Abstract
Perovskite solar cells (PSC) are promising next generation photovoltaic technologies, and there is considerable interest in the role of possible polarization of organic-inorganic halide perovskites (OIHPs) in photovoltaic conversion. The polarity of OIHPs is still hotly debated, however. In this review, we examine recent literature on the polarity of OIHPs from both theoretical and experimental points of view, and argue that they can be both polar and nonpolar, depending on composition, processing and environment. Implications of OIHP polarity to photovoltaic conversion are also discussed, and new insights gained through research efforts. In the future, integration of a local scanning probe with global macroscopic measurements in situ will provide invaluable microscopic insight into the intriguing macroscopic phenomena, while synchrotron diffractions and scanning transmission electron microscopy on more stable samples may ultimately settle the debate.
Keywords: perovskite solar cells, polarity, ferroelectricity, first-principles calculations, scanning probe, photovoltaic implication
The debates regarding polarity of organic-inorganic halide perovskites and its photovoltaic implications were discussed from both the experimental and theoretical aspects, bringing us new insights into this question.
INTRODUCTION
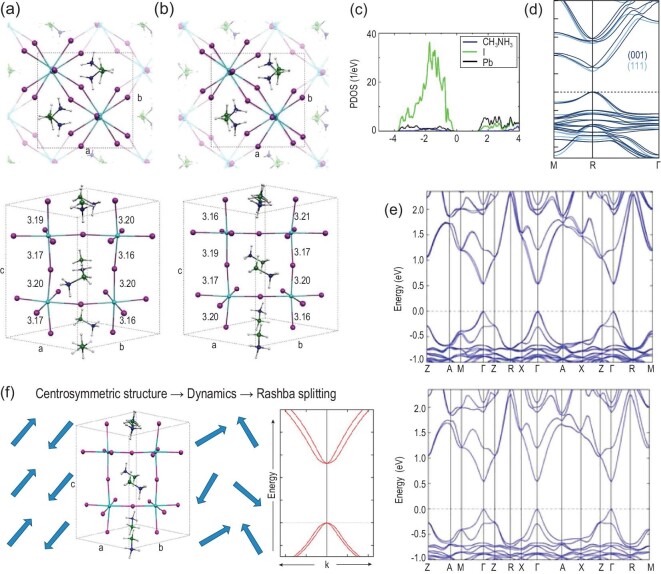
Ever since the spectacular rise of perovskite solar cells (PSCs), there have been suggestions on possible roles of ferroelectric polarization in their photovoltaic conversion. Perovskite materials, particularly oxides, are often ferroelectric, and early theoretical calculations indicated that polarization in organic-inorganic halide perovskites (OIHPs) may help charge separation and facilitate carrier transport [1]. However, the ferroelectricity of OIHPs has not been firmly established experimentally, and the possible polarity of OIHPs is still hotly debated [2,3]. There is considerable theoretical and experimental evidence to support either point of view [4]. Both nonpolar I4/mcm (Fig. 1a) and polar I4cm (Fig. 1b) space groups are possible for CH3NH3PbI3 (MAPI) [5–7], and the structural difference is very subtle, making it difficult to differentiate these by conventional structural characterization techniques such as diffraction. Indeed, the structure details of MAPI have not been fully resolved, and the poor stability of the materials exacerbates the problem. In this review, we examine recent literature on the polarity of OIHPs, and argue that they can be both polar and nonpolar, depending on composition, processing and environment. Implications to photovoltaic conversion, especially hysteresis, are also discussed.
Figure 1.

Schematic lattice of nonpolar I4/mcm (a) and polar I4cm (b) space groups for MAPI from side and top views. The hydrogen atoms are hidden for simplicity.
THEORETICAL CONSIDERATIONS
Differing from traditional perovskite, the component at the A site in OIHP is positioned by a molecule-type ion, which may have an intrinsic dipole and induce the deformation of the octahedron framework caused by the interatomic hydrogen bond. Therefore, the apparent polarization of OIHP is the collective polarization of each unit impacted by the orientation of the A-site molecule. In the case of MAPI, the major structural difference between the polar I4cm and nonpolar I4/mcm phases is the orientation of MA cations, which have an intrinsic dipole of ∼2.3 D [1]. In the polar phase, the C−N dipole shows a ‘head-tail’ alignment along the c axis and displays a large polarization of several μC/cm2 [8–11], whereas in the nonpolar counterpart, because of space group symmetry, each MA cation is usually described with partial occupancies with four identical positions and thus exhibits no net polarization [12]. Nevertheless, the orientation of an MA cation can distort the neighboring iodides from their centrosymmetric positions, leading to ferroelectricity [13].
The optimal orientation of MA in MAPbI3 bulk has not yet been determined through theoretical models and methods. Brivio et al. calculated the total energy of MA arrays along <100>, <110> and <111> directions in the cubic phase and found that <100> is the most stable orientation with energy difference less than 15 meV per atom [14]. Bechtel et al. calculated the full energy landscape for rigid-body rotations and translations of MA in the cubic phase and reached the same conclusion. They revealed that the preferential orientation is attributed to the strong N-H…I interactions between MA and the Pb-I framework along the <100> direction [15]. However, others reached different conclusions. Shimamura et al. used a cubic symmetry-assisted analysis and found that the prominent orientation of MA is in crystalline <110> directions, rather than the <100> and <111> directions [16]. Xu et al. studied MA orientation using the swarm intelligence-based structure prediction method combined with DFT calculations, but they found that the <012> orientation was most stable rather than the aforementioned directions [17].
Despite the puzzling optimal orientation, there is agreement that the orientation of MA tunes the strength and direction of the hydrogen bond between MA+ and I−, which is rather weak (∼0.09 eV/cation) [18]; there is only slight energy difference (< 0.1 eV/unit) between the two phases and the phase transition barrier is also quite small (about 0.2 eV/unit) [9]. Such a tiny difference makes for an easy transition between the polar and nonpolar phases at room temperature [19,20]. Furthermore, the subtlety between the two phases also makes the debate regarding polar and nonpolar nature of OIHPs notably dependent on method, model, size and time-scale in ab initio calculations [9,21]. We should not only focus on the origin of the polarity in its primitive cell, but also the long-range dynamics of the MA cations in a wider vision. The ab initio molecular simulation is a versatile method that can consider more operational conditions (such as temperature, long-range dynamics, etc.) with accuracy. The random order of MA and the phase transition between the two phases have been tracked, usually indicating an antiferroelectric nature of tetragonal OIHPs [19,20,22].
As mentioned, the phase transition causes reorientation of the MA cation and changes the hydrogen bond, which is very weak and has little contribution to the valence band maximum (VBM), conduction band minimum (CBM) or even band gap (∼0.1 eV fluctuation) [23,24] (Fig. 2c). However, the influence of the collective behavior of MA dynamics on the band structure cannot be neglected, as this will influence the photoelectric performance. Geng et al. designed several MA orientations in a supercell and tracked the band gap of MAPI. Their theoretical results showed that the band gap is tunable, ranging from 1.3 to 1.6 eV [25]. Mosconi et al. performed ab initio molecular dynamics simulations and also found a variation of ±0.1 to 0.2 eV of the electronic properties with the ion dynamics [22], which is consistent with Mladenović’s works [26]. Besides the value of the band gap, the orientation of MA can also cause transition from direct band gap to indirect band gap. Motta et al. performed van der Waals-corrected DFT calculations and revealed that the band gap will become indirect if MA orients along a <011>-like direction, causing dynamic change of the band structure which might be the origin of the slow carrier recombination of MAPI [27]. Later they found a similar direct–indirect transition in MAPbBr3 (MAPB). Their DFT calculations demonstrated that MAPB is a direct band gap semiconductor when MA is oriented along the <111> direction but turns indirect along the <100> direction (Fig. 2d) [28].
Figure 2.
Theoretical studies on photovoltaic properties of polar and nonpolar MAPI in bulk phase. The top and side views of (a) polar and (b) nonpolar phases of MAPI relying on the orientation of MA cation. The Pb, I, C, N, H atoms are colored in light blue, pink, blue, green and white. (c) Projected density of states of MAPI. The Pb and I instead of MA group mainly contribute to the CBM and VBM. (d) Band structure of MAPbBr3 (MAPB) with MA along different directions. A direct to indirect band transition is present when the orientation of MA changes from <111> to <001> direction. (e) The Rashba/Dresselhaus effect in the polar phases. Band splitting is present near the CBM and VBM in the polar phase, while in the nonpolar phase, the Rashba/Dresselhaus effect does not exist. (f) The dynamic Rashba effect in MAPI caused by random rotation of MA. Adapted with permission from Refs [9,24,28,31].
