Abstract
Aim
Based on the bibliometric method, the toxicity of aconite is analyzed and evaluated.
Methods
Studies on the toxicity of aconite were retrieved from CNKI, CQVIP, Chinese Biomedical Literature Service System, and PubMed, ranging from January 1985 to November 2020. All those studies were formed into the Database of Literature of Toxicity of Aconite (DLTA). Studies on the toxicity of aconite were retrieved from CNKI, CQVIP, SinoMed, and PubMed, respectively. Collecting relevant information in DLTA, we analyzed the hotspots, factors and mechanism of aconite toxicity, and attenuation methods.
Results
A total of 445 studies on the toxicity of aconite have been collected. “Compatibility attenuation” and “Processing attenuation” have been the hotspots of aconite toxicity in recent years. Many studies support that the main toxic reactions of aconite are heart damage, liver toxicity, nephrotoxicity, and neurotoxicity. The toxic effect of aconite is related to the effect on the central nervous system. Exciting the vagus nerve reduces the autonomy of the sinus node and damages myocardial cells. The decoction time, dosage, and administration of aconite are the main factors of the toxicity of aconite. There are few studies about the effect of the origin of aconite and the specifications of the medicinal materials on toxicity. Therefore, it is impossible to analyze its relevance. At present, the commonly used methods to reduce the toxicity of aconite mainly include three methods: drug compatibility, processing, and decoction. The most common compatibility with aconite medicines includes licorice, dried ginger, ginseng, and ephedra. Black sliced aconite, steamed slices, and fried slices are less toxic than other processed products. Aconite decoction for more than 60 minutes can basically reach the safe range, and more than 2 hours of decoction may cause the loss of active ingredients.
Conclusions
The research on the mechanisms of aconite dosage-efficacy-toxicity, compatibility, processing, liver toxicity, and nephrotoxicity is still not comprehensive and in-depth. Researchers should perfect toxicity studies of aconite, remove the constraints that affect its clinical application, and promote the clinical use of aconite safely and reasonably.
1. Introduction
Aconite is a processed product of the lateral root of Aconitum carmichaelii Debx. and a plant of the Ranunculaceae family. It is an authentic medicinal material produced in Sichuan. It was first published in “Shennong's Classic of Materia Medica,” which records its spicy, sweetness, hot property, and toxicity [1]. Aconite has a long history of application, and its clinical effect is remarkable. There are 23 proven recipes based on aconite in “Treatise on Febrile and Miscellaneous Diseases” by Zhang Zhongjing, a famous doctor in the Eastern Han Dynasty. Aconite is known as the most important medicine for reviving yang for resuscitation and is respected as one of the “four dimensions of medicine” [2]. The diester alkaloid contained in aconite is its main active ingredient, which can be used for the treatment of heart failure, rheumatic heart disease, coronary heart disease, hypotension, shock, etc. However, aconite is a type of famous toxic Chinese medicine, and the diester alkaloid is a toxic ingredient. This results in higher toxicity to the cardiovascular system, nervous system, digestive system, liver, and kidney [3]. Therefore, it is urgent to use the “double-edged sword” safely and rationally and remove the constraints that affect its clinical application. Based on the bibliometric method, this paper combed the studies on the toxicity of aconite in recent decades and evaluated and analyzed the research hotspots of aconite. In addition, we summarized the toxicity of aconite and related factors.
2. Methods
2.1. Literature Collection
Relevant studies on the toxicity of aconite were retrieved from CNKI (https://www.cnki.net/), CQVIP (http://www.cqvip.com/), Chinese Biomedical Literature Service System (http://www.sinomed.ac.cn/), and PubMed (https://www.ncbi.nlm.nih.gov/). The retrieval time was from January 1985 to November 2020. The retrieval formula used by each platform is as follows.
The following words are used as keywords or subject terms for literature retrieval in CNKI: “附子 or 附片 or 盐附子 or 白附片 or 黑附子 or 黑顺片 or 黑片 or 顺片 or 熟附子 or 熟片 or 制附子 or 制附片 or 川附子 or 淡附子 or 淡附片 or 炮附片 or 泥附子” with the publication years from 1985 to 2020.
The following words are used as keywords or subject terms for literature retrieval in CQVIP : M = (附子 OR 附片 OR 盐附子 OR 白附片 OR 黑附子 OR 黑顺片 OR 黑片 OR 顺片 OR 熟附子 OR 熟片 OR 制附子 OR 制附片 OR 川附子 OR 淡附子 OR 淡附片 OR 炮附片 OR 泥附子) AND (副作用 OR 毒 OR 不良反应 OR 毒效 OR 毒理 OR 毒副作用 OR 不良事件 OR 毒 OR 安全性 OR 中毒).
The following words are used as keywords or subject terms for literature retrieval in Chinese Biomedical Literature Service System: (“附子” [All fields: intelligent] OR “附片” [All fields: intelligent] OR “盐附子” [All fields: intelligent] OR “白附片” [All fields: intelligent] OR “黑附子” [All fields: intelligent] OR “黑顺片” [All fields: intelligent] OR “黑片” [All fields: intelligent] OR “顺片” [All fields: intelligent] OR “熟附子” [All fields: intelligent] OR “熟片” [All fields: intelligent] OR “制附子” [All fields: intelligent] OR “制附片” [All fields: intelligent] OR “川附子” [All fields: intelligent] OR “淡附子” [All fields: intelligent] OR “淡附片” [All fields: intelligent] OR “炮附片” [All fields: intelligent] OR “泥附子” [All fields: intelligent] OR “炮天雄” [All fields: intelligent] OR “制天雄” [All fields: intelligent]) AND (“副作用” [All fields: intelligent] OR “毒” [All fields: intelligent] OR “不良反应” [All fields: intelligent] OR “毒效” [All fields: intelligent] OR “毒理” [All fields: intelligent] OR “毒副作用” [All fields: intelligent] OR “不良事件” [All fields: intelligent] OR “毒” [All fields: intelligent] OR “安全性” [All fields: intelligent] OR “中毒” [All fields: intelligent]).
The following words are used as keywords or subject terms for literature retrieval in PubMed: (Aconitum [MeSH Terms]) OR (Aconite [Title/Abstract]) OR (Aconites [Title/Abstract]) OR (Radix Aconiti [Title/Abstract])) OR (Aconiti, Radix [Title/Abstract]) OR (Aconitus, Radix [Title/Abstract]) OR (Radix Aconitus [Title/Abstract]) OR (Aconitum napellus [Title/Abstract]) OR (Monkshood [Title/Abstract]) OR (Aconitums [Title/Abstract]) AND (poisoning [MeSH Terms]) OR (Side effects [MeSH Terms]) OR (adverse effect [Title/Abstract]) OR (side reaction [Title/Abstract]) OR (toxic effect[Title/Abstract]) OR (toxicity [Title/Abstract]).
2.2. Establishment of Database of the Literature of Toxicity of Aconite (DLTA)
After deleting the duplicate studies, 445 studies related to the toxicity of aconite were obtained to form the Database of Literature of Toxicity of Aconite (DLTA).
We evaluated the research hotspot of toxicity literature, toxicity classification, mechanism analysis, influencing factors of toxicity, and detoxification methods of aconite by the bibliometric method. The collected information included the annual publication of aconite toxicity literature, coword analysis, toxicity classification, toxicity mechanism, aconite varieties, origin, aconite usage, dosage, processing situation, compatible herbs, decoction method, and decoction time. Studies in the DLTA were screened and classified one by one and were structured according to the information collection table. Finally, the extracted data information was classified and analyzed, as shown in Figure 1. Related results can be analyzed and graphed with VOSviewer.
Figure 1.

Flow chart of data collection and analysis of studies of aconite toxicity.
3. Results
3.1. Research Hotspots of Toxicity Literature of Aconite
3.1.1. Annual Quantity Curve of Literature
There were a total of 445 studies related to the toxicity of aconite in the DLTA. The annual number of publications on the toxicity of aconite from 1985 to 2020 was calculated, as shown in Figure 2. 2007 to 2015 is the peak period of the annual number of publications on the toxicity of aconite, and the annual number of publications has shown a downward trend since 2015.
Figure 2.
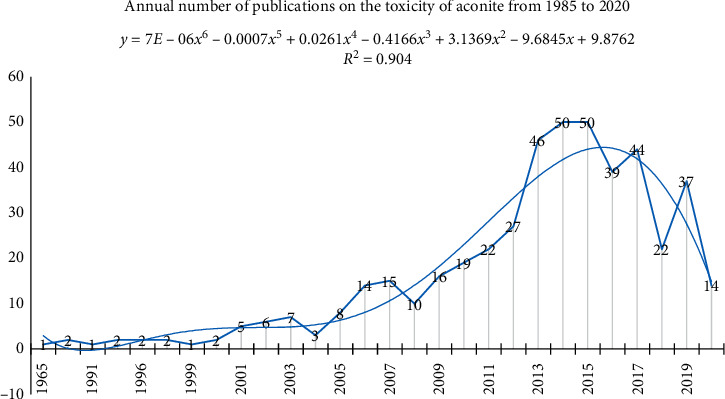
Annual number of publications on the toxicity of aconite from 1985 to 2020.
