Abstract
The dye-dilution assay is a powerful tool to study lymphocyte expansion dynamics. By combining time course dye-dilution experiments with computational analysis, quantitative information about cell biological parameters, such as percentage of cells dividing, time of division, and time of death, can be produced. Here, we describe the method to generate quantitative cell biological insights from dye-dilution experiments. We describe experimental methods for generating dye-dilution data with murine lymphocytes and then describe the computational data analysis workflow using a recently developed software package called FlowMax. The aim is to interpret the dye-dilution data quantitatively and objectively, such that cell biological parameters can be reported with an appropriate measure of confidence, which in turn depends on the quality and quantity of available data.
Keywords: Lymphocyte, B cell, Proliferation, Lymphocyte dynamics, CFSE assay, Quantitative dye dilution, Cell biological parameter, FlowMax
1. Introduction
Lymphocytes expansion dynamics are produced by the division and death of individual lymphocytes within the population. The effective immune response relies on the balance of division and death cell fate decisions. Classical methods to study lymphocyte proliferation are the incorporation of tritiated thymidine [1] or colorimetric assay [2] at a single time point. These methods produce relative information of prolife ration but do not produce a deep under standing of the proliferative response under different stimulation conditions. For example, less incorporation of tritiated thymidine in condition X compared to condition Y suggests the following possibilities: (1) a smaller number of cells enter the division cycle in condition X than Y, (2) cells are dividing slower in condition X than Y, (3) cells are dying faster in condition X than Y, (4) the time to the first division is longer in condition X than Y but division times of the following generations are the same, or (5) division rates are a little higher but death rates are much higher in condition X than Y.
These possibilities can be distinguished by time course dye-dilution experiments when followed by quantitative interpretation of dye-dilution data [3]. These dyes bind covalently to intracellular molecules and are fluorescent in nature. Cell divisions are measured by halving of the fluorescence in each division cycle and each peak in a log-fluorescence histogram represents corresponding generation number [4]. A number of theoretical models have been developed to interpret dye-dilution data. However, they do not provide information about the quality of fit to the experimental data [5, 6]. Recently, our laboratory developed an integrated computational tool “FlowMax,” which derives cell biological parameters from dye dilution data, provides information about the quality of fit, and thus allows objective interpretation of dye-dilution data to improve the rigor of dye dilution experiments. The FlowMax tool has a graphical user interface, provides the necessary functionality for preprocessing of raw fluorescence data, and is freely available upon request.
Here, we provide protocols for the experimental method of labeling lymphocyte and the computational method for quantitatively analyzing the data. Briefly, B cells are purified from splenocytes by negative selection (CD43 (Ly-48) MicroBeads) using magnetic assisted cell sorting. B cells are labeled with CellTrace™ Far Red (CTR). CTR labeled B cells are stimulated with CpG. 100 μL of culture volumes are acquired by flow cytometry at 14, 36, 48, 72, 96, and 120 h. Dead cells are excluded using dead cell marker (7AAD). The acquired data are exported as a FCS file and imported into “FlowMax.” The generation “0” peak is identified on the histogram and a broad range of the cell biological parameters are derived computationally.
2. Material
All reagents should be cell culture grade.
2.1. Isolation of B Cells
Phosphate buffer saline (PBS, pH 7.4): 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4.
Media: 1640 RPMI, 10% FBS, 1 pen-strep, 5 mM glutamine, 1 mM sodium pyruvate, 1 mM MEM non-essential amino acid, 20 mM HEPES, and 55 μM 2-mercaptoethanol (see Note 1).
Room temperature (RT) PBS (see Note 2).
RT media (see Note 3).
1.5 mL polypropylene centrifuge tube.
Table top centrifuge.
Cold FBS.
Cell Counter.
CD43 (Ly-48) Microbeads (Miltenyi Biotec GmbH, 130–049-801) mouse.
Red blood cell (RBC) lysis buffer (eBioscience, 00–4333-57).
Magnetic assisted cell sorting (MACS) buffer: PBS containing 0.5% BSA with 2 mM EDTA.
2.2. Labelling of Lymphocytes
37°C PBS.
RT media.
1.5 mL polypropylene centrifuge tube.
Rotator for mixing.
37 °C incubator.
Table top centrifuge.
Cold FBS.
