Abstract
Purpose
We aimed at describing for the first time peripheral small-fiber neurotoxicity and pain sensitization in survivors of pediatric acute lymphoblastic leukemia after stem cell transplantation (SCT).
Methods
In a cross-sectional, retrospective, single-center study, we assessed 25 relapse-free long-term survivors (median age at SCT: 11 ± 4.9 years; median time between SCT and testing: 8.25 years, 19 males) using a reduced version of the pediatric-modified total neuropathy score for clinical assessment and Quantitative Sensory Testing (QST). Inclusion criteria: 6 years old at testing, 18 years old at time of SCT, 1 year between SCT and testing.
Results
Nine patients (36%) had peripheral neuropathy as defined by the clinical red-pmTNS (≥ 4). The QST parameters mechanical pain sensitivity, mechanical detection threshold, thermal sensory limen, vibration detection threshold and pressure pain threshold were significantly abnormal in the survivor cohort (p < 0.0038). Except for one, all survivors showed at least one abnormal QST parameter. When using QST, signs of small and large fiber dysfunction were present in 22 (88%) and 17 (68%) survivors, respectively. More than half of all survivors were found to experience pathologic sensitization to pain.
Conclusions and implications for cancer survivors
Survivors of pediatric acute lymphoblastic leukemia after SCT are at high risk for long-term peripheral neuropathy with a dominating small-fiber and pain sensitization pattern.
Electronic supplementary material
The online version of this article (10.1007/s00432-020-03216-8) contains supplementary material, which is available to authorized users.
Keywords: Acute lymphoblastic leukemia, Chemotherapy-induced peripheral neuropathy, Quantitative sensory testing, Small- and large nerve fibers, Pain sensitization
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy in childhood (Siegel et al. (2014)) and hematopoietic stem cell transplantation (SCT), an established curative option for patients with high-risk biology or recurrent disease (Merli et al. 2019). Since survival rates after SCT consistently increased over the last decades, long-term sequelae have become a center of interest (Socie et al. 1999; Wingard et al. 2011; Gooley et al. 2010). Central and peripheral neurological complications are reported in up to 40% of patients undergoing SCT (Brabander et al. 2000).
First-line polychemotherapy of ALL includes neurotoxic components such as vincristine (VCR) and methotrexate (Board 2002; Gomber et al. 2010) and the most common adverse effect is acute chemotherapy-induced peripheral neuropathy (CIPN) affecting up to 85% of patients (Addington and Freimer 2016; Lavoie Smith et al. 2015). Despite recovery in most cases, CIPN is an important long-term sequel compromising sensory and motor function in survivors of pediatric ALL at clinically significant levels (Ness et al. 2012).
Peripheral polyneuropathy is subdivided into damage of large fibers, small fibers and a combination of both. Large-fiber neuropathy (LFN) affects Aα-and Aβ-fibers and is clinically characterized by loss of vibration perception, proprioception and motor control (Misra et al. 2008). Small-fiber neuropathy (SFN) involves the small Aδ- and C-fibers (Hovaguimian and Gibbons 2011) characterized by abnormal sensations of heat or cold, hypersensitivities to heat or cold, paresthesia, allodynia, spontaneous pain and abnormal perception of thermal stimuli or pain (Blackmore and Siddiqi 2017). Central pain sensitization is considered as increased synaptic function within the central nervous system by nociceptive inputs and a result of use-dependent plasticity of homosynaptic and predominantly heterosynaptic potentiation in the spinal cord (Woolf 2011). Inflammation or injury of peripheral nerves may contribute to peripheral pain sensitization (Bishop et al. 2010; Perl et al. 1976). More than half of the survivors of allogenic SCT report at least one chronic health condition, associated with diminished quality of life by impaired physical function (Schultz et al. 2017). Everyday impairments like sheet intolerance, burning feet or sensitive skin are claimed in 50–58.9% of patients with SFN (Bakkers et al. 2014).
Scores assessing symptoms and clinical signs in addition to Nerve Conduction Studies (NCS) are the most accurate diagnostic methods for large fiber CIPN (England et al. 2005). As NCS do not assess small-fiber function and are painful, they are inappropriate for detecting SFN in pediatric patients. Definitive diagnosis of SFN remains a challenge as skin biopsy determining intraepidermal fiber density is the current gold standard for diagnosing SFN (Devigili et al. 2008), but remains reserved for severe SFN causes due to its invasiveness and expense (Blackmore and Siddiqi 2017). Ridehalgh et al. and others examined clinical tests to determine their sensitivity to discover SFN and found that Quantitative Sensory Testing (QST) is a valid assessment to rule out SFN (Ridehalgh et al. 2018; Hansson et al. 2007; Magda et al. 2002), which is easily combined with neurological scoring for CIPN (Blackmore and Siddiqi 2017). QST is a psychophysical tool that covers almost all somatosensory aspects by investigating large- and small-fiber function. In 2006 and further approved in 2016, the German Research Network on Neuropathic Pain (DFNS) published a standardized protocol, which allows the comparison with reference values and reduces bias in children and adolescents (Rolke et al. 2006a,b; Vollert et al. 2016). Its cost-efficiency, non-invasiveness and applicability in children are substantial advantages (Lieber et al. 2018; Blankenburg et al. 2010,2012). QST detects pain sensitization as increased responses to noxious inputs, shifting the sensitivity of pain perception to activation by innocuous stimuli, continued pain after end of stimuli, expanding neuronal receptive fields and sensitizing normal tissue (Woolf 2011).
So far, no studies addressed peripheral neuropathy in survivors of pediatric ALL after SCT. We aimed at identifying (1) especially SFN besides LFN using a questionnaire for symptoms and clinical signs for the assessment of peripheral neuropathy as well as QST, (2) patients with signs of pain sensitization with QST, and (3) clinical risk factors for peripheral neuropathy. We hypothesized to reveal abnormal somatosensory function and pain sensitization in a substantial percentage of survivors of pediatric ALL after SCT.
