Abstract
Aerospace milestones in human history, including returning to the moon and manned Martian missions, have been implemented in recent years. Space exploration has become one of the global common goals, and to ensure the survival and development of human beings in the extraterrestrial extreme environment has been becoming the basic ability and technology of manned space exploration. For the purpose of fulfilling the goal of extraterrestrial survival, researchers in Nanjing University and the China Academy of Space Technology proposed extraterrestrial artificial photosynthesis (EAP) technology. By simulating the natural photosynthesis of green plants on the Earth, EAP converts CO2/H2O into fuel and O2 in an in-situ, accelerated and controllable manner by using waste CO2 in the confined space of spacecraft, or abundant CO2 resources in extraterrestrial celestial environments, e.g. Mars. Thus, the material loading of manned spacecraft can be greatly reduced to support affordable and sustainable deep space exploration. In this paper, EAP technology is compared with existing methods of converting CO2/H2O into fuel and O2 in the aerospace field, especially the Sabatier method and Bosch reduction method. The research progress of possible EAP materials for in-situ utilization of extraterrestrial resources are also discussed in depth. Finally, this review lists the challenges that the EAP process may encounter, which need to be focused on for future implementation and application. We expect to deepen the understanding of artificial photosynthetic materials and technologies, and aim to strongly support the development of manned spaceflight.
Keywords: solar energy, extraterrestrial survival, artificial photosynthesis, CO2 reduction, oxygen evolution
The extraterrestrial artificial photosynthesis in situ converts CO2 in spacecraft's confined space and in Martian atmosphere into O2 and fuels, thus supporting affordable and sustainable deep-space exploration and migration plans.
INTRODUCTION
Extraterrestrial survival is a prerequisite for humankind to achieve long-term space flight, extraterrestrial residence and immigration, and creates one of the greatest scientific and technological challenges of human deep space exploration. During human extraterrestrial exploration activities, a sustained supply of O2 and fuel is one of the essential abilities. Extraterrestrial artificial photosynthesis (EAP), i.e. CO2/H2O conversion into fuel and O2 from human respiration, combustion emissions and in-situ resources on outer-Earth planets, can greatly reduce the supply load of spacecraft and space stations, thus promoting affordable and sustainable human deep space exploration.
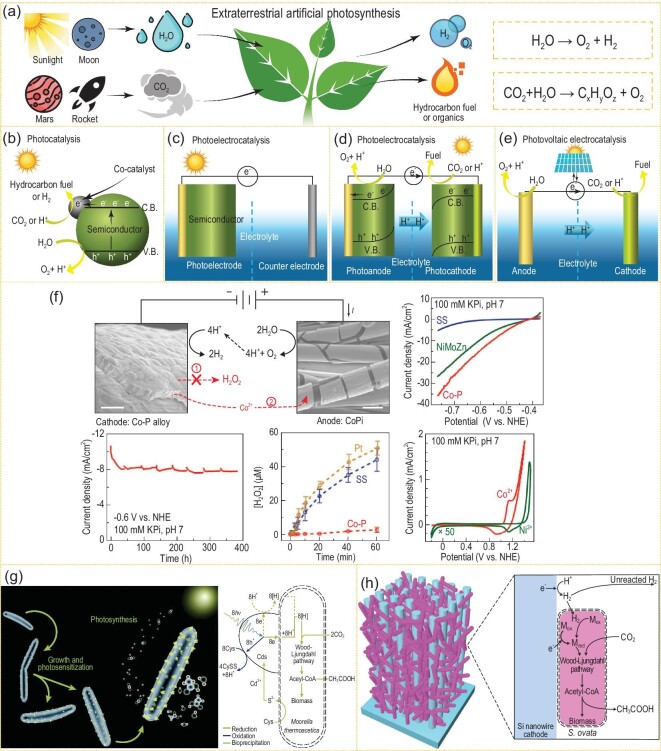
EAP (Fig. 1a) is a simulation of plant photosynthesis on Earth. Through photocatalysis [1,2] (Fig. 1b), photoelectrocatalysis [3,4] (Fig. 1c and d) or photovoltaic electrocatalysis [5] (Fig. 1e), EAP uses a controllable and accelerated chemical process to in-situ convert CO2/H2O into carbon-containing fuel and O2 by harnessing solar radiation. Compared with traditional CO2/H2O conversion techniques, such as the thermochemical or electrochemical method, EAP technology, which only uses solar energy and semiconductor materials, is usually carried out without consuming auxiliary energy inputs. EAP can be applied to in-situ convert CO2 waste in a confined space, which effectively reduces the supply demand of human space stations and deep space spacecraft, etc. Furthermore, it also makes use of abundant in-situ resources such as CO2 and H2O in the extraterrestrial atmospheric environments of the moon or Mars to meet the material demand. Through EAP, human beings can survive in extraterrestrial environments in the long term.
Figure 1.
Mechanism schemes of (a) EAP, (b) photocatalysis, (c and d) photoelectrocatalysis and (e) photovoltaic electrocatalysis. (f) A biocompatible inorganic water-splitting catalyst system (adapted from ref. [16] with permission from American Association for the Advancement of Science (AAAS)). (g) Scheme of CO2 reduction of the bacteria/semiconductor hybrid artificial photosynthetic system (adapted from ref. [17] with permission from AAAS). (h) Illustrations of a nanowire-bacteria hybrid system and the reaction mechanism (adapted from ref. [18] with permission from Elsevier).
In recent decades, for solving the key problems of supply demand for human space stations and deep space exploration, the USA, Japan and other countries have continuously carried out research on CO2 conversion technology as one of the crucial parts of in-situ resource utilization, developed a series of CO2 conversion systems based on the Sabatier method [6] and Bosch reduction method [7], and carried out experimental verification of CO2 reduction and O2 production on the International Space Station (ISS) [8,9]. In 2005, the National Aeronautics and Space Administration (NASA) in the USA proposed the Resource Prospector mission, planning to carry out in-situ utilization experiments for producing O2 from water in lunar surface soil in 2022. Russia raised a series of plans for lunar exploration and will launch a lander named Moon 27 in 2025 for in-situ lunar resource utilization. However, existing CO2 conversion devices adopting high-temperature and high-pressure reaction conditions always have high energy consumption. On the other hand, the extraterrestrial microgravity environment obviously promotes the formation of supersaturated layers of dissolved gas molecules near the electrode surface. These layers accelerate the formation and evolution of bubbles at the interface between electrode and electrolyte, and hinder the material transport rate at the microscopic scale [10–12]. The electrode reaction kinetics are thus significantly reduced, resulting in less than one-third of the working efficiency on the Earth. Furthermore, due to the lack of experimental data and related theoretical research, key scientific and technical challenges, such as in-situ preparation of photoelectrocatalytic materials, the heterogeneous catalytic process and the working parameters of materials and systems, have become major problems in CO2 conversion.
The ability to harvest light energy through artificial photosynthesis may create an essential foundation for technologies used in many areas, such as global carbon neutralization [13]. Thus, it is reasonable to expect the further development of EAP for human extraterrestrial survival. With regard to this, one example is demonstrated by an EAP device, developed by Qian Xuesen Laboratory of Space Technology, which reduces CO2 with water into a carbonaceous compound and produces O2. The feasibility of the reactor in CO2/H2O photo-conversion into carbonaceous compound and O2 was verified by ground experiment, which may provide a theoretical and practical foundation for subsequent device optimization, carbon dioxide conversion into variable hydrocarbon products with high selectivity, and in-orbit testing of artificial photosynthesis devices [14].
The research into artificial photosynthesis began in 1972 when Honda and Fujishima reported that H2 was produced by photolysis of water over titanium dioxide electrodes under ultraviolet light [4]. Then during the following few decades, numerous scientists carried out a series of research works on this specific area. In 2001, Zhigang Zou proposed a new theory and method to regulate the band structure of photocatalytic materials, and broadened the response range of photocatalytic materials. He realized visible-light-induced complete water decomposition [2] and CO2 reduction [15] under visible light, thus developing a new-generation visible-light-responsive photocatalytic material. In 2015, NASA started to focus on artificial photosynthesis and proposed the concept of microbial-assisted artificial photosynthesis. In 2016, Daniel G. Nocera devised a biocompatible-inorganic catalyst system to decompose water to get H2 and O2 at low voltages [16] (Fig. 1f). He utilized low concentrations of CO2 in the presence of O2 and H2 to generate biomass, fuel or chemical products. A 10% energy efficiency of CO2 reduction could be obtained when coupling this device with a photovoltaic system. Peidong Yang combined light-absorbing semiconductor nanomaterials with bacteria to produce biological-inorganic hybrid systems for CO2 fixation [17,18] (Fig. 1g and h). In 2017, Nanjing University and Qian Xuesen Laboratory at the China Academy of Space Technology carried out research into EAP materials and systems for the first time, aiming to resolve the requirements with regard to basic materials and energy during human extraterrestrial survival, and they have preliminarily completed the verification of materials and systems. This research progress provides the technical foundation for human survival during spaceflight missions and deep space exploration.
EXTRATERRESTRIAL ENVIRONMENTAL IMPACT
Space exploration activities are faced with various special environments, such as microgravity, strong radiation, extreme temperature and high vacuum, which bring about a series of challenges for realizing CO2/H2O conversion in outer space. Only through long-term and effective experiments, combined with ground simulation and in-orbit verification, can we investigate the mechanistic and process influences of outer space on EAP in the process of space exploration. However, it can be speculated that the external factors that mainly affect CO2 and H2O conversion may be, and are not limited to, the following aspects:
Microgravity. Under microgravity, the key problems of bubble formation, evolution and detachment at the reaction interface need to be solved urgently.
-
Cosmic radiation. Materials, especially semiconductors, on the lunar surface or in Earth orbit would be strongly affected by the impact of rays or particles such as electrons, protons, heavy ions and plasma. Material properties will be changed by electromagnetic radiation and charged particles, including X-rays, electrons, protons and heavy ions, mainly due to the internal interactions in materials during the bombardment caused by cosmic rays or high energy particles. These internal interactions are divided into coulomb interactions and electromagnetic effects.
Coulomb interactions include three cases: Coulomb scattering, bremsstrahlung and inelastic collision between particles and electrons. Coulomb scattering refers to the elastic collision process in which charged particles are incident to matter, then deflected and dispersed by the Coulomb electric field force of the atomic nucleus. When materials are incident by high-energy electrons, the high-speed electron suddenly slows down and produces the bremsstrahlung, which is an important process for charged particles to act on materials in space. When particles or electrons inelastically collide with material, the electron in the material is ionized or excited from the outer layer to the inner layer, causing primary ionization to produce a large number of secondary electrons. The total kinetic energy before and after collision is not equal.
Electromagnetic interaction mainly refers to the interaction between high-energy rays and materials. The whole, or part, of the initial energy of the high-energy ray can be transferred to electrons in the materials, and the incident particles disappear or scatter. Electromagnetic effects include the photoelectric effect, electron pair effect and Compton effect. The dominant effect of electromagnetic action is related to photon energy and atomic coefficient of absorbing material.
After the incident particles enter the material, the energy of the incident particles decreases and the velocity slows down. Finally, the incident particles are blocked or scattered. The charged particles enter the material and lose energy through two ways: the displacement effect and ionization effect. The displacement effect is when charged particles collide with the nucleus, making atoms leave their original positions; or the incident particles fill in the lattice gaps to form vacancies and interstitial atoms, leading to the corresponding changes in material structure and properties. Collision between incident high-energy particles and material atoms is the main source of energy loss. Further, a large number of recoil atoms are produced after collision, and the secondary reactions of recoil atoms with the surrounding atoms form a large number of Frenkel defects. Most of these defects are semi-permanent, leading to great damage to semiconductor materials and devices. The ionization effect is when the radiation of charged particles with a certain energy excites electrons outside the nucleus of materials to form free electrons. Material atoms thus become positive ions, forming electron-hole pairs. When electrons transition from valence band to conduction band, the electrical, chemical, physical and mechanical properties of materials can be affected. Small-dose and long-term steady-state radiation in space often leads to a cumulative ionization damage effect.
Extreme temperature. Extraterrestrial space, including the lunar surface, produces a huge temperature difference between day and night. During daytime, when the sun shines vertically, the temperature rises as high as 127ºC; at night, the temperature can be as low as −183ºC. As water evaporates or freezes easily, this poses a great challenge to CO2 reduction in aqueous systems. Furthermore, the thermal expansion and contraction caused by temperature switching generally accelerates the fatigue and aging of materials, which brings a series of system durability and reliability problems.
In addition, there are other problems caused by the atmospheric pressure and special atmospheric environments of extraterrestrial planets, although some of them, e.g. extreme temperatures and ultra-vacuums, can be resolved by aerospace engineering methods. For example, the Environmental Control and Life Support System (ECLSS) used on the ISS by NASA can maintain the space capsule pressure, temperature and humidity. Extreme conditions have brought great challenges for researchers in selecting and designing materials, and it will also become difficulties in our research of extraterrestrial artificial photosynthsis.
RECENT PROGRESS ON EXTRATERRESTRIAL CO2 AND H2O CONVERSION FOR SPACECRAFT
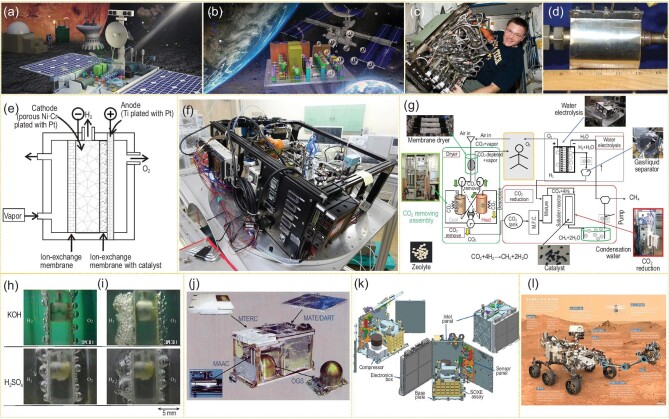
Since the 1960s, the Sabatier method [6] and Bosch reduction method [7] have been the main approaches in CO2 reduction technology. H2O electrolysis for H2 and O2 has also been widely anticipated [19]. Recently, the EAP technology proposed by Nanjing University and Qian Xuesen Laboratory realized CO2/H2O photo-conversion under mild conditions with low energy consumption (Fig. 2a and b).
Figure 2.
