Abstract
Objective:
Total joint arthroplasty (TJA) often reduces pain and improves function, but it is also a risk factor for the development of chronic pain and chronic opioid use. To protect against these untoward postsurgical outcomes, TJA patients need better, non-pharmacological pain management strategies. This study compared two, promising, mindfulness-based pain management techniques.
Method:
We conducted a single-site, three-arm, parallel-group randomized clinical trial conducted at an orthopedic clinic among patients undergoing TJA of the knee or hip. TJA patients (N=118, mean age=65, female=73, Caucasian=110) were randomized to either a preoperative mindfulness of breath (MoB), mindfulness of pain (MoP), or cognitive-behavioral pain psychoeducation (CB) intervention, approximately 3 weeks before surgery. Each intervention was delivered in a single, 20-minute session during a 2-hour, preoperative education program. Change in pain intensity was the sole preoperative outcome. The postoperative outcomes, pain intensity, pain interference, and opioid use, were assessed on the 2nd, 3rd, 7th, 14th, 21st, and 28th postoperative days.
Results:
MoB was found to most effectively decrease preoperative pain states (F2,89=5.28, p=.007), while MoP resulted in the least amount of postoperative pain intensity (F8,94=3.21, p=.003) and interference (F8,94=2.52, p=.016). Both MoB and MoP decreased postoperative opioid use relative to CB (F8,83=16.66, p<.001).
Conclusion:
A brief preoperative MBI may be able to prevent both postoperative pain and opioid use. Moreover, the MBIs used in this study are highly feasible, capable of being delivered by nearly any healthcare provider and requiring minimal clinic time given their brevity. As such, embedding MBIs in surgical care pathways has considerable potential.
Public Health Significance Statement:
This study suggests that a single, 20 minute, preoperative mindfulness-based intervention may be able to prevent knee and hip replacement patients’ postoperative pain and opioid use.
Keywords: Mindfulness, Pain, Opioid, Total joint arthroplasty, Surgery
Total joint arthroplasty (TJA) of the knee and hip are two of the most common inpatient surgeries performed in the U.S (Ethgen et al., 2004; Most Common Hospital Inpatient Operations - HCUP Fast Stats, n.d.) and are expected to increase over the next 20 years (Singh et al., 2019). Unfortunately, surgical outcomes can be highly variable and, too often, suboptimal. Despite improved surgical techniques (Kehlet et al., 2006), TJA is often severely painful (Kuo et al., 2019; Wylde et al., 2011) and can result in postoperative morbidity. Even after a technically sound TJA, 20% of patients report chronic postoperative pain in their replaced joint (Wylde et al., 2013), making TJA a risk factor for the development of chronic pain. TJA is also a risk factor for the development of chronic opioid use (Delaney et al., 2020; Goesling et al., 2016; Politzer et al., 2018). Opioids are the primary, postoperative pain management strategy for joint replacement patients despite no relationship between postoperative prescription of opioid medication and patient satisfaction with pain control (Roberts et al., 2019; Sabatino et al., 2018). Problematically, chronic postoperative opioid use is associated with multiple iatrogenic health risks, including risk for progression to opioid misuse (Bedard, Pugely, Dowdle, et al., 2017; Bedard, Pugely, Westermann, et al., 2017; Cancienne et al., 2018; Helmerhorst et al., 2014). Thus, TJA may serve as an important vector by which patients transition into chronic pain and opioid use. Clearly, non-pharmacological pain management is ideal for this patient group.
Cognitive behavioral and mindfulness-based interventions are promising non-pharmacological pain management strategies (e.g., Garland et al., 2020). Cognitive behavioral interventions (CBIs) are commonly considered the gold standard for behavioral pain management (Ehde et al., 2014), and have been shown to improve both pain and opioid-related outcomes (Garland et al., 2020). However, evidence supporting the efficacy of preoperative CBIs for TJA patients is limited. A pilot randomized controlled trial (RCT; N=91) utilizing a four session video-recorded CBI was not found to improve TJA patients’ clinical outcomes relative to a treatment as usual (TAU) control condition (Cooke et al., 2016). Additionally, a multi-week preoperative CBI for knee replacement patients (N=80) did not improve postsurgical outcomes relative to no intervention controls, but did decrease preoperative pain catastrophizing (Buvanendran et al., 2021). Comparatively, mindfulness-based interventions (MBIs) have also been shown to improve both pain and opioid-related outcomes (Garland et al., 2020), and emerging evidence indicates the preoperative delivery of an MBI can improve TJA outcomes. A day-long preoperative MBI accelerated pain and opioid cessation in veterans undergoing orthopedic surgery (N=88) relative to TAU (Dindo et al., 2018). More recently, a large scale RCT (N=285) found that a single, 15-minute preoperative MBI reduced preoperative pain and accelerated postoperative recovery of physical function following TJA relative to a single, 15-minute preoperative CBI (Hanley et al., 2021). Thus, preoperative MBIs may be superior to preoperative CBIs for TJA patients.
MBIs teach different mindfulness practice styles to alleviate pain. The majority of prior experimental studies have examined focused attention on the breath (i.e., mindfulness of breath, MoB), and found that this technique significantly reduces pain intensity and unpleasantness (Wells et al., 2020; Zeidan et al., 2015; Zeidan et al., 2011). Few studies have examined the mindfulness practices where pain is the target (i.e., mindfulness of pain, MoP). MoP practices provide a means of interoceptive exposure to pain (Flink et al., 2009) by using mindfulness to separate physical sensations from habituated emotional reactions and pain appraisals. Such practices are sometimes integrated into multi-week MBIs for the purpose of helping patients cope with chronic pain by attenuating affectively laden, catastrophic pain appraisals. For instance, Mindfulness-Oriented Recovery Enhancement (MORE; Garland, 2013) uses MoP as a core therapeutic technique to help patients reinterpret chronic pain as innocuous sensory information; and, indeed, the pain reductive effects of MORE are mediated by increases in this capacity to reinterpret pain (Garland et al., 2014). However, MORE and other MBIs teach both MoB and MoP, precluding a dismantling of the relative contributions of each technique. A recent pilot study found that 15 days of MoP via a mindfulness-based interoceptive exposure task was associated with significant reductions in chronic pain intensity (Cayoun et al., 2020); yet, this trial did not compare MoP to MoB.
To fill this lacuna in the literature, the present experiment compared two MBIs, MoB and MoP, with a CBI, all delivered in a very brief (i.e., 20 minute) single session for patients undergoing TJA of the knee or hip. We hypothesized (1) TJA patients receiving either MoB or MoP would report less pain intensity after the preoperative intervention as well as less postoperative pain intensity, pain interference, and opioid use relative to patients in the CBI condition. Furthermore, given that MoB, as a basic practice, is ostensibly easier to learn than MoP and may therefore produce more rapid analgesic benefits, we hypothesized that (2) MoB would outperform MoP on reducing preoperative pain intensity. In contrast, given the putative effects of MoP on prolonged pain by modifying schematized pain appraisals via interoceptive exposure, we hypothesized that (3) MoP would outperform MoB on reducing pain and opioid use in the postoperative period after participants had had more time to engage with the practice. Finally, insofar as a positive preoperative experience with mindfulness may increase patients’ abilities to manage postoperative pain, we hypothesized that preoperative pain decreases occasioned by mindfulness practice would predict less postoperative pain intensity, pain unpleasantness, and opioid use.
