Abstract
Maternal engineered nanomaterial (ENM) exposure during gestation has been associated with negative long-term effects on cardiovascular health in progeny. Here, we evaluate an epitranscriptomic mechanism that contributes to these chronic ramifications and whether overexpression of mitochondrial phospholipid hydroperoxide glutathione peroxidase (mPHGPx) can preserve cardiovascular function and bioenergetics in offspring following gestational nano-titanium dioxide (TiO2) inhalation exposure. Wild-type (WT) and mPHGPx (Tg) dams were exposed to nano-TiO2 aerosols with a mass concentration of 12.01 ± 0.50 mg/m3 starting from gestational day (GD) 5 for 360 mins/day for 6 nonconsecutive days over 8 days. Echocardiography was performed in pregnant dams, adult (11-week old) and fetal (GD 14) progeny. Mitochondrial function and global N6-methyladenosine (m6A) content were assessed in adult progeny. MPHGPx enzymatic function was further evaluated in adult progeny and m6A-RNA immunoprecipitation (RIP) was combined with RT-qPCR to evaluate m6A content in the 3′-UTR. Following gestational ENM exposure, global longitudinal strain (GLS) was 32% lower in WT adult offspring of WT dams, with preservation in WT offspring of Tg dams. MPHGPx activity was significantly reduced in WT offspring (29%) of WT ENM-exposed dams, but preserved in the progeny of Tg dams. M6A-RIP-qPCR for the SEC insertion sequence region of mPHGPx revealed hypermethylation in WT offspring from ENM-exposed WT dams, which was thwarted in the presence of the maternal transgene. Our findings implicate that m6A hypermethylation of mPHGPx may be culpable for diminished antioxidant capacity and resultant mitochondrial and cardiac deficits that persist into adulthood following gestational ENM inhalation exposure.
Keywords: Environmental exposure, GPx4, mitochondria, N6-methyladenosine, M6A
Introduction
While engineered nanomaterials (ENMs) and advanced materials, have enabled important innovations in biomedical engineering, the increased potential for their interactions with biological tissues warrants assessment of their ability to elicit undesirable toxicological effects (Bommarito Martin, and Fry 2017; Crispi, Miranda, and Gratacoset al. 2018; Pietroiusti et al. 2018). The application of nano-titanium dioxide (nano-TiO2), a commonly utilized ENM, escalated by 300% from 2011–2013 in consumer products (Vance et al. 2015). The primary route of exposure is inhalation, which allows particles to enter the bloodstream and systemically impact the body (Stebounova et al. 2012). The size of these particles (≥1 dimension that is ≤100 nm in diameter), plays a critical role in their ability to access systemic circulation and promote cardiovascular damage (Robichaud et al. 2009; Kessler 2011; Stebounova et al. 2012, Pietroiusti et al. 2018; Kunovac et al. 2020). Nano-TiO2 has been implicated in alterations to cardiovascular hemodynamics and mitochondrial dysregulation (Hathaway et al. 2017; Nichols et al. 2018; Hathaway et al. 2019a). Exposure to ENMs are of particular concern for vulnerable populations such as the developing fetus (Bommarito et al. 2017; Ferrari, Carugno, and Bollati 2019). Although research relating to particulate matter exposure during gestational development and the associated detriments are receiving more attention, therapeutic interventions are limited and the identification of mechanisms responsible for long term effects remain undefined.
Initial studies from our laboratory have determined that maternal ENM inhalation exposure during gestation elicits cardiac contractile dysfunction and bioenergetic disruption in the developing fetus (Kunovac et al. 2019), which are sustained into adulthood (Hathaway et al. 2017; Kunovac et al. 2019). At the fetal stage, the functional deficits were attributed to an increased production of reactive oxygen species (ROS) (marked by hydrogen peroxide (H2O2) levels) and downregulation of mitochondria phospholipid hydroperoxide glutathione peroxidase (mPHGPx) (Kunovac et al. 2019), an antioxidant enzyme that protects cells from oxidative stress. Because mPHGPx scavenges hydroperoxides, limiting diffusion of H2O2 from the mitochondrion into other cellular regions, its expression is critical for decreasing ROS influence on nuclear genome regulation. MPHGPx has the ability to mitigate cardiac dysfunction and bioenergetic dysregulation in several contexts (Hollander et al. 2003; Dabkowski et al. 2008; Baseler et al. 2013), including acute nano-TiO2 exposure (Nichols et al. 2018). However, whether enhanced antioxidant capacity can provide a protective role to fetal and adult offspring following maternal ENM inhalation exposure during gestation remains unexplored.
In a previous study from our laboratory, we reported mitochondrial and cardiac contractile decrements in the fetal stage that were sustained into adulthood following maternal ENM inhalation exposure (Kunovac et al. 2019). Though the mechanism by which these detriments are sustained is unclear, epitranscriptomic modifications resulting from maternal ENM exposure may be contributing to the persistent cardiac mitochondrial dysfunction observed. Of interest was our observation of increased ROS and concomitant diminution of mPHGPx protein expression at the fetal stage which may have been associated with epigenetic reprogramming that occurs following particulate exposure (Bommarito et al. 2017; Janssen et al. 2017; Kietzmann et al. 2017; Tanwar et al. 2017; Stapleton et al. 2018; Ferrari et al. 2019; Kunovac et al. 2019). Specifically, epitranscriptomic mechanisms may be responsible for degradation of mRNA and/or functional deficits in proteins (Engel et al. 2018; Min et al. 2018; Leonardi et al. 2019). N6-methyladenosine (m6A) is the most prevalent post-transcriptional mRNA modification in eukaryotes and has been associated with various pathologies (Ross-Innes et al. 2012; Engel et al. 2018; Zhong et al. 2018; Castellanos-Rubio et al. 2019; Cayir et al. 2019; Linder and Jaffrey 2019). Elevated stress levels have the ability to alter m6A machinery and therefore, modulate transcript stability by adding and removing methylation marks (Shi et al. 2017; Engel et al. 2018; Zhong et al. 2018; Leonardi et al. 2019; Berulava et al. 2020). M6A sites are known to be enriched near stop codons and in 3′-untranslated regions (UTR) of mRNA (Wan et al. 2015), thereby influencing translation (Meyer 2019). Investigation of m6A sites in crucial coding regions of nuclear genome-encoded mitochondrial mRNAs that are pivotal for antioxidant defense maintenance is of particular interest, as it may provide insight into mechanisms that result in the longitudinal outcomes of maternal ENM exposure during gestation.
We hypothesize that altered m6A status of the 3′-UTR of mPHGPx in the hearts of adult offspring contributes to mitochondrial and cardiac functional deficits and that overexpression of mPHGPx (in the mother or pup) can preserves these functional alterations following maternal ENM inhalation exposure. Through the use of differential breeding strategies, we were able to determine that the maternal transgene is protective for the fetus and the benefit is sustained in adult offspring, regardless of whether the progeny also possessed the transgene. Following gestational ENM inhalation exposure, mPHGPx enzymatic activity was entirely preserved only in offspring whose dams were mPHGPx transgenic. M6A methylation was highly enriched in the 3′-UTR of mPHGPx in wild-type (WT) offspring exposed in utero to nano-TiO2, but maternal mPHGPx overexpression prevented this modification. Our findings suggest that enhancing antioxidant defense in the pregnant dam may provide offspring with the most protection from epitranscriptomic remodeling and subsequent long-term cardiovascular repercussions that arise following gestational nano-TiO2 inhalation exposure.
Materials and methods
Animal model
The West Virginia University Animal Care and Use Committee approved all animal studies which conform to the most current National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals (8th edition) manual. Male and female transgenic (mPHGPx (+/+)) and wild-type (mPHGPx (−/−)) mice with a FVB/NJ genetic background were housed in the West Virginia University Health Sciences Center Animal Facility. All animals were given access to a rodent diet and water ad libitum. The mPHGPx transgenic mouse model has been previously described by our laboratory (Dabkowski et al. 2008; Baseler et al. 2013). Briefly, to achieve overexpression of mPHGPx, the gene was placed under the control of the cytomegalovirus promoter, which provides robust transgene expression. Transgene screening was performed on tail clip DNA of 3-week old mice using a qPCR approach in which we probed for mPHGPx using a fluorometric probe (Product no. Mm00515041_m1, Applied Biosystems, Foster City, CA). Increased mPHGPx protein content and improved scavenging ability for phosphatidylcholine hydroperoxide (PCOOH) and hydrogen peroxide (H2O2) in the mPHGPx transgenic mice indicated a functionally active enzyme (Dabkowski et al. 2008). MPHGPx overexpression as an antioxidant protective strategy was tested in multiple contexts utilizing complimentary breeding strategies (Figure 1(A,B)). Because mPHGPx mice are heterozygous transgenic, the transgene status of the donor parent (paternal vs. maternal) impacts the nature of the protective strategy provided to the offspring. The presence of the paternal mPHGPx transgene (Paternal mPHGPx Maternal Exposure (PPME)) enabled a portion of the litter (~50%) to be mPHGPx transgenic positive (Figure 1(A), right panel) and thus presented the opportunity to determine whether fetal mPHGPx transgene presence is protective in utero and adult offspring. The presence of the maternal mPHGPx transgene (Maternal mPHGPx maternal exposure (MPME)) enabled a portion of the litter (~50%) to be mPHGPx transgenic (Tg) as well, but with the added benefit of the maternal mPHGPx transgene (Figure 1(A), left panel). This breeding strategy allowed us to delineate whether mPHGPx transgene presence in the environment provided by the dam is beneficial to the non-transgenic progeny or of additional benefit to transgenic progeny. Pregnancy was verified by identifying the vaginal plug. Approximately 5 days after identification of the vaginal plug (gestational day 5 (GD 5)), the pregnant dams were designated into either sham or nano-TiO2 exposure groups. Echocardiographic assessments were performed on adult offspring (11 weeks old), as well as pregnant dams (12 weeks old) and fetal-stage progeny (GD 14). For the adult offspring study, pups remained with the singly-housed dams until weaning age (19 days) at which point they were housed with littermates and separated by sex, with no more than 5 animals per cage. Adult progeny was euthanized at ~11 weeks of age and hearts were excised for further analyses. From each adult litter, one animal of each genotype (WT/Tg) was randomly selected and designated N = 1, alternating selection of male and female offspring to control for sex as a biological variable. All offspring were genotyped using the qPCR method described above. Pregnant dams were euthanized one day following echocardiographic imaging (GD 15), the pups were removed and tail clips were collected for genotyping. Adult offspring and pregnant dams were euthanized through sedation with 5% isoflurane and subsequent cervical dislocation. A timeline of the study is provided in Figure 1(C).
Figure 1.
