Abstract
Preoperative characterization of parotid gland tumors using imaging or cytological examination (fine-needle aspiration cytology) has a strong clinical and therapeutic impact, but it is often difficult due to the tumors’ histological heterogeneity. The recent introduction of contrast-enhanced ultrasound (CEUS) and elastography, in the context of multiparametric ultrasound evaluation, contributed to improving the preoperative diagnosis of many diseases affecting the liver, breast, and thyroid, as well as other organs. However, limited evidence is available on the use and the diagnostic power of these techniques in parotid gland neoplasms. In this paper, we present a case of a parotid lesion that highlights the importance of a complete workup using multiparametric ultrasound evaluation, including CEUS and elastography, to identify malignant tumors of the parotid gland.
Keywords: Parotid gland, Head and neck, Ultrasound, CEUS, Elastography
Introduction
Parotid gland neoplasms are the most frequent salivary gland tumors, which are proved to be benign in about 60% of cases, usually affecting the superficial lobe of the gland. The overall incidence of parotid malignancies is 0.7–1% of all malignant tumors and 3% of tumors in the head and neck region [1]. These lesions have a wide variety of histological types and a distinct clinical and radiological behavior; so, the characterization of parotid tumors plays a crucial role in their treatment planning and in the prediction of possible surgical complications [2]. B-mode ultrasound (US) is usually used as a first step in the evaluation of parotid lesions, followed by magnetic resonance imaging (MRI) and computed tomography (CT) if there are any suspicious B-mode and color Doppler US patterns. CT and MRI are also used for staging and follow-up of the disease, although they cannot always guarantee an accurate differential diagnosis between benign lesions and malignancies [3]. Furthermore, fine-needle aspiration cytology (FNAC) is often nondiagnostic, requiring repetitions of the examination or further second-level examinations [4]. Therefore, to date, the gold standard in the diagnosis of salivary gland tumors remains histopathological examination. The introduction of new US tools, such as elastography and contrast-enhanced US (CEUS), has opened further opportunities to improve the differentiation between benign and malignant parotid lesions. However, limited evidence is available on the clinical use of these techniques in parotid gland neoplasms. In this paper, we present a case of a parotid tumor that highlights the importance of a complete workup using multiparametric US evaluation, including CEUS, to identify malignant tumors of the parotid gland and to avoid misleading diagnoses at first-level examination performed by conventional US alone.
Case presentation
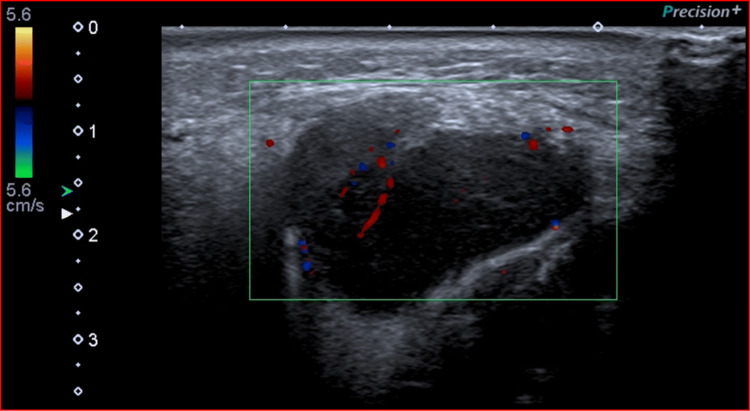
An 85-year-old male Caucasian nonsmoker presented to the Otolaryngology Department of our University Hospital with a painless, slowly growing swelling in the left parotid region, which appeared 6 months earlier. The overlying skin was normal, and there were no clinical signs of facial nerve injury. He had no palpable neck lymph nodes or fever. The patient’s anamnesis did not reveal other oncological diseases or a similar case in his family. He came to our Radiology Department to perform a B-mode US evaluation as a first-level assessment. We used a “high-end” machine (Aplio i800, Canon Medical Systems, Otawara, Japan) with a 5–14-MHz linear probe, which showed a hypoechoic mass within the right parotid gland of 2.4 × 2.7 cm, well circumscribed, with lobulated margins, without internal calcifications, and with posterior acoustic enhancement. Color Doppler evaluation highlighted poor vascularization within the mass. These US features were suggestive of a benign lesion, in particular of pleomorphic adenoma (Fig. 1).
