Abstract
Introduction:
We present lipidomic studies that have utilized cadaveric biological samples, including tissues and bodily fluids (excluding blood or serum). Analyses of lipids from cadaveric derived tissues play vital roles in many different fields, such as in anthropogeny to understand food habits of ancient people, in forensics for postmortem analyses, and in biomedical research to study human diseases.
Areas covered:
The goal of the review is to demonstrate how cadavers can be utilized for study of lipidome to get biological insight in different fields. Several important considerations need to be made when analyzing lipids from cadaver samples. For example, what important postmortem changes occur due to environmental or other intrinsic factors that introduce deviations in the observed differences versus true differences? Do these factors affect distinct classes of lipids differently? How do we arrive at a reasonable level of certainty that the observed differences are truly biological rather than artifacts of sample collection, changes during transportation, or variations in analytical procedures? These are pressing questions that need to be addressed when performing lipidomics investigations utilizing postmortem tissues, which inherently presents hurdles and unknowns beginning with harvesting methods, transportation logistics, and at analytical techniques. In our review, we have purposefully omitted blood and serum studies since they pose greater challenges in this regard. Several studies have been carried out with cadaveric tissues and fluids that support the successful use cases of these samples; however, many control studies are still necessary to provide insight into full potential of the cadaveric tissue and fluid resources. Most importantly, additional control studies will allow us to gain important insights into the opportunities lipidomics presents for biomedical studies of complex human disease and disorders. Another goal of the review is to generate awareness about limitations and pitfalls of use cadaver materials for study of lipidome.
Keywords: lipidomics, cadaveric, postmortem, mass spectrometry
Expert Opinion:
We comment on the current state of lipidomics studies that utilize cadaveric tissues, provide a few pertinent examples, and discuss perspectives on both future technological directions and the applications they will enable.
1. Introduction
Lipidomics is the study of all lipids simultaneously for a cell or a tissue. It is estimated that mammals have more than 150,000 lipids that folds into 78 classes. The study of total lipids or lipidome of a cell or a tissue simultaneously is referred as lipidomics. The central dogma of molecular biology postulated that all functional information within the cells is coded by deoxyribonucleic acids or DNA [1]. Indeed, DNA became the macromolecule of focus for many decades. Subsequently, proteins were recognized as the main functional molecules of the cell a long time ago [2,3]. In 1673, Techenius Otto discovered that alkali is neutralized by animal fat in the process of making soap and thus suggested the presence of an acidic compound in fat. It was initially postulated that the living cells are made up of lipoidal material, which consists of the substances readily soluble in hydrocarbons [4]. It has now been firmly established that lipids are the biomolecules that provide boundary to cells through the formation of bilayer cell membranes [5]. Moreover, lipids have far more pertinent functions as signaling molecules than many other biomolecules such as proteins [6]. Lipids are the biomolecules that: 1) provide cells with a boundary; 2) serve as signaling molecules; 3) are involved in intracellular transport; 4) are involved in drug delivery; and 5) are important for cardiovascular and neuronal health. Hence, lipids have diagnostic and prognostic biomarker abilities. Lipids occupy a central place in biology, they are an important bellwether for cardiovascular and neuronal health. They provide deep insight into the biology and health of living organisms. The ability of lipids to deliver DNA and RNA has been known for several decades now; however, recently, lipids have sparked a renewed interest due to their ability to be used in nearly all forms of drug delivery. The point of this description is that lipids have an important role in structure and function of cells just as nucleic acids and proteins. However, compared to nucleic acids and proteins they remain less studied in last 2–3 decades.
Beyond drug delivery, the diagnostic and prognostic abilities that result from lipid profiling have been well recognized for cardiovascular health. The central nervous system (CNS) also has a unique role for lipids resulting from its unique lipid composition. For example, 80% of the cholesterol in the CNS is synthesized de novo while only about 20% or less is from dietary distribution [7]. The mechanism by which dietary cholesterol affects de novo synthesized cholesterol in the CNS and vice versa remains poorly understood. Lipids are also the most diverse class of molecules within mammalian systems. It is estimated that there are approximately ~150,000 lipid species that fall into 78 classes [8–10]. They play critical roles in biological processes including metabolism, cell signaling, and the formation of cell membranes [11]. The study of lipids has provided significant insight into diseases involved in all organ systems. The lipids work in tandem with other biomolecules in the context biology of cell. Analyses of lipids as lipidome (lipidomics) expands our holistic understanding of composition and their changes and help bridge the compositional gap.
2. Analytical challengesand sources of variability
Studies of the lipidome using cadaveric biological samples involve organ harvesting as first step (Figure 1). The use of cadavers in lipidomic research is emerging and has not been fully explored. This review serves to showcase some of the studies using cadaveric lipidomic approaches and in turn promote the use of lipidomics in cadaver tissues and fluids. In this review we have not considered cadaveric blood or serum as they have received much more attention regarding issues in harvesting, collection, and analytical procedures [12].
Figure 1. Schematic representation of cadaveric lipidomic studies workflow and potential points of error introduction.
