Abstract
Purpose
Trilaciclib is an intravenous cyclin-dependent kinase 4/6 inhibitor indicated to decrease the incidence of chemotherapy-induced myelosuppression (CIM) by protecting hematopoietic stem and progenitor cells and immune system function from chemotherapy-induced damage (myeloprotection). Here, we investigated the myeloprotective effects of trilaciclib among patients at increased risk of CIM.
Patients and Methods
Data were pooled from three randomized, double-blind, placebo-controlled, phase 2 clinical studies of trilaciclib administered prior to chemotherapy in patients with extensive-stage small cell lung cancer (ES-SCLC). Myeloprotective outcomes were evaluated in patient subgroups based on age (<65 or ≥65 years), risk of chemotherapy-induced febrile neutropenia (FN), and risk of anemia or red blood cell (RBC) transfusions. For the FN and anemia analyses, risk factors were identified from published literature and used to classify patients into FN and anemia risk categories. Subgroup analysis based on age was also performed on patient reported outcome (PRO) measures.
Results
In total, 123 patients received trilaciclib and 119 patients received placebo. Myeloprotective benefits of trilaciclib were observed regardless of age, with greater effects observed among patients aged ≥65 years. Across FN risk factors and categories, trilaciclib had beneficial effects on neutrophil-related endpoints vs placebo, with greater effects observed in patients at higher risk of FN. Effects on RBC-related endpoints favored trilaciclib vs placebo, regardless of anemia risk factors and categories. Improvements in PROs with trilaciclib were observed irrespective of age group, but with greater improvements and less deterioration from baseline observed in older patients.
Conclusion
By both decreasing the incidence of CIM and improving quality of life, trilaciclib has the potential to allow patients receiving chemotherapy for ES-SCLC, including patients who are older or more vulnerable to CIM, to receive chemotherapy on schedule and at standard-of-care doses, and to improve the experience for patients receiving chemotherapy to treat ES-SCLC.
Clinical Trial Numbers
Keywords: trilaciclib, myelosuppression, myeloprotection, myelopreservation, chemotherapy, small cell lung cancer
Introduction
Standard-of-care chemotherapy regimens for small cell lung cancer (SCLC) are associated with clinically significant, multilineage myelosuppression that commonly manifests as neutropenia, anemia, and thrombocytopenia.1 Chemotherapy-induced myelosuppression (CIM) is associated with serious complications, including an increased risk of infection, sepsis, bleeding, and fatigue, and a reduction in quality of life (QoL).2 CIM is often managed with chemotherapy dose delays or reductions, which can reduce the dose intensity of chemotherapy and potentially impair therapeutic efficacy, and with supportive care interventions such as growth factors and blood cell transfusions.3–6 CIM and its management result in a substantial economic burden on the health care system, while also imposing a humanistic burden on patients and their caregivers.2,7
Older patients, who are more likely to have comorbid health conditions such as hypertension, cardiac disease, and diabetes are particularly vulnerable to the consequences of CIM.8–13 This is especially relevant in SCLC, wherein more than half of patients are aged ≥65 years at diagnosis, and patients often present as smokers with multiple comorbidities.8,10 The risk of developing chemotherapy-induced severe neutropenia (SN) or febrile neutropenia (FN) is increased by a number of patient characteristics, including older age, poor performance status, poor nutritional status (hypoalbuminemia), renal dysfunction, cardiovascular disease, chronic obstructive pulmonary disease, autoimmune disease, multiple comorbid conditions, and history of previous cytotoxic chemotherapy.14–20 In addition, the risk of chemotherapy-induced anemia and/or red blood cell (RBC) transfusions is reported to be increased in females and by older age, poor performance status, low baseline hemoglobin, and prior cytotoxic chemotherapy.21–25
The need to improve the safety of chemotherapy in patient populations who are older or more vulnerable to CIM is increasingly recognized as an important clinical issue. Owing to an increased risk of complications and heightened need for supportive care, the economic cost of cytotoxic chemotherapy appears to be higher in older and high-risk patients.9 This underscores the need to explore strategies that minimize the toxicities and costs of cytotoxic chemotherapy without compromising its effectiveness.
Trilaciclib is an intravenous cyclin-dependent kinase 4/6 inhibitor that protects hematopoietic stem and progenitor cells and immune system function from chemotherapy-induced damage (myeloprotection or myelopreservation). When administered prior to chemotherapy, trilaciclib transiently arrests bone marrow hematopoietic stem and progenitor cells in the G1 phase of the cell cycle during chemotherapy exposure, thus protecting them from the cytotoxic effects of chemotherapy.26,27 Trilaciclib is indicated to decrease the incidence of CIM in patients with extensive-stage SCLC (ES-SCLC), on the basis of data from three independent, phase 2, randomized, double-blind, placebo-controlled clinical studies.28–30 Analyses of the individual studies showed that the addition of trilaciclib to chemotherapy significantly reduced the percentage of patients with SN and the duration of SN, and reduced grade 3/4 hematologic toxicity (as measured by laboratory data and adverse events), resulting in fewer supportive care interventions, including RBC and platelet transfusions, and dose reductions.28–30 In addition, pooled data from these three studies showed that trilaciclib reduced CIM, while also reducing the need for supportive care interventions and improving QoL.31,32
In the present analysis, the myeloprotective effects of trilaciclib were evaluated in patients with ES-SCLC who were at increased risk of CIM.
Methods
Study Design
This retrospective analysis used pooled data from three phase 2, randomized, double-blind, placebo-controlled clinical studies (G1T28-05, NCT03041311; G1T28-02 [part 2 only], NCT02499770; and G1T28-03 [part 2 only], NCT02514447) that evaluated the myeloprotective effects of trilaciclib administered prior to chemotherapy in patients with ES-SCLC.28–30 In studies G1T28-05 and G1T28-02, respectively, patients with newly diagnosed, treatment-naive ES-SCLC received trilaciclib or placebo administered within 4 h prior to first-line treatment with carboplatin, etoposide and atezolizumab, or carboplatin and etoposide. In study G1T28-03, patients had received prior systemic treatment for ES-SCLC and were administered trilaciclib or placebo prior to topotecan in the second- or third-line setting. A list of investigational sites is provided in the Supplementary Material.
Definitions for Patients at High Risk of CIM
The myeloprotective effects of trilaciclib compared with placebo were evaluated in patient subgroups based on age (<65 and ≥65 years), risk factors for developing FN (including age ≥65 years), and risk factors for grade 3/4 decrease in hemoglobin (anemia) or needing an RBC transfusion.