Patients and methods
The Ethics Committee of Charité-Universitätsmedizin Berlin approved our study (EA2/105/16) in accordance with the Declaration of Helsinki. We identified survivors from the database of our pediatric SCT program at Charité-Universitätsmedizin Berlin.
Patients
We performed a single-center cross-sectional retrospective study with 25 of 84 eligible survivors of pediatric ALL after SCT performed between 2006 and 2016 at our center (Fig. 1). A minimum age of 6 years was obligatory for testing as both, clinical scores for CIPN and QST are validated as of this age (Blankenburg et al. 2010). Exclusion criteria for testing were age > 18 years at SCT, intellectual impairment, no relapse-free survival and < 1 year after SCT to exclude acute conditions of the treatment itself. Median recovery time between SCT and testing was 8.25 years (1.16–19 years). Patients were treated along current active protocols of first-line and SCT protocols for pediatric ALL approved by the German Society of Pediatric Oncology and Hematology (GPOH). Median cumulative vincristine dose was 12.28 mg/m2 (range 0–22.5 mg/m2). Cyclosporine A, prednisone, mycophenolate mofetil, and if needed corticosteroid pulses, were administered. Standard criteria defined acute or chronic Graft versus Host Disease (GvHD) (Filipovich et al. 2005; Martin et al. 2006, 2015). Almost all survivors had experienced acute GvHD. Grade I only affecting the skin was present in 13, grade II affecting skin and gut in 6 and grade III affecting skin, gut and liver in one survivor, respectively. Chronic GvHD was present in 3 survivors and still active during testing in one. Neurological complications during treatment were observed in 5 patients: Convulsions in 2, hemiplegia in one and sinus venous thrombosis in 2 patients. All these had resolved completely.
Fig. 1.
Enrollment of participants. Out of 100 ALL survivors 84 matched the inclusion criteria. Eventually, 25 survivors participated
Testing
We obtained written consent before examination and tested between 09/2016 and 04/2017 each patient for about one hour using standardized conditions as reported elsewhere (Rolke et al. 2006a).
Reduced pediatric-modified Total Neuropathy Score
To test for the presence of chronic pain conditions, all patients were asked to fill out the painDETECT questionnaire (Freynhagen et al. 2016) that we used in our previous study (Lieber et al. 2018). We used a reduced version of the pediatric-modified Total Neuropathy Score (red-pmTNS), consisting in 5 items (questionnaire on motor symptoms, sensory symptoms, autonomic symptoms and examination of muscle function, tendon reflexes) instead of the original 8 items to identify clinically relevant peripheral neuropathy as described elsewhere (Gilchrist and Tanner 2013). Due to double-testing, three items, light touch sensation, pin sensation and vibration, of the original pm-TNS were not included in our red-pmTNS as all 3 items were tested in QST too, but in a different approach when compared to pmTNS (Rolke et al. 2006a,b; Gilchrist and Tanner 2013; Gilchrist et al. 2014). Each category was scored from 0 to 4, with 0 given by no clinical symptoms/signs and four given by worst symptoms/signs. The scale of the red-pmTNS ranged from 0 to 20 points. Higher scores indicated worse severity of peripheral neuropathy. The cut-off score of 5 (16% of maximum score) for definition of clinical relevant peripheral neuropathy in the original pm-TNS was conservatively lowered to 4 (20% of maximum score).
Quantitative sensory testing
For QST, we chose the bilateral dermatomes L5/S1 on the dorsum of the feet since the longer nerve fibers on the lower limb are most likely to be impaired in peripheral neuropathy. QST was performed according to the amended DFNS protocol for children and adolescents identifying small and large fiber abnormalities and pain sensitization (Rolke et al. 2006a,b). For thermal testing, we used the TSA 2001-II (Thermal Sensory Analyzer, Medoc Ltd., Israel). In total, 13 parameters covering all somatosensory modalities were tested: Cold Detection Threshold (CDT), Warm Detection Threshold (WDT), Cold Pain Threshold (CPT), Heat Pain Threshold (HPT), Thermal Sensory Limen (TSL), Paradoxical Heat Sensations (PHS), Mechanical Detection Threshold (MDT), Mechanical Pain Threshold (MPT), Mechanical Pain Sensitivity (MPS), Wind Up Ratio (WUR), Allodynia (ALLO), Vibration Detection Threshold (VDT), Pressure Pain Threshold (PPT). According to Maier et al. we grouped QST results as combinations of losses and gains of somatosensory function (Maier et al. 2010).
Analysis
Results of QST were analyzed according to the standardized protocol using the published reference data for gender, age and body region (Magda et al. 2002; Rolke et al. 2006a; Gilchrist and Tanner 2013) and categorized into z scores as described elsewhere. Statistical analysis was performed using SPSS (version 23).
Results
Clinical characteristics of the survivor cohort
Out of 84 eligible subjects, 25 survivors were assessed (response rate 30%). Our assessed cohort did not differ significantly from non-participants except for an increase of males and patients treated with SCT in first remission (Table 1).
Table 1.
