Abstract
Femoral hernia is the protrusion of a peritoneal sac through the femoral ring into the femoral canal lying deep and inferior to the inguinal ligament. The hernia sac usually contains preperitoneal fat, omentum, bowel, or fluid. Ultrasound is recommended as the first-line investigation for diagnosing clinically occult femoral hernias in nonemergency settings, whereas CT is the imaging of choice in emergency settings. High accuracy of the ultrasound in clinically occult femoral hernia is further validated with further CT and MRI. In this article, we propose sonographic detection of the physiological peritoneal fluid herniating through capacious femoral ring manifesting as a “speech bubble/speech box appearance.” This is a potentially invaluable sonographic sign for clinically occult femoral hernias, differentiating them from inguinal hernias and cysts of the canal of Nuck in females and preventing inadvertent attempts to aspirate.
Keywords: Femoral hernia, Ultrasound, Speech bubble sign, Speech box sign
Introduction
Abdominal-wall hernias are usually found in the inguinal region, mostly in the form of direct and indirect inguinal hernias and less so as femoral hernias [1, 2] and can be associated with significant morbidity and mortality if misdiagnosed or left untreated [1, 3]. Hernias are usually diagnosed clinically; however, sometimes they can be difficult to identify [3]; this is where imaging—ultrasound, in particular—is helpful in diagnosing and showing the type of hernia [4].
A femoral hernia is defined as the protrusion of a peritoneal sac through the femoral ring into the femoral canal lying deep and inferior to the inguinal ligament and lateral to the pubic tubercle. The hernia sac usually contains omentum, bowel, or fluid. Femoral hernias may present as nonspecific vague groin discomfort, often nonpalpable on clinical examination or “occult” or misdiagnosed as inguinal hernias, leading to delayed surgical treatment [5, 6]. Early diagnosis and prompt surgical referral are crucial in femoral hernias because of the higher complication rate, especially strangulation under narrow femoral canal neck/femoral ring, compared with other abdominal or inguinal hernias [7]. Up to 40% of femoral hernias can be missed on presentation, leading to emergency surgical management when strangulated, which increases morbidity and mortality, including increased frequency of bowel resection [8].
Imaging is especially helpful in investigating clinically occult groin hernias. Computed tomography (CT) is the investigation of choice in detecting femoral hernias with high accuracy [9, 10] in emergency settings, especially in suspected strangulated inguinoscrotal hernias. However, despite the high accuracy and reliability of the CT scan in emergency settings, its elective use is limited because of cost and the degree of radiation exposure. In such cases, sonography remains the first-line imaging investigation for diagnosing inguinal hernias. Ultrasound can diagnose early occult femoral hernia with high accuracy [11, 12]. In diagnosing femoral hernia, ultrasound demonstrated a sensitivity of 80%, a specificity of 88%, a positive predictive value of 71%, and a negative predictive value of 92% [13].
Furthermore, ultrasound emerges as a preferred imaging tool for assessing abdominal-wall disorders beyond hernias including but not limited to diastasis of rectus abdominis muscles (DRAM), traumatic injuries, anterior abdominal-wall hematomas, endometriosis, vascular malformations, and tumors [14].
Ultrasound technique and findings
We have evaluated clinically occult femoral hernia cases referred by the surgical department as nonemergency cases. Institutional ethics board review was not required, as this was a retrospective analysis, and all the imaging had already been performed based on clinical details. Ultrasound (LOGIQ9; GE Healthcare, Waukesha, WI using a linear transducer (16-5 MHz) was performed by a musculoskeletal radiologist with 11 years of experience. Standardised ultrasound technique was followed in all cases. With the patient lying supine, the linear transducer was placed just inferior to the inguinal ligament, the femoral vessels were viewed in the transverse plane just inferior to the inguinal canal, and the femoral vein was identified. The femoral canal lies just medial to the femoral vein at this location. The femoral canal was assessed with the patient at rest and during straining (coughing or performing a Valsalva maneuver) while maintaining light transducer pressure. A protrusion of the intra-abdominal bowel or fat into the femoral canal is usually defined as a femoral hernia. The canal was reviewed in an orthogonal long axis to confirm that the hernia passed deep to the inguinal ligament.
We found that sonographic appearance of the early or clinically occult femoral hernia can be very subtle. The peritoneal sac was noted to herniate through the femoral canal just beneath the inguinal ligament with a narrow neck, which was dilated significantly distally as a fluid-filled hernial sac into the superficial aspect of the groin, giving a “speech bubble/speech box appearance.” No bowel or peritoneal fat was seen, and none of these patients had ascitis. Intraoperative findings correlated with sonographic findings and confirmed femoral hernia in all cases.
