Abstract
Objective:
To synthesize reported long-term outcomes in patients undergoing tracheostomy after severe acute brain injury (SABI).
Methods:
We systematically searched Pubmed, EMBASE, and Cochrane Library for studies in English, German, and Spanish between 1990–2019, reporting outcomes in patients with SABI who underwent tracheostomy. We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and the Meta-analyses Of Observational Studies in Epidemiology guidelines. We excluded studies reporting on less than 10 patients, mixed populations with other neurological diseases, or studies assessing highly select subgroups defined by age or procedures. Data were extracted independently by two investigators. Results were pooled using random effects modeling. The primary outcome was long-term functional outcome (mRS or GOS) at 6–12 months. Secondary outcomes included hospital and long-term mortality, decannulation rates, and discharge home rates.
Results:
Of 1,405 studies identified, 61 underwent full manuscript review and 19 studies comprising 35,362 patients from 10 countries were included in the meta-analysis. The primary outcome was available from five studies with 451 patients. At 6–12 months, about one third of patients (30%; 95% confidence interval [CI] 17–48) achieved independence, and about one third survived in a dependent state (36%, 95% CI 28–46%). The pooled short-term mortality for 19,048 patients was 12%, (95% CI 9–17%) with no significant difference between stroke (10%) and TBI patients (13%), and the pooled long-term mortality was 21% (95% CI 11–36). Decannulation occurred in 79% (95% CI 51–93%) of survivors. Heterogeneity was high for most outcome assessments (I2>75%).
Conclusions:
Our findings suggest that about one in three patients with SABI who undergo tracheostomy may eventually achieve independence. Future research is needed to understand the reasons for the heterogeneity between studies and to identify those patients with promising outcomes as well as factors influencing outcome.
Keywords: Severe acute brain injury, tracheostomy, ischemic stroke, intracranial hemorrhage, subarachnoid hemorrhage, traumatic brain injury
Introduction
Patients with severe acute brain injury (SABI) follow a distinct illness trajectory characterized by sudden, unexpected presentation and uncertain prognosis (1). Long-term trajectories depend on early treatment decisions, most of which are made by surrogate decision makers in concert with neurologists, neurosurgeons and intensivists. Patients who survive the first days often face another phase of crucial treatment decisions as the transition to longer-term life-sustaining treatments is considered. The indications for tracheostomy include facilitating the wean from mechanical ventilation or long-term airway protection, typically in the setting of persistent uncertainty to provide more time for a clearer prognosis to emerge (2).
In the general critical care population, approximately 5–15% of patients with acute respiratory failure requiring mechanical ventilation receive a tracheostomy (3,4). A study of mixed critical care patients who were either ventilated for more than 14 days or underwent a tracheostomy found high mortality rates: one third of patients died before hospital discharge and another third within one year (5). In addition, health care expenditures for these patients are substantial (6,7). In mechanically ventilated patients with SABI, tracheostomy rates tend to be even higher, ranging between 21 and 47% (3, 8–11). Mortality and functional recovery in this distinct subgroup are insufficiently investigated but crucial to know given that the decision for or against tracheostomy after SABI is often a life or death decision that follows sensitive goals of care conversations.
The aim of this meta-analysis was to synthesize data about the long-term outcome of patients with tracheostomy after SABI. This information may provide guidance for clinicians and families as they make critical patient-centered treatment decisions and identify opportunities to optimize resource utilization for this population.
Material and Methods
Search strategy and selection criteria
We developed a prespecified protocol in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (12) and the Meta-analyses Of Observational Studies in Epidemiology (MOOSE) checklist (13). The data supporting the findings of this study are available within the supplementary material, including a detailed list of search terms and quality assessment of each study.
We systematically searched PubMed, EMBASE and the Cochrane Library with keywords and controlled vocabulary, combining the search terms ‘stroke’, ‘traumatic brain injury’, ‘hypoxic-ischemic encephalopathy’, ‘tracheostomy’ and ‘outcomes’. We additionally hand screened references of published reviews for relevant content.
We focused our search on the following populations: adult (age > 18 years) patients who underwent tracheostomy after SABI defined as 1) stroke, including acute ischemic stroke (AIS), intracranial hemorrhage (ICH) and aneurysmal subarachnoid hemorrhage (SAH); 2) traumatic brain injury (TBI); and 3) hypoxemic-ischemic encephalopathy following cardiac arrest (HIE). Acknowledging important differences between these three diagnoses, we deliberately pooled them as one entity (SABI) as they all render the patient acutely neurologically devastated with impaired airway protection and high risk of both central and obstructive hypoventilation.
To minimize clinically relevant heterogeneity, we excluded studies, which a) focused on highly selective populations (e.g. defined by age groups or procedures); b) reported selective outcomes; and c) reported pooled outcomes of mixed neurological populations with diagnoses other than those listed in the inclusion criteria, for example seizures, neuromuscular disease, or CNS infections. Studies assessing the effect of timing of tracheostomy (early vs. late) were included, and outcomes were pooled between groups. To assess whether the timing of tracheostomy was a confounding factor, we also compared outcomes between the early vs. late populations.
