Abstract
Background
Organic dust is a complex mixture of particulate matter from microbial, plant or animal origin. Occupations with exposure to animal products have been associated with an increased lung cancer risk, while exposure to microbial components (e.g. endotoxin) has been associated with a decreased risk. To date there has not been a comprehensive evaluation of the possible association between occupational organic dust exposure (and its specific constituents) and lung cancer risk in the general population.
Methods
The SYNERGY project has pooled information on lifetime working and smoking from 13,300 lung cancer cases and 16,273 controls from 11 case-control studies conducted in Europe and Canada. A newly developed general population job-exposure matrix (assigning no, low, or high exposure to organic dust, endotoxin, and contact with animals or fresh animal products) was applied to determine level of exposure. Odds ratios for lung cancer were estimated by logistic regression, adjusted for age, sex, study, cigarette pack-years, time-since-quitting smoking, and ever employment in occupations with established lung cancer risk.
Results
Occupational organic dust exposure was associated with increased lung cancer risk. The second to the fourth quartile of cumulative exposure showed significant risk estimates ranging from 1.12 to 1.24 in a dose-dependent manner (p-value <.001). This association remained in the highest quartile after restricting analyses to subjects without COPD or asthma. No association was observed between lung cancer and exposure to endotoxin or contact with animals or animal products.
Conclusion
Occupational exposure to organic dust was associated with increased lung cancer risk in this large pooled case-control study.
Keywords: case-control study, endotoxin exposure, lung neoplasms, risk factors, wood dust exposure
INTRODUCTION
Organic dust consists of particulate matter from microbial, plant or animal origin. Its specific agents include: viruses, bacteria (and endotoxins from Gram-negative bacteria), actinomycete, spores from moss, fern or fungi (and mycotoxins and glucans from fungi), cells from algal or plant sources, proteins from plant or animal sources, enzymes, antibiotics and other products created through biotechnological processes, and insects and mites (and their fragments and excreta). Organic dust is present in many work environments, such as in agriculture, sawmills, or the meat industry.[1]
In addition to causing several infectious diseases, exposure to organic dust at the workplace is known to lead to an increased risk of occupational respiratory diseases, such as chronic obstructive pulmonary disease (COPD), asthma, hypersensitivity pneumonitis and organic dust toxic syndrome.[1–3] Much less is known about the risk of lung cancer due to organic dust exposure. Increased lung cancer risks have been reported in certain occupations exposed to organic materials, for instance in meat and wood workers.[4, 5] Alternatively, decreased risks among workers exposed to organic dust have also been described. A recent meta-analysis showed a reduction in lung cancer risk among agricultural and (cotton) textile workers who were exposed to organic dust containing endotoxin.[6] Stimulation of the immune system, in particular macrophages, by endotoxin has been hypothesised to be the mechanistic pathway.[7] However, many of the epidemiological studies were not adequately controlled for smoking and therefore residual confounding due to differences in smoking habits between exposed and non-exposed subjects could not be excluded.
The SYNERGY population (recently described by Olsson et al. 2010 [8]) provided the opportunity to explore in more detail the possible effects of organic dust exposure and some of its specific constituents (endotoxin and contact with animals and/or animal products) on the risk of lung cancer. Since SYNERGY is a community-based study, it entails the whole spectrum of possible workplaces. Moreover, extensive information regarding smoking habits is available to appropriately adjust for possible differences in tobacco consumption.
METHODS
The SYNERGY project started in 2007 and is a pooled analysis of case-control studies on the joint effects of occupational carcinogens in the development of lung cancer. It currently contains pooled data from 11 population or hospital based case-control studies conducted between 1985 and 2005 in 12 European countries and Canada. Altogether, these studies include 13,479 lung cancer cases and 16,510 controls. For all subjects, detailed lifetime occupational and smoking history is available. For MORGEN, which is a nested case-control study, smoking and occupational information is lacking for the time interval between enrolment and diagnosis or end of follow-up (mean interval less than 10 years). More information about the SYNERGY project can be found at http://synergy.iarc.fr. Ethical approvals were obtained in accordance with legislation in each country, and in addition by the institutional review board at the International Agency for Research on Cancer (IARC).
Exposure assessment
The occupational exposure assessment was performed by applying a general population job-exposure matrix (JEM) for biological exposures, created based on ISCO 1968 coding (International Standard Classification of Occupations (ISCO), 1968[9]). This JEM is an extension of the previously described DOM-JEM.[10] Biological exposure levels were rated by three occupational exposure experts (HK, RV, and SP) for ‘organic dust’ and for some of its constituents, namely ‘organic dust containing endotoxin’ (hereafter called ‘endotoxin’) and ‘contact with animals or fresh animal products’ (hereafter called ‘contact with animals’). The assigned intensity score was ordinal (0=no exposure; 1=low exposure; and 4=high exposure). No measurement data or population specific information was used for the exposure assessment. The three experts assigned intensity scores independently from each other, with initial agreements of 89% (organic dust), 70% (endotoxin) and 97% (contact with animals). In the online material the complete JEM can be found.