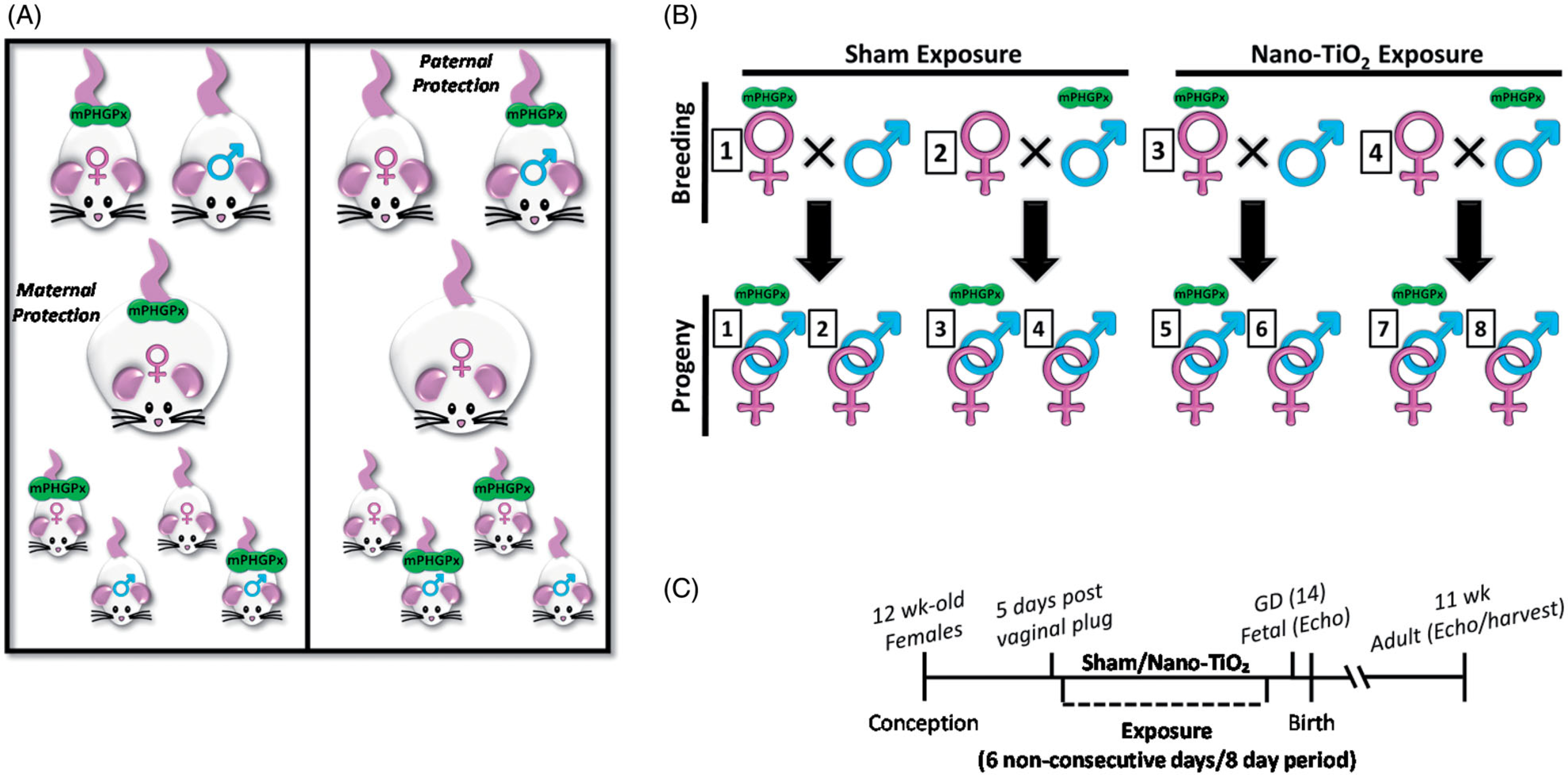
MPHGPx mouse breeding strategy. (A) Schematic of mPHGPx breeding strategies with transgene effect on offspring from maternal (left) or paternal (right) transgene; (B) Schematic of exposure paradigm that was implemented for each group, representing all groups that were utilized in the study; (C) A timeline of the study. MPHGPx: mitochondrial phospholipid hydroperoxide glutathione peroxidase.
Engineered nanomaterial inhalation exposure
The ENM inhalation exposure paradigm utilized in the current study has been previously described (Hathaway et al. 2019a). Nano-TiO2 P25 powder containing anatase (80%) and rutile (20%) TiO2 was purchased from Evonik (Aeroxide TiO2, Parsipanny, NJ) and prepared by drying, sieving, and storing (Nurkiewicz et al. 2008; Knuckles et al. 2012; Hathaway et al. 2019a). The primary particle characteristics have been previously reported including the size (21 nm), the specific surface area (48.08 m2/g), and the Zeta potential (−56.6 mV) (Nurkiewicz et al. 2008; Sager, Kommineni, and Castranova 2008; Nichols et al. 2018). Nano-TiO2 was aerosolized with a high-pressure acoustical generator (HPAG; IEStechno, Morgantown, WV), and has been detailed in previous studies (Hathaway et al. 2019a) involving rodent inhalation exposure. Figure 2 provides data outlining the aerosol characterization of nano-TiO2. In order to recapitulate a lung burden typically seen in a manufacturing setting exposure (Kunovac et al. 2019), a target aerosol mass concentration of 12 mg/m3 of engineered nano-TiO2 was chosen, for 360 min per day for 6 nonconsecutive days over an 8-day period, with a whole-body exposure chamber. This level was based on human equivalent alveolar doses during pregnancy and is explained in more detail later in the manuscript. The real-time TiO2 aerosol mass concentration readings (pDR-1500; Thermo Environmental Instruments Inc., Franklin, MA) over a typical exposure day were sampled from an exposure chamber (Figure 2(A)) and verified by gravimetric measurements during each exposure. Final gravimetric measurements indicated a daily 360-min equivalent average mass concentration of 12.01 ± 0.50 mg/m3. A high-resolution electrical low-pressure impactor (ELPI+; Dekati, Tampere, Finland), a scanning particle mobility sizer (SMPS 3938; TSI Inc., St. Paul, MN), and a Nano Micro-Orifice Uniform Deposit Impactor (MOUDI 115 R; MSP Corp, Shoreview, MN) were used to measure the size of the nano-TiO2 aerosols. A log-normal fit of the data from the ELPI + indicated a geometric count median diameter (CMD) of 0.163 μm with a geometric standard deviation (GSD) of 1.77 (Figure 2(B)). A log-normal fit of the data from the SMPS indicated a CMD of 0.190 μm with a GSD of 1.97 (Figure 2(C)). A log-normal fit of the data from the MOUDI indicated a mass median aerodynamic diameter (MMAD) of 0.968 μm with a GSD of 2.56 (Figure 2(D)). Transmission and scanning electron micrographs (TEM and SEM) of nano-TiO2 aerosolized particles, sampled from the exposure chamber have been previously published (Kunovac et al. 2019). The dose required to match the appropriate lung deposition was calculated based on the previously described mouse methodology modified to reflect alveolar deposition only (Nichols et al. 2018). The formula D = F × V × C × T, where F is the alveolar deposition fraction (4.40%) ((Multiple Path Particle Dosimetry Model (MPPD v3.04)) (Anjilvel and Asgharian 1995), V is the minute ventilation based on body weight (36.4 ml) (Bide, Armour, and Yee 1997), C is the mass concentration (12.01 mg/m3) and T is the exposure duration (6 h), was employed (Nurkiewicz et al. 2008; Stapleton et al. 2012). This resulted in a daily deposited nano-TiO2 alveolar dose of 6.92 μg (total six exposure dose = 41.55 μg). When this alveolar burden is normalized with respect to alveolar surface area (mousesa = 0.05 m3, humansa = 102 m3) and matched to human breathing the same aerosol, it corresponds to a human alveolar deposition of 84.76 mg. This is equivalent to an approximately 79-day exposure at the NIOSH recommended exposure limit (REL) of 2.4 mg/m3 for fine TiO2 established in 2011 (Department of Health and Human Services, 2011) (F = 12.24% (MMPD), V = 7.6 L). Since factory workers are exposed to airborneTiO2 levels of ~0.65 mg/m3 (Pelclova et al. 2015) and the human gestational period is 9 months, we feel that our exposure concentration levels are highly relevant to the female worker population. To maintain a comfortable humidity during the exposure, bedding material was soaked in water and placed in the exposure chamber. Control animals (sham) were exposed to HEPA filtered air, rather than nano-TiO2, in a designated chamber with similar chamber conditions. The final exposure was administered 48 h prior to sacrifice and tissue harvesting.
Figure 2.
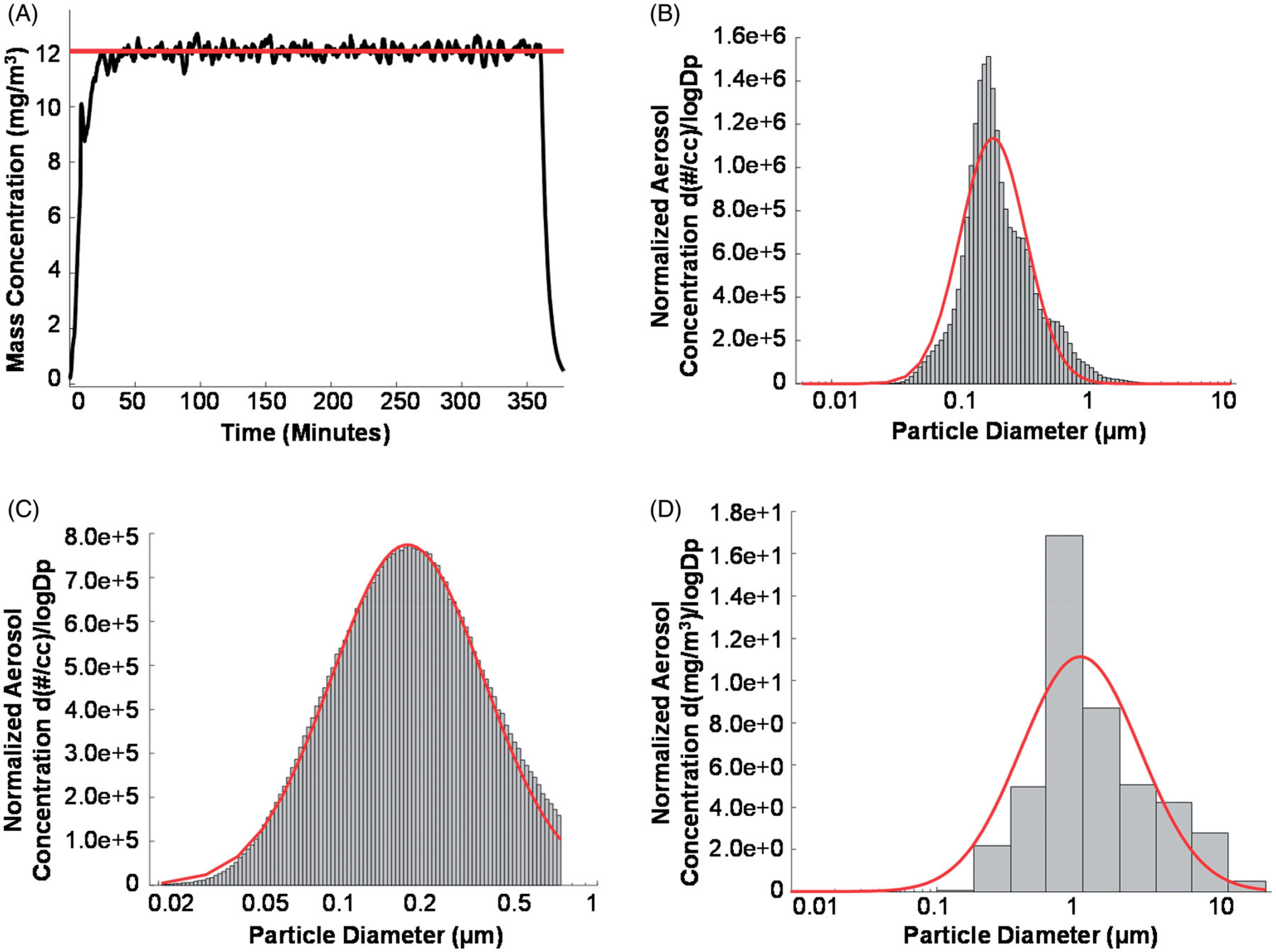
Maternal nano-TiO2 inhalation exposure characteristics. (A) Real-time (black line) aerosol mass concentration measurements with the red line indicating the target concentration (12 mg/m3) of a typical 360 min maternal nano-TiO2 inhalation exposure; (B) Count size distribution of the nano-TiO2 aerosols measured with a high resolution electric low-pressure impactor (ELPI+). The red line designates the log normal distribution obtained with the log probability plot method (CMD = 0.163 μm, with a GSD = 1.77); (C) Count size distribution of nano-TiO2 aerosols measured with a scanning mobility particle sizer (SMPS) with the red line representing a log normal fit of the data (CMD = 0.190 μm, GSD = 1.97); (D) Mass size distribution of nano-TiO2 aerosols measured with a nano micro-orifice uniform deposit impactor (MOUDI). The red line represents a log normal fit of the data indicating a mass median aerodynamic diameter (MMAD) of 0.968 μm and a GSD of 2.56. CMD: count median diameter; GSD: geometric standard deviation.
