Abstract
Introduction:
Human induced pluripotent stem cell (iPSC)-derived cardiomyocytes is one of the most widely used cell-based models that resulted from the discovery of how non-embryonic stem cells can be differentiated into multiple cell types. In just one decade, iPSC-derived cardiomyocytes went from a research lab to widespread use in biomedical research and preclinical safety evaluation for drugs and other chemicals.
Areas covered:
This manuscript reviews data on toxicology applications of human iPSC-derived cardiomyocytes. We detail the outcome of a systematic literature search on their use (i) in hazard assessment for cardiotoxicity liabilities, (ii) for risk characterization, (iii) as models for population variability, and (iv) in studies of personalized medicine and disease.
Expert opinion:
iPSC-derived cardiomyocytes are useful to increase the accuracy, precision, and efficiency of cardiotoxicity hazard identification for both drugs and non-pharmaceuticals, with recent efforts beginning to demonstrate their utility for risk characterization. Notable limitations include the needs to improve the maturation of cells in culture, to better understand their potential use identifying structural cardiotoxicity, and for additional case studies involving population-wide and disease-specific risk characterization. Ultimately, the greatest future benefits are likely for non-pharmaceutical chemicals, filling a critical gap where no routine testing for cardiotoxicity is currently performed.
1. Cardiovascular disease and cardiotoxicity
Cardiovascular diseases, such as coronary heart disease, stroke, hypertension, and congestive heart failure, are the most common non-communicable illnesses that contribute to the global burden of disease [1]. Worldwide, almost 18 million deaths were due to cardiovascular causes in 2017; more than three quarters of these deaths were in low-income and middle-income countries [2]. In the United States, it is estimated that 48% (or ~121.5 million) of adults are affected by cardiovascular illnesses [3]. A number of risk factors for cardiovascular disease in the general population have been identified through large-scale epidemiological studies. These range from so-called “unhealthy lifestyles” [4], including obesity, smoking, lack of exercise, and high sodium intake, to genetic factors [5] and exposures to environmental pollutants [5–7].
The term “cardiotoxicity” generally encompasses three major categories of adverse effects – structural (e.g., cell death, hypertrophy, or replacement of the functional tissue with connective tissue), functional (e.g., effects on electrophysiology and/or contractility), and vascular (e.g., effects on the blood flow to/from the heart) [8]. The adverse effects of drugs on the cardiovascular system are well documented [9, 10]. A large number of drug classes, from chemotherapeutics (anthracyclines, tyrosine kinase inhibitors, and alkylating agents) to antihistamines and antidepressants, are known to affect ion channels and, therefore, can induce any number of these cardiotoxic effects. The most frequently reported adverse effect is the prolongation of the QT interval that ultimately predisposes patients to lethal ventricular arrhythmias such as Torsades de pointes (TdP) [11].
Pharmaceutical- and non-pharmaceutical chemical-associated cardiotoxicity is of concern to both industry and the regulators [12] because it represents a potentially preventable cause of the overall morbidity and mortality from cardiovascular disease. Rigorous testing for potential cardiotoxicity of drugs occurs in preclinical studies [13, 14], during clinical trials [15], and through post-marketing surveillance [16]. By contrast, testing for potential cardiovascular effects is not part of standard regulatory requirements for approval of non-pharmaceutical chemicals (e.g., pesticides, flame retardants, industrial chemicals, etc.), and almost all of the information available on the potential causative involvement of environmental factors in cardiovascular disease comes from human epidemiological studies [17]. For example, pesticides such as organophosphates and carbamates have been associated with increased morbidity and mortality from myocardial infarction, findings that were confirmed in follow-up studies of cardiovascular adverse effects in animal models [18, 19]. Chemical-associated cardiotoxicity can also exacerbate the risk of development, progression, and mortality of cardiovascular diseases, especially in patients with pre-existing risk factors [20]. Due to the increasing prevalence of cardiovascular diseases and the increasing complexity of human exposures, it is ever more important to consider and adequately test for the potential cardiovascular adverse effects of pharmaceuticals and environmental compounds. However, for both drugs and other chemicals, currently used animal models do not provide sufficient information to meet both the throughput and human relevance needed to lower the preventable risks of cardiovascular disease [21, 22].
2. Models for cardiotoxicity testing
Cardiovascular toxicity, in the form of electrophysiological effects, hemodynamic changes, and/or histological findings, accounts for ~27% of drug attrition during the investigational new drug-enabling phase of preclinical development and ~18% of drug termination during the late discovery phase [23]. Evaluation of the potential cardiotoxicity liabilities in drug development is directed by the comprehensive guidelines from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) [24, 25]. A combination of in silico and in vitro (ion channel binding and cell-based assays) approaches, preclinical animal models, human clinical trials, and post-marketing surveillance is used to de-risk potential cardiovascular liabilities of pharmaceutical candidates. The approaches that are frequently used in cardiotoxicity testing of pharmaceuticals are summarized in Table 1.
Table 1.
Nonclinical and clinical models used to evaluate cardiotoxicity potential of drugs and chemicals.
| Strengths | Limitations | |
|---|---|---|
| In silico predictions [26, 27] |
|
|
| Ion channel assays [30] |
|
|
| Microelectrode array (MEA) field potential measurements [148] |
|
|
| Measurement of intracellular Ca2+ dynamics [51, 52] |
|
|
| Tissue slices [34] |
|
|
| Tissue chips [36] |
|
|
| Zebrafish [149, 150] |
|
|
| Rodents [38] |
|
|
| Dog [40] |
|
|
| Swine [151, 152] |
|
|
| Non-human primates [43] |
|
|
| Thorough QT/QTc (TQT) study [45] |
|
|
In the past 20 years, many publications have proposed and characterized a variety of quantitative structure-activity relationship (QSAR) models for predicting cardiotoxicity [26, 27]. The vast majority of these in silico models were developed for predictions of hERG (potassium channel Kv11.1, the human ether-a-go-go-related gene) activity. Drug-associated blockade of hERG has been associated with prolongation of the QT interval, type 2 Long QT Syndrome (LQTS), and increased risk of life-threatening cardiac arrhythmias [28, 29]. Because of the recognized importance of hERG binding, datasets containing up to tens of thousands of compounds have been developed for use in both the public domain and internally at large pharmaceutical companies to facilitate QSAR model development.
Cell-based assays are widely used for cardiotoxicity screening [30]; they are featured extensively in the Comprehensive In Vitro Proarrhythmia Assay (CiPA) initiative [22]. These assays typically include both non-heart-derived cells engineered to express one or more ion channels (e.g., hERG), as well as functional cardiomyocytes, either primary or those differentiated from induced pluripotent stem cells (iPSC) or embryonic stem cells (ESC). The former models have been used for almost 40 years in patch clamp-based manual [31] or automated [32] assays that provide quantitative data on the ability of test compounds to modulate ion channel behavior. Assays based on beating cardiomyocytes in tissue culture allow for comprehensive evaluation of cell viability, electrophysiology, and contractility. These methods are widely used and are considered to be high-throughput, because experiments can be easily performed in multi-well plates, cell culture is fairly straightforward, and imaging equipment for kinetic assays is commercially available [33].
Tissue slices have been widely used as in vitro models for multiple organs to maintain cellular infrastructure, and methods for culturing human and porcine heart slices have been developed [34]. This methodology is a form of 3D multicellular system that more closely reflects the myocardium in physiological or pathological conditions and allows for studies of both functional (e.g. electrophysiology, contractile force) and structural effects. While promising [35], this assay system has not been widely adopted because of the limited availability of human heart tissues and technical difficulties associated with establishing and maintaining organ function without perfusion. A large variety of additional microphysiological 3D models of the heart have been developed in the past decade [36] and are rapidly progressing from being proof of principle devices to multiplexed systems that can be used for screening in low- to medium-throughput formats [37].
In vivo animal studies of the cardiovascular risk of drugs and chemicals traditionally include a single-dose functional assessment conducted in instrumented animals and a histopathology evaluation in repeat-dose toxicity studies [38]. Studies in rodents (typically rat and/or mouse) are often utilized and the primary phenotyping is conducted through pathological evaluation [13, 38]. Even though there are numerous physiological and electrophysiological differences in the cardiovascular systems of rodents and humans [39, 40], the availability of genetically-defined populations and genetic engineering tools, and the lower cost compared to other mammalian models, make studies in rodents an invaluable part of the overall testing portfolio.
Dogs are the most commonly used preclinical animal model for evaluating the potential of pharmaceutical candidates to cause disruptions of heart electrophysiology [41]. The similarities between dogs and humans in terms of body size, heart weight, structural characteristics of the myocardium, and functional contractile characteristics make canine models more ideal and biologically relevant than rodent models [39, 40]. However, disadvantages of canine models include higher expense than rodent models, limited sample size during preclinical studies, ethical concerns, and difficulty obtaining approvals for animal use early in pharmaceutical development.
Non-human primates are the most human-relevant model for studies of cardiovascular disease [42]. Their similarities with humans in terms of genetic, metabolic, and physiologic factors bring unique strengths for non-human primates as models to better understand human cardiovascular disease and exogenous risks. Various primate species have been used extensively in preclinical drug development to assess the pharmacology and cardiovascular safety of new chemical entities and biopharmaceuticals, especially for their risk to prolong the corrected QT interval (QTc) [43]. However, both government agencies and industry are actively working to replace studies in non-human primates with alternative models due to ethical concerns [44].
There are also extensive cardiotoxicity testing strategies implemented for pharmaceuticals during human clinical trials. The ICH guideline E14 recommends thorough QT/QTc (TQT) studies in healthy volunteers to determine the threshold of pharmacologic effect on cardiac repolarization [45]. The data from a TQT study is generally used as the most useful outcome with respect to the potential liability of a drug to have off-target proarrhythmic effects in humans due to delayed cardiac repolarization. Even though TQT is a primary study for many drugs before they are approved for subsequent phases of clinical trials, it also presents a number of challenges, including a narrow focus on the QTc interval, ethical issues with performing these assessments in healthy volunteers, and the resource-intensive nature of these studies. The lack of integration between ICH guideline-required nonclinical and clinical risk assessments to streamline the determination of QT prolongation liability has also been identified as a major challenge with human studies of cardiotoxic risks [46].
Overall, due to the limitations of current models and the large number of drugs and chemicals for which safety needs to be assessed, there is an increasing interest in alternative methods for cardiotoxicity hazard assessment. Expanding the use of human-relevant models such as human iPSC-derived cardiomyocytes is one sensible path to address the challenges of assessing cardiovascular safety liabilities and to enable evaluation of a large number of chemicals [23].
