Abstract
Belatacept results in improved kidney transplant outcomes, but utilization has been limited by logistical barriers related to monthly (q1m) intravenous infusions. Every 2-month (q2m) belatacept has potential to increase utilization, therefore we conducted a randomized non-inferiority trial in low immunologic risk renal transplant recipients greater than one-year post-transplant. Patients on belatacept were randomly assigned to q1m or q2m therapy. The primary objective was a non-inferiority comparison of renal function (eGFR) at 12 months with a non-inferiority margin (NIM) of 6.0 ml/min/1.73m2. 166 participants were randomized to q1m (n=82) or q2m (n=84) belatacept, 163 patients received treatment, and 76 q1m and 77 q2m subjects completed the 12-month study period. Every 2-month belatacept was non-inferior to q1m, as the difference in mean eGFR adjusted for baseline renal function did not exceed the NIM. Two-month dosing was safe and well tolerated, with no patient deaths or graft losses. Four rejection episodes and three cases of donor-specific antibodies (DSA) occurred amongst q2m subjects; however, only one rejection and one instance of DSA was observed in subjects adherent to the study protocol. Every two-month belatacept therapy may facilitate long-term utilization of costimulation blockade, but future multicenter studies with longer-term follow-up will further elucidate immunologic risk. (ClinicalTrials.gov NCT02560558)
1. Introduction
Kidney transplantation is the treatment of choice for patients with ESRD resulting in clear survival and quality of life benefits (1, 2). While the field has experienced notable gains in short-term outcomes, significant improvements in long-term outcomes remain elusive (3). Barriers to improving long-term outcomes include immunosuppression-related cardiovascular morbidity, calcineurin inhibitor (CNI)-induced nephrotoxicity and immunologic injury resulting from HLA antibodies directed against the donor. The costimulation blocker belatacept has shown great promise as a CNI-alternative for maintenance immunosuppression with demonstrated improvements in long-term kidney transplant outcomes (4). In the phase III BENEFIT study, belatacept led to a 43% reduction in the risk of death and graft loss along with enhanced renal function seven years post-transplant in comparison to the CNI cyclosporine. However, utilization of belatacept has been low as 93% of adult kidney transplant recipients continue to be initiated on CNI-based regimens (5). The slow uptake of belatacept has been largely a result of higher early acute rejection rates and logistical challenges related to its monthly i.v. access and infusion requirement, as well as concerns regarding cost and its impact on protective immunity against viral pathogens (6). Thus, overcoming these obstacles will enable increased use of belatacept and provide more kidney transplant recipients with an opportunity at improved outcomes.
Several strategies to circumvent the higher incidence of rejection with belatacept have achieved acceptable acute rejection rates (7, 8), paving the way for long-term belatacept maintenance. Moreover, belatacept-based therapy still results in better renal allograft function and improved patient and graft survival irrespective of early rejection (4, 7). Therefore, there is an unmet need to develop strategies that facilitate greater use of belatacept as standard long-term maintenance immunosuppression following kidney transplantation. Limited vascular access is common in ESRD patients, and the need for perpetual i.v. infusion administration with belatacept often presents logistical challenges at both patient and institutional levels. In the absence of a subcutaneous formulation, less frequent i.v. belatacept dosing has the potential to minimize the burden of monthly healthcare encounters for patients and healthcare systems, reduce infectious complications and cost, and improve patient and provider satisfaction along with long-term compliance with belatacept-based regimens.
In the phase II randomized clinical trial evaluating the efficacy of belatacept, study subjects were first randomized to belatacept (either more intense or less intense)- or cyclosporine-based immunosuppression and then belatacept-treated patients were re-randomized to receive belatacept every 4 weeks or every 8 weeks (9). After ten years, subjects receiving belatacept every 8 weeks experienced higher cumulative acute rejection rates, but similar to those receiving belatacept every 4 weeks, maintained superior renal function compared to cyclosporine-treated patients without differences in patient death or graft loss (10). This study was not powered to measure differences between the 4-week and 8-week belatacept groups, and because of the double randomization design it was not clear whether the increased incidence of rejections in the 8-week group was a result of more intense vs. less intense belatacept or the prolonged dosing interval. Additionally, the vast majority of rejections occurred early within the first-year post-transplant, and renal function and the rejection rate were stable and similar between the 4-week and 8-week groups beyond the first post-transplant year. Thus, it is possible that in stable kidney transplant recipients greater than one-year post-transplant less frequent belatacept maintenance dosing could provide adequate prophylaxis against rejection yet reduce cost and logistical barriers to sustained long-term utilization and still provide the benefits of better renal function, less alloantibodies and off-target toxicities, and improved patient and graft survival.
In the current study we tested the hypothesis that belatacept administered every two months would be non-inferior to standard monthly dosing at maintaining renal function in renal transplant recipients. We designed a randomized controlled non-inferiority trial with the primary objective to assess renal function as measured by estimated glomerular filtration rate (eGFR) twelve months post-randomization in stable, low immunologic risk patients receiving belatacept every 2 months as compared to reference monthly dosing. A non-inferiority approach was selected based on prior use of this trial design to establish efficacy of belatacept relative to CNIs for the prevention of acute rejection and patient and graft survival after renal transplantation (11, 12), along with the expectation that non-inferiority of belatacept administered every 2 months would be sufficient to tip the risk-benefit ratio in its favor. Determination of non-inferiority was based on a pre-specified difference in mean eGFR of 6 ml/min/1.73m2 between treatment groups.
2. Methods
2.1. Design
This non-inferiority trial is a 12-month, randomized, parallel-group, single center study conducted between October 2015 and August 2019. Patients were allocated 1:1 into intervention groups. No changes were made to the study design after trial commencement. A data and safety monitoring board (DSMB) assessed cumulative safety and efficacy data at regular intervals during the course of the study. This trial was conducted in accordance with the ethical principles founded in the current Declaration of Helsinki and was consistent with International Conference on Harmonization Good Clinical Practice guidelines and applicable regulatory requirements. The study protocol and any amendments were reviewed and approved by the Emory Institutional Review Board. This trial is registered with ClinicalTrials.gov (NCT02560558).
2.2. Patients
Eligible patients were first-time living or deceased donor kidney transplant recipients 18 or older initiated on belatacept-based immunosuppression at the time of transplant and at least one-year post-transplant. Because the ETC has employed belatacept-based regimens with (belatacept 2.0) and without (belatacept 1.0) transient CNI therapy (7), study participants initiated on belatacept 2.0 regimens with short-term tacrolimus treatment were required to be off CNI for a minimum of six months. Additional inclusion criteria consisted of stable renal function with eGFR >35 ml/min/1.73m2 (for safety in case of deterioration), low immunologic risk (cPRA <50, no donor-specific antibody (DSA), ≤1 prior rejection episode) and minimum maintenance immunosuppression of belatacept (5 mg/kg monthly), mycophenolate mofetil (1000 mg daily) and prednisone (5 mg daily). Exclusion criteria included presence of a nonrenal solid organ transplant, uncontrolled diabetes (Hgb A1c >8%), proteinuria (protein/creatinine ratio >1), and active infection or viremia. The study took place at the Emory Transplant Center (ETC; Atlanta, GA, USA) and all patients provided signed informed consent prior to any study interventions.