We estimated cumulative exposure by summing the product of intensity and duration (years) for all reported job periods over the entire working career. The exposure intensity scores of none, low and high were arbitrarily assigned values of 0, 1 and 4 to reflect the log-normal (multiplicative) nature of occupational exposure concentrations. The weighting was based on reported levels for semi-quantitatively scored exposure.[3, 11] As such, a balanced weighting between intensity and duration in the calculation of cumulative exposure can be achieved. Estimated cumulative exposure was consequently categorised to the quartiles of the cumulative exposure distribution among controls.
Statistical analyses
Logistic regression models were fitted to calculate odds ratios (OR) and 95% confidence intervals (95% CI) of lung cancer associated with biological exposures. The risk estimates were calculated for the quartiles of cumulative exposure, compared to the never-exposed. P-values for trend were obtained by applying a logistic regression model including the respective continuous exposure variable. Adjustment was made for age group (<45; 45–49; 50–54; 55–59; 60–64; 65–69; 70–74; 75+), sex, study, tobacco smoking (log(cigarette pack-years+1)), time since quitting smoking cigarettes (current smokers; stopping smoking 2–7 years; 8–15; 16–25; 26+ years before interview/diagnosis; never smokers), and ever employment in a ‘list A job’. Current smokers were persons that had smoked ≥1 cigarette per day for ≥1 year, and included those that had stopped smoking in the last 2 years before diagnosis/interview. The cigarette pack-year was calculated as follows: Σ duration x average intensity per day / 20. ‘List A jobs’ are occupations and industries known to present an excess risk of lung cancer as identified by Ahrens and Merletti in 1998 and updated by Mirabelli et al. in 2001.[12, 13] Study-specific ORs were explored using meta-analytic methods and visualised in a forest plot. The extent of heterogeneity between ORs was expressed as a percentage (I2). Crude ORs and ORs only adjusted for potential confounders other than smoking are provided in the online material.
We investigated the effect among never smokers and subjects who have never been employed in a ‘list A job’ separately. We repeated the analyses stratified by sex and tested possible interaction between sex and exposure to organic dust. In addition, we stratified by subjects with and without a history of non-malignant respiratory diseases in order to investigate if a possible effect of biological exposures was mediated by these diseases. Information on these diseases was only available for a subset of the studies (AUT-Munich, EAGLE, HdA, INCO and MONTREAL). We also estimated the risks for specific histological subtypes of lung cancer in the full study population. Squamous and small cell carcinomas were combined for analyses and adenocarcinoma was analysed separately. Pearson correlations were calculated to describe the correlation between organic dust exposure and its specific constituents in the population.
All analyses were performed using SAS version 9.2 software (SAS Institute Inc.), except for the meta-analysis, which was conducted in Stata v.10.2 using the procedure ‘metan’.
RESULTS
The SYNERGY population comprised 29,989 lung cancer cases and controls. Subjects providing incomplete information for calculating duration of exposure or smoking status were excluded from the analyses, leaving 29,573 subjects for analyses (13,300 cases and 16,273 controls). Table 1 shows the characteristics of this population. The mean age was 62.4 with 81% of the population being male. Most frequent subtypes of lung cancer were squamous cell carcinoma (41%), adenocarcinoma (26%), and small cell carcinoma (17%; data not shown).
Table 1.