Characteristics of participants and non-participants
| Participants (n = 25) | Non-participants (n= 59) | |
|---|---|---|
| Median age (range) in years | 18 (8–33) | 18 (3–34) |
| Gender | ||
| Male | 19 | 30 |
| Female | 6 | 29 |
| Malignancy | ||
| Precursor-B-ALL | 17 | 46 |
| T-ALL | 6 | 9 |
| Unclassified | 2 | 4 |
| Median age at SCT (range) | 11 (2–18) | 7 (1–18) |
| Source of stem cells | ||
| Bone marrow | 17 | 38 |
| Peripheral blood stem cells | 8 | 21 |
| Donor | ||
| Related | 4 | 22 |
| Unrelated | 21 | 37 |
| Stage of disease | ||
| CR1 | 16 | 18 |
| CR2 | 9 | 36 |
| CR3 | - | 5 |
Ratio male: female of participants was 3.2:1 in comparison to all eligible subjects of 1.4:1. The ratio CR:CR2 of participants was 1.77:1 in comparison to all eligible subjects of 0.74:1
Symptoms and clinical signs of peripheral neuropathy
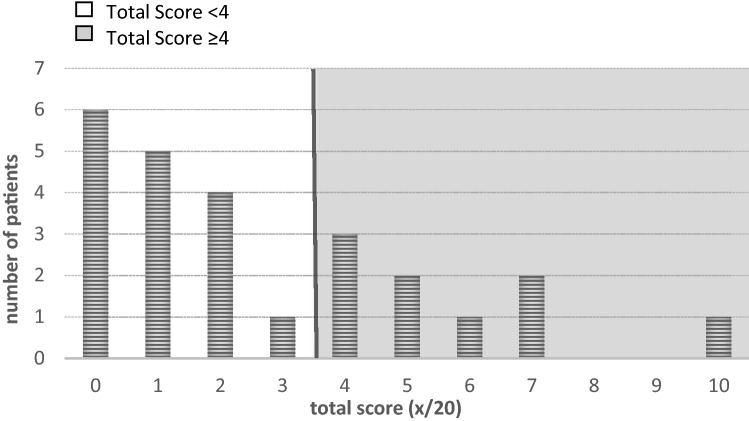
The median of the total reduced pediatric-modified Total Neuropathy Score was 2 (0–10) (Fig. 2). The median was zero for each item, except for autonomic symptoms with a median value of one. Autonomic symptoms were noted in 15 survivors (60%), 8 patients (32%) reported on sensory symptoms, and 3 (12%) on motor symptoms. Clinical signs like reduced muscle strength or loss of deep tendon reflexes were noted in 8 (32%). Nine survivors (36%) scored on or above the cut-off level of 4 in total. All of them reported autonomic symptoms; whereas, the other items were varying. No survivor reported chronic pain conditions in the painDETECT questionnaire.
Fig. 2.
Distribution of total scores in the reduced pediatric-modified Total Neuropathy Score. Total scores of 4 or higher (grey colored background) indicated peripheral neuropathy. y-axis: number of patients; x-axis: total score in red-pmTNS
QST reveals somatosensory deficits and pain sensitization in the survivor cohort
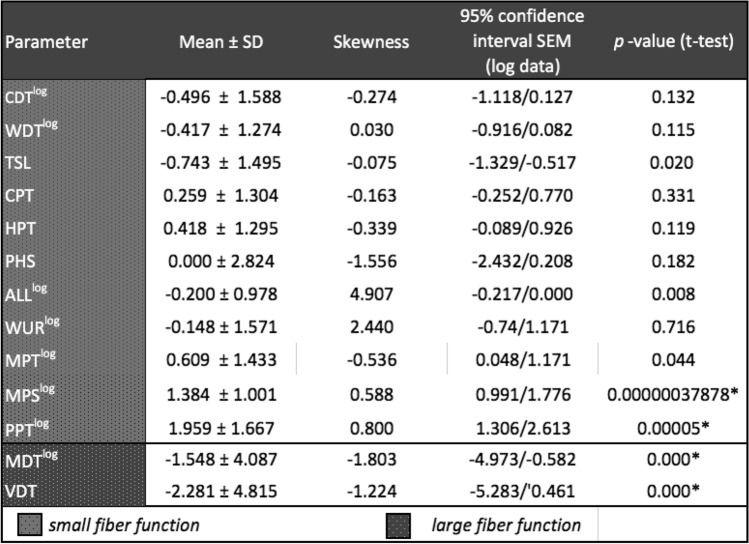
In total, 24/25 (96%) survivors showed at least one significant abnormal parameter (i.e., z-score above or below 1.96) in QST, 17 (68%) at least two and 14 (56%) three or more parameters (Table 2). Nearly, half of the patients had increased thresholds for vibration (14 patients; 56%) and tactile detection (10 patients; 40%). One-quarter of patients had increased thermal detection thresholds for thermal distinction (7 patients, 28%) and cold (2 patients, 8%).
Table 2.
QST-results of survivors for all 13 parameters presented in Z-Scores
p values were analyzed with one-sided t-test for normally distributed parameters and with one-side non-parametric Wilcoxon-test for parameters with a skewness > 1 or < − 1 after transformation. We performed Bonferroni-adjustment for statistical-significance due to multiple testing and defined p < 0.0038 as statically significant
One-third of survivors had decreased thresholds for mechanical nociceptive stimulation regarding pressure (8 patients, 32%) and mechanical pain (6 patients, 24%). Only few survivors showed decreased nociceptive thermal perception for cold (2 patients, 8%) and warm (3 patients, 12%). Nine (32%) survivors showed paradox heat sensations (PHS). A patient was diagnosed with the phenomenon of pain sensitization when showing at least one abnormal finding such as hyperalgesia to pressure, mechanical stimuli, allodynia or when testing wind-up ratio. This meant that the perception of a stimulus that is not painful in the healthy age-matched population was already perceived as painful. Half of our cohort fulfilled this criterium (13/25), i.e., that survivors had at least one abnormal finding in the four above-mentioned tests. Among them, seven survivors showed pain sensitization in one QST parameter, five in two and one in three, respectively.
Isolated positive symptoms like hyperalgesia to thermal or mechanical stimuli, allodynia and PHS were found in 4 patients (16%). Isolated negative symptoms like hypoesthesia and hypoalgesia to thermal or mechanical stimuli were detected in 7 patients (28%). Thirteen survivors (52%) presented a combination of positive and negative symptoms. The median number of abnormalities in QST was 3 (range: 0–7, SD 1.915).
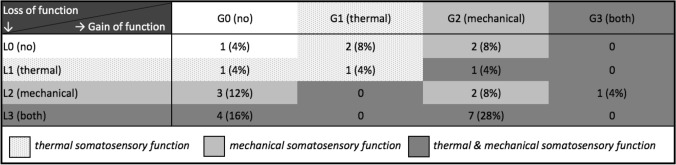
LoGa-Classification of the survivor cohort
When grouped into the LoGa scheme of losses and gains of function, the most frequent somatosensory deficit combination was L3G2 found in 7 patients (28%) (Table 3). Within the L3G2-combination, 5 out of 7 patients (71%) scored a total of 4 or higher in the red-pmTNS.