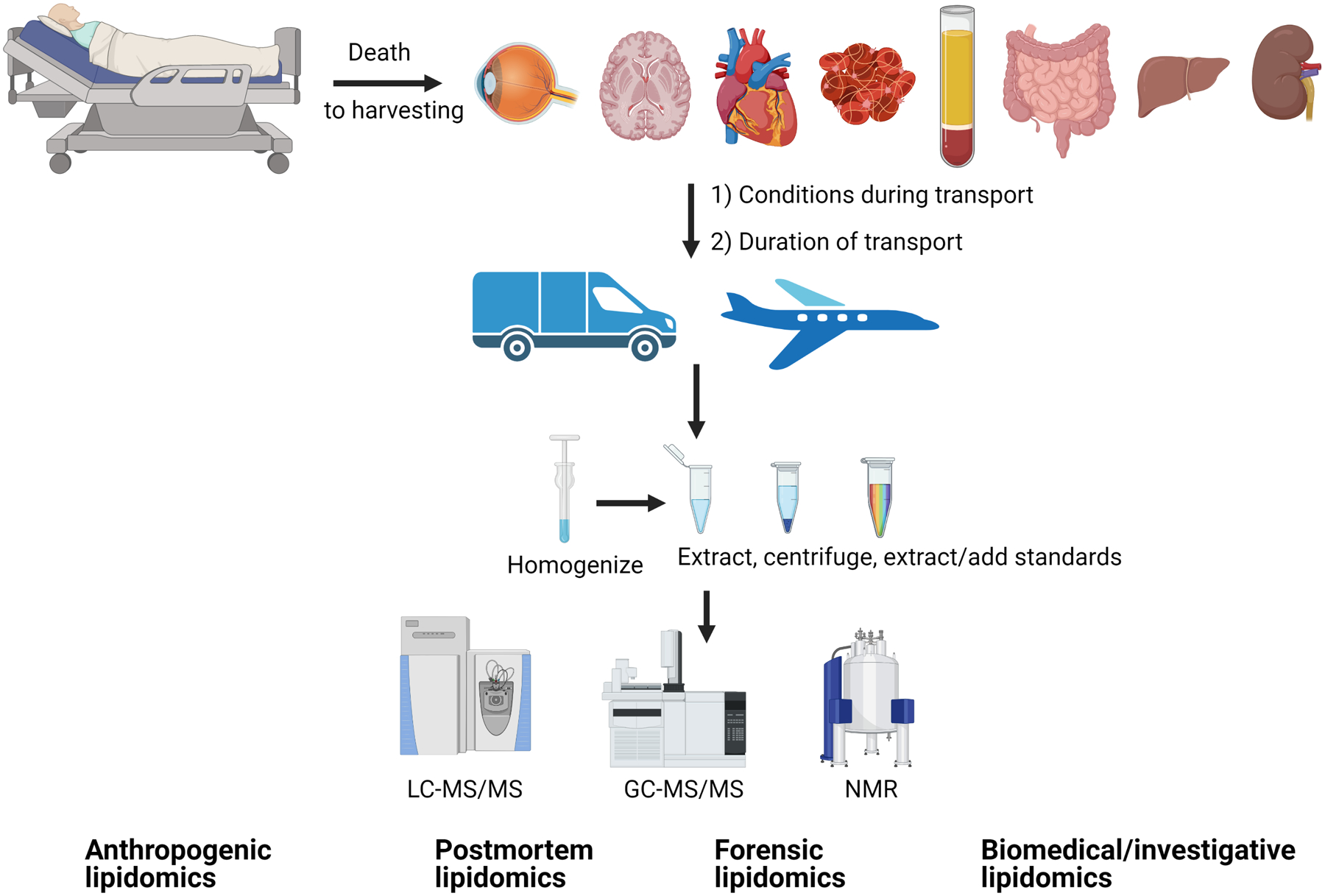
After death, organs/fluids are harvested. Depending upon conditions, small incisions biopsies are be performed on fresh cadavers to minimize tissue disruption. Biological samples (harvested organs/fluids) are then transported by various means. Transport conditions (such as temperature, humidity, duration) affect tissue modifications (for example, oxidation of lipids or enzymatic actions). Upon arrival to analytical laboratories, samples are homogenized (as needed for certain tissues) and extracted. The extraction process depends upon ultimate analytical technique. Various analytical instruments, such as NMR (nondestructive process) and/or mass spectrometry (destructive process), are utilized to obtain lipid profile and quantification. Figure bottom outlines some fields where such lipidomics data are utilized.
The following are the sources of variability:
The harvesting process. Harvesting may alter the lipidome significantly and whether these possible changes mask the actual differential data that the experiments are aiming to reveal.
The Transportation methods. The method for transportation of tissue or fluid samples are contributory to changes and mitigation techniques can be employed to diminish these effects.
Sample preparation techniques. Protocols such as homogenization and extraction can add significant errors particularly for quantitative studies.
Analytical techniques. The use of standards, analytical techniques, and finally,
Analytical software can introduce significant errors for whole lipidomes, length-selective lipidomes (based on carbon chain length), or class-selective lipidomes (based on constituents, double bond variation) (Figure 1). For example, a software that only matches precursor ion mass (do not use MS/MS fingerprint) may not distinguish two different lipids with same precursor ion mass. Similarly for identification of double bonds ion mobility data prior to mass spectrometry should be integrated to the software. The software algorithm may assign double bonded lipids simplistically and erroneously just based on precursor ion or one precursor and one MS/MS fingerprint.
In studies of the cornea [13,14], aqueous humor fluid [15–17], trabecular meshwork [18] and optic nerve [19] lipids extraction standards were shown to be effective for normalization. In general, we found that phospholipids undergo minimal changes due to the previously noted sources of error. We performed control studies subjecting freshly harvested cadaver tissue to conditions mimicking transport and/or different storage conditions [20]. These findings are consistent with prior studies involving human retina, optic nerve, or other ocular tissues [21–23].
Extraction methods:
The largest variability in quantification can be introduced by the method of extraction. Three commonly utilized extraction methods are:
Methyl tertiary-butyl ether (MTBE) method [24]; This method offers the advantage of quick extraction. The upper phase is extracted so that no interference from any other liquid or interphase layer is experienced.
Bligh-Dyer method [25]; This is one of the most commonly used method for extraction of lipids from bodily fluids and tissues.
The Folch method [26]; considered the gold standard for lipid extraction but a relatively time-consuming procedure.
Addition of internal standards prior to homogenization or during extraction allows for data normalization as well as improved quantification. Additionally, using internal standards for extraction enables early recognition of differences in extraction efficiency of different samples (for example, samples extracted with 95% efficiency versus 65% efficiency due to differences in technique or operator). The concentration of internal standards varies between studies. Ideally, each class of lipid being measured should include a low, high, and medium mass range lipid standard for extraction. However, such a range or varied inclusion of standards of many classes is not practical and thus only 1–2 standards are added. Currently, addition of standards for the most abundant lipid class is the norm; however, this does not accurately capture the low abundance and more difficult to extract lipids [18].
