Abstract
Background
A new inactivated whole-virion QazCovid-in® vaccine against COVID-19 was developed from SARS-CoV-2 isolated in Kazakhstan, inactivated by formaldehyde, and adjuvanted with aluminium hydroxide. Phase 1 and 2 clinical trials aimed at assessing the vaccine's safety, immunogenicity, and the duration of immunity induced by the QazCovid-in® vaccine after one or two immunisations.
Methods
From 23.09.2020 to 19.03.2021 we performed a randomised, single-blind, placebo-controlled phase 1 clinical trial and from 18.10.2020 to 17.04.2021 an open-label phase 2 clinical trials of the QazCovid-in® vaccine with a 6 months follow-up at a single centre in Almaty, the Republic of Kazakhstan. Eligible healthy adults aged 18 years and older with no history of laboratory-confirmed SARS-CoV-2 infection were randomly assigned to the treatment groups using a computerised randomisation scheme generator. In the phase 1 clinical trial, two doses of the vaccine (5 μg each) or placebo (0·9% NaCl) were administered intramuscularly to 44 subjects aged 18–50 years, 21 days apart. In the phase 2 trial, 200 healthy participants were randomised into four equal-sized groups according to the age (18–49 or ≥50 years) and either single (day 1) or double (day 1 and 21) vaccination protocol. The primary outcomes were safety and tolerability. The secondary outcome was immunogenicity. The cellular response was measured by a whole-blood cytokine release assay (phase 1 only). The trials were registered with ClinicalTrials.gov NCT04530357.
Findings
The QazCovid-in® vaccine was safe and well-tolerated and induced predominantly mild adverse events; no serious or severe adverse events were recorded in both trials. In the phase 1 trial, the percentage of subjects with a fourfold increase of antibody titres (sero conversion) in MNA was 59% after one vaccine dose and amounted to 100% after two doses. Neutralizing antibody titres reached the geometric mean titre (GMT) of 100 after administration of two doses. A statistically significant increase in the levels of pro-inflammatory cytokines after vaccination indicated the Th1-biased response. On day 180, 40% of placebo-treated subjects demonstrated a statistically significant increase in the levels of antibodies measured by both ELISA and MNA, which suggests the infection with SARS-CoV-2. In the phase 2 trial, 100% of subjects aged 18–49 years seroconverted for SARS-CoV-2 on day 21 after the first dose, as indicated by MNA yielding the GMTs of 32 or 30 in the one- and two-dose groups, respectively. Amongst ≥50-year-old subjects, the number of sero conversions in the two- and one-dose groups on day 21 was 94% and 92% with the respective GMTs of 25 and 24. After the second dose, the sero conversion rate reached 100%; however, the GMT was significantly lower when compared with the corresponding value measured in subjects aged 18–49 years (83 vs 143). In both trials, specific antibodies were detected in MNA and ELISA on study day 180, but the titres dropped in comparison to day 42. The results of this study serve as the rationale for the phase 3 study.
Interpretation
The QazCovid-in® vaccine is safe and well-tolerated and promotes pronounced humoral immunity which lasts for at least 6 months after double intramuscular immunisation.
Funding
The work was funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan within the framework of the Scientific and Technical Program "Development of a vaccine against coronavirus infection COVID-1900 . State registration number ?.0927.
Research in context.
Evidence before this study
The PubMed, Medline, and server medRxiv search for publications on clinical trials of COVID-19 vaccines, conducted on the 15th of March 2021, showed that 5 inactivated whole-virion candidate vaccines formulated with an aluminium adjuvant were in clinical development. Only the study of the BBV152 vaccine, which was formulated with aluminium and a Toll-like receptor 7/8 agonist molecule (IMDG) and manufactured in India, investigated the durability of immune response over a 3 month follow-up period after the second dose. Our study included a 6-month follow-up after the first immunization.
Added value of this study
After two immunizations, the inactivated whole-virion vaccine against COVID-19 induced humoral immunity with high titres of neutralizing antibodies persisting for 6 months, and T-cell immunity which was biased to Th1. Vaccinated volunteers did not show any increase in antibody titers to SARS-CoV-2 from day 42 to day 180 of the phase 1 study in contrast to the participants treated with placebo.
Implication of all the available evidence
The data obtained support the phase 3 clinical trial of the QazCovid-in® inactivated whole-virion vaccine in Kazakhstan, recruiting 3000 subjects aged 18 years and older. On January 5, 2021, the QazCovid-in® vaccine has been granted emergency use authorisation in Kazakhstan.
Alt-text: Unlabelled box
Introduction
At the end of 2019, a new severe acute respiratory syndrome coronavirus (SARS-CoV-2) emerged in China (Wuhan) and spread throughout the globe in several months inducing the disease named COVID-19 [1,2]. On March 11, 2020, the World Health Organization declared the COVID-19 pandemic. The infection can manifest in different forms, from asymptomatic (predominantly in children) and mild/medium to severe one which can lead to a lethal outcome. The case fatality rate for COVID-19 is 4·16% and 3·26% in men and women, and it rises to 56·82% and 41·10%, respectively, for people over 80 [3]. In one year, the number of laboratory-confirmed cases of SARS-CoV-2 exceeded 112 million, with more than 2.5 million deaths worldwide [1]. As there is no specific treatment that can effectively neutralise SARS-CoV-2 and prevent the virus spread, the world population is confronted with a substantial threat.
Preventive vaccination is the only effective measure to counteract the pandemic. Various companies have begun to develop vaccines based on different platforms, rapidly progressing through phase 1/3 clinical trials for evaluation. Amongst the first vaccines, several vector-based (Gamaleya National Research Centre [4], University of Oxford/AstraZeneca [5]) and mRNA-based vaccines (Moderna/National Institute of Allergy and Infectious Diseases [6], BioNTech/Fosun Pharma/Pfizer [7]) have been developed and produced. These research centres are amongst the first who managed to show vaccine's safety and high efficacy in phase 3 clinical trials, to pass the official registration, and to initiate the immunisation programs in different countries. Although community vaccination programmes are already running in many countries, there are still concerns about vaccine shortage, and a big part of the world population might not receive a vaccine in time. Therefore, conventional whole-virion inactivated vaccines or vaccines comprising recombinant proteins with the addition of an adjuvant are also considered [8], [9], [10].
In Kazakhstan, about 250,000 cases of SARS-CoV-2 have been registered up to date, with more than 2950 lethal cases. Despite the strict sanitary quarantine measures and widespread laboratory diagnostics, the number of people infected with SARS-CoV-2 continues to grow. To counter the pandemic, the Scientific Research Institute for Biological Safety Problems of the Republic of Kazakhstan has developed technology to produce QazCovid-in®, an inactivated whole-virion vaccine. The vaccine is produced in Vero cells, inactivated by formaldehyde, and adjuvanted with aluminium hydroxide.