Table 3.
Frequency of combinations of somatosensory abnormalities
L0 no somatosensory dysfunction; L1 loss of thermal detection; L2 loss of mechanical detection; L3 loss of thermal and mechanical detection; G0 no gain of function (hyperalgesia); G1 thermal hyperalgesia; G2 mechanical hyperalgesia; G3 mixed thermal and mechanical hyperalgesia
The other way around, survivors with pathologic red-pmTNS showed a L3G2-pattern in 30%.
Small and large fiber neuropathy in the survivor cohort
Isolated LFN was shown in 2 patients (8%), whereas 7 patients (28%) revealed a dominant SFN. A combination of LFN and SFN was found in 15 patients (60%).
Risk factors associated with peripheral neuropathy
We identified no significant clinical risk factor for abnormal red-pmTNS score and only one for QST parameters. Using Pearson’s correlation shorter recovery time after SCT was associated with increased sensitivity to pinprick stimuli (Pearson’s correlation coefficient: -0.573, p-value 0.003) and decreased VDT (Pearson’s correlation coefficient: 0.507, p-value 0.009).
Discussion
The red-pmTNS uncovered signs and symptoms of functional impairment in about one out of three survivors. In 2013, Gilchrist introduced the pmTNS and demonstrated its validity and reliability in pediatric cancer patients (Gilchrist and Tanner 2013). In total, 86% and 68% displayed signs and symptoms of CIPN using the pmTNS and the CTCAE criteria at the end of therapy, respectively. When using the CTCAE criteria, about 40% of the children with pathological scores of 5 or higher in the pmTNS were missed (Lavoie Smith et al. 2015; Gilchrist et al. 2014). In our previous study in survivors after pediatric ALL treated with chemotherapy alone (median recovery time 2.5 years), 33% of survivors showed pathological results in red-pmTNS (Lieber et al. 2018) when compared to 36% of the survivors in our cohort (median recovery time 8.25 years). We suggest that SCT does not add clinical signs of PN investigated by scoring systems like red-pmTNS. Genetic variations, i.e., in CYP3A5, may change VCR metabolisms and, therefore, critically influence CIPN development (Egbelakin et al. 2011). Similarly, previous studies using comparable clinical CIPN scores in childhood ALL survivors also identified 27–41% showing peripheral neuropathy (Tay et al. 2017; Jain et al. 2014; Varedi et al. 2018a) and are in line with our observations. On the contrary, Ramchandren et al. uncovered clinical signs of peripheral neuropathy in all survivors 7.4 years after end of therapy using the Neuropathy Impairment Scale (NIS) (Tay et al. 2017). The prevailing motor function within NIS when compared to red-pmTNS may explain the discrepancy. In a longitudinal study, Gilchrist et al. showed high recovery rates with abnormal pmTNS ratings of 85% during treatment and 12%, six months after chemotherapy (Gilchrist et al. 2017). In a long-term follow-up of childhood ALL survivors (median time of 29.9 years since diagnosis), Ness et al. outlined long-lasting negative impacts of antileukemic treatment on neuromuscular function in almost 50% of survivors (Ness et al. 2012): Patients performed poorer in balance, 6-min walking distance, deep tendon reflexes, and vibration detection. Several studies have shown associations between higher scores in pmTNS and poorer performance in either static or dynamic balance or 6-min walking distance (Gilchrist and Tanner 2013; Varedi et al. 2018a). Further studies should investigate these associations by adding performance tests to trial scores.
Thirty-six percent of our cohort showed abnormalities in the red-pmTNS, but 96% had at least one abnormality in QST, highlighting the broad silent LFN and SFN in our survivor group when using the clinical scoring system only. The American Academy of Neurology reported a definition for diagnosing distal symmetric polyneuropathies using combinations of symptoms, clinical signs and electrodiagnostic tests (England et al. 2005; England and Asbury 2004; Gewandter et al. 2019), indicating the use of scoring systems as additional but not sole diagnostic criterion for PN (Cavaletti et al. 2010,2013; Gilchrist 2012).
QST as our main testing modality covers almost all somatosensory functions. When compared to our previous study in ALL survivors treated with chemotherapy only, similar distribution of significant pathologic QST parameters representing large- and small-fiber deficits were found, i.e., Mechanical Detection Threshold, Pressure Pain Threshold and Thermal Sensory Limen (Lieber et al. 2018). The similarity could be due to neurotoxic vincristine that leads to axonal degeneration and was used at varying cumulative doses before undergoing SCT (Gomber et al. 2010; Lavoie Smith et al. 2015; Mora et al. 2016). We detected signs of LFN in about two-thirds of ALL survivors with and without SCT (Lieber et al. 2018). In contrast, signs of SFN were discovered in 88% of the current cohort, compared to only a third ALL survivors having received chemotherapy only (Lieber et al. 2018) indicating additional damage associated with SCT. The similar cumulative doses of VCR in survivors after SCT and chemotherapy only, i.e., 12.3 and 12 mg/sqm, respectively, do not explain the difference. Bilic et al. used QST in a prospective study on adults with chronic GvHD following SCT and found an isolated SFN in 11%, isolated LFN in 15% and mixed SFN and LFN in 67% of survivors. These data corroborate our findings of neuropathy type distribution, i.e., isolated SFN in 28%, isolated LFN in 8% and a mixed SFN and LFN in 60% of survivors, although we could not find any association between GvHD and neuropathy in our study. As chronic GvHD most likely resembles an autoimmune mediated pathology plus reduced intensity conditioning in contrast to myeloablative conditioning was associated with a higher incidence of SFN and peripheral nerve damage following SCT (Hoeijmakers et al. 2012), peripheral neuropathy following SCT may be caused by immune-mediated mechanisms like altered dermal and epidermal immune cell and cytokine composition and keratinocyte activation damaging particularly small fibers (Grauer et al. 2010; Hoeijmakers et al. 2012). Furthermore, immunosuppressive drugs following SCT like Cyclosporine A or Tacrolimus may cause additional nerve damage (Arnold et al. 2013).