To identify the risk factors for FN, published guidelines (including those of the American Society of Clinical Oncology, the European Organisation for Research and Treatment of Cancer, the National Comprehensive Cancer Network, and the European Society for Medical Oncology) on the use of hematopoietic growth factors to reduce the incidence of FN in patients with cancer receiving chemotherapy were reviewed.14–18 Six baseline factors (or conditions) associated with an increased risk of FN that were described in more than one set of guidelines were included as subgroups to evaluate their impact on the effect of trilaciclib on neutrophil-related endpoints: (1) age group (≥65 years); (2) poor nutritional status (baseline albumin <3.5 g/dL); (3) renal dysfunction; (4) cardiovascular disease; (5) multiple comorbid conditions; and (6) previous cytotoxic chemotherapy (study G1T28-03 only). Some factors identified using this approach were not included for the following reasons: (1) advanced stage of disease because all patients enrolled in the included studies had ES-SCLC; (2) HIV infection because patients with HIV infection were not eligible for enrollment; (3) preexisting neutropenia or bone marrow involvement with tumor because most patients were treated in the first-line setting and the relevant data were not collected; and (4) open wounds or recent surgery because ES-SCLC is not a surgical disease, so no patients were predicted to fall into this category. Full details of the criteria used to define risk factor subgroups for FN are provided in the Supplementary Material. To account for patients having more than one risk factor at baseline, patients were classified into four FN risk categories: no risk; 1/2 risk factors; 3/4 risk factors; and 5/6 risk factors.
To identify the risk factors for anemia and/or the use of RBC transfusions for treating anemia in patients with cancer undergoing chemotherapy treatment, a review of the published literature was performed.21–25 Risk factors identified in more than one publication were included as subgroups to evaluate their impact on RBC-related endpoints. Four baseline risk factors associated with an increased risk of anemia that were described in more than one publication were identified: (1) female sex; (2) Eastern Cooperative Oncology Group performance status (ECOG PS) of 2; (3) baseline hemoglobin <12 g/dL; and (4) prior cytotoxic chemotherapy (study G1T28-03 only). Having lung cancer was the only category identified in the literature review that was not included because all the patients in the included studies had lung cancer. To account for patients having more than one risk factor at baseline, patients were classified into three anemia risk categories: no risk; 1/2 risk factors; and 3/4 risk factors.
Myeloprotection Endpoints
The impact of age, and of FN risk factors and categories on the effects of trilaciclib vs placebo on the neutrophil-related endpoints of duration of severe (grade 4) neutropenia (DSN) in cycle 1 and the percentage of patients with SN was evaluated. SN was defined as absolute neutrophil count <0.5×109 cells/L. The impact of age, and of anemia risk factors and categories on the effects of trilaciclib vs placebo on RBC-related endpoints (percentage of patients with grade 3/4 anemia [defined as at least one hemoglobin value <8.0 g/dL during the treatment period], percentage of patients with RBC transfusions on/after week 5 of treatment, and total number of RBC transfusions on/after week 5) was assessed. RBC transfusions before week 5 were excluded to ensure that analyses of potential benefit were not confounded by the residual effect of previous treatment.
Patient-Reported Outcomes
The impact of age (<65 and ≥65 years) on the effects of trilaciclib vs placebo on patients’ QoL was evaluated on five patient-reported outcome (PRO) endpoints derived from the validated Functional Assessment of Cancer Therapy—Anemia (FACT-An) questionnaire to assess specific QoL concerns related to anemia and fatigue in patients receiving chemotherapy: physical wellbeing (PWB), functional wellbeing (FWB), fatigue subscale (fatigue), anemia trial outcome index (anemia TOI), and FACT-An total scores. Confirmed deterioration in PROs was based on thresholds of within-patient change per the literature33–36 and was defined as a decrease from baseline by a clinically meaningful threshold for two consecutive visits (ie, decrease from baseline of ≥3 points for PWB, FWB, and fatigue; ≥6 points for anemia TOI; and ≥7 points for FACT-An total scores).
Statistical Analyses
Data from the three trials were pooled and analyses conducted using intention-to-treat (ITT) principles. To account for potential variability among patients and studies when assessing the effects of trilaciclib vs placebo on subgroups, ECOG PS (0/1 or 2), presence of brain metastases (yes or no), and study (G1T28-05, G1T28-02, or G1T28-03) were used as common factors in all statistical models. Corresponding baseline values were included as covariates where appropriate. A chi-square test was used to assess differences in the patient distribution at baseline across the FN and anemia risk categories and the treatment groups.
To evaluate the impact of age, FN risk factors, and FN risk categories on the effect of trilaciclib on neutrophil-related endpoints, subgroup analyses were performed for DSN in cycle 1 and occurrence of SN. Treatment effect on DSN in cycle 1 was evaluated using a non-parametric analysis of covariance model, with mean group difference (trilaciclib minus placebo) and its 95% confidence interval (CI) generated using a Satterthwaite t-test. For the percentage of patients with SN and other binary endpoints, a modified Poisson model37 was used, and the adjusted relative risk (aRR) for trilaciclib vs placebo, its 95% CI, and 2-sided P-value were generated for each subgroup, controlling for the three factors mentioned above. The impact of a specific risk factor on the treatment outcome for occurrence of SN in the ITT population was assessed by testing the treatment-by-risk-factor interaction, using the modified Poisson model described above with additional terms of subgroup and treatment-by-subgroup interaction. Testing for interaction using the non-parametric analysis of covariance model was not permitted; therefore, no interaction analysis was performed for DSN in cycle 1. Forest plots were generated to show the mean difference (95% CI) for DSN in cycle 1 and the relative risk reduction (RRR [95% CI]) for the percentage of patients with SN. The RRR (%) was calculated as (1–aRR)×100, and its 95% lower and upper bound obtained as 1 minus the 95% upper and lower bound for the aRR, respectively. To evaluate the impact of age, risk factors and risk categories on the number of RBC transfusions on/after week 5, treatment group difference (trilaciclib vs placebo) was assessed using a negative binomial regression model, and the aRR, its 95% CI, and 2-sided P-value were generated.
