Abstract
Several studies suggest a role for the peripheral immune system in the pathophysiology of amyotrophic lateral sclerosis. However, comprehensive studies investigating the intrathecal immune system in amyotrophic lateral sclerosis are rare. To elucidate whether compartment-specific inflammation contributes to amyotrophic lateral sclerosis pathophysiology, we here investigated intrathecal and peripheral immune profiles in amyotrophic lateral sclerosis patients and compared them with controls free of neurological disorders (controls) and patients with dementia or primary progressive multiple sclerosis. Routine CSF parameters were examined in 308 patients, including 132 amyotrophic lateral sclerosis patients. In a subgroup of 41 amyotrophic lateral sclerosis patients, extensive flow-cytometric immune cell profiling in peripheral blood and CSF was performed and compared with data from 26 controls, 25 dementia and 21 multiple sclerosis patients. Amyotrophic lateral sclerosis patients presented with significantly altered proportions of monocyte subsets in peripheral blood and increased frequencies of CD4+ and CD8+ T cells expressing the activation marker HLA-DR in peripheral blood (CD8+) and CSF (CD4+ and CD8+) compared with controls. While dementia and multiple sclerosis patients exhibited a comparable increase in intrathecal CD8+ T-cell activation, CD8+ T-cell activation in the peripheral blood in amyotrophic lateral sclerosis was higher than in multiple sclerosis patients. Furthermore, intrathecal CD4+ T-cell activation in amyotrophic lateral sclerosis surpassed levels in dementia patients. Intrathecal T-cell activation resulted from in situ activation rather than transmigration of activated T cells from the blood. While T-cell activation did not correlate with amyotrophic lateral sclerosis progression, patients with rapid disease progression showed reduced intrathecal levels of immune-regulatory CD56bright natural killer cells. The integration of these parameters into a composite score facilitated the differentiation of amyotrophic lateral sclerosis patients from patients of all other cohorts. To conclude, alterations in peripheral monocyte subsets, as well as increased peripheral and intrathecal activation of CD4+ and CD8+ T cells concomitant with diminished immune regulation by CD56bright natural killer cells, suggest an involvement of these immune cells in amyotrophic lateral sclerosis pathophysiology.
Keywords: amyotrophic lateral sclerosis, immune system, neuroinflammation, immune phenotyping, cerebrospinal fluid
Rolfes et al. reported increased frequencies of activated T cells in the peripheral blood and cerebrospinal fluid of amyotrophic lateral sclerosis patients. They provided evidence that patients with rapid disease progression have reduced intrathecal levels of immune-regulatory CD56bright NK cells, suggest an involvement of these cells in disease pathophysiology.
Graphical Abstract
Graphical Abstract.
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive, paralytic disorder characterized by the degeneration of motor neurons in the brain and spinal cord.1 To date, the pathophysiology of ALS is widely elusive, although vast knowledge has been accumulated that elucidates the intracellular and molecular mechanisms involved in motor neuron loss.2,3 Although primarily described as neurodegenerative disease, recent evidence suggests that inflammatory mechanisms likely affect the progression and extent of the neurodegenerative process as well.4,5 In this context, increased leukocyte and CD8+ T-cell counts in the peripheral blood (PB) of ALS patients have been linked to rapid disease progression.5 Correspondingly, neuroprotective effects have been attributed to both regulatory T cells and the amount of natural killer (NK) cells in PB.6,7
T cells are the central players in adaptive immunity and specifically recognize antigens presented by major histocompatibility complex (MHC) molecules with their T-cell receptor, resulting in clonal expansion, differentiating, cytokine production or initiation of cytolytic processes.4,8 Whereas, CD4+ T cells recognize antigens presented by MHC class II molecules by antigen presenting cells and activate other immune cells, including CD8+ T cells, B cells as well as myeloid cells via cytokines, cytotoxic CD8+ T cells recognize peptides presented by MHC class I molecules, resulting in cytolytic activity directed against the presenting cell.
NK cells are lymphocytes of the innate immune system that play an important role in the control of viral infections and cancer by the production of cytokines and cytolytic processes.9–12 In addition to the well-known players of the immune-regulatory network like regulatory T cells and tolerogenic dendritic cells, NK cells have been described to also contribute to T-cell homeostasis.13–15 In humans, two major NK-cell subsets are distinguished based on the expression of CD56 and the Fcγ receptor III (CD16). Whereas CD56dimCD16+ NK cells dominate in the PB, CD56brightCD16dim/− NK cells are enriched in lymphoid tissues and the CSF.16–18
Regarding the CNS, pathological studies observed both CD4+ and CD8+ T cells in the brain and spinal cord of ALS patients.8,19 Of note, in a mouse model of ALS, the antigen-specific invasion of CD8+ T cells into the CNS directly resulted in the death of motoneurons.20 In contrast to other neurological diseases, the potential role of immune regulation of activated neurotoxic T cells by NK cells in ALS is still elusive.5
These observations suggest neuro-inflammation as an important factor in ALS pathogenesis and prompt further investigation of the intrathecal compartment to understand disease pathophysiology and identify novel biomarkers for diagnosis and prognosis. To date, only a few studies have investigated the immune-cell profile of ALS patients, with the main focus on PB.5,7,21–25 While a peripheral increase of CD4+ and CD8+ T cells in ALS was reported compared with healthy controls,23,24 intrathecal changes have not been observed so far.7 Also, several studies described changes of monocyte subpopulations in the PB21,22,25 comparable with patients with Alzheimer’s disease.26 Furthermore, the microRNA pattern of monocytes of ALS patients resembles the inflammatory signature identified in relapsing-remitting multiple sclerosis (MS) patients, thus suggesting an inflammatory component in the ALS pathophysiology.27
Here, we investigate both the peripheral and intrathecal immune-cell profile of ALS patients to identify specific factors related to pathophysiology, severity and progression of the disease to facilitate diagnosis and prognosis. We compare the extent and origin of immune-cell alterations of ALS patients with the results from another primary neurodegenerative disease and an autoimmune disorder.
