Abstract
We recently reported that adoptive transfer of cytolytic Natural Killer cells (cNKs) from the Reduced Uterine Perfusion Pressure (RUPP) rat induces a preeclampsia (PE)-like phenotype in pregnant rats, accompanied by increased TNF-α. The purpose of this study was to investigate a role for increased TNF-α to induce oxidative stress (ROS), decrease nitric oxide (NO) bioavailability, and induce vascular dysfunction as mechanisms of hypertension and intrauterine growth restriction in RUPPs. Pregnant Sprague Dawley rats underwent the RUPP or a Sham procedure on GD14. On GDs15 and 18, a subset of Sham and RUPP rats received i.p.injections of vehicle or 0.4 mg/kg of Etanercept (ETA), a soluble TNF-α receptor (n=10/group). On GD18, Uterine Artery Resistance Index (UARI) was measured, and on GD19, mean arterial pressure (MAP), fetal and placental weights were measured, and blood and tissues were processed for analysis. TNF-α blockade normalized the elevated MAP observed RUPP. Additionally, both fetal and placental weights were decreased in RUPP compared to Sham, and were normalized in RUPP+ETA. Placental ROS was also increased in RUPP rats compared to Sham, and remained elevated in RUPP+ETA. Compared to Sham, UARI was elevated in RUPPs while plasma total nitrate was reduced, and these were normalized in ETA treated RUPPs. In conclusion, TNF-α blockade in RUPPs reduced MAP and UARI, improved fetal growth, and increased NO bioavailability. These data suggest that TNF-α regulation of NO bioavailability is a potential mechanism that contributes to PE pathophysiology and may represent a therapeutic target to improve maternal outcomes and fetal growth.
Keywords: Preeclampsia, Inflammation, Tumor Necrosis Factor-alpha, Intrauterine Growth Restriction
INTRODUCTION
Preeclampsia (PE) is a hypertensive obstetric disorder that is a leading contributor of maternal and fetal morbidity and mortality worldwide.[1] While the precise etiology of PE is not fully known, the most prevalent hypothesis suggests that PE pathophysiology occurs in two phases.[2] The initial phase is marked by insufficient invasion of cytotrophoblasts into the maternal myometrium resulting in poorly remodeled spiral arteries and impaired placental blood flow.[3, 4] The second phase is a result of these ischemic conditions with the placenta releasing inflammatory mediators into the maternal circulation [3, 5] leading to systemic maternal immune activation, endothelial dysfunction and subsequent manifestations of hypertension (HTN), intrauterine organ restriction (IUGR), and end organ dysfunction.[3, 4, 6] Natural Killer cells (NKs) are among the immune cells activated in PE [7–9], and upon activation NKs secrete excess tumor necrosis factor alpha (TNF-α).[7, 10] As serum TNF-α has been shown to be significantly increased in PE women [11, 12] the actions of this inflammatory mediator may be a potential mechanism of NK involvement in PE development and progression.
TNF-α is a well-studied cytokine whose signaling acts as a central mediator of inflammation and has been implicated in the pathophysiology of several pregnancy disorders including spontaneous miscarriage, fetal growth restriction, preterm birth, and PE.[13, 14] A number of clinical studies have reported an association between increased TNF-03B1 and PE development. Therefore, the role of TNF-α in pregnancy has been the subject of enhanced research focus.[15, 16] Indeed, several studies in pregnant animals have shown that TNF-α infusion causes a PE- like phenotype with increased blood pressure and fetal growth restriction.[17, 18] Potential mechanisms whereby TNF-α may contribute to PE pathophysiology are by (1) decreasing nitric oxide (NO) bioavailability, (2) increasing vascular tone, and (3) inducing excessive reactive oxygen species (ROS) generation to cause endothelial dysfunction.[14, 19, 20] These mechanisms of TNF-α to mediate endothelial dysfunction leading to the development of HTN in PE have not been previously examined in a PE animal model and require further investigation.
The Reduced Uterine Perfusion Pressure (RUPP) rat model of placental ischemia displays many characteristics of PE women including HTN, IUGR, and vascular dysfunction.[21–25] We have previously shown that TNF-α is increased in the circulation and placentas of RUPP rats similar to that in PE women [23, 24], and depletion of placental NKs reduced circulating TNF-α and improved the HTN observed in RUPP rats.[24] More recently, we demonstrated that adoptive transfer of RUPP cytolytic NKs (cNKs) into pregnant rats resulted in HTN, IUGR, increased Uterine Artery Resistance, reduced NO bioavailability, and increased circulating TNF-α.[23] These data suggest that cNKs are a significant source of circulating TNF-α in RUPP rats and that enhanced TNF-α secretion may be a mechanism of cNK-induced pathophysiology. While previous investigations of TNF-α inhibition in RUPP rats demonstrated that blockade of TNF-α reduced endothelin expression, mitochondrial ROS, and NK activation [26, 27], the purpose of this study was to investigate a role for TNF-α to induce placental ROS, reduce NO bioavailability, and increase vascular resistance as mechanisms to promote vascular dysfunction leading to the development of HTN and IUGR in RUPP rats. Thus, we hypothesized that TNF-α blockade in RUPP rats would improve vascular function leading to improved HTN and IUGR.
MATERIALS AND METHODS
12–13 week old, timed pregnant Sprague-Dawley rats purchased from Envigo RMS, Inc. (Indianapolis, IN) were used in this study. The animals were delivered to the Center for Comparative Research at the University of Mississippi Medical Center on gestations day (GD) 10 or 11 and weighed approximately 250–260g upon arrival. The animals were housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle and maintained on Teklad 8640 diet (Envigo). The rats were group housed until surgery was performed and rats were randomly assigned to experimental groups. All experimental procedures conducted in this study were in accordance with the National Institute of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
Reduction in Uterine Perfusion Pressure
On GD14, a subset of timed pregnant Sprague Dawley rats, underwent RUPP or Sham surgery under isoflurane anesthesia delivered by an anesthesia apparatus (Ohio Medical Products, Madison, WI) as previously described.[28–30] Briefly, a midline incision was made, and a constrictive silver clip (.203mm) was placed on the abdominal aorta superior to the iliac bifurcation. To prevent compensatory blood flow via the ovarian arteries, restrictive silver clips (0.100 mm) were applied to the bilateral uterine arcades at the ovarian end. The animals received carprofen (5 mg/kg) immediately following surgery and 24 hours post-surgery to control for post-operative pain. Rats were excluded when the procedure resulted in total reabsorption of all fetuses.
Etanercept Treatment
Experiments were performed in four groups of rats: Sham+vehicle, Sham+etanercept (ETA) RUPP+vehicle, and RUPP+ETA (n=10/group). Vehicle treated rats received intraperitoneal (i.p.) injections of saline while the ETA treated group received 0.4 mg/kg Enbrel (a soluble TNF-α receptor) i.p. on GD15 and GD18. This dose been previously shown to decrease circulating TNF-α levels and decrease MAP in RUPP rats when given only on GD18.[27]
Measurement of Mean Arterial Pressure in Conscious Rats
Measurement of conscious MAP was performed as previously described by our group.[28–30] Under isoflurane anesthesia, 0.58 mm I.D. × 0.99 mm O.D vinyl catheter tubing (Scientific Commodities Inc., Lake Havasu City, AZ) was implanted into the carotid arteries and tunneled to the back of the neck on GD18 for the measurement of mean arterial pressure. On GD19, rats were placed in individual restrainers and conscious MAP was monitored with a pressure transducer (Powerlab, AD Instruments, Colorado Springs, CO). The MAP was recorded for 30 minutes following a 30-minute stabilization period.