Methods
Trial Design and Randomization
This was a single site, three-arm, parallel group mechanistic study associated with a randomized clinical trial (Trial Registry: NCT03665727). Given evidence of the analgesic effects of the MBI in NCT03665727, we conducted a follow-up mechanistic study to determine the relative efficacy of two different mindfulness techniques (Trial Registry: NCT04520958). All procedures were approved by the local IRB. Participant consent was obtained after reviewing an informed consent study cover letter with study personnel.
Participants were patients at an academic orthopedic center scheduled for knee or hip TJA. Before surgery, all knee and hip TJA patients were encouraged to attend a 2-hour preoperative education program, Joint Academy. During this program, medical professionals, including nurses, physical therapists, and psychologists, taught patients about what to expect before, during, and after surgery. First, a nurse discussed health optimization, anesthesia choice, typical length of hospital stay, and wound care. Second, a physical therapist discussed prehabilitation exercises, the physical therapy timeline, and the process of functional recovery. Finally, the psychologist delivered the pain management intervention. Randomization sequence was generated by a researcher uninvolved with intervention delivery or data collection before the trial began. Randomization was stratified by surgical procedure (knee or hip) and any differences in allocation (1:1:1) were purely due to chance as Joint Academy attendance varied from week to week (Average number of participants per week = 7, SD = 4.06).
Participants
All English-speaking adult (18+) patients scheduled for knee or hip TJA who attended Joint Academy preoperatively were eligible to participate. To be offered surgery at the academic orthopedic center where this study was conducted, patients must have failed reasonable attempts at non-operative joint treatments (e.g., anti-inflammatory medication, acetaminophen, activity modification, icing, bracing, injections, physical therapy) and medical optimization is prioritized (e.g., well controlled diabetes, < BMI 40, quit smoking > four weeks before surgery). Additionally, patients with a history of substance use disorder are required to provide a negative drug screen before surgery; patients taking prescription opioid medication preoperatively are requested to decrease their dose by at least 50% before surgery; and, patients with mental health concerns (e.g., anxiety, depression) are encouraged to proactively communicate with their mental health provider to coordinate postoperative services should they be needed. Finally, patients experiencing surgical complications and those scheduled for multiple, consecutive surgeries were excluded from the study due to the possibility that additional surgeries or procedures would confound their postoperative assessments.
Interventions
Study interventions were delivered according to a manualized protocol and were matched in terms of duration (20 minutes) and frequency (once). All three interventions began with a 5-minute presentation about biopsychosocial aspects of pain followed by a 15-minute, scripted, pain management intervention. MoB consisted of instruction in focused attention on the breath and metacognitive monitoring and acceptance of discursive thoughts, negative emotions, and body sensations. This script closely followed a standardized mindfulness induction script (see supplementary materials) validated in prior research on mindfulness in medical settings (Garland et al., 2017). MoP consisted of instruction in how to (1) zoom in to deconstruct pain into its constituent physical sensations and precisely map each sensation’s spatial location, (2) use mindful breathing to zoom out and broaden the field of attention to include previously neglected sensory elements (i.e., spaces within the body that were absent of sensation and pleasant sensations), and (3) shift attention from unpleasant sensations to neutral/pleasant sensations or experiences. This script closely followed the core MORE script (see supplementary materials; Garland, 2013) validated in prior research with pain patients (Garland et al., 2019; Garland et al., 2014, 2019). CB consisted of psychoeducation about the link between thoughts, emotions, and behavior and provided instruction in the use logic to dispute maladaptive thoughts about pain that might otherwise exacerbate pain and distress. This script (see supplementary materials) accorded with standard cognitive restructuring techniques employed in CBT interventions for pain (Jamison et al., 2010; Thorn & Kuhajda, 2006). Participants were provided audio recordings of the therapeutic scripts so they could continue practicing on their own in the pre- and postoperative periods.
All participants received standard medical care for TJA of the knee or hip. All TJAs were performed by one of four surgeons working at the same orthopedic center, so surgical and anesthetic protocols were highly consistent. For all primary TJAs of the knee or hip an intra-operative periarticular joint infiltration was used, consisting of a standardized cocktail of Clonidine, Morphine, Epinephrine, Toradol, Ropivicaine, and Saline. Some patients also received a pre- or post-operative adductor canal single shot nerve block. Post-operatively, patients received a standardized pain management regiment that included ASA 81 mg BID for deep venous thrombosis prophylaxis, Tylenol, an NSAID (e.g., Naproxen, Celebrex, Mobic), and Tramadol. For patients still experiencing pain, a breakthrough orally administered narcotic (Oxycodone) was used. Intravenous narcotics were avoided and PCA opioids are never used at this orthopedic center.
Measures
Sociodemographic and diagnostic information was obtained from patient medical records. Preoperative pain intensity was measured immediately before and after the psychosocial intervention, which comprised a 20-minute interval. The acceptability of the pain management technique introduced during the psychosocial intervention was also assessed immediately after the psychosocial intervention. The Qualtrics survey platform was used to email participants the postoperative self-report measures during the month following surgery. A total of 6 postoperative surveys were administered over the month following surgery. Surveys were administered on the 2nd, 3rd, 7th, 14th, 21st, and 28th postoperative days. Each survey response was time stamped to ensure that if it was not immediately completed, it was could be appropriately analyzed. We elected to use weekly surveys in this study to reduce participant burden while also capturing critical postoperative assessment points in the early recovery period (e.g., Houle et al., 2017). As patients at the academic orthopedic center where this study was conducted are told they should not need prescription opioids for surgical pain management by two weeks after surgery and substantive postoperative gains are not expected before one month after surgery (e.g., Knee Replacement - Mayo Clinic, n.d.), assessments on the 14th and 28th days were strongly indicated.
Clinical Symptoms.
Pain intensity and pain interference were measured with individual items rated on a numeric rating scale (0–10), a widely used and validated approach to measuring clinical pain and related symptomology (Garland et al., 2017; Hawker et al., 2011). Opioid use was measured with a single dichotomously scored item: “Did you take opioid medications in the last 24 hours?”
Technique acceptability.
Patients’ confidence (“How confident are you that you could effectively use this pain management technique?”; e.g. Irvin, 2007) in using the pain management technique introduced during Joint Academy and intent to use the technique (“How likely are you to use this pain management technique?”; e.g., Wallace et al., 2001) was assessed immediately after the preoperative psychosocial intervention with individual items rated on a numeric rating scale (0–10).
Power Calculation
For linear mixed modeling, a priori power analysis was conducted using Optimal Design (Spybrook et al., 2006). An estimated total sample size of 118 participants was determined necessary to detect an overall between-group effect on baseline-adjusted outcomes (d=0.50, or of medium size) with 80% power, two-sided, p < 0.05. This effect size estimate was derived from our prior study of knee and hip TJA patients in which we used a very similar design and observed effects sizes ranging from d=.40 to d=.54 (Hanley et al., 2021). A sample size of 118 provided >5 participants per parameter (k=21) in our path model, consistent with both Bollen’s (1989) and Bentler’s (1990) recommendations.