CellTrace™ Far Red (CTR) Cell Proliferation Kit (Thermo Fisher Scientific, C34572). Add 20 μL of DMSO to one vial of CellTrace™ Far Red. Mix it by either mild vortexing or pipetting up-and-down, followed by a short spin (see Note 4).
Cell counter.
2.3. Proliferation and Stimulation
Prepare 2.5 × 105 CTR label cells/mL in media.
Stimulus (CpG).
48-well tissue culture plate.
Dead cell marker (7AAD, 7-Aminoacetinomycin D).
Flow cytometry (Accuri C6).
2.4. FlowMax Analysis
PC with minimum configuration i3 processor, 4 GB RAM, 100 GB hard disk and either Linux, MacOSX, or Windows 7/10.
Install latest version of Java (https://java.com/en/download/).
Download FlowMax as a standalone JAR file (http://signalingsystems.ucla.edu/models-and-code/).
3. Methods
3.1. Isolation of B Cells
1. Isolate spleen from 10 to 12 weeks old C57BL/6 mice (see Note 5).
Immediately keep the spleen in cold media on ice.
Isolate splenocytes by macerate using strainer and plunger.
Centrifuge at 450 rcf, 4 °C for 5 min and discard the media.
Resuspend the pellet in 5 mL RBC lysis buffer (see Notes 6 and 7) and keep in RT for 5 min.
Add 5 mL of RT PBS and centrifuge at 450 rcf, 25 °C for 5 min (see Note 8).
Resuspend the cell pellet in 10 mL MACS buffer and count the splenocytes.
Centrifuge at 450 rcf, 4 °C for 5 min and discard the media.
Resuspend 107 splenocytes in 90 μL cold MACS buffer and add 10 μL CD43 (Ly-48) MicroBeads mouse (see Note 9).
Incubate for 15 min in ice or cold chamber (4 °C) with continuous shaking.
Adjust the volume to 10 mL and centrifuge at 450 rcf, 4 °C for 5 min.
Equilibrate the LS column with 3 mL cold MACS buffer at the time of centrifugation.
Discard the MACS buffer after centrifugation (step 11) and resuspend in 500 μL MACS buffer for 108 splenocytes in step 7 (see 10).
Pass it through the LS column and collect in a 15 mL polypropylene centrifuge tube. Wash the column three times with 1 mL cold MACS buffer.
Centrifuge the flow through at 450 rcf, 4 °C for 5 min.
Resuspend the pellet in media and count the cell.
Verify the purity of the B cell by B220-FITC and 7AAD [7].
3.2. Labelling of Lymphocytes
Prepare 1 mM CellTrace™ Far Red (CTR) dye stock solution in DMSO. Aliquot 5 μL of stock solution in a 500 μL polypropylene centrifuge tube and store it in −80 °C (see Note 11).
-
A. Thaw 1 mM CTR stock at RT and prepare 2 μM working concentration by diluting in warm PBS (37 °C) (see Note 12). The volume of working concentration is 500 μL.
B. Prepare 10 × 106 cells/mL of working concentration in warm PBS (37 °C). Make single-cell suspension by pipetting up and down.
Add 500 μL of cells (step 2B) to 500 μL of CTR solution (step 2A) in a 1.5 mL polypropylene centrifuge tube. Mix immediately by inverting the tube 3–4 times. If necessary, mildly vortex the tube for 15–30 s. Final concentration of CTR and cells should be 1 μM and 5 × 106 cells/mL respectively (see Note 13).
Incubate the cells at 37 °C for 20 min with constant mixing. Alternatively, incubate the cells at RT for 25 min with constant mixing.
Quench the excess unreacted CTR by adding 500 μL (1 volume) cold FBS and mix well by inverting the tube.
Pellet the cells by centrifuge at 450 rcf, 4 °C for 5 min and resuspend the cells pellet in 1 mL pre-warm (37 °C) media.
Repeat step 6.
Keep the cells 10 min in pre-warm media. Pellet and resuspend the cells as described in step 6.
Check the efficiency and quality of CTR labeling by flow cytometry (Fig. 1). If the histogram shows multiple peak or wide distribution discard the labeled cells (Fig. 1b) and start to label new cells to achieve a log-fluorescence histogram with single peak and narrow distribution (Fig. 1a).
Count the cells.
Fig. 1.