Discussion
Eighty percent of all abdominal-wall/groin hernias are inguinal hernias, and 5% are femoral hernias. The remaining 15% include incisional, umbilical, epigastric, and miscellaneous varieties. Inguinal hernias demonstrate male predominance at about 7:1, whereas female predominance is at about 1.8:1 in femoral hernias [9].
Preoperative clinical assessment of occult femoral hernia is difficult, as the hernial sac is not always palpable. Even in a patient with a clinically palpable inguinal hernia, other differentials including inguinal lymphadenopathy, lipomas, femoral artery aneurysm, psoas abscess, hydrocele, and cyst of the canal of Nuck in females should be considered. CT scan can be useful in differentiating these pathologies in conjunction with ultrasound [9]. For soft-tissue lesions other than hernias, multiparametric ultrasound, including high-resolution grayscale ultrasound, color-power-Doppler, spectral-Doppler, and elastography, remains a powerful tool to discriminate various pathologies. When posed with indeterminate soft-tissue mass, ultrasound in conjunction with MRI followed by ultrasound-guided biopsy or fine needle aspiration (FNA) is a standard practice of management [15].
Differentiating a femoral hernia from an inguinal hernia depends on the relationship of the hernia neck with the pubic tubercle and the medial end of the inguinal ligament. In femoral hernias, the neck of the hernia sac lies inferior (deep) and lateral to the pubic tubercle, whereas in inguinal hernias, the neck of the hernia sac lies superior and medial to the pubic tubercle. Furthermore, compression of the femoral vein by the hernia sac can also be utilized to differentiate femoral hernias from inguinal hernias [9].
Anatomical basis of the sonographic “speech bubble/speech box sign”
In the inguinal region, three lateral abdominal wall muscles (external oblique, internal oblique, and transversus abdominis) and associated fascia form an aponeurosis that extends toward the midline to the rectus abdominis muscle and its lateral fascial condensation, linea semilunaris and bordered posteriorly by transversalis fascia. The inguinal canal traverses through these layers, which contain neurovascular bundles, and through the spermatic cord in males or round ligament in females. The external oblique aponeurosis demonstrates further folding and thickening at its lower border, attaching to the anterior superior iliac spine laterally and to the pubic tubercle medially, forming the inguinal ligament.
The inguinal ligament and the inferior epigastric artery in the inguinal region divides it into three different anatomical compartments. The first is known as the Hesselbach’s strangle, which is lined inferiorly by the inguinal ligament, medially by the lateral border of the ipsilateral rectus abdominis, and laterally by the inferior epigastric artery. Second, the femoral region is inferior to the medial end of the inguinal ligament and lateral to the pubic tubercle. The third area lies just lateral to the inferior epigastric artery above the inguinal ligament (site for direct inguinal hernia) (Fig. 1).
Fig. 1.
Graphical representation of the inguinal and femoral region anatomy. Inferior epigastric vessels and inguinal ligaments are the key structures dividing three distinct anatomical site for direct and indirect inguinal hernia and femoral hernia. Direct inguinal hernia lying medial to inferior epigastric vessels and superior to inguinal ligament protruding through Hesselbach’s triangle, whereas indirect inguinal hernia enters through deep inguinal ring, follows inguinal canal structures and may emerge through superficial inguinal ring entering scrotum in males and labia majora in females. Femoral hernia protrudes through femoral ring inferior to inguinal ligament and lateral to femoral vein
Imaging—particularly ultrasound—relies on the anatomical relationships of the inguinal ligament and femoral vein to ascertain the position of the femoral canal and diagnose femoral hernia. Usually, the peritoneum secretes trace amounts of physiological fluid, and a femoral hernia, by definition, being a peritoneal outpouching through the weakened femoral ring, may only contain such fluid in the hernia sac (Figs. 2 and 3) during the temporal evolution of the hernia. Eventually, the hernia sac expands, containing properitoneal fat (Fig. 4) and bowel loops which may incarcerate or strangulate. Hence, on ultrasound, a peritoneal recess containing peritoneal fluid can be the only manifestation of occult femoral hernia. In our series, we found that the sac enters the femoral canal and extends distally, forming a fluid-distended hernia sac, and this appearance of a narrow neck at the inguinal ligament and further rapid dilatation of the hernia sac into the femoral canal mimics the “speech bubble/speech box,” which can be further exaggerated by Valsalva maneuver. The appearance was further confirmed with cross-sectional imaging CT (Fig. 5b) and MRI (Fig. 6b) when in doubt.
