Graphical abstract
Keywords: Aspergillus species, Mycotoxins, Foodstuffs, Environment, OMICS, Aptamers, Point of care (PoC)
Highlights
-
•
Aspergillus species are the ubiquitous fungi that contaminate various food substrates and produce mycotoxins.
-
•
It is furthermost environmental contaminant defile lives in patients with conceded immunity.
-
•
OMICS approaches are important for the precise detection mycotoxins.
-
•
Aptamers are fast, in-expensive, and field-deployable rapid point of care detection of toxins.
Abstract
Aspergillus species are the paramount ubiquitous fungi that contaminate various food substrates and produce biochemicals known as mycotoxins. Aflatoxins (AFTs), ochratoxin A (OTA), patulin (PAT), citrinin (CIT), aflatrem (AT), secalonic acids (SA), cyclopiazonic acid (CPA), terrein (TR), sterigmatocystin (ST) and gliotoxin (GT), and other toxins produced by species of Aspergillus plays a major role in food and human health. Mycotoxins exhibited wide range of toxicity to the humans and animal models even at nanomolar (nM) concentration. Consumption of detrimental mycotoxins adulterated foodstuffs affects human and animal health even trace amounts. Bioaerosols consisting of spores and hyphal fragments are active elicitors of bronchial irritation and allergy, and challenging to the public health. Aspergillus is the furthermost predominant environmental contaminant unswervingly defile lives with a 40–90 % mortality risk in patients with conceded immunity. Genomics, proteomics, transcriptomics, and metabolomics approaches useful for mycotoxins’ detection which are expensive. Antibody based detection of toxins chemotypes may result in cross-reactivity and uncertainty. Aptamers (APT) are single stranded DNA (ssDNA/RNA), are specifically binds to the target molecules can be generated by systematic evolution of ligands through exponential enrichment (SELEX). APT are fast, sensitive, simple, in-expensive, and field-deployable rapid point of care (POC) detection of toxins, and a better alternative to antibodies.
1. Introduction
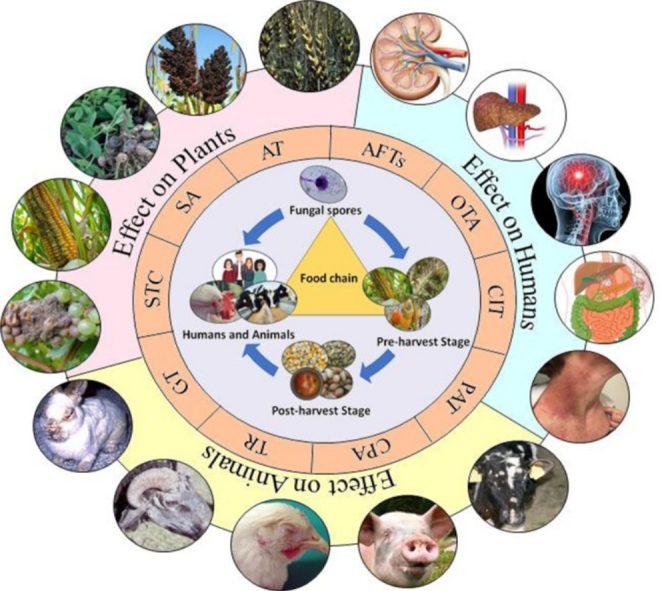
Fungi are the second largest group of eukaryotes that play a significant role in human health. The widespread prevalence of fungi in the environment and food chain makes them hazardous for humans. Mycotoxins contamination of agricultural produce is a serious threat to human health [1]. The ingestion of mycotoxins contaminated food, results acute and chronic toxicity to the humans and animals. Food and Agricultural Organization (FAO) suggested that about 25 % of the global food crops were contaminated by mycotoxins [2]. Approximately 300–400 mycotoxins have been identified, nevertheless, Aspergillus-derived mycotoxins have attracted the greatest attention to human, animal, and plant health (Fig. 1). The assessment of hazardous mycotoxin production and toxigenic fungal species is critical in assessing food safety and quality [3,4]. World Health Organization (WHO) and FAO, addressed global problem of mycotoxin contamination in food by adopting strict regulatory guidelines [5,6]. The joint scientific advisory committee expressed the responsibility for the evaluation of health risks from mycotoxins (WHO/FAO) [[2], [3], [4], [5]]. Aspergillus species produce various life-threatening biotoxins such as aflatoxins (AFTs), ochratoxins (OTA), patulin (PAT), citrinin (CIT), aflatrem (AT), secalonic acids (SA), cyclopiazonic acid (CPA), terrein (TR), sterigmatocystin (ST) and gliotoxin (GT), and other characteristic molecules [6,7].
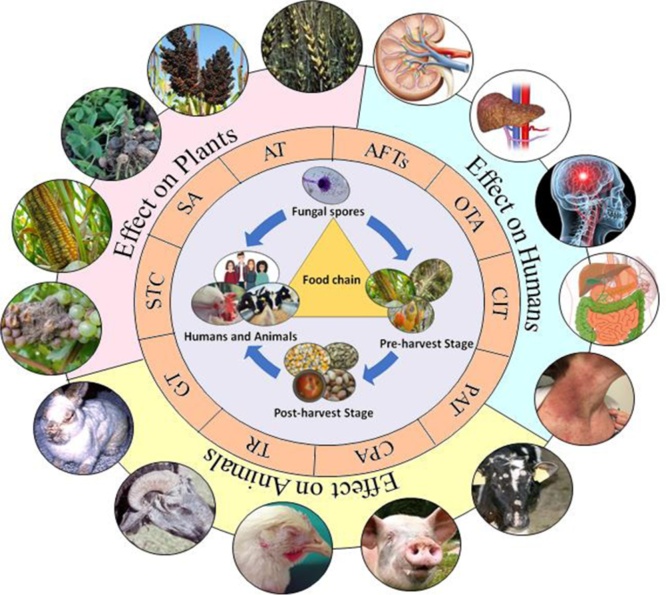
Fig. 1.
Aspergillus-derived mycotoxin contamination in food and the environment and its effects on humans, animals, and plants.
AFTs outbreaks was reported in India and stands for the 30 % food contamination globally. AFTs are thermostable, genotoxic, hepatotoxic, mutagenic, teratogenic, and carcinogenic, even nanogram levels. AFB1, AFB2, AFG1, and AFG2 that tainting various agronomic crops, food and feed and pose a potential risk to wellbeing’s. AFB1 metabolized as AFM1 in mammals. AFG2 and AFB2 are metabolized AFG1 and AFB1 after ingestion, respectively. OTA and CIT synergistically causative agent of Balkan Endemic Nephropathy (BEN), attenuate the RNA synthesis in renal disorders. AT reported to cause staggers syndromes, and neurodegenerative disorders in both animals and humans. Moreover, PAT produced by Penicillium, Aspergillus, Paecilomyces, and Byssochlamys, and contaminates various food and fruits. PAT prompts ulcers, inflammation, and intestinal hemorrhage. Similarly, CPA, GT, STC, TA and other small molecules/natural metabolites produced by species of Aspergillus exhibited extended toxicity to the humans and animals. Regrettably, various countries have failed to regulate the presence of toxins in food and feed.
Aspergillus species are widely distributed, grow on almost all humid substrates, and threaten public health in indoor environments. More than 600 fungal species are in human contact and about 50 species are widely recognized and characterized in epidemiological studies. Inhalation is a primary route of human exposure to fungal propagules. Indoor fungi cause irritative disorders such as allergy and asthma. Biological airborne particles such as fungi, bacteria, viruses, allergens, and biological fragments are present abundantly known as bioaerosols. Filamentous fungi is a significant genera of Aspergillus, Fusarium, Penicillium, Mucor, and Scedosporium present in the environment causing acute and chronic toxicity in humans. Fungal bioaerosols are readily breathable, consisting of spores and hyphal fragments, and are active elicitors of bronchial irritation and allergy. Besides, specific antigens from this pathogenic fungus in the environment induce hypersensitivity (HST). The fungal spores or occupational contaminants mediate HST and activate signs of pneumonia, inducing acute or chronic lung disease. Also, these spores are ingested along with food, and they can even come in contact with skin, leading to several conditions. Influenza-like fever, respiratory symptoms, organic dust toxic syndrome (ODTS), bronchopulmonary aspergillosis, invasive aspergillosis, pulmonary aspergilloma are some of the infectious diseases caused by large fungal spores in the atmosphere.
Furthermore, several researchers are working on methods ranging from traditional densitometer thin-layer chromatography (TLC) to advanced and precise detection of mycotoxins. Researchers have developed aptamer (APT)-based diagnostics in recent years, there have been no attempts to adapt current technologies to prepare POC diagnostic platforms. APTs are single-stranded oligonucleotides (ssDNA) that bind to their targets in a precise manner, ranging from small organic molecules to biological macromolecules. Specific APT are generated by systematic evolution of ligands through exponential enrichment (SELEX) processes. The word "rapid process" generally refers to a method that is significantly faster than the reference method and has a proclivity to promote the method. Many PoC test instruments are made up of simple membrane-based test strips that come with a test cassette for the rapid detection of various toxigenic fungi and their mycotoxins.
In this current review, authors are highlighted the Aspergillus derived mycotoxins in various agrarians produce, processing foods, fruits, meat, milk, alcoholic beverages, oil seeds, and indoor air. Besides its global occurrence, various toxicity such as liver, HCC, human hepatocytes, esophageal epithelial cells, alveolar type II (AT‐II) cells, AC3F1 mouse, and AC3F1 mouse models, Kidney, liver cells, neurotoxic cell lines (SHSY5Y, neuro 2 a, HepG2 Cells), HT29, Caco-2, HEK293, yeast model, fibroblasts, a pulmonary tumor cell line, and human gastric epithelial cells (Chk1) was reviewed for the understanding the toxicity of mycotoxins. Furthermore, proteomics, genomics, transcriptomics, and other OMICS approaches for the characterization, and precise detection of various toxins also discussed. Interestingly, aptamer-based methods, and other new technologies used in the study of mycotoxins was also discussed.
2. Literature review methodology
The current reviews the published research and review literature about the Aspergillus derived mycotoxins such as effects of aflatoxins (AFTs), ochratoxins (OTA), patulin (PAT), citrinin (CIT), aflatrem (AFT), secalonic acids (SA), cyclopiazonic acid (CPA), terrein (TR), sterigmatocystin (ST) and gliotoxin (GT).
2.1. Inclusion and exclusion criteria
This systematic review included fungal contamination of food and feed and effects of Aspergillus-derived toxins on food safety and human health. For inclusion criteria, the data obtained from diverse toxigenic species of Aspergillus is tabulated with specific sources of world-wide occurrence production of mycotoxins in food and environmental samples. The pivotal aim of the present review is to understand the prevalence and toxicity of Aspergillus in the food feed. Moreover, the non-toxic Aspergillus were excluded in this review.
2.2. Information sources
Owing to the medical nature of the question, the search was confined to PubMed, Scopus, Web of Science, and Google Scholar. About 300 abstracts, full text, Graphical abstract published from 1960 to 2020. Samples of various forms of foodstuffs, outdoor air for the isolation of toxigenic fungi in this study.
3. Literature review findings
3.1. Aspergillus toxins in food chain and toxicity
3.1.1. Aflatoxins (AFTs)
Mold poisoning due to ergot alkaloid is known to occur for many decades, although acquisition notoriety only after the epoch-making discovery of AFTs in 1961. The severity of the toxin and its effects are possibly shared by nutrient scarcity, caloric deficit, alcoholism abuse, and contagious disease. Susceptibility to microbial infections augments the effects of malnutrition. Many countries have established permissible confines for such toxins in food and feed intended for consumption because of the life-threatening implications of these toxins. The Scientific Committee on Food (SCF) provided a very similar strategy to the European Union (EU), which resulted in scientific opinions for the regulation of mycotoxins. Nevertheless, no specific maximum limits are defined in major markets globally such as India. Along with the high stability and accumulation of mycotoxins during grain storage and processing, the lack of identification of these toxins at the point of care (PoC) centers is of great concern. PoC are basic medical diagnoses (tests) performed close to the patient's point of treatment in order to obtain real-time, lab-quality outcomes in minutes. It provides users with fast, convenient transportation that should accurate and affordable.
AFTs are the world’s leading food and environmental species of Aspergillus which account for approximately 30 % contamination on their own [8]. In India, there have been many mycotoxicosis outbreaks over the past 40 years, such as AFTs hepatitis [9]. Entero-ergotism outbreak in Rajasthan, Maharashtra, and Gujarat in 1976 due to the ingestion of bajra infected with ergot alkaloids caused degnala disease in buffaloes and cattle. Childhood cirrhosis [10], inclusion body hepatitis was found in poultry in Northern India. The extreme aflatoxicosis outbreak in chicken occurred in Himachal Pradesh. Gujarat and Rajasthan were affected in western India, and 106 died during 1974 from the consumption of contaminated grains with toxins. A survey reported a total of 19,757 mycotoxins in feedstuffs in 2004, whereas, AFTs were more widespread in Southeast Asia [11].
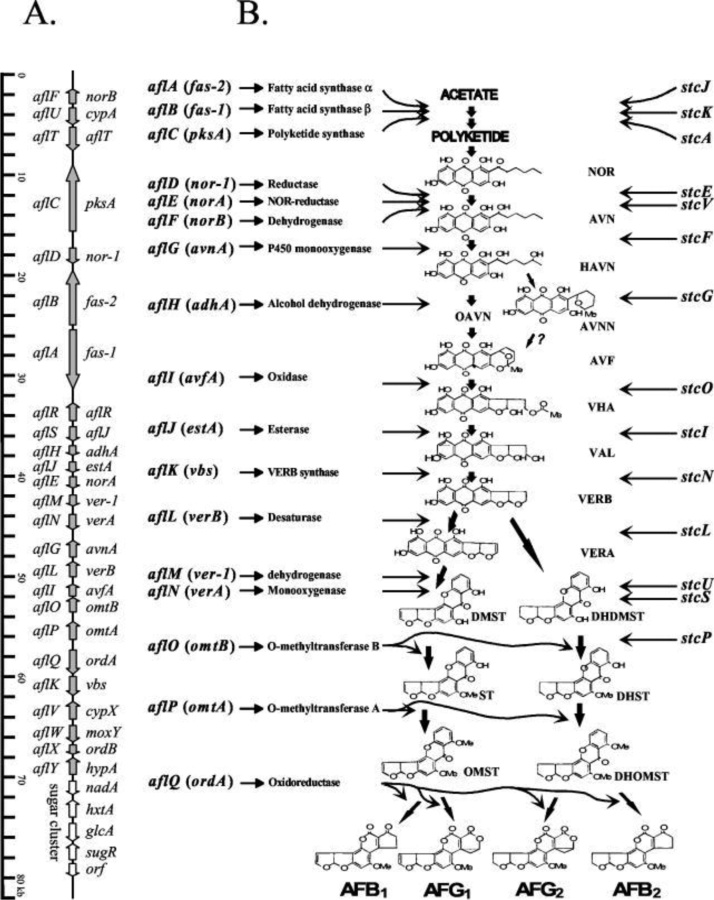
Of all major mycotoxins, AFTs, which are a group of furanocoumarins derived polyketides, are the most toxic and carcinogenic. AFB1, AFB2, AFG1, and AFG2 are major AFTs that contaminate various agricultural produce and pose a potential risk to livestock and human health. The biosynthetic pathway of AFTs is a complex system coded by a 70 kb DNA sequence (Fig. 2) with 25 genes or open reading frames (ORFs) representing a well-defined AFTs biosynthetic pathway gene cluster. The cluster of genes for the pathway is completely annotated in A. parasiticus containing 2.8 kb of chromosomal DNA [9]. So far, 15 structurally and well-established intermediates and at least 23 enzymatic reactions have been reported for AFTs biosynthetic pathway [12,5].
Fig. 2.
Biosynthetic gene cluster A, and the aflatoxin biosynthetic pathway (B) gene cluster in Aspergillus parasiticus and Aspergillus flavus [198].
3.1.2. Aflatoxin B1 (AFB1) and B2 (AFB2)
Aflatoxins are toxic metabolites produced by various Aspergillus species, primarily A. flavus and A.parasiticus that pose a serious threat to humans and livestock health [13]. AFTs were first discovered as a causative agent for the disease “turkey X” in 1960 in England. These toxins also occur naturally in foodstuffs such as groundnuts [14], maize, rice [15,16], cotton seeds, legumes, cereals [17], spices [18], crude vegetable oils [19], and cocoa beans [20]. AFTs are classified into aflatoxin B1, B2, G1, and G2 [21].
AFT-induced epidemics have killed many turkeys, pheasants, and ducklings alike. Originally, traces of AFTs were found in the chick feed, which included Brazilian groundnut food [22]. Afterward, the term AFTs was coined, symbolizing A. flavus [23]. In Kenya, too, few acute aflatoxicosis patients were recorded in 1981, when a drought was followed by heavy rainfall [24]. The AFTs' classification is based on their fluorescence property. AFB1 and AFB2 belong to the blue fluorescent (B) group in which the lactone ring of the coumarin moiety is fused to the cyclopentenone ring. Whereas the members of the green fluorescent (G) AFG1 and AFG2 toxins are formed by the fusion of lactone ring with cyclopentenone ring [23].
AFG2 and AFB2 are both harmless until they are metabolized after ingestion into AFG1 and AFB1, respectively. AFB1 in mammals is further metabolized to various hydroxylation products, such as AFM1 (“milk toxin”), which is further excreted in milk [24,25]. AFB1 can also be converted into AFQ1, AFP1, AFB2a, AFH1, and AFL [26,27]. AFTs are genotoxic, hepatotoxic [28], mutagenic [29], teratogenic [30], and carcinogenic [31]. Specifically, the transversion mutation, G→T is caused by AFB1 activity. Patients who are already diagnosed with the hepatitis B virus (HBV) are more likely to develop hepatocellular carcinoma (HCC) when exposed to AFB1 [32]. However, for longer periods, even lower doses of AFTs can result in both immunosuppression and nutritional interference [5].
Genotoxicity of AFTs was evaluated using the γH2AX assay in HepG2 human hepatoblastoma cells, human renal cell adenocarcinoma cells, and LS-174 T human epithelial colorectal adenocarcinoma cells. AFB1 can increase the expression of transcription factor Nrf2 and decrease the expression of liver Acc, disrupting hepatic mitochondrial lipid production and antioxidant capability [33]. AFB1 may change the proportion of DNA bases and may cause G: C to T: A and G: C to A: T transversions and transitions mutations, respectively in the liver cells [34].
A study found that 11 % of the 480 Chinese spices comprised noticeable amounts of AFTs, especially at higher levels in chili, prickly ash, and pepper [35]. Contamination with AFTB1 has been observed in Korean meju (crushed fermented soybean cake) at concentrations of 6.9 μg/kg [36]. The average tolerance for AFTs in soybean, as well as groundnut, is 4 μg/kg according to the European Union (EU). The concentration of AFB1 found in feed ranged from 4.22 and 10.54 μg/kg [37]. Climate change leads to an increase in the AFB1 concentration in maize and dairy feed samples [38,39]. AFB1 exhibits carcinogenic, teratogenic, and mutagenic effects when compared with other toxins [40]. Mice deficient in the xerodermapigmentosum (XPA) gene exposed to AFB1 are more susceptible to both natural tumor development and hepatocarcinogenesis (HCC) [41]. AFTs are at the highest degree of contamination in butter, sugar, and dark chocolate [42]. The food fermenting A. oryzae M2040 strain isolated from Korean Meju may inhibit the development of AFB1 and the spread of toxigenic A. flavus in vitro and peanuts [43]. Yeasts, bacteria cells, proteins, etc. most widely used in animal feed as a binder to mitigate the harm caused by AFTs [44].
3.1.3. Aflatoxin G1 (AFG1) and G2 (AFG2)
AFTs are commonly found in maize and other crops which are grown in warmer climates and present a serious threat to health in many regions of the world [45]. AFG1 and AFG2 are produced by several species of Aspergillus. In most cases, AFG1 is documented to be at higher levels of contamination than AFB2 and AFG2. Interestingly, no production of AFB2, as observed during the sense of AFG1, AFG2 [46]. AFB1 exhibits higher toxicity and carcinogenicity followed by AFG1, AFB2, and AFG2. These contaminants are not eliminated during industrial food processing, due to their high-temperature stability, as a result, they harbor even in bakery products and foods for infants and children [47]. Across China, a higher amount of AFG1 is detected in the population with elevated cases of gastric carcinoma and esophageal carcinoma [48]. AFG1 oral administration to mice, rodents, and hamsters has reduced altered hepatocytes, hepatocellular adenomas, carcinomas, and renal-cell tumors of the kidney cells. Severe outcomes, including elevated serum triglycerides, excessive inflammation in the liver cells, neutral fat accumulation, sudden autolysis of cells, death due to ingestion of food contaminated with AFG1 have been reported [49,50]. Feeding animals, affected by the consumption of toxin-contaminated groundnut cake (AFB1, AFB2, AFG1, and AFG2) showed the spread of connective tissue destroying hepatocytes and liver damage following clinical diagnosis [51,52]. AFG1 induced inflammatory response of the TNF-α in lung cells and caused in vitro oxidative DNA damage that may lead to lung carcinogenicity [53]
3.1.4. Aflatoxin M1 (AFM1) and M2 (AFM2)
AFM1 and AFM2 are usually detected after the ingestion of contaminated milk and milk products by humans. AFM1 is a by-product of AFB1 that is also reported to be toxic and carcinogenic and functions as a hydroxylated AFB1 metabolite in humans and animals [54,55]. Since cytochrome P450 (in microsome) is abundant in the liver, M1 is derived from AFB1 and is present in animal milk [56]. It is known to be hazardous to humans and is associated with carcinogenicity, genotoxicity, mutagenicity [57]. AFB1 and AFB2 metabolites are hydroxylated into AFM1 and AFM2 and are usually detected in milk and milk products derived from animals fed on infected grains [58]. Ingestion of excessive quantities of AFTs or long-term intake has led to the death of milch animals. Also, AFTs have been reported for milk production defects, immune system suppression followed by reproductive efficiency, and resulting in the development of bovine cancers [59].