Sample Collection
After MAP measurement, the animals were anesthetized for blood and tissue collection. Total litter size as well as the number of live pups were recorded. Placental and fetal weights were recorded for each dam and averaged. Randomly selected placenta tissues were snap frozen in liquid nitrogen and stored at −80°C until analyses.
Placental Reactive Oxygen Species and cyclic Guanosine Monophosphate (cGMP) Measurements
Superoxide production in the placenta was measured using the lucigenin technique, as previously described by our lab.[23, 28, 29] Briefly, placentas chosen at random from all groups were snap frozen in liquid nitrogen immediately after collection and stored at −80 °C until further processing. Placentas were homogenized using the Bio-Rad Cell Lysis Kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. The tissue lysate was incubated with lucigenin (Sigma-Aldrich, St. Louis, MO) at a concentration of 5 μM. The samples were allowed to equilibrate for 15 min in the dark, and the luminescence was measured for 10 s with a BioTek Plate Reader (BioTek, Winooski, VT). Luminescence was recorded as relative light units per minute (RLUs/min). An assay blank containing lucigenin with no homogenate was subtracted from the reading before transformation of the data. Each sample was run in triplicate and the average was used for data transformation. Levels of cGMP were measured in placental homogenates in duplicate using the Cyclic GMP ELISA Kit (# 581021, Cayman Chemical) according to the manufacturer’s instructions. The protein concentration of placental homogenates was measured using a protein assay with BSA standards (Pierce, Rockford, IL). All placental data were normalized to protein concentration and are expressed as RLU/min/mg protein.
Determination of placental and circulating cytokines and angiogenic factors
Circulating and placental TNF-α, IFN-γ, IL-6, and placental VEGF were measured in collected serum using a custom Bio-Plex Pro Rat Cytokine Immunoassay Kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions Circulating VEGF was measured in collected serum via ELISA (Boster Biological Technology, Pleasanton, CA; Catalog# EK054) according to the manufacturer’s instructions. Circulating soluble Fms-like Tyrosine kinase (sFlt-1) levels were measured in collected plasma via ELISA (R&D systems, Minneapolis, MN; Catalog# MVR100) according to the manufacturer’s instructions. All sample analyses were performed in duplicate. Protein concentration of the placental homogenates was measured using a protein assay with BSA standards. All placental data were normalized to protein and are expressed as pg/mg.
Determination of Circulating Total Nitrate/Nitrite and 8-Isoprostane Levels
Blood was collected in EDTA tubes and spun at 3000 × g for 10 minutes at 4o C. The collected plasma was assessed for total nitrate/nitrite in duplicate using the Nitrate/Nitrite Colorimetric Assay Kit (Catalog# 780001, Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions. Circulating levels of 8-isoprostane were assessed in plasma using the 8-Isoprostane ELISA Kit (Catalog# 516351, Cayman Chemical) according to the manufacturer’s instructions.
Determination of Uterine Artery Resistance Index
On GD 18, rats from all 4 groups were shaved and all abdominal hair was removed with a depilation cream. Power Doppler velocimetry measurements were performed on anesthetized pregnant dams at an imaging station with a Vevo 770 unit (Visual Sonics, Toronto, Canada) using a 30 Hz transducer and an insonating angle <30° as previously described.[31] The peak systolic flow velocity (PSV) and end diastolic flow velocity (EDV) were measured bilaterally. The uterine artery resistance index was calculated using the following formula: UARI= (PSV- EDV)/PSV. Uterine artery resistance index was determined using the mean measurements of three waveforms per side.
Western blot analysis of endothelial nitric oxide synthase (eNOS)
Placental eNOS expression and phosphorylation at S1176 were assessed in placental tissue via Western blot as previously described.[25, 31] Briefly, placentas were homogenized in cold RIPA-buffer and protein extracts, separated by SDS-PAGE using a polyacrylamide gel (4–20%). Proteins were transferred onto nitrocellulose membranes (Bio-Rad) and blocked with Blocking Buffer (LI-COR Biosciences, Lincoln, NE) for 1 h at room temperature. Membranes were incubated overnight at 4°C with primary antibody directed against eNOS (1:250; BD Transduction Laboratories, San Jose, CA. Cat# 610296) or Ser1177(human)/ Ser1176 (rodent) peNOS (BD Transduction Laboratories Cat# 612392). This was followed by anti-mouse IgG IRDye® 800Dx conjugated secondary antibody (1:10,000; Rockland, Gilbertsville, PA; Cat# 610731002) for 1 hr at room temperature and scanned using the Odyssey CLx Imager (LI-COR Biosciences, Lincoln, NE). The intensity of specific bands was quantified by densitometry using Image J (National Institutes of Health, USA) and the expression of eNOS or eNOS pS1176was normalized to β-actin (1:2,000; Miilipore Sigma, Darmstadt, Germany Cat# A1978).
Statistical analysis
All of the data are expressed as mean ± standard error mean for each group. Statistical analyses were performed using two-way ANOVA followed by multiple comparisons with Tukey’s post-hoc correction via GraphPad Prism 8 software. A value of p<0.05 was considered statistically significant.
RESUTS
Effects of TNF-α Blockade on Mean Arterial Pressure and Intrauterine Growth Restriction on Pregnant Rats
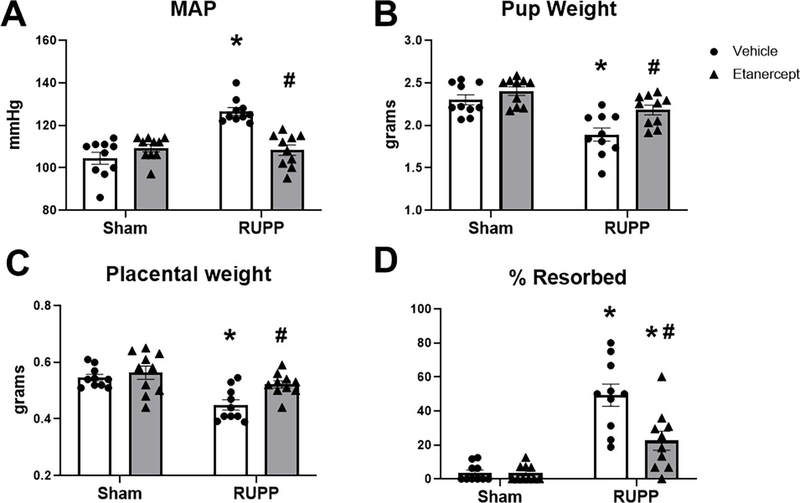
MAP was 105±3 mmHg in Sham and was unchanged at 109±2 mmHg in Sham+ETA (p=0.46 vs Sham). MAP was elevated to 127±2 mmHg in RUPP (p<0.0001 vs Sham) and was normalized to 109±3 mmHg in RUPP+ETA (p<0.0001 vs RUPP; Figure 1A). Mean fetal weight was 2.3±0.06 grams in Sham, 2.4±0.05 grams in Sham+ETA (p=0.65 vs Sham), and was reduced to 1.9±0.08 grams in RUPP (p=0.0002 vs Sham). Mean fetal weight was normalized to 2.2±0.06 grams in RUPP+ETA (p=0.011 vs RUPP; Figure 1B). Similarly, placental weight was 0.55±0.01 grams in Sham, 0.56±0.02 grams in Sham+ETA (p=0.90 vs Sham), and was reduced to 0.45±0.02 grams in RUPP (p=0.0015 vs Sham). Placental weight was normalized to 0.52±0.01 grams in RUPP+ETA (p=0.02 vs RUPP; Figure 1C). The percentage of resorbed fetuses was 4±2% in Sham and 3±1% in Sham+ETA (p>0.9999 vs Sham). This was elevated to 49±7% in RUPP (p<0.0001 vs Sham) and reduced to 23±6% in RUPP+ETA (p=0.0008 vs RUPP) although it remained elevated compared to Sham (p=0.02 vs Sham; Figure 1D).
