Abstract
Objective:
Excessive response to deviant stimuli during infancy and early childhood represents an early risk marker for anxiety disorders. However, research has yet to delineate the specific brain regions underlying the neonatal response to deviant stimuli near birth and the relation to risk for anxiety disorders.
Methods:
The authors used task-based functional magnetic resonance imaging (fMRI) to measure response to deviant auditory stimuli in N=45 sleeping neonates (mean age 27.8 days, 60% female, 64% African American). In 41 of the infants, neural response to deviant stimuli was examined in relation to maternal trait anxiety on the State-Trait Anxiety Inventory (STAI-T), a familial risk factor for offspring anxiety.
Results:
Neonates manifest a robust and widespread neural response to deviant stimuli that resembles patterns found previously in adults. Higher maternal trait anxiety relates to higher responses within multiple brain regions including bilateral anterior insula, ventrolateral prefrontal cortex, and multiple areas within anterior cingulate cortex. These areas overlap with brain regions previously linked to anxiety disorders and other psychiatric illnesses in adults.
Conclusion:
The neural architecture sensitive to deviant stimuli robustly functions in newborns. Excessive responsiveness of some circuitry components at birth may signal risk for anxiety and other psychiatric disorders.
INTRODUCTION
Anxiety disorders are common impairing conditions (1) that can endure following treatments in up to 50% of affected individuals (2). Evidence suggests that the altered neurodevelopmental trajectory associated with anxiety may start as early as birth (3–5) and include an early appearing excessive responsivity to unexpected or ‘deviant’ stimuli. However, little is known about the specific neural systems underlying this excessive responsivity. The current study uses task-based functional magnetic resonance imaging (fMRI) to delineate the neonatal response to deviant stimuli and its relationship to maternal trait anxiety, a risk factor for problematic anxiety in offspring (6, 7). An altered neonatal response to unexpected stimuli could signal increased risk for later anxiety, and the associated brain systems may provide targets for preventative interventions.
Prior research relates anxiety in children and adults to increased behavioral and physiological responses to environmental change (8). Similar hyper-responsivity manifests in infants at risk for anxiety disorders (3). Infants born to mothers with an anxiety disorder face a five-fold increased risk for anxiety disorders (9). This increased risk exists along a continuum, as normative variation in maternal trait anxiety is associated with variation in offspring trait anxiety (6), which in turn relates to later risk for an anxiety disorders (7). In non-clinical samples, higher antenatal maternal anxiety is associated with increased electroencephalographic (EEG) evoked responses to deviant sounds in offspring near birth (10) and at age 9 months (11). Such increased EEG evoked responses are also associated with early childhood temperaments that predict risk for adult anxiety disorders (3). Behavioral inhibition, an early appearing temperament that includes increased reactivity to novel or deviant stimuli, is a potent risk factor for anxiety disorders (3). Despite this behavioral and physiological evidence, the relevant brain regions remain unknown in infants.
The brain response to deviant stimuli and its relationship to anxiety are better understood in older children and adults than infants. In adults, deviant stimuli engage numerous brain regions, including the bilateral anterior insula (AI), the dorsal anterior cingulate (dACC), the inferior frontal junction (IFJ), and the ventrolateral prefrontal cortex (vlPFC) (12). These regions derive from multiple different ‘functional brain networks’ that connect regions into distinct maps, such as the salience (SN) (13), cingulo-opercular (CON) (14), dorsal attention (DAN), and ventral attention (VAN) networks (15). Although the functional brain network architecture of the neonatal brain is incompletely understood, evidence indicates that network connectivity is reduced in neonates, suggesting that networks are in a more immature form (16, 17). Anxiety disorders in older children and adults have been robustly associated with increased activity in regions within the SN, CON, and VAN in response to salient stimuli as well as salient task conditions such as making an error (18). Beyond this increased salience response, recent meta-analyses indicate that anxiety and other psychiatric disorders are additionally associated with activity alterations in the AI, dACC, vlPFC, subgenual anterior cingulate (sgACC), and pregenual anterior cingulate (pgACC) during a range of cognitive (19) and emotional (20) tasks.
Examining brain function in neonates may illuminate risk markers that could be obscured in older samples due to compensatory adaptations. Prior research suggests that some of the neural correlates of pediatric anxiety disorders represent adaptive changes, such as increased prefrontal regulatory processes (21). Measuring brain activity differences during infancy may uncover ‘core’ deficits that occur before these secondary changes. Uncovering these early risk profiles is important given anxiety prevalence (22) and clinical impact (23). Moreover, children with anxiety disorders face elevated risks in adulthood for many other disorders, including depression (24), substance use disorders (25), eating disorders (26), and bipolar disorder (27). Therefore, uncovering early risk profiles could inform attempts to identify mechanism-based targets that reduce the burden from many mental disorders.
To address these issues, we use fMRI to measure brain activity evoked by deviant auditory stimuli in n=45 sleeping neonates. Following a commonly used strategy, we assess the ‘oddball’ response by playing loud white noise bursts at irregular intervals in the context of a repeating sequence of background sounds (12); in this case the ongoing fMRI scanner sounds provide the background. Prior work has established the feasibility and utility of measuring fMRI responses to sounds in neonates (28–30). In addition, we relate variation in the evoked response to normative variation in maternal trait anxiety in a subset of 41 neonates. We relate neonatal activity to maternal trait anxiety, thereby modelling risk associated with mothers’ stable tendencies to experience anxiety in various situations (31), a known familial risk factor (6). We focus on familial risk as it might be transmitted by genetic or environmental effects, through in utero exposure to mothers’ biological stress response (32). In post hoc analyses, we additionally control for confounding influences from maternal depression, reported stress, and state anxiety.