(a) Space exploration experiment device using in-situ resources to produce O2. (b) Partial enlargement of (a). (c) Sabatier reaction system loaded into the ISS (adapted from ref. [6] with permission from American Institute of Aeronautics and Astronautics (AAIA)). (d) The core unit of the reactor (adapted from ref. [20] with permission from AIAA). (e) The principle of the reactor (adapted from ref. [21] with permission from AIAA) and (f) exterior feature of the JAXA water electrolyzer (adapted from ref. [9] with permission from AIAA). (g) New conceptual ECLSS in space station (adapted from ref. [9] with permission from AIAA). (h) Comparison of electrode in the environments of normal gravity on Earth (adapted from ref. [10] with permission from Elsevier). (i) Microgravity in space during the water electrolyzing process (adapted from ref. [11] with permission from Elsevier). (j) MIP CO2 converting system (adapted from ref. [22] with permission from AIAA). (k) MOXIE CO2 converting system (adapted from ref. [24] with permission from Elsevier). (l) Set-up diagram of ‘Mars 2020’ (adapted from ref. [24] with permission from Elsevier).
For solving the key problems of manned space stations and deep space exploration, the USA and other countries carried out research on CO2 and H2O conversion based on traditional ground technology, e.g. by using H2O electrolysis to supply O2 for astronauts in the ISS. To realize the recycling of CO2 released by astronauts, NASA and Japan Aerospace Exploration Agency (JAXA) have developed a set of CO2 reduction and O2 evolution devices, in which CO2 reduction is obtained by converting CO2 and H2 into methane with H2 obtained by H2O electrolysis. The Sabatier reactor contains a gas-solid two-phase process with a core unit temperature of 250–450ºC and a minimum gas pressure of 55 kPa. The mass of the ground experimental unit is around 41 kg and the total power is >100 W. The in-orbit test was completed for this system in October 2010 (Fig. 2c and d) [6,20]. The water electrolyzer developed by JAXA has also been tested in orbit [8,9]. This device was obtained by modifying the more technically mature proton exchange membrane electrolytic cell, which consists of an electrolytic unit and a gas-liquid separation unit [9,21] (Fig. 2e and f). In the ISS, a combination of the above two devices was tested to support the ECLSS to convert CO2 into O2 and methane by in-orbit reaction (Fig. 2g) [9]. JAXA researchers used parabolic flight and drop tower tests to make a series of research and improvement works on the water electrolysis device, including tests on the working temperature of the electrolytic cells, the pressure of the gas-liquid separation membranes, the electrolyte component, and the working voltage and current. However, even after various optimizations, the water electrolytic device’s efficiency under microgravity was less than a third of that under usual gravity environments. The supersaturated layers of dissolved gas molecules formed by the aggregation of over-dissolved gas on the electrode highly hindered the transport rate and reaction efficiency of electrolyte [10] (Fig. 2h and i). Matsushima et al. found that the interaction between electrodes and electrolytes in the microgravity environment has a significant impact on the formation and evolution of bubbles, and the electrolytic performance [12]. In order to improve the material transport rate and reaction efficiency under microgravity, Nanjing University and Qian Xuesen Laboratory used liquid shearing force to compel the generated gas from the electrode surface, to prevent bubble gathering near the electrode surface under microgravity conditions [14].
For the more challenging manned deep space exploration missions, the USA first proposed the scheme of producing O2 and fuel by using in-situ resources such as water and carbon dioxide on the moon or Mars. NASA proposed a Mars in-situ-propellant-production precursor (MIP) plan in 2001 to deoxidize carbon dioxide into O2 using high-temperature electrolysis (Fig. 2j) [22]. In 2013, NASA also proposed a Mars in-situ resource utilization landing mission, MARCO POLO [23], which would utilize Mars’s atmospheric and soil resources to produce H2, O2 and CH4 by the Sabatier method and water electrolytic technology. They further proposed the Mars Oxygen ISRU Experiment (MOXIE) load in 2014 to deoxidize carbon dioxide in the Martian atmosphere to generate O2 with a solid oxide electrolytic cell at 800ºC to achieve 10 g h–1 O2 production (Fig. 2k) [24]. The load project was launched in 2020, with about 2 h of experiments on Mars. If this in-situ resource utilization technology is validated, NASA will plan to follow up with a 100-fold magnificated scale device to support the 2033 manned Mars mission. In 2018, NASA supported a plan named the CO2 Conversion Challenge to develop novel synthesis technologies that use carbon dioxide to generate molecules that can be used to manufacture a variety of products. However, the selected projects have remained at the laboratory stage and do not show feasibility for application to spacecraft. The USA is extracting O2 from the Martian atmosphere as part of the ‘Mars 2020’ rover project (Fig. 2l) [24]. Generally, the American space mission in CO2 utilization and transformation mainly uses the relatively mature thermal or electrical chemical conversion technology found in industry. Although the technical route has high maturity and stability, it needs to be carried out under extremely high temperature conditions (900–1600ºC), with harsh operating conditions and large energy consumption, which is not conducive to manned deep space exploration. In 2020, Qian Xuesen Laboratory developed a demo of an EAP device for reducing carbon dioxide with water into carbonaceous compound and producing O2 [14]. The feasibility of the reactor in reducing carbon dioxide to O2 and carbonaceous compound was verified by ground experiment, which may provide a theoretical and practical foundation for subsequent device optimization, carbon dioxide conversion to variable hydrocarbon products with high selectivity, and on-orbit testing of artificial photosynthesis devices.
MATERIALS FOR EAP
Even on the moon or Mars, the energy provided by the sun is considerable. The light intensity on the moon approximates 1.4 times that of the Earth. Recent research shows that the permanent light area on the moon is adjacent to the ice area, which may be an ideal place for human beings to set up bases on the moon [25]. Solar power on Mars’s surface is nearly 40% of that found on Earth. Because there is no atmospheric absorption, the solar spectrum on the surface of the moon is similar to that in Earth orbit, which is about equal to AM0 (1366.1 W m–2). The transmitted sunlight wavelength on the surface of Mars increases with the increase of solar zenith angle and optical depth, and the energy at shorter wavelengths is more easily exhausted, because the cross section of dust particles suspended in the atmosphere at shorter wavelengths is larger. The 60-degree spectrum of sunlight on the surface of Mars is reduced to one-sixth of AM0, and further reduced in the blue part of the spectrum [26]. The moon has no atmosphere, thus there is no CO2 on the moon. It is conservatively estimated that 0.3% to 1% of the water on the moon is buried in the form of ice under 40 cm of dry regolith [27]. On Mars, 95% of the atmosphere (the average pressure is <1% of one atmosphere) is CO2. It has been estimated that Martian meteorites contain carbonates in low abundances (<1 vol.%) [28], and calcium carbonate has been identified in the soils at the Mars Phoenix landing site [29]. Thus, it is important to evaluate the feasibility of utilizing such mineralized carbon in
future research. At this stage this review mainly focuses on the EAP process of CO2/H2O. Moreover, ground ice and hydration water exist at the near-surface subsurface of Mars [27]. The above preconditions are propitious to EAP in extraterrestrial environments such as the moon and Mars.
Most of the practical applications of EAP are based on photocatalytic materials. Photocatalysts are an important component to support artificial photosynthesis. Catalysts suitable for EAP are herein classified as photocatalysts, photoanodes, photocathodes and photovoltaic-electrochemical catalysts. The evaluation parameters for the performance assessment of the above catalysts mainly contain product rate, catalytic current density (for photoelectrocatalysts), turnover number and turnover frequency (evaluating the activity of catalytic active sites), quantum yield (assessing the performance of photocatalysts) and faradaic efficiency (assessing the selectivity for photoelectrocatalysts). In the following text, we will mainly analyze photocatalytic/photoelectrocatalytic materials for EAP.
EAP processes mainly involve the conversion of CO2 and H2O into hydrocarbon fuel and O2, and water splitting to produce H2 and O2. CO2/H2O photo-conversion involves two parts of the half reaction, including CO2 reduction by photo-generated electrons, and H2O oxidation by photo-generated holes, respectively. The standard chemical potentials required in thermodynamics for these processes are shown in the following reactions (1–8), respectively:
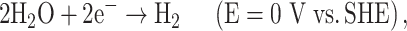 |
(1) |
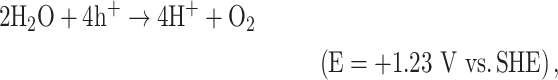 |
(2) |
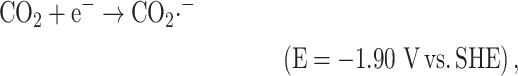 |
(3) |
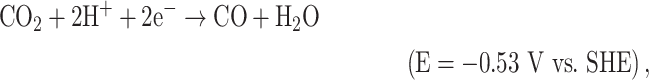 |
(4) |
 |
(5) |
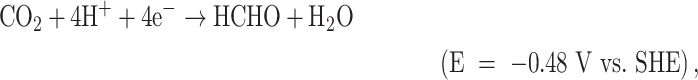 |
(6) |
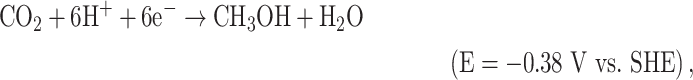 |
(7) |
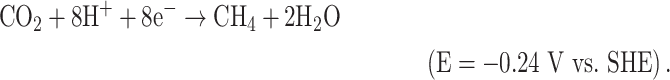 |
(8) |
In consideration of the extreme environmental conditions that EAP materials would be applied in, EAP materials should meet the following requirements: (i) an appropriate band structure, which is conducive for extraterrestrial sunlight absorption with different spectra and higher intensity, and reaches the chemical potentials needed to achieve the corresponding photo-redox reaction; (ii) adequate surface catalytic active sites to support effective photo-redox reaction; (iii) fast carrier transport and separation at the interface; (iv) stable activity under microgravity; (v) excellent survivability under intense cosmic radiations; (vi) great capability of resisting impact during take-off and landing; (vii) low cost. Additionally, CO2/H2O photocatalysts require excellence in reactant adsorption, product desorption and product selectivity. During CO2/H2O photo-conversion, H2 production is the major competitive side reaction. Therefore, avoiding H2 production is also one of the key points in improving CO2/H2O photo-conversion. A major way to hinder the H2 production is to adjust the surface adsorption behavior of protons during the EAP process. Typical methods [30] include adjusting adsorption properties of the catalyst surface on carbon intermediate species, realizing proper surface modification and hydrating or alloying the surface with cocatalysts.
EAP materials for overall photo-conversion
Most of the reported photocatalytic materials are thermodynamically unfavorable to complete both the reductive half reaction (CO2 reduction) and the oxidative half reaction (O2 production), since the overall CO2 conversion reaction generally needs a band gap of over 3.1 eV when taking account of the CO2 adsorption on the electrode surface (reaction (3)). Catalysts with suitable band structure supporting overall CO2/H2O conversion are mainly TiO2, ZnO, CdS, ZnS, ZnIn2S4, Ta3N5, TaON, graphitic carbon nitride (g-C3N4), SrTiO3 [31–35] (in which TiO2, ZnO and ZnS can only use ultraviolet light). SrTiO3 and CdS can theoretically carry out full conversion under visible light, however, their intrinsic properties are restricted by low carrier separation rate. Thus, modification by cocatalysts or construction of composite materials is needed for the practical application of overall CO2/H2O conversion. Most of the cocatalysts in photocatalysis are derived from electrocatalysts. The role of cocatalysts is to reduce the activation energy required for surface reactions. Cocatalysts for oxygen evolution reaction (OER) mainly include cobalt phosphate (CoPi) [36], iron hydroxide oxide (FeOOH) [37], nickel oxide (NiOx) [38], ceria (CeOx) [39], RuO2 [40], cobalt oxide (CoOx) [41] and manganese oxide (MnOx) [42]. Highly active cocatalysts for CO2 reduction reaction (CO2RR) primarily contain Au [43], Ag [44], Cu [45], Mn [46], MoS2 [47] and CdS [48]. Moreover, adjusting the composition and ratio of metals, metallic alloy or other composite cocatalysts can be helpful in improving the selectivity of CO2 reduction products [49–51].
At present, EAP materials are restricted by the low efficiency of photo-generated charge separation, the slow surface reaction rate and the serious inverse reaction of the products. A variety of methods have been developed to regulate the charge separation and surface reaction performance, as well as restrain the inverse reaction of the artificial photosynthetic catalysts, including crystal engineering, built-in electric field, polarization effect, effective mass reduction of photo-generated carriers, a single crystal with low structural defects, molecular composites, the Z-scheme strategy and surface modification with cocatalysts. Conformation of solid solution is one of the effective means of regulating the electronic structure of catalysts and promoting photosynthetic performance. Zhigang Zou’s group showed in their theoretical calculation results that Zn2GeO4 phase transformation from pseudo-cubic phase into cubic phase can effectively narrow the band gap. This is because the introduction of s and p orbitals of Ge enhances the repulsion of p-d (O 2p-Zn 3d) and raises the valence band position, while the s orbitals of Ge with low energy effectively lower the conduction band position. The solid solution photocatalysts composed of cubic ZnGa2O4 and pseudo-cubic Zn2GeO4 have light hole effective mass, and higher hole mobility. A narrow band gap is beneficial for absorbing sunlight with a wider wavelength, and high hole mobility is beneficial for improving the reaction rate of water oxidation. Therefore, the solid solution photocatalytic materials show higher photocatalytic performance with regard to reducing CO2 to hydrocarbon fuel [52]. Based on a similar mechanism, Kazunari Domen’s group also reported that (Ga1−xZnxN1−xOx) has excellent performance for visible-light-driven complete water splitting [53].
It is an effective method to separate photo-generated charges by forming a built-in electric field on the interface of materials. Commonly, the built-in electric field exists between two different semiconductor materials or between different phase structures of the same semiconductor. The electrons and holes in this type of built-in electric field separate to opposite directions under the electric field, thus improving the separation efficiency. Zhigang Zou et al. synthesized a CeO2 octahedral structure with vertical growth of hexahedron prism [54] (Fig. 3a). By adjusting the length and number of CeO2 prisms with exposed surface of {1 0 0} facets on octahedron with surface of {1 1 1} facets, the separation and transmission efficiency of photo-generated carriers were improved, leading to the highly improved efficiency of photocatalytic CO2 reduction to produce CH4. Theoretical calculation showed that for CeO2, the effective mass of electrons on {1 0 0} facet is much larger than that on {1 1 1} facet, while the effective mass of holes on {1 1 1} facet is larger than that on {1 0 0} facet. Therefore, photo-generated electrons easily migrate to the {1 1 1} facets, while photo-generated holes easily migrate to the {1 0 0} facets, which is conducive to the separation of photo-generated e– and h+. Thus, the CeO2 homojunction materials achieved the overall CO2/H2O photo-conversion efficiency with 0.86 μmol h–1 g–1 CH4 yield, and 0.2% quantum yield at 380 nm. The O2 yield was also detected in accord with ∼2 : 1 molar ratio to CH4.
Figure 3.
(a) Schematic illustration of CO2 photoreduction into CH4 over hexahedron prism anchored octahedronal CeO2 (adapted from ref. [54] with permission from ACS Publications). (b) An overall CO2/H2O photo-conversion system with the CotpyP-loaded SrTiO3:La,Rh|Au|RuO2-BiVO4:Mo photocatalysts (adapted from ref. [58] with permission from Springer Nature). (c) Sketch map of Z-scheme model and light-induced charge transfer path over SiC@MoS2 photocatalyst (adapted from ref. [59] with permission from ACS Publications). (d) CO2/H2O photo-conversion reaction route of the heterometallic cluster-based organic frame photocatalyst (adapted from ref. [60] with permission from Wiley-VCH). (e) Diagrammatic sketch of water splitting on Pt nanoparticle (gray) decorated CdS (yellow) (adapted from ref. [31] with permission from Springer Nature).