Statistical Analysis
Following our pre-specified analysis plan, we used an intent-to-treat framework to assess between group differences by fitting separate linear mixed models for the pre- and postoperative outcomes, using maximum likelihood estimation and the Holm–Bonferroni method to adjust alpha for the multiple comparisons (Holm, 1979). In all mixed models, outcome variables were regressed on intervention group (MoB vs. MoP vs. CB) and baseline values in accordance with the classical ANCOVA approach for analyzing clinical trial outcomes (Frison & Pocock, 1992). Adjusting for baseline values performs statistical matching on the prerandomization scores and ensures that comparisons of postrandomization values by treatment group are independent of baseline differences. For the analysis of preoperative pain relief, the pain intensity rating immediately before the intervention served as the baseline measurement point. For the analysis of postoperative outcomes, the 2nd postoperative day served as the baseline measurement point for that period. In the mixed models examining postoperative outcomes, outcome variables were also regressed on time (3rd postoperative day -> 28th postoperative day) and surgery type (knee vs. hip). Sensitivity analyses were also performed adjusting for sociodemographic characteristics found to differ by group: history of opioid use, BMI, and utilization of postoperative physical therapy sessions. Finally, path analysis was used to examine whether change in pain intensity during the preoperative psychosocial intervention mediated the relationship between condition and changes in the postoperative outcomes. We selected preoperative pain relief as a mediator based on the assumption that patients who experience greater preoperative pain relief may be more responsive to mindfulness interventions and/or more likely to engage in mindfulness practice following surgery and therefore experience enhanced postoperative outcomes. Condition was dummy coded into two variables, MoB condition and MoP condition, with the CB condition as the criterion and coded 0,0; the MoB condition coded 1,0; and the MoP condition coded 0,1. All tests and CIs were 2-sided and statistical significance was defined as a p value less than .05. All linear models were conducted using SPSS version 25 and the path model was conducted in R using the Lavaan package.
Results
Of the 195 patients scheduled to attend the preoperative information session, 172 attended. Of these, 11 declined to participate and 43 were excluded from the study due to surgery cancellation (n=38) or rescheduling (n=5). A total of 118 patients agreed to participate and underwent TJA of the knee or hip during the study period. Postoperative scores were obtained from 75% of participants (Figure 1). No intervention related harms or unintended effects occurred during this study.
Figure 1.
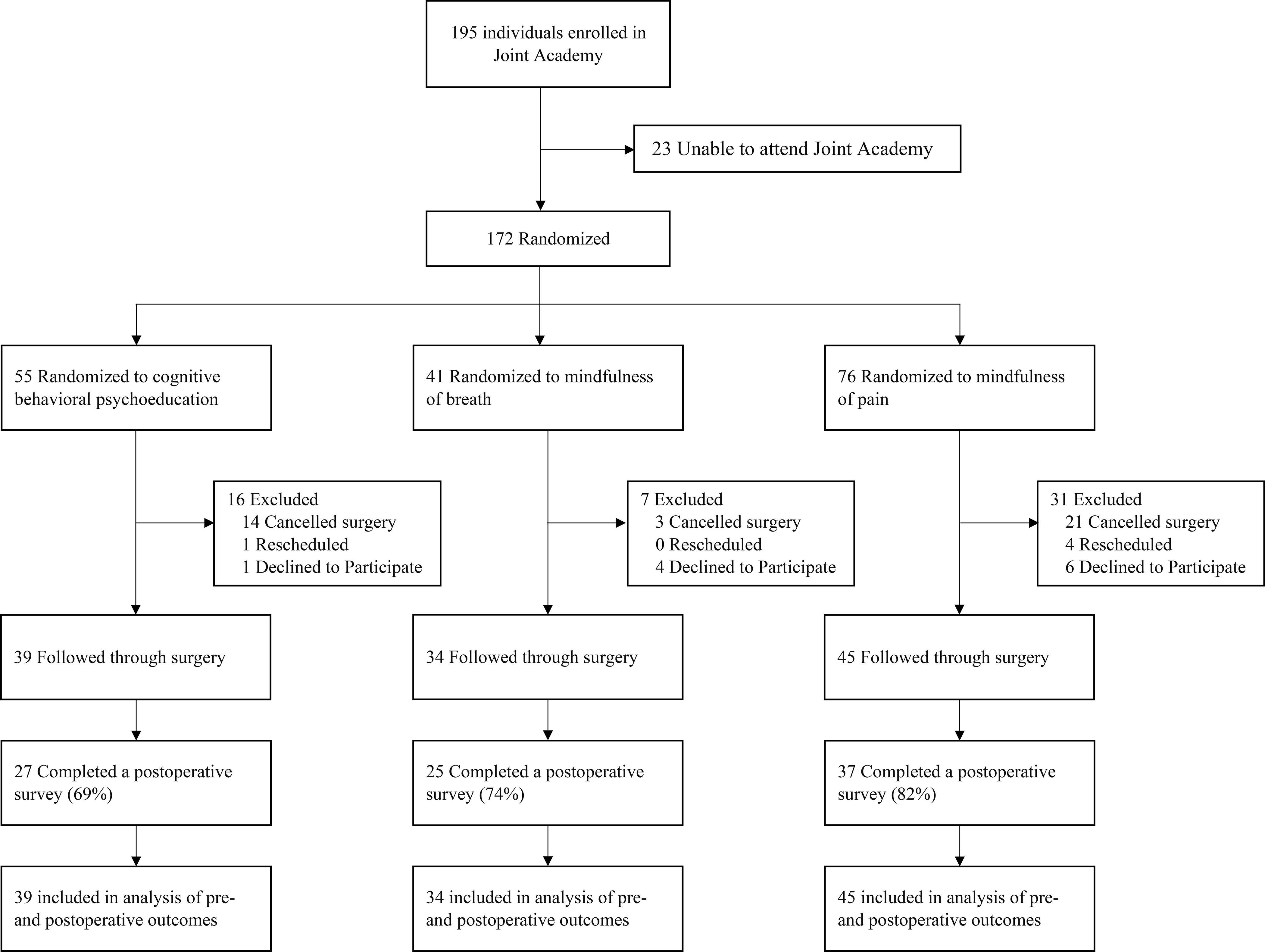
CONSROT FlowChart. Flow of participants through trial comparing the effects of preoperative mindfulness of breath, mindfulness of pain, and cognitive-behavioral pain psychoeducation interventions on patients undergoing total joint arthroplasty of the knee or hip.
Table 1 reports participants’ sociodemographic characteristics along with summary descriptive statistics for the groups at baseline. Treatment conditions were largely similar with respect to all investigated characteristics. Missing data analysis on postoperative outcomes revealed that the observed missing data pattern was consistent with the data being missing completely at random (MCAR) (Little’s MCAR test: χ2(473)=458.01, p=.68).
