Abstract
Background and objective
Pancreatic cancer tumors are difficult to access for biopsy. The use of coaxial needles during ultrasound (US)-guided coarse needle biopsy (CNB) may help to improve specimen collection yields and avoid tissue damage. In this retrospective study, the safety, efficacy, and clinical benefits of US-guided percutaneous coaxial CNB of pancreatic masses were evaluated and compared to those of non-coaxial CNB.
Methods
A total sample of 220 biopsies performed from August 2015 to August 2019 were analyzed, including 114 performed with a coaxial needle (17-gauge coaxial coarse needle combined with an 18-gauge coarse biopsy needle) and 106 performed with a non-coaxial needle (18-gauge coarse biopsy needle without a coaxial sheath). The coaxial CNB group was stratified by lesion location to further evaluate the applicability of coaxial core needles. The satisfactory specimen rate, diagnostic efficiency, operating time, and complication rate were compared statistically between groups and subgroups.
Results
Compared to the non-coaxial CNB group, the coaxial CNB group had a greater satisfactory specimen rate (98.3% vs. 92.3%; p = 0.048), a lesser mean operating time (8.9 ± 3.27 min vs. 16.8 ± 5.77 min; p < 0.001), and a lower complication rate (2.6% vs. 9.6%, p =0 .04). A better diagnostic efficiency was obtained for coaxial CNBs in the head of pancreas (98.7%) than in the body or tail of the pancreas (90%, p = 0.047).
Conclusion
For pancreatic masses, coaxial CNB can yield a higher satisfactory sample rate, lower complication rate, and shorter operating time than non-coaxial biopsy. US-guided percutaneous coaxial CNB is a safe and efficient puncture technique for pancreatic lesion diagnosis.
Keywords: Ultrasound-guided biopsy, Pancreatic lesion, Coaxial core biopsy
Introduction
Pancreatic cancer (PC) is among the most lethal malignant diseases and one of the leading causes of cancer mortality worldwide. In the USA alone, where it is the 10th most common cancer, some 57,600 people are expected to be diagnosed with PC in 2020. With an estimated 5-year survival rate of less than 5%, more than 47,050 people are expected to die of PC in the USA in 2020, where it accounts for 3.3% of all deaths, ranking as the fourth leading cause of cancer death overall [1–3]. Although surgery remains the only potentially curative-intent treatment for PC, most PC diagnoses are made at an advanced stage. Early diagnosis of PC is essential for improving treatment options and prognosis. Conventionally, needle biopsy has been the principle pathological diagnosis method for PC, and can be used during treatment monitoring and follow-up, as well.
Previous studies have suggested that ultrasound (US) image-guided percutaneous biopsy may offer a highly efficient and low-cost minimally invasive alternative mode of histological lesion evaluation [4, 5]. Given that the pancreas is relatively difficult to access, being situated in the retro-peritoneum, the improved accuracy afforded by US guidance can help to avoid damage to essential blood vessels and vital organ tissues, while also providing the advantages of radiation-sparing, flexibility, and real-time operation feedback [6, 7]. Although US-guided endoscopic fine-needle aspiration biopsy of the pancreas is a standard practice, it is a complicated procedure with a high false-negative rate [7, 8]. The use of larger-diameter cutting needles with multiple image guides has been reported to reduce the false-negative rate [9–11]. In recent years, coaxial coarse needle biopsy (CNB) procedures have been applied to the puncture of pancreatic masses, offering the advantages of enabling repeat sampling, reducing complication risk, and producing samples that are of adequate volume for histopathology and genetic analysis.
Theoretically, the safety of US-guided percutaneous coaxial CNB may make it an ideal technique for obtaining pathology tissues [12]. However, a few studies have assessed the clinical value and risk of coaxial CNB puncture for pancreatic lesions. Thus, the aim of the present study was to evaluate the safety, efficacy, and clinical utility of coaxial CNB, compared to that of non-coaxial CNB, for pancreatic lesions.
Methods
Inclusion and exclusion criteria
The institutional review committee approved this retrospective study. A hospital records search was performed for all US-guided percutaneous CNBs of pancreatic lesions performed at Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College between August 1, 2015 and August 1, 2019. The following inclusion criteria were applied: diagnostic pathology biopsy performed for an undiagnosed primary or secondary pancreatic mass; provision of informed written consent; and a safe and feasible puncture route to the target lesion was confirmed by US. Patients with any of the following circumstances were excluded from this study: other biopsy method used; coagulation function did not meet biopsy standard; or incomplete imaging before or after the procedure.