We limited our search to studies published between 1990–2019 to ensure our results reflect today’s standards of care. Review articles, correspondence, abstracts, case reports and case series with <10 patients relevant to the analysis were screened for references but not included in our results. We restricted our search to studies published in English, Spanish and German.
Trial selection and risk of bias
Two reviewers (SW and CC) independently screened titles and abstracts of the identified studies for eligibility. Disagreements were resolved by consensus, and if necessary, in consultation with a third reviewer (WL). We applied a customized Newcastle-Ottawa Scale (NOS) with seven methodological domains to assess risk of bias and study quality. We defined a high-quality study as one that met criteria for 6 or 7 domains.
Outcomes of interest
The primary outcome was long-term functional outcome, defining ‘long-term’ as at least six months after the initial injury. Functional outcome was typically described as a modified Rankin Scale (mRS) or Glasgow Outcome Scale (GOS) score. We divided these outcomes into three categories of independent, defined as mRS 0–3 or GOS 4–5; dependent, (mRS 4–5 or GOS 2–3); and dead (mRS 6, GOS 1). We also evaluated the proportion of patients with very good outcome, which we defined as mRS 0–2 or GOS 5.
Secondary outcomes were (a) mortality; (b) decannulation rates; (c) proportion of patients discharged home from the hospital. We distinguished between hospital mortality (death during the initial stay in the hospital) and long-term mortality (death reported between 6–12 months after injury). We also searched for other outcome assessments such as costs of care, quality of life and palliative care needs.
Statistical Analysis
We analyzed data in accordance with the Cochrane Handbook, MOOSE checklist, and PRISMA statement. We used a random effects model of single proportions with binomial exact confidence intervals (CI) to summarize results. Proportions were stabilized using logistic transformation. Statistical heterogeneity was quantified using the I2 statistic (14). High heterogeneity was defined as I2>75%, indicating that outcomes differed substantially across studies. Sensitivity analyses were conducted to examine the influence of each study on the pooled results. Analyses were conducted in R software using the metaprop function in the meta package (15,16). Pooled study characteristics, including age, gender, and GCS were generated by calculating weighted averages by study sample size.
Results
Of the 1,405 publications screened (Figure 1), 25 articles contained relevant information about at least one outcome of interest. Nineteen studies were conducted in acute care hospitals and included in the meta-analysis. The six studies conducted in post-acute care facilities were excluded from the statistical analysis, because these patients were thought to represent a select subpopulation. A separate meta-analysis of the post-acute care studies was not feasible due to the use of different outcome scales (Barthel Index, Disability Rating Scale, Functional Independence Measure), high variability of weaning protocols, large variations in follow-up intervals, and overlap in patients between studies from the same institutions. Therefore, summary results were extracted and reported separately below.
Figure 1 –
Study Flow Chart
The 19 studies included in the meta-analysis comprised 35,362 patients from 10 countries (Table 1). The majority of patients had a diagnosis of stroke (n=29,986 with AIS, ICH or SAH across 9 studies, 85%); all others had TBI (n=5,376 across 12 studies, 15%); 2 studies (n=247) combined stroke and TBI. Despite a systematic search, no acute care studies reported outcomes in tracheotomized patients with HIE. Table 2 displays pooled data for patient characteristics. Eleven of the 19 studies were considered high-quality studies according to the NOS, including all 6 studies assessing the primary outcome. Two of the 19 studies were randomized controlled trials (n=112), both comparing early vs late tracheostomy. Four articles were prospective cohort studies (n=414 patients), and the remaining 13 were retrospective observational studies (n=34,948). Thirteen studies (n=18,125) in our analysis investigated outcome measures based on the timing of tracheostomy (‘early’ vs ‘late’), with varying definitions of early tracheostomy (cutoff varied between 3–10 days), only 2 of these studies provided information on the primary outcome.
Table 1: Study characteristics.