Figure 1: Effect of TNF-α Blockade on Mean Arterial Pressure and Intrauterine Growth Restriction in Pregnant Rats.
On Gestation Day (GD) 14, either the Reduced Uterine Perfusion Pressure (RUPP) procedure or a Sham procedure was performed on pregnant Sprague Dawley rats. On GD 15 and 18, vehicle or 0.4 mg/kg Etanercept (ETA) was injected i.p. in a subset of Sham and RUPP rats. On GD 19, conscious Mean Arterial Pressure (A) was measured and fetal weights, (B) placental weights (C),and fetal resorptions (D) were recorded under isoflurane anesthesia. Sham n=10; RUPP n=10; Sham+ETA n=10; RUPP+ETA n=10. All data are expressed as mean ± SEM. Statistical analyses were performed using two-way ANOVA with multiple comparisons followed by Tukey’s post-hoc test. *p<0.05 versus Sham; #p<0.05 vs RUPP.
Effects of TNF-α Blockade on Uterine Artery Resistance, Plasma total Nitrate, and Placental ROS in Pregnant Rats
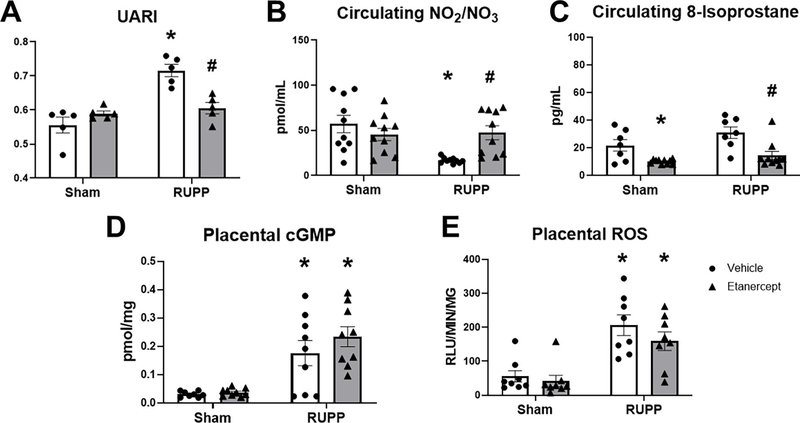
Uterine Artery Resistance Index was 0.56±0.02 in Sham and 0.59±0.01 in Sham+ETA (p=0.54 vs Sham). This was elevated to 0.72±0.02 in RUPP (p<0.0001 vs Sham) and was normalized to 0.61±0.02 in RUPP+ETA (p= 0.002 vs RUPP; Figure 2A). Plasma total nitrate was 57±10 pmol/mL and remained 45±7 pmol/mL in Sham+ETA (p= 0.65 vs Sham). This was reduced to 17±1 pmol/mL in RUPP (p=0.0014 vs Sham) and was normalized to 47±8 pmol/mL in RUPP+ETA (p= 0.02 vs RUPP; Figure 2B). Circulating 8-isoprostane, a biomarker of oxidative stress was 22±4 pg/mL in Sham and was significantly decreased to 10±0.5 pg/mL in Sham+ETA (p=0.048 vs Sham). In RUPP, circulating 8-isoprostane was 31±4 pg/mL (p= 0.22 vs Sham) and this was significantly reduced to 14±3 pg/mL in RUPP+ETA (p= 0.003 vs RUPP; Figure 2C). Placental cGMP was 0.031±0.004 pmol/mg in Sham and 0.037±0.004 pmol/mg in Sham+ETA (p=0.9989 vs Sham). This was elevated to 0.176±0.045 pmol/mg in RUPP (p=0.0089 vs Sham) and remained elevated at 0.234±0.035 pmol/mg in RUPP+ETA (p=0.50 vs RUPP; Figure 2D).Levels of placental ROS were 56±17 RLU/min/mg in Sham and remained at 42±17 RLU/min/mg in Sham+ETA (p=0.98 vs Sham). The levels were elevated to 206±30 RLU/min/mg in RUPP (p=0.0006 vs Sham) and remained elevated in RUPP recipients of ETA at 159±28 RLU/min/mg (p=0.51 vs RUPP; Figure 2E).
Figure 2: Effect of TNF-α Blockade on Uterine Artery Resistance Index (UARI), Circulating Total Nitrate and 8-Isoprostane, and Placental cGMP and Reactive Oxygen Species (ROS).
On Gestation Day (GD) 14, either the Reduced Uterine Perfusion Pressure (RUPP) procedure or a Sham procedure was performed on pregnant Sprague Dawley rats. On GD 15 and 18, vehicle or 0.4 mg/kg Etanercept (ETA) was injected i.p. in a subset of Sham and RUPP rats. On GD18, UARI (A) was measured via Doppler Ultrasound and on GD19, blood and placentas were collected under isoflurane anesthesia and frozen for analysis. Plasma was used to measure circulating total nitrate (B) and circulating 8-isoprostane (C) levels. Additionally, placental cGMP (D) and placental ROS (E) were measured. Sham: n=5–10; RUPP: n=5–10; Sham+ETA: n=5–10; RUPP+anti-IFNγ: n=5–10. All data are expressed as mean ± SEM. Statistical analyses were performed using two-way ANOVA with multiple comparisons followed by Tukey’s post-hoc test. *p<0.05 versus Sham; #p<0.05 vs RUPP.