Here, we report the results of two clinical trials, both of which included a 6-month follow-up period and aimed to evaluate the safety and immunogenicity of the QazCovid-in® vaccine. A single-centre, randomised, single-blind, placebo-controlled phase 1 clinical trial was conducted in subjects aged 18–50 years who received two doses of the vaccine. An open-label phase 2 clinical trial was performed within a single or double vaccination protocol in the subjects of two age groups, 18–49 and ≥50 years.
Methods
Study design and participants
From 23.09.2020 to 19.03.2021 we conducted a randomised, single-blind, placebo-controlled (2 groups in a 1:1 ratio for vaccine or placebo, 44 subjects) phase 1 clinical trial and from 18.10.2020 to 17.04.2021 a randomised, open-label (4 groups divided in a 1:1:1:1 ratio depending on the age and regimen of vaccination, 200 subjects) phase 2 trial of the QazCovid-in® vaccine at the National Scientific Centre for Phthisiopulmonology (Almaty, Kazakhstan).
Participants were recruited through ethics-approved advertising on the study site's website. Eligible participants were male or female adults aged 18 years and older who were healthy at enrolment based on their medical history, vital signs, and physical examination. Participants were screened to meet the inclusion criteria. Subjects were enrolled in the trials if they had no history of COVID-19, their serology tests for IgM and IgG antibodies to SARS-CoV-2 were negative, and if they had no close contact with individuals suspected of being infected with SARS-CoV-2 14 days before the enrolment. Female participants of childbearing potential had to agree to use reliable forms of contraception throughout the whole study period. Volunteers were screened for the absence of chronic viral infections, such as human immunodeficiency virus (HIV), hepatitis B (HBV) and hepatitis C (HCV) viruses. Routine biochemical blood test, hematology test panel, electrocardiogram (ECG), and pregnancy test for women were also performed. Main exclusion criteria included allergic history, drug intolerance including hypersensitivity to any vaccine component, an axillary temperature of more than 37·0 °C, abnormalities in laboratory tests, and positive urine pregnancy test for women. Subjects with any mental disease or serious chronic illness were also excluded. A detailed list of the inclusion and exclusion criteria can be found in the Supplement. The trials were approved by the National Regulatory Authority and the ethics committee of the National Scientific Centre for Phthisiopulmonology of the Republic of Kazakhstan (No. KZ78VMX00000211) and conducted in compliance with the International Council for Harmonization Good Clinical Practice guidelines. An investigator or a staff member explained the investigational nature and the purpose of the study to a subject in sufficient detail to allow the subject to make an informed decision about participating. Written informed consent was obtained from the study subjects before enrolment and before any study procedure, including screening. The trials were registered with ClinicalTrials.gov NCT04530357.
Procedures
The QazCovid-in® vaccine was produced from the SARS-CoV-2 strain, isolated in Kazakhstan, at the Scientific Research Institute for Biological Safety Problems according to the Good Manufacturing Practice guidelines. The SARS-CoV-2/human /KAZ/KZ_Almaty/2020 strain was sequenced, and the complete genome sequence was deposited in GenBank (accession number MZ379258.1). The nucleotide sequence of the genome of the SARS-CoV-2 /human/KAZ/KZ_Almaty/2020 strain is almost identical to the sequence of the Wuhan-Hu-1 isolate (accession number NC_045512.2). The SARS-CoV-2/human/KAZ/KZ_Almaty/2020 virus was grown in Vero cells (WHO). The harvested virus was inactivated by formaldehyde, clarified by low-speed centrifugation, purified, and concentrated. Sterilising filtration was carried out through cascades of filters with a pore diameter of 0·45/0·22 μm. Then, 2% gel of aluminium hydroxide was added to a final concentration of 0·5 µg/0·5 ml. The total protein concentration was 5 µg per dose of the vaccine. The vaccine was stored at 2–8 C in single-use glass vials and was provided as a sterile liquid ready for intramuscular injection into the upper arm (deltoid muscle) at a volume of 0·5 ml per dose. The vaccine was administered in a one- or two-dose regimen on day 1 and day 21. Both clinical trials included a screening stage and 16 inpatient visits during the whole study period. The phase 2 trial started after the 7-day safety observation period following the second vaccination during the phase 1 trial. The interval between the screening and the day 1 visit did not exceed five days. Follow-up visits were scheduled on days 1–7, 21–27, 42, and 180. Blood and urine samples for the laboratory testing (hematological and biochemical data, urinary test data) were collected on study days 1, 3, 21, 27, 42, and 180. The humoral immune response was analyzed on study day 1 and 21 (before the first and second vaccination, respectively) and on days 27, 42, and 180. The whole-blood cytokine release assay was done only in the phase 1 study: on study day 1 (before first vaccination), days 7, 21 (before second vaccination), days 27, 42, and 180. A detailed description of all assay methods can be found in the Supplement.
Randomisation and masking
In the phase 1 clinical trial, 44 participants aged 18–50 years were randomly assigned (1:1) to receive the QazCovid-in® vaccine or placebo. In the phase 2 clinical trial, 200 participants from the two age groups (18–49 years and 50 years and older) were randomly divided into four equal-sized groups to receive the QazCovid-in® vaccine in either a single-dose or two-dose regimen. The randomization was carried out with complete concealment and using SAS Programming (version 9.4). The randomization codes were available to the Principal Investigator, the sponsor's designee, and an independent statistician. Unblinding of randomization codes was scheduled on day 90 according to the protocol. Premature unblinding was possible in case of a serious adverse event. Eligible participants were sequentially assigned a study number at enrolment and were vaccinated according to the randomisation list prepared by an independent statistician. Study medications were allocated to codes by block randomisation using a computerised randomisation scheme generator. In the phase 1 trial, the unblinded study personnel prepared the vaccine or placebo out of sight of the participants, using opaque syringes to ensure masking since the vaccine and placebo are visually different. An independent monitor assigned by the funder's representative closely monitored the process to confirm adherence to the randomisation scheme.
Outcomes
The primary safety outcomes were the incidence of solicited adverse reactions within 7 days after each dose and the incidence of unsolicited adverse events (AEs) for 21 days after each vaccination. The secondary safety outcomes included the occurrence of adverse events from day 43 to day 180, and serious adverse events from day 1 through day 180. The primary endpoints for immunogenicity were the geometric mean titres (GMTs) of S-specific antibodies and virus neutralizing antibodies measured on days 21 and 42 after vaccination. The secondary endpoints for immunogenicity were antibody responses 6 months after vaccination, quantified by ELISA and MNA, and specific cell-mediated response on study days 7, 21, 27, 42 and 180. Seroconversion of the humoral immune responses was also a secondary endpoint, and it was defined as at least a fourfold increase in post-vaccination antibody titre compared to the baseline.