Polar semiconductors with asymmetric positive and negative electron centers can induce the polarization effect for effective separation of photo-generated charges. Zhigang Zou et al. proposed that single crystals growing along the polarization axis of a polar semiconductor could maximize the polarization field effect of the polar semiconductor, and efficiently separate photo-generated charges [55]. Because of the periodic potential field, the photo-generated charges are separated effectively along the polarization axis. And the photo-generated electrons are transmitted preferentially along the direction perpendicular to the polarization axis, forming a specific two-dimensional transport path, which greatly reduced the e––h+ recombination probability. Thus, the activity and selectivity of reducing CO2 to CH4 are greatly improved. Li's group also found that the surface electric field induced by intrinsic polarity of GaN nanoarrays can effectively enhance carriers’ spatial separation and greatly promote the photocatalytic overall water splitting. Based on the photo-generated charge separation effect between polar and non-polar surfaces, the quantum efficiency was improved from 0.9% to 6.9% with the redox cocatalysts constructed on polar and non-polar surfaces, respectively [56].
Carbon dots synthesized by microwave can quickly extract holes from carbon nitride and prevent the surface adsorption of methanol, which is beneficial to water oxidation and improves the ability of selective CO2 reduction to alcohols. Tang and Guo's group found that the carbon dots synthesized by microwave method have unique hole-accepting properties, which can extend the electron lifetime of carbon nitride 6-fold [57]. It is thus beneficial to the stable production of stoichiometric O2 and methanol from water and CO2, respectively. The selectivity of CH3OH is close to 100% and the inherent quantum efficiency is 2.1% under visible light. This work paves the way for the sustainable production of metal-free catalytic methanol, providing a unique strategy that can efficiently and selectively reduce CO2 to high-value chemicals via artificial synthesis.
Nanostructured single-crystal photocatalysts with few structural defects and the appropriate cocatalysts were shown to be excellent in overall solar water splitting. For example, although Ta3N5 photocatalysts have excellent visible light absorption and almost ideal energy band structure, non-single crystal or haploid Ta3N5 can barely achieve overall water splitting due to the strong charge recombination at defects. Domen's group fabricated Ta3N5 nanorods growing on lattice-matched cubic KTaO3 particles, combined with the Rh/Cr2O3 cocatalyst. Since the single-crystal Ta3N5 nanorod crystals had few grain boundaries, the materials presented high water-splitting efficiency under simulated sunlight [33].
Molecular composites are also effective photocatalytic materials for achieving scalable and sustainable carbon dioxide reduction. Reisner's group prepared a photocatalytic sheet by integrating La and Rh co-doped SrTiO3, Mo-doped BiVO4, phosphonated Co(ii) bis(terpyridine) and RuO2 catalysts onto a gold layer [58] (Fig. 3b). This device achieves a solar energy conversion (from CO2 to formate) efficiency of 0.08 ± 0.01% and a selectivity of 97 ± 3%, respectively. When the device was exposed to simulated sunlight, e––h+ pairs were produced in both SrTiO3: La, Rh and BiVO4: Mo. Electrons were transformed from BiVO4:Mo conduction band to the SrTiO3:La,Rh donor level through the Au layer. With the aid of molecular compounds, e– in SrTiO3: La,Rh reduced CO2 into HCOO–, simultaneously h+ in the BiVO4:Mo oxidized H2O to O2 with RuO2 cocatalyst for O2 generation.
The Z-scheme heterojunction can be propitious to the transfer balance of photo-generated e––h+ pairs. Li's group realized the overall CO2/H2O conversion through marigold-like SiC@MoS2 nanoflower materials with 323 μL g–1 h–1 methane yield and 620 μL g–1 h–1 O2 release under λ ≥ 420 nm visible light irradiation. This photocatalytic performance can be ascribed to the following aspects: (i) the direct Z-scheme heterostructure with negative SiC conduction band for CO2 reduction and positive MoS2 valence band for O2 evolution, (ii) the high e– mobility of SiC and high h+ mobility of MoS2, (iii) the marigold flower-like microstructure of SiC@MoS2 making the surface of catalyst completely exposed to reactants and (iv) the gas–solid reaction beneficial to adsorption/desorption behavior on the Z-scheme heterostructure surface [59] (Fig. 3c).
Through reasonable material design, appropriate doping modification and deposition of cocatalysts, the feasibility of free charge recombination losses for efficient overall water splitting can be achieved. Domen's group demonstrated overall water splitting, evolving H2 and O2 in a 2 : 1 stoichiometric ratio at an external quantum efficiency up to 96% (350 nm < λ < 360 nm), using Al doped SrTiO3 photocatalysts [35]. By selectively photo-depositing the cocatalysts Rh/Cr2O3 for H2 evolution and cobalt hydroxide oxide (CoOOH) for O2 generation respectively, the H2 and O2 evolution could be enhanced separately by anisotropic charge transport on different crystallographic planes of the catalyst particles.
A system with clear structure to clarify the relationship between structure and photosynthesis is very important in promoting the development of artificial photosynthesis. Lan and Liu synthesized metal organic framework (MOF) photocatalysts based on heterometallic Fe2M clusters. The catalysts converted carbon dioxide and water into formate and O2 without additional sacrificial agents and photosensitizers. Visible light excited heterometallic clusters and photosensitive ligands to produce photo-generated electron-hole pairs. Low-cost metals accepted electrons to reduce carbon dioxide, while high-price metals used holes to oxidize water [60] (Fig. 3d). Lan and Liu's work proposed a novel strategy of designing crystalline catalysts for overall artificial photosynthesis.
The combination of catalysts that are of nanometer and molecular scale is of great significance in visible-light-induced overall artificial photosynthesis. Stolarczyk's group put forward the design idea of ‘all in one’. Spatial separation of oxidation and reduction sites respectively on CdS nanorods and the co-modification parts of the both sites, i.e. Pt nanoparticles and Ru(tpy)(bpy)Cl2-based molecules, was realized [31] (Fig. 3e). Pt nanoparticles at the tip of CdS nanorods acted as electron receptors and were responsible for H2 production (20 μmol gcat–1 h–1). Ru(tpy)(bpy)Cl2-based molecular cocatalysts were fixed to the periphery of CdS nanorods and are responsible for O2 production (170 μmol gcat–1 h–1). The catalyst has both reduction and oxidation catalytic functions, so that visible light can drive the total water splitting without sacrificial agents.
Generally speaking, in the last decade, great progress has been made on photocatalytic CO2RR. The solar energy conversion efficiency of photocatalytic materials has gradually improved; the methods to improve the photocatalytic reaction efficiency tend to be clear; the understanding of the photocatalytic mechanism has gradually deepened; the characterization methods are developing rapidly; and photocatalytic materials based on novel physical mechanisms are emerging. However, in order to achieve the practical goals of EAP, the research on CO2RR still needs a leap forward. First of all, one of the key problems is how to greatly improve the photocatalytic CO2RR performance. In addition to the requirement to develop new materials, how to match the band gap of photocatalytic materials with the extraterrestrial solar spectrum, how to match the conduction/valence band position of EAP materials with the potential of reactants, how to reduce electron-hole recombination and improve quantum efficiency, and how to improve the stability of photocatalytic materials are still key scientific issues that must be solved in this field. Secondly, existing characterization techniques cannot fully facilitate an understanding of the catalytic mechanism. Thus, some advanced in-situ and/or atomic level characterization methods are still required to reveal the key factors affecting the photocatalytic reaction process. As for realizing efficient and stable EAP, researchers need to deepen the understanding of the photocatalytic reaction mechanism from a macroscopic and qualitative description to microscopic and quantitative research, to comprehensively study the process of light absorption, electron-hole excitation and transport, and interface dynamics, and to clarify the mechanism of energy transfer and conversion. These approaches can guide researchers when it comes to developing EAP materials with high quantum efficiency, by breaking through the existing theoretical framework and actively promoting the cross-integration of photochemistry and other disciplines.
Photoelectrocatalysis materials for EAP
Photocatalytic overall CO2/H2O conversion materials are mainly in the form of powders. There are problems such as the undesirable recombination of photo-generated electron-hole pairs in the particles and difficult separation of products from the system. Thus, photoelectrocatalysis, especially non-biased photoelectrocatalysis, in which the catalysts are in the form of film, is more beneficial to the practical applications of EAP.
Photoelectrochemical artificial photosynthesis is generally carried out by photoelectrodes composed of conductive substrates, semiconductors and cocatalysts in the aqueous environment. In this system, the excited photoelectrodes generate electrons or holes that migrate to the surface of the photocathodes or photoanodes for a reductive or oxidation reaction, respectively. Compared with the fact that the charge separation driving force of photocatalysts is the built-in electric field, charge separation in photoelectrocatalytic systems is promoted not only by a built-in electric field but also through external bias. Therefore, some thermodynamically insufficient catalytic reactions can be carried out under photoelectrocatalytic systems with proper bias. In addition, photoelectrocatalysis realizes the spatial separation of the reduction reaction and oxidation reaction to avoid the inverse reaction. According to the module composition of each part of the system, the photoelectrocatalysis system can be divided into a photocathode-to-electrode system, photoanode-to-electrode system, photocathode-photoanode system and photovoltaic coupled photoelectrocatalysis system.
A photocathode usually consists of p-type semiconductors. The conductive band of a p-type semiconductor bends downward at the interface of semiconductor and solution. This band bending allows photo-generated electrons to migrate to the electrode/solution interface and the electrons participate in the CO2 reduction reaction to produce hydrocarbon fuel. According to the thermodynamic requirements, the main photocathode materials are Cu2O [49], Cu2ZnSnS4 [50], Co2P and p-Si [51].
Correspondingly, a photoanode usually consists of n-type semiconductors. The valence band of an n-type semiconductor bends upward at the semiconductor/solution interface, allowing photo-generated holes to transfer to the electrolyte for O2 evolution. The main photoanode materials are TiO2, Fe2O3, WO3, ZnO, BiVO4, Ta3N5 and n-Si [3,61–66].
It is usually difficult to construct a device that has an individual photoelectrode and can achieve both CO2 reduction and water oxidation conversion for overall artificial photosynthesis (Fig. 1d). The construction of a dual photoelectrode system with both the photocathode and photoanode is beneficial for realizing the full conversion of artificial photosynthesis without bias. The difference in Fermi level between two photoelectrodes determines the theoretical maximum of the photo-generated voltage between them [58,59], which should be larger than the thermodynamic and kinetic requirements.
The photovoltaic coupling system is also used to couple photovoltaic cells to the photoelectrocatalytic system to make effective artificial photosynthetic systems, in which the photovoltaic cell provides bias to assist in driving electrocatalytic reactions. This kind of system includes photovoltaic-photocathode, photovoltaic-photoanode and photovoltaic-electrocatalytic coupling systems (Fig. 1e).
As mentioned above, the photocathode catalysts, photoanode catalysts and photovoltaic electrodes together form the most important material basis in photo-electrocatalytic systems for EAP. Thus, the research progress of photocathode catalysts, photoanode catalysts and photovoltaic electrodes appropriate for EAP will be discussed as follows.
Photocathode materials
Numerous p-type semiconductors have been exploited and investigated for photocathodes, including p-type silicon [67], oxides [68–73], sulfides [74], phosphides [67] and selenides [75]. Tellurides [76] have been investigated for CO2 reduction or H2O reduction. In addition, profiting from remarkable CO2/H2O molecular adsorption and activation, cocatalysts (e.g. Pd, Au, Ag and Cu) [77] usually serve as photocathodes. However, their performance is still limited by high overpotential, low selectivity and long-term operational instability [78]. Rational design is needed to optimize the material interface to achieve efficient charge transfer at low overpotential while maintaining high selectivity.
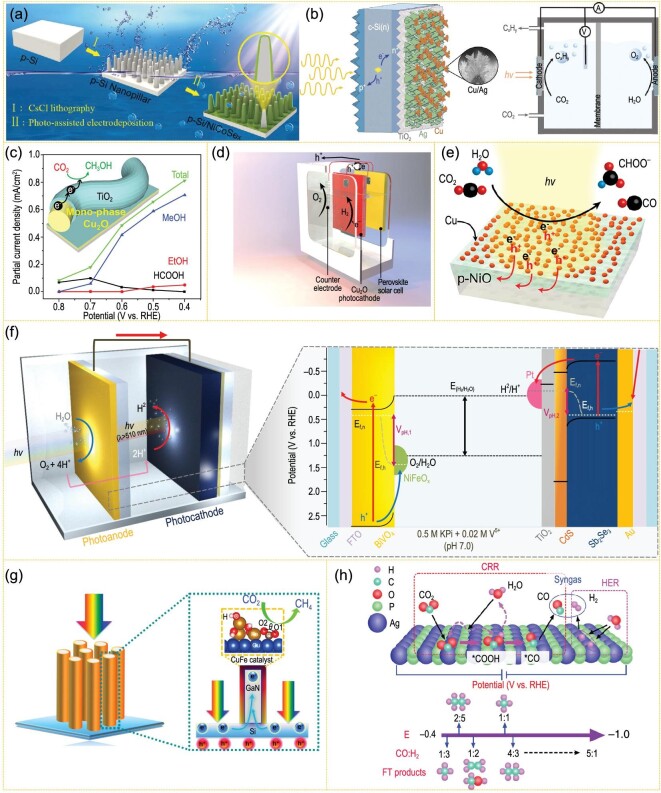
P-Si is one of the most popular materials for photocathodes, not only because of its high earth reservation, but more importantly, Si has a proper valence band position, with a 1.1 eV band gap. However, its CO2 reduction activity is low, and it lacks a suitable cocatalyst to improve the effect. For planar Si electrodes, the overpotential of CO2 reduction on the surface is mainly reduced by loading metal cocatalysts [79,80]. Recently, the loading of non-noble metal cocatalysts such as MoS2 [81] reduced graphene oxide (rGO) [82], NiOx [83] and Al2O3 [84] has been found to further improve the stability of Si in the water decomposition process of photoelectrochemical cells (PECs). For example, Bench et al. deposited a MoS2 thin layer on the surface of an Si photocathode with n+p planar structure, and the photocathode could be stable in electrolyte for >100 h [81]. Si nanowires are also widely used in the construction of photocathodes. The main reason is that Si is a typical indirect band gap semiconductor, and its high light absorption efficiency can be ensured when the penetration depth of light reaches 200 μm. The nanowire structure can reduce the migration distance of minority carriers, and multiple reflections of light can ensure the effective absorption of light by Si [85]. Photolithography technology was used to etch and form Si nanopillar arrays on Si wafers, which increased the contact area between photocathode and electrolyte. After Mo3S4 nanoclusters were deposited on the surface of p-Si, the light absorption range of the material was widened to over 620 nm, and reached a highly increased current density [86]. Recently, Chen et al. modified MoS2 with band gap position matching p-Si arrays as the cocatalyst, and used it to collect photo-generated electrons to reduce carrier recombination. Therefore, the initial H2 evolution potential of an Si@MoS2 photocathode is positively shifted to 0.122 V vs. reversible hydrogen electrode (RHE). The researchers further doped MoS2 with metal atoms to construct an Si@MMoSx (M = Fe, Co, Ni) photocathode. It was found that the initial H2 evolution potential of the material moved positively to 0.192 V vs. RHE after Co doping, and the current density at 0 V vs. RHE reached −17.2 mA cm–2 [87]. Jin et al. supported a transparent NiCoSex thin layer on the surface of a bamboo-shoot-like Si nano-array by photo-assisted electrodeposition (Fig. 4a) [88]. Under 100 mW cm–2 sunlight intensity, the current density of the catalyst at 0 V vs. RHE reached −37.5 mA cm–2. In addition, Hu Xile's research group combined Mo2C with amorphous Si and tested it as photocathode in 1 M KOH and 0.1 M H2SO4. The stable photocurrent density of this material in both electrolytes can reach −11 mA cm–2. This is the first time that the Si-based photocathode has been used in strong alkaline electrolyte, and is also the Si-based photocathode material with the widest pH range so far [89]. Besides, n-type doping on the surface can control the band bending of p-Si and form an n+p depletion layer, thus increasing the photovoltage of the p-Si photocathode. However, the direct contact between n+p-Si and metal catalysts generally causes serious surface recombination and reduced photovoltage. Inserting a metal oxide layer between the Si and catalysts to form a heterojunction can effectively adjust the charge transfer from semiconductor to cocatalysts. CO2 reduction in a silicon photocathode was reported to be realized by depositing TiO2 through p+ implantation and n+ implantation on the illumination side, and then loading the Ag/dendritic Cu catalyst. A self-powered CO2 reduction device is formed by tandemly coupling the silicon photocathode with two series of translucent CH3NH3PbI3 perovskite solar cells, and the conversion efficiency from sunlight to hydrocarbon and oxygen-containing compounds is 1.5%. Under simulated sunlight conditions, the efficiency of photocathode to hydrocarbons and oxygenated compounds (mainly ethylene, ethanol and propanol) is kept above 60%, which can last for more than several days (Fig. 4b) [90].