The carcinogenicity of in-vivo AFM1 is about 10 % higher than that of AFB1, AFM1 is characterized by DNA-damage, one-third compared to that of AFB1 [60]. Similarly, in dairy products, AFM2 is a toxic metabolite, which is a 4-dihydroxy derivative of AFB2 [61]. The AFM2 residue levels which are not governed by dairy cattle regulations was around 15 times higher than AFM1 residue levels. On the other hand, the AFM2 toxicity spectrum may be lower than that of AFM1 [62]. Acute aflatoxicosis causes persistent symptoms of fatigue, loss of appetite, fatty liver, jaundice, decreased milk production in dairy cattle. Nonetheless, the US Food and Drug Administration (USFDA), set the maximum amount of AFM1 in milk as 0.05 μg/kg, [63].
3.1.5. Aflatrem (AT)
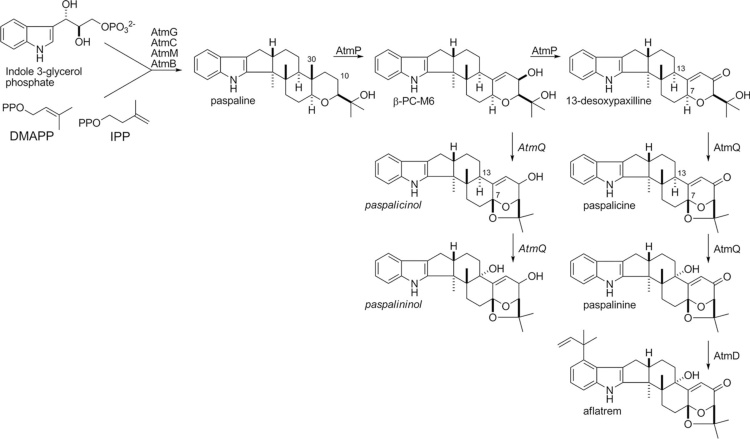
Aflatrem (AT) is a potent tremorgenic toxin (indole-diterpene) produced by A. flavus and the diminutive amount by A. minisclerotigenes. These strains are capable to produce paspalitrems, paspaline, terpendoles, shearinines, penitrems, lolitrems, janthitrems, paxilline, and supinates [64]. AT is known to cause neurological disorders and is visible as noxious food decay that grows on a variety of products [65]. Geranylgeranyl diphosphate (GGPP) is a precursor of all of these metabolites and they also have an indole moiety derived from tryptophan [66]. AT has been described to cause staggers syndromes, which include a variety of neurodegenerative disorders characterized by muscle tremors and hyperexcitability, and therefore is a health hazard for both animals and humans [67]. Sequences of genes that are highly similar to paxillin synthesis were found in the sequence data consisting of complete genomes of A.flavus NRRL3357 and A. oryzae RIB40. The genes atmG, atmC, and atmM which are homologous to paxG, paxC, and paxM respectively are required for AT production [68]. Nonetheless, the ATM1 locus expresses putative orthologs of only three of the seven genes required for paxilline biosynthesis. The remaining genes similar to the paxilline biosynthesis genes paxA, paxB, paxP, and paxQ were identified at the second locus, ATM2, and constituted a 25 kb gene cluster [69] (Fig. 3).
Fig. 3.
Proposed biosynthesis scheme for aflatrem biosynthesis in Aspergillus flavus [70].
3.1.6. Ochratoxins
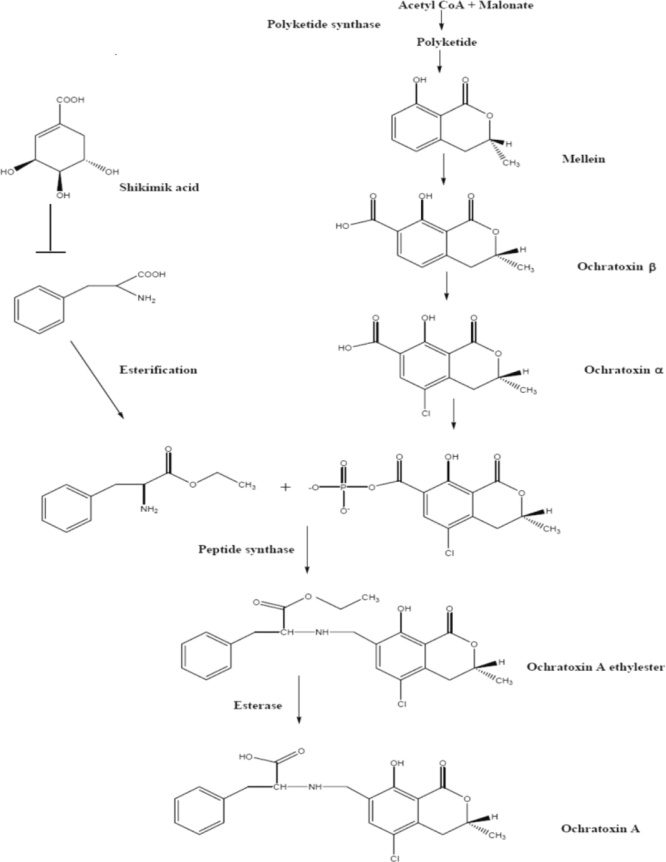
Ochratoxins are secondary metabolites of fungal origin which are chemically known as OTA, OTB, and OTC. The OTB and OTC are less toxic, because of the absence of chlorine [70]. OTA is derived from a β-phenylalanine-associated polyketide synthase (PKS) (dihydrocoumarin family) [71]. While there is a great deal of knowledge about toxigenic properties of OTA, unlike other important mycotoxins, A detailed biosynthetic pathway of OTA in any fungal species (Fig. 4). The isocoumarin group is widely believed to be a pentapeptide formed from acetate and malonate throughsynthetic PKS pathways. OTA biosynthesis requires a PKS gene which is known to be the main enzyme [72]. The heterocyclic component of OTA is similar in structure to mullein produced by A. ochraceus, A. westerdijkiae, and A. melleus.
Fig. 4.
Hypothetical OTA biosynthetic pathway [72].
OTA is produced primarily by the species of Aspergillus and Penicillium, which are distributed worldwide [53].It is a significant and detrimental toxin [54], which contaminates a variety of foodstuffs, such as grapes [55], coffee [56], cocoa, nuts [57], infant food [58], wine [59], corn [60], rice [61], wheat [62], meat [63], cheese [64], beer [65], feedstuffs [66], oilseeds [62] and indoor air [1]. OTA is produced during crop storage and is responsible for various cyanogenic effects in animals. This toxin primarily affects the kidneys and also harms the immune system. Contrary to strong evidence of renal organ toxicity and renal organ cancer in animals exposed to OTA, there is no evidence of damage of renal organs in humans, but effects on the renal organs are incontestable. Furthermore, OTA is also confirmed to be, neurotoxic, hepatotoxic, immunotoxic, genotoxic, embryotoxic, teratogenic, and carcinogenic in animals and humans [67,68]. Humans are exposed to OTA by numerous routes such as dietary route, dermal contact, and by inhalation [69]. International Agency for Research on Cancer (IARC), classified OTA to a human Group 2B carcinogen (IARC, 1993). OTA has also been detected in animal and human body fluids and kidneys [69,70]. OTA is not completely removed during cooking [72]. It can also withstand 3 h of sterilization at 121 °C and higher temperatures and can be only partially degraded at 250 °C [73] OTA absorption occurs in the stomach, which plays a crucial role in enterohepatic circulation. Moreover, OTA decreases the ability of the kidney to absorb the filtrate and has detrimental effects on the glomerular filtration rate which impairs the function of the kidneys epithelial cells [74]. The majority of animals affected by the consumption of contaminated feed from OTA are horses, cattle, goats, pigs, sheep, poultry, swine [75], and birds [76]. Laying chicks had exhibited medical symptoms of OTA intoxication. In poultry birds, reduction as well as delay in egg and weight development, increased number of discolored eggs, decalcification of eggshells, shape change, high urates content resulting diarrhea, refusal of feed, frailty, and even death [77].
Geno-toxicity of OTA treatment was induced increased levels of apoptosis, expression of oxidative stress genes studied on male Wistar rat’s kidney [78,79]. Also, an increased percentage of DNA in the tail observed in Vero-E6 cell lines [80]. The combined toxicity of OTA, AFTs, and OTA, PAT exhibited the significant DNA strand break upon exposure of 60 min studied on human peripheral blood lymphocytes [81,82].
OTA has been isolated from A. ochraceus, but studies have shown that diverse fungal species are also capable of producing OTA [83]. A. carbonarius, A. ochraceus, and A. westerdijkiae produce a huge quantity of OTA in grape products [84,85] and coffee beans [73,86,87]. Also, A. niger aggregates have been reported as OTA producers on natural substrates and section Nigri produces a smaller amount of OTA than A. carbonarius [88,89]. A. lacticoffeatus and A. sclerotioniger also produce OTA [90]. A. pseudoelegans, A.cretensis, A. roseoglobulosus, A. flocculosus, A. sclerotiorum, A. sulphurous, A.ochraceushave been reported as OTA producers [[91], [92], [93]]. And details of OTA producing Aspergillusspecies (94–158) are depicted in Table 1.
Table 1.
Worldwide occurrence of Aspergillus species and production of mycotoxins in food and the environment.
| Name of Toxin | Aspergillus species | Occurrence | References |
|---|---|---|---|
Aflatoxin G1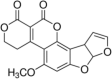 Molecular Formula: C17H12O7 Molecular Weight: 328.27 g/mol Aflatoxin G2 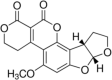 Molecular Formula: C17H14O7 Molecular Weight: 330.29 g/mol |
A. arachidicola A. arachidicola sp. nov A.minisclerotigenes sp. nov. A. bombycis A. flavus A. minisclerotigenes A. nomius A. parasiticus A. parvisclerotigenus A. pseudotamarii A. toxicarus A. rambellii A. korhogoensis Aspergillus pseudocaelatus A. caelatus A. novoparasiticus A. terreus |
Brazilian corn kernels Argentinean peanuts Poultry feed Blackand whitepepper Peanuts Arachis burkartii leaf Corrientes province, Argentina |
[94] [95] [23] [96] [97] [98] [99] [100] [101] |
Aflatoxin B1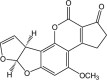 Molecular Formula: C17H12O6 Molecular Weight:312.2798 g/mol Aflatoxin B2 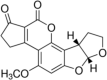 Molecular Formula: C17H14O6 Molecular Weight: 314.29 g/mol |
A. arachidicola A. arachidicola sp. nov A.minisclerotigenes sp. nov. A. bombycis A. flavus A. minisclerotigenes A. nomius A. parasiticus A. parvisclerotigenus A. pseudotamarii A. toxicarus A. rambellii Emericellavenezuelensis (A. venezuelensis) A. korhogoensis A. tamarii Aspergillus pseudocaelatus A. pseudonomius A. caelatus A. novoparasiticus |
Brazilian corn kernels Argentinean peanuts Poultry feed Black and white pepper Peanuts Arachis burkartii leaf Corrientes province, Argentina Insects and soil in the USA |
[102] [95] [63] [96] [97] [103] [104] [98] [105] |
Aflatoxin M1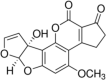 Molecular Formula:C17H12O7 Molecular Weight:328.27 g/mol Aflatoxin M2 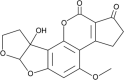 Molecular Formula:C17H14O7 Molecular Weight:330.29 g/mol |
A. arachidicola A. arachidicola sp. nov A.minisclerotigenes sp. nov. A. bombycis A. flavus A. minisclerotigenes A. nomius A. parasiticus A. parvisclerotigenus A. pseudotamarii A. toxicarus A. rambellii A. korhogoensis A. novoparasiticus |
Brazilian corn kernels Argentinean peanuts Poultry feed Black and White Pepper Peanuts |
[100] [95] [23] [96] [97] [98] [105] |
Ochratoxin A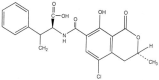 Molecular Formula: C20H18O6ClN Molecular Weight: 403.8 g/mol |
A. ochraceus | – | [71] |
| A. affinis | Italy | [106] [107] |
|
| A. alliaceus | Australia Worldwide (Argentina, Australia, Canada, Egypt, France, Greece, Hungary, Lybia, Mexico, Netherlands, New Zealand, Russia, Saudi Arabia, Spain, Tunisia, Turkey, UK, USA) |
[100] [108] [109] [110] |
|
| A. auricomus | Queensland, Australia | [102] | |
| A. albertensis | Edmonton, Canada | ||
| A. carbonarius | Southern Thailand Food products and tropical soil |
[111] [112] [113] |
|
| A. lanosus | India | [6] [114] |
|
| A. niger | Southern Thailand, Northern Thailand | [111] [112] |
|
| A. ochraceus | Australia | [102] [109] [107] [115] |
|
| A. ostianus | – | [109] | |
| A.petrakii | – | ||
| A. melleus | – | ||
| A. sclerotiorum | Chaing Mai, Thailand | [102] [109] |
|
| A. sulphureus | Mysore, India | ||
| A. sclerotioniger | – | [112] | |
| A. lacticoffeatus | – | ||
| A. cretensis | – | [107] | |
| A. fresenii | – | ||
| A. muricatus | – | ||
| A. occultus | – | ||
| A. pseudoelegans | – | ||
| A. pulvericola | – | ||
| A. roseoglobulosus | – | ||
| A. sesamicola | – | ||
| A. steynii | – | ||
| A. westerdijkiae | – | ||
| A. ochraceopetaliformis | – | ||
Ochratoxin B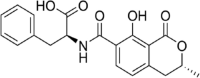 Molecular Formula: C20H19NO6 Molecular Weight: 369.37 g/mol |
A. alliaceus | Australia Worldwide (Argentina, Australia, Canada, Egypt, France, Greece, Hungary, Lybia, Mexico, Netherlands, New Zealand, Russia, Saudi Arabia, Spain, Tunisia, Turkey, UK, USA) |
[102] [108] [109] [110] |
| A. auricomus | Queensland, Australia | [102] | |
| A. wentii | Indonesia | ||
| A. carbonarius | Southern Thailand | [111] [112] [113] |
|
| A. lanosus | India | [6] [114] |
|
| A. niger | Southern Thailand, Northern Thailand | [111] [112] |
|
| A. ochraceopetaliformis | – | [107] | |
| A. ochraceus | Australia | [102] [109] [107] [115] |
|
| A. sclerotiorum | Chaing Mai, Thailand | [102] [109] |
|
| A. sulphureus | Mysore, India | ||
| A. melleus | – | [109] | |
| A. ostianus | – | ||
| A. petrakii | – | ||
Ochratoxin C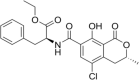 Molecular Formula: C22H22ClNO6 Molecular Weight: 431.9 g/mol |
A. ochraceopetaliformis | – | [107] |
| A. ochraceus | Australia | [102] [109] [107] [103] |
|
Citrinin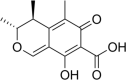 Molecular Formula: C13H14O5 Molecular Weight: 250.25 g/mol |
A.alabamensis A.allahabadii A.carneus A.floccosus A.hortai A.neoindicus A.niveus A.pseudoterreus A.oryzae A.terreus A.candidus A.flavipes A.iranicus A.urmiensis A.aventil A.ostianus A.fumigatus A.awemori A.parasiticus A.niger |
Soil Soil Soil Soil Soil Soil Soil Soil Soil Soil |
[94] [116] [117] [118] |
Patulin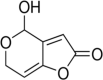 Molecular Formula: C7H6O4 Molecular Weight: 154.12 g/mol |
A.clavitus A.giganteus A.terreus A.longivesica A.amstelodami A.candidus A.echinulatus A.flavus A.fumigatus A.manginii A.ochraceus A.oryzae A.parasiticus A.petrakii A.repens A.sydowii A.tamarii A.variecolor |
Cereals,malt,Apple, Pear,Cherry Soil |
[119] [120] [121] [122] [123] [124] |
Cyclopiazonic acid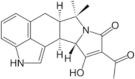 Molecular formulae:C20H20N203 Molecular Weight: 336.39 g/mol |
A. aflatoxiformans A. oryzae A. austwickii A. bertholletius A. cerealis A. flavus A. minisclerotigenes A. mottae A. pseudocaelatus A. pseudotamarii A. sergii A. tamarii A. aculeatus A. lentulus A. parvisclerotigenus A. versicolor A. pseudocaelatus A. phoenicis A. fumigatus A. pipericola A. hancockii A. korhogoensis A. caelatus A. fumisynnematus |
China Japan Argentina Nigeria USA |
[116] [125] [126] [127] [128] [95] [129] [130] [131] [99] [95] [132] [133] [134] [98] |
Secalonic acid A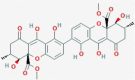
|
A. Ochraceous C. purpurea Pyrenochaetaterrestris Cetraria ornate Parmeliaeutotheiochroa Pseudoparmeliasphaerospora P. hypomilta |
Brasil Canada |
[135] [136] [137] [137] [138] |
Secalonic acid B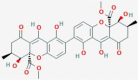
|
A. homomorphus Clavicepspurpurea |
– | [103] [122] |
Secalonic acid C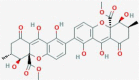
|
C. purpurea Cetrariaornata |
– | [122] [137] |
Secalonic acid D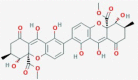
|
Aspergillus labruscus A. aculeatinus A. aculeatus A. homomorphus A. uvarum A. pseudoheteromorphus A. japonicus Penicilliumovalicum |
Brazil Thailand Europe |
[139] [140] [141] [103] [111] [142] |
Secalonic acid E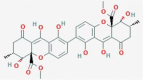
|
P. terrestris | – | [136] |
Secalonic acid F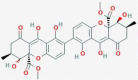
|
A. aculeatinus A. aculeatus A. homomorphus A. uvarum A. japonicus |
Thailand Europe |
[140] [141] [112] [111] |
Secalonic acid G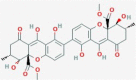
|
P. terrestris | – | [136] |
| Secalonic acid H | P.oxalicum | – | [143] |
| Secalonic acid I | P. oxalicum | – | [143] |
Aflatrem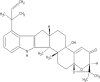 Molecular formulae:C32H39NO4 Molecular Weight: 501.67 g/mol |
A. flavus A. oryzae A.minisclerotigenes A. aflatoxiformans A. austwickii A. cerealis A. pipericola A. sergii A. fumigatus A. korhogoensis A. parvisclerotigenus |
New Jersey USA Nashville Germany |
[144] [126] [127] [116] [95] [145] [98] |
Terrein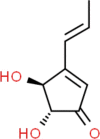 Molecular Formula: C8H10O3 Molecular Weight: 154.16 g/mol |
A.terreus A.lentulus A.novofumigatus A.fischeri A.stellatus A.monodii A.olivicola E.enezuelensis E.variecolour |
– | [146] [147] [131] [148] |
| Sterigmatocystin | A. versicolor | Unknown | |
| A. ustus | Unknown | [149] | |
| A. fructus | Date fruit | [150] | |
| A. protuberus | Indoor Air | [150] | |
| A. tennesseensis | Chestnut Seed | [150] | |
| A. cvjetkovicii | Soil | [150] | |
| A. griseoaurantiacus | House Dust Sample | [107] | |
| A. hongkongensis | Human | [151] | |
| A. pepii sp. Nov | Indoor Air | [152] | |
| A. chevalieri | Unknown | [153] | |
| A. amstelodami | Unknown | [153] | |
| A. multicolour | Unknown | [149] | |
| A. aurantio-brunneus | Unknown | [149] | |
| A. amstelodami | Unknown | [149] | |
| A. parasiticus | Unknown | [149] | |
| A. flavus | Unknown | [149] | |
| A. rambellii sp. nov. | Unknown | [99] | |
| A. askiburgiensis | Cave sediment | [154] | |
| A.croceus | Invertebrate and Vertebrate excreta, Soil | [154] | |
| A.fruticulosus | Unknown | [155] | |
| A.venezuelensis | Marine origin | [103] | |
Gliotoxin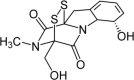 Molecular Formula: C13H14N2O4S2 Molecular Weight: 326.4 g/mol |
A.fumigatus A.clavatus A.thermonutatus A.niger A.flavus A.terreus A.chevalieri |
– | [156] [157] [158] |
OTA is a causative agent of Balkan Endemic Nephropathy (BEN), a progressive disorder that results in irreversible human kidney failure. Moreover, due to its strong binding to human serum macromolecules, it has a prolonged serum half-life which delays its removal from the body [159]. OTA biotransformation can be brought about by Cytochrome P450 3A4 (CYP 3A4), Cytochrome P450 Family 1 Subfamily A Member 1 (CYP 1A1), and Cytochrome P450 2C9 (CYP 2C9-1) [160]. DNA adducts may also occur in animals that are exposed to OTA [161]. Due to OTA toxicity; oxidative stress, liver toxicity, nephropathy, cell apoptosis, cell autophagy, calcium homeostasis, protein synthesis inhibition, etc. have been observed [75]. The mTOR/AKT pathways are remarkably decontrolled in rodents after exposure to OTA and this contributes to the carcinogenicity of kidney cells [162].