Fig. 1.
A hypoechoic, well circumscribed, poorly vascularized mass of the parotid gland at Color Doppler
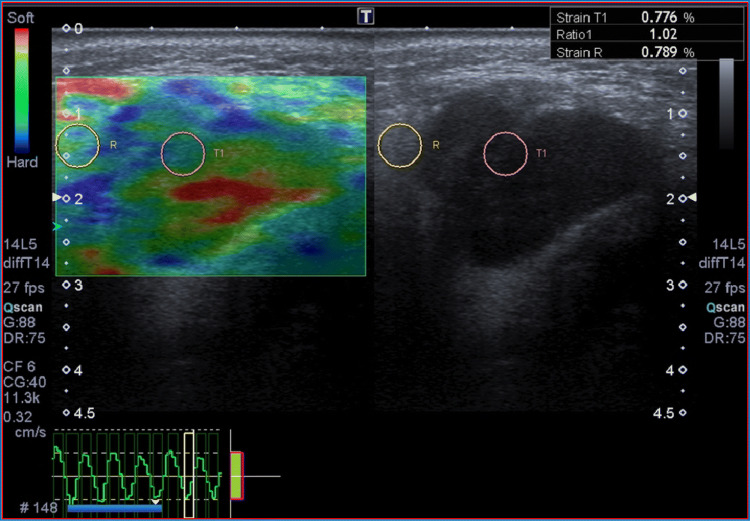
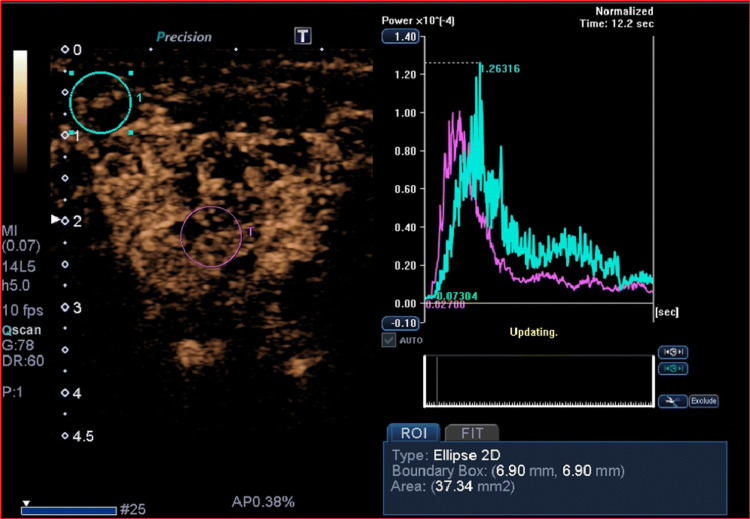
Despite the benign features, we continued the evaluation using other additional tools of multiparametric US. First, a semiquantitative quasistatic US elastography was done, which proved that the lesion was soft (strain ratio [SR] = 1.02, cut-off SR > 3), thus apparently confirming the hypothesis of benignity (Fig. 2). Subsequently, CEUS was performed by administering 2.4 ml of intravenous contrast medium (Sonovue, Bracco, Milan, Italy), followed by 10 ml of isotonic saline solution. The study was carried out continuously for two minutes, using a low mechanical index (MI) of 0.05–0.07, which does not cause significant destruction of microbubbles in the contrast medium. The procedure was recorded digitally to perform qualitative and quantitative analysis. CEUS examination revealed a heterogeneous enhancement with fast arterial uptake and venous wash-out of contrast agent, indicative of malignancy (Fig. 3). Consequently, the patient underwent MRI and FNAC, which confirmed the suspicion of cancer; in particular, cytology showed clusters of atypical squamoid cells. Finally, our patient underwent total parotidectomy, and the histopathological result clinched the diagnosis of primary squamous cell carcinoma. After surgery, the patient received adjuvant radiation therapy. At a 6-month follow-up, there were no signs of recurrence.