Figure 4.
(a) Schematic diagram of the construction process of the p-Si/NiCoSex photocathode (adapted from ref. [88] with permission from the Royal Society of Chemistry). (b) Left: schematic of the Si/TiO2/Ag/Cu photocathode. Right: schematic of the membrane-separated photoelectrochemical cell (PEC) for overall CO2/H2O conversion (adapted from ref. [90] with permission from the Royal Society of Chemistry). (c) Diagrammatic sketch of the Cu2O nanofiber electrode with a Cu2O underlayer and a TiO2 passivation layer and its CO2 photo-reduction performance (adapted from ref. [94] with permission from ACS Publications). (d) A photovoltaic (PV)-PEC system based on CuSCN/Cu2O photocathode and perovskite solar cell/IrOx anode for overall H2O splitting (adapted from ref. [97] with permission from Springer Nature). (e) Plasmonic Cu/p-NiO photocathodes for CO2/H2O conversion (adapted from ref. [98] with permission from ACS Publications). (f) Scheme of the NiFeOx/Mo: BiVO4/FTO||Pt/TiO2/CdS/Sb2Se3/Au/FTO cell for total water splitting (adapted from ref. [100] with permission from Springer Nature). (g) Spatial decoupling of CO2 reduction from light absorption and charge separation over CuFe@GaN NWs/Si (adapted from ref. [101] with permission from the National Academy of Sciences, USA). (h) Schematic diagram of selective CO2-to-syngas on AgP2 (211) (adapted from ref. [102] with permission from Springer Nature).
Metal oxide semiconductors (such as binary oxide Cu2O (2.0 eV)) and ternary oxides (for instance, CaFe2O4 (1.9 eV), CuNb3O8 (1.5 eV), CuFeO2 (1.5 eV) and LaFeO3) are typical p-type semiconductors due to metal vacancies in the structure. Metal oxide semiconductors are extensively utilized for the design and synthesis of photocathode materials because of their easy preparation and low cost. However, their unsatisfactory optical absorption coefficient, carrier mobility and stability make the energy conversion efficiency of oxide photocathodes relatively low [68–73]. Cu2O is a typical representative of this type of material and considered as a promising photocathode material to replace Si [91–93]. However, poor stability is the main problem for Cu2O-based photocathodes (Fig. 4c) [94]. Grätzel’s group has done a series of work to improve the stability of Cu2O-based photocathodes. They used MoS2+x film as a hydrogen evolution reaction (HER) catalyst on the TiO2-protected Cu2O photocathode, and the current density of the composite electrode could reach −5.7 mA cm–2 (0 V vs. RHE) [95]. After that, they deposited a double layer Al :ZnO/TiO2 film on the Cu2O surface and its improved activity was mainly attributed to the matched conduction band position of Cu2O, ZnO and TiO2, leading to quick electron migration from electrode to electrolyte [96]. As the best-performing oxide photocathode, the Cu2O photocathode’s activity surpasses that of many photocathodes after continuous research and development. However, Cu2O photocathodes employing Au as the back contact generally caused considerable e––h+ recombination. By employing CuSCN as the h+ transport material, h+ transport between Cu2O and CuSCN is expedited by band-tail states, delivering a 4.55% solar-to-hydrogen (STH) efficiency (Fig. 4d) [97].
p-NiO is also a type of electrode material for CO2 reduction PECs. DuChene et al. investigated the light-induced modulation of catalytic selectivity over Cu/p-type NiO photocathodes. According to their analysis, the optical excited hot e– of Cu nanoparticles were mainly used for CO2 reduction, while hot h+ injection from Cu nanoparticles into p-type NiO leads to charge separation. Thus, the optical excitation of plasmonic Cu/p-type NiO photocathodes enhanced CO2 reduction and inhibited H2 evolution, driving increased production of CO and HCOOH. This work demonstrated a plasmon-driven photocathode for CO2 reduction PECs (Fig. 4e) [98].
CuInxGa1−xSe2 (CIGS, 1.0–1.68 eV) and Cu2ZnSnS4 (CZTS, 1.0–1.5 eV) perform outstandingly in the field of solar cells due to their high light absorption coefficient (∼105 cm–1) and adjustable direct band gap. The modulation of chemical composition (I = Cu, Ag; II = Al, In, Ga; VI = S, Se, Te) makes the band gap of such a semiconductor adjustable within 1.0–2.4 eV. Furthermore, because of the inherent metal defects (such as Cu vacancy), this type of semiconductors are typical p-type semiconductors, which can be used as a photocathode material. CIGS photocathodes with high current density, such as CuGaSe2 (1.7 eV), CuGa3Se5 (1.8 eV) and CuInS2 (1.5 eV), have also been reported in recent years. However, the lattice mismatch at the interface limits the energy conversion efficiency of this kind of material. CdS film prepared by chemical bath deposition (CBD) is one of the best n-type semiconductors reported to compound CIGS to form a p–n junction. However, these materials were difficult in terms of application because of their slow surface reaction process and poor stability in aqueous solution [99]. Therefore, depositing a protective layer on the surface is the most important means to solve the above problems. Sb2Se3 can also be a promising material for artificial photosynthesis. Yang et al. reported an Sb2Se3 photocathode material with low cost, small band gap and good photoelectric properties and photo-corrosion stability, which also showed a high current density of almost 30 mA cm–2 at 0 V vs. RHE. The optimized Sb2Se3 photocathode achieved 1.5% solar-to-hydrogen efficiency for unassisted water splitting under the condition of 1 sun simulation when combined with a BiVO4 photoanode, and the stability exceeded 10 hours (Fig. 4f) [100].
Some traditional cocatalysts (like Ag) are great electrocatalysts for CO2-to-CO conversion. However, high overpotential limits the efficiency and the lack of efficient and highly selective cocatalysts restricts the PEC performance of photocathode catalysts. To solve the problem of unsatisfied efficiency resulted from high overpotential, a few ingenious cocatalysts have been designed and present a promising avenue for photocathode catalysts, such as CuFe alloy and AgP2. The designed CuFe was reported to exhibit a −38.3 mA cm–2 current density with CH4 faradaic efficiency up to 51%, resulting in a 2176 h–1 turnover frequency using silicon as photocathode under one sun illumination (AM 1.5G) (Fig. 4g) [101]. Integrating AgP2 nanocrystal cocatalysts on n+p-Si to construct an n+p-Si/Al2O3/AgP2 hybrid photocathode and separating the n+p-Si and AgP2 by the Al2O3 layer led to a highly improved CO2 conversion efficiency. Compared with the Ag cocatalyst, the overpotential of AgP2 nanocrystals for CO2 reduction to CO is reduced by 0.3 V, and the maximum faradaic efficiency is 82% (Fig. 4h) [102].
Photoanode materials
Generally, the anodic reaction is an OER in a PEC, which is a vital reaction in extraterrestrial space for human respiration. The photoanode OER is a four-electron transfer process with slow reaction kinetics, which is the rate control step of the artificial synthesis process. Therefore, photoanode materials with high activity and stability are indispensable in improving the energy conversion efficiency of PEC systems for EAP. Numerous n-type semiconductors have been exploited and investigated, including n-type silicon [66], oxides [61–64], nitrides [103] and sulfides [104], as photoanodes for O2 production. In addition, finely dispersed cocatalyst nanoparticles (e.g. Co [61], Fe [66]), phosphides [105] and hydroxyapatite [106], or conformal thin layers of cocatalysts (e.g. oxyhydroxide, sulfides) [107] on the photoanode surface are also an effective approach for remarkable O2 generation. Among the diverse catalysts, TiO2, WO3, Fe2O3 and BiVO4 with befitting band structure and plummy stability of aqueous solution, are good n-type semiconductor photoanode materials for O2 production through water oxidation [3,61–63]. At present, the O2 production of unmodified individual photoanode materials still needs to be improved, mainly due to poor surface reaction kinetics and weak carrier separation and transport. Many studies have improved the above problems by surface modification or defect regulation, design of heterojunction, rational design of cocatalyst, and so forth.
Recent research progress on the study of TiO2 and BiVO4 photoanodes is introduced below. Surface modification and heterojunction construction have been used for improving TiO2 photoanode performance. The Ti-OH surface states produced by electrochemical doping on the photoanode of TiO2 nanotubes, leading to charge-separation-efficiency increase, contributes to the water oxidation [108]. Heterojunction construction of the g-C3N4 film on the TiO2 nanorod array exhibits obvious advantages in PEC performance (Fig. 5a) [103].
Figure 5.
(a) Schematic of the energy diagrams and PEC system of the TiO2@g-C3N4 (adapted from ref. [103] with permission from the Royal Society of Chemistry). (b) Diagram of the construction of Fe2O3/interfacial VO/FexS photoanode and its catalytic mechanism (adapted from ref. [104] with permission from the Royal Society of Chemistry). (c) Illustration of charge transfer during the catalytic process on β-FeOOH/BiVO4 photoanode (adapted from ref. [110] with permission from Wiley-VCH). (d) Catalytic mechanism on BiVO4/FexNi1−xOOH photoanode (adapted from ref. [111] with permission from Wiley-VCH). (e) Mechanism of solar H2O splitting over BiVO4/AgOx/NiOx photoanode (adapted from ref. [113] with permission from the Royal Society of Chemistry).
The design of heterojunction and oxygen vacancy defects is demonstrated to be a synergetic method of producing a high performance in photoanodes. Integration of FexS and the synchronous generation of interfacial oxygen vacancies (VO) synergistically reduced the carrier recombination, increased the number of active sites and facilitated the participation of photo-generated holes in water oxidation for the Fe2O3 photoanode (Fig. 5b) [104]. Lianzhou Wang et al. reported a synergetic BiVO4 film rich in in-situ-formed oxygen vacancy defects converted from Bi2S3 precursor films through a sulfur oxidation method, in which the electron-hole separation rate of the bulk phase was significantly improved. NiFeOx O2-evolution cocatalysts were supported on the photocatalyst surface to facilitate surface O2 evolution. A 5.54 mA cm–2 photocurrent density was obtained under 1.23 V vs. RHE and simulated sunlight (AM 1.5), with a stability over 80 h. A 6.24 mA cm–2 photocurrent density can be obtained by stacking two BiVO4/NiFeOx electrodes, and the photoelectric conversion efficiency reaches 2.76% [109]. Bi et al. also enhanced the O2 production activity of the BiVO4 photoanode under a similar mechanism by depositing a 2 nm β-FeOOH film with abundant oxygen vacancies (Fig. 5c) [110].
In addition, for most of the photoanode materials, ingenuity in cocatalyst design has been proven to ameliorate the OER. The selective growth of FeNi cocatalysts on the BiVO4 photoanode surface obviously raised the photocurrent density to 5.8 mA cm–2 under 1.23 V vs. RHE and simulated sunlight (AM 1.5) (Fig. 5d) [111]. The selective formation of interfacial bonds between Fe, Ni in FeNi cocatalysts and Bi, V on the surface of the BiVO4, implied that after optical excitation, Fe-O-Bi interface bonding can effectively transfer the photo-induced holes from BiVO4 to Fe active sites. The photo-induced electronic injection will be delivered from Ni atoms to V sites through the Ni-O-V, thus effectively avoiding the V5+ ion dissolution. The introduction of Fe species can significantly improve its water-oxidation activity, while Ni can effectively enhance its photoelectric catalytic stability. Inserting black phosphorene (BP) between the OER cocatalyst (NiOOH, MnOx or CoOOH) and BiVO4 was reported to improve the PEC performance by 1.2–1.6-fold [112], and a 4.48 mA cm–2 photocurrent density at 1.23 V vs. RHE was achieved by the NiOOH/BP/BiVO4 photoanode. The intrinsic p-type BP can enhance h+ extraction and the h+ trapping lifetime on the BiVO4 surface, while the OER cocatalyst overlayer can suppress catalyst self-oxidation for achieving a high durability. This research presents an advantageous nexus between cocatalyst and semiconductor. AgOx/NiOx composite cocatalysts can be exploited to hoist the H2O oxidation kinetics, as well as the carrier separation of BiVO4 photoanodes, because of the high-valence-state stabilization of the metal ions, the formation of H2O oxidation active sites and the extension of the band bending region induced by AgOx/NiOx (Fig. 5e) [113].