Echocardiography
Cardiac contractile function was assessed in adult offspring (11 weeks old) using the Vevo2100 High-frequency Ultrasound system (Visual Sonics, Toronto, Canada) to obtain Motion mode (M-mode) images as previously described (Kunovac et al. 2019). Anesthesia was induced using inhalant isoflurane, which was then maintained at ~1.5% in order to achieve a physiologically relevant heart rate. Images were acquired with a linear array transducer at 32–40 MHz, with a frame rate of 233–401 frames/s. M-mode images were also obtained for pregnant dams (12 weeks old) and their fetal progeny (GD 14). For fetal echocardiographic imaging, pups in the right and left uterine horns were imaged sequentially, which allowed us to correlate the assessed cardiac function with the genotype of that specific pup. For adult offspring analyses, one animal of each genotype (WT/Tg) from each exposed (sham/nano-TiO2) dam (PPME (WT mom) or MPME (Tg mom) was selected at random. Measurements were calculated over three cardiac cycles and averaged.
Speckle-tracking-based strain evaluations were performed using parasternal long and short axis B-mode images as previously described by our laboratory (Nichols et al. 2015; Shepherd et al. 2016; Hathaway et al. 2017; Kunovac et al. 2019). A speckle-tracking algorithm in Visual Sonics VevoStrain software (Toronto, Canada) was employed to trace the endocardium and epicardium walls. Data were analyzed for three cardiac cycles using time-to-peak analysis for curvilinear data. Measures of systolic strain including deformation, strain rate, displacement, and velocity were obtained. For adult progeny, speckle-tracking was performed on both long- and short-axis images. For fetal progeny, speckle-tracking was performed on short-axis images. All echocardiographic measurements were acquired by one analyst blinded to the animal exposure group and genotype.
Electron transport chain (ETC) complex activities
ETC Complex activities (I, III, IV, and V) were measured in hearts of adult offspring as previously described (Barrientos, Fontanesi, and Diaz 2009; Baseler et al. 2013; Hathaway et al. 2017). Whole tissue was homogenized using the Polytron PowerGen 500 S1 tissue homogenizer (Fisher Scientific, Hampton, NH) in NP-40 buffer (20 mM Tris, 137 mM NaCl, 10% Glycerol, 1% Triton x100, 2 mM EDTA) (Hathaway et al. 2019b). Samples were centrifuged for 10 min at 10 000 xg (4 °C) and the supernatant was used for the activity assays. The protein homogenates were used to measure activities of ETC complexes I, III, IV, and V (ATP synthase). ETC complex I and III activities were determined by measuring the reduction of decylubiquinone (I) and cytochrome c (III) in cardiac protein lysate of adult offspring. ETC complex IV activity was determined by measuring the oxidation of reduced cytochrome c, while complex V activity was determined by measuring oligomycin-sensitive ATPase activity through pyruvate kinase and phosphoenolpyruvate in cardiac protein lysate of adult offspring. The Molecular Devices Flex Station 3 Multi-Mode microplate reader (Sunnyvale, CA) was used to measure all assays spectrophotometrically. Protein content was normalized using the Bradford method, with bovine serum albumin protein assay standards. Final values were expressed as Unit/nanogram (I-IV) or milligram (V) of protein, where Unit = nanomoles of substrate oxidized (minute−1).
Total glutathione peroxidase (GPx) activity
Total GPx activity was measured using a GPx Activity Kit from Cayman Chemical (item no. 703102, Ann Arbor, MI). The Cayman Chemical GPx Activity Kit determines all glutathione-dependent peroxidase activity indirectly through a coupled reaction with glutathione reductase (GR). The cumene hydroperoxide in this assay is reduced by GPx producing oxidized glutathione (GSSG), which can then be recycled to its reduced state by GR and NADPH. The oxidation of NADPH results in a decrease in absorbance at 340 nm that is proportional to the GPx activity when it is the rate-limiting factor. The experiment was carried out using the manufacturer’s protocol, with minor modifications: Heart lysate (in NP-40) samples were diluted with sample buffer and 20 μl of the diluted heart lysate was used for the assay. Absorbance was read once every minute for 6 min at 340 nm using the Molecular Devices Flex Station 3 Multi-Mode microplate reader. Absorbance values were retrospectively normalized to protein content with the Bradford assay (Bradford 1976).
MPHGPx activity
To determine mitochondrial phospholipid hydroperoxide glutathione peroxidase (GPx4) activity, phosphatidylcholine hydroperoxide (PCOOH), a specific substrate of mPHGPx, was synthesized as previously described (Stolwijk et al. 2020). l-α-Phosphatidylcholine Type III/S (PC) (item no. P3782; Sigma-Aldrich, St. Louis, MO) was slowly added to a Tris/Base buffer (0.2 M, pH 8.8, 3 mM sodium deoxycholate (item no. D6750, Sigma-Aldrich)) while stirring at medium-high speed. Once a cloudy emulsion of small droplets was visible, additional Tris/Base buffer was introduced with continuous stirring until the solution was clear. A volume of soybean lipoxidase Type V (item no. L6632, Sigma-Aldrich) was added that equaled 250 000 U to initiate the synthesis reaction followed by continuous stirring for 1 h. A Sep-Pak C-18 cartridge (item no. 020515, Waters, Milford, MA) was activated with methanol and equilibrated with ddH2O, then used to purify the PCOOH with a 30 ml glass syringe (item no. Z314374, Sigma-Aldrich). The cartridge was washed with water to remove water-soluble substances and the PCOOH was eluted from the C18 resin with methanol. The concentration of PCOOH was determined at A234 using the NanoDrop ND-100 (Thermo Fisher Scientific, Waltham, MA) and diluted to the desired concentration with methanol. The PCOOH was immediately used as the substrate (in place of cumene hydroperoxide) with the Glutathione Peroxidase Assay Kit (Cayman) as described above, with absorbance readings every 30 s for 6 min at 340 nm with the Molecular Devices Flex Station 3 Multi-Mode microplate reader. All samples were blanked with background wells (no sample) and normalized to protein content.
Mitochondrial isolation
Adult progeny were sacrificed at 11 weeks of age, and hearts were excised through a midsagittal cut in the thoracic cavity. Isolation of mitochondrial subpopulations was achieved through differential centrifugation as previously described (Palmer, Tandler, and Hoppel 1977; Baseler et al. 2013). The two subpopulations were combined and utilized as a total mitochondrial fraction for subsequent analyses. KME buffer (100 mM KCl, 50 mM MOPS and 0.5 mM EGTA pH 7.4) was used to resuspend isolated mitochondria. Protein concentrations were determined using the Bradford method with bovine serum albumin as a standard (Bradford 1976).
Hydrogen peroxide (H2O2) production
Hydrogen peroxide (H2O2) production was evaluated in total tissue lysate and isolated mitochondria from adult offspring in order to determine mitochondrial ROS production (Starkov 2010; Nichols et al. 2018; Kunovac et al. 2019). The Invitrogen™ Amplex™ Red Hydrogen Peroxide Assay Kit (product no. A2218; Thermo Fisher) was utilized per the manufacturer’s instructions. In the presence of horseradish peroxidase (HRP), the Amplex™ Red reagent produces a red fluorescent oxidation product when the reagent reacts with H2O2. The oxidation product, resorufin, was detected spectrophotometrically at 560 nm and data were normalized to protein content.
Global M6A methylation
Total RNA was purified from heart samples that were digested with QIAzol lysis reagent (item no. 79306, Qiagen, Hilden, Germany) from one adult offspring of each genotype (WT/Tg) from each dam (PPME/MPME) from each exposure group (sham/nano-TiO2) using the miRNeasy Mini Kit (item no. 217004, Qiagen). Global m6A methylation was quantified in total RNA using the EpiQuik™ m6A RNA Methylation Quantification Kit (item no. P-9005; EpiGentek, Farmingdale, NY) per manufacturer’s instructions. Briefly, total RNA samples were diluted to a standard concentration of 200 ng with IDTE buffer. Dilution of the provided positive control allowed for the generation of a standard curve. RNA was bound to the assay wells through incubation with the binding solution. An optimized m6A capture antibody was added to the wells, incubated, and washed, followed by the addition of the detection antibody and enhancer solution. The color developing solution was then introduced and incubated for 2 min, until the color changed to blue, at which point the stop solution was added. Absorbance was read at 450 nm using the Molecular Devices Flex Station 3 Multi-Mode microplate reader. A standard curve was used to determine global m6A levels in each sample.
Immunoprecipitation (m6A-RIP)
Total RNA was purified from whole heart samples from PPME and MPME WT adult offspring from each exposure group (sham/nano-TiO2) using the miRNeasy Kit (Qiagen) described above.
RNA fragmentation
Before the immunoprecipitation was carried out, the RNA samples were fragmented using the NEBNext Magnesium RNA Fragmentation kit (item no. E6150s; New England Biolabs) (Zeng et al. 2018; Barros-Silva et al. 2020). To determine the necessary length of incubation to achieve desired fragmentation (~130 nt), samples were incubated for 1–10 min at 94 °C. The 10 samples were analyzed with the Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA) to determine the size distribution of the fragments.
M6A-RIP
The EpiMark N6-Methyladenosine Enrichment Kit (item no. E1610S; New England Biolabs, Ipswich, MA) was used to enrich m6A modified RNA per manufacturer’s instructions, with minor modifications. RNA samples were fragmented at 94 °C for 3 min (Barros-Silva et al. 2020). Dynabeads™ Protein G magnetic beads from Thermo Fisher Scientific (product no. 10003 D) were washed and resuspended in Reaction Buffer. An anti-N6-Methyladenosine (m6A) rabbit monoclonal antibody (product no. 56593, Cell Signaling Technology, Danvers, MA) was bound to the beads, followed by the addition of the fragmented total RNA (3.7 μg) and RNasin ® Plus RNase Inhibitor (product no. N2611; Promega, Madison, WI). The normal Rabbit IgG control (product no. 2729, Cell Signaling Technology), an unconjugated rabbit polyclonal antibody, was used as a nonspecific IgG control for the immunoprecipitation. After the immunoprecipitation, RNA was eluted with Buffer RLT (product no. 79216; Qiagen) then cleaned and concentrated using Dynabeads™ MyOne™ Silane (product no. 37002 D; Life Technologies, Carlsbad, CA). The RNA samples from both input and immunoprecipitated samples were examined by RT-qPCR to quantify m6A enrichment.
RT-qPCR
WT adult offspring heart samples (from WT/Tg and sham/nano-TiO2 exposed dams) were prepared for downstream analysis by reverse-transcribing the input and immunoprecipitated RNA using the Applied Biosystems High-Capacity cDNA Reverse Transcription Kit (product no. 4368814; Thermo Fisher Scientific) per manufacturer’s protocol. RT-qPCR was utilized to evaluate m6A enrichment of the mPHGPx 3′-UTR, specifically the region containing the crucial selenocysteine (Sec) insertion sequence (SECIS) (Ingold et al. 2018; Castellanos-Rubio et al. 2019). Primers for mPHGPx and a control, GAPDH, were designed using Primer3Plus (Baseler et al. 2011) and the sequences are listed in Table S1 (Additional file 1). Quantification was achieved with SYBR® Green master mix (product no. A25742, Thermo Fisher Scientific) using the Applied Biosystems 7500 Fast Real-Time PCR system. Samples were run in triplicate. The enrichment fold was calculated using the ΔCt value of the IP normalized to the ΔCt of the input, relative to the dilution factor (20% input; dilution factor = 5), and normalized to GAPDH, as illustrated below (Marmisolle, Garcia, and Reyes 2018).