The Rashba/Dresselhaus effect is a phenomenon in solid-state physics in which spin-orbit interaction causes energy bands to split, especially in a crystal system lacking inversion symmetry. The polar OIHP is a typical case in which to present such effect. In the I4cm polar phase (shown in Fig. 2a), the Rashba effect can be detected by ab initio calculations, resulting in splitting of frontier orbitals near Fermi level along the M-Γ-Z direction and creation of an indirect band gap (Fig. 2e) [9,29], while in the I4/mcm nonpolar phase (Fig. 2b), the Rashba effect does not exist (Fig. 2e) [12]. Niesner et al. used angle-resolved photoelectron spectroscopy and detected the Rashba/Dresselhaus effect in MAPB [30], which is consistent with theoretical prediction. Furthermore, a ‘dynamic Rashba effect’ was proposed by Etienne et al. through the rotation of MA or the deformation of the framework when the thermal movement of MA was tracked by van der Waals-corrected ab initio simulations (Fig. 2f) [31]. Such an effect might lead to reduced recombination rate caused by a spin-forbidden transition [32]. Niesner et al. resonantly excited photocurrents in single-crystalline tetragonal MAPI with circularly polarized light to clarify the existence of such effect. Further studies showed that the energy splitting between the spin-polarized transition and the direct optical transition, as well as the amplitude of the circular photogalvanic effect, increased with temperature [33]. Wu et al. used a broad range of temperature-dependent and time-resolved optical spectroscopies, correlated with density functional theory (DFT) and molecular dynamics (MD) calculations and electrical characterizations, and proved the existence of indirect tail states below the direct transition edge in MAPB arising from a dynamical Rashba splitting effect [34]. Recalling the general features of Rashba/Dresselhaus splitting, Kepenekian et al. used symmetry analysis and DFT calculations and discussed the possibility of designing spintronic devices. They found even in the centrosymmetric system, the Rashba effect can be present under the external electric field [35]. The polarization can also impact electronic properties of the surface structure apart from the bulk. The orientation of MA cations can give rise to strong bending in the valence and conduction bands of polar phases, as exhibited by a gradient in density of states (DOS) along the [001] direction (Fig. 3a). Such band bending may reduce the carrier recombination and assist charge separation [9]. For the nonpolar phase (Fig. 3b), on the other hand, DOS along both [110] and [001] directions are nearly constant. In the mesoscopic or macroscopic system, the domain wall can be formed in OIHP and display different electronic properties compared with the bulk. Chen et al. studied the formation and band gap vs the domain width. As shown in Fig. 4a, they reported that the domain is stable with rather low formation energy and that increasing the domain width can decrease the electronic band gap from ∼1.4 eV to 0 [36]. The MA orientation can tune the charge aggregation near CBM and VBM [37] (shown in Fig. 4c), which might act as the ‘ferroelectric highway’ and profit the carrier separation [1]. The polarization in ferroelectric domains can suppress the nonradiative electron-hole recombination based on the time-domain ab initio study (Fig. 4b) [38]. Here, the pristine system is pure I4cm polar phase with aligned C−N bonds, the mixed system refers to a mixture of aligned and anti-aligned C−N bond pairs, presenting nonpolar characteristics, while the ferro-system contains two domains with opposite C−N polar bonds. Charge separation is clearly observed in mixed and ferro-systems with opposite polar axes, beneficial for recombination suppression. Furthermore, when the domain wall is charged, the band gap can be reduced by 20–40%, and there is a strong potential step that facilitates electron-hole separation (Fig. 4d), providing segregated channels for photoexcited charge carriers [39], which are desirable for high conversion efficiency [1]. Summarizing these theoretical studies, there is general agreement that polarization may be beneficial for photovoltaic conversion.
Figure 3.

Density of states (DOS) of the valence and conduction bands for the surface constructed from (a) polar and (b) nonpolar phases along [110] and [001] directions. Adapted with permission from Ref. [9].
Figure 4.
Theoretical studies on the properties of the domain wall in MAPI. (a) Domain wall energy and electronic band gap as a function of domain width in MAPI. (b) The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) charge densities in pristine MAPI, mixed and ferro systems. (c) The charge density of the CBM and VBM states in the MAPI supercell with MA randomly oriented. Both the CBM and VBM charge densities are strongly localized. (d) The charged (top) and uncharged (bottom) domain walls formed by the orientation of MA cation. Head-to-head and tail-to-tail charge domain walls attract electron and hole, respectively, facilitating charge separation. Adapted with permission from Refs [36–39].
EXPERIMENTAL EVIDENCE
Given the uncertainty associated with two possible tetragonal lattices for MAPI (Fig. 1), it was natural to carry out detailed structure analysis via X-ray and neutron diffraction techniques [40–45]. However, the subtle structural difference has proven difficult to resolve, and the data can be fit by either polar [20,38,46–58] or nonpolar [8,19,59–68] space groups. Attempts have also been made using transmission electron microscopy (TEM) [69–71], although the materials are prone to degradation [72] and it is virtually impossible to get an atomically resolved image with one exception, wherein an HRTEM image acquired from more stable MAPbBr3 (MAPB) showed polar domains [73]. As a result, much effort has been devoted to functional probing, as the properties of polar and nonpolar groups are drastically different. Macroscopic ferroelectric, pyroelectric and dielectric measurements have also been carried out [48,50,59,60,64,66,74–76], although leakage current and ionic migration often make the data interpretation ambiguous. While polar structure, with the breaking of inversion symmetry, is expected to be active in optical second harmonic generation (SHG) [12], the experimental data are inconclusive because of the strong background from other nonlinearities [62,77]. Absence of macroscopic SHG was first reported by Yamada et al. (Fig. 5a) [67,77], who did not observe any SHG signal under excitation at 1.03 eV (1204 nm) after application of a poling electric field around 1 kV/cm to the sample, while third harmonic generation and PL signals were clear because of two-photon absorption. To exclude the possibility that the second harmonic generated at wavelengths <800 nm would be strongly absorbed by MAPI in view of its small band gap, Govinda et al. adopted 1800 nm to perform SHG experiments, but the absence of a SHG response at 900 nm was still evident (Fig. 5b) [62]. It remains possible that ferroelectric domain size is below laser wavelength. Indeed, spatially resolved SHG mapping (Fig. 5c) provided strong evidence on polar domains in MAPI [76], and local polarity can be averaged out at macroscopic scale, which highlights the importance of spatially resolved local probing. Piezoresponse force microscopy (PFM) is a powerful tool to probe local electromechanical coupling at the nanoscale [78,79], and it has been widely applied to study OIHPs. Not surprisingly, PFM data reported largely fall into two categories, supporting either polar [46,47,49–54,56–58] or nonpolar [8,61,63,65,68,80] structure. In fact, even with quite similar experimental observations, for example characteristic lamellar domain patterns reported by different groups [53,81], the interpretations can be completely opposite. This is because electromechanical responses as probed by PFM can arise from complex microscopic mechanisms [82], especially ionic activities, making PFM data analysis for OIHPs nontrivial. This is best illustrated by the recent debates in Nature Materials [2,3] on the chemical nature of ferroelastic domains in MAPI reported by Liu et al. [68], and there is no agreement on whether it is ferroelectric or not. The latest publication from Liu et al., however, raised an alternative interpretation, that chemical and strain gradients induce flexoelectric polarization in MAPI [83]. This latest study seems to suggest symmetry breaking in MAPI more aligned with polar structure, although its microscopic origin is different.
Figure 5.

Second harmonic generation (SHG) response. (a) Emission spectra of MAPI under excitation at 1.03 eV. (b) Spectra of second harmonic generated at 900 nm, with incident 1800 nm laser pulse measured on urea (in black) at 1.75 mW incident power, quartz (in red) at 14.9 mW, and MAPI (in blue) at 14.9 mW after subtracting the detector background, which is shown in green. (c) A polar plot of the SHG signal (right) from the marked point in the middle SHG mapping, the area of which is approximately marked by the red box in the bright-field transmission image (left) of a crystal fragment. Adapted with permission from Refs [62,67,76].