For PRO endpoints, change from baseline to the end of a cycle (1–4) within each age group was analyzed using a mixed-effect, maximum likelihood–based, repeated measures analysis model, with treatment, ECOG PS, presence of brain metastases, study (G1T28-02, G1T28-05, or G1T28-03), and time point of measurement as fixed effects, and baseline value as a covariate. The treatment-by-age-group interaction was tested using a separate model in the ITT population, with additional terms of age group and treatment-by-age-group interaction. The Kaplan–Meier method was used to estimate the median time to confirmed deterioration (TTCD), and a Cox proportional hazard regression model with treatment, ECOG PS, presence of brain metastases, and study as fixed effects was used to estimate the hazard ratio of deterioration and its 95% CI. Treatment-by-age-group interaction was tested using a separate Cox proportional hazard model that contained the fixed terms mentioned above, with the additional terms of age group and treatment-by-age-group interaction. The treatment-by-subgroup interaction was considered to be statistically significant if the interaction P-value was greater than 0.20 for all interaction testing.
Results
Patients
Overall, 123 patients received trilaciclib prior to chemotherapy, and 119 patients received placebo prior to chemotherapy. As described previously,31 patient demographics and baseline disease characteristics were generally comparable between the treatment groups, although there was a slightly higher proportion of male patients and current smokers in the trilaciclib group vs the placebo group. Overall, 127 patients were aged <65 years (trilaciclib, n=66 [53.7%]; placebo, n=61 [51.3%]), and 115 were aged ≥65 years (trilaciclib, n=57 [46.3%]; placebo, n=58 [48.7%]). Compared with the studies in newly diagnosed patients (G1T28-02/-05), fewer patients aged ≥65 years were enrolled in the study of trilaciclib/placebo prior to second-/third-line topotecan (G1T28-03), possibly due to concern regarding higher susceptibility to treatment toxicity. Patient distribution in FN and anemia/RBC transfusion risk categories was comparable between the two treatment groups, as indicated by nonsignificant Chi-square test results (P=0.7632 and P=0.6870, respectively) (Table 1). No patients were categorized as having 5/6 FN risk factors.
Table 1.
Distribution of Risk Factors for FN, and Anemia or RBC Transfusions by Treatment Group
| Risk Category, n (%) | Trilaciclib (n=123) | Placebo (n=119) |
|---|---|---|
| FN | ||
| No risk factors | 32 (26.0) | 35 (29.4) |
| 1/2 risk factors | 85 (69.1) | 77 (64.7) |
| 3/4 risk factors | 6 (4.9) | 7 (5.9) |
| 5/6 risk factors | 0 | 0 |
| Chi-square P-value* | 0.7632 | |
| Anemia or RBC transfusions | ||
| No risk factors | 48 (39.0) | 47 (39.5) |
| 1/2 risk factors | 68 (55.3) | 62 (52.1) |
| 3/4 risk factors | 7 (5.7) | 10 (8.4) |
| Chi-square P-value* | 0.6870 | |
Note: *Calculated to test the treatment-by-risk-category association.
Abbreviations: FN, febrile neutropenia; RBC, red blood cell.
Myeloprotection Efficacy by Subgroup
Age
Administering trilaciclib prior to chemotherapy significantly reduced most measures of CIM in the ITT population (Table 2). These findings were consistently observed across both age groups (as shown by nonsignificant treatment-by-age-group interactions for binary endpoints); however, trilaciclib appeared to have a greater magnitude of effect among patients aged ≥65 years compared with placebo.
Table 2.
Myeloprotective Effects According to Age Subgroups
| ITT Population | Age <65 Years | Age ≥65 Years | |||||
|---|---|---|---|---|---|---|---|
| Trilaciclib (n=123) | Placebo (n=119) | P-value | Trilaciclib (n=66) | Placebo (n=61) | Trilaciclib (n=57) | Placebo (n=58) | |
| Mean DSN in cycle 1, days (SD)* | 0 (1.8) | 4 (5.1) | P<0.001 | 0 (1.7) | 3 (4.5) | 0 (2.1) | 5 (5.6) |
| Patients with SN, n (%)* | 14 (11.4) | 63 (52.9) | P<0.001 | 7 (10.6) | 26 (42.6) | 7 (12.3) | 37 (63.8) |
| Treatment-by-age-group interaction P=0.3765† | |||||||
| Patients with grade 3/4 decreased hemoglobin, n (%) | 25 (20.3) | 38 (31.9) | P=0.0279 | 12 (18.2) | 16 (26.2) | 13 (22.8) | 22 (37.9) |
| Treatment-by-age-group interaction P=0.6957† | |||||||
| Patients with RBC transfusions on/after week 5, n (%) | 18 (14.6) | 31 (26.1) | P=0.0252 | 8 (12.1) | 11 (18.0) | 10 (17.5) | 20 (34.5) |
| Treatment-by-age-group interaction P=0.6791† | |||||||
| Number of RBC transfusions on/after week 5, event rate (per week) | 0.015 | 0.031 | P=0.0027 | 0.011 | 0.018 | 0.019 | 0.045 |
Notes: *Primary endpoint; two-sided P-value for treatment effect. †A nonsignificant treatment-by-age-group interaction indicates that trilaciclib benefits were comparable in both age groups.
Abbreviations: DSN, duration of severe (grade 4) neutropenia; ITT, intention-to-treat; RBC, red blood cell; SD, standard deviation; SN, severe (grade 4) neutropenia.
FN Risk Factors and Categories
Evaluation of the percentage of placebo-treated patients who had SN by FN risk factor categories indicated that, as a patient’s risk of FN increased, so did the likelihood of SN (ie, 31.4% for patients with no risk factors to 59.7% for patients with 1/2 risk factors, and 85.7% for patients with 3/4 risk factors). This observation supports the use of the selected risk factors to identify those patients at increased risk for FN.