Statistics
All statistical analyses were performed using GraphPad Prism Software Version 8 for Windows (GraphPad Software, La Jolla CA). For adult offspring analyses, from each MPME or PPME cohort, one animal of each genotype (WT/Tg) from a given exposure (sham and nano-TiO2) was randomly selected and designated N = 1. For fetal analyses, each pregnant dam that was exposed (sham/nano-TiO2) was considered one observation, with each genetic group (WT/Tg) considered as N = 1. Data were analyzed using a two-way analysis of variance (ANOVA) method to evaluate mPHGPx presence and nano-TiO2 maternal exposure. Normality was determined using the D’Agostino-Pearson test. Tukey’s multiple comparison method was performed following the two-way ANOVA to evaluate effects within the PPME and MPME groups. Statistical difference was defined by p ≤ 0.05. The presence of a letter above a specific group denotes statistical significance between those two groups as determined by Tukey’s multiple comparisons test. For functional assays performed using adult hearts, a two-tailed Student’s t-test was also employed to evaluate whether maternal nano-TiO2 inhalation exposure during gestation elicited change between offspring of the same genotype. The presence of a dagger (†) above a given group indicates statistical significance between WT Sham and WT TiO2 or Tg Sham and Tg TiO2 of the same maternal genetic group (MPME or PPME). For qPCR data, a one-way ANOVA was employed followed by Tukey’s multiple comparisons test to determine significant differences in m6A fold enrichment across WT offspring groups. All data are presented as the mean ± the standard error of the mean (SEM).
Results
Cardiac function
Deleterious changes in cardiac structure and function have previously been identified in offspring following maternal nano-TiO2 inhalation exposure during gestation (Hathaway et al. 2017; Kunovac et al. 2019). Conventional M-mode evaluations determined that adult offspring of WT dams (PPME) who were gestationally exposed to nano-TiO2 presented with higher systolic diameter and volume, as well as lower ejection fraction and fractional shortening than those whose dams were sham-exposed (Table 1). Additionally, adult offspring whose dams were mPHGPx Tg (MPME) overall had significantly lower diastolic diameter and volume, stroke volume, and cardiac outputs than PPME offspring regardless of exposure (Table 1). Adult offspring heart rates were unchanged regardless of exposure or genotype (Additional file 1: Table S2).
Table 1.
M-mode echocardiography for adult progeny.
| Parameter | PPME WT Shama |
PPME WT TiO2b |
PPME Tg Shamc |
PPME Tg TiO2d |
MPME WT Shame |
MPME WT TiO2f |
MPME Tg Shamg |
MPME Tg TiO2h |
|---|---|---|---|---|---|---|---|---|
| Diameter; s (mm) | 0.61 ± 0.05 | 0.85 ± 0.08 a,d | 0.66 ± 0.07 | 0.51 ± 0.06 | 0.63 ± 0.05 | 0.60 ± 0.02 | 0.57 ± 0.02 | 0.72 ± 0.06 |
| Diameter; d (mm) | 2.12 ± 0.04 | 2.27 ± 0.08 | 2.15 ± 0.09 | 2.07 ± 0.07 | 1.98 ± 0.08 | 1.98 ± 0.07 | 1.88 ± 0.06 | 2.07 ± 0.07 |
| Volume; s (uL) | 0.58 ±0.13 | 1.47 ± 0.35 a,c,d | 0.73 ± 0.19 | 0.38 ± 0.11 | 0.60 ± 0.11 | 0.56 ± 0.02 | 0.46 ± 0.05 | 0.94 ± 0.24 |
| Volume;d (uL) | 14. 78 ± 0.61 | 17.85 ± 1.66 | 15.63 ± 1.56 | 14.09 ± 1.25 | 12.61 ± 1.31 | 12.65 ± 1.11 | 11.00 ± 0.92 | 14.21 ± 1.31 |
| Stroke volume (uL) | 14. 19 ± 0.63 | 16.38 ± 1.34 | 14.90 ± 1.43 | 13.71 ±1.16 | 12.01 ± 1.21 | 12.08 ± 1.12 | 10.54 ± 0.87 | 13.27 ± 1.50 |
| Ejection fraction (%) | 96.02 ± 0.94 | 92.22 ± 1.29 a,d | 95.55 ± 0.91 | 97.48 ± 0.59 | 95.34 ± 0.41 | 95.43 ± 0.53 | 95.82 ± 0.13 | 93.35 ± 2.30 |
| Fractional shortening (%) | 71.25 ±2.37 | 62.99 ± 2.24 a,d | 69.84 ± 2.37 | 75.72 ± 2.37 | 68.64 ± 1.24 | 69.64 ± 1.92 | 69.52 ± 0.35 | 65.40 ± 4.27 |
| Cardiac output (mL/min) | 10.08 ± 0.56 | 10.32 ± 0.77 | 9.67 ± 1.07 | 9.06 ±0.81 | 7.60 ± 1.44 | 8.00 ± 0.71 | 6.80 ± 0.78 | 9.19 ± 1.20 |
| Relative wall thickness | 1.23 ± 0.06 | 1.20 ± 0.06 | 1.17 ± 0.12 | 1.36 ± 0.06 | 1.18 ± 0.06 | 1.27 ± 0.06 | 1.35 ± 0.05 | 1.15 ± 0.10 |
M-mode echocardiography was performed in adult offspring (11 weeks of age) following maternal inhalation exposure. Bold text indicates significant data defined by p ≤ 0.05. A two-way ANOVA determined statistical difference between PPME and MPME denoted with underlined values for that parameter. A letter next to a given group denotes statistical significance between the marked group and the group represented by each letter based on a Tukey’s multiple-comparisons test. All data are presented as the mean ± the standard error of the mean (SEM). PPME WT Sham, n = 7; PPME WT TiO2, n = 6; PPME Tg Sham, n = 6; PPME Tg TiO2, n = 5; MPME WT Sham, n = 4; MPME WT TiO2, n = 5; MPME Tg Sham, n = 5; MPME Tg TiO2, n = 4.
PPME: paternal mPHGPx maternal exposure; MPME: maternal mPHGPx maternal exposure; WT Sham: wild-type offspring whose dam was exposed to control air; Tg Sham: mPHGPx transgenic offspring whose dam was exposed to control air; WT TiO2: wild-type offspring whose dam was exposed to nano-TiO2; Tg TiO2: mPHGPx transgenic offspring whose dam was exposed to nano-TiO2; s: systolic; d: diastolic.
When examining the dams, WT nano-TiO2-exposed mice had significantly decreased diastolic diameter and volume when compared to WT sham-exposed dams, as well as reduced pump function indicated by stroke volume and cardiac output reductions (Additional file 1: Table S3). Furthermore, relative wall thickness was significantly increased following ENM exposure in WT dams, but not in the mPHGPx Tg dams (Additional file 1: Table S3). Maternal heart rate remained unchanged between all exposure groups (Additional file 1: Table S4). At the fetal stage, in order to correlate the assessed cardiac function with the genotype of that specific pup, pups in the right and left uterine horns were sequentially imaged (Figure 3(A)). A representative trace of the fetal pup’s left ventricle (LV) in M-mode is shown in Figure 3(B). A group-dependent effect was found, with the MPME fetal offspring demonstrating higher diastolic diameter and volume, as well as higher stroke volume and cardiac output than the PPME offspring (Additional file 1: Table S5). MPME fetal offspring also had significantly higher heart rates (Additional file 1: Table S2). Decreased function in the PPME WT offspring following gestational ENM exposure confirms previous findings (Kunovac et al. 2019) and substantiates the susceptibility of WT offspring to cardiovascular changes due to maternal nano-TiO2 exposure, which may be a result of limited antioxidant capacity.
Figure 3.

Assessment of fetal pup cardiac function in utero. (A) Graphical depiction of the uterine horn and the pup identification system; (B) Representative M-mode echocardiographic scan of a fetal pup with the short-axis left ventricular trace (blue); (C–D) Representative B-mode scan of a fetal pup with the left and right ventricles identified by blue arrows at end diastole (C) and end systole (D); (E–F) Representative B-mode scan in the short-axis of a fetal pup at with green outlines indicating tracking of the epicardium and endocardium borders through (E) end diastole and (F) end systole. R: right; L: left.
Speckle-tracking-based stress strain assessments were performed during systole in fetal and adult offspring groups of all dams (Table 2 and Additional file 1: Tables S6,7). Long-axis measurements in adult offspring demonstrated that endocardial global longitudinal strain (GLS) was significantly lower in PPME WT offspring whose dams were exposed to nano-TiO2 compared to sham-exposed (Table 2). The PPME WT pups also had a significantly lower GLS than PPME Tg adults, who were both progeny of nano-TiO2 exposed dams. (Table 2). A group-dependent effect was seen with MPME progeny having higher radial and longitudinal strain rates, and diminished longitudinal displacement compared to PPME progeny. Group-dependent effects were also observed in the parasternal short-axis between the MPME (Additional file 1: Table S6). Figures 3(C) and D provide representative B-mode images of a fetal pup in utero during end diastole and end-systole, respectively, as well as speckle-tracking traces correlating to each of these frames (Figure 3(E,F)). At the fetal stage, short-axis radial and circumferential strain rates were significantly lower in both the PPME WT and Tg groups whose dams were exposed to nano-TiO2 than the pups of the same respective genotype whose dams were sham-exposed (Additional file 1: Table S7). Further, these data also revealed a group-dependent effect based on maternal genotype (MPME vs. PPME). Overall, these changes appear to suggest that enhanced maternal antioxidant protection may provide sufficient protection to thwart cardiac maladaptation that is precipitated by ENM exposure.
Table 2.
