Abstract
Background
Hyperpolarized 129Xe magnetic resonance imaging (MRI) provides a non‐invasive assessment of regional pulmonary gas exchange function. This technique has demonstrated that chronic obstructive pulmonary disease (COPD) patients exhibit ventilation defects, reduced interstitial barrier tissue uptake, and poor transfer to capillary red blood cells (RBCs). However, the behavior of these measurements following therapeutic intervention is unknown.
Purpose
To characterize changes in 129Xe gas transfer function following administration of an inhaled long‐acting beta‐agonist/long‐acting muscarinic receptor antagonist (LABA/LAMA) bronchodilator.
Study Type
Prospective.
Population
Seventeen COPD subjects (GOLD II/III classification per Global Initiative for Chronic Obstructive Lung Disease criteria) were imaged before and after 2 weeks of LABA/LAMA therapy.
Field Strength/Sequences
Dedicated ventilation imaging used a multi‐slice 2D gradient echo sequence. Three‐dimensional images of ventilation, barrier uptake, and RBC transfer used an interleaved, radial, 1‐point Dixon sequence. Imaging was acquired at 3 T.
Assessment
129Xe measurements were quantified before and after LABA/LAMA treatment by ventilation defect + low percent (vendef + low) and by barrier uptake and RBC transfer relative to a healthy reference population (bar%ref and RBC%ref). Pulmonary function tests, including diffusing capacity of the lung for carbon monoxide (DLCO), were also performed before and after treatment.
Statistical Tests
Paired t‐test, Pearson correlation coefficient (r).
Results
Baseline vendef + low was 57.8 ± 8.4%, bar%ref was 73.2 ± 19.6%, and RBC%ref was 36.5 ± 13.6%. Following treatment, vendef + low decreased to 52.5 ± 10.6% (P < 0.05), and improved in 14/17 (82.4%) of subjects. However, RBC%ref decreased in 10/17 (58.8%) of subjects. Baseline measurements of bar%ref and DLCO were correlated with the degree of post‐treatment change in vendef + low (r = −0.49, P < 0.05 and r = −0.52, P < 0.05, respectively).
Conclusion
LABA/LAMA therapy tended to preferentially improve ventilation in subjects whose 129Xe barrier uptake and DLCO were relatively preserved. However, newly ventilated regions often revealed RBC transfer defects, an aspect of lung function opaque to spirometry. These microvasculature abnormalities must be accounted for when assessing the effects of LABA/LAMA therapy.
Level of Evidence
1
Technical Efficacy Stage
4
Keywords: xenon‐129, magnetic resonance imaging, lung, bronchodilator agents, chronic obstructive pulmonary disease
Hyperpolarized (HP) 129Xe gas magnetic resonance imaging (MRI) is a promising tool for 3D visualization and quantification of regional gas exchange function in the lung. It offers faster imaging and superior spatial resolution compared to the radionuclide ventilation‐perfusion scan. Such functional pulmonary MRI techniques can complement the detailed structural information provided by computed tomography (CT), and because they do not expose patients to ionizing radiation, can be performed as a means of longitudinal monitoring.1
HP 129Xe is sensitive to ventilation defects in both chronic obstructive pulmonary disease (COPD)2, 3 and asthma.4 In asthma, it has been used to visualize the regional reversal of defects after bronchodilator administration.5 Beyond ventilation imaging, 129Xe MRI also provides the unique ability to image the diffusive transfer of gas into the alveolar‐capillary barrier tissue and red blood cell (RBC) compartments6 and recent work has provided a model to relate this technique to the observed DLCO.7 129Xe MRI has been used to demonstrate that patients with idiopathic pulmonary fibrosis (IPF) exhibit increased 129Xe uptake into the barrier tissues consistent with thickened lung interstitium, and these 129Xe‐MRI‐derived metrics correlated significantly with pulmonary function tests (PFTs) but not with CT fibrosis scores.8 Notably, the combination of both increased barrier uptake and poor RBC transfer has been associated with poor outcomes in patients with IPF.9 129Xe gas exchange MRI in COPD patients has demonstrated a combination of ventilation defects, low barrier uptake, and poor transfer of 129Xe to RBCs compared to other lung diseases.10 However, 129Xe MRI measures of gas transfer in COPD have not been evaluated in the context of a therapeutic intervention. We thus have little understanding of which measures are affected by therapy or whether any markers of 129Xe gas exchange are predictive of positive therapy response.
Among the major treatment options for COPD are the dual bronchodilator preparations that have been shown to increase forced expiratory volume in 1 second (FEV1) and decrease the frequency of acute exacerbation.11, 12 However, it remains unclear how improvement in FEV1, a global measurement of lung mechanics, may affect regional gas exchange function.
Thus, the aim of this study was to use HP 129Xe MRI to assess gas transfer function in patients with COPD before and after treatment with an inhaled long‐acting beta‐agonist/long‐acting muscarinic receptor antagonist (LABA/LAMA) bronchodilator preparation (glycopyrrolate/formoterol fumarate). We tested whether baseline measurements, including clinical variables, spirometry, and 129Xe gas exchange, were predictive of ventilation improvement as measured using 129Xe MRI following the dual LABA/LAMA bronchodilator preparation.
Materials and Methods
Patient Population
We recruited 17 subjects age ≥40 of any sex with a pulmonologist diagnosis of COPD. Diagnosis was based on the clinical and spirometric criteria stated in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 guidelines13: dyspnea, chronic cough or sputum production, a history of recurrent lower respiratory tract infections, and/or a history of exposure to risk factors for the disease, together with a post‐bronchodilator FEV1/FVC < 0.70. Subjects were classified as either GOLD II (FEV1 50–79%) or GOLD III (FEV1 30–49%). All subjects had a smoking history of at least 10 pack‐years.