METHODS
Sample
This study was approved by the Human Studies Committees at Washington University and informed consent was obtained from a parent of all participants. We recruited participants from a separate study at Washington University: the Early Life Adversity, Biological Embedding, and Risk for Developmental Precursors of Mental Disorders (eLABE) study. Participants in eLABE were themselves recruited during the 2nd or 3rd trimester of pregnancy, and eLABE collected a battery of maternal assessments as well as structural MRI in neonates (average age 21.4 days, range 3 to 40 days). Note that participants recruited for the current study comprised a normative sample and were not enriched for psychiatric symptoms. Parents of infants born full term (36 weeks gestational age or older) were asked to participate in the current study, which involved a subsequent task-based fMRI visit. This session occurred on average 6.8 days (range 0 to 18) following the structural scan. Additional recruitment details, comparison of participants in the current study relative to all of eLABE, and inclusion/exclusion criteria are in the Supplemental Methods.
Maternal and Family Assessments
We utilized questionnaires from eLABE pertaining to psychiatric symptoms and demographic data. The primary measure of maternal anxiety was the trait scale from the State-Trait Anxiety Inventory (STAI), which provides a stable metric of enduring trait-like anxiety (31). The STAI was completed by n=41 women, including 34 who completed the measure within a 5-week period following birth and 7 who completed the measure within an 8-week period following the child’s first birthday. Results did not change substantively when restricting to mothers who had completed the STAI near birth (Supplemental Figure 10). Two women who completed the STAI near birth had missing items on the state subscale only, resulting in n=41 women with trait scores (STAI-T) and n=39 women with state scores (STAI-S). Additional assessments of psychiatric and demographic variables are detailed in the Supplemental Methods.
Imaging Data Acquisition and Pre-processing
Imaging was performed without sedating medications using a Siemens 3T Prisma scanner and 64-channel head coil. Details of procedures, fMRI parameters, and pre-processing are in the Supplemental Methods. We acquired between one and eleven fMRI blood-oxygen level dependent (BOLD) scans, depending on how the infant tolerated the scan. All BOLD scans were collected on the same day. The scans were 5.7 minutes in length for the first 37 infants and 6.7 minutes in length for the next 8 infants.
During each BOLD run, we played white noise auditory stimuli lasting 400 ms at irregular intervals, using E-prime (Psychology Software Tools, Sharpsburg, PA) and played via the external speaker on the Prisma. Infants wore MiniMuff ear protection to attenuate sounds to safe levels. Each run began with 56 seconds of background scanner noise (no white noise pulses). Next, a total of 24 oddball auditory stimuli were presented at random intervals every 9 to 14 seconds; the first oddball was always presented at exactly 56 seconds after scanner onset. In the first 47 infants, scanning ended after the last oddball; in the last 8, an additional 56 seconds of background scanner noise was played. Estimates were not substantively affected by the addition of this time (Supplemental Figure 14). The background scanner noise from the BOLD sequence was regular and did not vary throughout the scan. The task paradigm is illustrated in Supplemental Figure 1.
We censored frames with framewise displacement (FD) greater than 0.9 mm (in Talairach atlas space) to reduce motion artifact. This FD cutoff has been empirically demonstrated to be optimal for task-based fMRI (33); we repeated the primary analyses using an FD cut-off of 0.2 mm and obtained similar results (Supplemental Figure 11). Task runs with fewer than 150 remaining frames after censoring were excluded from analysis, resulting in a total of 2 runs eliminated from a single subject. We obtained a median of 33 minutes of data in each participant (see Supplemental Figure 1), including a median of 31 minutes (SD 14 minutes) after censoring.
Statistical Analyses
Pre-processed BOLD data were analyzed with a general linear model (GLM) as implemented using in-house software (www.nil.wustl.edu/labs/fidl/). Details of modeling are presented in the Supplemental Materials. Importantly, the auditory response was modeled by using separate finite impulse response (FIR) regressors (34) for each of the 40 BOLD frames following white noise onset (40 frames × 0.8 seconds TR yielding = 32 seconds modeled).
We computed a whole-brain repeated measures ANOVA with timepoint (1–40 frames after stimulus onset) as a within-subject factor using all subjects (n=45). The main effect of timepoint in this analysis indicates voxels with significant activity changes in response to the stimulus. Next, we computed a repeated measures ANOVA in the subset of participants with maternal trait anxiety data (n=41). Factors were timepoint (1–40 frames after the stimulus onset), maternal trait anxiety on the STAI, and timepoint × maternal anxiety interaction. Maternal anxiety was treated as a continuous variable, though 2 participants with high maternal anxiety scores were winsorized (see Supplemental Methods). Supplemental Figure 2 provides a histogram of maternal trait anxiety scores. We performed additional supplemental statistical analyses relating neonatal brain activity to maternal trait anxiety based on median split (Supplemental Figures 7 and 8) as well as maternal state anxiety (Supplemental Figure 9).
Data were spatially smoothed using a 6mm FWHM Gaussian kernel. All results were multiple comparisons corrected to achieve a whole-brain cluster-wise error rate of p<0.01. Details on multiple comparisons correction, derivation of regions-of-interest (ROIs) and timecourses from statistical maps, network characterization, assessments of movement during scans, control for confounding variables, and tests to determine how the neural responses varied over the course of each run are provided in the Supplemental Methods.
RESULTS
Sample demographics are in Table 1 and zero-order relations among variables are in Supplemental Table 2. Maternal trait anxiety overall was in the normative range (mean STAI-T score 31.2, SD 7.5, range 20–53) and unrelated to socioeconomic status, infant sex, or gestational age. Based on a score of 40 or higher on the STAI-S (35), 10.3% had clinically significant anxiety. As expected, maternal trait anxiety was significantly related to maternal state anxiety as measured with the STAI-S (r=0.71, p<0.001), stress as measured with the Perceived Stress Scale (r=0.60, p<0.001), and depression as measured with the Edinburgh Postnatal Depression Scale (r=0.50, p=0.001). Head movement overall was low (mean FD after censoring 0.12mm, SD 0.13mm) and was no more likely to occur at the onset of the sound relative to other times in the scan (F(39, 1716)=1.12, p=0.28).
Table 1.