Adult progeny systolic stress-strain in the long-axis.
| Parameter | Units | PPME WT Shama |
PPME WT TiO2b |
PPME Tg Shamc |
PPME Tg TiO2d |
MPME WT Shame |
MPME WT TiO2f |
MPME Tg Shamg |
MPME Tg TiO2h |
|---|---|---|---|---|---|---|---|---|---|
| Radial velocity | Pk cm/s | 2.01 ±0.17 | 1.89 ± 0.12 | 1.82 ±0.10 | 2.52 ± 0.16 c | 2.03 ± 0.21 | 2.46 ± 0.23 | 2.23 ± 0.15 | 2.08 ± 0.26 |
| Radial displacement | Pk mm | 0.47 ± 0.03 | 0.43 ± 0.03 | 0.44 ± 0.04 | 0.61 ± 0.02 a,b,c | 0.40 ± 0.00 | 0.55 ± 0.04 | 0.47 ± 0.05 | 0.52 ± 0.03 |
| Radial strain | Pk % | 27.82 ± 1.84 | 25.95 ± 2.85 | 25.54 ± 3.37 | 31.55 ± 2.74 | 26.48 ± 2.58 | 32.90 ± 2.72 | 30.69 ± 3.08 | 23.67 ± 1.49 |
| Radial strainrRate | Pk 1/s | 9.10 ±0.83 | 8.20 ± 0.67 | 8.62 ± 1.13 | 11.12 ± 1.16 | 11.34 ± 1.55 | 11.80 ± 1.02 | 11.31 ± 0.34 | 8.50 ± 0.97 |
| Longitudinal velocity | Pk deg/s | 1.14 ± 0.16 | 1.19 ± 0.18 | 1.00 ± 0.18 | 1.07 ± 0.20 | 1.16 ± 0.36 | 1.09 ± 0.28 | 1.28 ± 0.60 | 0.95 ± 0.15 |
| Longitudinal displacement | Pk deg | 0.18 ± 0.01 | 0.12 ± 0.04 | 0.15 ± 0.06 | 0.11 ± 0.02 | 0.04 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.12 ± 0.04 |
| Longitudinal strain | Pk % | −10.99 ± 2.13 | −9.51 ± 2.00 | −9.16 ± 0.90 | −15.81 ± 3.41 | −5.38 ± 2.61 | −7.98 ± 1.52 | −12.99 ± 2.78 | −10.68 ± 4.73 |
| Longitudinal strain rate | Pk 1/s | −7.63 ± 0.58 | −6.86 ± 0.76 | −7.06 ± 1.01 | −9.34 ± 0.76 | −7.45 ± 0.85 | −8.93 ± 1.35 | −14.26 ± 1.35 e,f,h | −7.49 ± 1.18 |
| Global longitudinal strain (GLS) | AU | 24.26 ± 1.54 | 16.49 ± 1.97 a,d | 19.85 ± 2.20 | 28.51 ± 1.88 c | 20.07 ± 2.25 | 23.58 ± 3.52 | 22.67 ± 1.86 | 22.29 ± 2.44 |
Peak strain and strain-rate values acquired from B-mode images for longitudinal and radial dimensions in adult progeny (11 weeks of age) following maternal inhalation exposure. Bold text indicates significant data defined by p ≤ 0.05. A two-way ANOVA determined statistical difference between PPME and MPME denoted with underlined values for that parameter. A letter next to a given group denotes statistical significance between the marked group and the group represented by each letter based on a Tukey’s multiple-comparisons test. All data are presented as the mean ± the standard error of the mean (SEM). PPME WT Sham, n = 7; PPME WT TiO2, n = 6; PPME Tg Sham, n = 6; PPME Tg TiO2, n = 5; MPME WT Sham, n = 4; MPME WT TiO2, n = 5; MPME Tg Sham, n = 5; MPME Tg TiO2, n = 4.
PPME: paternal mPHGPx maternal exposure; MPME: maternal mPHGPx maternal exposure; WT Sham: wild-type offspring whose dam was exposed to control air; Tg Sham: mPHGPx transgenic offspring whose dam was exposed to control air; WT TiO2: wild-type offspring whose dam was exposed to nano-TiO2; Tg TiO2: mPHGPx transgenic offspring whose dam was exposed to nano-TiO2; GLS: global longitudinal strain; Pk: peak.
ETC complex activities
ETC complex activity assays were performed on protein lysate samples of adult offspring (Figure 4). Two-way ANOVA analysis revealed no significant difference between PPME and MPME offspring for complex I (Figure 4(A)) and complex III activities (Figure 4(B)), but MPME offspring had significantly higher activities for both complex IV (Figure 4(C)) and V (Figure 4(D)). Individual t-tests were implemented to better delineate exposure specific differences between offspring of the same genotype (WT or Tg) and the same cohort (PPME or MPME). The PPME WT offspring of ENM-exposed dams had significantly diminished complex I (Figure 4(A)) and IV (Figure 4(C)) activities than PPME WT offspring whose dams were sham-exposed. These findings are consistent with previously published data from our laboratory (Kunovac et al. 2019).
Figure 4.

Electron transport chain (ETC) complex activities of adult PPME and MPME offspring. (A–D) Electron transport chain (ETC) activities assessed in cardiac protein lysate of adult offspring for (A) complex I, (B) complex III, (C) complex IV, and (D) complex V (ATP Synthase). PPME WT Sham, n = 7; PPME WT TiO2, n = 6; PPME Tg Sham, n = 6; PPME Tg TiO2, n = 5; MPME WT Sham, n = 4; MPME WT TiO2, n = 5; MPME Tg Sham, n = 7; MPME Tg TiO2, n = 4. Adult = 11 weeks old. Statistical difference was defined by p ≤ 0.05. * = group difference determined by a two-way ANOVA and ns=no statistical difference. A letter above a group denotes statistical significance between those groups based on a Tukey’s multiple-comparisons test. A dagger (†) above a group indicates statistical significance between WT Sham and WT TiO2 or Tg Sham and Tg TiO2 of the same maternal genetic group (MPME or PPME) based on a Student’s t-test. All data are presented as the mean ± the standard error of the mean (SEM). Adult = 11 weeks of age, mPHGPx=mitochondrial phospholipid hydroperoxide glutathione peroxidase, PPME = Paternal mPHGPx maternal exposure, MPME = Maternal mPHGPx maternal exposure, WT Sham = wild-type offspring whose dam was exposed to control air, Tg Sham = mPHGPx transgenic offspring whose dam was exposed to control air, WT TiO2 = wild-type offspring whose dam was exposed to nano-TiO2, Tg TiO2 = mPHGPx transgenic offspring whose dam was exposed to nano-TiO2, Unit = nanomoles of substrate oxidized (minute−1).
GPx activity and H2O2 production
Total GPx enzymatic activity (including all isoforms) was measured in adult offspring. MPME offspring had a significantly higher level of total GPx activity than the PPME offspring (Figure 5(A)). Within the PPME group, Tg offspring of sham-exposed dams had elevated enzymatic activity compared to their WT littermates (Figure 5(A)). To assess specific mPHGPx activity, phosphatidylcholine (PCOOH) was used as a substrate. Within the PPME group, the Tg sham adult offspring had the highest level of mPHGPx activity and was significantly higher than the other three PPME groups, including the PPME Tg offspring whose dams were nano-TiO2 exposed (Figure 5(B)). In the MPME group, the Tg sham and Tg nano-TiO2 groups were both significantly higher than their littermate controls (Figure 5(B)). Student’s t-tests were used to delineate exposure-specific differences between offspring with the same genotype from the same cohort (PPME or MPME). These analyses revealed that in the PPME group, gestational exposure to ENM resulted in lower mPHGPx activity regardless of the offspring’s genotype (Figure 5(B)).
Figure 5.

Antioxidant activity and H2O2 production in adult offspring following gestational inhalation exposure. (A) Total GPx activity in cardiac protein lysate determined by cumene hydroperoxide as substrate and normalized to protein content; (B) Mitochondrial GPx (mPHGPx) activity in cardiac protein lysate determined by phosphatidylcholine hydroperoxide (PCOOH) as substrate and normalized to protein content; (C) Total hydrogen peroxide (H2O2) concentration in cardiac protein lysate normalized to protein content; (D) Hydrogen peroxide (H2O2) concentration in cardiac isolated mitochondria of adult offspring normalized to protein content. PPME WT Sham, n = 7 (protein) and n = 6 (mitochondria); PPME WT TiO2, n = 6; PPME Tg Sham, n = 6 (protein) and n = 5 (mitochondria); PPME Tg TiO2, n = 5; MPME WT Sham, n = 4; MPME WT TiO2, n = 5; MPME Tg Sham, n = 7; MPME Tg TiO2, n = 4. Statistical difference was defined by p ≤ 0.05. * = group difference determined by a two-way ANOVA and ns = no statistical difference. A letter above a group denotes statistical significance between those groups based on a Tukey’s multiple-comparisons test. A dagger (†) above a group indicates statistical significance between WT Sham and WT TiO2 or Tg Sham and Tg TiO2 of the same maternal genetic group (MPME or PPME) based on a Student’s t-test. All data are presented as the mean ± the standard error of the mean (SEM). Adult = 11 weeks of age, mPHGPx = Mitochondrial phospholipid hydroperoxide glutathione peroxidase, PPME = Paternal mPHGPx maternal exposure, MPME = Maternal mPHGPx maternal exposure, WT Sham = wild-type offspring whose dam was exposed to control air, Tg Sham = mPHGPx transgenic offspring whose dam was exposed to control air, WT TiO2 = wild-type offspring whose dam was exposed to nano-TiO2, Tg TiO2 = mPHGPx transgenic offspring whose dam was exposed to nano-TiO2.
Previously published data from our laboratory have indicated that maternal ENM exposure during gestation resulted in increased H2O2 levels at the fetal stage, which was not sustained into adulthood (Kunovac et al. 2019). In the current study, H2O2 levels were unchanged in adult offspring (Figure 5(C)), which was consistent with our previous findings. H2O2 levels were also evaluated in isolated mitochondria. H2O2 levels were elevated in PPME WT adult offspring whose dams were gestationally exposed to ENM when compared to PPME WT adult offspring whose dams were gestationally-sham-exposed (Figure 5(D)). These data overall indicate that maternal exposure to nano-TiO2 during gestation results in decreased hydroperoxide scavenging and increased ROS, while maternal antioxidant protection has the potential to prevent these detriments.
Global and mPHGPx M6A methylation
Alterations in the relative abundance of m6A modifications can affect RNA metabolism and are associated with various diseases, including metabolic diseases affecting the heart (Li et al. 2020). The SEC insertion sequence (SECIS), which is present in the 3′-UTR of mPHGPx and allows for the insertion of the crucial selenocysteine, contains potential m6A sites (Xuan et al. 2018, Liu et al. 2020). To investigate whether altered total m6A methylation plays a role in the diminished mPHGPx activity in offspring whose dams were exposed to nano-TiO2, we quantified global m6A in RNA isolated from the hearts of adult offspring (Figure 6(A)). Initial analyses indicated that global m6A status was statistically unchanged between the groups, but individual t-tests revealed that MPME Tg offspring whose dams were exposed to nano-TiO2 had significantly lower levels of m6A methylation than MPME Tg offspring whose dams were sham-exposed (Figure 6(A)).
Figure 6.

Epitranscriptomic mechanism contributing to diminished antioxidant scavenging ability following maternal nano-TiO2 inhalation exposure. (A) Global m6A content was determined as a percentage (%) of total RNA isolated from adult offspring cardiac tissue. PPME WT Sham, n = 6; PPME WT TiO2, n = 5; PPME Tg Sham, n = 6; PPME Tg TiO2, n = 5; MPME WT Sham, n = 4; MPME WT TiO2, n = 5; MPME Tg Sham, n = 6; MPME Tg TiO2, n = 4; (B) Isolated RNA with 3 min fragmentation utilized to achieve ~130 nt fragments (top) compared to no fragmentation (bottom) (C) Schematic of the predicted m6A site in the 3′-UTR of the mouse mPHGPx mRNA, specifically within the SECIS region; (D) M6A-RIP-qPCR for mPHGPx (3′-UTR region including potential m6A site). N = 4 per group (ran in triplicate). Statistical difference was defined by P ≤ 0.05 (ns = no significance) based on a one or two-way ANOVA, where appropriate. A letter above a group denotes statistical significance between those groups based on a Tukey’s multiple-comparisons test. A dagger (†) above a group indicates statistical significance between WT Sham and WT TiO2 or Tg Sham and Tg TiO2 of the same maternal genetic group (MPME or PPME) based on a Student’s t-test. All data are presented as the mean ± the standard error of the mean (SEM). Adult = 11 weeks old, mPHGPx = mitochondrial phospholipid hydroperoxide glutathione peroxidase, M6A = N6-Methyladenosine, PPME = Paternal mPHGPx maternal exposure, MPME = Maternal mPHGPx maternal exposure, WT Sham = wild-type offspring whose dam was exposed to control air, Tg Sham = mPHGPx transgenic offspring whose dam was exposed to control air, WT TiO2 = wild-type offspring whose dam was exposed to nano-TiO2, Tg TiO2 = mPHGPx transgenic offspring whose dam was exposed to nano-TiO2, RIN = RNA integrity number, SECIS = selenocysteine insertion sequence.
