Abstract
Purpose:
To investigate the effects of 1,25-Vit D3 and 24,25-Vit D3 on corneal fibroblast expression of the vitamin D-associated enzymes CYP27B1 and CYP24A1 and the roles of the vitamin D receptor (VDR) and protein disulfide isomerase, family A, member 3 (Pdia3) in these cells.
Methods:
CYP24A1, CYP27B1, VDR, and Pdia3 expression in corneas was detected using immunohistochemistry. Western blotting was used to measure protein expression in human and mouse fibroblasts, including VDR KO mouse cells, treated with 1,25-Vit D3 (20 nM) and 24,25-Vit D3 (100 nM). The Pdia3 inhibitor LOC14 was used to explore the role of Pdia3 as a Vit D3 receptor in these cells.
Results:
CYP24A1, CYP27B1, VDR, and Pdia3 were all expressed in mouse and human corneal fibroblasts. 1,25-Vit D3 significantly increased VDR expression in human and mouse fibroblasts. 1,25-Vit D3 and 24,25-VitD3 significantly increased CYP24A1 and CYP27B1 expression level in human, VDR WT mouse, and VDR KO mouse corneal fibroblasts. CYP24A1 and CYP27B1 expression was unchanged in VDR KO mouse fibroblasts treated with 1,25-Vit D3 or 24,25-Vit D3 plus LOC14. Human fibroblast VDR, CYP24A1, and CYP27B1 expression were unaffected by LOC14.
Conclusions:
Vitamin D metabolic enzymes, VDR, and Pdia3 are all expressed in mouse and human corneal fibroblasts. 1,25-Vit D3 modulates fibroblast vitamin D enzymes through both the VDR and Pdia3 pathways in a species-dependent manner. 24,25-Vit D3 can increase expression of fibroblast CYP24A1 and CYP27B1 in the absence of VDR and is likely involved in fibroblast regulation independent of 1,25-Vit D3 or VDR.
Keywords: Vitamin D, Keratocyte, VDR, CYP24A1, CYP27B1, Pdia3
Introduction
As a prohormone, vitamin D has pleiotropic effects on many different cell types.1,2 These vitamin D effects regulate a wide range of physiological and pathological processes including mineral ion homeostasis, cell growth, migration, immune response modulation, and differentiation.3–7 Vitamin D deficiency is a widespread medical health problem worldwide.8 Epidemiological evidence associates vitamin D deficiency with the prevalence of metabolic or chronic diseases such as type 1 and type 2 diabetes.9–11 Multiple studies have implicated the relationship between vitamin D deficiency and the prevalence of dry eye disease.12–14 In addition, 1,25-Vit D3 has been found to inhibit neovascularization, modulate angiogenesis, myopia,15 diabetic retinopathy,16,17 and antitumor activity in retinoblastoma.18
Vitamin D is mainly produced from 7-dehydrocholesterol in the skin following UV-B exposure. Vitamin D metabolism involves several enzymes,19 including the three key cytochrome P450 enzymes 25-hydroxylase (CYP2R1), 1α-hydroxylase (CYP27B1), and 24-hydroxylase (CYP24A1).20,21 Vitamin D is initially 25-hydroxylated in the liver by CYP2R1, followed by 1α-hydroxylation in the kidney by CYP27B1 to its active hormonal form 1α, 25-dihydroxyvitamin D (1,25-Vit D3).22 Catabolism of both 25-Vit D3 and 1,25-Vit D3 is primarily through CYP24A1 to its 24 (and 23) hydroxy forms, 24,25-Vit D3, and 1,24,25-Vit D3 (or 1,23,25-Vit D3).21
Extra-hepatic and extra-renal tissue-specific local production of vitamin D has been demonstrated in a number of tissues.23 Our lab has previously demonstrated that corneal epithelium, which is directly exposed to the sun in the same manner as the skin, has the enzymatic components and ability to generate and activate vitamin D. The vitamin D receptor (VDR), CYP24A1, CYP27B1, and five different vitamin D metabolites have all been detected in the cornea and anterior segment.24,25 To our knowledge, there have been no published studies examining vitamin D metabolism in corneal keratocytes. Quiescent keratocytes constitute the primary cell type of the corneal stroma and occupy approximately 3% of its volume.26 Corneal keratocytes are involved in the synthesis of collagens, proteoglycans, growth factors and related proteins, and are the primary cell type involved in the corneal stroma wound repair process. A number of protein and lipid-associated growth factors have been shown to influence these processes in keratocytes. The presence and activity of the VDR and vitamin D-associated metabolic enzymes have not been studied in corneal keratocytes.
VDR is critical for most of the actions of vitamin D.27 VDR is a member of the nuclear receptor superfamily, and it regulates the expression of the numerous genes involved in downstream vitamin D signaling. Although initially identified in the small intestine, VDR is widespread in nearly all tissues,28,29 as are the key vitamin D metabolizing enzymes CYP27B1 and CYP24A1.21 While VDR is primarily a nuclear receptor,30,31 its expression has also been found in the cell membrane32 and mitochondria.33 Furthermore, the protein disulfide isomerase family member 3 (Pdia3) has been identified as an additional membrane-associated vitamin D receptor.34,35
Renal CYP27B1 is responsible for generating circulating levels of active 1,25-Vit D3 and is also widely distributed in other organs, including dendritic cells, parathyroid cells, osteoblasts, osteoclasts, keratinocyte, mammary epithelial cells, renal tubular cells, pancreatic beta cells, vascular endothelial cells, and prostate epithelial cells.21,36 CYP24A1 plays an important role in the regulation of vitamin D action and reduces the pool of 25-Vit D3 available for 1α-hydroxylation. Mutations of CYP27B1 result in the lack of 1,25-Vit D3 synthesis, whereas mutations of CYP24A1 lead to excess 1,25-Vit D3.20 In the corneal epithelium, 1,25-Vit D3 and 24,25-Vit D3 stimulate CYP24A1 in a VDR-dependent manner, while 24,25-Vit D3 stimulates CYP27B1 via a VDR independent pathway.3 However, the effects of vitamin D on corneal fibroblasts are uncertain, and the control and feedback pathways of vitamin D metabolism in corneal fibroblasts are unclear.
The aims of this study were to verify the presence of VDR, Pdia3, and the vitamin D metabolic enzymes in corneal fibroblasts, and to investigate the effects of 1,25-Vit D3 and 24,25-Vit D3 on the expression of VDR, CYP27B1, and CYP24A1 in human and mouse corneal fibroblasts. VDR knockout (VDR KO) corneal fibroblasts and a Pdia3 inhibitor were utilized to examine the role of VDR and Pdia3 in these responses.