2.3. Interventions
Patients underwent 1:1 randomization stratified for prior history of rejection to receive belatacept maintenance therapy monthly (q1m) or every two months (q2m). Participant screening criteria were entered into REDCap software that allocated patients into intervention groups according to a computer-generated randomization list. Allocation sequence was concealed from the research team. Study participants, healthcare providers, data collectors and outcome adjudicators were not blinded. Patients allocated to the q1m reference group were maintained on standard monthly belatacept (5 mg/kg i.v.) infusions (12), and those allocated to the q2m group were transitioned to less frequent bimonthly infusions at the same 5 mg/kg i.v. dose (9). Infusions were administered on site at the ETC or a certified local infusion center. Each group had 12 monthly study visits, and q2m patients had 3 additional lab checks between months 1–4 for safety purposes that were not included in data analyses. Patients experiencing acute rejection were treated in accordance with an established rejection grade-based protocol (corticosteroids for grades <1B and thymoglobulin for grades ≥1B), and q2m participants were converted back to monthly belatacept dosing.
2.4. Outcomes
The primary objective of this study was to assess whether q2m belatacept dosing is non-inferior to standard monthly maintenance therapy as measured by renal function (eGFR) 12 months after randomization. Renal function was estimated from serum creatinine values using the CKD-EPI equation monthly. Secondary outcome measures included assessment of rejection, graft loss, patient death, DSA formation and incidence of infections. Biopsies were performed for cause and rejections defined as grade 1A or greater as determined by a staff pathologist according to standard Banff criteria (13). HLA antibodies were evaluated at baseline (screening), 6 and 12 months per protocol, and at the time of any for cause biopsies. At baseline, flow PRA screening and single antigen bead (SAB) testing were performed on all subjects, while 6- and 12-month flow PRA screening was done with reflex SAB and DSA testing when visible changes in flow PRA architecture were present per clinical protocol. Adverse events were captured through active surveillance via open ended questions by the research coordinator at each study visit in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) (14) with a focus on serious adverse events, malignancies, and infections. Cumulative clinical infections were assessed at 12 months and viremias were detected for cause. No changes were made to the primary or secondary outcomes after the trial was initiated.
2.5. CD86 Receptor Saturation
Under an independent Emory IRB-approved protocol (IRB00006248) fresh whole blood from healthy controls (n=11) and a representative random subset of q1m (n=18) and q2m (n=17) belatacept-treated study patients was tested to determine CD86 receptor saturation. For belatacept-treated patients, samples were collected before (trough) and 30 minutes after (peak) i.v. infusion. As previously described (15), CD86 receptor occupancy (RO) was determined by capturing the saturation of an anti-CD86 mAb (HA5.2B7, Beckman Coulter) that competes with belatacept for CD86 binding on CD14+ monocytes via flow cytometry.
2.6. Statistics
At the time of trial design, the mean eGFR one-year post-transplant in all renal transplant recipients with renal function >35 ml/min/1.73m2 receiving monthly belatacept at the ETC was 61.5 ml/min/1.73m2 (S.D. 13.1 ml/min/1.73m2). Based on these data, a sample size of 166 patients (83 per group) was determined necessary and sufficient (with a two-sided 95% CI and 80% power) to exclude a difference in mean eGFR between treatment groups of more than 6.0 ml/min/1.73m2. The non-inferiority margin (NIM) of 6.0 ml/min/1.73m2 was selected based on reported superiority margins of >12 ml/min in belatacept over CNIs (7, 12), use of a margin of 5.7 ml/min to determine superiority of belatacept over CNI in a previous conversion study (16), and standard clinical practice/judgement as well as cost and feasibility considerations to perform the study. No formal interim analyses were planned and the DSMB periodically reviewed study data. Stopping rules were defined for threshold incidences of biopsy proven acute rejection of 20% on a rolling basis consistent with pre-specified numbers of subjects with rejection per enrolled patients (modified Simon’s two-stage design strategy for 75–90% probability).
For the primary outcome of eGFR at 12 months, the unadjusted and adjusted mean difference in eGFR were utilized to determine non-inferiority. Renal function at 12 months was adjusted for baseline eGFR with analysis of covariance (ANCOVA). The adjusted means of q1m and q2m dosing intervals and their difference (least square means and 95% CI of the means) were calculated by using baseline eGFR as a covariate in ANCOVA. If the upper bound of the 95% CI of the difference in mean eGFR between the q1m and q2m groups at 12 months was less than the pre-specified NIM of 6 ml/min/1.73m2, then q2m belatacept was considered non-inferior to standard monthly belatacept. These analyses were conducted per protocol on all randomized patients that received treatment and achieved the primary endpoint, and intent-to-treat (ITT) with imputation of missing data for sensitivity analysis utilizing a multiple imputation method to account for missing values that included randomized subjects that did not receive intervention or complete the study. We observed a monotone missing data pattern in chronologically ordered eGFR values for q1m and q2m patients. Using the MCMC method, an ANCOVA model with treatment as a fixed effect and baseline eGFR value as a covariate was fit using SAS PROC GLM for 10 imputations. For each imputation, mean eGFR at 12 months adjusted for the baseline and the eGFR difference between the adjusted means were computed.
The secondary outcomes of rejection, patient death and graft loss were analyzed ITT including all randomized subjects utilizing Kaplan-Meier time to event comparisons between groups and the log rank test to determine statistical significance. The incidence of DSA and infections was compared between groups by the two-sample independent t-test statistic. The Mann–Whitney U nonparametric t test was performed for analysis of unpaired groups using GraphPad Prism (GraphPad Software, Inc.) for the CD86 receptor saturation assay. Statistical significance was attributed to p values <0.05.
3. Results
3.1. Participant flow and patient characteristics
Participant flow is illustrated in Figure 1 and consisted of initial screening of approximately 850 transplant recipients on belatacept for eligibility from October 2015 to August 2018. Of subjects screened, 258 were approached for participation and 176 were consented and screened (82 declined to participate), resulting in 10 screen failures for subclinical DSA, proteinuria, uncontrolled diabetes and CMV viremia. One hundred sixty-six were randomized to receive belatacept monthly (q1m, n=82) or every two months (q2m, n=84). Three subjects randomized to the q2m group did not receive treatment. One suffered a stroke 2 weeks after randomization and 2 due to reluctance immediately after randomization. Of those receiving treatment, six q1m and 4 q2m patients did not complete the study (Supp. Table 1). In the q1m group, 4 were lost to follow-up and 2 died, while in the q2m group 1 became reluctant to participate and 3 were withdrawn from the study as a result of protocol deviations for immunosuppression noncompliance.
