Abstract
Background
Superficial venous thrombosis (SVT) is a common clinical problem across various treatment settings. SVT shares risk factors with deep venous thrombosis (DVT) and carries a risk of thromboembolic complications, greater than previously expected. Little is known about the pathophysiology, resolution and recurrence of this disease.
Objectives
The objective of the present study was to describe the natural course of SVT, and factors correlated with the progression or resolution of the thrombus.
Methods
We included 218 patients with a recent diagnosis of SVT that were consecutively referred to a thrombosis clinic from the Emergency Department (ED) between January 2016 and April of 2018.
Results
The resolution of the thrombus prior to discharge was correlated to gender (female 73.8% vs. male 57.5%, p = 0.015), presence of varicose veins (62.4% vs. 46.4, p = 0.026), absence of family or personal history of thrombosis (98% vs. 91.3%, p = 0.021). The factor most correlated to thrombus resolution prior to discharge was the result of the 2nd ultrasound (improvement 83.9% vs. 16.1%, p < 0.001) immediately after initiation of heparin treatment. In the multivariate analysis, a high thrombus burden in the early follow-up ultrasound was the most significant predictive variable with prior to discharge recanalization (B = 20.9, 95% CI 9.8–44.7; p < 0.001).
Conclusion
The follow-up of SVT with duplex lower extremity ultrasound allows us to monitor the evolution and early identify residual thrombosis, as a marker of hypercoagulability and recurrence. This study offers new perspectives for future research, necessary to improve the management of this disease, to reduce long-term complications.
Keywords: Ultrasound (US), Venous thromboembolism (VTE), Deep vein thrombosis (DVT), Superficial vein thrombosis (SVT), Duplex lower extremity ultrasound
Introduction
Venous thromboembolism (VTE) includes deep vein thrombosis (DVT), superficial vein thrombosis (SVT) and pulmonary embolism (PE).
Superficial vein thrombosis (SVT, Fig. 1), also known as phlebitis or thrombophlebitis, usually affects varicose veins (enlarged superficial veins beneath the skin) of the lower extremities, is a common clinical problem across various treatment settings. Although it has traditionally been considered a benign disorder, it is currently known that shares risk factors with DVT and risk of thromboembolic complications (OR for DVT 6.3, OR for PE 3.9) [1]. According to previous studies, SVT has been reported to be simultaneous with DVT in up to 25% and 5% of PE [2]. The isolated presence of a SVT carries a risk of DVT of 4–8.3% [3, 4]. Therefore, it is considered, as part of the VTE disease, and its management must go beyond the mere symptomatic resolution, but oriented in the monitoring and prevention of VTE complications [5], such as DVT, PE or post-thrombotic syndrome.
Fig. 1.
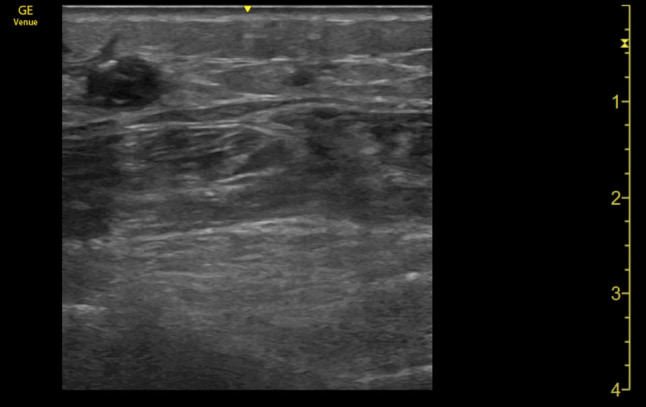
Linear probe. Superficial thrombosis (above muscular fascia) of the great saphenous vein with internal echoes associated with increased echogenicity within the vein and failure to compress
The thrombus, in its recanalization process undergoes a fragmentation that begins at the margins. At the same time, in the center of the lumen, pores are formed, which allow their subsequent communication and recanalization [5, 6].
In clinical practice there is a wide range of treatments for SVT (including oral anticoagulants, low molecular weight heparins, oral and topical non-steroidal anti-inflammatory drugs, compression stockings), but there is little evidence on the efficacy of each therapy in the recanalization process of the thrombus. Duplex lower extremity ultrasound is a useful tool to study changes in the venous system, since it is useful to locate the thrombus, measuring change over time, as well as identify recanalization.