Methods
Material and methods
Materials
1,25-Vit D3 and 24,25-Vit D3 were purchased from Enzo Life Sciences (Catalog# BML-DM200-0050, BML-DM300-0050, Farmingdale, NY). Antibodies for CYP24A1 and CYP27B1 were purchased from ABCAM (Catalog# ab203308, ab206655, Cambridge, MA). VDR and Thy1/CD90 antibodies were purchased from Cell Signaling Technology (Catalog# 12550S and 13801S, Danvers, MA). MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) and keratocan antibodies were purchased from ThermoFisher Scientific (Catalog# M6494, and PA5-79552, Waltham, MA). The Pdia3 (Protein disulfide isomerase family A member 3) antibody (Ab099) was a kind gift of Dr. Ilka Nemere. Pdia3 inhibitor LOC14 was purchased from Tocris, Bio-Techne Corporation (Catalog# 5606, Minneapolis, MN). Pre-stained protein markers were obtained from Bio-Rad (Catalog# LC5699, Hercules, CA). Polyvinylidene difluoride (PVDF) membrane and the enhanced chemiluminescence (ECL) detection system were obtained from Bio-Rad (Catalog# 1704272, 1705060, Hercules, CA).
Isolation and cultivation of primary corneal fibroblasts
Human primary corneal keratocytes were isolated from three de-identified donor corneal rims (two male and one female, ages 49, 71, and 76, respectively) provided by local ophthalmologists. Corneal epithelium and endothelium were removed from the stroma by scraping with a razor blade. The stromal tissue was cut into small pieces and placed in 35 mm plates (four or five pieces of 2 × 2 mm tissue per well) in Dulbecco’s modified Eagle’s medium (DMEM) (ThermoFisher Scientific, Catalog # 11965118, Waltham, MA) plus 1% fetal bovine serum (ThermoFisher Scientific, Catalog# SH3007103HI, Waltham, MA), and incubated at 37°C with 5% CO2 until a sufficient amount of keratocytes migrated from the explants. Use of the donor tissue was in accordance with the Declaration of Helsinki. This study was deemed exempt by the Augusta University IRB committee. Informed consent was obtained from all donors or their families in accordance with tissue donation protocols.
Primary mouse corneal fibroblasts were cultured from four 4-week-old WT and VDR KO C57BL/6 mice as above. Cells were routinely screened using western blotting for the presence of the corneal stromal cell-specific marker keratocan37 and fibroblast marker Thy1/CD9038 to confirm the cells were from a keratocyte lineage. Culturing keratocytes in a serum-containing medium changes them to a fibroblast phenotype, which is a transitional phenotype between the keratocyte and epithelial–mesenchymal transition (EMT)-resulting myofibroblast phenotype.
All protocols for animal use and euthanasia were reviewed and approved by the Augusta University Institutional Animal Care and Use Committee and followed the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Immunofluorescence studies
Donor human corneal rims and mouse eyes were prepared for cryosectioning and were flash frozen in liquid nitrogen and embedded in optimal cutting temperature compound (Tissue-Tek, Sakura Finetek, Torrance, CA). Ten micrometer-thick cryosections were fixed for 10 minutes in 4% paraformaldehyde (4% PFA) and blocked with 10% goat serum in 0.1% Triton X-100/PBS for 1 hour at room temperature. Cryosections were incubated with primary antibodies at a dilution of 1:200 in block solution at 4°C overnight (anti-CYP24A1, anti-CYP27B1, anti-VDR, and anti-Pdia3), followed by incubation with goat anti-rabbit IgG (H + L) alexa fluor 488 secondary antibody (ThermoFisher Scientific, Catalog # A-11034, Waltham, MA) at a dilution of 1:800 in block solution at room temperature for 1 hour. Cryosections were examined using a Zeiss LSM 780 inverted laser-scanning confocal microscope (Zeiss Microscopy, Jena, Germany).
For cultured human fibroblasts, cells were isolated and cultured in 24-well cell culture plates with glass coverslips (ThermoFisher Scientific, Catalog # 12-545-81, Waltham, MA), and treated with vitamin D as described below. The immunofluorescence protocol for cells cultured on glass coverslides was the same as described above for cryosections.
Protein extraction and western blot analysis
Protein was isolated from human corneal fibroblasts (HCF), VDR WT, and VDR KO mouse corneal fibroblasts (MCF) grown on 35 mm dishes. After washing cells with PBS, they were exposed to lysis buffer (Cell Signaling Technology, Catalog# 2881S, Danvers, MA). Cell lysates were collected and western blotting was performed as previously described.39,40 Briefly, protein samples (30 μg/well) and dual plus molecular weight ladders (Bio-Rad Laboratories, Catalog# 1610394, Hercules, CA) were separated by SDS-PAGE on Stain-free Gels with a 4–15% gradient (Bio-Rad, Catalog# 4568084) for 40 min at 180 V in running buffer. Stain-free gels were activated by exposure to UV for 1 min. Proteins were transferred to PVDF membranes (Bio-Rad, Catalog# 1704272) using the Bio-Rad Trans-Blot Turbo Transfer System for 10 min. Total proteins on membranes were detected using the Stain-free method.41,42 All Western blotting procedures (except primary antibody incubation) were carried out at room temperature. Membranes were blocked with 10% non-fat milk in TBST for 60 min. Membranes were then incubated with primary antibodies at a dilution of 1:1000 in TBST with 10% non-fat milk at 4°C overnight. Removal of excess primary antibody was carried out by washing the membranes in TBST three times for 10 min each. The secondary antibody peroxidase-conjugated anti-rabbit IgG secondary antibody (ThermoFisher Scientific, Catalog# 31460, Waltham, MA) diluted 1:3000 was incubated with the membrane in TBST with 10% non-fat dry milk for one 1 h at room temperature. Excess secondary antibody was removed by washing the membranes in TBST three times for 10 min each. Membranes were exposed to clarity enhanced chemiluminescence (ECL) reagent (Bio-Rad) for 5 min at room temperature and visualized using a ChemiDoc MP (Bio-Rad). Detection and quantification of band intensities was conducted using Image Lab 6.1 software (Bio-Rad). Bands were normalized to total protein by dividing the intensity of the band by the intensity of the total protein from the same sample on the same blot.
Corneal fibroblasts treated with vitamin D
1,25-Vit D3 and 24,25-Vit D3 were dissolved in dimethyl sulfoxide (DMSO). Both human and mouse corneal fibroblasts were treated with 1,25-Vit D3 (20 nM) or 24,25-Vit D3 (100 nM) for 24 hours. Vitamin D concentrations are the final concentration in the culture medium. Control groups were treated only with DMSO.