Study population characteristics
| Overall (n=29,573) |
Cases
(n=13,300) |
Participation rate cases |
Controls
(n=16,273) |
Participation rate controls | Data collection (between yrs) | Organic dust exposure (between yrs) | Source of controls | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | 23,837 | (81%) | 10,810 | (81%) | 13,027 | (80%) | |||||
| Female | 5,736 | (19%) | 2,490 | (19%) | 3,246 | (20%) | ||||||
| Mean age (SD) | 62.4 | (±9.0) | 62.3 | (±8.9) | 62.4 | (±9.0) | ||||||
| Smoking status | Non-smoker | 5,568 | (19%) | 799 | (6%) | 4,759 | (30%) | |||||
| Former smoker | 10,193 | (34%) | 3,827 | (29%) | 6,366 | (39%) | ||||||
| Smoker | 13,801 | (47%) | 8,670 | (65%) | 5,131 | (32%) | ||||||
| Unknown/missing | 21 | (<1%) | 4 | (<1%) | 17 | (<1%) | ||||||
| Study | AUT-Munich (Germany) | 6,429 | (22%) | 3,180 | (24%) | 77% | 3,249 | (20%) | 41% | 1990–1995 | 1931–1995 | Population |
| EAGLE (Italy) | 4,000 | (14%) | 1,917 | (14%) | 87% | 2,083 | (13%) | 72% | 2002–2005 | 1931–2005 | Population | |
| HdA (Germany) | 2,006 | (7%) | 1,004 | (8%) | 69% | 1,002 | (6%) | 68% | 1988–1993 | 1926–1993 | Population | |
| INCO (Czech Rep.) | 756 | (3%) | 304 | (2%) | 94% | 452 | (3%) | 80% | 1998–2002 | 1937–2002 | Hospital | |
| INCO (Hungary) | 696 | (2%) | 391 | (3%) | 90% | 305 | (2%) | 100% | 1998–2001 | 1931–1999 | Hospital | |
| INCO (Poland) | 1,628 | (6%) | 793 | (6%) | 88% | 835 | (5%) | 88% | 1999–2002 | 1937–2001 | Hospital + population | |
| INCO (Romania) | 404 | (1%) | 179 | (1%) | 90% | 225 | (1%) | 99% | 1998–2001 | 1936–2002 | Hospital | |
| INCO (Russia) | 1,179 | (4%) | 599 | (5%) | 96% | 580 | (4%) | 90% | 1998–2000 | 1932–2003 | Hospital | |
| INCO (Slovakia) | 630 | (2%) | 345 | (3%) | 90% | 285 | (2%) | 84% | 1998–2002 | 1936–2002 | Hospital | |
| INCO (UK) | 1,359 | (5%) | 442 | (3%) | 78% | 917 | (6%) | 84% | 1998–2005 | 1933–2003 | Population | |
| LUCA (France) | 589 | (2%) | 297 | (2%) | 98% | 292 | (2%) | 98% | 1989–1992 | 1927–1992 | Hospital | |
| LUCAS (Sweden) | 3,321 | (11%) | 1,014 | (8%) | 87% | 2,307 | (14%) | 85% | 1985–1990 | 1923–1990 | Population | |
| MONTREAL (Canada) | 2,681 | (9%) | 1,176 | (9%) | 85% | 1,505 | (9%) | 69% | 1996–2002 | 1935–2002 | Population | |
| MORGEN (NL) | 251 | (1%) | 64 | (0.5%) | NA | 187 | (1%) | NA | 1993–1997 | 1945–1997 | Population | |
| PARIS (France) | 396 | (1%) | 169 | (1%) | 95% | 227 | (1%) | 95% | 1988–1992 | 1924–1992 | Hospital | |
| ROME (Italy) | 652 | (2%) | 328 | (3%) | 74% | 324 | (2%) | 63% | 1993–1996 | 1926–1996 | Hospital | |
| TURIN/VENETO (Italy) | 2,596 | (9%) | 1,098 | (8%) | 79% | 1,498 | (9%) | 80% | 1990–1994 | 1922–1994 | Population | |
Table 2 shows the ORs for lung cancer associated with cumulative exposure to organic dust, endotoxin, and contact with animals. Occupational exposure to organic dust was associated with an increase in lung cancer risk. The second to fourth quartile of cumulative exposure showed significant ORs ranging from 1.12 to 1.24 in a dose dependent matter (p-value for trend <.001). The observed trend remained significant even after excluding the never exposed from the analyses (p-value for trend 0.001; data not shown). Results were similar for exposure specifically to endotoxin, but only the two highest quartiles showed significantly increased risk estimates. Exposure to endotoxin was moderately correlated with exposure to organic dust (Pearson’s correlation=0.68; p<.001) and the effect of exposure to endotoxin disappeared when both organic dust and endotoxin were in the statistical model. Nor was there an effect observed when exposure to endotoxin was analysed within subjects exposed to organic dust (data not shown). Contact with animals showed no excess of lung cancer risk except for the highest exposure category (OR 1.15; 95% CI 1.00–1.32). This finding disappeared when both organic dust and contact with animals were in the statistical model.
Table 2.
