Abstract
Plant diseases caused by plant viruses and pathogens seriously affect crop yield and quality, and it is very difficult to control them. The discovery of new leads based on natural products is an important way to innovate pesticides. Based on the resveratrol is a kind of natural phytoalexin, but it cannot be used as candidate for the development of new drug due to its poor druggability. The phenolic hydroxyl groups in the resveratrol structure are easily destroyed by oxidation, in order to improve its stability, ester formation is the most commonly used modification method in drug design. Their structures were characterized by 1H NMR, 13C NMR and HRMS. The activity against tobacco mosaic virus (TMV) of these ester derivatives has been tested for the first time. The bioassay results showed part of the target compounds exhibited good to excellent in vivo activities against TMV. The optimum compounds III-2 (inhibitory rates of 50, 53, and 59% at 500 μg/mL for inactivation, curative, and protection activities in vivo, respectively), III-4 (inhibitory rates of 57, 59, and 51% at 500 μg/mL, respectively), and II-5 (inhibitory rates of 54, 52, and 51% at 500 μg/mL, respectively) displayed higher activity than commercial plant virucide ribavirin (inhibitory rates of 38, 37, and 40% at 500 μg/mL, respectively). Compounds I-9 and I-10 also showed excellent activities. The systematic study provides strong evidence that these simple resveratrol derivatives could become potential TMV inhibitors. The novel concise structure provides another new template for antiviral studies.
Subject terms: Chemical modification, Medicinal chemistry, Organic chemistry, Chemical synthesis
Introduction
There are many different kinds of plant viruses, which have very extensive distribution; virus diseases rank only second to the fungi categories in agricultural plant diseases. Most commercial crops are subjected to production or quality decline because of the detriment of the plant virus. Plant virus are absolute parasitic in plant cells; the required material, energy, places are provided by the host cell. Because plants do not have the complete immune system, it is difficult to prevent plant virus disease completely, therefore plant virus disease is also known as the "plant cancer"1. Tobacco mosaic virus (TMV) was the first discovered plant virus and is widely used as a model virus in the study of new antiviral agents. Its host is very wide, and it can infect many plants including capsicum, cucumber, tomato, eggplant, solanum nigrum, and so on, which brings great harm to agricultural production2. Although several commercial antiviral agents against TMV have been used, efficient and practical varieties are few. The most widely used antiviral agent ribavirin only gave an inhibition rate of no more than 50% anti-TMV effect at 500 μg/mL, and the most effective antiviral agent ningnanmycin has an inhibitory effect of 50–60% at 500 μg/mL. Plant diseases caused by TMV are difficult to control. Therefore, it is urgent to develop novel antiviral agents with a simple structure and outstanding activities3.
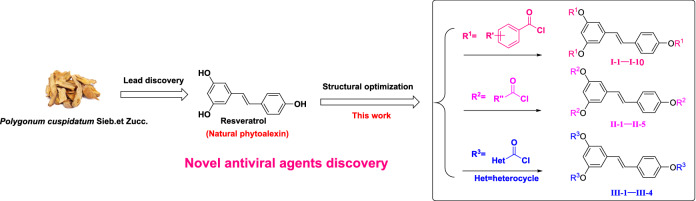
Lead discovery and optimization based on natural products are crucial means to develop novel pesticides, because their novel scaffolds can provide modes of action different from those of existing agents. Some natural products have been commercialized or used as lead compounds for plant-virus control; however, there are only a few reported economically viable antiviral chemicals available for practical application in plant protection. The naturally occurring resveratrol (3,5,4′-trihydroxy-trans-stilbene, Fig. 1) is a phytoalexin which can be activated by adverse conditions of plants, protecting against fungal infections4–11. First isolated in 1940 from Veratrum grandiflorum by Takaoka12,13, and later, it has been obtained in larger quantities from the roots of Polygonum cuspidatum14, a plant used in traditional Chinese medicine. It attracted wider attention only in 1992 when its presence in wine was suggested as the explanation for cardioprotective effects15. Resveratrol is the most representative compound of stilbene analogues, resveratrol and its derivatives exhibits a wide range of intriguing biological activities, such as antibacterial16, antitumour17,18, antiviral19, antioxidant20, and antihypertensive activities21. Furthermore, stilbene moieties may have numerous agrochemical applications, such as in herbicides22–25. Recently, considerable attention has been focused on resveratrol derivatives. Although resveratrol possesses a series of pharmacological activities, its applications in the field of pesticide development have not been reported.
Figure 1.