3.1.7. Citrinin (CIT)
Citrinin (CIT) is a polyketide metabolite of P. citrinum and is characterized as an antibiotic that acts against bacteria, bacteriophages, sarcomas, protozoa, and animal cells [163]. It is a renal toxin that affects poultry birds, domestic animals, and humans [164], CIT is involved in the etiology of endemic nephropathy, and is also genotoxic, embryocidal, and fetotoxic although the CIT molecular mechanism of toxicity is not completely unknown [165]. It shows structural similarity to OTA. Several species can produce CIT, among them are A. alabamensis, A. carneus, A. floccose A. allahabadii, A. hortai, A. neoindicus, A. pseudoterreus, A. niveus, and A. flavipesare important species [94,103].
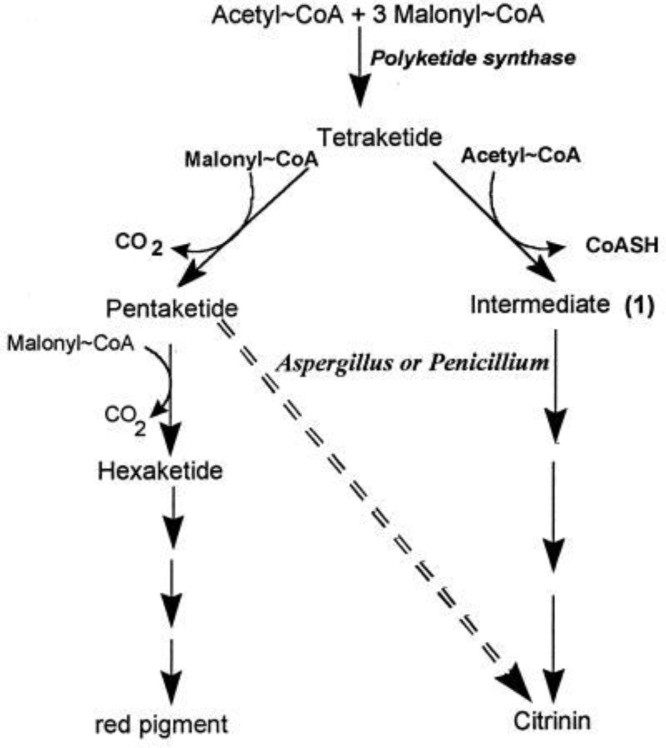
CIT and OTA both function synergistically to attenuate the activity of RNA synthesis in renal tissue [166,167] and causes renal disorders as a result of the development of DNA adduct with an increase in the formation of C-C8dG-OTA adduct [168]. Interestingly, the possibility of carcinogenesis in humans is significantly increased by CIT and OTA [169]. M. ruber is capable of biosynthesis of CIT along with natural red dye [170]. Moreover, Aspergillus and Penicillium, synthesizing CIT indicate a potential difference in the CIT biosynthetic pathway from that of M. ruber (Fig. 5), but they don’t produce any pigment. CIT is formed by the condensation of a single acetyl-CoA molecule with four malonyl CoA accompanied by the addition of three methyl units in Aspergillus species [171].
Fig. 5.
Biosynthesis of citrinin (CIT) in Aspergillus and Penicillium is indicated by the dashed arrow and red pigment in M. ruber [304].
3.1.8. Patulin (PAT)
Patulin (PAT) is a toxic metabolites produced by about 30 genera belonging to Penicillium, Aspergillus, Paecilomyces, and Byssochlamys, etc which in food and fruits [124,172]. Genus Aspergillus, Clavati group: A. clavatus, A. giganteus, and A. longivesica can producePAT [120]. It was evaluated for human medicinal purposes under the drug forename, Tercinin as a probable antibiotic but had been abandoned due to its toxicity to humans and animals [173]. Due to its suspected toxicity, PAT has been included in the list of mycotoxins and its level in foods is limited in several countries. Permissible amounts of PAT in juices (50 μg/L), solid apple products (25 μg/L) have been laid down, and foods intended for infants and children [174]. Structurally PAT has a strong affinity to the sulfhydryl groups (S—H) which describes its inhibition of various enzymes [175]. It induces ulcers, inflammation, and intestinal hemorrhage [176], as well as reduction of TEER (trans-endothelial electrical resistance), mediated inactivation of protein tyrosine phosphatase in human intestinal cell lines HT29 and Caco-2 [175]. PAT affects human embryonic renal cell development (HEK293) resulting in increased oxidative stress and eventually apoptosis [172,177]. It also results in increased levels of Th2 cytokine and elevated production of IFN-gamma, causes airway hyperactivity, hemorrhage, enlarged interstitial tissue, cortex dilation and fibrosis [178], increased serum ALT (alanine transaminase), AST (aspartate transaminase), lipid peroxidation and leads to cell damage [179].
On another hand, it leads to reduced activity of glutathione peroxidase and glutathione reductase activity [179] which leads to type 2 diabetes and is further associated with diabetic nephropathy. Mitochondrial ATP depletion and lysosomal failure were diagnosed with PAT exposure [180]. Expression of p53, bax, and cytochrome C was observed when rats were exposed to PAT, along with the downregulation of bcl2 in kidney cells [181]. PAT also leads to glomeruli disintegration and hemorrhage of the kidney [182].
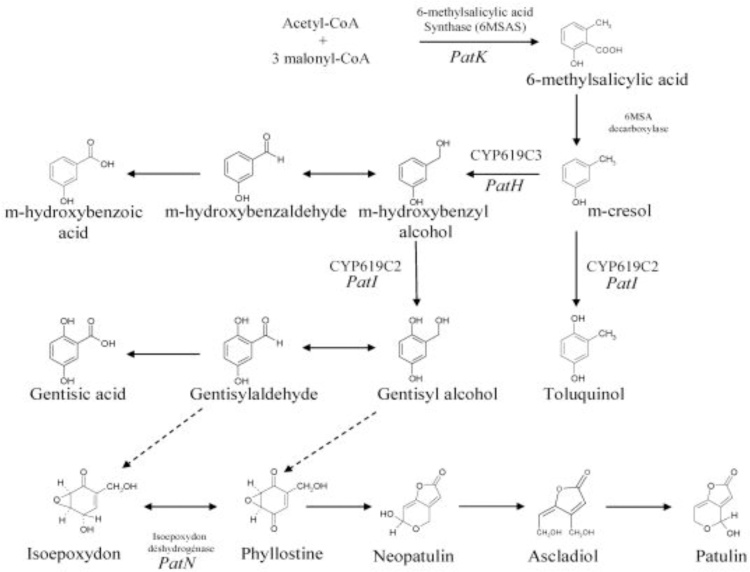
Biosynthetic gene clusters linked to PAT biosynthesis have been established. PAT biosynthesis is catalyzed by 6-methyl salicylic acid synthase (6MSAS). The isoepoxydon dehydrogenase (IDH) gene which is encoding the seventh enzyme involved in PAT biosynthesis in P. griseofulvum. A cluster of 15 genes located in the 40 kb region is involved in PAT biosynthesis in A. clavatus [119]. The formation of 6-methyl salicylic acid (6MSA) by condensation of one acetyl-CoA and three malonyl-CoA units is carried out by acetyl and malonyl transferase, ketoacyl synthase, ketoreductase, and dehydratase [183], and the complete biosynthetic pathway is depicted in Fig. 6.
Fig. 6.
Scheme of patulin biosynthetic pathways [303].
3.1.9. Terrein (Ter A)
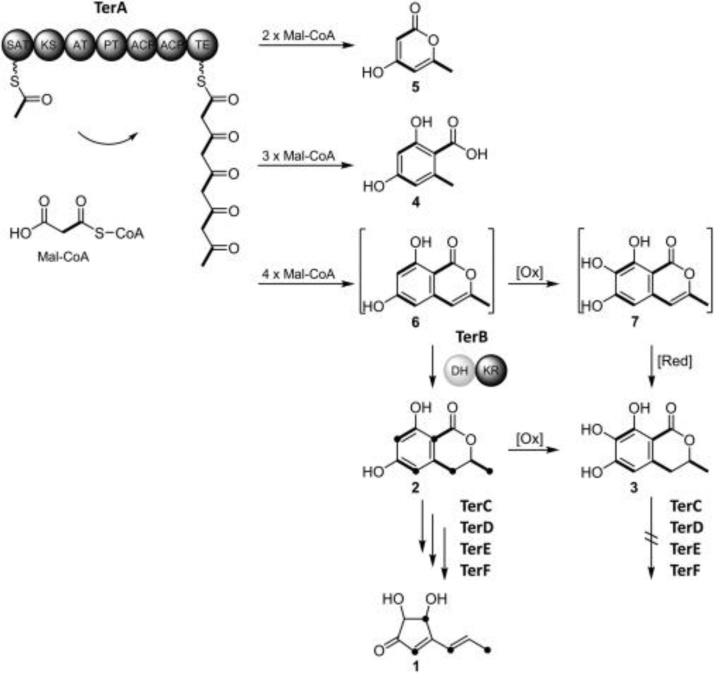
Terrein (Ter A) is a secondary, toxic metabolite isolated from several species of Aspergillus viz, A. terreus, A. lentulus, A. novofumigatus, A. fischeri, A. stellatus [147,148]. The complete biosynthetic pathway is not clearly defined, but there are 11 putative genes and their role in TR biosynthesis was elucidated [184]. Ter A has several effects, including anti-inflammatory anti-oxidant [63], anti-proliferative, and skin-whitening properties [35]. Ter A demonstrated angiogenesis inhibition in the androgen-dependent cell line (LNCaP-CR) of prostate cancer [185]. The biosynthetic pathway of Ter A is shown in Fig. 7. Initially, TerA produces compounds 5 (4-HMP; 4 hydroxy 6 methylpyranone), 4 (OA; orsellinic acid), and 6 (2,3-dehydro-6-HM; 6-hydroxymellein) by condensing acetyl-CoA with two, three, or four malonyl-CoA units. 6-HM (6-hydroxymellein) serves as a precursor for terrein production [184]. Ter A also functions as a proteasome inhibitor by reducing chymotrypsin activity and encourages apoptotic cell fatality in tumoral cell lines of the human lung (NCI-H292) [186]. It also inhibits human breast cancer cell proliferation [187]. The Ter A toxicity study of human lung and Zaehleenocarcinoma epithelial cell line showed inhibition of cell viability, proliferation, and morphological changes [177]. It also leads to the secretion of vascular endothelial growth factor (VEGF) and phosphorylation of STAT3, ERK1/2, and JNK1/2 in HGFs [188].
Fig. 7.
Terrein biosynthetic pathway in Aspergillus terreus [184].
3.1.10. Gliotoxin (GT)
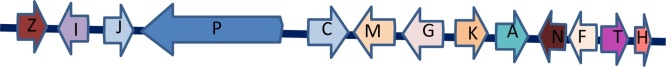
Gliotoxin (GT) belongs to a class of cyclic dipeptides of fungal metabolites epipolythiodioxopiperazin (ETP) initially discovered in Gliocladium fimbriatum [[189], [190], [191]]. Later production of GT was identified in A. fumigatus, Eurotium chevalieri, Trichoderma, and Penicillium. GT biosynthetic gene is responsible for ETP biosynthesis [192] and currently, 12 biosynthetic pathway genes that are responsible for GT biosynthesis have been identified in A. fumigatus. The ETP biosynthesis genes code for sirodesmin biosynthesis and other ETP-type toxins are gliovirin, epicoccin A, sirodesmin A, and sporidesmin A. The GT gene cluster comprises 13 genes consisting of gliZ, gliI, gliJ, gliP, gliC, gliF, gliM, gliG, gliK, gliA, gliN, gliT, gliH [193]. However, GT biosynthesis continues to revolve around several open issues in GT biosynthesis (Fig. 8). The GT has various adverse reactions including genotoxicity, immunosuppression, apoptosis, and cytotoxicity. This toxin also demonstrated strenuous genotoxic effects, DNA damage, and inhibition of human lymphocyte development [194].
Fig. 8.
Gliotoxin (GT) biosynthetic gene cluster of Aspergillus fumigatus [193].
3.1.11. Sterigmatocystin (STC)
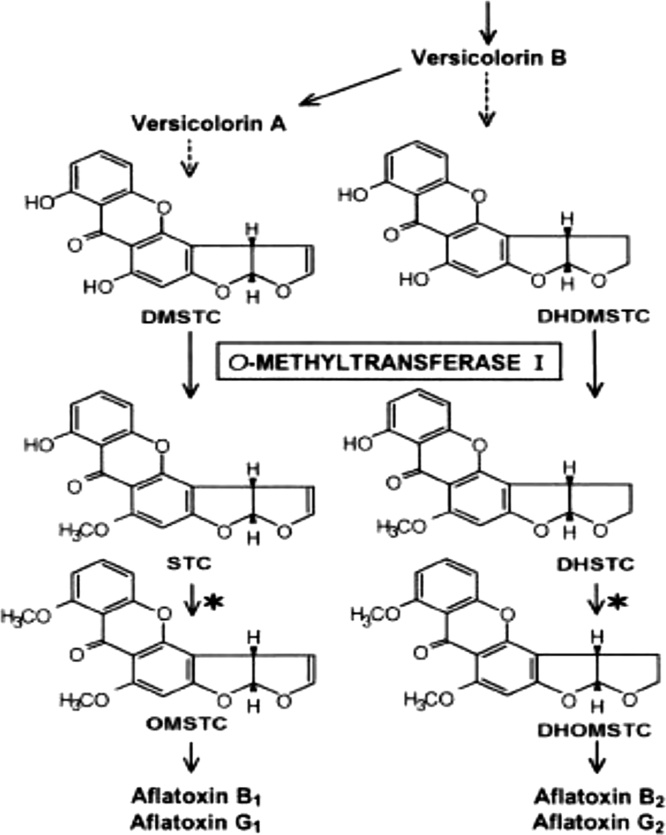
Sterigmatocystin (STC) is a toxic metabolite belonging to fungal polyketides, a precursor for AFB1, and is one of the most significant carcinogenic toxins. STC was isolated in 1957 for the first time from A. versicolor but it is also produced by several Aspergillus species. STC contamination occurs in several crops and is detected frequently in food grains, green coffee beans, spices, and dairy products [195,196]. Furthermore, agricultural commodities contaminated with these fungi contain elevated concentrations of STC. A. flavus and A. parasiticus infestation are responsible for trace amounts of STC, which is converted into AFTs [87]. The structure of the STC is closely related to AFB1, but the lethal potency of the STC is about one-tenth (1/10th) of AFB1 [197]. The STC biosynthesis pathway includes a 60-kb genome region containing a cluster of 25 genes required for STC biosynthesis (Fig. 9) [198]. It has many toxic effects such as immuno-modulatory activity [193], mutagenic [200], chromosomal damages [201,202], cytotoxicity [203], inhibition of cell-cycle [204,205], oxidative stress in liver of chicks [206]. DNA damage in the human liver [207] and reactive oxygen species (ROS) that leads to lipid peroxidation [208]. There is no regulation for the maximum allowed limit of STC in the food chain. Nevertheless, from a scientific point of view, the European Food Safety Authority (EFSA) acknowledged the possibilities of STC contamination in food and feed [209]. Besides, STC contamination of rice has been reported globally [196].
Fig. 9.
Biosynthesis of sterigmatocystin (STC) and, depending on the fungal species, further to aflatoxins [196].
3.1.12. Cyclopiazonic acid (CPA)
Cyclopiazonic acid (CPA) is a secondary metabolite (indoletetrameric acid) isolated from species of Aspergillus and Penicillium [210], originally discovered in P. cyclopium Westling (Synonyms: P. griseofulvum Dierckx). P. cyclopium was isolated from groundnuts that induced acute toxicosis in ducklings and rats [134,211]. Historically, CPA has also been reported to occur in food commodities of plant origin and is frequently detected in peanuts and maize. Prevalence of CPA has been observed in corn, peanuts, Kodo millet, sunflower seed, rice, cheese, poultry quail feed, and is associated with alleged field outbreaks of CPA toxicity [212,213,210]. CPA is a common mycotoxin and co-occurs with AFTs and is identified to be the causative agent of Turkey X disease. Apart from being harmful to rats, pigs, guinea pigs, chickens, dogs, CPA is also carcinogenic to humans. In addition to CPA, P. cyclopium also produces non-toxic indole derivatives, such as α-cyclopiazonic acid-imine (α-CPA-imine), and bissecodehydrocyclopiazonic acid (β-CPA) [204]. CPA has been isolated from the Kodo millet seed (Paspalum scrobiculatum) which causes symptoms of ‘Kodua poisoning’ in humans [214]. It results in sleepiness and tremors. Kodo millet is a staple food in North India, when contaminated with A. flavus and A. tamari, its ingestion was often found to cause intoxication and poisoning [215,216]. Unintended household CPA consumption in Uttar Pradesh, India has expressed signs of giddiness and nausea [217]. Intra-peritoneal injection of CPA showed depression and mobility defects. Also, cattle poisoning is characterized by signs of deficiency in muscle strength, overwhelming gait, and depressive disorders. Cattle usually recover after a few days but sometimes CPA poisoning is fatal [218]. Upon ingesting contaminated feed with CPA, animals displayed rigorous gastrointestinal upsets and neurodegenerative disorders [128].CPA also showed adverse effects on the liver, kidney, heart, digestive tract along with degenerative changes, necrosis, accumulation in the skeletal muscle of rats and chickens [213].
Humans are exposed to this toxin through the ingestion of dairy products such as cheese, milk, eggs, and meat showing toxic effects [219]. Contaminated milk affects weight loss, necrosis and viscera, seizure, and death [220,221]. Synergistic activity of AFTs and CPA could adversely affect the performance of broiler chicken causing high mortality [222]. CPA is harmful when given orally to swine, guinea pigs, and dogs that target the alimentary tract, liver, kidneys, and skeletal muscle.CPA is a major contaminant of maize (51 %) in the USA with an average level of up to 2.8 mg/kg. About 90 % of the peanut samples showed visible damage [223]. P. camemberti is a major food and feed contaminants and 20 commercial cheese product which is capable of producing CPA. P. camemberti and A. oryzae are used globally for the production of fermented foods [224].
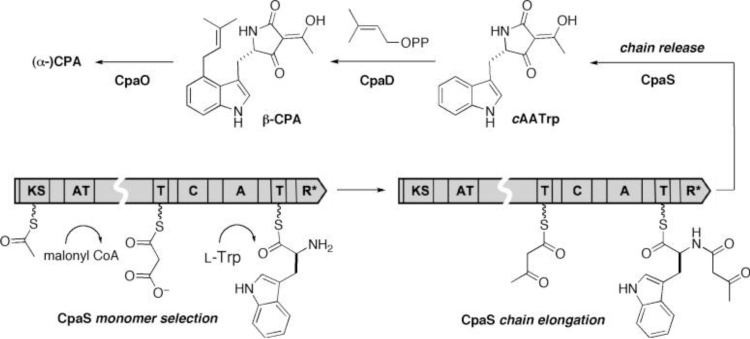
The tetramide biosynthetic gene cluster is responsible for CPA biosynthesis. CPA-related cyclopiazonate scaffold is derived from cyclo-acetoacetyl L-tryptophan (cAATrp) and β-CPA, which are assembled in a three-enzyme pathway Fig. 10 [225]. The immediate precursor of α-CPA is the tricyclic β-CPA, this conversion is catalyzed by oxidocyclase (CpaO), whereas dimethyl allyltransferase (CpaD) catalyzes the conversion of cyclo-acetayl -L-tryptophan (cAATrp) to β-CPA. The first enzyme is a hybrid two-module PKS-NRPS (CpaS), homologous to other sequenced fungal tetramate synthetases, that is responsible for the synthesis of tetramate cAATrp [226] is shown (Fig. 11).
Fig. 10.
Cyclopiazonic acid biosynthetic enzymes (CpaS, CpaD and CpaO) and their role tailor intermediates cyclo-acetayl -L-tryptophan (cAATrp) and B-CPA to afford ((alpha-CPA).