Sample Demographics
| Neonatal Characteristics (n=45) | n | Mean | SD |
|
| |||
| Sex | |||
| Male | 18 | ||
| Female | 27 | ||
|
| |||
| Gestational Age at Birth in Weeks | 38.2 | 1.0 | |
|
| |||
| Age at Scan in Days | 27.9 | 9.8 | |
|
| |||
| Birthweight in Grams | 3115 | 488 | |
|
| |||
| Area Deprivation Index | 72.0 | 22.5 | |
|
| |||
| Child’s Race | |||
| African American | 29 | ||
| White | 16 | ||
|
| |||
| Ethnicity | |||
| Non-Hispanic | 45 | ||
|
| |||
| Maternal Characteristics | n | Mean | SD |
|
| |||
| Psychotropic Medicine | |||
| Sertraline | 2 | ||
| Dextroamphetamine | 1 | ||
| Dextroamphetamine & Topiramate | 1 | ||
|
| |||
| STAI Trait Anxiety | 31.2 | 7.5 | |
|
| |||
| STAI State Anxiety | 28.5 | 7.6 | |
|
| |||
| Edinburgh Postnatal Depression Scale | 4.0 | 3.1 | |
|
| |||
| Perceived Stress Scale | 12.8 | 5.7 | |
Neonatal Brain Response to Deviant Auditory Stimuli
As depicted in Figure 1, the deviant auditory stimuli elicited activity across a large portion of the neonatal brain. Specific regions included those that respond to auditory stimuli, including the thalamus, putamen, and auditory cortex. In addition, activity increased in regions of cortex that respond to deviant stimuli in adults, including the bilateral dACC, AI, and several discrete regions along the precentral gyrus; as well as in the right superior temporal sulcus extending posteriorly into the temporal-parietal junction (complete list of regions in Supplemental Table 3). Somewhat unexpectedly, the left motor cortex also demonstrated robust activity increases. All activity changes were highly statistically significant, and the shape of the fMRI response across these regions resembled fMRI responses to stimuli in older samples (see Figure 1). Of the 177 regions demonstrating significant activity changes, 176 remained significant when controlling for residual head motion (see Supplemental Table 3). In most brain regions, neural activity changes were highest in magnitude for stimuli that were presented early in each fMRI run, intermediate for stimuli presented in the middle of each run, and lowest for auditory stimuli that were presented late in each run (see Supplemental Figure 3).
Figure 1.
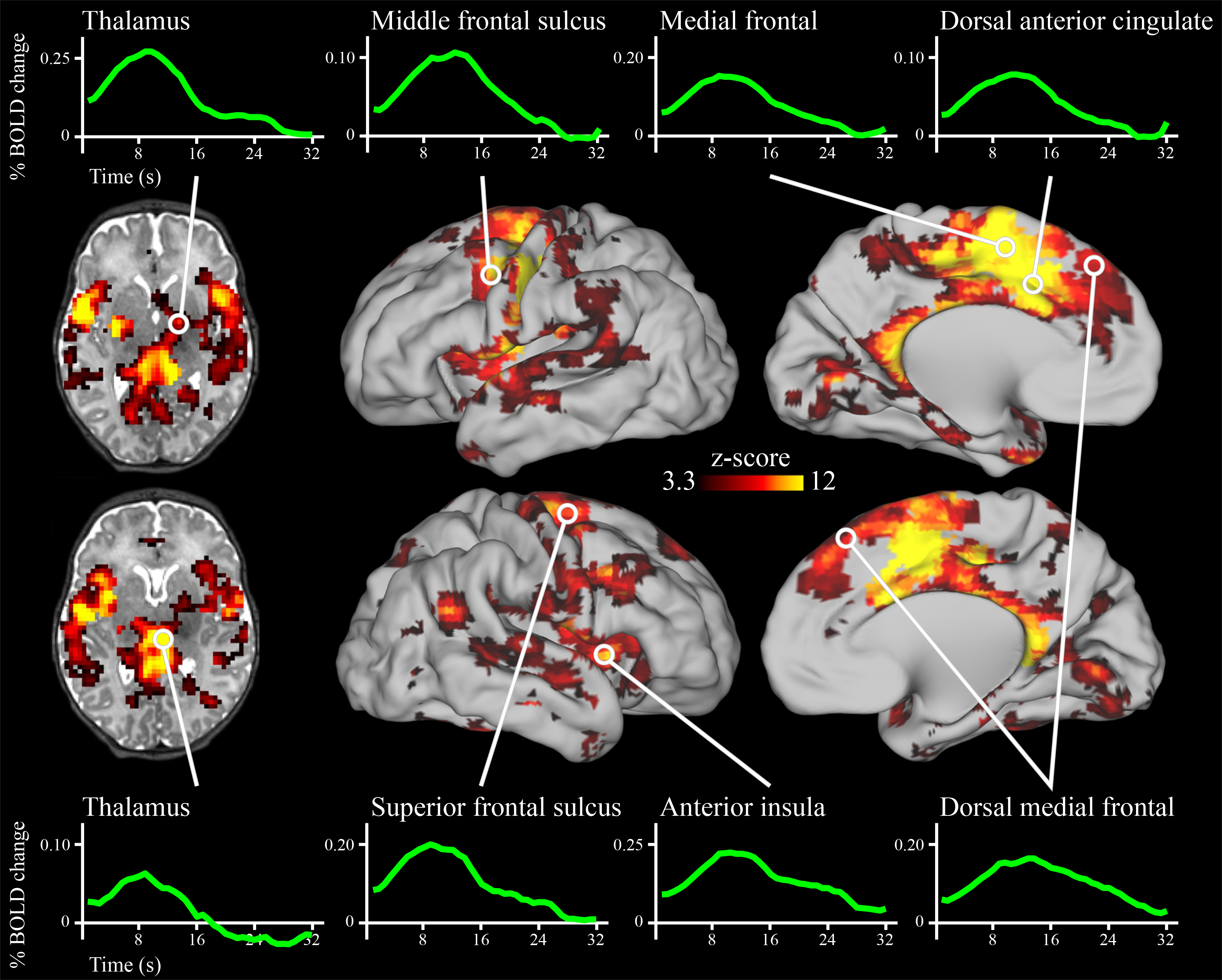
Brain regions with significant activity modulations in neonates following the onset of deviant sounds. Results are whole-brain multiple comparisons corrected at p<0.01, with each significant cluster comprised of a volume of at least 756mm3 in which each voxel is significant at p<0.001 (45, 46). A full list of regions is provided in Supplemental Table 3.