Fluorescence profile of CTR label B cells. Peak representing CTR label B cells at “0” h is indicated by an arrow. (a) Single peak with narrow distribution, (b) two peaks with wide distribution
3.3. Stimulation and Proliferation
Add 3 mL media to 7.5 × 105 CTR label cells and prepare single-cell suspension. The working concentration of cells 2.5 × 105/mL.
Mix CpG, to a final concentration of 50 nM, to the single-cell suspension. Invert the tubes 3–4 times to thoroughly mix. Seed 250 μL of cells/ well in a 48-well plate and culture the cells in 37 °C with 5% CO2 in humidified atmosphere for 14, 36, 48, 72, 96, and 120 h. For all the time point seed in duplicate.
Gently pipette the cells in the respective well. Transfer the complete contents of each well to a new 1.5 mL polypropylene centrifuge tube. Add 10 μL of 7AAD (see Note 14) to each tube and incubate for 5 min. Pass the cells through a 40 μm strainer. Acquire 100 μL (see Note 15) at each time point using the Accuri C6 flow cytometer (Fig. 2 and see Note 16). Ensure to independently acquire cells from two replicate wells for each time point. Measure proliferation at 14 h (see Note 17), 36, 48, 72, 96, and 120 h (see Note 18).
Export the acquired file to Flow cytometry standard (FCS) file (one file for each flow cytometry run, clearly labeled by time from the start of stimulation and replicate number).
Fig. 2.
B cell proliferation time course. CTR label B cells stimulated 50 nM CpG. Dead cells were excluded by staining with 7AAD. B cell proliferation measured at 14, 36, 48, 72, 96, and 120 h
3.4. FlowMax
FlowMax is written in java 1.6 language. Make sure java is installed on your computer (see Note 19).
Startup FlowMax either by double clicking on the downloaded jar file or by running it from a terminal or command prompt using command “java –jar FlowMax.jar” (see Note 20).
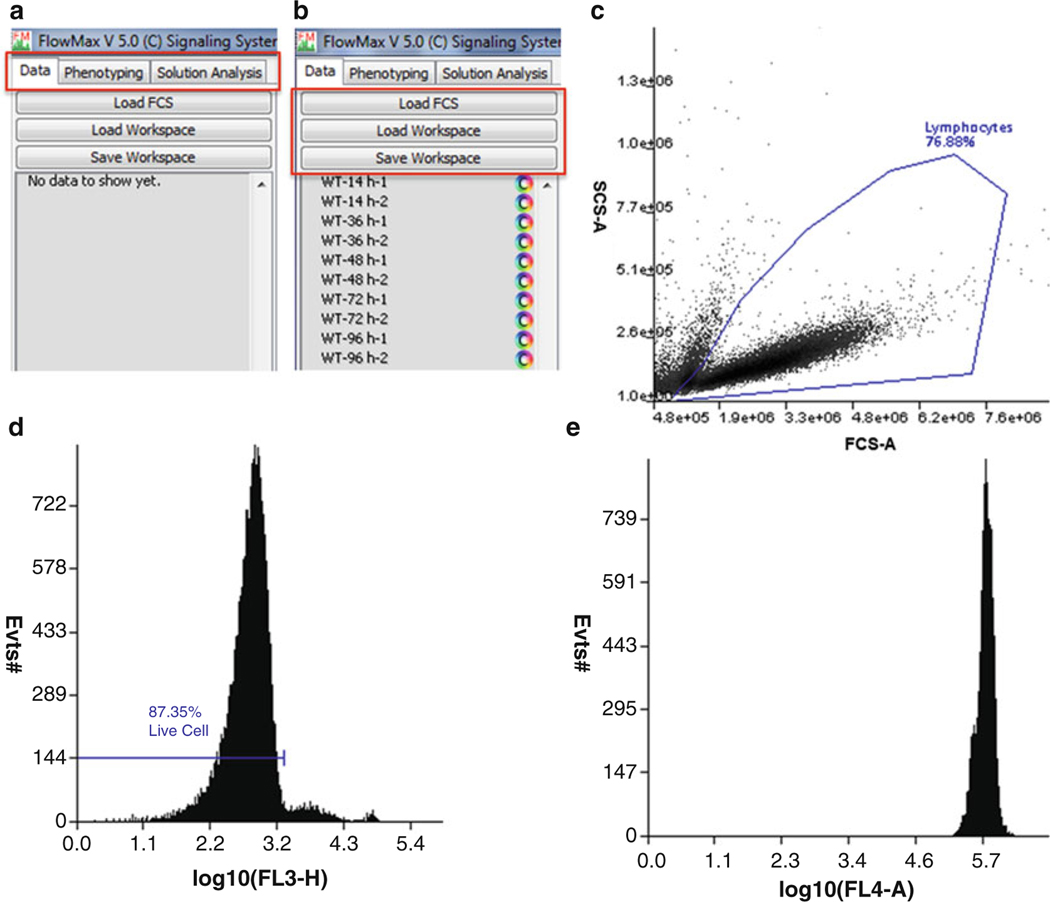
The FlowMax user interface is shown in Fig. 3a. FlowMax has three tabs: “Data,” “Phenotyping,” and “Solution Analysis.” The “Data” tab provides functionality for importing FCS files (raw data), performing in silico compensation, gating on scattering and fluorescence. The data tab also enables annotating each sample according to stimulation time, approximate location of the generation 0 peak, and total cell count (estimated automatically if run on Accuri C6). The “Phenotyping” tab is used for the model fitting of the experimental data using the total simulation time of each sample, and the total cell count information provided by the user on. The “Solution Analysis” tab visualizes the cell biological parameters obtained by fitting the mathematical model of proliferating lymphocytes. The functions of the tabs are discussed below in more detail.
