylation of BAG3 at Ser187 site induces epithelial-mesenchymal transition and enhances invasiveness in thyroid cancer FRO cells. Oncogene. 2013;32(38):4539–4548. doi: 10.1038/onc.2012.466. [DOI] [PubMed] [Google Scholar]
- 69.Colvin TA, et al. Proteotoxicity is not the reason for the dependence of cancer cells on the major chaperone Hsp70. Cell Cycle. 2014;13(14):2306–2310. doi: 10.4161/cc.29296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boiani M, et al. The stress protein BAG3 stabilizes Mcl-1 protein and promotes survival of cancer cells and resistance to antagonist ABT-737. J Biol Chem. 2013;288(10):6980–6990. doi: 10.1074/jbc.M112.414177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacobs AT, Marnett LJ. HSF1-mediated BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated colon cancer cells via stabilization of anti-apoptotic Bcl-2 proteins. J Biol Chem. 2009;284(14):9176–9183. doi: 10.1074/jbc.M808656200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sugio A, et al. BAG3 upregulates Mcl-1 through downregulation of miR-29b to induce anticancer drug resistance in ovarian cancer. Gynecol Oncol. 2014;134(3):615–623. doi: 10.1016/j.ygyno.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 73.Verdecchia A, et al. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8(9):784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 74.Pacini F, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154(6):787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 75.Nucera C, Pontecorvi A. Clinical outcome, role of BRAF(V600E), and molecular pathways in papillary thyroid microcarcinoma: is it an indolent cancer or an early stage of papillary thyroid cancer? Front Endocrinol (Lausanne) 2012;3:33. doi: 10.3389/fendo.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiappetta G, et al. BAG3 down-modulation reduces anaplastic thyroid tumor growth by enhancing proteasome-mediated degradation of BRAF protein. J Clin Endocrinol Metab. 2012;97(1):E115–E120. doi: 10.1210/jc.2011-0484. [DOI] [PubMed] [Google Scholar]
- 77.Liao Q, et al. The anti-apoptotic protein BAG-3 is overexpressed in pancreatic cancer and induced by heat stress in pancreatic cancer cell lines. FEBS Lett. 2001;503(2-3):151–157. doi: 10.1016/S0014-5793(01)02728-4. [DOI] [PubMed] [Google Scholar]
- 78.Rosati A, et al. Expression of the antiapoptotic protein BAG3 is a feature of pancreatic adenocarcinoma and its overexpression is associated with poorer survival. Am J Pathol. 2012;181(5):1524–1529. doi: 10.1016/j.ajpath.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 79.Falco A, et al. BAG3 is a novel serum biomarker for pancreatic adenocarcinomas. Am J Gastroenterol. 2013;108(7):1178–1180. doi: 10.1038/ajg.2013.128. [DOI] [PubMed] [Google Scholar]
- 80.Rosati A, et al. BAG3 promotes pancreatic ductal adenocarcinoma growth by activating stromal macrophages. Nat Commun. 2015;6:8695. doi: 10.1038/ncomms9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cairns RA, et al. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 82.Li C, et al. BAG3 regulates stability of IL-8 mRNA via interplay between HuR and miR-4312 in PDACs. Cell Death Dis. 2018;9(9):863. doi: 10.1038/s41419-018-0874-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li J, et al. Regulation and function of autophagy in pancreatic cancer. Autophagy. doi: 10.1080/15548627.2020.1847462. [published online November 20, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ellinor PT, et al. A novel locus for dilated cardiomyopathy, diffuse myocardial fibrosis, and sudden death on chromosome 10q25-26. J Am Coll Cardiol. 2006;48(1):106–111. doi: 10.1016/j.jacc.2006.01.079. [DOI] [PubMed] [Google Scholar]
- 85.Norton N, et al. Genome-wide studies of copy number variation and exome sequencing identify rare variants in BAG3 as a cause of dilated cardiomyopathy. Am J Hum Genet. 2011;88(3):273–282. doi: 10.1016/j.ajhg.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chami N, et al. Nonsense mutations in BAG3 are associated with early-onset dilated cardiomyopathy in French Canadians. Can J Cardiol. 2014;30(12):1655–1661. doi: 10.1016/j.cjca.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 87.Franaszczyk M, et al. The BAG3 gene variants in Polish patients with dilated cardiomyopathy: four novel mutations and a genotype-phenotype correlation. J Transl Med. 2014;12:192. doi: 10.1186/1479-5876-12-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feldman AM, et al. Decreased levels of BAG3 in a family with a rare variant and in idiopathic dilated cardiomyopathy. J Cell Physiol. 2014;229(11):1697–1702. doi: 10.1002/jcp.24615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Toro R, et al. Familial dilated cardiomyopathy caused by a novel frameshift in the BAG3 gene. PLoS One. 2016;11(7):e0158730. doi: 10.1371/journal.pone.0158730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah S, et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. 