Methods
Study population
Definition of ALS cohort
One hundred thirty-two patients presenting with sporadic ALS at the University Hospitals Muenster and Magdeburg (Germany) between 2011 and 2020 were retrospectively identified. Patients meeting the diagnosis of probable or definite ALS according to the revised El Escorial criteria were eligible for study inclusion.28 Patients were excluded if they: (i) had a family history of ALS; (ii) had a diagnosis of neurodegenerative disease in addition to ALS; (iii) had a concomitant chronic inflammatory disease; (iv) used immunomodulatory medication; or (v) had a fever or acute illness reported at the time of sampling. Disease severity was assessed using the revised ALS Functional Rating Scale (ALS-FRS-R).29 According to the average monthly decline of the ALS-FRS-R sum score from symptom onset through sampling [calculated as Δ score = (48 − ALS-FRS-R)/months from disease onset], patients were stratified as slow (Δ score < 0.47/month) or fast progressing patients (Δ score ≥ 1.11/month).30,31 Owing to a better comparability, we oriented our analysis to the cut-off values used in Labra et al.30 Disease characteristics are summarized in Table 1. The study was conducted according to the Declaration of Helsinki and approved by the local ethics committees (Muenster: 2010-262-f-S/2016-053-f-S; Magdeburg: No 07/17). All participants gave their written informed consent.
Table 1.
Patient baseline characteristics of disease groups
| Total cohort |
Flow cytometry sub-cohort |
|||||||
|---|---|---|---|---|---|---|---|---|
| Group | ALS | CTRL | DEM | PPMS | ALS | CTRL | DEM | PPMS |
| Patients, N (%) | 132 | 33 | 122 | 21 | 41 | 26 | 25 | 21 |
| Age, years, median (IQR) | 64 (56–71)ns | 62 (47–72) | 65 (59–71) | 59 (49–63) | 62 (55–70)ns | 59 (47–72) | 66 (60–71) | 59 (49–63) |
| Female patients (%) | 45.5ns | 57.6 | 58.2 | 57.1 | 43.9ns | 61.5 | 52.0 | 57.1 |
| Months from onset to sampling, median (IQR) | 17.6 (4–116) | 79.0 (44–127) | 10.0 (6.0–17.0) | 79.0 (44–127) | ||||
Onset site
|
|
|||||||
| ALS-FRS-R total score, median (IQR) | 41 (18–47) | 43 (39–45) | ||||||
| ALS-FRS-R total slope, median (IQR) | 0.71 (0.01–3.83) | 0.50 (0.10–0.91) | ||||||
| ALS-FRS-R motor subscore, median (IQR) | 19 (4–24) | 21 (17–22) | ||||||
| ALS-FRS-R motor subscore slope, median (IQR) | 0.52 (0.0–3.0) | 0.27 (0.08–1.07) | ||||||
| ALS-FRS-R bulbar subscore, median (IQR) | 11 (6–12) | 11 (9–12) | ||||||
| ALS-FRS-R bulbar subscore slope, median (IQR) | 0.16 (0.0–1.63) | 0.06 (0.0–0.26) | ||||||
| EDSS, median (IQR) | 4.5 (3.0–6.5) | 4.5 (3.0–6.5) | ||||||
ALS, amyotrophic lateral sclerosis; ALS-FRS-R, revised Amyotrophic Lateral Sclerosis Functional Rating Scale; CTRL, non-inflammatory/neurodegenerative controls; DEM, dementias; IQR, interquartile range; N, number; PPMS, primary progressive multiple sclerosis; ns, not significant; Kruskal–Wallis test with Dunn’s post-test, α = 0.05.
Definition of control groups
Patients with ALS were compared with a control group of 33 patients free of neurological disease who were either diagnosed with somatoform disorders32 or who provided sample material during spinal anaesthesia (controls, Table 1, left). Furthermore, 122 patients with dementia (Alzheimer’s disease)32 were investigated as an example of a primary neurodegenerative CNS disease (Table 1, left). Moreover, 21 patients with primary progressive MS (PPMS)33 were examined as this disease is characterized by both autoimmune and neurodegenerative features and is usually diagnosed at a similar age as ALS (Table 1, left). Control patients had not been treated with immunotherapies.
Multiparameter flow cytometry and CSF analysis
Flow cytometric measurements were done in one study centre (Muenster). For this purpose, PB and CSF were analysed within one hour after sampling. The CSF was centrifuged and treated with VersaLyseTM (Beckman Coulter, Krefeld, Germany) in parallel to PB, according to the manufacturer’s instructions. Cells were incubated with fluorochrome-conjugated monoclonal antibodies [CD14-FITC (clone RM052), CD138-PE (clone B-A38), HLA-DR-ECD (clone Immu-357), CD3-PC5.5 (clone UCHT1), CD56-PC7 (clone N901), CD4-APC (clone 13B8.2), CD19-APC A700 (clone J3-119), CD16-APC A750 (clone 3G8), CD8-PacificBlue (clone B9.11), CD45-KromeOrange (clone J.33); all Beckman Coulter, dilution 1:200] for 30 min, washed and analysed by flow cytometry on a NaviosTM (Beckman Coulter) flow cytometer.32 Data were analysed with KaluzaTM 2.1 software (Beckman Coulter; for gating strategy, see Supplementary Fig. 1). Routine CSF parameters were investigated in addition to flow cytometry in both study centres (Magdeburg and Muenster). Cells were counted using a Fuchs-Rosenthal chamber. The CSF/serum IgG, IgA, IgM, and albumin ratio and the blood/CSF-barrier integrity were determined by nephelometry (BN ProSpecTM, Siemens Healthcare). IgG oligoclonal band (OCB) patterns were analysed by isoelectric focussing in gel-electrophoresis and subsequent silver staining (Processor PlusTM, GE Healthcare).
Mathematical modelling
A mathematical model for migration and activation of lymphocytes has been introduced using Markov jump processes.34 Here, its derivation is not repeated, but a modification to quantify migration and activation patterns in ALS patients is proposed. As in the previous work,34 the distribution of lymphocytes of different stages (naïve/activated) is modelled in distinct compartments (PB/CSF) as a time-independent process (Fig. 1C left).35 Time independence is presumed since sufficient numbers of lymphocytes are expected to be present within each compartment and with each stage. The mathematical model describes the cell distribution in four states for each cell and each patient, labelled 1–4 (Fig. 1C left).35 The transitions between those states describe either the process of CNS trans-migration (α) or activation (β), where the individual cells are assumed to not have any memory of their prior state. The transitions at any given stage are considered to be independent of each other. This assumption is justified, provided that sufficient amounts of cells are available within each stage. Given cell distribution data for T and NK cell populations in all four stages, the corresponding transition rates can be computed analytically by the following set of equations:
| (1) |
| (2) |
| (3) |
| (4) |
Figure 1.