Proliferation assay
HCF and MCF were seeded onto 24-well cell culture plates (2 × 104 cells/dish), incubated at 37°C until plates reached 55–65% confluence, and then placed in DMEM plus 0.1% fetal bovine serum overnight at 37°C. Cells were stimulated with 1,25-Vit D3 (20 nM) or 24,25-Vit D3 (100 nM). The MTT reduction assay was used to measure the density of viable cells and was performed according to the manufacturer’s instructions. Control and vitamin D-treated cells were incubated in serum-free medium containing 0.4 mg/mL MTT. After 2 hours, the MTT solution was aspirated and dimethylsulfoxide was added (0.3 mL/well). Optical densities of the supernatant were read at 540 nm using a microplate spectrophotometer (BioTek, Winooski, VT). Growth ratios were calculated as the ratio of the absorbance of vitamin D-treated cells versus control cells. Absorbances were normalized to the control cultures, which represented 100% viability.
Statistical analysis
Western blot data are provided as the mean ± SE of at least three experiments, and proliferation data presented as mean ± SD. Data were analyzed using the unpaired Student’s t-test comparing experimental groups against controls. P < .05 was considered statistically significant. GraphPad Prism 8.4.3 was used for the data analysis.
Results
VDR, CYP24A1, and CYP27B1 immunostaining
To verify the presence of vitamin D signaling proteins in the corneal stroma, immunofluorescence microscopy was used to visualize VDR (Figure 1a,b,g,h), CYP24A1 (Figure 1c,d,i,j), and CYP27B1 (Figure 1e,f,k,l) in mouse and human corneas. In mouse corneas (Figure 1a–f), all three proteins were detected throughout the cornea, including in the epithelium, keratocytes, and endothelium. As expected, VDR immunostaining was primarilly nuclear, while CYP24A1 and CYP27B1 were distributed in both the cytoplasm and nucleus, with CYP24A1 heavilly staining the epithelium with more punctate staining in keratocytes. Human keratocytes were stained in a similar fashion (Figure 1g–l), although staining was not as punctate. Epithelium and endothelium were not included in human immunostaining samples.
Figure 1.

VDR (mouse a,b; human g,h), CYP24A1 (mouse c,d; human i,j), and CYP27B1 (mouse e,f; human k,l) protein immunostaining in representative mouse corneas including epithelium (Ep), stroma (S), and endothelium (En) (a-f) and donor human corneal stroma (g-l). Protein staining was visualized with secondary FITC conjugated antibodies (green). Nuclei were stained with DAPI (blue). Negative controls shown in b, d, f, h, j, and l. The insets show magnified portions of the images at the position of the arrows
1,25-Vit D3 and 24,25-Vit D3 effects on human fibroblast protein expression
Treatment of cells with 1,25-Vit D3 resulted in significantly increased VDR protein expression in HCF (p < .01) with no significant change in VDR protein expression following 24,25-Vit D3 treatment (Figure 2a). HCF CYP24A1 (Figure 2b) and CYP27B1 (Figure 2c) were both increased by 1,25-Vit D3 and 24,25-Vit D3 treatment.
Figure 2.

Effect of vitamin D on human fibroblast VDR (a), CYP24A1 (b), and CYP27B1 (c) protein expression. Western blots from HCF and corresponding average blot density graphs demonstrating that 1,25-Vit D3 stimulates VDR expression, and both 1,25-Vit D3 and 24,25-Vit D3 stimulate CYP24A1 and CYP27B1 expression (n = 3; **p < .01, *p < .05 compared to control, t-test). There was no significant change of VDR expression in HCF treated with 24,25-Vit D3.
Immunohistochemical examination of HCF treated in the same manner with 1,25-Vit D3 and 24,25-Vit D3 showed similar results (Figure 3). Figure 3a–d demonstrates increased nuclear VDR immunostaining following 1,25-Vit D3 treatment, with little or no increase following 24,25-Vit D3 treatment. CYP24A1 (Figure 3e–h) and CYP27B1 (Figure 3i–l) immunostaining were increased following 1,25-Vit D3 and 24,25-Vit D3 treatment, with both cytoplasmic and nuclear staining.
Figure 3.
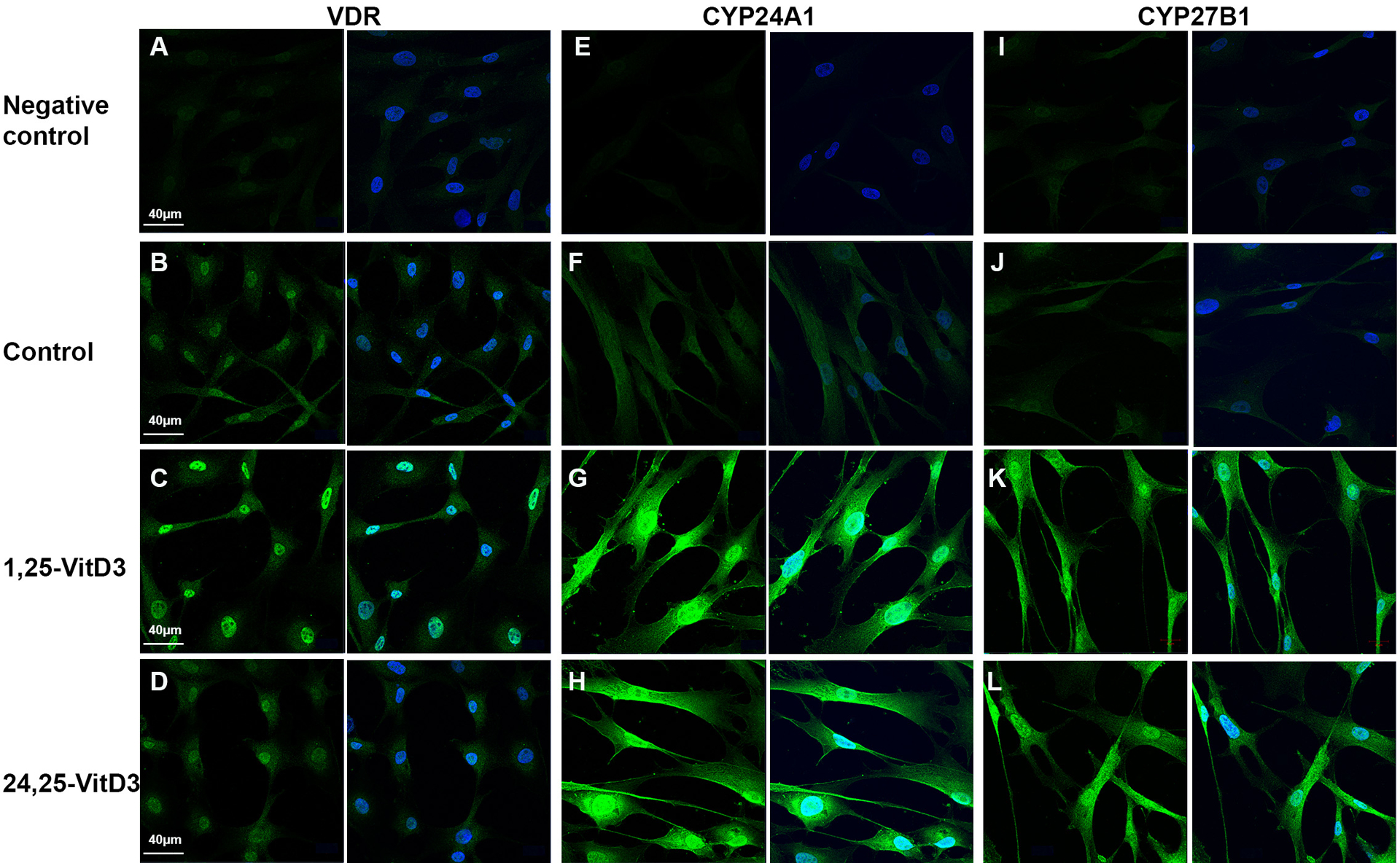
Effect of vitamin D on human fibroblast VDR (a-d), CYP24A1 (e-h), and CYP27B1 protein immunofluorescence (i-l). Each pair of representative photos shows target protein immunofluorescence in green (left) and target protein plus DAPI nuclear staining (blue, right). Results were similar to western blot results in Figure 2
1,25-Vit D3 and 24,25-Vit D3 effects on mouse fibroblast protein expression