Effects of TNF-α Blockade on Angiogenic Factors in Pregnant Rats
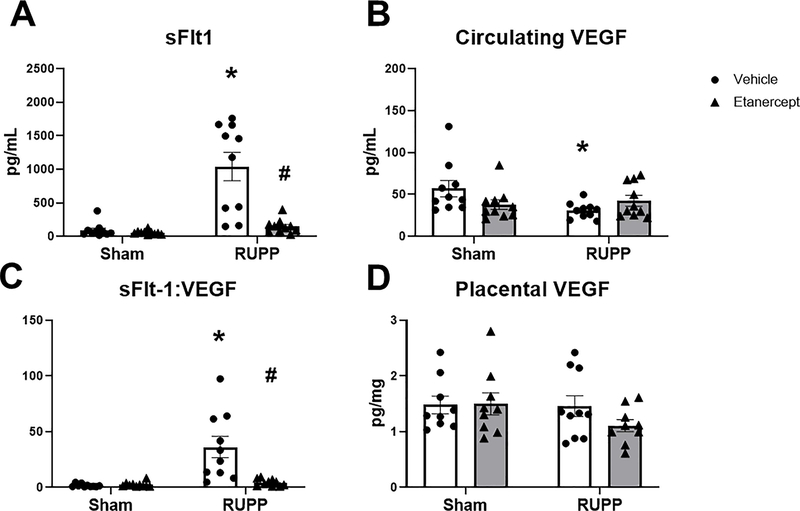
sFlt-1 was 92±34 pg/mL in Sham and 53±9 pg/mL in Sham+ETA (p=0.99 vs Sham). This was elevated to 1039±210 pg/mL in RUPP (p<0.0001 vs Sham) and was reduced to 145±33 pg/mL in RUPP+ETA (p<0.0001 vs RUPP; Figure 3A). Circulating VEGF was 57±10 pg/mL in Sham and 38±6 pg/mL in Sham+ETA (p=0.20 vs Sham) Circulating VEGF was reduced to 31±3 pg/mL in RUPP (p=0.04 vs Sham) and was 42±6 in RUPP+ETA (p=0.60 vs RUPP; Figure 3B). The mean sFlt-1:VEGF ratio was 2±0.4 and 2±0.7 in Sham and Sham+ETA respectively (p=0.9997 vs Sham), was elevated to 36±10 in RUPP (p<0.0001 vs Sham) and was normalized to 4±1 in RUPP+ETA (p=0.0002 vs RUPP; Figure 3C). Placental VEGF was unchanged in all groups at 1.5±0.2 pg/mg in Sham, 1.5±0.2 pg/mg in Sham+ETA, 1.5±0.2 pg/mg in RUPP and 1.1±0.1 pg/mg in RUPP+ETA (p>0.05 in all group comparisons; Figure 3D).
Figure 3: Effect of TNF-α Blockade on Circulating and Placental Angiogenic Factors.
On Gestation Day (GD) 14, either the Reduced Uterine Perfusion Pressure (RUPP) procedure or a Sham procedure was performed on pregnant Sprague Dawley rats. On GD 15 and 18, vehicle or 0.4 mg/kg Etanercept was injected i.p. in a subset of Sham and RUPP rats. On GD 19, blood and placentas were collected under isoflurane anesthesia and processed for further analysis. Circulating sFlt-1(A) and VEGF(B) were measured via ELISA and the sFlt-1:VEGF ratio (C) is shown. Placental VEGF (D) was measured using the Bio-Plex Pro Rat Cytokine Immunoassay Kit. Sham: n=9–10; RUPP: n=10; Sham+ETA: n=9–10; RUPP+ETA: n=9–10. All data are expressed as mean ± SEM. Statistical analyses were performed using two-way ANOVA with multiple comparisons followed by Tukey’s post-hoc test. *p<0.05 versus Sham; #p<0.05 vs RUPP.
Effects of TNF-α Blockade on Circulating and Placental Cytokines in Pregnant Rats
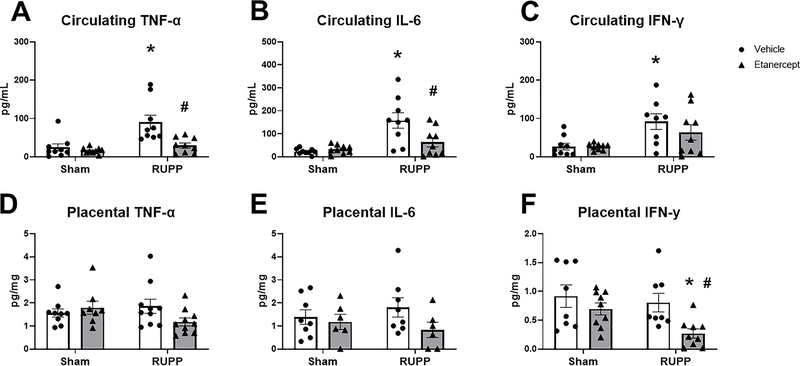
Circulating TNF-α was 24±9 pg/mL in Sham and 16±3 pg/mL in Sham+ETA (p=0.95 vs Sham), elevated to 91±18pg/mL in RUPP (p=0.0007 vs Sham), and normalized to 29±7 pg/mL in RUPP+ETA (p=0.0018 vs RUPP: Figure 4A). Similarly, circulating IL-6 was 23±4 pg/mL in Sham and 32±6 pg/mL in Sham+ETA (p=0.99 vs Sham). This was elevated to 158±33 pg/mL in RUPP (p=0.0002 vs Sham) and was reduced to 64±20 pg/mL in RUPP+ETA (p=0.011 vs RUPP; Figure 4B). Circulating IFN-γ was 27±9 pg/mL in Sham and was 26±3 pg/mL (p>0.9999 vs Sham). This was increased to 92±21 pg/mL in RUPP (p=0.023 vs Sham) and remained at 64±20 pg/mL in RUPP+ETA (p=0.55 vs RUPP; Figure 4C).
Figure 4: Effect of TNF-α Blockade on Circulating and Placental Cytokines.
On Gestation Day (GD) 14, either the Reduced Uterine Perfusion Pressure (RUPP) procedure or a Sham procedure was performed on pregnant Sprague Dawley rats. On GD 15 and 18, vehicle or 0.4 mg/kg Etanercept was injected i.p. in a subset of Sham and RUPP rats. On GD19, blood and placentas were collected under isoflurane anesthesia and frozen for analysis of circulating TNF-α (A), circulating IL-6 (B), circulating IFNγ (C), placental TNF-α (D), placental IL-6 (E), and placental IFNγ (F). Sham: n=8–9; RUPP: n=8–10; Sham+ETA: n=7–9; RUPP+ETA: n=7–10. All data are expressed as mean ± SEM. Statistical analyses were performed using two-way ANOVA with multiple comparisons followed by Tukey’s post-hoc test. *p<0.05 versus Sham; #p<0.05 vs RUPP.
Placental TNF-α was 1.6±0.2 pg/mg in Sham, 1.8±0.3 pg/mg in Sham+ETA, 1.9±0.3 pg/mg in RUPP, and 1.2±0.2 pg/mg in RUPP+ETA (p>0.05 in all group comparisons; Figure 4D). Similarly, placental IL-6 was unchanged at 1.4±0.3 pg/mg, 1.0±0.3 pg/mg, 1.8±0.4 pg/mg, and 0.7±0.3 pg/mg in Sham, Sham+ETA, RUPP, and RUPP+ETA respectively (p>0.05 in all group comparisons; Figure 4E). Placental IFN-γ was 0.9±0.2 pg/mg in Sham, 0.7±0.1 pg/mg (p=0.66 vs Sham), and 0.8±0.2 pg/mg in RUPP (p=0.94 vs Sham). This was significantly reduced to 0.3±0.1 pg/mg in RUPP+ETA (p=0.01 vs Sham; p=0.047 vs RUPP; Figure 4F).
Effects of TNF-α Blockade on Placental expression and phosphorylation of eNOS in Pregnant Rats
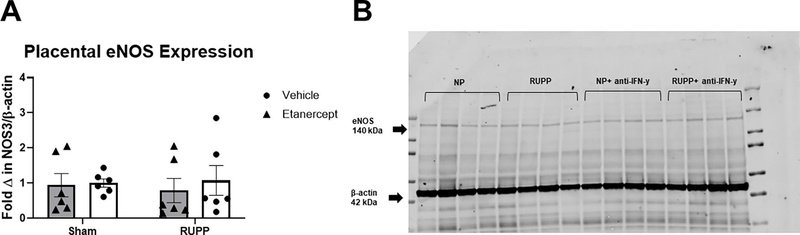
Placental eNOS expression was unchanged in all groups with a mean fold change of 1±0.1 in Sham, 0.9±0.3 in Sham+ETA, 1.1±0.4 in RUPP and 0.8±0.3 in RUPP+ETA (p> 0.05 in all group comparison; Figure 5A). A representative blot is shown in Figure 5B.