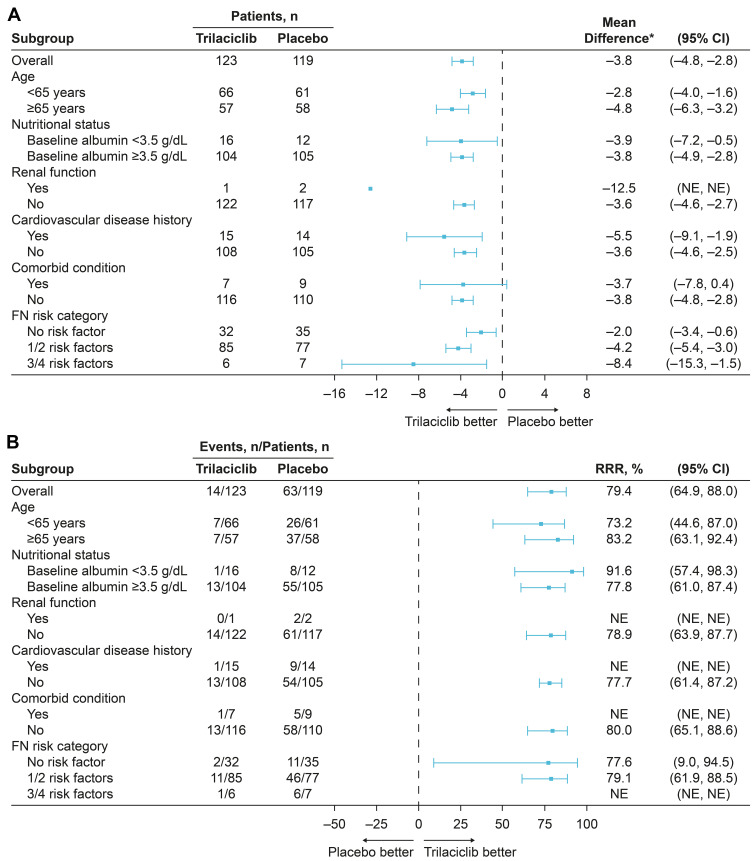
When evaluating the effects of trilaciclib by FN risk factors and categories, treatment group differences on neutrophil-related endpoints (DSN in cycle 1 and percentage of patients with SN) consistently favored trilaciclib vs placebo, with trilaciclib having greater effects relative to placebo in patients at higher risk of FN; effects were aligned with the ITT population (Table 3; Figure 1). For DSN in cycle 1, the 95% CI for the mean difference between trilaciclib and placebo was not estimable for patients with renal dysfunction because there were too few patients in that subgroup. For the percentage of patients with SN, the RRR and 95% CIs were not estimable for patients with renal dysfunction, cardiovascular disease, or comorbid conditions, or for those with 3/4 FN risk factors, owing to there being too few patients in each of the subgroups to allow the statistical model to converge. With the exception of renal dysfunction, for which the treatment-by-subgroup interaction was not estimable owing to the low number of patients, the treatment-by-subgroup interaction for each of the FN risk factors was nonsignificant, indicating no difference in treatment effects on reducing the occurrence of SN between patients with or without specific risk factors for FN.
Table 3.
FN Risk Category Results
| ITT Population | FN Risk Category | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1/2 | 3/4 | ||||||
| Trilaciclib | Placebo | Trilaciclib | Placebo | Trilaciclib | Placebo | Trilaciclib | Placebo | |
| Mean DSN in cycle 1, days (SD) | 0 (1.8) | 4 (5.1) | 0 (1.2) | 2 (3.8) | 1 (2.1) | 5 (5.1) | 0 (0.8) | 9 (7.5) |
| Patients with SN, n (%) | 14 (11.4) | 63 (52.9) | 2 (6.3) | 11 (31.4) | 11 (12.9) | 46 (59.7) | 1 (16.7) | 6 (85.7) |
Abbreviations: DSN, duration of severe (grade 4) neutropenia; FN, febrile neutropenia; ITT, intention-to-treat; SD, standard deviation; SN, severe (grade 4) neutropenia.
Figure 1.
Subgroup analysis of (A) DSN in cycle 1 and (B) percentage of patients with SN by risk factor and category.
Note: *Trilaciclib minus placebo.
Abbreviations: CI, confidence interval; DSN, duration of severe (grade 4) neutropenia; FN, febrile neutropenia; NE, not estimable (statistical model did not converge); RRR, relative risk reduction; SN, severe (grade 4) neutropenia.
Anemia Risk Factors and Categories
The percentage of placebo-treated patients who experienced at least one grade 3/4 decrease in hemoglobin increased with increasing risk of anemia (ie, from 14.9% for patients with no risk factors to 40.3% for patients with 1/2 risk factors, and 60.0% for patients with 3/4 risk factors). As for FN, this observation supports the use of the selected risk factors to identify patients at increased risk for anemia/RBC transfusions.
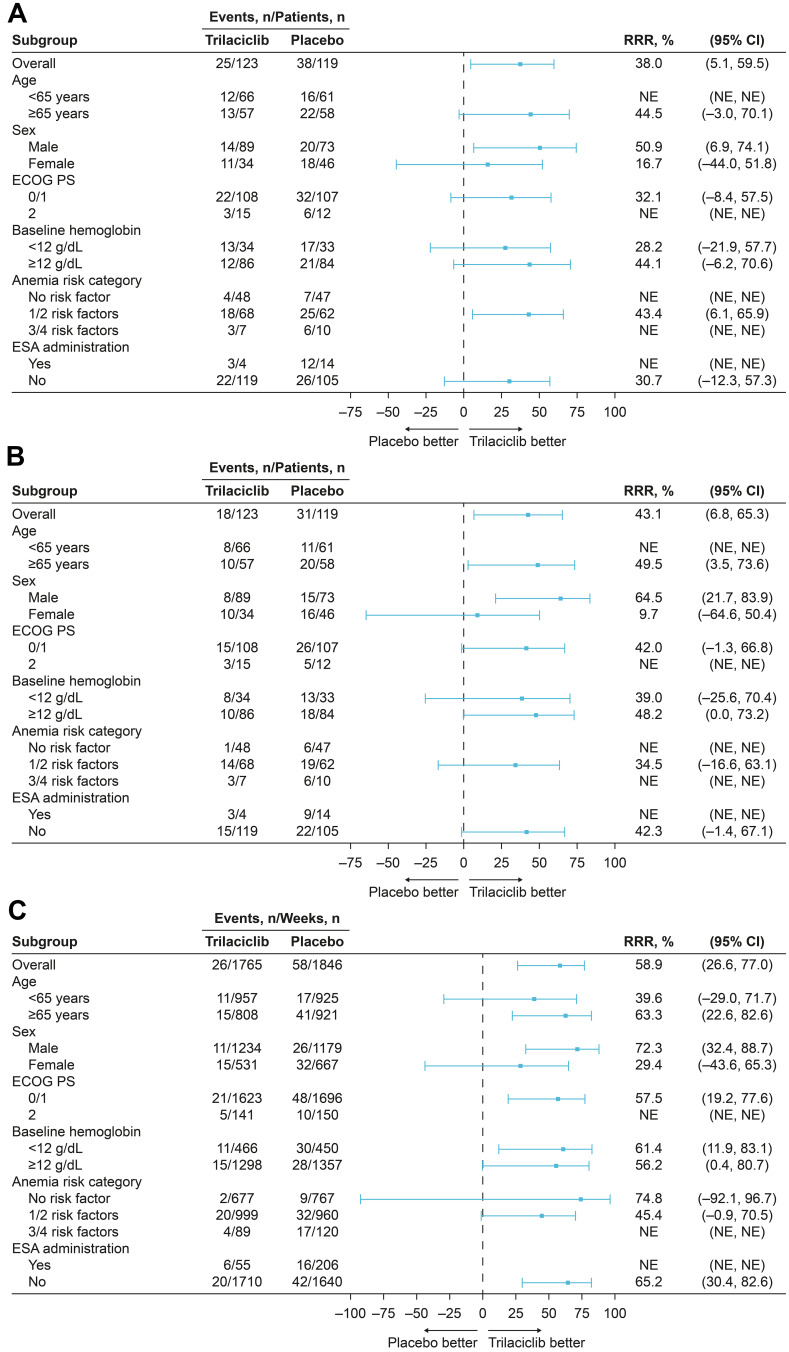
When evaluating the effect of trilaciclib by anemia risk factors and categories, effects on RBC-related endpoints (percentage of patients with grade 3/4 anemia and RBC transfusions on/after week 5) consistently favored trilaciclib vs placebo, and were aligned with the treatment effects in the ITT population (Table 4; Figure 2). The treatment-by-subgroup interaction for each of the anemia risk factors was nonsignificant for the percentage of patients with grade 3/4 anemia, and for the percentage of patients with, and total number of, RBC transfusions on/after week 5 (except for the risk factor of sex, where the interaction P-value was not estimable), providing statistical evidence of consistent treatment effects between patients with or without specific risk factors for anemia.