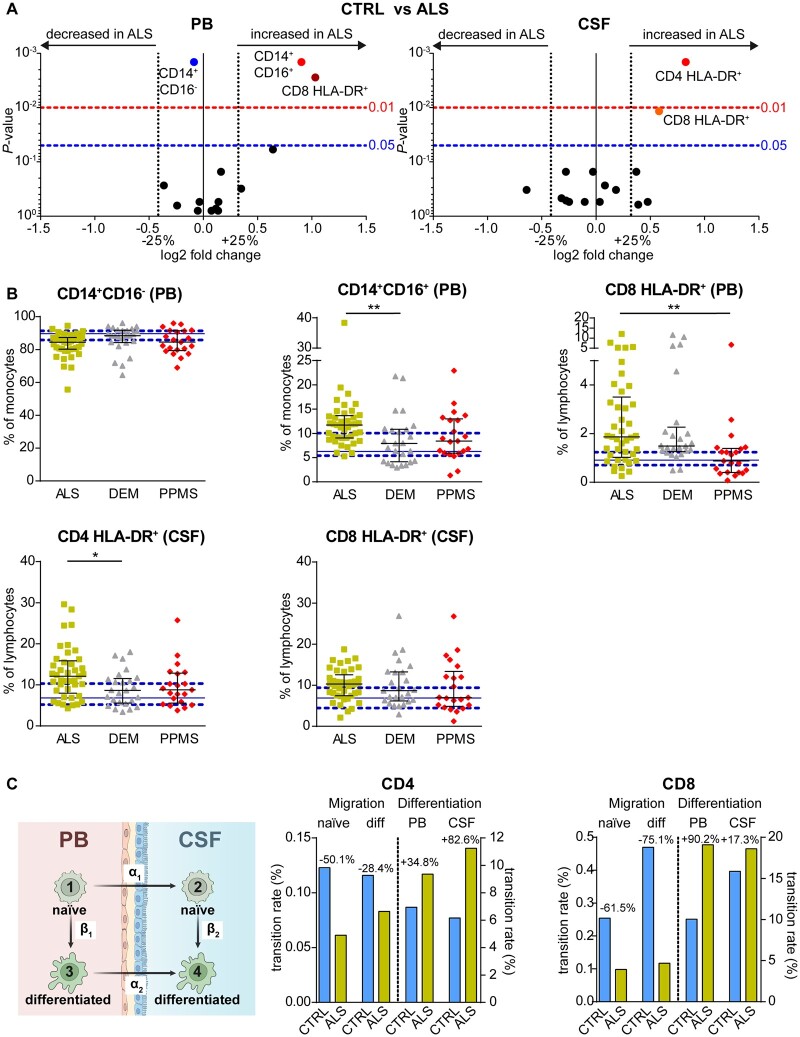
Distinct immune signature of ALS patients. (A) Data derived from the flow-cytometric investigation of PB and CSF were visualized by volcano plots representing the median fold change in parameters between ALS (n = 41) and controls (CTRL, n = 26) patients in the PB (left) and CSF (right). P-values were calculated using the Mann–Whitney test with Benjamini–Hochberg correction for multiple testing. Only the significantly altered parameters (P < 0.05) are labelled. (B) Proportions of immune cells altered between ALS (yellow squares) and CTRL (solid blue line: median, dashed blue lines: 25/75% quartiles) compared with dementia (DEM, grey triangles, n = 25) and PPMS (red diamonds, n = 21) patients. P-values were calculated by Kruskal–Wallis test with Dunn’s post-test, *P < 0.05, **P < 0.01. Error bars indicate the median and interquartile range. (C) Cohort- and cell-specific transition rates derived by mathematical modelling of the activation of naïve CD4 and CD8 T cells in the PB and CSF as well as of the CNS trans-migration of naïve and activated T cells by Markov jump processes.
The components of the vector X are the normalized cell distributions in stages 1–4. A0 is the total amount of cells available. The model consists of the equations (1)–(4). They can be heuristically explained as balance of cells within the four difference compartments. For example, equation (2) states that α1 A0 cells enter compartment 2 while at the same time β1 X2 leave this compartment. They in fact appear as inflow to the compartment 4 as seen in equation (4). The explanation for the balance equations for the other compartments is analogous. The previous model therefore depends on the transition rates (α1, α2, β1, β2) which govern the balance of cells. The only four mono-directional transition rates are a simplistic model for complex immunological processes, such as cell activation/differentiation and CNS transmigration. Those are in general unknown and may be dependent on each individual patient.
Given the heterogeneity of the patients, we propose to determine all rates by solving an unbiased and non-weighted regression problem. This amounts to solve the following nonlinear regression problem the unknown rates35:
| (5) |
Here, = (X1, X2, X3, X4) is the solution to the set of linear equations given by ((1)–(4)). Furthermore, we denote by are the measured cell distributions in all four compartments of the kth patient. Since co-variance information for the measured cell distribution is not available, a non-weighted least-square estimate is used in formula (5). The solution to problem (5) will yield optimal transition rates (α1, α2, β1, β2) for the given set of patient data This problem can only be solved numerically and it has been solved for all computational tests up to machine precision. It did further did have a numerical solution for all data sets considered.
Statistical analysis
Statistical analysis was performed using R3.5.3 via RStudio 1.1.442, GraphPad Prism V6.01, and Microsoft Excel 2016. Volcano plots were constructed by plotting log2 values of the relative difference between the medians (continuous) or means (categorical parameters) against the P-values, calculated using the Mann–Whitney test with Benjamini–Hochberg correction for multiple testing. In the case of division by zero, the log2 fold change is assumed as ALS/(Max–Min), whereas in the case of division of zero, the log2 fold change is set to [controls]/(Max–Min). Kruskal–Wallis test with Dunn’s post-test was used to analyse more than two groups.
Receiver operating characteristics (ROC) analyses were performed using package pROC (1.15.3) in Rstudio. Odds ratios and corresponding P-values were calculated with Fisher’s exact test, and for all statistical tests, the applied significance levels were set at *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Data availability statement
Individual participant data (including demographic and laboratory measures) collected during the trial will be available, after de-identification. Moreover, we will share statistical analysis, including analytic codes and informed consent forms. Data will be available for all types of analyses immediately following publication, without end date, and will be shared with qualified investigators upon request. Please contact marc.pawlitzki@ukmuenster.de.