Figure 5: Western Blot analysis of placental eNOS expression.
On Gestation Day (GD) 14, either the Reduced Uterine Perfusion Pressure (RUPP) procedure or a Sham procedure was performed on pregnant Sprague Dawley rats. On GD 15 and 18, vehicle or 0.4 mg/kg Etanercept was injected i.p. in a subset of Sham and RUPP rats. On GD19, placentas were collected following sacrifice and processed for analysis of placental eNOS expression. (A).Quantified fold change of placental eNOS expression normalized to β-actin. (B) A representative blot of eNOS and β-actin expression in all groups is shown. Sham: n=6; RUPP: n=6; Sham+ETA: n=6; RUPP+ETA: n=6. All data are expressed as mean ± SEM. Statistical analyses were performed using two-way ANOVA with multiple comparisons followed by Tukey’s post-hoc test. *p<0.05 versus Sham; #p<0.05 vs RUPP.
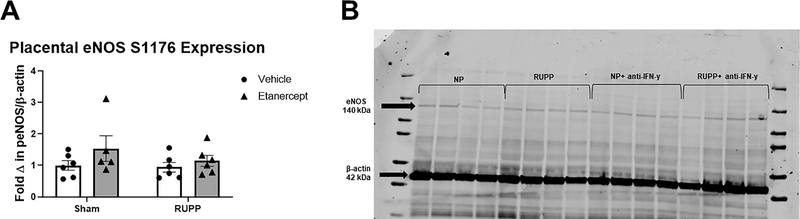
Fold change in placental eNOS pS1176 expression was 1 ±0.1 in Sham, 1.5±0.4 in Sham+ETA, 0.9±0.2 in RUPP, and 1.1±0.1 in RUPP+ETA (p>0.05 in all group comparisons; Figure 6A). A representative blot is shown in Figure 6B.
Figure 6: Western Blot analysis of placental eNOS S1176 phosphorylation.
On Gestation Day (GD) 14, either the Reduced Uterine Perfusion Pressure (RUPP) procedure or a Sham procedure was performed on pregnant Sprague Dawley rats. On GD 15 and 18, vehicle or 0.4 mg/kg Etanercept was injected i.p. in a subset of Sham and RUPP rats. On GD19, placentas were collected following sacrifice and processed for analysis of placental eNOS expression. (A).Quantified fold change of placental eNOS expression normalized to β-actin. (B) A representative blot of eNOS pS1176 and β-actin expression in all groups is shown. Sham: n=6; RUPP: n=6; Sham+ETA: n=5; RUPP+ETAγ: n=6. All data are expressed as mean ± SEM. Statistical analyses were performed using two-way ANOVA with multiple comparisons followed by Tukey’s post-hoc test. *p<0.05 versus Sham; #p<0.05 vs RUPP.
DISCUSSION
TNF-α is a potent inflammatory cytokine that has been observed to be elevated in PE women.[11, 12] It is also released by cNKs [32, 33] which previous adoptive transfer experiments have shown to induce a PE-like phenotype in pregnant rats.[23] Therefore, the purpose of this study was to investigate a role for TNF-α to induce ROS, decrease NO bioavailability, and promote vascular dysfunction as mechanism to promote HTN and IUGR in RUPP rats. We hypothesized that TNF-α blockade in RUPP rats would improve vascular function leading to improved HTN and IUGR. UARI and circulating sFlt-1 were reduced and plasma total nitrate was elevated in RUPPs treated with ETA compared to untreated RUPPs, and this was accompanied by a normalization of MAP and fetal growth. These data suggest that cNK secretion of TNF-α may mediate vascular dysfunction, HTN, and IUGR observed in RUPPs and is a potential mechanism of cNK involvement in PE.
In this study, we observed a normalization in MAP, fetal growth, and reduced fetal resorptions in RUPP+ETA compared to RUPP. These results differ somewhat from previous TNF-α inhibition studies in RUPP rats, which also saw improved MAP, but no improvements in fetal growth or live pups following ETA treatment.[26, 27, 34] These observed differences in fetal outcomes are likely due to differences in intervention. In previous studies, ETA was only administered on GD 18 [26, 27, 34], while in the present study ETA treatment was administered on GDs 15 and 18. The purpose of this change in the treatment timeline was to evaluate the effects of earlier TNF-α blockade on RUPP pathophysiology. A potential mechanism of these improvements is reduced vascular resistance which is reflected by reduced UARI observed in RUPP+ETA compared to RUPP. UARI is a measure of uterine artery function and is often used as a diagnostic tool to predict pregnancy disorders including IUGR and PE.[35, 36] UARI normally decreases as pregnancy progresses, indicating proper spiral artery remodeling and vasodilation.[35] Increased UARI is often observed in women with PE and IUGR and is an indicator of increased vascular resistance, decreased placental perfusion and subsequently decreased fetal blood supply.[35, 37] TNF-α has previously been shown to promote vasoconstriction in certain microvascular beds[38] thereby acting as a regulator of perfusion and blood pressure. As previous studies performed by LaMarca et al found increased renal vascular resistance following TNF-α infusion into pregnant rats[17], we examined uterine artery resistance in RUPPs following TNF-α blockade to further analyze the role of TNF-α in RUPP vascular dysfunction. The observed reduction of UARI observed in RUPP+ETA suggests that reduced vascular resistance and improved placental perfusion is a mechanism of the improved MAP and IUGR seen in RUPP+ETA rats.
In this study, we also examined levels of plasma total nitrate as an indicator of NO bioavailability to investigate the role of TNF-a in NO signaling in PE. NO is a potent vasodilator mainly produced by endothelial nitric oxide synthase (eNOS) and it freely diffuses into vascular smooth muscle cells leading to vasorelaxation and reduced peripheral resistance.[39, 40] PE is characterized by maternal endothelial dysfunction, and this is further demonstrated by the reduced NO bioavailability observed in PE women.[41–43] NO induced vasorelaxation is an important factor involved in the adaptation of maternal vasculature required to provide adequate blood flow to the fetal-placental unit.[44] Therefore, NO reduction is suggested to contribute to PE by causing increased vascular tone, which promotes increased blood pressure and reduced fetal blood flow.[45, 46] Infusion of the eNOS inhibitor L-NAME into pregnant rats and mice causes a PE like phenotype with HTN and fetal growth restriction, further supporting the importance of NO signaling in PE pathophysiology [47]. TNF-α has been recognized to decrease NO bioavailability by both increasing NO clearance and decreasing eNOS expression [19]. While previous studies by our collaborators have found a decrease in renal NOS expression in TNF-α infused pregnant rats there were no observed changes in placental eNOS expression or phosphorylation between groups in this study [48]. Furthermore, placental levels of cGMP, a NO second messenger, were elevated in both RUPP groups. While this appears to be paradoxical with the decreased plasma nitrate/nitrite levels, cGMP is also a second messenger of atrial natriuretic peptide (ANP) which has also been shown to be elevated in placental ischemic pregnant rats [49, 50]. ANP levels have also been reported to be elevated in preeclamptic women; however, the mechanism driving this elevation is still unknown and represents an area of further investigation [51, 52]. Importantly, in this study we also observed that ETA administration in RUPP rats significantly decreased circulating levels of 8-isoprostane, which is indicative of reduced circulating oxidative stress. Reactive oxygen species are known to quench NO resulting in reduced bioavailability; [53, 54] thus, the reduced circulating oxidative stress in RUPP+ETA compared to RUPP may contribute to the normalization of plasma nitrate in this group. This increased NO bioavailability can lead to reduced vascular resistance and a subsequent reduction in MAP. NO-induced vasorelaxation can also improve placental perfusion[55, 56], thus the increased plasma nitrate may contribute to the improved UARI and fetal growth observed in RUPP+ETA. These data suggest that TNF-α-induced NO deficiency is a mechanism of HTN, IUGR, and increased UARI in RUPP rats.