Identifying factors that predict a higher risk of progression or lack of resolution of SVT would help identify a subgroup of patients who would benefit from a closer monitoring or more aggressive therapy. To better clarify the pathophysiology of the disease, we decided to study the evolution of occluded veins after the acute phase of thrombosis. The objective of this study was to follow the natural course of SVT, as well as to know the factors correlated to the progression or resolution of the thrombus.
Patients and methods
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local Research Ethics Committee (PI-3306). The Ethics Committee waived the need to obtain informed consent for the collection, analysis and publication of the retrospectively obtained and anonymized data for this non-interventional study.
Study design
In this observational, retrospective and single-center study, the aim was to assess the baseline characteristics and evolution of the thrombus of patients with a recent diagnosis of SVT that were consecutively referred to a thrombosis clinic from the Emergency Department (ED) between January 2016 and April 2018. In total, 218 patients were included in this study.
Patient selection
All patients who went to the ED and were diagnosed with an acute SVT were recruited and referred to a thrombosis clinic. For this purpose, they had to present an acute symptomatic SVT (< 7 days after the onset of symptoms), confirmed by duplex lower extremity ultrasound (either in the ED or in the first follow-up ultrasound in the clinic). Those who did not have sonographic confirmation of SVT were excluded, as well as patients with contraindication for anticoagulant therapy.
They were scheduled for a follow-up at day 15 and 45 from diagnosis in the ED, in case of isolated SVT. In those patients, where a synchronous DVT was identified, the sapheno-femoral or popliteal junction were affected, a monthly follow-up was scheduled, at least during 3 months.
Initial patient assessment
Demographic data (age, sex, weight), medical history (comorbidities, medications), risk factors for VTE (previous personal or family history of VTE, pregnancy/puerperium, oral contraceptives, hormone replacement therapy, autoimmune disease, recent surgery—last 3 months—, recent immobilization, thrombophilia—lupus anticoagulant, beta2 glycoprotein, anticardiolipin, factor V Leiden, prothrombin gene mutation, antithrombin deficiency, protein C, protein S and methyl tetrahydrofolate reductase -, malignancy—active or in remission—, obesity—the WHO definition was used, that is, obesity is defined as a BMI > 30, venous insufficiency, active chemotherapy—, imaging tests performed (duplex lower extremity ultrasound performed in the ED, during follow-up and prior to discharge; other radiology exams), physical exam, laboratory tests (creatinine, urea, hemoglobin, white blood cells, platelets, D-dimer, INR), variables correlated to therapy (type of anticoagulation, dose, duration), as well as variables correlated to follow-up (end of symptoms, destination and date of discharge).
Ultrasound data collection
Radiologists in the department of radiology performed initial whole-leg US examination in patients with the suspicion of having a SVT. A senior radiologist with more than 10 years of experience performed this exam. The follow up US was performed by an internist from the thrombosis clinic with long-standing experience in vascular US (more than 5 years). In both cases, a whole-leg US protocol was performed, and the following veins were scanned transversally over their entire length: common femoral vein, femoral vein, popliteal veins, anterior and posterior tibial veins, peroneal veins, medial and lateral gastrocnemius veins, soleal veins, the saphenofemoral/popliteal junctions, the trunk of the great saphenous vein (GSV) and small saphenous vein (SSV), and where thrombosis was suspected based on physical exam (pain and swelling). The initial study was performed using a Phillips iU22 ultrasound machine, with a linear transducer (5–17 MHz) (Phillips España, Madrid, Spain) and the follow up study by a GE LOGIQ-e ultrasound system, with a linear transducer (5–10 MHz) (General Electrics Healthcare, Madrid, Spain). Which demonstrates both exams were performed by equally experienced physicians, followed same protocol and machines were of similar quality.
The diagnosis of venous thrombosis was confirmed by the presence of a hypoechoic area (non-compressible or partially compressible) in the course of an identified vein.
Outcome measures and definitions
Our objective was to know the evolution of superficial venous thrombosis (SVT) in a thrombosis clinic of patients referred from the ED. The risk factors associated with the extension or resolution of thrombosis were assessed, as well as the presence of residual venous thrombosis. Partial sonographic improvement was defined as the decrease of the size of the thrombus or vein caliber, partially responding to compression. The presence of flow within the thrombus of a previous non-occlusive vein or compressibility > 50% was considered as recanalization. Baseline and follow-up studies were compared by report.