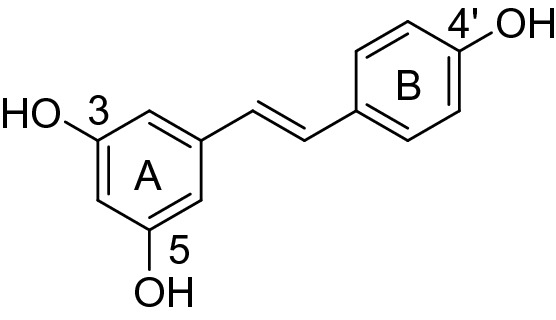
Structure of resveratrol.
Studies have shown that modification of the structure of natural compounds can improve their biological activities. To improve the stability of resveratrol, many researchers have undertaken the synthesis and activity evaluation of resveratrol derivatives and analogues. They have modified the phenolic hydroxyl groups, double bonds and benzene ring of resveratrol so as to further understand the interactions among functional groups and its structure–activity relationship. Grow evidence indicates that this compound could be used as a lead compound in the design of drugs and resveratrol derivatives synthesized from the lead compound resveratrol or stilbene have a higher efficacy and lower toxicity26–29.
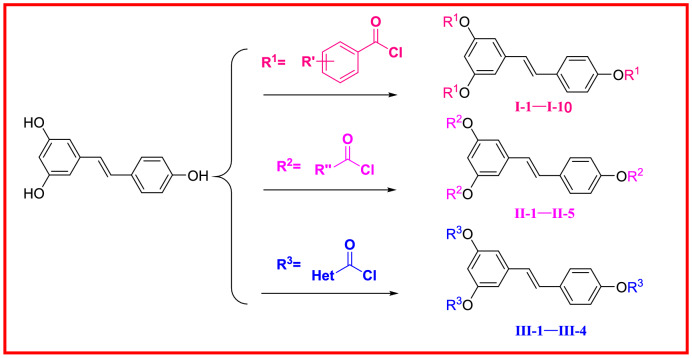
Whilst the antiviral activity of resveratrol has been extensively studied, little is known about the activity against plant viruses of resveratrol and resveratrol derivatives. Ester formation is the most commonly used modification method in drug design. A few resveratrol ester derivatives had been synthesized and tested for their antitumor activity. However, reports of the anti-TMV activity of the resveratrol ester derivatives are rather rare, and no examples are documented in the recent literature. In this paper, in order to increase the stability and druggability of resveratrol, we synthesized a series of resveratrol ester derivatives and their activities against TMV were evaluated (Fig. 2). The structure–activity relationships of these derivatives were discussed as well. Additionally, the synthesized derivatives were also investigated for their potential as fungicidal, or insecticidal agents. Resveratrol derivatives will become a research focus in future in agricultural applications.
Figure 2.
The design and synthesis of compounds I-1–I-10, II-1–II-5 and III-1–III-4.
Materials and methods
Instruments
All other commercial reagents and solvents were used as received without further purification. Reaction solvents were distilled from calcium hydride for dichloromethane and from sodium metal and benzophenone for tetrahydrofuran. E-resveratrol (98%) was purchased from Shanxi Sciphar Hi-tech Industry Co., Ltd. (Shanxi, China). Reaction progress was monitored by thin-layer chromatography on silica gel GF254 with detection by ultraviolet (UV). Melting points were obtained using an X-4 binocular microscope melting point (mp) apparatus and are uncorrected. Yields were not optimized. 1H-NMR spectra and 13C-NMR spectra were recorded utilizing a Bruker AV400 spectrometer with CDCl3 as solvent and tetramethylsilane as internal standard. Chemical shifts (δ) were given in parts per million (ppm). High-resolution mass spectra (HRMS) data were obtained with a Fourier transform ion cyclotron resonance mass spectrometry (FTICR-MS) spectrometer (ionspec, 7.0 T).
General synthetic procedure for the target compounds I-1–I-10, II-1–II-5, and III-1–III-4 (Scheme 1)
Scheme 1.
The synthetic route of target compounds.
E-resveratrol (0.1 g, 0.44 mmol) was dissolved in dichloromethane (20 mL), and Et3N (0.22 g, 2.19 mmol) was added. Then, PhCOCl (0.31 g, 2.19 mmol) were slowly added and the reaction mixture was stirred at room temperature. The progress of the reaction was monitored by TLC until the reaction was complete. Then the reaction mixture was added dichloromethane and water. The aqueous phase was separated and then extracted with dichloromethane twice. The combined organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and filtered. After the solvent was removed in vacuo, the residue was purified by column chromatography on silica gel to give the target compound I-1 as white solid.