Fig. 2.
The lesion appeared soft at Strain US-Elastography (Strain Ratio = 1.02)
Fig. 3.
The parotid lesion showed heterogeneous enhancement at CEUS evaluation, with fast wash-in and wash-out of the contrast agent as shown by intensity/time curves
Discussion and conclusions
Presurgical diagnosis of parotid gland tumors using imaging and cytological examination (FNAC) is often difficult due to the tumors’ histological heterogeneity [4], but it has a strong therapeutic impact. Although malignancies represent only 3% of all malignant tumors of the head and neck region, the characterization of parotid tumors plays an important role in the choice of surgery and the prediction of possible complications, as parotid gland neoplasms have different clinical behavior. Malignant tumors require an aggressive therapeutic approach; on the other hand, among benign tumors, discriminating pleomorphic adenoma allows planning the right timing of surgery, considering that this histological type can present with a malignant transformation in 2–3% of cases [2].
The most frequent benign parotid gland neoplasms are pleomorphic adenoma, monomorphic adenoma, papillary cystadenoma or Warthin tumor, ductal papilloma, cystadenoma, sialoblastoma, and myoepithelioma. The most frequent malignant tumors are adenocarcinoma, mucoepidermoid carcinoma, adenoid cystic carcinoma, malignant mesenchymal neoplasms, and metastases [1].
Squamous cell carcinoma of the parotid gland is an extremely rare tumor, accounting for less than 1% of all salivary gland neoplasms. It is an aggressive tumor in the elderly, with a mean age of presentation of 64 years [5]. The male–female ratio is approximately 2:1. Prior radiation therapy is considered a predisposing factor for this tumor. Patients typically present in an advanced stage with a rapidly enlarging mass in the parotid region, often accompanied by cervical lymphadenopathy and facial nerve involvement. Primary squamous cell carcinoma of the salivary glands is considered a high-grade aggressive malignancy, which carries a worse prognosis than cutaneous squamous cell carcinoma.
B-mode US, used routinely in clinical practice, allows the identification and location of parotid lesions and their structure. However, a more accurate and detailed representation of the microvascular pattern (crucial in the differential diagnosis) can be achieved by CEUS, allowing a quasiquantitative analysis of the perfusion in solid tumor tissue. CEUS is easy to perform, and the contrast injections can be repeated, as the microbubbles have a high safety profile with a low risk of adverse events, lower than that of the contrast media used for CT or MRI [6–8].
There is still limited evidence on the use of CEUS in parotid gland neoplasms. In the latest edition of the guidelines of the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB), no clinical recommendation was stated, even though it was recognized as a research topic [8]. Nevertheless, recent studies in the literature showed a promising perspective of CEUS. A study by Wei et al. in 2013 defined the usefulness of CEUS in the evaluation of microvascularization of the lesion according to the morphological and distribution characteristics of the vascular pattern [9].
Elastography can add information regarding the elastic properties of tissues, correlating them with the composition and structural organization of macromolecules [10, 11]. Elastographic techniques have already been evaluated for the characterization of head and neck lesions, with different results. Dimitriu et al. [12] reported the absence of any elastographic pattern that could define the malignant or benign nature of a lesion, observing little benefits in differentiating between benign and malignant parotid tumors, particularly with regard to the quantitative values of pleomorphic adenomas, which presented similar results to those of malignant tumors. In contrast, a meta-analysis from 2015 [13] evaluated the results of nine studies without publication bias regarding the use of elastography for the differentiation of 581 benign and malignant salivary gland lesions, highlighting good sensitivity and specificity values (76% and 73%, respectively).