3.1.2. Coword Analysis
We performed clustering analysis through VOSviewer1.6.15 for coword analysis. Cluster analysis is a multivariate index statistical method that classifies multiple data according to the degree of distance relationship between objects [4]. All studies in DLTA were imported into VOSviewer for coword clustering analysis. They were divided into eight groups with distinguished colors. The size of the node indicates the word frequency, and the number of connections among lines indicates its tightness. As shown in Figure 3, “aconite,” “aconitine,” “cardiomyocyte,” “compatibility,” “licorice,” “poisoning,” “processing,” and “arrhythmia” are the words with the highest frequency. Combined with the coword analysis, the arrhythmia caused by aconite damage to myocardial cells is a common toxic reaction of aconite. Thus, the use of licorice or processing to reduce toxicity is a research hotspot in aconite (Table 1).
Figure 3.
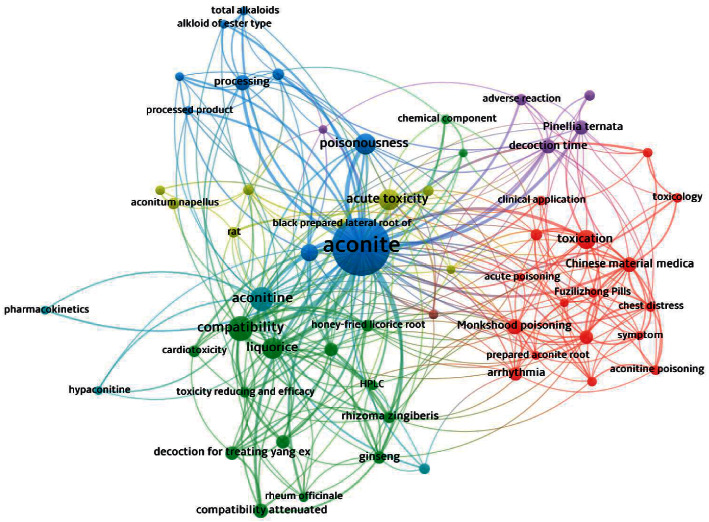
Network visualization in DLTA.
Table 1.
Top 59 representative keywords in terms of occurrences and total link strength.
| No. | Label | Occurrences | Total link strength |
|---|---|---|---|
| 1 | Aconite | 206 | 326 |
| 2 | Compatibility | 39 | 95 |
| 3 | Aconitine | 36 | 65 |
| 4 | Acute toxicity | 29 | 40 |
| 5 | Poisonousness | 29 | 46 |
| 6 | Liquorice | 29 | 81 |
| 7 | Toxication | 23 | 35 |
| 8 | Alkaloid | 19 | 41 |
| 9 | Processing | 15 | 28 |
| 10 | Monkshood poisoning | 15 | 16 |
| 11 | Chinese material medica | 13 | 44 |
| 12 | Pinellia ternata | 13 | 26 |
| 13 | Decoction time | 13 | 26 |
| 14 | Compatibility attenuated | 13 | 22 |
| 15 | Metabonomics | 12 | 26 |
| 16 | Decoction for treating yang exhaustion | 12 | 19 |
| 17 | Reduction of toxicity | 11 | 23 |
| 18 | Arrhythmia | 11 | 15 |
| 19 | Patient | 11 | 33 |
| 20 | Ginseng | 10 | 27 |
| 21 | Rhizoma zingiberis | 9 | 31 |
| 22 | Adverse reaction | 8 | 13 |
| 23 | Aconitum napellus | 8 | 6 |
| 24 | Ester alkaloids | 8 | 18 |
| 25 | Cardiotoxicity | 8 | 11 |
| 26 | Honey-fried licorice root | 8 | 24 |
| 27 | Lateral root slices | 8 | 15 |
| 28 | Toxicity reducing and efficacy enhancing | 7 | 15 |
| 29 | Rat | 7 | 10 |
| 30 | Security | 7 | 12 |
| 31 | Myocardial cell | 7 | 8 |
| 32 | Crude lateral root of aconite | 7 | 13 |
| 33 | Rhizoma typhonii | 7 | 6 |
| 34 | HPLC | 6 | 12 |
| 35 | Clinical application | 6 | 8 |
| 36 | Aconitine poisoning | 6 | 12 |
| 37 | Chemical component | 6 | 10 |
| 38 | Eighteen incompatibilities | 6 | 13 |
| 39 | Rheum officinale | 6 | 14 |
| 40 | Mouse | 6 | 16 |
| 41 | Electrocardiograph | 6 | 15 |
| 42 | Toxicology | 6 | 11 |
| 43 | Neurovirulence | 6 | 5 |
| 44 | Chest distress | 6 | 27 |
| 45 | Fuzi Lizhong pills | 6 | 8 |
| 46 | Black prepared lateral root of aconite | 6 | 9 |
| 47 | Compatibility of Chinese medicine | 5 | 8 |
| 48 | Prepared aconite root | 5 | 14 |
| 49 | Heart rate | 5 | 17 |
| 50 | Acute poisoning | 5 | 10 |
| 51 | Total alkaloids | 5 | 12 |
| 52 | Hypaconitine | 5 | 6 |
| 53 | Poisonous components | 5 | 9 |
| 54 | Processed product | 5 | 12 |
| 55 | Symptom | 5 | 17 |
| 56 | Sliced white aconite | 5 | 11 |
| 57 | Pharmacokinetics | 5 | 5 |
| 58 | Alkloid of ester type | 5 | 13 |
| 59 | High-efficiency liquid chromatography | 5 | 14 |
3.2. Toxicity Classification and Mechanism Analysis
3.2.1. Classification of Toxicity of Aconite
In Table 2, the toxic effects of aconite in DLTA include cardiac damage (including arrhythmia, atrial damage, ventricular toxicity, atrioventricular block, and Al-Syndrome), liver toxicity, renal toxicity, and neurotoxicity [9, 13, 14]. 180 studies mentioned the cardiac damage of aconite. Thus, the main toxic effect of aconite is cardiotoxicity, and arrhythmia is more common in clinical practice.
Table 2.
Classification of toxicity of aconite.
| Toxicity classification | Reference |
|---|---|
| Cardiac damage | Sheng et al. [5] |
| Liver toxicity | Hao et al. [6] |
| Renal toxicity | Hao et al. [6] |
| Neurotoxicity | Pan and Peng [7] |
| Gastrointestinal toxicity | Han and Hou [8] |
| Respiratory system damage | Jiang. [9] |
| Endocrine dysfunction | Hao et al. [6] |
| Neurotoxicity of brain | Han et al. [10] |
| Hematopoietic system damage | Liu. [11] |
| Embryotoxicity/reproductive organ damage | Xiao et al. [12] |
3.2.2. Analysis of the Toxicity Mechanism of Aconite
A total of 223 studies on the action mechanism of aconite were collected. The toxic action mechanism of aconite was extracted and classified. Then, the statistical analysis was carried out. The toxic action mechanism of aconite that was reported only in one study was eliminated. The classification of its toxic mechanism is shown in Table 3. The toxic reaction mechanisms of aconite reported in many studies are as follows. (1) Aconite has the effect of initial excitement and then inhibition on the central nervous system and peripheral nerves. It mainly acts on the medulla oblongata, causing shock or respiratory depression due to bulbar paralysis, respiratory failure, hypoxic encephalopathy, and death in severe cases [7]. (2) Aconite can excite the vagus nerve, inhibit voltage-dependent sodium channels, increase the sodium ion permeability of nerve cells and myocardial cells, and cause arrhythmia [29]. (3) Aconitum in aconite can inhibit most of the mitochondrial enzymes in the myocardium and cause damage to myocardial cells [30]. (4) Aconite can adjust the activity of L-calcium channels to relatively prolong the repolarization, increase the calcium ion concentration, cause calcium ion overload in myocardial cells, and damage cardiomyocytes [31]. (5) Aconite can excite cholinergic nerves and inhibit cholinesterase activity, thereby inhibiting the heart [31].
Table 3.
The classification of the toxicity action mechanism of aconite and the number of studies.
| The toxicity action mechanism of aconite | Occurrence | Reference |
|---|---|---|
| The effect on various nerve fiber endings and the central nervous system is initial excitement and then inhibition (the effect of the central nervous system may be related to the promotion of the release of β-endorphin by aconite) | 42 | Han et al. [10] |
| Exciting the vagus nerve reduces the autonomy of the sinus node | 40 | Li [15] |
| Inhibiting voltage-dependent sodium channels, increasing the sodium ion permeability of nerve cells and myocardial cells, and causing arrhythmia | 27 | Sheng et al. [5] |
| Inhibiting the acid cycle of myocardial tricarboxylic and the acidification of oxidative phosphorylation of the respiratory chain, causing myocardial cell damage and necrosis to release myocardial enzymes | 19 | Strzelecki et al. [16] |
| Adjusting L-calcium channel activity to relatively prolong repolarization, increasing calcium ion concentration, and causing calcium ion overload in cardiac myocytes | 12 | Zhou et al. [17] |
| Exciting cholinergic nerves, inhibiting cholinesterase activity | 11 | Wang et al. [18] |
| Inhibiting the vasomotor center | 10 | Zhang [19] |
| Inhibiting the Na-K-ATPase activity of the myocardial cell membrane, leading to a large amount of depletion of myocardial high-energy phosphate bonds, and causing damage to myocardial cells | 9 | Sun et al. [20] |
| Causing damage to peripheral nerve | 7 | Wang [21] |
| Increasing RyR2 protein expression level | 4 | Peng [22] |
| β2 adrenergic receptor agonism | 4 | Lu et al. [23] |
| Increasing the release of active substances such as prostaglandins and catecholamines | 4 | Gao and Huang [24] |
| Promoting the expression of NCX and SERCA2a genes to increase cellular Ca2+ concentration | 2 | Fu [25] |
| Causing damage to the DNA of lung fibroblasts | 2 | Xie [26] |
| Downregulating gene expression level of Bcl-2 and upregulating gene expression levels of bax | 2 | Wang et al. [27] |
| Significantly reducing the mRNA and protein expression levels of PGC-1α in cardiomyocytes | 2 | Zhao et al. [28] |
From the statistical analysis of studies (Table S1), there are many studies on the action mechanism of aconite toxicity on the heart, with in-depth research and a broader perspective. There are few studies on the action mechanism of aconite toxicity on the liver and kidney. However, these toxic reactions are common in clinical research. If there is no exact description of these toxicity mechanisms, it is impossible to avoid toxic side effects and conduct reasonable treatment.