As in HCF, 1,25-Vit D3 increased VDR protein expression in WT MCF (p < .01), while 24,25-Vit D3 had no effect (Figure 4a). Also, as in HCF, 1,25-Vit D3, and 24,25-Vit D3 increased both CYP24A1 (Figure 4b, p < .05) and CYP27B1 (Figure 4c, p < .01) protein expression in MCF. Interestingly, both CYP24A1 (Figure 4d) and CYP27B1 (Figure 4e) protein expression were also increased in VDR KO MCF by 1,25-Vit D3 and 24,25-Vit D3 (p < .05).
Figure 4.

Effect of vitamin D on VDR WT mouse fibroblast VDR (a), CYP24A1 (b), CYP27B1 (c) protein expression, and on VDR KO mouse fibroblast CYP24A1 (d) and CYP27B1 (e) protein expression. Western blots from VDR WT MCF and VDR KO MCF with corresponding average blot density graphs demonstrating that 1,25-Vit D3 stimulates VDR expression and both 1,25-Vit D3 and 24,25-Vit D3 stimulate CYP24A1 and CYP27B1 expression (n = 3; **p < .01, *p < .05 compared to control, t-test). There was no significant change of VDR expression in VDR WT MCF treated with 24,25-Vit D3
Pdia3 in mouse and human corneas
The results above indicate that corneal fibroblast CYP24A1 and CYP27B1 protein levels can be mediated by vitamin D through VDR or through a secondary vitamin D receptor. Such a secondary vitamin D receptor, Pdia3, has been identified in other tissues.34,43 Figure 5a demonstrates positive cytoplasmic Pdia3 immunohistochemical staining in all mouse cell types of the cornea, including keratocytes. Figure 5c,e show positive cytoplasmic Pdia3 immunohistochemical staining in human corneal epithelium, keratocytes, and endothelium, with strong staining around the nucleus in epithelial cells, and an indication of cell membrane staining in keratocytes and endothelial cells.
Figure 5.

Pdia3 protein expression in (a,b) mouse and (c,d) human corneal epithelium and keratocytes and (e,f) human corneal keratocytes and endothelium. Protein staining was visualized with secondary FITC conjugated antibodies (green). Nuclei were stained with DAPI (blue), b d and fare negative controls. The abbreviations of Ep, S, and En stand for epithelium, stroma, and endothelium, respectively
Influence of Pdia3 inhibition on Vit D3-influenced mouse fibroblast protein expression
VDR WT and KO MCF and HCF were treated with 1,25-Vit D3 and 24,25-Vit D3 in the presence of the Pdia3 inhibitor LOC14 (10 μM for human and 20 μM for mouse) for 24 h. A lower LOC14 concentration was used in human fibroblasts due to an apparent toxic response with the higher concentration. VDR expression was significantly increased in LOC14-treated HCFs and VDR WT MCFs cultured with 1,25-Vit D3 (Figure 6a,f), although that increase was much less than that observed in cells with no LOC14 present (Figure 2a, 4a). There was no difference in VDR expression in LOC14-treated HCF or VDR WT MCF cultured with 24,25-Vit D3, which was similar to cells with no LOC14 present.
Figure 6.

The Pdia3 inhibitor LOC14 attenuates vitamin D-stimulated VDR, CYP24A1 and CYP27B1 protein production in mouse but not human fibroblasts. Controls in all experiments included LOC14 in the culture medium. (a) Western blots from VDR WT MCF with corresponding average blot density graphs demonstrate that in the presence of LOC14, 1,25-Vit D3 increases VDR expression while 24,25-Vit D3 has no effect. (b,c) In the presence of LOC14, 24,25-Vit D3 decreases CYP24A1 and CYP27B1 expression while 1,25-Vit D3 has no effect on CYP24A1 and CYP27B1 expression (n = 3; **p < .01, *p < .05 compared to control, t-test). (d,e) Western blots from VDR KO MCF with corresponding averaged blot density graphs demonstrate that neither 1,25-Vit D3 or 24,25-Vit D3 affect CYP24A1 or CYP27B1 expression. (f-h) Representative western blots from HCF with corresponding average blot density graphs demonstrate that in the presence of LOC14, only 1,25-Vit D3 increases VDR expression, while 1,25-Vit D3 and 24,25-Vit D3 both increase CYP24A1 and CYP27B1 expression.
In VDR WT MCF treated with LOC14, the 1,25-Vit D3-induced increase in CYP24A1 and CYP27B1 protein expression seen with no LOC14 present (Figure 4b,c) was attenuated (Figure 6b,c). 24,25-Vit D3 plus LOC14 resulted in significantly decreased CYP24A1 (p < .05) and CYP27B1 (p < .01) protein expression. In HCF, 1,25-Vit D3 and 24,25-Vit D3 resulted in significantly increased CYP24A1 and CYP27B1 expression (Figure 6g,h), indicating no effect of LOC14 (compare to Figure 2b,c).
To further analyze the contribution of VDR and Pdia3 signaling to Vit D3 induced CYP24A1 and CYP27B1 protein expression, VDR KO MCFs were treated with 1,25-Vit D3 and 24,25-Vit D3 in the presence of LOC14. Neither 1,25-Vit D3 nor 24,25- Vit D3 had any effect on or CYP27B1 protein expression in cultured mouse VDR KO or LOC14-treated corneal fibroblasts (Figure 6d,e).