Subjects were required to be at least 6 weeks removed from an upper respiratory tract infection or acute exacerbation. The following exclusion criteria were applied: 1) daily use of >10 mg of systemic steroids; 2) chronic oxygen therapy; 3) a history of lung surgery including resection, decortication, or pneumothorax; 4) diagnosis of asthma‐COPD overlap syndrome; 5) a history of exposure to occupational hazards known to cause lung disease; 6) a history of myocardial infarction, unstable angina, cardiac arrhythmias, cardiomyopathy, uncontrolled hypertension, uncontrolled diabetes mellitus, diabetic ketoacidosis, thyrotoxicosis, seizures, hypokalemia, and/or narrow‐angle glaucoma; 7) interstitial or chronic infectious lung disease confirmed by imaging studies; and/or 8) pregnancy.
Protocol
All participants signed a written, informed consent prior to enrollment and the HIPAA‐compliant study protocol was approved by the Institutional Review Board of Duke University. All subjects who were on regular inhalation therapies for COPD underwent a washout period of 7–10 days, during which they stopped all inhaled corticosteroids, LABA and LAMA. Use of inhaled albuterol was allowed as needed. At the end of the washout period, subjects received a pre‐treatment 129Xe MRI scan and PFTs together with an assessment of secondary outcomes (described below). They were then given a two‐week sample of glycopyrrolate/formoterol aerosphere (Bevespi®, AstraZeneca, Cambridge, United Kingdom) at a dose of 2 puffs twice a day, and were trained on correct administration technique. After 2 weeks of therapy, subjects returned for a post‐treatment 129Xe MRI scan, PFTs, and assessment of secondary outcomes. A follow‐up phone call was conducted after an additional 2‐week period to assess any potential adverse events.
129Xe Polarization and Dose Administration
129Xe was hyperpolarized via continuous flow spin‐exchange optical pumping and cryogenic accumulation using commercially available systems (Model 9820 and 9810; Polarean plc., Durham, NC) and dispensed into a Tedlar™ dose delivery bag as previously described.14 HP 129Xe imaging and spectroscopy were acquired on a 3‐T scanner (Magnetom Trio; Siemens, Erlangen, Germany) during three separate breath‐holds, one each for calibration, dedicated ventilation, and gas exchange scans. Subjects received a small dose (target 129Xe dose equivalent [DE] ≥ 65, actual 64.9 ± 16.8 mL) for calibration followed by two larger doses for the gas exchange (target DE ≥150 mL, actual 180.9 ± 33.1 mL) and dedicated ventilation scans (DE ≥80 mL, actual 96.5 ± 23.2 mL) with the scans performed in that order. The dose equivalent represents the magnetization that would be provided by the indicated volume of 100% enriched, 100% polarized 129Xe.14 These dose equivalents were achieved with xenon volumes ranging from 250 mL to 715 mL expanded with the same 89% helium blend used in 129Xe polarization to achieve a net 1‐liter volume, which subjects inhaled from functional residual capacity. Patients remained supine on the table during the entirety of the approximately 20‐minute imaging session. Heart rate and oxygen saturation were continuously monitored with an MR‐compatible monitoring system (Nonin 7500 Pulse Oximeter, Nonin Medical Inc., Plymouth, MN).
The calibration scan was performed over a 16 s breath‐hold, during which 600 129Xe free induction decays were acquired at 20 msec intervals (echo time 0.45 msec, flip angle target 20°, dwell time 37 μs, 512 points).15 Dedicated ventilation images were acquired during an 8.5 s breath‐hold using a multi‐slice gradient echo (GRE) sequence at 4 × 4 × 15 mm3 resolution (TR/TE = 7.65/3 msec, 10° flip angle, BW = 170 Hz/pixel).16 Subsequent 3D images of ventilation, barrier uptake, and RBC transfer (the latter two of which constitute the “dissolved phase”) were then acquired during a 15 s breath‐hold using an interleaved radial acquisition with an effective repetition time (TR) of 15 msec, gas/dissolved‐phase flip angle of 0.5°/20° and 1000 radial views per phase (2000 total views). The signal was acquired at an echo time (approximately 0.47 msec) that allowed the two dissolved‐phase compartments to be decomposed using the 1‐point Dixon method.17 This process generated 3D images of the gas, barrier, and RBC components with a nominal isotropic resolution of 6.25 mm.
Although both 129Xe acquisitions provide ventilation images, the 3D dissolved‐phase sequence is significantly undersampled relative to the 2D GRE acquisition and may not resolve smaller defects.16 For this reason, the quantitative ventilation metrics were derived from the 2D GRE acquisition. Dedicated ventilation images as well as gas‐exchange images were deemed acceptable for analysis if the gas‐phase SNR was ≥5 based on previous work by He et al.18
Anatomical 1H MRI Scan Parameters
To facilitate quantitative analysis of the gas exchange and dedicated ventilation 129Xe images, two separate anatomical 1H MRI scans were acquired. Each scan was acquired during a single breath‐hold of a 1‐liter airbag dose in order to match lung inflation volume with their corresponding 129Xe scans. An isotropic radial image was acquired with an FOV matched to the gas exchange 129Xe scan, and a 2D steady‐state fast spin‐echo scan was acquired with an FOV and number of slices matched to the dedicated ventilation 129Xe scan.
Image Analysis
Dedicated 129Xe ventilation images were rendered into quantitative maps by rescaling by their top percentile of intensities and assigning each voxel into one of six classification “bins” using pre‐existing thresholds derived from a healthy reference cohort described previously.19 The percent of voxels falling in the lowest bin was designated the “ventilation defect percent,” or VDP, and the percent of voxels in the next‐lowest bin was designated the “low ventilation percent,” or LVP. Ventilation deficit was quantified as the sum of VDP and LVP, or “vendef + low.” This metric was chosen to capture potentially meaningful changes in partially obstructed (i.e., hypoventilated) as well as fully defected regions, an approach suggested previously by Myc et al.20 However, to provide a bridge to the existing literature, we also analyzed the change in the well‐established VDP metric.
