Abstract
Dunbar syndrome, also known as median arcuate ligament syndrome, is a rare clinical condition due to the external compression of the celiac trunk by the median arcuate ligament causing abdominal angina. We report a case of Dunbar syndrome and its borderline imaging findings focused on the crucial diagnostic role of color-Doppler ultrasound. We also reviewed the current literature, delineating the clinical manifestations and the diagnostic workup of the Dunbar syndrome with the objective to increase the knowledge of this clinical entity as a cause of postprandial abdominal pain and to underline the pivotal role of color-Doppler ultrasound to avoid incorrect or delayed diagnosis.
Keywords: Celiac trunk compression, Median arcuate ligament, Dunbar syndrome, Color-Doppler ultrasound
Introduction
Dunbar syndrome, also known as median arcuate ligament syndrome, is a rare clinical condition due to the external compression of the celiac trunk by the median arcuate ligament [1, 2]. The syndrome was first described in a case report by Harjola in 1963, and then in a case series by Dunbar et al. in 1965 [1–4]. This syndrome affects about 2/100,000 patients, especially women (female to male ratio is 3:1), aged between 18 and 30 years, who clinically present with postprandial epigastric pain (80%), nausea (9.7%), weight loss (48%), and diarrhea (7.5%) [5, 6]. The median arcuate ligament is a fibrous arch that connects the two cruras of the diaphragm at the 1st lumbar vertebra forming the anterior part of the aortic hiatus, through which the aorta, thoracic duct and azygos vein pass [7, 8]. Generally, this ligament lies superior to the origin of celiac artery; however, in some cases, it may have a lower localization, crossing over the proximal portion of the celiac axis [9]. The abnormally low-lying ligament may cause celiac trunk compression, potentially resulting in clinical manifestations, especially abdominal angina [1, 2, 7].
The diagnosis of Dunbar syndrome is made after exclusion of other diseases causing similar symptoms, such as cholelithiasis, esophagitis and food intolerance [1, 2]. The treatment consists only of surgical decompression, performed using either a traditional or, preferably, a laparoscopic approach, and characterized by the section of the median arcuate ligament and of the fibers of the celiac plexus [10, 11]. We report a case of Dunbar syndrome with borderline imaging findings, diagnosed through color-Doppler ultrasound (US) examination, with the objective to increase the knowledge of this condition as a cause of postprandial abdominal pain, and to underline the pivotal role played by color-Doppler US evaluation, to avoid incorrect or delayed diagnosis.
Case report
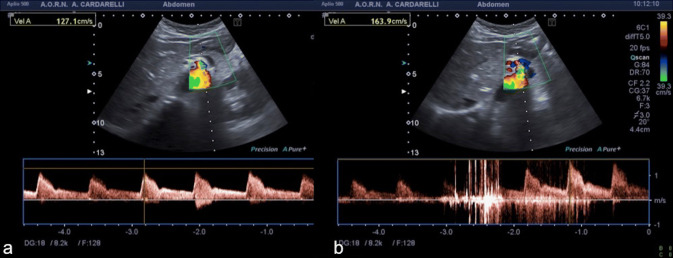
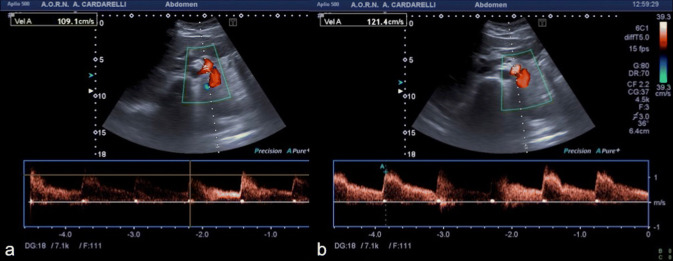
A 37-year-old female patient presented at the emergency department with a 2-month history of abdominal pain, nausea, vomiting and dyspepsia. Pain was located in the epigastrium, continuous in nature, with severity of 6/10 on a pain scale of 0–10, and was also aggravated with eating. The patient also reported anorexia with weight loss. She had a poor symptomatology response to high dose proton-pump inhibitors, prescribed by the primary care physician. She denied other medical conditions. Laboratory blood tests were normal. The initial abdominal US exam performed was inconclusive. Therefore, abdominal computed tomography (CT) angiography was performed, which was also considered substantially inconclusive. For this reason, under the clinical suspicion of Dunbar syndrome, color-Doppler US evaluation with spectral analysis of the celiac trunk was performed showing a normal (obtuse) celiac trunk deflection-angle and a normal calibre and color signal of the vessel during the inspiratory phase, with a peak systolic velocity (PSV) of 127.1 cm/s (Fig. 1a). However, during the expiratory phase, there was the appearance at the proximal celiac trunk of an abnormal (acute) deflection-angle, a color aliasing in the vessel lumen and a moderate increase of PSV (163.9 cm/s) and of the end diastolic velocity, with widening of the spectral window (Fig. 1b). Color-Doppler US findings, especially the color aliasing in the vessel lumen during expiration, were strongly suggestive for Dunbar syndrome. Thus, the CT examination previously performed was reviewed, showing only a slight compression of the proximal celiac trunk due to the median arcuate ligament (Fig. 2). Surgical laparoscopic section of the ligament was made and symptom relief was achieved (Fig. 3). Color-Doppler US examination performed 2 weeks after surgery did not reveal any color aliasing or elevated velocity at the celiac artery origin during either inspiration or expiration. The peak systolic velocities of the celiac artery on expiration, post surgery, measured 121.4 cm/s (Fig. 4).