Instruments for analysis:
Nuclear magnetic resonance (NMR) offers nondestructive identification and quantification of lipids [27]. NMR is also readily able to distinguish lipid isomers [28,29]. A critical issue for lipidomics using NMR is the availability of pulse sequence and methods [30]. Lipids are special metabolites soluble in organic solvents [9,10]. Mass spectrometry (MS) is a destructive but relatively easier analytical method used for profiling, identification, and quantification [11]. The approaches to lipidomics using mass spectrometry involve shotgun lipidomics [31] and extensive fragmentation based automated identification [32,33]. Various chromatographic approaches such as ion exchange chromatography, reverse phase chromatography and Hydrophilic Interaction liquid chromatography have also been applied to lipidomics [34,35]. The mass spectrometry offers higher sensitivity, accuracy, and resolution when combined with chromatography as compared to chromatographic methods alone. Usually, mass spectrometry is combined with fractionation methods such as capillary electrophoresis and chromatography. For some lipid classes such as free cholesterol, gas chromatography (GC) offers fractionation, while for other classes such as esterified cholesterol, liquid chromatography (LC) is the method of choice for fractionation prior to mass spectrometry. Thus, gas-chromatography-mass spectrometry (GC-MS) is generally the method of choice for profiling cholesterol species. Tandem mass spectrometry (MS/MS) offers higher resolution than simpler mass spectrometers even when lipids are fractionated [33]. Analyses using non-destructive methods such as NMR followed by destructive mass spectrometry provides more comprehensive investigation and higher confidence levels for identification and quantification. These combined approaches are not uncommon for studies in biomedical research [36,37]. A common issue for mass spectrometry is identification of isomers. The application of ion mobility (generally prior to mass spectrometry) has allowed for great improvements with these identifications [38,39], and is expected to be refined further in future. Similarly, the use of high-resolution magic angle spinning (HR-MAS) [40–42] combined with sample processing through cryogenically cooled microcapillary probes [43] is another technological advancement expected to revolutionize future NMR protocols for lipidomics and metabolomics.
Software and lipid nomenclature:
Several approaches have been applied to develop algorithms for software for specific applications in methods [31,32]. These software have evolved over time to enhance high throughput identification and analyses of lipids by mass spectrometry [32]. As noted above, NMR pulse sequence, databases, and software developments are also under continuous development for lipidomics. Lipid nomenclature and database preparation has also undergone significant evolution through different approaches [8,9,44]. The Lipidsearch™ (commercially available from Thermo Fischer Scientific) and LipidXplorer remains some of the major software for identification and quantification of lipids.
The chemical events modifying the lipidome is also a source of variability. This includes oxidative modification as well as other changes such as enzymatic degradation that should be prevented. These changes introducing variabilities have been extensively reviewed elsewhere [45,46].
Three main technologies: NMR, LC-MS/MS and GC-MS or GC-MS/MS, are used for analyses of lipids, they provide independent datasets. However, they often generate complementary dataset and the identities or information can be integrated. For example, GC-MS is able to quantify free cholesterol while LC-MS/MS is able to quantify esterified cholesterol. Only when these two datasets are combined, they render the complete integrated picture of total cholesterol species. Now GC-MS data alone is free cholesterol lipidome and they can be viewed and analyzed alone. However, use of the three technologies provide overlapping (higher confidence and confirmatory data) and they can be used for integrated and complementary information on different species. However, each technology has independent issues that need to be considered for each technology.
3. Application fields with examples
Lipidomics investigations using human cadaveric tissues and fluids can be divided into a few different categories:
Anthropogenic lipidomics:
Study of lipidome and their changes surrounding the environments related to human activities is termed anthropogenic lipidomics. Lipids are ubiquitous and abundant in nearly all organisms, and they are sensitive to changes in environment. A well-preserved lipid provides unique insights into how humans have interacted with other organisms over different geographic regions and times and the different environmental conditions that existed concurrently [47].A particular of study of interest examined ceramic lipid residues to shed light on the diversity of food in the diet of the Indus civilization in the northwest Indian subcontinent [48]. In this study, lipid residue analysis was performed by isolating ceramic fragments of eating vessels (or pottery). Lipids were extracted and methylated through the acidified methanol technique and analyzed using gas chromatography-mass spectrometry, then subjected to gas-chromatography-combustion-isotope-ratio mass spectrometry. Interpretable lipid concentrations were obtained; however, the overall level was relatively low. The low level of lipids in the residual material samples can be attributed to the heavy rain and hot temperature of the region. The result showed an abundance of animal fat and absence of aquatic fat. Interestingly, biomarkers for millet were also absent from the samples found within ceramic utensil fragments, although millet is present during summertime in the region. The lipid identification results/lipid profile can be interpreted in many different ways, especially when other data (such as DNA and protein profiles) are obtained from the same materials. At a minimum, this kind of study suggests the utility of studying lipidomics and important insight it may provide when examined in conjunction with other high-throughput experimental evidence in anthropogenic studies. It is extremely important to recognize the inherent stability of lipids and stable lipid modifications (that occur in a defined manner) for these types of studies when they involve cadaver/mummified sample derived lipids.
Forensic lipidomics and Postmortomics:
The establishment of a timeline is extremely important in the solving of fatal crimes. The current method to calculate Post-Mortem Interval (PMI) has significant shortfalls as it relies heavily on the experience and the opinion of the investigator, often rendering this metric as subjective and unreliable. Similar to the use of lipidomics for anthropogenic studies, lipidomics are a powerful addition to the arsenal of investigative and corroborative tools in the repertoire of modalities used for the collection of forensic evidence. This is also relevant for holistic postmortem “Omics” or “multi-omics” studies. Of course, since they utilize cadaveric tissues, all the questions raised above regarding error in the data (Fig. 1) are relevant for these studies as well. It is imperative to find biomarkers that provide better, more accurate estimates of the time of death in an objective manner [49].
Generally, to minimize disruption of sample integrity, a tissue biopsy is performed using the smallest possible incision. In a case study, tissue was collected via incision, put on dry ice immediately, and stored at −80°C. Six cadavers were separated into two experimental groups: one under refrigeration and one exposed to the natural environment (with average temperatures of 23.3 and 17.8°C) to maximize the reliability of the result. Extraction and analysis utilized a chloroform/methanol/water solution and hydrophilic interaction mass spectrometry, respectively. PMI calculation was reported in cumulative degree hours (CDH) to account for the temperature effect on body decomposition. Several amino acids, particularly histidine and lysine, showed a similar trend of increase and are expected to be candidates for reliable calculation of PMI upon further validation. Choline phosphate, a metabolic product of phosphatidylcholine has also shown positive linear relationship with PMI [49] and is a potential candidate to serve as a biomarker for PMI.