3.3. Factors Analysis of Aconite Toxicity
We analyzed the correlation between aconite toxicity and several factors, such as the origin, the specification, the decoction time, and the dosage of aconite. The bibliometric method was used to analyze the relationship between the origin of aconite, the specifications of medicinal materials, the decoction time, the dosage, and the toxicity of aconite.
3.3.1. The Origin of Aconite
The sources of aconite medicinal materials in all studies of DLTA were analyzed, and the classification statistics were performed (Figure 4). Most of the studies reported that the source of aconite is decoction pieces companies or hospitals. It is difficult to trace its source. Therefore, it is impossible to evaluate the relationship between the aconite source and toxicity objectively. In DLTA, 55 studies reported that the aconite is from Jiangyou City, Sichuan. Jiangyou aconite is a well-known authentic medicinal material produced in Sichuan, where it has sophisticated and complicated root-repairing and tipping cultivation techniques [32]. It is a national product of geographic indication. The medicinal ingredients of Jiangyou aconite are more recognized by academia. Therefore, the use of Jiangyou aconite not only ensures clinical safety but also improves the reliability of experimental results.
Figure 4.
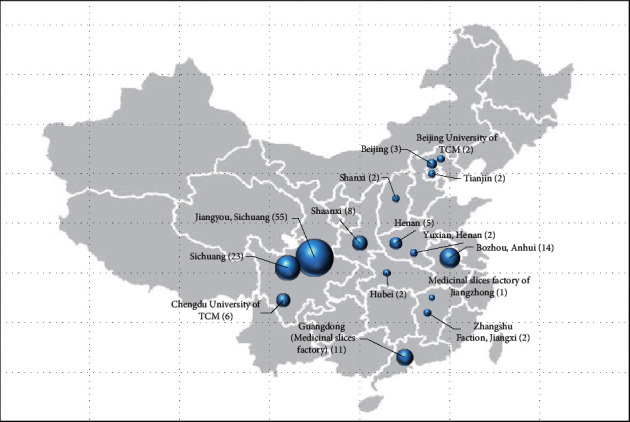
The source of aconite medicinal materials in studies of DLTA (Note: the number in parentheses is the number of studies involved).
3.3.2. Aconite Medicinal Material Specifications
In DLTA, the extracted specifications of aconite medicinal materials include aconite, black aconite/sliced aconite, cooked aconite, prepared aconite, and prescriptions and medicines containing aconite (Table S2). The classification statistics are shown in Table 4 (Figure 5). Huang and Wang [32] summarized the specifications of aconite medicinal materials, including two kinds of aconite and 18 kinds of sliced aconite based on the systematic literature survey, real estate survey, and expert consultation. However, most of the specifications of medicinal materials, such as Linjiang slice, Yangfu slice, Yinfu slice, Liuye slice, and Gugu slice, have gradually disappeared and lost due to various reasons. Therefore, we have not made a statistical analysis on the specifications of medicinal materials with smaller yields.
Table 4.
The occurrence of aconite medicinal material specifications.
| Specification | Occurrences | Reference |
|---|---|---|
| Aconite | 103 | Li et al. [33] |
| Prepared aconite | 45 | Zhang et al. [34] |
| Drug combination containing aconite | 24 | Xu et al. [35] |
| Black sliced aconite | 21 | Peng et al. [36] |
| Cooked aconite | 21 | Xie et al. [37] |
| Fuzi Lizhong pills | 12 | Wang and Liu [38] |
| Salted aconite | 11 | Guo et al. [39] |
| Pills containing aconite | 9 | Jin et al. [40] |
| The wine soaked with aconite | 5 | Wu [41] |
Figure 5.
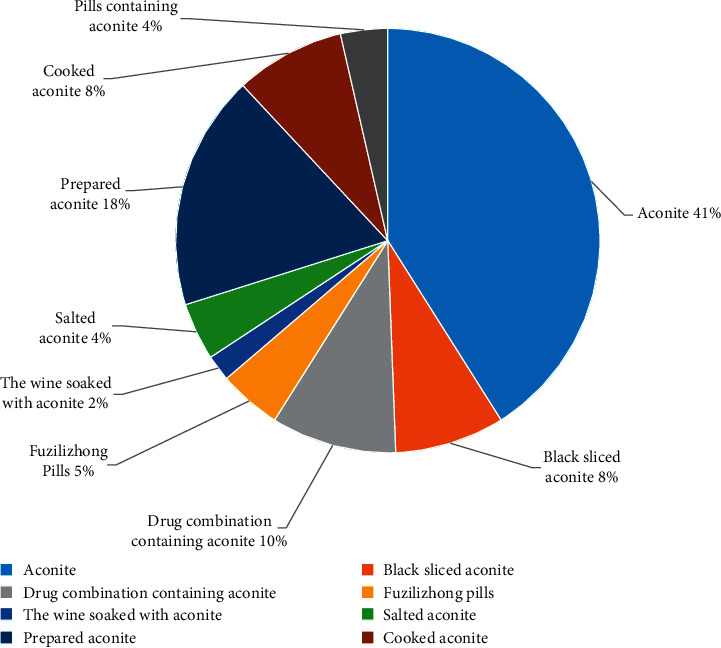
The number of studies of various specifications of aconite in DLTA.
Literature studies have shown that aconite medicinal materials have many specifications. Most of those specifications are toxic. However, in general, raw aconite has a higher toxicity than other specifications of aconite. Therefore, studies that use “raw aconite or aconite” as the research object are mostly aimed at analyzing its toxicity mechanism or reducing toxicity by compatibility, etc., and there are few clinical studies. The literature with “processed aconite” as the research object involves more clinical research.
3.3.3. Decoction Time of Aconite
This study analyzed the decoction time of aconite in various studies (Table S3), as shown in Table 5 (Figure 6). The preparation method with decoction time of half an hour or one hour has been widely used. Too short decoction time [47] of aconite or no decoction [48] in the prescription is the common cause of toxicity.
Table 5.
The occurrence of decoction time.
Figure 6.
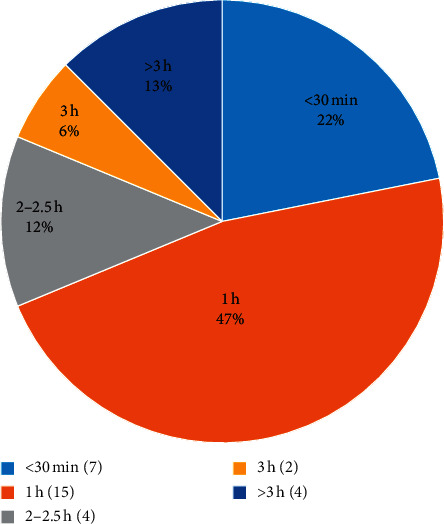
The number of studies of decoction time in the DLTA.
Scholars generally believe that a decoction time of less than 30 minutes is likely to cause clinical toxic events. The decoction time should be extended as the dose of aconite increases. However, most clinicians rely on their own experience about the corresponding relationship between dosage and decoction time, and there is no standard for it. This is one of the reasons for the prone to poisoning reaction when using large doses of aconite [49]. Usually, when the dosage of aconite is less than 30 g, it can be decocted for half an hour. When the dosage is 40∼70 g, it should be decocted for 1 h. If the dosage is more than 70 g, it should be decocted for more than 2 h [50].
3.3.4. Clinical Dosage of Aconite
The current textbooks and pharmacopeias stipulate that the common dosage of aconite is 3–15 g/d [51], and aconite is not suitable for long-term use. However, 16 reports mentioned that patients still have poisoning reactions even after reasonably taking aconite and its medicine preparations at normal doses [52], especially the toxicity caused by Aconite Lizhong Pills [38, 53]. The textbooks and pharmacopeias stipulate that the usual amount of aconite is 3–15 g/d [54], which is not suitable for long-term use. However, 16 articles mentioned that patients still had toxic reactions when taking aconite and its medicine preparations at normal doses reasonably [55], especially the toxicity caused by Aconite Lizhong Pills [56, 57]. There are also many cases of overdose or long-term use in clinical practice. Daily doses exceeding 50 g are very common (Figure 7, Table 6). Ultralarge doses of aconite are mostly used to treat intractable diseases such as rheumatoid arthritis and heart block. Although overdose use will increase the probability of aconite poisoning, doctors will mostly reduce toxicity by extending the decoction time and compatibility of medicinal materials. Therefore, the clinical use of aconite does not have strict limits on the dose, and the dose distribution range is relatively wide. There are individual differences in the dose of aconite in clinical application. Even if it is taken according to the instructions or pharmacopeia methods, toxic reactions may still occur. The clinical dose of aconite is positively correlated with toxicity. The use of overdose can also be recognized by doctors in the context of measuring the risk-benefit ratio for some difficult and miscellaneous diseases. The results of the classification are shown in Table S4.