Figure 1. CONSORT diagram of participant flow.
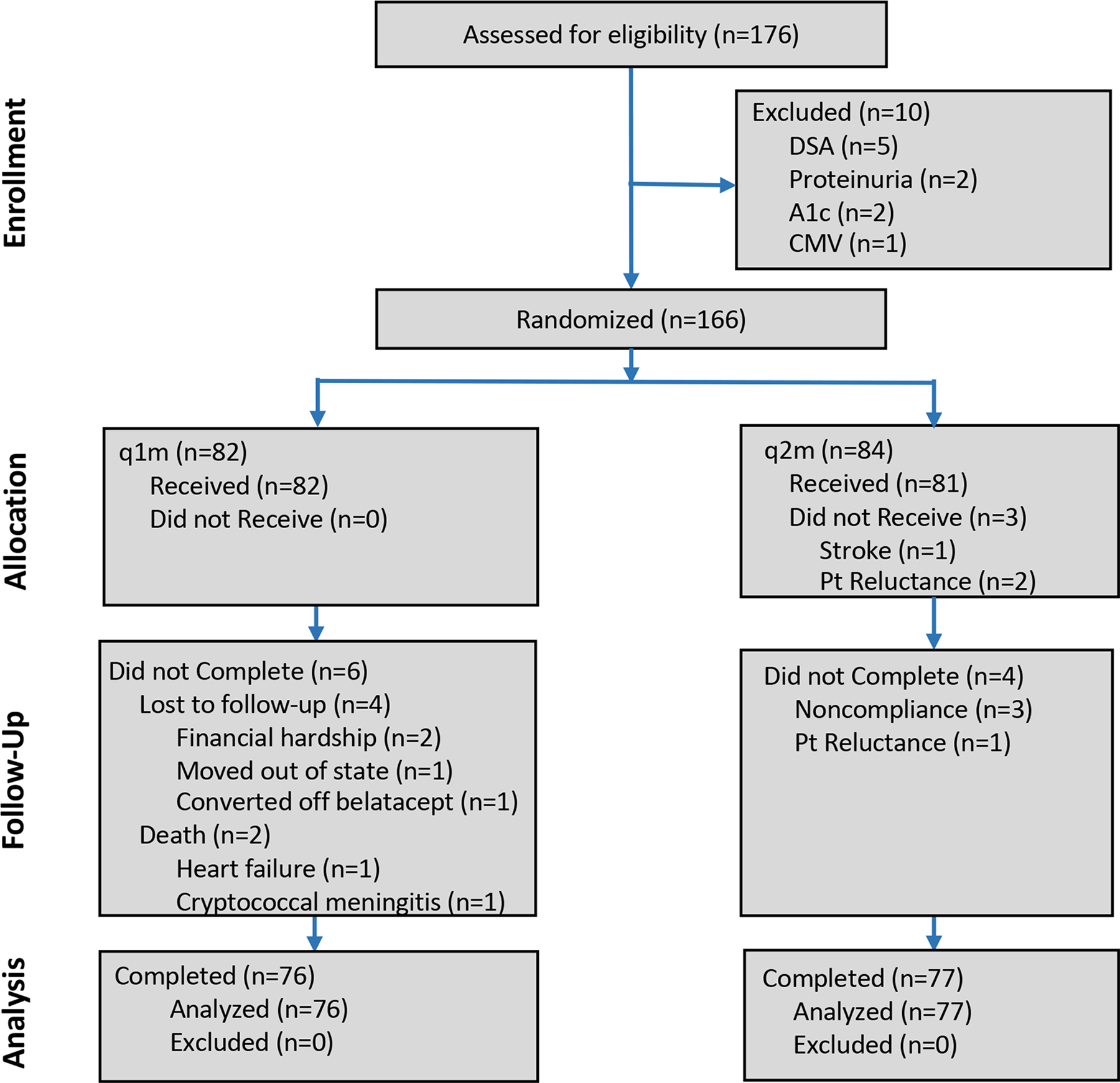
Study participant flow in this single center, randomized non-inferiority trial evaluating every two-month (q2m) belatacept maintenance therapy in comparison to monthly (q1m) reference dosing in kidney transplant recipients.
Baseline demographic and clinical characteristics are outlined in Table 1. Patient age, sex, race and etiology of ESRD were similar between the groups, with a greater proportion of living donor recipients in the q1m arm. Study subjects were a median of 26- and 22-months post-transplant and a median of 393 and 332 days off CNI therapy in the q1m and q2m groups, respectively. In regard to immunologic risk, prior sensitization as indicated by cPRA at the time of transplant along with for cause allograft biopsies and rejection history were also similar between treatment arms. Baseline renal function as measured by eGFR at the time of randomization and initiation of study was slightly better in q1m recipients (72.4 +/− 17.7 ml/min/1.73m2) relative to q2m recipients (69.3 +/− 16.4 ml/min/1.73m2).
Table 1.
Patient baseline demographic and clinical characteristics
| q1m (n=82) | q2m (n=81) | |
|---|---|---|
| Age, years (SD) | 52 (12) | 50 (13) |
| Sex | ||
| Male | 63 (77) | 54 (67) |
| Female | 19 (23) | 27 (33) |
| Race | ||
| Black | 32 (39) | 36 (44) |
| Non-black | 50 (61) | 45 (56) |
| Etiology of ESRD | ||
| Hypertension | 19 (23) | 22 (27) |
| Diabetes | 17 (21) | 18 (22) |
| PKD | 14 (17) | 11 (14) |
| Glomerulonephritis | 7 (9) | 2 (3) |
| FSGS | 5 (6) | 6 (7) |
| Other | 20 (24) | 22 (27) |
| Donor type | ||
| Living | 46 (56) | 37 (46) |
| Deceased | 36 (44) | 44 (54) |
| Time post-transplant, months (IQR) | 26 (20–47) | 22 (19–34) |
| CMV risk | ||
| Low | 20 (24) | 18 (22) |
| Moderate | 56 (68) | 54 (67) |
| High | 6 (7) | 9 (11) |
| cPRA at transplant | ||
| 0 | 71 (87) | 67 (83) |
| <20 | 6 (7) | 6 (7) |
| ≥20 | 5 (6) | 8 (10) |
| Induction immunosuppression | ||
| Thymoglobulin | 1 (1) | 3 (4) |
| Basiliximab | 81 (99) | 78 (96) |
| Maintenance immunosuppression | ||
| Belatacept 1.0 | 12 (15) | 7 (9) |
| Belatacept 2.0 | 70 (85) | 74 (91) |
| Time off CNI, days (IQR) | 393 (237–592) | 332 (245–700) |
| eGFR, ml/min/1.73m2 (SD) | 72.4 (17.7) | 69.3 (16.4) |
| Biopsy history | ||
| 0 | 43 (52) | 33 (41) |
| ≥1 | 39 (48) | 48 (59) |
| Borderline | 8 (10) | 15 (19) |
| Rejection history | ||
| Total | 8 (10) | 12 (15) |
| IA, IB | 4 (5) | 7 (9) |
| IIA | 4 (5) | 5 (6) |
Belatacept 1.0, belatacept-based CNI-free regimen; Belatacept 2.0, belatacept-based regimen with transient CNI therapy; Data are means (SD), numbers (%) or median (IQR)
3.2. Renal function and patient/graft survival
The primary endpoint of mean eGFR at 12 months was evaluated per protocol and included all randomly assigned patients that received treatment and completed the study (93% (n=76 of 82) and 92% (n=77 of 84) of subjects in the q1m and q2m groups, respectively). At 12 months, the unadjusted mean eGFR for patients in the q1m group was 74.11 (SD, 18.25) and 70.69 (SD, 16.66) ml/min/1.73m2 in the q2m group, resulting in a mean difference of 3.41 ml/min/1.73m2 (95% CI −2.17, 9.00) (Table 2). Based on the pre-specified margin of 6.0 ml/min/1.73m2, unadjusted renal function in the q2m group is inconclusive for non-inferiority because the upper 95% CI of 9.00 exceeds 6.0. However, mean eGFR at 12 months adjusted for the random baseline difference observed between the two groups prior to intervention was 72.65 (95% CI 70.64, 74.65) and 72.13 (95% CI 70.14, 74.12) in the q1m and q2m groups, respectively (Table 2). The eGFR difference between these adjusted means (0.52, −2.32, 3.35) demonstrates non-inferiority, as the 95% CI are within the NIM of 6.0 (Figure 2). ITT sensitivity analysis with imputation also demonstrated non-inferiority between the adjusted means (Supp. Table 2). Additionally, renal function over the course of the 12-month study period was similar between the q1m and q2m groups along with mean change in eGFR between baseline and end of study.