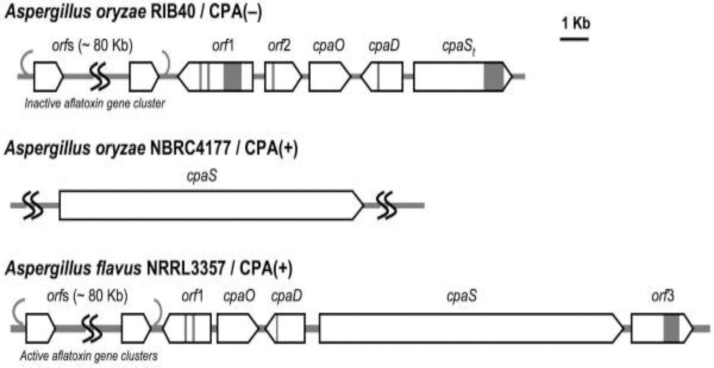
Fig. 11.
Biosynthetic gene cluster identified in Aspergillus species including two CPA producers and non-producers [226].
3.1.13. Secalonic acids
Secalonic acid (SA) is an important natural and versatile secondary metabolite of fungal species. SA has been isolated from the ergot fungus Claviceps purpurea in 1906 and reported by Kraft in 1906. Twenty-two members of SA were structurally identified as dimers of six monoxanthones, A–F [227,228]. SAA, SAB, SAC, SAD were isolated from Claviceps purpurea, P. oxalicum, and A. ochraceus [228,229]. SAA attenuates the cytotoxicity of colchicine in rat cortical neurons [230]. SAA, SAE, and SAG are known as yellow pigments isolated from P. terrestris. SA’s exhibits extensive biological activity such as anticancer, antimicrobial, antitumor, and anti-HIV [[231], [232], [233]]. Furthermore, SA is closely related to ergofalvin and ergochrysin A that are collectively referred to as ergochromes which grow on ryegrasses [231]. Antitumor activities of SA against Ehrlich ascites carcinoma, sarcoma-180, and mice tumors induced by Rous sarcoma virus have been documented [234]. SAD is highly toxic, teratogenic, and weakly mutagenic mycotoxin and is a common contaminant in the United States. SAD, a teratogenic toxin has effects on pregnant mice and its progeny and acts in a dose-dependent response that leads to neurotoxicity [235]. SA with dimeric tetrahydroxanthenone skeleton is less toxic to humans but has a wide variety of anticancer and antimicrobial activities, including the inhibition of topoisomerase 1 and Protein kinase C [236,61]. SAD could inhibit VEGF-mediated angiogenesis through the Akt/mTOR pathway in breast cancer [237].
SAD not only inhibits responsive cell growth and induced leukemia but also inhibits multidrug-resistant cells [139,238]. Moreover, SAD produces symmetrical tetrahydroxanthone derivatives, which are part of novel DNA topoisomerase I inhibitors [239], which regulates the conformational alterations in DNA topology. SAD a comparatively low toxic molecule shows a promising antitumor, anticancer activity, and relatively safe for exploration [240]. SAF has anticancer activity, prevents proliferation, and promotes the apoptosis of HepG2 cells. Nevertheless, the underlying mechanisms and other biological activities of SAF remain unknown.
4. OMICS tools for mycotoxin detection
Mycotoxins produced mainly by Aspergillus, Penicillium, and Fusarium have intensive toxic effects on humans, plants, and animals. Owing to the dynamic biosynthetic pathway genes, these toxins are diverse in chemical structures and biological activities and can be integrated into several ways. Such mycotoxins are often involved in the entire food chain therefore they have to be systematically identified and monitored. The co-occurrence of the various toxins in agricultural fields, which makes it necessary for the development of coherent detection methods. Conventional approaches such as LC–MS, HPLC, HRMS, etc. must be replaced with modern methodologies that can easily detect the toxins. OMICs tools like genomics, proteomics, transcriptomics, and metabolomics have been used to classify and quantify the biosynthetic genes for mycotoxins and can be used for their detection in the food chain [241].
4.1. Metabolomics
Metabolomics is the study of chemical processes and products of metabolisms that includes small molecules and other various metabolites. It is based on targeted or non-targeted approaches [242,243] and analyzed with the help of several databases such as METLIN, ChemSpider, and PubChem. In 1970 Scott et al. performed thin layer chromatography (TLC) as the first traditional method in which 18 metabolites including aflatoxins B1, B2, G1, G2, and OTA were identified [244]. Mycotoxins can be identified quantitatively as well as qualitatively using LC–MS/MS [245], due to their high selectivity and sensitivity in nanogram levels (ng) [246].
Later, ultra-high-pressure liquid chromatography (UHPLC) coupled with SCIEX triple quad MS system (QDMS) was performed to detect 26 mycotoxins in commercially available finished grain or nut products [247]. LC–MS/MS and LC-HRMS were used to detect 24 mycotoxins that were identified from pigs, broiler chickens, and biological matrices [248]. Time-of-Flight (TOF) and Orbitrap HR-MS are the most widely used techniques for the evaluation of untargeted toxin-metabolites [249] with the utmost sensitivity, selectivity, and precise mass resolution. Furthermore, HRMS has been used to classify 28 different toxins and 245 fungal, as well as bacterial metabolites in pet food [250]. The MS-based protein analysis technique is very effective and improves rapidly in terms of specificity and accuracy [251]. Several researchers have identified a wide range of microbes such as bacteria, yeast, and fungi, and their role in toxicity [252].
4.2. Genomics
The analysis of mycotoxin-producing species involves the study of the genomes of these organisms, the comparison of the sequenced genome with the related organisms, and the identification of the corresponding homologous proteins [253]. The first analysis was performed by using USDA/ARS (United States Department of Agriculture/ Agriculture Research Service) Expressed Sequence Tags (EST) genome of A. flavus [254]. Recently, the full mitochondrial genome analysis of AFB and AFG producing A. parasiticus has been achieved using sophisticated bioinformatics tools [255]. The J. Craig Venter Institute, USA could identify more than 12,000 functional genomes in A. flavus by using their bioinformatics software [256].
In recent years, the use of different genomic tools such as microarray, Ion Torrent Personal Genome Machine (PGM), quantitative reverse transcription-PCR (qRT-PCR), and whole-genome sequencing (WGS), have been used to pursue various research [257]. Genome sequencing of AFTs producing fungi A. arachidicola of Argentinian peanut was rendered using BLAST2GO [258]. Besides, genome analysis of clinically isolated A. flavus was performed in Japan [259]. Furthermore, a comparative genomic analysis of A. flavus as a model for understanding mycotoxin biosynthesis and plant pathogenicity has been reported [257]. Genome sequencing helped to identify the target genes for the production of AFTs in field fungal isolates. The genomic study was conducted using a Next-Generation Sequencing analysis of 240 Aspergillus strains in peanut seeds where nine clades were identified, three clades were non-aflatoxigenic and five were aflatoxigenic [260]. Gilbert et al. [261] developed a methodology through a functional genomics study that can assist to forecast the impact of climatic change on A. flavus, AFTs production, and expression of biosynthetic regulatory genes [260].
More than 50 secondary polyketide synthase (PKS) and non-ribosomal peptide synthase (NRPS) genes were located in the OTA biosynthetic gene cluster genome of A. westerdijkiae [262]. Genes including 633 carbohydrate-active enzymes, 716 cytochrome P450 enzymes, and 377 proteases along with two-hybrid t1pks-nrps gene clusters were involved in OTA biosynthesis in A. westerdijkiae [263]. Recently, PAT biosynthesis has shown that all the 15 genes, including VelA, VelB, and VelC, but not VosA in the cluster, are involved in PAT biosynthesis and allow localization of subcellular proteins [264].
4.3. Transcriptomics
Transcriptomics is highly useful in the field of agriculture, in medicine for early-stage diagnosis and treatment, as well as an understanding of complex biological systems [265]. It is also useful in crop advancement to study the regulation of large networks of biological processes [266,267]. Genes transcribed from mycotoxins-infected plant cells are different from not-affected cells; hence they can be subjected to transcriptional profiling. It involves quantifying the gene expression of several genes in given conditions. Numerous techniques such as serial analysis of the gene (SAGE), RT-qPCR, nylon membrane arrays, and northern blots are prominently used for gene profiling. Also, whole transcriptome shotgun sequencing (WTSS) and gene expression microarray are rapidly being used for food safety consideration [268]. Certain approaches that have been recently reported are shotgun analysis (RNA-seq) and RT-qPCR [269].
The transcriptomic analysis of various mycotoxins producing strains has helped to understand the mechanism of plant-fungus crosstalk of mycotoxin toxicity as well as the abiotic factors affecting mycotoxin production. The induction of resistance genes in Z. mays by A. flavusemploys a variety of mechanisms [270]. Which are likely to contribute to the development of resistant varieties. Transcriptomic profiling resulting in differentiation of A. flavus gene response and susceptible peanut genotypes [271]. Analysis of transcriptomics of A. flavus isolates containing higher levels of AFTs showcased few differentially expressed genes under environmental stress [272]. Furthermore, transcriptomics does not elucidate the mode of action of mycotoxin but has bestowed appropriate toxicological details [273]. In an attempt to identify biomarkers for AFTs production in A. flavus during oxidative stress, 220 out of about 1000 proteins were found to be differentially expressed [274].
4.4. Proteomics
Protein signals in response to mycotoxin production have been used as biomarkers for the enhancement of plant resistance as well as fungal stress tolerance that contributes to interactions between host-pathogen [275,276]. Degola et al. [277] documented the modification of sclerotial production of cuminaldehyde thiosemicarbazone, an inhibitor of AFTs biosynthesis in A. flavus. However, there were several problems with the detection of undefined peptides, as well as the proteins. The use of 2D gel electrophoresis in proteomics addresses the complexity of the sample protein mixtures by separating the proteins into smaller groups or individual proteins [278]. Tryptic digestion is also used to achieve a high output of less complex or length optimum peptides for the analysis. Recently, relative and absolute protein quantitation was achieved using gel electrophoresis (2D) along with isobaric tags (iTRAQ) to classify biomarkers using the number of determined proteins [279]. Following the digestion, the identification is performed using tandem mass spectrometry (MS/MS) with the aid of UniProt and NCBI applications. Proteomics helps to boost other types of omics such as transcriptomics and genomics [280].
5. Field-deployable rapid point of care (POC) diagnostics
Globally, several researchers are actively engaged in the development of methods ranging from conventional densitometer thin-layer chromatography (TLC) to advanced immunosensors for the detection of mycotoxins in food and other matrices. Chromatographic methods such as HPLC, HPTLC, GC, LC–MS/MS, Fluorescence Spectrophotometry, Frontier Infrared Spectroscopy, fluorometer, Fourier-transform infrared spectroscopy (FTIR), radioimmunoassay (RIA), ELISA, lateral flow devices (Immunodipstick), surface plasmon resonance (SPR), electrochemical immunosensors are commonly used for the detection and quantification of mycotoxins in agricultural food crops [281]. Monoclonal/ polyclonal antibodies have been used to identify several toxins, but cross-reactivity is a major obstacle, resulting in uncertainty. The detection of mycotoxins in various cereal-based foods using immunoassay techniques has increased over the last decade [282], including field-based immune-chromatographic toxin interaction test strips and antibody-coated nano-materials.
Lateral-flow Devices (LFDs) are emerging and are used commercially in agricultural produce to detect toxins. Advances in portable photometric strip readers have enhanced the quantitative detection of mycotoxins in food and feedstuffs [130]. Moreover, several researchers targeting AFB1, FB1, and OTA have also developed aptamer-based diagnostic in recent years, but no attempts have been made to adapt existing technologies to prepare POC diagnostic platforms [283]. Rather than conventional methods, detection of these enigmatic toxins is more feasible using sensitive and field-deployable rapid POC diagnostics using aptamers.
5.1. Aptamers(APT)
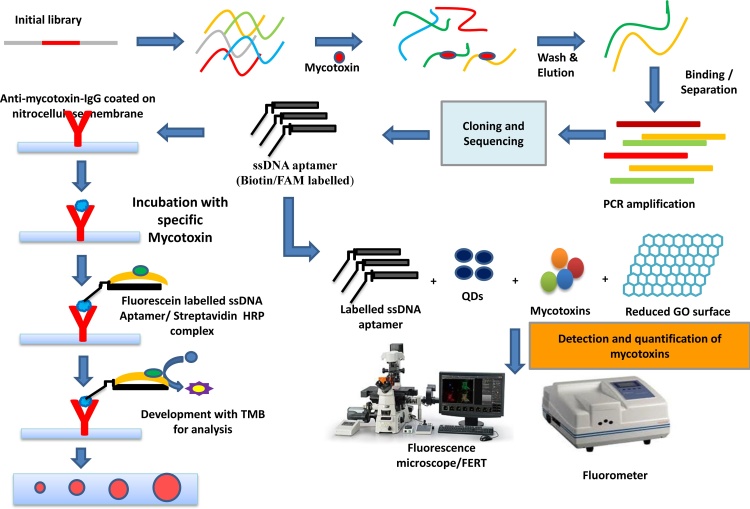
They are short single-stranded oligonucleotides that bind to their targets ranging from tiny organic molecules to biological macromolecules in a precise manner. APTs are synthetic DNA/RNA/XNA molecules and consist of short strands of oligonucleotides sequences. Specific APT was developed by systematic evolution of ligands by exponential enrichment (SELEX) processes for synthetic materials [284]. APTs are an alternative to antibody-based systems and have several advantages, particularly thermal and chemical stability, practical synthesis, as well as the increased binding affinity of the target, are also flexible to modification with functional groups such as thiols and amines. These unique characteristics provide great potential for the detection of biomolecules. Such novel properties make APTs the material of choice for highly sensitive biosensing platforms. The APT is conjugated with nano-materials with highly specific recognition abilities and a unique way to analyze target analytes in food and feed samples (Fig. 12).
Fig. 12.
Development of novel ssDNA aptamer-based bio-sensing platforms for biothreat fungal toxins in food and feed chain using nano-biotechnology approaches. Modified from Front Pharmacol. 2018; 9: 271. doi: 10.3389/fphar.2018.00271 [305].
APTs are third-generation molecular probes and can be easily synthesized in the laboratory. At one time, several billion copies of selected aptamers can be generated. Animals or high-end in vitro animal cell culture facilities are not required. Fungal toxins are non-protein organic compounds for which generating precise and adaptive antibodies is often difficult and has limitations. On the other hand, APT probes for these molecules are easy to generate in the lab in a limited period and are very cost-effective when compared to the current probes such as antibodies and sensors. These are versatile and minimize the cross-reactions that can lead to false results. Until now, only a few detection methods and no POC detection methods for fungal toxins are available in India. Since these platforms are easy to fabricate, replication is possible and user-friendly to use and interpret the results. In the present scenario, the detection of FDA approved aflatoxins kits such as Aflatest (VICAM Co), Agriscreen (NEOGEN Crop), AflaCup 10 (ROMER LABS Inc), AflaCup 20 (ROMER LABS Inc.), EZ-Screen (MEDTOX) are available in the market and are very expensive. Most of the food industries prefer to use other toxin determination tests as the above kits are uneconomical. It is therefore important to establish simple techniques that are open to all kinds of food manufacturing industries.
6. Toxigenic Aspergillus in environment and its effects on humans
Environmental opportunistic pathogens (EOPs) are a wide class of pathogens capable of persisting and evolving outside most environments and entering the host under favorable. EOPs are highly contagious, that which normal circumstances do not affect the host, but leads to adverse conditions when host resistance declines. Human aspergillosis results in systemic and localized immune-suppression mainly by A. flavus and A. fumigatus. Aspergillomas and invasive pulmonary disease are severe Aspergillosis-related infections [285]. Approximately 300 species of Aspergillus can cause various diseases including invasive aspergillosis (IA), rhinosinusitis, bronchopulmonary and chronic pulmonary aspergillosis, keratitis, otomycos, and infections in trauma or burn wounds [286]. Many species of the Aspergillusgenera such as A. fumigatus, A. flavus, A. terreus, A. lentulus, A. nidulans, A. ustus, A. fischeri, A. versicolor, A. glaucus, A. niger, and A. oryzae which are capable of causing aspergillosis have been studied [287]. A. fumigatus is a widespread species responsible for human aspergillosis with a mortality rate of 40–90 % in immune-compromised patients. Approximately 90 % of human IA infections have been identified [288].Furthermore, A. flavus causes about 30 % of all aspergillosis cases in the United States [289], affecting around 11 million patients annually.
Many therapies have also emerged for these infections. Human aspergillosis occurs in about one in 100,000 In the United States, which means that seven people are infected every day, and patients suffering from this infection spend an average of $95,000 on medications. A. fumigatus is an environmental fungal pathogen that may cause severe asthma, sinusitis, hematological malignancies, hematopoietic stem cell transplantation, leukemia, or lymphoma [290]. The spores of A. fumigatus can reach the respiratory system quickly and trigger an infection that can evolve into an angioinvasive disease by penetrating the liver, kidneys, and brain [291]. In extreme cases, systemic infections may lead to death in patients previously diagnosed with hematological malignancies, organ transplants, cancer, prolonged steroid treatments, and HIV. A. fumigatus produce virulence, surprisingly this strain is capable of producing 226 metabolites that act as an immunosuppressant [292]. However, the extension of the infection by crossing the blood-brain barrier (BBB) to the central nervous system (CNS). Certain obstructions may include edema, alveolar flooding, and lack of oxygen supply, which causes the fungus to develop a hypoxic environment for its survival in the inflamed tissue and different organs.
Complementary systems and phagocytic cells aid in defensive mechanisms against various invasions by the pathogen. EOPs bind to the host complement regulators thereby overriding the immune system and downregulating the complement system which can also contribute to many specific diseases. Luis et al. [293] isolated the environmental strains which are more virulent than the clinical strains. The water used in the hospital in which patients with hematological malignancies were admitted, showed the presence of Aspergillus and other fungal species at the concentration of 16.1 CFU/m3 in bathrooms, 7 CFU/m3 in inpatient rooms, and 8.6 CFU/m3 in hallways, which symbolizes that the aerosol present in the hospitals was created from the infected water and can adversely infect the patients as they emerge [286].
Furthermore, the widespread occurrence of various filamentous fungal spores in the environment of allergic patients and persons with impaired immunity makes them more vulnerable to secondary inhalation infections. Pulmonary tuberculosis and pulmonary fungal infections have similar clinical characteristics due to which pulmonary tuberculosis is misdiagnosed. Patients with prior tuberculosis are affected by A. fumigatus and A. flavus causing various secondary diseases. A recent study showed that 12.3 % of positive patients with tuberculosis (TB) had co-infected TB with secondary fungal pulmonary infections [294]. Sivasankari et al. [295] reported that 8 out of 80 sputum samples obtained from patients with TB were positive for Aspergillus species, indicating the extent of the secondary fungal invasion infection in Kanchipuram state, India. In Uganda, patients who were diagnosed with residual chest x-ray cavitation pulmonary TB were resurveyed after 2 years using computed tomography, the results showed that 14 out of 285 patients were positive for cyclopiazonic acid (CPA) [296].
7. Effect of mycotoxins on children’s health
The continuous subjection to mycotoxins is creating havoc in human life. Nowadays infants and children are exposed to diverse types of toxins through various forms of food supplements. Consumption of such highly contaminated food results in different toxic effects right from the infant stage, including growth impairment, stunting, underweight, which could be further exacerbated immune deficiencies, and other infectious diseases. The co-occurrence of the mycotoxins in infant foods such as wheat, barley, corn, banana, apples, and sweet potatoes leads to episodes of toxicity. According to the European Commission (EC), the overall allowable level for the specific toxins in baby food is 0.5–200 ppb [172]. An infant cereal, one with cocoa has demonstrated the highest level of AFTs. Cereals containing dehydrated fruits and gluten-free cereals have shown an intermediate level in both milk and honey-based cereals. Control of the aflatoxin during the production and processing of infant food is very important for infant food safety [297]. AFTs and CIT have been found in relatively high amounts in processed foods as well as in native homemade formulated foodstuffs such as Tom bran (native Nigerian food) leading to health risks in infants and young children (IYC) [33].
In young infants near Cleveland, Ohio, a study reported idiopathic pulmonary hemorrhage (PH) due to indoor air exposure to Stachybotrys chartarum. This fungus has been thriving in flooding, plumbing leakage, or even roof leaks in homes as it requires water-soaked cellulose to grow. It is known, infants are highly susceptible to the mycotoxins spores as their lungs expand very rapidly. The prevalence of environmental tobacco smoke that has prominently triggered hemorrhage, had also led to the worsening of the disease. About 101 cases of this disease occurred in the United States between 1993–1998 [298]. The presence of molds on the indoor walls of the homes in Poland led to a deficiency in the intelligence quotient of infants who were residing in these homes for more than 2 years. These cases provide an insight into the magnitude of the effect of mycotoxins, especially on infants [299]. Another study included the occurrence of AFT-albumin adducts up to 720 pg AFT-lysine equivalent per mg albumin in Gambian children serum samples for malaria patients. In the children with hepatitis B surface antigen (HBsAg) and Plasmodium falciparum, the amount of AFT-albumin adducts was much higher than their control groups [300]. AFM1 measured 0.16–0.33 μg/kg in the breast milk of the lactating mothers in Nigeria’s Ogun State. Also, 82 % of the breast milk collected from the state samples was positive for AFM1 [301].