Table 1.
Baseline Characteristics of Initial Participant Sample by Treatment Group and Usual Care Patients
| Total | Cognitive Behavioral Psychoeducation | Mindfulness of Breath | Mindfulness of Pain | Test Statistic | Statistical Significance | |
|---|---|---|---|---|---|---|
| N | 118 | 39 | 34 | 45 | ||
| Age, mean (SD) | 64.84 (9.86) | 64.26 (10.87) | 64.97 (10.37) | 65.25 (8.68) | F=0.10 | p=.90 |
| Women, No. (%) | 73 (61.9) | 23 (59.0) | 21 (61.8) | 29 (64.4) | χ2=1.12 | p=.89 |
| Race, No. (%) | χ2=7.98 | p=.44 | ||||
| Asian | 1 (0.8) | - | 1 (2.9) | - | ||
| Native Hawaiian or Other Pacific Islander | 1 (0.8) | 1 (2.6) | - | - | ||
| White or Caucasian | 110 (93.0) | 35 (89.7) | 31 (91.2) | 44 (97.8) | ||
| Other | 4 (3.4) | 3 (7.6) | 2 (5.9) | 1 (2.2) | ||
| Hispanic Ethnicity, No. (%) | 3 (2.5) | 2 (5.1) | 1 (2.9) | 1 (2.2) | χ2=7.36 | p=.29 |
| Admitting Diagnosis, No. (%) | χ2=1.68 | p=.43 | ||||
| Hip Arthroplasty | 39 (33.1) | 16 (41.0) | 10 (29.4) | 13 (28.9) | ||
| Knee Arthroplasty | 79 (66.9) | 23 (59.0) | 24 (70.6) | 32 (71.1) | ||
| BMI, mean (SD) | 30.83 (6.69) | 30.80 (6.54) | 33.01 (7.13) | 29.18 (6.16) | F=3.27 | p=.04 |
| Tobacco Use, No. (%) | 27 (22.9) | 7 (17.9) | 9 (26.5) | 11 (24.4) | χ2=1.61 | p=.81 |
| Alcohol Use, No. (%) | 53 (44.9) | 15 (38.5) | 15 (44.1) | 23 (51.1) | χ2=3.14 | p=.54 |
| Drug Use, No. (%) | 2 (1.7) | 1 (2.6) | 1 (2.9) | - | χ2=3.23 | p=.52 |
| Nonsteroidal Anti-Inflammatory Drug Use, No. (%) | 75 (63.6) | 25 (64.1) | 21 (61.8) | 29 (64.4) | χ2=0.18 | p=.92 |
| Prescription Opioid Use, No. (%) | 27 (22.9) | 13 (33.3) | 3 (8.8) | 11 (24.4) | χ2=6.59 | p=.04 |
| Psychotropic Medication Use, No. (%) | 42 (35.6) | 13 (33.3) | 10 (29.4) | 19 (42.2) | χ2=1.67 | p=.43 |
| Days from Preoperative Intervention to Surgery (SD) | 23.26(16.63) | 24.72 (15.05) | 25.53 (18.06) | 19.67 (15.85) | F=1.44 | p=.24 |
| Surgeon, No. (%) | χ2=9.93 | p=.27 | ||||
| Surgeon 1 | 15 (12.7) | 3 (7.7) | 5 (14.7) | 7 (15.6) | ||
| Surgeon 2 | 32 (27.1) | 12 (30.8) | 6 (17.6) | 14 (31.1) | ||
| Surgeon 3 | 35 (29.7) | 9 (23.1) | 16 (47.1) | 10 (22.2) | ||
| Surgeon 4 | 34 (28.8) | 14 (35.9) | 7 (20.6) | 13 (28.9) | ||
| Spinal Anesthesia, No. (%) | 102 (86.4) | 36 (92.3) | 28 (82.4) | 38 (84.4) | χ2=3.60 | p=.46 |
| Physical Therapy Sessions, mean (SD) | 3.02 (4.17) | 1.26 (2.00) | 5.68 (5.57) | 2.48 (3.27) | F=12.82 | p<.01 |
| Readmissions, mean (SD) | 0.10 (0.31) | 0.05 (0.23) | 0.15 (0.36) | 0.11 (0.32) | F=0.89 | p=.41 |
Preoperative Results
Condition (MoB vs. MoP vs. CB) had a significant effect on baseline adjusted pain intensity immediately after the preoperative intervention (F2,89=5.28, p=.007). Pairwise comparisons indicated participants in the MoB condition reported significantly less pain relative to participants in the CB condition (Contrast Estimate = −0.93, CI: −1.51 to −0.36, p=.002). Pain reports from participants in the MoP condition did not statistically differ from reports from participants in the MoB (Contrast Estimate = 0.50, CI: −0.17 to 1.17, p=.14) or the CB (Contrast Estimate = −0.43, CI: −1.01 to 0.15, p=.14) conditions.
Patients did not differ by condition in their confidence (F2,81=1.20, p=.31; MoB =6.40, SE=0.57; MoP =5.13, SE=0.70; CB =6.29, SE=0.46) in using the pain management technique introduced during the preoperative education program or intent to use the technique (F2,81=0.76, p=.47; MoB =6.40, SE=0.62; MoP =5.63, SE=0.74; CB =6.71, SE=0.48).
Postoperative Results
A significant condition (MoB vs. MoP vs. CB) by time (3rd postoperative day -> 28th postoperative day) interaction was observed for baseline adjusted, pain interference (F8,94=2.52, p=.016), and opioid use (F8,94=5.50, p<.001). See Figure 1. Sensitivity analyses, adjusting for between group differences in opioid use history at baseline, BMI, and postoperative physical therapy session usage, did not substantively alter these results: pain intensity (F8,83=3.00, p=.005), pain interference (F8,83=2.52, p=.017), and opioid use (F8,83=16.66, p<.001). Simple contrasts revealed patients in the MoP condition reported significantly less pain intensity on postoperative days 14, 21, and 28 as well as significantly less pain interference on postoperative days 21 and 28, relative to patients in the CB condition (Table 2). Patients receiving either preoperative MBI reported significantly less opioid use 21 days after surgery, relative to patients in the CB condition.
Table 2.