Two current methods are commonly used to calculate PMI [49,50]: (1) Potassium content in the vitreous humor [50,51]; however, it is only accurate before body decomposition. Longer PMI calculation comes from visual inspection and is a much more subjective, making it vastly unreliable. (2) The total body score (TBS) to calculate accumulated degree days (ADD) is a more reliable method; however, it has not been fully validated. A preliminary study has shown through direct infused lipidomics that the degradation of glycerophospholipids in skeletal muscle seems to be a reliable indicator for PMI. In this study, two sets of samples were obtained from donors to test the validity of this process. Lipids were extracted using water and methanol. Internal standards were added. Constant infusion lipidomics was performed and various phospholipids were quantified through MS/MS, including ethanolamine plasmalogens (PlsE), phosphatidylethanolamines (PtdE), choline plasmalogens (PlsC), phosphatidylcholines (PtdC), and phosphatidylglycerols (PG). The most noticeable difference was observed in PtdE and the most consistent differences were observed in PG and PtdE. A regression model was developed and was able to evaluate PMI within a high confidence interval using PtdE and PG; however, accurate ADD could not be validated independently and hence the method is yet not recommended for forensic use. These findings suggest the powerful potential for the use of lipidomics within forensic and postmortem sciences. Additional studies in these areas are warranted to develop protocols for regular use in forensic and postmortomic investigations.
Biomedical investigations:
The biomedical investigation here refers to investigation into the biology of disease, disorder or related aspects. The use of cadaver tissue for research has long been utilized for anatomy, histology, pathology, and in the invention of new surgical techniques. With the recent advances made in the field of chromatography, mass spectrometry, NMR, and other techniques we are now poised to generate molecular data utilizing cadaver tissues; thus, allowing for more extensive research within biomedical sciences. The use of cadaveric tissue for lipidomic purposes is poised to yield great insights, for research such as investigating novel biomarkers for disease evaluation and potential targets for new treatments. The recent application of cadaveric lipidomics has made significant contributions to research of brain, eye, liver, and heart disease; however, given the large number of lipids and their vast diversity, substantial control studies (Figure 1) need to be performed to learn limitations and proper interpretation.
Postmortem brain tissues of patients with Alzheimer’s Disease (AD) [52] demonstrated differences in the brain transcriptomes and lipidomes of AD patients as compared to control, suggesting APOE allele-associated differences in the pathogenic mechanism of Alzheimer’s. Differences in myelination detected through lipidomics in this study point to issues possibly arising due to dysregulated proteostasis in these tissues. Another investigation of lipidomics differences in phospholipids (PL) between postmortem prefrontal cortex of individuals with AD compared to control individuals without AD [53] noted minimal changes in PL species between the AD and control brain tissues; however, it demonstrated choline plasminogen 18:0/22:6 and stearic acid (18:0) in total PLs were reduced by 26–27% in AD prefrontal cortex tissues compared to controls.
Subcortical ischemic vascular dementia (SIVD), one of the leading causes of elderly memory loss, has not been fully explored despite vast array of studies on AD [54]. Understanding the full etiology and the process of SIVD may offer insights for the prevention and treatment for this disease. Due to the role lipids play in the vascular formation and pathways, the group decided to examine the full lipidome of the patients, including those with mixed dementia (resulting due to the co-occurrence of the infarction and cognitive impairment). White and grey matter of the patients were collected postmortem temporal lobe tissue. Lipid extraction was performed using a modified Bligh and Dyer method and samples were analyzed through LC/MS. The results showed an overall reduction of the sphingolipidome and an increase of phospholipids in the white matter in pure SIVD patients. What is interesting is that the opposite happened in the grey matter of patients with mixed dementia. The reduction of sphingolipid correlated with reduced myelination by oligodendrocytes. Available data suggested preferential death of oligodendrocytes. The increase of phospholipid has been speculated to be an adaptive protection against the oxidative environment resulting from ischemia. This is consistent with independent observations that ischemia tends to start in the frontal and parietal lobe and progresses to temporal lobe and with observations from an independent better controlled study on mouse models with a defective ceremide synthase 2 [55]. Authors postulated that the damaged endolysosomal function led to an inability to metabolize sphingolipid containing lipoprotein, thus increasing this class of lipids while phospholipids were reduced due to neurodegenerative defects in mixed dementia.
Moving from brain to eye, we find a number of ocular studies have utilized cadaveric tissues from anterior to posterior eye chambers [13,18,19,51,56–58]. In diabetic retinopathy, sphingolipid is extracted with a combination of ethanol/methanol/water solution with the 7:2:1 ratio including internal standards. The sphingolipids between type 2 diabetics and nondiabetic donors were analyzed through LC-MS/MS. There was a 3% increase in the ceramide concentration and subsequent decrease in hexosylceramide and sphingomyelin composition. The most significant difference was in long chain sphingolipid where dihydro-sphingosine-1-phosphate level increased significantly. The effect on or of the increase of ceramide and long chain sphingolipid containing ceramide seems to be independent of the control of glucose level in patients. More studies are warranted for the further investigation of the human vitreous humor sphingolipids including other disease pathologies that involve the retina and retinal vessels.
To assess the metabolomic profiles of donor tissues and their associations with allograft function in transplant recipients, a lipidomics approach was utilized on liver graft donors [59]. The liver biopsies of 124 donors were collected before transplantation and analyzed using LC-MS. Donor liver grafts were classified into two groups, either showing early allograft dysfunction (EAD) or immediate graft function (IGF). The study identified a set of dysfunction-associated biomarkers, with several key changes noted including significantly increased levels of bile acids, lysophospholipids, phospholipids, sphingomyelins, and histidine metabolism products. These biomolecules were thought to suggest disruptions in lipid homeostasis and altered histidine pathway within donor tissues that led to early allograft dysfunction following liver transplantation. A predictive EAD model was developed based on these molecules as biomarkers, which yielded 91% sensitivity and 82% specificity. In this case, the use of lipidomics successfully deciphered a biosignature that accurately differentiates donor livers that later showed EAD or IGF. This approach thus has a potential to become a predictive clinical tool for donor liver quality assessment and for anticipating graft function prior to transplantation of donor livers.