8. Conclusions and future research
Mycotoxin contamination has been a global concern since the early days of human existence and management of mycotoxin production is limited and challenging. Environmental factors play an important role in the development of mycotoxins that enters the food chain and the interaction of toxigenic fungi is a major concern. The continuous and over, period of exposure of toxin developing pandemonium in human life. Also, various forms of food supplements highly contaminated with toxins right from the infant stage are lead to outbreaks of toxicity. However, no quantitative data available for several mycotoxins, and more studied are needed globally to know the exact degree of contamination of mycotoxins in food and feed. Unpredictably, the metabolic pathway, and toxicity of numerous molecules are yet to understand, which are urgently considered for the for safety and public health concerns. As described Aspergillus is the one of the major genera of filamentous fungi in terms of food, agriculture, and pharma industries for their wide ranges of applications. In addition to the mycotoxins producers, also most prevalent EOPs that directly affect individuals with weakened immune system leading to mortality risk. Also, pulmonary TB and fungal infections had similar clinical characteristics, and is misdiagnosed. Coronavirus pandemic, pulmonary diseases caused by fungal bioaerosols might misdiagnosed as COVID-19 which misleads to the physicians for wrong prescription of the medicines. AFTs, OTA, CIT, PAT, CPA, Ter A, STC, TA and other induced toxins, and their combined toxicity was studied in various mammalian cell line models, and animals species exhibited Geno-toxicity, and increased levels of apoptosis, expression of oxidative stress genes.
The technological capability of toxin chemotypes for detection using various analytical tools are commonly used for the detection and quantification. Genome, proteomes, and transcriptional studies are imperative for the genome level organization. Till date, no POC detection methods have been developed for mycotoxins are available in India to detect mycotoxin in agricultural food and environmental samples. APTs may complement to the traditional methods, and open up new technological solutions to the rapid and robust detection of mycotoxins. The early, cost effective detection of mycotoxins and eco-friendly management approaches are very imperative. Paper-based APT test kits are quick, inexpensive and no equipment or laboratory facilities are required which are used for early detection of mycotoxins need to be explored. Of various management approaches, such as physical, and chemical, biological control agents (BCA), help to neutralization and/ or degradation of mycotoxins in food.
Ethical statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
This work was supported by the Department of Biotechnology Ministry of Science (BT/PR27494/NNT/28/1549/2018), SERB, Department of Science and Technology, India (EEQ/2017/000502), Council of Scientific and Industrial Research (CSIR), New Delhi, India, and CSIR-National Chemical Laboratory, Pune, India by providing the necessary facilities.
Handling Editor: Dr. Aristidis Tsatsakis
Contributor Information
Vishwambar Navale, Email: vd.navale@ncl.res.in.
Koteswara Rao Vamkudoth, Email: v.koteswara@ncl.res.in.
References
- 1.Koteswara Rao V., Shilpa P., Girisham S., Reddy S.M. Incidence of mycotoxigenic penicillia in feeds of Andhra Pradesh, India. Int. J. Biotechnol. Mol. Biol. Res. 2016;2:46–50. [Google Scholar]
- 2.FAO (Food and Agriculture Organization) Information Division, FAO; Rome: 2003. Worldwide Regulations for Mycotoxins in Food and Feed in 2003. [Google Scholar]
- 3.Suanthie Y., Cousin M.A., Woloshuk C.P. Multiplex real-time PCR for detection and quantification of mycotoxigenic Aspergillus, Penicillium and Fusarium. J. Stored Prod. Res. 2008;45:139–145. [Google Scholar]
- 4.Ruben R.G., Soares A.R., Corrado F., Domenico C., Giampiero C., Virginia C., Raquel Aires-Barros M. Advances, challenges and opportunities for point-of- need screening of mycotoxins in foods and feeds. Analyst. 2018;143:1015–1035. doi: 10.1039/c7an01762f. [DOI] [PubMed] [Google Scholar]
- 5.Bennett J.W., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16(3):497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker J.L., Bayman P., Mahoney N.E., Klich M.A., Palumbo J.D., Campbell B.C. Ochratoxigenic A. lanosusand A. alliaceus isolates from California tree nut orchards. Proceedings of the 3rd Fungal Genomics, 4th Fumonisin, and 16th Aflatoxin Elimination Workshops; Savannah, Georgia; 2003. [Google Scholar]
- 7.Ráduly Z., Szabó L., Madar A., Pócsi I., Csernoch L. Toxicological and medical aspects of aspergillus-derived mycotoxins entering the feed and food chain. Front. Microbiol. 2020;10:2908. doi: 10.3389/fmicb.2019.02908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RASFF (The rapid alert system for food and feed) Annual report European communities implications of mycotoxin in animal feed. Pak. J. Nature. 2008;5:398–403. [Google Scholar]
- 9.Cleveland T.E., Bhatnagar D. Molecular regulation of aflatoxin biosynthesis. In: Bray G.A., Ryan D.H., editors. Vol. 1. Pennington Center Nutrition Series, LSU Press; Baton Rouge, La: 1991. pp. 270–287. (Mycotoxins, Cancer and Health). [Google Scholar]
- 10.Bhandari P.C., Bhandari B. Aflatoxin and indian childhood cirrhosis. Indian Pediatr. 1980;17:593–596. [PubMed] [Google Scholar]
- 11.Gruber-Dorninger C., Jenkins T., Schatzmayr G. Global mycotoxin occurrence in feed: a ten-year survey. Toxins (Basel) 2019;11:375. doi: 10.3390/toxins11070375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minto R.E., Townsend C.A. Enzymology and molecular biology of aflatoxin biosynthesis. Chem. Rev. 1997;97:2537–2556. doi: 10.1021/cr960032y. [DOI] [PubMed] [Google Scholar]
- 13.Safwan A.A.M., Bacha N., Alharazi T. Detection of total aflatoxins in groundnut and soybean samples in Yemen using enzyme-linked immunosorbent assay. J. Food Qual. 2019;1:7. [Google Scholar]
- 14.Moore G.G., Mack B.M., Beltz S.B., Puel O. Genome sequence of an aflatoxigenic pathogen of Argentinian peanut, Aspergillus arachidicola. BMC Genomics. 2018;19:1. doi: 10.1186/s12864-018-4576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-zupir A.O., Alamer A.S., Dutton M.F. The occurrence of aflatoxin in rice worldwide: a review. Toxin. 2015;34:37–42. [Google Scholar]
- 16.Ozluoymak O., Guzel E. Prediction of aflatoxin contamination on dried fig Ficus Carica samples by spectral image analysis in comparison with laboratory results. Fresenius Environ. Bull. 2018;27 [Google Scholar]
- 17.Andrade P., Caldas E. Aflatoxins in cereals: worldwide occurrence and dietary risk assessment. World Mycotoxin J. 2015;8(4):415–431. [Google Scholar]
- 18.Makhlouf J., Carvajal-Campos A., Querin A., Tadrist S., Puel O., Lorber S. Morphologic, molecular and metabolic characterization ofAspergillus section flavi in spices marketed in Lebanon. Sci. Rep. 2019;9:1. doi: 10.1038/s41598-019-41704-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keliani B., Sawada M.M., Rodrigues C.E.D.C., Fonseca C.R.D., Oliveira C.A.F. Incidence of aflatoxins in oil seeds and possible transfer to oil: a review. Food Environ. Rev. 2014;6:20–28. [Google Scholar]
- 20.Pires P.N., Vargas E.A., Gomes M.B., Vieira C.B.M., Santos E.A.D., Bicalho A.A.C., Silva S.C. Aflatoxins and ochratoxin a: occurrence and contamination levels in cocoa beans from Brazil. Food Addit. Contam. 2019;36:815–824. doi: 10.1080/19440049.2019.1600749. [DOI] [PubMed] [Google Scholar]
- 21.Okoth S. CTA Working Paper; 2016. Improving the Evidence Base on Aflatoxin Contamination and Exposure in Africa. [Google Scholar]
- 22.Blount W.P. Turkey “X” disease. J. Brit. Turkey Fedr. 1961;9:55–58. [Google Scholar]
- 23.Nesbitt B.F., Okelly J., Sargeant K., Sheridan A. Aspergillus flavus and Turkey X disease: toxic metabolites of Aspergillus flavus. Nature. 1962;195:1062–1063. doi: 10.1038/1951062a0. [DOI] [PubMed] [Google Scholar]
- 24.Ngindu A., Kenya P., Ocheng D., Omondi T., Ngare W., Gatei D. An outbreak of acute hepatitis caused by aflatoxin poisoning in Kenya. Lancet. 1982;319:1346–1348. doi: 10.1016/s0140-6736(82)92411-4. [DOI] [PubMed] [Google Scholar]
- 25.Asao T., Buchi G., Abdel-Kader M.M., Chang S.B., Emily L.W., Wogan G.N. Aflatoxins B and G. J. Am. Chem. Soc. 1963;85(11):1706–1707. doi: 10.1021/ja01082a031. [DOI] [PubMed] [Google Scholar]
- 26.Cullen J.M., Ruebner B.H., Hsieh L.S., Hyde D.M., Hsieh D.P. Carcinogenicity of dietary aflatoxin M1 in male fischer rats compared to aflatoxin B1. Cancer Res. 1987;47:1913–1917. [PubMed] [Google Scholar]
- 27.Sinnhuber R.O., Lee D., Wales J.H., Landers M.K., Keyl A.C. Hepatic carcinogenesis of aflatoxin M1 in rainbow trout Salmo gairdneri and its enhancement by cyclopropene fatty acids. JNCI. 1974:1285–1288. doi: 10.1093/jnci/53.5.1285. [DOI] [PubMed] [Google Scholar]
- 28.Galvano F., Galofaro V., Galvano G. Occurrence and stability of aflatoxin M1 in milk and milk products: a worldwide review. J. Food Prot. 1996;59:1079–1090. doi: 10.4315/0362-028X-59.10.1079. [DOI] [PubMed] [Google Scholar]
- 29.Amanda K.M., Stephen R.L. Mechanisms underlying aflatoxin-associated mutagenesis–implications in carcinogenesis. DNA Repair. 2019;77:76–86. doi: 10.1016/j.dnarep.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdulrazzaq Y.M., Padmanabhan R., Bastaki S., Kochyil J., Shafiullah M. Teratogenic effects of aflatoxin B1 in mice exposed in early and late gestation. Pediatr. Res. 2011;70 405–405. [Google Scholar]
- 31.Rushing B.R., Selim M.I. Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019;124:81–100. doi: 10.1016/j.fct.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 32.Zain M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011;15:129–144. [Google Scholar]
- 33.Oluwaseun T.O., Chibundu N.E., Mari K.E., Bojan S., Babalola D., Michael S., Jana H., Christopher E., Rudolf K. Mycotoxin co-exposures in infants and young children consuming household- and industrially-processed complementary foods in Nigeria and risk management advice. Food Control. 2019;98:312–322. [Google Scholar]
- 34.Leslie L.W., Egner P.A., Belanger C.L., Wattanawaraporn R., Trudel L.J., Croy R.G., Groopman J.D. Aflatoxin B1-DNA adduct formation and mutagenicity in livers of neonatal male and female B6C3F1. Med. Toxicol. Sci. 2011;122:38–44. doi: 10.1093/toxsci/kfr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerald N.W., Kensler T.W., Groopman J.D. Present and future directions of translational research on aflatoxin and hepatocellular carcinoma. A review. Food Addtive Cont. Part A. 2012;29:249–257. doi: 10.1080/19440049.2011.563370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim E.K., Shon D.H., Yoo J.Y., Ryu D., Lee C., Kim Y.B. Natural occurrence of aflatoxins in korean meju. Food Addtive Cont. 2001;18:151–156. doi: 10.1080/02652030010006104. [DOI] [PubMed] [Google Scholar]
- 37.Maryann E.S., Currier S.S., Bailey E.A., Essigmann J.M. The chemistry and biology of aflatoxin B1: from mutational spectrometry to carcinogenesis. Carcinogenesis. 2001;22:535–545. doi: 10.1093/carcin/22.4.535. [DOI] [PubMed] [Google Scholar]
- 38.Xubo Z., Schaffner D.W., Yue T. Quantification of aflatoxin risk associated with chinese spices: point and probability risk assessments for aflatoxin B1. Food Control. 2013;33:366–377. [Google Scholar]
- 39.Yohannes B., Ayalew W., Getachew A. Analysis to ascertain the determination for aflatoxin contamination of milk and feeds from Gurage Zone, Ethiopia. Int. J. Agric. Res. 2018;13:1–11. [Google Scholar]
- 40.Kathleen G., Chinnici J.P., M.Lewellyn G.C. Effects of aflatoxin B1, aflatoxin B2, aflatoxin G1, and sterigmatocystin on viability, rates of development, and body length in two strains of Drosophila melanogaster Diptera. J. Invertebrate Pathol. 1982;39:388–394. doi: 10.1016/0022-2011(82)90064-7. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi Y., Yoko N., Shaomin Z., Yasuhito S., Haruki K., Kiyoji T., Ide Fumio. Enhanced spontaneous and aflatoxin-induced liver tumorigenesis in xeroderma pigmentosum group a gene-deficient mice. Carcinogenesis. 2002;23:627–633. doi: 10.1093/carcin/23.4.627. [DOI] [PubMed] [Google Scholar]
- 42.Van der Fels-Klerx H.J., Vermeulen L.C., Gavai A.K., Liu C. Climate change impacts on aflatoxin B1 in maize and aflatoxin M1 in milk: a case study of maize grown in Eastern Europe and imported to the Netherlands. PLoS One. 2019;14:6. doi: 10.1371/journal.pone.0218956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Nahla S., Imam H., Moussa E., Ibrahim A., Ghanam A. Teratogenic effects of aflatoxin in rabbits Oryctolagus Cuniculus. J. Vet. Anat. 2013;6(2):67–85. [Google Scholar]
- 44.Marina V.C., Iamanaka B.T., Pereira J.L., Lemes D.P., Nakano F., Taniwaki M.H. Co-occurrence of ochratoxin a and aflatoxins in chocolate marketed in Brazil. Food Control. 2012;26:36–41. [Google Scholar]
- 45.Ahmad F.A., Gibbons J.G., Lee M.K., Han K.H., Hong S.B., Yu Y.H. Controlling aflatoxin contamination and propagation of Aspergillus flavus by a soy-fermenting Aspergillus oryzae strain. Sci. Rep. 2018;8:16871. doi: 10.1038/s41598-018-35246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weidenborner M. Springer Publisher Berlin; New York, London: 2001. Encyclopedia of Food Mycotoxins. [Google Scholar]
- 47.Ahlberg S., Randolph D., Okoth S., Lindahl J. Aflatoxin binders in foods for human consumption-can this be promoted safely and ethically? Toxins. 2019;11(7):410. doi: 10.3390/toxins11070410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marina V.C., Iamanaka B.T., Pereira J.L., Lemes D.P., Nakano F., Taniwaki M.H. Co-occurrence of ochratoxin a and aflatoxins in chocolate marketed in Brazil. Food Control. 2012;26:36–41. [Google Scholar]
- 49.RASFF (The rapid alert system for food and feed) Annual report European communities implications of mycotoxin in animal feed. Pak. J. Nat. 2008;5:398–403. [Google Scholar]
- 50.Zengning L., Jinfeng C., Xianghang Z., Weijun K. Aflatoxin G1 reduces the molecular expression of HLA-I, TAP-1 and LMP-2 of adult esophageal epithelial cells in vitro. Toxicol. Lett. 2010;195:169–173. doi: 10.1016/j.toxlet.2010.03.1118. [DOI] [PubMed] [Google Scholar]
- 51.Yassein N., Zghair Z.R. Study of toxicity and pathogenicity of aflatoxin B1 and G1 in mice. Al-Anbar J. Vet. Sci. 2012;5:1999–6527. [Google Scholar]
- 52.Yassein N., Zghair Z.R. Study of toxicity and pathogenicity of aflatoxin B1 and G1 in mice. Al-Anbar J. Vet. Sci. 2012;5:1999–6527. [Google Scholar]
- 53.Peilu S., Ningfei G., Can W., Mei Z., Li Y., Chunping L., Kan L. Aflatoxin G1 induced TNF‐α‐Dependent lung inflammation to enhance DNA damage in alveolar epithelial cells. J. Cell Physiol. 2018;234:9194–9206. doi: 10.1002/jcp.27596. [DOI] [PubMed] [Google Scholar]
- 54.Asi M., Iqbal S., Ariño A., Hussain A. Effect of Seasonal variations and lactation times on aflatoxin M1 contamination in milk of different species from Punjab, Pakistan. Food Control. 2012;25:34–38. [Google Scholar]
- 55.Lien K.W., Wang X., Pan M.H., Ling M.P. Assessing aflatoxin exposure risk from peanuts and peanut products imported to Taiwan. Toxins. 2019;11:80. doi: 10.3390/toxins11020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang N.Y., Qi M., Zhao L., Zhu M.K., Guo J., Liu J., Gu C.Q., Rajput S.A., Krumm C.S., Qi D.S., Sun L.H. Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in Chick Liver. Toxins. 2016;8:327. doi: 10.3390/toxins8110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaid J., Dawra R.K., Sharma O.P., Negi S.S. Chronic aflatoxicosis in cattle. Vet. Hum. Toxicol. 1981;23:436–438. [PubMed] [Google Scholar]
- 58.Ramon, Gerardo, Aflatoxin Biochemistry and Molecular Biology Book, ISBN 978-953-307-395-398.