Patients
We collected data from patients who underwent pancreatic mass biopsy during the aforementioned 4-year study period in our hospital for retrospective analysis. All patients had US-detectable pancreatic masses (maximal diameter range, 1.2–13.9 cm) and were without contraindications for biopsy. All patients were informed of the operational risks and provided signed informed consent before the procedure.
Patients receiving coaxial or non-coaxial CNB were assigned to the coaxial and non-coaxial groups, respectively. Generally, the decision for which type of CNB was performed was made by the operator in each case without any notable tendency. Each patient remained in the hospital for observation for at least 24 h after the biopsy procedure, and complications were given timely treatment. Each biopsy was performed independently by one of two experienced interventional radiologists. Both of the operators had been conducting US-guided biopsies for more than 8 years. Imaging data and electronic medical records were collected from our hospital's picture archiving and communication system for retrospective analysis.
Biopsy
Before the biopsy procedure, US images and contrast computed tomography (CT) images were reviewed for each patient to clarify the locations of each lesion and of lesion-adjacent vital blood vessels (e.g., superior mesenteric arteriovenous vein and splenic vein) and organs (e.g., the stomach and duodenum). Pre-biopsy scans were done with a Doppler US instrument with a Supersonic Aixplorer system equipped with an abdominal convex ultrasonic 1–6 MHz probe (SuperSonic Imagine, France). The shortest and most direct route that avoided critical vessels, suspected necrosis, and cavitation was considered to be the optimal puncture route. In addition, US guidance was used to determine the most suitable skin entry site and path length, to visualize blood flow around the target, and to identify necrosis and cavities. Local disinfectant and 2% lidocaine anesthetic were applied prior to the biopsy.
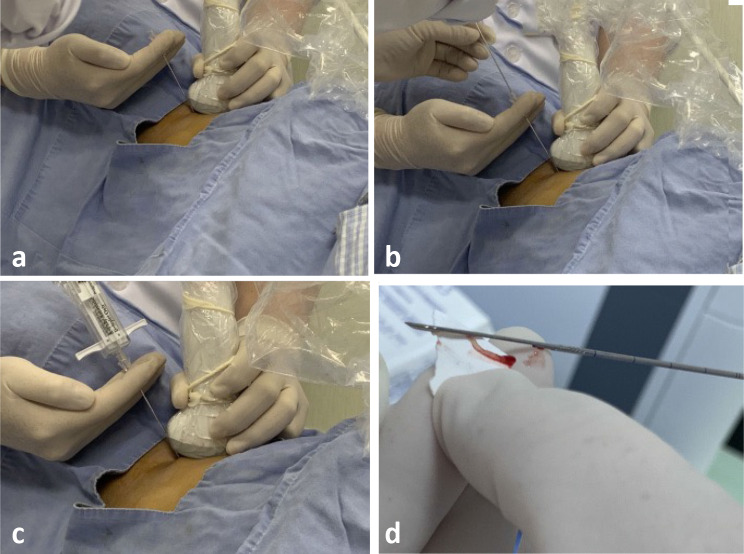
In the coaxial CNB group, the biopsy was implemented with a 17-gauge (1.4-mm) coaxial introducer system (Tru-Guide Disposable Coaxial Needle; C. R. Bard, Tempe, AZ) combined with an 18-gauge (1.2-mm) coarse biopsy needle (Magnum Needle; C. R. Bard, Tempe, AZ) for core specimens (Fig. 1). In the non-coaxial CNB group, following the same position, confirmation procedure and an 18-gauge cutting needle were also used, but without a coaxial sheath. In the presence of necrosis or cystic changes within a large pancreatic mass, we used contrast-enhanced US (CEUS) to reveal highly enhanced components for biopsy guidance, thereby reducing false-negative rates and improving puncture accuracy. In each case, 2–4 tissue samples were obtained, while target mass and biopsy needle were visible by US imaging. Lesion specimens were cut into fragments (1.5–2.2 cm long) with the biopsy needle. Biopsy complications were classified and recorded in accordance with the Society of Interventional Radiology guidelines.
Fig. 1.
Photographs of BNP equipment and procedures. a Coaxial biopsy needle: 17-gauge (1.4-mm) coaxial introducer system. b Non-coaxial biopsy needle: 18-gauge (1.2 mm) coarse biopsy needle; c 18-gauge cutting needle without a coaxial sheath. d Biopsy specimen
Diagnostic assessment
Samples were collected and stored according to standard techniques in the pathology department of our hospital. Biopsy specimens were considered satisfactory if there was enough tissue obtained for diagnosis. Diagnosis outcomes were benign (“negative”), malignant (“positive”), or undiagnosed (due to undiagnosable pathological tissue due to sample insufficiency or termination of the operation). Each patient was followed until there was an ultimate pathology diagnosis. If the final determination was benign disease, then the original CNB diagnostic result was designated as a true or false negative. If the definitive diagnosis based on a subsequent surgical specimen was malignant disease, then the positive diagnostic result was stratified as a false positive or true positive. For patients who did not receive surgery, the final determination of malignancy was determined based on clinical features such as metastasis, lesion regression, or an increasing or stable lesion size.