From the “Data” tab, click the “Load FCS” button to import the FCS file—it will then appear in the left panel (Fig. 3b). Each time point is shown in a row and double clicking on each row opens the respective FCS file. Fluorescence channel compensation can be performed if needed (see Fig. 4 and Note 21). Gate on the population of interest (Fig. 3c) based on the Forward scattered (FSC) and Side scatter (SSC). The gated population will appear underneath the sample in the left panel. Double click on the gated population name on the left to display a new plot. Change the Y-axis variable to the viability dye channel (7AAD) by right clicking and selecting this channel from the menu. Now create a gate to distinguish the viable cells from the dead cells (Fig. 3d). From the left panel, double-click the viable cell population. A third plot will appear containing the viable cells. Generate a log fluorescence histogram of CTR by toggling log x, right-clicking on the X-axis, and selecting CTR (Fig. 3e).
Define generation “0” in the histogram of CTR (see Note 22), input sample name and condition (cell type, genotype, stimulus), and define the time since stimulation in hours. The sample name must be identical for samples to be associated together. Replicates should be defined as having the same stimulation duration. Save your progress by clicking on “Save Workspace” button in the “Data” tab (Fig. 3b).
Repeat steps 4 and 5 for each collected sample. Multiple conditions/stimulations can be loaded and processed simultaneously but will require additional computational memory.
Go to the “Phenotype” tab and select the condition in the left side panel of Fig. 5a. To get a quick estimate of parameters, set “Solutions” to 5, which means that five separate model fitting attempts will be made (see Note 23). Click “Phenotype FCyton” to start model fitting. Solution number and fitting statistics appear at the bottom of the left side panel. Green line indicates fitting of the entire population, blue line indicates fitting of each generation, and background color indicates the goodness of fit. Green, yellow, and red background of each sample corresponds to <10%, 10–20%, and 20–30% error of the fit respectively (Fig. 5b). For the best model fit, all background colors should be green, and generations should line up with center of peaks. If a model peak appears to be misaligned, or if most samples are fit poorly (red background), please adjust the fluorescence and fcyton parameter ranges (see Note 24). If satisfied with the preliminary 5 solutions repeat the same run with 500–1000 solution to build a comprehensive sampling of the solution space (see Note 25).
Go to “Solution Analysis” tab to visualize the model solutions (Phenotypes). Phenotypes automatically appear after phenotyping has finished. To visualize previous phenotypes, click on “Load Phenotype,” and browse to the saved .csv file (Fig. 6a).
Click “Draw” in the tab “Visualize Parameter” to plot cell biological parameters, e.g., Fs (progressor fraction, i.e., % of cells dividing in each generation), Tdiv (Time to division), and Tdie (Time to death). Suffix indicates the cell generation, e.g., F0 means F of generation 0, Tdiv1+ means Tdiv from 1 to terminal generation Parameters on the right are used to change the plot parameters. Plots can be saved by right-clicking and selecting copy to clipboard (see Note 26). To export the data for plotting in other software use the appropriate save button for the data required at the bottom “visualize parameter” tab.