3. iPSC-derived cardiomyocytes: history and applications in toxicology
The discovery that mature cells can be reprogrammed to become pluripotent, recognized by the Nobel Prize in Physiology or Medicine (2012), and the subsequent rapid developments in iPSC technology brought monumental advancements to cell biology, medical science, disease modeling, drug discovery, and personalized medicine [47, 48]. The demonstration that somatic cells (fibroblasts or peripheral blood mononuclear cells) can be reprogrammed to express transcription factors for pluripotency, resulting in iPSCs, enabled the generation of an unlimited cell source which retains the genetic information of the individual donor from which the somatic cells were derived. Interestingly, one of the first functional cell types into which iPSCs and embryonic stem cells (ESC) could be successfully reprogrammed was cardiomyocytes [48, 49].
iPSC-derived cardiomyocytes in culture mimic both the ultrastructural and functional features of human native cardiac tissue, with gene expression patterns most closely resembling fetal left ventricular cardiac tissue and action potentials reflecting a heterogeneous population of mixed atrial, nodal, and ventricular cells [50]. Because some differentiation is required even when using commercial preparations [51, 52], they transition in culture from fetal phenotype (small size, lack of organization of structural proteins and some functional machinery, and reliance on glycolysis for energy production) [53–55] to cells that are more mature in their morphology, gene expression patterns, and functionality [53–56]. Still, iPSC-derived cardiomyocytes exhibit an immature phenotype, and many recent studies have aimed to enhance their maturation through biochemical [57] or mechanical [58] stimulation, co-culture with other cell types [59], prolonged culture time [60], or culture in a 3D geometry [61].
In culture, iPSC-derived cardiomyocytes self-organize into monolayers and express hallmark cardiac proteins including sarcomeric myosin light and heavy chains (MLC-2v, MYH-6, MYH-7, MYL-7, MYL-2), troponin (cTnT, TNNT2), connexin 43 (Cx43), N-cadherin, and α-actinin [55, 56, 62, 63]. These cells also express proteins responsible for the function of the major ion channels, including transient outward and inward rectifier potassium channels (KCNH2, KCNQ1), sodium channels (SCN5A), and L-type calcium channels (CACNA1C, CACNA1D) [56, 62–65], and IP3R2, RyR2, and SERCA2a that are responsible for transients of Ca2+ [65]. These cells begin to spontaneously beat regularly as a synchronous monolayer within one to two weeks of cell culture and can also be paced to contract with different rhythm [51–55, 66]. Their contractile characteristics mimic the function of human cardiomyocytes and exhibit the standard features of the action potential waveform, including rapid depolarization, a plateau phase, and rapid repolarization [23]. Further, the duration of the action potential in these cells generally falls within the normal range expected for the human QT interval measured from the electrocardiogram [63].
Not only was the discovery of iPSC-derived cardiomyocytes important for cell biology, but the potential for these cells to address a large gap in drug and chemical safety testing was almost immediately recognized (Figure 1), with the earliest examples of the use of human iPSC-derived cardiomyocytes in toxicology appearing in 2011, specifically for drug safety evaluation [63, 67, 68]. In parallel, a number of investigators demonstrated that iPSC-derived cardiomyocytes retain in vivo-relevant phenotypes linked to familial syndromes that result in cardiovascular disease [69–72]. Very quickly, iPSC-derived cardiomyocytes were recognized as a useful model by the pharmaceutical industry [73–75], and robust medium- to high-throughput methods for ion channel activity measurements, through both MEA [64, 76] and Ca2+ flux [51, 52], were developed. Studies that focused on the systematic evaluation of various classes of environmental chemicals in iPSC-derived cardiomyocytes did not appear until 2017 [66], albeit some earlier publications included non-pharmaceutical compounds among large libraries of drugs [51, 77].
Figure 1.
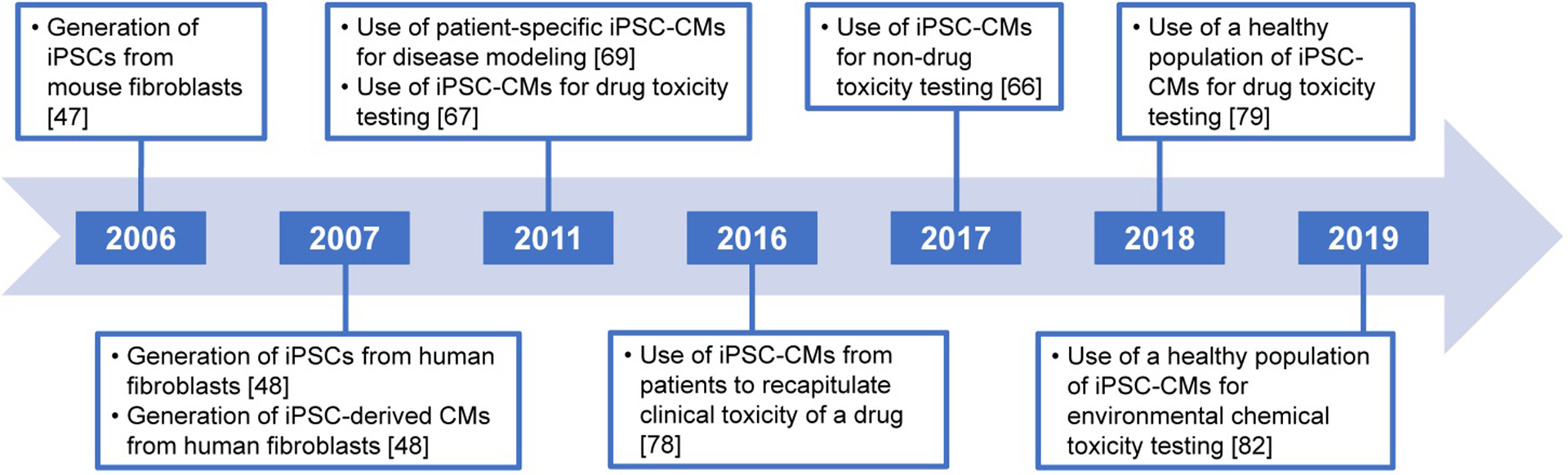
Timeline showing the progression of iPSC technology and the use of iPSC-derived cardiomyocytes for toxicity testing.
The application of iPSC-derived cardiomyocytes to personalized medicine was first demonstrated for doxorubicin-associated cardiotoxicity [78]. Studies using cells from numerous individual donors without known cardiovascular diseases have demonstrated robustness and reproducibility in in vitro phenotypes [79]. Subsequent work demonstrated the potential to use this population-based model to evaluate hazard [80–82], mechanisms [79], and risk [81, 83] associated with exposure to both drugs and environmental chemicals. While the most common platform to test iPSC-derived cardiomyocytes is a monolayer in the multi-well plate, methods for phenotyping beating spheroids from these cells are also available [84].
Although a number of reviews detailing the use of cell-based models in drug safety testing have been published [23, 85–87], few conducted a systematic evaluation of the applications of iPSC-derived cardiomyocytes in hazard identification and risk characterization of both drugs and non-pharmaceutical compounds. Here, we performed a systematic literature search to collect, evaluate, and catalog published studies on this topic. We followed best practices of systematic evidence evaluation in toxicology [88] and created an online database of studies where the abstracts, search terms, inclusion/exclusion criteria, and the interactive literature tree (Figure 2) are publicly accessible (https://hawcproject.org/assessment/1061/). A total of 258 publications were identified. Of these, 192 references met the pre-defined inclusion criteria and were synthesized for use in this review; 66 references were excluded, most frequently for being a review article and not a primary publication. Included studies were further divided into pre-defined categories, namely (i) assessing hazard, (ii) population variability, (iii) disease models, (iv) 3D tissues and spheroids, (v) enhancing maturation in culture, and (vi) no chemical treatment. The hazard category was further subdivided to describe the types of hazards that can be assessed using iPSC-derived cardiomyocytes, namely (i) electrophysiology, (ii) contractility, (iii) structural toxicity, (iv) metabolic function, and (v) mechanisms of cardiotoxicity.
Figure 2.

Literature tree visualization of the 256 studies returned during the search in HAWC and their assignment to binned categories. The numbers in each circle indicate how many studies apply to that category. Circles colored in blue indicate subcategories, while circles colored in white indicate a terminal category. Included categories subdivide into bins describing the purpose of the study (i.e. hazard assessment, population variability, 3D models, enhancing cell maturity in culture, etc.). Further, the hazard assessment category subdivides into bins describing the types of hazards assessed (i.e. electrophysiology, contractility, structural toxicity, metabolic function, mechanisms, etc.) and the methods available to assess these hazards (e.g. impedance/MEA, patch clamp, ion channels, etc.). The terminal bins in each category are subdivided by drugs and non-drug chemicals, as shown for the “Structural Toxicity” bin.
4. Hazard assessment with iPSC-derived cardiomyocytes
4.1. Overview of hazard assessment for drugs and chemicals
iPSC-derived cardiomyocytes are an attractive model for cardiotoxicity testing and serve as an informative tool for characterizing the electrophysiological, contractile, structural, and metabolic effects of drugs and chemicals (Table 2). Often, a multiparametric approach (i.e. combining readouts from a complementary panel of these studies) is taken to more comprehensively understand and describe the bioactivity profiles of compounds. The majority of studies identified in this review used iPSC-derived cardiomyocytes to assess the hazards presented by drugs, most often in concentration-response format, while a comparatively smaller number addressed the cardiotoxic potential of non-drug compounds (e.g. environmental chemicals such as pesticides, flame retardants, and PAHs, food/flavor agents, cosmetic ingredients, etc.).
Table 2.