The loss of cardiac tetralinoleoyl cardiolipin (L4CL), a lipid, which is a mitochondrial metabolic product, was investigated in humans with heart failure (HF) [60]. Left ventricle (LV) tissues were obtained from explant hearts (cadaver derived tissue) of HF patients diagnosed with idiopathic dilated cardiomyopathy (IDC) and from non-failing (NF) patients with no history of cardiac or pulmonary disease as a control. The L4CL content was found to be significantly lower in LV samples obtained from the IDC hearts as compared to NF hearts (p<0.05). Additionally, a significant increase in minor cardiolipin (CL) species containing alternate acyl side chains was found in the IDC versus NF hearts. Overall decrease in CL mass was observed in the IDC vs NF hearts. The loss of CL mass in IDC samples was not attributable to a decrease in mitochondrial density because no significant difference in cytochrome oxidase (COx) protein was found between the groups (IDC vs NF). Overall, alongside rat model experiments, the study suggested that a progressive loss of cardiac L4CL occurs in response to chronic cardiac overload, but not aging alone. These findings are consistent in both interfibrillar and subsarcolemmal cardiac mitochondria. These cadaveric human tissue lipidomics contributed to expanding our understanding of mitochondrial respiratory dysfunction during the pathogenesis of heart failure. They also offer potential biotargets for the development of novel treatments to prevent the progression of heart failure.
4. Expert opinion
This review presents how cadavers have been utilized for lipidomic studies in different field to draw biological insight, rendering the readership aware of limitations and pitfalls of such studies. All the examples noted above suggested that whether by design, or through the use of residual cadaveric byproducts or tissues, cadaveric tissue lipidomics has an important place in biomedical investigations of human disease and disorders. Lipidomics studies alone can provide important insights and may add complementary or corroborative knowledge to adjacent studies. Biological lipids are a repertoire with tremendous promise. They are a new frontier for developing novel intervention strategies inherently, as endogenous molecules, or following appropriate modifications. Lipid pathways are concerted targets for cardiovascular and neuronal health. Important insights can be derived through anthropogenic studies from lipidomics. Lipid analyses in isolation or combined with proteomics or metabolomics as well as other biological investigations can expand our understanding of anthropogeny, postmortomics, and biomedical science. Such combinations can also help corroborate important findings.
Quadomics:
An important feature required in all high throughput analyses is reproducibility. The concept of “Quadomics” has been developed [61] to evaluate the reproducibility of “-Omics” findings with a particular focus on utilizing them for potential clinical applications in the future. Quadomics stands for quality assessment of omics studies for the evaluation of the methodological quality. Over-interpretation as well as statistical over-fitting of data is minimized due to Quadomics considerations in -omics studies. The low sample size of the current studies can mask the data variability and present validation issues. Most lipidomics studies using cadaveric tissues report specific method details such as lipid extraction procedure, mass spectrometer type, and operating/identification software. As noted, validation studies are necessary to mitigating the biological parameters of variability. Small sample size in the reported studies and other issues remain to be addressed to be fully compliant with recommended Quadomics parameters[61]. Due to inherent variability in samples, all high throughput analyses have reproducibility limits. Often the reproducibility is ensured with respect to a limited “molecular” outcome such as one or more key molecules or a range of quantitative value for a lipid class or molecules (for example, low density lipoprotein for cardiovascular disease risk). However, overall parametric assessment for reproducibility is highly helpful for normalization and for these studies.
Cadaveric lipidomic studies have enriched us with a better understanding of civilizations, given us a more accurate estimation of postmortem interval, and provided insights into the underlying physiological changes in disease pathology. Cadaveric lipidomic findings may well offer important additional clinical insight into the understanding and treatment of human health and disease. More studies are necessary to confirm the validity of the results due to known and unknown confounding variables (ie. tissue procurement/preservation protocols, transport conditions, oxidative modifications, degradations, other modifications). With the huge progress made in the analytical techniques such as NMR, mass spectrometry, and chromatography, it is evident that more holistic “-omics” studies including lipidomics would benefit the research greatly. Application of Quadomics concepts in lipidomics studies will be helpful to address issues with reproducibility and reliability of these studies. A number of developments are occurring in all omics fields and enabling their use for machine learning and deep learning to predict the disease susceptibility, progression, and treatment efficacy. The advancement of ion mobility [38,39] and magic angle spinning [40,41] for mass spectrometry and high-resolution NMR, respectively, will greatly expand the analytical ability, reproducibility, and sensitivity of analyses for lipidomics. These advances along with control experiments are expected to fuel the broad utilization of cadavers for lipidomics as a source to gain insight into biological processes and expand our understanding of normal function and the aberrations that results in disease. Late-onset and progressive diseases are likely to see the most benefit from cadaver lipidomics. Given the strong, growing technological foundation and broad potential applications of these studies, it is likely that cadaveric lipidomics with receive greater momentum in the future for research in the biomedical sciences.
Table 1.