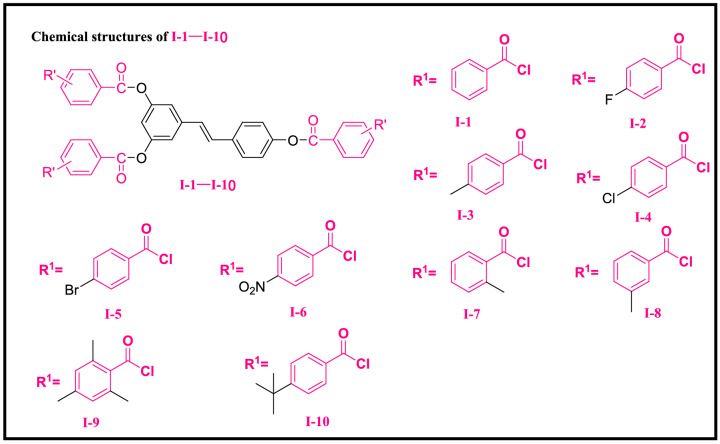
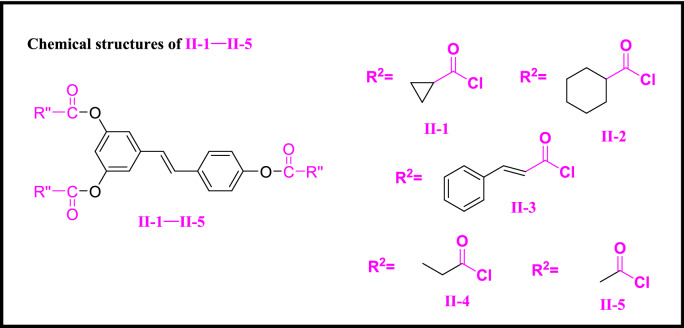
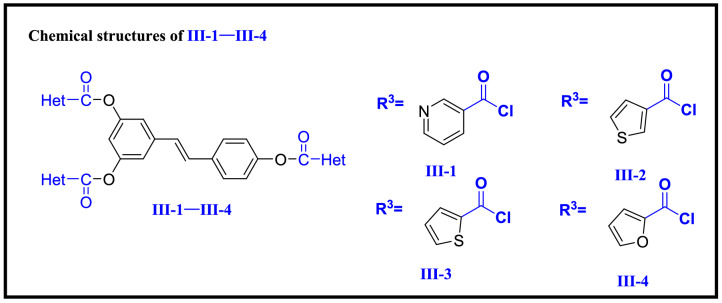
Compounds I-2–I-10 (Scheme 2), II-1–II-5 (Scheme 3) and III-1–III-4 (Scheme 4) were synthesized according to the method used for compound I-1.
Scheme 2.
Chemical structures of I-1–I-10.
Scheme 3.
Chemical structures of II-1–II-5.
Scheme 4.
Chemical structures of III-1–III-4.
Data for (E)-5-(4-(benzoyloxy)styryl)-1,3-phenylene dibenzoate (I-1). White solid; yield 96%; mp 170–172 °C. 1H NMR (400 MHz, CDCl3) δ8.22 (m, 6H), 7.71–7.61 (m, 3H), 7.60–7.48 (m, 8H), 7.36–7.30 (m, 2H), 7.15 (m, 5H); 13C NMR (100 MHz, CDCl3) δ165.13, 164.86, 151.70, 150.73, 139.75, 134.56, 133.83, 133.70, 130.27, 130.23, 129.80, 129.45, 129.25, 128.68, 128.63, 127.78, 127.27, 122.09, 117.24, 114.82; HRMS (ESI) (m/z): calcd for C35H24O6 [M + NH4]+558.1911, found 558.1911.
Data for (E)-5-(4-((4-fluorobenzoyl)oxy)styryl)-1,3-phenylene bis(4-fluorobenzoate) (I-2). White solid; yield 81%; mp 167–169 °C. 1H NMR (400 MHz, CDCl3) δ8.28–8.20 (m, 6H), 7.56 (d, J = 8.0 Hz, 2H), 7.31 (d, J = 2.0 Hz, 2H), 7.25–7.17 (m, 8H), 7.15–7.04 (m, 3H); 13C NMR (100 MHz, CDCl3) δ164.45 (d, J = 119 Hz, 1C), 164.14, 151.56, 150.62, 139.80, 134.59, 132.89 (d, J = 9 Hz, 1C), 132.90, 132.80, 129.87, 127.80, 127.24, 125.47, 122.05, 117.24, 116.05, 115.97, 115.83, 115.74, 114.72; HRMS (ESI) (m/z): calcd for C35H21F3O6 [M + NH4]+ 612.1628, found 612.1630.
Data for (E)-5-(4-((4-methylbenzoyl)oxy)styryl)-1,3-phenylene bis(4-methylbenzoate) (I-3). White solid; yield 80%; mp 159–161 °C. 1H NMR (400 MHz, CDCl3) δ8.14–8.07 (m, 6H), 7.55 (d, J = 8.4 Hz, 2H), 7.36–7.28 (m, 8H), 7.24–7.02 (m, 5H), 2.46 (s, 9H); 13C NMR (100 MHz, CDCl3) δ165.16, 164.89, 151.76, 150.79, 144.64, 144.50, 139.66, 134.50, 130.29, 130.26, 129.73, 129.38, 129.32, 127.72, 127.30, 126.72, 126.54, 122.10, 117.16, 114.86, 21.80; HRMS (ESI) (m/z): calcd for C38H30O6 [M + NH4]+ 600.2381, found 600.2379.