In order to evaluate the SEC region of mPHGPx in WT offspring, RNA was fragmented and used to perform an m6A RNA immunoprecipitation, followed by qPCR for a specific region of the mPHGPx 3′-UTR. RNA fragmentation incubation time was determined using the Bioanalyzer 2100. A 3-minute incubation was deemed the most appropriate in order to achieve ~130 nt fragments (Figure 6(B)) (Barros-Silva et al. 2020). RMBase v2.0 software predicted the presence of m6A sites at the 3′-UTR of mPHGPx mRNA specifically within the SECIS region (Figure 6(C)). RT-qPCR revealed that PPME WT adult offspring whose dams were exposed to nano-TiO2 had significantly higher m6A levels in the 3′-UTR region of mPHGPx than the control group (PPME WT sham) (Figure 6(D)), as well as both the sham and nano-TiO2 MPME WT groups. These data indicate that gestational exposure in WT dams, has the ability to modulate epitranscriptomic reprogramming that is present in offspring at adulthood. The increase in m6A enrichment in the PPME WT offspring of ENM-exposed dams may be responsible for the persistent mitochondrial bioenergetic and cardiac functional detriments. Additionally, enhanced maternal antioxidant capacity may provide protection from the initial stimuli responsible for the altered m6A status of mPHGPx.
Discussion
With the inevitable increase in production and application of ENM-enabled products in manufacturing, biomedicine, and general consumer use, a thorough understanding of the longitudinal repercussions is necessary for the development of preventative and protective strategies for workers and consumers. This is of particular importance during fetal growth, where a toxicological insult may elicit immediate and long-term deleterious effects. In this study, we have demonstrated that increased antioxidant defense in the maternal environment is capable of providing preservation to cardiac contractile function potentially through the maintenance of mitochondrial bioenergetics. Furthermore, the presence of the maternal transgene preserved m6A status at the 3′-UTR of mPHGPx in offspring despite gestational ENM inhalation exposure. Our data point to a maladaptation in the epitranscriptomic signature as a contributing mechanism responsible for diminished bioenergetics and cardiac contractile deficits in adult offspring following maternal exposure.
Left ventricular ejection fraction (LVEF) has been widely used as a key measure of cardiac contractile function and as a prognostic tool for heart failure (Ponikowski et al. 2016). The dependence of this parameter on load, can limit reproducibility, necessitating a more reliable marker of cardiac function (Kalam, Otahal, and Marwick et al. 2014; Amzulescu et al. 2019). Global longitudinal strain (GLS), a measure of myocardial deformation, is the most commonly utilized clinical application of strain imaging (Ersboll et al. 2013; Mani 2019). GLS appears to be a better predictor of cardiovascular outcomes than LVEF, with better reproducibility (Ashish et al. 2019; Karlsen et al. 2019). Decreases in GLS, as seen in the PPME WT offspring following exposure, highlight the susceptibility of this group to future cardiovascular events (Table 2). The preservation of GLS in the MPME group is therefore noteworthy as the antioxidant protective strategy provided by the mother was able to sufficiently protect her offspring from long-term adverse cardiovascular complications.
Maternal ENM exposure during gestation interferes with fetal development and has long-term consequences for offspring that persist into adulthood (Sun et al. 2013; Hougaard et al. 2015; Bommarito, Martin, and Fry 2017; Crispi, Miranda, and Gratacos 2018). The importance of the maternal environment are outlined by the developmental origins of health and disease (DOHaD) hypothesis and supported by an increasing number of studies (Swanson et al. 2009). The current study highlights how amplifying antioxidant capacity in the maternal environment can deter the maladaptive cardiovascular changes that persist into adulthood, regardless of the progeny’s genotype. Although transgenic expression provides a limitation to clinical uses of maternal antioxidant protection, we establish a basis for investigating other potential antioxidant protective strategies. One example is mitoquinone mesylate (MitoQ), a mitochondrial-targeted antioxidant that has shown promise in improving mitochondrial dysfunction, reducing hydrogen peroxide production, and enhancing antioxidant enzyme activity (Ribeiro Junior et al. 2018; Park et al. 2020). MitoQ also has the ability to prevent the long-term impacts of maternal cigarette smoke exposure on progeny by mitigating renal total ROS in the mothers and offspring at adulthood (Sukjamnong et al. 2018). Future investigations should aim to determine whether supplementation with antioxidant-boosting therapeutics during pregnancy can provide the same protective effect to progeny as maternal mPHGPx overexpression.
As a relatively new area of research in ENM inhalation exposure toxicology, there are a few studies that investigate the detailed epitranscriptomic mechanisms associated with the exposures. However, a recent study demonstrated that in A549 cells, global m6A was decreased following particulate matter exposure as a result of altered m6A modulator genes (Cayir et al. 2019). Although this study was performed using lung epithelial cells, it is one of the first studies to highlight the concept that exposure to environmental toxicants has the ability to alter m6A RNA methylation profiles. M6A has previously been established to preferentially occur around stop codons, with high enrichment in 3′-UTRs of human and mouse transcriptomes (Dominissini et al. 2012; Meyer 2019). Further, the 3′-UTR of mPHGPx is a critical region as it is the location of the Sec incorporation machinery, which enables the catalytic activity of this peroxide scavenging protein (Ingold et al. 2018). To ensure proper function of mPHGPx as a peroxide scavenger, proper selenocysteine (Sec) incorporation is required, which involves the recoding of a UGA codon from “Stop” to “Sec” via a complex that is assembled at a specific region of the 3′-UTR known as the SECIS (Shetty and Copeland 2015; Ingold et al. 2018). Recoding occurs through an eEFSec/Sec-tRNASec complex binding to Sec binding protein 2 (SBP2), which is anchored to the SECIS in the 3′-UTR. Alterations to the SECIS region, may affect the efficiency of Sec incorporation and thus alter the catalytic activity and/or expression of mPHGPx by retaining the premature termination codon, potentially resulting in decay (Squires et al. 2007; Shetty and Copeland 2015). Modulation of this critical region may be the mechanism that results in the persistent adverse outcomes of maternal ENM inhalation exposure into adulthood.
Early studies of m6A modifications determined that m6A primarily occurs in two possible sequences, Gm6AC or Am6AC (Wei and Moss 1977), but have further been specified as RRACH (R = A/G and H = A/C/U) (Dominissini et al. 2012; Batista 2017; Xuan et al. 2018). Interestingly, RMBase v2.0 software, which was utilized as a way of scanning for RRACH motifs, predicted m6A sites at the 3′-UTR of mPHGPx mRNA specifically within the SECIS region (Xuan et al. 2018), as demonstrated in Figure 6(C). The presence of m6A sites in this region was also confirmed in a recent study that aimed to assess the methylome of human and mouse tissue (Liu et al. 2020). Papp et al. reported that inefficient selenoprotein synthesis can increase sensitivity to oxidative stress, which is one potential explanation for the alterations seen at the fetal stage following gestational ENM inhalation exposure (Papp et al. 2006). Moreover, increased methylation, shown in Figure 6, may alter Sec incorporation by changing the secondary structure of the SECIS hairpin (Roundtree et al. 2017) and preventing SBP2 from anchoring the incorporation complex to the RNA (Fletcher et al. 2001). This in turn may be leading to inefficient mPHGPx hydroperoxide scavenging (Ingold et al. 2018) at the adult stage. The decrease in mPHGPx protein expression at the fetal stage compared to the maintenance of expression, but the diminution of mPHGPx activity at the adult stage will require further investigation. A possible explanation is that the epitranscriptomic remodeling occurring at the adult stage may differ from the initial reprogramming that occurred in utero following maternal nano-TiO2 inhalation exposure.
Figure 7 provides an overview of the mechanisms involved in the long-term ramifications of maternal ENM inhalation exposure during gestation on offspring. Overexpression of mPHGPx in the pregnant dam limits H2O2 (ROS) production, thereby mitigating changes to mitochondrial bioenergetics and cardiac contractile function in adult progeny. However, without the overexpression of mPHGPx in the maternal environment, ROS production is not controlled following nano-TiO2 exposure during gestation, resulting in downstream effects on cardiac function and mitochondrial bioenergetics. This occurs, in part, as a result of elevated m6A methylation at the SECIS region of mPHGPx, which disrupts Sec incorporation and diminishes mPHGPx catalytic activity. The current study highlights the therapeutic potential of enhanced antioxidant protection in the maternal environment during gestation (Figure 7). Increased hydroperoxide scavenging through this approach can provide protection for offspring into adulthood from the deleterious effects of maternal particulate exposure during gestation, without the need for supplementation by the progeny. Overexpression of mPHGPx in the pregnant dam likely circumvents the initial surge of oxidative stress that is elicited by the xenobiotic exposure, through a mechanism that involves epitranscriptomic reprogramming.
Figure 7.

Physiological and molecular consequences of gestational nano-TiO2 inhalation exposure in adult offspring. Following gestational exposure, elevated ROS levels in the progeny are concomitant with cardiac functional alterations that can be mitigated by overexpression of maternal mPHGPx. Augmented mitochondrial ROS plays a key role in the persistence of deficits into adulthood (11 weeks of age) characterized by diminished GLS and ETC complex activities. A mechanism that could promote the sustained consequences is initiated by high oxidative stress, which increases m6A methylation at the SECIS in the 3′-UTR of mPHGPx. Ultimately, this may tamper with the SECIS binding region for the SBP2 that is vital for the incorporation of selenocysteine and the catalytic activity of mPHGPx. Decreased mPHGPx activity then propagates mitochondrial bioenergetic deficits, limiting overall cardiac performance in the adult offspring. MPHGPx = mitochondrial phospholipid hydroperoxide glutathione peroxidase, ROS = reactive oxygen species, GLS = global longitudinal strain, ETC = electron transport chain, m6A = N6-methyladenosine, SECIS = Selenocysteine insertion sequence, SBP2 = SECIS binding protein 2.
At the adult stage, maternal ENM exposure during gestation was associated with altered m6A methylation of mPHGPx that contributes to diminished enzymatic activity and persistent cardiac contractile changes. These findings highlight a specific targeted modification to the epitranscriptome that may be mechanistically linked to sustained bioenergetic and cardiac dysfunction associated with gestational ENM exposure. Alterations to the epitranscriptome presented in this study provide a new perspective on the role of m6A methylation changes in the fields of cardiovascular ENM inhalation exposure toxicology. Our study introduces a protective strategy that can be implemented to safeguard developing progeny against long-term cardiovascular ramifications.
Supplementary Material
Acknowledgements
We would like to thank Sherri A. Friend and the National Institute for Occupational Safety and Health, Morgantown, WV, USA for contributing in the physicochemical characterization of the nano-TiO2 aerosolized particles.
Funding
This work was supported by The National Heart, Lung, and Blood Institute (NHLBI) under Grant [R01 HL-128485] (JMH), the National Institute of Environmental Health Sciences (NIEHS) under Grant [R01 ES-015022] (TRN), American Heart Association under Grant [AHA-20PRE35080170] (AK), American Heart Association under Grant [AHA-17PRE33660333] (QAH), National Institute on Aging (NIA) under Grant [5 T32 AG 52375-3] (KLG), WVU Genomics Core Facility support by CTSI Grant from the National Institute of General Medical Sciences (NIGMS) [U54GM104942], WVU Animal Models & Imaging Facility supported by the WVU Cancer Institute and NIH grants [P20 RR016440] and [P30 RR032138/GM103488], and the Community Foundation for the Ohio Valley Whipkey Trust (JMH).
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplemental data for this article can be accessed here.