Lipidomics studies utilizing cadaveric or related biological samples
| Anthropogenic investigations | ||||||
|---|---|---|---|---|---|---|
| Research Focus | Source | Extraction Method | Internal Standard | Analytical Method | Instrument | Citations |
| Use of biomarkers in archaelogical context to define early manuring practice | Anthropogenic Soil (Scotland) | Bligh and Dyer | 5β-pregnan-3α-ol, hyocholic acid | High temperature-GC (HT-GC) | Hewlett-Packard 5890 Series II GC | Simpson et al. 1988 |
| investigate foodstuffs used in ceramic vessels by populations of the Indus Civilization in northwest India | Pottery fragments (Northwest India) | Acidified methanol, n-hexanes | Not specified | GC-MS, GC-C-IRMS | Agilent 7890 B GC, Agilent 5977 B MS; Hewlett Packard 7890B GC | Suryanaran et al.2020 |
| Faecal biomarkers for species identification | Faeces, soil | Solid phase extraction (ENVY disks) | 5α-cholestane | GC-MS | Shimadzu QP2010plus | Harrault et al. 2019 |
| Forensic/postmortem investigations | ||||||
| Membrane phospholipids as a biomarker for longer term PMI | Cadaveric Skeletal Muscle (quadriceps) | MTBE | ([2H31]PtdE 34:1, [2H54]PtdE 28:0, [2H31]PtdC 34:1, [2H54]PtdC 28:0) | ESI+MS/MS | Thermo Q Exactive | Langley et al. 2019 |
| Metabolites as biomarkers for PMI | Rat, Human muscle (biceps femoris) | chloroform/methanol/water solution 20:60:20 | Not specified | LC-MS | Thermo Q Exactive | Pesko et al. 2020 |
| Lipodomic analyses of postmortem tissues suitable to provide quantitative, objective PMI estimates | Cadaveric Skeletal Muscle (quadriceps) | MTBE | [2H8]arachidonic acid, [2H4]hexacosanoic acid, [2H31] PtdEtn 34:1, [2H31]PtdCh 34:1, and [2H31]PG 34:1 | ESI+MS/MS | Thermo Q Exactive | Wood et al. 2013 |
| Biomedical/Human health and disease investigations | ||||||
| Phospholipid profiles of human trabecular meshwork in glaucoma vs control | Cadaveric trabecular meshwork | Modified Bligh and Dyer | 1,2-ditridecanoyl-sn-glycero-3-phosphocholine, 1,2-dioleoyl-sn-glycero-3-phospho-L-serine, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, 1,2-dioleoyl-sn-glycero-3-phospho-(10-myo-inositol) | LC-MS/MS | TSQ Quantum Access Max | Arbindi et al. 2013 |
| Lipidomics reveal impaired glucosylspingosine lipids pathway in glaucoma | Cadaveric optic nerve | Modified Bligh and Dyer | Not specified | LC-MS | Thermo Q Exactive | Chauhan et al. 2019 |
| Sphingolipid profile of diabetic cornea | Cadaveric cornea | Not specified | Not specified | LC-MS/MS | Shimadzu Nexera UPLC | Priyadarsini et al. 2015 |
| Lipid and protein profile in diabetic vitreous | Human vitreous | Acidified methanol, solid-phase extraction | 500 pg each of d4-PGE2, d8-12-HETE, d11-11(12)- DiHETrE (dihydroxyeicosatrienoic acid), d11-8(9)-EET, d6-20-HETE, and d8-11(12)-EET | LC-MS/MS | Q-trap 3200 | Schwartzman et al. 2010 |
| Sphingolipid and ceramide profiles of human trabecular meshwork | Cadaveric trabecular meshwork | Bligh and Dyer | N-oleoyl-D-erythro-sphingosylphosphorylcholine, D-er- ythro-sphingosine, D-erythro-sphingosine-1-phosphate, and N- oleoyl-D-erythro-sphingosine | GC-MS | TSQ Quantum Access Max | Aljohani et al. 2014 |
| Spingolipid profile in Small Ischemic Vascular Dementia (SIVD) | Cadaveric brain (temporal lobe) | Modified Bligh and Dyer | PC-14:0/14:0, PE-14:0/14:0, PS-14:0/14:0, PA-17:0/17:0, PG-14:0/14:0, PI-34:1/d31, LPC-C20:0, LPE-C17:0, C8-GluCer, C17-Cer, C14-LBPA, C12-SM, C12-SL | LC-MS/MS | Q-trap 3200 | Lam et al. 2013 |
| Donor liver biomarkers associated with early allograft dysfunction | Human Liver | Methanol | Not specified | LC-MS and MALDI | Xevo G2 | Li et al. 2017 |
| Aberrant lipid metabolism in Hepatocellular Varcinoma | Human Liver | Not specified | Not specified | LC-MS | Not specified | Cortes et al. 2014 |
| Changes in phospholipid profile in Alzheimer’s Disease | Cadaveric brain (prefrontal cortex) | MTBE | Not specified | MS | Xcalibur and nano me | Lefterov et al. 2014 |
| Loss of cardiac tetralinoleoyl cadiolipin in Heart Failure | Cadaveric heart (left ventricle) | Modified Bligh and Dyer | Not specified | not specified | Not specified | Sparagna et al. 2007 |
| APOE2 orchestrated differences in transcriptomic and lipidomic profiles in Alzheimer’s Disease | Cadaveric brain (parietal love) | Folch | PlsCho 16:0/18:1, 16:0/18:2, 16:0/20:4 and 16:0/22:6 | LC-MS/MS | Agilent 1290 | Otoki et al. 2021 |
Article highlights.
Postmortem to harvesting time, transport conditions, transport duration, extraction methods, use of extraction standards and internal standards for quantification are sources of common indeterministic errors.
Substantial technological advances have been made in analytical platforms (both mass spectrometry and NMR) for lipidomics. Use of ion mobility prior to mass spectrometry and magic angle spinning for high resolution NMR combined with cryogenic cooling probes enables greater sensitivity and increases reproducibility in analyses.
Lipidomics opens up the use of machine-learning and deep-learning to identify predictive value for susceptibility, progression and intervention efficacy for non-communicable, late-onset, and complex diseases.
Lipidomics combined with other “-omics” is poised to provide important insights into normal biological processes and mechanisms of disease, in addition to valuable information in anthropogenic and forensic studies.
Cadaver-derived biological samples utilized for lipidomics (and other -omics) are expected to gain tremendous momentum in the near future.