Data analysis
Demographic variables (age, height, and weight) were analyzed with parametric or non-parametric tests depending on distribution normality. Categorical variables were compared with independent Chi-squared tests or Fisher’s exact tests. Quantitative variables were compared across the two groups (binary comparison) with independent t tests or Mann–Whitney tests. Means are reported with standard deviations (SDs). All data analyses were performed in SPSS version 21 software, with a significance criterion of p < 0.05.
Results
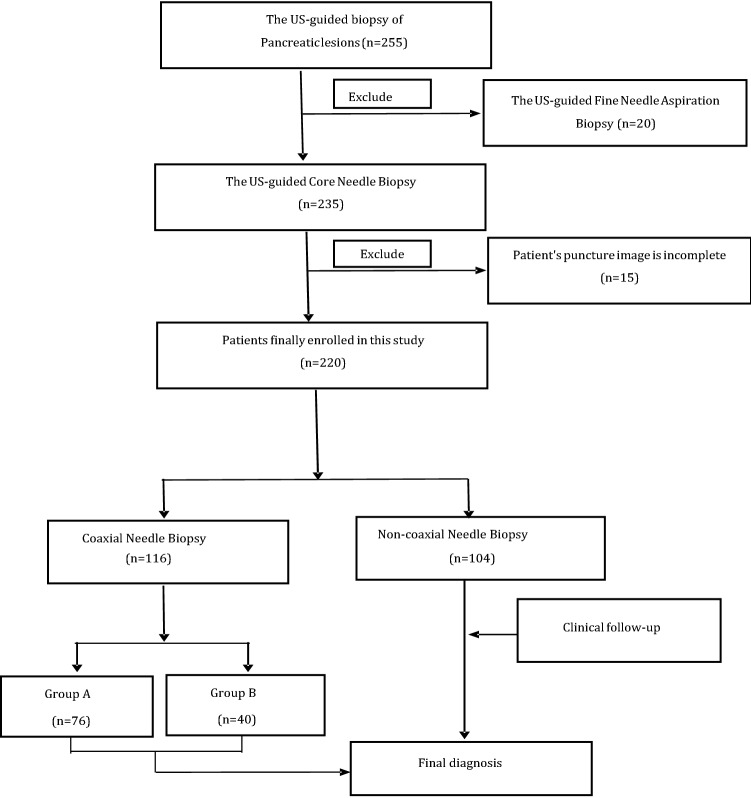
Case records were collected for a total of 255 eligible patients, of which 35 patients were excluded (Fig. 2). Thus, the final study sample consisted of 220 patients (137 males, 62.27%; 83 females, 37.73%) with a mean age of 56.2 ± 15.3 years (median 58; range 14–87 years). Lesion sizes ranged from 1.2 to 13.9 cm in their maximum dimension diameter. The clinical characteristics of the patients and CNB procedural outcomes are reported in Table 1. Notably, very high satisfactory specimen and diagnostic efficiency rates were achieved. In all cases, the biopsies were conducted under US guidance, including 20 CEUS-guided biopsies. Each target lesion received an average of 2.5 punctures (range, 2–4). The pathology diagnosis results for all biopsies are reported in Table 2.
Fig. 2.
Flowchart of patient inclusion in the study population
Table 1.