Click “Draw” in the tab “Visualize Count” to plot total and generation-specific cell count during time course (Fig. 6a). Experimental cell count is indicated by a red dot within the black line at each time point and a black line indicates the total cell count achieved by model fitting (see Note 27).
Comparison of multiple conditions can be achieved through the “Compare Phenotypes” tab (Fig. 6a) (see Note 28).
Cell biological parameters (F0, Tdiv0, Tdie0, Tdiv1+, Tdie1+) appear in the right side of the plot (Fig. 6b). These values are automatically saved as csv files in the working directory. Fs refers to the percentage of cells dividing. The black curve under Fs shows the percentage of cells dividing for each generation. F0 = 0.596 indicates ~59% of initial cells will divide. The green curve under undivided cells shows the probability density distribution of Tdiv0 within the B cell population. Tdiv0 = 42.462|4.727 indicates time of division of “generation 0” is 42.462 with standard deviation 4.727. Similarly, Tdie0, Tdiv1+, and Tdie1+ indicate the time to die of the initial generation, time of division of generations after the initial generation, and time to die of generations after the initial generation respectively. A curve is plotted for each model fit to indicate how well the distributions are constrained by the data. If the distributions are poorly constrained, represented by multiple non-overlapping curves, this may indicate that there is not enough information to accurately determine that biological process.
Fig. 3.
Using FlowMax to build log-fluorescence histograms. (a) FlowMax has tabs for data preprocessing (Data), for model fitting (Phenotyping), and for solution analysis and visualization (Solution Analysis). (b) Begin by loading all of the FCS raw datasets, or a previously saved workspace. Datasets and gate information will be listed at left below the buttons. (c) Double click a sample to show a plot of the data. Use FCS and SSC to select the viable cell population. (d) Use another gate to select the viable cells. (e) A log fluorescence histogram showing CTR fluorescence on the log scale. For this time point, cells are still largely undivided
Fig. 4.
Manual compensation for fluorescence spillover between channels. (a) Click on the multi-colored circle next to a sample to open the compensation dialog box. (b) The compensation dialog box can be used to subtract a percentage of one channel from another (in this case 1% of the viability stain (FL3) channel is subtracted from the CTR (FL4) channel)
Fig. 5.
FlowMax model fitting of CTR labeled B cells. (a) The parameter panel in the “Phenotyping” tab is used to set the number of solutions, the parameter ranges, and whether solutions should be visualized while fitting. (b) Model fit (green and blue traces) overlapping the CTR log-fluorescence histograms. The two rows are duplicates and each column indicates a different time point. The condition and time point are shown on the top left corner of each box. Black histogram is the experimental data. The green line is the optimal fit when the data of all generations is considered; the blue line is the optimal fit to each generation. The red line indicates the fluorescence of generation “0” or undivided cells. Green and yellow backgrounds indicate the quality of the fits: <10% and <20% deviation from experimental data, respectively. The deviation between experimental data and fit is shown in blue at the top left corner (below the condition and time point) of the each box
Fig. 6.
Analyzing the cell biological parameter. (a) After the completion of the phenotyping go to the tab “Solution Analysis” and click load phenotype. Select the CSV file and click “Draw” (Draw is present at the top right corner) to visualize the cell biological parameters. (b) Cell biological parameter (F0, Tdiv0, Tdie0, Tdiv1 +, Tdie1+) obtained by FlowMax fitting. X axis refers to % of cells dividing and Y axis refers generation of cells. Fs: The bullet point in the black curve shows the % of cells dividing (Fs) in each generation. Undivided Cells: The distribution of the green curve shows the probability of time of division of generation “0” (Tdiv0) cells and distribution of the red curve shows the probability of time of die of generation “0” (Tdie0) cells. Dividing Cells: The distribution of the blue curve shows the probability of time of division of all the generation after “0” (Tdiv1+) cells and distribution of the red curve shows the probability of time of die of all the generation after “0” (Tdie1+) cells. The values of the cell biological parameters are shown in the right side. Though, it is better not to consider the Tdiv/Tdie value rather consider the distribution of Tdiv/Tdie in the curve respective curve
4. Notes
Prepare fresh media before (maximum 1 day earlier) isolation of B cells.
Keep the cold PBS at 37 °C water bath for 1 h to achieve (RT). Check the temperature of the media by touching the container using the palm of your hand. Only use it if the temperature is close to RT.