2020;11(1):163. doi: 10.1038/s41467-019-13690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Esslinger U, et al. Exome-wide association study reveals novel susceptibility genes to sporadic dilated cardiomyopathy. PLoS One. 2017;12(3):e0172995. doi: 10.1371/journal.pone.0172995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mazzarotto F, et al. Reevaluating the genetic contribution of monogenic dilated cardiomyopathy. Circulation. 2020;141(5):387–398. doi: 10.1161/CIRCULATIONAHA.119.037661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Choquet H, et al. Meta-analysis of 26 638 individuals identifies two genetic loci associated with left ventricular ejection fraction. Circ Genom Precis Med. 2020;13(4):e002804. doi: 10.1161/CIRCGEN.119.002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aung N, et al. Genome-wide analysis of left ventricular image-derived phenotypes identifies fourteen loci associated with cardiac morphogenesis and heart failure development. Circulation. 2019;140(16):1318–1330. doi: 10.1161/CIRCULATIONAHA.119.041161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jordan E, et al. An evidence-based assessment of genes in dilated cardiomyopathy. Circulation. doi: 10.1161/circulationaha.120.053033. [published online May 5, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morales A, et al. Variant interpretation for dilated cardiomyopathy: refinement of the American College of Medical Genetics and Genomics/ClinGen guidelines for the DCM Precision Medicine Study. Circ Genom Precis Med. 2020;13(2):e002480. doi: 10.1161/CIRCGEN.119.002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Denus S, et al. A genetic association study of heart failure: more evidence for the role of BAG3 in idiopathic dilated cardiomyopathy. ESC Heart Fail. 2020;7(6):4384–4389. doi: 10.1002/ehf2.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dominguez F, et al. Dilated cardiomyopathy due to BLC2-associated athanogene 3 (BAG3) mutations. J Am Coll Cardiol. 2018;72(20):2471–2481. doi: 10.1016/j.jacc.2018.08.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Selcen D, et al. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann Neurol. 2009;65(1):83–89. doi: 10.1002/ana.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adriaenssens E, et al. BAG3 Pro209 mutants associated with myopathy and neuropathy relocate chaperones of the CASA-complex to aggresomes. Sci Rep. 2020;10(1):8755. doi: 10.1038/s41598-020-65664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fang X, et al. P209L mutation in Bag3 does not cause cardiomyopathy in mice. Am J Physiol Heart Circ Physiol. 2019;316(2):H392–H399. doi: 10.1152/ajpheart.00714.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meister-Broekema M, et al. Myopathy associated BAG3 mutations lead to protein aggregation by stalling Hsp70 networks. Nat Commun. 2018;9(1):5342. doi: 10.1038/s41467-018-07718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Quintana MT, et al. Cardiomyocyte-specific human Bcl2-associated anthanogene 3 P209L expression induces mitochondrial fragmentation, Bcl2-associated anthanogene 3 haploinsufficiency, and activates p38 signaling. Am J Pathol. 2016;186(8):1989–2007. doi: 10.1016/j.ajpath.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Myers VD, et al. Association of variants in BAG3 with cardiomyopathy outcomes in African American individuals. JAMA Cardiol. 2018;3(10):929–938. doi: 10.1001/jamacardio.2018.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Homma S, et al. BAG3 deficiency results in fulminant myopathy and early lethality. Am J Pathol. 2006;169(3):761–773. doi: 10.2353/ajpath.2006.060250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hishiya A, et al. BAG3 and Hsc70 interact with actin capping protein CapZ to maintain myofibrillar integrity under mechanical stress. Circ Res. 2010;107(10):1220–1231. doi: 10.1161/CIRCRESAHA.110.225649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iwasaki M, et al. BAG3 directly associates with guanine nucleotide exchange factor of Rap1, PDZGEF2, and regulates cell adhesion. Biochem Biophys Res Commun. 2010;400(3):413–418. doi: 10.1016/j.bbrc.2010.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Feldman AM, et al. BAG3 regulates contractility and Ca(2+) homeostasis in adult mouse ventricular myocytes. J Mol Cell Cardiol. 2016;92:10–20. doi: 10.1016/j.yjmcc.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McClung JM, et al. BAG3 (Bcl-2-associated athanogene-3) coding variant in mice determines susceptibility to ischemic limb muscle myopathy by directing autophagy. Circulation. 2017;136(3):281–296. doi: 10.1161/CIRCULATIONAHA.116.024873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Su F, et al. Bcl-2-associated athanogene 3 protects the heart from ischemia/reperfusion injury. JCI Insight. 2016;1(19):e90931. doi: 10.1172/jci.insight.90931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Knezevic T, et al. Adeno-associated virus serotype 9–driven expression of BAG3 improves left ventricular function in murine hearts with left ventricular dysfunction secondary to a myocardial infarction. JACC Basic Transl Sci. 2016;1(7):647–656. doi: 10.1016/j.jacbts.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Myers VD, et al. Haplo-insufficiency of Bcl2-associated athanogene 3 in mice results in progressive left ventricular dysfunction, β-adrenergic insensitivity, and increased apoptosis. J Cell Physiol. 