In 2018, we reported an in-depth PFM study [4] on single crystalline MAPI [84], with the goal to resolve the microscopic mechanisms of piezoresponse probed. We acquired the most compelling domain patterns (Fig. 6a), and established distinct mechanisms underlying the piezoresponse in adjacent domains (Fig. 6b) suggesting the coexistence of alternating polar and nonpolar structures. In particular, polar domains exhibit predominantly first harmonic linear response that arises from piezoelectricity, while nonpolar domains possess predominantly second harmonic quadratic response arising from ionic motion induced electrochemical dipoles [85]. This interpretation is supported by the drastically different thermal variation of piezoresponses in polar and nonpolar domains, one increasing with temperature, the other decreasing with temperature (Fig. 6c), which converge above cubic-tetragonal transition temperature. When the temperature is reduced, the original domain configuration is recovered (Fig. 6d), demonstrating a strong memory effect. In our view, this set of data unambiguously establishes alternating polar and nonpolar domains in our crystal, and this observation can reconcile all the inconsistent experimental data and theoretical analysis reported in the literature. Other PFM studies rarely examine the linear versus quadratic piezoresponses, and thus it is difficult to identify the dominant mechanisms critical for the differentiation. Theoretical calculation suggested that the energetic difference between polar and nonpolar lattice is tiny, <100 meV [9], and thus depending on composition, processing and environment, the balance can be easily tipped, making both structures possible in experiments. As summarized in Table 1, OIHPs with various compositions are reported to be either polar or nonpolar [5,6,12,19,39,41,42,44,45,47–60,62–68,73,76,83,86–101]. In addition, the processing methods may also affect the polarity of MAPI. A comparison of the representative preparation methods for MAPI reported to be polar [52–54,57] and nonpolar [60,68,102] presents that treating MAPI with dimethylformamide (DMF) vapor on a hotplate after general synthesis procedure and inclusion of methylammonium chloride (MACl) or PbCl2, in which chlorine was shown to be beneficial for obtaining MAPI with large grains [103], together with methylammonium iodide (MAI) during synthesis might be favorable for forming polar MAPI. In a sense, we all are both wrong and right that OIHPs can be both polar and nonpolar.
Figure 6.
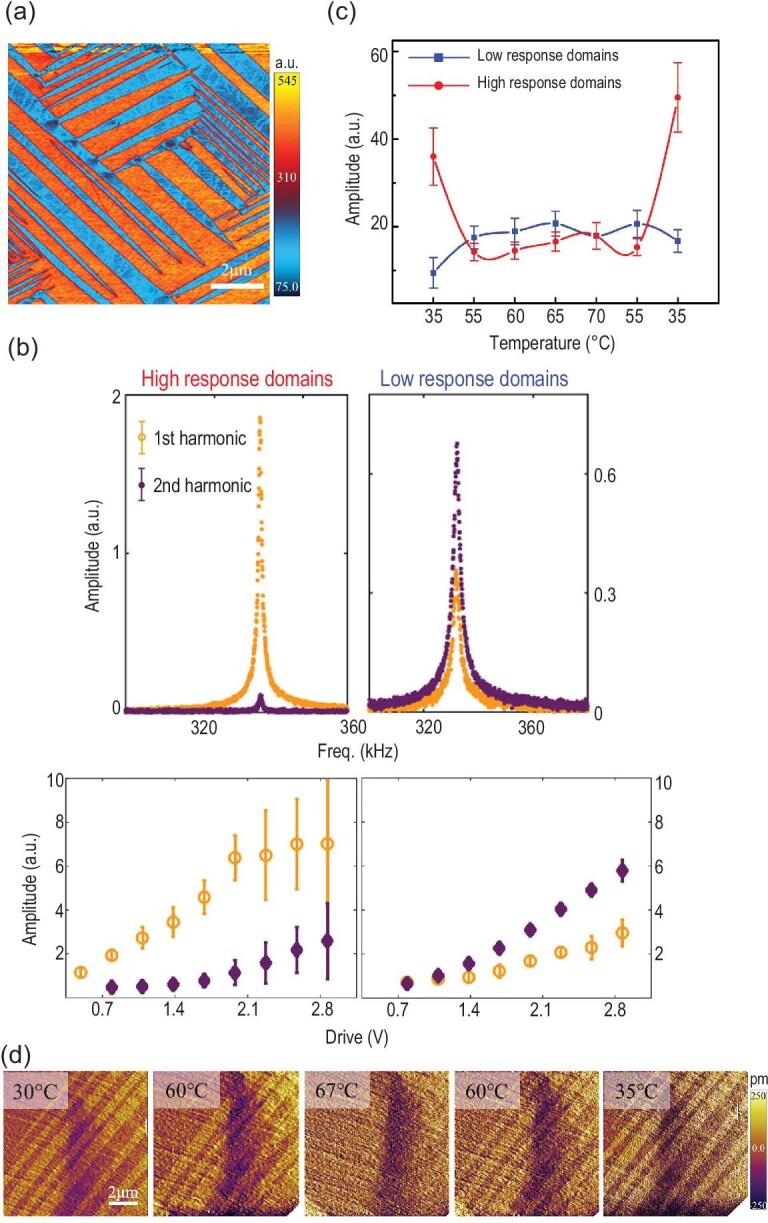
Alternating polar and nonpolar domains in MAPI. (a) Ferroic domain patterns of a MAPI crystal revealed by the vertical PFM. (b) The first row: point-wise tuning of piezoresponse versus frequency showing a point in high-response polar domain has dominant first harmonic response and negligible second harmonic one, while a point in low-response nonpolar domain has higher second harmonic response; the second row: comparison of first and second harmonic responses versus alternating current voltages averaged over a number of points in high- and low-response domains. (c) Piezoresponses averaged in high-response polar and low-response nonpolar domains showing opposite trend with respect to temperature, yet convergence beyond phase transition. (d) AFM topography mappings under a sequence of temperature across phase transition showing appearance and reemergence of ferroic domains. Adapted with permission from Ref. [4].
Table 1.
Literature survey on the polarity of OIHPs with various compositions.
| Composition | Non-polar | Polar |
|---|---|---|
| MAPbCl3 | Refs [66,86] | Ref. [39] |
| MAPbBr3 | Refs [19,66,87–89] | Refs [39,73,90] |
| MAPbI3 | Refs [5,12,19,42,44,45,59,60,62,68,91] | Refs [6,41,47–58,76,83,92] |
| FAPbBr3 | Ref. [94] | |
| FAPbI3 | Ref. [95]10.1021/acs.jpclett.7b03296 | Ref. [93] |
| CsPbCl3 | Refs [96–98] | |
| CsPbBr3 | Ref. [99] | Refs [100,101] |
| CsFAMAPbIxBr3−x | Ref. [63] | |
If MAPI is polar, can its polarity be switched? Macroscopically this is difficult to resolve, as the data are often smeared by leakage current, ionic migration as well as spatial averaging. Nevertheless, a number of recent reports indicate that electric field can indeed manipulate the domain structures [47,54,104], pointing toward a ferroelectric nature of the domain. The unambiguous switching of MAPI domains, however, requires further studies. We also note that ferroelectricity has been reported in MAPB [90], CsPbBr3 [101] and mixed perovskites [93,105,106].
PHOTOVOLTAIC IMPLICATIONS
It is also important to examine the implications of OIHPs’ polarity, or lack of it, to photovoltaic conversion, otherwise the problem remains largely academic. We have indeed observed photo-induced domain switching in MAPI via PFM [58], and a similar phenomenon has been observed under photo-excited scanning tunneling microscopy (STM) [107]. The light-domain interactions have been studied by Liu et al. [91,108], and poling has been shown to shift diode characteristic of MAPI [54]. Furthermore, piezoelectric modulations of photocurrent have also been observed [109,110]. All these studies suggest that polar structure may influence the photovoltaic conversion process, and to the very least, band bending induced by spontaneous polarization can either promote or hinder carrier transport, depending on its direction. Our study in 2018 indeed revealed that a polar domain possesses smaller photocurrent compared to a nonpolar one [4] (Fig. 7a), and upon heating and cooling across phase transition, a memory effect in photocurrent analogue to ferroic domains is also observed (Fig. 7b), confirming modulation of photocurrent by domains.
Figure 7.

Regulation of photocurrent by polar domains. (a) Correlation between PFM (left) and photocurrent (middle) of the same area with reduced photocurrent in polar domains; and surface potential distribution under light follows ferroic domain pattern in Fig. 6a with negatively shifted potential in polar domains. (b) Photocurrent distribution in a separate domain pattern at different temperatures across phase transition, showing the disappearing domain pattern at 70°C upon heating and its reemergence at 35°C after cooling. Adapted with permission from Ref. [4].
Nevertheless, there remains controversy about the effect of polarization on photovoltaic hysteresis. Unfavorable hysteresis is usually observed in the current-voltage (I−V) curve at various scanning rates or directions [111], casting doubts on the validity of the performance of solar cells and making it hard to compare stability data among them. Despite booming research and significant progress on the efficiency of perovskite solar cells, fundamental understanding of frequently observed hysteresis is still inadequate.