Safety evaluation data included the results of physical examination, vital signs, and laboratory tests, which were obtained at specified time points throughout the trial. All participants were first observed for at least 2 h after each vaccination. They were then closely monitored for 7 days after each vaccination, having their temperature, blood pressure, pulse, and other symptoms recorded during their daily visits to the study centre. In addition, from day 8 to 20 (self-observation Diary 1) and from 28 to 41 (self-observation Diary 2) of the study, the volunteers filled out self-observation diaries. From day 43 to day 180 of the study, the subjects reported any adverse events (local and systemic reactions) to the investigator by phone, as specified in the informed consent form. Adverse events were graded according to a severity score (mild, moderate, severe) and whether they were related or unrelated to the investigational vaccine, as detailed in the Protocol (appendix 1). Clinical safety evaluation data and all laboratory test data were transferred to the CRF from the clinical test report forms. CRF data were input into the database, verification of data accuracy, identification of the study subject by the individual number, and the entered data processing were carried out using special database management software.
Statistical analysis
While the sample size was not based on any statistical hypothesis, it was adjusted to adequately assess the vaccine's safety in each study group. Qualitative characteristics of a study group were presented as percentages; quantitative parameters were presented as median with interquartile range (IQR) and min-max range. The safety analysis set included all randomised participants who received at least one dose of the study vaccine or placebo. Descriptive summary data were provided as numbers and per group percentages and included the participants who reported at least one solicited local reaction or systemic adverse event, any unsolicited adverse events, serious adverse events, or adverse events of special interest after the first or second dose. The attributable risk was calculated for particular groups and presented with 95% CI calculated by the Newcombe-Wilson method with continuity correction. The difference in IgE levels between placebo and vaccine groups was assessed by repeated-measures ANOVA (RM-ANOVA) applied to logarithmic data values. Immunogenicity analysis included the participants who received at least one dose of the study vaccine or placebo and provided blood samples according to their allocated vaccination schedule. Immunogenicity metrics were presented as geometric mean titres (GMTs) with 95% CIs. A seroconversion rate was assessed as the percentage of subjects with a fourfold or greater increase in antibody titre compared to day 1 and was presented with 95% Wilson CI. Post hoc statistical analyses were performed for MNA data, ELISA data, and Cytokine data. For each dataset, we performed a two-way RM-ANOVA followed by the Bonferroni multiple comparison test. Sources of variation included: 1) type of immunisation (vaccine vs placebo in phase 1 study; single vs double immunisation in phase 2 study); 2) day after immunisation (time point). Dependent variables were: log2 antibody titre in ELISA data set analysis; log2 neutralising antibody titre in MNA data set analysis; log10 cytokine concentration, independently for each cytokine data set. The analysis implied that values were log-normally distributed. The Greenhouse-Geisser correction was applied, assuming the unequal variation of the differences between both the types of immunisation and the time points. To verify the difference in antibody response between age groups, separate data analyses were performed using the two-way RM-ANOVA with age group and time point as the two sources of variance. The immunogenicity data were missing for 2 subjects in the phase 1 study and 30 subjects in the phase 2 study, as the subjects failed to attend the follow-up on day 180. The data omission procedure was run, and the data from these subjects were excluded from the analysis. Statistical analysis was performed using GraphPad Prism 8 (version 8.4.3) software.
Role of funding
The work was funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan. The sponsor had no role in the design of the study, as well as in the collection, analyses, or interpretation of data, or in the writing of the manuscript, and in the decision to publish the results.
Results
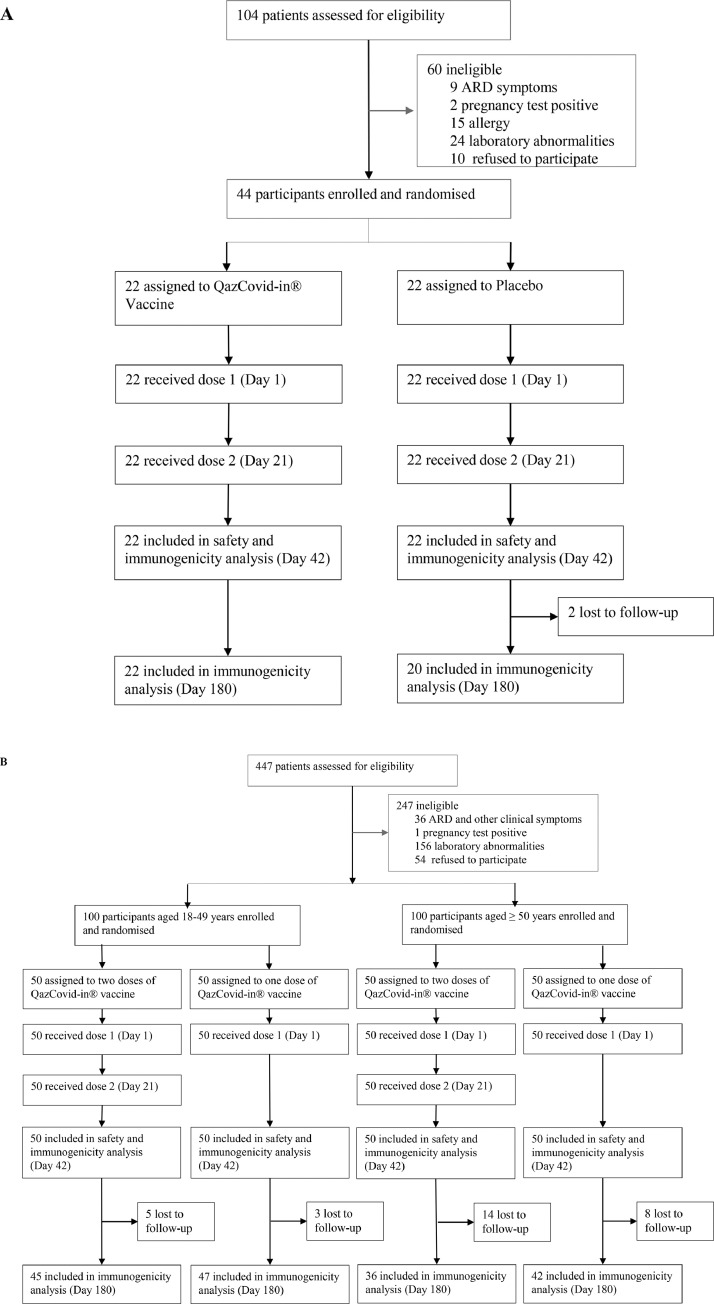
Between Sept 19 and Sept 23, 2020, 104 potential participants aged 18–50 years were screened, of whom 44 were enrolled into the phase 1 clinical trial and randomly assigned to receive the QazCovid-in® vaccine or placebo (1:1, 22 subjects per group). The process of study subjects’ distribution is summarised in Fig. 1A. The majority of screen failures (50 [83·3%] out of 60) were due to the exclusion criteria. The subjects meeting the inclusion criteria were randomised, received the assigned treatment on day 1 and day 21, and completed all scheduled visits, except for the two subjects from the placebo group who missed their last visit on day 180. The baseline characteristics of the participants enrolled in the phase 1 study are shown in table S1. The median age was 28·0 years (23·3–32·8) for the vaccine recipients and 28·0 years (24·0–42·5) for the placebo recipients; 17 (77·3%) and 12 volunteers (54·5%) were male in the vaccine and placebo groups, respectively. The population was homogeneous with a slight predominance of males.