Results
ALS patients exhibit routine CSF characteristics comparable with dementia patients
Data from routine CSF analysis of 132 ALS patients were compared with an age-matched cohort of 33 controls, 122 dementia and 21 PPMS patients (Table 2). ALS patients showed non-pathological (<5/µl) leukocyte counts in the CSF (1, IQR 0–1) comparable to controls (0, IQR 0–1, P = 0.493), dementia (1, IQR 0–1, P = 0.999) and PPMS (1, IQR 1–10, P = 0.065) patients. Furthermore, ALS patients exhibited increased levels of intrathecal total protein (480 mg/l, IQR 362–628) and increased albumin quotients (6.3, IQR 4.5–8.6) as well as frequency of blood/CSF barrier dysfunction (30.0%) compared with controls (total protein 387 mg/l, IQR 340–448, P = 0.005; albumin quotient 4.9, IQR 4.0–5.6, P = 0.008; blood/CSF barrier dysfunction 0%, P = 0.004). However, except for a higher proportion of ALS patients with blood/CSF barrier dysfunction compared with dementia (14.8%, P = 0.016), those parameters did not differ between ALS, dementia (total protein 453 mg/l, IQR 360–597, p = 0.999; albumin quotient 5.4, IQR 4.1–7.2, P = 0.241), and PPMS (total protein 461 mg/l, IQR 360–583, P = 0.999; albumin quotient 5.7, IQR 4.0–8.0, P = 0.999; blood/CSF barrier dysfunction 18.2%, P = 0.999) patients. Similarly, ALS [OCB type 2/3 4.6%; IgG (Reiber) 0%; IgA (Reiber) 0%; IgM (Reiber) 0.8%] patients as well as controls [OCB type 2/3 0%; IgG/A/M (Reiber) 0%, P = 0.999; 0.999; 0.999; 0.999] and dementia [OCB type 2/3 2.5%; IgG (Reiber) 1.6%; IgA (Reiber) 0%; IgM (Reiber) 2.5%, P = 0.999; 0.999; 0.999; 0.999] patients rarely exhibited intrathecal IgG- and IgA synthesis, clearly differentiating them from PPMS patients [OCB type 2/3 77.3%; IgG (Reiber) 45.5%; IgA (Reiber) 4.5%; IgM (Reiber) 4.5%, P < 0.001; <0.001; 0.003; 0.999].
Table 2.
Routine CSF parameters compared between disease groups (total cohort)
| Group | ALS | CTRL | DEM | PPMS | Statistics |
|---|---|---|---|---|---|
| Leukocytes [cells/µl], median (IQR) | 1 (0–1) | 0·0 (0–1) | 1 (0–1) | 1 (1–10) | ns |
| Total protein [mg/l], median (IQR) | 480 (362–628) | 387 (340–448) | 453 (360–597) | 461 (360–583) | CTRL** |
| Albumin quotient, median (IQR) | 6.3 (4.5–8.6) | 4.9 (4.0–5.6) | 5.4 (4.1–7.2) | 5.7 (4.0–8.0) | CTRL** |
| Blood/CSF-barrier dysfunction (%) | 30.0 | 0 | 14.8 | 18.2 | CTRL**/DEM* |
| Lactate [mmol/l], median (IQR) | 1.72 (1.54–1.90) | 1.64 (1.47–1.75) | 1.76 (1.60–2.03) | 1.72 (1.59–1.96) | ns |
| OCB type 2/3 (%) | 4.6 | 0 | 2.5 | 77.3 | PPMS**** |
| IgG (Reiber, %) | 0 | 0 | 1.6 | 45.5 | PPMS**** |
| IgA (Reiber, %) | 0 | 0 | 0 | 4.5 | PPMS** |
| IgM (Reiber, %) | 0.8 | 0 | 2.5 | 4.5 | ns |
ALS, amyotrophic lateral sclerosis; CSF, cerebrospinal fluid; CTRL, non-inflammatory/neurodegenerative controls; DEM, dementias; Ig, immunoglobulin; IQR, interquartile range; ns, not significant; OCB, oligoclonal bands; Statistics, ALS patients were compared with other cohorts using Kruskal–Wallis test with Dunn’s post-test. Significance levels are indicated after the respective cohort abbreviations; ns P ≥ 0.05; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
In summary, ALS patients exhibited a routine CSF parameter profile distinct from controls and PPMS patients but comparable with dementia patients.
ALS patients show increased CD4+ and CD8+ T-cell activation in PB and CSF
In addition to routine CSF parameters, available immune cell profiles in PB and CSF were analysed in a sub-cohort of 41 ALS, 26 controls, 25 dementia and 21 PPMS patients (Table 1, right) to gain deeper insights into the immune-pathophysiology of ALS. Notably, baseline characteristics, including time from onset to sampling, ALS-FRS-R scores and slope values, as well as routine CSF parameters, did not differ between this sub-cohort and the overall ALS study cohort mentioned above (Tables 1 and 2). While monocyte frequencies, in general, remained unaltered in the PB of ALS patients compared with controls (P = 0.793), ALS patients showed decreased proportions of the CD14+CD16− monocyte-subset in the PB (ALS: 84.46%, IQR 80.25–87.36; controls: 89.67%, IQR 85.79–91.37, P = 0.001), whereas CD14+CD16+ monocytes were increased (ALS: 11.71%, IQR 9.05–13.66; controls: 6.26%, IQR 5.4–10.06, P = 0.002) and CD14lowCD16+ monocyte remained unaltered (P = 0.311; Fig. 1A, left). Intrathecal changes in monocyte subsets were not observed. In contrast, ALS patients exhibited increased proportions of CD4+ (12.10%, IQR 7.96–15.86) and CD8+ T cells (10.32%, IQR 7.46–12.55) expressing the activation marker HLA-DR in the CSF and in the case of CD8+ T cells also in PB (1.87%, IQR 1.03–3.51) compared with controls (CD4+HLA-DR+ CSF 6.83%, IQR 5.18–10.33, P = 0.001; CD8+HLA-DR+ PB 0.92%, IQR 0.71–1.24, P = 0.003; CD8+HLA-DR+ CSF 6.90%, IQR 4.44–9.41, P = 0.012; Fig. 1A).