Multiparametric US evaluation seems the most promising approach to the preoperative diagnosis of parotid neoplasms. In 2015, Mansour et al. [14] studied 202 patients with parotid lesions, concluding that there was a correlation between the micro- and the macrovascularization of the lesions evaluated with CEUS and color Doppler, respectively, but the predictive value of each technique was small, and a multiparametric assessment was required to increase the specificity and the positive predictive value in the diagnosis of malignant parotid lesions.
Primary squamous cell carcinoma of the salivary glands is a very rare aggressive malignancy. The differential diagnosis of any tumor with squamous differentiation in the parotid region should include high-grade mucoepidermoid carcinoma and metastasis from primary skin squamous cell carcinomas located elsewhere. In our case, we avoided the incorrect diagnosis of a benign lesion by the tools offered by multiparametric US evaluation. In particular, CEUS demonstrated heterogeneous enhancement of the lesion, with fast wash-in and wash-out, suggesting the malignant nature of the lesion [15–18]. Nevertheless, the final diagnosis of squamous cell carcinoma of the salivary glands should, because of its rarity, always be offered only as a diagnosis of exclusion.
In conclusion, our case report highlights the importance of a multiparametric US examination of parotid lesions, as already reported in other organs [19, 20], to obtain the greatest number of exact diagnoses, as timely identification of aggressive tumors is of the utmost importance in deciding treatment options and predicting outcomes. Therefore, further multicenter studies are encouraged to confirm the clinical use of these techniques in parotid gland neoplasms, as there is still only limited evidence available.
Compliance with ethical standards
Conflict of interest
Nothing to declare.
Ethical statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Informed consent
Duly signed informed consent was taken from the patient in question to include personal case details and clinical images. Confidentiality was ensured at all stages.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Classification of Head and Neck Tumours (2017) Edited by El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. IARC (ISBN-13 978-92-832-2438-9)
- 2.Lewis AG, Tong T, Maghami E. Diagnosis and management of malignant salivary gland tumors of the parotid gland. Otolaryngol Clin N Am. 2016;49(2):343–380. doi: 10.1016/j.otc.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 3.David E, Cantisani V, De Vincentiis M, Sidhu PS, Greco A, Tombolini M, Drudi FM, Messineo D, Gigli S, Rubini A, Fresilli D, Ferrari D, Flammia F, D’Ambrosio F. Contrast-enhanced ultrasound in the evaluation of parotid gland lesions: an update of the literature. J Ultrasound. 2016;24(2):104–110. doi: 10.1177/1742271X15626611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seethala RR, LiVolsi VA, Baloch ZW. Relative accuracy of fine-needle aspiration and frozen section in the diagnosis of lesions of the parotid gland. Head Neck. 2005;27:217–223. doi: 10.1002/hed.20142. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JE, Olsen KD. Squamous cell carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 245–246. [Google Scholar]
- 6.Bartolotta TV. Contrast media in ultrasonography: basic principles and clinical applications. Berlin: Springer; 2005. [Google Scholar]
- 7.Trimboli P, Castellana M, Virili C, Havre RF, Bini F, Marinozzi F, D'Ambrosio F, Giorgino F, Giovanella L, Prosch H, Grani G, Radzina M, Cantisani V. Performance of contrast-enhanced ultrasound (CEUS) in assessing thyroid nodules: a systematic review and meta-analysis using histological standard of reference. Radiol Med. 2020;125(4):406–415. doi: 10.1007/s11547-019-01129-2. [DOI] [PubMed] [Google Scholar]