Fig. 2.

Transverse grey scale ultrasound image of an early occult femoral hernia sac forming a deformed ‘speech box’ sonographically. Hernia sac with a narrow neck (white speech bubble) and lying in close to (FV) femoral vein
Fig. 3.

Classical ‘speech box’ appearance of the femoral hernia (outlined in white): Transverse grey scale ultrasound image of an early occult femoral hernia sac containing peritoneal fluid only. (H) hernia sac with a narrow neck (white arrow) and (V) femoral vein
Fig. 4.
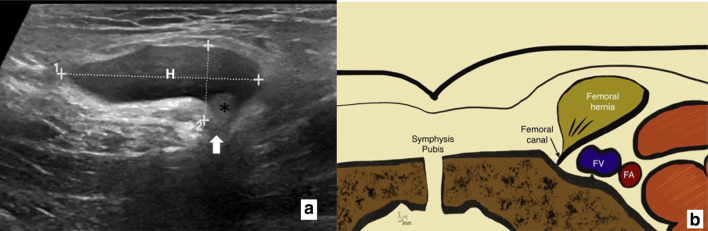
On the left a transverse grey scale ultrasound image of an early occult femoral hernia sac giving a ‘speech box’ appearance sonographically. (H) hernia sac with a narrow neck (white arrow) containing peritoneal fat (asterisk). On the right b graphical representation of the relationship between pubic tubercle, femoral canal containing femoral hernia and femoral vessels lying most laterally
Fig. 5.
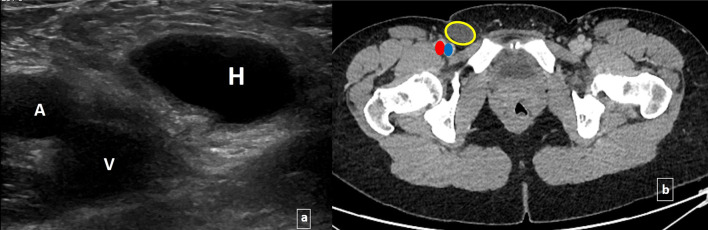
a Transverse grey scale ultrasound image of an early occult femoral hernia sac containing peritoneal fluid only; Hernia sac (H), femoral vein (V), femoral artery (A), and b corresponding axial contrast-enhanced CT image demonstrating hernia sac (yellow circle), femoral vein (blue oval) and femoral artery (red oval)
Fig. 6.
Confirming with an MRI: Transverse greyscale ultrasound image (left) demonstrating fluid-filled occult femoral hernia sac and corresponding T2-weighted MR axial image (right) confirming femoral hernia sac (white rectangle) and its relation with femoral vessels
Importance of recognizing the “speech bubble/speech box” sign
Failure to understand this sonographic finding of fluid-only-filled sac and its anatomy may lead to delayed or missed diagnosis of femoral hernia. It can also help avoid further imaging, saving time and cost in its management. Understanding the sonographic “speech bubble/speech box” appearance may aid sonographers, radiology trainees, and early-career radiologists to arrive at a correct diagnosis in a timely manner.
Recognizing this appearance can be extremely important in differentiating femoral hernia from the cyst/hydrocele of the canal of Nuck in females in which the patent processus vaginalis does not communicate with the peritoneum, does not change after Valsalva maneuver, and extends into the labia majora rather than the medial groin, lying more medially from the femoral canal structures (Fig. 7).
If such appearance of femoral hernia is misdiagnosed as a cyst of the canal of Nuck, there may be requests for aspiration of the “cyst” with a risk of local infection or complication such as peritonitis.
Fig. 7.
Mimicker Cyst of canal of Nuck: Transverse greyscale ultrasound image demonstrating irregular fluid-filled structure (white arrow) lying far medially to the femoral vein (FV) and femoral artery (FA). Anatomically, it is a fluid collected within an enclosed space (processus vaginalis) having no communication to the peritoneal cavity. Hence, it is remarkably irregular in outline, does not change dimentions on Valsalva manoeuvre and extends into labia majora far medially to the femoral vein
Conclusions
To summarize, the “speech bubble/speech box” appearance on an ultrasound is created by an extremely narrow neck of the femoral hernia with an expanded hernia sac and is a crucial sonographic sign in diagnosing occult femoral hernia, where there is no herniation of peritoneal fat or bowel, and in differentiating it from the cyst/hydrocele of the canal of Nuck and inguinal hernia, guiding appropriate management and preventing complications.