Photovoltaic (photo)electrocatalytic materials
Compared with powder photocatalysis, photoelectrocatalysis constructs the macro-space separation of oxidation and reduction half reactions, which is beneficial to the separation of catalytic products. However, for higher catalytic performance, photoelectrocatalysis depends on the input of external electric energy. Recently, photovoltaic cells have been applied for photoelectric conversion, and the photovoltaic-electrocatalytic CO2/H2O conversion technology has also been greatly developed. Photovoltaic (photo)electrocatalytic overall CO2/H2O conversion with high efficiency provides an effective avenue for storing solar energy, which can greatly benefit EAP.
Solar cells are mainly categorized into silicon-based, III–V-based or perovskite-based photovoltaic electrocatalytic devices [114]. As high operational current densities are demanded in photovoltaic electrocatalytic devices, high-quality electrocatalysts (e.g. Au, Pt, IrOx) are preferred. Commercial noble-metal-based electrodes such as IrO2 (for O2 evolution) and Au (for CO2 reduction) [115] were successfully used for overcoming the sluggish kinetics of CO2 reduction and O2 evolution, respectively, which hinder the extensive implementation of overall CO2/H2O conversion. However, the moderate performance limits the total efficiency, and resource scarcity increases the cost. Therefore, taking further consideration of economy, resource abundance and high product selectivity, more cost-effective materials, such as Fe, Ni, Zn, W, Cu, Co or Ti-based materials, are research hotspots. The products of artificial photosynthesis are developed gradually to obtain non-toxic fuels rather than CO.
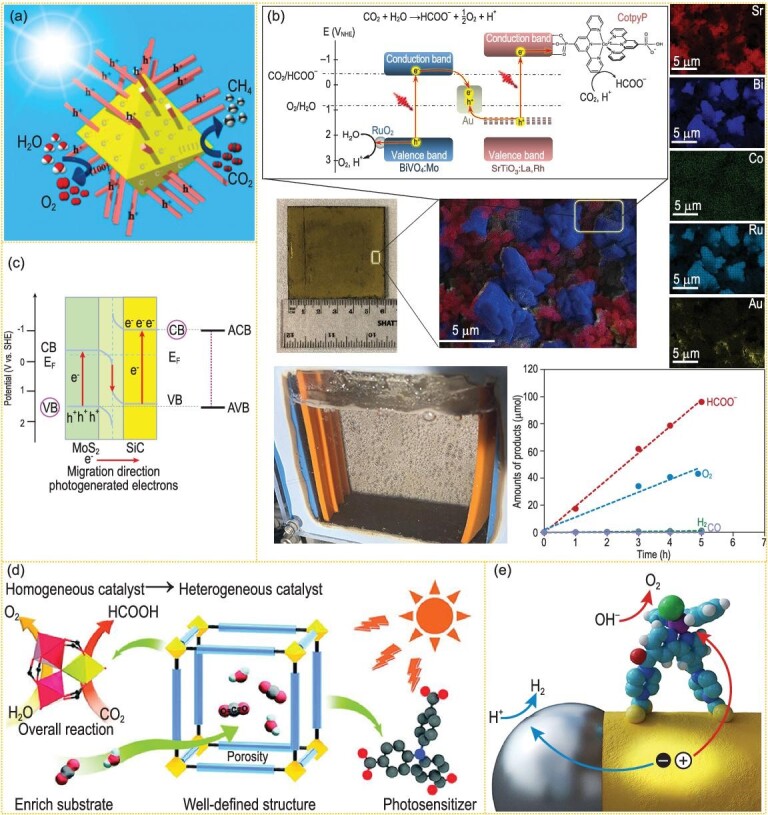
Recently, Zheng et al. constructed a GaAs solar cell with a nickel-iron hydroxide electrode for H2O oxidation, and Au nanocatalysts to reduce CO2 to CO, between which a Zn/Zn2+ redox medium was used for optimized charge transfer. This redox medium auxiliary system can achieve 15.6% solar energy-carbon monoxide photoelectric conversion efficiency and 63% electric energy efficiency under one sun intensity (Fig. 6a) [116]. Park et al. constructed a WO3 photoanode and CuxO photocathode system coupled with a dye-sensitized solar cell for driving CO2 reduction on CuxO and water oxidation on WO3 with zero external bias. In this dual-light-absorbing cell, the solar-to-chemical energy efficiency of CO2 reduction for CO is 2.5%, while it is 0.7% for H2 and 0.25% for HCOOH, respectively (Fig. 6b) [117]. By using Cu-based catalysts at both anode and cathode coupled with a perovskite photovoltaic mini-module, Fontecave et al. reported a 21% energy efficiency and a 2.3% solar-to-hydrocarbon efficiency of CO2 reduction to CH2=CH2 and CH3CH3 (Fig. 6c) [118]. By using Co2FeO4 nanosheet arrays for both cathode and anode, driven by a GaInP2/GaAs/Ge photovoltaic cell, Cao et al. showed a complete overall CO2/H2O conversion system with 13.1 mA cm–2 photocurrent density, corresponding to 15.5% solar-to-CO efficiency (Fig. 6d) [119]. The dual functional attributes can be attributed to the formation of *COOH and *O intermediates that originated from the Co sites in Co2FeO4. Lee et al. reported an enzyme—W-containing formate dehydrogenase (FDH) from Clostridium ljungdahlii (ClFDH)-conjugated direct electron transfer-type biocathode based on TiN nanoshell—and applied it to a PV-PEC system with free bias, which showed promising and stable solar-driven CO2-to-HCOOH conversion at a rate of 0.78 μmol h–1 for 24 h and a 77.3% faraday efficiency (Fig. 6e) [120].
Figure 6.
(a) Schematic and photograph of the redox-medium-assisted CO2 photovoltaic electrocatalytic reduction system containing a nickel-iron hydroxide electrode and a zinc/zincate redox with a gold nanocatalyst (adapted from ref. [116] with permission from Springer Nature). (b) Illustration of a WO3 (photoanode)||CuxO (photocathode) system coupling with a dye-sensitized solar cell (adapted from ref. [117] with permission from Elsevier). (c) Schematic view of the dendritic nanostructured CuO material before (top) and after (bottom) CO2 photovoltaic electrocatalytic reduction in 0.1 M CsHCO3 (adapted from ref. [118] with permission from the National Academy of Sciences, USA). (d) Illustration of the solar-driven overall CO2 splitting system with Co2FeO4 nanoarrays both as anode and cathode (adapted from ref. [119] with permission from Wiley-VCH). (e) Schematic illustration of the BiVO4/perovskite/3D TiN-ClFDH biocatalytic tandem PEC system for unbiased, solar-driven formate production (adapted from ref. [120] with permission from Wiley-VCH).
Although the research history of the photoelectrocatalytic CO2 reduction reaction has been developed over tens of years with great progress, there are still many problems and challenges. Because of the low catalytic efficiency, it is far from meeting the requirements of EAP application:
The selectivity of high carbon products is still very low. In CO2RR, although the faraday efficiency of CO and HCOOH can reach >90%, the highest faraday efficiencies of other reduction products, which are much more desired for EAP technology, such as CH4, CH3OH, C2H5OH, CH3COOH and C2H4, are only at the level of ∼50%.
The catalysts have poor stability. Stability is an important parameter to characterize the advantages and disadvantages of a catalyst material, and it is also a problem that must be overcome to realize EAP in harsh extraterrestrial environments. Although some catalytic materials remain stable for hundreds of hours, they are still far from the EAP goals. In the field of photoelectrocatalysis especially, due to the complex structure of photocatalysts/cocatalysts, high-energy radiation corrosion and other factors, the stability of photoelectrocatalytic CO2RR is a great challenge to overcome.
The reaction process of (photo)electrocatalytic CO2RR requires a deeper understanding. Light absorption, charge separation and interfacial reaction in the process of (photo)electrocatalytic CO2RR not only have differences on the time scale, but are also often localized at the atomic or molecular level on the spatial scale. Therefore, for observing the (photo)electrocatalytic CO2RR process in order to provide a reference for future theoretical analysis, transient, microregion and in-situ analysis methods should be utilized and developed.
With regard to the selection of the most suitable EAP technology, photocatalysis, photoelectrocatalysis and photovoltaic electrocatalysis have their individual advantages and disadvantages. EAP based on photocatalysis without an external complex device system is portable and easy to work, and therefore more adaptable to the complex extraterrestrial environment. However, its low reaction efficiency leads to low concentration of the products, with difficulties in separation and enrichment for CO2RR and OER products. Photoelectrocatalysis with two-electrode systems has the obvious advantage of efficient product separation. However, the very low stability of semiconductor photoelectrocatalysts under high-energy radiation makes the photoelectrocatalytic process difficult to adopt currently. In comparison, the photovoltaic coupled with electrocatalysis process can realize energy storage by separating photoelectric conversion and energy-to-chemical conversion, thus being more suitable for application in the extraterrestrial environment in recent times. With the further development of semiconductor/cocatalyst materials, we believe that composite technology with two or more systems will be the trend of EAP applications. More specific and detailed information for each system can be seen in Table 1.
Table 1.
More specific and detailed information about the example references.
| Method | Catalyst | Reactant | Light source/intensity | Current densities | Product/selectivity | TOF | Efficiency | Ref. |
|---|---|---|---|---|---|---|---|---|
| Photocatalysis | 4.5(ZnGa2O4):(Zn2GeO4) | CO2, 0.4 mL deionized water | A UV-enhanced (200 to 350 nm) 300 W xenon arc lamp | - | 0.5 μmol h–1 CH4, 3 μmol h–1 O2 | - | - | [52] |
| CeO2 octahedron textured with nanorods | CO2, 0.4 mL deionized water | 300 W Xe lamp (full spectrum) | - | 0.86 μmol h–1 g–1 CH4, ∼2 : 1 molar ratio of O2 to CH4 | - | CH4 quantum efficiency (%) = 0.2% at 380 nm | [54] | |
| Rh-CoOx/GaN nanorod arrays | Pure aqueous solution | 300 W Xe lamp (full spectrum) | - | H2 and O2 in stoichiometric ratio (2 : 1) | - | OER quantum efficiency (incident light wavelength range of 250–400 nm) reaches 81.1%, quantum efficiency of overall water splitting reaches 6.9% | [56] | |
| Microwave-synthesized carbon-dots decorated carbon- nitride | CO2, 10 mL water | 300 W Xe lamp (λ > 420 nm) | - | Methanol 13.9 μmol h–1 g–1, CO 0.05 μmol h–1 g–1, O2 to methanol is ∼1.45 : 1; 99.6% selectivity from CO2 to methanol | - | Internal quantum efficiency (IQY) of 2.1% in the visible region | [57] | |
| CotpyP-loaded SrTiO3:La,Rh|Au|RuO2-BiVO4:Mo photocatalyst sheet | CO2-saturated 0.1 M KHCO3 | AM 1.5 G (1 sun) illumination (100 mW cm–2) | - | O2 1.3 μmol h–1 cm–2, HCOO– 16.1 μmol in 5 h | TON for HCOO− was 305 after 4 h | A solar-to-formate conversion efficiency of 0.08 ± 0.01% with a selectivity for formate of 97 ± 3% | [58] | |
| SrTiO3:Al photo-deposited with Rh/Cr2O3/CoOOH | Xe lamp (300 W, full arc) | - | Overall water splitting, evolving H2 and O2 in a 2 : 1 stoichiometric ratio | - | An IQE of 100% and an external quantum efficiency (EQE) >50% | [35] | ||
| Photoelectrochemical cells | Si@CoMoSx photocathode | 0.5 M H2SO4 | 500 W xenon lamp equipped with an AM 1.5 filter (100 mW cm–2) | −17.2 mA cm–2 at 0 VRHE | H2 3.86 μmol min –1 | - | Faradaic efficiency 81.0% | [87] |
| p-Si/NiCoSx core/shell NP arrays | 0.5 M H2SO4 | AM 1.5G illumination (100 mW cm–2) | -37.5 mA cm –2 at 0 VRHE | H2 | - | - | [88] | |
| a-Si/Mo2C | 0.1 M H2SO4 and 1.0 M KOH | AM 1.5G illumination (100 mW cm–2) | -11.2 mA cm–2 at 0 VRHE | H2 | - | - | [89] | |
| An Si photocathode with two series-connected CH3NH3PbI3 perovskite solar cells | 0.1–0.5 M CsHCO3 | AM 1.5G illumination (100 mW cm–2) | No bias | Ethylene, ethanol and 1-propanol | - | Total solar-to-chemical conversion efficiency of 3.5% to all products and 1.5% to hydrocarbons and oxygenates | [90] | |
| MoS2+x film as the HER catalyst on TiO2-protected Cu2O photocathode | 1 M Na2SO4 buffered with 0.1 M K3PO4 (pH = 5.0) | AM 1.5G illumination (100 mW cm–2) | −5.7 mA cm–2 at 0 VRHE (pH 1.0) | - | - | 7% STH efficiency in a tandem cell configuration | [91] | |
| Cu2O photocathode using solution-processed CuSCN as hole transport material | 0.5 M Na2SO4 (pH = 5.0) | AM 1.5 G illumination (100 mW cm–2) | 6.4 mA cm–2 at 0 VRHE | - | - | STH efficiency of 4.55% | [97] | |
| Sb2Se3 photocathode with a BiVO4 photoanode | 0.5 M KPi buffer + 0.01 M V2O5 (pH = 7.0) | AM 1.5 G illumination (100 mW cm–2) | - | - | - | STH efficiency of 1.5% with stability over 10 h | [100] | |
| CuFe catalyst coupled with GaN nanowires on n+-p silicon wafer | CO2-purged 0.5 M KHCO3 aqueous solution (pH ≈ 8.0) | AM 1.5 G illumination (100 mW cm–2) | −38.3 mA cm–2 at −1.2 VRHE | CH4 88.8 μmol h–1 cm–2 | 2176 h–1 | Faradaic efficiency of 51% | [101] | |
| A photocathode consisting of a n+p-Si wafer coated with ultrathin Al2O3 and AgP2 nanocrystals | CO2-saturated 0.5 M KHCO3 solution | AM 1.5 G illumination (100 mW/cm2) | −3.2 mA cm–2 at −0.11 VRHE | - | - | FECO of 67% at −0.2 V vs. RHE | [102] | |
| BiVO4/NiFeOx | 1 M borate buffer electrolyte containing 0.2 M Na2SO3 | AM 1.5 G (100 mW cm–2) | 5.54 mA cm–2 at 1.23 VRHE | - | - | Photon-to-current efficiency of 2.76% | [109] | |
| Ultrathin FeOOH nanolayers with abundant oxygen vacancies on BiVO4 photoanode | 0.2 m Na2SO4 (pH = 7.0) | AM 1.5 G illumination (100 mW cm–2) | 4.3 mA cm–2 at 1.23 VRHE | H2 92.3 μmol after 120 min | - | - | [110] | |
| BiVO4/FexNi1−xOOH photoanode | 0.5 m K3BO3 electrolyte (pH = 9.5) | AM 1.5 G (100 mW cm–2) | 5.8 mA cm–2 at 1.23 VRHE | H2 388 μmol, O2 172 μmol after 3 h | - | The average incident photon to current efficiency (IPCE) of 90% at 420 nm | [111] | |
| NiOOH/BP/BiVO4 photoanode | 0.5 M phosphate buffer (KPi, pH = 7.1) | AM 1.5 G (100 mW cm–2) | 4.48 mA cm–2 at 1.23 VRHE | - | - | - | [112] | |
| BiVO4/AgOx/NiOx photoanode | 0.2 M KBi aqueous solution (pH = 9.25) | AM 1.5 G illumination (100 mW cm–2) | 1.24 mA cm–2 at 0.6 VRHE | - | The average IPCE values in the 380–450 nm range increased from 22.4% to 49.3%. | [113] | ||
| Photovoltaic cell + electrocatalysis | A photovoltaic and electrocatalytic system consisting of GaAs solar cell, nano-Au catalyst cathode and NiFe hydroxide anode and Zn/Zn(II) redox medium | 0.5 M CO2-saturated KHCO3 (pH = 7.2) | AM 1.5 G illumination (100 mW cm–2) | 10 mA cm–2 at 1.96 VRHE | - | - | FEco of ∼92%, a solar-to-CO photo-conversion efficiency of 15.6%, and an electric energy efficiency of 63% | [116] |
| A PEC composed of WO3/dye-sensitized solar cell and CuxO (x = 1 and 2) wire arrays as a dual-absorber photoanode and cathode | CO2-purged 0.1 M bicarbonate aqueous solution | AM 1.5 G illumination (100 mW cm–2) | - | CO, H2 and formate | - | The primary CO2 conversion product is CO, with a solar-to-chemical energy efficiency of ∼2.5%; H2 and formate are obtained with energy efficiencies of 0.7% and 0.25%, respectively in 5 h (overall efficiency ∼3.45%) | [117] | |
| A system coupling a photovoltaic cell to an electrochemical cell using the dendritic nanostructured copper oxide material at both the anode and cathode | Cathodic electrolyte: 0.1 M CO2-saturated CsHCO3 (pH = 6.8); anodic electrolyte: 0.2 M Cs2CO3 (pH = 11) | Without external bias and AM 1.5 G (1 sun) illumination (100 mW cm–2) | A stable current of 6.0 ± 0.2 mA and a potential of 2.8 ± 0.02 V | C2H4, C2H6, CO, HCOOH, H2 | - | C2H4 and C2H6 as the main products with an average faradaic yield (FY) of 40.5% (34% for C2H4 and 6.5% for C2H6), together with CO and HCOOH in 4.8% and 6.4% FY, respectively Concomitant H2 production was 42.2% FY | [118] | |
| GaInP/GaAs/Ge/Co2FeO4 driven by a triple junction GaInP2/GaAs/Ge photovoltaic cell | 0.1 M CO2-saturated KHCO3 | AM 1.5 G illumination (100 mW cm–2) | 13.1 mA cm –2 at −1.2 VRHE | - | CO | Solar-to-CO efficiency of 15.5% | [119] | |
| W‐containing formate dehydrogenase from Clostridium ljungdahlii (ClFDH) on the 3D TiN nanoshell | CO2 saturated phosphate buffer | Xe lamp (photon energy flux of 100 mW cm–2, 420 nm cut‐off filter) | Bias‐free | Formate 0.78 μmol h–1 | - | Faradaic efficiency 77.3% | [120] |
CONCLUSION AND PERSPECTIVE
In summary, the development of EAP materials is highly prospected. Looking at the future of space science and technology, how to achieve extraterrestrial survival and affordable and sustainable deep space exploration through EAP technology under extreme conditions, has become the common pursuit of humanity. On this frontier, there are still a series of concomitant scientific problems, such as low concentrations of CO2/H2O, different solar radiation intensities, ultrahigh gravity or microgravity, extreme temperatures, intense cosmic radiation, extreme pressure and ultimate vacuums. The problems that urgently need to be resolved are listed below:
CO2/H2O photo-conversion materials with normal working ability at low atmospheric density are the linchpin for EAP. At present, research into CO2/H2O photo-conversion mainly focuses on atmospheric conditions with a high concentration of, or pure, CO2. However, the concentration of CO2 or H2O in the extraterrestrial environment or the enclosed space inside the capsule is usually at an ultralow level or unstable state. Therefore, how to realize the enrichment and further utilization of CO2 is one of the critical problems. Development of artificial photosynthetical materials in low CO2 concentration environments or CO2 collection technology may be considered as the solutions.