With all this, we intended to identify areas of improvement in the care of patients with VTE, specifically, with SVT.
Statistical analysis
Baseline characteristics are presented as mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables and count and proportions for categorical variables. For group comparisons, we used t-test for continuous variables and the Chi-square or Fisher exact test for categorical one. Variables with non-normal distribution were log-transformed before analyzing their differences. To analyze the relationships between ultrasound evolution and other clinical or analytical parameters, a multivariate regression analysis was performed, in which it was adjusted for predictive variables. Statistical significance was established at p < 0.05. Mean values were reported along with 95% confidence intervals computed using bootstrap resampling (1000 repetitions). Statistical significance was set at p value < 0.05. Statistical analyses were conducted with IBM SPSS software v20.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 218 patients admitted to the ED with a final diagnosis of SVT were included. The baseline characteristics are shown in Table 1. More than half of the patients were female (66.5%, 145 patients). The mean age (SD) was 62.6 (16.4) years. The most common risk factors for SVT were the presence of varicose veins (57.3%, 125 patients), BMI ≥ 30 kg/m2 (25.2%, 55 patients), personal history of VTE (22%, 48 patients) and malignancy (13.8%, 30 patients). 17% had immobilization or surgery prior to the thrombosis episode. 7.8% of the patients were on antiplatelet therapy at the time of inclusion and 5 patients were with anticoagulant therapy. The average D-dimer was 3124.5 ng/mL (SD 5034.4). The most affected vein was the great saphenous vein (GSV), in any of its segments (171 patients, 85.5%). 7.3% had a synchronous affected SVT, and 4.1% DVT (see Table 2). 7 patients (3.2%) had a negative ultrasound exam in the ED.
Table 1.
Demographic and clinical characteristics of the patients included
| Demographic characteristics (N = 218) | N (%) |
|---|---|
| Gender, woman (%) | 145 (66.5) |
| Age, years (SD) | 62.6 (16.4) |
| Weight, kg (SD) | 81.5 (9.5) |
| Therapy with LMWH (days) mean (SD) | 61.1 (49.2) |
| Therapy with Acenocoumarol (days) mean (SD) | 103 (53.0) |
| Interval between first and last US exam, days (SD) | 58.3 (43.3) |
| Laboratory parameters (SD) | |
| Hb, g/dL (SD) | 14.1 (1.5) |
| WBC, × 109/L (SD) | 8.7 (9.5) |
| Platelets, × 109/L (SD) | 243.7 (81.3) |
| PT (s) | 11.6 (3.0) |
| INR (SD) | 1.0 (0.2) |
| D-dimer, ng/mL (SD) | 3124.5 (5034.4) |
| aPTT, s. (SD) | 24.4 (4.3) |
| Creatinine, mg/dL (SD) | 1.5 (5.3) |
| Urea (mg/dL) | 40.6 (18.7) |
| Risk factors for VTE | |
| Obesity | 55 (25.2) |
| Varicose veins | 125 (57.3) |
| Malignancy (total) | 30 (13.8) |
| Cancer remission | 11 (5.1) |
| Active malignancy | 19 (8.7) |
| Malignancy type | |
| Breast | 11 (5) |
| Ovary/uterine | 3 (1.4) |
| Urinary Tract | 8 (3.7) |
| Hematologic | 3 (1.4) |
| CNS | 1 (0.5) |
| GI tract | 2 (0.9) |
| ENT/lung | 1 (0.5) |
| Skin | 1 (0.5) |
| Active Chemotherapy | 11 (5) |
| Personal history of VTE | 48 (22) |
| SVT | 30 (13.8) |
| DVT | 8 (3.7) |
| SVT + DVT | 5 (2.3) |
| PE | 2 (0.9) |
| PE + SVT/DVT | 3 (1.4) |
| Family history of VTE | 9 (4.1) |
| Pregnancy or puerperium | 9 (4.1) |
| Oral contraception | 6 (2.8) |
| Hormone replacement therapy | 1 (0.5) |
| Systemic autoimmune disease | 9 (4.1) |
| Recent Surgery | 15 (6.9) |
| Recent immobilization | 22 (10.1) |
| Positive thrombophilia | 20 (9.2) |
| Negative thrombophilia | 13 (6.0) |
| Medication at inclusion | |
| Antiaggregant at inclusion | 17 (7.8) |
| ASA | 14 (6.4) |
| Clopidogrel | 2 (0.9) |
| Double antiaggregation | 1 (0.5) |
| Anticoagulation at inclusion | 5 (2.2) |
| Acenocoumarol | 3 (1.4) |
| LMWH (anticoagulant) | 1 (0.5) |
| Dabigatran | 1 (0.5) |
| Type of LMWH | 206 (94.5) |
| Bemiparin | 77 (35.3) |
| Enoxaparin | 129 (59.2) |
| Dosage of LMWH | 206 (94.5) |
| Prophylaxis | 121 (58.7) |
| Therapeutic | 85 (41.3) |
aPTT activated partial thromboplastin time; ASA acetylsalicylic acid; CNS central nervous system; DVT deep vein thrombosis; ENT Ear, Nose and Throat; GI gastrointestinal; INR International normalized ratio; LMWH low molecular weight heparin; PE pulmonary embolism; PT prothrombin time; SD standard deviation; SVT superficial vein thrombosis; US ultrasound; VTE venous thromboembolism; WBC white blood cells
Table 2.