Dissolved‐phase images were divided on a voxel‐by‐voxel basis by the gas‐phase intensities from the same acquisition in order to create normalized images of barrier uptake and RBC transfer. These were rendered into quantitative maps within the ventilated region of the thoracic cavity (i.e., the region within the thoracic cavity mask not classified as VDP on the corresponding gas‐phase image), thereby omitting voxels where the ventilation signal is most likely to be noise‐driven. Normalization to gas‐phase was performed on a voxel‐by‐voxel basis and then averaged to get the mean signal value, which was then divided by the corresponding mean value of the healthy reference population to produce the metrics bar%ref and RBC%ref.21
Pulmonary Function Tests
Spirometry and plethysmography were referenced using the Crapo/Hsu prediction equations.22, 23 Total lung capacity (TLC) and residual volume (RV) were measured using plethysmography for all but one subject. Diffusing capacity of the lung for carbon monoxide (DLCO) was measured using Vmax® Encore System (CareFusion, Yorba Linda, CA) and referenced using the Gaensler‐Smith equation.24 All tests met the technical criteria set by the American Thoracic Society and European Respiratory Society.25, 26, 27
Secondary Outcome Measures
Secondary outcome measures included the COPD Assessment Test (CAT),28 St. George's Respiratory Questionnaire (SGRQ),29 and the modified Borg Dyspnea Scale and 6‐minute walk test (6MWT).30 These measurements were performed at both the pre‐ and post‐treatment 129Xe MRI visits.
Statistical Analysis
A paired Student's t‐test was used to assess changes in vendef + low and conventional clinical measures following therapy. Pearson's correlation coefficient was used to assess the association between changes in 129Xe ventilation and PFTs/secondary outcomes, and to evaluate baseline measures of interstitial barrier and PFTs as potential predictors of 129Xe ventilation response to therapy. All statistical analysis was performed using R version 3.6.0.31 A P‐value of <0.05 was considered statistically significant. Note that this definition of significance is agnostic to a specific threshold for clinically meaningful changes in ventilation on 129Xe MRI, as such a threshold has yet to be empirically established.32
Results
Twenty subjects were initially recruited. One subject was excluded from data analysis due to poor image signal‐to‐noise ratio (SNR), and two elected not to return for a post‐treatment visit; thus, a total of 17 subjects (8M 9F) were included in the data analysis. Clinical data and pre‐treatment pulmonary function for this study population are shown in Table 1. Nine subjects were classified as GOLD II and eight as GOLD III. Figure 1 shows images of ventilation, barrier uptake, and RBC transfer from five example COPD subjects along with a representative healthy subject for reference. These COPD subjects exhibited clear regions of vendef + low (red and yellow), which were especially extensive in subjects 2, 4, and 5. Subjects 2–5 also exhibited clear regions of reduced barrier uptake (red and yellow) and all five subjects exhibited regions of reduced RBC transfer, with subjects 3–5 appearing devoid of any normal RBC transfer. Note that in the barrier and RBC maps, the dark areas within the thoracic cavity represent unventilated regions (defects) where analysis of gas exchange is not possible. Overall, subjects at baseline (i.e., prior to treatment) had vendef + low of 57.8 ± 8.4%, bar%ref of 73.2 ± 19.6%, and RBC%ref of 36.5 ± 13.6%.
TABLE 1.
Clinical Characteristics and Pre‐Treatment Pulmonary Function of the Study Population
| Patient | Sex | Age (years) | FEV1% pred | FVC% pred | TLC% pred | RV% pred | DLCO% pred |
|---|---|---|---|---|---|---|---|
| 01 | F | 55 | 29 | 74 | 124 | 206 | 42 |
| 02 | F | 67 | 33 | 74 | 113 | 155 | 52 |
| 03 | F | 71 | 75 | 98 | 84 | 64 | 51 |
| 04 | M | 64 | 66 | 97 | 88 | 72 | 39 |
| 05 | F | 58 | 29 | 66 | 104 | 150 | 36 |
| 06 | M | 66 | 61 | 77 | 92 | 121 | 75 |
| 07 | F | 64 | 61 | 91 | 96 | 98 | 76 |
| 08 | M | 74 | 64 | 96 | 101 | 104 | 34 |
| 09 | M | 69 | 43 | 76 | 96 | 134 | 71 |
| 10 | F | 61 | 51 | 73 | 95 | 123 | 64 |
| 11 | M | 59 | 68 | 94 | 95 | 98 | 66 |
| 12 | F | 66 | 48 | 60 | 96 | 143 | 104 |
| 13 | F | 66 | 42 | 89 | 125 | 173 | 32 |
| 14 | F | 67 | 57 | 90 | 129 | 167 | 67 |
| 15 | M | 67 | 38 | 67 | NA | NA | 54 |
| 16 | M | 58 | 45 | 97 | 112 | 144 | 56 |
| 17 | M | 61 | 70 | 90 | 99 | 121 | 64 |
| Mean | 64.3 | 52 | 83 | 103 | 127 | 58 | |
| SD | 4.9 | 15 | 13 | 12 | 29 | 19 |
All pulmonary function tests expressed as percent predicted (%).
DLCO = diffusion capacity of the lung for carbon monoxide; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; RV = residual volume; TLC = total lung capacity.
FIGURE 1.