To analyse the specificity of these observations, monocyte subset frequencies in PB and T-cell activation in PB and CSF in ALS patients were compared with dementia and PPMS patients (Fig. 1B). CD14+CD16− monocytes in PB and CD8+ HLA-DR+ T-cell frequencies in the CSF did not differ between those cohorts (P = 0.050/0.999, P = 0.999/0.651), whereas the intrathecal level of T-cell activation was increased for CD4+ T cells compared with dementia (8.65%, IQR 5.58–11.55, P = 0.035) but not with PPMS (8.79%, IQR 5.58–12.86, P = 0.110). With regard to PB changes, CD14+CD16+ monocyte levels were increased in ALS patients compared with dementia (7.94%, IQR 4.14–10.87, P = 0.007) patients, but not in PPMS patients (8.42%, IQR 5.92–12.97, P = 0.113). In contrast, CD8+ HLA-DR+ T-cell proportions in the PB were elevated in ALS patients compared with PPMS (0.88%, IQR 0.41–1.40, P = 0.002) patients, whereas there was no difference compared with dementia patients (1.49%, IQR 1.26–2.27, P = 0.999).
The intrathecal increase of activated T-cell subsets might either be due to an elevated migration of activated T cells from PB or in situ activation. To quantify the effect of the possible causes, a mathematical model was applied to estimate migration and activation rates from circulating naïve cell subsets [Fig. 1C left (1)] to circulating activated cells [Fig. 1C left (β1)], their migration rates [Fig. 1C left (α1 and α2] into the CSF, and intrathecal activation [Fig. 1C left (β2)]. Results showed no increase of CNS migration of both naïve and activated CD4+ and CD8+ T cells in ALS patients compared with controls (Fig. 1C). In contrast, activation processes were increased in both compartments for CD4+ (PB +34.8%, CSF +82.6%) as well as CD8+ (PB +90.2%, CSF +17.3%) T cells, indicating distinct and independent T-cell activation processes in PB and CNS, rather than migration of activated peripheral T cells, as the reason for increased intrathecal T-cell proportions in ALS (Fig. 1C).
ALS patients with rapid disease course exhibit reduced numbers of CD56bright NK cells in the CSF
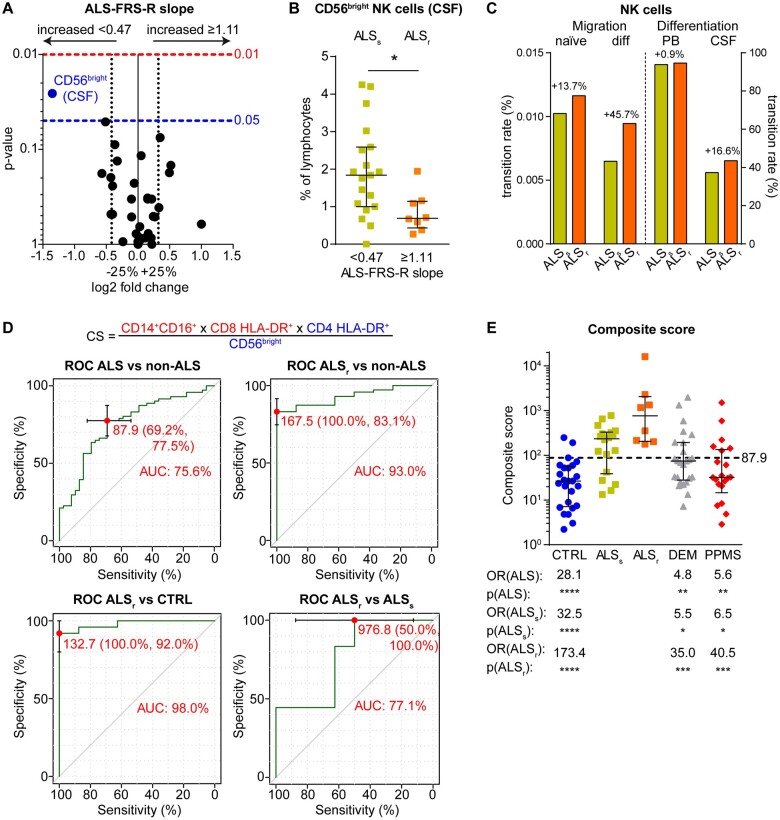
To identify markers for ALS prognosis, both routine CSF and flow-cytometric parameters were investigated in ALS patients stratified by disease progression, as measured by the ALS-FRS-R-slope (Fig. 2A). ALS patients with lower ALS-FRS-R slope, i.e. slower disease progression (ALSs, ALS-FRS-R-slope <0.47), showed increased proportions of immune-regulatory CD56bright NK cells in the CSF (1.84%, IQR 1.00–2.59, P = 0.023) compared with patients with rapid disease progression (ALSr, ALS-FRS-R-slope >1.11; 0.69%, IQR 0.43–1.14) (Fig. 2A and B). Next, the transition rates of naïve CD56bright NK cells for CNS trans-migration and differentiation into CD56dim NK cells were compared between ALS patients with slow and rapid disease progression (Fig. 2C). While ALSr patients showed increased migration of both naïve CD56bright (+13.7%) and differentiated CD56dim NK cells (+45.7%), this cannot explain reduced CD56bright NK cell proportions in the CSF. In contrast, ALSr patients exhibited increased differentiation of intrathecal CD56bright into CD56dim NK cells (+16.6%) as a likely reason for reduced frequencies in the CSF of ALSr patients (Fig. 2C).
Figure 2.
Differential immune phenotype of ALS patients with high disease severity and progression. (A) ALS patients were grouped into patients with slow progression (ALS-FRS-R slope <0.47, n = 19) and patients with fast progression (ALS-FRS-R slope >1.11, n = 8), and classical CSF parameters, as well as flow cytometric parameters, were investigated. Volcano plots represent the median fold change in parameters between the groups, and the corresponding P-values were calculated with the Mann–Whitney test. Only significantly altered parameters (P < 0.05) are labelled. Parameter labelling provides information on the respective compartment (red—PB; blue—CSF). (B) Frequency of CD56bright NK cells in the CSF of ALS patients with slower disease progression (ALSs, yellow) and with rapid progression (ALSr, orange). P-values are calculated with the Mann–Whitney test. (C) Cohort- and cell-specific transition rates derived by mathematical modelling of the differentiation of naïve CD56bright into CD56dim NK cells in the PB and CSF as well as of their CNS trans-migration by Markov jump processes. (D) Composite score (cs; top) calculated by division of putatively pathologic parameters by putatively protective parameters. The optimal cut-off for differentiation of all ALS patients (left top) as well as ALSr patients (right top) from non-ALS patients and of ALSr from controls (CTRL, left bottom) and ALSs (right bottom) was determined by receiver operating characteristic (ROC) analysis, showing specificity, sensitivity, and area under the curve (AUC) for the determined cut-offs. (E) Composite scores of individual patients. Error bars indicate median and IQR. P-values and odds ratio (OR) for the differentiation of all ALS patients (ALS) as well as ALSs and ALSr patients were calculated by Fisher’s exact test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. The dashed line indicates the cut-off of 87.9.