Proliferation assay
1,25-Vit D3 and 24,25-Vit D3 had no significant effects on human or mouse corneal fibroblast proliferation (Table 1).
Table 1.
MTT Proliferation Assay
| Time(h)╲Cell | Human Control | Human 1,25-VitD3 | Human 24,25-VitD3 | WT Mouse Control | WT Mouse 1,25-VitD3 | WT Mouse 24,25-VitD3 | KO Mouse Control | KO Mouse 1,25-VitD3 | KO Mouse 24,25-VitD3 |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.32±0.03 | 0.28±0.05 | 0.27±0.01 | 0.17±0.02 | 0.12±0.03 | 0.2±0.01 | 0.32±0.01 | 0.23±0.01 | 0.25±0.03 |
| 24 | 0.29±0.04 | 0.34±0.05 | 0.34±0.06 | 0.25±0.03 | 0.29±0.05 | 0.32±0.03 | 0.37±0.06 | 0.40±0.04 | 0.51±0.02 |
| 48 | 0.41±0.06 | 0.43±0.02 | 0.41±0.03 | 0.31±0.03 | 0.36±0.08 | 0.55±0.08 | 0.49±0.07 | 0.47±0.06 | 0.57±0.12 |
| 72 | 0.66±0.06 | 0.66±0.08 | 0.49±0.05 | 0.84±0.06 | 1.01±0.06 | 1.19±0.06 | 1.18±0.2 | 1.17±0.04 | 1.36±0.05 |
| 96 | 0.71±0.02 | 0.72±0.06 | 0.69±0.05 | 0.72±0.03 | 0.84±0.07 | 0.99±0.08 | 1.10±0.12 | 1.10±0.09 | 1.21±0.06 |
O.D. values at 540 nm of human and mouse corneal fibroblasts (WT and KO) cultured with 1,25-Vit D3 (20 nM) or 24,25-Vit D3 (100 nM). No significant differences found between groups examined.
Discussion
Our lab previously demonstrated that the corneal epithelium has the enzymatic components (CYP24A1, CYP27B1) and ability to generate and activate vitamin D.24,39 UV-B exposure of cultured corneal epithelial cells in the presence of 7-dehydrocholesterol leads to increased levels of vitamin D3, 1,25-Vit D3 and 24,25-Vit D3.24 We have also detected the vitamin D receptor,39 Pdia344 (also called protein disulfide isomerase, ERp57, GR58, and 1,25D3-MARRS), and five different vitamin D metabolites in the cornea and anterior segment.24,25,39 Several other labs have also examined VDR and vitamin D metabolizing components in the cornea and anterior segment,44–46 although no studies have previously examined these components specifically in corneal stromal cells. VDR has also been observed in the rat ciliary body epithelium, corneal epithelium, and corneal stroma,45 and was found in the corneal endothelium, basal epithelium, and lens epithelium of the human eye.46 The presence of CYP24A1 and CYP27B1 has been confirmed in human corneal endothelium, non-pigmented ciliary body epithelium, and adult retinal pigment epithelial cell lines, and human scleral fibroblasts.47 This current study demonstrates that human and mouse corneal fibroblasts express VDR, Pdia3, CYP24A1, and CYP27B1, which indicates that corneal fibroblasts, like corneal epithelium, have the ability to produce 1,25-Vit D3, and that vitamin D likely has a role in the regulation and function of keratocytes. We chose to focus on fibroblasts for the cell culture portion of these studies, as opposed to keratocytes, because many documented actions of vitamin D in fibroblast-like cells involve suppressing EMT, and fibroblasts are that transitional phenotype in the keratocyte to myofibroblast transition.
The VDR is critical for most actions of vitamin D, which is found in nearly all cells and acts primarily as a nuclear gene transcription factor.19 Vitamin D initiates or influences a host of VDR-dependent cellular activities, including metabolism, cell and tissue morphology, cell junction and adhesion, differentiation and development, angiogenesis, and epithelial to mesenchymal transition.27 While most actions of VDR involve its role as a transcription factor, VDR has also been shown to have rapid non-genomic actions via receptors likely associated with plasma membrane-associated caveolae.32 1,25-Vit D3 has also been shown to initiate rapid effects on cells through Pdia3,48 which has been localized to caveolae, the cell membrane, and within the cytoplasm.49,50 Our previous work examining the function of 1,25-Vit D3 in corneal epithelial cells found that it influences gap junctions and tight junctions,51,52 migration, proliferation,3 wound healing,2 and epithelial desmosomal and hemidesmosomal protein expression.53 The current study focused on corneal keratocytes and fibroblasts, where both VDR and Pdia3 were detected in mouse and human cells, which is similar to what we previously found in corneal epithelium.44 Moreover, we determined that 1,25-Vit D3 increases VDR protein expression in human and mouse fibroblasts. This is also similar to what we found in corneal epithelium.3 1,25-Vit D3 has also been shown to increase VDR expression in bone cells, but not in the intestine.54
The CYP27B1 enzyme, which activates 1,25-Vit D3, has been identified as a VDR target.55 In the current study, we found that CYP27B1 protein expression was increased in human, VDR WT, and VDR KO mouse primary fibroblasts cultured with 1,25-Vit D3 and 24,25-Vit D3. Interestingly, as in kidney and skin cells, our previous work found that 1,25-Vit D3 decreases CYP27B1 protein expression in VDR WT mouse primary corneal epithelium.3
It is interesting that we found 1,25-Vit D3 stimulated CYP27B1 in VDR KO MCF. This is similar to our findings in VDR KO mouse primary corneal epithelium, where 1,25-Vit D3 and 24,25-Vit D3 both promoted CYP27B1 protein expression.3 As we concluded in corneal epithelium, our data indicate that VDR-independent pathways are involved in the regulation of 1,25-Vit D3 and 24,25-Vit D3-induced corneal stromal CYP27B1 protein expression in MCF, along with the expression of additional corneal proteins.
CYP24A1, which converts 1,25-Vit D3 to 24,25-Vit D3, has also been shown to have cell-specific regulation in kidney and non-renal target tissues. The pathway leading to the increase of CYP24A1 in keratocytes is unknown, but could occur by Vit D3 acting at the gene activation level as has been shown in kidney cells.56 Previous studies determined that CYP24A1 expression can be controlled by 1,25-Vit D3, calcium, and fibroblast growth factor 23 (FGF23).22 We found CYP24A1 protein expression was significantly increased by 1,25-Vit D3 and 24,25-Vit D3 in human, VDR WT, and VDR KO mouse primary corneal fibroblasts, which is similar to what we described in corneal epithelial cells.3 The 24 hour time point used in the current study to examine the influence of 1,25-Vit D3 and 24,25-Vit D3 on vitamin D metabolizing enzymes is based on this previous work in corneal epithelial cells. We have not examined additional time points.