Interestingly, QST parameters reflecting pain sensitization like MPS, WUR and ALLO were more pronounced with 50% in the current cohort compared to 30% of ALL survivors with chemotherapy only. Among them, seven showed pain sensitization in one QST parameter, five in two and one in three, respectively. Most importantly, pain sensitization does not imply a chronic pain condition by itself. According to Woolf et al. repetitive Aβ-inputs can trigger hyperalgesia via conditioned C-fibers (Woolf 2011). Also, mechanical and thermal loss of detection may be compensated by reduced thresholds for other stimuli, such as pain, resulting in hyperalgesia (Baron et al. 2017; Simone et al. 1991). In our cohort, 76% of survivors showing symptoms of hyperalgesia also had signs of thermal or mechanical hypoesthesia at the same time. Still, pathomechanisms of SFN and pain sensitization are still unknown (Terkelsen et al. 2017). Previous investigations suggest that elevation of macrophages and mast cells in immune-mediated SFN leads via chemokines and other mediators to microglia cell activation (Marchand et al. 2005; Karl et al. 2019). This peripheral activation together with altered sensory stimuli processing in the CNS may contribute to pain sensitization (Hoeijmakers et al. 2012). Moreover, gene variations in SCN9A encoding Nav1.7 sodium channel which carries out a gain-of-function by increased spontaneous firing and sensitivity to depolarizing stimuli may contribute to additional SFN (Faber et al. 2012). Nevertheless, it is important to keep in mind that survivors were burdened with long hospital stays and may have reacted differently to the QST testing as healthy individuals due to sensitization towards the environment (Vaudre et al. 2005). Still interestingly in a study by Schultz et al., SCT survivors categorized their quality of life in a self-assessment as excellent/very good/good, although more than half of them suffered at least one chronic health conditions (Schultz et al. 2017), highlighting survivors’ changed perception of health and its links.
When categorizing deficit patterns of our current cohort as well as ALL survivors with chemotherapy only into the LoGa-classification, the most frequent somatosensory deficit combinations were L3G2 and L3G0. This is in line with Maier et al. who found patients with central pain sensitization, peripheral neuropathy and peripheral nerve injury most frequently displayed a L3G2 and L3G0 pattern (Maier et al. 2010). The similarity of LoGa-patterns may indicate the same principal underlying cause for somatosensory deficits, i.e., chemotherapy. Still, several studies showed the multifactorial genesis of CIPN, including genetic factors like CYP3A5 metabolism, treatment-related factors like drug concentration, and concomitant treatment with interacting medications like azoles (Egbelakin et al. 2011; Mora et al. 2016; Velde et al. 2017), outlining the difficulty to elucidate CIPN’s underlying pathomechanisms. Among the most affected survivors (L3G2 pattern), almost all reported difficulties in daily living concerning difficulties with zipping zippers, tripping more often, going up the stairs or decreased strength, which underlines the impact on daily living.
Three survivors after chemotherapy only reported chronic pain (Lieber et al. 2018), whereas all survivors after SCT negated it. As our cohort of survivors showed considerably more SFN and pain sensitization, we expected different results. Bakkers et al. showed that 60% of SFN patients suffer burning feet symptoms at least occasionally (Bakkers et al. 2014). Our small sample size of n = 25 may cause our controversial results.
We found a negative correlation between recovery time and severity of hyperalgesia for pinprick stimuli as well as hypoesthesia for vibration. Few studies support our findings of improvement in peripheral nerve function and chronic pain conditions over time following ALL treatment (Jain et al. 2014; Ramchandren et al. 2009; Miltenburg and Boogerd 2014), and yet impairment in peripheral nerve function and chronic pain conditions may still be present years after treatment (Lavoie Smith et al. 2015; Mora et al. 2016; Ramchandren et al. 2009). Treatment options for pediatric cancer survivors suffering from long-term CIPN are understudied. Data on gabapentin and glutamic acids as protective agents against VCR toxicity are inconsistent (Kandula et al. 2016; Anghelescu et al. 2011; Rao et al. 2007).
QST as a psychophysical assessment tool is prone to errors, especially in children as testing lasts for about one hour and attention and collaboration are essential (Rolke et al. 2006a). The participation rate of only 30% of eligible survivors and the shifted gender and clinical remission ratio (see Table 1) may reflect a selection bias. Nevertheless, age- and sex-matched reference data moderated its impact on our results. Our red-pmTNS is another limitation of our study. Still, with our study we aimed to compare our QST results with a clinical score. Survivors were examined after SCT. Hence, differentiation between CIPN prior to SCT or due to SCT is limited. (Lavoratore et al. 2009; Wei et al. 2018). Investigating survivors of SCT receiving no neurotoxic drugs before SCT, e.g., patients with metabolic disorders or hemoglobinopathies may help identifying contributing factors to small and large fiber damage in the SCT setting. So far, studies on survivors of SCT during childhood due to, i.e., sickle cell disease examined long-term central nervous system deficits, but not somatosensory functions (Walters et al. 1995,2010). Here, further longitudinal studies are needed to elucidate the contribution of SCT to long-term neuropathies, particularly SFN. The wide range of recovery time and age at testing among our patients may also affect our findings, as different developmental levels during treatment and at testing may influence this psychophysical assessment (Hirschfeld et al. 2012; Blankenburg et al. 2011). QoL may have given more insights into the clinical relevance of our study (Vetsch et al. 2018; Corella Aznar et al. 2019; Ness et al. 2015; Goebel et al. 2019), but was not measured due to already extensive testing. In future studies, large- and small-fiber neuropathy leading to motor function impairment could be tested by, e.g., 6-min walking test, and timed up-and-go test (Varedi et al. 2018b).
In conclusion, we firstly uncovered that survivors of pediatric acute lymphoblastic leukemia after SCT are at high risk for long-term peripheral neuropathy with a dominating small-fiber and pain sensitization pattern by applying scoring systems and QST as a reliable and valid diagnostic device bearing in mind its limitations. Mechanisms of nerve damage during antileukemic treatment and SCT as well as genetic variations influencing dimensions of sequelae should be the center of interest for future investigations to elucidate the contribution of the varying factors to CIPN in ALL survivors after SCT.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all participants and parents for their effort and commitment to our study.