To examine similarity with adults, we overlaid the neonatal brain response with results from a published meta-analysis of the response to deviant stimuli in adults (see Supplemental Figure 4). Nearly all brain regions demonstrating a response to deviant stimuli in adults also responded to deviant stimuli in the neonates. To probe how the neonatal brain functional architecture relates to the adult architecture, we registered adult network definitions (36) to the neonatal brain (see Supplemental Figure 5). While infant brain organization may vary from adults, we used adult network definitions because the organization of the neonatal brain is less well understood. For each adult-defined cortical network, we quantified the percentage of cortical surface area within that network that responded to the deviant stimuli in neonates (see Figure 2). The adult-defined SN, CON, and VAN were among the networks with the highest percentage of surface area responsive to the stimuli in neonates.
Figure 2.
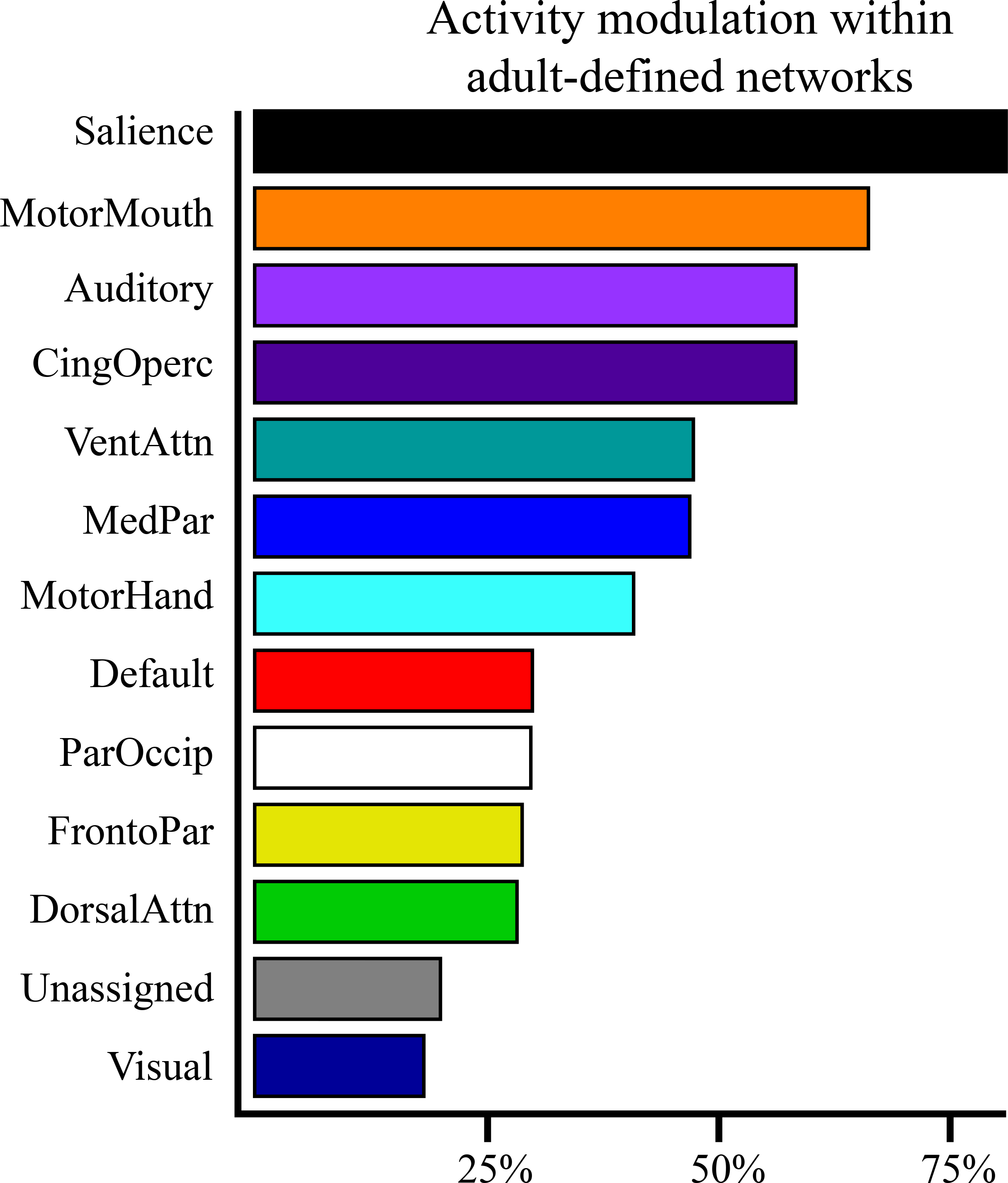
Proportion of adult-defined networks with significant activity modulations in neonates following the onset of the deviant sounds. Bar length indicates the percentage of cortical surface area for each network in which z>3.3 (p<0.001), the threshold used for multiple comparisons correction in the whole-brain analyses. MotorMouth: mouth representation within motor network; CingOperc: cingulo-opercular; VentAttn: ventral attention; MedPar: medial parietal; MotorHand: hand representation within motor network; Default: default mode; ParOccip: parietal occipital; FrontoPar: fronto-parietal; DorsalAttn: dorsal attention.
Relation of Neural Response to Maternal Trait Anxiety
The activity in many different neonatal brain regions in response to deviant stimuli varied as a function of maternal trait anxiety (Figure 3). Infants born to mothers with higher trait anxiety had higher activity in response to the deviant stimuli in the bilateral AI, dACC, subgenual anterior cingulate, and vlPFC (Figure 4). In addition, in many regions in the occipital and posterior parietal cortex, activity following deviant stimuli was lower in neonates born to mothers with higher trait anxiety (Figure 4). Of the 86 regions in which activity varied with maternal anxiety, 82 remained significant in post-hoc sensitivity analyses that separately controlled for residual head motion, amount of retained data, state anxiety, depression, and stress (see Supplemental Table 4).
Figure 3.