Previous studies by our collaborators have shown increased sFlt-1 levels in RUPP rats that were normalized following TNF-α blockade.[34] The data in this study mirror these results and reinforce a role of TNF-α in induction of sFlt-1 production. sFlt-1 is a splice variant of the vascular endothelial growth factor (VEGF) receptor Flt-1 that is released into the maternal circulation where it binds proangiogenic factors VEGF and placental growth factor PLGF.[57–59] sFlt-1 acts as an antiangiogenic factor by sequestering VEGF and PLGF and preventing them from reaching their cellular receptors.[60] Elevated sFlt-1 has been observed in PE women and animal models andis implicated in PE pathophysiology.[18, 58, 61, 62] Furthermore, infusion of sFlt-1 into pregnant animals causes a PE like phenotype with HTN and renal injury.[63–65] Although VEGF remained unchanged in RUPP+ETA, the reduced sFlt-1 resulted in a normalized sFlt-1:VEGF ratio. The reduced sFlt-1:VEGF ratio observed in response to TNF-α blockade likely contributed to the improved pathophysiology observed in the RUPP+ETA group.
An additional downstream action of TNF-α signaling is to stimulate production of other inflammatory mediators including IL-6.[66, 67] Previous studies have shown that circulating IL-6 is elevated in PE women [68, 69] and RUPP rats. [70] This was also observed in this study, and circulating IL-6 was significantly reduced in RUPP+ETA. IL-6 is known to induce endothelial dysfunction [71] and has been shown to decrease eNOS activity by reducing phosphorylation at S1177 in isolated human endothelial cells.[72] We did not observe any changes in placental eNOS phosphorylation at S1176 (the equivalent site in rodents) in this study, nor did we observed decreases in placental IL-6. However, there may have been effects on vascular endothelial eNOS that contributed to reduction in plasma nitrate in RUPPs and the normalization seen in RUPP+ETA. Additionally, a study by Lamarca, et al demonstrated that chronic infusion of IL-6 increased blood pressure in pregnant rats[73], indicating that IL-6 is a mediator of HTN during pregnancy. Thus, decreased circulating IL-6 is another potential mechanism of the reduced MAP seen in RUPP+ETA. These data suggest that TNF-α blockade may show additional benefits in PE by decreasing plasma IL-6 and limiting additional systemic inflammatory signaling.
Although the results of this study identified additional mechanisms of TNF-α in mediating HTN, IUGR, and vascular dysfunction in placental ischemia and PE, there are some limitations. While we observed reduced levels plasma nitrate in RUPP compared to Sham and normalized levels in RUPP+ETA, we did not detect any changes in placental eNOS expression or phosphorylation at S1176. This suggests that further investigation surrounding the mechanisms of TNF-α-mediated reductions in NO bioavailability in RUPPs is required. We also observed that TNF-α blockade did not reduce placental ROS, despite previous studies showing decreased placental mitochondrial ROS in RUPP rats that received ETA on GD 18.[26] These data suggest that future studies of TNF-α induced oxidative stress will need to differentiate between ROS production in different cellular compartments. Finally, the administration of ETA inhibited the TNF-α signaling from all cellular sources, therefore the improvement observed in RUPP+ETA cannot be solely attributed to inhibition of cNK secreted TNF-α. However, these studies combined with our previous adoptive transfer experiments support this hypothesis, and future studies by our lab will further examine this mechanism. Despite these limitations, this study reveals that TNF-α blockade in RUPPs increased plasma total nitrate, decreased sFlt-1, reduced MAP and UARI, and improved fetal growth.
SOURCES OF FUNDING
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award NumberP20GM104357and the National Heart, Lung and Blood Institute under Award NumberP01HL51971. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of HealthThis work was funded by National Institute of Health Grants: F31HL149257 awarded to OKT, R01DK109133 awarded to JMW, and R00HL130456 and R01HL151407 awarded to DCC.
Footnotes
DECLARATIONS OF INTEREST
No conflicts of interest, financial or otherwise, are declared by the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this paper as all supporting data related to the study are included in the manuscript.
REFERENCES
- [1].Jeyabalan A, Epidemiology of preeclampsia: impact of obesity, Nutr Rev 71Suppl 1 (2013) S18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Roberts JM, Preeclampsia: what we know and what we do not know, Semin Perinatol 24(1) (2000) 24–8. [DOI] [PubMed] [Google Scholar]
- [3].Hladunewich M, Karumanchi SA, Lafayette R, Pathophysiology of the clinical manifestations of preeclampsia, Clin J Am Soc Nephrol 2(3) (2007) 543–9. [DOI] [PubMed] [Google Scholar]
- [4].Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM, Pre-eclampsia: pathophysiology, diagnosis, and management, Vasc Health Risk Manag 7 (2011) 467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Young BC, Levine RJ, Karumanchi SA, Pathogenesis of preeclampsia, Annu Rev Pathol 5 (2010) 173–92. [DOI] [PubMed] [Google Scholar]
- [6].Ives Christopher W, Sinkey R, Rajapreyar I, Tita Alan TN, Oparil S, Preeclampsia— Pathophysiology and Clinical Presentations, J Am Coll Cardiol 76(14) (2020) 1690–1702. [DOI] [PubMed] [Google Scholar]
- [7].Fukui A, Yokota M, Funamizu A, Nakamua R, Fukuhara R, Yamada K, Kimura H, Fukuyama A, Kamoi M, Tanaka K, Mizunuma H, Changes of NK cells in preeclampsia, Am J Reprod Immunol 67(4) (2012) 278–86. [DOI] [PubMed] [Google Scholar]
- [8].Fukui A, Funamizu A, Yokota M, Yamada K, Nakamua R, Fukuhara R, Kimura H, Mizunuma H, Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia, J Reprod Immunol 90(1) (2011) 105–10. [DOI] [PubMed] [Google Scholar]
- [9].Yougbare I, Tai WS, Zdravic D, Oswald BE, Lang S, Zhu G, Leong-Poi H, Qu D, Yu L, Dunk C, Zhang J, Sled JG, Lye SJ, Brkic J, Peng C, Hoglund P, Croy BA, Adamson SL, Wen XY, Stewart DJ, Freedman J, Ni H, Activated NK cells cause placental dysfunction and miscarriages in fetal alloimmune thrombocytopenia, Nat Commun 8(1) (2017) 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y, The role of the immune system in preeclampsia, Mol Aspects Med 28(2) (2007) 192–209. [DOI] [PubMed] [Google Scholar]
- [11].Trisnawati E, Nontji W, Nurasni S, Tumour necrosis factor-alpha (TNF-alpha) serum levels in preeclampsia pregnant women and pregnant women at risk with preeclampsia, Enferm Clin 30Suppl 2 (2020) 27–30. [Google Scholar]