Biological exposures and lung cancer risk
| Exposure | Cumulative exposure | Cases | Controls | OR* | 95% CI |
|---|---|---|---|---|---|
| Organic dust | Never | 6,487 | 8,940 | 1.00 | Reference |
| 1st Quartile | 1,549 | 1,770 | 1.05 | 0.96–1.15 | |
| 2nd Quartile | 1,875 | 1,914 | 1.15 | 1.06–1.25 | |
| 3rd Quartile | 1,634 | 1,804 | 1.12 | 1.03–1.22 | |
| 4th Quartile | 1,755 | 1,845 | 1.24 | 1.14–1.35 | |
| Test for trend, p-value† | <.001 | ||||
| Endotoxin | Never | 8,509 | 11,305 | 1.00 | Reference |
| 1st Quartile | 1,241 | 1,276 | 1.08 | 0.98–1.19 | |
| 2nd Quartile | 1,113 | 1,175 | 1.08 | 0.98–1.20 | |
| 3rd Quartile | 1,230 | 1,268 | 1.12 | 1.02–1.23 | |
| 4th Quartile | 1,207 | 1,249 | 1.23 | 1.12–1.36 | |
| Test for trend, p-value† | <.001 | ||||
| Contact with animals | Never | 11,158 | 14,001 | 1.00 | Reference |
| 1st Quartile | 661 | 627 | 1.10 | 0.97–1.26 | |
| 2nd Quartile | 463 | 512 | 1.05 | 0.90–1.21 | |
| 3rd Quartile | 488 | 563 | 1.07 | 0.93–1.24 | |
| 4th Quartile | 530 | 570 | 1.15 | 1.00–1.32 | |
| Test for trend, p-value† | 0.081 | ||||
ORs are adjusted for age, sex, study, cigarette pack-years, time-since-quitting smoking, and ever employment in a ‘list A job’
P-values for trend result from logistic regression model with exposure as continuous variable
When analyses were based on duration of exposure only, we observed a positive association with duration of occupational exposure to organic dust and lung cancer risk. This was found for ever exposure, only low exposure, and high exposure (data not shown). The observed association between ever occupational exposure to organic dust and lung cancer risk differed significantly between studies with ORs ranging from 0.92 (EAGLE) to 1.85 (MORGEN) and a summary risk estimate of 1.12 (95% CI 1.02–1.24) (Figure 1; I2=58.6% (p for heterogeneity=0.001)).
Figure 1.
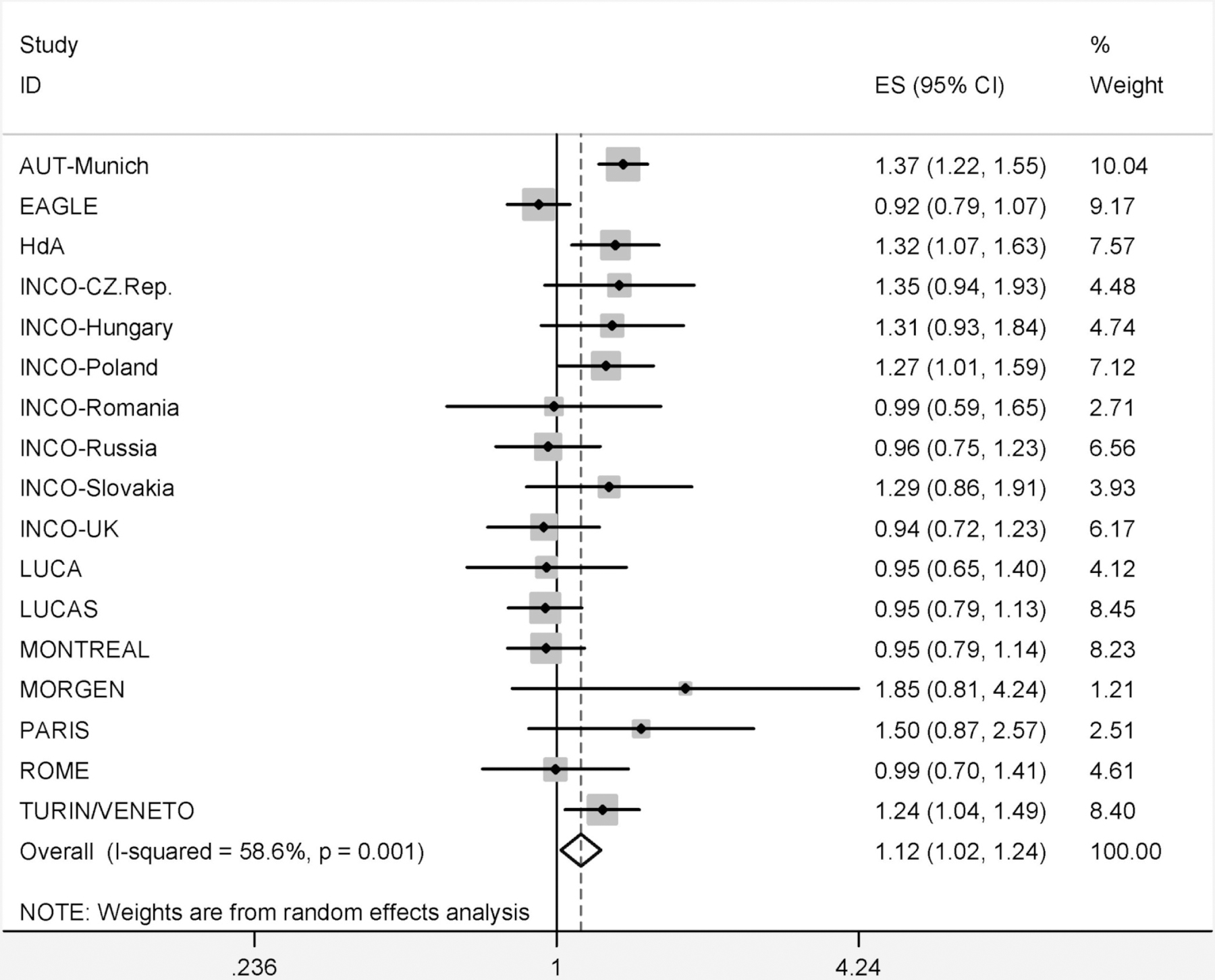
Forest plot showing study specific ORs (ES) and 95% confidence intervals (95%-CI) for organic dust exposure and lung cancer, adjusted for age, sex, cigarette pack-years, time since quitting smoking, and ever employment in a ‘list A job’. Weight indicates the study specific statistical weight based on a random effects model.