Figure 7.

The number of studies on different dosages of aconite in DLTA.
Table 6.
The occurrence of dosage.
3.3.5. Other Factors
In addition, the factors affecting the toxicity of aconite are shown in Table 7 (Figure 8). (1) Drug-induced side effects or unidentified reason for toxicity: some studies did not clearly explain the cause of poisoning, or the poisoning reaction still occurred without the occurrence of poisoning incentives [63]. (2) Improper processing: for example, the black aconite used excessive bile water in the process [64], causing damage to yang and diarrhea in patients, which are completely unrelated to the symptoms of aconite. “Short decoction time” is also the main cause of poisoning in patients [47]. (3) Drug accumulation [61, 65]: if the patient does not follow the doctor's prescription or take an overlong course of treatment, it may cause poisoning. (4) Aconite medicated with wine or food [62, 66]: six reports in DLTA mentioned that patients took medicinal liquor or medicine containing aconite, which caused toxic reactions. (5) Mistaken eating or self-prescribing [67, 68]: these mostly occur in rural areas and early times. With the improvement of public health awareness, mistaken eating has become rare. The results of the classification are shown in Table S5.
Table 7.
The occurrence of other influencing factors.
Figure 8.

The number of reports on the causes of aconite poisoning in DLTA.
3.4. Analysis of Detoxification Methods of Aconite
Through the literature survey, it is found that the commonly used ways to reduce the toxicity of aconite mainly include three methods: drug compatibility, processing, and decoction.
3.4.1. Drug Compatibility
In DLTA, we collected the results and causes of the effects of compatible medicinal materials on the toxicity of aconite and analyzed them statistically, as shown in Table 8. The most commonly compatible drugs with aconite are Glycyrrhiza uralensis Fisch, Zingiber officinale Roscoe, Panax ginseng C. A. Meyer, and Ephedra sinica Stapf, etc. The toxicity studies of the compatibility of other drugs with aconite are few. The chemical reaction between aconite and codecocting drugs can reduce toxicity by compatibility, thereby changing the structure of diester alkaloids and converting them into compounds with weaker toxicity [87], or improving enzyme activity to increase the metabolism of aconite [88].
Table 8.
The effect of compatible drugs on aconite toxicity and its causes.
| Compatible drugs | Number of articles | Toxic effect on aconite | Reason | Reference |
|---|---|---|---|---|
| Licorice | 28 | Reduce toxicity | ① Glycyrrhizic acid and glycyrrhetinic acid in licorice can neutralize with the aconite alkaloids in aconite, and the flavonoids in licorice can also combine with aconitum alkaloids to form a precipitate, both of which can delay or reduce the absorption of toxic alkaloids such as aconitine. ② Glycyrrhizic acid in the gastrointestinal tract can be converted into glycyrrhetinic acid and flavonoids. Licorice flavonoids contain multiple hydroxyl groups, which can combine with alkaloids in aconite to form ester alkaloid precipitation, reduce the content of toxic alkaloids. | Yang et al. [69] |
| Dried ginger | 11 | Reduce toxicity | The chemical components in dried ginger can convert the more toxic diester alkaloids in aconite into less toxic ester alkaloids and can antagonize the central inhibitory effect of aconitine, thereby achieving the purpose of detoxification | Yue et al. [70] |
| Ginseng | 8 | Reduce toxicity | Ginsenosides in ginseng can increase the SOD activity of cardiomyocytes, reduce MDA content and LDH release rate, and can inhibit the apoptosis of aconite on cardiomyocytes and effectively inhibit its toxic effects. | Wang et al. [27] |
| Pinellia | 6 | Toxic increase/decrease | ① The compatibility of aconite with qing pinellia, pinellia ginger, and raw pinellia can inhibit the hydrolysis reaction of the alkaloids in aconite, resulting in a significant increase in the content of diester alkaloids. ② The compatibility of aconite and pinellia ternata is attenuated and can make the toxicity more toxic. Large diester alkaloids are transformed into less toxic monoester alkaloids. ③ Compatibility of aconite and pinellia can inhibit CYP1A2 and CYP3A1 enzyme activity, inhibit drug metabolism, and enhance the toxicity. | Huang [71] Jin et al. [72] |
| Ephedra and Fuzi licorice soup | 5 | Reduce toxicity | It can significantly reduce the content of diester alkaloids, and the codecocting effect of the three is the best. | Wang and Wan [73] |
| Ephedra | 5 | Reduce toxicity | After the two are compatible, the content of monoester alkaloids-benzoyl neoaconitine and benzoyl hypoaconitine is reduced, thereby generating a new ester alkaloid-8-linoleoyl-14- benzoyl hypoaconitine and 8-linoleyl-14-benzoyl aconitine reduces the toxicity. | Pi et al. [74] |
| Rhubarb | 4 | Reduce toxicity | During the decoction, the tannins and aconite alkaloids contained in rhubarb produce aconitine salt of tannic acid that is not absorbed by the intestine, thereby reducing the toxicity of aconite, and the content of aconitine decreases as the dose of rhubarb increases. Those are linearly related, and its attenuation effect also increases with the increasing dose of rhubarb. | Wang et al. [75] |
| Fritillaria Zhejiang/Fritillaria Chuan | 4 | Toxic increase/decrease | ① After codecoction of aconite and fritillaria, the content of aconitine, hypoaconitine, and neoaconitine increased significantly, and the dissolution rate of toxic components of aconitine increased. ② After the compatibility of aconite and fritillaria cirrhosa, the amount of the three diester alkaloids aconitine, mesaconitine, and hypoaconitine was significantly reduced or undetectable, and the toxicity was reduced. | Bian et al. [76] Dong et al. [77] |
| Trichosanthes kirilowii | 2 | Increase toxicity | The combination of aconite and Trichosanthes kirilowii showed serious toxic effects, including promoting heart and kidney inflammation, increasing myocardial fibrosis, and activating β2-AR/PKA signal. | Sun et al. [78] |
| Astragalus | 2 | Reduce toxicity | ① Compatible with astragalus can reduce the 6 alkaloids of aconite to varying degrees (benzoyl hypoaconitine BHA, benzoyl neoaconitine BMA, benzoyl aconitine BAC, hypoaconitine HA, new aconitine MA, aconitine AC) plasma concentration. ② Astragalus inhibits the absorption of aconite alkaloids that may be related to the expression of astragalus-induced efflux transporter. ③ Astragalus promotes the clearance of aconite that may be related to the induction of corresponding metabolic enzymes (CYP3A4, CYP3A4, astragalus, CYP1A1, CYP2E1); activity is related. | Liu et al. [79] Zhang et al. [80] Lou et al. [81] |
| White Peony | 1 | Reduce toxicity | The diester-type alkaloids in aconite react with the chemical components in the white peony root, so that hypoaconitine, which is not easily hydrolyzed, generates lipid alkaloids. The lipid exchange reaction leads to a decrease in the content of hypoaconitine, thereby achieving attenuation. | Yue et al. [70] |
| Guizhi | 1 | Reduce toxicity | The compatibility of Aconite with Guizhi can reduce the total alkaloids and ester alkaloids of aconite, thereby reducing the poisonousness of aconite, and may be able to guide aconite to dispel cold and relieve pain and warm meridians and improve the pulse. | Ye et al. [82] |
| Cinnamon | 1 | Unknown | The compatibility of aconite with cinnamon can promote the dissolution of the effective components of aconite and can better guide the aconite to play the role of warming yang and igniting fire. | Ye et al. [82] |
| Windproof | 1 | Reduce toxicity | Improve LD50 and TD50 | Zhang et al. [83] |
| Polygala | 1 | Reduce toxicity | Improve LD50 and TD50 | Zhang et al. [83] |
| Dogwood | 1 | Reduce toxicity | Enhance the effect of “Wen tongxinyang” of aconite, and reduce its cardiotoxicity. | Jin et al. [84] |
| Trichosanthin | 1 | Reduce toxicity | Subacute toxicity experiments in mice show that the toxicity of aconite and Trichosanthes is less than that of aconite single decoction. | Yang et al. [85] |
| Rifampin | 1 | Reduce toxicity | Rifampicin is a liver drug enzyme inducer, which induces aconite metabolism to accelerate and significantly reduces the acute toxicity of aconite. | Chen et al. [86] |
| Dry Rehmannia | 1 | Reduce toxicity | Induces CYP1A2 and CYP3A4 enzyme activity, increases CYP450 enzyme content, accelerates the metabolism of toxic components of aconite, and achieves aconite attenuation [53]. | Li et al. [33] |
3.4.2. Processing Method
A total of 38 studies on the processing of aconite were collected in this study, and information analysis was performed (Table 9). Studies have shown that because of its high toxicity, raw aconite is rarely taken directly. Most of the processed products of aconite are used clinically. Aconite processing products have many specifications. In recent years, techniques such as puffing and dry heat baking have been introduced in the processing of aconite and have good effects of reducing toxicity [99, 100]. Based on the results of various studies, the toxicity of black sliced aconite, steamed aconite slice, and cooked aconite slice are weaker than that of other processed products of aconite.