Immune phenotyping may be helpful in classifying ALS patients with rapid disease progression
Finally, the identified parameters were analysed for their potential to differentiate ALS patients from controls, dementia and PPMS patients by constructing a composite score. For this purpose, putatively pathologic parameters, i.e. altered cell populations versus controls that were differentially expressed also in comparison with dementia or PPMS, were divided by putatively protective parameters, i.e. CD56bright NK cells in the CSF (Fig. 2D, bottom). The optimal cut-off (87.9) for differentiation of ALS versus non-ALS patients was calculated by ROC analysis (Fig. 2D, top left), which showed a composite score specificity of 77.5% with a sensitivity of 69.2% and an area under the curve of 75.6%. Furthermore, ROC analysis showed high AUC values for the differentiation of ALSr patients from non-ALS patients (Fig. 2D, top right), controls (Fig. 2D, bottom left), and ALSs (Fig. 2D, bottom right). Using the determined cut-off of 87.9, other cohorts (controls: 26.49, IQR 7.14–55.87, P < 0.0001; dementia: 74.76, IQR 27.87–193.9, P = 0.005; PPMS: 32.01, IQR 14.51–135.3, P = 0.003) could be differentiated from ALS (16.05, IQR 5.77–32.87) with significant odds ratios (Fig. 2E), which was also true for ALSs patients (235.4, IQR 38.62–329.9, P < 0.001/0.014/0.010). ALS patients with rapid disease progression (764.1, IQR 205.7–2061) could distinguished from other cohorts with even higher odds ratios and significance (P < 0.001/0.001/0.001).
Discussion
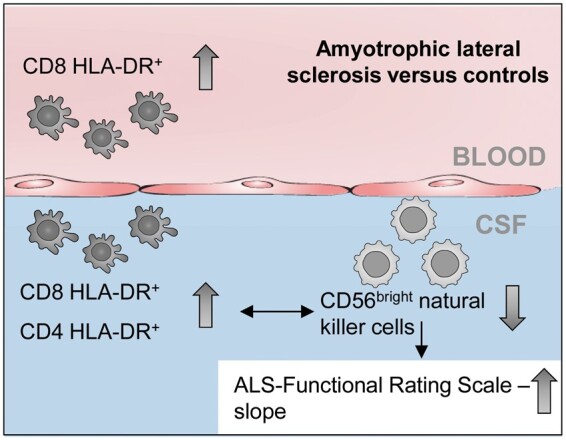
Three important observations emerged from the present study: (i) ALS patients exhibit altered monocyte subset ratios in PB as well as increased levels of activated CD4+ (CSF) and CD8+ T cells (PB and CSF) compared with controls; (ii) decreased intrathecal levels of T-cell regulating CD56bright NK cells are associated with faster disease progression of ALS; and (iii) immunophenotyping may have diagnostic and prognostic value.
Against the background of the emerging role of inflammation in various neurodegenerative diseases,36 we here investigate the immune profile in PB and CSF of ALS patients. In accordance with previous studies, we confirm alterations in monocyte subsets in the periphery of ALS patients.5,22 In detail, we showed an increase of CD14+CD16+ monocytes in the PB of ALS patients, as described by Murdock et al.5 However, in contrast to the mentioned study, our data further described a decrease in CD14+CD16− monocyte levels compared with CLTR, which is consistent with an earlier study of the same research group and other investigations.22,25,27 In contrast to previous findings, we did not find any relation between monocyte levels in PB and clinical decline of ALS patients,22,26 and no differences were apparent in the CD14lowCD16+ population.21,25 Also, intrathecal changes were not observed, in line with a previous study.7
Importantly, in our cohort, the extent of T-cell activation in PB and CSF in ALS patients significantly exceeded the level observed in controls. T-cell activation was not restricted to the periphery but coincided with increased intrathecal T-cell activation. Thus, in ALS, CD8+ T cells might be activated in the periphery and contribute to the dysfunction of the blood/CSF-barrier. Furthermore, intrathecal T cells get activated in an independent process and may ultimately contribute to the degeneration of motor neurons.20 This assumption is supported by the results of our mathematical model, demonstrating an increased activation process in both compartments for CD4+ as well as CD8+ T cells.
Of note, the identification of peripheral alterations in the T-cell population is consistent with recent preclinical and clinical studies, indicating an individual time-dependent change of peripheral T-cell counts in ALS5 as well as a shift towards pro-inflammatory T-cell subsets.7 Interestingly, Murdock et al.5 showed that individual decreasing CD4+ T-cell levels over time correlate with ALS disease progression. They postulated that a reduced number of peripheral CD4+ T cells might be attributable to a reduced amount of regulatory T cells within the CD4 population, likely representing a time-dependent decrease in neuroprotective response.5 Since our study was not designed to obtain longitudinal PB and CSF data, we were not able to confirm this observation. Moreover, we did not investigate the CD4+ regulatory T-cell counts. However, ALS patients with rapid disease course showed reduced proportions of CD56brightNK cells in the CSF. Interestingly, previous studies reported that CD56bright NK cells control activated T cells in the periphery and the CSF, thereby attenuating adaptive immune responses and exerting protective effects in autoimmune CNS diseases.13 Though we did not provide mechanistic investigations, similar mechanism of impaired immune regulation in the CSF of ALS patients might be suggested. Therefore, in ALS, increased T-cell activation might coincide with impaired intrathecal T-cell regulation by CD56bright NK cells, resulting in increased disease progression.14
Notably, activated T cells in the CSF do not appear to be a specific hallmark of ALS but rather a common phenomenon in CNS diseases with neuro-degenerative pathophysiology (Fig. 1B).32,37,38 However, exceeding levels of peripheral and intrathecal T-cell activation paralleled by reduced intrathecal CD56bright NK cell levels might be a characteristic feature of ALS, as demonstrated by the integration of these parameters in a composite score capable of differentiating ALS from controls as well as from dementia and PPMS.