Result of the first, second and third (prior to discharge) ultrasound exam of the lower extremity venous system
| Ultrasound results (N = 218) | 1st US exam—N (%) | 2nd US Exam—N (%) | 3rd US Exam—N (%) |
|---|---|---|---|
| Negative US in the ED | 7 (3.2) | – | – |
| SVT | 211 (96.8) | 114 (52.3) | – |
| Tributaries of the GSV | 35 (16.1) | 36 (16.5) | – |
| Tributaries of the SSV | 12 (5.5) | 6 (2.8) | – |
| Distal GSV | 14 (6.4) | 11 (5.0) | – |
| Distal SSV | 4 (1.8) | 3 (1.4) | – |
| Medial 1/3 of GSV | 1 (0.5) | 1 (0.5) | – |
| Medial 1/3 of SSV | 1 (0.5) | 1 (0.5) | – |
| Proximal 1/3 of GSV | 10 (4.6) | 3 (1.4) | – |
| Proximal 1/3 of SSV | 4 (1.8) | 4 (1.8) | – |
| Saphenofemoral junction | 24 (11.0) | 8 (3.7) | – |
| Saphenopopliteal junction | 1 (0.5) | 1 (0.5) | – |
| Complete GSV | 3 (1.4) | 3 (1.4) | – |
| Complete SSV | 0 (0) | 0 (0) | – |
| Non-specified extension of GSV | 72 (33.0) | 24 (11.0) | – |
| Non-specified extension of SSV | 19 (8.7) | 11 (5.0) | – |
| Non-specified SVT | 11 (5.0) | 2 (0.9) | – |
| Synchronous SVT in different location | 16 (7.3) | 14 (6.4) | |
| Medial 1/3 of GSV | 4 (1.8) | 3 (0.5) | – |
| Proximal 1/3 of SSV | 1 (0.5) | 1 (0.5) | – |
| Complete GSV | 1 (0.5) | 1 (0.5) | – |
| Non-specified extension of SSV | 10 (4.6) | 1 (0.5) | – |
| No other SVT | 202 (92.7) | 1 (0.5) | – |
| Synchronous DVT | 9 (4.1) | 12 (4.6) | |
| Proximal DVT | 4 (1.8) | 3 (1.4) | – |
| Distal DVT | 3 (1.4) | 7 (3.2) | – |
| Proximal + distal DVT | 2 (0.9) | 2 (0.9) | – |
| Differences between 1st and 2nd US | |||
| Without change | – | 55 (25.2) | – |
| Resolution | – | 88 (40.4) | – |
| Partial improvement | – | 40 (18.3) | – |
| New SVT | – | 9 (4.1) | – |
| Progression of affected territory | – | 3 (1.4) | – |
| New proximal DVT | – | 2 (0.9) | – |
| New distal DVT | – | 5 (2.3) | – |
| US prior to discharge | |||
| Without change | – | – | 13 (6.0) |
| Resolution | – | – | 149 (68.3) |
| Residual thrombus | – | – | 56 (25.7) |
DVT deep vein thrombosis; GSV great saphenous vein; SSV small saphenous vein; SVT superficial vein thrombosis; US ultrasound, VTE venous thromboembolism
Of the 202 patients that had an early follow-up ultrasound (day 14), 128 patients (58.7%) presented a partial improvement or resolution of the thrombus. 74 patients presented worsening or lack of improvement on ultrasound (See Table 2). Prior to discharge from the clinic, an ultrasound exam was repeated (Table 2), with a resolution of the thrombus observed in 149 patients (68.3%).