Types of hazards assessed using iPSC-derived cardiomyocytes.
| Hazard Type | Examples with Drugs | Examples with Non-Drugs |
|---|---|---|
| Electrophysiology | ||
| Impedance/MEA | [67, 89–91, 101, 103, 114, 123] | [122, 153, 154] |
| Patch Clamp | [63, 90, 92, 93, 101, 104, 106] | [153] |
| Ca2+ Channels | [78, 82, 84, 89, 92, 95, 96, 102, 104] | [66, 80, 82, 84] |
| Na+/K+ Channels | [90, 91, 96–98, 104] | None |
| Optogenetics | [99–101, 155] | None |
| Contractility | ||
| Beat Rate | [78, 82, 84, 89, 92, 95–98, 102, 104] | [66, 80, 82, 84] |
| Arrhythmia | [78, 81, 82, 84, 89, 92, 95, 97, 98, 102, 103] | [66, 82, 84] |
| Contractile Force | [104–106] | None |
| Structural Toxicity | [78, 82, 84, 92, 102, 105, 109–113, 117] | [66, 80, 82, 84, 122] |
| Metabolic Function | [74, 78, 106, 110, 111, 113, 114] | |
| Mechanisms | ||
| Gene Expression | [78, 90, 92, 105, 110–114, 117] | [122] |
| Protein Expression | [92, 105, 110–114, 116, 117] | [153] |
| miRNA Expression | [117] | None |
4.2. Electrophysiology
Most studies identified in this review tested for the potential electrophysiological hazards presented by drugs, including effects on ion channels and the waveform of the action potential. Common methods for assessing electrophysiological liabilities in iPSC-derived cardiomyocytes include microelectrode array (MEA) and impedance measurement, patch clamp, and calcium- or voltage-sensitive dyes.
4.2.1. Impedance/MEA
MEA uses an array of microelectrodes etched onto a cell culture surface to detect the extracellular field potential across a monolayer of iPSC-derived cardiomyocytes. The action potential waveforms generated by the beating cell layer are recorded and quantified by the electrodes. Parameters such as the field potential duration or action potential duration (in vitro surrogates for the in vivo QT interval), wave amplitude, beat frequency, and presence of early afterdepolarizations or arrhythmic beating provide insight into the electrophysiological and arrhythmogenic effects of compounds. Many studies have shown the reliability and utility of MEA for testing the effects of drugs on the cardiac action potential of iPSC-derived cardiomyocytes, such as antiarrhythmics known to induce clinical prolongation of the QT interval [89–91]. For example, a study utilizing MEA was conducted to examine the dose-dependent effects of dofetilide (a class III antiarrhythmic drug and selective blocker of the hERG channel) and quinidine (a class IA antiarrhythmic drug and blocker of the INa current), two compounds associated with arrhythmic beating. These drugs were found to prolong the FPD at concentrations equivalent to or exceeding human maximal blood concentrations [90]. Further, not only the type of effect, but also the concentration at which the effect occurs in the MEA is often consistent with clinical observations [90], demonstrating the predictive capability of this system.
4.2.2. Patch clamp
Patch clamp works by sealing a glass pipette filled with electrolyte solution onto the cell membrane, allowing currents passing through the membrane to be recorded by an electrode. The changes in membrane potential elicited by chemical exposure are detected and quantified, informing the function of specific ion channels that contribute to the action potential waveform. Studies using the patch clamp technique have demonstrated that iPSC-derived cardiomyocytes exhibit action potential durations within the range of the normal QT interval in humans and show the expected sensitivities to various drugs [63]. Multiple studies have implemented patch clamp to test for the effects of drugs on specific ion channels [63, 92, 93]. For example, tyrosine kinase inhibitors such as vandetanib elicit prolongation of the action potential duration, corresponding to decreases in the beat rate at clinically-relevant concentrations [92]. Further, the use of patch clamp elucidated the mechanism of vandetanib toxicity, which is blockade of both the IKr and INa currents. These results may also inform the mechanism by which other tyrosine kinase inhibitors induce cardiotoxic effects. Further, these studies on vandetanib using patch clamp correlate with clinical observations, which show an increased incidence and risk of QT interval prolongation in patients taking the drug [94]. Thus, the patch clamp technique can provide insight into the electrophysiological hazards and specific ion channels affected by exposure to drugs and chemicals.
4.2.3. Ca2+ channels
Calcium (Ca2+) is a critical molecule for propagation of electrical signal in heart tissue, so the influx of Ca2+ into the intracellular space can be used as a surrogate for electrophysiological determination of action potential waveforms in iPSC-derived cardiomyocytes. Numerous studies have employed fluorescent Ca2+-sensitive dyes (e.g. Fluo-4, FLIPR Calcium 6, Fura-2) to test for the potential effects of compounds on intracellular calcium homeostasis and beating patterns. Various drugs and chemicals have been found to elicit effects on the function of iPSC-derived cardiomyocytes that could be measured through intracellular Ca2+ signaling [82, 95, 96]. Cardioactive drugs known to induce effects on beat rate such as isoproterenol (a β-adrenergic receptor agonist and positive chronotrope) and diltiazem (a Ca2+ channel antagonist and negative chronotrope) exhibit the expected effects, while non-cardioactive drugs (acetaminophen and aspirin) and chemicals (food constituents leucine and sorbitol) show no activity, confirming the specificity of this in vitro test model [82, 84]. Ca2+ flux assays have proven instrumental in demonstrating the potential of numerous environmental chemicals of different classes (e.g. pesticides, flame retardants, and industrial chemicals) to affect the beating parameters of iPSC-derived cardiomyocytes [66, 82]. Over half of the environmental compounds tested using this assay show activity for at least one phenotype at varying degrees of potency, the most common of which is positive chronotropy [66, 82, 83]. This increasing evidence of bioactivity in vitro warrants future studies focusing on the cardiovascular hazards presented by environmental chemicals. Further, these studies demonstrate the utility of iPSC-derived cardiomyocytes for rapid, high-throughput hazard identification of drugs and chemicals and suggest that this model may be able to sustain the large volume of environmental chemical testing needs.
4.2.4. Na+/K+ channels
The voltage-gated sodium (Na+) and potassium (K+) ion channels are critical for propagation of electrical signal in heart tissue. Voltage-sensitive dyes are different from intracellular Ca2+-sensitive dyes in that they bind the membrane and fluoresce when changes in membrane potential occur. Changes in fluorescence are recorded through high-resolution microscopy and optical mapping techniques. These recordings of the membrane potential from voltage-sensitive dyes are highly correlated to the waveform patterns, duration, and beat rate as measured by Ca2+-sensitive dyes, both at baseline and in drug-induced effects [96]. Many studies have used voltage-sensitive dyes (e.g. FluoVolt, di-4-ANEPPS) to test for the potential effects of drugs on cardiomyocyte electrophysiology. For example, a panel of drugs of known proarrhythmic risk included in the CiPA initiative has been tested for effects using this method [97]. Many CiPA drugs categorized as high risk for TdP arrhythmia elicit the expected prolongation of the APD in assays using voltage-sensitive dyes [97]. Further, the presence of EAD events and arrhythmic beating are detected following exposure to some high and intermediate risk CiPA compounds, whereas no TdP arrhythmias are observed for any low risk compounds in the panel [96–98]. These studies demonstrate the utility of voltage-sensitive dyes as tools to assess the electrophysiological and arrhythmogenic hazards presented by compounds.
4.2.5. Optogenetics
A rapidly emerging method for hazard assessment in iPSC-derived cardiomyocytes is optogenetics, in which specific ion channels are genetically modified to be light-sensitive. Optical mapping is often used to track and quantify the changes to fluorescence, which inform characteristics of cell contraction and electrophysiology [99]. The ability to tailor optogenetic encoding to specific ion channels provides the ability to assess the contribution of different ion channels to the action potential waveform. Optogenetic methods can be used in combination with other methods such as MEA and patch clamp to assess the electrophysiological effects of compounds [100, 101].
4.3. Contractility
Potential contractile hazards of drugs include effects on beat rate, rhythmicity, and force of contraction. Common methods for assessing contractile liabilities in iPSC-derived cardiomyocytes include measurement of Ca2+ flux and optical motion tracking of cardiomyocyte beating through high-resolution video microscopy. More sophisticated methods for assessing contractile characteristics are available for 3D/microfluidic culture models and often rely on measuring the physical displacement of wires, cantilevers, or silicon posts due to the force of cardiomyocyte beating.
4.3.1. Beating frequency (rate)
One primary characteristic that describes the contractile function of iPSC-derived cardiomyocytes is the beat rate (peak frequency). The beat rate can be recorded through high-resolution video microscopy or determined by measuring intracellular Ca2+ flux as a surrogate marker for contractility. Methods for assessing contractility are often different from electro-physiological methods in that they do not rely on direct recording of the membrane potential through electrical signaling, but instead on surrogate markers for the action potential or physical contractility to inform the rate of beating. Simultaneous recordings of Ca2+ flux and physical contraction as measured through cell edge detection (tracking the physical movement of cardiomyocytes during beating by high-resolution imaging) demonstrate similar beating patterns both at baseline and in drug-induced effects [102]. For example, verapamil (an antihypertensive drug and blocker of the ICaL current) inhibits both the Ca2+ transient and physical contraction of cardiomyocytes in a dose-dependent manner, though the edge detection method may be more sensitive to drug-induced effects than is the measurement of Ca2+ flux [102]. In addition to beat rate, Ca2+ flux measurements can also inform other characteristics of cardiomyocyte contraction waveforms, including peak amplitude, width, rise time, decay time, and spacing [51, 102]. This ability to measure regularity in the waveform pattern and changes to rise and decay times of the waves can inform the arrhythmogenicity of compounds, discussed further in the section below.
4.3.2. Beating rhythmicity (arrhythmia)
iPSC-derived cardiomyocytes are an attractive model for assessing the arrhythmogenic hazards of compounds through methods such as Ca2+ flux measurement and motion vector prediction based on video microscopy [81, 103]. The assessment of wave propagation patterns, beating regularity, peak spacing, and surrogate phenotypes for QT prolongation such as decay to rise time ratio can provide insight into the possible arrhythmic effects of compounds. For example, using a population of iPSC-derived cardiomyocytes enables the ability to accurately predict clinical prolongation of the QTc following exposure to various drugs, based on increases in the decay to rise time ratio as measured by Ca2+ flux in combination with Bayesian population concentration-response modeling [81]. Drugs known to induce clinical QT prolongation, such as disopyramide (a class IA antiarrhythmic drug and blocker of the INa current), result in dose-dependent increases to the decay to rise time ratio in iPSC-derived cardiomyocytes, at concentrations which recapitulate clinical dose-response relationships [81]. The model also accurately predicts drugs that do not induce clinical prolongation of the QT interval, as in vitro effects occur only at concentrations that are multiple orders of magnitude higher than maximal blood concentrations [81]. Importantly, this study showed that in vitro-derived point-of-departure values for the population median were able to predict clinical concentration-response relationships more accurately than the use of cells from a single donor, highlighting the utility of population-based models for hazard assessment. This study demonstrated the promise of accurately assessing the proarrhythmic liabilities of drugs in vitro, suggesting the potential for “Thorough QT/QTc (TQT) studies in a dish” that would greatly reduce the burden of traditional human TQT studies for assessing drug-induced effects on cardiac repolarization.