Because CYP24A1 protein expression increased in VDR KO fibroblasts treated with 1,25-Vit D3, it is likely that VDR-independent pathways are involved in the regulation of 1,25-Vit D3-induced corneal stromal CYP24A1 protein expression. As noted earlier, 1,25-Vit D3 was found to stimulate cell signaling via the Pdia3 membrane receptor.57, 58 In the current study, we have demonstrated the presence of cytoplasmic Pdia3 in all cornea cell types using immunohistochemistry. In epithelial cells, Pdia3 stains heavily around the nucleus as was previously reported using the same antibody in osteoblast-like MC3T3-E1 cells.59 In keratocytes and endothelial cells, the staining is a bit more diffuse throughout the cytoplasm with an indication of membrane staining.
To determine if 1,25-Vit D3 can regulate CYP24A1 and CYP27B1 expression using the Pdia3 signaling pathway, the Pdia3 inhibitor LOC14 was used in this study. LOC14 has previously been used to inhibit Pdia3 signaling in mouse lung epithelial cells60 and brain slices.61 To our knowledge, there are no published data examining the effects of LOC14 on VDR. While 1,25-Vit D3 increased both CYP27B1 and CYP24A1 expression in VDR KO MCFs, it had no effect on CYP24A1 and CYP27B1 expression in these same cells when treated with LOC14. On the other hand, LOC14 did not affect 1,25-Vit D3-induced CYP24A1 or CYP27B1 expression in HCF. These results demonstrate species-specific differences and indicate that vitamin D3 metabolite-initiated feedback control of the key vitamin D enzymes CYP24A1 and CYP27B1 can occur through both the VDR and Pdia3 signaling pathways in corneal fibroblasts. Knocking down or knocking out Pdia3 in these cells will be necessary to confirm these pharmacological results.
It is generally believed that 24,25-Vit D3 is an inactive form of vitamin D3. However, previous work from our laboratory demonstrated that 24,25-Vit D3 stimulates HCEC cell proliferation and migration, and that it enhances corneal epithelial CYP24A1 and CYP27B1 expression.3 A 24,25-Vit D3 positive feedback loop has also been reported in osteoblasts, where 24,25-Vit D3 markedly enhances CYP24A1 levels while not effecting CYP27B1 mRNA, which likely results in a higher production of 24,25-Vit D3.62 Our current findings demonstrate that 24,25-Vit D3 significantly increases CYP24A1 and CYP27B1 expression in HCF, VDR WT MCF, and VDR KO MCF. VDR expression was unaffected by 24,25-Vit D3 in HCF and VDR WT MCF. These results lead us to conclude that as with 1,25-Vit D3, 24,25-Vit D3-initiated feedback control of CYP24A1 and CYP27B1 acts through both VDR-dependent and -independent signaling in corneal fibroblasts. We did not explore the alternate signaling pathway being used by 24,25-Vit D3, although it was recently reported that the endoplasmic reticulum-associated gene product of the Tlcd3b2 gene can act as a 24,25-Vit D3 receptor in chondrocytes.63,64 A limitation of the current study is the use of cells from a single human donor for each of the human fibroblast experiments.
Proliferation of activated keratocytes following corneal injury or insult typically begins 12 to 24 hours after injury.65 Both the corneal epithelium and keratocytes are intimately involved in this wound healing process. Given the presence of VDR and the same vitamin D enzymatic machinery and metabolites in the corneal epithelium and the positive influence of vitamin D on corneal epithelial proliferation and wound healing, it is possible that vitamin D and vitamin D metabolites can exert a similar influence on keratocytes. On the other hand, if vitamin D inhibits fibrotic responses in the cornea as it does in other tissues, we would expect either no stimulation of proliferation or inhibited proliferation following treatment with vitamin D. We found no change in corneal fibroblast proliferation following treatment with either 1,25-Vit D3 or 24,25-Vit D3.
In summary, VDR, CY24A1, and CYP27B1, and Pdia3 are all present in corneal fibroblasts. 1,25-Vit D3 and 24,25-Vit D3 can both modulate the levels of VDR and the metabolic enzymes through VDR-dependent and -independent signaling pathways, with 1,25-Vit D3 likely working, at least in part, and in a species-dependent manner, through the Pdia3 signaling pathway. As in the corneal epithelium, vitamin D is likely a significant regulator of corneal keratocyte physiological processes. The contributions of vitamin D to overall corneal function and health call attention to the importance for ophthalmologists to be aware of patient vitamin D status and to work with primary care physicians to correct it when patients are vitamin D deficient.
Acknowledgments
We are grateful to Dr. Amy Estes (Department of Ophthalmology, Medical College of Georgia at Augusta University, Augusta, GA) and The Eye Guys, Eye Physicians and Surgeons of Augusta, GA for providing donor corneal rims.
Funding
This work was supported by NIH Grant [EY021747-06].
Footnotes
Disclosure statement. The authors have no financial interests in this work.