Table 4.
Anemia/RBC Transfusions Risk Category Results
| ITT Population | Anemia Risk Category | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1/2 | 3/4 | ||||||
| Trilaciclib | Placebo | Trilaciclib | Placebo | Trilaciclib | Placebo | Trilaciclib | Placebo | |
| Patients with grade 3/4 decreased hemoglobin levels, n (%) | 25 (20.3) | 38 (31.9) | 4 (8.3) | 7 (14.9) | 18 (26.5) | 25 (40.3) | 3 (42.9) | 6 (60.0) |
| Patients with RBC transfusions on/after week 5, n (%) | 18 (14.6) | 31 (26.1) | 1 (2.1) | 6 (12.8) | 14 (20.6) | 19 (30.6) | 3 (42.9) | 6 (60.0) |
| Total number of RBC transfusions on/after week 5, event rate per week | 0.015 | 0.031 | 0.003 | 0.012 | 0.020 | 0.033 | 0.045 | 0.142 |
Abbreviations: ITT, intention-to-treat; RBC, red blood cell.
Figure 2.
Subgroup analysis of (A) percentage of patients with grade 3/4 anemia, (B) percentage of patients with RBC transfusions on/after week 5, and (C) total number of RBC transfusions on/after week 5 by risk factor and category.
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; ESA, erythropoiesis-stimulating agent; NE, not estimable (statistical model did not converge); RBC, red blood cell; RRR, relative risk reduction.
Effects of Trilaciclib on QoL by Age
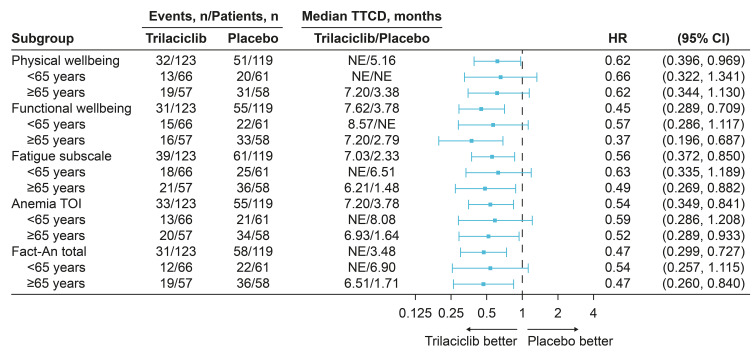
In the ITT population, greater improvements in PRO endpoints (PWB, FWB, fatigue, anemia TOI, and FACT-An scores) were observed in all patients receiving trilaciclib compared with those receiving placebo, irrespective of age group (<65 and ≥65 years). For each of the PRO endpoints, median TTCD for patients receiving trilaciclib was longer than that for patients receiving placebo regardless of age group, with greater improvements (smaller hazard ratios) observed among older patients (Figure 3).
Figure 3.
Subgroup analysis of TTCD.
Abbreviations: CI, confidence interval; FACT-An, Functional Assessment of Cancer Therapy–Anemia; HR, hazard ratio; NE, not evaluable; TOI, trial outcome index; TTCD, time to confirmed deterioration.
Discussion
Although treatment with curative intent is medically appropriate in many oncology cases, evidence suggests that some patients with SCLC who are older or more vulnerable to CIM are not receiving it for a number of reasons, including having a higher risk of experiencing the consequences of CIM, and thus may miss an opportunity to benefit from treatment.38,39 Particular consideration of the potential benefits and adverse effects of treatment is therefore needed when managing patient populations who are older or more vulnerable to CIM.40 In this regard, an intervention that could decrease the incidence of unwanted side effects of CIM and improve QoL could be meaningful to patients, clinicians, and payers, and may enable chemotherapy to be delivered on schedule and at standard-of-care doses, thus allowing patients to gain the maximum benefit from treatment. Previously reported findings from the three phase 2 studies of trilaciclib in patients with ES-SCLC have shown that the addition of trilaciclib to chemotherapy provides multilineage myeloprotection, resulting in an improved safety profile, better QoL, and a reduced need for supportive care interventions and dose delays/reductions.28–32 In line with findings in the ITT populations, the current pooled analysis of data from the three SCLC studies showed that myeloprotective benefits with trilaciclib were observed in all patients, irrespective of age, and across all FN and anemia risk factors and categories.
The magnitude of the effects of trilaciclib compared with placebo on neutrophil- and anemia-/RBC transfusion-related endpoints was greater among patients aged ≥65 years, which is important because both the incidence and consequences of CIM can be more profound in older/vulnerable patients. For example, chemotherapy-induced grade 3/4 hematologic adverse events have been reported in 50–80% of older patients with SCLC.39,41,42 Older patients are also more likely to have comorbid cardiovascular and pulmonary conditions, which may lead to more serious consequences of chemotherapy-induced anemia by worsening symptoms and reducing tolerance to hemoglobin decreases.13,43 Notably, in this pooled analysis, administering trilaciclib prior to chemotherapy in patients aged ≥65 years reduced the percentages of patients with SN, with grade 3/4 anemia, and needing RBC transfusions on/after week 5, to be more in line with those seen in younger patients receiving trilaciclib.