Lung cancer risk associated with occupational exposure to organic dust was stratified by subgroups in table 3. Results showed the same pattern as observed for the full population when restricted to subjects who never worked in a ‘list A job’. Confidence intervals were wide for analyses among never smokers due to small numbers. No clear monotonic trends between occupational exposure to organic dust and lung cancer were observed in these subgroups. Risk estimates were essentially equivalent for women and men (p-value for interaction between sex and exposure to organic dust =0.23; data not shown). For part of the population with information on previous non-malignant respiratory diseases (n=21,655), the analyses for exposure to organic dust are shown in table 4. Regardless of the history of COPD or asthma, the risk of lung cancer due to occupational exposure to organic dust was increased albeit that the association seemed stronger among subjects with a history of COPD or asthma (p-value for interaction=0.12). Analyses on specific histological subtypes of lung cancer showed that the increased risk of organic dust was limited to squamous and small cell carcinoma (table 5). When restricted to never smokers, the same pattern was observed.
Table 3.
Exposure to organic dust and lung cancer risk
| Subjects | Cumulative exposure to organic dust | Cases | Controls | OR* | 95% CI |
|---|---|---|---|---|---|
| All | Never | 6,487 | 8,940 | 1.00 | Reference |
| 1st Quartile | 1,549 | 1,770 | 1.05 | 0.96–1.15 | |
| 2nd Quartile | 1,875 | 1,914 | 1.15 | 1.06–1.25 | |
| 3rd Quartile | 1,634 | 1,804 | 1.12 | 1.03–1.22 | |
| 4th Quartile | 1,755 | 1,845 | 1.24 | 1.14–1.35 | |
| Test for trend, p-value† | <.001 | ||||
| Never smokers | Never | 487 | 2,971 | 1.00 | Reference |
| 1st Quartile | 105 | 498 | 0.98 | 0.76–1.26 | |
| 2nd Quartile | 128 | 576 | 1.07 | 0.84–1.35 | |
| 3rd Quartile | 129 | 540 | 1.20 | 0.95–1.51 | |
| 4th Quartile | 115 | 592 | 0.98 | 0.76–1.25 | |
| Test for trend, p-value† | 0.440 | ||||
| Workers never employed in a ‘list A job’ | Never | 6,018 | 8,598 | 1.00 | Reference |
| 1st Quartile | 1,335 | 1,586 | 1.06 | 0.97–1.17 | |
| 2nd Quartile | 1,607 | 1,694 | 1.19 | 1.09–1.30 | |
| 3rd Quartile | 1,397 | 1,604 | 1.14 | 1.04–1.24 | |
| 4th Quartile | 1,576 | 1,706 | 1.25 | 1.14–1.36 | |
| Test for trend, p-value† | <.001 | ||||
ORs are adjusted for age, sex, study, cigarette pack-years, time-since-quitting smoking (where appropriate), and ever employment in a ‘list A job’
P-values for trend result from logistic regression model with exposure as continuous variable
Table 4.