Among various interpretations of hysteresis, ferroelectricity was suggested as one plausible origin at the very beginning. For example, Wei et al. attributed the hysteresis to the ferroelectric effect and built a ferroelectric diode model to explain the dependence of hysteresis on the scan range as well as the velocity [112]. They pointed out that special attention should be paid to optimization of power conversion efficiency. Recently, Ma et al. investigated correlations between the interfacial ferroelectricity and the hysteresis of specific heterojunctions by simulations [113]. They found that ferroelectricity is suppressed at the FAPbI3/TiO2 and MAPbI3/phenyl-C61-butyric-acid-methyl-ester (PCBM) interfaces. The substitution of strong polar MA (dipole moment: 2.29 D) by weak polar FA ions (dipole moment: 0.29 D) and interface passivation could eliminate the interfacial electric field between perovskite and TiO2, leading to consistent interfacial electronic dynamics and the absence of hysteresis [113]. Although it is now generally accepted that ions play a more important role in hysteresis [60,114,115], the separation of ionic effect and polar order is not trivial. For example, it has been reported that a dipolar Frenkel pair can be induced by ionic migration [116]. Meloni et al. claimed that hysteresis results from polarization of halide ion (vacancy) migration in the perovskite layer under the influence of the built-in and applied potential. The mobility of the other possible ionic species (MA+ and Pb2+) is much lower and not expected to give any significant contribution to polarization of devices [114]. We also found that while illumination may enhance polar response in Cs0.05FA0.81MA0.14PbI2.55Br0.45 (CsFAMA), only small photovoltaic hysteresis is observed at both the nano- and macroscale, demonstrating that the presence of strong polarization plays a negligible role in photovoltaic hysteresis. Based on multi-harmonic measurements, our study supports the concept that the primary mechanism responsible for photovoltaic hysteresis is ionic migration rather than polarization for this material [117].
CONCLUSION AND OUTLOOK
Theoretical calculation is a versatile tool to reveal the interaction of MA with the Pb-I framework, and study the influence of MA orientation on optoelectronic properties at the nanoscale. Adequate achievements have been reported and some common views have been reached: (i) the orientation of MA is determined by the hydrogen bond and usually faces towards the low-index direction; (ii) the orientation of MA can cause deformation of the Pb-I framework because of the H…I hydrogen bond, which can break the symmetry of the system and cause polarization; (iii) just tuning the orientation of MA, namely, polarization or not has little influence on the value of band gap, can cause direct-indirect band transition, as well as the Rashba/Dresselhaus effect or even dynamic Rashba/Dresselhaus effect, which may reduce the recombination of carrier, increase the carrier lifetime and enhance the optoelectronic performance.
Experimental observations on the ferroic properties of perovskite solar cells were systematically reviewed, along with photovoltaic implications: (i) a subtle difference between polar and nonpolar structure cannot be resolved by diffraction techniques, TEM, conventional macroscopic measurements and SHG in a conclusive way because of sample damage or an averaging effect; (ii) a powerful scanning probe can capture spatially resolved functional response from different structures, although caution must be exercised to distinguish complex microscopic mechanisms among polarity, ionic motion and defect; (iii) modulation of photocurrent by polar and nonpolar domains is confirmed, while ions may play a more important role in hysteresis, which is crucial for the performance of solar cells.
We may find that polarization, whatever its exact origins, plays only marginal roles in PSCs, but the endeavor often brings in unexpected twists. As shown in Fig. 8, giant electrostriction has been reported [116], which was attributed to defect dipoles of Frenkel pairs induced by ionic migration. Here, it seems impossible to distinctly separate ionic migration, defect and polarity, all of which will be reflected in the experimental observations.
Figure 8.
Electrostrictive response of MAPI single crystal. (a) Schematic illustration of AFM measurement of strain induced by electric field. (b) Electrostrictive strain of a 40 μm MAPI single crystal under a.c. bias at 10 Hz. (c) Thickness change measured by Mach-Zehnder interferometer resulting from first-order piezoelectricity and second-order electrostriction for a 40 μm MAPI single crystal under 100 Hz a.c. with different field amplitudes. Adapted with permission from Ref. [116].
Although the beam-induced damage of synchrotron diffraction and scanning transmission electron microscopy on perovskite samples is inevitable, with continuous improvement in characterization as well as material stability [118–121], it is hoped that these techniques will ultimately settle the debate as shown in Table 2, by resolving the structure details of OIHPs. For example, Breternitz et al. recently presented crystallographic evidence that the symmetry breaking on MAPI comes from interaction of polar cation MA with the anion framework via synchrotron diffraction [13]. Besides, tentative efforts have been made to mitigate the damage for acquiring atomically resolved imaging [118], including but not limited to using cryo-conditions for higher dose tolerance of sample [122], increasing acceleration voltage to decrease radiolysis [123], and taking advantage of facet-dependent electron beam sensitivity [119]. Although further investigation is required to examine their validity, these methods have provided promising directions for future characterizations. In addition, macroscopic techniques, such as impedance spectroscopy [124,125], in combination with modeling and simulation, may provide valuable supporting data on the microscopic mechanisms. In this regard, integrating a local scanning probe with global macroscopic measurements in situ will provide invaluable microscopic insight into the macroscopic phenomena, which we are trying to develop, and it is particularly important to examine different and often competing dynamical processes from local relaxation studies [126]. From a theoretical perspective, as the energy difference between the polar and nonpolar phases is tiny and might varies with different functionals or methods, calculations with higher accuracy should be performed. In parallel, a study on the polarization should proceed not only microscopically but also mesoscopically considering the long-range interaction of the ferroelectric domains. Therefore, ab initio calculations of the larger-scale system are also needed. To mimic the real experimental conditions, other factors including temperature, strain and light should also be taken into account to investigate the dynamics of OIHP. Combined with the machine learning and artificial intelligence algorithm [127], the classical molecular dynamic simulation with accurate potential energy surface also needs to be improved. So are OIHPs polar or nonpolar? That might not be the question, but efforts made to answer it continue to deliver new insights.
Table 2.
Literature survey on the polarity of OIHPs.
| Technique | Nonpolar I4/mcm | Polar I4cm | Noncommittal |
|---|---|---|---|
| X-ray and neutron diffractions | Refs [5,42–45] | Refs [6,41] | Ref. [40] |
| Optic SHG | Refs [12,19,62,67] | Refs [76,93] | |
| Macroscopic measurements | Refs [59,60,64] | Refs [48,50,76,90,101] | Ref. [75] |
| Microscopic PFM | Refs [8,63,65,68,91] | Refs [46,47,49–58,83] | Refs [61,80,81,104] |
| TEM | Ref. [73] | Ref. [70] | |
| DFT and MD simulations | Refs [12,19,66] | Ref. [92] | Refs [8,21] |
Acknowledgements
We acknowledge the support of the Center for Computational Science and Engineering at Southern University of Science and Technology.
Contributor Information
Boyuan Huang, Department of Materials Science and Engineering, Southern University of Science and Technology, Shenzhen 518055, China; Guangdong Key Laboratory of Functional Oxide Materials and Devices, Southern University of Science and Technology, Shenzhen 518055, China.
Zhenghao Liu, Shenzhen Key Laboratory of Nanobiomechanics, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China.
Changwei Wu, Shenzhen Key Laboratory of Nanobiomechanics, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China.
Yuan Zhang, Shenzhen Key Laboratory of Nanobiomechanics, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China.
Jinjin Zhao, School of Materials Science and Engineering, Key Laboratory of Smart Materials and Structures Mechanics of Hebei Province, Shijiazhuang Tiedao University, Shijiazhuang 050043, China.
Xiao Wang, Shenzhen Key Laboratory of Nanobiomechanics, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China.
Jiangyu Li, Department of Materials Science and Engineering, Southern University of Science and Technology, Shenzhen 518055, China; Guangdong Key Laboratory of Functional Oxide Materials and Devices, Southern University of Science and Technology, Shenzhen 518055, China; Shenzhen Key Laboratory of Nanobiomechanics, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China.