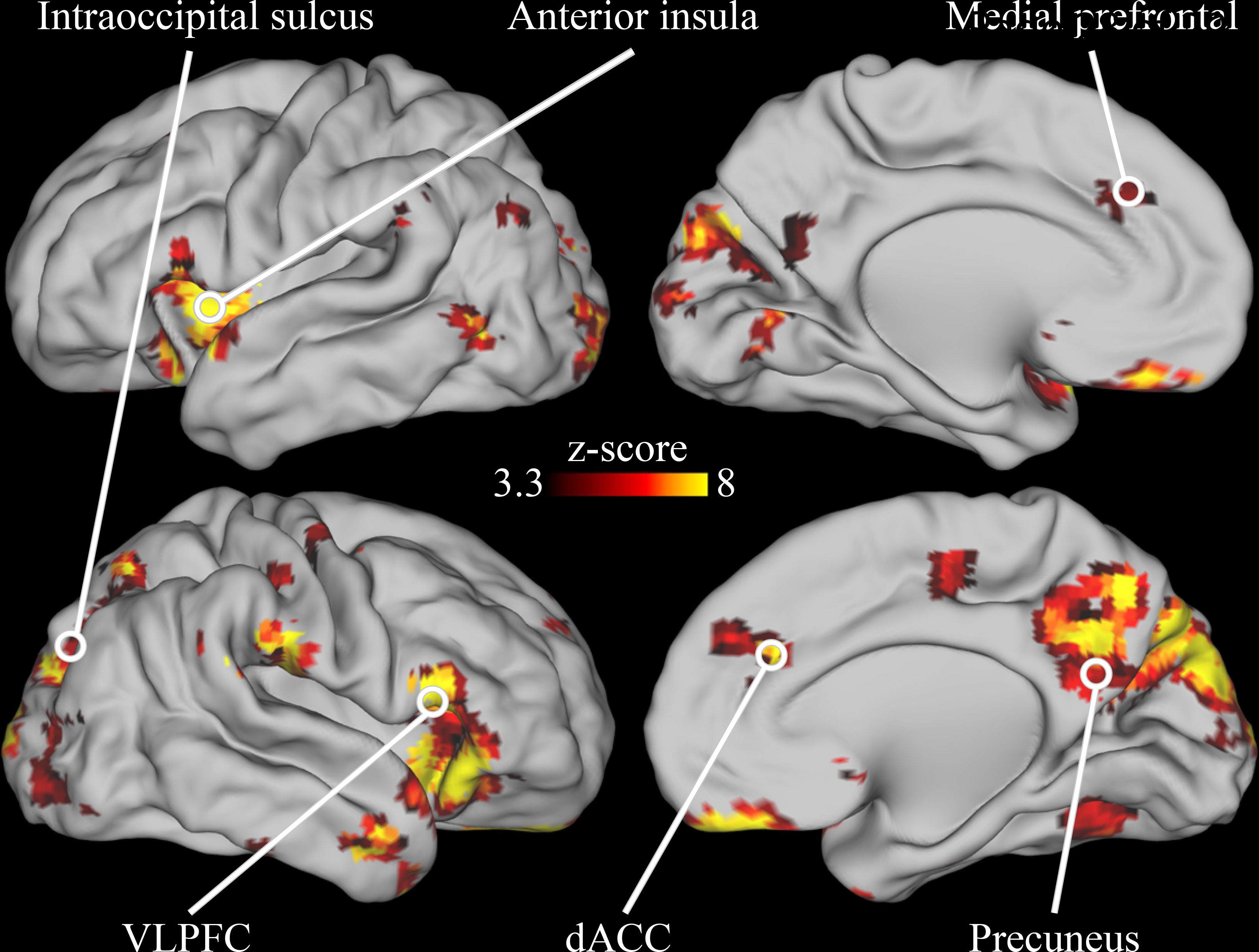
Brain areas in which neonatal neural activity following the onset of deviant sounds varied depending on maternal trait anxiety. Results are whole-brain multiple comparisons corrected at p<0.01, with each significant cluster comprised of a volume of at least 756mm3 in which each voxel is significant at p<0.001. A complete list of regions is provided in Supplemental Table 4.
Figure 4.
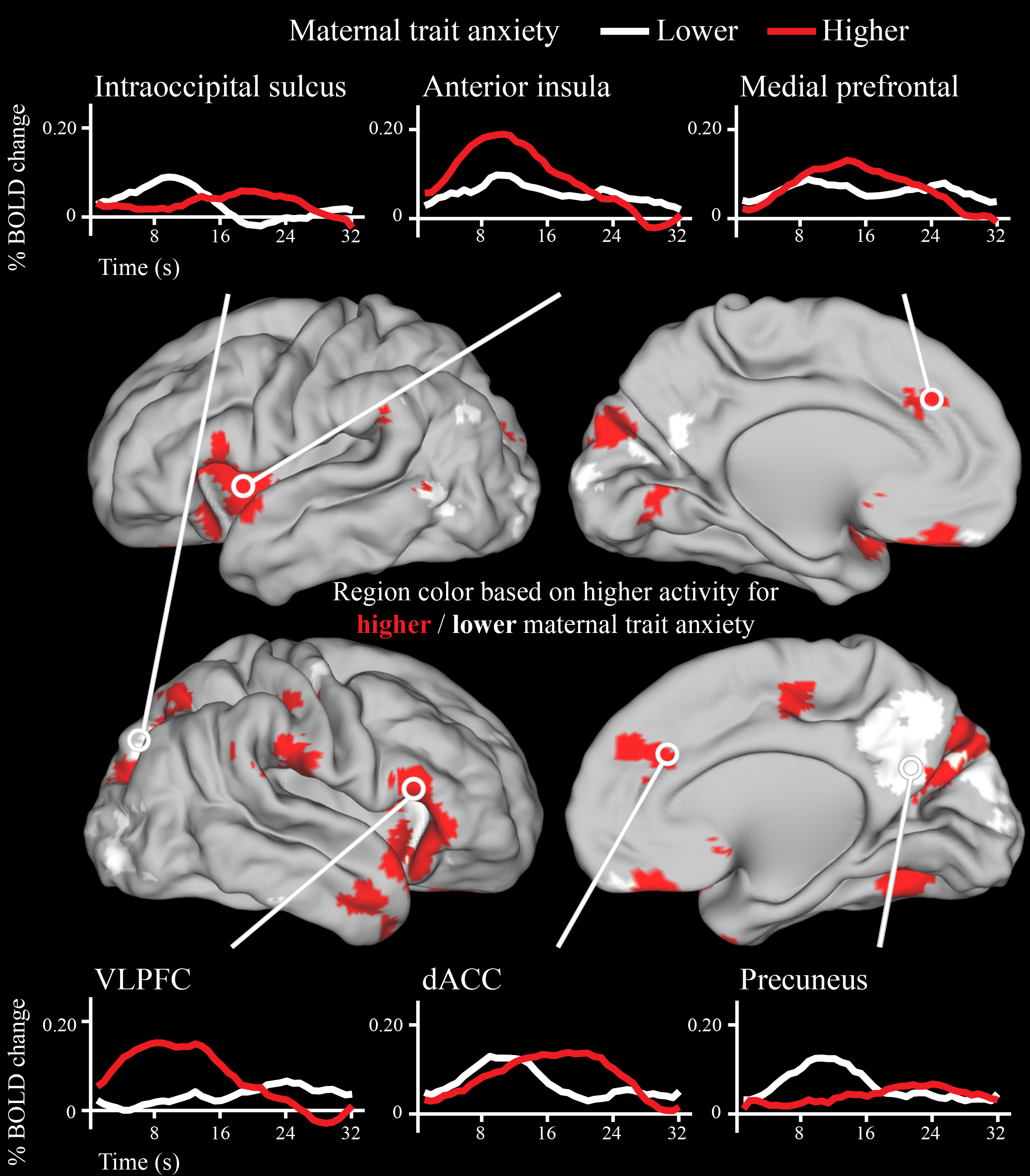
Timecourses for a subset of brain regions in which neonatal neural activity following onset of deviant sounds varied as a function of maternal trait anxiety. Note that the brain regions depicted here are identical to the brain regions in Figure 3, which is a statistical map of the same data. Trait anxiety was treated as a continuous measure in the statistical analyses, and a median split was used to generate the timecourses above solely for display purposes. Areas of cortex in red had higher peak activity in neonates born to mothers with higher trait anxiety, while areas of cortex in white had higher peak activity in neonates born to mothers with lower trait anxiety.
We next characterized the adult network definitions of these neonatal brain regions in which activity varied with maternal trait anxiety (Supplemental Figure 6 and Supplemental Table 4). Brain regions with higher activity for neonates born to mothers with higher trait anxiety typically fell within the CON, SN, VAN and anterior default mode network (DMN). There were however some exceptions to this general pattern of results, with some CON, SN, VAN, and DMN regions demonstrating lower activity for infants born to mothers with higher anxiety (see Supplemental Table 4). Brain regions with lower activity for neonates born to mothers with higher trait anxiety tended to fall in the visual network and posterior portions of the DAN and DMN.
DISCUSSION
This study reveals that the neonatal brain exhibits a robust response to deviant auditory stimuli. The specific regions that respond are similar to prior work in adults and include the dACC, AI, the precentral gyrus, the right superior temporal sulcus, and the right temporal-parietal junction; in adults, these regions comprise primarily the SN, CON, and VAN. In a subset of regions, including portions of the vlPFC, AI, dACC, and sgACC, activity following deviant stimuli is higher in neonates born to mothers with higher relative to lower trait anxiety. In other regions, including nearby portions of the AI, precuneus, and visual cortex, activity following deviant stimuli is lower in neonates born to mothers with higher relative to lower trait anxiety. Relations to maternal trait anxiety remain significant when controlling for maternal state anxiety, depression, and reported stress.
The current results are consistent with other research on early childhood risk. This research suggests that neural stimulus-response properties near birth signal risk for cascades generating later-life anxiety and other psychiatric illnesses (3–5). Infants in the current study born to mothers with higher trait anxiety had increased neural responses in a subset of the brain regions that respond to deviant stimuli. Previous work suggests that early increased neural responsivity to novel stimuli continues through childhood and interacts with other