References
- 1.Valle YL, Almalki SG, Agrawal DK. Vitamin D machinery and metabolism in porcine adipose-derived mesenchymal stem cells. Stem Cell Res Ther. 2016;7(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu X, Vick S, Chen Z, Chen J, Watsky MA. Effects of vitamin D receptor knockout and vitamin D deficiency on corneal epithelial wound healing and nerve density in diabetic mice. Diabetes. 2020;69(5):1042–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu X, Chen Z, Mylarapu N, Watsky MA. Effects of 1,25 and 24,25 vitamin D on corneal epithelial proliferation, migration and vitamin D metabolizing and catabolizing enzymes. Sci Rep. 2017;7(1):16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reins RY, Hanlon SD, Magadi S, McDermott AM. Effects of topically applied vitamin D during corneal wound healing. PLoS One. 2016;11(4):e0152889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wimalawansa SJ, Razzaque MS, Al-Daghri NM. Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol. 2018;180:4–14. [DOI] [PubMed] [Google Scholar]
- 6.Oda Y, Hu L, Nguyen T, Fong C, Zhang J, Guo P, Bikle DD. Vitamin D receptor is required for proliferation, migration, and differentiation of epidermal stem cells and progeny during cutaneous wound repair. J Invest Dermatol. 2018;138(11):2423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouillon R Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13(8):466–79. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18(2):153–65. [DOI] [PubMed] [Google Scholar]
- 9.Latacz M, Snarska J, Kostyra E, Fiedorowicz E, Savelkoul HF, Grzybowski R, Cieslinska A. Single nucleotide polymorphisms in 25-hydroxyvitamin D3 1-alpha-hydroxylase (CYP27B1) gene: the risk of malignant tumors and other chronic diseases. Nutrients. 2020;12(3):801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Belle TL, Gysemans C, Vitamin MC. D and diabetes: the odd couple. Trends Endocrinol Metab. 2013;24(11):561–68. [DOI] [PubMed] [Google Scholar]
- 11.Norris JM, Lee HS, Frederiksen B, Erlund I, Uusitalo U, Yang J, Lernmark A, Simell O, Toppari J, Rewers M, et al. Plasma 25-hydroxyvitamin D concentration and risk of islet autoimmunity. Diabetes. 2018;67(1):146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galor A, Gardener H, Pouyeh B, Feuer W, Florez H. Effect of a mediterranean dietary pattern and vitamin D levels on dry eye syndrome. Cornea. 2014;33(5):437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khamar P, Nair AP, Shetty R, Vaidya T, Subramani M, Ponnalagu M, Dhamodaran K, D’Souza S, Ghosh A, Pahuja N, et al. Dysregulated tear fluid nociception-associated factors, corneal dendritic cell density, and vitamin D levels in evaporative dry eye. Invest Ophthalmol Vis Sci. 2019;60(7):2532–42. [DOI] [PubMed] [Google Scholar]
- 14.Jin KW, Ro JW, Shin YJ, Hyon JY, Wee WR, Park SG. Correlation of vitamin D levels with tear film stability and secretion in patients with dry eye syndrome. Acta Ophthalmol. 2017;95(3):e230–e235. [DOI] [PubMed] [Google Scholar]
- 15.French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res. 2013;114:58–68. [DOI] [PubMed] [Google Scholar]
- 16.Aksoy H, Akcay F, Kurtul N, Baykal O, Serum AB. 1,25 dihydroxy vitamin D (1,25(OH)2D3), 25 hydroxy vitamin D (25(OH)D) and parathormone levels in diabetic retinopathy. Clin Biochem. 2000;33(1):47–51. [DOI] [PubMed] [Google Scholar]
- 17.Reins RY, McDermott AM. Vitamin D: implications for ocular disease and therapeutic potential. Exp Eye Res. 2015;134:101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verhoeff FH. Retinoblastoma undergoing spontaneous regression. Calcifying agent suggested in treatment of retinoblastoma. Am J Ophthalmol. 1966;62(3):573–74. [DOI] [PubMed] [Google Scholar]
- 19.Bikle DD. Vitamin D: newer concepts of its metabolism and function at the basic and clinical level. J Endocr Soc. 2020;4(2):bvz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones G, Kottler ML, Schlingmann KP. Genetic diseases of vitamin D metabolizing enzymes. Endocrinol Metab Clin North Am. 2017;46(4):1095–117. [DOI] [PubMed] [Google Scholar]
- 21.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D3–1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103(3–5):316–21. [DOI] [PubMed] [Google Scholar]
- 24.Lin Y, Ubels JL, Schotanus MP, Yin Z, Pintea V, Hammock BD, Watsky MA. Enhancement of vitamin d metabolites in the eye following vitamin D3 supplementation and UV-B irradiation. Curr Eye Res. 2012;37(10):871–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu X, Elizondo RA, Nielsen R, Christensen EI, Yang J, Hammock BD, Watsky MA. Vitamin D in tear fluid. Invest Ophthalmol Vis Sci. 2015;56(10):5880–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kowtharapu BS, Stachs O. Corneal cells: fine-tuning nerve regeneration. Curr Eye Res. 2020;45(3):291–302. [DOI] [PubMed] [Google Scholar]
- 27.Saccone D, Asani F, Bornman L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene. 2015;561(2):171–80. [DOI] [PubMed] [Google Scholar]
- 28.Stumpf WE, Sar M, Reid FA, Tanaka Y, DeLuca HF. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979;206(4423):1188–90. [DOI] [PubMed] [Google Scholar]
- 29.Berger U, Wilson P, McClelland RA, Colston K, Haussler MR, Pike JW, Coombes RC. Immunocytochemical detection of 1,25-dihydroxyvitamin D receptors in normal human tissues. J Clin Endocrinol Metab. 1988;67(3):607–13. [DOI] [PubMed] [Google Scholar]
- 30.Carlberg C Molecular endocrinology of vitamin D on the epigenome level. Mol Cell Endocrinol. 2017;453:14–21. [DOI] [PubMed] [Google Scholar]
- 31.Pike JW, Meyer MB, Lee SM, Onal M, Benkusky NA. The vitamin D receptor: contemporary genomic approaches reveal new basic and translational insights. J Clin Invest. 2017;127(4):1146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25(4):543–59. [DOI] [PubMed] [Google Scholar]
- 33.Silvagno F, Pescarmona G. Spotlight on vitamin D receptor, lipid metabolism and mitochondria: some preliminary emerging issues. Mol Cell Endocrinol. 2017;450:24–31. [DOI] [PubMed] [Google Scholar]
- 34.Nemere I, Farach-Carson MC, Rohe B, Sterling TM, Norman AW, Boyan BD, Safford SE. Ribozyme knockdown functionally links a 1,25(OH)2D3 membrane binding protein (1,25D3-MARRS) and phosphate uptake in intestinal cells. Proc Natl Acad Sci U S A. 2004;101(19):7392–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mesbah M, Nemere I, Papagerakis P, Nefussi JR, Orestes-Cardoso S, Nessmann C, Berdal A. Expression of a 1,25-dihydroxyvitamin D3 membrane-associated rapid-response steroid binding protein during human tooth and bone development and biomineralization. J Bone Miner Res. 2002;17(9):1588–96. [DOI] [PubMed] [Google Scholar]