Fig. 1.
Trial profile for phase 1 (A) and phase 2 (B).
For the phase 2 clinical trial, 447 participants were recruited and screened for eligibility between Oct 15 and Oct 20, 2020. Two hundred healthy adults were enrolled and assigned to their respective age groups: 18–49 years, 100 participants, and ≥50 years, 100 participants. Within the age groups, 100 participants were randomly assigned to receive the QazCovid-in® vaccine in a single-dose or two-dose regimen (21 days apart). The trial profile for the phase 2 study is given in Fig. 1B. Demographics were similar across the groups (table 1). In the 18–49 years group, the median age of participants was 35·0 years (25·3–41·8) for the two-dose vaccine recipients and 32·0 years (24·3–41·0) for the one-dose vaccine recipients. In the ≥50 years age group, the median age was 54·5 years (52·0–57·3) and 55·0 years (52·0–61·0) for the one-dose and two-dose vaccine recipients, respectively. The population was homogeneous. None of the subjects enrolled in both trials had any underlying disease or history of allergic reactions.
Table 1.
Demographic characteristics of subjects who participated in the phase 2 trial.
| Vaccine (n = 50)18–49 years2 Doses | Vaccine (n = 50)≥ 50 years2 Doses | Vaccine (n = 50)18–49 years1 Dose | Vaccine (n = 50)≥ 50 years1 Dose | |
|---|---|---|---|---|
| Sex | ||||
| Female | 30 (60·0%) | 31 (62·0%) | 28 (56·0%) | 34 (68·0%) |
| Male | 20 (40·0%) | 19 (38·0%) | 22 (44·0%) | 16 (32·0%) |
| Ethnicity | ||||
| Caucasian | 12 | 2 | 14 | 7 |
| Asian | 38 | 48 | 36 | 43 |
| Age, years | 35·0 (25·3, 41·8) | 55·0 (52·0, 61·0) | 32·0 (24·3, 41·0) | 54·5 (52·0, 57·3) |
| 20·0–49·0 | 50·0–66·0 | 19·0–49·0 | 51·0–70·0 | |
| Height, cm | 168·5 (165·0, 173·5) | 169·0 (164·3, 171·0) | 169·5 (165·5, 174·0) | 168·0 (164·0, 171·0) |
| 156·0–191·0 | 152·0–180·0 | 155·0–179·0 | 153·0–175·0 | |
| Body weight, kg | 71·0 (61·8, 75·8) | 83·0 (76·8, 87·0) | 71·5 (61·0, 82·8) | 79·0 (73·0, 85·0) |
| 51·0–95·0 | 58·0–97·0 | 53·0–110·0 | 53·0–95·0 |
Data are presented as Median (IQR) and min-max range.
The safety analysis set included the randomised subjects who received at least one dose of the study vaccine and for whom safety data were available.
The QazCovid-in® vaccine induced predominantly mild adverse events; no serious or severe adverse events were recorded for studied subjects in both trials. No immediate reactions were reported within the first 2 h after either single or double administration of the vaccine. During 7 days after the first vaccination, solicited local adverse events of mild severity (grade 1) were observed in 6 vaccinated subjects (27%), which included pain at the injection site (table S2). One mild systemic AE (increased body temperature, up to 37·1 °C) was observed on day 1 after the first vaccination. No AEs were observed after the second vaccination or in the placebo group. The routine laboratory safety tests that were performed on the scheduled study days showed no abnormalities according to the FDA Toxicity Grading Scale, as detailed in the protocol (appendix 1, p. 93–99). No statistically significant difference between the placebo and vaccine groups was revealed in the level of IgE antibodies (ELISA) in the serum specimens obtained before vaccination (day 1), 21 days after the first and second immunisations (day 21 and 42, respectively, two-way RM-ANOVA: p [Group] = 0·1641; p [Day] = 0·003; p [Group*Day] =0·4220) (table 2). No serious AEs or AEs of special interest were reported within the 180-day follow-up period.
Table 2.
Total IgE in serum on different time points from the Phase 1 study.
| Assay | Placebo (n = 22) |
Vaccine (n = 22) |
||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 21 | Day 27 | Day 42 | Day 1 | Day 21 | Day 27 | Day 42 | |
| GMT (95% CI) | 4·5 (2·6, 7·6) |
11·5 (7·1, 18·6) | 12 (9·2, 15·7) | 12·7 (8·3, 19·6) | 4·7 (2·7, 8·4) | 15·3 (10·4, 22·6) | 8·9 (6·1, 12·7) | 10·5 (6·7, 16·4) |
| Median (IQR) | 3·3 (1·6, 15) | 10·5 (6·8, 38·8) | 9·8 (8, 17·3) | 13·8 (7·3, 30·3) | 3·5 (1·1, 8·8) | 16·6 (8·5, 27·7) | 7·5 (5·1, 20·2) | 13·9 (5·5, 28·4) |
Values shown represent IgE concentrations measured in IU/ml. Presented data are geometric mean with geometric SD. No statistically significant difference was observed between Placebo and Vaccine groups at any time points (two-way RM-ANOVA results: p [Group] = 0.1641; p[Day] = 0.003; p[Group*Day] =0.4220).
In the phase 2 study, 77% of participants (77 of 100) aged 18–49 years and 39% of ≥50-year-old participants (39 of 100) reported solicited local and systemic AEs after the first dose of the QazCovid-in® vaccine (table 3). Local reactions were represented by transient (1–3 days) cases of pain, hyperaemia, induration, and itch at the injection site, which were of grade 1 (mild severity) almost exclusively. Only six and two cases of grade 2 (moderate severity) injection site pain were reported in the 18–49 and ≥50 years age groups, respectively.
Table 3.