The reduction in the thrombus size in the 2nd ultrasound (14 days) was greater in non-obese patients (72.7% vs. 27.3%, p = 0.071), although it did not reach statistical significance. Those who had a history of VTE had a less favorable evolution (15.6 vs. 84.4%, p = 0.001).
The resolution of the thrombus on prior to discharge ultrasound was correlated with gender (female 73.8% vs. male 57.5%, p = 0.015), presence of varicose veins (62.4% vs. 46.4, p = 0.026), absence of a personal history of VTE (98% vs. 91.3%, p = 0.021). The factor most correlated to thrombus resolution prior to discharge was the result of the 2nd ultrasound (improvement 83.9% vs. 16.1%, p < 0.001) immediately after initiation of heparin treatment. Although a trend to recanalization was observed in tributaries and distal vein thrombosis, it did not reach statistical significance (p = 0.054). In the multivariate analysis, after adjustment for age, sex, obesity and location of the SVT, we observed that a high thrombus burden in the early follow-up ultrasound exam was the most significant predictive variable with prior to discharge recanalization (B = 20.9, 95% CI 9.8–44.7; p < 0.001).
A D-dimer value > 1000 ng/mL at diagnosis was correlated to the presence of residual thrombosis prior to discharge (52.5% vs. 30.3%, p = 0.039).
No relationship was found between the evolution of the thrombus with previous antiaggregant (p = 0.379) or anticoagulant (p = 0.571) therapy. After the beginning of low molecular weight heparin (LMWH), 24 patients (11%) required a change of the dosage according to the drug data sheet. Those patients in whom the LMWH was modified from therapy or prophylaxis doses (12.5% vs. 71.4%, p < 0.001), or a change to acenocoumarol or rivaroxaban (35.3% vs. 71.5%, p = 0.002) achieved a lesser extent recanalization.
Correlations were made between the continuous variables (age, weight, laboratory tests, days of treatment), without obtaining significant results.
Discussion
In the diagnosis of SVT, we know that physical exam is not specific; we might find classic signs such as edema, erythema and pain in many other diseases [7, 8]. In addition, there is a poor correlation between physical exam and thrombus extension [7, 8], which is a key element in the management of this pathology. Another pillar of the diagnosis, the D-dimer, which is a product of clot degradation, widely used in the evaluation of DVT and PE, is of little clinical relevance for the diagnosis of SVT [9]. All these reflect the diagnosis of this pathology can be challenging.
For more than 20 years, duplex lower extremity ultrasound has proven to be a tool that allows us to diagnose SVT with high sensitivity and specificity [10]. However, its widespread use in this pathology remains controversial [11], not being considered by many, a cost-efficient technique [12].
In the POST study (prospective observational superficial thrombophlebitis study) [13], 844 patients diagnosed with symptomatic SVT were followed, performing a full venous examination by duplex lower extremity ultrasound, finding that synchronous DVT was present in 23.5% [13]. In addition, after the initial diagnosis of a SVT, especially in the thigh region, it is advisable to perform an ultrasound follow-up to objectify the progression of the disease after the beginning treatment, which would justify switch to a more aggressive treatment plan [14].
In a previous study of our group [15], we showed that in the ultrasound follow-up of patients with suspected DVT or SVT, with a negative initial study, it allowed us to diagnose a SVT in 11.1% of patients.
This would justify that duplex lower extremity ultrasound should be the initial diagnostic technique of choice in suspected SVT, as well as a tool for monitoring the response to treatment.
According to previous studies, the recanalization of SVT is a prolonged process over time, with recanalization being observed in less than 50% of the patients at the end of the year [5], being even lower than the recanalization rate of DVT [16, 17].
There are different factors that correlate with the duration of the recanalization process. Similar to previous studies, our data showed that the evolution towards recanalization was more frequent in women, obese and patients with varicose veins, which could mean that they also tend to have less thrombus burden, since there is a more localized exogenous factor for its appearance, against endogenous causes, such as patients with thrombophilia or systemic autoimmune disease [5, 18].