- 36.Adams JS, Rafison B, Witzel S, Reyes RE, Shieh A, Chun R, Zavala K, Hewison M, Liu PT. Regulation of the extrarenal CYP27B1-hydroxylase. J Steroid Biochem Mol Biol. 2014;144 Pt A:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278(46):45629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pei Y, Sherry DM, McDermott AM. Thy-1 distinguishes human corneal fibroblasts and myofibroblasts from keratocytes. Exp Eye Res. 2004;79(5):705–12. [DOI] [PubMed] [Google Scholar]
- 39.Yin Z, Pintea V, Lin Y, Hammock BD, Watsky MA. Vitamin D enhances corneal epithelial barrier function. Invest Ophthalmol Vis Sci. 2011;52(10):7359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilda JE, Ghosh R, Cheah JX, West TM, Bodine SC, Gomes AV. Western blotting inaccuracies with unverified antibodies: need for a western blotting minimal reporting standard (wbmrs). PLoS One. 2015;10(8):e0135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilda JE, Gomes AV. Stain-free total protein staining is a superior loading control to beta-actin for western blots. Anal Biochem. 2013;440(2):186–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilda JE, Gomes AV. Western blotting using in-gel protein labeling as a normalization control: stain-free technology. Methods Mol Biol. 2015;1295:381–91. [DOI] [PubMed] [Google Scholar]
- 43.Nemere I, Garbi N, Hammerling GJ, Khanal RC. Intestinal cell calcium uptake and the targeted knockout of the 1,25D3-MARRS (membrane-associated, rapid response steroid-binding) receptor/Pdia3/ERP57. J Biol Chem. 2010;285(41):31859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu X, Chen Z, Vick S, Watsky MA. Vitamin D receptor and metabolite effects on corneal epithelial cell gap junction proteins. Exp Eye Res. 2019;187:107776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson JA, Grande JP, Roche PC, Campbell RJ, Kumar R. Immunolocalization of calcitriol receptor, plasma membrane calcium pump and calbindin-d28k in the cornea and ciliary body of the rat eye. Ophthalmic Res. 1995;27:42–47. [DOI] [PubMed] [Google Scholar]
- 46.Johnson JA, Grande JP, Roche PC, Campbell RJ, Kumar R. Immuno-localization of the calcitriol receptor, calbindin-D28K and the plasma membrane calcium pump in the human eye. Curr Eye Res. 1995;14:101–08. [DOI] [PubMed] [Google Scholar]
- 47.Alsalem JA, Patel D, Susarla R, Coca-Prados M, Bland R, Walker EA, Rauz S, Wallace GR. Characterization of vitamin D production by human ocular barrier cells. Invest Ophthalmol Vis Sci. 2014;55(4):2140–47. [DOI] [PubMed] [Google Scholar]
- 48.Nemere I, Yoshimoto Y, Norman AW. Calcium transport in perfused duodena from normal chicks: enhancement within fourteen minutes of exposure to 1,25-dihydroxyvitamin D3. Endocrinology. 1984;115(4):1476–83. [DOI] [PubMed] [Google Scholar]
- 49.Doroudi M, Chen J, Boyan BD, Schwartz Z. New insights on membrane mediated effects of 1alpha,25-dihydroxy vitamin D3 signaling in the musculoskeletal system. Steroids. 2014;81:81–87. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Doroudi M, Cheung J, Grozier AL, Schwartz Z, Boyan BD. Plasma membrane Pdia3 and VDR interact to elicit rapid responses to 1alpha,25(OH)(2)D(3). Cell Signal. 2013;25(12):2362–73. [DOI] [PubMed] [Google Scholar]
- 51.Elizondo RA, Yin Z, Lu X, Watsky MA. Effect of vitamin D receptor knockout on cornea epithelium wound healing and tight junctions. Invest Ophthalmol Vis Sci. 2014;55(8):5245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu X, Watsky MA. Effects of vitamin D receptor knockout on cornea epithelium gap junctions. Invest Ophthalmol Vis Sci. 2014;55(5):2975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu X, Watsky MA. Influence of vitamin D on corneal epithelial cell desmosomes and hemidesmosomes. Invest Ophthalmol Vis Sci. 2019;60(13):4074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wood RJ, Fleet JC, Cashman K, Bruns ME, Deluca HF. Intestinal calcium absorption in the aged rat: evidence of intestinal resistance to 1,25(OH)2 vitamin D. Endocrinology. 1998;139(9):3843–48. [DOI] [PubMed] [Google Scholar]
- 55.Bikle DD, Vitamin PS. D, calcium, and epidermal differentiation. Endocr Rev. 1993;14(1):3–19. [DOI] [PubMed] [Google Scholar]
- 56.Meyer MB, Lee SM, Carlson AH, Benkusky NA, Kaufmann M, Jones G, Pike JW. A chromatin-based mechanism controls differential regulation of the cytochrome p450 gene CYP24A1 in renal and non-renal tissues. J Biol Chem. 2019;294(39):14467–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boyan BD, Sylvia VL, McKinney N, Schwartz Z. Membrane actions of vitamin D metabolites 1alpha,25(OH)2D3 and 24R,25(OH)2D3 are retained in growth plate cartilage cells from vitamin d receptor knockout mice. J Cell Biochem. 2003;90(6):1207–23. [DOI] [PubMed] [Google Scholar]
- 58.Khanal RC, Nemere I. The ERP57/GRP58/1,25D3-MARRS receptor: multiple functional roles in diverse cell systems. Curr Med Chem. 2007;14(10):1087–93. [DOI] [PubMed] [Google Scholar]
- 59.Chen J, Olivares-Navarrete R, Wang Y, Herman TR, Boyan BD, Schwartz Z. Protein-disulfide isomerase-associated 3 (Pdia3) mediates the membrane response to 1,25-dihydroxyvitamin D3 in osteoblasts. J Biol Chem. 2010;285(47):37041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chamberlain N, Korwin-Mihavics BR, Nakada EM, Bruno SR, Heppner DE, Chapman DG, Hoffman SM, Van Der Vliet A, Suratt BT, Dienz O, et al. Lung epithelial protein disulfide isomerase a3 (Pdia3) plays an important role in influenza infection, inflammation, and airway mechanics. Redox Biol. 2019;22:101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou X, Li G, Kaplan A, Gaschler MM, Zhang X, Hou Z, Jiang M, Zott R, Cremers S, Stockwell BR, et al. Small molecule modulator of protein disulfide isomerase attenuates mutant huntingtin toxicity and inhibits endoplasmic reticulum stress in a mouse model of Huntington’s disease. Hum Mol Genet. 2018;27(9):1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Der Meijden K, Lips P, Van Driel M, Heijboer AC, Schulten EA, Den Heijer M, Bravenboer N. Primary human osteoblasts in response to 25-hydroxyvitamin D3, 1,25-dihydroxyvitamin D3 and 24R,25-dihydroxyvitamin D3. PLoS One. 2014;9(10):e110283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Antonyan L, Martineau C, St-Arnaud R. The er protein TLC domain 3b2 and its enzymatic product lactosylceramide enhance chondrocyte maturation. Connect Tissue Res. 2021;62(2):176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martineau C, Naja RP, Husseini A, Hamade B, Kaufmann M, Akhouayri O, Arabian A, Jones G, St-Arnaud R. Optimal bone fracture repair requires 24R,25-dihydroxyvitamin D3 and its effector molecule FAM57B2. J Clin Invest. 2018;128(8):3546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torricelli AA, Santhanam A, Wu J, Singh V, Wilson SE. The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res. 2016;142(110–118):110–18. [DOI] [PMC free article] [PubMed] [Google Scholar]