Fig. 1.
Spectral Doppler US with patient in supine decubitus, during inspiration (a) and expiration (b) at the proximal celiac trunk. Significantly elevated peak systolic velocity (163.9 cm/s) is seen on expiration with aliasing artifact at color-Doppler mode (b)
Fig. 2.
Computed tomography angiography in sagittal (a) and axial (b) planes, showing a slight notch (a marker) along the superior border of the proximal portion of the celiac artery wall and the median arcuate ligament (b marker)
Fig. 3.

Dissection of median arcuate ligament by laparoscopy. The laparoscopic view shows the median arcuate ligament (arrows) at the level of celiac trunk and the crus of diaphragm (double-headed arrow)
Fig. 4.
Postoperative spectral Doppler US with patient in supine decubitus, during inspiration (a) and expiration (b), showing the change in the peak systolic velocity during expiration after surgery, that became normal
Discussion
Dunbar syndrome is a condition characterized by external compression of the proximal celiac trunk by the median arcuate ligament in an abnormal lower localization. In most cases this is an asymptomatic finding [12]. According to the recent clinical practice guidelines of the European Society of Vascular Surgery, Dunbar syndrome also includes symptoms due to postprandial intestinal ischaemia, such as abdominal angina, nausea, weight loss and diarrhea [12]. Pathophysiology of Dunbar syndrome remains controversial [12, 13].
Two main etiological theories have been proposed to explain the symptoms, which include a vascular and a neurogenic cause, respectively. According to the vascular theory, mesenteric vascular compression produces mesenteric ischemia, creating abdominal angina and other related symptoms. In contrast, the neurogenic theory posits that the splanchnic vasoconstriction is due to the stimulation of the celiac ganglion and the celiac plexus [10]. For these reasons, Dunbar syndrome remains a diagnosis of exclusion [1, 5]. Therefore, guided by clinical suspicion, the correct diagnosis of Dunbar syndrome can be made through the use of color-Doppler US, CT angiography, Magnetic Resonance (MR) angiography, selective catheter angiography, or a combination of these exams [1, 14]. Moreover, other causes of abdominal pain should be excluded by endoscopy of the upper gastrointestinal tract, testing for Helicobacter pylori infection, electrocardiography and laboratory tests ruling out myocardial ischemia [11].
In the past, selective catheter angiography was the exam that commonly detected the Dunbar syndrome, showing a typical superior indentation along the proximal tract of the celiac trunk usually about 5 mm from its origin at the abdominal aorta, more evident during expiration rather than during inspiration [15].
Currently, color-Doppler US evaluation, in combination with CT angiography (preferred) or with MR angiography, allows a non-invasive diagnosis of the median arcuate ligament syndrome [8, 14–17]. CT angiography and MR angiography, especially in the sagittal plane, allow for direct evidence of an abnormally low-lying median arcuate ligament, the aorto-mesenteric angle (6°–25°, unlike the normal angle of 28°–65°), the aorto-mesenteric distance (less than 8 mm, unlike the normal distance of 10–34 mm), a typical hooked narrowing in the proximal celiac trunk, eventual atherosclerotic disease and any collateral circulation developed following compression [15].