- 59.Afum C., Cudjoe L., Hills J., Hunt R., Padilla L., Elmore S., Afrije A. Association between aflatoxin M1 and liver disease in HBV/HCV infected persons in Ghana. Int. J. Environ. Res. Public Health. 2016;13(4):377. doi: 10.3390/ijerph13040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marchese S., Polo A.A., Ariano S., Velotto S., Costantini S., Severino L. Aflatoxin B.1 and M1: biological properties and their involvement in cancer development. Toxins. 2018;10:214. doi: 10.3390/toxins10060214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie L., Li M., Liu D., Wang X., Wang P., Dai H. Secalonic acid-F, a novel mycotoxin, represses the progression of hepatocellular carcinoma via MARCH 1 regulation of the PI3K/AKT/β-catenin signaling pathway. Molecules. 2019;24:393. doi: 10.3390/molecules24030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tozzi B., Liponi G., Meucci V., Casini L., Dall’Asta C., Intorre L., Gatta D. Aflatoxins M1 and M2 in the milk of donkeys fed with naturally contaminated diet. Dairy Sci. Technol. 2016;96:513–523. [Google Scholar]
- 63.Lee D., Lee L.G. Analysis of aflatoxin M1 and M2 in commercial dairy products using high-performance liquid chromatography with a fluorescence detector. Food Control. 2015;50:467–471. [Google Scholar]
- 64.Barikbin B., Allahresani A., Khosravi R., Khodadadi M. Detection of aflatoxin M1 in dairy products marketed in Iran. J. Health Scope. 2015;4:1. [Google Scholar]
- 65.Saeger S.D., Logrieco A. Belgium, Toxins; 2017. p. 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calvo, Cary J. Association of fungal secondary metabolism and sclerotial biology. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reddy P., Guthridge K., Vassiliadis S., Hemsworth J., Hettiarachchige I., Spangenberg G., Rochfort S. Tremorgenic mycotoxins: structure diversity and biological activity. Toxins. 2019;11:302. doi: 10.3390/toxins11050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kozák L., Szilágyi Z., Tóth L., Pócsi I., Molnár I. Tremorgenic and neurotoxic paspaline-derived indole-diterpenes: biosynthetic diversity, threats and applications. Appl. Microbiol. Biotechnol. 2019;103:1599–1616. doi: 10.1007/s00253-018-09594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang S., Monahan B.J., Tkacz J.S., Scott B. An indolediterpene gene cluster from Aspergillus flavus. Appl. Environ. Microbiol. 2004;70:6875–6883. doi: 10.1128/AEM.70.11.6875-6883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matthew J.N., Albert B.K., Monahan J., Beth L.P., Gary A., Scott B. Identification of two aflatrem biosynthesis gene loci in Aspergillus flavus and metabolic engineering of Penicillium paxilli to elucidate their function. Appl. Environ. Microbiol. 2009;75:7469–7481. doi: 10.1128/AEM.02146-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van der Merwe K.J., Steyn P.S., Fourie L. Ochratoxin A., a toxic metabolite produced by Aspergillus ochraceusWilh. Nature. 1965;205:1112–1113. doi: 10.1038/2051112a0. [DOI] [PubMed] [Google Scholar]
- 72.Huff W.E., Hamilton P.B. Mycotoxins – their biosynthesis in fungi: ochratoxins – metabolites of combined pathways. J. Food Prot. 1979;42:815–820. doi: 10.4315/0362-028X-42.10.815. [DOI] [PubMed] [Google Scholar]
- 73.Ana L.L. Occurrence of ochratoxin a in coffee: threads and solutions-a mini-review. Beverages. 2019;5:36. [Google Scholar]
- 74.Van Walbeek W., Scott P.M., Harwig J., Lawrence J.W. Penicillium viridicatum westling: a new source of Ochratoxin A. Can. J. Microbiol. 1969;15:1281–1285. doi: 10.1139/m69-232. [DOI] [PubMed] [Google Scholar]
- 75.Liye Z., Boyang Z., Yaqi D., Hongyu L., Wentao X. A review: epigenetic mechanism in Ochratoxin A toxicity studies. Toxins. 2017;9:113. doi: 10.3390/toxins9040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jørgensen K. Survey of pork, poultry, coffee, beer and pulses for Ochratoxin A. Food Addit. Contam. 1998;15:550–554. doi: 10.1080/02652039809374680. [DOI] [PubMed] [Google Scholar]
- 77.Dongmei W., Xiaohu W., Jun X., Fenshou D., Yongquan Z., Ji M. Determination of ochratoxin a contamination in grapes, processed grape products and animal-derived products using ultra-performance liquid chromatography-tandem mass spectroscopy system. Sci. Rep. 2018;8:20–51. doi: 10.1038/s41598-018-20534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ülger T.G., Uçar A., Çakıroğlu F.P., Yilmaz S. Genotoxic effects of mycotoxins. Toxicon. 2020;185:104–113. doi: 10.1016/j.toxicon.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 79.Domijan A.D., Gajski G., Jovanovi I.N., Geri M., Garaj-Vrhovac V. In vitro genotoxicity of mycotoxins ochratoxin A and fumonisin B1 could be prevented by sodium copper chlorophyllin–implication to their genotoxic mechanism. Food Chem. 2015;170:455–462. doi: 10.1016/j.foodchem.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 80.Luhe A., Hildebrand H., Bach U., Dingermann T., Ahr H.J. A new approach to studying ochratoxin A (OTA)-induced nephrotoxicity: expression profiling in vivo and in vitro employing cDNA microarrays. Toxicol. Sci. 2003;73(2):315–328. doi: 10.1093/toxsci/kfg073. [DOI] [PubMed] [Google Scholar]
- 81.Costa J.G., Saraiva N., Guerreiro P.S., Louro H., Silva M.J., Miranda J.P. Ochratoxin A-induced cytotoxicity, genotoxicity and reactive oxygen species in kidney cells: an integrative approach of complementary endpoints. Food Chem. Toxicol. 2016;87:65–76. doi: 10.1016/j.fct.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 82.Assunçao R., Pinhao M., Loureiro S., Alvito P., Silva M.J. A multi-endpoint approach to the combined toxic effects of patulin and ochratoxin a in human intestinal cells. Toxicol. Lett. 2019;313:120–129. doi: 10.1016/j.toxlet.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Marta H.T., John I.P., Marina C.C., Aldir A.T., Beatriz T.I. Understanding mycotoxin contamination across the food chain in Brazil: challenges and opportunities. Toxins. 2019;11:411. doi: 10.3390/toxins11070411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jack C., Lauren J., Hyun J.L., Wei Z., Fadwa A.T., Jerry Z., Ryu D. Occurrence of ochratoxin a in infant foods in the United States. J. Food Prot. 2017;80:251–256. doi: 10.4315/0362-028X.JFP-16-339. [DOI] [PubMed] [Google Scholar]
- 85.Andrea A.A., Pablo M.M.A., Francisco P.F., Gabriela C., Cinthia C., Kohil M.M., Peralta I. Occurrence of deoxynivalenol and Ochratoxin A in beers and wines commercialized in Paraguay. Toxins. 2019;11(6):308. doi: 10.3390/toxins11060308. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Catootjie L.N., Angi A.H., Supit M.A.J., Ambarwati S. Aflatoxin and Ochratoxin A contamination in corn grains and sago putak meal from different sources for poultry in West Timor, Indonesia. Int. J. Poult. Sci. 2019;18:353–360. [Google Scholar]
- 87.Terenzio B., Marco R., Silvia R., Paola G. Mycotoxins and related Fungi in Italian paddy rice during the growing season and storage. Toxins. 2019;11:151. doi: 10.3390/toxins11030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi H., Schwab W., Yu P. Natural occurrence and co-contamination of twelve mycotoxins in industry-submitted cool-season cereal grains grown under a low heat unit climate condition. Toxins. 2019;11:160. doi: 10.3390/toxins11030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Halil D.K., Canan H., Beyza U. Mycotoxin hazard in meat and meat products. Atatürk Univ. J. Vet. Sci. 2019;14(1):90–97. [Google Scholar]
- 90.Cagla T., Erhan K. Determination of aflatoxin M1 and ochratoxin a in raw, pasteurized and UHT milk in Turkey. Acta Sci. Veterin. 2019;47:1626. [Google Scholar]
- 91.Jan G., Robert K., Magdalena T., Anna B.K. Occurrence and risk assessment of mycotoxins through polish beer consumption. Toxins. 2019;11:254. doi: 10.3390/toxins11050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mohamed F.A., Gözde G., Terken B. Mycotoxin detection in maize, commercial feed and raw dairy milk samples from Assiut City. Egypt. J. Vet. Sci. 2019;6:57. doi: 10.3390/vetsci6020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Juan-García A., Tolosa J., Juan C., Ruiz M.J. Cytotoxicity, genotoxicity,y and disturbance of cell cycle in HepG2 cells exposed to OTA and BEA: single and combined actions. Toxins. 2019;11:341. doi: 10.3390/toxins11060341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Samson R., Peterson S., Frisvad J., Varga J. New species in Aspergillus section terrei. Stud. Mycol. 2011;69:39–55. doi: 10.3114/sim.2011.69.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pildain M.B., Frisvad J.C., Vaamonde G., Cabral D., Varga J., Samson R.A. Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts. Int. J. Syst. Evol. Microbiol. 2008;58:725–735. doi: 10.1099/ijs.0.65123-0. [DOI] [PubMed] [Google Scholar]
- 96.Kurtzman C.P., Horn B.W., Hesseltine C.W. Aspergillus nomius, a new aflatoxin-producing species related to Aspergillus flavus and Aspergillus tamarii. Antonie Van Leeuwenhoek. 1987;53:147–158. doi: 10.1007/BF00393843. [DOI] [PubMed] [Google Scholar]
- 97.Madhyastha M.S., Bhat R.V. Aspergillus parasiticus growth and aflatoxin production on black and white pepper and the inhibitory action of their chemical constituents. Appl. Environ. Microbiol. 1984;48:376–379. doi: 10.1128/aem.48.2.376-379.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carvajal-Campos A., Manizan A., Tadrist S., Akaki D., Koffi-Nevry R., Moore G., Stephen O.F. Aspergillus korhogoensis, a novel aflatoxin producing species from the Côte d’Ivoire. Toxins. 2017;9(11):353. doi: 10.3390/toxins9110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frisvad J.C., Skouboe P., Samson R.A. Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-O-methylsterigmatocystin, Aspergillus rambellii sp. nov. Syst. Appl. Microbiol. 2005;28(5):442–453. doi: 10.1016/j.syapm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 100.Varga J., Frisvad J., Samson R. A reappraisal of fungi producing aflatoxins. World Mycotoxin J. 2009;2:263–277. [Google Scholar]
- 101.Moore G.G., Olarte R.A., Horn B.W., Elliott J.L., Singh R., Oneal C.J. Global population structure and adaptive evolution of aflatoxin-producing Fungi. Ecol. Evol. 2017;7:9179–9191. doi: 10.1002/ece3.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Varga J., Frisvad J., Samson R. A reappraisal of fungi producing aflatoxins. World Mycotoxin J. 2009;2:263–277. [Google Scholar]
- 103.Frisvad J.C., Samson R.A. b. Emericella venezuelensis, a new species with stellate ascospores producing sterigmatocystin and Aflatoxin B1. Syst. Appl. Microbiol. 2004;27:672–680. doi: 10.1078/0723202042369910. [DOI] [PubMed] [Google Scholar]
- 104.Goto T., Wicklow D.T., Ito Y. Aflatoxin and cyclopiazonic acid production by a sclerotium-producing Aspergillus tamarii strain. Appl. Environ. Microbiol. 1996;62(11):4036–4038. doi: 10.1128/aem.62.11.4036-4038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Viaro H.P., Silva J.J.D., Ferranti L.D.S., Bordini J.G., Massi F.P., Fungaro M.H.P. The first report of A. novoparasiticus, A. arachidicola and A. pseudocaelatus in Brazilian Corn Kernels. Int. J. Food Microbiol. 2017;243:46–51. doi: 10.1016/j.ijfoodmicro.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 106.Domenico D., Anna M.P., Biancamaria P., Alessandra R., Oriana M. Aspergillus affinis sp. nov., a novel ochratoxin A-producing Aspergillus species section circumdati isolated from decomposing leaves. Int. J. Syst. Evol. Microbiol. 2012;62:1007–1015. doi: 10.1099/ijs.0.034785-0. [DOI] [PubMed] [Google Scholar]
- 107.Visagie C.M., Varga J., Houbraken J., Meijer M., Kocsube S., Yilmaz N., Fotedar R. Ochratoxin production and taxonomy of the yellow aspergilli Aspergillus section circumdati. Stud. Mycol. 2014;78:1–61. doi: 10.1016/j.simyco.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paul B., James L.B., Mark A.D., Themis J.M., Noreen E.M. Ochratoxin production by the Aspergillus ochraceus group and Aspergillus alliaceus. Appl. Environ. Microbiol. 2002;68:2326–2329. doi: 10.1128/AEM.68.5.2326-2329.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hesseltine C.W., Vandegraft E.E., Fennell D.I., Smith M.L., Shotwell O.L. Aspergilli as ochratoxin producers. Mycologia. 1972;64:539–550. [PubMed] [Google Scholar]
- 110.Ciegler A. Bioproduction of ochratoxin a and penicillc acid by members of the Aspergillus ochraceus group. Can. J. Infect. Dis. Med. Microbiol. 1972;18:631–636. doi: 10.1139/m72-100. [DOI] [PubMed] [Google Scholar]
- 111.Perrone G., Susca A., Cozzi G., Ehrlich K., Varga J., Frisvad J.C. Biodiversity of Aspergillus species in some important agricultural products. Stud. Mycol. 2007;59:53–66. doi: 10.3114/sim.2007.59.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Samson R., Houbraken J., Kuijpers A., Frank J., Frisvad J. New ochratoxin a or sclerotium producing species in Aspergillus section nigri. Stud. Mycol. 2004;50:45–61. [Google Scholar]
- 113.Ángel M., Mateo R., Valle-Algarra F.M., Mateo E.M., Jiménez M. Effect of Carbendazim and physicochemical factors on the growth and ochratoxin a production of aspergilluscarbonarius isolated from grapes. Int. J. Food Microbiol. 2007;119(3):230–235. doi: 10.1016/j.ijfoodmicro.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 114.Palumbo J.D., O’Keeffe a T.L., Mahoney N.E. Inhibition of ochratoxin a production and growth of Aspergillus species by phenolic antioxidant compounds. Mycopathologia. 2007;164:241–248. doi: 10.1007/s11046-007-9057-0. [DOI] [PubMed] [Google Scholar]
- 115.Frisvad J.C., Frank M., Houbraken J.A.M.P., Kuijpers A.F.A., Samson R.A. a. New ochratoxin A producing species of Aspergillus section circumdati. Stud. Mycol. 2004;50:23–43. [Google Scholar]
- 116.Frisvad J., Hubka V., Ezekiel C., Hong S.B., Nováková A., Chen A., Arzanlou M. Taxonomy of Aspergillus section flavi and their production of aflatoxins, ochratoxins, and other mycotoxins. Stud. Mycol. 2019;93:1–63. doi: 10.1016/j.simyco.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arzanlou M., Samadi R., Frisvad J.C., Houbraken J., Ghosta Y. Two novel Aspergillus Species from hypersaline soils of the national park of Lake Urmia, Iran. Mycol. Prog. 2016;15:1081–1092. [Google Scholar]
- 118.Li Y.N., Wang Y.Y., Zheng Y.Q., Guo Y.H. Preparation and characterization of the high specificity monoclonal antibodies against citrinin. Prog. Biochem. Biophys. 2011;37:1248–1253. [Google Scholar]
- 119.Artigot M.P., Loiseau N., Laffitte J., Mas-Reguieg L., Tadrist S., Oswald I.P., Puel O. Molecular cloning and functional characterization of two CYP619 cytochrome P450s involved in biosynthesis of patulin in Aspergillus clavatus. Microbiology. 2009;155(5):1738–1747. doi: 10.1099/mic.0.024836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Varga J., Due M., Frisvad J., Samson R. Taxonomic revision of Aspergillus section clavati based on molecular, morphological and physiological data. Stud. Mycol. 2007;59:89–106. doi: 10.3114/sim.2007.59.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steiman R., Seigle-Murandi F., Sage L., Krivobok S. Production of patulin by micromycetes. Mycopathology. 1989;105:129–133. doi: 10.1007/BF00437244. [DOI] [PubMed] [Google Scholar]
- 122.Frank H.K. Occurrence of Patulin in fruit and vegetables. Annales de la Nutrition et de L’Alimentation. 1977;31:459–465. [PubMed] [Google Scholar]
- 123.Luque M.I., Rodríguez A., Andrade M.J., Gordillo R., Rodríguez M., Córdoba J.J. Development of a PCR protocol to detect patulin producing moulds in food products. Food Control. 2011;22:1831–1838. [Google Scholar]
- 124.Frisvad J. A critical review of producers of small lactone mycotoxins: patulin, penicillic acid, and moniliformin. World Mycotoxin J. 2018;11:73–100. [Google Scholar]
- 125.Chang P.K., Ehrlich K., Fujii I. Cyclopiazonic acid biosynthesis of Aspergillus flavus and Aspergillus oryzae. Toxins. 2009;1(2):74–99. doi: 10.3390/toxins1020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rank C., Klejnstrup M.L., Petersen L.M., Kildgaard S., Frisvad J.C., Gotfredsen C.H., Thomas O.L. Comparative chemistry of Aspergillus oryzae RIB40 and A. flavus NRRL 3357. Metabolites. 2012;2:39–56. doi: 10.3390/metabo2010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun K., Li Y., Guo L., Wang Y., Liu P., Zhu W. Indole diterpenoids and Isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei. Mar. Drugs. 2014;12:3970–3981. doi: 10.3390/md12073970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Uka V., Moore G., Arroyo-Manzanares N., Nebija D., Saeger S.D., Mavungu J.D.D. Unravelling the diversity of the cyclopiazonic acid family of mycotoxins in Aspergillus flavus by UHPLC Triple-TOF HRMS. Toxins. 2017;9:35. doi: 10.3390/toxins9010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ito Y., Peterson S.W., Wicklow D.T., Goto T. Aspergillus pseudotamarii, a new aflatoxin producing species in Aspergillus section flavi. Mycol. Res. 2001;105:233–239. [Google Scholar]
- 130.Krska R., ährer K., Richard L., Rodrigues I., Schuhmacher R., Slate B., Whitaker W.B. In: Guide to Mycotoxins Featuring Mycotoxin Risk Management in Animal Production. biomin edition. Binder E.M., Krasha R., editors. Romar labs Division holding GmbH; Australia: 2012. ISBN 9780-9573721-1-5. [Google Scholar]
- 131.Larsen T.O., Smedsgaard J., Nielsen K.F., Hansen M.A.E., Samson R.A., Frisvad J.C. Production of mycotoxins by Aspergillus lentulus and other medically important and closely related species in section fumigati. Med. Mycol. 2007;45:225–232. doi: 10.1080/13693780601185939. [DOI] [PubMed] [Google Scholar]
- 132.Orth R. Mycotoxins of Aspergillus oryzae strains for use in the food industry as starters and enzyme producing molds. Annales de la Nutrition et de L’alimentation. 1977;31:617–624. [PubMed] [Google Scholar]
- 133.Cole R.J. Cyclopiazonic acid and related toxins. In: Betina V., editor. Mycotoxins- Production, Isolation, Separation, and Purification. Elsevier Science Publishers; Amsterdam, the Netherlands: 1984. pp. 405–414. [Google Scholar]
- 134.Vinokurova N.G., Ivanushkina N.E., Khmel’Nitskaya I.I., Arinbasarov M.U. Synthesis of α-cyclopiazonic acid by fungi of the genus Aspergillus. Appl. Biochem. Microbiol. 2007;43:435–438. [PubMed] [Google Scholar]
- 135.Yamazaki M., Maebayashi Y., Miyaki K. The isolation of secalonic acid a from Aspergillus ochraceus cultured on rice. Chem. Pharm. Bull. 1971;19:199–201. doi: 10.1248/cpb.19.199. [DOI] [PubMed] [Google Scholar]
- 136.Kurobane I., Vining L.C., McInnes A.G. A new secalonic acid linkage between tetrahydroxan- thone units determined from deuterium isotope 13C chemical shifts. Tetrahedron Lett. 1978:4633–4636. [Google Scholar]
- 137.Yosioka I., Yamauchi H., Murata K., Kitagawa I. Coloring substances of a lichen cetrariaornata. Chem. Pharm. Bull. 1972;20:1082–1084. [Google Scholar]
- 138.Yosioka I., Nakanishi T., Izumi S., Kitagawa I. Structure of a lichen pigment entothein and its identity with secalonic acid a, a major ergot pigment. Chem. Pharm. Bull. 1968;16:2090–2091. doi: 10.1248/cpb.16.2090. [DOI] [PubMed] [Google Scholar]
- 139.Ren H., Tian L., Gu Q., Zhu W. Secalonic acid d; a cytotoxic constituent from marine lichen-derived fungus gliocladium sp. T31. Arch. Pharm. Res. 2006;29:59–63. doi: 10.1007/BF02977469. [DOI] [PubMed] [Google Scholar]
- 140.Samson R., Noonim P., Meijer M., Houbraken J., Frisvad J., Varga J. Diagnostic tools to identify black aspergilli. Stud. Mycol. 2007;59:129–145. doi: 10.3114/sim.2007.59.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Andersen R., Buechi G., Kobbe B., Demain A.L. Secalonic acids d and F are toxic metabolites of Aspergillus aculeatus. J. Org. Chem. 1977;42(2):352–353. doi: 10.1021/jo00422a042. [DOI] [PubMed] [Google Scholar]
- 142.Steyn P., Isolation The. Structure and absolute configuration of secalonic acid d, the toxic metabolite of Penicillium oxalicum. Tetrahedron. 1970;26:51–57. doi: 10.1016/0040-4020(70)85006-2. [DOI] [PubMed] [Google Scholar]
- 143.Du L., Zhang Q., Chen L., Bi Y., Li Y.P., Li X.X., Qin-Yan L. Secalonic acids H and I, two new secondary metabolites from the marine-derived fungus Penicillium oxalicum. Heterocycles. 2017;94(9):17–66. [Google Scholar]