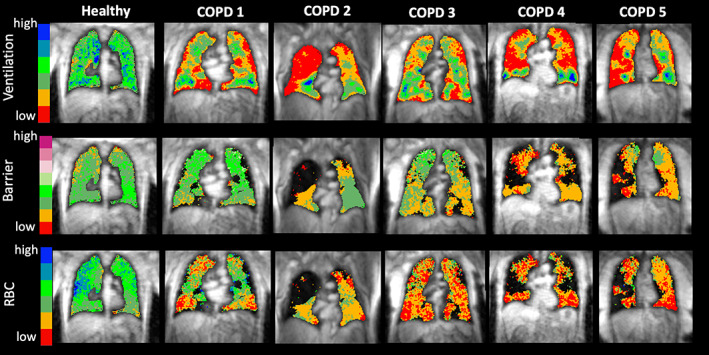
Example baseline129Xe magnetic resonance imaging (MRI) maps of ventilation, barrier uptake, and red blood cell (RBC) transfer from five chronic obstructive pulmonary disease (COPD) subjects and a healthy subject for reference. All maps are overlaid onto an anatomical proton MRI scan for reference. On the ventilation map, the lowest two of the color bins correspond to the vendef + low measurement. Barrier and RBC images are also binned to aid in visual interpretation. Note that black areas are associated with regions of ventilation defect where analysis of barrier and RBC is not possible. The healthy subject underwent informed consent and imaging as part of another study in our lab and is presented here solely for visual context.
Change in Measurements Following Therapy
Representative 129Xe ventilation images from subjects with a range of therapeutic responses are shown in Fig. 2. Some subjects exhibited a clear decrease in vendef + low (top row, left and right) after LABA/LAMA; green arrows indicate newly ventilated regions. However, other subjects showed no change in vendef + low or ventilation pattern (bottom row, left), while others showed an increase in vendef + low with newly emergent ventilation defects (bottom row, right), indicated by the red arrow.
FIGURE 2.
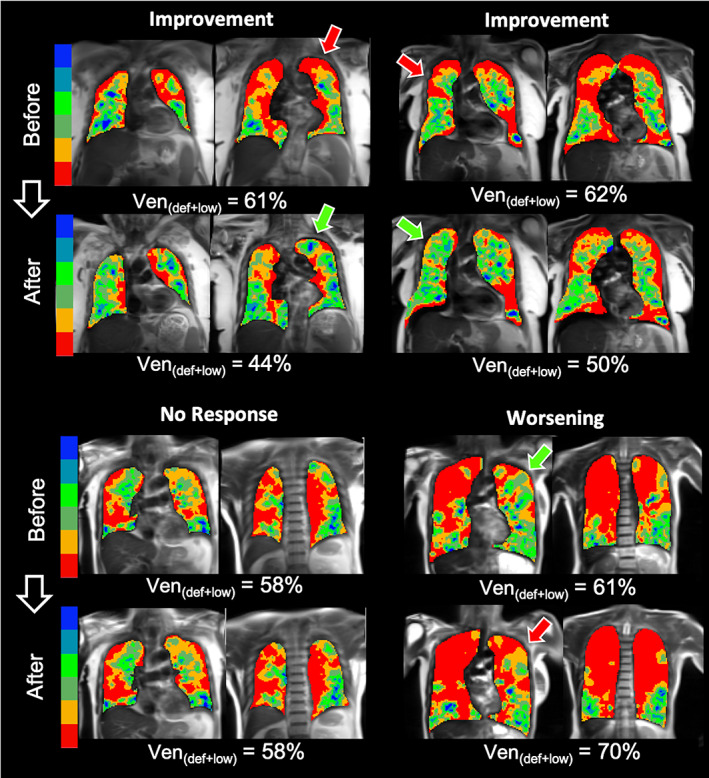
129Xe magnetic resonance imaging (MRI) ventilation maps before and after therapy from four representative subjects. The subjects in the top row showed improved (i.e., reduced) vendef + low following therapy, while subjects in the bottom row exhibited no change (left) or worsening (right). Arrows indicate prominent regions where ventilation changed following therapy.
In the overall study population, ventilation improved, with vendef + low decreasing from 57.8 ± 8.4% to 52.5 ± 10.6% following treatment (P < 0.05), as did VDP from 33.7 ± 8.9% to 29.5 ± 11.4% (P < 0.05). Barrier uptake did not change, with bar%ref values of 73.2 ± 19.6% before and 75.1 ± 20.6% after treatment (P = 0.23). Similarly, RBC%ref did not change, with values of 36.5 ± 13.6% before and 35.1 ± 14.0% after treatment (P = 0.21). Therapy also improved several conventional pulmonary function metrics: specifically, FEV1% and FVC% increased significantly following therapy (P < 0.05 in both cases). However, most metrics did not change significantly, including FEV1/FVC (P = 0.10), DLCO% (P = 0.80), TLC% (P = 0.16), or RV% (P = 0.24). Among clinical measures, CAT score decreased significantly (P < 0.05), but there was no significant change in Borg dyspnea score (P = 0.28), 6MWT (P = 0.12), or SGRQ (P = 0.44). The four metrics that changed significantly after therapy, including vendef + low, FEV1, FVC, and CAT score, are shown in Fig. 3.
FIGURE 3.
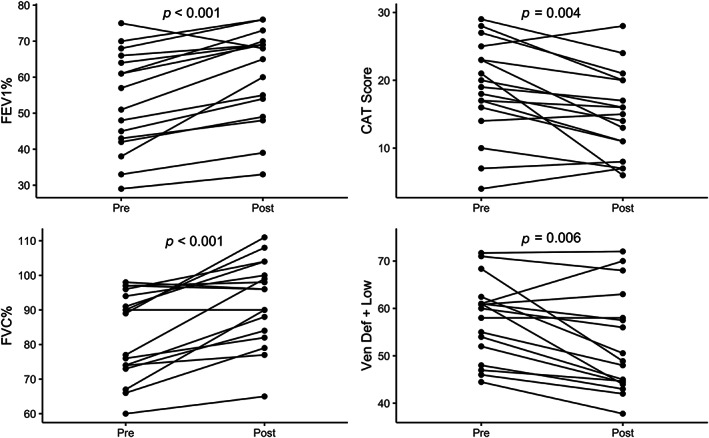
Clinical and ventilation metrics with significant changes following therapy. Each quadrant contains one of the four metrics that changed significantly following therapy. Values from each individual subject are shown before and after therapy, connected by a line. Significance: FEV1%, P < 0.001; CAT, P = 0.004; FVC%, P < 0.001, vendef + low, P < 0.006).