However, these exams do not allow functional and direct evaluation of the hemodynamic changes in the celiac trunk blood flow following the variation of the respiratory phase, since this compression is respiratory-dependent. The compression appears during expiration and disappears during deep inspiration [18]. Color-Doppler US evaluation allows for dynamic and direct documentation of the variations of calibre, color signal, aorto-mesenteric angle, spectral window and PSV of celiac trunk following the variation of the respiratory phase [18–20]. During the deep inspiratory phase, normal color-Doppler features are generally detected. Yet, during the expiratory phase, there typically appears an abnormal (acute) deflection-angle, a color aliasing in the proximal lumen of the celiac trunk, with an increase of PSV and of the end diastolic velocity [16–20]. According to existing literature, a PSV over 200 cm/s during expiratory phase or a ratio more than 3:1 of PSV of celiac artery to aorta in expiratory phase is a Doppler criterion for diagnosis of Dunbar syndrome [16–20]. In our case, color-Doppler US detected the color aliasing in the proximal lumen of the celiac trunk during the expiratory phase with a slight increase of the PSV (163.9 cm/s). Even if the PSV has not reached a PSV value over 200 cm/s as documented in the literature, neither morphological CT angiography findings were pathognomonic, patient was symptomatic and her symptoms resolved after resection of the median arcuate ligament.
This underlines the pivotal role of the color-Doppler US evaluation for the diagnosis of Dunbar syndrome allowing a dynamic study of the involved anatomical site. Furthermore, other studies have demonstrated the crucial role of the color-Doppler US in challenging cases involving abdominal vessels in emergency settings. [21–23].
Some authors have also observed the role of contrast-enhanced ultrasound (CEUS) in the diagnosis of celiac trunk compression, remarking on a characteristic hooked appearance and dynamic nature of celiac artery compression syndrome with respiration [21, 24]. The introduction of microbubbles as ultrasonographic contrast agents has rendered CEUS an evolving valuable technique, additional to color-Doppler US and complementary to CT with markedly increased diagnostic accuracy for certain vascular applications as well as aortic abdominal and great vessel pathology or follow-up after endovascular aneurysm repair [25–27].
In conclusion, Dunbar syndrome may present with imaging features of variable entity. Color-Doppler US examination plays a crucial role in diagnosis due to the opportunity to examine the patient both during inspiration and expiration showing suggestive findings. The knowledge of borderline conditions at imaging, as in the presented case, is key to avoiding misdiagnosis or delayed diagnosis [14].
Compliance with ethical standards
Conflict of interest
The Authors declare that they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and its late amendments. Additional informed consented was obtained from all patients for which identifying information is not included in this article.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the Authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torres O, Pereira Gama-Filho O, Torres C, Medeiros R, et al. Laparoscopic treatment of Dunbar syndrome: a case report. Int J Surg Case Rep. 2017;37:230–232. doi: 10.1016/j.ijscr.2017.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubinkiewicz M, Ramakrishnan PK, Henry BM, Roy J, Budzynski A. Laparoscopic decompression as treatment for median arcuate ligament syndrome. Ann R Coll Surg Engl. 2015;97:e96–e99. doi: 10.1308/rcsann.2015.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunbar JD, Molnar W, Beman FF, Marable SA. Compression of celiac trunkand abdominal angina. Am J Roentgenol Radium Ther Nucl Med. 1965;95:731–744. doi: 10.2214/ajr.95.3.731. [DOI] [PubMed] [Google Scholar]
- 4.Harjola PT. A rare obstruction of the coeliac artery; report of case. Ann Chir Gynecol Fenn. 1963;52:547–550. [PubMed] [Google Scholar]
- 5.Duran M, Simon F, Ertas N, Schelzig H, et al. Open vascular treatment of median arcuate ligament syndrome. BMC Surg. 2017;17(1):95. doi: 10.1186/s12893-017-0289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim EN, Lamb K, Relles D, Moudgill N, Di Muzio PJ, Eisenberg JA. Median arcuate ligament syndrome-review of this rare disease. JAMA Surg. 2016;151(5):471–477. doi: 10.1001/jamasurg.2016.0002. [DOI] [PubMed] [Google Scholar]
- 7.Lambda R, Tanner DT, Sekhon S, McGahan JP, Corwin MT, Lall CG. Multidetector CT of vascular compression syndromes in the abdomen and pelvis. Radiographics. 2014;34:93–115. doi: 10.1148/rg.341125010. [DOI] [PubMed] [Google Scholar]