Safety of phase 2 trial: local and systemic AEs observed within 7 days after the first or the second vaccinations.
| AEs after the first vaccination |
AEs after the second vaccination |
|||
|---|---|---|---|---|
| Vaccine (n = 100)18–49 years | Vaccine (n = 100)≥ 50 years | Vaccine (n = 50)18–49 years | Vaccine (n = 50)≥ 50 years | |
| Any foreseen local and systemic AEs | ||||
| Any | 77 (77%)* | 39 (39%) | 9 (18%)* | 12 (24%) |
| Mild (grade 1) | 71 (71%) | 37 (37%) | 9 (18%) | 12 (24%) |
| Moderate (grade 2) | 6 (6%) | 2 (2%) | 0 | 0 |
| Local reactions | ||||
| Pain | 75 (75%) | 39 (39%) | 6 (12%) | 7 (14%) |
| Mild (grade 1) | 69 (69%) | 37 (37%) | 6 (12%) | 7 (14%) |
| Moderate (grade 2) | 6 (6%) | 2 (2%) | 0 | 0 |
| Hyperaemia | 14 (14%) | 6 (6%) | 2 (4%) | 8 (16%) |
| Mild (grade 1) | 14 (14%) | 6 (6%) | 2 (4%) | 8 (16%) |
| Induration | 7 (7%) | 1 (1%) | 0 | 0 |
| Mild (grade 1) | 7 (7%) | 1 (1%) | 0 | 0 |
| Itch | 1 (1%) | 1 (1%) | 0 | 0 |
| Mild (grade 1) | 1 (1%) | 1 (1%) | 0 | 0 |
| Systemic reactions | ||||
| Fever | 4 (4%) | 1 (1%) | 1 (2%) | 0 |
| Mild (grade 1) | 4 (4%) | 1 (1%) | 1 (2%) | 0 |
| General weakness | 1 (1%) | 1 (1%) | 0 | 1 (2%) |
| Mild (grade 1) | 1 (1%) | 1 (1%) | 0 | 1 (2%) |
| Muscle pain | 0 | 1 (1%) | 0 | 0 |
| Mild (grade 1) | 0 | 1 (1%) | 0 | 0 |
| Drowsiness | 1 (1%) | 1 (1%) | 0 | 0 |
| Mild (grade 1) | 1 (1%) | 1 (1%) | 0 | 0 |
| Fatigue | 1 (1%) | 0 | 1 (2%) | 1 (2%) |
| Mild (grade 1) | 1 (1%) | 0 | 1 (2%) | 1 (2%) |
| Malaise | 0 | 0 | 1 (2%) | 1 (2%) |
| Mild (grade 1) | 0 | 0 | 1 (2%) | 1 (2%) |
Data are presented as n (%). Subjects who developed more than one AE were only counted once. Adverse reactions were graded according to the Guidance for industry: Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials (US Food and Drug Administration, 2007). *Difference in Any AEs detected after first vs second immunizations is significant in 18–49 years age group. Attributable risk is 0.59 (95%CI 0.42–0.71), calculated by Newcombe/Wilson method with continuity correction.
The frequency of AEs decreased after the second dose. Grade 1 (mild severity) solicited local and systemic AEs were reported by 18% of the participants (9 of 50) aged 18–49 years and by 24% of the participants (12 of 50) aged ≥50 years. The recorded systemic reactions (fever, general weakness, muscle pain, drowsiness, fatigue, malaise) were relatively infrequent (up to 4% of subjects in each age group) and graded as mild, transient (1–2 days), and self-limiting.
Unsolicited AEs following the first and second dose of the study vaccine were associated with minor changes in urine and blood testing results (based on complete blood count and blood chemistry analysis) and were graded as mild following the FDA Toxicity Grading Scale (table 4). No serious AEs were reported within the 6-month follow-up period.
Table 4.
Laboratory Abnormalities on Day 21 in the phase 2 study.
| Laboratory parameters | Vaccine (n = 100)18–49 years | Vaccine (n = 100)≥50 years |
|---|---|---|
| Blood chemistry | 4 (4%) | 3 (3%) |
| ALT | 2 (2%) | – |
| Grade 1 | 2 (2%) | – |
| AST | 2 (2%) | – |
| Grade 1 | 2 (2%) | – |
| Alkaline phosphatase | – | 2 (2%) |
| Grade 1 | – | 2 (2%) |
| Urea | – | 1 (1%) |
| Grade 1 | – | 1 (1%) |
| clinical blood test | 4 (4%) | 5 (5%) |
| hemoglobin | 4 (4%) | 2 (2%) |
| Grade 1 | 4 (4%) | 2 (2%) |
| Lymphocytes | – | 3 (3%) |
| Grade 1 | – | 3 (3%) |
Data are n (%). ALT = alanine aminotransferase. AST = aspartate aminotransferase.
The immunological effectiveness of the QazCovid-in® vaccine was estimated in phase 1/2 clinical trials by the post-vaccination increase in virus-specific antibody titres. The antibody titres were measured by MNA and ELISA on serum samples collected during the phase 1 study before vaccination (day 1), 21 days after the first and second vaccine administration (day 21 and 42, respectively), and in 6 months (day 180). The number of subjects with a fourfold increase of antibody titres and the dynamics of GMTs of SARS-CoV-2-specific antibodies in the serum samples, measured in the phase 1 study, are shown in table S3 and figure S1. 59% of subjects demonstrated seroconversion in MNA after the first dose, and the number reached 100% after two doses of the vaccine. An increase in the titres of neutralising antibodies was statistically significant, reaching GMT of 5.1 (95% CI 3·5–7·6) on day 21 and GMT of 100 (95% CI 77–129) on day 42 (see table S3, figure S1A). On day180 after the first immunisation, the GMT dropped to 7 (95% CI 5–7). In volunteers who received the placebo, no virus-neutralising antibodies were detected in the serum samples collected on days 21 and 42. However, by day 180, 25% of them demonstrated antibody titre increase with GMT of 2 (1, 3) according to MNA. These volunteers declared the absence of COVID-19 within this period, which suggests asymptomatic infection.
In the subjects treated with the QazCovid-in® vaccine, GMTs of Spike-specific IgG antibodies were much higher than those measured by MNA and showed a statistically significant increase on days 21 and 42 (see table S3, figure S1B). On day180 after the first vaccination, titres of Spike-specific IgG decreased below the day 42 level. The subjects who received the placebo did not show any increase of Spike-specific IgG on days 21 and 42, but 40% of the subjects had Spike-specific IgG antibodies on day 180 after the first immunisation.
The data of the phase 1 study indicate that the QazCovid-in® vaccine was immunogenic in individuals aged from 18 to 50 years and induced a fourfold increase of neutralising antibody in 100% of subjects. None of them shown an increase in Spike-specific IgG on day 180. The subjects who participated in phase 1 clinical trial remained clinically healthy throughout the observation period of 180 days during the active development of the SARS-CoV-2 epidemic in Almaty. This can imply that the QazCovid-in® vaccine prevented infection with SARS-CoV-2 within 180 days following vaccination.