- [12].Udenze I, Amadi C, Awolola N, Makwe CC, The role of cytokines as inflammatory mediators in preeclampsia, Pan Afr Med J 20 (2015) 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Azizieh FY, Raghupathy RG, Tumor necrosis factor-alpha and pregnancy complications: a prospective study, Med Princ Pract 24(2) (2015) 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, Llurba E, Gris JM, Tumor Necrosis Factor-Alpha and Pregnancy: Focus on Biologics. An Updated and Comprehensive Review, Clin Rev Allergy Immunol 53(1) (2017) 40–53. [DOI] [PubMed] [Google Scholar]
- [15].Cornelius DC, Preeclampsia: From Inflammation to Immunoregulation, Clin Med Insights Blood Disord 11 (2018) 1179545X17752325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Raghupathy R, Cytokines as key players in the pathophysiology of preeclampsia, Med Princ Pract 22Suppl 1 (2013) 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP, Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha, Hypertension 46(4) (2005) 1022–5. [DOI] [PubMed] [Google Scholar]
- [18].Sunderland NS, Thomson SE, Heffernan SJ, Lim S, Thompson J, Ogle R, McKenzie P, Kirwan PJ, Makris A, Hennessy A, Tumor necrosis factor alpha induces a model of preeclampsia in pregnant baboons (Papio hamadryas), Cytokine 56(2) (2011) 192–9. [DOI] [PubMed] [Google Scholar]
- [19].Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, Dellsperger KC, Zhang C, Role of TNF-alpha in vascular dysfunction, Clin Sci (Lond) 116(3) (2009) 219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Picchi A, Gao X, Belmadani S, Potter Barry J, Focardi M, Chilian William M, Zhang C, Tumor Necrosis Factor-a Induces Endothelial Dysfunction in the Prediabetic Metabolic Syndrome, Circ. Res. 99(1) (2006) 69–77. [DOI] [PubMed] [Google Scholar]
- [21].Walsh SK, English FA, Johns EJ, Kenny LC, Plasma-mediated vascular dysfunction in the reduced uterine perfusion pressure model of preeclampsia: a microvascular characterization, Hypertension 54(2) (2009) 345–51. [DOI] [PubMed] [Google Scholar]
- [22].Li J, LaMarca B, Reckelhoff JF, A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model, Am J Physiol Heart Circ Physiol 303(1) (2012) H1–H8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Travis OK, Baik C, Tardo GA, Amaral L, Jackson C, Greer M, Giachelli C, Ibrahim T, Herrock OT, Williams JM, Cornelius DC, Adoptive transfer of placental ischemia-stimulated natural killer cells causes a preeclampsia-like phenotype in pregnant rats, Am J Reprod Immunol (2020) e13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Elfarra J, Amaral LM, McCalmon M, Scott JD, Cunningham MW Jr., Gnam A, Ibrahim T, LaMarca B, Cornelius DC, Natural killer cells mediate pathophysiology in response to reduced uterine perfusion pressure, Clin Sci (Lond) 131(23) (2017) 2753–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Amaral LM, Faulkner JL, Elfarra J, Cornelius DC, Cunningham MW, Ibrahim T, Vaka VR, McKenzie J, LaMarca B, Continued Investigation Into 17-OHPC: Results From the Preclinical RUPP Rat Model of Preeclampsia, Hypertension 70(6) (2017) 1250–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cunningham MW, Jayaram A, Deer E, Amaral LM, Vaka VR, Ibrahim T, Cornelius DC, LaMarca B, Tumor necrosis factor alpha (TNF-alpha) blockade improves natural killer cell (NK) activation, hypertension, and mitochondrial oxidative stress in a preclinical rat model of preeclampsia, Hypertens Pregnancy 39(4) (2020) 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP, Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade, Hypertension 52(6) (2008) 1161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Travis OK, White D, Baik CH, Giachelli C, Thompson W, Stubbs C, Greer M, Lemon JP, Williams JM, Cornelius DC, Interleukin-17 Signaling Mediates Cytolytic Natural Killer Cell Activation in Response to Placental Ischemia, Am J Physiol Regul Integr Comp Physiol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shields CA, McCalmon M, Ibrahim T, White DL, Williams JM, LaMarca B, Cornelius DC, Placental ischemia-stimulated T-helper 17 cells induce preeclampsia-associated cytolytic natural killer cells during pregnancy, Am J Physiol Regul Integr Comp Physiol 315(2) (2018) R336–R343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cornelius DC, Amaral LM, Wallace K, Campbell N, Thomas AJ, Scott J, Herse F, Wallukat G, Dechend R, LaMarca B, Reduced uterine perfusion pressure T-helper 17 cells cause pathophysiology associated with preeclampsia during pregnancy, Am J Physiol Regul Integr Comp Physiol 311(6) (2016) R1192–R1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Amaral LM, Cornelius DC, Harmon A, Moseley J, Martin JN Jr., LaMarca B, 17-hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model, Hypertension 65(1) (2015) 225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang R, Jaw JJ, Stutzman NC, Zou Z, Sun PD, Natural killer cell-produced IFN-gamma and TNF-alpha induce target cell cytolysis through up-regulation of ICAM-1, J Leukoc Biol 91(2) (2012) 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jewett A, Gan XH, Lebow LT, Bonavida B, Differential secretion of TNF-alpha and IFN-gamma by human peripheral blood-derived NK subsets and association with functional maturation, J Clin Immunol 16(1) (1996) 46–54. [DOI] [PubMed] [Google Scholar]
- [34].Murphy SR, LaMarca BB, Parrish M, Cockrell K, Granger JP, Control of soluble fms-like tyrosine-1 (sFlt-1) production response to placental ischemia/hypoxia: role of tumor necrosis factor-alpha, Am J Physiol Regul Integr Comp Physiol 304(2) (2013) R130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Barati M, Shahbazian N, Ahmadi L, Masihi S, Diagnostic evaluation of uterine artery Doppler sonography for the prediction of adverse pregnancy outcomes, J Res Med Sci 19(6) (2014) 515–9. [PMC free article] [PubMed] [Google Scholar]
- [36].Cnossen JS, Morris RK, ter Riet G, Mol BW, van der Post JA, Coomarasamy A, Zwinderman AH, Robson SC, Bindels PJ, Kleijnen J, Khan KS, Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis, CMAJ 178(6) (2008) 701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Alfirevic Z, Stampalija T, Dowswell T, Fetal and umbilical Doppler ultrasound in high-risk pregnancies, Cochrane Database Syst Rev 6 (2017) CD007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kroetsch JT, Levy AS, Zhang H, Aschar-Sobbi R, Lidington D, Offermanns S, Nedospasov SA, Backx PH, Heximer SP, Bolz SS, Constitutive smooth muscle tumour necrosis factor regulates microvascular myogenic responsiveness and systemic blood pressure, Nat Commun 8 (2017) 14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hermann M, Flammer A, Luscher TF, Nitric oxide in hypertension, J Clin Hypertens (Greenwich) 8(12 Suppl 4) (2006) 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen K, Pittman RN, Popel AS, Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective, Antioxid Redox Signal 10(7) (2008) 1185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Seligman SP, Buyon JP, Clancy RM, Young BK, Abramson SB, The role of nitric oxide in the pathogenesis of preeclampsia, Am J Obstet Gynecol 171(4) (1994) 944–8. [DOI] [PubMed] [Google Scholar]