Abbreviations
- ALL
Acute lymphoblastic leukemia
- Allo
Allodynia
- CDT
Cold detection threshold
- CIPN
Chemotherapy-induced peripheral neuropathy
- CPT
Cold pain threshold
- GvHD
Graft versus host disease
- HPT
Heat pain threshold
- DFGN
German Research Network on Neuropathic Pain
- LFN
Large-fiber neuropathy
- MDT
Mechanical detection threshold
- MPT
Mechanical pain threshold
- MPS
Mechanical pain sensitivity
- NCS
Nerve conduction studies
- QST
Quantitative sensory testing
- pm-TNS
Pediatric-modified Total Neuropathy Score
- PHS
Paradoxical heat sensations
- PPT
Pressure pain threshold
- red-pmTNS
Reduced pediatric-modified Total Neuropathy Score
- SD
Standard deviation
- SFN
Small-fiber neuropathy
- TSL
Thermal sensory limen
- SCT
Stem cell transplantation
- VCR
Vincristine
- VDT
Vibration detection threshold
- WDT
Warm detection threshold
- WUR
Wind-up-ratio
Funding
Open Access funding enabled and organized by Projekt DEAL.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
The original online version of this article was revised due to a retrospective Open Access order.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sascha Lieber and Pablo Hernáiz Driever equally contributed to this manuscript
Change history
8/13/2021
A Correction to this paper has been published: 10.1007/s00432-021-03751-y
References
- Addington J, Freimer M (2016) Chemotherapy-induced peripheral neuropathy: an update on the current understanding. F1000Res. 10.12688/f1000research.8053.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anghelescu DL, Faughnan LG, Jeha S, Relling MV, Hinds PS, Sandlund JT et al (2011) Neuropathic pain during treatment for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 57(7):1147–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R, Pussell BA, Pianta TJ, Lin CSY, Kiernan MC, Krishnan AV (2013) Association between calcineurin inhibitor treatment and peripheral nerve dysfunction in renal transplant recipients. Am J Transplant 13(9):2426–2432 [DOI] [PubMed] [Google Scholar]
- Bakkers M, Faber CG, Hoeijmakers JG, Lauria G, Merkies IS (2014) Small fibers, large impact: quality of life in small-fiber neuropathy. Muscle Nerve 49(3):329–336 [DOI] [PubMed] [Google Scholar]
- Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G et al (2017) Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain 158(2):261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop T, Marchand F, Young AR, Lewin GR, McMahon SB (2010) Ultraviolet-B-induced mechanical hyperalgesia: a role for peripheral sensitisation. Pain 150(1):141–152 [DOI] [PubMed] [Google Scholar]
- Blackmore D, Siddiqi ZA (2017) Diagnostic criteria for small fiber neuropathy. J Clin Neuromuscul Dis 18(3):125–131 [DOI] [PubMed] [Google Scholar]
- Blankenburg M, Boekens H, Hechler T, Maier C, Krumova E, Scherens A et al (2010) Reference values for quantitative sensory testing in children and adolescents: developmental and gender differences of somatosensory perception. Pain 149(1):76–88 [DOI] [PubMed] [Google Scholar]
- Blankenburg M, Meyer D, Hirschfeld G, Kraemer N, Hechler T, Aksu F et al (2011) Developmental and sex differences in somatosensory perception–a systematic comparison of 7- versus 14-year-olds using quantitative sensory testing. Pain 152(11):2625–2631 [DOI] [PubMed] [Google Scholar]
- Blankenburg M, Kraemer N, Hirschfeld G, Krumova EK, Maier C, Hechler T et al (2012) Childhood diabetic neuropathy: functional impairment and non-invasive screening assessment. Diabetes Med 29(11):1425–1432 [DOI] [PubMed] [Google Scholar]
- Board PP (2002) Childhood acute lymphoblastic leukemia treatment (PDQ(R)): health professional version. PDQ cancer information summaries. National cancer institute, Bethesda [Google Scholar]
- Cavaletti G, Frigeni B, Lanzani F, Mattavelli L, Susani E, Alberti P et al (2010) Chemotherapy-induced peripheral neurotoxicity assessment: a critical revision of the currently available tools. Eur J Cancer 46(3):479–494 [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Cornblath DR, Merkies IS, Postma TJ, Rossi E, Frigeni B et al (2013) The chemotherapy-induced peripheral neuropathy outcome measures standardization study: from consensus to the first validity and reliability findings. Ann Oncol 24(2):454–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corella Aznar EG, Ayerza Casas A, Carbone Baneres A, Calvo Escribano MAC, Labarta Aizpun JI, Samper VP (2019) Quality of life and chronic health conditions in childhood acute leukaemia survivors. Med Clin (Barc) 152(5):167–173 [DOI] [PubMed] [Google Scholar]
- de Brabander C, Cornelissen J, Smitt PA, Vecht CJ, van den Bent MJ (2000) Increased incidence of neurological complications in patients receiving an allogenic bone marrow transplantation from alternative donors. J Neurol Neurosurg Psychiatry 68(1):36–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G et al (2008) The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 131(Pt 7):1912–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbelakin A, Ferguson MJ, MacGill EA, Lehmann AS, Topletz AR, Quinney SK et al (2011) Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 56(3):361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- England JD, Asbury AK (2004) Peripheral neuropathy. Lancet 363(9427):2151–2161 [DOI] [PubMed] [Google Scholar]
- England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT et al (2005) Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 64(2):199–207 [DOI] [PubMed] [Google Scholar]
- Faber CG, Hoeijmakers JG, Ahn HS, Cheng X, Han C, Choi JS et al (2012) Gain of function Nanu1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 71(1):26–39 [DOI] [PubMed] [Google Scholar]
- Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al (2005) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transpl 11(12):945–956 [DOI] [PubMed] [Google Scholar]