Table 2.
Primary outcome: renal function
| n | q1m | n | q2m | |
|---|---|---|---|---|
| Unadjusted | ||||
| eGFRb, mean at baseline (SD) | 82 | 72.43 (17.70) | 81 | 69.25 (16.41) |
| eGFR12, mean at 12 months (SD) | 76 | 74.11 (18.25) | 77 | 70.69 (16.66) |
| Change in eGFR (eGFR12 – eGFRb) | - | 1.24 (9.86) | - | 1.12 (8.14) |
| Mean | 95% CI | |||
| Difference in eGFR12 (q1m – q2m) | 3.41 | −2.17, 9.00 | ||
| Adjusted (for baseline) | ||||
| eGFR12, mean at 12 months (95% CI) | 76 | 72.65 (70.64, 74.65) | 77 | 72.13 (70.14, 74.12) |
| Mean | 95% CI | |||
| Difference in eGFR12 (q1m – q2m) | 0.52 | −2.32, 3.35 | ||
eGFR, estimated glomerular filtration rate (ml/min/1.73m2)
Figure 2. Renal function with q2m belatacept maintenance therapy is non-inferior to standard q1m administration.
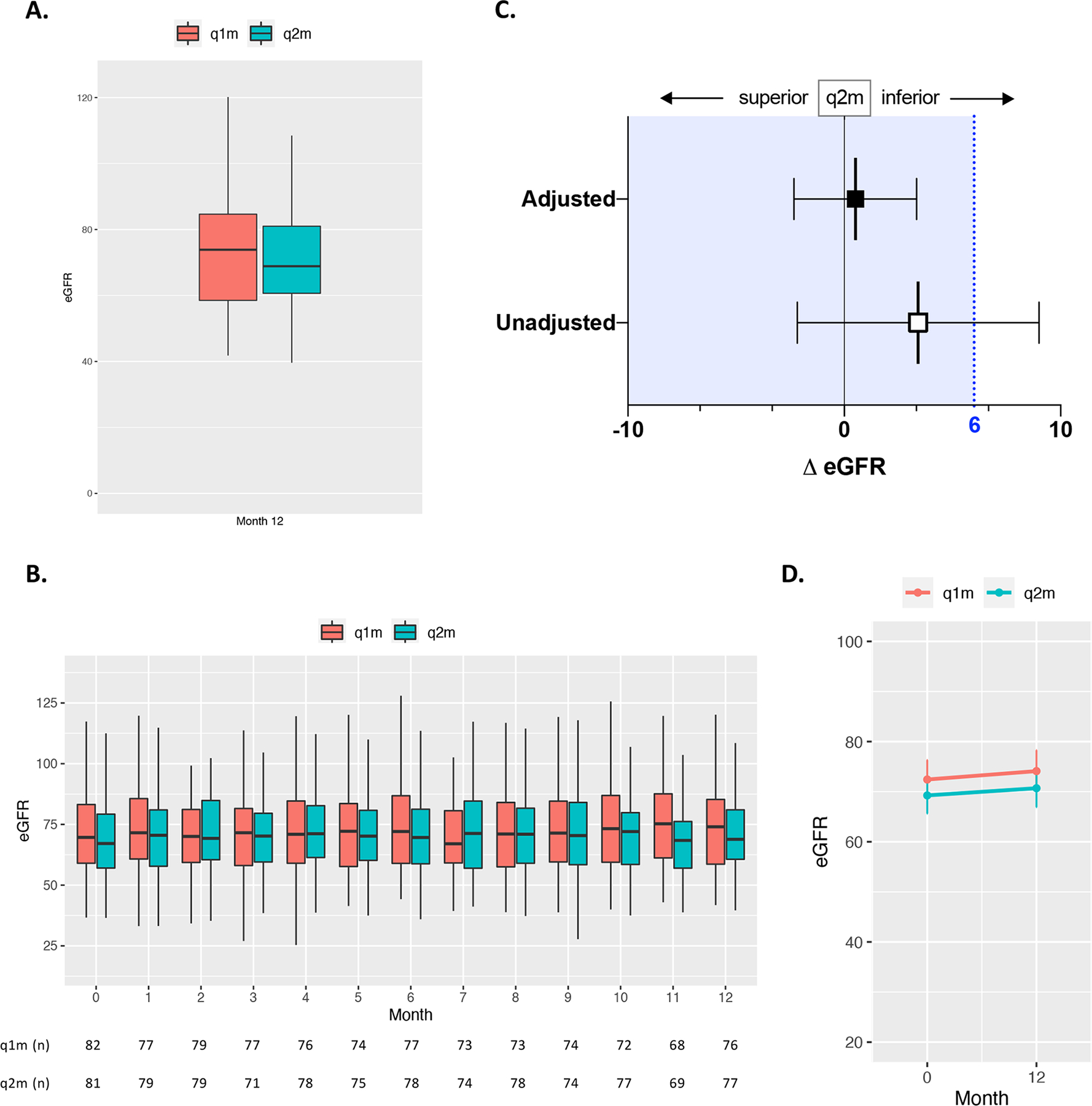
Kidney transplant recipients were randomized to receive q1m or q2m belatacept (5 mg/kg) maintenance therapy and observed over a 12-month study period. (A) Unadjusted mean renal function as measured by eGFR (ml/min/1.73m2) at 12 months post-randomization between groups. Box and whiskers indicate IQR. (B) Unadjusted mean eGFR between groups over the 12-month study period with numbers at risk for each group. Box and whiskers indicate IQR. (C) Observed unadjusted and adjusted difference in mean eGFR between q1m and q2m treatment groups 12 months after randomization. The blue dashed line indicates the pre-specified non-inferiority margin of a mean difference (Δ) in eGFR of 6 ml/min/1.73m2, and the blue tinted region to the left of this line indicates the zone of non-inferiority. The error bars indicate 2-sided 95% CIs for the mean difference in eGFR between groups at 12 months (Table 2). (D) Change in eGFR from baseline (month 0) to end of study (month 12) for each treatment group.