The absolute change in vendef + low after therapy was not significantly correlated with changes in FEV1% (P = 0.15), FVC% (P = 0.81), FEV1/FVC (P = 0.08), TLC% (P = 0.50), RV% (P = 0.26), or DLCO% (P = 0.66), nor with changes in 6MWT (P = 0.60) or with any of the questionnaires, including CAT (P = 0.73), SGRQ (P = 0.45), or Borg dyspnea score (P = 0.91).
Changes in 129Xe Ventilation, Barrier Uptake, and RBC Transfer
Absolute changes in 129Xe vendef + low, bar%ref, and RBC%ref following therapy are shown together in Fig. 4. Change in RBC%ref is shown on the vertical axis and change in bar%ref on the horizontal. Numbers next to each point (and the associated color scale) indicate the improvement (or worsening) in vendef + low for that particular subject. Only 3 of 17 (17.6%) subjects exhibited worsening ventilation after therapy as indicated by increasing vendef + low. Therapy caused mean barrier uptake to increase in 13/17 (76.5%) of subjects (i.e., right of the origin), whereas it revealed a decrease in RBC%ref (below the origin) in 10/17 (58.8%) subjects. The trend toward increased mean barrier uptake with a concomitant decrease in mean RBC transfer is illustrated by the plurality of subjects (7/17, 41.2%) falling in the lower‐right quadrant of Fig. 4. Indeed, only one subject (1/17, 5.9%) is in the upper‐left quadrant, indicating decreased barrier signal but increased RBC signal. An example of a subject with increased barrier uptake and decreased RBC transfer following therapy is shown in Fig. 5.
FIGURE 4.
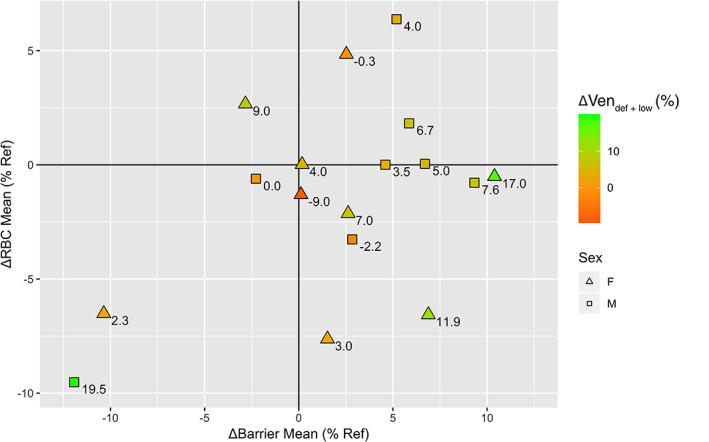
Changes in 129Xe gas exchange following therapy. Change in bar%ref (x‐axis) vs. change in RBC%ref (y‐axis) for each subject in the cohort following therapy. The value next to each point indicates the change in vendef + low following therapy, where a positive value (green color) indicates a decrease in the amount of ventilation defect.
FIGURE 5.
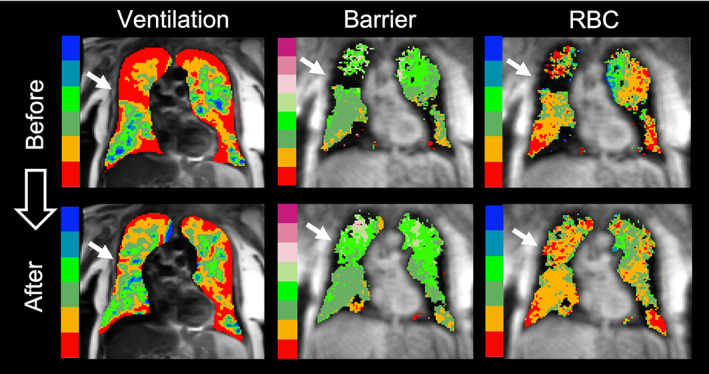
A newly ventilated region with poor red blood cell (RBC) transfer. Example of a subject where a region of improved ventilation following therapy (white arrows) revealed areas of normal barrier uptake but poor RBC transfer.
Baseline Measurements Associated with 129Xe Ventilation Change
As seen in Fig. 6, two metrics measured at baseline were found to be associated with post‐therapy changes in vendef + low: bar%ref (r = −0.49, P < 0.05) and DLCO% (r = −0.52, P < 0.05), although RBC%ref was just short of the threshold of significance (r = −0.47 [95% CI −0.77 to 0.01], P = 0.057). None of the other baseline PFTs or clinical markers under consideration were significantly associated with change in vendef + low, including FEV1% (P = 0.44), FVC% (P = 0.40), FEV1/FVC (P = 0.43), TLC% (P = 0.69), RV% (P = 0.82), CAT (P = 0.92), SGRQ (P = 0.80), 6MWT (0.83), or Borg dyspnea score (P = 0.08).
FIGURE 6.