Clinical information for all pancreatic masses and inter-group comparisons of lesion and procedural variables between CNB method groups and between mass locations within the pancreas
| Variable | All masses (N = 220) | CNB method | Mass location | ||||
|---|---|---|---|---|---|---|---|
| Coaxial | Non-coaxial | P | Head | Body/tail | P | ||
| Lesion characteristics, cm | |||||||
| Long axis | 5.07 ± 1.69 | 4.89 ± 1.11 | 5.26 ± 2.13 | 0.11 | 4.8 ± 1.15 | 5.0 ± 1.02 | 0.46 |
| Short axis | 3.25 ± 1.06 | 3.12 ± 1.13 | 3.38 ± 0.95 | 0.70 | 3.0 ± 1.10 | 3.3 ± 1.18 | 0.23 |
| Depth | 8.47 ± 1.62 | 8.64 ± 1.75 | 8.29 ± 1.42 | 0.11 | 9.10 ± 1.67 | 7.76 ± 1.55 | <0 .0001 |
| Puncture needles, n | 2.46 ± 0.65 | 2.5 ± 0.66 | 2.4 ± 0.63 | 0.38 | 2.56 ± 0.73 | 2.38 ± .48 | 0.14 |
| Procedure time, min | 12.66 ± 6.11 | 8.9 ± 3.27 | 16.8 ± 5.77 | < 0.001 | 9.39 ± 3.52 | 8.00 ± 2.55 | 0.02 |
| Satisfactory samples | 95.5% | 98.3 | 92.3 | 0.048 | 100 | 95 | 0.11 |
| Complications | 5.9% | 2.6 | 9.6 | 0.04 | 0 | 7.5 | 0.074 |
| Diagnostic efficiency | 93.6% | 95.7% | 91.3% | 0.29 | 98.7 | 90 | 0.047 |
Means are reported with SDs
Table 2.
Summary of final case diagnoses
| Neoplasm type subtype | N | % |
|---|---|---|
| Malignant biopsies (N = 200) | ||
| Pancreatic ductal adenocarcinoma | 182 | 82.73 |
| Neuroendocrine carcinoma | 15 | 6.82 |
| Sarcoma | 1 | 0.45 |
| Metastasis | 2 | 0.91 |
| Benign biopsies (N = 20) | ||
| Insulinoma | 3 | 1.36 |
| Cyst | 3 | 1.36 |
| Fibrosis | 6 | 2.73 |
| Inflammation | 8 | 3.64 |
| Total | 220 | 100 |
The study sample included 116 coaxial CNBs and 104 non-coaxial CNBs, with similar gender (68 males, 58.6% vs. 69 males, 66.3%, respectively; p = 0.30) and age (mean age ± SD, 54.63 ± 16.48 years vs. 57.97 ± 13.46 years, respectively; p = 0.10) compositions. Outcome parameters are compared between the two groups in Table 1. Notably, compared to the non-coaxial CNB group, the coaxial CNB group had a significantly higher satisfactory specimen rate and a significantly shorter mean operation time (Table 1). As shown in Fig. 3, operating times tended to increase as the number of puncture needles used increased, with the coaxial CNB group having significantly shorter operating times than the non-coaxial CNB group with 2, 3, or 4 needles. There were no major complications in the puncture process in either group. Direct minor complications in this study included hematoma, clinically considerable pain, and hemorrhage. The incidence of complications in the coaxial CNB group was significantly lower than that in the non-coaxial CNB group (Table 1).
Fig. 3.
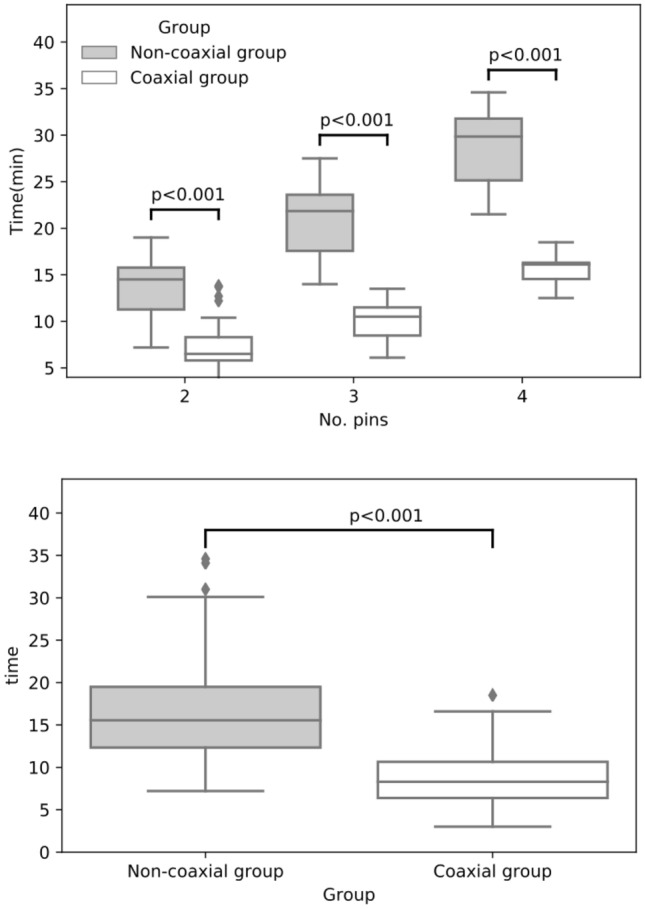
Inter-group comparison of biopsy operating times, overall (above), and relative to the number of needles used (below)
The diagnostic efficiency rates of both groups were high, with only one and three false negatives in the non-coaxial CNB and coaxial CNB groups, respectively. In four of the false-negative cases, the masses were surgically removed and pathologically confirmed to be malignant. Ten biopsies were without diagnosis, 7 of which were ultimately determined to be malignant in follow-up and 3 of which were benign.