Overview of all studies included in the statistical analysis
| Study Type | n | Study population | Early vs late, cutoff | Duration of follow-up | Relevant outcome measures | Study Quality (NOS) | |
|---|---|---|---|---|---|---|---|
|
Ahmed 2007 USA 2002–2005 |
Single center, retrospective cohort study | 55 | Severe TBI (GCS≤8), expected to survive > 3 days | Yes, ≤7d vs >7d | dc hospital | Mortality at dc Cost of Hospitalization |
5 |
|
Alali 2014 Canada 2009–2011 |
Multicenter, retrospective cohort study | 1142 | TBI (AIS 3 or greater) | Yes, ≤8d vs >8d | dc hospital | Mortality at dc | 6 |
|
Baron 2016 Austria 1998–2010 |
Multicenter, retrospective cohort study | 553 | Moderate-severe isolated TBI (GCS ≤ 12, AIS 2–5) |
No | dc hospital | ICU mortality Mortality at dc |
6 |
|
Bösel 2013 Germany 2009–2011 |
Single Center, prospective RCT | 60 | Stroke (AIS, ICH, SAH) admitted to the ICU, intubated on admission | Yes, 1–3d vs 7–10d |
6 months | Neurological outcome (mRS) at dc ICU and 6 months ICU mortality Mortality at 6 months Decannulation rates Cost of Hospitalization |
7 |
|
Bouderka 2004 Morocco (no time period defined) |
Single Center, prospective RCT | 31 | Severe TBI (GCS≤8 on hospital day 1 and 5), plus contusion on CT | Yes, day 5 or 6 * | dc hospital | Mortality at dc | 5 |
|
Gandia-Martinez 2010 Spain 2004–2007 |
Single Center, prospective observational study | 118 | ‘Severe’ TBI [no GCS limit] (60.2%) or stroke (39.8%), all pts intubated on admission | Yes, ≤9d vs >9d | dc hospital | ICU mortality Mortality at dc |
5 |
|
Gessler 2015 Germany 2006–2011 |
Multicenter, retrospective observational cohort study | 148 | Aneurysmal SAH, HH3–5 | Yes, ≤7d vs >7d | 6 months | Neurological outcome (mRS) at 6 months ICU mortality Mortality at 6 months |
6 |
|
Khalili 2017 Iran 2014–2015 |
Single Center, prospective cohort study | 152 | Severe TBI (GCS≤8) | Yes, ≤6d vs >6d | dc hospital | Mortality at dc | 5 |
|
Lahiri 2015 USA 2005–2012 |
Multicenter, retrospective review of administrative state data | 16,264 | Stroke (AIS, ICH and SAH) | No | dc hospital | Discharge home | 5 |
|
Rabinstein 2004 USA 1976–2000 |
Single Center, retrospective chart review | 97 | Stroke (AIS, ICH and SAH) | No | At dc hospital and at 1 year | Neurological outcome (GOS) at 1yr Mortality at dc Mortality at 1 year Decannulation |
7 |
|
Rizk 2011 USA 1990–2005 |
Multicenter (trauma registry), retrospective observational cohort study | 3104 | Severe TBI (GCS≤8), excluded isolated head injury | Yes, ≤7d vs >7d | dc hospital | Mortality at dc | 6 |
|
Roch 2003 USA 1997–2000 |
Single Center, retrospective chart review; prospective follow-up | 50 | ICH | No | 27+/− 14 mo | Decannulation rate** | 7 |
|
Schneider 2017 Germany 2014–2015 |
Single Center, prospective observational cohort study | 53 | Stroke (AIS, ICH and SAH) | No | 1 year | Neurological outcome (mRS) at 10 days, 3 months and 1 year Mortality at 10 days, 3 months and 1 year Decannluation rate Discharge destination |
6 |
|
Shibahashi 2017 Japan 2010–2014 |
Single center, retrospective review | 91 | Severe TBI (AIS ≥4****) | Yes, 2–3 vs 4–6 days | dc hospital | Mortality at dc | 6 |
|
Siddiqui 2015 Pakistan 2002–2009 |
Single center, both retro-and prospective data | 49 | Isolated severe TBI (GCS<8) | Yes, ≤7d* | dc hospital | Mortality at dc*** Cost of Care |
4 |
|
Sugarman 1997 USA (no time period defined) |
Multicenter, prospective cohort study | 35 | Severe TBI (GCS≤8), as part of cohort (other are NTBI) | Yes, 3–5d vs 10–14d |
dc hospital | Mortality at dc | 5 |
|
Villwock 2014 USA 2008–2010 |
Multicenter, retrospective review of NIS registry data | 13,165 | Stroke (AIS, ICH, SAH) | Yes, ≤10d vs >10d | dc hospital | Mortality at discharge Discharge destination Cost of Care |
4 |
|
Wabl 2018 USA 2008–2013 |
Single center, both retro-and prospective data | 129 | Severe TBI (GCS≤8), AIS, ICH< SAH | No | 36 months | Neurological outcome | 7 |
| Wang 2011 Taiwan 2005–2008 |
Single center, retrospective review | 66 | Severe TBI (GCS≤8) | Yes, ≤10d vs >10d | 1 year | Mortality at 1 month and 1 year | 6 |
NOS= Newcastle-Ottawa Scale, RCT = randomized controlled trial, dc = discharge, NIS = National Inpatient Service
compared to group with prolonged MV, the latter was not included as not specified whether the whole group was tracheotomized
Mortality not analyzed due to selection bias
long-term neurological outcome not reported due to time interval (average)
study excluded patients that died
severe chest injury with AIS≥4 excluded
Table 2:
Pooled patient characteristics
| All studies | Studies of patients with TBI | Studies of patients with stroke | |
|---|---|---|---|
| Age (years) * | 59.6 (1.2) | 52.84 (2.39) | 61.1(0.52) |
| Female (%) ** | 38.9 (4.4) | 23.78 (1.24) | 45.42 (0.84) |
| GCS on admission *** | 4.4 (0.8) | 4.27 (0.84) | 5.92 (1.73) |
| Timing of Tracheostomy (Day) **** | 12 (2.3) | 12.5 (1.59) | 12 (2.80) |
Data are mean (SD). GCS = Glasgow Coma Scale. TBI = Traumatic Brain Injury. Stroke = Acute Ischemic Stroke, Intracranial Hemorrhage, Aneurysmal Subarachnoid Hemorrhage.