- 8.Sunkara T, Caughey ME, Zhen KC, Chiong B, Gaduputi V. Dunbar syndrome-A rare cause of foregut ischemia. J Clin Diagn Res. 2017;11(7):OD13–OD14. doi: 10.7860/jcdr/2017/28142.10267). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coelho J, Silva J, Domingos M, Paulin J, et al. Laparoscopic treatment of celiac axis compression syndrome: case report. ABCD Arq Bras Cir Dig. 2015;28(4):295. doi: 10.1590/S0102-6720201500030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bobadilla JL. Mesenteric ischemia. Surg Clin North Am. 2013;93(4):925–940. doi: 10.1016/j.suc.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Michalik M, Dowgiałło-Wnukiewicz N, Lech P, Majda K, Gutowski P. Hybrid (laparoscopy + stent) treatment of celiac trunk compression syndrome (Dunbar syndrome, median arcuate ligament syndrome (MALS) Videosurgery Miniinv. 2016;11(4):236–239. doi: 10.5114/wiitm.2016.64070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjorck M, Koelemay M, Acosta S, et al. Editor’s choice– management of the diseases of mesenteric arteries and veins: clinical practice guidelines of the European Society of Vascular Surgery (ESVS) Eur J Vasc Endovasc Surg. 2017;53:460–510. doi: 10.1016/j.ejvs.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Gander S, Mulder DJ, Jones S, Ricketts JD, Soboleski DA, Justinich CJ. Recurrent abdominal pain and weight loss in an adolescent: celiac arterycompression syndrome. Can J Gastroenterol. 2010;24:91–93. doi: 10.1155/2010/534654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karavelioglu Y, Kalcik M, Sarak T. Dunbar Syndrome as an unusual case of exercise-induced retrosternal pain. Arch Turk Soc Cardiol. 2014;43(5):465–476. doi: 10.5543/tkda.2015.52563. [DOI] [PubMed] [Google Scholar]
- 15.Horton MK, Talamini MA, Fishman EK. Median arcuate ligament syndrome: evaluation with CT angiography. RadioGraphics. 2005;25:1177–1182. doi: 10.1148/rg.255055001. [DOI] [PubMed] [Google Scholar]
- 16.Ozel A, Toksoy G, Ozdogan O, et al. Ultrasonographic diagnosis of median arcuate ligament syndrome: a report of two cases. Med Ultrason. 2012;14:154–157. [PubMed] [Google Scholar]
- 17.Aswani Y, Thakkar H, Anandpara KM. Imaging in median arcuate ligament syndrome. BMJ Case Rep. 2015 doi: 10.1136/bcr-2014-207856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruber H, Loizides A, Peer S, Gruber I. Ultrasound of the median arcuate ligament syndrome: a new approach to diagnosis. Med Ultrason. 2012;14:5–9. [PubMed] [Google Scholar]
- 19.Erden A, Yurdakul M, Cumhur T. Marked increase in flow velocities during deep expiration: a duplex Doppler sign of celiac artery compression syndrome. Cardiovasc Intervent Radiol. 1999;22:331–333. doi: 10.1007/s002709900399. [DOI] [PubMed] [Google Scholar]
- 20.Wolfman D, Bluth EI, Sossaman J. Median arcuate ligament syndrome. J Ultrasound Med. 2003;22:1377–1380. doi: 10.7863/jum.2003.22.12.1377. [DOI] [PubMed] [Google Scholar]
- 21.Ferrara D, Esposito F, Blasio R, et al. Role of color Doppler ultrasound in the early diagnosis of a major complication after percutaneous renal biopsy: two case reports. J Ultrasound. 2018;21(4):343–349. doi: 10.1007/s40477-018-0326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youssef AT. Intrauterine arterial pseudoaneurysm, a rare cause of per vaginal bleeding. J Ultrasound. 2018;21(4):333–337. doi: 10.1007/s40477-018-0295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Serafino M, Verde F, Ferro F, et al. Ultrasonography of the pediatric spleen: a pictorial essay. J Ultrasound. 2019;22(4):503–512. doi: 10.1007/s40477-018-0341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang XM, Hua XP, Zheng GL. Celiac artery compression syndrome evaluated with 3-D contrast-enhanced ultrasonography: a new approach. Ultrasound Med Biol. 2018;44(1):243–250. doi: 10.1016/j.ultrasmedbio.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Cantisani V, David E, Ferrari D, Fanelli F, Di Marzo L, Catalano C, et al. Color Doppler ultrasound with superb microvascular imaging compared to contrast enhanced ultrasound and computed tomography angiography to identify and classify endoleaks in patients undergoing EVAR. Ann Vasc Surg. 2017;40:136–145. doi: 10.1016/j.avsg.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 26.Cantisani V, Grazhdani H, Clevert DA, Iezzi R, Aiani L, Martegani A, et al. EVAR: Benefits of CEUS for monitoring stent-graft status. Eur J Radiol. 2015;84(9):1658–1665. doi: 10.1016/j.ejrad.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 27.David E, Cantisani V, Grazhdani H, et al. What is the role of contrast-enhanced ultrasound in the evaluation of the endoleak of aortic endoprostheses? A comparison between CEUS and CT on a widespread scale. J Ultrasound. 2016;19(4):281–287. doi: 10.1007/s40477-016-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]