The coaxial CNB group was divided into two lesion-location subgroups: pancreatic head (N = 76) and pancreatic body/tail (N = 40). The head and body/tail subgroups had statistically similar gender (41 males, 53.9% vs. 27 males, 67.5%, respectively; p = 0.22) and age (mean age ± SD, 52.67 ± 18.23 years vs. 58.35 ± 11.64 years, respectively; p = 0.08). The results of outcome parameter comparisons between the head and body/tail subgroups are reported in Table 1. Notably, we observed a significantly higher diagnostic efficiency rate and a significantly lower complication rate in the head subgroup than in the body/tail subgroup of patients subjected to coaxial CNB. As expected, mean lesion depth and, accordingly, mean procedure time were significantly greater in the head subgroup than in the body/tail subgroup.
Discussion
The results of the present retrospective study aimed at assessing the clinical value of US-guided coaxial CNB for pancreatic lesions show that our patient group that underwent coaxial CNB had a higher satisfactory sample rate, a lower complication rate, and a shorter mean operating time than patients who underwent non-coaxial CNB. The results provide support for the adoption of US-guided coaxial CNB based on overall safety outcomes and the very high diagnostic accuracy observed.
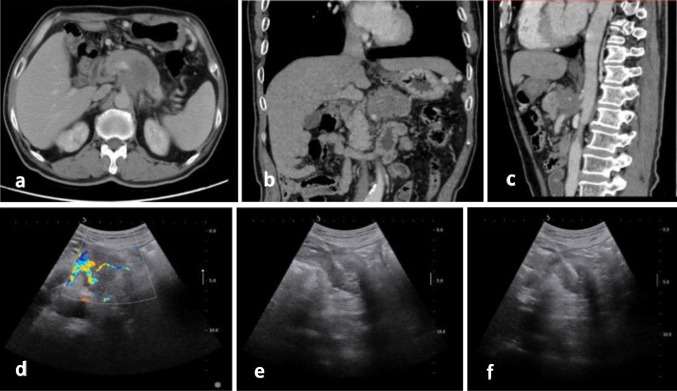
Based on our experience, it is our view that our findings of a higher satisfactory sample rate and shorter procedure time with coaxial CNB than with non-coaxial CNB can be attributed to the former allowing for more complete tissue cutting (Fig. 4). Our results fit with Babaei Jandaghi et al.’s findings in a prior prospective study of renal biopsy [12], in which coaxial biopsies required less time than non-coaxial procedures.
Fig. 4.
CT and US of a 63-year-old man with pancreatic ductal adenocarcinoma. a–c Contrast-enhanced CT shows a 4-cm low-intensity tumor in the pancreas. d US image showing a hypoechoic mass in the pancreas. e, f US-guided coaxial core biopsy with the patients lying in a supine position and the biopsy needle inside the lesion
The values of CNB for pancreatic tumor diagnosis, grading, and histological subtyping far outweigh the risks. Our finding of a lower complication rate in the coaxial CNB group than in the non-coaxial CNB group could be consequent to the blunt needle tip of the coaxial sheath reducing tissue damage. Additionally, the use of a coaxial needle enables the operator to use the same needle channel to make multiple purposeful tissue cuts by changing the angle of the needle, thereby reducing the risk of bleeding. An embolization drug can be incorporated into the coaxial biopsy sheath to augment hemostasis in coaxial CNBs and the coaxial biopsy needle used in our clinic is a disposable semi-automatic cutting needle, which has been shown to improve biopsy safety [13, 14]. Notwithstanding, our findings differ from those of some previous studies reporting similar complication rates for coaxial and non-coaxial techniques [15–18]. Importantly, the relatively large bi-institutional study by Berger-Richardson and colleagues suggested that CNBs of retroperitoneal masses have overall low rates of needle tract seeding and early minor complications [19].
Our comparison of coaxial CNBs between regions of the pancreas suggested that the procedure results in a better diagnostic efficiency rate and lower complication rate for masses located in the head of the pancreas than for masses located in the tail or body of the pancreas. We suspect that these differences are due to pancreatic malignancies occurring predominantly in the head of the pancreas, whereas masses in the tail are more often cystic masses that are less well defined by the conventional US, thereby increasing the difficulty of puncture [20, 21]. These results may provide guidance to clinicians in the process of choosing a suitable puncture method depending on the characteristics of each pancreatic lesion.