Contrast estimates by condition for each postoperative outcome at each assessment point.
| Day | Contrast | Pain Intensity | Pain Interference | Opioid Use | |||
|---|---|---|---|---|---|---|---|
| Contrast Estimate | p | Contrast Estimate | p | Contrast Estimate | p | ||
| 3 | MoB vs. CB | 0.13 (−2.07 to 2.33) | .91 | 0.58 (−1.89 to 3.05) | .64 | 0.08 (−0.20 to 0.36) | .57 |
| MoP vs. CB | −1.00 (−2.76 to 0.76) | .26 | −0.63 (−3.14 to 1.88) | .62 | 0.07 (−0.21 to 0.35) | .60 | |
| 7 | MoB vs. CB | 0.49 (−1.32 to 2.29) | .59 | −0.15 (−2.00 to 1.70) | .87 | 0.23 (−0.62 to 1.07) | .60 |
| MoP vs. CB | 0.00 (−1.56 to 1.56) | 1.00 | −0.49 (−2.21 to 1.23) | .57 | −0.39 (−1.68 to 0.90) | .55 | |
| 14 | MoB vs. CB | −1.58 (−3.50 to 0.34) | .11 | −1.43 (−3.29 to 0.43) | .13 | −0.10 (−0.85 to 0.65) | .79 |
| MoP vs. CB | −2.06 (3.76 to −0.35) | .02 | −1.48 (−3.27 to 0.30) | .10 | −0.19 (−0.57 to 0.18) | .31 | |
| 21 | MoB vs. CB | −0.92 (−3.43 to 1.58) | .47 | −1.01 (2.75 to 0.74) | .26 | −0.49 (−0.92 to −0.06) | .027 |
| MoP vs. CB | −2.22 (−3.84 to −0.60) | .008 | −1.85 (−3.39 to −0.31) | .019 | −0.37 (−0.69 to −0.04) | .027 | |
| 28 | MoB vs. CB | −0.88 (−2.05 to 0.28) | .14 | −1.60 (−3.22 to 0.02) | .053 | −0.39 (−0.86 to 0.08) | .11 |
| MoP vs. CB | −1.80 (−2.84 to −0.76) | .001 | −2.40 (−4.03 to −0.76) | .005 | −0.38 (−0.82 to 0.06) | .09 | |
Path Model
The path model (Figure 2) examined whether the relationships between condition and baseline-adjusted postoperative pain intensity, pain interference, and opioid use were mediated by the preoperative change in pain intensity occasioned by mindfulness practice. The model fit the data well: χ2=0.21, p=.65, CFI=1.00, RMSEA<.001, SRMR=.006. The MoB (β=−.24, p=.019) and MoP (β=−.22, p=.042) conditions had significant direct effects on preoperative pain intensity, relative to the CB condition. Both mindfulness conditions decreased preoperative pain intensity. Additionally, the MoP condition had significant direct effects on postoperative pain intensity (β=−.36, p=.026) and pain interference (β=−.67, p<.001); the MoB condition had a significant direct effect on postoperative pain interference (β=−.28, p=.037); and, preoperative pain intensity had a significant direct effect on postoperative pain intensity (β=.46, p=.029). No significant indirect effects were observed, but total effects of preoperative mindfulness training (i.e., direct effect of condition plus indirect effect of condition on outcome via preoperative pain intensity) on postoperative pain intensity (MoP: β=−.46, p=.008) and pain interference (MoB: β=−.33, p=.010; MoP: β= −.71, p<.001) were significant.
Figure 2.

Line graphs depicting baseline adjusted estimated marginal means by condition for pain intensity, pain interference, and opioid use sensitivity analyses. MoB = Mindfulness of Breath. MoP = Mindfulness of Pain. CB = Cognitive Behavioral Pain Psychoeducation.
Discussion
While intended to reduce pain and improve function, TJA too often results in chronic postoperative pain and opioid use (Delaney et al., 2020; Goesling et al., 2016; Politzer et al., 2018; Vikki Wylde et al., 2013). Instead of being part of the solution, in some cases, TJA may actually be contributing to the chronic pain/opioid crisis, albeit unintentionally. Therefore, improving TJA outcomes necessitates better pain management focused on non-opioid strategies. This study investigated the pre- and postoperative effects of three, brief non-opioid pain management strategies (MoB vs. MoP vs. CB) in patients undergoing TJA of the knee or hip. Results indicated that by teaching knee and hip replacement patients a mindfulness-based pain management strategy before surgery, patients reported immediate pain relief along with less postoperative pain, pain interference, and opioid use. Moreover, greater preoperative pain relief predicted greater postoperative pain relief, a finding consistent with our previous study of a brief preoperative MBI for knee and hip replacement patients (in press). It may be that those patients experiencing preoperative pain relief during mindfulness training were more likely to continue using a mindful pain management technique before and after surgery and recovered more rapidly as a result. Alternatively, the degree of analgesia achieved by brief mindfulness practice may serve as an individual difference factor predicting mindfulness treatment response. That is, patients who experience immediate pain relief following brief mindfulness training may be more responsive to mindfulness interventions and therefore may be more likely to experience positive postoperative outcomes. Future research is needed to test these hypotheses.
Results also indicated the different mindfulness practice styles had differential impacts on the pre- and postoperative outcomes. MoB was found to most effectively decrease preoperative pain states, decreasing joint replacement patients’ self-reported pain by 49%. Comparatively, MoP and CB decreased preoperative pain by 30% and 19%, respectively. As such, all three conditions provided a considerable degree of pain relief during the brief, preoperative intervention. With respect to postoperative outcomes, the MBIs outperformed the CB intervention. Preoperative MoP was found to result in the least amount of postoperative pain (MoP =2.78, SE=0.41 vs. MoB =3.25, SE=0.46 vs. CB =4.28, SE=0.48), with patients in the MoP condition reporting 45% less pain than patients in the CB condition at 1-month follow-up. Patients in the MoB condition reported 24% less pain than patients in the CB condition. Similarly, preoperative MoP was found to result in the least amount of postoperative pain interference (MoP =1.60, SE=0.81 vs. MoB =1.81, SE=0.43 vs. CB =3.51, SE=0.85), with patients in the MoP condition reporting 64% less pain interference at 1-month follow-up than patients in the CB condition. Patients in the MoB condition reported 48% less pain interference than patients in the CB condition. Additionally, only 2% of patients in the MoP and MoB conditions were taking opioids at the 1-month follow-up, compared to 40% of patients in the CB condition. Taken together, these results suggest MoB may be the most effective of the three techniques for immediate pain relief, whereas MoP appears to have the most substantial postoperative impact.
These findings are largely consistent with the only known studies examining the impact of a preoperative MBI on postoperative functioning (Dindo et al., 2018; Kiran et al., 2017). Congruent with our results, Dindo et al. (2018) also found a preoperative MBI decreased postoperative pain and opioid use in veterans undergoing orthopedic surgery. However, the present study extended Dindo et al.’s (2018) work in significant ways. Specifically, the 20 minute MBIs used in the present study were considerably briefer than MBI used in Dindo et al.’s (2018) study, which included a 5 hour one day preoperative intervention as well as a postoperative booster session. Our results suggest an even less resource intensive intervention may be efficacious for a civilian sample of TJA patients and future research is needed to determine whether this very brief MBI model could also benefit veterans and military service members. The present study also used an active control condition to account for non-specific therapeutic factors, whereas treatment as usual was the control condition in Dindo et al.’s (2018) study. As such, the present study provided a more rigorous test of the effects of a preoperative MBI. Findings from the present study are also consistent with recent evidence that a preoperative MBI can accelerate recovery of postoperative physical functioning in knee and hip replacement patients (Hanley et al., 2021). However, in this prior study, joint replacement patients were not tracked during the early recovery phase, leaving an open question as to why patients receiving a preoperative MBI recovered faster. Now, interpreting the results from these two studies together suggests a working hypothesis. If a preoperative MBI can decrease pain, pain interference and opioid use in the early recovery phase, then it is not surprising that patients receiving a preoperative MBI would more quickly return to an active lifestyle. Future research is needed to empirically examine this hypothesis.