Keep the cold media for 2 h at RT. Check the temperature of the media by touching the container using the palm of your hand. Only use it if the temperature is close to RT.
Prepare freshly and store it at −80 °C in a 5 μL aliquot.
Usually the color of the spleen is reddish black. In some conditions it is common to see that a portion of spleen is black in color. The black part of the spleen is primarily dead cells. Do not take the black part of the spleen for cell isolation to increase the proportion of viable B cells.
Use 5 mL/mice, if spleen size is normal, i.e., total splenocytes <250 × 106.
Do not use cold RBC lysis buffer. Keep the RBC lysis buffer fora maximum of 30 min in 37 °C water bath to reach RT. Check the temperature of the RBC lysis buffer by touching the container using the palm of your hand. Only use it if the temperature is close to RT.
If the pellet color is still red then repeat steps 5 and 6 of Subheading 3.1.
Make a homogeneous suspension of the microbeads by vortexing the bottle.
Scale up the volume of the MACS buffer depending on the number of splenocytes.
CellTrace™ Violet/CellTrace™ Yellow/CFSE can be used as an alternate to CellTrace™ Far Red depending on the configuration of flow cytometry to be used. We observe that CellTrace™ conjugated dye shows more distinct peaks of different generations than CFSE. Also, the fluorescence decay rate of CFSE label is faster than CTR. CFSE labeled B cells lose fluorescence intensity rapidly in the first 48 h and more slowly thereafter.
The freezing point of DMSO 18.5 °C. If the RT is below 20 °C thaw it in >20 °C in a water bath.
Adjust the number of cells and CTR solution depending on the experimental need; e.g., if the experiment needs 10 × 106 then add 1 mL of working concentration of cells to 1 mL CTR solution.
Propidium Iodide (PI) can also be used as a dead cell marker.
It is important to count cells in the same volume for each timepoint. If 100 μL contains too many or too few cells adjust the count volume to get a significant number of cells.
FlowMax can work with FCS3.0 and FCS2.0 input files; however, it uses Accuri C6 metadata tags on the total volume collected to determine cell count automatically. For flow cytometers without accurate volume uptake measurement, we recommend manually measuring the volume of sample acquired and then manually normalizing the count from within FlowMax.
The 14 h time point is critical to get the correct value of F0. Lymphocytes isolation from tissue, such as spleen, bone marrow, etc., requires a mechanical tissue dissociation process that leads to cell death termed mechanical cell death [8]. Mechanical cell death occurs before 12 h [3]. To exclude mechanical cell death from the downstream analysis, the 14 h time point is critical. To get consistency of the data (F0, Tdiv0, and Tdie0) the first time point (14 h) has to be consistent in each replicate experiment.
The time points near the first division when cells have started dividing (usually between 24 and 48 h) and when most cells have died (usually 120–140 h after stimulation) are important for constraining the parameters during fitting. The time to first division and time when most cells are dead varies depending on the stimulus. Optimization of time points is required to achieve good FlowMax fitting.
Type “java” in the command prompt and a description of expected inputs and outputs should be displayed. If the “java” is not recognized, you may need to add its location to the $PATH variable of your operating system of use the full path to java (e.g., “/usr/bin/java”).
FlowMax needs to load all FCS files into memory. This may require allocating additional memory for FlowMax. If analysis fails with larger datasets run FlowMax from command prompt using command “java –jar –Xmx1024m FlowMax.jar.” Also, make sure that FlowMax is not running from a restricted directory such as “Program Files” in Windows.
To minimize fluorescence “spillover” from one channel into the next, a percentage of the fluorescence signal from one channel can be subtracted from another channel. First double-click on the sample you would like to compensate on the left. Then change the X- and Y-axis variables to the two fluorescence channels in which “spillover” is suspected. Then click on the multi-colored C next to each FCS sample in the left panel (Fig. 4a), adding the appropriate rules and clicking “Compensate” (Fig. 4b). Repeat until you see a separation in the signals.
CTR label B cells gradually lose fluorescence of CTR during an experimental time course, probably due to catabolism of the CTR labeled protein. The result of which is a slight decrease in the intensity of generation “0” (undivided cell) peak as the time point increases. Accurately defining the generation “0” peak is very important to reliably estimate cell biological parameters. The best control, to correctly define generation “0,” is unstimulated B cells. The rate of loss of fluorescence decay of unstimulated B cells is similar to stimulated B cells [5, 9].