Association between lung cancer risk and exposure to organic dust, stratified by history of COPD or asthma
| Exposure to organic dust | Overall | No history of COPD or asthma | With history of COPD or asthma |
|---|---|---|---|
| N=21,655* | N=16,737 | N=4,918 | |
| OR † (95% CI) | OR † (95% CI) | OR † (95% CI) | |
| Never | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 1st Quartile | 1.08 (0.98–1.19) | 1.09 (0.86–1.22) | 1.04 (0.85–1.28) |
| 2nd Quartile | 1.12 (1.02–1.23) | 1.06 (0.95–1.18) | 1.32 (1.08–1.62) |
| 3rd Quartile | 1.12 (1.01–1.24) | 1.09 (0.97–1.22) | 1.27 (1.02–1.58) |
| 4th Quartile | 1.32 (1.19–1.46) | 1.27 (1.13–1.42) | 1.49 (1.18–1.87) |
| Test for trend, p-value‡ | <.001 | <.001 | <.001 |
Subset of total study population for which information on non-malignant respiratory diseases was available
ORs are adjusted for age, sex, study, cigarette pack-years, time-since-quitting smoking, and ever employment in a ‘list A job’
P-values for trend result from logistic regression model with exposure as continuous variable
Table 5.
Association between lung cancer risk and exposure to organic dust, stratified by histology
| Exposure to organic dust | Controls | Squamous and small cell carcinoma | Adenocarcinoma | |||
|---|---|---|---|---|---|---|
| N | N | OR* (95% CI) | N | OR* (95% CI) | ||
| Total population | Never | 8,940 | 3,520 | 1.00 (ref) | 1,816 | 1.00 (ref) |
| 1st Quartile | 1,770 | 868 | 1.10 (0.99–1.22) | 382 | 0.92 (0.81–1.05) | |
| 2nd Quartile | 1,914 | 1,115 | 1.25 (1.13–1.38) | 455 | 1.00 (0.88–1.13) | |
| 3rd Quartile | 1,804 | 996 | 1.23 (1.11–1.36) | 392 | 1.01 (0.88–1.15) | |
| 4th Quartile | 1,845 | 1,146 | 1.39 (1.26–1.54) | 347 | 0.97 (0.84–1.11) | |
| Test for trend, p-value† | <.001 | 0.950 | ||||
| Never smokers | Never | 2,971 | 144 | 1.00 (ref) | 251 | 1.00 (ref) |
| 1st Quartile | 498 | 36 | 1.17 (0.78–1.76) | 44 | 0.76 (0.54–1.09) | |
| 2nd Quartile | 576 | 41 | 1.18 (0.80–1.75) | 54 | 0.84 (0.60–1.16) | |
| 3rd Quartile | 540 | 47 | 1.50 (1.04–2.16) | 57 | 0.95 (0.69–1.31) | |
| 4th Quartile | 592 | 44 | 1.16 (0.78–1.75) | 51 | 0.87 (0.61–1.21) | |
| Test for trend, p-value† | 0.106 | 0.819 | ||||
ORs are adjusted for age, sex, study, pack-years, time-since-quitting smoking (where appropriate), and ever employment in a ‘list A job’
P-values for trend result from logistic regression model with exposure as continuous variable
DISCUSSION
Our results have shown that occupational exposure to organic dust is associated with an increased risk of lung cancer. These results were consistent among people who never worked in a ‘list A job’ and in subjects with or without a history of COPD or asthma. Subgroup analyses among never smokers showed evidence of an increased risk, but numbers were small precluding meaningful analyses of trends between occupational exposure to organic dust and lung cancer. No protective effect from exposure to endotoxin was observed. Additionally, having had contact with animals or animal products showed no effect on lung cancer risk.
Endotoxin has been proposed to be protective for the development of lung cancer. This hypothesis is mainly based on studies performed among two occupational groups, namely farmers and cotton-textile workers. In a recent meta-analysis a decreased lung cancer risk was found, with risk estimates of 0.62 (95% CI 0.52–0.75) for agricultural workers and 0.72 (95% CI 0.57–0.90) for cotton textile workers.[6] However, many of these studies did not adequately correct for smoking, which might have resulted in residual confounding. Our study has very detailed information on smoking and as such would be better equipped to correct for smoking habits. We did not observe a protective effect of exposure to endotoxin on the risk of lung cancer with or without correction for smoking habits. Interestingly, when we looked at the risk of lung cancer associated with ever being a farmer, an increased risk was observed (OR=1.13 (95% CI 1.04–1.22)). This would suggest that exposure misclassification is probably not the main explanation for the lack of an association between endotoxin and lung cancer risk. As such this large pooled case-control study on lung cancer does not seem to support the previous findings, especially among farmers, on a possible protective effect of endotoxin.
For textile workers, no effect was found in our study (OR=1.02 (95% CI 0.87–1.20)), but we have to recognize that we were not able to separate cotton textile workers from non-cotton textile workers in our study population because of the job coding used. Working with cotton will have lead to potentially high endotoxin exposure levels while other textile workers will not have been exposed to endotoxin. As such we cannot exclude that the absence of a protective effect among textile workers is caused by the inability to separate cotton from non-cotton textile workers in these analyses.