Two patients in the q1m group died with functioning grafts while on study (Figure 3A), one due to heart failure and the other from cryptococcal meningitis. Of the four q1m subjects lost to follow-up, there were no additional deaths and one graft loss (Supp. Table 1). There were no deaths or graft losses in the q2m group. Of q2m subjects lost to follow-up, the patient that did not receive intervention due to stroke died off study. Otherwise, there were no additional deaths or graft losses amongst those that did not complete the study (Supp. Table 1).
Figure 3. Kaplan-Meier time to event curves for patient death/graft loss and acute rejection.
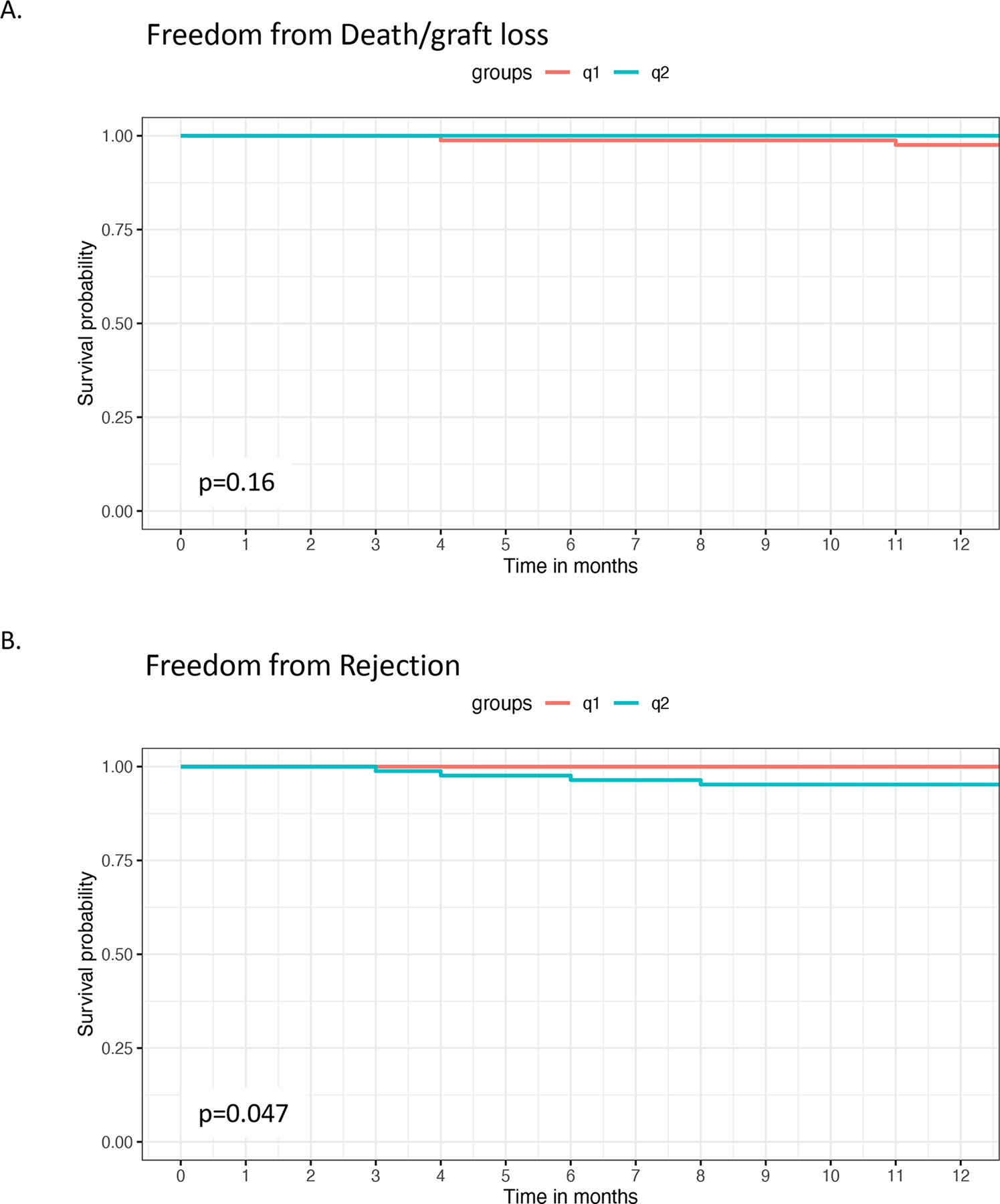
Kaplan-Meier plots for freedom from (A) patient death/graft loss and (B) rejection on the randomized intention to treat population (q1m n=82, q2m n=84).
3.3. Acute rejection and DSA
For cause allograft biopsies were performed on one q1m patient and five q2m patients, demonstrating four total rejections in the q2m group (Figure 3B, Table 3). Two biopsies at different times were performed on the same q1m patient, both diagnosed as borderline for acute cellular rejection (ACR). In the q2m patients, four were performed for elevations in creatinine and one due to the detection of de novo DSA at 6 months per study protocol with normal renal function. Two grade 1A ACRs, one grade 1B ACR, and 1 mixed ACR/AMR were diagnosed in the four patients with renal dysfunction, and the biopsy in the patient with subclinical DSA was normal. Notably, three of the four rejections were associated with documented non-compliance with immunosuppressive medication, and three rejectors were earlier post-transplant than the median times for both q1m and q2m groups (Supp. Table 3). Overall, there were two borderline for ACR in the q1m group and four ACRs in the q2m group, 3 of 4 (75%) of which were associated with non-compliance.
Table 3.