Reported in 15 studies overall (n=16,512): 8 TBI studies, 5 stroke studies, and 2 mixed TBI/stroke cohort
Reported in 15 studies overall (n=19,567): 8 TBI studies, 5 stroke studies, and 2 mixed TBI/stroke cohort
Reported in 13 studies overall (n=6,157): 9 TBI studies, 2 stroke studies, and 2 mixed TBI/stroke cohort
Time from Initial Brain Injury to Tracheostomy Placement, Reported in 11 studies overall (n=16,162): 5 TBI studies, 4 stroke studies, and 2 mixed TBI/stroke cohort
Long-term functional outcome (Figure 2a):
Figure 2 -.
Forest plots displaying a) Long-term Functional Outcomes, b) Mortality, and c) Decannulation rates.
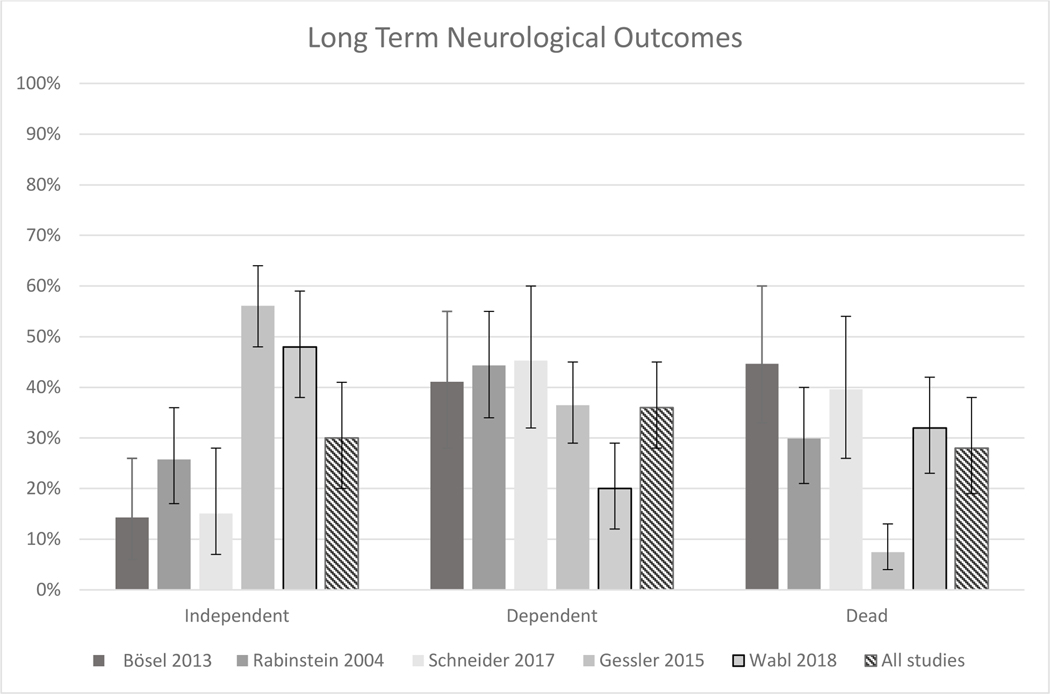
Bösel, Rabinstein and Schneider report outcomes on mixed stroke cohorts (AIS, ICH, SAH). Gessler reports outcomes on SAH cohorts only. Wabl reports outcomes on a mixed cohort of patients with AIS, ICH, SAH and TBI.
Five studies (n=451 patients) reported on the primary outcome of long-term neurological function (17–21), with the following diagnoses: SAH (51.4%), AIS (25.4%), ICH (24.6%), and TBI (4%).
One third (30%, 95% CI 17–48) of all patients and 44% of survivors (95% CI 28–62) achieved independence at 6–12 months. Ten percent (95% CI 2–35) of all patients and 15% of survivors (95% CI 4–44) had very good outcomes. The percentage of patients who remained dependent was 36% (95% CI 36–46) overall and 56% (95% CI 38–72) amongst survivors. Statistical heterogeneity was high across all analyses.
Most studies did not distinguish outcomes between SABI subgroups. One study exclusively investigating patients with SAH (17) reported higher proportions of independence (56%) and very good outcomes (44%) compared the 3 other studies looking at mixed stroke populations (19% and 4%, supplement) (18–20). The only study separating outcomes by subgroup (21) suggested that patients with SAH had the highest rate of independence (66%) and very good outcomes (50%) at 6–12 months, followed by patients with TBI (44% and 28%), ICH (38 and 21%) and AIS (29% and 12%). Figure 3 displays the proportion of independent, dependent and dead patients within all five studies reporting the primary outcome.
Figure 3 –
Distribution of Long-term Functional Outcomes.
Bösel,, Rabinstein and Schneider report outcomes on mixed stroke cohorts (AIS, ICH, SAH). Gessler reports outcomes on SAH cohorts only. Wabl reports outcomes on a mixed cohort of patients with AIS, ICH, SAH and TBI.