It is necessary to develop a photocatalytic material system suitable for withstanding different solar radiation intensities, strong cosmic radiation and Frenkel defects under extraterrestrial conditions. Due to the difference in solar radiation intensity and spectral distribution between outer space and the Earth’s surface, photocatalytic materials developed for the solar spectral conditions on Earth may not work effectively and stably in the outer space environment. Therefore, it is necessary to develop new artificial photosynthetic materials suitable for cosmic radiation with a long working life, high stability, wide spectrum and responsiveness to AM0 spectra, to meet the requirements of long-term space exploration tasks. Ceramics or composites can be selected to achieve thermostability and thermal shock resistance. Metal oxides such as PbO, BaO and Bi2O3 with a high atomic number, or rare metallic elements, can be added to the material for radiation resistance [121].
Research into the effects of extreme temperature, ultra-vacuum and microgravity on the photochemical reaction, as well as multi-photon processes in the photochemical reaction, will give clues as to how to plan the EAP process. The increase of interfacial resistance (ohmic drop) caused by bubbles produced in the photocatalytic process will severely affect the surface coverage of electrodes. The mass transfer process under microgravity will become more difficult, greatly reducing the energy efficiency of the system. Supersaturated gas layers formed by gas reactants and products gathered near the three-phase interfaces of electrodes have a very important negative influence on the reaction process, material transport and reaction efficiency. Therefore, it is necessary to deeply explore the diffusion and transfer process of reaction media in electrolyte under microgravity, as well as the key mechanisms of bubble nucleation, growth, interface separation, gas-liquid two-phase flow and gas-liquid separation and their influence on photocatalytic processes. In addition, the state-of-the-art technologies of ECLSS architecture as a subsystem typical of a crewed space vehicle, which provides all the necessary conditions, can be used for the control of steady temperature and pressure inside the space capsule to support the artificial photosynthetic material, devices or systems.
The photo/thermo/electric coupling catalysis mechanism needs to be deeply understood and realized by utilizing the full spectrum of solar energy, 99.9% of which is in the infrared region (43%), visible region (50%) and ultraviolet region (7%). The band gap of existing photocatalytic materials is extensively large, and most of them absorb the ultraviolet or near-ultraviolet spectrum. As a result, the utilization rate of solar energy is not high and the overall efficiency of photocatalysis is relatively low. It is necessary to explore the mechanism of solar photo/thermo/electric coupling catalysis, develop a composite photocatalytic system with multi-spectral absorption and full-spectral utilization, and improve the reaction rate of photosynthesis and photochemical conversion efficiency.
Recently, many new artificial photosynthesis processes by microorganism/semiconductor composite systems have been developed. The core problem of the artificial photosynthesis system based on microorganisms is the interaction between microorganisms and inorganic materials, the key of which lies in the transfer of energy and charge at the interface. The interface interaction not only affects the expression of microorganisms, but also has an important impact on the properties of materials. Therefore, it is very important to improve the solar energy to chemical energy conversion efficiency of artificial photosynthesis systems based on microorganisms by increasing the interface charge transfer rate.
Advanced in-situ and atomic-scale analysis, and computational simulation techniques will play an important role in EAP. Through advanced in-situ micro-analysis and computational simulation, scientists can fully understand the complex reaction process and intermediate products in CO2/H2O photo-conversion, and thus help overcome the obstacles in energy efficiency, reaction selectivity and total conversion rate.
How to convert CO2 from human respiration into O2? How to use CO2/H2O on Mars or in other extraterrestrial atmospheric environments to generate O2 and fuel? These are the core missions of human beings with regard to achieving extraterrestrial survival and sustainable exploration. In recent years, driven by sustainable development on Earth, the technology of artificial photosynthesis has rapidly developed. In space exploration activities, it will become a core ability to in-situ transform CO2/H2O at room temperature into the basic material needed for human beings to survive outside the Earth. Chinese scientists put forward the concept of EAP, took the lead in developing artificial photosynthesis devices and space experiments that will greatly promote the development of this field, and will guide research in the fields of materials, physics, chemistry, energy, aerospace science and technology.
Contributor Information
Liuqing Yang, Eco-Materials and Renewable Energy Research Center (ERERC), Jiangsu Key Laboratory for Nano Technology, National Laboratory of Solid State Microstructures, School of Physics, Nanjing University, Nanjing 210093, China.
Ce Zhang, Qian Xuesen Laboratory of Space Technology, China Academy of Space Technology, Beijing 100094, China.
Xiwen Yu, Eco-Materials and Renewable Energy Research Center (ERERC), Jiangsu Key Laboratory for Nano Technology, National Laboratory of Solid State Microstructures, School of Physics, Nanjing University, Nanjing 210093, China; Collaborative Innovation Center of Advanced Microstructures, College of Engineering and Applied Sciences, Nanjing University, Nanjing 210093, China.
Yingfang Yao, Eco-Materials and Renewable Energy Research Center (ERERC), Jiangsu Key Laboratory for Nano Technology, National Laboratory of Solid State Microstructures, School of Physics, Nanjing University, Nanjing 210093, China; Collaborative Innovation Center of Advanced Microstructures, College of Engineering and Applied Sciences, Nanjing University, Nanjing 210093, China; Kunshan, Innovation Institute of Nanjing University, Suzhou 215347, China; School of Science and Engineering, The Chinese University of Hong Kong, Shenzhen 518172, China; Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology, Wuhan 430074, China.
Zhaosheng Li, Eco-Materials and Renewable Energy Research Center (ERERC), Jiangsu Key Laboratory for Nano Technology, National Laboratory of Solid State Microstructures, School of Physics, Nanjing University, Nanjing 210093, China; Collaborative Innovation Center of Advanced Microstructures, College of Engineering and Applied Sciences, Nanjing University, Nanjing 210093, China.
Congping Wu, Eco-Materials and Renewable Energy Research Center (ERERC), Jiangsu Key Laboratory for Nano Technology, National Laboratory of Solid State Microstructures, School of Physics, Nanjing University, Nanjing 210093, China; Kunshan, Innovation Institute of Nanjing University, Suzhou 215347, China.
Wei Yao, Qian Xuesen Laboratory of Space Technology, China Academy of Space Technology, Beijing 100094, China.
Zhigang Zou, Eco-Materials and Renewable Energy Research Center (ERERC), Jiangsu Key Laboratory for Nano Technology, National Laboratory of Solid State Microstructures, School of Physics, Nanjing University, Nanjing 210093, China; Qian Xuesen Laboratory of Space Technology, China Academy of Space Technology, Beijing 100094, China; Collaborative Innovation Center of Advanced Microstructures, College of Engineering and Applied Sciences, Nanjing University, Nanjing 210093, China; School of Science and Engineering, The Chinese University of Hong Kong, Shenzhen 518172, China; Macau Institute of Systems Engineering, Macau University of Science and Technology, Macau 999078, China.
FUNDING
This work was supported primarily by the National Key Research and Development Program of China (2020YFA0710302 and 2018YFE0208500), the Major Research Plan of the National Natural Science Foundation of China (91963206), the National Natural Science Foundation of China (52072169, 51627810, 51972164 and 51972167), the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2019ZT08L101), the Natural Science Foundation of Jiangsu Province (SBK2018022120), the open fund of Wuhan National Laboratory for Optoelectronics (2018WNLOKF020), the Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX19_0043), the Fundamental Research Funds for the Central Universities (14380180), the Civil Aerospace Technology Research Project (B0108), and the Foshan Xianhu Laboratory of the Advanced Energy Science and Technology Guangdong Laboratory.
Conflict of interest statement. None declared.