- Freynhagen R, Tolle TR, Gockel U, Baron R (2016) The painDETECT project-far more than a screening tool on neuropathic pain. Curr Med Res Opin 32(6):1033–1057 [DOI] [PubMed] [Google Scholar]
- Gewandter JS, Gibbons CH, Campagnolo M, Lee J, Chaudari J, Ward N et al (2019) Clinician-rated measures for distal symmetrical axonal polyneuropathy: ACTTION systematic review. Neurology 93(8):346–360 [DOI] [PubMed] [Google Scholar]
- Gilchrist L (2012) Chemotherapy-induced peripheral neuropathy in pediatric cancer patients. Semin Pediatr Neurol 19(1):9–17 [DOI] [PubMed] [Google Scholar]
- Gilchrist LS, Tanner L (2013) The pediatric-modified total neuropathy score: a reliable and valid measure of chemotherapy-induced peripheral neuropathy in children with non-CNS cancers. Support Care Cancer 21(3):847–856 [DOI] [PubMed] [Google Scholar]
- Gilchrist LS, Marais L, Tanner L (2014) Comparison of two chemotherapy-induced peripheral neuropathy measurement approaches in children. Support Care Cancer 22(2):359–366 [DOI] [PubMed] [Google Scholar]
- Gilchrist LS, Tanner LR, Ness KK (2017) Short-term recovery of chemotherapy-induced peripheral neuropathy after treatment for pediatric non-CNS cancer. Pediatr Blood Cancer 64(1):180–187 [DOI] [PubMed] [Google Scholar]
- Goebel AM, Koustenis E, Rueckriegel SM, Pfuhlmann L, Brandsma R, Sival D et al (2019) Motor function in survivors of pediatric acute lymphoblastic leukemia treated with chemotherapy-only. Eur J Paediatr Neurol 23(2):304–316 [DOI] [PubMed] [Google Scholar]
- Gomber S, Dewan P, Chhonker D (2010) Vincristine induced neurotoxicity in cancer patients. Indian J Pediatr 77(1):97–100 [DOI] [PubMed] [Google Scholar]
- Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M et al (2010) Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 363(22):2091–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grauer O, Wolff D, Bertz H, Greinix H, Kuhl JS, Lawitschka A et al (2010) Neurological manifestations of chronic graft-versus-host disease after allogeneic haematopoietic stem cell transplantation: report from the Consensus Conference on Clinical Practice in chronic graft-versus-host disease. Brain 133(10):2852–2865 [DOI] [PubMed] [Google Scholar]
- Hansson P, Backonja M, Bouhassira D (2007) Usefulness and limitations of quantitative sensory testing: clinical and research application in neuropathic pain states. Pain 129(3):256–259 [DOI] [PubMed] [Google Scholar]
- Hirschfeld G, Zernikow B, Kraemer N, Hechler T, Aksu F, Krumova E et al (2012) Development of somatosensory perception in children: a longitudinal QST-study. Neuropediatrics 43(1):10–16 [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JG, Faber CG, Lauria G, Merkies IS, Waxman SG (2012) Small-fibre neuropathies–advances in diagnosis, pathophysiology and management. Nat Rev Neurol 8(7):369–379 [DOI] [PubMed] [Google Scholar]
- Hovaguimian A, Gibbons CH (2011) Diagnosis and treatment of pain in small-fiber neuropathy. Curr Pain Headache Rep 15(3):193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P, Gulati S, Seth R, Bakhshi S, Toteja GS, Pandey RM (2014) Vincristine-induced neuropathy in childhood ALL (acute lymphoblastic leukemia) survivors: prevalence and electrophysiological characteristics. J Child Neurol 29(7):932–937 [DOI] [PubMed] [Google Scholar]
- Kandula T, Park SB, Cohn RJ, Krishnan AV, Farrar MA (2016) Pediatric chemotherapy induced peripheral neuropathy: a systematic review of current knowledge. Cancer Treat Rev 50:118–128 [DOI] [PubMed] [Google Scholar]
- Karl F, Wussmann M, Kress L, Malzacher T, Fey P, Groeber-Becker F et al (2019) Patient-derived in vitro skin models for investigation of small fiber pathology. Ann Clin Transl Neurol 6:1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie Smith EM, Li L, Chiang C, Thomas K, Hutchinson RJ, Wells EM et al (2015) Patterns and severity of vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. J Peripher Nerv Syst 20(1):37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoratore SR, Navarro OM, Grunebaum E, Ali M, Koo A, Schechter T et al (2009) Cyclosporine-induced pain syndrome in a child undergoing hematopoietic stem cell transplant. Ann Pharmacother 43(4):767–771 [DOI] [PubMed] [Google Scholar]
- Lieber S, Blankenburg M, Apel K, Hirschfeld G, Hernaiz Driever P, Reindl T (2018) Small-fiber neuropathy and pain sensitization in survivors of pediatric acute lymphoblastic leukemia. Eur J Paediatr Neurol 22(3):457–469 [DOI] [PubMed] [Google Scholar]
- Magda P, Latov N, Renard MV, Sander HW (2002) Quantitative sensory testing: high sensitivity in small fiber neuropathy with normal NCS/EMG. J Peripher Nerv Syst 7(4):225–228 [DOI] [PubMed] [Google Scholar]
- Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F et al (2010) Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 150(3):439–450 [DOI] [PubMed] [Google Scholar]
- Marchand F, Perretti M, McMahon SB (2005) Role of the immune system in chronic pain. Nat Rev Neurosci 6(7):521–532 [DOI] [PubMed] [Google Scholar]
- Martin PJ, Weisdorf D, Przepiorka D, Hirschfeld S, Farrell A, Rizzo JD et al (2006) National institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-Host Disease: VI. Design of Clinical Trials Working Group report. Biol Blood Marrow Transpl 12(5):491–505 [DOI] [PubMed] [Google Scholar]
- Martin PJ, Lee SJ, Przepiorka D, Horowitz MM, Koreth J, Vogelsang GB et al (2015) National institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-Host disease: VI. The 2014 Clinical Trial Design Working Group Report. Biol Blood Marrow Transpl 21(8):1343–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merli P, Algeri M, Del Bufalo F, Locatelli F (2019) Hematopoietic Stem Cell Transplantation in Pediatric Acute Lymphoblastic Leukemia. Curr Hematol Malig Rep 14(2):94–105 [DOI] [PubMed] [Google Scholar]