Moreover, a combination of both routine CSF analysis and intrathecal immune cell profiling helps to differentiate between ALS and PPMS, as both diseases share clinical signs resulting from the degeneration of the long cerebral and spinal cord tracts in the central motor system.39 While the extent of intrathecal T-cell activation was similar between both groups, B-cell immunity seems to be a major hallmark of PPMS reflected by the presence of OCB and a higher degree of plasma-cell reactivity.40
Our study has several limitations. First, we do not describe immunological changes over time. To support our findings, additional cytokine measurements, as well as a more detailed T-cell profiling, might have been favourable, but the tiny amount of biomaterial generated during the diagnostic process did not allow for additional measures of (rare) cell populations. Moreover, the applied mathematical model only considered four-cell stages and four transition rates. This approach is certainly not a very detailed description of the underlying complex biological process but was justified by and corresponded to the available data.
To conclude, we identified altered monocyte subset proportions as well as increased peripheral and intrathecal T-cell activation as factors associated with motor neuron degeneration in ALS, a pattern that is partially shared with other neurodegenerative diseases. Moreover, impaired intrathecal T-cell regulation by CD56bright NK cells might be associated with ALS progression, thus potentially providing a marker for ALS prognosis. Further prospective studies to thoroughly characterize T-cell immunity and regulation in ALS are needed to better understand the pathophysiology and heterogeneity of the disease. In the long-term, those studies might help to translate our findings into clinical practice and explore novel therapeutic options to restore T-cell regulation in ALS.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
The authors thank Kirsten Weiß, Gabriele Behrens, Arne Seeger and Christiane Schulze-Weppel for excellent technical assistance. Information presented within this manuscript is part of a filed patent.
Funding
The authors received financial support from the Deutsche Forschungsgemeinschaft (DFG; projects: 333849990/IRTG-2379 and HIDSS-004) and the federal institute for risk assessment [Bundesinstitut für Risikobewertung (BfR), project 60–0102-01.P585]. Funders had no role in study design, data collection, data analyses, interpretation or writing of the report.
Competing interests
L.R. received travel reimbursements from Merck Serono and Sanofi Genzyme. A.S.-M. received travel expenses and research support from Novartis. S.P. received travel reimbursements from Sanofi-Genzyme and Merck Serono, honoraria for lecturing from Sanofi Genzyme, Biogen and Mylan Healthcare, and research support from Merck Serono, Diamed and the German Multiple Sclerosis Society. T.R. reports grants from German Ministry of Education, Science, Research and Technology, during the conduct of the study; grants and personal fees from Sanofi-Genzyme; personal fees from Biogen; personal fees and nonfinancial support from Merck Serono; personal fees from Roche and Teva, outside the submitted work. H.W. receives honoraria for acting as a member of Scientific Advisory Boards of Biogen, Evgen, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Roche Pharma AG and Sanofi-Aventis as well as speaker honoraria and travel support from Alexion, Biogen, Cognomed, F. Hoffmann-La Roche Ltd, Gemeinnützige Hertie-Stiftung, Merck Serono, Novartis, Roche Pharma AG, Genzyme, TEVA and WebMD Global. He is acting as a paid consultant for Abbvie, Actelion, Biogen, IGES, Johnson & Johnson, Novartis, Roche, Sanofi-Aventis, and the Swiss Multiple Sclerosis Society. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgesellschaft (DFG), European Union (Horizon 2020), Else Kröner Fresenius Foundation, Fresenius Foundation, Hertie Foundation, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (IZKF) Muenster and RE Children’s Foundation, Biogen, GlaxoSmithKline GmbH, Roche Pharma AG and Sanofi-Genzyme. C.G. received speaker honoraria from Mylan, Bayer Healthcare and Sanofi-Genzyme and travel/accommodation/meeting expenses from Bayer Healthcare, Biogen, EUROIMMUN, Novartis and Sanofi-Genzyme. She also received research support from Biogen and Novartis; her research is funded by the European Union (Horizon 2020), the German Ministry for Education and Research (BMBF; KKNMS), Deutsche Forschungsgemeinschaft (DFG; SFB128A09, Susac) and the Interdisciplinary Center for Clinical Studies (IZKF) Muenster. C.C.G. received speaker honoraria from Mylan, Bayer Healthcare and Sanofi-Genzyme and travel/accommodation/meeting expenses from Bayer Healthcare, Biogen, EUROIMMUN, Novartis and Sanofi-Genzyme. She also received research support from Biogen and Novartis. S.G.M. received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology and by Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, ONO Pharma, Roche and Teva. M.B. received speaker honoraria and financial research support from Desitin, UCB, Sanofi-Genzyme and Löwenstein Medical. M.P. received speaker honoraria and travel/accommodation/meeting expenses and research funding from Novartis. His research is founded by the German Multiple Sclerosis Society North Rhine-Westphalia (DMSG) and the programme ‘Innovative Medizinische Forschung (IMF)’ of the Medical Faculty of the University of Muenster. And the other authors have no competing of interest to declare.