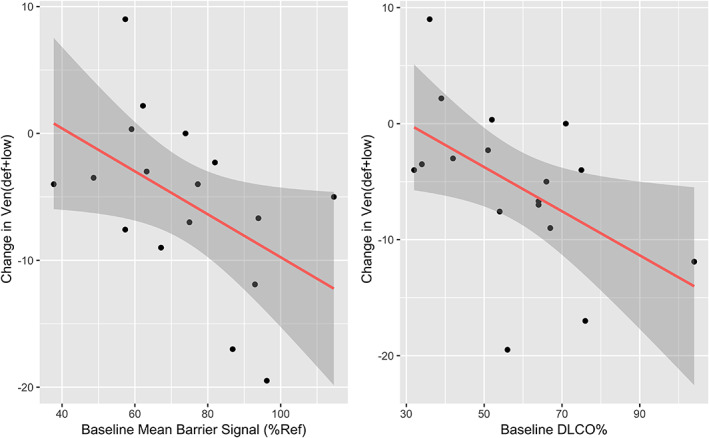
Barrier uptake and diffusing capacity of the lung for carbon monoxide (DLCO) at baseline are correlated with ventilation change. Change in vendef + low was correlated with both baseline bar%ref (shown at left; r = 0.49, P = 0.048) and DLCO% (right; r = −0.52, P = 0.032). None of the other baseline pulmonary function tests (PFTs) or clinical markers under consideration were significantly predictive of change in vendef + low. Linear regression line is shown with the shaded area representing the 95% confidence interval of the model.
Discussion
In this study, we found that each patient in this cohort of GOLD II/III COPD patients exhibited significant ventilation and RBC transfer defects, with barrier uptake that ranged from low to normal. As expected given our exclusion criteria, no subjects exhibited the high barrier uptake that has been previously associated with interstitial lung disease.8, 9, 10 After 2 weeks of LABA/LAMA therapy, regional ventilation was significantly improved across the cohort, reflected in decreasing vendef + low. These changes were also accompanied by significant improvements in FEV1%, FVC%, and CAT score. The improvement in ventilation enabled barrier uptake and RBC transfer to be analyzed in previously inaccessible regions of the lung. This revealed modestly higher barrier uptake after LABA/LAMA, but also exposed regions of reduced or absent RBC transfer. Ventilation improved in most but not all patients, with the degree of response being associated with baseline 129Xe barrier uptake and DLCO. Specifically, patients who had a more preserved level of barrier uptake, or a higher DLCO, exhibited greater improvement in 129Xe ventilation following therapy. This is consistent with the finding of Han et al that COPD subjects with higher DLCO were more likely to exhibit bronchoreversibility.33
Reduced 129Xe barrier signal and DLCO are both consistent with an emphysema‐predominant COPD phenotype in which the alveolar septa have been destroyed.34 This reduces the alveolar surface area available for gas diffusion into the blood and leads to airway collapse.34 Conversely, patients with relatively preserved measures of barrier uptake and DLCO may have airway obstruction that is caused by a bronchitis‐predominant phenotype.34 It is this subset who appeared more likely to respond to the LABA/LAMA treatment as measured by 129Xe ventilation MRI. This is further supported by the observation that mean barrier uptake increased after treatment, suggesting that newly exposed regions of the lung had preserved surface area for gas exchange. This is consistent with earlier findings by Baldi et al, in which bronchodilation in COPD was associated with a small but significant increase in DLCO. 35
By contrast, the consistently poor RBC transfer in this cohort both before and after therapy is striking mean 129Xe RBC transfer signal actually tended to decrease following therapy. Since 129Xe RBC transfer is a measure of raw RBC signal normalized by local ventilation, the observed decrease may be the result of newly ventilated regions exposing an underlying architecture with significant microvascular abnormalities.36, 37, 38 Our observations of increased barrier uptake coupled with decreasing RBC transfer after dual bronchodilator therapy suggest that these underlying regions had relatively preserved surface area for gas exchange, but significant underlying vascular abnormalities or lack of capillary blood volume. The finding that therapy‐induced redistribution of ventilation revealed additional regions of impaired gas exchange indicates a need for further studies to determine whether additional treatments targeting the pulmonary vasculature can recover more functionality in those regions.
These results suggest that a comprehensive assessment of therapeutic response in COPD requires functional measurements of gas exchange beyond those afforded by measurements of airway limitation and obstruction alone. Thus, 129Xe MRI is uniquely positioned to address this question by not only visualizing regional ventilation response but also revealing the functionality of the underlying tissue and vasculature that has been exposed.
In addition to improved regional 129Xe ventilation, we observed significant improvements in FEV1%, FVC%, and CAT scores. This is consistent with prior randomized controlled trials of the LABA/LAMA inhaler used in this study in moderate‐to‐severe COPD patients. Specifically, the PINNACLE‐1 and PINNACLE‐2 trials of glycopyrrolate/formoterol showed that after 24 weeks of therapy, the pre‐dose trough FEV1 increased by 153 mL and 105 mL respectively.39 In another study, peak FEV1 increased by approximately 300 mL after 7 days of therapy.40 Similarly, after 24 weeks of glycopyrrolate/formoterol MDI, prior studies have shown a change in CAT score of −3.41 This is similar to the change of −3.7 that we observed.
Thus, while numerous conventional metrics are capable of measuring a significant response to LABA/LAMA therapy, 129Xe MRI is able to measure that response regionally, and to directly observe the underlying gas exchange characteristics of newly ventilated regions. Of note is the relatively poor RBC transfer in newly ventilated regions. This appears to suggest that truly improving the trajectories of these patients will require addressing the underlying vascular abnormalities that currently prevent these newly ventilated regions from contributing meaningfully to gas exchange.