Immunologic outcomes: biopsies and rejection
| Subject | Time (m) | Pathology | DSA | Action/Comment | Study Completion |
|---|---|---|---|---|---|
| q1m | |||||
| 009 | 6 | Borderline | N | Oral steroid pulse | Y |
| 009 | 10 | Borderline | N | Oral steroid pulse | Y |
| q2m | |||||
| 078 | 6 | 1A ACR | N | IV steroid pulse, converted to q1m | N |
| NC with MMF, prednisone, belatacept | |||||
| 098 | 8 | 1B ACR | Y | Thymoglobulin, converted to q1m | N |
| NC with MMF, prednisone | |||||
| 130 | 4 | 1A ACR | N | Converted to q1m | Y |
| 135 | 4 | 1B ACR, AMR | Y | Thymoglobulin, plasmapheresis/IVIG | N |
| Converted to q1m | |||||
| NC with MMF | |||||
| 151 | 6 | Normal | Y | Converted to q1m | Y |
m, months; ACR, acute cellular rejection; Oral steroid pulse, 100 mg prednisone PO for 5 days; IV steroid pulse, 10 mg/kg methylprednisolone IV daily for 3 days followed by an oral prednisone taper over 6 weeks; NC, non-compliant; MMF, mycophenolate mofetil; AMR, antibody mediated rejection
HLA antibody testing was performed on all subjects at 6 and 12 months and for cause with allograft biopsies. Per study protocol, we observed increases in PRA in two q1m patients that were not donor-specific, and two q2m subjects, one of which did develop DSA at 6 months (Table 4). Interestingly, the development of de novo DSA in the q2m subject was preceded by an episode of sepsis from a urinary tract infection and was associated with normal renal function and a normal allograft biopsy as detailed above (Table 3). Amongst the patients that had HLA antibody assessments with their allograft biopsies for renal dysfunction, two of the four q2m subjects developed concomitant de novo DSA and one had associated AMR (Table 4). These patients were among those with ACR that had documented non-compliance (Table 3). Of note, the DSA in all three subjects was directed against DQ antigens (Supp. Table 4).
Table 4.
Immunologic outcomes: alloantibodies
| n | q1m | n | q2m | P value | |
|---|---|---|---|---|---|
| Change in PRA | |||||
| 0 months | 82 | - | 81 | - | |
| 6 months | 77 | 0 | 77 | 1 | 0.32 |
| 12 months | 69 | 2 | 75 | 1 | 0.56 |
| Biopsy | 2 | 0 | 4 | 2 | |
| Total | 2 | 4 | 0.41 | ||
| DSA | |||||
| 0 months | 82 | 0 | 81 | 0 | |
| 6 months | 77 | 0 | 77 | 1 | 0.32 |
| 12 months | 69 | 0 | 75 | 0 | - |
| Biopsy | 2 | 0 | 4 | 2 | |
| Total | 0 | 3 | 0.08 |
m, month; PRA, panel reactive antibodies; DSA, donor-specific antibodies
3.4. Safety
The frequency of adverse events was not significantly different between the q1m and q2m groups throughout the course of the study (Table 5). The cumulative incidence of infections, non-melanoma skin cancers, and serious adverse events were similar. No hematologic or solid organ malignancies occurred in either group. Low level CMV and/or BK viremias were detected in 19 q1m and 18 q2m patients and resolved with immunosuppression reduction and/or oral antiviral therapy.
Table 5.
Adverse events
| q1m (n=82) | q2m (n=81) | P value | |||
|---|---|---|---|---|---|
| subjects | events | subjects | events | ||
| Patient deaths, n (%) | 2 (2) | 2 | 0 (0) | 0 | - |
| Graft losses*, n (%) | 0 (0) | 0 | 0 (0) | 0 | - |
| Serious adverse events, n (%) | 10 (12) | 14 | 9 (11) | 10 | 0.40 |
| Infectious | 6 (7) | 8 | 5 (6) | 6 | 0.60 |
| Non-infectious | 4 (5) | 6 | 4 (5) | 4 | 0.53 |
| Adverse events, n (%) | 27 (33) | 34 | 23 (28) | 37 | 0.59 |
| Infectious | 24 (29) | 30 | 19 (23) | 27 | 0.67 |
| Urinary tract | 4 (5) | 5 | 5 (6) | 9 | - |
| Respiratory tract | 15 (18) | 17 | 8 (10) | 9 | - |
| Other infection | 6 (7) | 8 | 8 (10) | 9 | - |
| Malignancy | 1 (1) | 1 | 4 (5) | 5 | 0.09 |
| Skin cancer (nm) | 1 (1) | 1 | 4 (5) | 5 | - |
| Other cancer | 0 (0) | 0 | 0 () | 0 | - |
| Other | 3 (4) | 3 | 4 (5) | 5 | - |
, death-censored; nm, non-melanoma
3.5. CD86 Receptor Occupancy
To evaluate the pharmacodynamics of q2m belatacept relative to q1m dosing, CD86 receptor saturation was measured in healthy controls and random study subjects receiving q1m or q2m belatacept. Every 2-month belatacept achieved peak levels of CD86 RO equivalent to q1m belatacept as measured by a CD86 RO assay (Figure 4), and slight yet statistically significant reduced trough CD86 saturation. However, both dosing frequencies led to significant reductions in the amount of unbound CD86 compared to healthy controls.
Figure 4. CD86 receptor occupancy.