Funding
This paper was funded by the U.S. Department of Health and Human Services, National Institutes of Health: EY031292; and the U.S. Department of Defense, W81XWH-19-1-0845.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Funding
This paper was in part supported by grants from National Institutes of Health: EY031292, EY14801 and US Department of Defense grant W81XWH-19-1-0845.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Crick F. Central dogma of molecular biology. Nature. 1970August8;227(5258):561–3. 10.1038/227561a0 [DOI] [PubMed] [Google Scholar]
- 2.Mulder GJ. Sur la composition de quelques substances animales Bulletin des Sciences Physiques et Naturelles en Néerlande. 1838;104. [Google Scholar]
- 3.Hartley H. Origin of the word ‘protein’. Nature. 1951August11;168(4267):244. 10.1038/168244a0 [DOI] [PubMed] [Google Scholar]
- 4.Danielli JF, Davson H. A contribution to the theory of permeability of thin films. J Cellular and Comp Physiol. 1935;5(4):495–508. [Google Scholar]
- 5.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972February18;175(4023):720–31. 10.1126/science.175.4023.720 [DOI] [PubMed] [Google Scholar]
- 6.Piomelli D, Astarita G, Rapaka R. A neuroscientist’s guide to lipidomics. Nat Rev Neurosci. 2007October;8(10):743–54. [DOI] [PubMed] [Google Scholar]
- 7.Lin JB, Mast N, Bederman IR, et al. Cholesterol in mouse retina originates primarily from in situ de novo biosynthesis. J Lipid Res. 2016February;57(2):258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fahy E, Subramaniam S, Murphy RC, et al. Update of the LIPID MAPS comprehensive classification system for lipids. J Lipid Res. 2009April;50Suppl:S9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahy E, Subramaniam S, Brown HA, et al. A comprehensive classification system for lipids. J Lipid Res. 2005May;46(5):839–61. 10.1194/jlr.E400004-JLR200 [DOI] [PubMed] [Google Scholar]
- 10.Fahy E, Cotter D, Sud M, et al. Lipid classification, structures and tools. Biochim Biophys Acta. 2011November;1811(11):637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang K, Han X. Lipidomics: Techniques, Applications, and Outcomes Related to Biomedical Sciences. Trends Biochem Sci. 2016November;41(11):954–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villanueva J, Philip J, Chaparro CA, et al. Correcting common errors in identifying cancer-specific serum peptide signatures. J Proteome Res. 2005Jul-Aug;4(4):1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crane AM, Hua HU, Coggin AD, et al. Mass spectrometric analyses of phosphatidylcholines in alkali-exposed corneal tissue. Invest Ophthalmol Vis Sci. 2012;53(11):7122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parikh T, Eisner N, Venugopalan P, et al. Proteomic analyses of corneal tissue subjected to alkali exposure. Invest Ophthalmol Vis Sci. 2011March;52(3):1819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aljohani AJ, Munguba GC, Guerra Y, et al. Sphingolipids and ceramides in human aqueous humor. Mol Vis. 2013;19:1966–84. [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards G, Aribindi K, Guerra Y, et al. Phospholipid profiles of control and glaucomatous human aqueous humor. Biochimie. 2014June;101:232–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards G, Aribindi K, Guerra Y, et al. Sphingolipids and ceramides of mouse aqueous humor: Comparative profiles from normotensive and hypertensive DBA/2J mice. Biochimie. 2014July9;105:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aribindi K, Guerra Y, Lee RK, et al. Comparative phospholipid profiles of control and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2013;54(4):3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chauhan MZ, Valencia AK, Piqueras MC, et al. Optic Nerve Lipidomics Reveal Impaired Glucosylsphingosine Lipids Pathway in Glaucoma. Invest Ophthalmol Vis Sci. 2019April1;60(5):1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milbeck SM, Bhattacharya SK. Alteration in Lysophospholipids and Converting Enzymes in Glaucomatous Optic Nerves. Invest Ophthalmol Vis Sci. 2020June3;61(6):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagy K, Brahmbhatt VV, Berdeaux O, et al. Comparative study of serine-plasmalogens in human retina and optic nerve: identification of atypical species with odd carbon chains. J Lipid Res. 2012April;53(4):776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acar N, Berdeaux O, Gregoire S, et al. Lipid composition of the human eye: are red blood cells a good mirror of retinal and optic nerve fatty acids? PLoS One. 2012;7(4):e35102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saab S, Mazzocco J, Creuzot-Garcher CP, et al. Plasmalogens in the retina: from occurrence in retinal cell membranes to potential involvement in pathophysiology of retinal diseases. Biochimie. 2014December;107Pt A:58–65. 10.1016/j.biochi.2014.07.023 [DOI] [PubMed] [Google Scholar]
- 24.Matyash V, Liebisch G, Kurzchalia TV, et al. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res. 2008May;49(5):1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959August;37(8):911–7. 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- 26.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957May;226(1):497–509. [PubMed] [Google Scholar]
- 27.Mahrous EA, Lee RB, Lee RE. A rapid approach to lipid profiling of mycobacteria using 2D HSQC NMR maps. J Lipid Res. 2008February;49(2):455–63. 10.1194/jlr.M700440-JLR200 [DOI] [PubMed] [Google Scholar]
- 28.Szulc ZM, Bai A, Bielawski J, et al. Synthesis, NMR characterization and divergent biological actions of 2’-hydroxy-ceramide/dihydroceramide stereoisomers in MCF7 cells. Bioorg Med Chem. 2010November1;18(21):7565–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vetica F, Sansone A, Meliota C, et al. Free-Radical-Mediated Formation of Trans-Cardiolipin Isomers, Analytical Approaches for Lipidomics and Consequences of the Structural Organization of Membranes. Biomolecules. 2020August15;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma YJ, Fan S, Shao H, et al. Use of Multiplied, Added, Subtracted and/or FiTted Inversion Recovery (MASTIR) pulse sequences. Quant Imaging Med Surg. 2020June;10(6):1334–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang K, Cheng H, Gross RW, et al. Automated lipid identification and quantification by multidimensional mass spectrometry-based shotgun lipidomics. Anal Chem. 2009June1;81(11):4356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzog R, Schwudke D, Schuhmann K, et al. A novel informatics concept for high-throughput shotgun lipidomics based on the molecular fragmentation query language. Genome Biol. 2011;12(1):R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuhmann K, Herzog R, Schwudke D, et al. Bottom-up shotgun lipidomics by higher energy collisional dissociation on LTQ Orbitrap mass spectrometers. Anal Chem. 2011July15;83(14):5480–7. 10.1021/ac102505f [DOI] [PubMed] [Google Scholar]