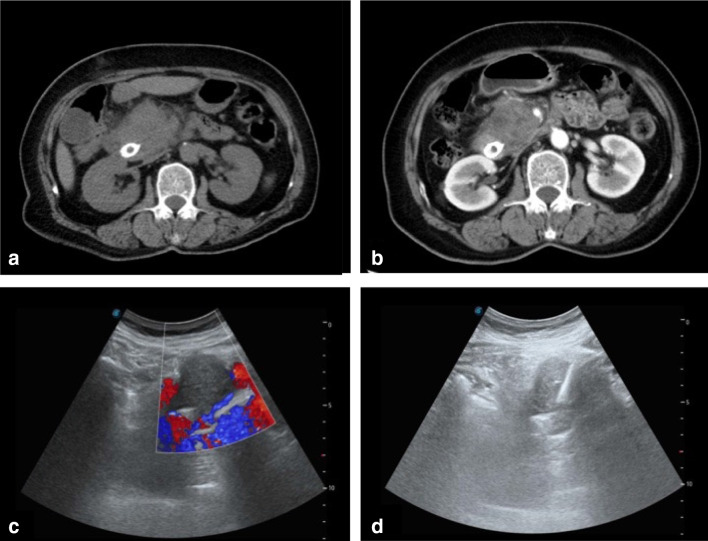
Conventional fine-needle biopsy has the disadvantage of providing a small amount of tissue, which increases the possibility of a false negative [22, 23]. Endoscopic US-guided biopsy is generally the first-choice method for obtaining biopsies from pancreatic head lesions, but this operation requires assiduous cooperation of patients while the operator has limited visualization of the probe. Although CT-guided puncture biopsy is technically and practically feasible, it is more costly than US-guided procedures and it is less safe due to radiation exposure, a long operating time, and an inability to view the lesion in real time. Magnetic resonance imaging-guided biopsy has been reported, but its clinical application is rare due to the high cost and complex technology involved [24–26]. As shown in Fig. 5, color Doppler can be used to visualize vascular structures, while US imaging allows the entire puncture path to be traced in real time.
Fig. 5.
CT and US of a 65-year-old man with a pancreatic head mass detected after biliary stent implantation. The pathology diagnosis of the mass was pancreatic ductal adenocarcinoma. a Transverse CT image showing iso-density of the pancreatic head mass. b Contrast-enhanced CT showing a low-intensity tumor in the pancreatic head. c, d US-guided puncture of the pancreatic head hypoechoic mass
US-guided biopsy is safe and has the benefits of portability, low cost, and non-exposure to ionizing radiation [27–29]. CEUS-guided CNB was performed in 20 of the presently evaluated 220 cases. CEUS-guided core biopsy has been widely applied for other abdominal organs, especially the liver [30–33]. CEUS enables the operator to distinguish the target mass from surrounding healthy tissues owing to microbubble contrast and local blood flow imaging. The practicability and safety of CEUS-guided biopsy for abdominal lesions are worthy of further study and evaluation.
This retrospective study has some limitations. First, the number of case samples was small and all of the samples came from a single central hospital. The repeatability and reproducibility of coaxial CNB need to be examined in a broader population. Second, patients were not randomized to the biopsy approaches, because the study was retrospective; the choice of biopsy method may be affected by the subjective experience of the operator. Therefore, our conclusions should be confirmed in a well-designed and controlled prospective, case-randomized multicenter clinical trial. Furthermore, because we did have specimen size data, it was impossible to determine whether needle type was associated with specimen size, which is a critical factor for satisfactory sampling. We need to plan to use different types of needles and continuously optimize puncture techniques to improve accuracy further.
In conclusion, this single-center retrospective study showed that, compared to non-coaxial CNB, coaxial CNB yields a higher satisfactory sample rate, similar diagnostic efficiency, shorter operating time, and a lower post-procedural complication rate. Thus, our findings support the view that US-guided percutaneous coaxial CNB is an efficient and safe biopsy technique for the diagnosis of pancreatic masses.
Acknowledgements
We would like to thank all participants for their support in this study. No grant support needs to be reported.