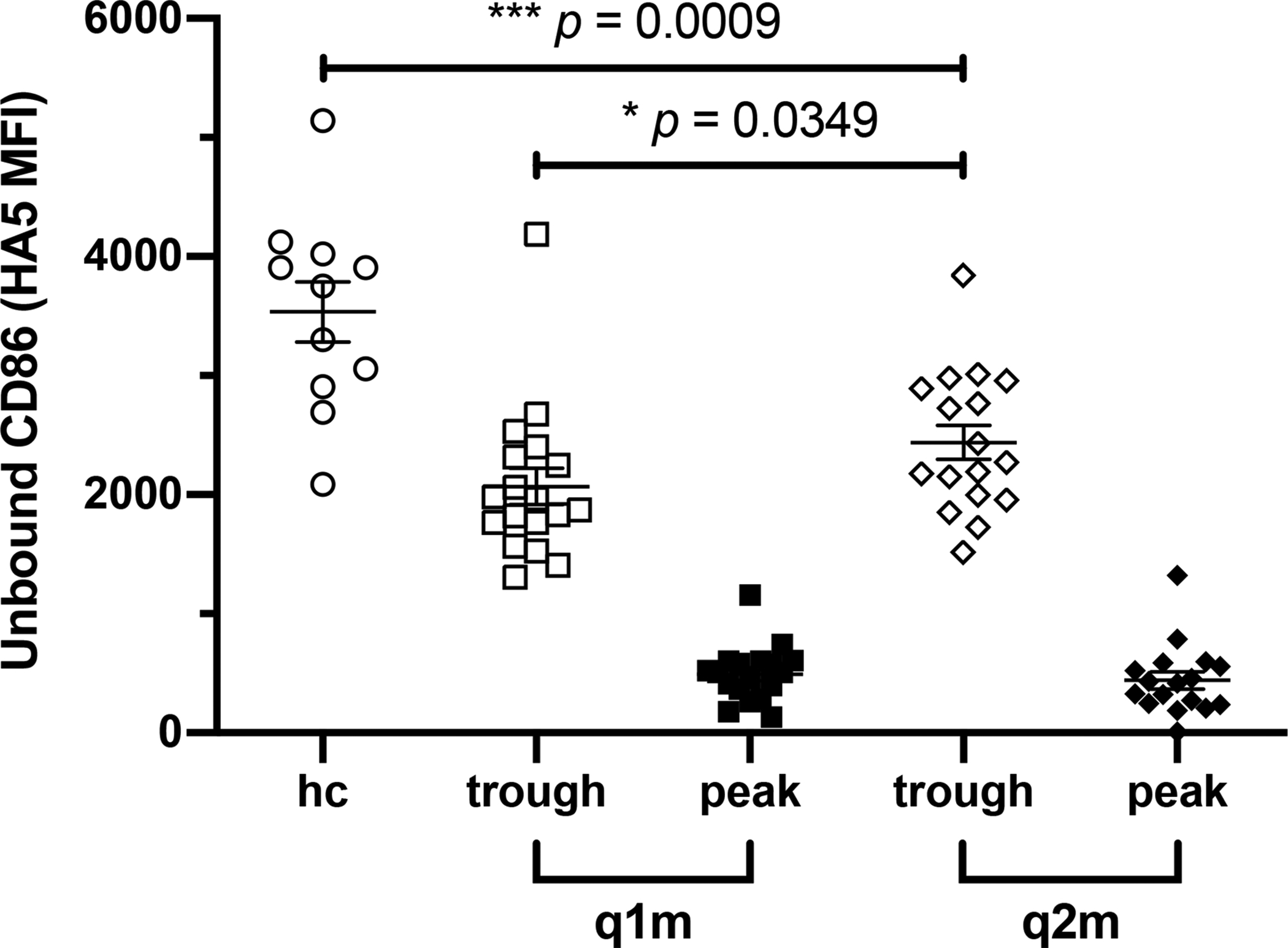
Whole blood was collected from healthy controls (HC), and q1m and q2m belatacept-treated transplant recipients before i.v. infusion (trough) and 30 minutes after infusion (post-dose). Samples were stained with HA5, an anti-CD86 mAb that competes with CTLA-4-Ig for CD86, and the amount of CD86 receptor saturation by belatacept on CD14+ monocytes was measured by HA5 MFI. Summary data represent mean (SE). * p <0.05, *** p <0.001.
4. Discussion
In this randomized controlled trial, we demonstrate that q2m belatacept maintenance dosing is non-inferior to standard q1m administration in kidney transplant recipients as measured by mean eGFR twelve months post-randomization. The difference in renal function between groups adjusted for baseline eGFR did not exceed the pre-specified NIM with similar changes in eGFR over the course of the study. Two-month dosing was safe and well tolerated, with no patient deaths or graft losses. We observed a total of four rejections and 3 cases of de novo DSA. However, only a single episode of rejection and one case of DSA occurred amongst q2m subjects compliant with the study protocol and their immunosuppressive medications.
The biologic composition of belatacept confers a relatively long half-life and underlies its reduced dosing requirement. The CD86 RO assay was developed as a pharmacodynamic measure of costimulation blockade-mediated inhibition of the alloimmune response and has been demonstrated to correlate with serum concentrations of belatacept (15). Consistent with belatacept’s pharmacokinetic properties and results from the phase II trial (9), we observed less frequent q2m dosing to result in slightly reduced CD86 RO compared to monthly dosing, but significantly greater receptor saturation relative to untreated healthy controls (Figure 4). While q1m kinetics reliably achieve target serum concentrations and RO in the induction and early maintenance period (17), less is known regarding optimal dosing goals in stable kidney transplant recipients more than 12 months after transplant. Although trough CD86 saturation was comparatively less, our findings support that q2m belatacept achieves clinically sufficient costimulation blockade to prevent rejection as long-term maintenance therapy.
Our findings extend and validate the observations of the phase II belatacept study. In that trial, study recipients on 8-week belatacept dosing experienced higher acute rejection rates early after transplant but similar renal function over the 10-year follow-up period (9, 12). Given that the vast majority of rejections occurred with the first year post-transplant, we expected to observe few episodes of rejection amongst the low immunologic risk patients enrolled in this study. Although 4 total rejections occurred in the q2m group, 3 of these were observed in subjects with documented non-compliance. Upon questioning, all 3 patients admitted to missing immunosuppression leading up to the episodes of rejection and their medication non-adherence was verified by medication administration and pharmacy fill records. As such, the single rejection that occurred among patients compliant with the q2m study protocol is consistent with the phase II study and anticipated low incidence of rejection after the first transplant year.
The single center trial design, non-compliance associated with rejections and relatively short 12-month follow-up are limitations of our study. Our center adopted a belatacept-based regimen in 2011 that has evolved to consist of transient CNI therapy without depletional induction within the first post-transplant year (7). As a result, Emory has a large single center experience with belatacept along with a unique protocol that differs from more prevalent depletion-based induction and tacrolimus maintenance regimens with “for cause” belatacept conversions later in the transplant course (16, 18). Alternative immunomodulatory exposure up front could impact downstream tolerance of q2m belatacept and result in different outcomes at centers with less costimulation blockade experience. Additionally, 12-month follow-up was feasible but relatively short for this maintenance phase study. Future multicenter studies evaluating q2m belatacept therapy are warranted and should consider protocol biopsies and longer-term follow-up. Nevertheless, the 10-year phase II outcomes albeit limited are encouraging (10).
It is not clear why we observed a disproportionate amount of medication non-adherence and protocol violations in the q2m study arm. It is probable that similar numbers of patients with non-compliance were randomized into both treatment groups as the majority of participant characteristics were well balanced between study arms and medication non-adherence is a well-recognized and common problem in transplantation (19, 20). It is also possible that the nature of this trial offering a chance at immunosuppression reduction and greater patient convenience due to less frequent i.v. infusions may have attracted unbalanced interest from patients with a propensity for non-adherence. We speculate that while adequate and non-inferior to q1m dosing for the majority of patients, q2m belatacept dosing may result in less margin for tolerance of missed immunosuppression and carries a greater risk of rejection in the setting of non-compliance than monthly administration. Therefore, transitioning renal transplant recipients to q2m belatacept as with any immunosuppression reduction strategy should only be performed in carefully screened, reliable and compliant transplant recipients that fully understand the risk of non-adherence. Furthermore, any rejection risk associated with lowering the immunosuppressive burden should also be weighed against the risk of lethal infectious complications, as observed in one patient in the q1m group that died of cryptococcal meningitis.