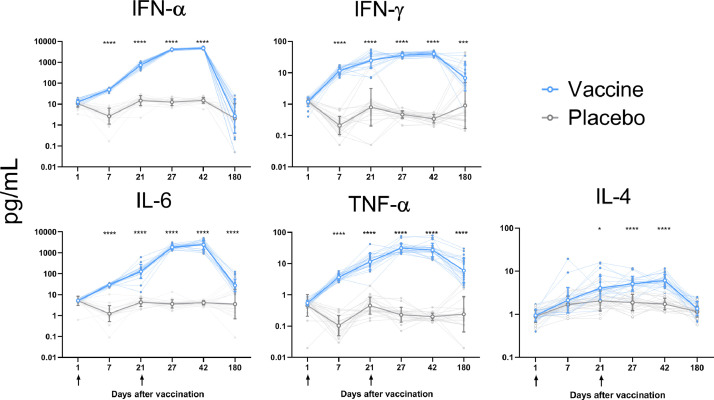
To characterise post-vaccination cellular immune response,[11] the whole-blood cytokine release assay was used. In response to stimulation with Nucleocapsid and Spike proteins of SARS-CoV-2 virus, the level of cytokine production on day 7, 21, 27, 42, and 180 exceeded the baseline, and a consistent increase in IL-6, IFN-α, IFN-γ, TNF-α, and IL-4 levels was observed till study day 42 (Fig. 2, table S4). By study day 180, the levels of the studied cytokines dropped significantly compared to the level observed at day 42 (p < 0.0001). At the same time, cases of cytokine response were observed only occasionally in the placebo group at all time points. The IFN-γ/IL-4 ratio indicated the Th1-biased response in the vaccine group (table 5).
Fig. 2.
Cytokine profiles in placebo and vaccine groups
Individual values for each subject are shown as small closed circles. Open circles with error bars represent geometric mean with 95% CI for each time point. All concentrations are presented as pg/mL after the background subtraction. Groups were compared by two-way RM-ANOVA (applied to the log10 values), followed by Bonferroni's multiple comparison test; * p < 0·05, *** p–0·001, and **** p–0·0001- indicate statistically significant differences between groups is.
Table 5.
IFN-γ to IL-4 cytokine response ratio on different time points from the Phase 1 study.
| Day | IFN-γ / IL-4 ratio |
|
|---|---|---|
| Placebo | Vaccine | |
| Day 1 | 1·4 (0·9, 1·9) | 1·3 (1·1, 1·6) |
| Day 7 | 0·1 (0·1, 0·2) | 6·2 (4·8, 8·1) |
| Day 21 | 0·4 (0·2, 0·7) | 7·2 (4·5, 9·2) |
| Day 27 | 0·3 (0·2, 0·4) | 6·6 (6, 8·8) |
| Day 42 | 0·2 (0·2, 0·3) | 6·4 (5·1, 9·1) |
| Day 180 | 0·4 (0·3, 1·8) | 4·1 (2·6, 9·1) |
Data are group Median (IQR).
In the phase 2 study, the immunogenicity of the QazCovid-in® vaccine was estimated in 200 subjects of two age groups, 18–49 and ≥50 years, after a single and double vaccination. Antibody titres were measured by MNA and ELISA in serum samples collected before vaccination (day 1), after the first (days 21 and 27), the second vaccination (day 42), and 6 months after the first vaccination (day 180).
Amongst the volunteers aged 18–49 years, the number of subjects with seroconversion in MNA was 100% on day 21 after the first dose, with the GMT of 32 (95% CI 22 −47) in the one-dose group and of 30 (95% CI 21- 42) in the two-dose group (see tables 6, 7 figure S2). Remarkably, volunteers immunised once did not show any increase of the GMT (20, 95% CI 16–26) on day 42. The volunteers who received the second vaccine dose showed a statistically significant increase in antibody titres, reaching GMTs of 143 (95% CI 115–178) on day 42 (see table 6, figure S2).
Table 6.
Immunogenicity of phase 2 study: Humoral immune response after double vaccination.
| Assay | Double vaccination |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 18–49 years |
≥ 50 years |
|||||||||
| Day 1 | Day 21 | Day 27 | Day 42 | Day 180 | Day 1 | Day 21 | Day 27 | Day 42 | Day 180 | |
| MNA | ||||||||||
| GMT (95% CI) | 1.0 (n/a) | 32 (22, 47) | 32 (23, 44) | 143* (115, 178) | 10 (7, 13) | 1.0 (n/a) | 25 (16, 38) | 32 (24, 43) | 83* (66, 104) | 9 (6, 13) |
| % seroconversion (95% CI) | n/a | 100 (93, 100) | 98 (90, 100) | 100 (93, 100) | 89 (77, 95) | n/a | 94 (84, 98) | 100 (93, 100) | 100 (93, 100) | 79 (65, 88) |
| ELISA | ||||||||||
| GMT (95% CI) | 50 (n/a) | 1452 (787, 2679) | 3940 (2116, 7334) | 10,254 (5520, 19,048) | 1866 (1021, 3410) | 50 (n/a) | 1715 (985, 2986) | 4987 (2707, 9187) | 10,254 (5464, 19,242) | 2802 (1296, 6061) |
| % seroconversion (95% CI) | n/a | 78 (65, 87) | 92 (81, 97) | 98 (90, 100) | 89 (77, 95) | n/a | 84 (71, 92) | 94 (84, 98) | 96 (87, 99) | 81 (67, 90) |
Data are GMTs and 95% CIs,% of sero conversion is the percentage of subjects with ≥ fourfold antibody titre increase compared to Day 1, n/a – not applicable *In the post hoc data analyses the statistically significant difference in GMTs on day 42 between the age groups (18–49 vs ≥50 years) was revealed with p = 0·04 estimated in Bonferroni's multiple comparison test after two-way RM-ANOVA with age group and time point as two sources of variance.
Table 7.
Immunogenicity of phase 2 study: Humoral immune response after the single vaccination.
| Assay | Single vaccination |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 18–49 years |
≥ 50 years |
|||||||||
| Day 1 | Day 21 | Day 27 | Day 42 | Day 180 | Day 1 | Day 21 | Day 27 | Day 42 | Day 180 | |
| MNA | ||||||||||
| GMT (95% CI) | 1.0 (n/a) | 30 (21, 42) | 25 (19, 23) | 20 (16, 26) | 4 (3, 6) | 1.0 (n/a) | 24 (16, 36) | 30 (23, 40 | 27 (21, 35) | 5 (3, 7) |
| % sero conversion (95% CI) | n/a | 100 (93, 100) | 98 (90, 100) | 98 (90, 100) | 72 (56, 84) | n/a | 92 (81, 97) | 100 (93, 100) | 100 (93, 100) | 52 (38, 67) |
| ELISA | ||||||||||
| GMT (95% CI) | 50 (n/a) | 822 (471, 1436) | 1997 (1066, 3741) | 3478 (1855, 6519) | 1345 (544, 3325) | 50 (n/a) | 1041 (572, 1894) | 2263 (1183, 4329) | 3336 (1660, 6706) | 472 (207, 1077) |
| % seroconversion (95% CI) | n/a | 80 (67, 89) | 88 (76, 94) | 90 (79, 96) | 67 (50, 80) | n/a | 76 (63, 86) | 80 (67, 89) | 84 (71, 92) | 45 (31, 60) |
Data are GMTs and 95% CIs,% of sero conversion is the percentage of subjects with ≥ fourfold antibody titre increase compared to Day 1, n/a – not applicable.