FUNDING
This work was supported by the Key-Area Research and Development Program of Guangdong Province (2018B010109009), the Shenzhen Science and Technology Innovation Committee (JCYJ20180507182257563), the National Natural Science Foundation of China (11772207 and 22003074), the Instrument Developing Project of Chinese Academy of Sciences (ZDKYYQ20180004), the Natural Science Foundation of Guangdong Province (2017A030313342 and 2020A1515110580), the State Key Laboratory of Mechanics and Control of Mechanical Structures (Nanjing University of Aeronautics and Astronautics) (MCMS-E-0420G01), the Guangdong Provincial Key Laboratory Program from the Department of Science and Technology of Guangdong Province (2021B1212040001), and the SIAT Innovation Program for Excellent Young Researchers.
Conflict of interest statement. None declared.
REFERENCES
- 1.Frost JM, Butler KT, Brivio Fet al. Atomistic origins of high-performance in hybrid halide perovskite solar cells. Nano Lett 2014; 14: 2584–90. 10.1021/nl500390f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Collins L, Proksch Ret al. Reply to: on the ferroelectricity of CH3NH3PbI3 perovskites. Nat Mater 2019; 18: 1051–3. 10.1038/s41563-019-0481-6 [DOI] [PubMed] [Google Scholar]
- 3.Schulz AD, Röhm H, Leonhard Tet al. On the ferroelectricity of CH3NH3 PbI3 perovskites. Nat Mater 2019; 18: 1050. 10.1038/s41563-019-0480-7 [DOI] [PubMed] [Google Scholar]
- 4.Huang B, Kong G, Esfahani ENet al. Ferroic domains regulate photocurrent in single-crystalline CH3NH3PbI3 films self-grown on FTO/TiO2 substrate. npj Quant Mater 2018; 3: 30. 10.1038/s41535-018-0104-5 [DOI] [Google Scholar]
- 5.Baikie T, Fang Y, Kadro JMet al. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J Mater Chem A 2013; 1: 5628–41. 10.1039/c3ta10518k [DOI] [Google Scholar]
- 6.Stoumpos CC, Malliakas CD, Kanatzidis MG. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg Chem 2013; 52: 9019–38. 10.1021/ic401215x [DOI] [PubMed] [Google Scholar]
- 7.Yin WJ, Yang JH, Kang Jet al. Halide perovskite materials for solar cells: a theoretical review. J Mater Chem A 2015; 3: 8926–42. 10.1039/C4TA05033A [DOI] [Google Scholar]
- 8.Fan Z, Xiao J, Sun Ket al. Ferroelectricity of CH3NH3PbI3 perovskite. J Phys Chem Lett 2015; 6: 1155–61. 10.1021/acs.jpclett.5b00389 [DOI] [PubMed] [Google Scholar]
- 9.Quarti C, Mosconi E, De Angelis F. Interplay of orientational order and electronic structure in methylammonium lead iodide: implications for solar cell operation. Chem Mater 2014; 26: 6557–69. 10.1021/cm5032046 [DOI] [Google Scholar]
- 10.Stroppa A, Quarti C, De Angelis Fet al. Ferroelectric polarization of CH3NH3PbI3: a detailed study based on density functional theory and symmetry mode analysis. J Phys Chem Lett 2015; 6: 2223–31. 10.1021/acs.jpclett.5b00542 [DOI] [PubMed] [Google Scholar]
- 11.Zheng F, Takenaka H, Wang Fet al. First-principles calculation of the bulk photovoltaic effect in CH3NH3PbI3 and CH3NH3PbI3−xClx. J Phys Chem Lett 2015; 6: 31–7. 10.1021/jz502109e [DOI] [PubMed] [Google Scholar]
- 12.Frohna K, Deshpande T, Harter Jet al. Inversion symmetry and bulk Rashba effect in methylammonium lead iodide perovskite single crystals. Nat Commun 2018; 9: 1829. 10.1038/s41467-018-04212-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breternitz J, Lehmann F, Barnett SAet al. Role of the iodide-methylammonium interaction in the ferroelectricity of CH3NH3PbI3. Angew Chem Int Ed 2020; 59: 424–8. 10.1002/anie.201910599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brivio F, Walker AB, Walsh A. Structural and electronic properties of hybrid perovskites for high-efficiency thin-film photovoltaics from first-principles. APL Mater 2013; 1: 042111. 10.1063/1.4824147 [DOI] [Google Scholar]
- 15.Bechtel JS, Seshadri R, Van der Ven A. Energy landscape of molecular motion in cubic methylammonium lead iodide from first-principles. J Phys Chem C 2016; 120: 12403–10. 10.1021/acs.jpcc.6b03570 [DOI] [Google Scholar]
- 16.Shimamura K, Hakamata T, Shimojo Fet al. Rotation mechanism of methylammonium molecules in organometal halide perovskite in cubic phase: an ab initio molecular dynamics study. J Chem Phys 2016; 145: 224503. 10.1063/1.4971791 [DOI] [PubMed] [Google Scholar]
- 17.Xu Q, Stroppa A, Lv Jet al. Impact of organic molecule rotation on the optoelectronic properties of hybrid halide perovskites. Phys Rev Mater 2019; 3: 125401. 10.1103/PhysRevMaterials.3.125401 [DOI] [Google Scholar]
- 18.Svane KL, Forse AC, Grey CPet al. How strong is the hydrogen bond in hybrid perovskites? J Phys Chem Lett 2017; 8: 6154–9. 10.1021/acs.jpclett.7b03106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Govinda S, Kore BP, Bokdam Met al. Behavior of methylammonium dipoles in MAPbX3 (X = Br and I). J Phys Chem Lett 2017; 8: 4113–21. 10.1021/acs.jpclett.7b01740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Behtash M, Wong Jet al. Enhancing ferroelectric dipole ordering in organic-inorganic hybrid perovskite CH3NH3PbI3: strain and doping engineering. J Phys Chem C 2018; 122: 177–84. 10.1021/acs.jpcc.7b10413 [DOI] [Google Scholar]
- 21.Mattoni A, Filippetti A, Saba Met al. Methylammonium rotational dynamics in lead halide perovskite by classical molecular dynamics: the role of temperature. J Phys Chem C 2015; 119: 17421–8. 10.1021/acs.jpcc.5b04283 [DOI] [Google Scholar]
- 22.Mosconi E, Quarti C, Ivanovska Tet al. Structural and electronic properties of organo-halide lead perovskites: a combined IR-spectroscopy and ab initio molecular dynamics investigation. Phys Chem Chem Phys 2014; 16: 16137–44. 10.1039/C4CP00569D [DOI] [PubMed] [Google Scholar]
- 23.Carignano MA, Kachmar A, Hutter J. Thermal effects on CH3NH3PbI3 perovskite from ab initio molecular dynamics simulations. J Phys Chem C 2015; 119: 8991–7. 10.1021/jp510568n [DOI] [Google Scholar]
- 24.Long R, Fang W, Prezhdo OV. Moderate humidity delays electron-hole recombination in hybrid organic-inorganic perovskites: time-domain ab initio simulations rationalize experiments. J Phys Chem Lett 2016; 7: 3215–22. 10.1021/acs.jpclett.6b01412 [DOI] [PubMed] [Google Scholar]
- 25.Geng W, Hu Q, Tong CJet al. The Influence of dipole moments induced by organic molecules and domain structures on the properties of CH3NH3PbI3 perovskite. Adv Theory Simul 2019; 2: 1900041. 10.1002/adts.201900041 [DOI] [Google Scholar]
- 26.Mladenović M, Vukmirović N. Effects of thermal disorder on the electronic structure of halide perovskites: insights from MD simulations. Phys Chem Chem Phys 2018; 20: 25693–700. 10.1039/C8CP03726D [DOI] [PubMed] [Google Scholar]
- 27.Motta C, El-Mellouhi F, Kais Set al. Revealing the role of organic cations in hybrid halide perovskite CH3NH3PbI3. Nat Commun 2015; 6: 7026. 10.1038/ncomms8026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motta C, El-Mellouhi F, Sanvito S. Exploring the cation dynamics in lead-bromide hybrid perovskites. Phys Rev B 2016; 93: 235412. 10.1103/PhysRevB.93.235412 [DOI] [Google Scholar]