Glossary
- ALS =
amyotrophic lateral sclerosis
- ALS-FRS-R =
ALS Functional Rating Scale
- MHC =
major histocompatibility complex
- PB =
peripheral blood
- PPMS =
primary progressive multiple sclerosis
- ROC =
receiver operating characteristic
- NK cells =
natural killer cells
References
- 1.Beers DR, Appel SH.. Immune dysregulation in amyotrophic lateral sclerosis: Mechanisms and emerging therapies. Lancet Neurol. 2019;18(2):211–220. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell JD, Borasio GD.. Amyotrophic lateral sclerosis. Lancet. 2007;369(9578):2031–2041. [DOI] [PubMed] [Google Scholar]
- 3.Mejzini R, Flynn LL, Pitout IL, Fletcher S, Wilton SD, Akkari PA.. ALS genetics, mechanisms, and therapeutics: Where are we now? Front Neurosci. 2019;13:1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooten KG, Beers DR, Zhao W, Appel SH.. Protective and toxic neuroinflammation in amyotrophic lateral sclerosis. Neurotherapeutics. 2015;12(2):364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murdock BJ, Zhou T, Kashlan SR, Little RJ, Goutman SA, Feldman EL.. Correlation of peripheral immunity with rapid amyotrophic lateral sclerosis progression. JAMA Neurol. 2017;74(12):1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beers DR, Henkel JS, Zhao W, et al. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain. 2011;134(Pt 5):1293–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin M, Gunther R, Akgun K, Hermann A, Ziemssen T.. Peripheral proinflammatory Th1/Th17 immune cell shift is linked to disease severity in amyotrophic lateral sclerosis. Sci Rep. 2020;10(1):5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmoy T.T cells in amyotrophic lateral sclerosis. Eur J Neurol. 2008;15(4):360–366. [DOI] [PubMed] [Google Scholar]
- 9.Cooper MA, Fehniger TA, Caligiuri MA.. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. [DOI] [PubMed] [Google Scholar]
- 10.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA.. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155(6):1823–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanier LL.NK cell recognition. Annu Rev Immunol. 2005;23:225–274. [DOI] [PubMed] [Google Scholar]
- 12.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S.. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. [DOI] [PubMed] [Google Scholar]
- 13.Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103(15):5941–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross CC, Schulte-Mecklenbeck A, Runzi A, et al. Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation. Proc Natl Acad Sci U S A. 2016;113(21):E2973–E2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laroni A, Armentani E, Kerlero de Rosbo N, et al. Dysregulation of regulatory CD56(bright) NK cells/T cells interactions in multiple sclerosis. J Autoimmun. 2016;72:8–18. [DOI] [PubMed] [Google Scholar]
- 16.Han S, Lin YC, Wu T, et al. Comprehensive immunophenotyping of cerebrospinal fluid cells in patients with neuroimmunological diseases. J Immunol. 2014;192(6):2551–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamann I, Dorr J, Glumm R, et al. Characterization of natural killer cells in paired CSF and blood samples during neuroinflammation. J Neuroimmunol. 2013;254(1-2):165–169. [DOI] [PubMed] [Google Scholar]
- 18.Lunemann A, Lunemann JD, Munz C.. Regulatory NK-cell functions in inflammation and autoimmunity. Mol Med. 2009;15(9-10):352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelhardt JI, Tajti J, Appel SH.. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch Neurol. 1993;50(1):30–36. [DOI] [PubMed] [Google Scholar]
- 20.Coque E, Salsac C, Espinosa-Carrasco G, et al. Cytotoxic CD8(+) T lymphocytes expressing ALS-causing SOD1 mutant selectively trigger death of spinal motoneurons. Proc Natl Acad Sci U S A. 2019;116(6):2312–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGill RB, Steyn FJ, Ngo ST, et al. Monocytes and neutrophils are associated with clinical features in amyotrophic lateral sclerosis. Brain Commun. 2020;2(1):fcaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murdock BJ, Bender DE, Kashlan SR, et al. Increased ratio of circulating neutrophils to monocytes in amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3(4):e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafson MP, Staff NP, Bornschlegl S, et al. Comprehensive immune profiling reveals substantial immune system alterations in a subset of patients with amyotrophic lateral sclerosis. PLoS One. 2017;12(7):e0182002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantovani S, Garbelli S, Pasini A, et al. Immune system alterations in sporadic amyotrophic lateral sclerosis patients suggest an ongoing neuroinflammatory process. J Neuroimmunol. 2009;210(1-2):73–79. [DOI] [PubMed] [Google Scholar]
- 25.Zondler L, Muller K, Khalaji S, et al. Peripheral monocytes are functionally altered and invade the CNS in ALS patients. Acta Neuropathol. 2016;132(3):391–411. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R, Gascon R, Miller RG, et al. Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol. 2005;159(1-2):215–224. [DOI] [PubMed] [Google Scholar]
- 27.Butovsky O, Siddiqui S, Gabriely G, et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122(9):3063–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–299. [DOI] [PubMed] [Google Scholar]
- 29.Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169(1-2):13–21. [DOI] [PubMed] [Google Scholar]
- 30.Labra J, Menon P, Byth K, Morrison S, Vucic S.. Rate of disease progression: A prognostic biomarker in ALS. J Neurol Neurosurg Psychiatry. 2016;87(6):628–632. [DOI] [PubMed] [Google Scholar]
- 31.Hamidou B, Marin B, Lautrette G, et al. Exploring the diagnosis delay and ALS functional impairment at diagnosis as relevant criteria for clinical trial enrolment. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(7-8):519–527. [DOI] [PubMed] [Google Scholar]
- 32.Lueg G, Gross CC, Lohmann H, et al. Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer's disease. Neurobiol Aging. 2015;36(1):81–89. [DOI] [PubMed] [Google Scholar]
- 33.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. [DOI] [PubMed] [Google Scholar]
- 34.Ruck T, Bittner S, Meuth SG, Herty M.. Insights from mathematical modelling for T cell migration into the central nervous system. Math Med Biol. 2017;34(1):39–58. [DOI] [PubMed] [Google Scholar]
- 35.Gross CC, Pawlitzki M, Schulte-Mecklenbeck A, et al. Generation of a model to predict differentiation and migration of lymphocyte subsets under homeostatic and CNS autoinflammatory conditions. Int J Mol Sci. 2020;21(6):2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gate D, Saligrama N, Leventhal O, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer's disease. Nature. 2020;577(7790):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroder JB, Pawlowski M, Meyer Zu Horste G, et al. Immune cell activation in the cerebrospinal fluid of patients with Parkinson's disease. Front Neurol. 2018;9:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heneka MT.An immune-cell signature marks the brain in Alzheimer's disease. Nature. 2020;577(7790):322–323. [DOI] [PubMed] [Google Scholar]
- 39.Abdelhak A, Junker A, Brettschneider J, et al. Brain-specific cytoskeletal damage markers in cerebrospinal fluid: Is there a common pattern between amyotrophic lateral sclerosis and primary progressive multiple sclerosis? Int J Mol Sci. 2015;16(8):17565–17588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(Pt 5):1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data (including demographic and laboratory measures) collected during the trial will be available, after de-identification. Moreover, we will share statistical analysis, including analytic codes and informed consent forms. Data will be available for all types of analyses immediately following publication, without end date, and will be shared with qualified investigators upon request. Please contact marc.pawlitzki@ukmuenster.de.