altered processes, such as increased attention to threat or errors, to increase risk for an anxiety disorder later in life (37). Of note, early childhood anxiety disorders are associated with additional psychiatric disorders later in life (25–27), which also have been linked to altered neural responses to deviant stimuli (38–40). Thus, the current data may inform the developmental neurobiology of other mental illnesses. Somewhat unexpectedly, infants born to mothers with higher trait anxiety additionally had lower activity in a separate set of brain regions; the functional significance of this lower activity is an important topic for future work.
Taken in the context of prior work, results from the current study may inform the development of biomarkers for use in early risk stratification and as specific neural targets for preventative efforts. Maternal anxiety in the current study largely varied across the normal range on the trait-anxiety scale. Prior work indicates that such normative variation in maternal anxiety is associated with risk for psychiatric and neurocognitive outcomes in offspring during childhood (41). This familial risk likely reflects impact from both genes and environmental factors, possibly transmitted though in utero exposure to maternal physiology. Results from the current study suggest that some of the increased familial risk for higher trait anxiety in neonates may manifest as alterations in basic stimulus-response properties. While similar altered stimulus-response associations have been previously reported using EEG, the current study may clarify the specific brain regions and systems underlying risk. Regionally specific brain activity as measured with fMRI may inform specific targets that are more easily identified longitudinally across development relative to less regionally specific EEG-based measures. Such findings inform basic research in other species attempting to localize neural processes associated with risk.
The current study found that neonatal neural activity varies as a function of maternal trait anxiety in the same brain regions that have been linked to expression of anxiety in adults. A meta-analysis of 283 experiments that totaled over 10,000 participants identified a series of brain regions that robustly show differential brain activity in cognitive tasks between controls and participants with major psychiatric illnesses, including anxiety disorders, depression, schizophrenia, and substance use disorders (19). Regions identified by this meta-analysis included the bilateral anterior cingulate and insular cortices and right vlPFC. A more recent meta-analysis using the same approach but examining emotional rather than cognitive tasks additionally identified the subgenual and pregenual medial prefrontal cortices (20). In the current study, we discovered that these same regions have higher activity in response to simple deviant sounds in sleeping newborn infants born to mothers with higher trait anxiety. This observation suggests that either prior reported results in children and adults pertain to alterations in basic stimulus-response properties rather than higher-level processes; or that variation in simple stimulus-response mechanisms at birth serves as the developmental foundation for disruption in higher order processes later in life.