- 29.Leppert L, Reyes-Lillo SE, Neaton JB. Electric field- and strain-induced Rashba effect in hybrid halide perovskites. J Phys Chem Lett 2016; 7: 3683–9. 10.1021/acs.jpclett.6b01794 [DOI] [PubMed] [Google Scholar]
- 30.Niesner D, Wilhelm M, Levchuk Iet al. Giant Rashba splitting in CH3NH3PbBr3 organic-inorganic perovskite. Phys Rev Lett 2016; 117: 126401. 10.1103/PhysRevLett.117.126401 [DOI] [PubMed] [Google Scholar]
- 31.Etienne T, Mosconi E, De Angelis F. Dynamical origin of the Rashba effect in organohalide lead perovskites: a key to suppressed carrier recombination in perovskite solar cells? J Phys Chem Lett 2016; 7: 1638–45. 10.1021/acs.jpclett.6b00564 [DOI] [PubMed] [Google Scholar]
- 32.Zheng F, Tan LZ, Liu Set al. Rashba spin-orbit coupling enhanced carrier lifetime in CH3NH3PbI3. Nano Lett 2015; 15: 7794–800. 10.1021/acs.nanolett.5b01854 [DOI] [PubMed] [Google Scholar]
- 33.Niesner D, Hauck M, Shrestha Set al. Structural fluctuations cause spin-split states in tetragonal (CH3NH3)PbI3 as evidenced by the circular photogalvanic effect. Proc Natl Acad Sci USA 2018; 115: 9509–14. 10.1073/pnas.1805422115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu B, Yuan HF, Xu Qet al. Indirect tail states formation by thermal-induced polar fluctuations in halide perovskites. Nat Commun 2019; 10: 484. 10.1038/s41467-019-08326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kepenekian M, Robles R, Katan Cet al. Rashba and Dresselhaus effects in hybrid organic-inorganic perovskites: from basics to devices. ACS Nano 2015; 9: 11557–67. 10.1021/acsnano.5b04409 [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Paillard C, Zhao HJet al. Tailoring properties of hybrid perovskites by domain-width engineering with charged walls. NPJ Comput Mater 2018; 4: 75. 10.1038/s41524-018-0134-3 [DOI] [Google Scholar]
- 37.Ma J, Wang L-W. Nanoscale charge localization induced by random orientations of organic molecules in hybrid perovskite CH3NH3PbI3. Nano Lett 2015; 15: 248–53. 10.1021/nl503494y [DOI] [PubMed] [Google Scholar]
- 38.Qiao L, Fang W-H, Long R. Ferroelectric polarization suppresses nonradiative electron-hole recombination in CH3NH3PbI3 perovskites: a time-domain ab initio study. J Phys Chem Lett 2019; 10: 7237–44. 10.1021/acs.jpclett.9b02931 [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Zheng F, Koocher NZet al. Ferroelectric domain wall induced band gap reduction and charge separation in organometal halide perovskites. J Phys Chem Lett 2015; 6: 693–9. 10.1021/jz502666j [DOI] [PubMed] [Google Scholar]
- 40.Baikie T, Barrow NS, Fang YAet al. A combined single crystal neutron/X-ray diffraction and solid-state nuclear magnetic resonance study of the hybrid perovskites CH3NH3PbX3 (X = I, Br and Cl). J Mater Chem A 2015; 3: 9298–307. 10.1039/C5TA01125F [DOI] [Google Scholar]
- 41.Dang YY, Liu Y, Sun YXet al. Bulk crystal growth of hybrid perovskite material CH3NH3PbI3. CrystEngComm 2015; 17: 665–70. 10.1039/C4CE02106A [DOI] [Google Scholar]
- 42.Fang HH, Raissa R, Abdu-Aguye Met al. Photophysics of organic-inorganic hybrid lead iodide perovskite single crystals. Adv Funct Mater 2015; 25: 2378–85. 10.1002/adfm.201404421 [DOI] [Google Scholar]
- 43.Sewvandi GA, Kodera K, Ma Het al. Antiferroelectric nature of CH3NH3PbI3-xClx perovskite and its implication for charge separation in perovskite solar cells. Sci Rep 2016; 6: 30680. 10.1038/srep30680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weller MT, Weber OJ, Henry PFet al. Complete structure and cation orientation in the perovskite photovoltaic methylammonium lead iodide between 100 and 352 K. Chem Commun 2015; 51: 4180–3. 10.1039/C4CC09944C [DOI] [PubMed] [Google Scholar]
- 45.Whitfield PS, Herron N, Guise WEet al. Structures, phase transitions and tricritical behavior of the hybrid perovskite methyl ammonium lead iodide. Sci Rep 2016; 6: 35685. 10.1038/srep35685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen B, Shi J, Zheng Xet al. Ferroelectric solar cells based on inorganic-organic hybrid perovskites. J Mater Chem A 2015; 3: 7699–705. 10.1039/C5TA01325A [DOI] [Google Scholar]
- 47.Garten LM, Moore DT, Nanayakkara SUet al. The existence and impact of persistent ferroelectric domains in MAPbI3. Sci Adv 2019; 5: eaas9311. 10.1126/sciadv.aas9311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo H, Liu P, Zheng Set al. Re-entrant relaxor ferroelectricity of methylammonium lead iodide. Curr Appl Phys 2016; 16: 1603–6. 10.1016/j.cap.2016.09.016 [DOI] [Google Scholar]
- 49.Kim H-S, Kim SK, Kim BJet al. Ferroelectric polarization in CH3NH3PbI3 perovskite. J Phys Chem Lett 2015; 6: 1729–35. 10.1021/acs.jpclett.5b00695 [DOI] [PubMed] [Google Scholar]
- 50.Kim Y-J, Dang T-V, Choi H-Jet al. Piezoelectric properties of CH3NH3PbI3 perovskite thin films and their applications in piezoelectric generators. J Mater Chem A 2016; 4: 756–63. 10.1039/C5TA09662F [DOI] [Google Scholar]
- 51.Kutes Y, Ye L, Zhou Yet al. Direct observation of ferroelectric domains in solution-processed CH3NH3PbI3 perovskite thin films. J Phys Chem Lett 2014; 5: 3335–9. 10.1021/jz501697b [DOI] [PubMed] [Google Scholar]
- 52.Leonhard T, Schulz AD, Röhm Het al. Probing the microstructure of methylammonium lead iodide perovskite solar cells. Energy Technol. 2019; 7: 1800989. 10.1002/ente.201800989 [DOI] [Google Scholar]
- 53.Röhm H, Leonhard T, Hoffmann Met al. Ferroelectric domains in methylammonium lead iodide perovskite thin-films. Energy Environ Sci 2017; 10: 950–5. 10.1039/C7EE00420F [DOI] [Google Scholar]
- 54.Röhm H, Leonhard T, Hoffmann MJet al. Ferroelectric poling of methylammonium lead iodide thin films. Adv Funct Mater 2020; 30: 1908657. 10.1002/adfm.201908657 [DOI] [Google Scholar]
- 55.Rohm H, Leonhard T, Schulz ADet al. Ferroelectric properties of perovskite thin films and their implications for solar energy conversion. Adv Mater 2019; 31: 1806661. 10.1002/adma.201806661 [DOI] [PubMed] [Google Scholar]
- 56.Seol D, Jeong A, Han MHet al. Origin of hysteresis in CH3NH3PbI3 perovskite thin films. Adv Funct Mater 2017; 27: 1701924. 10.1002/adfm.201701924 [DOI] [Google Scholar]
- 57.Vorpahl SM, Giridharagopal R, Eperon GEet al. Orientation of ferroelectric domains and disappearance upon heating methylammonium lead triiodide perovskite from tetragonal to cubic phase. ACS Appl Energy Mater 2018; 1: 1534–9. 10.1021/acsaem.7b00330 [DOI] [Google Scholar]
- 58.Wang P, Zhao J, Wei Let al. Photo-induced ferroelectric switching in perovskite CH3NH3PbI3 films. Nanoscale 2017; 9: 3806–17. 10.1039/C6NR09310H [DOI] [PubMed] [Google Scholar]
- 59.Anusca I, Balčiūnas S, Gemeiner Pet al. Dielectric response: answer to many questions in the methylammonium lead halide solar cell absorbers. Adv Energy Mater 2017; 7: 1700600. 10.1002/aenm.201700600 [DOI] [Google Scholar]
- 60.Beilsten-Edmands J, Eperon G, Johnson Ret al. Non-ferroelectric nature of the conductance hysteresis in CH3NH3PbI3 perovskite-based photovoltaic devices. Appl Phys Lett 2015; 106: 173502. 10.1063/1.4919109 [DOI] [Google Scholar]