Mortality (Figure 2b):
Of the 17 studies (n=19,048 pts) reporting mortality data (17–33) all reported hospital mortality, whereas only 6 studies (n=517) also assessed late mortality (17–21, 30). The pooled hospital mortality was 12%, (95% CI 9–17%), and similar for patients with stroke (10%; 95% CI 4–21%) and TBI (13%; 95% CI 9–18%). The pooled long-term mortality was 21% (95% CI 11–36), with half of those (10%; 95% CI 5–19%) patients having died before hospital discharge (supplement). Only three studies specified the cause of death (18, 24, 29), and one study (18) provided information on how withhold or withdraw life-sustaining treatment impacted mortality.
Decannulation (Figure 2c):
Relevant information on decannulation rates was extracted from 4 studies (n=256) (18–20, 35), all of which were conducted in stroke patients. One half (51%, 95% CI 31–71) of all patients and 79% (95% CI 51–93) of survivors had been decannulated when assessed at 6–12 months (supplement). One study reported that 82.9% of decannulations occurred during the initial hospitalization (35). Another study comparing different stroke types reported that two-thirds of patients with SAH were decannulated vs. only one-third of patients with AIS and ICH (20). Factors associated with decannulation included age ≤ 65, absence of vasospasm, tracheostomy ≤ 7 days from admission, absence of sepsis, and supratentorial lesions, whereas stroke severity on admission or presence of chronic obstructive pulmonary disease did not significantly influence decannulation. Decannulated patients had better functional outcomes at one year (17, 20).
Discharge to home:
Based on information from 2 large studies (n=29,429) (31, 34), 4% (SD 0.04) of patients with SABI who received a tracheostomy were discharged home directly from the hospital. One additional study reported that 19% were at home one year after their initial injury (19).
Finally, when comparing pooled hospital mortality between the early vs late subgroups (11 studies), no differences were found in hospital mortality (supplement). Four studies reported the costs of the patients’ hospitalization (18, 22, 29, 31) (supplement). We found no studies addressing long-term cost, quality of life, or palliative care needs specific to these patients and their families.
Post-Acute Care Studies:
A select group of SABI patients are admitted to a rehabilitation unit or weaning faculty after tracheostomy. A consortium of 29 Italian rehabilitation facilities (925 patients) reported a mean GOS of 2.75 on admission (mean time from inciting event: 49 days), which improved to 2.95 (after a mean length of stay: 150 days) (36). The greatest improvement was seen in TBI patients (mean admission GOS 2.44 → 3.2 at discharge; mortality 3.4%), compared to patients with AIS (GOS 2.4 → 2.74, mortality 7.3%), and HIE (GOS 1.79 → 2.17, mortality 10.7%) [unpublished data provided by the authors in direct correspondence]. Two studies from a German neurorehabilitation center reported similar mortality rates (6% and 7.7%) among stroke patients (37, 38).
Decannulation rates ranged between 54.7% and 73.6% (36, 39, 40). Rates were higher among patients with TBI (68%), compared to those with stroke (51.4%) or HIE (26.4%) (36); with age ≤ 65 years (76.5%) compared to > 65 years (55.1%) (41); and patients with supratentorial (82%) versus infratentorial (61%) lesions (40).
Approximately half of SABI patients were eventually discharged home from the rehabilitation unit (36, 37, 39). In one cohort, 48% of patients with AIS (50% of those fully independent), 59% of patients with ICH (32% fully independent) and 54% of patients with SAH (50% fully independent) were able to live at home again (37).
Discussion
Our meta-analysis suggests that among SABI patients who undergo tracheostomy, a substantial proportion may eventually achieve independence (30%) or even survive with minimal deficits (10%); one in five patients die within one year after tracheostomy; and three quarters of long-term survivors are decannulated within a year after their initial injury. While difficult to interpret, it is noteworthy that among patients who were admitted to a rehabilitation unit with a tracheostomy, mortality rates ranged between 3–10% and the chances of neurological recovery differed markedly between patients with TBI (highest), stroke, and HIE (lowest).
The proportion of patients with good outcomes in our study is surprisingly high, especially in comparison to studies in the general critical care population: In a prospective cohort study of 126 critically ill patients (23% with neurological disorders), only 9% were functionally independent at 12 months and 44% died (6). A recent meta-analysis examining mixed critically ill populations requiring prolonged MV or tracheostomy reported a hospital and one-year mortalities of 29% and 62% (5), compared to 12% and 21% (respectively) in our cohort. These differences may be attributable to the distinct indications for mechanical ventilation and tracheostomy in SABI patients, where intubation is typically guided by level of consciousness rather than by cardio-pulmonary pathology.
For clinicians and family members, the decision to undergo tracheostomy in any individual patient largely depends on estimated prognosis and goals of care. Unfortunately, our ability to accurately predict outcome after SABI remains limited, and published prognostic models are met with a variety of concerns (42–43). Decisions to withhold or withdraw life-sustaining treatment after SABI are commonly driven by the fear of a patient surviving with a poor quality of life (high disability, frequent rehospitalizations, medical complications) as well as suffering (emotional distress, financial burden) for patients and their families. Our analysis does not ease this fear, suggesting that while a surprisingly large proportion of patients can achieve good outcomes, namely independence, the majority of tracheotomized stroke patients will have a poor outcome (dependence or death). These findings highlight crucial skills for clinicians to master – such as communicating uncertainty and helping families balance hope with realism - while maintaining humility in our prognostication.