Limitations
Our study has several limitations. First, our sample size is relatively small and the study was conducted over a short time period. A larger population would allow us to better detect overall patterns of changes in gas exchange function following therapy, and enable multivariate models directly comparing DLCO and 129Xe as predictors of outcomes. A longer follow‐up period would enable the characterization of transient vs. long‐term changes in gas exchange following treatment, possibly as a result of ventilation‐perfusion matching. Second, CT was not acquired as part of the protocol. A contemporaneous CT could provide confirmation of disease phenotype and aid in the interpretation of 129Xe findings, as illustrated by recent work by Myc et al.20 Third, the repeatability of 129Xe MRI ventilation measurements in COPD has not been well characterized, limiting our ability to determine a clinically meaningful change in ventilation metrics. Previous studies have showed high repeatability for 129Xe measurements of VDP in asthma4 and cystic fibrosis,42 and analogous studies are needed for COPD. Further, while the RBC to barrier ratio is known to be more robust to lung inflation than those measurements taken individually,43 it is not possible to tell whether changes in this ratio are the result of changes in RBC, barrier, or both; thus we have elected here to report the two as separate measurements. Finally, in order to compare 129Xe MRI with conventional clinical metrics, we employed measures derived from the whole lung. However, additional insights may be gained by more regional analysis such as illustrated by Matin et al, who evaluated lobar correlations between CT measures of emphysema with 129Xe ventilation and apparent diffusion coefficient (ADC). A similar approach could be used with dissolved‐phase measures of gas exchange.44
Conclusion
In this study, LABA/LAMA therapy tended to preferentially improve ventilation in those subjects with relatively preserved measures of 129Xe barrier uptake and DLCO. However, even in subjects with improved ventilation, newly ventilated lung regions often revealed persistent 129Xe RBC transfer defects, an aspect of LABA/LAMA therapy response that is opaque to spirometry. Taken together, these results add to the body of knowledge regarding COPD phenotypes and indicate a possible role for 129Xe gas transfer MRI as a tool for both patient selection and measuring treatment response in future COPD clinical trials. As we develop therapies that demonstrably improve not only ventilation but also RBC transfer, 129Xe MRI may ultimately develop into a tool that can guide individualized patient care.
Conflict of Interest
D.G.M. is a consultant with Polarean Imaging, plc. B.D. is a founder and shareholder with Polarean Imaging, plc.
Author Contributions
B.D. and Y.‐C.H. designed and implemented the study. D.G.M., E.M.C., Z.W., and E.A.B. collected the data. D.G.M., E.M.C., Z.W., E.A.B., and J.L. were responsible for data analysis. All authors contributed to the writing of the manuscript.
Acknowledgments
The study was supported by an investigator‐initiated grant from AstraZeneca (ESR‐17‐12722) and NHLBI R01HL105643. The authors acknowledge Alexander Church for operating the hyperpolarizer equipment for these studies and Tatiana Johnson for editing and proofreading the manuscript.
D.G.M. and E.M.C. are co‐first authors.
References
- 1.Roos JE, McAdams HP, Kaushik SS, Driehuys B. Hyperpolarized gas MR imaging: Technique and applications. Magn Reson Imaging Clin 2015;23:217‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirby M, Svenningsen S, Kanhere N, et al. Pulmonary ventilation visualized using hyperpolarized helium‐3 and xenon‐129 magnetic resonance imaging: Differences in COPD and relationship to emphysema. J Appl Physiol 2013;114:707‐715. [DOI] [PubMed] [Google Scholar]
- 3.Kirby M, Svenningsen S, Owrangi A, et al. Hyperpolarized 3He and 129Xe MR imaging in healthy volunteers and patients with chronic obstructive pulmonary disease. Radiology 2012;265:600‐610. [DOI] [PubMed] [Google Scholar]
- 4.Ebner L, He M, Virgincar RS, et al. Hyperpolarized 129Xenon magnetic resonance imaging to quantify regional ventilation differences in mild to moderate asthma: A prospective comparison between semiautomated ventilation defect percentage calculation and pulmonary function tests. Invest Radiol 2017;52:120‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svenningsen S, Kirby M, Starr D, et al. Hyperpolarized 3He and 129Xe MRI: Differences in asthma before bronchodilation. J Magn Reson Imaging 2013;38:1521‐1530. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Robertson SH, Wang J, et al. Quantitative analysis of hyperpolarized 129Xe gas transfer MRI. Med Phys 2017;44:2415‐2428. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Rankine L, Bier EA, et al. Using hyperpolarized 129Xe gas exchange MRI to model the regional airspace, membrane and capillary contributions to diffusing capacity. J Appl Physiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang JM, Robertson SH, Wang Z, et al. Using hyperpolarized 129Xe MRI to quantify regional gas transfer in idiopathic pulmonary fibrosis. Thorax 2018;73:21‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rankine LJ, Wang Z, Wang JM, et al. 129Xenon gas exchange magnetic resonance imaging as a potential prognostic marker for progression of idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2020;17:121‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Bier EA, Swaminathan A, et al. Diverse cardiopulmonary diseases are associated with distinct xenon magnetic resonance imaging signatures. Eur Respir J 2019;54:1900831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deas SD, Huprikar N. Dual bronchodilator therapy for chronic obstructive pulmonary disease: Evidence for the efficacy and safety of fixed dose combination treatments in the setting of recent guideline updates. Curr Opin Pulm Med 2018;24:130‐137. [DOI] [PubMed] [Google Scholar]
- 12.Beeh KM, Burgel P‐R, Franssen FME, et al. How do dual long‐acting bronchodilators prevent exacerbations of chronic obstructive pulmonary disease? Am J Respir Crit Care Med 2017;196:139‐149. [DOI] [PubMed] [Google Scholar]