Table 9.
Determination results and comparison of specifications and toxic content of processing products of aconite.
| Aconite processing specifications | The determination results | Conclusions | Reference |
|---|---|---|---|
| Black sliced aconite, baifupian, mud aconite | The LD50 of alcohol extracts of black sliced aconite, baifupian, and mud aconite is 49.853 g kg−1, 42.550 g kg−1, and 22.169 g kg−1, respectively | Toxicity: black sliced aconite < baifupian < mud aconite | Xie et al. [89] |
| Bafupian, black sliced aconite, salted aconite | The LD50 of baifupian and black sliced aconite is 20.529 g kg−1. Salted aconite is more toxic with LD50 of 11.301 g kg−1 | Toxicity: black sliced aconite = baifupian < salted aconite | Chai et al. [90] |
| Shengfupian, baifupian, Heifupian, Paofupian | The LD50 of the water extract and alcohol extract of shengfupian is 22.4 g kg−1 and 13.2 g kg−1, respectively; the maximum tolerable dosages of baifupian, heifupian, and paofupian are 533 g, 666 g, and 266 g, respectively | Toxicity: water extract of shengfu pian < alcohol extract of shengfu pian; heifupian < baifupian < paofupian | Zhou et al. [91] |
| Aconite puffed decoction pieces | The content of diester-type alkaloids of aconite: 0.13952% for raw aconite, 0.03771% for black sliced aconite, 0.05896% for baifupian, and 0.05024% for aconite puffed decoction pieces | Toxicity: black sliced aconite < aconite puffed decoction pieces < baifupian < raw aconite | Cheng. [92] |
| dry heat baking, moist heat baking | After processing, the content of diester alkaloids of aconite significantly decreases or disappears, and the content of monoester alkaloids increases significantly | Reducing toxicity | Tang et al. [93] |
| High voltage sliced aconite | The content of monoester alkaloids is higher, and the content of toxic components of diester alkaloids significantly decreases. High-pressure aconite tablets have the advantages of high efficiency and low toxicity | Active ingredients: high voltage sliced aconite > high temperature sliced aconite, microwave sliced aconite, black sliced aconite | Jia et al. [94] |
| Fried sliced aconite | Compared with other processed aconite products, fried sliced aconite has a lower content of diester alkaloids, the highest content of monoester alkaloids, and the lowest content of ephedra alkaloids | Toxicity: fried sliced aconite < black sliced aconite, danfupian, baifupian, paofupian | Qiu [95] |
| Raw aconite, prepared aconite | Raw aconite has a better effect than processed aconite on Rhubarb aconite decoction | Rhubarb aconite decoction using raw aconite has a better therapeutic effect and is safe to use | Guo [96] |
| Black sliced aconite, fried sliced aconite, steamed sliced aconite | The maximum tolerated amount of fried sliced aconite is 170 g kg−1, that of steamed sliced aconite is 268 g kg−1, and the LD50 of Heishunian is 138.13 g | Toxicity: steamed sliced aconite < fried sliced aconite < black sliced aconite | Zhang et al. [97] |
| Danba preparation | Among different salted products, aconite with 40% and 45% concentration of danba preparation has lower toxicity, and the former one also has an obvious neuroprotective effect | 40% danba should be used for salted aconite | Liu [98] |
In addition, although the safety of aconite is guaranteed, the medicine materials for disease treatment decrease due to overprocessing. Many experimental data supported this view [101, 102]. Only reasonable processing can both reduce the toxicity of raw aconite and give full play to the efficacy. Thus, it is urgent to establish scientific standards of aconite processing. We studied the control of the toxic components of aconite for the safety of the medication and the better curative effect. Therefore, we maintain a balance between toxicity and effect.
3.4.3. Decoction Time
The diester alkaloids in aconite, such as aconitine, hypaconitine, and neoaconitine, are both effective and toxic components [3]. The diester alkaloids can be hydrolyzed by heating or have a lipid exchange reaction with fatty acids to generate fat base and reduce its content, thereby reducing toxicity. Therefore, the pyrolysis reaction caused by aconite heating is also one of the effective ways of aconite detoxification [103].
Through literature surveys (Table 10), it was found that aconitum decoction for more than 60 minutes reached the safe range, and more than 2 hours of decoction may cause the loss of effective components. The toxicity of aconite is closely related to the decocting time, which is a direct factor for the toxicity of aconite.
Table 10.
The effect of decocting time on aconite toxicity.
| Type | Detection indicator | Conclusion | Reference |
|---|---|---|---|
| Acute toxicity test in mice | Biochemical indicators, mortality, adverse reactions, etc. | The toxicity of aconite decoction for 60 minutes is relatively low, and the pharmacological activity is the strongest; when the decoction exceeds 105 minutes, the animals in each group behave normally without death | Kao and Zhang [104] |
| Clinical safety experiment | Nausea, vomiting, dizziness, salivation, and other adverse reactions | The normal dose decoction time should be controlled within 1-2 h; but if the dose is more than 200 g, an additional 1 h decoction time should be added | Liang et al. [105] |
| HPLC content determination | Aconitine, neoaconitine, hypoaconitine | Both the 0 min and 30 min water decoctions of aconite are toxic, but the 60 min toxicity is not significant, and it can basically be defined as nontoxic | Sun et al. [106] |
| HPLC and UV methods | Neoaconitine, hypoaconitine, aconitine, benzoyl neoaconitine, benzoyl hypoaconitine, benzoyl aconitine | After 0.5 h of decoction, the content of diester alkaloids basically disappeared. After 1 h of decoction, the content of monoester alkaloids and total alkaloids reached the maximum | Gong et al. [107] |
| HPLC content determination | Aconitine, hypoaconitine, neoaconitine, benzoyl neoaconitine, benzoyl hypoaconitine, benzoyl aconitine | After decoction of black sliced aconite for 3.5 hours, the content of monoester alkaloids gradually disappeared | Lin et al. [108] |
| HPLC content determination | 13 kinds of alkaloids including aconitine, neoaconitine and hypoaconitine | After decoction of shengfu tablets for 2–4 hours, the content of diester alkaloids is already very low, which can ensure the safety of clinical medication | Zhang et al. [109] |
| HPLC content determination | Neoaconitine, hypoaconitine, aconitine, benzoyl neoaconitine, benzoyl hypoaconitine, benzoyl aconitine | The diester alkaloids in raw aconite are extremely unstable in water decoction. Hypoaconitine was detected within 0.5 h of decoction | Chen et al. [110] |
| HPLC content determination | Aconitum alkaloids | After decocting the aconite in water for 30 minutes, the content of aconitine and hypoaconitine became 10.5% and 41.9% of the peak value, respectively, and aconitine was completely undetectable; after the aconite microwave heating for 150s, the content of aconitine, neoaconitine, and hypoaconitine was 59.2%, 41.4%, and 86.6% of the peak value, respectively | Sui et al. [111] |
| HPLC and UV methods | Total alkaloids, ester alkaloids, polysaccharide components, diester alkaloid components | The best decocting time is within 1 hour | Yu et al. [112] |
4. Discussion
Based on the bibliometric method, this study evaluated the publication trend and research hotspots of aconite toxicity in the past 35 years. In addition, this paper analyzed the common toxic reactions, toxic mechanisms, factors, and commonly used detoxification methods of aconite. This can provide a useful reference for clinical rational use of aconite and related research on its toxicity.
Since the beginning of 1985, there have been research reports on the toxicity of aconite. The decoction time, dosage, and mode of administration of aconite are the main factors affecting the toxicity of aconite. There are few studies on the effect of the origin of aconite and the specifications of medicinal materials on toxicity, so it is impossible to analyze their relevance. At present, the commonly used methods to reduce the toxicity of aconite include three methods: drug compatibility, concoction, and decoction. The most common drugs compatible with aconite are licorice, dried ginger, ginseng, and ephedra. Black sliced aconite, steamed slices, and fried slices are less toxic than other processed products. Aconite decoction for more than 60 minutes can basically reach the safe range, and more than 2 hours of decoction may cause the loss of active ingredients.
One problem is that the number of studies on the toxicity of aconite has declined in recent years. However, the toxicity of aconite has not been fully revealed. More experimental data and research are needed to confirm and answer: (1) overdose use of aconite (exceeding the pharmacopeia recommendation) is common, but there are few studies on the relationship between quantity and toxicity effect of aconite. When clinical use is caused, there is no uniform reference standard for indication dose, which could easily cause toxic reactions. (2) The reasonable decoction time of aconite is 30 minutes to 1 hour. This has been supported by many studies. However, there are also 2–4 hours of decoction cases in clinical practice, especially in the case of large-dose use. Although long-term decoction reduces the incidence of toxic reactions, does the effect of aconite change after long-term decoction? There are too few research data. The decoction time of large-dose aconite lacks a reference standard. (3) Although there are many studies about the processing and compatibility of aconite, there is a large convergence, the processing method is rarely innovative, and the compatibility research is mostly concentrated on several drugs such as licorice, and the compatibility evaluation with other drugs is less, leading to the lack of precise evidence to guide doctors when prescribing drugs in the clinic, and it is also easy to cause toxic events. (4) More and more reports show that aconite can cause adverse reactions such as kidney and liver damage, but the mechanism of its hepatotoxicity and nephrotoxicity is less. The exact toxicity mechanism needs further study. The researchers should be committed to improve the research of toxicity of aconite, remove the constraints for application, and promote the safe and reasonable use of aconite in the clinic. Researchers should improve various toxicity studies of aconite, remove the constraints that affect its clinical application, and promote the rational use of aconite.