These results reveal an urgent need for more research to optimize decision-making after SABI. We need to better understand the heterogeneity underlying the studies analyzed and the implied variation in current clinical practice. We need large, prospective studies, to help us map the trajectory of recovery over time, which may help physicians and families determine how long to continue a time-limited trial of aggressive treatment. We need to identify specific clinical, radiographic and therapeutic factors that influence these outcomes, and improve our ability to understand which patients will benefit from life-sustaining treatment. Subgroup analysis of data that may already exist from major interventional stroke and TBI trials may inform prognostication after tracheostomy in patients with SABI. In addition, results from an ongoing randomized, multicenter study (44) investigating the optimal timing of tracheostomy, and benefits in functional outcome, will provide further clarification regarding potential benefits of early tracheostomy. In-depth qualitative research is needed to better understand the decision-making process to proceed with tracheostomy, the effect of family support and communication interventions, and ultimately, the well-being and quality of life of patients and family members.
Our study has several limitations: First, we found only few studies that assessed the primary outcome. The paucity of available data on long-term neurological function highlights the need for future studies with emphasis on this highly relevant outcome measure. Second, for most outcomes, including the primary outcome, statistical heterogeneity was high. This observation may be due to differences in study designs and variation in clinical practice on an institutional and global level, including: indication and timing of tracheostomy, and variations in weaning protocols. We attempted to reduce heterogeneity by 1) selecting comparable patient populations with similar disease severity and excluding studies with highly selected patient subgroups that could confound outcomes, 2) assessing methodological quality according to a customized NOS, and 3) excluding studies conducted in a post-acute care setting. After careful review of each individual study, we believe that pooling of the data was reasonable as the main features we investigated were comparable. Combining the results in a meta-analysis was a pragmatic approach, allowing us to provide a summary of the available data on the subject. Third, a large number of studies compared early vs late tracheostomy, which represented the majority of published literature on the subject. One key question is whether the patients in our study truly needed the tracheostomy. Specifically, one might hypothesize that some patients in the early group might not have needed the tracheostomy and therefore skewed the results towards better outcomes. The largest randomized controlled trial of early vs late tracheostomy in a mixed ICU cohort (45) reported that 65% of patients randomized to the late group did not require a tracheostomy, reflecting persistent challenges in accurately predicting the need for a tracheostomy even among experts and despite validated prediction scores (9,46). We decided to include all cohorts because variation in timing of tracheostomy reflects the spectrum of clinical practice across institutions. Overall, meta-analyses specifically investigating early vs late tracheostomy have found mixed results (45, 47, 48). When comparing outcomes between the early and late groups across the 11 studies looking at hospital mortality, we found no difference (supplement). Fourth, the majority of studies in our analysis - all but one (18) - did not specify how withdrawal of life sustaining treatment impacted their results, thus ignoring a fundamental confounder in determining outcomes in these patients. Finally, our study suggests possible differences between SABI subtypes. While the small size of some subgroups limits generalizability, our results highlight a need for larger studies providing more outcome data for each of the disease entities separately.
Despite these limitations, this study synthesized long-term outcomes of tracheotomized SABI patients in a quantitative analysis and identified critical issues relevant to patients and their family members, to clinicians, investigators and policy makers, including decision making at the individual patient level, ethical and financial challenges emerging from a rising population of patients with chronic critical illness, and a critical gap in the literature that requires further research. In summary, our results underscore the need to determine indications and optimal timing of tracheostomy, and to develop better tools to prognosticate long-term neurologic outcome after tracheostomy, so that we can better assist patients and families reach patient-centered, goal-concordant treatment decisions.
Conclusions
Our findings suggest that a sizable minority of patients with SABI who undergo tracheostomy may eventually achieve independence, while the majority has a poor outcome. These results highlight the need for further, large-scale observational research as well as novel interventional studies evaluating ways to improve communication and decision-making after SABI and ultimately improve long-term outcomes and processes of care for patients and their families.
Supplementary Material
Acknowledgments
Dr. Claire J. Creutzfeldt receives funding from the NIH–National Institutes of Neurological Disease and Stroke (NINDS) (K23 NS099421).
We would like to thank Dr. Renato Avesani (Department of Rehabilitation, Sacro Cuore Don Calabria Hospital, Negrar, Verona, Italy) and Dr. Florian Gessler (Department of Neurosurgery, Universitaetsklinikum Frankfurt, Germany) for sharing unpublished data, outlining details of neurological outcomes within their studies, which were highly relevant for our analysis and this manuscript.
Footnotes
No reprints will be requested.
The authors report no relevant financial disclosures or conflict of interest.
Declaration of interests
We declare no competing interests or financial disclosures.
We confirm that the manuscript complies with all instruction to authors. Authorship Requirements have been met. The final manuscript was approved by all authors. This manuscript has not been published elsewhere and is not under consideration by another journal. IRB approval was not sought as this study was a review of the literature and meta-analysis, and did not involve any direct patient information. The authors do not have any relevant financial disclosures.
We developed a prespecified protocol and analyzed our data in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and the Meta-analyses Of Observational Studies in Epidemiology (MOOSE) checklist.