4.3.3. Contractile force
Advanced methods for assessing contractile function typically require 3D models and rely on measuring the physical displacement of wires, cantilevers, poles, or silicon posts due to the force of cardiomyocyte beating [104–106]. For example, custom 96-well plates with inserts holding a cell culture surface area between two flexible posts have been engineered from polydimethylsiloxane [105]. 3D organoids of iPSC-derived cardiomyocytes and supporting cell types are seeded into the device and fill the surface area around the posts. When the cardiomyocytes contract, the corresponding movement of the posts is tracked using high-content video microscopy and analyzed using tracking analysis software to measure changes in the contraction force over time. This approach yields force trace curves that look similar to Ca2+ flux traces and describe characteristics such as the contraction rate, relaxation time, and contraction force.
The pole deflection method has been used to screen a panel of more than 100 drug candidates for their potential contractile effects in iPSC-derived cardiomyocytes [105]. Exposure to some compounds decreased the force of contraction in a dose-dependent manner, indicating adverse effects on contractile machinery and cardiomyocyte function; these compounds were subsequently removed from the pool of drug candidates [105]. Studies utilizing deflection-based force measurements have also demonstrated that electrical conditioning (i.e. increasing the frequency of electrical stimulation in a stepwise manner) enhances the structural, metabolic, and functional maturity of cardiomyocyte tissues, resulting in increased contraction force, calcium signaling, and more physiological responses to drugs [58, 107, 108]. These studies highlight the advantage of using a 3D geometry for enhancing the maturity of iPSC-derived cardiomyocytes so as to more closely resemble the human adult myocardium. Further, these studies demonstrate the utility of 3D cardiomyocyte models in combination with force measurement systems to assess the contractile characteristics both at baseline and in drug-induced effects. This capability is unique, as other approaches discussed in this review cannot directly measure the physical contraction force of cardiomyocytes.
4.4. Structural toxicity
Structural toxicity is often assessed through biochemical assays measuring cell viability (e.g. ATP depletion, protease activity, mitochondrial membrane potential) or high-content microscopy approaches describing cell count, cell size, or organization of subcellular structures. A multiparametric approach is often used to more comprehensively inform the structural cardiotoxicity hazard of a compound. For example, the structural effects induced by chemotherapeutic drugs such as tyrosine kinase inhibitors (sorafenib, erlotinib) and anthracyclines (doxorubicin) have been investigated using a combination of these methods [78, 92, 109]. These chemotherapeutics elicit the expected dose-dependent structural and cytotoxic effects in iPSC-derived cardiomyocytes, based on readouts from both biochemical assays and high-content microscopy. These effects are often correlated with disruption of calcium handling, mitochondrial energetics, and critical signaling pathways and ultimately manifest as functional cardiotoxicity (i.e. a drastic reduction in beating parameters or quiescence) [78, 92, 109, 110]. By contrast, non-cardioactive drugs such as aspirin do not elicit any functional or structural effects in the tested dose range, confirming the specificity of this approach for in vitro hazard assessment [109]. iPSC-derived cardiomyocytes can also be used to investigate chemical-induced cardiac hypertrophy (i.e. increases in cell size and/or expression of hypertrophic signaling markers such as B-type natriuretic peptide [BNP]) [111–113].
4.5. Metabolic function
iPSC-derived cardiomyocytes can also be used to assess the metabolic hazards presented by chemicals. Metabolic effects are often characterized by measuring mitochondrial function, intracellular ATP content, glycolytic capacity, extent of oxidative phosphorylation, oxygen consumption rate, extracellular acidification rate, and/or lactate release into cell culture medium. Changes in metabolic function can be assessed independently or, often, are used in combination with structural toxicity assays as an early indicator of cytotoxicity. For example, effects on metabolic function were assessed following exposure to the chemotherapeutic drugs sorafenib and doxorubicin, in parallel with the structural toxicity assays discussed above [78, 110]. The cytotoxic effects of sorafenib are accompanied by dose-dependent decreases in the oxygen consumption rate and intracellular ATP content, downregulation of oxidative phosphorylation and upregulation of glycolysis as an adaptive mechanism, defects in mitochondrial energetics, and inhibition of cardiac metabolism [74, 110]. Similarly, the cytotoxic effects of doxorubicin correspond to impairments in mitochondrial function and endogenous cardiac metabolism, as well as an increased production of reactive oxygen species [78, 114]. In the case of both drugs, these metabolic effects are accompanied by changes to the beating rate, regularity, and amplitude following chemical exposure, demonstrating the utility of a multiparametric approach for characterizing and more thoroughly understanding the cardiotoxicity hazards of drugs.
4.6. Studies of mechanisms of chemical-induced cardiotoxicity
Another application of iPSC-derived cardiomyocytes is probing the molecular mechanisms by which chemicals may elicit cardiotoxic effects, often measured through changes in gene expression. For example, the mechanisms of toxicity for the chemotherapeutic drug class anthracyclines have been investigated using gene expression analysis in iPSC-derived cardiomyocytes [78, 114]. These studies demonstrate that exposure to doxorubicin induces the expression of cell stress-related genes (e.g., p53 signaling, apoptosis) and downregulates genes related to cardiac function and regulation of contraction (e.g. formation of sarcomere, myofibril organization) [114]. This information provides a mechanistic basis for the functional and structural effects observed following doxorubicin exposure (i.e. effects on ion channels and calcium handling, beat rate changes, presence of arrhythmic beating, and cytotoxicity) [78, 109, 114]. Interestingly, some doxorubicin-induced transcriptomic changes are reversible and return to basal levels following a washout period (e.g. sarcomere formation, nucleosome organization, apoptotic signaling), whereas other changes are irreversible (e.g. calcium ion binding, endoplasmic reticulum-nuclear signaling) [114]. Other anthracyclines such as daunorubicin and related drugs such as mitoxantrone (an anthracenedione) induce similar transcriptomic and functional changes, suggesting the ability to determine gene expression “signatures” for drugs with the same mode of action and to predict the cardiotoxicity liabilities of drugs within a class based on these mechanisms [114, 115]. Other readouts such as protein expression [114, 116], and less commonly, miRNA expression [117], have also been used to investigate mechanisms of anthracycline-induced toxicity (and the mechanisms of other drugs and a few chemicals). These studies demonstrate the utility of iPSC-derived cardiomyocytes for probing the molecular mechanisms by which chemicals induce cardiotoxic effects and for understanding key events in the cardiotoxicity adverse outcome pathway.
5. Risk characterization with iPSC-derived cardiomyocytes
Risk is defined as “the combination of the probability of occurrence of harm and the severity of that harm” [118], and risk characterization involves the integration of the exposure and the hazards associated with a particular agent [119]. However, there are varying approaches to risk characterization depending on decision context, each of which has different requirements in terms of accuracy and/or precision.
Most of the efforts with respect to utilizing in vitro studies in risk assessment involve applications to “prioritization” or “screening.” While often discussed together, and both involve down selecting the most “concerning” substances from a larger set, there are some important distinctions. In prioritization, one is typically seeking to characterize the relative degree of potential risk across a group of substances, whereas for screening, one is concerned about which substances have the potential to pose an unreasonable risk. In both cases, however, it is common to utilize the estimation of margins of safety (MOS) for pharmaceuticals or margins of exposure (MOE) for non-pharmaceuticals. When based on in vivo studies, the MOS or MOE is often defined as the ratio between a dose level associated with safety, such as a no-observed-adverse-effect-level (NOAEL) or other point-of-departure (POD), and the level of actual human exposure [120]. For in vitro studies, this ratio is defined in terms of an effective concentration (EC) and a human plasma concentration, such as a Cmax or AUC.
Analogously, several studies have used iPSC-derived cardiomyocytes to estimate MOS/MOEs [66, 80, 83, 95, 121–123], finding wide variation across different pharmaceutical compounds and between pharmaceutical and non-pharmaceutical compounds. For example, one study found MOS values for torsadogenic effects of pharmaceutical compounds to range from 0.0058 (ibutilide) to 3,727 (terfenadine), dividing compounds into low- and high-risk categories for torsadogenic effects [123]. Two studies that used iPSC-derived cardiomyocytes to assess multiple cardiophysiologic and cytotoxic effects evaluated MOS/MOEs in both drugs and environmental chemicals and found that MOEs for environmental chemicals were between one and five orders of magnitude, much higher than MOSs for most pharmaceutical compounds [66, 83]. These studies confirmed the relatively low MOSs for many pharmaceutical compounds, particularly those known to have cardiotoxic effects. Interestingly, for many environmental chemicals, while potentially presenting a hazard to cardiomyocytes in vitro, there appears to be little potential risk to the general population when put into the context of estimated exposures [66, 82, 83]; however, it should be acknowledged that environmental exposure estimates are highly uncertain due to the lack of widespread biomonitoring data on most compounds.
These studies suggest that iPSC-derived cardiomyocytes have the potential to be useful in quantifying risk in terms of ranking or screening for cardiotoxicity potential, through estimation of concentration-response relationships for cardiotoxic effects. However, to be useful in this regard, demonstration is needed that the concentration-response from iPSC-derived cardiomyocytes is sufficiently accurate so as to offer an advantage over other preclinical models for characterizing concentration-response. Direct comparisons of concentration-response relationships for QT prolongation from studies of iPSC-derived cardiomyocytes and those in vivo have been conducted [81, 124], finding that for known QTc-prolonging drugs, the central tendency and range of responses were consistent between iPSC-derived cardiomyocytes and clinical trials at clinically relevant plasma concentrations. These results, while promising, need to be further replicated with a larger set of compounds, iPSC-derived cardiomyocytes from additional individuals, and for a wider range of endpoints [125].