No association was found between contact with animals or animal products and lung cancer. Additional analyses showed the same result when restricted to contact with living animals only or ever having worked as a meat processor (OR=1.00 (95% CI 0.80–1.25)). Previous studies among meat processors, reviewed by McLean and colleagues, contained no smoking data, but the observed elevated risk of lung cancer among people occupationally exposed to meat or meat products was assumed to be greater than that which could be attributed to smoking.[4] After adjusting for smoking, the current analyses do not support these previous findings.
The finding that lung cancer risk is possibly associated with occupational exposure to organic dust is of interest. Given the complex and diverse nature of organic dust, however, more in depth analyses should be performed to identify specific constituents that might be related to the observed increased risk. Farmers and several types of woodworkers represented the majority (52%) of the individuals with high exposure to organic dust. Wood dust is classified by IARC as carcinogenic to humans (group 1), which is based on increased risks for cancer of the nasal cavities and paranasal sinuses.[14] Evidence for lung cancer is currently inconsistent.[5, 15] When we looked in more detail to workers exposed to wood dust (i.e. loggers, sawyers, cabinetmakers, and carpenters), we saw an increased lung cancer risk among subjects ever employed as a woodworker, with an OR of 1.19 (95% CI 1.07–1.32). This might suggest that wood dust is one of the components of organic dust responsible for the observed excess risk of lung cancer. As is the case for sinonasal cancer, there might be differences in carcinogenic potency between softwood and hardwood dust, as well as regarding histological subtype of lung cancer.[14] This will need further investigation. However, when woodworkers were eliminated from the analyses, an increased risk of lung cancer due to exposure to organic dust was still found. This indicates that other constituents may also be responsible for the observed risk.
Risk estimates were heterogeneous across studies, as shown in figure 1. We explored possible reasons for the observed heterogeneity. The heterogeneity was not driven by one study or region in particular. There was no trend in obtained risk estimates for rural versus urban study populations, nor was there a correlation between the occupational exposure prevalence to organic dust and the observed risk by studies. For example, the highest exposure prevalence was observed for the INCO-UK and HdA study from Germany (59% of the controls were ever exposed in both studies), but their risk estimates were 0.94 and 1.32, respectively. The lowest exposure prevalence was for INCO-Romania (32%), LUCAS from Sweden and INCO-Slovakia (both 33%) while risk estimates in these studies were also on different sides of the reference value of 1. The observed heterogeneity in risk estimates did not change after excluding farmers from the analyses (data not shown). As such, the observed heterogeneity does not seem to be driven by quantitative and/or qualitative differences in organic dust exposure although this cannot be fully excluded. When comparing lung cancer incidence rates for the different countries,[16] no pattern was observed with regard to the observed lung cancer risks.
Occupational exposure to organic dust is a known risk factor for COPD and asthma,[1–3, 17] and these diseases have shown to be related to an increased lung cancer risk.[18–20] Adjustment for COPD and asthma did not affect the observed risk estimates for occupational exposure to organic dust. When stratified by history of COPD or asthma, the association with exposure to organic dust remained in both strata albeit that the association was stronger among subjects with a history of COPD or asthma. These findings indicate that if lung cancer is associated with occupational exposure to organic dust, the association is not mediated by COPD or asthma only.
When analysing the main histological subtypes of lung cancer separately it appeared that the increased risk was only observed for squamous and small cell carcinomas and not for adenocarcinoma. It therefore seems that the effect of organic dust is limited to the more central part of the lung. This pattern is similar to those reported for smoking of non-filter cigarettes [21, 22] and might indicate that the observed effect is associated with relatively larger dust particles that do not reach the peripheral parts of the lung. The observation of similar risk estimates among never smokers points towards a possible association between organic dust and lung cancer risk, instead of residual confounding by smoking.
In conclusion, occupational exposure to organic dust appears to be associated with an increased risk of lung cancer.
Supplementary Material
Key messages.
What is the key question:
Is there an association between occupational exposure to organic dust and its specific constituents and lung cancer risk in the general population?
What is the bottom line:
Occupational exposure to organic dust was associated with an increased risk of lung cancer, while no effect was observed for endotoxin exposure or contact with animals or animal products.
Why read on:
Extensive information regarding smoking habits was available for the SYNERGY population of about 30,000 subjects, providing the opportunity to appropriately adjust for possible differences in tobacco consumption.
Acknowledgements
The authors wish to thank Veronique Luzon at IARC and Isabelle Gross at IPA for the data management, and Professor Franco Merletti and George Downward for their helpful comments on the manuscript.