Consistent with previous findings in the overall ITT populations of the pooled and individual studies,29–32 the myeloprotective benefits of trilaciclib translated into improvements in PROs vs placebo in both younger (<65 years of age) and older (≥65 years of age) patients. Greater improvements in QoL were observed among patients aged ≥65 years, a population for whom QoL improvements may be particularly relevant because current treatments are not expected to achieve significant advantages in terms of overall survival in older patients with ES-SCLC;44 in this context, the expected toxicity of treatment and its impact on QoL is an important consideration in driving treatment decisions.45 Indeed, in cases of metastatic cancer, treatments that improve QoL can be recommended even if they do not improve overall survival.45
The trilaciclib and placebo groups were balanced with respect to the percentage of patients within each FN and anemia risk factor category. Across both treatment groups, approximately 5–6% of patients had 3/4 FN risk factors, and approximately 6–8% of patients had 3/4 anemia risk factors. Despite the small sample sizes, subgroup analyses indicated that these highest-risk patients received comparable myeloprotective benefits from trilaciclib relative to placebo when compared with patients in lower-risk categories, and that patients in all risk categories benefited from trilaciclib in terms of a reduction in DSN in cycle 1, a reduction in the percentage of patients with SN, and improvements in RBC-related endpoints. Across all individual risk factors and categories, the percentages of patients with SN, with grade 3/4 anemia, or needing RBC transfusions were consistently lower in the trilaciclib group than in the placebo group, and nonsignificant treatment-by-subgroup interactions indicated no evidence of a difference in treatment effect between subgroups.
An important consideration is whether the addition of trilaciclib prior to chemotherapy has any negative effects on efficacy outcomes, particularly because ES-SCLC has a generally poor prognosis due to its acute progression and invasiveness. Based on the mechanism of action of trilaciclib, there is no scientific rationale for a negative impact on the efficacy of chemotherapy for ES-SCLC, as SCLC tumor cells replicate independently of CDK4/6 and therefore remain susceptible to the cytotoxic effects of chemotherapy when trilaciclib is administered.26,28 Moreover, preclinical studies have shown that, even in CDK4/6-dependent tumors, transient CDK4/6 inhibition with trilaciclib does not antagonize the intended antitumor effects of chemotherapy.46 The results of the pooled and individual trials of trilaciclib in patients with ES-SCLC also indicate that, while there was no improvement in antitumor efficacy with the addition of trilaciclib prior to chemotherapy, trilaciclib did not antagonize the effects of chemotherapy in patients with ES-SCLC in the first- or second-/third-line settings, with similar efficacy outcomes observed between treatment groups (trilaciclib versus placebo).28–31 Among response-evaluable patients in the pooled population who received trilaciclib or placebo, the objective response rate was 49.1% and 51.8%, (P=0.7879), respectively; median progression-free survival was 5.3 versus 5.0 months (HR, 0.80; 95% CI, 0.61–1.06; P=0.1404), and median OS was 10.6 versus 10.6 months, respectively (HR, 1.00; 95% CI 0.75–1.35; P=0.8136).31 Overall, these findings indicate that trilaciclib had no impact on the antitumor efficacy of three individual chemotherapy regimens used in the first- or second-/third-line treatment of ES-SCLC.
Overall, the administration of trilaciclib prior to chemotherapy decreased the incidence of CIM and resulted in an improved benefit–risk profile for chemotherapy used to treat patients with ES-SCLC, irrespective of patient age and risk of FN or grade 3/4 anemia. By both decreasing the incidence of CIM and improving symptoms and functional limitations associated with cancer and CIM, trilaciclib has the potential to allow patients receiving chemotherapy for ES-SCLC, including patients who are older or more vulnerable to CIM who might otherwise not receive treatment, to receive chemotherapy on schedule and at standard-of-care doses, and to improve patients’ experience of receiving chemotherapy to treat SCLC.
Acknowledgments
We thank and acknowledge all of the patients, their families, and study personnel for participating in the studies. We thank Jie Xiao and Julio Ruiz for statistical and programming support. Medical writing assistance was provided by Beth Demetrious-Seymour of Alligent Europe (Envision Pharma Group), funded by G1 Therapeutics, Inc.
Funding Statement
This work was supported by G1 Therapeutics, Inc. The sponsor was involved in the collection, analysis, and interpretation of data; in the editing of the manuscript; and in the decision to submit the article for publication.
Abbreviations
aRR, adjusted relative risk; CI, confidence interval; CIM, chemotherapy-induced myelosuppression; DSN, duration of severe (grade 4) neutropenia; ECOG PS, Eastern Cooperative Oncology Group performance status; ES-SCLC, extensive-stage small cell lung cancer; FACT-An, Functional Assessment of Cancer Therapy–Anemia; FN, febrile neutropenia; FWB, functional wellbeing; ITT, intention-to-treat; PRO, patient-reported outcome; PWB, physical wellbeing; QoL, quality of life; RBC, red blood cell; RRR, relative risk reduction; SCLC, small cell lung cancer; SN, severe (grade 4) neutropenia; TOI, trial outcome index; TTCD, time to confirmed deterioration.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The studies (NCT03041311; NCT02499770; NCT02514447) were designed and conducted in compliance with the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonisation. The study protocol and all study-related materials were approved by the institutional review board or independent ethics committee of each investigational site. Written informed consent was obtained from each patient before the initiation of study procedures.
Disclosure
Dr Maen Hussein reports personal fees from Integra Connect, Coherus Biosciences, Athenex, Karyopharm Therapeutics, Bristol-Myers Squibb, and AstraZeneca, outside the submitted work. Dr Alexander Spira has received research funding from G1 Therapeutics, Inc., has been a consultant to Amgen, AstraZeneca, Bristol-Myers Squibb, Merck, Mirati, and Novartis, and received personal fees from Sanofi, AstraZeneca and Bristol-Myers Squibb. Dr Yili Pritchett and Dr Rajesh Malik are paid employees and shareowners of G1 Therapeutics, Inc. Dr Joyce M. Antal was an employee of G1 Therapeutics, Inc. at the time of manuscript preparation. Dr J. Thaddeus Beck has received grants from Abbvie, Alliance, Amgen, Ascentage Pharma Group, AstraZeneca, Bayer, Biodesix, Boehringer Ingelheim, Boston Biomedical, Bristol-Myers Squibb, Calithera, Celgene, Daiichi Sankyo, EMD Serono, Eli Lilly, Evelo, Exact Sciences, G1 Therapeutics, Inc., Genentech-Roche, Hutchison, Immunomedics, Janssen, Laekna, Mirati Therapeutics, MT Group, Nektar, Novartis, Novocure, Oncopeptides, Pfizer, Polynoma, Seattle Genetics, Syneos Health, Tarveda Therapeutics, Tesaro, TG Therapeutics, Ultimovacs, and Vaccinex. The authors report no other conflicts of interest in this work.