As shown by MNA, 94% (two-dose group) and 92% (one-dose group) of the subjects aged 50 years and older demonstrated seroconversion on day 21 after the first vaccination, with respective GMTs of 25 (95% CI 16–38) and 24 (95% CI 16 – 36) (see tables 6, 7, figure S2). After the second dose, the number of subjects with seroconversion reached 100%, yielding the GMTs of 83 (95% CI 66–104) on day 42; however, the GMT value in this age group was significantly lower than in subjects aged 18–49 years (83 vs 143) (see table 6).
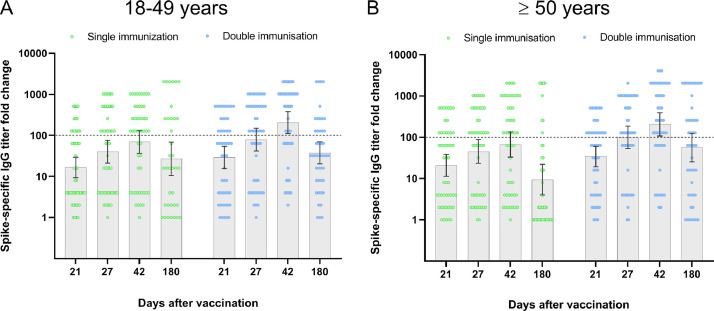
GMTs of Spike-specific IgG antibodies measured by ELISA increased on day 21 after the first dose of the vaccine and grew further, being higher on day 42 than on day 21. The increase was statistically significant, and it was observed irrespectively of the number of vaccine doses received or the age of participants (see table 6,7, figure S2). Nevertheless, only the double immunisation resulted in a more than 100-fold increase in antibody titre with the 95% confidence level (see Fig. 3, table S5). On study day 180, 72% of the participants from the 18–49 years age group and 52% – from ≥ 50 years age group still had neutralising antibodies after a single vaccination. After double vaccination, the titers of neutralising antibodies remained elevated in 89% and 79% of the subjects, according to the age group; however, the GMT decreased by five times or more (table 6,7).
Fig. 3.
Humoral immune response in phase 2 (ELISA results)
Fold change in serum Spike-specific antibody titres measured on days 21 and 27 (after the first vaccination), and on days 42 and 180 (after the second vaccination) in comparison to day 1 (before vaccination) are shown. Individual values for each subject are shown as circles. Grey bars with errors indicate geometric means with 95% CIs. The horizontal dotted line represents the 100-fold increase threshold as per the study protocol.
The immunogenicity data of the phase 2 study indicate that two doses of the QazCOVID-in® vaccine are required to induce titres of neutralising antibodies in the range comparable to that of convalescent COVID-19 patients. It applies to all study participants irrespectively of their age, although subjects aged 50 years and older develop lower titers of neutralising antibodies compared to the 18–49 age group.
Discussion
QazCovid-in® is an inactivated aluminium adjuvanted whole-virion vaccine against COVID-19. Our phase 1 and 2 clinical trials have proved the QazCovid-in® vaccine to be safe and well-tolerated by adults aged from 18 to 70 years, who receive one or two doses. The reported local and systemic AEs, associated with intramuscular administration of the inactivated vaccine, are predominantly mild and classified as foreseeable. Between the vaccine and placebo groups, no statistically significant difference has been shown in the level of total serum IgE antibodies which are known as specific triggers of allergic reactions [12]. A good safety profile is a big advantage of the QazCovid-in® vaccine which does not induce acute allergic reactions. However, the absence of direct comparison and a small number of subjects does not allow comparing it directly with other vaccines, such as the AstraZeneca vector vaccine or mRNA-based vaccines produced by Moderna and Pfizer-BioNTech, which have been shown to occasionally induce acute allergic reactions [5,13].
All 18–49-years old subjects administered with two doses of the QazCovid-in® vaccine have responded to the vaccination and produced neutralising antibodies with the GMT of 100 (phase 1) or 143 (phase 2). In the phase 2 trial, 100 out of 100 volunteers in the ≥50 years old age group have also responded to the vaccine; however, the GMTs of neutralising antibodies produced after two vaccine doses have been lower than in younger participants aged 18–49 years. In both age groups, single immunisation has promoted the production of neutralising antibodies with GMTs of 27 on day 21 which have not grown further, proving that the second vaccination is necessary. In both trials, Spike-specific IgG antibody titres have been much higher than antibody titres in MNA, and they have been increasing consistently after each vaccination. This indicates the presence of antibodies that are specific to the Spike protein but do not neutralise the virus. Non-neutralising antibodies, as have been shown in preclinical studies with the SARS-CoV-1 virus, can induce the antibody disease enhancement effect (ADE) that can influence the vaccine safety parameters. However, no ADE have been recorded so far in the subjects vaccinated with inactivated whole-virion vaccines made from SARS-CoV-2.
The whole-blood cytokine release assay is considered an easy and valid tool for the evaluation of SARS-CoV-2-specific response in COVID-19 patients, showing a good correlation with other approaches involving peripheral blood mononuclear cell stimulation [11]. We have applied this approach by using SARS-CoV-2 Nucleocapsid and Spike proteins for stimulation. The vaccine has been shown to induce the desirable Th1-biased cellular response, as indicated by the IFN-γ/IL-4 cytokine ratio. In terms of vaccine safety, the Th1-polarised immune response is preferred to the Th2-polarised response that can potentially induce disease enhancement. On the other hand, increased expression of IL-6 can stimulate both T- and B-cellular immune response, promoting the differentiation of follicular T-helpers and antibody-producing B-cells that contribute to protection [14].
The study of immune response durability has demonstrated that both neutralising and Spike-specific IgG antibody levels have dropped on study day 180. Nevertheless, using MNA and ELISA, we have identified 40% of subjects with seroconversion in the placebo group. This might indicate the SARS-CoV-2 infection in the placebo-treated subjects during the 6-month follow-up period. A high percentage of seropositive volunteers in the placebo group corresponds to the literature data showing the rate of 40–45% patients with asymptomatic infection [15]. Considering that neutralising and Spike-specific IgG antibody levels have dropped in the vaccinated subjects, we suggest their resistance to the infection with homologous/heterologous circulating strains. Immunologic correlates of protection against SARS-CoV-2 infection are not identified yet. Patients who recovered from COVID-19 are shown to produce neutralising antibodies with a mean GMT of 40 [16,17], which circulate for more than 6 months and decline by the 8th month [18].