Delineating the neural architecture that responds to deviant auditory stimuli in neonates may also illuminate the fundamental neural building blocks of later more complex processes relevant to risk for psychopathology. Prior work indicates that variation in lower order brain processes in the first year of life is associated with variation in more complex cognitive brain processes later in childhood (42). Brain regions that robustly responded to deviant stimuli in the current study, such as the AI and dACC, are involved in more complex operations in adults such as executive function and error monitoring (14). Future longitudinal studies could evaluate whether variation in the response to deviant stimuli at birth predicts variation in other functions performed by these regions later in life, such as executive function.
The current study should be considered in light of its limitations. The sample size was modest (N=45), though this was mitigated in part by a high amount of data per subject (~30 minutes after frame censoring), which may provide for more reliable estimates of responses from each individual (43). The sample included mothers primarily with non-clinical levels of anxiety; future studies can test whether these results extend to clinical samples. Maternal trait anxiety in the current study was highly correlated with other maternal symptoms including depression and perceived stress. Although results remained significant when controlling for these factors, future work is required to disambiguate the impact of these various maternal symptoms on neonatal brain activity. Results in the current study pertained to familial risk as assessed through maternal trait anxiety; future work is required to dissociate the impact of genetic versus environment risk, such as exposure to circulating maternal stress hormones. We were unable to determine sleep stage in our participants, which may affect BOLD activity (44). Finally, future longitudinal studies are required to clarify the relation between the infant oddball response near birth and relations to observed temperament during infancy and clinical symptoms in later childhood.
In summary, the current study reveals that the neonatal brain has a robust and specific response to deviant sounds and that the magnitude of neural activity in many brain regions is related to maternal trait anxiety. Furthermore, the specific brain regions that respond robustly to deviant stimuli, and the regional responses that vary with maternal trait anxiety, are the same regions implicated in the response to deviant stimuli and the pathophysiology of anxiety disorders in adults. These findings have significant implications for the developmental neurobiology of complex human behaviors and the origins of psychiatric disorders.
Supplementary Material
Acknowledgements
The authors thank Steven E. Petersen for comments on this manuscript and Joshua S. Shimony (J.S.S.) for reviewing the structural MRIs for neonatal injuries. This research was supported by the Brain and Behavior Research Foundation Grant Number 26735 (C.M.S.); National Institute of Health Grants K23 MH109983 (C.M.S.), R01MH122389 (C.M.S.), K02 NS089852 (C.D.S.), R01 MH113570 (C.D.S. and C.E.R.), R01 MH113883 (C.D.S. and J.L.L.), U54 HD087011 (C.D.S., C.E.R.), K23 MH105179 (C.E.R.), R01 MH090786 (D.M.B.), MH100019–06 (M.T.P.); the McDonnell Center for Systems Neuroscience (C.M.S.); the Taylor Family Institute (C.M.S.); the Parker Fund (C.M.S.). D.S.P. was supported by the NIMH Intramural Research Program through Project ZIAMH002782. The authors report no additional conflicts of interest.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. [DOI] [PubMed] [Google Scholar]
- 2.Piacentini J, Bennett S, Compton SN, Kendall PC, Birmaher B, Albano AM, March J, Sherrill J, Sakolsky D, Ginsburg G, Rynn M, Bergman RL, Gosch E, Waslick B, Iyengar S, McCracken J, Walkup J. 24- and 36-Week Outcomes for the Child/Adolescent Anxiety Multimodal Study (CAMS). Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral Inhibition: Linking Biology and Behavior within a Developmental Framework. Annual Review of Psychology. 2005;56:235–262. [DOI] [PubMed] [Google Scholar]
- 4.Sylvester CM, Smyser CD, Smyser T, Kenley J, Ackerman JJ, Shimony JS, Petersen SE, Rogers CE. Cortical Functional Connectivity Evident After Birth and Behavioral Inhibition at Age 2. The American journal of psychiatry. 2017:appiajp201717010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. Journal of child psychology and psychiatry, and allied disciplines. 2007;48:631–648. [DOI] [PubMed] [Google Scholar]
- 6.Savage JE, Sawyers C, Roberson-Nay R, Hettema JM. The genetics of anxiety-related negative valence system traits. Am J Med Genet B Neuropsychiatr Genet. 2017;174:156–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mundy EA, Weber M, Rauch SL, Killgore WD, Simon NM, Pollack MH, Rosso IM. Adult Anxiety Disorders in Relation to Trait Anxiety and Perceived Stress in Childhood. Psychol Rep 2015;117:473–489. [DOI] [PubMed] [Google Scholar]
- 8.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion (Washington, DC: ). 2007;7:336–353. [DOI] [PubMed] [Google Scholar]
- 9.Shimada-Sugimoto M, Otowa T, Hettema JM. Genetics of anxiety disorders: Genetic epidemiological and molecular studies in humans. Psychiatry and clinical neurosciences. 2015;69:388–401. [DOI] [PubMed] [Google Scholar]
- 10.Harvison KW, Molfese DL, Woodruff-Borden J, Weigel RA. Neonatal auditory evoked responses are related to perinatal maternal anxiety. Brain Cogn. 2009;71:369–374. [DOI] [PubMed] [Google Scholar]
- 11.Otte RA, Donkers FC, Braeken MA, Van den Bergh BR. Multimodal processing of emotional information in 9-month-old infants II: prenatal exposure to maternal anxiety. Brain Cogn. 2015;95:107–117. [DOI] [PubMed] [Google Scholar]