Funding
This work did not receive funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration, and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants prior to enrollment in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yi Yang and Yujing Xin have contributed equally to this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Chiaravalli M, Reni M, O'Reilly EM. Pancreatic ductal adenocarcinoma: State-of-the-art 2017 and new therapeutic strategies. Cancer Treat Rev. 2017;60:32–43. doi: 10.1016/j.ctrv.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Goral V. Pancreatic cancer: pathogenesis and diagnosis. Asian Pac J Cancer Prev. 2015;16(14):5619–5624. doi: 10.7314/apjcp.2015.16.14.5619. [DOI] [PubMed] [Google Scholar]
- 4.Solomon SB, Silverman SG. Imaging in interventional oncology. Radiology. 2010;257(3):624–640. doi: 10.1148/radiol.10081490. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y, Shi J, Chen YY, Li K. Ultrasound-guided percutaneous core needle biopsy for the diagnosis of pancreatic disease. Ultrasound Med Biol. 2018;44(6):1145–1154. doi: 10.1016/j.ultrasmedbio.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Yang RY, Ng D, Jaskolka JD, Rogalla P, Sreeharsha B. Evaluation of percutaneous ultrasound-guided biopsies of solid mass lesions of the pancreas: a center's 10-year experience. Clin Imaging. 2015;39(1):62–65. doi: 10.1016/j.clinimag.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Nabahati M, Moazezi Z, Fartookzadeh S, Mehraeen R, Ghaemian N, Sharbatdaran M. The comparison of accuracy of ultrasonographic features versus ultrasound-guided fine-needle aspiration cytology in diagnosis of malignant thyroid nodules. J Ultrasound. 2019;22(3):315–321. doi: 10.1007/s40477-019-00377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.VanderLaan PA. Fine-needle aspiration and core needle biopsy: an update on 2 common minimally invasive tissue sampling modalities. Cancer Cytopathol. 2016;124(12):862–870. doi: 10.1002/cncy.21742. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Wallace MJ, Cardella JF, et al. Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol. 2010;21(7):969–975. doi: 10.1016/j.jvir.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Verma S, Choyke PL, Eberhardt SC, et al. The current state of MR imaging-targeted biopsy techniques for detection of prostate cancer. Radiology. 2017;285(2):343–356. doi: 10.1148/radiol.2017161684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipnik AJ, Brown DB. Image-guided percutaneous abdominal mass biopsy: technical and clinical considerations. Radiol Clin North Am. 2015;53(5):1049–1059. doi: 10.1016/j.rcl.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Babaei Jandaghi A, Lebady M, Zamani AA, Heidarzadeh A, Monfared A, Pourghorban R. A randomised clinical trial to compare coaxial and noncoaxial techniques in percutaneous core needle biopsy of renal parenchyma. Cardiovasc Intervent Radiol. 2017;40(1):106–111. doi: 10.1007/s00270-016-1466-3. [DOI] [PubMed] [Google Scholar]
- 13.Lin CY, Ou MC, Liu YS, et al. A CT-guided fat transversing coaxial biopsy technique for pancreatic lesion biopsy that avoids major organs and vessels. Saudi J Gastroenterol. 2017;23(6):341–347. doi: 10.4103/sjg.SJG_199_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu H, Zhang C, Liu S, et al. Application value of coaxial biopsy system in needle cutting biopsy for focal ground glass-like density nodule. J Cancer Res Ther. 2018;14(7):1509–1514. doi: 10.4103/jcrt.JCRT_382_18. [DOI] [PubMed] [Google Scholar]
- 15.Nour-Eldin NE, Alsubhi M, Emam A, et al. Pneumothorax complicating coaxial and non-coaxial CT-guided lung biopsy: comparative analysis of determining risk factors and management of pneumothorax in a retrospective review of 650 patients. Cardiovasc Intervent Radiol. 2016;39(2):261–270. doi: 10.1007/s00270-015-1167-3. [DOI] [PubMed] [Google Scholar]
- 16.Cazzato RL, Garnon J, Shaygi B, et al. Performance of a New Blunt-Tip Coaxial Needle for Percutaneous Biopsy and Drainage of "Hard-To-Reach" Targets. Cardiovasc Intervent Radiol. 2017;40(9):1431–1439. doi: 10.1007/s00270-017-1663-8. [DOI] [PubMed] [Google Scholar]