In support of our a priori hypothesis, MoP produced the most robust improvements in postoperative pain and opioid use. These results might stem from the effect of MoP on cognitive and affective factors (e.g., pain catastrophizing) that prolong postoperative pain. From the perspective of “pain perception as inference” (Tabor et al., 2017; Wiech, 2016), inferences and predictions derived from past pain episodes (Labrenz et al., 2016; Wiech et al., 2014) may result in biased interpretations of the body’s current state that accumulate into cognitive schemas (e.g., “My pain is never going to go away”) that preserve and exacerbate pain. MoP practices, rooted in the Buddhist Abhidharma tradition, encourage mindful examination of phenomenological experience in a fine grained manner to deconstruct and parse experiential gestalts into a flux of cognitive, affective, and sensorial components (Nyanaponika, 1998). As such, they may be useful for disrupting maladaptive cognitive schemas and reducing affective bias during pain appraisal. Alternatively, MoP may increase awareness of pleasant sensations at body sites proximal or distal to the surgical site – a process theorized to enhance pain coping (Garland, 2020). Future studies should examine whether MoP reduces pain by modifying the aforementioned cognitive-affective processes.
Despite study strengths -- including being the first study to compare two preoperative MBIs in a surgical population -- limitations should be noted. First, we were unable to track intervention usage after preoperative training. Though all participants were explicitly instructed to continue practicing their pain management technique and there were no between group differences in patient reported confidence in using their pain management technique or intent to use their technique, it may have been that the MBIs proved more attractive or easily self-administered outside of the preoperative training context, thereby increasing the frequency with which patients continued using mindfulness skills to manage pain. In all likelihood, participants receiving the preoperative MBIs continued to practice mindfulness techniques in the postoperative period, which accounted for the observed decreases in postoperative pain and opioid use. Though we were unable to quantify home mindfulness practice, future studies should use ecological momentary assessment (EMA) to carefully characterize daily mindfulness practice and its relations with pain and opioid use in the moment. Alternatively, assessing the postoperative use and the perceived benefits of the study interventions, would have been useful for understanding whether continued practice occurred and whether it related to postoperative outcomes.
This was a pragmatic study conducted in a real-world orthopedic clinic. Thus, in an effort to balance participant burden and data integrity, this study was also limited by measurement decisions. Specifically, we did not assess psychological factors (e.g., depression, anxiety, pain catastrophizing) known to be associated with pain and which may potentially impact postoperative recovery. We also did not gather information on educational background or prior experience with mindfulness or related activities, which may have influenced intervention uptake. Additional measurement related limitations include (i) relying solely on self-reported, postoperative opioid use without triangulating this data with prescription refill counts or taking steps to ensure patients could accurately differentiate between opioid and non-opioid medications; (ii) using only single-item pain intensity and pain interference scores without including pain tolerance and/or behaviorally anchored functional status items, which could have provided more nuanced postoperative data; and (iii) a limited follow-up period. Future studies should incorporate a more comprehensive assessment strategy and extend the postoperative follow-up period to examine study outcomes over a longer period of time. In addition, although the experimental conditions did not differ by surgery type, it is possible that knee and hip patients may require distinct intervention strategies. Behavioral treatment development research may be needed to tailor pre-/postoperative MBIs for a variety of surgical procedures to meet patients’ individual needs while maximizing treatment adherence and transportability into medical settings. Moreover, participants were knee or hip replacement patients who voluntarily attended a preoperative education program and may represent a distinct subset of highly motivated TJA patients. Relatedly, the majority of participants were white and living in the mountain west. As such, these results may not generalize to all TJA patients. Given known racial disparities with respect to TJA utilization and outcomes (e.g., longer operative times and length of hospital stay, more frequent complications, and increased mortality for black patients), understanding the generalizability of these interventions is particularly salient (e.g., Amen et al., 2020). Finally, it should be noted that it may be more difficult to effectively present a CB intervention than a MBI in the 20 minute intervention period examined in the present study. As such, future brief mindfulness studies might employ progressive muscle relaxation as a matched comparison condition.
Innovative efforts to improve non-opioid pain management for patients undergoing TJA of the knee or hip are clearly needed as surgical rates are projected to increase 200% to 400% by 2040 (Singh et al., 2019). Without better pain management, vulnerable TJA patients will continue to develop chronic postoperative pain and transition from prescription opioid use for postoperative pain management to opioid misuse and addiction (Guy et al., 2017; Kolodny et al., 2015; Madras, 2017; Scholl, 2019; Vowles et al., 2015). If pain management practices do not improve, within two decades an estimated 1,000,000+ knee and hip replacement patients will emerge from their joint replacement surgeries with chronic postoperative pain and/or chronic postoperative opioid use per year. Results from this study suggest a brief preoperative MBI may be able to prevent both untoward outcomes. Moreover, the brief, scripted MBIs used in this study are highly feasible, capable of being delivered by nearly any healthcare provider and requiring minimal clinic time given their brevity. As such, embedding MBIs in surgical care pathways has considerable potential and continued research is needed to capitalize on this potential.
Supplementary Material
Figure 3.

Path model depicting study timeline and the effect of experimental condition (Mindfulness of Breath vs. Mindfulness of Pain vs. Cognitive Behavioral Pain Psychoeducation) on pain intensity immediately after the preoperative intervention as well as pain intensity, pain interference, and opioid use during the first month after surgery. For this analysis, experimental condition was dummy coded into two variables, Mindfulness of Breath and Mindfulness of Pain, with the Cognitive Behavioral Pain Psychoeducation condition as the criterion and coded 0,0; the Mindfulness of Breath condition coded 1,0; and the Mindfulness of Pain condition coded 0,1. Non-significant paths were removed from this figure to improve visual clarity.
Acknowledgments
Funding/support: A.W.H. was supported by a seed grant from the University of Utah College of Social Work during the preparation of this manuscript. E.L.G. was supported by grant number R01DA042033 from the National Institutes of Health during the preparation of this manuscript. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of interest disclosure: Eric Garland, PhD, LCSW is the Director of the Center on Mindfulness and Integrative Health Intervention Development. The Center provides Mindfulness-Oriented Recovery Enhancement (MORE), mindfulness-based therapy, and cognitive behavioral therapy in the context of research trials for no cost to research participants; however, Dr. Garland has received honoraria and payment for delivering seminars, lectures, and teaching engagements (related to training clinicians in mindfulness) sponsored by institutions of higher education, government agencies, academic teaching hospitals, and medical centers. Dr. Garland receives royalties from the sale of books related to MORE. Dr. Garland also is a consultant to BehaVR, LLC. The other authors declare that they have no competing interests.
Footnotes
Data Sharing Statement: De-identified study data will be made available to qualified parties upon request following manuscript publication.