6. iPSC-derived cardiomyocytes and population variability
For both drugs and non-pharmaceutical compounds, population variability in cardiotoxicity hazard is an important factor to consider. The availability of iPSC-derived cardiomyocytes from healthy individuals of diverse genetic backgrounds represents an ideal platform to model inter-individual variability in both baseline characteristics and in chemical-induced cardiophysiologic sensitivities. In a population of iPSC-derived cardiomyocytes from 27 healthy donors, it was shown that inter-individual variability in baseline beating parameters and drug-induced effects is highly reproducible [79]. A follow-up study demonstrated that the in vitro changes to QT prolongation from this population of 27 donors accurately predicts both hazard and dose for clinical QT changes across a panel of 13 pharmaceuticals [81]. These studies suggest that in vitro cardiotoxicity testing using iPSC-derived cardiomyocytes may serve as a viable alternative to the traditional TQT study conducted in clinical cardiovascular safety assessment, albeit many additional questions remain to be addressed and a larger set of compounds needs to be tested [125]. Another recent study that evaluated how closely iPSC-derived cardiomyocytes from healthy subjects can recapitulate a range of responses examined the concentration-response of two hERG‐blocking drugs, dofetilide and moxifloxacin, both in vivo in a phase I clinical trial setting and in iPSC-derived cardiomyocytes derived from the same 16 healthy volunteers [124]. The authors found no significant correlation between the subject‐specific in vitro effects and clinical QT response slopes to either drug, nor was there significant correlation between baseline QT and baseline in vitro measurements. While the results from these two studies [81, 124] are not directly comparable because the subjects did not overlap and preparation of iPSC-derived cardiomyocytes was conducted by different laboratories, further investigation into factors that may obscure in vitro to in vivo correlation are warranted.
Subsequent studies in iPSC-derived cardiomyocytes that used an even larger population of 43 healthy human donors examined both cardiotoxicity hazard and toxicodynamic population variability for a large number of drugs (n = 54) and environmental chemicals (n = 82) [82, 83]. These studies demonstrated that inter-individual variability in both baseline and chemical-induced effects on iPSC-derived cardiomyocytes was reproducible. Further, hierarchical Bayesian population concentration-response modeling of five phenotypes reflecting cardiomyocyte function or viability showed that many drugs were active across all phenotypes, while only about half of the tested environmental chemicals showed activity in at least one phenotype, most commonly positive chronotropy. Together, these studies demonstrate the feasibility of using iPSC-derived cardiomyocytes from a population of healthy human individuals to characterize “normal” toxicodynamic population variability of drugs and chemicals, which is important for both pharmaceuticals and non-pharmaceuticals. For instance, a high degree of human variation may indicate the need for a lower first-in-human dose and/or a higher therapeutic index [126]. Analogously for non-pharmaceuticals, uncertainty (or “safety”) factors used to calculate exposure limits may need to be increased from their default values if a chemical exhibits a high degree of variability in susceptibility [127].
Thus, a few studies have used populations of iPSC-derived cardiomyocytes to replace default uncertainty factors and enable chemical-specific estimates of human toxicodynamic variability. For example, one study screened a panel of 136 drugs and environmental chemicals in concentration-response in a population of iPSC-derived cardiomyocytes from 43 individuals and derived estimates of a “toxicodynamic variability factor” (TDVF) that expresses quantitatively the degree to which a “sensitive” individual is more susceptible than a “median” individual [82, 83]. These studies found that for functional phenotypes such as QT prolongation and positive or negative chronotropy, most test compounds (both drug and environmental chemical) had TDVF values above the default estimate of 3.16. By contrast, the TDVF values for cytotoxicity phenotypes were often below 3.16, consistent with other human in vitro data [128], suggesting that it is important to consider how functional phenotypes may be more sensitive to chemical-induced effects than phenotypes such as overt cytotoxicity [83].
7. iPSC-derived cardiomyocytes as models of disease and personalized medicine
In addition to being used to characterize variability across the population as a whole, iPSC-derived cardiomyocytes have also generated considerable interest for disease modeling and personalized medicine. For example, a common application of iPSC-derived cardiomyocytes in biomedical science is for recapitulating critical aspects of disease biology in vitro by using patient-specific cells [33]. A number of familial disease phenotypes have been successfully modeled in vitro, such as catecholaminergic polymorphic ventricular tachycardia [129–132], hypertrophic cardiomyopathy [133, 134], dilated cardiomyopathy [72, 135], and cardiac channelopathies including various types of long-QT syndromes [69, 71, 136]. The availability of these models opens opportunities for understanding disease mechanisms, performing genetic manipulations, and developing novel therapeutics for rare diseases [137].
Further, gene expression patterns in iPSC-derived cardiomyocytes cluster by the genetic background of the donors and can provide insight into genetic variants associated with normal cardiophysiology and disease susceptibility [138]. A number of studies have used iPSC-derived cardiomyocytes from different donors to evaluate inter-individual susceptibility in vitro. For example, iPSC-derived cardiomyocytes from 10 donors recapitulated the variability in clinical QT prolongation induced by exposure to moxifloxacin [139]. Further, a study of 8 breast cancer patients and 4 healthy controls showed that in vivo susceptibility to cardiotoxicity induced by doxorubicin can be faithfully replicated in the iPSC-derived cardiomyocytes from the same individuals [78]. These studies demonstrate the utility of using iPSC-derived cardiomyocytes in personalized medicine.
8. Expert opinion
The promise and utility of human iPSC-derived cardiomyocytes for the drug development pipeline was widely recognized almost immediately after robust differentiation protocols were developed [33, 73, 75]. In addition to the usual advantages of in vitro systems related to throughput and cost, another advantage of iPSC-derived cardiomyocytes is their commercial availability, which helps to ensure reproducibility, particularly across cells from large panels of donors. iPSC-derived cardiomyocytes from multiple individuals are established to be reproducible with respect to both baseline characteristics and treatment-related phenotypes [79, 82]. Another advantage of this model is that it is human-based and shows the appropriate expression profiles of ion channels [63]; thus, species differences in toxicodynamics need not be considered and hazard assessment can occur much more rapidly than with data from preclinical animal models.
The most common application of human iPSC-derived cardiomyocytes is in early and rapid hazard identification for drug candidates and environmental chemicals [23]. iPSC-derived cardiomyocytes have come to be used in a variety of modalities, including electrophysiological, contractile, imaging-based, and mechanistic assays, and in a variety of contexts for both pharmaceuticals and environmental chemicals. Their versatility in terms of compatibility with a variety of screening modalities allows for both targeted and non-targeted, rapid approaches to hazard assessment of compounds, while also enabling identification of both functional and mechanistic phenotypes.
Beyond hazard identification, a number of efforts have demonstrated that human iPSC-derived cardiomyocytes can be useful in several risk characterization contexts. Incorporation of exposure and toxicokinetic data, for instance, can provide quantitative insights into the margin of safety or margin of exposure that is likely to be achieved in vivo. Such applications, however, do require testing to be done in concentration-response format. Moreover, as with any in vitro system, the use of data from iPSC-derived cardiomyocytes for risk characterization requires in vitro-to-in vivo extrapolation, both in terms of the selection of relevant test concentrations accounting for freely available compound as well as developing appropriate metrics of response in order to facilitate interpretation of effective concentrations in the context of expected plasma levels. Additionally, because these cells can be derived from a multitude of different individuals, including potentially specific patients, iPSC-derived cardiomyocytes hold the promise of both population-based risk characterization and personalized medicine. However, in order to achieve this promise, a number of technical details need to be refined in order to ensure reproducible results while optimizing throughput and cost. For instance, while some studies have suggested that using iPSC-derived cardiomyocytes from a population of individuals, rather than a single individual, improves predictive accuracy, additional analyses examining design optimization in terms of the number of individuals and number of replicates needed would be beneficial. Additionally, only a handful of studies have examined general population variability, as opposed to variation in the context of personalized medicine, and validation of predictions as to inter-individual variability is challenging.
Together, these studies demonstrate that iPSC-derived cardiomyocytes constitute an attractive model for characterizing the hazard and risk of both pharmaceutical and non-pharmaceutical compounds. Nonetheless, there are notable limitations to this approach. Most iPSC-derived cardiomyocyte preparations available today remain largely immature when compared to the human adult myocardium, despite numerous efforts to improve the maturation state of cells in culture [140]. Such morphological and molecular differences between iPSC-derived and adult cardiomyocytes include differences in structure (organization of sarcomeres, sarcoplasmic reticulum, and transverse tubules), electrophysiology (mixed action potential and lack of IK1 that is essential for stabilization of the resting potential in iPSC-derived cardiomyocytes), calcium handling (differences in how intracellular Ca2+ concentrations that are important for the synchronized contraction in multiple sarcomeres are regulated), metabolism (glycolysis versus fatty acid oxidation), and gene expression (identifying transcriptomic signatures of advanced state of cardiomyocyte maturation). A number of experimental strategies have been proposed to drive iPSC-derived cardiomyocyte maturation. These include extensions of cell culture period from days to months [141], amendments to the cell culture media to modulate hormonal composition and facilitate utilization of fatty acids for energy production [142], modulation of extracellular matrices and substrates [143], and electro-mechanical stimulation [144]. One area that deserves special interest in this regard is the use of 3D cell culture techniques and tissue chips, models that are in active development [36]. An additional challenge lies in the scalability of production and efficient differentiation of iPSC-derived cardiomyocytes, as this process is time- and resource-intensive and technically challenging. Endeavors to the increase the efficiency and mass production of these cells will be critical for their widespread use in chemical hazard assessment [145, 146]. Another key challenge that remains in using iPSC-derived cardiomyocytes as a replacement for current in vivo models is the limitation of the types of cardiovascular effects that can be addressed with these cells. For example, vascular cardiotoxicity, hemodynamic changes, and vasomotor activity cannot be assessed in models of iPSC-derived cardiomyocytes alone. Further, chemical metabolism is not considered in systems of iPSC-derived cardiomyocytes alone, which is disadvantageous as metabolism often plays a key role in chemical toxicity [147].
Despite these limitations, human iPSC-derived cardiomyocytes serve as an attractive alternative approach to cardiotoxicity hazard assessment and risk characterization. Looking to the future, while incremental benefits in terms of increased accuracy, precision, and efficiency are likely to be achieved by routine use of iPSC-derived cardiomyocytes in pharmaceutical development, the greatest benefits are likely to be in addressing the potential cardiotoxicity risks for environmental chemicals. This is because, in contrast to pharmaceuticals, there is currently no routine testing required for the potential cardiovascular adverse effects of environmental chemicals. Even for environmental chemicals where testing is required, such as pesticides, the current toxicity testing paradigm relies primarily on in vivo guideline studies in rodents, which examine general biomarkers of toxicity in blood and urine and histopathology endpoints. These studies are generally either 28- or 90-day toxicity studies or 2-year cancer bioassays. While structural changes in the cardiovascular system are observed and recorded in these studies [13, 38], these studies are costly and time-consuming. In addition, such studies are not designed to detect many important cardiotoxic effects, such as arrhythmias and other functional endpoints. Moreover, for the vast majority of non-pharmaceutical substances in commerce, no routine testing is required at all. Therefore, there is a critical need for innovative, high-throughput testing approaches that can accurately assess the potential cardiotoxicity hazards posed by environmental chemicals, and iPSC-derived cardiomyocytes have been shown to be a useful model to address this gap.