- 34.Wenk MR. The emerging field of lipidomics. Nat Rev Drug Discov. 2005July;4(7):594–610. 10.1038/nrd1776 [DOI] [PubMed] [Google Scholar]
- 35.Avela HF, Siren H. Advances in lipidomics. Clin Chim Acta. 2020November;510:123–141. 10.1016/j.cca.2020.06.049 [DOI] [PubMed] [Google Scholar]
- 36.Myer C, Perez J, Abdelrahman L, et al. Differentiation of soluble aqueous humor metabolites in primary open angle glaucoma and controls. Exp Eye Res. 2020April1;194:108024. 10.1016/j.exer.2020.108024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myer C, Abdelrahman L, Banerjee S, et al. Aqueous humor metabolite profile of pseudoexfoliation glaucoma is distinctive. Mol Omics. 2020March9. 10.1039/c9mo00192a [DOI] [PubMed] [Google Scholar]
- 38.Rivera ES, Djambazova KV, Neumann EK, et al. Integrating ion mobility and imaging mass spectrometry for comprehensive analysis of biological tissues: A brief review and perspective. J Mass Spectrom. 2020December;55(12):e4614. 10.1002/jms.4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pathak P, Sarycheva A, Baird MA, et al. Delineation of Isomers by the (13)C Shifts in Ion Mobility Spectra. J Am Soc Mass Spectrom. 2021January6;32(1):340–345. 10.1021/jasms.0c00350 [DOI] [PubMed] [Google Scholar]
- 40.Yoon D, Kim YJ, Lee WK, et al. Metabolic Changes in Serum Metabolome of Beagle Dogs Fed Black Ginseng. Metabolites. 2020December19;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khattri RB, Kim K, Thome T, et al. Unique Metabolomic Profile of Skeletal Muscle in Chronic Limb Threatening Ischemia. J Clin Med. 2021February2;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hani A, Diserens G, Oevermann A, et al. Sampling Method Affects HR-MAS NMR Spectra of Healthy Caprine Brain Biopsies. Metabolites. 2021January6;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonnella NC. Chromatographic Separation and NMR An Integrated Approach in Pharmaceutical Development. Adv Chromatogr. 2012;50:93–138. [PubMed] [Google Scholar]
- 44.Pauling JK, Hermansson M, Hartler J, et al. Proposal for a common nomenclature for fragment ions in mass spectra of lipids. PLoS One. 2017;12(11):e0188394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fishman EK, Magid D, Brooker AF, et al. Fractures of the sacrum and sacroiliac joint: evaluation by computerized tomography with multiplanar reconstruction. South Med J. 1988February;81(2):171–7. 10.1097/00007611-198802000-00007 [DOI] [PubMed] [Google Scholar]
- 46.Saeed M, Kausar MA, Singh R, et al. The Role of Glyoxalase in Glycation and Carbonyl Stress Induced Metabolic Disorders. Curr Protein Pept Sci. 2020;21(9):846–859. 10.2174/1389203721666200505101734 [DOI] [PubMed] [Google Scholar]
- 47.Koelmel JP, Napolitano MP, Ulmer CZ, et al. Environmental lipidomics: understanding the response of organisms and ecosystems to a changing world. Metabolomics. 2020April19;16(5):56. 10.1007/s11306-020-01665-3 [DOI] [PubMed] [Google Scholar]
- 48.Suryanarayan A, Cubas M, Craig OE, et al. Lipid residues in pottery from the Indus Civilisation in northwest India. J Archaeol Sci. 2021January;125:105291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pesko BK, Weidt S, McLaughlin M, et al. Postmortomics: The Potential of Untargeted Metabolomics to Highlight Markers for Time Since Death. OMICS. 2020November;24(11):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langley NR, Wood P, Herling P, et al. Forensic Postmortem Interval Estimation from Skeletal Muscle Tissue: A Lipidomics Approach. Forensic Anthropology. 2019;2(3):152–157., DOI: 10.5744/fa.2019.1011 [DOI] [Google Scholar]
- 51.Wilmott LA, Grambergs RC, Allegood JC, et al. Analysis of sphingolipid composition in human vitreous from control and diabetic individuals. J Diabetes Complications. 2019March;33(3):195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lefterov I, Wolfe CM, Fitz NF, et al. APOE2 orchestrated differences in transcriptomic and lipidomic profiles of postmortem AD brain. Alzheimers Res Ther. 2019December30;11(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Otoki Y, Kato S, Nakagawa K, et al. Lipidomic Analysis of Postmortem Prefrontal Cortex Phospholipids Reveals Changes in Choline Plasmalogen Containing Docosahexaenoic Acid and Stearic Acid Between Cases With and Without Alzheimer’s Disease. Neuromolecular Med. 2021March;23(1):161–175. 10.1007/s12017-020-08636-w [DOI] [PubMed] [Google Scholar]
- 54.Lam SM, Wang Y, Duan X, et al. Brain lipidomes of subcortical ischemic vascular dementia and mixed dementia. Neurobiol Aging. 2014October;35(10):2369–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben-David O, Pewzner-Jung Y, Brenner O, et al. Encephalopathy caused by ablation of very long acyl chain ceramide synthesis may be largely due to reduced galactosylceramide levels. J Biol Chem. 2011August26;286(34):30022–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crane AM, Bhattacharya SK. The use of bromodeoxyuridine incorporation assays to assess corneal stem cell proliferation. Methods Mol Biol. 2013;1014:65–70. 10.1007/978-1-62703-432-6_4 [DOI] [PubMed] [Google Scholar]
- 57.Aribindi K, Guerra Y, Piqueras Mdel C, et al. Cholesterol and glycosphingolipids of human trabecular meshwork and aqueous humor: comparative profiles from control and glaucomatous donors. Curr Eye Res. 2013October;38(10):1017–1026. [DOI] [PubMed] [Google Scholar]
- 58.Milbeck SM, Bhattacharya SK. Alteration in lysophospholipids 1 and converting enzymes in glaucomatous optic nerves. Invest Ophthalmol Vis Sci. 2020:In press. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cortes M, Pareja E, Garcia-Canaveras JC, et al. Metabolomics discloses donor liver biomarkers associated with early allograft dysfunction. J Hepatol. 2014September;61(3):564–74. 10.1016/j.jhep.2014.04.023 [DOI] [PubMed] [Google Scholar]
- 60.Sparagna GC, Chicco AJ, Murphy RC, et al. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007July;48(7):1559–70. 10.1194/jlr.M600551-JLR200 [DOI] [PubMed] [Google Scholar]
- 61.Lumbreras B, Porta M, Marquez S, et al. QUADOMICS: an adaptation of the Quality Assessment of Diagnostic Accuracy Assessment (QUADAS) for the evaluation of the methodological quality of studies on the diagnostic accuracy of ‘-omics’-based technologies. Clin Biochem. 2008November;41(16–17):1316–25. 10.1016/j.clinbiochem.2008.06.018 [DOI] [PubMed] [Google Scholar]