References
- Amzulescu MS, De Craene M, Langet H, Pasquet A, Vancraeynest D, Pouleur AC, Vanoverschelde JL, and Gerber BL. 2019. “Myocardial Strain Imaging: Review of General Principles, Validation, and Sources of Discrepancies.” European Heart Journal Cardiovascular Imaging 20 (6): 605–619. doi: 10.1093/ehjci/jez041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjilvel S, and Asgharian B. 1995. “A Multiple-path Model of Particle Deposition in the Rat Lung.” Fundamental and Applied Toxicology 28 (1): 41–50. doi: 10.1093/toxsci/28.1.41. [DOI] [PubMed] [Google Scholar]
- Ashish K, Faisaluddin M, Bandyopadhyay D, Hajra A, and Herzog E. 2019. “Prognostic Value of Global Longitudinal Strain in Heart Failure Subjects: A Recent Prototype.” International Journal of Cardiology. Heart & Vasculature 22: 48–49. doi: 10.1016/j.ijcha.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos A, Fontanesi F, and Diaz F. 2009. “Evaluation of the Mitochondrial Respiratory Chain and Oxidative Phosphorylation System Using Polarography and Spectrophotometric Enzyme Assays.” Current Protocols in Human Genetics 63 (1): 19.3.1–19.3.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros-Silva D, Lobo J, Guimaraes-Teixeira C, Carneiro I, Oliveira J, Martens-Uzunova ES, Henrique R, and Jeronimo C. 2020. “VIRMA-dependent N6-methyladenosine Modifications Regulate the Expression of Long Non-coding RNAs CCAT1 and CCAT2 in Prostate Cancer.” Cancers 12 (4): 771. doi: 10.3390/cancers12040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseler WA, Dabkowski ER, Jagannathan R, Thapa D, Nichols CE, Shepherd DL, Croston TL, et al. 2013. “Reversal of Mitochondrial Proteomic Loss in Type 1 Diabetic Heart with Overexpression of Phospholipid Hydroperoxide Glutathione Peroxidase.” American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 304 (7): R553–65. doi: 10.1152/ajpregu.00249.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseler WA, Dabkowski ER, Williamson CL, Croston TL, Thapa D, Powell MJ, Razunguzwa TT, and Hollander JM. 2011. “Proteomic Alterations of Distinct Mitochondrial Subpopulations in the Type 1 Diabetic Heart: Contribution of Protein Import Dysfunction.” American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 300 (2): R186–200. doi: 10.1152/ajpregu.00423.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista PJ 2017. “The RNA Modification N(6)-methyladenosine and Its Implications in Human Disease.” Genomics Proteomics Bioinformatics 15 (3): 154–163. doi: 10.1016/j.gpb.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berulava T, Buchholz E, Elerdashvili V, Pena T, Islam MR, Lbik D, Mohamed BA, et al. 2020. “Changes in m6A RNA Methylation Contribute to Heart Failure Progression by Modulating Translation.” European Journal of Heart Failure 22 (1): 54–66. doi: 10.1002/ejhf.1672. [DOI] [PubMed] [Google Scholar]
- Bide RW, Armour SJ, and Yee E. 1997. Estimation of Human Toxicity From Animal Inhalation Toxicity Data:1. Minute Volume-Body Weight Relationships Between Animals And Man. A.N. Defence. [Google Scholar]
- Bommarito PA, Martin E, and Fry RC. 2017. “Effects of Prenatal Exposure to Endocrine Disruptors and Toxic Metals on the Fetal Epigenome.” Epigenomics 9 (3): 333–350. doi: 10.2217/epi-2016-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM 1976. “A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding.” Analytical Biochemistry 72: 248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castellanos-Rubio A, Santin I, Olazagoitia-Garmendia A, Romero-Garmendia I, Jauregi-Miguel A, Legarda M, and Bilbao JR. 2019. “A Novel RT-qPCR-based Assay for the Relative Quantification of Residue Specific m6A RNA Methylation.” Scientific Reports 9 (1): 4220. doi: 10.1038/s41598-019-40018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayir A, Barrow TM, Guo L, and Byun HM. 2019. “Exposure to Environmental Toxicants Reduces Global N6-methyladenosine RNA Methylation and Alters Expression of RNA Methylation Modulator Genes.” Environmental Research 175: 228–234. doi: 10.1016/j.envres.2019.05.011. [DOI] [PubMed] [Google Scholar]
- Crispi F, Miranda J, and Gratacos E. 2018. “Long-term Cardiovascular Consequences of Fetal Growth Restriction: Biology, Clinical Implications, and Opportunities for Prevention of Adult Disease.” American Journal of Obstetrics and Gynecology 218 (2): S869–S879. doi: 10.1016/j.ajog.2017.12.012. [DOI] [PubMed] [Google Scholar]
- Dabkowski ER, Williamson CL, and Hollander JM. 2008. “Mitochondria-specific Transgenic Overexpression of Phospholipid Hydroperoxide Glutathione Peroxidase (GPx4) Attenuates Ischemia/Reperfusion-associated Cardiac Dysfunction.” Free Radical Biology and Medicine 45 (6): 855–865. doi: 10.1016/j.freeradbiomed.2008.06.021. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services, C.F.D.C.a.P. 2011. Occupational Exposure to Titanium Dioxide. National Institute for Occupational Safety and Health. [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, et al. 2012. “Topology of the Human and Mouse m6A RNA Methylomes Revealed by m6A-seq.” Nature 485 (7397): 201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- Engel M, Eggert C, Kaplick PM, Eder M, Roh S, Tietze L, Namendorf C, et al. 2018. “The Role of m(6)a/m-RNA Methylation in Stress Response Regulation.” Neuron 99 (2): 389–403. doi: 10.1016/j.neuron.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersboll M, Valeur N, Mogensen UM, Andersen MJ, Moller JE, Velazquez EJ, Hassager C, Sogaard P, and Kober L. 2013. “Prediction of All-cause Mortality and Heart Failure Admissions from Global Left Ventricular Longitudinal Strain in Patients with Acute Myocardial Infarction and Preserved Left Ventricular Ejection Fraction.” Journal of the American College of Cardiology 61 (23): 2365–2373. doi: 10.1016/j.jacc.2013.02.061. [DOI] [PubMed] [Google Scholar]
- Ferrari L, Carugno M, and Bollati V. 2019. “Particulate Matter Exposure Shapes DNA Methylation through the Lifespan.” Clinical Epigenetics 11 (1): 129. doi: 10.1186/s13148-019-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JE, Copeland PR, Driscoll DM, and Krol A. 2001. “The Selenocysteine Incorporation Machinery: Interactions between the SECIS RNA and the SECIS-binding Protein SBP2.” RNA 7 (10): 1442–1453. [PMC free article] [PubMed] [Google Scholar]
- Hathaway QA, Durr AJ, Shepherd DL, Pinti MV, Brandebura AN, Nichols CE, Kunovac A, et al. 2019a. “miRNA-378a as a Key Regulator of Cardiovascular Health following Engineered Nanomaterial Inhalation Exposure.” Nanotoxicology 13 (5): 644–663. doi: 10.1080/17435390.2019.1570372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway QA, Nichols CE, Shepherd DL, Stapleton PA, Mclaughlin SL, Stricker JC, Rellick SL, et al. 2017. “Maternal-engineered Nanomaterial Exposure Disrupts Progeny Cardiac Function and Bioenergetics.” American Journal of Physiology-Heart and Circulatory Physiology 312 (3): H446–H458. doi: 10.1152/ajpheart.00634.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway QA, Roth SM, Pinti MV, Sprando DC, Kunovac A, Durr AJ, Cook CC, et al. 2019b. “Machinelearning to Stratify Diabetic Patients Using Novel Cardiac Biomarkers and Integrative Genomics.” Cardiovascular Diabetology 18 (1): 78. doi: 10.1186/s12933-019-0879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JM, Lin KM, Scott BT, and Dillmann WH. 2003. “Overexpression of PHGPx and HSP60/10 Protects against Ischemia/Reoxygenation Injury.” Free Radical Biology and Medicine 35 (7): 742–751. doi: 10.1016/S0891-5849(03)00400-3. [DOI] [PubMed] [Google Scholar]
- Hougaard KS, Campagnolo L, Chavatte-Palmer P, Tarrade A, Rousseau-Ralliard D, Valentino S, Park MV, et al. 2015. “A Perspective on the Developmental Toxicity of Inhaled Nanoparticles.” Reproductive Toxicology 56: 118–140. doi: 10.1016/j.reprotox.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, Roveri A, et al. 2018. “Selenium Utilization by GPX4 is required to Prevent Hydroperoxide-induced Ferroptosis.” Cell 172 (3): 409–422. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- Janssen BG, Madhloum N, Gyselaers W, Bijnens E, Clemente DB, Cox B, Hogervorst J, et al. 2017. “Cohort Profile: The ENVIRonmental Influence on Early AGEing (ENVIRONAGE): A Birth Cohort Study.” International Journal of Epidemiology 46 (5): 1387m–1387m. doi: 10.1093/ije/dyx033. [DOI] [PubMed] [Google Scholar]
- Kalam K, Otahal P, and Marwick TH. 2014. “Prognostic Implications of Global LV Dysfunction: A Systematic Review and Meta-analysis of Global Longitudinal Strain and Ejection Fraction.” Heart 100 (21): 1673–1680. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- Karlsen S, Dahlslett T, Grenne B, Sjoli B, Smiseth O, Edvardsen T, and Brunvand H. 2019. “Global Longitudinal Strain is a more Reproducible Measure of Left Ventricular Function than Ejection Fraction Regardless of Echocardiographic Training.” Cardiovascular Ultrasound 17 (1): 18. doi: 10.1186/s12947-019-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R 2011. “Engineered Nanoparticles in Consumer Products: Understanding a New Ingredient.” Environmental Health Perspectives 119 (3): a120–5. doi: 10.1289/ehp.119-a120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kietzmann T, Petry A, Shvetsova A, Gerhold JM, and Gorlach A. 2017. “The Epigenetic Landscape Related to Reactive Oxygen Species Formation in the Cardiovascular System.” British Journal of Pharmacology 174 (12): 1533–1554. doi: 10.1111/bph.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuckles TL, Yi J, Frazer DG, Leonard HD, Chen BT, Castranova V, and Nurkiewicz TR. 2012. “Nanoparticle Inhalation alters Systemic Arteriolar Vasoreactivity through Sympathetic and Cyclooxygenase-mediated Pathways.” Nanotoxicology 6 (7): 724–735. doi: 10.3109/17435390.2011.606926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunovac A, Hathaway QA, Pinti MV, Goldsmith WT, Durr AJ, Fink GK, Nurkiewicz TR, and Hollander JM. 2019. “ROS Promote Epigenetic Remodeling and Cardiac Dysfunction in Offspring following Maternal Engineered Nanomaterial (ENM) Exposure.” Particle and Fibre Toxicology 16 (1): 24. doi: 10.1186/s12989-019-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunovac A, Hathaway QA, Pinti MV, Taylor AD, and Hollander JM. 2020. “Cardiovascular Adaptations to Particle Inhalation Exposure: Molecular Mechanisms of the Toxicology.” American Journal of Physiology. Heart and Circulatory Physiology 319 (2): H282–H305. doi: 10.1152/ajpheart.00026.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi A, Evke S, Lee M, Melendez JA, and Begley TJ. 2019. “Epitranscriptomic Systems Regulate the Translation of Reactive Oxygen Species Detoxifying and Disease Linked Selenoproteins.” Free Radical Biology & Medicine 143: 573–593. doi: 10.1016/j.freeradbiomed.2019.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang J, Huang C, Shen M, Zhan H, and Xu K. 2020. “RNA N6-methyladenosine: A Promising Molecular Target in Metabolic Diseases.” Cell & Bioscience 10 (1): 19. doi: 10.1186/s13578-020-00385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder B, and Jaffrey SR. 2019. “Discovering and Mapping the Modified Nucleotides that Comprise the Epitranscriptome of mRNA.” Cold Spring Harbor Perspectives in Biology 11 (6): a032201. doi: 10.1101/cshper-spect.a032201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li K, Cai J, Zhang M, Zhang X, Xiong X, Meng H, et al. 2020. “Landscape and Regulation of m(6)a and m(6)Am Methylome across Human and Mouse Tissues.” Molecular Cell 77 (2): 426–440 e6. doi: 10.1016/j.molcel.2019.09.032. [DOI] [PubMed] [Google Scholar]