Article highlights.
iPSC-derived cardiomyocytes are a well-accepted model for preclinical drug testing
These cells have been shown to be useful as disease models
Availability of cells from multiple individuals opens their use in personalized medicine
Population variability testing using cells from normal individuals is also possible
There is an opportunity to use these cells for testing of environmental chemicals
Funding
This work was funded, in part, by grants from the National Institutes of Health (P42 ES027704 and T32 ES026568) and a cooperative agreement with the United States Environmental Protection Agency (STAR RD83580201). The views expressed in this manuscript do not reflect those of the funding agencies. The use of specific commercial products in this work does not constitute endorsement by the authors or the funding agencies.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.WHO Cvd Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health 2019;7:e1332–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YB, Pan XF, Chen J, et al. Combined lifestyle factors, all-cause mortality and cardiovascular disease: a systematic review and meta-analysis of prospective cohort studies. J Epidemiol Community Health 2020. [DOI] [PubMed] [Google Scholar]
- 5.Knowles JW, Ashley EA. Cardiovascular disease: The rise of the genetic risk score. PLoS Med 2018;15:e1002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrebola JP, Fernandez MF, Martin-Olmedo P, et al. Historical exposure to persistent organic pollutants and risk of incident hypertension. Environ Res 2015;138:217–23. [DOI] [PubMed] [Google Scholar]
- 7.Fu X, Xu J, Zhang R, et al. The association between environmental endocrine disruptors and cardiovascular diseases: A systematic review and meta-analysis. Environ Res 2020;187:109464. [DOI] [PubMed] [Google Scholar]
- 8.Gavila J, Segui MA, Calvo L, et al. Evaluation and management of chemotherapy-induced cardiotoxicity in breast cancer: a Delphi study. Clin Transl Oncol 2017;19:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 2009;53:2231–47. [DOI] [PubMed] [Google Scholar]
- 10.Ferri N, Siegl P, Corsini A, et al. Drug attrition during pre-clinical and clinical development: understanding and managing drug-induced cardiotoxicity. Pharmacol Ther 2013;138:470–84. [DOI] [PubMed] [Google Scholar]
- 11.Stockbridge N, Morganroth J, Shah RR, et al. Dealing with global safety issues : was the response to QT-liability of non-cardiac drugs well coordinated? Drug Saf 2013;36:167–82. [DOI] [PubMed] [Google Scholar]
- 12.Laverty H, Benson C, Cartwright E, et al. How can we improve our understanding of cardiovascular safety liabilities to develop safer medicines? Br J Pharmacol 2011;163:675–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berridge BR, Hoffmann P, Turk JR, et al. Integrated and translational nonclinical in vivo cardiovascular risk assessment: gaps and opportunities. Regul Toxicol Pharmacol 2013;65:38–46. [DOI] [PubMed] [Google Scholar]
- 14.Herman EH, Ferrans VJ. Preclinical animal models of cardiac protection from anthracycline-induced cardiotoxicity. Semin Oncol 1998;25:15–21. [PubMed] [Google Scholar]
- 15.Bonaca MP, Olenchock BA, Salem JE, et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation 2019;140:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: a systematic review. BMC Med 2016;14:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pruss-Ustun A, Wolf J, Corvalan C, et al. Diseases due to unhealthy environments: an updated estimate of the global burden of disease attributable to environmental determinants of health. J Public Health (Oxf) 2017;39:464–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgiadis N, Tsarouhas K, Tsitsimpikou C, et al. Pesticides and cardiotoxicity. Where do we stand? Toxicology and applied pharmacology 2018;353:1–14. [DOI] [PubMed] [Google Scholar]
- 19.Mills KT, Blair A, Freeman LE, et al. Pesticides and myocardial infarction incidence and mortality among male pesticide applicators in the Agricultural Health Study. Am J Epidemiol 2009;170:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark RA, Marin TS, Berry NM, et al. Cardiotoxicity and cardiovascular disease risk assessment for patients receiving breast cancer treatment. Cardiooncology 2017;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts RA, Kavanagh SL, Mellor HR, et al. Reducing attrition in drug development: smart loading preclinical safety assessment. Drug Discov Today 2014;19:341–7. [DOI] [PubMed] [Google Scholar]
- 22.Colatsky T, Fermini B, Gintant G, et al. The Comprehensive in Vitro Proarrhythmia Assay (CiPA) initiative - Update on progress. J Pharmacol Toxicol Methods 2016;81:15–20. [DOI] [PubMed] [Google Scholar]
- 23.Pang L, Sager P, Yang X, et al. Workshop Report: FDA Workshop on Improving Cardiotoxicity Assessment With Human-Relevant Platforms. Circ Res 2019;125:855–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.E14 Implementation Working Group. ICH E14 Guideline: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs Questions & Answers (R3). Geneva, Switzerland: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; 2015. [Google Scholar]
- 25.Arrigoni C, Crivori P. Assessment of QT liabilities in drug development. Cell Biol Toxicol 2007;23:1–13. [DOI] [PubMed] [Google Scholar]
- 26.Villoutreix BO, Taboureau O. Computational investigations of hERG channel blockers: New insights and current predictive models. Adv Drug Deliv Rev 2015;86:72–82. [DOI] [PubMed] [Google Scholar]
- 27.Ekins S Progress in computational toxicology. J Pharmacol Toxicol Methods 2014;69:115–40. [DOI] [PubMed] [Google Scholar]
- 28.Sanguinetti MC, Jiang C, Curran ME, et al. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 1995;81:299–307. [DOI] [PubMed] [Google Scholar]
- 29.Curran ME, Splawski I, Timothy KW, et al. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 1995;80:795–803. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Zhang R, Zhao B, et al. Cardiotoxicity screening: a review of rapid-throughput in vitro approaches. Archives of Toxicology 2016;90:1803–16. [DOI] [PubMed] [Google Scholar]
- 31.Hamill OP, Marty A, Neher E, et al. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 1981;391:85–100. [DOI] [PubMed] [Google Scholar]
- 32.Dunlop J, Bowlby M, Peri R, et al. High-throughput electrophysiology: an emerging paradigm for ion-channel screening and physiology. Nat Rev Drug Discov 2008;7:358–68. [DOI] [PubMed] [Google Scholar]
- 33.Mercola M, Colas A, Willems E. Induced pluripotent stem cells in cardiovascular drug discovery. Circ Res 2013;112:534–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ou Q, Jacobson Z, Abouleisa RRE, et al. Physiological Biomimetic Culture System for Pig and Human Heart Slices. Circ Res 2019;125:628–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller JM, Meki MH, Ou Q, et al. Heart slice culture system reliably demonstrates clinical drug-related cardiotoxicity. Toxicology and applied pharmacology 2020;406:115213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuppinger C 3D Cardiac Cell Culture: A Critical Review of Current Technologies and Applications. Front Cardiovasc Med 2019;6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marx U, Akabane T, Andersson TB, et al. Biology-inspired microphysiological systems to advance patient benefit and animal welfare in drug development. ALTEX 2020;37:365–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berridge BR, Mowat V, Nagai H, et al. Non-proliferative and Proliferative Lesions of the Cardiovascular System of the Rat and Mouse. J Toxicol Pathol 2016;29:1S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avram R, Tison GH, Aschbacher K, et al. Real-world heart rate norms in the Health eHeart study. NPJ Digit Med 2019;2:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milani-Nejad N, Janssen PM. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol Ther 2014;141:235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindgren S, Bass AS, Briscoe R, et al. Benchmarking safety pharmacology regulatory packages and best practice. J Pharmacol Toxicol Methods 2008;58:99–109. [DOI] [PubMed] [Google Scholar]
- 42.Cox LA, Olivier M, Spradling-Reeves K, et al. Nonhuman Primates and Translational Research-Cardiovascular Disease. Ilar Journal 2017;58:235–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen YT. Primate models for cardiovascular drug research and development. Curr Opin Investig Drugs 2010;11:1025–9. [PubMed] [Google Scholar]
- 44.Avila AM, Bebenek I, Bonzo JA, et al. An FDA/CDER perspective on nonclinical testing strategies: Classical toxicology approaches and new approach methodologies (NAMs). Regul Toxicol Pharmacol 2020;114:104662. [DOI] [PubMed] [Google Scholar]
- 45.Darpo B Clinical ECG Assessment. Handb Exp Pharmacol 2015;229:435–68. [DOI] [PubMed] [Google Scholar]
- 46.Vargas HM, Rolf MG, Wisialowski TA, et al. Time for a Fully Integrated Nonclinical-Clinical Risk Assessment to Streamline QT Prolongation Liability Determinations: A Pharma Industry Perspective. Clin Pharmacol Ther 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–76. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–72. [DOI] [PubMed] [Google Scholar]
- 49.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 2007;25:1015–24. [DOI] [PubMed] [Google Scholar]
- 50.Karakikes I, Ameen M, Termglinchan V, et al. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ Res 2015;117:80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sirenko O, Cromwell EF, Crittenden C, et al. Assessment of beating parameters in human induced pluripotent stem cells enables quantitative in vitro screening for cardiotoxicity. Toxicology and applied pharmacology 2013;273:500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sirenko O, Crittenden C, Callamaras N, et al. Multiparameter in vitro assessment of compound effects on cardiomyocyte physiology using iPSC cells. J Biomol Screen 2013;18:39–53. [DOI] [PubMed] [Google Scholar]
- 53.Lundy SD, Zhu WZ, Regnier M, et al. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev 2013;22:1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamakura T, Makiyama T, Sasaki K, et al. Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ J 2013;77:1307–14. [DOI] [PubMed] [Google Scholar]
- 55.Ivashchenko CY, Pipes GC, Lozinskaya IM, et al. Human-induced pluripotent stem cell-derived cardiomyocytes exhibit temporal changes in phenotype. Am J Physiol Heart Circ Physiol 2013;305:H913–22. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Wilson GF, Soerens AG, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res 2009;104:e30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang CY, Peres Moreno Maia-Joca R, Ong CS, et al. Enhancement of human iPSC-derived cardiomyocyte maturation by chemical conditioning in a 3D environment. J Mol Cell Cardiol 2020;138:1–11. [DOI] [PubMed] [Google Scholar]