Acknowledgments
This study was supported by National Basic Research Program of China (No. 2019YFC1604905) and National Natural Science Foundation (No. 81760717).
Contributor Information
Lihua Chen, Email: chlly98@163.com.
Meiying Ao, Email: 15870687101@163.com.
Data Availability
The datasets generated during and/or analyzed during the current study are available from CNKI (https://www.cnki.net/), CQVIP (http://www.cqvip.com/), Chinese Biomedical Literature Service System (http://www.sinomed.ac.cn/), and PubMed (https://www.ncbi.nlm.nih.gov/).
Conflicts of Interest
There are no conflicts of interest.
Authors' Contributions
Diyao Wu took charge of guiding the experiments and paper writing. Tielong Xu, Qian Chen, Yaling Wang, Hongfu Chen, and Zhengdong Huang wrote the manuscript and finished data mining research. Meiying Ao and Lihua Chen completed the experimental design and execution.
Supplementary Materials
Table S1: the classification of the toxicity action mechanism of aconite and the number of studies. Figure S2: the number of studies of various specifications of aconite in DLTA. Table S3: the number of studies of decoction time in the DLTA. Table S4: the number of studies on different dosages of aconite in DLTA. Table S5: the number of reports on the causes of aconite poisoning in DLTA.
References
- 1.Yang K., Yu G. Study on the development and evolution of Chinese medicinal materials from pre-Qin to Tang Dynasty. Chinese Journal of Chinese Materia Medica. 2017;42(23):4674–4678. doi: 10.19540/j.cnki.cjcmm.20171031.001. [DOI] [PubMed] [Google Scholar]
- 2.Qin K., Song J., Ye Q. Research on the efficacy of aconite. Chinese Materia Medica. 2015;38(1):185–187. [Google Scholar]
- 3.Gao Y., Xie L., Lu Z. Progress in the study of toxicology and synergistic compatibility of aconite. Tianjin Pharmacy. 2020;32(1):65–69. [Google Scholar]
- 4.Liu J., Zhai X., Liao X. Bibliometric analysis on cardiovascular disease treated by traditional Chinese medicines based on big data. International Journal of Parallel, Emergent and Distributed Systems. 2020;35(3):323–339. doi: 10.1080/17445760.2019.1606912. [DOI] [Google Scholar]
- 5.Sheng K., Tian Q., Wang H., Zhao Q. M. Effects of total alkaloids of Aconitum carmichaeli on rat heart function and its mechanism in vitro. Genomics and Applied Biology. 2019;38(10):4709–4714. [Google Scholar]
- 6.Hao L., Liang G., Hongxin W., Ning J., Gao S. Research progress on toxicological safety of Aconitum carmichaeli. Journal of Toxicology. 2020;34(6):435–440. [Google Scholar]
- 7.Pan X., Peng C. Research progress on neurotoxicity of Aconitum carmichaeli. World Journal of Traditional Chinese Medicine. 2017;12(11):2551–2554. [Google Scholar]
- 8.Han X., Hou Y. Research progress on pharmacological action and toxicity of aconitine. Chinese Prescription Drug. 2014;12(12):149–150. [Google Scholar]
- 9.Jiang X. Toxic components and adverse reactions of aconite. Journal of Applied Traditional Chinese Medicine Internal Medicine. 2018;32(12):73–75. [Google Scholar]
- 10.Han S., Lu L., Wang H., et al. Neurotoxicity of three Aconitum drugs in rats in vivo and in vitro. Journal of West China Pharmacy. 2007;3:286–288. [Google Scholar]
- 11.Liu H. Discussion on toxicity and prevention of traditional Chinese medicine. Beijing Traditional Chinese Medicine. 2004;4:232–234. [Google Scholar]
- 12.Xiao K., Li H., Wang Y., et al. Embryotoxicity and teratogenicity of aconitum. Journal of China Pharmaceutical University. 2005;6:567–571. [Google Scholar]
- 13.Qi Y., Chen J. Effect of atropine combined with lidocaine on ventricular arrh -ythmia induced by aconitine poisoning. Yunnan Medicine. 2017;38(4):357–358. [Google Scholar]
- 14.Sun J. Study on the Mechanism of Ginseng and Prepared Liquorice Reducing Toxicity of Aconite Based on CYP450 Enzyme. Dalian, China: Liaoning Normal University; 2019. [DOI] [Google Scholar]
- 15.Li Z. Study on the interaction regulation of Aconitum carmichaeli “effect toxicity network” in the compatible environment of Sini decoction. Chinese Journal of Traditional Chinese Medicine. 2015;40:733–738. [PubMed] [Google Scholar]
- 16.Strzelecki A., Pichon N., Gaulier J. M., Amiel J. B., Champy P., Clavel M. Acute toxic herbal intake in a suicide attempt and fatal refractory ventricular arrhythmia. Basic & Clinical Pharmacology & Toxicology. 2010;107(2):698–699. doi: 10.1111/j.1742-7843.2010.00566.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhou T., Yang J., Wan H., Zhang Y., Zhou H. Protective effect of compatibility of main components of aconitine Radix Aconiti and Glycyrrhiza uralensis Fisch. Journal of Beijing University of Traditional Chinese Medicine. 2014;37:22–26. [Google Scholar]
- 18.Wang R., Zhan X., Qiao Y. Experimental study on acute toxicity of total alkaloid extract of Aconitum carmichaeli. Chinese Journal of Experimental Prescriptions. 2009;15:102–103. [Google Scholar]
- 19.Zhang A. One case of arrhythmia and four phase complete right bundle branch block caused by Zhifu tablet poisoning. Journal of Modern Integrated Traditional Chinese and Western Medicine. 2003;12 [Google Scholar]
- 20.Sun Y., Wang Yu, Liu J., Wang S., Wang X. Research progress on effects of diester diterpenoid alkaloids from Aconitum carmichaeli on metabolic enzymes and myocardial toxicity. Journal of Tianjin University of Traditional Chinese Medicine. 2018;37(4):274–279. [Google Scholar]
- 21.Wang B. The Activity and Mechanism of Aconite Root in Diabetic Peripheral Neuropathy and Identification of Aconitine Constituents. Beijing, China: Beijing University of Chinese Medicine; 2016. [Google Scholar]
- 22.Peng W. A case of toxic myocarditis caused by aconite root. Diet Health Care. 2018;5:82–83. [Google Scholar]
- 23.Lu Z., Zhang Y., Zhuang P., et al. Effect of aconite root on hemodynamics in rats with acute heart failure and its mechanism. Chinese Herbal Medicine. 2015;46(21):3223–3227. [Google Scholar]
- 24.Gao K., Huang C. Study on reproductive toxicity of aconite root extract in rats. China Modern Drug Application. 2014;18(16):20–21. [Google Scholar]
- 25.Fu M. Experimental Study on Cellular and Molecular Mechanism of Aconitine Induced Arrhythmia. Beijing, China: Beijing University of Traditional Chinese medicine; 2007. [Google Scholar]
- 26.Xie X. Mechanism of Aconite Heart Toxicity. Chengdu, China: Chengdu University of Traditional Chinese Medicine; 2012. [Google Scholar]
- 27.Wang X., Li L., Li Y., Li C., Zhang D. Attenuation effect of different compatibility of aconite root and ginseng on myocardial cells. Chinese Journal of Experimental Prescriptions. 2015;21:153–158. [Google Scholar]
- 28.Zhao J., he J., Ma Z., et al. Toxic mechanism of aconite root on H9c2 myocardial cell line mitochondria. Chinese Journal of Pharmacology and Toxicology. 2015;29(5):816–824. [Google Scholar]
- 29.Wang X. Comparison and Cytological Mechanism of Arrhythmia Induced by Aconitine and Neoconitine. Shijiazhuang, China: Hebei Medical University; 2013. [Google Scholar]
- 30.Wang H., Tian Q., Zhao Q. Effects of total alkaloid of aconite on cardiac function and its mechanism in isolated rat heart. Genomics & Applied Biology. 2019;38(10):4709–4714. [Google Scholar]
- 31.Li S., Rui Li, Zeng Y., et al. Progress in studies on chemical constituents and pharmacological effects of Radix Polygoni. Chinese Journal of Chinese Materia Medica. 2019;44(12):2433–2443. doi: 10.19540/j.cnki.cjcmm.20190221.004. [DOI] [PubMed] [Google Scholar]
- 32.Huang Q. Z., Wang J. Index. Liberal Cosmopolitan. 2011;17(23):269–271. doi: 10.1163/ej.9789004192133.i-272.43. [DOI] [Google Scholar]
- 33.Li Q., wanting D., Ju A., Zhao J. Study on attenuation mechanism of Radix Aconiti Lateralis Preparata combined with Radix Rehmanniae based on liver drug metabolism enzymes. Chinese Journal of Traditional Chinese Medicine. 2020;45:3961–3966. doi: 10.19540/j.cnki.cjcmm.20200512.202. [DOI] [PubMed] [Google Scholar]
- 34.Zhang F., Li L., Wu R., Liu P. Comparative experiment on chemical constituents and toxicity of aconite root, aconite tablet and decoction. Yunnan Journal of Traditional Chinese Medicine. 2004;25:29–31. [Google Scholar]
- 35.Xu J., Wang H., Zhang W., Wu M. Effects of different combinations and decocting methods of dahuang fuzi decoction on aconitine content. Chinese Pharmacy. 2003;14:634–635. [Google Scholar]
- 36.Peng H., Wang Y., Zhang S., Ge T., Peng D., Chen W. Acute toxicity of different components of heishun tablet on normal mice. Chinese Patent Medicine. 2018;40:1842–1844. [Google Scholar]
- 37.Xie Y. F., Feng W. W., Liu M. C., et al. Investigation of efficacy enhancing and toxicity reducing mechanism of combination of Aconiti lateralis radix praeparata and paeoniae radix alba in adjuvant-induced arthritis rats by metabolomics. Evidence-based Complementary and Alternative Medicine. 2019;2019 doi: 10.1155/2019/9864841.9864841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J., Liu G. Report of 2 cases of poisoning induced by Fuzi Lizhong pill. Journal of Practical Chinese Medicine. 2001;17:p. 43. [Google Scholar]
- 39.Guo W., Tan P., Wu Y., Qin Y., Zhao L., Fei Li. Effects of processing with double excipients on traditional properties and ester alkaloids of Aconitum carmichaeli. Chinese Patent Medicine. 2015;37(6):1289–1293. [Google Scholar]
- 40.Jin L., Yang Y., Xinping W., Chu J. Dosage evaluation and supervision of Jinkuishenqi tablets. Chinese Modern Applied Pharmacy. 2016;33(1):97–101. [Google Scholar]
- 41.Wu Q. One case of arrhythmia induced by Fuzi wine. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine. 2002;12 [Google Scholar]
- 42.Wen Y., Li Y., Li J. A case of Baifu tablet poisoning. Practical Medicine and Clinical. 2009;12:p. 435. [Google Scholar]
- 43.Chen J., Chen H., Gao J., Tian J., Xi S. Ten cases of arrhythmia caused by Aconitum carmichaeli poisoning. 2016.