Fitting proceeds in two steps, first fluorescence parameters are fitted to each histogram to account for experimental variability and loss of dye labeling over time. Next, the population parameters are repeatedly determined, using a stochastic optimization approach. An initial quick fit is recommended to see if reasonable solutions can be found using default parameter ranges.
The default parameter ranges may or may not be optimal for all conditions, and may need to be fine-tuned. To do this, check the box “Advanced Options” and tune the default parameter ranges. Hovering over each box gives a brief description. Constraining or relaxing some parameter ranges can help guide the optimization algorithm, however, over-zealous constraining will bias the result, and is not recommended without biological justification. We recommend listing the parameter ranges used and justifying changes when reporting results. For a full description of the fcyton model parameters, please see ref. 6.
Running 500–1000 solutions typically produces at least one good solution, but is time consuming. While running a few solutions should be enough for testing if parameter ranges are acceptable, we recommend running at least 500 solutions prior to interpretation of results.
When overlaying multiple phenotypes in the graph, you can change the color of each curve manually by clicking on the appropriate Overlay button and selecting the color of your choice. Uncheck the plot clusters box to plot the individual clustered solutions (e.g., the 500 solutions obtained), instead of sampling from the maximum-likelihood cluster ranges for parameters (default behavior).
The time axis, cell count axis, number of generations plotted, number of samples plotted, and the size of the graph, and whether counts are sampled or taken directly from the best-fit solutions in the cluster can be changed using the right panel. Select the fcyton model to ensure the right counts are plotted.
To determine which population parameters are sufficient for describing the difference between two phenotypes, FlowMax can be used to generate “chimeric” phenotypes containing parameters from both phenotypes. Check the parameters you would like to copy from the second phenotype, and hit the “Generate intermediate phenotype” button. FlowMax will create a new phenotype containing the parameters from the first phenotype with the selected parameters taken from the second phenotype. You can now plot the total cell counts to visually compare if the changes are sufficient for describing the population behavior.
Acknowledgment
This work was supported by NIH grant R01 AI132731 (A.H.) and from NIH-NCI CCSG: P30 014195, and the Helmsley Trust (M.N.S.).
References
- 1.de Fries R, Mitsuhashi M (1995) Quantification of mitogen induced human lymphocyte proliferation: comparison of alamarBlue assay to 3H-thymidine incorporation assay. J Clin Lab Anal 9:89–95 [DOI] [PubMed] [Google Scholar]
- 2.Denizot F, Lang R (1986) Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89:271–277 [DOI] [PubMed] [Google Scholar]
- 3.Hawkins ED, Hommel M, Turner ML, Battye FL, Markham JF et al. (2007) Measuring lymphocyte proliferation, survival and differentiation using CFSE time-series data. Nat Protoc 2:2057–2067 [DOI] [PubMed] [Google Scholar]
- 4.Quah BJ, Warren HS, Parish CR (2007) Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc 2:2049–2056 [DOI] [PubMed] [Google Scholar]
- 5.Hasbold J, Gett AV, Rush JS, Deenick E, Avery D et al. (1999) Quantitative analysis of lymphocyte differentiation and proliferation in vitro using carboxyfluorescein diacetate succinimidyl ester. Immunol Cell Biol 77:516–522 [DOI] [PubMed] [Google Scholar]
- 6.Shokhirev MN, Hoffmann A (2013) FlowMax: a computational tool for maximum likelihood Deconvolution of CFSE time courses. PLoS One 8:e67620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teodorovic LS, Riccardi C, Torres RM, Pelanda R (2012) Murine B cell development and antibody responses to model antigens are not impaired in the absence of the TNF receptor GITR. PLoS One 7:e31632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein AB, Witonsky SG, Ahmed SA, Holladay SD, Gogal RM Jr et al. (2006) Impact of different cell isolation techniques on lymphocyte viability and function. J Immunoassay Immunochem 27:61–76 [DOI] [PubMed] [Google Scholar]
- 9.Lyons AB, Hasbold J, Hodgkin PD (2001) Flowcytometric analysis of cell division history using dilution of carboxyfluorescein diacetate succinimidyl ester, a stably integrated fluorescent probe. Methods Cell Biol 63:375–398 [DOI] [PubMed] [Google Scholar]