References
- Amen TB, Varady NH, Rajaee S, & Chen AF (2020). Persistent racial disparities in utilization rates and perioperative metrics in total joint arthroplasty in the US: A comprehensive analysis of trends from 2006 to 2015. JBJS, 102(9), 811–820. [DOI] [PubMed] [Google Scholar]
- Bedard NA, Pugely AJ, Dowdle SB, Duchman KR, Glass NA, & Callaghan JJ (2017). Opioid use following total hip arthroplasty: Trends and risk factors for prolonged use. The Journal of Arthroplasty, 32(12), 3675–3679. [DOI] [PubMed] [Google Scholar]
- Bedard NA, Pugely AJ, Westermann RW, Duchman KR, Glass NA, & Callaghan JJ (2017). Opioid use after total knee arthroplasty: Trends and risk factors for prolonged use. The Journal of Arthroplasty, 32(8), 2390–2394. [DOI] [PubMed] [Google Scholar]
- Bentler PM (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107(2), 238–246. [DOI] [PubMed] [Google Scholar]
- Buvanendran A, Sremac AC, Merriman PA, Della Valle CJ, Burns JW, & McCarthy RJ (2021). Preoperative cognitive–behavioral therapy for reducing pain catastrophizing and improving pain outcomes after total knee replacement: A randomized clinical trial. Regional Anesthesia & Pain Medicine. [DOI] [PubMed] [Google Scholar]
- Cancienne JM, Patel KJ, Browne JA, & Werner BC (2018). Narcotic use and total knee arthroplasty. The Journal of Arthroplasty, 33(1), 113–118. [DOI] [PubMed] [Google Scholar]
- Cayoun B, Simmons A, & Shires A (2020). Immediate and Lasting Chronic Pain Reduction Following a Brief Self-Implemented Mindfulness-Based Interoceptive Exposure Task: A Pilot Study. Mindfulness, 11(1), 112–124. 10.1007/s12671-017-0823-x [DOI] [Google Scholar]
- Cooke M, Walker R, Aitken LM, Freeman A, Pavey S, & Cantrill R (2016). Pre-operative self-efficacy education vs. usual care for patients undergoing joint replacement surgery: A pilot randomised controlled trial. Scandinavian Journal of Caring Sciences, 30(1), 74–82. [DOI] [PubMed] [Google Scholar]
- Delaney LD, Gunaseelan V, Rieck H, Dupree IV JM, Hallstrom B, & Waljee JF (2020). High-Risk Prescribing Increases Rates of New Persistent Opioid Use in Total Hip Arthroplasty Patients. The Journal of Arthroplasty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindo L, Zimmerman MB, Hadlandsmyth K, StMarie B, Embree J, Marchman J, Tripp-Reimer T, & Rakel B (2018). Acceptance and Commitment Therapy for Prevention of Chronic Post-surgical Pain and Opioid Use in At-Risk Veterans: A Pilot Randomized Controlled Study. The Journal of Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehde DM, Dillworth TM, & Turner JA (2014). Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. American Psychologist, 69(2), 153. [DOI] [PubMed] [Google Scholar]
- Ethgen O, Bruyère O, Richy F, Dardennes C, & Reginster J-Y (2004). Health-related quality of life in total hip and total knee arthroplasty: A qualitative and systematic review of the literature. JBJS, 86(5), 963–974. [DOI] [PubMed] [Google Scholar]
- Flink IK, Nicholas MK, Boersma K, & Linton SJ (2009). Reducing the threat value of chronic pain: A preliminary replicated single-case study of interoceptive exposure versus distraction in six individuals with chronic back pain. Behaviour Research and Therapy, 47(8), 721–728. 10.1016/j.brat.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Frison L, & Pocock SJ (1992). Repeated measures in clinical trials: Analysis using mean summary statistics and its implications for design. Statistics in Medicine, 11(13), 1685–1704. [DOI] [PubMed] [Google Scholar]
- Garland EL, Hanley AW, Riquino MR, Reese SE, Baker AK, Bryan MA, Salas K, Yack B, Bedford CE, Atchley RM, Nakamura Y, Froeliger B, & Howard MO (2019). Mindfulness-Oriented Recovery Enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: A randomized controlled trial. Journal of Consulting and Clinical Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL (2013). Mindfulness-oriented recovery enhancement for addiction, stress, and pain. NASW Press, National Association of Social Workers. [Google Scholar]
- Garland EL (2020). Psychosocial intervention and the reward system in pain and opioid misuse: New opportunities and directions. PAIN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Hanley AW, Kline A, & Cooperman NA (2019). Mindfulness-Oriented Recovery Enhancement reduces opioid craving among individuals with opioid use disorder and chronic pain in medication assisted treatment: Ecological momentary assessments from a stage 1 randomized controlled trial. Drug and Alcohol Dependence, 203, 61–65. 10.1016/j.drugalcdep.2019.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, & Howard MO (2014). Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: Results from an early-stage randomized controlled trial. Journal of Consulting and Clinical Psychology, 82(3), 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland Eric L., Baker AK, Larsen P, Riquino MR, Priddy SE, Thomas E, Hanley AW, Galbraith P, Wanner N, & Nakamura Y (2017). Randomized controlled trial of brief mindfulness training and hypnotic suggestion for acute pain relief in the hospital setting. Journal of General Internal Medicine, 32(10), 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland Eric L., Brintz CE, Hanley AW, Roseen EJ, Atchley RM, Gaylord SA, Faurot KR, Yaffe J, Fiander M, & Keefe FJ (2020). Mind-body therapies for opioid-treated pain: A systematic review and meta-analysis. JAMA Internal Medicine, 180(1), 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goesling J, Moser SE, Zaidi B, Hassett AL, Hilliard P, Hallstrom B, Clauw DJ, & Brummett CM (2016). Trends and predictors of opioid use following total knee and total hip arthroplasty. Pain, 157(6), 1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy JG, Zhang K, Bohm MK, Losby J, Lewis B, Young R, Murphy LB, & Dowell D (2017). Vital Signs: Changes in Opioid Prescribing in the United States, 2006–2015. MMWR. Morbidity and Mortality Weekly Report, 66(26), 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley AW, Gililland J, Erickson J, Pelt C, Peters C, Rojas J, & Garland EL (2021). Brief preoperative mind-body therapies for total joint arthroplasty patients: A randomized controlled trial. PAIN, Articles in Press. 10.1097/j.pain.0000000000002195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker GA, Mian S, Kendzerska T, & French M (2011). Measures of adult pain: Visual analog scale for pain (vas pain), numeric rating scale for pain (nrs pain), mcgill pain questionnaire (mpq), short-form mcgill pain questionnaire (sf-mpq), chronic pain grade scale (cpgs), short form-36 bodily pain scale (sf-36 bps), and measure of intermittent and constant osteoarthritis pain (icoap). Arthritis Care & Research, 63(S11). [DOI] [PubMed] [Google Scholar]
- Helmerhorst GT, Vranceanu A-M, Vrahas M, Smith M, & Ring D (2014). Risk factors for continued opioid use one to two months after surgery for musculoskeletal trauma. JBJS, 96(6), 495–499. [DOI] [PubMed] [Google Scholar]