- 13.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med 2017;195:557‐582. [DOI] [PubMed] [Google Scholar]
- 14.He M, Robertson SH, Kaushik SS, et al. Dose and pulse sequence considerations for hyperpolarized 129Xe ventilation MRI. Magn Reson Imaging 2015;33:877‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bier EA, Robertson SH, Schrank GM, et al. A protocol for quantifying cardiogenic oscillations in dynamic 129Xe gas exchange spectroscopy: The effects of idiopathic pulmonary fibrosis. NMR Biomed 2019;32:e4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He M, Wang Z, Rankine L, et al. Generalized linear binning to compare hyperpolarized 129Xe ventilation maps derived from 3D radial gas exchange versus dedicated multislice gradient echo MRI. Acad Radiol 2020;27:e193‐e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaushik SS, Robertson SH, Freeman MS, et al. Single‐breath clinical imaging of hyperpolarized 129Xe in the airspaces, barrier, and red blood cells using an interleaved 3D radial 1‐point Dixon acquisition. Magn Reson Med 2016;75:1434‐1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He M, Zha W, Tan F, Rankine L, Fain S, Driehuys B. A comparison of two hyperpolarized 129Xe MRI ventilation quantification pipelines: The effect of signal to noise ratio. Acad Radiol 2019;26:949‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He M, Driehuys B, Que LG, Huang Y‐CT. Using hyperpolarized 129Xe MRI to quantify the pulmonary ventilation distribution. Acad Radiol 2016;23:1521‐1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myc L, Qing K, He M, et al. Characterisation of gas exchange in COPD with dissolved‐phase hyperpolarised xenon‐129 MRI. Thorax 2021;76:178‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, He M, Virgincar R, Bier E, Luo S, Driehuys B. Quantifying hyperpolarized 129Xe gas exchange MRI across platforms, field strength, and acquisition parameters. Proc Intl Soc Magn Reson Med 2019:6608. [Google Scholar]
- 22.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis 1981;123:659‐664. [DOI] [PubMed] [Google Scholar]
- 23.Hsu KHK, Jenkins DE, Hsi BP, et al. Ventilatory functions of normal children and young adults—Mexican‐American, white, and black. I. Spirometry. J Pediatr 1979;95:14‐23. [DOI] [PubMed] [Google Scholar]
- 24.Gaensler EA, Smith AA. Attachment for automated single breath diffusing capacity measurement. Chest 1973;63:136‐145. [DOI] [PubMed] [Google Scholar]
- 25.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single‐breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720‐735. [DOI] [PubMed] [Google Scholar]
- 26.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511‐522. [DOI] [PubMed] [Google Scholar]
- 27.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319‐338. [DOI] [PubMed] [Google Scholar]
- 28.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Leidy NK. Development and first validation of the COPD assessment test. Eur Respir J 2009;34:648‐654. [DOI] [PubMed] [Google Scholar]
- 29.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self‐complete measure of health status for chronic airflow limitation. Am Rev Respir Dis 1992;145:1321‐1327. [DOI] [PubMed] [Google Scholar]
- 30.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur Respir J 2014;44:1428‐1446. [DOI] [PubMed] [Google Scholar]
- 31.R Core Team . R: A language and environment for statistical computing [online]. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 32.Eddy RL, Svenningsen S, McCormack DG, Parraga G. What is the minimal clinically important difference for helium‐3 magnetic resonance imaging ventilation defects? Eur Respir J 2018;51:1800324. [DOI] [PubMed] [Google Scholar]
- 33.Han MK, Wise R, Mumford J, et al. Prevalence and clinical correlates of bronchoreversibility in severe emphysema. Eur Respir J 2010;35:1048‐1056. [DOI] [PubMed] [Google Scholar]
- 34.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004;364:709‐721. [DOI] [PubMed] [Google Scholar]
- 35.Baldi S, Fracchia C, Bruschi C, et al. Effect of bronchodilatation on single breath pulmonary uptake of carbon monoxide in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2006;1:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peinado VI, Pizarro S, Barbera JA. Pulmonary vascular involvement in COPD. Chest 2008;134:808‐814. [DOI] [PubMed] [Google Scholar]
- 37.Matsuoka S, Washko GR, Dransfield MT, et al. Quantitative CT measurement of cross‐sectional area of small pulmonary vessel in COPD: Correlations with emphysema and airflow limitation. Acad Radiol 2010;17:93‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oelsner EC, Balte PP, Grams ME, et al. Albuminuria, lung function decline, and risk of incident chronic obstructive pulmonary disease. The NHLBI Pooled Cohorts Study. Am J Respir Crit Care Med 2019;199:321‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez FJ, Rabe KF, Ferguson GT, et al. Efficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using co‐suspension delivery technology in patients with COPD. Chest 2017;151:340‐357. [DOI] [PubMed] [Google Scholar]
- 40.Reisner C, Pearle J, Kerwin EM, St Rose E, Darken P. Efficacy and safety of four doses of glycopyrrolate/formoterol fumarate delivered via a metered dose inhaler compared with the monocomponents in patients with moderate‐to‐severe COPD. Int J Chron Obstruct Pulmon Dis 2018;13:1965‐1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maltais F, Ferguson GT, Feldman GJ, et al. A randomized, double‐blind, double‐dummy study of glycopyrrolate/formoterol fumarate metered dose inhaler relative to umeclidinium/vilanterol dry powder inhaler in COPD. Adv Ther 2019;36:2434‐2449. [DOI] [PubMed] [Google Scholar]
- 42.Smith LJ, Horsley A, Bray J, et al. The assessment of short‐ and long‐term changes in lung function in cystic fibrosis using 129Xe MRI. Eur Respir J 2020;56:2000441. [DOI] [PubMed] [Google Scholar]
- 43.Hahn AD, Kammerman J, Evans M, et al. Repeatability of regional pulmonary functional metrics of hyperpolarized 129Xe dissolved‐phase MRI. J Magn Reson Imaging 2019;50:1182‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matin TN, Rahman N, Nickol AH, et al. Chronic obstructive pulmonary disease: Lobar analysis with hyperpolarized 129Xe MR imaging. Radiology 2017;282:857‐868. [DOI] [PubMed] [Google Scholar]