Funding
The SYNERGY project is funded by the German Social Accident Insurance (DGUV), and is coordinated by the International Agency for Research on Cancer (IARC), the Institute for Prevention and Occupational Medicine of the DGUV, Institute of the Ruhr-University Bochum (IPA) and the Institute for Risk Assessment Sciences (IRAS) at Utrecht University.
Footnotes
Competing interests
None
References
- 1.Lacey J and Dutkiewicz J. Bioaerosols and occupational lung disease. J Aerosol Sci 1994;25:1371–404. [Google Scholar]
- 2.Douwes J, Thorne P, Pearce N, et al. Bioaerosol health effects and exposure assessment: progress and prospects. Ann.Occup.Hyg 2003;47:187–200. [DOI] [PubMed] [Google Scholar]
- 3.Matheson MC, Benke G, Raven J, et al. Biological dust exposure in the workplace is a risk factor for chronic obstructive pulmonary disease. Thorax 2005;60:645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLean D and Pearce N. Cancer among meat industry workers. Scand.J.Work Environ.Health 2004;30:425–37. [DOI] [PubMed] [Google Scholar]
- 5.Barcenas CH, Delclos GL, El-Zein R, et al. Wood dust exposure and the association with lung cancer risk. Am.J.Ind.Med 2005;47:349–57. [DOI] [PubMed] [Google Scholar]
- 6.Lenters V, Basinas I, Beane-Freeman L, et al. Endotoxin exposure and lung cancer risk: a systematic review and meta-analysis of the published literature on agriculture and cotton textile workers. Cancer Causes Control 2010;21:523–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liebers V, Raulf-Heimsoth M and Bruning T. Health effects due to endotoxin inhalation (review). Arch.Toxicol 2008;82:203–10. [DOI] [PubMed] [Google Scholar]
- 8.Olsson A, Gustavsson P, Kromhout H, et al. Exposure to diesel motor exhaust and lung cancer risk in a pooled analysis from case-control studies in Europe and Canada. Am. J. Respir. Crit. Care Med 2011;183:941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Labour Office. International Standard Classification of Occupations 1968;
- 10.Peters S, Vermeulen R, Cassidy A, et al. Comparison of exposure assessment methods for occupational carcinogens in a multi-centre lung cancer case-control study. Occup.Environ.Med 2011;68:148–53. [DOI] [PubMed] [Google Scholar]
- 11.Stewart PA and Herrick RF. Issues in Performing Retrospective Exposure Assessment. Appl Occup Environ Hyg 1991;6:421–7. [Google Scholar]
- 12.Ahrens W and Merletti F. A standard tool for the analysis of occupational lung cancer in epidemiologic studies. Int.J.Occup.Environ.Health 1998;4:236–40. [DOI] [PubMed] [Google Scholar]
- 13.Mirabelli D, Chiusolo M, Calisti R, et al. Database of occupations and industrial activities that involve the risk of pulmonary tumors. Epidemiol.Prev 2001;25:215–21. [PubMed] [Google Scholar]
- 14.Straif K, Benbrahim-Tallaa L, Baan R, et al. A review of human carcinogens--part C: metals, arsenic, dusts, and fibres. Lancet Oncol 2009;10:453–4. [DOI] [PubMed] [Google Scholar]
- 15.Demers PA, Boffetta P, Kogevinas M, et al. Pooled reanalysis of cancer mortality among five cohorts of workers in wood-related industries. Scand.J.Work Environ.Health 1995;21:179–90. [DOI] [PubMed] [Google Scholar]
- 16.Tyczynski JE, Bray F and Parkin DM. Lung cancer in Europe in 2000: epidemiology, prevention, and early detection. Lancet Oncol 2003;4:45–55. [DOI] [PubMed] [Google Scholar]
- 17.Toren K, Zock JP, Kogevinas M, et al. An international prospective general population-based study of respiratory work disability. Thorax 2009;64:339–44. [DOI] [PubMed] [Google Scholar]
- 18.Koshiol J, Rotunno M, Consonni D, et al. Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case-control study. PLoS One 2009;4:e7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H and Diepgen TL. Is atopy a protective or a risk factor for cancer? A review of epidemiological studies. Allergy 2005;60:1098–111. [DOI] [PubMed] [Google Scholar]
- 20.Purdue MP, Gold L, Jarvholm B, et al. Impaired lung function and lung cancer incidence in a cohort of Swedish construction workers. Thorax 2007;62:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morabia A and Wynder EL. Cigarette smoking and lung cancer cell types. Cancer 1991;68:2074–8. [DOI] [PubMed] [Google Scholar]
- 22.Yang CP, Gallagher RP, Weiss NS, et al. Differences in incidence rates of cancers of the respiratory tract by anatomic subsite and histologic type: an etiologic implication. J.Natl.Cancer Inst 1989;81:1828–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