- 144.Tepaske M.R., Gloer J.B., Wicklow D.T., Dowd P.F. Aflavarin and β-Aflatrem: new anti-insectan metabolites from the sclerotia of Aspergillus flavus. J. Nat. Prod. 1992;55:1080–1086. [Google Scholar]
- 145.Lan W.J., Wang K.T., Xu M.Y., Zhang J.J., Lam C.K., Zhong G.H., Yang De-Po. Secondary metabolites with chemical diversity from the marine-derived fungus pseudallescheria boydii F19-1 and their cytotoxic activity. R. Soc. Chem. Adv. 2016;6:76206–76213. [Google Scholar]
- 146.Yin W.B., Grundmann A., Cheng J., Li S.M. Acetylaszonalenin biosynthesis in Neosartorya fischeri. Identification of the biosynthetic gene cluster by genomic mining and functional proof of the genes by biochemical investigation. J. Biol. Chem. 2009;284:100–109. doi: 10.1074/jbc.M807606200. [DOI] [PubMed] [Google Scholar]
- 147.Boruta T., Bizukojc M. Culture-based and sequence-based insights into biosynthesis of secondary metabolites by Aspergillus terreus ATCC 20542. J. Biotechnol. 2014;175:53–62. doi: 10.1016/j.jbiotec.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 148.Qureshi I.H., Kamal A., Noorani R., Husain S.A. Studies in the biochemistry of microorganisms, Part vii. Terrein and kojic acid, metabolic products of Aspergillus stellatusCurzi. Pak. J. Biol. Sci. 1968;4:367. [Google Scholar]
- 149.Rabie C.J., Steyn M., van Schalkwyk C.G. New species of Aspergillus producing sterigmatocystin. Appl. Environ. Microbiol. 1997;33:1023–1025. doi: 10.1128/aem.33.5.1023-1025.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jurjević Ž., Peterson S.W., Solfrizzo M., Peraica M. Sterigmatocystin production by nine newly described Aspergillus species in section versicolores grown on two different media. Mycotoxicol. Res. 2013;29 doi: 10.1007/s12550-013-0160-4. [DOI] [PubMed] [Google Scholar]
- 151.Tsang C.C., Hui T.W.S., Lee K.C., Chen J.H.K., Ngan A.H.Y., Tam E.W.T., Chan J.F.W. Genetic diversity of Aspergillus species isolated from Onychomycosis and aspergillushongkongensis sp. Nov. with implications to antifungal susceptibility testing. Diagn. Microbiol. Infect. Dis. 2016;84:125–134. doi: 10.1016/j.diagmicrobio.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 152.Jakšić D.D., Kocsubé S., Bencsik O., Kecskeméti A., Szekeres A.A., Vágvölgyi C., Varga J. Species diversity and cytotoxic potency of airborne sterigmatocystin-producing Aspergilli from the section versicolores. Sci. Total Environ. 2016;562:296–304. doi: 10.1016/j.scitotenv.2016.03.183. [DOI] [PubMed] [Google Scholar]
- 153.Schroeder H.W., Kelton W.H. Production of Sterigmatocystin by some species of the genus Aspergillus and its toxicity to chicken embryos. Appl. Environ. Microbiol. 1975;30:589–591. doi: 10.1128/am.30.4.589-591.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hubka V., Nováková A., Peterson A.S.W. A reappraisal of Aspergillus section nidulantes with descriptions of two new sterigmatocystin-producing species. Plant Syst. Evol. 2016;302:1267–1299. [Google Scholar]
- 155.Frisvad J.C. Vol. 102. Plenum Press; New York: 1985. pp. 437–443. (Secondary Metabolites as an Aid to Emericella Classification. Advances in Penicillium and Aspergillus Systematics). [Google Scholar]
- 156.Glister G.A., Williams T.I. Production of Gliotoxin by Aspergillus fumigatus mut. Helvola Yuill. Nature. 1944;153 651–651. [Google Scholar]
- 157.Reeves E.P., Messina C., Doyle S., Kavanagh K. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathology. 2004;158:73–79. doi: 10.1023/b:myco.0000038434.55764.16. [DOI] [PubMed] [Google Scholar]
- 158.Wilkinson S., Spilsbury J.F. Gliotoxin from Aspergillus chevalierimangin thom et church. Nature. 1965;206 doi: 10.1038/206619a0. 619–619. [DOI] [PubMed] [Google Scholar]
- 159.Bui-Klimke T.R., Wu F. Ochratoxin a and human health risk: a review of the evidence. Crit. Rev. Food Sci. Nutr. 2015;55:1860–1869. doi: 10.1080/10408398.2012.724480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gautier J.C., Richoz J., Welti D.H. Metabolism of ochratoxin a: absence of formation of genotoxic derivatives by human and rat enzymes. Chem. Res. Toxicol. 2001;14(1):34–45. doi: 10.1021/tx000070j. [DOI] [PubMed] [Google Scholar]
- 161.Jennings-Gee J.E., Tozlovanu M., Manderville R., Miller M.S., Pfohl-Leszkowicz A., Schwartz G.G. Ochratoxin a: in utero exposure in mice induces adducts in testicular DNA. Toxins Basel. 2010;2:1428–1444. doi: 10.3390/toxins2061428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stemmer K., Ellinger-Ziegelbauer H., Ahr H.J., Dietrich D.R. Molecular characterization of preneoplastic lesions provides insight on the development of renal tumors. Am. J. Pathol. 2009;175:1686–1698. doi: 10.2353/ajpath.2009.081071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hetherington A.C., Raistrick H. On the Production and Chemical Constitution of a New Yellow Colouring Matter, Citrinin, Produced from Glucose by Penicillium citrinum Thom. Philosop. Royal Society B: Biological Sciences. 1931;220:269–295. [Google Scholar]
- 164.Barnett L.M.A., Cummings B.S. Nephrotoxicity and renal pathophysiology: a contemporary perspective. Toxicology Science. 2018;164:379–390. doi: 10.1093/toxsci/kfy159. [DOI] [PubMed] [Google Scholar]
- 165.Doughari J.H. The occurrence, properties and significance of citrinin mycotoxin. J. Plant Pathol. Microbiol. 2015;6:1–6. [Google Scholar]
- 166.Gayathri L., Dhivya R., Dhanasekaran D., Periasamy V.S., Alshatw A.A., Akbarsha M.A. Hepatotoxic effect of ochratoxin a and Citrinin, alone and in combination, and protective effect of vitamin e: in vitro study in HepG2 cell. Food Chem. Toxicol. 2015;83:151–163. doi: 10.1016/j.fct.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 167.Barnett L.M.A., Cummings B.S. Nephrotoxicity and renal pathophysiology: a contemporary perspective. Toxicology Science. 2018;164:379–390. doi: 10.1093/toxsci/kfy159. [DOI] [PubMed] [Google Scholar]
- 168.Ostry V., Malir F., Ruprich J. Producers and important dietary sources of ochratoxin a and citrinin. Toxins. 2013;5:1574–1586. doi: 10.3390/toxins5091574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Culig B., Berardi M., Bosnir J., Serdar S., Lasic D., Racs A., Galic A. Presence of Citrinin in grains and its possible health effects. Afr. J. Tradit. Complement. Altern. Med. 2017;14:22–30. doi: 10.21010/ajtcam.v14i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Blanc P.J., Laussac J.P., Le Bars J., Le Bars P., Loret M.O., Pareilleux A., Prome D. Characterization of monascidin a from Monascus as citrinin. Int. J. Food Microbiol. 1995;27:201–213. doi: 10.1016/0168-1605(94)00167-5. [DOI] [PubMed] [Google Scholar]
- 171.Hill R.A., Carter R.H., Staunton J. Biosynthesis of Fungal Metabolites. Terrein, A Metabolite ofAspergillus terreus Thom. J. Chem. Soc. Perkin Trans. I. 1981;1:2570–2576. [Google Scholar]
- 172.Erdoğan A., Ghimire D., Gürses M., Çetin B., Baran A. Patulin contamination in fruit juices and its control measures. European Journal of Science and Technology. 2018;14:39–48. [Google Scholar]
- 173.Chalmers I. Commentary: the 1944 patulin trial: the first properly controlled multicentre trial conducted under the aegis of the british medical research council. Int. J. Epidemiol. 2004;33:253–260. doi: 10.1093/ije/dyh162. [DOI] [PubMed] [Google Scholar]
- 174.Kim M., Shukla S., Oh Y., Chung S.H., Kim M. Comparative diminution of patulin content in apple juice with food-grade additives sodium bicarbonate, vinegar, mixture of sodium bicarbonate and vinegar, citric acid, baking powder, and ultraviolet irradiation. Front. Pharmacol. 2018;9:822. doi: 10.3389/fphar.2018.00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guerra-Moreno A., Hanna J. Induction of proteotoxic stress by the mycotoxin patulin. Toxicol. Lett. 2017;276:85–91. doi: 10.1016/j.toxlet.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Speijers G., Franken M., Leeuwen F.V. Sub-acute toxicity study of Patulin in the rat: effects on the kidney and the gastro-intestinal tract. Food Chem. Toxicol. 1988;26:23–30. doi: 10.1016/0278-6915(88)90037-3. [DOI] [PubMed] [Google Scholar]
- 177.Zhang B., Peng X., Li G., Xu Y., Xia X., Wang Q. Oxidative stress is involved in patulin induced apoptosis in HEK293 cells. Toxicon. 2015;94:1–7. doi: 10.1016/j.toxicon.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 178.Pal S., Singh N., Ansari K.M. Toxicological effects of patulin mycotoxin on the mammalian system: an overview. Toxicol. Res. 2017;6:764–771. doi: 10.1039/c7tx00138j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Song E., Xia X., Su C., Dong W., Xian Y., Wang W., Yong S. Hepatotoxicity and genotoxicity of Patulin in mice, and its modulation by green tea polyphenols administration. Food Chem. Toxicol. 2014;71:122–127. doi: 10.1016/j.fct.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 180.Malekinejad H., Aghazadeh-Attari J., Rezabakhsh A., Sattari M., Ghasemsoltani-Momtaz B. Neurotoxicity of mycotoxins produced in vitro by Penicillium roqueforti isolated from maize and grass silage. Hum. Exp. Toxicol. 2015;34:997–1005. doi: 10.1177/0960327114565493. [DOI] [PubMed] [Google Scholar]
- 181.Boussabbeh M., Prola A., Salem I.B., Guilbert A., Bacha H., Lemaire C., Abis-Essefi S. Crocin and quercetin prevent PAT-Induced apoptosis in mammalian cells: involvement of ROS-Mediated ER stress pathway. Environ. Toxicol. 2015;31:1851–1858. doi: 10.1002/tox.22185. [DOI] [PubMed] [Google Scholar]
- 182.Ayed-Boussema I., Pascussi J.M., Maurel P., Bacha H., Hassen W. Effect of aflatoxin B1 on nuclear receptors PXR, CAR, and AhR and their target cytochromes P450 mRNA expression in primary cultures of human hepatocytes. Int. J. Toxicol. 2012;31:86–93. doi: 10.1177/1091581811422453. [DOI] [PubMed] [Google Scholar]
- 183.Lynen F., Tada M. Die BiochemischenGrundlagen der polyacetat-regel. Angew. Chem. 1961;73:513–519. [Google Scholar]
- 184.Zaehle C., Gressler M., Shelest E., Geib E., Hertweck C., Brock M. Terrein biosynthesis in Aspergillus terreus and its impact on phytotoxicity. Chem. Biol. 2014;21:719–731. doi: 10.1016/j.chembiol.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 185.Arakawa M., Someno T., Kawada M., Lkeda D. A new terrein glucoside, a novel inhibitor of angiogenin secretion in tumor angiogenesis. J. Antibiot. 2008;61(2007):442–448. doi: 10.1038/ja.2008.60. [DOI] [PubMed] [Google Scholar]
- 186.Demasi M., Felicio A.L., Pacheco A.O., Leite H., Lima C., Andrade L.H. Studies on terrein as a new class of proteasome inhibitors. J. Braz. Chem. Soc. 2010;21:299–305. [Google Scholar]
- 187.Liao W.Y., Shen C.N., Lin L.H., Yang Y.L., Hsin-Ying H., Jing-Wei C., Kuo Sheng-Chu. Asperjinone, a nor-neolignan, and terrein, a suppressor of ABCG2- expressing breast cancer cells, from thermophilic Aspergillus terreus. J. Nat. Prod. 2012;75:630–635. doi: 10.1021/np200866z. [DOI] [PubMed] [Google Scholar]
- 188.Hiroki M., Kazuhiro O., Daisuke Y., Toki T., Kyouta M., Satoshi Y., Koichi M. Synthetic + - terrein suppresses Interleukin-6/Soluble Interleukin-6 receptor induced-secretion of vascular endothelial growth factor in human gingival fibroblasts. Bioorg. Med. Chem. 2014;22:5338–5344. doi: 10.1016/j.bmc.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 189.Weindling R., Emerson O.H. The isolation of a toxic substance from the culture filtrate of Trichoderma. Phytopathology. 1936;26:1068. [Google Scholar]
- 190.Weindling R. Experimental consideration of the mold toxins of Gliocladium and Trichoderma. Phytopathology. 1941;31:991. [Google Scholar]
- 191.Müllbacher A., Waring P., Tiwari-Palni U., Eichner R.D. Structural relationship of Epipolythiodioxopiperazines and their immunomodulating activity. Mol. Immunol. 1986;23:231–235. doi: 10.1016/0161-5890(86)90047-7. [DOI] [PubMed] [Google Scholar]
- 192.Gardiner D.M., Cozijnsen A.J., Wilson L.M., Pedras M.S.C., Howlett B.J. The sirodesmin biosynthetic gene cluster of the plant pathogenic fungus leptosphaeriamaculans. Mol. Microbiol. 2004;53:1307–1318. doi: 10.1111/j.1365-2958.2004.04215.x. [DOI] [PubMed] [Google Scholar]
- 193.Gardiner D.M., Howlett B.J. Bioinformatics and expression analysis of the putative gliotoxin biosynthetic gene cluster of Aspergillus fumigatus. FEMS Microbiol. Lett. 2005;248:241–248. doi: 10.1016/j.femsle.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 194.Nouri M.A., Al-Halbosiy M.M., Dheeb B.I., Hashim A.J. Cytotoxicity, and genotoxicity of Gliotoxin on human lymphocytes in vitro. J. King Saud Univ. Sci. 2015;27:193–197. [Google Scholar]
- 195.VersiIlovskis A., Saeger S.D. Sterigmatocystin occurrence in foodstuffs and analytical methods an overview. Mol. Nutr. Food Res. 2010;54:136–147. doi: 10.1002/mnfr.200900345. [DOI] [PubMed] [Google Scholar]
- 196.Rofiat A.S., Fanelli F., Atanda O., Sulyok M., Cozzi G., Bavaro S., Krska R., Logrieco A., Ezekiel C.N. Fungal and bacterial metabolites associated with natural contamination of locally processed rice in Nigeria. Food Addit. and Contam. 2015;32:950–959. doi: 10.1080/19440049.2015.1027880. [DOI] [PubMed] [Google Scholar]
- 197.Kobayashi N., Kubosaki A., Takahashi Y., Yanai M., Konuma R., Uehara S. Distribution of Sterigmatocystin-Producing Aspergilli in Japan. Food Saf. 2018;6:67–73. doi: 10.14252/foodsafetyfscj.2018001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yu J., Chang P.K., Ehrlich K.C., Cary J.W., Bhatnagar D., Cleveland T.E., Payne G.A. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004;703:1253–1262. doi: 10.1128/AEM.70.3.1253-1262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Noda K., Umeda M., Ueno Y. Cytotoxic and mutagenic effects of Sterigmatocystin on cultured chinese Hamster cells. Carcinogenesis. 1981;2:945–949. doi: 10.1093/carcin/2.10.945. [DOI] [PubMed] [Google Scholar]
- 201.Curry P.T., Reed R.N., Martino R.M., Kitchin R.M. Induction of sister-chromatid exchanges in vivo in mice by the mycotoxins Sterigmatocystin and griseofulvin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1984;137:111–115. doi: 10.1016/0165-1218(84)90099-5. [DOI] [PubMed] [Google Scholar]
- 202.Ueda N., Fujie K., Gotoh-Mimura K., Chattopadhyay S., Sugiyama T. Acute cytogenetic effect of Sterigmatocystin on rat bone-marrow cells in vivo. Mutat. Res. Lett. 1984;139:203–206. doi: 10.1016/0165-7992(84)90129-5. [DOI] [PubMed] [Google Scholar]
- 203.Zouaoui N., Mallebrera B., Berrada H., Abid-Essefi S., Bacha H., Ruiz M. Cytotoxic effects induced by Patulin, Sterigmatocystin, and Beauvericin on CHO–K1 cells. Food Chem. Toxicol. 2016;89:92–103. doi: 10.1016/j.fct.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 204.Huang S., Wang J., Xing L., Shen H., Yan X., Wang J., Zhang X. Impairment of cell cycle progression by Sterigmatocystin in human pulmonary cells in vitro. Food Chem. Toxicol. 2014;66:89–95. doi: 10.1016/j.fct.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 205.Jiang X., Wang J., Xing L., Shen H., Lian W., Yi L., Zhan D. Sterigmatocystin-induced checkpoint adaptation depends on Chk1 in immortalized human gastric epithelial cells in vitro. Arch. Toxicol. Suppl. 2016;91:259–270. doi: 10.1007/s00204-016-1682-2. [DOI] [PubMed] [Google Scholar]
- 206.Balogh K., Kövesi B., Zándoki E., Kulcsár S., Ancsin Z., Erdélyi M., Dobolyi C. Effect of sterigmatocystin or aflatoxin contaminated feed on lipid peroxidation and glutathione redox system and expression of glutathione redox system regulatory genes in broiler chicken. Antioxidants. 2019;8(7):201. doi: 10.3390/antiox8070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gao V., Jiang L., Ge L., Chen M., Geng C., Yang G., Qiujuan L. Sterigmatocystin-induced oxidative DNA damage in human liver-derived cell line through lysosomal damage. Toxicology. 2015;29:1–7. doi: 10.1016/j.tiv.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 208.Sivakumar V., Thanislass J., Niranjali S., Devaraj H. Lipid peroxidation as a possible secondary mechanism of sterigmatocystin toxicity. Hum. Exp. Toxicol. 2001;20:398–403. doi: 10.1191/096032701682692955. [DOI] [PubMed] [Google Scholar]
- 209.EFSA (European Food Safety Authority) Scientific opinion on the risk for public and animal health related to the presence of Sterigmatocystin in food and feed. Eur. Food Saf. Authority J. 2013;11:32–54. [Google Scholar]
- 210.Ostry V., Toman J., Grosse Y., Malir F. Cyclopiazonic acid: 50th anniversary of its discovery. World Mycotoxin J. 2018;11:135–148. [Google Scholar]
- 211.Okoth S., Boevre M.D., Vidal A., Mavungu J.D.D., Landschoot S., Kyallo M., Njuguna J., Harvey J., De Saeger S. Genetic and toxigenic variability within Aspergillus flavus population isolated from maize in two diverse environments in Kenya. Front. Microbiol. 2018;9:57. doi: 10.3389/fmicb.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Maragos C.M., Sieve K.K., Bobell J. Detection of cyclopiazonic acid CPA in maize by immunoassay. Mycotoxin Res. 2017;33:157–165. doi: 10.1007/s12550-017-0275-0. [DOI] [PubMed] [Google Scholar]
- 213.Heperkan D., Somuncuoglu S., Karbancioglu-Güler F., Mecik N. Natural contamination of cyclopiazonic acid in dried figs and co-occurrence of aflatoxin. Food Control. 2012;23:82–86. [Google Scholar]
- 214.Rao B.L., Husain A. Presence of cyclopiazonic acid in kodo millet paspalumscrobiculatum causing kodua poisoning in man and its production by associated Fungi. Mycopathology. 1985;89:177–180. doi: 10.1007/BF00447028. [DOI] [PubMed] [Google Scholar]
- 215.Hariprasanna K. Genetic improvement in kodo millet. In: Tonapi V.A., Patil J.V., editors. Book Millets: Ensuring Climate Resilience and Nutritional Security. Daya Publishing House; New Delhi: 2015. [Google Scholar]
- 216.Rasheed U., Wu H., Wei J., Ou X., Qin P., Yao X., Chen H. A polyphasic study of Aspergillus section flavi isolated from corn in Guangxi, China- a hot spot of aflatoxin contamination. Int. J. Food Microbiol. 2019;310:108–307. doi: 10.1016/j.ijfoodmicro.2019.108307. [DOI] [PubMed] [Google Scholar]
- 217.Goron T.L., Raizada M.N. Genetic diversity and genomic resources available for the small millet crops to accelerate a new green revolution. Front. Plant Sci. 2015;6 doi: 10.3389/fpls.2015.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dwivedi S., Upadhyaya H., Senthilvel S., Hash C., Fukunaga K., Diao X., Santra D. Millets RASFF: genetic and genomic resources. Plant Breed. Rev. 2011:247–375. [Google Scholar]
- 219.Gallo A., Giuberti G., Frisvad J., Bertuzzi T., Nielsen K. Review on mycotoxin issues in ruminants: occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins. 2005;7(8):3057–3111. doi: 10.3390/toxins7083057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Deabes M.M. Natural Co-occurrence of aflatoxins, cyclopiazonic acid, and their production by Aspergillus flavus isolates from corn grown in Egypt. Adv. Clin. Toxicol. 2018;3:3. [Google Scholar]
- 221.Maragos C. Complexation of the mycotoxin cyclopiazonic acid with lanthanides yields luminescent products. Toxins. 2018;10:285. doi: 10.3390/toxins10070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sugiharto S. A review of filamentous fungi in broiler production. Ann. Agric. Sci. 2019;64:1–8. [Google Scholar]
- 223.King E.D., Bassi A.B., Ross D.C., Druebbisch B. An industry perspective on the use of “Atoxigenic” strains of Aspergillus flavus as biological control agents and the significance of cyclopiazonic acid. Toxin Rev. 2011;30:33–41. doi: 10.3109/15569543.2011.588818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dupont J., Dequin S., Giraud T., Tacon F., Marsit S., Ropars J., Richard F. Fungi as a source of food. Microbiol. Spectrum. 2017;5(2):0030–2016. doi: 10.1128/microbiolspec.FUNK-0030-2016. [DOI] [PubMed] [Google Scholar]
- 225.Holzapfel C.W., Wilkins D.C. On the biosynthesis of cyclopiazonic acid. Phytochemistry. 1971;10:351–358. [Google Scholar]
- 226.Chang P.K., Ehrlich K.C., Hua S.S. Cladal relatedness among Aspergillus oryzae isolates and Aspergillus flavus S and l morphotype isolates. Int. J. Food Microbiol. 2006;108:172–177. doi: 10.1016/j.ijfoodmicro.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 227.Zhang W., Krohn K., Ullah Z., Flörke U., Pescitelli G., Di Bari L., Sandor A. New mono- and dimeric members of the secalonic acid family: blennolides a–g isolated from the fungus blennoria sp. Chem. Eur. J. 2008;14:4913–4923. doi: 10.1002/chem.200800035. [DOI] [PubMed] [Google Scholar]