In our study, partial improvement or resolution of the thrombus with signs of recanalization in a short period of time (14 days), immediately after therapy initiation, occurred in 58.3% of the patients, which proved to be the most significant predictor of recanalization on prior to discharge ultrasound.
The optimal treatment of SVT remains poorly defined, especially treatment to prevent or treat recurrence [19]. In our study, it is striking that there is a great variability in the management of this pathology, even in the same department, requiring at the time of the first visit to the thrombosis clinic, an adjustment of the treatment in 24 patients. However, according to our data, the dose and type of heparin or other anticoagulants, as well as the previous use of antiaggregant, does not seem to significantly influence thrombus resolution. These data would favor previous studies in which the recanalization of thrombosis correlates to the endogenous fibrinolysis system itself, playing a key role, beyond the treatment regimen itself [5, 17].
As far as we know, this is the first study that evaluates the potential benefit of ultrasound monitoring performed in a thrombosis clinic of patients with acute SVT referred from the ED. We believe that the evolution of the thrombus could be used in the future as a guide, to individualize the treatment plan and as a monitoring tool of the patient evolution. Thus, finding a residual thrombus after stopping anticoagulant treatment or a high D-dimer would indicate that the thrombus has not resolved completely. This could allow us to identify a subgroup of patients with a higher risk of recurrence. In addition, many authors support the idea that residual thrombosis, like elevated D-dimer [7], should be considered as a marker of hypercoagulability.
Among the strengths of our study are that all DVT or SVT were confirmed according to a rigorous sonographic protocol. The D-dimer test we used has high reproducibility and a negative predictive value in clinically symptomatic patients. In addition, only experienced physicians performed the initial and follow-up ultrasound study.
Limitations
There are several limitations to our study. We are aware of the possible reference bias; primary care physicians might have limited the patients they referred to the ED to those at higher risk of complicated DVT or SVT. However, we believe that this risk, although real, resembles our clinical practice, limited by easy access to emergency duplex lower extremity ultrasound. Another limitation is that the initial and follow-up ultrasound exam were performed by different physicians, on different machines and conditions. Although the impact of this limitation is reduced, since both groups had a similar experience, they followed the same protocol and the machines had a similar quality.
The small number of subjects and the single-center study design would limit external validity. Therefore, we believe that this study could be useful for the design of future multicenter studies, which could change our clinical practice.
Conclusion
The follow-up of acute SVT with duplex lower extremity ultrasound allows us to monitor the evolution and identify residual thrombosis early, as a marker of hypercoagulability and recurrence. This study offers new perspectives for future research, necessary to improve the management of this disease, thus reducing long-term complications.
Acknowledgements
All authors read and approved the final manuscript. This work has not been supported by public grants or financial support. No sources of funding were used to assist in the preparation of this study. Each author certifies that he has no commercial associations that might pose a conflict of interest in connection with the submitted article. We certify that the reporting of this case was conducted in conformity with ethical principles of our institution. Our tables have not been previously published and reproduced from another source.
Author contributions
Conception and design: YTC, ARN, AMV. Analysis and interpretation: YTC, ARN, AMV, CFC. Data collection: YTC, IPS. Writing the article: YTC, IPS. Critical revision of the article: YTC, ARN, AMV, CFC, ALH, TSB, GS. Final approval of the article: YTC, ARN, AMV, IPS, CFC, ALH, TSB, GS. Statistical analysis: YTC. Overall responsibility: YTC, ARN, AMV.
Compliance with ethical standards
Conflict of interest
The authors have declared no conflicts of interest.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Research Ethics Committee of our University Hospital.