References
- 1.Creutzfeldt CJ, Longstreth WT, Holloway RG. Predicting decline and survival in severe acute brain injury: the fourth trajectory. BMJ. 2015;351:h3904. [DOI] [PubMed] [Google Scholar]
- 2.Quill TE, Holloway R. Time-limited trials near the end of life. JAMA. 2011;306(13):1483–4. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz P, Fitts V, Sumer Z, et al. How does care differ for neurological patients admitted to a neurocritical care unit versus a general ICU? Neurocritical care. 2011;15(3):477–80. [DOI] [PubMed] [Google Scholar]
- 4.Mehta AB, Syeda SN, Bajpayee L, et al. Trends in Tracheostomy for Mechanically Ventilated Patients in the United States, 1993–2012. Am J Respir Crit Care Med. 2015;192(4):446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damuth E, Mitchell JA, Bartock JL, et al. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(7):544–53. [DOI] [PubMed] [Google Scholar]
- 6.Unroe M, Kahn JM, Carson SS, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010;153(3):167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skolarus LE, Morgenstern LB, Zahuranec DB, et al. Acute care and long-term mortality among elderly patients with intracerebral hemorrhage who undergo chronic life-sustaining procedures. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2013;22(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelosi P, Ferguson ND, Frutos-Vivar F, et al. Management and outcome of mechanically ventilated neurologic patients. Critical care medicine. 2011;39(6):1482–92. [DOI] [PubMed] [Google Scholar]
- 9.Steidl C, Boesel J, Suntrup-Krueger S, et al. Tracheostomy, extubation, reintubation: Airway Management Decisions in Intubated Stroke Patients. Cerebrovascular diseases (Basel, Switzerland). 2017;44(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 10.Anderson CD, Bartscher JF, Scripko PD, et al. Neurologic examination and extubation outcome in the neurocritical care unit. Neurocritical care. 2011;15(3):490–7. [DOI] [PubMed] [Google Scholar]
- 11.Krishnamoorthy V, Hough CL, Vavilala MS, et al. Tracheostomy After Severe Acute Brain Injury: Trends and Variability in the USA. Neurocritical care. 2019;30(3):546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. https://www.rdocumentation.org/packages/meta/versions/4.9-2/topics/metaprop.
- 16.RStudio Team (2016). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA: URL http://www.rstudio.com/. [Google Scholar]
- 17.Gessler F, Mutlak H, Lamb S, et al. The Impact of Tracheostomy Timing on Clinical Outcome and Adverse Events in Poor-Grade Subarachnoid Hemorrhage. Critical care medicine. 2015;43(11):2429–38. [DOI] [PubMed] [Google Scholar]
- 18.Bosel J, Schiller P, Hook Y, et al. Stroke-related Early Tracheostomy versus Prolonged Orotracheal Intubation in Neurocritical Care Trial (SETPOINT): a randomized pilot trial. Stroke; a journal of cerebral circulation. 2013;44(1):21–8. [DOI] [PubMed] [Google Scholar]
- 19.Rabinstein AA, Wijdicks EF. Outcome of survivors of acute stroke who require prolonged ventilatory assistance and tracheostomy. Cerebrovascular diseases (Basel, Switzerland). 2004;18(4):325–31. [DOI] [PubMed] [Google Scholar]
- 20.Schneider H, Hertel F, Kuhn M, et al. Decannulation and Functional Outcome After Tracheostomy in Patients with Severe Stroke (DECAST): A Prospective Observational Study. Neurocritical care. 2017;27(1):26–34. [DOI] [PubMed] [Google Scholar]
- 21.Wabl R, Williamson CA, Pandey AS, et al. Long-term and delayed functional recovery in patients with severe cerebrovascular and traumatic brain injury requiring tracheostomy. J Neurosurg. 2018;131(1):114–21. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed N, Kuo YH. Early versus late tracheostomy in patients with severe traumatic head injury. Surgical infections. 2007;8(3):343–7. [DOI] [PubMed] [Google Scholar]
- 23.Alali AS, Scales DC, Fowler RA, et al. Tracheostomy timing in traumatic brain injury: a propensity-matched cohort study. J Trauma Acute Care Surg. 2014;76(1):70–6; discussion 6–8. [DOI] [PubMed] [Google Scholar]
- 24.Baron DM, Hochrieser H, Metnitz PG, et al. Tracheostomy is associated with decreased hospital mortality after moderate or severe isolated traumatic brain injury. Wien Klin Wochenschr. 2016;128(11–12):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouderka MA, Fakhir B, Bouaggad A, et al. Early tracheostomy versus prolonged endotracheal intubation in severe head injury. The Journal of trauma. 2004;57(2):251–4. [DOI] [PubMed] [Google Scholar]
- 26.Gandia-Martinez F, Martinez-Gil I, Andaluz-Ojeda D, et al. [Analysis of early tracheostomy and its impact on development of pneumonia, use of resources and mortality in neurocritically ill patients]. Neurocirugia (Astur). 2010;21(3):211–21. [PubMed] [Google Scholar]