- 44.Luo C., Zhang R. Discussion on toxicity of Aconitum carmichaeli in large dose application. Chinese Journal of Traditional Chinese Medicine. 2009;34(18):2423–2424. [Google Scholar]
- 45.Fu W. Wu Peiheng’s experience in the application of Aconitum carmichaeli. Henan Traditional Chinese Medicine. 2011;31(4):339–341. [Google Scholar]
- 46.Tan W., Xia Y., Li Y., Qiangzhong P. I., Luo S. Study on the cardiotoxic substance basis and related mechanism of aconite root. Pharmacology and Clinic of Traditional Chinese Medicine. 2019;35(3):95–101. [Google Scholar]
- 47.Yang C. Clinical toxic reaction and treatment analysis of 37 cases of Aconitum traditional Chinese medicine. Clinical Practice of Integrated Traditional Chinese and Western Medicine. 2017;17(3):161–162. [Google Scholar]
- 48.Luo H., Wang X., Hua Y. Clinical application and safety evaluation of aconite root. Journal of Clinical Rational Drug Use. 2019;12(23):105–106. [Google Scholar]
- 49.Lin J., Xu J., Huang Q. Large dose of Aconitum carmichaeli should be decocted for a long time. Contemporary Medicine. 2012;18(17):36–37. [Google Scholar]
- 50.Huang Y. Study on Dosage and Related Problems of Treatise on Febrile Diseases. Beijing, China: Beijing University of traditional Chinese medicine; 2007. [Google Scholar]
- 51.National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Beijing, China: China Medical Science and Technology Press; 2020. [Google Scholar]
- 52.Wang R. One case of malignant arrhythmia caused by conventional dose of aconite poisoning. Sichuan Medical Journal. 2001;22(5):p. 509. doi: 10.3969/j.issn.1004-0501.2001.05.098. [DOI] [Google Scholar]
- 53.Cao C. Aconite poisoning: a case report. Journal of Practical Chinese Medicine. 2012;28(8):p. 705. [Google Scholar]
- 54.Wang R. S. A case of malignant arrhythmia caused by conventional dose of adhesion poisoning. Sichuan Medical Journal. 2001;22(5):p. 509. [Google Scholar]
- 55.Cao C. A case report of aconite poisoning. Journal of Practical Traditio-Nal Chinese Medicine. 2012;28(8):p. 705. [Google Scholar]
- 56.Wang J., Liu G. A report of 2 cases of toxic reaction caused by aconit li zhong pill. Journal of Practical Traditional Chinese Medicine. 2001;1:p. 43. [Google Scholar]
- 57.Luo C., Zhang R. The application of super large dose aconite in recent 20 years. Chinese Journal of Traditional Chinese Medicine. 2005;2:17–20. [Google Scholar]
- 58.Hou S., Zhang X., Lin C. One case of acute aconite poisoning. Journal of Clinical Rational Drug Use. 2011;4:p. 73. [Google Scholar]
- 59.Zhang J., Zeng Z., Zhang B., Yu S. Analysis of clinical adverse reactions of Aconitum carmichaeli. Chinese Journal of Experimental Prescriptions. 2014;20(18):228–231. [Google Scholar]
- 60.Wang J., Cai X. Report of three cases of Baifu tablet poisoning. Northern Pharmacy. 2011;8:p. 128. [Google Scholar]
- 61.Wang X. Analysis of aconite accumulation poisoning cases. Correspondence of Traditional Chinese Medicine. 1997;2:p. 13. [Google Scholar]
- 62.Wu Q. A case of arrhythmia induced by aconite wine. Journal of Zhejiang Integrated Traditional and Western Medicine. 2002;1:12–14. [Google Scholar]
- 63.Li C., Peng W. A case of second degree atrioventricular block caused by conventional dose of aconite poisoning. Basic Medical Forum. 2020;24(10):1467–1468. [Google Scholar]
- 64.Wang X. M. Report and analysis of 4 cases of adverse reactions to decoction pieces of Chinese medicine. Proceedings of 2015 Annual Conference of Beijing Society of Traditional Chinese Medicine; December 2016; Beijing, China. pp. 303–304. [Google Scholar]
- 65.Chen T. L., Liao J. L., Zhong Z. L. Transforming inpatient care. Transforming Health Care. 2010;17(6):111–127. doi: 10.3969/j.issn.1674-4659.2010.06.111. [DOI] [Google Scholar]
- 66.Zhang C., Yang H., Yang C. Treatment of rapid ventricular arrhythmias caused by severe aconitine poisoning in eight cases. Yunnan Medicine. 2018;39(3):273–279. [Google Scholar]
- 67.Huang Z. A case report of aconite poisoning in Chinese medicine. Qinghai Medical Journal. 2007;37(11):p. 13. doi: 10.3969/j.issn.1007-3795.2007.11.041. [DOI] [Google Scholar]
- 68.Wang Y., Gu Z. Five cases of aconitum poisoning treated by traditional Chinese medicine combined with traditional Chinese medicine and western medicine. Chinese Journal of Integrated Traditional and Western Medicine. 1994;S1:p. 406. [Google Scholar]
- 69.Yang M., Liu X., Huang Q. Mechanism of reducing toxicity and increasing efficiency of aconite root and licorice. Shi Zhen, Guo Yi Guo Yao. 2003;14(4):197–198. [Google Scholar]
- 70.Yue H., Zi F., Song F., et al. Electrospray ionization mass spectrometry analysis of alkaloids in different combinations of aconite roots. Journal of Pharmaceutical Sciences. 2007;42(2):201–205. [Google Scholar]
- 71.Huang C. Changes of Alkaloids in Compatibility of Radix Aconiti Lateralis Preparata and Rhizoma Pinelliae. Jinan, China: Shandong University of traditional Chinese medicine; 2012. [Google Scholar]
- 72.Jin K., Shi S., Shen J., Gao Y. Regulatory effect of Pinellia and Aconitum on CYP450 in rat liver. Chinese Journal of Traditional Chinese Medicine. 2007;07:1358–1361. [Google Scholar]
- 73.Wang C., Wan L. Effects of different compatibility methods of Mahuang Fuzi Gancao Decoction on its toxic components. Chinese Medical Guide. 2016;14(10):37–38. [Google Scholar]
- 74.P. Z., Yue H., Meng X., et al. Study on the changes of alkaloids in radix Aconiti lateralis preparata during compatibility of mahuang fuzi gancao decoction. World Science and Technology Modernization of Traditional Chinese Medicine. 2009;11(2):269–273. [Google Scholar]
- 75.Wang X., Sun W., Zhang T. Research progress on compatibility of Aconitum toxic traditional Chinese medicine. Chinese Journal of Experimental Prescriptions. 2012;18(18):327–331. [Google Scholar]
- 76.Bian B., Nan Si, Wang H., et al. Study on the content changes of aconitine, hypaconitine, neoaconitine and other toxic components after aconite single decoction and co Decoction with Fritillaria thunbergii. Chinese Journal of Experimental Prescriptions. 2006;12(4):9–10. [Google Scholar]
- 77.Dong X., Wang S., Zhu E., Song F., Liu Z., Liu S. Chemical Study on compatibility of Aconitum and its processed products with Fritillaria thunbergii and Fritillaria thunbergii. Chinese Herbal Medicine. 2012;43(2):265–269. [Google Scholar]
- 78.Sun F., Sheng Y., fan S., Zhang Y., Zhuang P. Han Juan β-2-Ar/PKA signal to investigate the toxic effect of aconite root and Trichosanthes kirilowii on inflammatory response and myocardial fibrosis in pressure overload rats. Chinese Journal of Traditional Chinese Medicine. 2019;44(19):4212–4218. doi: 10.19540/j.cnki.cjcmm.20190505.402. [DOI] [PubMed] [Google Scholar]
- 79.Liu Y., Jiang Q., Huang G., Chen X., Liao Z. Study on the effect of Astragalus membranaceus on the pharmacokinetics of six Aconitum alkaloids in Aconitum carmichaeli in rats by LC-MS. Acta Pharmaceutica Sinica. 2019;54(12):2289–2295. [Google Scholar]