- 17.Schaffler-Schaden D, Birsak T, Zintl R, Lorber B, Schaffler G. Risk of needle tract seeding after coaxial ultrasound-guided percutaneous biopsy for primary and metastatic tumors of the liver: report of a single institution. Abdom Radiol (NY) 2019 doi: 10.1007/s00261-019-02120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moulton JS, Moore PT. Coaxial percutaneous biopsy technique with automated biopsy devices: value in improving accuracy and negative predictive value. Radiology. 1993;186(2):515–522. doi: 10.1148/radiology.186.2.8421758. [DOI] [PubMed] [Google Scholar]
- 19.Berger-Richardson D, Burtenshaw SM, Ibrahim AM, et al. Early and late complications of percutaneous core needle biopsy of retroperitoneal tumors at two tertiary sarcoma centers. Ann Surg Oncol. 2019;26(13):4692–4698. doi: 10.1245/s10434-019-07656-6. [DOI] [PubMed] [Google Scholar]
- 20.Sur YK, Kim YC, Kim JK, Lee JH, Yoo BM, Kim YB. Comparison of ultrasound-guided core needle biopsy and endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesions. J Ultrasound Med. 2015;34(12):2163–2169. doi: 10.7863/ultra.14.11030. [DOI] [PubMed] [Google Scholar]
- 21.D'Onofrio M, De Robertis R, Barbi E, et al. Ultrasound-guided percutaneous fine-needle aspiration of solid pancreatic neoplasms: 10-year experience with more than 2,000 cases and a review of the literature. Eur Radiol. 2016;26(6):1801–1807. doi: 10.1007/s00330-015-4003-x. [DOI] [PubMed] [Google Scholar]
- 22.Matsubayashi H, Sasaki K, Ono S, et al. Pathological and molecular aspects to improve endoscopic ultrasonography-guided fine-needle aspiration from solid pancreatic lesions. Pancreas. 2018;47(2):163–172. doi: 10.1097/MPA.0000000000000986. [DOI] [PubMed] [Google Scholar]
- 23.Tyng CJ, Almeida MF, Barbosa PN, et al. Computed tomography-guided percutaneous core needle biopsy in pancreatic tumor diagnosis. World J Gastroenterol. 2015;21(12):3579–3586. doi: 10.3748/wjg.v21.i12.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu MY, Pan KT, Chen CM, et al. CT-guided percutaneous core-needle biopsy of pancreatic masses: comparison of the standard mesenteric/retroperitoneal versus the trans-organ approaches. Clin Radiol. 2016;71(6):507–512. doi: 10.1016/j.crad.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Wang L, He X, et al. 1.0T MR-guided percutaneous coaxial cutting needle biopsy in pancreatic lesion diagnosis. J Magn Reson Imaging. 2018;48(2):382–388. doi: 10.1002/jmri.25952. [DOI] [PubMed] [Google Scholar]
- 26.Donofrio M, Beleù A, De Robertis R. Ultrasound-guided percutaneous procedures in pancreatic diseases: new techniques and applications. Eur Radiol Exp. 2019;3(1):2. doi: 10.1186/s41747-018-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francica G, Meloni MF, Riccardi L, et al. Ablation treatment of primary and secondary liver tumors under contrast-enhanced ultrasound guidance in field practice of interventional ultrasound centers. A multicenter study. Eur J Radiol. 2018;105:96–101. doi: 10.1016/j.ejrad.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 28.Jeong S, Park SB, Kim SH, Hwang JH, Shin J. Clinical significance of contrast-enhanced ultrasound in chronic kidney disease: a pilot study. J Ultrasound. 2019;22(4):453–460. doi: 10.1007/s40477-019-00409-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcovich M, Faccia M, Meloni F, et al. Contrast-enhanced ultrasound patterns of hepatocellular adenoma: an Italian multicenter experience. J Ultrasound. 2019;22(2):157–165. doi: 10.1007/s40477-018-0322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutherland EL, Choromanska A, Al-Katib S, Coffey M. Outcomes of ultrasound guided renal mass biopsies. J Ultrasound. 2018;21(2):99–104. doi: 10.1007/s40477-018-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sconfienza LM, Mauri G, Grossi F, et al. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology. 2013;266(3):930–935. doi: 10.1148/radiol.12112077. [DOI] [PubMed] [Google Scholar]
- 32.Miller DL, Abo A, Abramowicz JS, et al. Diagnostic ultrasound safety review for pointof-care ultrasound practitioners. J Ultrasound Med. 2019 doi: 10.1002/jum.15202. [DOI] [PubMed] [Google Scholar]
- 33.Francica G, Meloni MF, de Sio I, et al. Biopsy of Liver Target Lesions under Contrast-Enhanced Ultrasound Guidance—A Multi-Center Study. Biopsie von Leber-Zielläsionen unter kontrastverstärkter Ultraschall-Führung—eine multizentrische Studie. Ultraschall Med. 2018;39(4):448–453. doi: 10.1055/s-0043-12249. [DOI] [PMC free article] [PubMed] [Google Scholar]