- 61.Coll M, Gomez AS, Mas-Marza Eet al. Polarization switching and light-enhanced piezoelectricity in lead halide perovskites. J Phys Chem Lett 2015; 6: 1408–13. 10.1021/acs.jpclett.5b00502 [DOI] [PubMed] [Google Scholar]
- 62.Govinda S, Mahale P, Kore BPet al. Is CH3NH3PbI3 polar? J Phys Chem Lett 2016; 7: 2412–9. 10.1021/acs.jpclett.6b00803 [DOI] [PubMed] [Google Scholar]
- 63.Gómez A, Wang Q, Goñi ARet al. Ferroelectricity-free lead halide perovskites. Energy Environ Sci 2019; 12: 2537–47. 10.1039/C9EE00884E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoque MNF, Yang M, Li Zet al. Polarization and dielectric study of methylammonium lead iodide thin film to reveal its nonferroelectric nature under solar cell operating conditions. ACS Energy Lett 2016; 1: 142–9. 10.1021/acsenergylett.6b00093 [DOI] [Google Scholar]
- 65.Liu Y, Collins L, Belianinov Aet al. Dynamic behavior of CH3NH3PbI3 perovskite twin domains. Appl Phys Lett 2018; 113: 072102. 10.1063/1.5041256 [DOI] [Google Scholar]
- 66.Šimėnas M, Balčiūnas S, Mączka Met al. Exploring the antipolar nature of methylammonium lead halides: a Monte Carlo and pyrocurrent study. J Phys Chem Lett 2017; 8: 4906–11. 10.1021/acs.jpclett.7b02239 [DOI] [PubMed] [Google Scholar]
- 67.Yamada Y, Yamada T, Phuong LQet al. Dynamic optical properties of CH3NH3PbI3 single crystals as revealed by one- and two-photon excited photoluminescence measurements. J Am Chem Soc 2015; 137: 10456–9. 10.1021/jacs.5b04503 [DOI] [PubMed] [Google Scholar]
- 68.Liu Y, Collins L, Proksch Ret al. Chemical nature of ferroelastic twin domains in CH3NH3PbI3 perovskite. Nat Mater 2018; 17: 1013–9. 10.1038/s41563-018-0152-z [DOI] [PubMed] [Google Scholar]
- 69.Chen S, Zhang X, Zhao Jet al. Atomic scale insights into structure instability and decomposition pathway of methylammonium lead iodide perovskite. Nat Commun 2018; 9: 4807. 10.1038/s41467-018-07177-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rothmann MU, Li W, Zhu Yet al. Direct observation of intrinsic twin domains in tetragonal CH3NH3PbI3. Nat Commun 2017; 8: 14547. 10.1038/ncomms14547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rothmann MU, Li W, Zhu Yet al. Structural and chemical changes to CH3NH3PbI3 induced by electron and gallium ion beams. Adv Mater 2018; 30: 1800629. 10.1002/adma.201800629 [DOI] [PubMed] [Google Scholar]
- 72.Saliba M, Stolterfoht M, Wolff CMet al. Measuring aging stability of perovskite solar cells. Joule 2018; 2: 1019–24. 10.1016/j.joule.2018.05.005 [DOI] [Google Scholar]
- 73.Zhang D, Zhu Y, Liu Let al. Atomic-resolution transmission electron microscopy of electron beam-sensitive crystalline materials. Science 2018; 359: 675–9. 10.1126/science.aao0865 [DOI] [PubMed] [Google Scholar]
- 74.Birkhold ST, Hu H, Höger PTet al. Mechanism and impact of cation polarization in methylammonium lead iodide. J Phys Chem C 2018; 122: 12140–7. 10.1021/acs.jpcc.8b00631 [DOI] [Google Scholar]
- 75.Cordero F, Craciun F, Trequattrini Fet al. Competition between polar and antiferrodistortive modes and correlated dynamics of the methylammonium molecules in MAPbI3 from anelastic and dielectric measurements. J Phys Chem Lett 2018; 9: 4401–6. 10.1021/acs.jpclett.8b01761 [DOI] [PubMed] [Google Scholar]
- 76.Rakita Y, Bar-Elli O, Meirzadeh Eet al. Tetragonal CH3NH3PbI3 is ferroelectric. Proc Natl Acad Sci USA 2017; 114: E5504–12. 10.1073/pnas.1702429114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Govinda S, Kore BP, Mahale Pet al. Can SHG measurements determine the polarity of hybrid lead halide perovskites? ACS Energy Lett 2018; 3: 1887–91. 10.1021/acsenergylett.8b00999 [DOI] [Google Scholar]
- 78.Bonnell DA, Kalinin SV, Kholkin Aet al. Piezoresponse force microscopy: a window into electromechanical behavior at the nanoscale. MRS Bull 2009; 34: 648–57. 10.1557/mrs2009.176 [DOI] [Google Scholar]
- 79.Li J, Li J-F, Yu Qet al. Strain-based scanning probe microscopies for functional materials, biological structures, and electrochemical systems. J Materiomics 2015; 1: 3–21. 10.1016/j.jmat.2015.03.001 [DOI] [Google Scholar]
- 80.Hermes IM, Bretschneider SA, Bergmann VWet al. Ferroelastic fingerprints in methylammonium lead iodide perovskite. J Phys Chem C 2016; 120: 5724–31. 10.1021/acs.jpcc.5b11469 [DOI] [Google Scholar]
- 81.Strelcov E, Dong Q, Li Tet al. CH3NH3PbI3 perovskites: ferroelasticity revealed. Sci Adv 2017; 3: e1602165. 10.1126/sciadv.1602165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen QN, Ou Y, Ma Fet al. Mechanisms of electromechanical coupling in strain based scanning probe microscopy. Appl Phys Lett 2014; 104: 242907. 10.1063/1.4884422 [DOI] [Google Scholar]
- 83.Liu Y, Ievlev AV, Collins Let al. Strain-chemical gradient and polarization in metal halide perovskites. Adv Electron Mater 2020; 6: 1901235. 10.1002/aelm.201901235 [DOI] [Google Scholar]
- 84.Zhao J, Kong G, Chen Set al. Single crystalline CH3NH3PbI3 self-grown on FTO/TiO2 substrate for high efficiency perovskite solar cells. Sci Bull 2017; 62: 1173–6. 10.1016/j.scib.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 85.Yu J, Esfahani EN, Zhu Qet al. Quadratic electromechanical strain in silicon investigated by scanning probe microscopy. J Appl Phys 2018; 123: 155104. 10.1063/1.5023407 [DOI] [Google Scholar]
- 86.Bari M, Bokov AA, Ye ZG. Ferroelasticity, domain structures and phase symmetries in organic-inorganic hybrid perovskite methylammonium lead chloride. J Mater Chem C 2020; 8: 9625–31. 10.1039/D0TC02124E [DOI] [Google Scholar]
- 87.Gonzalez-Carrero S, Frances-Soriano L, Gonzalez-Bejar Met al. The luminescence of CH3NH3PbBr3 perovskite nanoparticles crests the summit and their photostability under wet conditions is enhanced. Small 2016; 12: 5245–50. 10.1002/smll.201600209 [DOI] [PubMed] [Google Scholar]
- 88.Mashiyama H, Kawamura Y, Kubota Y. The anti-polar structure of CH3NH3PbBr3. J Korean Phys Soc 2007; 51: 850–3. 10.3938/jkps.51.850 [DOI] [Google Scholar]
- 89.Bari M, Bokov AA, Ye Z-G. Ferroelastic domains and phase transitions in organic–inorganic hybrid perovskite CH3NH3PbBr3. J Mater Chem C 2021; 9: 3096–107. 10.1039/D0TC05618A [DOI] [Google Scholar]
- 90.Gao Z-R, Sun X-F, Wu Y-Yet al. Ferroelectricity of the orthorhombic and tetragonal MAPbBr3 single crystal. J Phys Chem Lett 2019; 10: 2522–7. 10.1021/acs.jpclett.9b00776 [DOI] [PubMed] [Google Scholar]
- 91.Liu Y, Ievlev AV, Collins Let al. Light-ferroic interaction in hybrid organic-inorganic perovskites. Adv Optical Mater 2019; 7: 1901451. 10.1002/adom.201901451 [DOI] [Google Scholar]
- 92.Frost JM, Butler KT, Walsh A. Molecular ferroelectric contributions to anomalous hysteresis in hybrid perovskite solar cells. APL Mater 2014; 2: 081506. 10.1063/1.4890246 [DOI] [Google Scholar]
- 93.Ahmadi M, Collins L, Puretzky Aet al. Exploring anomalous polarization dynamics in organometallic halide perovskites. Adv Mater 2018; 30: 1705298. 10.1002/adma.201705298 [DOI] [PubMed] [Google Scholar]
- 94.Ding R, Liu H, Zhang XLet al. Flexible piezoelectric nanocomposite generators based on formamidinium lead halide perovskite nanoparticles. Adv Funct Mater 2016; 26: 7708–16. 10.1002/adfm.201602634 [DOI] [Google Scholar]