- Holm S (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 65–70. [Google Scholar]
- Houle TT, Miller S, E.Lang J, Booth JL, Curry RS, Harris L, Aschenbrenner CA, & Eisenach JC (2017). Day-to-day experience in resolution of pain after surgery. Pain, 158(11), 2147–2154. 10.1097/j.pain.0000000000001015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R (2007). Information and communication technology (ICT) literacy: Integration and assessment in higher education. Journal of Systemics, Cybernetics and Informatics, 5(4), 50–55. [Google Scholar]
- Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, & Wasan AD (2010). Substance misuse treatment for high-risk chronic pain patients on opioid therapy: A randomized trial. PAIN, 150(3), 390–400. 10.1016/j.pain.2010.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehlet H, Jensen TS, & Woolf CJ (2006). Persistent postsurgical pain: Risk factors and prevention. The Lancet, 367(9522), 1618–1625. [DOI] [PubMed] [Google Scholar]
- Kiran U, Ladha S, Makhija N, Kapoor PM, Choudhury M, Das S, Gharde P, Malik V, & Airan B (2017). The role of Rajyoga meditation for modulation of anxiety and serum cortisol in patients undergoing coronary artery bypass surgery: A prospective randomized control study. Annals of Cardiac Anaesthesia, 20(2), 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knee replacement—Mayo Clinic. (n.d.). Retrieved June 22, 2020, from https://www.mayoclinic.org/tests-procedures/knee-replacement/about/pac-20385276
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, & Alexander GC (2015). The Prescription Opioid and Heroin Crisis: A Public Health Approach to an Epidemic of Addiction. Annual Review of Public Health, 36(1), 559–574. 10.1146/annurev-publhealth-031914-122957 [DOI] [PubMed] [Google Scholar]
- Kuo AC, Raghunathan K, Lartigue AM, Bryan III WE, Pepin MJ, Takemoto S, & Wallace AW (2019). Freedom From Opioids After Total Knee Arthroplasty. The Journal of Arthroplasty, 34(5), 893–897. [DOI] [PubMed] [Google Scholar]
- Labrenz F, Icenhour A, Schlamann M, Forsting M, Bingel U, & Elsenbruch S (2016). From Pavlov to pain: How predictability affects the anticipation and processing of visceral pain in a fear conditioning paradigm. NeuroImage, 130, 104–114. 10.1016/j.neuroimage.2016.01.064 [DOI] [PubMed] [Google Scholar]
- Madras BK (2017). The Surge of Opioid Use, Addiction, and Overdoses: Responsibility and Response of the US Health Care System. JAMA Psychiatry, 74(5), 441–442. 10.1001/jamapsychiatry.2017.0163 [DOI] [PubMed] [Google Scholar]
- Most Common Hospital Inpatient Operations—HCUP Fast Stats. (n.d.). Retrieved December 23, 2018, from https://www.hcup-us.ahrq.gov/faststats/NationalProceduresServlet
- Nyanaponika. (1998). Abhidhamma studies: Buddhist explorations of consciousness and time. Simon and Schuster. [Google Scholar]
- Politzer CS, Kildow BJ, Goltz DE, Green CL, Bolognesi MP, & Seyler TM (2018). Trends in opioid utilization before and after total knee arthroplasty. The Journal of Arthroplasty, 33(7), S147–S153. [DOI] [PubMed] [Google Scholar]
- Roberts K, Moser S, Collins AC, McCardel B, Schultz K, Tramer JS, Carpenter C, Urquhart AG, Pierce J, & Edwards A (2019). Prescribing and Consumption of Opioids After Primary, Unilateral Total Hip and Knee Arthroplasty in Opioid Naive Patients. The Journal of Arthroplasty. [DOI] [PubMed] [Google Scholar]
- Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, & Jevsevar DS (2018). Excess Opioid Medication and Variation in Prescribing Patterns Following Common Orthopaedic Procedures. JBJS, 100(3), 180. 10.2106/JBJS.17.00672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L (2019). Drug and Opioid-Involved Overdose Deaths—United States, 2013–2017. MMWR. Morbidity and Mortality Weekly Report, 67. 10.15585/mmwr.mm6751521e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JA, Yu S, Chen L, & Cleveland JD (2019). Rates of Total Joint Replacement in the United States: Future Projections to 2020–2040 Using the National Inpatient Sample. The Journal of Rheumatology, jrheum-170990. [DOI] [PubMed] [Google Scholar]
- Spybrook J, Raudenbush SW, Liu X, Congdon R, & Martínez A (2006). Optimal design for longitudinal and multilevel research: Documentation for the “Optimal Design” software. Ann Arbor: University of Michigan School of Education, Hierarchical Models Project. [Google Scholar]
- Tabor A, Keogh E, & Eccleston C (2017). Embodied pain—Negotiating the boundaries of possible action. Pain, 158(6), 1007–1011. [DOI] [PubMed] [Google Scholar]
- Thorn BE, & Kuhajda MC (2006). Group cognitive therapy for chronic pain. Journal of Clinical Psychology, 62(11), 1355–1366. [DOI] [PubMed] [Google Scholar]
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, & van der Goes DN (2015). Rates of opioid misuse, abuse, and addiction in chronic pain: A systematic review and data synthesis. Pain, 156(4), 569–576. [DOI] [PubMed] [Google Scholar]
- Wallace LM, Freeman T, Latham L, Walshe K, & Spurgeon P (2001). Organisational strategies for changing clinical practice: How trusts are meeting the challenges of clinical governance. BMJ Quality & Safety, 10(2), 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells RE, Collier J, Posey G, Morgan A, Auman T, Strittmatter B, Magalhaes R, Adler-Neal A, McHaffie JG, & Zeidan F (2020). Attention to breath sensations does not engage endogenous opioids to reduce pain. Pain, 161(8), 1884–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K (2016). Deconstructing the sensation of pain: The influence of cognitive processes on pain perception. Science, 354(6312), 584–587. 10.1126/science.aaf8934 [DOI] [PubMed] [Google Scholar]
- Wiech K, Vandekerckhove J, Zaman J, Tuerlinckx F, Vlaeyen JW, & Tracey I (2014). Influence of prior information on pain involves biased perceptual decision-making. Current Biology, 24(15), R679–R681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylde V, Rooker J, Halliday L, & Blom A (2011). Acute postoperative pain at rest after hip and knee arthroplasty: Severity, sensory qualities and impact on sleep. Orthopaedics & Traumatology: Surgery & Research, 97(2), 139–144. [DOI] [PubMed] [Google Scholar]
- Wylde Vikki, Bruce J, Beswick A, Elvers K, & Gooberman-Hill R (2013). Assessment of chronic postsurgical pain after knee replacement: A systematic review. Arthritis Care & Research, 65(11), 1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, & Coghill RC (2015). Mindfulness Meditation-Based Pain Relief Employs Different Neural Mechanisms Than Placebo and Sham Mindfulness Meditation-Induced Analgesia. Journal of Neuroscience, 35(46), 15307–15325. 10.1523/JNEUROSCI.2542-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan Fadel, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, & Coghill RC (2011). Brain mechanisms supporting the modulation of pain by mindfulness meditation. Journal of Neuroscience, 31(14), 5540–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