- 228.Wezeman T., Bräse S., Masters K.S. Xanthone Dimers: A Compound Family which is both Common and Privileged. Nat. Prod. Rep. 2015;32:1–104. doi: 10.1039/c4np00050a. [DOI] [PubMed] [Google Scholar]
- 229.Franck B., Flasch H. Progress in the Chem.of Org. Nat. Prodt.; 1973. Die ErgochromePhysiologie, Isolierung, Struktur und Biosynthese; pp. 151–206. [Google Scholar]
- 230.Zhai A., Zhu X., Wang X., Chen R., Wang H. Secalonic acid a protects dopaminergic neurons from 1-methyl-4-phenylpyridinium MPP + -Induced cell death via the mitochondrial apoptotic pathway. Eur. J. Pharmacol. 2013;71:58–67. doi: 10.1016/j.ejphar.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 231.Masters K.S., Bräse S. Xanthones from Fungi, lichens, and Bacteria: the natural products and their synthesis. Chem. Rev. 2012;112:3717–3776. doi: 10.1021/cr100446h. [DOI] [PubMed] [Google Scholar]
- 232.Qin T., Porco J.A. Total syntheses of secalonic acids a and d. AngewandteChemie. 2014;126:3171–3174. [Google Scholar]
- 233.Barbero M., Artuso E., Prandi C. Fungal anticancer metabolites: synthesis towards drug discovery. Curr. Med. Chem. 2017;25 doi: 10.2174/0929867324666170511112815. [DOI] [PubMed] [Google Scholar]
- 234.Kanematsu S., Franz A.S., Miyono M.S. Antitumor activity of cytoxan. Cancer Res. 1961;21:1412–1420. [PubMed] [Google Scholar]
- 235.Flieger M., Stodůlková E., Wyka S.A., Černý J., Grobárová V., Píchová K., Novak P. Ergochromes: heretofore neglected side of ergot toxicity. Toxins. 2019;11(8):439. doi: 10.3390/toxins11080439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ganapathy D., Reiner J.R., Loeffler L.E., Ma L., Gnanaprakasam B., Niepoetter B., Koehne I. ChemInform abstract: enantioselective total synthesis of secalonic acid E. Cheminform. 2015;47:15. doi: 10.1002/chem.201503593. [DOI] [PubMed] [Google Scholar]
- 237.Guru S.K., Pathania A.S., Kumar S., Ramesh D., Kumar M., Rana S., Kumar A. Secalonic Acid-D represses HIF1/VEGF-Mediated angiogenesis by regulating the Akt/mTOR/p70S6K signaling cascade. Cancer Res. 2015;75(14):2886–2896. doi: 10.1158/0008-5472.CAN-14-2312. [DOI] [PubMed] [Google Scholar]
- 238.Guo Z., She Z., Shao C., Wen L., Liu F., Zheng Z. 1H and 13C NMR signal assignments of paecilin A and B, two new chromone derivatives from mangrove endophytic fungus Paecilomycessp. Tree 1–7. Magn. Reson. Chem. 2007;45(9):777–780. doi: 10.1002/mrc.2035. [DOI] [PubMed] [Google Scholar]
- 239.Cho H.J., Jung M.J., Woo S., Kim J., Lee E.S., Kwon Y., Younghwa N. New benzoxanthone derivatives as topoisomerase inhibitors and DNA cross-linkers. Bioorg. Med. Chem. 2010;18(3):1010–1017. doi: 10.1016/j.bmc.2009.12.069. [DOI] [PubMed] [Google Scholar]
- 240.Chen L., Li Y.P., Li X., Lu Z.H., Zheng Q.H., Liu Q.Y. Isolation of 4,4′-Bond secalonic acid d from the marine-derived fungus Penicillium oxalicum with inhibitory property against hepatocellular carcinoma. J. Antibiot. 2018;72(1):34–44. doi: 10.1038/s41429-018-0104-5. [DOI] [PubMed] [Google Scholar]
- 241.Farhana R.P., David J.B., Amy M.P., Konstantinos K., Sanjay S., Horst J.S., David W. Systems biology and multi-omics integration: viewpoints from the metabolomics research community. Metabolites. 2019;9:76. doi: 10.3390/metabo9040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Castro-Puyana M., Pérez-Míguez R., Montero L., Herrero M. Application of mass spectrometry-based metabolomics approaches for food safety, quality, and traceability. TrAC Trends Anal. Chem. 2017;93:102–118. [Google Scholar]
- 243.Righetti L., Dall’Asta C., Hajslova J., Rubert J. IntechOpen; 2016. Metabolomics Approaches and Their Hidden Potential for Explaining the Mycotoxin Contamination Problem, Metabolomics-fundamentals and Applications. [Google Scholar]
- 244.Scott P.M., Lawrence J.W., Walbeek W. Detection of mycotoxins by thin-layer chromatography: application to screening of fungal extracts. Appl. Microbiol. 1970;20:839–842. doi: 10.1128/am.20.5.839-842.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Malachová A., Stránská M., Václavíková M., Elliott C.T., Black C., Meneely J., Hajslova J., Ezekiel C.N., Schuhmacher R., Krska R. Advanced LC-MS-based methods to study the co-occurrence and metabolization of multiple mycotoxins in cereals and cereal-based food. Anal. Bioanal. Chem. 2018;410:801–825. doi: 10.1007/s00216-017-0750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hird S.J., Lau B.P.Y., Schuhmacher R., Krska R. Liquid chromatography-mass spectrometry for the determination of chemical contaminants in food. TrAC Trends Anal. Chem. 2014;59:59–72. [Google Scholar]
- 247.Liao C.D., Wong J.W., Zhang K., Yang P., Wittenberg J.B., Trucksess M.W., Hayward D.G. Multi-mycotoxin analysis of finished grain and nut products using ultrahigh-performance liquid chromatography and positive electrospray ionization-quadrupole orbital ion trap high-resolution mass spectrometry. J. Agric. Food Chem. 2015;63:8314–8332. doi: 10.1021/jf505049a. [DOI] [PubMed] [Google Scholar]
- 248.Marianne L., Siegrid D.B., Ben L., Michael R., Siska, Mathias D. Multi LC-MS/MS and LC-HRMS methods for determination of 24 mycotoxins including major phase I and II biomarker metabolites in biological matrices from pigs and broiler chickens. Toxins. 2019;11:171. doi: 10.3390/toxins11030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Righetti L., Paglia G., Galaverna G., Dall’Asta C. Recent advances and future challenges in modified mycotoxin analysis: why HRMS has become a key instrument in food contaminant research. Toxins. 2016;8:361. doi: 10.3390/toxins8120361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Luigi C., Luana G., Josefa I. Target analysis and retrospective screening of multiple mycotoxins in pet food using UHPLC-Q-Orbitrap HRMS. Toxin. 2019;11:434. doi: 10.3390/toxins11080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.João N., Catarina C., Judit M., João R., Sofi A.P., Alexandra M.M.A. Mass spectrometry-based methodologies for targeted and untargeted identification of protein covalent adducts adductomics: current status and challenges. HighThroughput. 2019;8:9. doi: 10.3390/ht8020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Abouseada N., Raouf M., EI-Attar E., Moez P. Matrix-assisted laser desorption ionisation time-of-flight mass spectrometry rapid detection of carbapenamase activity in Acinetobacter baumannii isolates. Ind. J. Med. Microbiol. 2017;35(1):85–89. doi: 10.4103/0255-0857.202335. [DOI] [PubMed] [Google Scholar]
- 253.Tuuli L., Alexandra J., Scott M.B., Ira M.H. Genomic analysis in the age of human genome sequencing. Cell. 2019;177 doi: 10.1016/j.cell.2019.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mahmoud M.A. Detection of Aspergillus flavus in stored peanuts using real-time PCR and the expression of aflatoxin genes in toxigenic and atoxigenic A. Flavus isolates. Foodborne Pathog. Dis. 2015;12:289–296. doi: 10.1089/fpd.2014.1854. [DOI] [PubMed] [Google Scholar]
- 255.Jongsun P., Woochan L., Bohan Z., Anbazhagan M., In-Beom H., Jong-Hwa K., Hap-Hoon H. Complete mitochondrial genome sequence of an aflatoxin B and g producing fungus, Aspergillus parasiticus. Mitochondrial Dna Part B. 2019;4:947–948. [Google Scholar]
- 256.Zhang J., Chiodini R., Badr A., Zhang G. The impact of next-generation sequencing on genomics. J. Genet. Genom. 2011;38:95–109. doi: 10.1016/j.jgg.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Moore G.G., Mack B.M., Beltz S.B. Genomic sequence of the aflatoxigenic filamentous fungus Aspergillus nomius. BMC Genomics. 2015;16:551. doi: 10.1186/s12864-015-1719-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Geromy G.M., Brian M.M., Shannon B.B., Olivier P. Genome sequence of an aflatoxigenic pathogen of argentinian peanut, Aspergillus arachidicola. BMC Genomics. 2018;19:189. doi: 10.1186/s12864-018-4576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Takahito T., Saho H., Satoe Y., Hiroki T., Daisuke K., Masahiko T., Kondoh D. Comparative genome analysis of Aspergillus flavus clinically isolated in Japan. Dna Res. 2018;26:95–103. doi: 10.1093/dnares/dsy041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Faustinelli P.C., Wang X.M., Palencia E.R., Arias R.S. Genome sequences of eight Aspergillus flavus spp. and One A. parasiticus sp. isolated from Peanut Seeds in Georgia. Genome Announcment. 2016;4 doi: 10.1128/genomeA.00278-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gilbert M.K., Mack B.M., Payne G.A., Bhatnagar D. Use of functional genomics to assess the climate change impact on Aspergillus flavus and aflatoxin production. World Mycotoxin J. 2016;9:665–672. [Google Scholar]
- 262.Chakrabortti A., Li J., Liang Z.X. Complete genome sequence of the filamentous fungus Aspergillus westerdijkiae reveals the putative biosynthetic gene cluster of ochratoxin a. Genome Announc. 2016;4 doi: 10.1128/genomeA.00982-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Han X., Chakrabortti A., Zhu J., Liang Z.X., Li J. Sequencing and functional annotation of the whole genome of the filamentous fungus Aspergillus westerdijkiae. BMC Genomics. 2016;17:633. doi: 10.1186/s12864-016-2974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Boqiang L., Yong C., Yuanyuan Z., Yanjiao S., Zhanquan Z., Xiaodi X. Dissection of patulin biosynthesis, spatial control and regulation mechanism in Penicillium expansum. Environ. Microbiol. 2019;21:1124–1139. doi: 10.1111/1462-2920.14542. [DOI] [PubMed] [Google Scholar]
- 265.Sayanthooran S., Magana-Arachchi D.N., Gunerathne L., Abeysekera T. Potential diagnostic biomarkers for chronic kidney disease of unknown EtiologyCKDu in Sri Lanka: a pilot study. BMC Nephrol. 2017;18:31. doi: 10.1186/s12882-017-0440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Vercruysse J., Van Bel M., Osuna-Cruz C.M., Kulkarni S.R., Storme V., Nelissen H., Gonzalez N. Comparative transcriptomics enables the identification of functional orthologous genes involved in early leaf growth. Plant Biotechnol. J. 2019;18:553–567. doi: 10.1111/pbi.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Christine N.S., Benjamin J.C., Doina C., Junyan L., Yuko Y., Mona G., Gaina M. High-throughput single-cell transcriptome profiling of plant cell types. Cell Rep. 2019;27(7):2241–2247. doi: 10.1016/j.celrep.2019.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lowe R., Shirley N., Bleackley M., Dolan S., Shafee T. Transcriptomics technologies. PLoS Comput. Biol. 2017;13 doi: 10.1371/journal.pcbi.1005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Castellá G., Bragulat M.R., Puig L., Sanseverino W., Cabañes F.J. Genomic diversity in ochratoxigenic and non ochratoxigenic strains of Aspergillus carbonarius. Sci. Rep. 2018;8:5439. doi: 10.1038/s41598-018-23802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Musungu B.M., Bhatnagar D., Brown R.L., Payne G.A., OBrian G., Fakhoury A.M., Matt G. A network approach of gene Co-expression in the Zea mays/aspergillus flavusPathosystem to map Host/Pathogen interaction pathways. Front. Genet. 2016;7:206. doi: 10.3389/fgene.2016.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nayak S.N., Agarwal G., Pandey M.K., Sudini H.K., Jayale A.S., Purohit S., Desai A. Aspergillus flavus infection triggered immune responses and host-pathogen cross-talks in groundnut during in-vitro seed colonization. Sci. Rep. Ist. Super. Sanita. 2017;7:9659. doi: 10.1038/s41598-017-09260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Bhatnagar D., Rajasekaran K., Gilbert M., Cary J.W., Magan N. Advances in molecular and genomic research to safeguard food and feed supply from aflatoxin contamination. World Mycotoxin J. 2018;11:47–72. [Google Scholar]
- 273.Bo W., Vivek K., Andrew O., Doreen W. Reviving the transcriptome studies: an insight into the emergence of single-molecule transcriptome sequencing. Front. Genet. 2019;10:384. doi: 10.3389/fgene.2019.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Fountain J.C., Koh J., Yang L., Pandey M.K., Nayak S.N., Bajaj P., Wei-Jian Z. Proteome analysis of Aspergillus flavus isolate-specific responses to oxidative stress in relationship to aflatoxin production capability. Sci. Rep. 2018;8:3430. doi: 10.1038/s41598-018-21653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Xia X., Li H., Liu F., Zhang Y., Zhang Q., Wang Y., Li P. Proteome changes in Penicillium expansum grown in a medium derived from host plant. J. Microbiol. Biotechnol. 2017;27:624–632. doi: 10.4014/jmb.1605.05022. [DOI] [PubMed] [Google Scholar]
- 276.Lai T., Wang Y., Fan Y., Zhou Y., Bao Y., Zhou T. The response of growth and patulin production of postharvest pathogen Penicillium expansum to exogenous potassium phosphite treatment. Int. J. Food Microbiol. 2017;244:1–10. doi: 10.1016/j.ijfoodmicro.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 277.Degola F., Bisceglie F., Pioli M., Palmano S., Elviri L., Pelosi G., Lodi T. Structural modification of cuminaldehyde thiosemicarbazone increases inhibition specificity toward aflatoxin biosynthesis and sclerotia development in Aspergillus flavus. Appl. Microbiol. Biotechnol. 2017;101:6683–6696. doi: 10.1007/s00253-017-8426-y. [DOI] [PubMed] [Google Scholar]
- 278.O’Farrell P.H. High-resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 279.Mohammadi M., Anoop V., Gleddie S., Harris L.J. Proteomic profiling of two maize inbreds during early gibberella ear rot infection. Proteomics. 2011;11:3675–3684. doi: 10.1002/pmic.201100177. [DOI] [PubMed] [Google Scholar]
- 280.Bilal A., Madiha B., Muhammad A.N., Mohsin K., Muhammad H.R. Proteomics: technologies and their applications. J. Chromatogr. B Biomed. Sci. Appl. 2017;55(2):182–196. doi: 10.1093/chromsci/bmw167. [DOI] [PubMed] [Google Scholar]
- 281.Alex P.W., Wendiro D., Vuzi P.C., Hawumba J.F. Methods for detection of aflatoxins in agricultural food crops. J. Appl. Chem. 2014:1–15. [Google Scholar]
- 282.Goryacheva I.Y., De Saeger S., Eremin S.A., Van Peteghem C. Immunochemical methods for rapid mycotoxin detection: evolution from single to multiple analyte screening. A review. Food Add. Cont. 2007:1169–1183. doi: 10.1080/02652030701557179. [DOI] [PubMed] [Google Scholar]
- 283.Saraf N. Development of in vitro point of care diagnostics (IVPCD) based on aptamers integrated biosensors. Electronic. 2019:6618. [Google Scholar]
- 284.Sefah K. In vitro selection with artificial expanded genetic information systems. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1449–1454. doi: 10.1073/pnas.1311778111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bazaz R., Denning D.W. Aspergillosis: causes, types, and treatment. Pharm. J. 2019;303:7927. doi: 10.1211/PJ.2019.20206738. [DOI] [Google Scholar]
- 286.Malcolm R., Riina R. Exposure to Aspergillus in home and healthcare facilities’ water environments: focus on biofilms. Microorganisms. 2019;7:7. doi: 10.3390/microorganisms7010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Vivek-Ananth R.P., Mohanraj K., Vandanashree M., Jhingran A., James Craig P., Samal Areejit. Comparative systems analysis of the secretome of the opportunistic pathogen Aspergillus fumigatus and other Aspergillus species. Sci. Rep. 2017:1–31. doi: 10.1038/s41598-018-25016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Raffa N., Keller Nancy P. A call to arms: mustering secondary metabolites for success and survival of an opportunistic pathogen. PLoS Pathog. 2019;15:4. doi: 10.1371/journal.ppat.1007606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Salah H., Lackner M., Houbraken J., Theelen B., Lass-Flörl C., Boekhout T., Almaslamani M., Taj-Aldeen S.L. The emergence of rare clinical Aspergillus species in Qatar: molecular characterization and antifungal susceptibility profiles. Front. Microbiol. 2019;10:1677. doi: 10.3389/fmicb.2019.01677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Barnes P.D., Marr K.A. Aspergillosis: spectrum of disease, diagnosis, and treatment. Infect. Dis. Clin. N Am. 2006;20:545–561. doi: 10.1016/j.idc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 291.Oguma T., Taniguchi M., Shimoda T., Kamei K., Matsuse H., Hebisawa A., Takayanagi N., Konno S., Fukunaga K., Harada K., Tanaka J., Tomomatsu K., Asano K. Allergic bronchopulmonary aspergillosis in Japan: a nationwide survey. Allergol. Int. 2018;67:79–84. doi: 10.1016/j.alit.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 292.Nepal B., Myers R., Lohmar J.M., Puel O., Thompson B., Van Cura M., Calvo A.M. Characterization of the putative polysaccharide synthase CpsA and its effects on the virulence of the human pathogen Aspergillus fumigatus. PLoS One. 2019:1–18. doi: 10.1371/journal.pone.0216092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Luis A.R.C., Ana P.T.O., José L.A.R., Bayman P. An opportunistic human pathogen on the fly: strains of Aspergillus flavus vary in virulence in Drosophila melanogaster. Med. Mycol. 2014;52:211–219. doi: 10.1093/mmy/myt008. [DOI] [PubMed] [Google Scholar]
- 294.Amiri M.J., Karami P., Chichaklu A.H., Jangan E.H. Identification and isolation of Mycobacterium tuberculosis from Iranian patients with recurrent TB using different staining methods. J Res. Med. Dent Sci. 2018;6:409–414. [Google Scholar]
- 295.Sivasankari S. Prevalence of Invasive Aspergillosis Among (PTB) Patients in Kanchipuram, India. J. Clin. Diagn. Res. 2014;8:22–23. doi: 10.7860/JCDR/2014/7957.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Page L.D. School of Medicine / Institute of Inflammation and Repair; 2021. Pulmonary Aspergillosis in Association With Tuberculosis and HIV in Uganda. [Google Scholar]
- 297.Hernández-Martínez R., Navarro-Blasco I. Aflatoxin levels and exposure assessment of Spanish Infant Cereals. Food Addit. Contam.: Part B. Surveill. 2010;3(4):275–288. doi: 10.1080/19393210.2010.531402. [DOI] [PubMed] [Google Scholar]
- 298.Dorr G.D., Iwona Y., Sorenson W.G., Martha J.M., Ruth A.E. Overview of investigations into pulmonary haemorrhage among infants in Cleveland, Ohio. Environ. Health Perspect. 1999;107:495–499. doi: 10.1289/ehp.99107s3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Jedrychowski W., Maugeri U., Perera F., Stigter L., Jankowski J., Butscher M., Maria B. Cognitive function of 6-year-old children exposed to mold-contaminated homes in early postnatal period. Prospective birth cohort study in Poland. Physiol. Behav. 2011;104:989–995. doi: 10.1016/j.physbeh.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Allen S.J., Wild C.P., Wheeler J.G., Riley E.M., Montesano R., Bennett S. Aflatoxin exposure, malaria and hepatitis B infection in rural Gambian children. Trans. R. Soc. Trop. Med. Hyg. 1992;86(4):426–430. doi: 10.1016/0035-9203(92)90253-9. [DOI] [PubMed] [Google Scholar]
- 301.Oloyede A., Olusegun A., Assunta R., Yinka S., Ranajit B., Alberto R. Correlation between aflatoxin M1 content of breast milk, dietary exposure to aflatoxin B1 and socioeconomic status of lactating mothers in Ogun State, Nigeria. Food Chem. Toxicol. 2013;56:171–177. doi: 10.1016/j.fct.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 303.Puel O., Galtier P., Oswald P.I. Biosynthesis and toxicological effects of patulin. Toxins. 2010:2. doi: 10.3390/toxins2040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Hajjaj H., Klaebe A., Loret M.O., Goma G., Blanc P.J., Francois J.F. Biosynthetic pathway of citrinin in the filamentous fungus Monascusruber as revealed by 13C nuclear magnetic resonance. Appl. Environ. Microbiol. 1999:311–314. doi: 10.1128/aem.65.1.311-314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Aswani Kumar Y.V.V., Renuka R.M., Achuth J., Venkataramana M., Ushakiranmayi M., Sudhakar P. Development of hybrid IgG-Aptamer sandwich immunoassay platform for aflatoxin B1 detection and its evaluation onto various field samples. Front. Pharmacol. 2018;9:271. doi: 10.3389/fphar.2018.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]