- 58.Ronaldson-Bouchard K, Ma SP, Yeager K, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018;556:239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giacomelli E, Meraviglia V, Campostrini G, et al. Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell 2020;26:862–79 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ross GR, Rizvi F, Emelyanova L, et al. Prolonged post-differentiation culture influences the expression and biophysics of Na(+) and Ca(2+) channels in induced pluripotent stem cell-derived ventricular-like cardiomyocytes. Cell Tissue Res 2019;378:59–66. [DOI] [PubMed] [Google Scholar]
- 61.Kolanowski TJ, Busek M, Schubert M, et al. Enhanced structural maturation of human induced pluripotent stem cell-derived cardiomyocytes under a controlled microenvironment in a microfluidic system. Acta Biomater 2020;102:273–86. [DOI] [PubMed] [Google Scholar]
- 62.Zwi L, Caspi O, Arbel G, et al. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation 2009;120:1513–23. [DOI] [PubMed] [Google Scholar]
- 63.Ma J, Guo L, Fiene SJ, et al. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol 2011;301:H2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Navarrete EG, Liang P, Lan F, et al. Screening drug-induced arrhythmia [corrected] using human induced pluripotent stem cell-derived cardiomyocytes and low-impedance microelectrode arrays. Circulation 2013;128:S3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Itzhaki I, Rapoport S, Huber I, et al. Calcium handling in human induced pluripotent stem cell derived cardiomyocytes. PLoS One 2011;6:e18037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sirenko O, Grimm FA, Ryan KR, et al. In vitro cardiotoxicity assessment of environmental chemicals using an organotypic human induced pluripotent stem cell-derived model. Toxicology and applied pharmacology 2017;322:60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo L, Abrams RM, Babiarz JE, et al. Estimating the risk of drug-induced proarrhythmia using human induced pluripotent stem cell-derived cardiomyocytes. Toxicological sciences 2011;123:281–9. [DOI] [PubMed] [Google Scholar]
- 68.Cohen JD, Babiarz JE, Abrams RM, et al. Use of human stem cell derived cardiomyocytes to examine sunitinib mediated cardiotoxicity and electrophysiological alterations. Toxicology and applied pharmacology 2011;257:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Itzhaki I, Maizels L, Huber I, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 2011;471:225–9. [DOI] [PubMed] [Google Scholar]
- 70.Huang HP, Chen PH, Hwu WL, et al. Human Pompe disease-induced pluripotent stem cells for pathogenesis modeling, drug testing and disease marker identification. Hum Mol Genet 2011;20:4851–64. [DOI] [PubMed] [Google Scholar]
- 71.Egashira T, Yuasa S, Suzuki T, et al. Disease characterization using LQTS-specific induced pluripotent stem cells. Cardiovasc Res 2012;95:419–29. [DOI] [PubMed] [Google Scholar]
- 72.Sun N, Yazawa M, Liu J, et al. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med 2012;4:130ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anson BD, Kolaja KL, Kamp TJ. Opportunities for use of human iPS cells in predictive toxicology. Clin Pharmacol Ther 2011;89:754–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rana P, Anson B, Engle S, et al. Characterization of human-induced pluripotent stem cell-derived cardiomyocytes: bioenergetics and utilization in safety screening. Toxicological sciences 2012;130:117–31. [DOI] [PubMed] [Google Scholar]
- 75.Mordwinkin NM, Burridge PW, Wu JC. A review of human pluripotent stem cell-derived cardiomyocytes for high-throughput drug discovery, cardiotoxicity screening, and publication standards. J Cardiovasc Transl Res 2013;6:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Asai Y, Tada M, Otsuji TG, et al. Combination of functional cardiomyocytes derived from human stem cells and a highly-efficient microelectrode array system: an ideal hybrid model assay for drug development. Curr Stem Cell Res Ther 2010;5:227–32. [DOI] [PubMed] [Google Scholar]
- 77.Grimm FA, Iwata Y, Sirenko O, et al. High-Content Assay Multiplexing for Toxicity Screening in Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Hepatocytes. Assay Drug Dev Technol 2015;13:529–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burridge PW, Li YF, Matsa E, et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med 2016;22:547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grimm FA, Blanchette A, House JS, et al. A human population-based organotypic in vitro model for cardiotoxicity screening. ALTEX 2018;35:441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grimm FA, Klaren WD, Li X, et al. Cardiovascular Effects of Polychlorinated Biphenyls and Their Major Metabolites. Environ Health Perspect 2020;128:77008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blanchette AD, Grimm FA, Dalaijamts C, et al. Thorough QT/QTc in a Dish: An In Vitro Human Model That Accurately Predicts Clinical Concentration-QTc Relationships. Clin Pharmacol Ther 2019;105:1175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burnett SD, Blanchette AD, Grimm FA, et al. Population-based toxicity screening in human induced pluripotent stem cell-derived cardiomyocytes. Toxicology and applied pharmacology 2019;381:114711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blanchette AD, Burnett SD, Grimm FA, et al. A Bayesian Method for Population-wide Cardiotoxicity Hazard and Risk Characterization Using an In Vitro Human Model. Toxicological sciences 2020;178:391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sirenko O, Hancock MK, Crittenden C, et al. Phenotypic Assays for Characterizing Compound Effects on Induced Pluripotent Stem Cell-Derived Cardiac Spheroids. Assay Drug Dev Technol 2017;15:280–96. [DOI] [PubMed] [Google Scholar]
- 85.Pinheiro EA, Magdy T, Burridge PW. Human In Vitro Models for Assessing the Genomic Basis of Chemotherapy-Induced Cardiovascular Toxicity. J Cardiovasc Transl Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fonoudi H, Burridge PW. Cellular model systems to study cardiovascular injury from chemotherapy. J Thromb Thrombolysis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Apati A, Varga N, Berecz T, et al. Application of human pluripotent stem cells and pluripotent stem cell-derived cellular models for assessing drug toxicity. Expert Opin Drug Metab Toxicol 2019;15:61–75. [DOI] [PubMed] [Google Scholar]
- 88.Shapiro AJ, Antoni S, Guyton KZ, et al. Software Tools to Facilitate Systematic Review Used for Cancer Hazard Identification. Environ Health Perspect 2018;126:104501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takasuna K, Asakura K, Araki S, et al. Comprehensive in vitro cardiac safety assessment using human stem cell technology: Overview of CSAHi HEART initiative. J Pharmacol Toxicol Methods 2017;83:42–54. [DOI] [PubMed] [Google Scholar]
- 90.Blinova K, Stohlman J, Vicente J, et al. Comprehensive Translational Assessment of Human-Induced Pluripotent Stem Cell Derived Cardiomyocytes for Evaluating Drug-Induced Arrhythmias. Toxicological sciences 2017;155:234–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blinova K, Dang Q, Millard D, et al. International Multisite Study of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Drug Proarrhythmic Potential Assessment. Cell Rep 2018;24:3582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma A, Burridge PW, McKeithan WL, et al. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Transl Med 2017;9:pii: eaaf2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haechl N, Ebner J, Hilber K, et al. Pharmacological Profile of the Bradycardic Agent Ivabradine on Human Cardiac Ion Channels. Cell Physiol Biochem 2019;53:36–48. [DOI] [PubMed] [Google Scholar]
- 94.Zang J, Wu S, Tang L, et al. Incidence and risk of QTc interval prolongation among cancer patients treated with vandetanib: a systematic review and meta-analysis. PLoS One 2012;7:e30353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kopljar I, Lu HR, Van Ammel K, et al. Development of a Human iPSC Cardiomyocyte-Based Scoring System for Cardiac Hazard Identification in Early Drug Safety De-risking. Stem Cell Reports 2018;11:1365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bedut S, Seminatore-Nole C, Lamamy V, et al. High-throughput drug profiling with voltage- and calcium-sensitive fluorescent probes in human iPSC-derived cardiomyocytes. Am J Physiol Heart Circ Physiol 2016;311:H44–53. [DOI] [PubMed] [Google Scholar]
- 97.da Rocha AM, Creech J, Thonn E, et al. Detection of Drug-Induced Torsades de Pointes Arrhythmia Mechanisms Using hiPSC-CM Syncytial Monolayers in a High-Throughput Screening Voltage Sensitive Dye Assay. Toxicological sciences 2020;173:402–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.da Rocha AM, Campbell K, Mironov S, et al. hiPSC-CM Monolayer Maturation State Determines Drug Responsiveness in High Throughput Pro-Arrhythmia Screen. Sci Rep 2017;7:13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bjork S, Ojala EA, Nordstrom T, et al. Evaluation of Optogenetic Electrophysiology Tools in Human Stem Cell-Derived Cardiomyocytes. Front Physiol 2017;8:884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Quach B, Krogh-Madsen T, Entcheva E, et al. Light-Activated Dynamic Clamp Using iPSC-Derived Cardiomyocytes. Biophys J 2018;115:2206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lapp H, Bruegmann T, Malan D, et al. Frequency-dependent drug screening using optogenetic stimulation of human iPSC-derived cardiomyocytes. Sci Rep 2017;7:9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pointon A, Harmer AR, Dale IL, et al. Assessment of cardiomyocyte contraction in human-induced pluripotent stem cell-derived cardiomyocytes. Toxicological sciences 2015;144:227–37. [DOI] [PubMed] [Google Scholar]
- 103.Kawatou M, Masumoto H, Fukushima H, et al. Modelling Torsade de Pointes arrhythmias in vitro in 3D human iPS cell-engineered heart tissue. Nat Commun 2017;8:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao Y, Rafatian N, Feric NT, et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 2019;176:913–27 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mills RJ, Parker BL, Quaife-Ryan GA, et al. Drug Screening in Human PSC-Cardiac Organoids Identifies Pro-proliferative Compounds Acting via the Mevalonate Pathway. Cell Stem Cell 2019;24:895–907 e6. [DOI] [PubMed] [Google Scholar]