Compliance with ethical standards
Conflict of interest
All the authors declare no conflict of interest related to this article.
Ethical approval
This is not a Study but a Pictorial Essay where images and data has been retrospectively obtained from already performed radiological examinations. The examinations were been performed with complete ethical standards and patient consent.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kulah B, Duzgun AP, Moran M, Kulacoglu IH, Ozmen MM, Coskun MF. Emergency hernia repairs in elderly patients. Am J Surg. 2001;182(5):455–459. doi: 10.1016/S0002-9610(01)00765-6. [DOI] [PubMed] [Google Scholar]
- 2.McIntosh A, Hutchinson A, Roberts A, Withers H. Evidence-based management of groin hernia in primary care—a systematic review. Fam Pract. 2000;17(5):442–447. doi: 10.1093/fampra/17.5.442. [DOI] [PubMed] [Google Scholar]
- 3.Askew G, Williams GT, Brown SC. Delay in presentation and misdiagnosis of strangulated hernia: prospective study. J R Coll Surg Edinb. 1992;37(1):37–38. [PubMed] [Google Scholar]
- 4.Korenkov M, Paul A, Troidl H. Color duplex sonography: diagnostic tool in the differentiation of inguinal hernias. J Ultrasound Med. 1999;18(8):565–568. doi: 10.7863/jum.1999.18.8.565. [DOI] [PubMed] [Google Scholar]
- 5.Whalen HR, Kidd GA, O’Dwyer PJ. Femoral hernias. BMJ. 2011;8(343):d7668. doi: 10.1136/bmj.d7668. [DOI] [PubMed] [Google Scholar]
- 6.McEntee GP, O’Carroll A, Mooney B, Egan TJ, Delaney PV. Timing of strangulation in adult hernias. Br J Surg. 1989;76(7):725–726. doi: 10.1002/bjs.1800760724. [DOI] [PubMed] [Google Scholar]
- 7.Gallegos NC, Dawson J, Jarvis M, Hobsley M. Risk of strangulation in groin hernias. Br J Surg. 1991;78(10):1171–1173. doi: 10.1002/bjs.1800781007. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson H, Nilsson E, Angerås U, Nordin P. Mortality after groin hernia surgery: delay of treatment and cause of death. Hernia. 2011;15(3):301–307. doi: 10.1007/s10029-011-0782-4. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S, Furui S, Okinaga K, Sakamoto T, Murata J, Furukawa A, Ohnaka Y. Differentiation of femoral versus inguinal hernia: CT findings. Am J Roentgenol. 2007;189(2):W78–W83. doi: 10.2214/AJR.07.2085. [DOI] [PubMed] [Google Scholar]
- 10.Kitami M, Takase K, Tsuboi M, Rikimaru Y, Hakamatsuka T, Yamada T, Takahashi S. Differentiation of femoral and inguinal hernias on the basis of anteroposterior relationship to the inguinal ligament on multidimensional computed tomography. J Comput Assist Tomogr. 2009;33(5):678–681. doi: 10.1097/RCT.0b013e3181977a0a. [DOI] [PubMed] [Google Scholar]
- 11.Robinson P, Hensor E, Lansdown MJ, Ambrose NS, Chapman AH. Inguinofemoral hernia: accuracy of sonography in patients with indeterminate clinical features. Am J Roentgenol. 2006;187(5):1168–1178. doi: 10.2214/AJR.05.1251. [DOI] [PubMed] [Google Scholar]
- 12.Gupta H, Subedi N, Robinson P. Effectiveness of sonography in detecting clinically occult femoral hernias. J Ultrasound Med. 2016;35(8):1675–1679. doi: 10.7863/ultra.15.09045. [DOI] [PubMed] [Google Scholar]
- 13.Brandel DW, Girish G, Brandon CJ, Dong Q, Yablon C, Jamadar DA. Role of sonography in clinically occult femoral hernias. J Ultrasound Med. 2016;35(1):121–128. doi: 10.7863/ultra.15.02061. [DOI] [PubMed] [Google Scholar]
- 14.Draghi F, Cocco G, Richelmi FM, Schiavone C. Abdominal wall sonography: a pictorial review. J Ultrasound. 2020;3:1–4. doi: 10.1007/s40477-020-00435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catalano O, Varelli C, Sbordone C, Corvino A, De Rosa D, Vallone G, Wortsman X. A bump: what to do next? Ultrasound imaging of superficial soft-tissue palpable lesions. J Ultrasound. 2019;30:1–4. doi: 10.1007/s40477-019-00415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]