- 95.Taylor VCA, Tiwari D, Duchi Met al. Investigating the role of the organic cation in formamidinium lead iodide perovskite using ultrafast spectroscopy. J Phys Chem Lett 2018; 9: 895–901. 10.1021/acs.jpclett.7b03296 [DOI] [PubMed] [Google Scholar]
- 96.Voloshinovskii A, Myagkota S, Levitskii R. Luminescence of ferroelastic CsPbCl3 nanocrystals. Ferroelectrics 2005; 317: 311–5. 10.1080/00150190590963570 [DOI] [Google Scholar]
- 97.Lim AR, Jeong S-Y. Ferroelastic phase transition and twin structure by 133Cs NMR in a CsPbCl3 single crystal. Physica B Condens Matter 2001; 304: 79–85. 10.1016/S0921-4526(01)00485-9 [DOI] [Google Scholar]
- 98.Hirotsu S. Experimental studies of structural phase transitions in CsPbCl3. J Phys Soc Jpn 1971; 31: 552–60. 10.1143/JPSJ.31.552 [DOI] [Google Scholar]
- 99.Marçal LA, Oksenberg E, Dzhigaev Det al. In situ imaging of ferroelastic domain dynamics in CsPbBr3 perovskite nanowires by nanofocused scanning X-ray diffraction. ACS Nano 2020; 14: 15973–82. 10.1021/acsnano.0c07426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao Y-Q, Ma Q-R, Liu Bet al. Pressure-induced strong ferroelectric polarization in tetra-phase perovskite CsPbBr3. Phys Chem Chem Phys 2018; 20: 14718–24. 10.1039/C8CP01338A [DOI] [PubMed] [Google Scholar]
- 101.Li X, Chen S, Liu P-Fet al. Evidence for ferroelectricity of all-inorganic perovskite CsPbBr3 quantum dots. J Am Chem Soc 2020; 142: 3316–20. 10.1021/jacs.9b12254 [DOI] [PubMed] [Google Scholar]
- 102.Chen Q, Zhou HP, Hong ZRet al. Planar heterojunction perovskite solar cells via vapor-assisted solution process. J Am Chem Soc 2014; 136: 622–5. 10.1021/ja411509g [DOI] [PubMed] [Google Scholar]
- 103.Luo SQ, Daoud WA. Crystal structure formation of CH3NH3PbI3-xClx perovskite. Materials 2016; 9: 123. 10.3390/ma9030123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim D, Yun JS, Sharma Pet al. Light- and bias-induced structural variations in metal halide perovskites. Nat Commun 2019; 10: 444. 10.1038/s41467-019-08364-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xiao J, Chang J, Li Bet al. Room temperature ferroelectricity of hybrid organic-inorganic perovskites with mixed iodine and bromine. J Mater Chem A 2018; 6: 9665–76. 10.1039/C7TA09967C [DOI] [Google Scholar]
- 106.Zhang Q, Solanki A, Parida Ket al. Tunable ferroelectricity in Ruddlesden-Popper halide perovskites. ACS Appl Mater Interfaces 2019; 11: 13523–32. 10.1021/acsami.8b21579 [DOI] [PubMed] [Google Scholar]
- 107.Hsu H-C, Huang B-C, Chin S-Cet al. Photodriven dipole reordering: key to carrier separation in metalorganic halide perovskites. ACS Nano 2019; 13: 4402–9. 10.1021/acsnano.8b09645 [DOI] [PubMed] [Google Scholar]
- 108.Liu Y, Li M, Wang Met al. Twin domains modulate light-matter interactions in metal halide perovskites. APL Mater 2020; 8: 011106. 10.1063/1.5127866 [DOI] [Google Scholar]
- 109.Khorramshahi F, Woughter AG, Ram MKet al. Apparent piezo-photocurrent modulation in methylammonium lead iodide perovskite photodetectors. Adv Electron Mater 2019; 5: 1900518. 10.1002/hskipz@aelm.201900518 [DOI] [Google Scholar]
- 110.Lai Q, Zhu L, Pang Yet al. Piezo-phototronic effect enhanced photodetector based on CH3NH3PbI3 single crystals. ACS Nano 2018; 12: 10501–8. 10.1021/acsnano.8b06243 [DOI] [PubMed] [Google Scholar]
- 111.Ravishankar S, Gharibzadeh S, Roldan-Carmona Cet al. Influence of charge transport layers on open-circuit voltage and hysteresis in perovskite solar cells. Joule 2018; 2: 788–98. 10.1016/j.joule.2018.02.013 [DOI] [Google Scholar]
- 112.Wei J, Zhao Y, Li Het al. Hysteresis analysis based on the ferroelectric effect in hybrid perovskite solar cells. J Phys Chem Lett 2014; 5: 3937–45. 10.1021/jz502111u [DOI] [PubMed] [Google Scholar]
- 113.Ma W, Zhang X, Xu Zet al. Reducing anomalous hysteresis in perovskite solar cells by suppressing the interfacial ferroelectric order. ACS Appl Mater Interfaces 2020; 12: 12275–84. 10.1021/acsami.9b20988 [DOI] [PubMed] [Google Scholar]
- 114.Meloni S, Moehl T, Tress Wet al. Ionic polarization-induced current-voltage hysteresis in CH3NH3PbX3 perovskite solar cells. Nat Commun 2016; 7: 10334. 10.1038/ncomms10334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shi JJ, Li YM, Li YSet al. From ultrafast to ultraslow: charge-carrier dynamics of perovskite solar cells. Joule 2018; 2: 879–901. 10.1016/j.joule.2018.04.010 [DOI] [Google Scholar]
- 116.Chen B, Li T, Dong Qet al. Large electrostrictive response in lead halide perovskites. Nat Mater 2018; 17: 1020–6. 10.1038/s41563-018-0170-x [DOI] [PubMed] [Google Scholar]
- 117.Xia G, Huang B, Zhang Yet al. Nanoscale insights into photovoltaic hysteresis in triple-cation mixed-halide perovskite: resolving the role of polarization and ionic migration. Adv Mater 2019; 31: 1902870. 10.1002/adma.201902870 [DOI] [PubMed] [Google Scholar]
- 118.Chen S, Gao P. Challenges, myths, and opportunities of electron microscopy on halide perovskites. J Appl Phys 2020; 128: 010901. 10.1063/5.0012310 [DOI] [Google Scholar]
- 119.Chen S, Zhang Y, Zhao Jet al. Transmission electron microscopy of organic-inorganic hybrid perovskites: myths and truths. Sci Bull 2020; 65: 1643–9. 10.1016/j.scib.2020.05.020 [DOI] [PubMed] [Google Scholar]
- 120.Ono LK, Qi YB, Liu SZ. Progress toward stable lead halide perovskite solar cells. Joule 2018; 2: 1961–90. 10.1016/j.joule.2018.07.007 [DOI] [Google Scholar]
- 121.Zhou YY, Sternlicht H, Padture NP. Transmission electron microscopy of halide perovskite materials and devices. Joule 2019; 3: 641–61. 10.1016/j.joule.2018.12.011 [DOI] [Google Scholar]
- 122.Li Y, Zhou W, Li Yet al. Unravelling degradation mechanisms and atomic structure of organic-inorganic halide perovskites by cryo-EM. Joule 2019; 3: 2854–66. 10.1016/j.joule.2019.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dang Z, Shamsi J, Palazon Fet al. In situ transmission electron microscopy study of electron beam-induced transformations in colloidal cesium lead halide perovskite nanocrystals. ACS Nano 2017; 11: 2124–32. 10.1021/acsnano.6b08324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jeong S-H, Park J, Han T-Het al. Characterizing the efficiency of perovskite solar cells and light-emitting diodes. Joule 2020; 4: 1206–35. 10.1016/j.joule.2020.04.007 [DOI] [Google Scholar]
- 125.Pitarch-Tena D, Ngo TT, Vallés-Pelarda Met al. Impedance spectroscopy measurements in perovskite solar cells: device stability and noise reduction. ACS Energy Lett 2018; 3: 1044–8. 10.1021/acsenergylett.8b00465 [DOI] [Google Scholar]
- 126.Yu JX, Huang BY, Li ALet al. Resolving local dynamics of dual ions at the nanoscale in electrochemically active materials. Nano Energy 2019; 66: 104160. 10.1016/j.nanoen.2019.104160 [DOI] [Google Scholar]
- 127.Correa-Baena JP, Hippalgaonkar K, van Duren Jet al. Accelerating materials development via automation, machine learning, and high-performance computing. Joule 2018; 2: 1410–20. 10.1016/j.joule.2018.05.009 [DOI] [Google Scholar]