- Mani A 2019. “Global Longitudinal Strain Imaging and its Utility in Assessing Risk in Early Stages of Hypertension.” The Journal of Clinical Hypertension 21 (11): 1711–1712. doi: 10.1111/jch.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmisolle FE, Garcia ML, and Reyes CA. 2018. “RNA-binding Protein Immunoprecipitation as a Tool to nvestigate Plant miRNA Processing Interference by Regulatory Proteins of Diverse Origin.” Plant Methods 14 (1): 9. doi: 10.1186/s13007-018-0276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD 2019. “m(6)A-mediated Translation Regulation.” Biochimica et Biophysica Acta (Bba) - Gene Regulatory Mechanisms 1862 (3): 301–309. doi: 10.1016/j.bbagrm.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KW, Zealy RW, Davila S, Fomin M, Cummings JC, Makowsky D, Mcdowell CH, et al. 2018. “Profiling of m6A RNA Modifications Identified an Age-associated Regulation of AGO2 mRNA Stability.” Aging Cell 17 (3): e12753. doi: 10.1111/acel.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CE, Shepherd DL, Hathaway QA, Durr AJ, Thapa D, Abukabda A, Yi J, Nurkiewicz TR, and Hollander JM. 2018. “Reactive Oxygen Species Damage Drives Cardiac and Mitochondrial Dysfunction following Acute Nano-titanium Dioxide Inhalation Exposure.” Nanotoxicology 12 (1): 32–48. doi: 10.1080/17435390.2017.1416202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CE, Shepherd DL, Knuckles TL, Thapa D, Stricker JC, Stapleton PA, Minarchick VC, et al. 2015. “Cardiac and Mitochondrial Dysfunction following Acute Pulmonary Exposure to Mountaintop Removal Mining Particulate Matter.” American Journal of Physiology. Heart and Circulatory Physiology 309 (12): H2017–30. doi: 10.1152/ajpheart.00353.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Hubbs AF, Cumpston JL, Chen BT, Frazer DG, and Castranova V. 2008. “Nanoparticle Inhalation Augments Particle-Dependent Systemic Microvascular Dysfunction.” Particle and Fibre Toxicology 5 (1): 1. doi: 10.1186/1743-8977-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JW, Tandler B, and Hoppel CL. 1977. “Biochemical Properties of Subsarcolemmal and Interfibrillar Mitochondria Isolated from Rat Cardiac Muscle.” The Journal of Biological Chemistry 252 (23): 8731–8739. doi: 10.1016/S0021-9258(19)75283-1. [DOI] [PubMed] [Google Scholar]
- Papp LV, Lu J, Striebel F, Kennedy D, Holmgren A, and Khanna KK. 2006. “The Redox State of SECIS Binding Protein 2 Controls its Localization and Selenocysteine Incorporation Function.” Molecular and Cellular Biology 26 (13): 4895–4910. doi: 10.1128/MCB.02284-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Pekas EJ, Headid RJ, Son WM, Wooden TK, Song J, Layec G, Yadav SK, Mishra PK, and Pipinos II. 2020. “Acute Mitochondrial Antioxidant Intake Improves Endothelial Function, Antioxidant Enzyme Activity, and Exercise Tolerance in Patients with Peripheral Artery Disease.” American Journal of Physiology-Heart and Circulatory Physiology 319 (2): H456–H467. doi: 10.1152/ajpheart.00235.2020. [DOI] [PubMed] [Google Scholar]
- Pelclova D, Barosova H, Kukutschova J, Zdimal V, Navratil T, Fenclova Z, Vlckova S, et al. 2015. “Raman Microspectroscopy of Exhaled Breath Condensate and Urine in Workers Exposed to Fine and Nano TiO2 Particles: A Cross-sectional Study.” Journal of Breath Research 9 (3): 036008. doi: 10.1088/1752-7155/9/3/036008. [DOI] [PubMed] [Google Scholar]
- Pietroiusti A, Stockmann-Juvala H, Lucaroni F, and Savolainen K. 2018. “Nanomaterial Exposure, Toxicity, and Impact on Human Health.” Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 10 (5): e1513. doi: 10.1002/wnan.1513. [DOI] [PubMed] [Google Scholar]
- Ponikowski P,. Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, et al. 2016. “ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC.” European Heart Journal 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- Ribeiro Junior RF, Dabkowski ER, Shekar KC, Ka OC, Hecker PA, and Murphy MP. 2018. “MitoQ Improves Mitochondrial Dysfunction in Heart Failure Induced by Pressure Overload.” Free Radical Biology & Medicine 117: 18–29. doi: 10.1016/j.freeradbiomed.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud CO, Uyar AE, Darby MR, Zucker LG, and Wiesner MR. 2009. “Estimates of Upper Bounds and Trends in Nano-TiO2 Production as a Basis for Exposure Assessment.” Environmental Science & Technology 43 (12): 4227–4233. doi: 10.1021/es8032549. [DOI] [PubMed] [Google Scholar]
- Ross-Innes CS, Stark R, Teschendorff AE, Holmes KA, Ali HR, Dunning MJ, Brown GD, et al. 2012. “Differential Oestrogen Receptor Binding is Associated with Clinical Outcome in Breast Cancer.” Nature 481 (7381): 389–393. doi: 10.1038/nature10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundtree IA, Evans ME, Pan T, and He C. 2017. “Dynamic RNA Modifications in Gene Expression Regulation.” Cell 169 (7): 1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager TM, Kommineni C, and Castranova V. 2008. “Pulmonary Response to Intratracheal Instillation of Ultrafine versus Fine Titanium Dioxide: Role of Particle Surface Area.” Particle and Fibre Toxicology 5 (1): 17. doi: 10.1186/1743-8977-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd DL, Nichols CE, Croston TL, Mclaughlin SL, Petrone AB, Lewis SE, Thapa D, Long DM, Dick GM, and Hollander JM. 2016. “Early Detection of Cardiac Dysfunction in the Type 1 Diabetic Heart Using Speckle-tracking Based Strain Imaging.” Journal of Molecular and Cellular Cardiology 90: 74–83. doi: 10.1016/j.yjmcc.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty SP, and Copeland PR. 2015. “Selenocysteine Incorporation: A Trump Card in the Game of mRNA Decay.” Biochimie 114: 97–101. doi: 10.1016/j.biochi.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, and He C. 2017. “YTHDF3 Facilitates Translation and Decay of N(6)-methyladenosine-modified RNA.” Cell Research 27 (3): 315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires JE, Stoytchev I, Forry EP, and Berry MJ. 2007. “SBP2 Binding Affinity is a Major Determinant in Differential Selenoprotein mRNA Translation and Sensitivity to Nonsense-mediated Decay.” Molecular and Cellular Biology 27 (22): 7848–7855. doi: 10.1128/MCB.00793-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton PA, Hathaway QA, Nichols CE, Abukabda AB, Pinti MV, Shepherd DL, Mcbride CR, et al. 2018. “Maternal Engineered Nanomaterial Inhalation during Gestation Alters the Fetal Transcriptome.” Particle and Fibre Toxicology 15 (1): 3. doi: 10.1186/s12989-017-0239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton PA, Minarchick VC, Cumpston AM, Mckinney W, Chen BT, Sager TM, Frazer DG, et al. 2012. “Impairment of Coronary Arteriolar Endothelium-dependent Dilation after Multi-walled Carbon Nanotube Inhalation: A Time-course Study.” International Journal of Molecular Sciences 13 (11): 13781–13803. doi: 10.3390/ijms131113781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA 2010. “Measurement of Mitochondrial ROS Production.” Methods in Molecular Biology 648: 245–255. [Database] doi: 10.1007/978-1-60761-756-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebounova LV, Morgan H, Grassian VH, and Brenner S. 2012. “Health and Safety Implications of Occupational Exposure to Engineered Nanomaterials.” Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 4 (3): 310–321. doi: 10.1002/wnan.174. [DOI] [PubMed] [Google Scholar]
- Stolwijk JM, Falls-Hubert KC, Searby CC, Wagner BA, and Buettner GR. 2020. “Simultaneous Detection of the Enzyme Activities of GPx1 and GPx4 Guide Optimization of Selenium in Cell Biological Experiments.” Redox Biology 32: 101518. doi: 10.1016/j.redox.2020.101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukjamnong S, Chan YL, Zakarya R, Nguyen LT, Anwer AG, Zaky AA, Santiyanont R, et al. 2018. “MitoQ Supplementation Prevent Long-term Impact of Maternal Smoking on Renal Development, Oxidative Stress and Mitochondrial Density in Male Mice Offspring.” Scientific Reports 8 (1): 6631. doi: 10.1038/s41598-018-24949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Zhang Q, Wang Z, and Yan B. 2013. “Effects of Nanotoxicity on Female Reproductivity and Fetal Development in Animal Models.” International Journal of Molecular Sciences 14 (5): 9319–9337. doi: 10.3390/ijms14059319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Entringer S, Buss C, and Wadhwa PD. 2009. “Developmental Origins of Health and Disease: Environmental Exposures.” Seminars in Reproductive Medicine 27 (05): 391–402. doi: 10.1055/s-0029-1237427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanwar V, Gorr MW, Velten M, Eichenseer CM, Long VP, Bonilla IM, Shettigar V, et al. 2017. “In Utero Particulate Matter Exposure Produces Heart Failure, Electrical Remodeling, and Epigenetic Changes at Adulthood.” Journal of the American Heart Association 6 (4): e005796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance ME, Kuiken T, Vejerano EP, Mcginnis SP, Hochella MF Jr., Rejeski D, and Hull MS. 2015. “Nanotechnology in the Real World: Redeveloping the Nanomaterial Consumer Products Inventory.” Beilstein Journal of Nanotechnology 6: 1769–1780. doi: 10.3762/bjnano.6.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Tang K, Zhang D, Xie S, Zhu X, Wang Z, and Lang Z. 2015. “Transcriptome-wide High-throughput Deep m(6)A-seq Reveals Unique Differential m(6)a Methylation Patterns between Three Organs in Arabidopsis thaliana.” Genome Biology 16 (1): 272. doi: 10.1186/s13059-015-0839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CM, and Moss B. 1977. “Nucleotide Sequences at the N6-methyladenosine Sites of HeLa Cell Messenger Ribonucleic Acid.” Biochemistry 16 (8): 1672–1676. doi: 10.1021/bi00627a023. [DOI] [PubMed] [Google Scholar]
- Xuan JJ, Sun WJ, Lin PH, Zhou KR, Liu S, Zheng LL, Qu LH, and Yang JH. 2018. “RMBase v2.0: Deciphering the Map of RNA Modifications from Epitranscriptome Sequencing Data.” Nucleic Acids Research 46 (D1): D327–D334. doi: 10.1093/nar/gkx934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Wang S, Gao S, Soares F, Ahmed M, Guo H, Wang M, et al. 2018. “Refined RIP-seq Protocol for Epitranscriptome Analysis with Low Input M.” PLOS Biology 16 (9): e2006092. doi: 10.1371/journal.pbio.2006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Yu J, Frazier K, Weng X, Li Y, Cham CM, Dolan K, et al. 2018. “Circadian Clock Regulation of Hepatic Lipid Metabolism by Modulation of m(6)a mRNA Methylation.” Cell Reports 25 (7): 1816–1828. doi: 10.1016/j.celrep.2018.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