In terms of the number of subjects with antibody response, our phase 1 and phase 2 clinical trials of the inactivated QazCOVID-in® vaccine are comparable to the studies of other vaccines against COVID-19, developed on different platforms. A similar inactivated whole-virion BBIBP-CorV vaccine adjuvanted with aluminium hydroxide, developed in China, is shown to induce the response in 97·7% of subjects with the GMTs of 244 for neutralising antibodies after two immunisations [19]. A similar dose-dependant level of neutralising antibody titres with GMTs of 92·5–160 is shown for the Indian BBV152 inactivated whole-virion vaccine, which is formulated with a Toll-like receptor agonist IMDG adsorbed to Algel [20]. Similar immunogenicity is shown for the simian replication-deficient adenovirus-vectored vaccine ChAdOx1 nCoV 19 (100% subjects with GMT of 136 after two immunisations) [21], while the Ad5-based CanSino (GMT of 19·5) [22] and Russian Gam-COVID-Vac vectored vaccines comprising Ad5 and Ad26 vectors (GMT of 49·25) have slightly lower immunogenicity [17]. Stronger immune response is promoted by a novel mRNA-based mRNA-1273 vaccine which induces neutralising antibodies with the GMT of 339·7 after double immunisation [23]. Most of these vaccines demonstrate high protective efficacy ranging from 70% to 96% in phase 3 clinical trials. Thus, one can expect that double intramuscular immunisation with the inactivated QazCovid-in® vaccine adjuvanted with aluminium will protect ≥18-year-old adults from the disease induced by SARS-CoV-2.
The production technology of the QazCovid-in® inactivated whole-virion vaccine is simple, enabling easy replacement of a vaccine strain by a new one when a SARS-CoV-2 mutant with changed antigenic properties emerges. Inactivated vaccines have been used for many decades and are shown to be safe upon repeated administration. In addition, the QazCovid-in® vaccine is stable when stored at 2 to 8 °C, which gives it an advantage over the vaccines developed by Moderna/National Institute of Allergy and Infectious Diseases [6] and BioNTech/Fosun Pharma/Pfizer that require the cold chain storage. The implementation of Kazakhstan's QazCovid-in® vaccine for the prevention of COVID-19 should be a significant contribution to the fight against the pandemic.
The limited number of the elderly in the ≥50-year-olds group with a median age of 56·3 years may be considered as a study limitation. The second limitation is that the study was performed in a single centre. The results of this study serve as the rationale for the next, more expanded phase 3 study. Phase 3 clinical trial, which is in progress, recruits 3000 subjects aged 18 years and older in several centres and aims to evaluate the QazCovid-in® vaccine preventive efficacy against COVID-19. On January 5, 2021, the QazCovid-in® vaccine has been granted emergency use authorisation in Kazakhstan.
Declaration of Competing Interest
All authors declare no competing interests.
Acknowledgments
Acknowledgements
We would like to thank the study participants, the staff of the Research Centre, as well as members of the test management groups, and the leadership of the Ministry of Education and Science of the Republic of Kazakhstan.
Funding
The work was funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan within the framework of the Scientific and Technical Program "Development of a vaccine against coronavirus infection COVID-19″. State registration number О.0927. The sponsor had no role in the design of the study, as well as in the collection, analyses, or interpretation of data, or in the writing of the manuscript, and in the decision to publish the results.
Author's contribution
The study was carried out at the clinical base of the National Scientific centre of Phthisiopulmonology of the Republic of Kazakhstan, Ministry of Health of the Republic of Kazakhstan by a contract organization centre for Clinical Medicine and Research. The director Ilyas Kulmagambetov is the Principal Investigator who organized and conducted the studies and made the final decision to publish the results. The corresponding author confirms that he had full access to all study data and had final responsibility for the decision to submit the paper for publication. GS, MSt, MSe, and BKh coordinated the studies, contributed to the data analysis and interpretation, and edited the report. KZ, LK, MO, YA, KS, MK, and BKh contributed to the vaccine development, implementation of the studies, and data collection. TD, LK, MO, YA, KS, and MK were responsible for the laboratory research. All authors critically reviewed the report and approved the final version.
Data sharing
The Individual details of a participant will be available upon request to BKh. Once the request is approved, the data can be transferred via a secure online platform.
Footnotes
Funding: The work was funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101078.
Appendix. Supplementary materials
References
- 1.World Health Organization. Available at: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed March 2, 2021).
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: summary of a Report of 72314 cases from the Chinese Center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Undurraga E.A., Chowell G., Mizumoto K. COVID-19 case fatality risk by age and gender in a high testing setting in Latin America: chile, March-August 2020. Infect Dis Poverty. 2021;10(1):11. doi: 10.1186/s40249-020-00785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomized controlled phase 3 trial in Russia. Lancet. 2021;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voysey M., Clemens S.A.C., Madhi S.A. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden L.R., El Sahly H.M., Essink B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia S., Duan K., Zhang Y. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: interim Analysis of 2 Randomized Clinical Trials. JAMA. 2020;324(10):951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khairkhah N., Aghasadeghi M.R., Namvar A., Bolhassani A. Design of novel multi-epitope constructs-based peptide vaccine against the structural S, N and M proteins of human COVID-19 using immuno-informatics analysis. PLoS ONE. 2020;15(10) doi: 10.1371/journal.pone.0240577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Zeng G., Pan H. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomized, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrone L., Petruccioli E., Vanini V. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin Microbiol Infect. 2021;27(2) doi: 10.1016/j.cmi.2020.09.051. 286 e7- e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castells M.C., Phillips E.J. Maintaining Safety with SARS-CoV-2 Vaccines. N Engl J Med. 2021;384(7):643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumenthal K.G., Robinson L.B., Camargo C.A., Jr. Acute allergic reactions to mRNA COVID-19 Vaccines. JAMA. 2021 doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16(5):448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 15.Oran D.P., Topol E.J. Prevalence of Asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X., Guo X., Xin Q. Neutralizing antibodies responses to SARS-CoV-2 in COVID-19 inpatients and convalescent patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logunov D.Y., Dolzhikova I.V., Zubkova O.V. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomiZed phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dan J.M., Mateus J., Kato Y. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529) doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia S., Zhang Y., Wang Y. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomized, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ella R., Reddy S., Jogdand H. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomized, multi-centre, phase 2 trial, and 3-month follow-up of a double-blind, randomized phase 1 trial. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folegatti P.M., Ewer K.J., Aley P.K. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomized controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu F.C., Guan X.H., Li Y.H. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomized, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson L.A., Anderson E.J., Rouphael N.G. An mRNA Vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.