2018;233(9):6319–6326. doi: 10.1002/jcp.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Judge LM, et al. A BAG3 chaperone complex maintains cardiomyocyte function during proteotoxic stress. JCI Insight. 2017;2(14):e94623. doi: 10.1172/jci.insight.94623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fang X, et al. Loss-of-function mutations in co-chaperone BAG3 destabilize small HSPs and cause cardiomyopathy. J Clin Invest. 2017;127(8):3189–3200. doi: 10.1172/JCI94310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Villard E, et al. A genome-wide association study identifies two loci associated with heart failure due to dilated cardiomyopathy. Eur Heart J. 2011;32(9):1065–1076. doi: 10.1093/eurheartj/ehr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.De Marco M, et al. Detection of soluble BAG3 and anti-BAG3 antibodies in patients with chronic heart failure. Cell Death Dis. 2013;4:e495. doi: 10.1038/cddis.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kirk JA, et al. Pacemaker-induced transient asynchrony suppresses heart failure progression. Sci Transl Med. 2015;7(319):319ra207. doi: 10.1126/scitranslmed.aad2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blair CA, et al. Heart failure in humans reduces contractile force in myocardium from both ventricles. JACC Basic Transl Sci. 2020;5(8):786–798. doi: 10.1016/j.jacbts.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martin TG, Kirk JA. Under construction: the dynamic assembly, maintenance, and degradation of the cardiac sarcomere. J Mol Cell Cardiol. 2020;148:89–102. doi: 10.1016/j.yjmcc.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martin TG, et al. Cardiomyocyte contractile impairment in heart failure results from reduced BAG3-mediated sarcomeric protein turnover. Nat Commun. 2021;12(1):2942. doi: 10.1038/s41467-021-23272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martin TG, et al. BAG3 expression and sarcomere localization in the human heart are linked to HSF-1 and are differentially affected by sex and disease. Am J Physiol Heart Circ Physiol. 2021;320(6):H2339–H2350. doi: 10.1152/ajpheart.00419.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palikaras K, Tavernarakis N. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp Gerontol. 2014;56:182–188. doi: 10.1016/j.exger.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 123.Selivanov VA, et al. Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. PLoS Comput Biol. 2011;7(3):e1001115. doi: 10.1371/journal.pcbi.1001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tahrir FG, et al. Evidence for the role of BAG3 in mitochondrial quality control in cardiomyocytes. J Cell Physiol. 2017;232(4):797–805. doi: 10.1002/jcp.25476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McDermott-Roe C, et al. Investigation of a dilated cardiomyopathy-associated variant in BAG3 using genome-edited iPSC-derived cardiomyocytes. JCI Insight. 2019;4(22):e128799. doi: 10.1172/jci.insight.128799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ghasemi Tahrir F, et al. Role of Bcl2-associated athanogene 3 in turnover of gap junction protein, connexin 43, in neonatal cardiomyocytes. Sci Rep. 2019;9(1):7658. doi: 10.1038/s41598-019-44139-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fang X, et al. The BAG3-dependent and -independent roles of cardiac small heat shock proteins. JCI Insight. 2019;4(4):e126464. doi: 10.1172/jci.insight.126464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Siegel RL, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 129.Fraunhoffer NA, et al. Evidencing a pancreatic ductal adenocarcinoma subpopulation sensitive to the proteasome inhibitor carfilzomib. Clin Cancer Res. 2020;26(20):5506–5519. doi: 10.1158/1078-0432.CCR-20-1232. [DOI] [PubMed] [Google Scholar]
- 130.Garcia-Pavia P, et al. Genetic variants associated with cancer therapy-induced cardiomyopathy. Circulation. 2019;140(1):31–41. doi: 10.1161/CIRCULATIONAHA.118.037934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Narayan V, et al. Mechanistic biomarkers informative of both cancer and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(21):2726–2737. doi: 10.1016/j.jacc.2020.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guo X, et al. Cardioprotective role of tumor necrosis factor receptor-associated factor 2 by suppressing apoptosis and necroptosis. Circulation. 2017;136(8):729–742. doi: 10.1161/CIRCULATIONAHA.116.026240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cristofani R, et al. A crucial role for the protein quality control system in motor neuron diseases. Front Aging Neurosci. 2020;12:191. doi: 10.3389/fnagi.2020.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ravikumar B, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36(6):585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 135.Lei Z, et al. BAG3 facilitates the clearance of endogenous tau in primary neurons. Neurobiol Aging. 2015;36(1):241–248. doi: 10.1016/j.neurobiolaging.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]