REFERENCES
- 1.Tu W, Zhou Y, Zou Z.. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: state-of-the-art accomplishment, challenges, and prospects. Adv Mater 2014; 26: 4607–26. 10.1002/adma.201400087 [DOI] [PubMed] [Google Scholar]
- 2.Zou ZG, Ye JH, Sayama Ket al. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001; 414: 625–7. 10.1038/414625a [DOI] [PubMed] [Google Scholar]
- 3.Kim TW, Choi KS.. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 2014; 343: 990–4. 10.1126/science.1246913 [DOI] [PubMed] [Google Scholar]
- 4.Inoue T, Fujishima A, Konishi Set al. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979; 277: 637–8. 10.1038/277637a0 [DOI] [Google Scholar]
- 5.Han H, Li C. Photocatalysis in solar fuel production. Natl Sci Rev 2015; 2: 145–7. 10.1093/nsr/nwv016 [DOI] [Google Scholar]
- 6.Junaedi C, Hawley K, Walsh Det al. Compact and lightweight sabatier reactor for carbon dioxide reduction. In: 41st International Conference on Environmental Systems (ICES), Portland, OR, USA, 17–21 July 2011. [Google Scholar]
- 7.Chevallier F, Feng L, Bösch Het al. On the impact of transport model errors for the estimation of CO2 surface fluxes from GOSAT observations. Geophys Res Lett 2010; 37: L21803. 10.1029/2010GL044652 [DOI] [Google Scholar]
- 8.Sakurai M, Oguchi M, Yoshihara Set al. Water electrolysis cell that are free liquid-gas separation system for microgravity conditions in order to establish circulated life support system. J Jpn Soc Microgravity Appl 2008; 25: 653–6. [Google Scholar]
- 9.Sakurai M, Terao T, Sone Y.. Development of water electrolysis system for oxygen production aimed at energy saving and high safety. In: 45th International Conference on Environmental Systems (ICES), Bellevue, USA, 12–16 July 2015. [Google Scholar]
- 10.Matsushima H, Fukunaka Y, Kuribayashi K.. Water electrolysis under microgravity: part II. Description of gas bubble evolution phenomena. Electrochim Acta 2006; 51: 4190–8. 10.1016/j.electacta.2005.11.046 [DOI] [Google Scholar]
- 11.Matsushima H, Nishida T, Konishi Yet al. Water electrolysis under microgravity: part 1. Experimental technique. Electrochim Acta 2003; 48: 4119–25. 10.1016/S0013-4686(03)00579-6 [DOI] [Google Scholar]
- 12.Kiuchi D, Matsushima H, Fukunaka Yet al. Ohmic resistance measurement of bubble froth layer in water electrolysis under microgravity. J Electrochem Soc 2006; 153: 138–43. 10.1149/1.2207008 [DOI] [Google Scholar]
- 13.Hammarstrom L, Hammes-Schiffer S.. Artificial photosynthesis and solar fuels. Acc Chem Res 2009; 42: 1859–60. 10.1021/ar900267k [DOI] [PubMed] [Google Scholar]
- 14.Feng DQ, Zhang C, Jiang WJet al. Design and trial of extraterrestrial artificial photosynthesis device. Chinese Space Sci Technol 2020; 40:13–22. [Google Scholar]
- 15.Yan SC, Ouyang SX, Gao Jet al. A room-temperature reactive-template route to mesoporous ZnGa2O4 with improved photocatalytic activity in reduction of CO2. Angew Chem Int Ed 2010; 49: 6400–4. 10.1002/anie.201003270 [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Colon BC, Ziesack Met al. Water splitting-biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 2016; 352: 1210–3. 10.1126/science.aaf5039 [DOI] [PubMed] [Google Scholar]
- 17.Sakimoto KK, Wong AB, Yang PD.. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 2016; 351: 74–7. 10.1126/science.aad3317 [DOI] [PubMed] [Google Scholar]
- 18.Su YD, Cestellos-Blanco S, Kim JMet al. Close-packed nanowire-bacteria hybrids for efficient solar-driven CO2 fixation. Joule 2020; 4: 800–11. 10.1016/j.joule.2020.03.001 [DOI] [Google Scholar]
- 19.Tong W, Forster M, Dionigi Fet al. Electrolysis of low-grade and saline surface water. Nat Energy 2020; 5: 367–77. 10.1038/s41560-020-0550-8 [DOI] [Google Scholar]
- 20.Samplatsky DJ, Grohs K, Edeen Met al. Development and integration of the flight sabatier assembly on the ISS. In: 41st International Conference on Environmental Systems (ICES), Portland, OR, USA, 17–21 July 2011. [Google Scholar]
- 21.Sakurai M, Oguchi M, Hoshino Tet al. Water electrolysis cells designed for microgravity conditions in order to establish air revitalization system. In: 35th International Conference on Environmental Systems (ICES), Rome, Italy, 11–14 July2005. [Google Scholar]
- 22.Kaplan D, Baird R, Flynn Het al. The 2001 Mars in-situ-propellant-production precursor (MIP) flight demonstration-project objectives and qualification test results. In: Space 2000 Conference and Exposition, Long Beach, USA, 19–21 September2000. [Google Scholar]
- 23.Interbartolo MA, Sanders GB, Oryshchyn Let al. Prototype development of an integrated Mars atmosphere and soil-processing system. J Aerosp Eng 2013; 26: 57–66. 10.1061/(ASCE)AS.1943-5525.0000214 [DOI] [Google Scholar]
- 24.Hinterman E, Hoffman JA.. Simulating oxygen production on Mars for the Mars oxygen in-situ resource utilization experiment. Acta Astronaut 2020; 170: 678–85. 10.1016/j.actaastro.2020.02.043 [DOI] [Google Scholar]
- 25.NASA Space Science Data Coordinated Archive . Ice on the Moon: A Summary of Clementine and Lunar Prospector Results. https://nssdc.gsfc.nasa.gov/planetary/ice/ice_moon.html (10 December 2012, date last accessed). [Google Scholar]
- 26.Edmondson K, Joslin D, Fetzer Cet al. Simulation of the Mars surface solar spectra for optimized performance of triple junction solar cells. In: 19th Space Photovoltaic Research and Technology Conference, Brookpark, OH, USA, 20–22 September2005. [Google Scholar]
- 27.Rapp D.Use of Extraterrestrial Resources for Human Space Missions to Moon or Mars. New York: Springer, 2018. [Google Scholar]
- 28.Niles PB, Catling DC, Berger Get al. Geochemistry of carbonates on Mars: implications for climate history and nature of aqueous environments. Space Sci Rev 2013; 174: 301–28. 10.1007/s11214-012-9940-y [DOI] [Google Scholar]
- 29.Boynton WV, Ming DW, Kounaves SPet al. Evidence for calcium carbonate at the Mars Phoenix landing site. Science 2009; 325: 61–4. 10.1126/science.1172768 [DOI] [PubMed] [Google Scholar]
- 30.Fu J, Jiang K, Qiu Xet al. Product selectivity of photocatalytic CO2 reduction reactions. Mater Today 2020; 32: 222–43. 10.1016/j.mattod.2019.06.009 [DOI] [Google Scholar]
- 31.Wolff CM, Frischmann PD, Schulze Met al. All-in-one visible-light-driven water splitting by combining nanoparticulate and molecular co-catalysts on CdS nanorods. Nat Energy 2018; 3: 862–9. 10.1038/s41560-018-0229-6 [DOI] [Google Scholar]
- 32.Wang J, Lin S, Tian Net al. Nanostructured metal sulfides: classification, modification strategy, and solar-driven CO2 reduction application. Adv Funct Mater 2021; 31: 2008008. 10.1002/adfm.202008008 [DOI] [Google Scholar]
- 33.Wang Z, Inoue Y, Hisatomi Tet al. Overall water splitting by Ta3N5 nanorod single crystals grown on the edges of KTaO3 particles. Nat Catal 2018; 1: 756–63. 10.1038/s41929-018-0134-1 [DOI] [Google Scholar]
- 34.Zhang W, Mohamed AR, Ong WJ. Z-scheme photocatalytic systems for carbon dioxide reduction: where are we now? Angew Chem Int Ed 2020; 59: 22894–915. 10.1002/anie.201914925 [DOI] [PubMed] [Google Scholar]
- 35.Takata T, Jiang JZ, Sakata Yet al. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020; 581: 411–4. 10.1038/s41586-020-2278-9 [DOI] [PubMed] [Google Scholar]
- 36.Chen LF, Wang Z, Kang P. Efficient photoelectrocatalytic CO2 reduction by cobalt complexes at silicon electrode. Chin J Catal 2018; 39: 413–20. 10.1016/S1872-2067(17)62993-0 [DOI] [Google Scholar]
- 37.Zhong Z, Li R, Lin Wet al. One-dimensional nanocrystals of cobalt perylene diimide polymer with in-situ generated FeOOH for efficient photocatalytic water oxidation. Appl Catal B 2020; 260: 118135. 10.1016/j.apcatb.2019.118135 [DOI] [Google Scholar]
- 38.Melo MA, Centurion HA, Lucas TTAet al. Pseudobrookite Fe2TiO5 nanoparticles loaded with earth-abundant nanosized NiO and Co3O4 cocatalysts for photocatalytic O2 evolution via solar water splitting. ACS Appl Nano Mater 2020; 3: 9303–17. 10.1021/acsanm.0c01957 [DOI] [Google Scholar]
- 39.Ahmed MG, Zhang MY, Tay YFet al. Surface modification of hematite photoanodes with CeOx cocatalyst for improved photoelectrochemical water oxidation kinetics. ChemSusChem 2020; 13: 5489–96. 10.1002/cssc.202001135 [DOI] [PubMed] [Google Scholar]
- 40.Zhang GW, Wang B, Li Let al. Tailoring the electronic structure by constructing the heterointerface of RuO2-NiO for overall water splitting with ultralow overpotential and extra-long lifetime. J Mater Chem A 2020; 8: 18945–54. 10.1039/D0TA06565J [DOI] [Google Scholar]
- 41.Mine S, Lionet Z, Shigemitsu Het al. Design of Fe-MOF-bpdc deposited with cobalt oxide (CoOx) nanoparticles for enhanced visible-light-promoted water oxidation reaction. Res Chem Intermed 2020; 46: 2003–15. 10.1007/s11164-019-04077-8 [DOI] [Google Scholar]
- 42.Tian L, Zhai XH, Wang Xet al. Advances in manganese-based oxides for oxygen evolution reaction. J Mater Chem A 2020; 8: 14400–14. 10.1039/D0TA05116K [DOI] [Google Scholar]
- 43.Zeng S, Vahidzadeh E, VanEssen CGet al. Optical control of selectivity of high rate CO2 photoreduction via interband- or hot electron Z-scheme reaction pathways in Au-TiO2 plasmonic photonic crystal photocatalyst. Appl Catal B 2020; 267: 118644. 10.1016/j.apcatb.2020.118644 [DOI] [Google Scholar]
- 44.Ishii T, Anzai A, Yamamoto Aet al. Calcium zirconate photocatalyst and silver cocatalyst for reduction of carbon dioxide with water. Appl Catal B 2020; 277: 119192. 10.1016/j.apcatb.2020.119192 [DOI] [Google Scholar]
- 45.Li Y, Li BH, Zhang DNet al. Crystalline carbon nitride supported copper single atoms for photocatalytic CO2 reduction with nearly 100% CO selectivity. ACS Nano 2020; 14: 10552–61. 10.1021/acsnano.0c04544 [DOI] [PubMed] [Google Scholar]
- 46.Yang J, Wang ZY, Jiang JCet al. In-situ polymerization induced atomically dispersed manganese sites as cocatalyst for CO2 photoreduction into synthesis gas. Nano Energy 2020; 76: 105059. 10.1016/j.nanoen.2020.105059 [DOI] [Google Scholar]
- 47.Li K, Lin YZ, Wang Ket al. Rational design of cocatalyst system for improving the photocatalytic hydrogen evolution activity of graphite carbon nitride. Appl Catal B 2020; 268: 118402. 10.1016/j.apcatb.2019.118402 [DOI] [Google Scholar]
- 48.Jian QY, Hao XQ, Jin ZLet al. Amorphous tungsten phosphosulphide-modified CdS nanorods as a highly efficient electron-cocatalyst for enhanced photocatalytic hydrogen production. Phys Chem Chem Phys 2020; 22: 1932–43. 10.1039/C9CP04724G [DOI] [PubMed] [Google Scholar]
- 49.Lee I, Kim WJ, Lee DC.. Design of metallic cocatalysts in heterostructured nanoparticles for photocatalytic CO2-to-hydrocarbon conversion. J Phys D: Appl Phys 2020; 53: 123001. 10.1088/1361-6463/ab5cb7 [DOI] [Google Scholar]
- 50.Li P, Liu L, An Wet al. Ultrathin porous g−C3N4 nanosheets modified with AuCu alloy nanoparticles and C–C coupling photothermal catalytic reduction of CO2 to ethanol. Appl Catal B 2020; 266: 118618. 10.1016/j.apcatb.2020.118618 [DOI] [Google Scholar]
- 51.Ran J, Jaroniec M, Qiao SZ.. Cocatalysts in semiconductor-based photocatalytic CO2 reduction: achievements, challenges, and opportunities. Adv Mater 2018; 30: 1704649. 10.1002/adma.201704649 [DOI] [PubMed] [Google Scholar]
- 52.Yan SC, Wang JJ, Gao HLet al. Zinc gallogermanate solid solution: a novel photocatalyst for efficiently converting CO2 into solar fuels. Adv Funct Mater 2013; 23: 1839–45. 10.1002/adfm.201202484 [DOI] [Google Scholar]
- 53.Maeda K, Teramura K, Lu DLet al. Photocatalyst releasing hydrogen from water. Nature 2006; 440: 295. 10.1038/440295a [DOI] [PubMed] [Google Scholar]
- 54.Li P, Zhou Y, Zhao Zet al. Hexahedron prism-anchored octahedronal CeO2: crystal facet-based homojunction promoting efficient solar fuel synthesis. J Am Chem Soc 2015; 137: 9547–50. 10.1021/jacs.5b05926 [DOI] [PubMed] [Google Scholar]
- 55.Zhou P, Wang X, Yan SCet al. Solid solution photocatalyst with spontaneous polarization exhibiting low recombination toward efficient CO2 photoreduction. ChemSusChem 2016; 9: 2064–8. 10.1002/cssc.201600512 [DOI] [PubMed] [Google Scholar]
- 56.Li Z, Zhang L, Liu Yet al. Surface polarity-induced spatial charge separation boosts photocatalytic overall water splitting on GaN nanorod arrays. Angew Chem Int Ed 2020; 59: 935–42. 10.1002/anie.201912844 [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Liu X, Han Xet al. Unique hole-accepting carbon-dots promoting selective carbon dioxide reduction nearly 100% to methanol by pure water. Nat Commun 2020; 11: 2531. 10.1038/s41467-020-16227-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Q, Warnan J, Rodríguez-Jiménez Set al. Molecularly engineered photocatalyst sheet for scalable solar formate production from carbon dioxide and water. Nat Energy 2020; 5: 703–10. 10.1038/s41560-020-0678-6 [DOI] [Google Scholar]
- 59.Wang Y, Zhang ZZ, Zhang LNet al. Visible-light driven overall conversion of CO2 and H2O to CH4 and O2 on 3D-SiC@2D-MoS2 heterostructure. J Am Chem Soc 2018; 140: 14595–8. 10.1021/jacs.8b09344 [DOI] [PubMed] [Google Scholar]
- 60.Dong LZ, Zhang L, Liu Jet al. Stable heterometallic cluster-based organic framework catalysts for artificial photosynthesis. Angew Chem Int Ed 2020; 59: 2659–63. 10.1002/anie.201913284 [DOI] [PubMed] [Google Scholar]
- 61.Okazaki M, Suganami Y, Hirayama Net al. Site-selective deposition of a cobalt cocatalyst onto a plasmonic Au/TiO2 photoanode for improved water oxidation. ACS Appl Energy Mater 2020; 3: 5142–6. 10.1021/acsaem.0c00857 [DOI] [Google Scholar]