- 106.Mosqueira D, Mannhardt I, Bhagwan JR, et al. CRISPR/Cas9 editing in human pluripotent stem cell-cardiomyocytes highlights arrhythmias, hypocontractility, and energy depletion as potential therapeutic targets for hypertrophic cardiomyopathy. Eur Heart J 2018;39:3879–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feric NT, Pallotta I, Singh R, et al. Engineered Cardiac Tissues Generated in the Biowire II: A Platform for Human-Based Drug Discovery. Toxicological sciences 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ma Z, Huebsch N, Koo S, et al. Contractile deficits in engineered cardiac microtissues as a result of MYBPC3 deficiency and mechanical overload. Nat Biomed Eng 2018;2:955–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maddah M, Mandegar MA, Dame K, et al. Quantifying drug-induced structural toxicity in hepatocytes and cardiomyocytes derived from hiPSCs using a deep learning method. J Pharmacol Toxicol Methods 2020;105:106895. [DOI] [PubMed] [Google Scholar]
- 110.Wang H, Sheehan RP, Palmer AC, et al. Adaptation of Human iPSC-Derived Cardiomyocytes to Tyrosine Kinase Inhibitors Reduces Acute Cardiotoxicity via Metabolic Reprogramming. Cell Syst 2019;8:412–26 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ng KM, Lau YM, Dhandhania V, et al. Empagliflozin Ammeliorates High Glucose Induced-Cardiac Dysfuntion in Human iPSC-Derived Cardiomyocytes. Sci Rep 2018;8:14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carlson C, Koonce C, Aoyama N, et al. Phenotypic screening with human iPS cell-derived cardiomyocytes: HTS-compatible assays for interrogating cardiac hypertrophy. J Biomol Screen 2013;18:1203–11. [DOI] [PubMed] [Google Scholar]
- 113.Johansson M, Ulfenborg B, Andersson CX, et al. Cardiac hypertrophy in a dish: a human stem cell based model. Biol Open 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chaudhari U, Nemade H, Wagh V, et al. Identification of genomic biomarkers for anthracycline-induced cardiotoxicity in human iPSC-derived cardiomyocytes: an in vitro repeated exposure toxicity approach for safety assessment. Archives of Toxicology 2016;90:2763–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buza V, Rajagopalan B, Curtis AB. Cancer Treatment-Induced Arrhythmias: Focus on Chemotherapy and Targeted Therapies. Circ Arrhythm Electrophysiol 2017;10. [DOI] [PubMed] [Google Scholar]
- 116.Atwal M, Swan RL, Rowe C, et al. Intercalating TOP2 Poisons Attenuate Topoisomerase Action at Higher Concentrations. Mol Pharmacol 2019;96:475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gupta SK, Garg A, Avramopoulos P, et al. miR-212/132 Cluster Modulation Prevents Doxorubicin-Mediated Atrophy and Cardiotoxicity. Molecular Therapy 2019;27:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.International Conference on Harmonisation. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guideline Q9 on quality risk management. Geneva, Switzerland: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; 2006. [Google Scholar]
- 119.National Research Council. Risk assessment in the federal government: Managing the process. Washington, D.C.: National Academies Press, 1983. [PubMed] [Google Scholar]
- 120.U.S. EPA. A Review of the Reference Dose and Reference Concentration Processes. Wahington, DC: US Environmental Protection Agency; 2002. [Google Scholar]
- 121.Redfern WS, Carlsson L, Davis AS, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res 2003;58:32–45. [DOI] [PubMed] [Google Scholar]
- 122.Chaudhari U, Nemade H, Sureshkumar P, et al. Functional cardiotoxicity assessment of cosmetic compounds using human-induced pluripotent stem cell-derived cardiomyocytes. Archives of Toxicology 2018;92:371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ando H, Yoshinaga T, Yamamoto W, et al. A new paradigm for drug-induced torsadogenic risk assessment using human iPS cell-derived cardiomyocytes. J Pharmacol Toxicol Methods 2017;84:111–27. [DOI] [PubMed] [Google Scholar]
- 124.Blinova K, Schocken D, Patel D, et al. Clinical Trial in a Dish: Personalized Stem Cell-Derived Cardiomyocyte Assay Compared With Clinical Trial Results for Two QT-Prolonging Drugs. Clin Transl Sci 2019;12:687–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vargas HM. “Thorough QT/QTc in a Dish”: Can Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Predict Thorough QT Outcomes? Clin Pharmacol Ther 2019;105:1064–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muller PY, Milton MN. The determination and interpretation of the therapeutic index in drug development. Nat Rev Drug Discov 2012;11:751–61. [DOI] [PubMed] [Google Scholar]
- 127.Meek ME, Renwick A, Ohanian E, et al. Guidelines for application of chemical-specific adjustment factors in dose/concentration-response assessment. Toxicology 2002;181–182:115–20. [DOI] [PubMed] [Google Scholar]
- 128.Chiu WA, Wright FA, Rusyn I. A tiered, Bayesian approach to estimating of population variability for regulatory decision-making. ALTEX 2017;34:377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Di Pasquale E, Lodola F, Miragoli M, et al. CaMKII inhibition rectifies arrhythmic phenotype in a patient-specific model of catecholaminergic polymorphic ventricular tachycardia. Cell Death Dis 2013;4:e843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kujala K, Paavola J, Lahti A, et al. Cell model of catecholaminergic polymorphic ventricular tachycardia reveals early and delayed afterdepolarizations. PLoS One 2012;7:e44660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Novak A, Barad L, Lorber A, et al. Functional abnormalities in iPSC-derived cardiomyocytes generated from CPVT1 and CPVT2 patients carrying ryanodine or calsequestrin mutations. J Cell Mol Med 2015;19:2006–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jung CB, Moretti A, Mederos y Schnitzler M, et al. Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol Med 2012;4:180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Han L, Li Y, Tchao J, et al. Study familial hypertrophic cardiomyopathy using patient-specific induced pluripotent stem cells. Cardiovasc Res 2014;104:258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tanaka A, Yuasa S, Mearini G, et al. Endothelin-1 induces myofibrillar disarray and contractile vector variability in hypertrophic cardiomyopathy-induced pluripotent stem cell-derived cardiomyocytes. J Am Heart Assoc 2014;3:e001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu H, Lee J, Vincent LG, et al. Epigenetic Regulation of Phosphodiesterases 2A and 3A Underlies Compromised beta-Adrenergic Signaling in an iPSC Model of Dilated Cardiomyopathy. Cell Stem Cell 2015;17:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lahti AL, Kujala VJ, Chapman H, et al. Model for long QT syndrome type 2 using human iPS cells demonstrates arrhythmogenic characteristics in cell culture. Dis Model Mech 2012;5:220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang G, McCain ML, Yang L, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 2014;20:616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Panopoulos AD, D’Antonio M, Benaglio P, et al. iPSCORE: A Resource of 222 iPSC Lines Enabling Functional Characterization of Genetic Variation across a Variety of Cell Types. Stem Cell Reports 2017;8:1086–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shinozawa T, Nakamura K, Shoji M, et al. Recapitulation of Clinical Individual Susceptibility to Drug-Induced QT Prolongation in Healthy Subjects Using iPSC-Derived Cardiomyocytes. Stem Cell Reports 2017;8:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ahmed RE, Anzai T, Chanthra N, et al. A Brief Review of Current Maturation Methods for Human Induced Pluripotent Stem Cells-Derived Cardiomyocytes. Front Cell Dev Biol 2020;8:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lewandowski J, Rozwadowska N, Kolanowski TJ, et al. The impact of in vitro cell culture duration on the maturation of human cardiomyocytes derived from induced pluripotent stem cells of myogenic origin. Cell Transplant 2018;27:1047–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang X, Rodriguez ML, Leonard A, et al. Fatty Acids Enhance the Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Stem Cell Reports 2019;13:657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Talman V, Kivela R. Cardiomyocyte-Endothelial Cell Interactions in Cardiac Remodeling and Regeneration. Front Cardiovasc Med 2018;5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kroll K, Chabria M, Wang K, et al. Electro-mechanical conditioning of human iPSC-derived cardiomyocytes for translational research. Prog Biophys Mol Biol 2017;130:212–22. [DOI] [PubMed] [Google Scholar]
- 145.Kempf H, Kropp C, Olmer R, et al. Cardiac differentiation of human pluripotent stem cells in scalable suspension culture. Nature Protocols 2015;10:1345–61. [DOI] [PubMed] [Google Scholar]
- 146.Halloin C, Schwanke K, Lobel W, et al. Continuous WNT Control Enables Advanced hPSC Cardiac Processing and Prognostic Surface Marker Identification in Chemically Defined Suspension Culture. Stem Cell Reports 2019;13:366–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oleaga C, Riu A, Rothemund S, et al. Investigation of the effect of hepatic metabolism on off-target cardiotoxicity in a multi-organ human-on-a-chip system. Biomaterials 2018;182:176–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Harris K, Aylott M, Cui Y, et al. Comparison of electrophysiological data from human-induced pluripotent stem cell-derived cardiomyocytes to functional preclinical safety assays. Toxicological sciences 2013;134:412–26. [DOI] [PubMed] [Google Scholar]
- 149.Eimon PM, Rubinstein AL. The use of in vivo zebrafish assays in drug toxicity screening. Expert Opin Drug Metab Toxicol 2009;5:393–401. [DOI] [PubMed] [Google Scholar]
- 150.Sarmah S, Marrs JA. Zebrafish as a Vertebrate Model System to Evaluate Effects of Environmental Toxicants on Cardiac Development and Function. Int J Mol Sci 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Swindle MM, Makin A, Herron AJ, et al. Swine as models in biomedical research and toxicology testing. Vet Pathol 2012;49:344–56. [DOI] [PubMed] [Google Scholar]
- 152.van der Laan JW, Brightwell J, McAnulty P, et al. Regulatory acceptability of the minipig in the development of pharmaceuticals, chemicals and other products. J Pharmacol Toxicol Methods 2010;62:184–95. [DOI] [PubMed] [Google Scholar]
- 153.Nystoriak MA, Kilfoil PJ, Lorkiewicz PK, et al. Comparative effects of parent and heated cinnamaldehyde on the function of human iPSC-derived cardiac myocytes. Toxicol In Vitro 2019;61:104648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Matschke V, Piccini I, Schubert J, et al. The Natural Plant Product Rottlerin Activates Kv7.1/KCNE1 Channels. Cell Physiol Biochem 2016;40:1549–58. [DOI] [PubMed] [Google Scholar]
- 155.Dempsey GT, Chaudhary KW, Atwater N, et al. Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J Pharmacol Toxicol Methods 2016;81:240–50. [DOI] [PubMed] [Google Scholar]