- 12.Kim H. Involvement of the dorsal and ventral attention networks in oddball stimulus processing: A meta-analysis. Human Brain Mapping. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyser CD, Neil JJ. Use of resting-state functional MRI to study brain development and injury in neonates. Seminars in Perinatology. 2015;39:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grayson DS, Fair DA. Development of large-scale functional networks from birth to adulthood_ A guide to the neuroimaging literature. NeuroImage. 2017:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, Zorumski CF, Lenze EJ. Functional network dysfunction in anxiety and anxiety disorders. Trends in Neurosciences. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mcteague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of Common Neural Circuit Disruptions in Cognitive Control Across Psychiatric Disorders. The American journal of psychiatry. 2017;174:676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McTeague LM, Rosenberg BM, Lopez JW, Carreon DM, Huemer J, Jiang Y, Chick CF, Eickhoff SB, Etkin A. Identification of Common Neural Circuit Disruptions in Emotional Processing Across Psychiatric Disorders. Am J Psychiatry. 2020:appiajp201918111271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Blair RJR, Chen G, Charney DS, Ernst M, Pine DS. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. The American journal of psychiatry. 2006;163:1091–1097. [DOI] [PubMed] [Google Scholar]
- 22.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodward LJ, Fergusson DM. Life course outcomes of young people with anxiety disorders in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:1086–1093. [DOI] [PubMed] [Google Scholar]
- 24.Kessler RC, Avenevoli S, McLaughlin KA, Green JG, Lakoma MD, Petukhova M, Pine DS, Sampson NA, Zaslavsky AM, Merikangas KR. Lifetime co-morbidity of DSM-IV disorders in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A). Psychological Medicine. 2012;42:1997–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glantz MD, Anthony JC, Berglund PA, Degenhardt L, Dierker L, Kalaydjian A, Merikangas KR, Ruscio AM, Swendsen J, Kessler RC. Mental disorders as risk factors for later substance dependence: estimates of optimal prevention and treatment benefits. Psychological Medicine. 2009;39:1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaumberg K, Zerwas S, Goodman E, Yilmaz Z, Bulik CM, Micali N. Anxiety disorder symptoms at age 10 predict eating disorder symptoms and diagnoses in adolescence. J Child Psychol Psychiatry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nurnberger JI, McInnis M, Reich W, Kastelic E, Wilcox HC, Glowinski A, Mitchell P, Fisher C, Erpe M, Gershon ES, Berrettini W, Laite G, Schweitzer R, Rhoadarmer K, Coleman VV, Cai X, Azzouz F, Liu H, Kamali M, Brucksch C, Monahan PO. A high-risk study of bipolar disorder. Childhood clinical phenotypes as precursors of major mood disorders. Archives of general psychiatry. 2011;68:1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science (New York, NY: ). 2002;298:2013–2015. [DOI] [PubMed] [Google Scholar]
- 29.Perani D, Saccuman MC, Scifo P, Spada D, Andreolli G, Rovelli R, Baldoli C, Koelsch S. Functional specializations for music processing in the human newborn brain. Proceedings of the National Academy of Sciences. 2010;107:4758–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arichi T, Fagiolo G, Varela M, Melendez-Calderon A, Allievi A, Merchant N, Tusor N, Counsell SJ, Burdet E, Beckmann CF, Edwards AD. Development of BOLD signal hemodynamic responses in the human brain. NeuroImage. 2012;63:663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA: Manual for the State-Trait Anxiety Inventory. Palo Alto, CA, Consulting Psychologists Press; 1983. [Google Scholar]
- 32.Rees S, Channon S, Waters CS. The impact of maternal prenatal and postnatal anxiety on children's emotional problems: a systematic review. Eur Child Adolesc Psychiatry. 2019;28:257–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, Petersen SE. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Human Brain Mapping. 2014;35:1981–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI I. The Method. Neuroimage. 2001;13:210–217. [DOI] [PubMed] [Google Scholar]
- 35.Tendais I, Costa R, Conde A, Figueiredo B. Screening for depression and anxiety disorders from pregnancy to postpartum with the EPDS and STAI. Span J Psychol. 2014;17:E7. [DOI] [PubMed] [Google Scholar]
- 36.Power Jonathan D, Cohen Alexander L, Nelson Steven M, Wig Gagan S, Barnes Kelly A, Church Jessica A, Vogel Alecia C, Laumann Timothy O, Miezin Fran M, Schlaggar Bradley L, Petersen Steven E. Functional Network Organization of the Human Brain. Neuron. 2011;72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henderson HA, Pine DS, Fox NA. Behavioral inhibition and developmental risk: a dual-processing perspective. Neuropsychopharmacology. 2015;40:207–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nan C, Wang G, Wang H, Wang X, Liu Z, Xiao L, Bai H, Wu S. The P300 component decreases in a bimodal oddball task in individuals with depression: An event-related potentials study. Clin Neurophysiol. 2018;129:2525–2533. [DOI] [PubMed] [Google Scholar]
- 39.Stevens MC, Pearlson GD, Kiehl KA. An FMRI auditory oddball study of combined-subtype attention deficit hyperactivity disorder. The American journal of psychiatry. 2007;164:1737–1749. [DOI] [PubMed] [Google Scholar]
- 40.Collier AK, Wolf DH, Valdez JN, Turetsky BI, Elliott MA, Gur RE, Gur RC. Comparison of auditory and visual oddball fMRI in schizophrenia. Schizophr Res. 2014;158:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–508. [DOI] [PubMed] [Google Scholar]
- 42.Scott LS, Pascalis O, Nelson CA. A Domain-General Theory of the Development of Perceptual Discrimination. Curr Dir Psychol Sci. 2007;16:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, Ortega M, Hoyt-Drazen C, Gratton C, Sun H, Hampton JM, Coalson RS, Nguyen AL, McDermott KB, Shimony JS, Snyder AZ, Schlaggar BL, Petersen SE, Nelson SM, Dosenbach NUF. Precision Functional Mapping of Individual Human Brains. NEURON. 2017;95:791–807.e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tagliazucchi E, Laufs H. Decoding wakefulness levels from typical fMRI resting-state data reveals reliable drifts between wakefulness and sleep. Neuron. 2014;82:695–708. [DOI] [PubMed] [Google Scholar]
- 45.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain connectivity. 2017;7:152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