- Miltenburg NC, Boogerd W (2014) Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev 40(7):872–882 [DOI] [PubMed] [Google Scholar]
- Misra UK, Kalita J, Nair PP (2008) Diagnostic approach to peripheral neuropathy. Ann Indian Acad Neurol 11(2):89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora E, Smith EM, Donohoe C, Hertz DL (2016) Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res 6(11):2416–2430 [PMC free article] [PubMed] [Google Scholar]
- Ness KK, Hudson MM, Pui CH, Green DM, Krull KR, Huang TT et al (2012) Neuromuscular impairments in adult survivors of childhood acute lymphoblastic leukemia: associations with physical performance and chemotherapy doses. Cancer 118(3):828–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness KK, DeLany JP, Kaste SC, Mulrooney DA, Pui CH, Chemaitilly W et al (2015) Energy balance and fitness in adult survivors of childhood acute lymphoblastic leukemia. Blood 125(22):3411–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl ER, Kumazawa T, Lynn B, Kenins P (1976) Sensitization of high threshold receptors with unmyelinated (C) afferent fibers. Prog Brain Res 43:263–277 [DOI] [PubMed] [Google Scholar]
- Ramchandren S, Leonard M, Mody RJ, Donohue JE, Moyer J, Hutchinson R et al (2009) Peripheral neuropathy in survivors of childhood acute lymphoblastic leukemia. J Peripher Nerv Syst 14(3):184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA et al (2007) Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer 110(9):2110–2118 [DOI] [PubMed] [Google Scholar]
- Ridehalgh C, Sandy-Hindmarch OP, Schmid AB (2018) Validity of clinical small-fiber sensory testing to detect small-nerve fiber degeneration. J Orthop Sports Phys Ther 48(10):767–774 [DOI] [PubMed] [Google Scholar]
- Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A et al (2006a) Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 123(3):231–243 [DOI] [PubMed] [Google Scholar]
- Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F et al (2006b) Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain 10(1):77–88 [DOI] [PubMed] [Google Scholar]
- Schultz KA, Chen L, Kunin-Batson A, Chen Z, Woods WG, Gamis A et al (2017) Health-related Quality of Life (HR-QOL) and Chronic Health Conditions in Survivors of Childhood Acute Myeloid Leukemia (AML) with Down Syndrome (DS): a Report From the Children's Oncology Group. J Pediatr Hematol Oncol 39(1):20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J (2014) Cancer incidence rates and trends among children and adolescents in the United States, 2001–2009. Pediatrics 134(4):e945–e955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, LaMotte RH et al (1991) Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J Neurophysiol 66(1):228–246 [DOI] [PubMed] [Google Scholar]
- Socie G, Stone JV, Wingard JR, Weisdorf D, Henslee-Downey PJ, Bredeson C et al (1999) Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med 341(1):14–21 [DOI] [PubMed] [Google Scholar]
- Tay CG, Lee VWM, Ong LC, Goh KJ, Ariffin H, Fong CY (2017) Vincristine-induced peripheral neuropathy in survivors of childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer. 10.1111/j.1529-8027.2009.00230.x [DOI] [PubMed] [Google Scholar]
- Terkelsen AJ, Karlsson P, Lauria G, Freeman R, Finnerup NB, Jensen TS (2017) The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol 16(11):934–944 [DOI] [PubMed] [Google Scholar]
- Varedi M, Lu L, Howell CR, Partin RE, Hudson MM, Pui CH et al (2018a) Peripheral neuropathy, sensory processing, and balance in survivors of acute lymphoblastic leukemia. J Clin Oncol 36(22):2315–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varedi M, Ness KK, McKenna RF (2018b) Balance deficits in long-term pediatric all survivors. Oncotarget 9(66):32554–32555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudre G, Trocme N, Landman-Parker J, Maout F, Tabone MD, Tourniaire B et al (2005) Quality of life of adolescents surviving childhood acute lymphoblastic leukemia. Arch Pediatr 12(11):1591–1599 [DOI] [PubMed] [Google Scholar]
- van de Velde ME, Kaspers GL, Abbink FCH, Wilhelm AJ, Ket JCF, van den Berg MH (2017) Vincristine-induced peripheral neuropathy in children with cancer: a systematic review. Crit Rev Oncol Hematol 114:114–130 [DOI] [PubMed] [Google Scholar]
- Vetsch J, Wakefield CE, Robertson EG, Trahair TN, Mateos MK, Grootenhuis M et al (2018) Health-related quality of life of survivors of childhood acute lymphoblastic leukemia: a systematic review. Qual Life Res 27(6):1431–1443 [DOI] [PubMed] [Google Scholar]
- Vollert J, Attal N, Baron R, Freynhagen R, Haanpaa M, Hansson P et al (2016) Quantitative sensory testing using DFNS protocol in Europe: an evaluation of heterogeneity across multiple centers in patients with peripheral neuropathic pain and healthy subjects. Pain 157(3):750–758 [DOI] [PubMed] [Google Scholar]
- Walters MC, Sullivan KM, Bernaudin F, Souillet G, Vannier JP, Johnson FL et al (1995) Neurologic complications after allogeneic marrow transplantation for sickle cell anemia. Blood 85(4):879–884 [PubMed] [Google Scholar]
- Walters MC, Hardy K, Edwards S, Adamkiewicz T, Barkovich J, Bernaudin F et al (2010) Pulmonary, gonadal, and central nervous system status after bone marrow transplantation for sickle cell disease. Biol Blood Marrow Transpl 16(2):263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Zhao M, Li Q, Xiao X, Zhu L (2018) Tacrolimus-induced pain syndrome after bone marrow transplantation: a case report and literature review. Transplant Proc 50(10):4090–4095 [DOI] [PubMed] [Google Scholar]
- Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D et al (2011) Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol 29(16):2230–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152(3 Suppl):S2–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.