- 80.Zhang G., Ou R., Li F., et al. Regulation of drug-metabolizing enzymes and efflux transporters by Astragali radix decoction and its main bioactive compounds: Implication for clinical drug-drug interactions. Journal of Ethnopharmacology. 2016;180:104–113. doi: 10.1016/j.jep.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 81.Lou Y., Guo Z., Zhu Y., et al. Astragali radix and its main bioactive compounds activate the Nrf2-mediated signaling pathway to induce P-glycoprotein and breast cancer resistance protein. Journal of Ethnopharmacology. 2019;228:82–91. doi: 10.1016/j.jep.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 82.Ye Q., Shi Y., Cheng P., Guo Li. Study on the regulation of aconite root compatibility with Cinnamomum cassia. Journal of Chengdu University of Traditional Chinese Medicine. 2011;34(3):65–68. [Google Scholar]
- 83.Zhang G., Suhua J., Zhu X., Zhang S., ye Z. Experimental study on compatibility of Aconitum carmichaeli with Xiangsha and Xiangwei. Chinese Journal of Traditional Chinese Medicine. 2012;37(15):2215–2218. [PubMed] [Google Scholar]
- 84.Jin Z., Qin K., Ye Q., Song J. Study on the synergism and attenuation of Fructus Corni on “warming and tongxinyang” of aconite. Chinese Herbal Medicine. 2015;38(3):576–579. [Google Scholar]
- 85.Yang Z., Ma J., Lifu W., Cui J. Subacute toxicity of radix Aconiti lateralis preparata and radix trichosanthis in mice. Chinese Folk Medicine. 2018;27(20):7–8. [Google Scholar]
- 86.Chen Y. X., Zhou K., Fan L., et al. Study on induced degradation of acute toxic substances in Aconitum carmichaeli by rifampicin. Chinese Modern Traditional Medicine. 2019;21(6):786–790. [Google Scholar]
- 87.Tang H., Yan Y., Tang B., et al. World science and technology-modernization. Traditional Chinese Medicine. 2018;20(10):1867–1875. [Google Scholar]
- 88.Li Z., Miao Y., Zhang B., et al. Progress in the study of toxicity reduction mechanism of licorice compatibility based on pharmacokinetics. Chinese Journal of Herbal Medicine. 2015;46(23):3611–3616. [Google Scholar]
- 89.Xie X., Fang P., Cheng Y., Huhai Y., Zheng W. Comparative study on acute toxicity of different processed extracts of aconite. Chinese Medicine and Clinical. 2012;3(3):29–51. [Google Scholar]
- 90.Chaiyushuang W., Hualei Z., Xiao C., et al. Comparison of toxic effects of aconitus aconitus and its processed products. World Science and Technology (Modernization of Traditional Chinese Medicine) 2011;13(5):847–851. [Google Scholar]
- 91.Zhou Z., Xiong Y., Huang Q., Yang M. Acute toxicity of different processed products and their parts of epiphytes. Journal of Chengdu University of Traditional Chinese Medicine. 2012;35(3):63–65. [Google Scholar]
- 92.Cheng F. The Effect of Expanded Processing Technology on the Toxic Chinese Medicine Aconite/Pinellia Ternata. Chengdu, China: Southwest Jiaotong University; 2013. [Google Scholar]
- 93.Tangxiaolong Y., Xiayanli H., Chenyan L. The influence of different processing methods on the content of six ester alkaloids in aconite. Chinese Journal of Experimental Prescription. 2013;19(21):96–100. [Google Scholar]
- 94.Jia X., Lin H., Shen Y., Deng G. Determination of aconitine alkaloids in new processed products of Aconitum carmichaeli and Study on its cardiotonic effect. Drug Evaluation Research. 2016;39(02):224–229. [Google Scholar]
- 95.Qiu L. Study on the Relationship of “Disease Syndrome Dose Effect/Toxicity” of Mahuang Xixin Fuzi Decoction. Jinan, China: Shandong University of Chinese medicine; 2019. [Google Scholar]
- 96.Guo H. Study on Serum Pharmacochemistry of Dahuang Fuzi Decoction. Nanjing, China: Nanjing University of traditional Chinese medicine; 2013. [Google Scholar]
- 97.Zhang X., Huang W., Zhao K., et al. Effects of different processing techniques on toxicity and efficacy of aconite. Pharmacology and Clinic of Traditional Chinese Medicine. 2019;35(01):103–107. [Google Scholar]
- 98.Liu H. Effects of Danba Processing on Chemical Constituents and Neurotoxicity of Aconitum Carmichaeli. Chengdu, China: Chengdu University of traditional Chinese medicine; 2018. [Google Scholar]
- 99.Huang Z., Zhang Y., Yang C., et al. Effects of steaming and baking on alkaloid content of aconite. Chinese Journal of Traditional Chinese Medicine. 2014;39(24):4798–4803. [PubMed] [Google Scholar]
- 100.He Y., Lu M., Huang W., et al. Study on influencing factors of processing technology of aconite with microwave cannons. Chinese Traditional and Herbal Medicine. 2020;51(12):3157–3164. [Google Scholar]
- 101.Liu Y., Liu H., Ye Q., et al. Study on the effects of the processing of gallba on the alkaloids of aconite. New Drugs and Clinical Pharmacology of Chinese Materia Medica. 2019;30(04):472–477. [Google Scholar]
- 102.Ye Q., Guo Y., Cheng P. Order pholidota. Mammals of China. 2013;28(03):275–277. doi: 10.1515/9781400846887.275. [DOI] [Google Scholar]
- 103.Zhou Y. Detoxification of Aconitum carmichaeli from hydrolysis of diester alkaloids (2) Pharmacology and Clinic of Traditional Chinese Medicine. 2014;30(03):154–157. [Google Scholar]
- 104.Kao Y., Zhang H. Study on the safety and efficacy of large dose edible aconite. Shaanxi Traditional Chinese Medicine. 2013;34(04):478–480. [Google Scholar]
- 105.Liang B., Zhong W., Shang Li, Li X., Wang X. Relationship between adverse reaction of aconite root and decocting time and dosage. China New Clinical Medicine. 2015;8(09):864–866. [Google Scholar]
- 106.Sun W., Liu F., Yuan Q., Shan L., Xiao L., Huang J. Study on the effect of decocting time on toxicity of Aconitum carmichaeli. Chinese Medical Emergency. 2018;27(05):761–764. [Google Scholar]
- 107.Gong Y., Deng G., Zheng X., Qin J., Luo M., Wang F. Content change and hydrolysis mechanism of alkaloids in Radix Aconiti Lateralis Preparata during decocting. China Pharmaceutical. 2017;26(04):9–15. [Google Scholar]
- 108.Lin H., Shen Y., Deng G., Gong Y. Effects of decocting time and compatibility on alkaloid content in different doses of Aconitum carmichaeli Decoction. Chinese Journal of Traditional Chinese Medicine. 2016;31(01):265–268. [Google Scholar]
- 109.Zhang Y., Yang C., Huang Z., et al. Study on the dynamic changes of 13 alkaloids in the process of Aconite Decoction. Journal of Pharmaceutical Analysis. 2015;35(01):16–23. [Google Scholar]
- 110.Chen D., Jin Y., Huang Z., Liang L. X., Wu Y. Dynamic changes of ester alkaloids in Aconitum carmichaeli Decoction. Chinese Journal of Experimental Prescriptions. 2011;17(03):64–68. [Google Scholar]
- 111.Sui Z., Chen M., Liu Z., Zifeng P. I., Liu Z. Changes and significance of Aconitum alkaloids in Aconitum carmichaeli decoction and compatibility. Journal of Jilin University (Medical Edition) 2009;35(02):226–229. [Google Scholar]
- 112.Yu C., Guo Li, Cheng P. Study on quality control of toxic components and toxic components of Aconitum carmichaeli with different decocting time. Chinese Society of Traditional Chinese Medicine Basic Theory Branch: Chinese Society of Traditional Chinese Medicine. 2008;6 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: the classification of the toxicity action mechanism of aconite and the number of studies. Figure S2: the number of studies of various specifications of aconite in DLTA. Table S3: the number of studies of decoction time in the DLTA. Table S4: the number of studies on different dosages of aconite in DLTA. Table S5: the number of reports on the causes of aconite poisoning in DLTA.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from CNKI (https://www.cnki.net/), CQVIP (http://www.cqvip.com/), Chinese Biomedical Literature Service System (http://www.sinomed.ac.cn/), and PubMed (https://www.ncbi.nlm.nih.gov/).