References
- 1.Povsic M, Enstone A, Wyn R, Kornalska K, Penrod JR, Yuan Y. Real-world effectiveness and tolerability of small-cell lung cancer (SCLC) treatments: a systematic literature review (SLR). PLoS One. 2019;14(7):e0219622. doi: 10.1371/journal.pone.0219622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein RS, Aapro MS, Basu UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37(8):3606–3618. doi: 10.1007/s12325-020-01419-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100(2):228–237. doi: 10.1002/cncr.11882 [DOI] [PubMed] [Google Scholar]
- 4.Bryer E, Henry D. Chemotherapy-induced anemia: etiology, pathophysiology, and implications for contemporary practice. Int J Clin Transfus Med. 2018;6:21–31. doi: 10.2147/IJCTM.S187569 [DOI] [Google Scholar]
- 5.Weycker D, Hatfield M, Grossman A, et al. Risk and consequences of chemotherapy-induced thrombocytopenia in US clinical practice. BMC Cancer. 2019;19(1):151. doi: 10.1186/s12885-019-5354-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford J, Denduluri N, Patt D, et al. Relative dose intensity of first-line chemotherapy and overall survival in patients with advanced non-small-cell lung cancer. Support Care Cancer. 2020;28(2):925–932. doi: 10.1007/s00520-019-04875-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liou SY, Stephens JM, Carpiuc KT, Feng W, Botteman MF, Hay JW. Economic burden of haematological adverse effects in cancer patients: a systematic review. Clin Drug Investig. 2007;27(6):381–396. doi: 10.2165/00044011-200727060-00002 [DOI] [PubMed] [Google Scholar]
- 8.Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist. 2000;5(3):224–237. doi: 10.1634/theoncologist.5-3-224 [DOI] [PubMed] [Google Scholar]
- 9.Balducci L, Hardy CL, Lyman GH. Hemopoietic reserve in the older cancer patient: clinical and economic considerations. Cancer Control. 2000;7(6):539–547. doi: 10.1177/107327480000700605 [DOI] [PubMed] [Google Scholar]
- 10.Lyman GH, Kuderer NM. Epidemiology of febrile neutropenia. Support Cancer Ther. 2003;1(1):23–35. doi: 10.3816/SCT.2003.n.002 [DOI] [PubMed] [Google Scholar]
- 11.Lyman GH, Lyman CH, Agboola O. Risk models for predicting chemotherapy-induced neutropenia. Oncologist. 2005;10(6):427–437. doi: 10.1634/theoncologist.10-6-427 [DOI] [PubMed] [Google Scholar]
- 12.Talcott JA, Siegel RD, Finberg R, Goldman L. Risk assessment in cancer patients with fever and neutropenia: a prospective, two-center validation of a prediction rule. J Clin Oncol. 1992;10(2):316–322. doi: 10.1200/jco.1992.10.2.316 [DOI] [PubMed] [Google Scholar]
- 13.Aarts MJ, Aerts JG, van den Borne BE, Biesma B, Lemmens VE, Kloover JS. Comorbidity in patients with small-cell lung cancer: trends and prognostic impact. Clin Lung Cancer. 2015;16(4):282–291. doi: 10.1016/j.cllc.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 14.Chao C, Page JH, Yang SJ, Rodriguez R, Huynh J, Chia VM. History of chronic comorbidity and risk of chemotherapy-induced febrile neutropenia in cancer patients not receiving G-CSF prophylaxis. Ann Oncol. 2014;25(9):1821–1829. doi: 10.1093/annonc/mdu203 [DOI] [PubMed] [Google Scholar]
- 15.Chia VM, Page JH, Rodriguez R, Yang SJ, Huynh J, Chao C. Chronic comorbid conditions associated with risk of febrile neutropenia in breast cancer patients treated with chemotherapy. Breast Cancer Res Treat. 2013;138(2):621–631. doi: 10.1007/s10549-013-2454-9 [DOI] [PubMed] [Google Scholar]
- 16.Morrison VA, Picozzi V, Scott S, et al.; Oncology Practice Pattern Study Working Group. The impact of age on delivered dose intensity and hospitalizations for febrile neutropenia in patients with intermediate-grade non-Hodgkin’s lymphoma receiving initial CHOP chemotherapy: a risk factor analysis. Clin Lymphoma. 2001;2(1):47–56. doi: 10.3816/clm.2001.n.011 [DOI] [PubMed] [Google Scholar]
- 17.Lyman GH, Morrison VA, Dale DC, et al.; OPPS Working Group; ANC Study Group. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma. 2003;44(12):2069–2076. doi: 10.1080/1042819031000119262 [DOI] [PubMed] [Google Scholar]
- 18.Intragumtornchai T, Sutheesophon J, Sutcharitchan P, Swasdikul D. A predictive model for life-threatening neutropenia and febrile neutropenia after the first course of CHOP chemotherapy in patients with aggressive non-Hodgkin’s lymphoma. Leuk Lymphoma. 2000;37(3–4):351–360. doi: 10.3109/10428190009089435 [DOI] [PubMed] [Google Scholar]
- 19.Lyman GH, Abella E, Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol. 2014;90(3):190–199. doi: 10.1016/j.critrevonc.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 20.Seo SH, Kim SE, Kang YK, et al. Association of nutritional status-related indices and chemotherapy-induced adverse events in gastric cancer patients. BMC Cancer. 2016;16(1):900. doi: 10.1186/s12885-016-2934-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincent M, Dranitsaris G, Verma S, et al. The development and validation of a prediction tool for chemotherapy-induced anemia in patients with advanced nonsmall cell lung cancer receiving palliative chemotherapy. Support Care Cancer. 2007;15(3):265–272. doi: 10.1007/s00520-006-0154-2 [DOI] [PubMed] [Google Scholar]
- 22.Barrett-Lee PJ, Ludwig H, Birgegård G, et al.; European Cancer Anaemia Survey Advisory Board and Participating Centers. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology. 2006;70(1):34–48. doi: 10.1159/000091675 [DOI] [PubMed] [Google Scholar]
- 23.Coiffier B, Guastalla JP, Pujade-Lauraine E, Bastit P; Anemia Study Group. Predicting cancer-associated anaemia in patients receiving non-platinum chemotherapy: results of a retrospective survey. Eur J Cancer. 2001;37(13):1617–1623. doi: 10.1016/s0959-8049(01)00169-1 [DOI] [PubMed] [Google Scholar]