Alternatively, less frequent q2m dosing may diminish the positive reinforcement stemming from monthly healthcare encounters for infusions. It is also possible that patients with rejection on the q2m regimen were under-immunosuppressed independent of noncompliance, especially in potentially higher risk subjects less far out from transplant mismatched at DQ with their donor (Supp. Tables 3, 4). A better understanding of the pharmacodynamics of q2m dosing in all study subjects, particularly those experiencing immunologic events, is of interest and should be considered in future studies. Additional knowledge gained on CD86 RO may support hybrid approaches such as every 6-week belatacept administration.
The non-inferiority of q2m belatacept in stable, low immunologic risk kidney transplant recipients has significant implications on the future of costimulation blockade in the clinic. Safe reduction in the frequency of belatacept administration has potential to overcome cost, vascular access, patient convenience and logistical barriers to increased belatacept utilization (6). Moreover, maintaining improved renal function with a low risk of rejection on q2m belatacept is one strategy that could be employed to manage transplant recipients with infectious complications in need of immunosuppression reduction, or CMV naïve recipients at risk for inferior outcomes in the event of CMV infection (21). The observed clinical efficacy of q2m belatacept despite reduced CD86 RO supports studying alternative costimulation blockade methods (e.g. subcutaneous abatacept) perceived as less mechanistically potent but potentially more accessible for long-term maintenance therapy (22). While gaining a better understanding of longer-term outcomes and immunologic risk of a q2m belatacept maintenance strategy will require larger, multicenter studies, reducing belatacept dosing to enhance its long-term use promises to help continue to improve long-term outcomes in kidney transplantation.
Supplementary Material
Acknowledgments
This study was funded by the Carlos and Marguerite Mason Trust and the James C. Kennedy and James M. Cox Foundation. The authors would like to acknowledge the Georgia Clinical & Translational Science Alliance (CTSA) for assistance with trial design and statistical analysis. The CTSA is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378.
Abbreviations:
- ACR
acute cellular rejection
- ANCOVA
analysis of covariance
- CNI
calcineurin inhibitor
- DSA
donor-specific antibody
- DSMB
data and safety monitoring board
- eGFR
estimated glomerular filtration rate
- ETC
Emory Transplant Center
- ITT
intention to treat
- NIM
non-inferiority margin
- RO
receptor occupancy
- SAB
single antigen bead
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Data availability statement
No shared data have been utilized in this manuscript and the data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Evans RW, Manninen DL, Garrison LP Jr., Hart LG, Blagg CR, Gutman RA et al. The quality of life of patients with end-stage renal disease. N Engl J Med 1985;312(9):553–559. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999;341(23):1725–1730. [DOI] [PubMed] [Google Scholar]
- 3.Lodhi SA, Lamb KE, Meier-Kriesche HU. Solid organ allograft survival improvement in the United States: the long-term does not mirror the dramatic short-term success. Am J Transplant 2011;11(6):1226–1235. [DOI] [PubMed] [Google Scholar]
- 4.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med 2016;374(4):333–343. [DOI] [PubMed] [Google Scholar]
- 5.Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Castro S et al. OPTN/SRTR 2017 Annual Data Report: Kidney. Am J Transplant 2019;19Suppl 2:19–123. [DOI] [PubMed] [Google Scholar]
- 6.Heher E, Markmann JF. The Clearer BENEFITS of Belatacept. N Engl J Med 2016;374(4):388–389. [DOI] [PubMed] [Google Scholar]
- 7.Adams AB, Goldstein J, Garrett C, Zhang R, Patzer RE, Newell KA et al. Belatacept Combined With Transient Calcineurin Inhibitor Therapy Prevents Rejection and Promotes Improved Long-Term Renal Allograft Function. Am J Transplant 2017;17(11):2922–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodle ES, Kaufman DB, Shields AR, Leone J, Matas A, Wiseman A et al. Belatacept-based immunosuppression with simultaneous calcineurin inhibitor avoidance and early corticosteroid withdrawal: A prospective, randomized multicenter trial. Am J Transplant 2020;20(4):1039–1055. [DOI] [PubMed] [Google Scholar]
- 9.Vincenti F, Blancho G, Durrbach A, Friend P, Grinyo J, Halloran PF et al. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol 2010;21(9):1587–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincenti F, Blancho G, Durrbach A, Grannas G, Grinyo J, Meier-Kriesche HU et al. Ten-year outcomes in a randomized phase II study of kidney transplant recipients administered belatacept 4-weekly or 8-weekly. Am J Transplant 2017;17(12):3219–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med 2005;353(8):770–781. [DOI] [PubMed] [Google Scholar]
- 12.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 2010;10(3):535–546. [DOI] [PubMed] [Google Scholar]
- 13.Roufosse C, Simmonds N, Clahsen-van Groningen M, Haas M, Henriksen KJ, Horsfield C et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation 2018;102(11):1795–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Common Terminology Criteria for Adverse Events (CTCAE). 2020March27, 2020 [cited 2020 Aug 26]; v5.0:[Available from: https://ctep.cancer.gov
- 15.Latek R, Fleener C, Lamian V, Kulbokas E 3rd, Davis PM, Suchard SJ et al. Assessment of belatacept-mediated costimulation blockade through evaluation of CD80/86-receptor saturation. Transplantation 2009;87(6):926–933. [DOI] [PubMed] [Google Scholar]
- 16.Rostaing L, Massari P, Garcia VD, Mancilla-Urrea E, Nainan G, del Carmen Rial M et al. Switching from calcineurin inhibitor-based regimens to a belatacept-based regimen in renal transplant recipients: a randomized phase II study. Clin J Am Soc Nephrol 2011;6(2):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen J, Townsend R, You X, Shen Y, Zhan P, Zhou Z et al. Pharmacokinetics, pharmacodynamics, and immunogenicity of belatacept in adult kidney transplant recipients. Clin Drug Investig 2014;34(2):117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darres A, Ulloa C, Brakemeier S, Garrouste C, Bestard O, Del Bello A et al. Conversion to Belatacept in Maintenance Kidney Transplant Patients: A Retrospective Multicenter European Study. Transplantation 2018;102(9):1545–1552. [DOI] [PubMed] [Google Scholar]
- 19.Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation 2004;77(5):769–776. [DOI] [PubMed] [Google Scholar]
- 20.Denhaerynck K, Dobbels F, Cleemput I, Desmyttere A, Schafer-Keller P, Schaub S et al. Prevalence, consequences, and determinants of nonadherence in adult renal transplant patients: a literature review. Transpl Int 2005;18(10):1121–1133. [DOI] [PubMed] [Google Scholar]
- 21.Karadkhele G, Hogan J, Magua W, Zhang W, Badell IR, Mehta A et al. CMV high-risk status and posttransplant outcomes in kidney transplant recipients treated with belatacept. Am J Transplant 2021;21(1):208–221. [DOI] [PubMed] [Google Scholar]
- 22.Badell IR, Karadkhele GM, Vasanth P, Farris AB 3rd, Robertson JM, Larsen CP. Abatacept as rescue immunosuppression after calcineurin inhibitor treatment failure in renal transplantation. Am J Transplant 2019;19(8):2342–2349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