- 62.Zhang N, Wang X, Feng Jet al. Paving the road toward the use of β-Fe2O3 in solar water splitting: Raman identification, phase transformation and strategies for phase stabilization. Natl Sci Rev 2020; 7: 1059–67. 10.1093/nsr/nwaa039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shao C, Malik AS, Han Jet al. Oxygen vacancy engineering with flame heating approach towards enhanced photoelectrochemical water oxidation on WO3 photoanode. Nano Energy 2020; 77: 105190. 10.1016/j.nanoen.2020.105190 [DOI] [Google Scholar]
- 64.Lim FS, Tan ST, Zhu Yet al. Tunable plasmon-induced charge transport and photon absorption of bimetallic Au–Ag nanoparticles on ZnO photoanode for photoelectrochemical enhancement under visible light. J Phys Chem C 2020; 124: 14105–17. 10.1021/acs.jpcc.0c03967 [DOI] [Google Scholar]
- 65.Xiao Y, Feng C, Fu Jet al. Band structure engineering and defect control of Ta3N5 for efficient photoelectrochemical water oxidation. Nat Catal 2020; 3: 932–40. 10.1038/s41929-020-00522-9 [DOI] [Google Scholar]
- 66.Oh K, Dorcet V, Fabre Bet al. Dissociating water at n-Si photoanodes partially covered with Fe catalysts. Adv Energy Mater 2020; 10: 1902963. 10.1002/aenm.201902963 [DOI] [Google Scholar]
- 67.Thalluri SM, Wei B and Welter K et al. Inverted pyramid textured p-silicon covered with Co2P as an efficient and stable solar hydrogen evolution photocathode. ACS Energy Lett 2019; 4: 1755–62. 10.1021/acsenergylett.9b00964 [DOI] [Google Scholar]
- 68.Matsumoto Y, Sugiyama K, Sato E. New photocathode materials for hydrogen evolution: calcium iron oxide (CaFe2O4) and strontium iron oxide (Sr7Fe10O22). J Phys Chem 1987; 91: 577–81. 10.1021/j100287a018 [DOI] [Google Scholar]
- 69.Viswanathan B, Subramanian VR, Lee JS.. Materials and Processes for Solar Fuel Production. New York: Springer, 2014. [Google Scholar]
- 70.Prévot MS, Guijarro N, Sivula K.. Enhancing the performance of a robust sol–gel-processed p-type delafossite CuFeO2 photocathode for solar water reduction. ChemSusChem 2015; 8: 1359–67. 10.1002/cssc.201403146 [DOI] [PubMed] [Google Scholar]
- 71.Joshi UA, Maggard PA.. CuNb3O8: a p-type semiconducting metal oxide photoelectrode. J Phys Chem C 2012; 3: 1577–81. [DOI] [PubMed] [Google Scholar]
- 72.Liu Z, Zhao Z-G, Miyauchi M.. Efficient visible light active CaFe2O4/WO3 based composite photocatalysts: effect of interfacial modification. J Phys Chem C 2009; 113: 17132–7. 10.1021/jp906195f [DOI] [Google Scholar]
- 73.Nian J-N, Hu C-C, Teng H.. Electrodeposited p-type Cu2O for H2 evolution from photoelectrolysis of water under visible light illumination. Int J Hydrogen Energy 2008; 33: 2897–903. 10.1016/j.ijhydene.2008.03.052 [DOI] [Google Scholar]
- 74.Feng K, Huang D, Li Let al. MoSx-CdS/Cu2ZnSnS4-based thin film photocathode for solar hydrogen evolution from water. Appl Catal B 2020; 268: 118438. 10.1016/j.apcatb.2019.118438 [DOI] [Google Scholar]
- 75.Kuehnel MF, Creissen CE, Sahm CDet al. ZnSe nanorods as visible-light absorbers for photocatalytic and photoelectrochemical H2 evolution in water. Angew Chem Int Ed 2019; 58: 5059–63. 10.1002/anie.201814265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang QL, Wang XK, Yu ZHet al. Artificial photosynthesis of ethanol using type-II g-C3N4/ZnTe heterojunction in photoelectrochemical CO2 reduction system. Nano Energy 2019; 60: 827–35. 10.1016/j.nanoen.2019.04.037 [DOI] [Google Scholar]
- 77.Chang X, Wang T, Yang Pet al. The development of cocatalysts for photoelectrochemical CO2 reduction. Adv Mater 2019; 31: 1804710. 10.1002/adma.201804710 [DOI] [PubMed] [Google Scholar]
- 78.Zhang N, Long R, Gao Cet al. Recent progress on advanced design for photoelectrochemical reduction of CO2 to fuels. Sci China Mater 2018; 61: 771–805. 10.1007/s40843-017-9151-y [DOI] [Google Scholar]
- 79.Maier CU, Specht M, Bilger G.. Hydrogen evolution on platinum-coated p-silicon photocathodes. Int J Hydrogen Energy 1996; 21: 859–64. 10.1016/0360-3199(96)00023-7 [DOI] [Google Scholar]
- 80.Feng J, Gong M, Kenney MJet al. Nickel-coated silicon photocathode for water splitting in alkaline electrolytes. Nano Res 2015; 8: 1577–83. 10.1007/s12274-014-0643-4 [DOI] [Google Scholar]
- 81.Benck JD, Lee SC, Fong KDet al. Designing active and stable silicon photocathodes for solar hydrogen production using molybdenum sulfide nanomaterials. Adv Energy Mater 2014; 4: 1400739. 10.1002/aenm.201400739 [DOI] [Google Scholar]
- 82.Zhong X, Wang G, Papandrea Bet al. Reduced graphene oxide/silicon nanowire heterostructures with enhanced photoactivity and superior photoelectrochemical stability. Nano Res 2015; 8: 2850–8. 10.1007/s12274-015-0790-2 [DOI] [Google Scholar]
- 83.Kargar A, Cheung JS, Liu C-Het al. NiOx-Fe2O3-coated p-Si photocathodes for enhanced solar water splitting in neutral pH water. Nanoscale 2015; 7: 4900–5. 10.1039/C4NR07074G [DOI] [PubMed] [Google Scholar]
- 84.Choi MJ, Jung J-Y, Park M-Jet al. Long-term durable silicon photocathode protected by a thin Al2O3/SiOx layer for photoelectrochemical hydrogen evolution. J Mater Chem A 2014; 2: 2928–33. 10.1039/c3ta14443g [DOI] [Google Scholar]
- 85.Stelzner T, Pietsch M, Andrä Get al. Silicon nanowire-based solar cells. Nanotechnology 2008; 19: 295203. 10.1088/0957-4484/19/29/295203 [DOI] [PubMed] [Google Scholar]
- 86.Hou Y, Abrams BL, Vesborg PCKet al. Bioinspired molecular co-catalysts bonded to a silicon photocathode for solar hydrogen evolution. Nat Mater 2011; 10: 434–8. 10.1038/nmat3008 [DOI] [PubMed] [Google Scholar]
- 87.Chen CJ, Yang KC, Liu CWet al. Silicon microwire arrays decorated with amorphous heterometal-doped molybdenum sulfide for water photoelectrolysis. Nano Energy 2017; 32: 422–32. 10.1016/j.nanoen.2016.12.045 [DOI] [Google Scholar]
- 88.Zhang H, Ding Q, He Det al. A p-Si/NiCoSex core/shell nanopillar array photocathode for enhanced photoelectrochemical hydrogen production. Energy Environ Sci 2016; 9: 3113–9. 10.1039/C6EE02215D [DOI] [Google Scholar]
- 89.Morales-Guio CG, Thorwarth K, Niesen Bet al. Solar hydrogen production by amorphous silicon photocathodes coated with a magnetron sputter deposited Mo2C catalyst. J Am Chem Soc 2015; 137: 7035–8. 10.1021/jacs.5b03417 [DOI] [PubMed] [Google Scholar]
- 90. Gurudayal, Beeman JW, Bullock Jet al. Si photocathode with Ag-supported dendritic Cu catalyst for CO2 reduction. Energy Environ Sci 2019; 12: 1068–77. 10.1039/C8EE03547D [DOI] [Google Scholar]
- 91.Lin CY, Lai YH, Mersch Det al. Cu2O|NiOx nanocomposite as an inexpensive photocathode in photoelectrochemical water splitting. Chem Sci 2012; 3: 3482–7. 10.1039/c2sc20874a [DOI] [Google Scholar]
- 92.Yang C, Tran PD, Boix PPet al. Engineering a Cu2O/NiO/Cu2MoS4 hybrid photocathode for H2 generation in water. Nanoscale 2014; 6: 6506–10. 10.1039/C4NR00386A [DOI] [PubMed] [Google Scholar]
- 93.Yang Y, Xu D, Wu Qet al. Cu2O/CuO bilayered composite as a high-efficiency photocathode for photoelectrochemical hydrogen evolution reaction. Sci Rep 2016; 6: 35158. 10.1038/srep35158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stepek IA, Cao T, Koetemann Aet al. Antibiotic discovery with synthetic fermentation: library assembly, phenotypic screening, and mechanism of action of beta-peptides targeting penicillin-binding proteins. ACS Chem Biol 2019; 14: 1030–40. 10.1021/acschembio.9b00227 [DOI] [PubMed] [Google Scholar]
- 95.Morales-Guio CG, Tilley SD, Vrubel Het al. Hydrogen evolution from a copper(I) oxide photocathode coated with an amorphous molybdenum sulphide catalyst. Nat Commun 2014; 5: 3059. 10.1038/ncomms4059 [DOI] [PubMed] [Google Scholar]
- 96.Paracchino A, Laporte V, Sivula Ket al. Highly active oxide photocathode for photoelectrochemical water reduction. Nat Mater 2011; 10: 456–61. 10.1038/nmat3017 [DOI] [PubMed] [Google Scholar]
- 97.Pan L, Liu Y, Yao Let al. Cu2O photocathodes with band-tail states assisted hole transport for standalone solar water splitting. Nat Commun 2020; 11: 318. 10.1038/s41467-019-13987-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.DuChene JS, Tagliabue G, Welch AJet al. Optical excitation of a nanoparticle Cu/p-NiO photocathode improves reaction selectivity for CO2 reduction in aqueous electrolytes. Nano Lett 2020; 20: 2348–58. 10.1021/acs.nanolett.9b04895 [DOI] [PubMed] [Google Scholar]
- 99.Zhang L, Minegishi T, Kubota Jet al. Hydrogen evolution from water using AgxCu1−xGaSe2 photocathodes under visible light. Phys Chem Chem Phys 2014; 16: 6167–74. 10.1039/c3cp54590c [DOI] [PubMed] [Google Scholar]
- 100.Yang W, Kim JH, Hutter OSet al. Benchmark performance of low-cost Sb2Se3 photocathodes for unassisted solar overall water splitting. Nat Commun 2020; 11: 861. 10.1038/s41467-020-14704-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou BW, Ou PF, Pant Net al. Highly efficient binary copper-iron catalyst for photoelectrochemical carbon dioxide reduction toward methane. Proc Natl Acad Sci USA 2020; 117: 1330–8. 10.1073/pnas.1911159117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li H, Wen P, Itanze DSet al. Colloidal silver diphosphide (AgP2) nanocrystals as low overpotential catalysts for CO2 reduction to tunable syngas. Nat Commun 2019; 10: 5724. 10.1038/s41467-019-13388-8 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Wang RY, Liu HD, Fan ZWet al. Unconventional gas-based bottom-up, meter-area-scale fabrication of hydrogen-bond free g-CN nanorod arrays and coupling layers with TiO2 toward high-efficiency photoelectrochemical performance. Nanoscale 2018; 10: 3342–9. 10.1039/C7NR09244J [DOI] [PubMed] [Google Scholar]
- 104.Liao AZ, Chen RT, Fan FTet al. Integration of FexS electrocatalysts and simultaneously generated interfacial oxygen vacancies to synergistically boost photoelectrochemical water splitting of Fe2O3 photoanodes. Chem Commun 2018; 54: 13817–20. 10.1039/C8CC08350A [DOI] [PubMed] [Google Scholar]
- 105.Song ZM, Zhu XD, Zeng YSet al. Cobalt phosphate cocatalyst loaded-CdS nanorod photoanode with well-defined junctions for highly efficient photoelectrochemical water splitting. Catal Lett 2020; 150: 1878–89. 10.1007/s10562-019-03084-z [DOI] [Google Scholar]
- 106.Chong RF, Du YQ, Chang ZXet al. 2D co-incorporated hydroxyapatite nanoarchitecture as a potential efficient oxygen evolution cocatalyst for boosting photoelectrochemical water splitting on Fe2O3 photoanode. Appl Catal B 2019; 250: 224–33. 10.1016/j.apcatb.2019.03.038 [DOI] [Google Scholar]
- 107.Bedin KC, Muche DNF, Melo MAet al. Role of cocatalysts on hematite photoanodes in photoelectrocatalytic water splitting: challenges and future perspectives. ChemCatChem 2020; 12: 3156–69. 10.1002/cctc.202000143 [DOI] [Google Scholar]
- 108.Zhu H, Zhao MM, Zhou JKet al. Surface states as electron transfer pathway enhanced charge separation in TiO2 nanotube water splitting photoanodes. Appl Catal B 2018; 234: 100–8. 10.1016/j.apcatb.2018.04.040 [DOI] [Google Scholar]
- 109.Wang S, He T, Chen Pet al. In situ formation of oxygen vacancies achieving near-complete charge separation in planar BiVO4 photoanodes. Adv Mater 2020; 32: 2001385. [DOI] [PubMed] [Google Scholar]
- 110.Zhang BB, Wang L, Zhang YJet al. Ultrathin FeOOH nanolayers with abundant oxygen vacancies on BiVO4 photoanodes for efficient water oxidation. Angew Chem Int Ed 2018; 57: 2248–52. 10.1002/anie.201712499 [DOI] [PubMed] [Google Scholar]
- 111.Zhang B, Huang X, Zhang Yet al. Unveiling the activity and stability origin of BiVO4 photoanodes with FeNi oxyhydroxides for oxygen evolution. Angew Chem Int Ed 2020; 59: 18990–5. 10.1002/anie.202008198 [DOI] [PubMed] [Google Scholar]
- 112.Zhang K, Jin B, Park Cet al. Black phosphorene as a hole extraction layer boosting solar water splitting of oxygen evolution catalysts. Nat Commun 2019; 10: 2001. 10.1038/s41467-019-10034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hu YF, Wu YQ, Feng JYet al. Rational design of electrocatalysts for simultaneously promoting bulk charge separation and surface charge transfer in solar water splitting photoelectrodes. J Mater Chem A 2018; 6: 2568–76. 10.1039/C7TA10361A [DOI] [Google Scholar]
- 114.He J, Janaky C.. Recent advances in solar-driven carbon dioxide conversion: expectations versus reality. ACS Energy Lett 2020; 5: 1996–2014. 10.1021/acsenergylett.0c00645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schreier M, Curvat L, Giordano Fet al. Efficient photosynthesis of carbon monoxide from CO2 using perovskite photovoltaics. Nat Commun 2015; 6: 7326. 10.1038/ncomms8326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Y, Liu J, Wang Yet al. Efficient solar-driven electrocatalytic CO2 reduction in a redox-medium-assisted system. Nat Commun 2018; 9: 5003. 10.1038/s41467-018-07380-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nath NCD, Choi SY, Jeong HWet al. Stand-alone photoconversion of carbon dioxide on copper oxide wire arrays powered by tungsten trioxide/dye-sensitized solar cell dual absorbers. Nano Energy 2016; 25:51–9. 10.1016/j.nanoen.2016.04.025 [DOI] [Google Scholar]
- 118.Huan TN, Corte DAD, Lamaison Set al. Low-cost high-efficiency system for solar-driven conversion of CO2 to hydrocarbons. Proc Natl Acad Sci USA 2019; 116: 9735–40. 10.1073/pnas.1815412116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mi Y, Qiu Y, Liu Yet al. Cobalt−iron oxide nanosheets for high-efficiency solar-driven CO2-H2O coupling electrocatalytic reactions. Adv Funct Mater 2020; 30: 2003438. [Google Scholar]
- 120.Kuk SK, Ham Y, Gopinath Ket al. Continuous 3D titanium nitride nanoshell structure for solar-driven unbiased biocatalytic CO2 reduction. Adv Energy Mater 2019; 9: 1900029. 10.1002/aenm.201900029 [DOI] [Google Scholar]
- 121.Shen Z, Xia Y, Yang Yet al. Protection of materials and structures from space radiation environments on spacecraft. Aerosp Mater Technol 2020; 50: 1–7. [Google Scholar]