- [42].Choi JW, Im MW, Pai SH, Nitric oxide production increases during normal pregnancy and decreases in preeclampsia, Ann Clin Lab Sci 32(3) (2002) 257–63. [PubMed] [Google Scholar]
- [43].Tuteja N, Chandra M, Tuteja R, Misra MK, Nitric Oxide as a Unique Bioactive Signaling Messenger in Physiology and Pathophysiology, J Biomed Biotechnol 2004(4) (2004) 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Osol G, Ko NL, Mandala M, Altered Endothelial Nitric Oxide Signaling as a Paradigm for Maternal Vascular Maladaptation in Preeclampsia, Curr Hypertens Rep 19(10) (2017) 82. [DOI] [PubMed] [Google Scholar]
- [45].Sutton EF, Gemmel M, Powers RW, Nitric oxide signaling in pregnancy and preeclampsia, Nitric Oxide 95 (2020) 55–62. [DOI] [PubMed] [Google Scholar]
- [46].Kashiwagi M, Zimmermann R, Beinder E, Pathophysiology of pre-eclampsia: Update on the role of nitric oxide, Curr. Hypertens. Rep. 5(6) (2003) 493–497. [DOI] [PubMed] [Google Scholar]
- [47].Yallampalli C, Garfield RE, Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia, Am J Obstet Gynecol 169(5) (1993) 1316–20. [DOI] [PubMed] [Google Scholar]
- [48].Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP, Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression, Am J Hypertens 15(2 Pt 1) (2002) 170–5. [DOI] [PubMed] [Google Scholar]
- [49].Casper FW, Seufert RJ, Atrial natriuretic peptide (ANP) in preeclampsia-like syndrome in a rat model, Exp Clin Endocrinol Diabetes 103(5) (1995) 292–6. [DOI] [PubMed] [Google Scholar]
- [50].Theilig F, Wu Q, ANP-induced signaling cascade and its implications in renal pathophysiology, American journal of physiology. Renal physiology 308(10) (2015) F1047–F1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Malee MP, Malee KM, Azuma SD, Taylor RN, Roberts JM, Increases in plasma atrial natriuretic peptide concentration antedate clinical evidence of preeclampsia, The Journal of Clinical Endocrinology & Metabolism 74(5) (1992) 1095–1100. [DOI] [PubMed] [Google Scholar]
- [52].Adam B, Malatyalio gbreve, lu E, Alvur M, Kökçü A, Bedir A, Plasma Atrial Natriuretic Peptide Levels in Preeclampsia and Eclampsia, J Matern Fetal Investig 8(2) (1998) 85–8. [PubMed] [Google Scholar]
- [53].Pacher P, Beckman JS, Liaudet L, Nitric oxide and peroxynitrite in health and disease, Physiol Rev 87(1) (2007) 315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Schulz E, Gori T, Munzel T, Oxidative stress and endothelial dysfunction in hypertension, Hypertens Res 34(6) (2011) 665–73. [DOI] [PubMed] [Google Scholar]
- [55].Abdel-Razik M, El-Berry S, Mostafa A, The Effects of Nitric Oxide Donors on Uterine Artery and Sub-endometrial Blood Flow in Patients with Unexplained Recurrent Abortion, J Reprod Infertil 15(3) (2014) 142–6. [PMC free article] [PubMed] [Google Scholar]
- [56].Kulandavelu S, Whiteley KJ, Qu D, Mu J, Bainbridge SA, Adamson SL, Endothelial nitric oxide synthase deficiency reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant mice, Hypertension 60(1) (2012) 231–8. [DOI] [PubMed] [Google Scholar]
- [57].Roberts JM, Rajakumar A, Preeclampsia and soluble fms-like tyrosine kinase 1, J Clin Endocrinol Metab 94(7) (2009) 2252–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondai S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA, Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia, J Clin Invest 111(5) (2003) 649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Eichmann A, Simons M, VEGF signaling inside vascular endothelial cells and beyond, Curr Opin Cell Biol 24(2) (2012) 188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kendall RL, Thomas KA, Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor, Proc Natl Acad Sci U S A 90(22) (1993) 10705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y, Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia, J Clin Endocrinol Metab 88(5) (2003) 2348–51. [DOI] [PubMed] [Google Scholar]
- [62].Zhu M, Ren Z, Possomato-Vieira JS, Khalil RA, Restoring placental growth factor-soluble fms-like tyrosine kinase-1 balance reverses vascular hyper-reactivity and hypertension in pregnancy, Am J Physiol Regul Integr Comp Physiol 311(3) (2016) R505–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gilbert JS, Babcock SA, Granger JP, Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression, Hypertension 50(6) (2007) 1142–7. [DOI] [PubMed] [Google Scholar]
- [64].Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP, Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats, Am J Hypertens 22(5) (2009) 564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Murphy SR, LaMarca BB, Cockrell K, Granger JP, Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats, Hypertension 55(2) (2010) 394–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tanabe K, Matsushima-Nishiwaki R, Yamaguchi S, Iida H, Dohi S, Kozawa O, Mechanisms of tumor necrosis factor-a-induced interleukin-6 synthesis in glioma cells, J Neuroinflammation 7(1) (2010) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Webb SJ, McPherson JR, Pahan K, Koka S, Regulation of TNF-alpha-induced IL-6 production in MG-63 human osteoblast-like cells, J Dent Res 81(1) (2002) 17–22. [DOI] [PubMed] [Google Scholar]
- [68].Conrad KP, Miles TM, Benyo DF, Circulating levels of immunoreactive cytokines in women with preeclampsia, Am J Reprod Immunol 40(2) (1998) 102–11. [DOI] [PubMed] [Google Scholar]
- [69].Xiao JP, Yin YX, Gao YF, Lau S, Shen F, Zhao M, Chen Q, The increased maternal serum levels of IL-6 are associated with the severity and onset of preeclampsia, Cytokine 60(3) (2012) 856–60. [DOI] [PubMed] [Google Scholar]
- [70].Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP, Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6, Hypertension 48(4) (2006) 711–6. [DOI] [PubMed] [Google Scholar]
- [71].Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP, IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy, Arterioscler Thromb Vasc Biol 27(12) (2007) 2576–81. [DOI] [PubMed] [Google Scholar]
- [72].Hung MJ, Cherng WJ, Hung MY, Wu HT, Pang JH, Interleukin-6 inhibits endothelial nitric oxide synthase activation and increases endothelial nitric oxide synthase binding to stabilized caveolin-1 in human vascular endothelial cells, J Hypertens 28(5) (2010) 940–51. [DOI] [PubMed] [Google Scholar]
- [73].Lamarca B, Speed J, Ray LF, Cockrell K, Wallukat G, Dechend R, Granger J, Hypertension in response to IL-6 during pregnancy: role of AT1-receptor activation, Int J Interferon Cytokine Mediat Res 2011(3) (2011) 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this paper as all supporting data related to the study are included in the manuscript.