- 24.Lyman CH, Crawford J, Kuderer NM, et al. A conditional risk model for chemotherapy-induced anemia (CIA) in cancer patients. Blood. 2007;110(11):372. doi: 10.1182/blood.V110.11.372.372 [DOI] [Google Scholar]
- 25.Ray-Coquard I, Le Cesne A, Rubio MT, et al. Risk model for severe anemia requiring red blood cell transfusion after cytotoxic conventional chemotherapy regimens. The Elypse 1 Study Group. J Clin Oncol. 1999;17(9):2840–2846. doi: 10.1200/jco.1999.17.9.2840 [DOI] [PubMed] [Google Scholar]
- 26.Bisi JE, Sorrentino JA, Roberts PJ, Tavares FX, Strum JC. Preclinical characterization of G1T28: a novel CDK4/6 inhibitor for reduction of chemotherapy-induced myelosuppression. Mol Cancer Ther. 2016;15(5):783–793. doi: 10.1158/1535-7163.Mct-15-0775 [DOI] [PubMed] [Google Scholar]
- 27.He S, Roberts PJ, Sorrentino JA, et al. Transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-induced exhaustion. Sci Transl Med. 2017;9(387):eaal3986. doi: 10.1126/scitranslmed.aal3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss JM, Csoszi T, Maglakelidze M, et al.; G1T28-02 Study Group. Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: a phase Ib/randomized Phase II trial. Ann Oncol. 2019;30(10):1613–1621. doi: 10.1093/annonc/mdz278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniel D, Kuchava V, Bondarenko I, et al. Trilaciclib prior to chemotherapy and atezolizumab in patients with newly diagnosed extensive-stage small cell lung cancer: a multicentre, randomised, double-blind, placebo-controlled Phase II trial. Int J Cancer. 2020;148(10):2557–2570. doi: 10.1002/ijc.33453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hart LL, Ferrarotto R, Andric ZG, et al. Myelopreservation with trilaciclib in patients receiving topotecan for small cell lung cancer: results from a randomized, double-blind, placebo-controlled phase II study. Adv Ther. 2021;38(1):350–365. doi: 10.1007/s12325-020-01538-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss J, Goldschmidt J, Andric Z, et al. Effects of trilaciclib on chemotherapy-induced myelosuppression and patient-reported outcomes in patients with extensive-stage small cell lung cancer: pooled results from three phase II randomized, double-blind, placebo-controlled studies. Clin Lung Cancer. 2021;2:64. doi: 10.1016/j.cllc.2021.03.010 [DOI] [PubMed] [Google Scholar]
- 32.Weiss JM, Skaltsa K, Gwaltney C, et al. Positive effects of trilaciclib on patient myelosuppression-related symptoms and functioning: results from three phase 2 randomized, double-blind, placebo-controlled small cell lung cancer trials. Presented at: MASCC/ISOO Annual Meeting: San Francisco, CA, USA; 2019. [Google Scholar]
- 33.Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) study 5592. J Clin Epidemiol. 2002;55(3):285–295. doi: 10.1016/s0895-4356(01)00477-2 [DOI] [PubMed] [Google Scholar]
- 34.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547–561. doi: 10.1016/s0885-3924(02)00529-8 [DOI] [PubMed] [Google Scholar]
- 35.Yost KJ, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: the FACIT experience. Eval Health Prof. 2005;28(2):172–191. doi: 10.1177/0163278705275340 [DOI] [PubMed] [Google Scholar]
- 36.Butt Z, Webster K, Eisenstein AR, et al. Quality of life in lung cancer: the validity and cross-cultural applicability of the Functional Assessment of Cancer Therapy-Lung scale. Hematol Oncol Clin North Am. 2005;19(2):389–420. doi: 10.1016/j.hoc.2005.02.009 [DOI] [PubMed] [Google Scholar]
- 37.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 38.Ludbrook JJ, Truong PT, MacNeil MV, et al. Do age and comorbidity impact treatment allocation and outcomes in limited stage small-cell lung cancer? A community-based population analysis. Int J Radiat Oncol Biol Phys. 2003;55(5):1321–1330. doi: 10.1016/s0360-3016(02)04576-5 [DOI] [PubMed] [Google Scholar]
- 39.Janssen-Heijnen MLG, Maas HAAM, van de Schans SAM, Coebergh JWW, Groen HJM. Chemotherapy in elderly small-cell lung cancer patients: yes we can, but should we do it? Ann Oncol. 2011;22(4):821–826. doi: 10.1093/annonc/mdq448 [DOI] [PubMed] [Google Scholar]
- 40.Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;7(4):249–257. doi: 10.1016/j.jgo.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuen AR, Zou G, Turrisi AT, et al. Similar outcome of elderly patients in intergroup trial 0096: cisplatin, etoposide, and thoracic radiotherapy administered once or twice daily in limited stage small cell lung carcinoma. Cancer. 2000;89(9):1953–1960. doi: [DOI] [PubMed] [Google Scholar]
- 42.Schild SE, Stella PJ, Brooks BJ, et al. Results of combined-modality therapy for limited-stage small cell lung carcinoma in the elderly. Cancer. 2005;103(11):2349–2354. doi: 10.1002/cncr.21034 [DOI] [PubMed] [Google Scholar]
- 43.Crawford J, Kosmidis PA, Hirsch FR, Langer CJ. Targeting anemia in patients with lung cancer. J Thorac Oncol. 2006;1(7):716–725. [PubMed] [Google Scholar]
- 44.Kalemkerian GP, Akerley W, Bogner P, et al.; National Comprehensive Cancer Network. Small cell lung cancer. J Natl Compr Canc Netw. 2013;11(1):78–98. doi: 10.6004/jnccn.2013.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Maio M, Perrone F. Quality of life in elderly patients with cancer. Health Qual Life Outcomes. 2003;1:44. doi: 10.1186/1477-7525-1-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorrentino JA, Bisi JE, Thompson D, et al. Trilaciclib, a CDK4/6 inhibitor, does not impair the efficacy of chemotherapy in CDK4/6-dependent tumor models. presented at: EORTC, NCI, and AACR symposium. Eur J Cancer. 2018;103(suppl 1):e23–e147. [Google Scholar]