- 8.Sidhu PS, Cantisani V, et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (short version) Ultraschall Med. 2018;39(2):154–180. doi: 10.1055/s-0044-101254. [DOI] [PubMed] [Google Scholar]
- 9.Wei X, Li Y, Zhang S, Li X, Wang H, Yong X, Wang X, Li X, Gao M. Evaluation of microvascularization in focal salivary gland lesions by contrast-enhanced ultrasonography (CEUS) and Color Doppler sonography. Clin Hemorheol Microcirc. 2013;54(3):259–271. doi: 10.3233/CH-131732. [DOI] [PubMed] [Google Scholar]
- 10.Cantisani V, David E, Grazhdani H, Rubini A, Radzina M, Dietrich CF, Durante C, Lamartina L, Grani G, Valeria A, Bosco D, Di Gioia C, Frattaroli FM, D'Andrea V, De Vito C, Fresilli D, D'Ambrosio F, Giacomelli L, Catalano C. Prospective evaluation of semiquantitative strain ratio and quantitative 2d ultrasound shear wave elastography (SWE) in association with TIRADS classification for thyroid nodule characterization. Ultraschall Med. 2019;40(4):495–503. doi: 10.1055/a-0853-1821. [DOI] [PubMed] [Google Scholar]
- 11.Bădărînză M, Serban O, Maghear L, Bocsa C, Micu M, Damian L, Felea I, Fodor D. Shear wave elastography as a new method to identify parotid lymphoma in primary Sjögren syndrome patients: an observational study. Rheumatol Int. 2020 doi: 10.1007/s00296-020-04548-x. [DOI] [PubMed] [Google Scholar]
- 12.Dumitriu D, Dudea SM, Botar-Jid C, Baciut G. Ultrasonographic and sonoelastographic features of pleomorphic adenomas of the salivary glands. Med Ultrason. 2010;12(3):175–183. [PubMed] [Google Scholar]
- 13.Liu Y, Li J, Tan Y-R, Xiong P, Zhong L-P. Accuracy of diagnosis of salivary gland tumors with the use of ultrasonography, computed tomography and magnetic resonance imaging: a meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(2):238–245.e2. doi: 10.1016/j.oooo.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Mansour N, Bas M, Stock KF, et al. Multimodal ultrasonographic pathway of parotid gland lesions. Ultraschall Med. 2015;38(2):166–173. doi: 10.1055/s-0035-1553267. [DOI] [PubMed] [Google Scholar]
- 15.Rzepakowska A, Osuch-Wójcikiewicz E, Maria Sobo M, Cruz R, Niemczyk S-B. The differential diagnosis of parotid gland tumors with high-resolution ultrasound in otolaryngological practice. Eur Arch Otorhinolaryngol. 2017;274(8):3231–3240. doi: 10.1007/s00405-017-4636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knopf A, Mansour N, Chaker A, et al. Multimodal ultrasonographic characterisation of parotid gland lesions. A pilot study. Eur J Radiol. 2012;81:3300–3305. doi: 10.1016/j.ejrad.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Karaman CZ, Başak S, Polat YD, Ünsal A, Taşkın F, Kaya E, Günel C. The role of real-time elastography in the differential diagnosis of salivary gland tumors. J Ultrasound Med. 2019;38(7):1677–1683. doi: 10.1002/jum.14851. [DOI] [PubMed] [Google Scholar]
- 18.Cantisani V, David E, Sidhu P, Sacconi B, Greco A, Pandolfi F, Tombolini M, Lo ML, Calliada F, Brunese L, Catalano C, De Vincentiis M, Di Leo N, Ascenti G, D'Ambrosio F. Parotid gland lesions: multiparametric ultrasound and MRI features. Ultraschall Med. 2016;37(5):454–471. doi: 10.1055/s-0042-109171. [DOI] [PubMed] [Google Scholar]
- 19.Fresilli D, Grani G, De Pascali ML, Alagna G, Tassone E, Ramundo V, Ascoli V, Bosco D, Biffoni M, Bononi M, D'Andrea V, Frattaroli F, Giacomelli L, Solskaya Y, Polti G, Pacini P, Guiban O, Gallo Curcio R, Caratozzolo M, Cantisani V. Computer-aided diagnostic system for thyroid nodule sonographic evaluation outperforms the specificity of less experienced examiners. J Ultrasound. 2020 doi: 10.1007/s40477-020-00453-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidili G, De Sio I, D'Onofrio M, Mirk P, Bertolotto M, Schiavone C, SIUMB experts committee SIUMB guidelines and recommendations for the correct use of ultrasound in the management of patients with focal liver disease. J Ultrasound. 2019;22(1):41–51. doi: 10.1007/s40477-018-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]