Informed consent
Written informed consent was obtained from each enrolled patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van Langevelde K, Lijfering WM, Rosendaal FR, Cannegieter SC. Increased risk of venous thrombosis in persons with clinically diagnosed superficial vein thrombosis: results from the MEGA study. Blood. 2011;118(15):4239–4241. doi: 10.1182/blood-2011-05-356071. [DOI] [PubMed] [Google Scholar]
- 2.Frappé P, Buchmuller-Cordier A, Bertoletti L, Bonithon-Kopp C, Couzan S, Lafond P, et al. Annual diagnosis rate of superficial vein thrombosis of the lower extremities: the STEPH community-based study. J Thromb Haemost. 2014;12(6):831–838. doi: 10.1111/jth.12575. [DOI] [PubMed] [Google Scholar]
- 3.Dewar C, Panpher S. Incidence of deep vein thrombosis in patients diagnosed with superficial thrombophlebitis after presenting to an emergency department outpatient deep vein thrombosis service. Emerg Med J EMJ. 2010;27(10):758–761. doi: 10.1136/emj.2009.079517. [DOI] [PubMed] [Google Scholar]
- 4.Decousus H. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med. 2010;152(4):218. doi: 10.7326/0003-4819-152-4-201002160-00006. [DOI] [PubMed] [Google Scholar]
- 5.Spirkoska A, Jezovnik MK, Poredos P. Time course and the recanalization rate of superficial vein thrombosis treated with low-molecular-weight heparin. Angiology. 2015;66(4):381–386. doi: 10.1177/0003319714533183. [DOI] [PubMed] [Google Scholar]
- 6.Sevitt S. The mechanisms of canalisation in deep vein thrombosis. J Pathol. 1973;110(2):153–165. doi: 10.1002/path.1711100207. [DOI] [PubMed] [Google Scholar]
- 7.Scovell S. Phlebitis and thrombosis of the superficial lower extremity veins. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. https://www.uptodate.com Accessed 18 Dec 2019
- 8.Becciolini M, Galletti S, Vallone G, et al. Sonographic diagnosis of clinically unsuspected thrombosis of the medial marginal vein and dorsal arch of the foot. J Ultrasound. 2020 doi: 10.1007/s40477-019-00421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillet J-L, Ffrench P, Hanss M, Allaert F-A, Chleir F. Predictive value of D-dimer assay in superficial thrombophlebitis of the lower extremities. J Mal Vasc. 2007;32(2):90–95. doi: 10.1016/j.jmv.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 10.Lutter KS, Kerr TM, Roedersheimer LR, Lohr JM, Sampson MG, Cranley JJ. Superficial thrombophlebitis diagnosed by duplex scanning. Surgery. 1991;110(1):42–46. [PubMed] [Google Scholar]
- 11.ACCF/ACR/AIUM/ASE/IAC/SCAI/SCVS/SIR/SVM/SVS/SVU Appropriate use criteria for peripheral vascular ultrasound and physiological testing. Part II: Testing for venous disease and evaluation of hemodialysis access. Vasc Med Lond Engl. 2013;18(4):215–231. doi: 10.1177/1358863X13497637. [DOI] [PubMed] [Google Scholar]
- 12.Quenet S, Laroche J-P, Bertoletti L, Quere I, Decousus H, Becker F, et al. Value of a planned compression ultrasonography after an isolated superficial vein thrombosis: results from a prospective multicentre study. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2012;43(2):233–237. doi: 10.1016/j.ejvs.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Quere I, Leizorovicz A, Galanaud J-P, Presles E, Barrellier M-T, Becker F, et al. Superficial venous thrombosis and compression ultrasound imaging. J Vasc Surg. 2012;56(4):1032–1038.e1. doi: 10.1016/j.jvs.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Wichers IM, Haighton M, Buller HR, Middeldorp S. A retrospective analysis of patients treated for superficial vein thrombosis. Neth J Med. 2008;66(10):423–427. [PubMed] [Google Scholar]
- 15.Tung-Chen Y, Pizarro I, Rivera-Núñez A, Martínez-Virto A, Lorenzo-Hernández A, Sancho-Bueso T, et al. Reaffirmation of the importance of follow-up ultrasound studies in patients with high D-dimers and clinical suspicion of vein thrombosis. Ultrasound. 2019 doi: 10.1177/1742271X19865000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jezovnik MK, Poredos P. Factors influencing the recanalisation rate of deep venous thrombosis. Int Angiol J Int Union Angiol. 2012;31(2):169–175. [PubMed] [Google Scholar]
- 17.Poredos P, Spirkoska A, Jezovnik MK. In patients with superficial vein thrombosis the inflammatory response is increased and related to the recanalization rate. Arch Med Sci AMS. 2019;15(2):393–401. doi: 10.5114/aoms.2019.83292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiezia L, Tormene D, Pesavento R, Salmaso L, Simioni P, Prandoni P. Thrombophilia as a predictor of persistent residual vein thrombosis. Haematologica. 2008;93(3):479–480. doi: 10.3324/haematol.12205. [DOI] [PubMed] [Google Scholar]
- 19.Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg Cochrane Vascular Group, editor. Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858.CD004982.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]