- 27.Khalili H, Paydar S, Safari R, et al. Experience with Traumatic Brain Injury: Is Early Tracheostomy Associated with Better Prognosis? World Neurosurg. 2017;103:88–93. [DOI] [PubMed] [Google Scholar]
- 28.Rizk EB, Patel AS, Stetter CM, et al. Impact of tracheostomy timing on outcome after severe head injury. Neurocritical care. 2011;15(3):481–9. [DOI] [PubMed] [Google Scholar]
- 29.Siddiqui UT, Tahir MZ, Shamim MS, et al. Clinical outcome and cost effectiveness of early tracheostomy in isolated severe head injury patients. Surgical neurology international. 2015;6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang HK, Lu K, Liliang PC, et al. The impact of tracheostomy timing in patients with severe head injury: an observational cohort study. Injury. 2012;43(9):1432–6. [DOI] [PubMed] [Google Scholar]
- 31.Villwock JA, Villwock MR, Deshaies EM. Tracheostomy timing affects stroke recovery. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2014;23(5):1069–72. [DOI] [PubMed] [Google Scholar]
- 32.Sugerman HJ, Wolfe L, Pasquale MD, et al. Multicenter, randomized, prospective trial of early tracheostomy. The Journal of trauma. 1997;43(5):741–7. [DOI] [PubMed] [Google Scholar]
- 33.Shibahashi K, Sugiyama K, Houda H, et al. The effect of tracheostomy performed within 72 h after traumatic brain injury. British journal of neurosurgery. 2017;31(5):564–8. [DOI] [PubMed] [Google Scholar]
- 34.Lahiri S, Mayer SA, Fink ME, et al. Mechanical Ventilation for Acute Stroke: A Multi-state Population-Based Study. Neurocritical care. 2015;23(1):28–32. [DOI] [PubMed] [Google Scholar]
- 35.Roch A, Michelet P, Jullien AC, et al. Long-term outcome in intensive care unit survivors after mechanical ventilation for intracerebral hemorrhage. Critical care medicine. 2003;31(11):2651–6. [DOI] [PubMed] [Google Scholar]
- 36.Avesani R, Roncari L, Khansefid M, et al. The Italian National Registry of severe acquired brain injury: epidemiological, clinical and functional data of 1469 patients. Eur J Phys Rehabil Med. 2013;49(5):611–8. [PubMed] [Google Scholar]
- 37.Ponfick M, Wiederer R, Nowak DA. Outcome of Intensive Care Unit-Dependent, Tracheotomized Patients with Cerebrovascular Diseases. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2015;24(7):1527–31. [DOI] [PubMed] [Google Scholar]
- 38.Ponfick M, Wiederer R, Bosl K, et al. The influence of weaning duration on rehabilitative outcome in early neurological rehabilitation. NeuroRehabilitation. 2014;34(3):493–8. [DOI] [PubMed] [Google Scholar]
- 39.Formisano R, Azicnuda E, Sefid MK, et al. Early rehabilitation: benefits in patients with severe acquired brain injury. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2016. [DOI] [PubMed] [Google Scholar]
- 40.Mitton K, Walton K, Sivan M. Tracheostomy weaning outcomes in relation to the site of acquired brain injury: A retrospective case series. Brain Inj. 2017;31(2):267–71. [DOI] [PubMed] [Google Scholar]
- 41.Chiavaroli F, Derraik JG, Zani G, et al. Epidemiology and clinical outcomes in a multicentre regional cohort of patients with severe acquired brain injury. Disabil Rehabil. 2016;38(20):2038–46. [DOI] [PubMed] [Google Scholar]
- 42.Becker KJ, Baxter AB, Cohen WA, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001;56(6):766–72. [DOI] [PubMed] [Google Scholar]
- 43.Creutzfeldt CJ, Becker KJ, Weinstein JR, et al. Do-not-attempt-resuscitation orders and prognostic models for intraparenchymal hemorrhage. Critical care medicine. 2011;39(1):158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schonenberger S, Niesen W, Fuhrer, et al. Early tracheostomy in ventilated stroke patients: Study protocol of the international multicentre randomized trial SETPOINT2 (Stroke-related Early Tracheostomy vs. Prolonged Orotracheal Intubation in Neurocritical care Trial 2). Int J Stroke. 2016April;11(3):368–79. [DOI] [PubMed] [Google Scholar]
- 45.Young D, Harrison DA, Cuthbertson BH, et al. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121–9. [DOI] [PubMed] [Google Scholar]
- 46.Schonenberger S, Al-Suwaidan F, Kieser M, et al. The SETscore to Predict Tracheostomy Need in Cerebrovascular Neurocritical Care Patients. Neurocritical care. 2016;25(1):94–104. [DOI] [PubMed] [Google Scholar]
- 47.McCredie VA, Alali AS, Scales DC, et al. Effect of Early Versus Late Tracheostomy or Prolonged Intubation in Critically Ill Patients with Acute Brain Injury: A Systematic Review and Meta-Analysis. Neurocritical care. 2017;26(1):14–25. [DOI] [PubMed] [Google Scholar]
- 48.Elkbuli A, Narvel RI, Spano PJ, et al. Early versus Late Tracheostomy: Is There an Outcome Difference? The American surgeon. 2019;85(4):370–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.