Data for (E)-5-(4-((4-chlorobenzoyl)oxy)styryl)-1,3-phenylene bis(4-chlorobenzoate) (I-4). White solid; yield 97%; mp 165–167 °C. 1H NMR (400 MHz, CDCl3) δ8.14–8.11 (m, 4H), 8.07 (d, J = 8.4 Hz, 2H), 7.55–7.47 (m, 9H), 7.30 (d, J = 2.0 Hz, 2H), 7.21 (d, J = 8.4 Hz, 2H), 7.13–7.04 (m, 2H); 13C NMR (100 MHz, CDCl3) δ164.24, 163.96, 161.30, 151.51, 150.58, 141.42, 140.43, 140.26, 139.83, 134.61, 131.89, 131.60, 129.89, 129.38, 129.08, 129.00, 127.81, 122.00, 117.23, 114.62; HRMS (ESI) (m/z): calcd for C35H21Cl3O6 [M + NH4]+ 660.0742, found 660.0731.
Data for (E)-5-(4-((4-bromobenzoyl)oxy)styryl)-1,3-phenylene bis(4-bromobenzoate) (I-5). White solid; yield 85%; mp 183–185 °C. 1H NMR (400 MHz, CDCl3) δ8.08–8.05 (m, 4H), 7.99 (d, J = 8.0 Hz, 2H), 7.68–7.65 (m, 6H), 7.55 (d, J = 8.4 Hz, 2H), 7.31 (d, J = 2.0 Hz, 2H), 7.22 (d, J = 8.4 Hz, 2H), 7.17–7.03 (m, 3H); 13C NMR (100 MHz, CDCl3) δ164.43, 164.13, 161.46, 151.48, 150.55, 139.84, 134.61, 132.41, 132.10, 132.01, 131.95, 131.70, 129.90, 129.18, 128.08, 127.82, 127.20, 122.01, 117.25, 114.63; HRMS (ESI) (m/z): calcd for C35H21Br3O6[M + NH4]+793.9206, found 793.9171.
Data for (E)-5-(4-((4-nitrobenzoyl)oxy)styryl)-1,3-phenylene bis(4-nitrobenzoate) (I-6). Pale yellow solid; yield 79%; mp 237–239 °C. 1H NMR (400 MHz, CDCl3) δ8.42–8.27 (m, 12H), 7.59 (d, J = 8.4 Hz, 2H), 7.38 (d, J = 2.0 Hz, 2H), 7.27 (d, J = 8.0 Hz, 2H), 7.23–7.06 (m, 3H); 13C NMR (100 MHz, CDCl3) δ162.97, 151.28, 151.09, 150.39, 140.11, 134.79, 134.46, 131.40, 131.37, 130.95, 130.20, 128.86, 127.97, 127.15, 123.89, 123.80, 123.72, 121.91, 117.40, 114.37; HRMS (ESI) (m/z): calcd for C35H21N3O12 [M-OH]- 658.1092, found 657.9651.
Data for (E)-5-(4-((2-methylbenzoyl)oxy)styryl)-1,3-phenylene bis(2-methylbenzoate) (I-7). White solid; yield 81%; mp 192–193 °C. 1H NMR (400 MHz, CDCl3) δ8.21–8.15 (m, 3H), 7.57 (d, J = 8.4 Hz, 2H), 7.52–7.47 (m, 3H), 7.36–7.31 (m, 8H), 7.22 (d, J = 8.4 Hz, 2H), 7.15–7.05 (m, 3H), 2.70 (s, 6H), 2.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ165.74, 165.38, 151.70, 150.68, 141.57, 141.43, 139.72, 134.54, 132.96, 132.83, 132.06, 132.01, 131.30, 131.21, 129.75, 128.18, 127.75, 127.29, 126.02, 125.96, 122.19, 117.26, 114.98, 22.07, 22.00; HRMS (ESI) (m/z): calcd for C38H30O6 [M + NH4]+ 600.2381, found 600.2379.
Data for (E)-5-(4-((3-methylbenzoyl)oxy)styryl)-1,3-phenylene bis(3-methylbenzoate) (I-8). White solid; yield 78%; mp 107–108 °C. 1H NMR (400 MHz, CDCl3) δ8.04–8.00 (m, 6H), 7.56 (d, J = 8.4 Hz, 2H), 7.47–7.38 (m, 6H), 7.32 (d, J = 2.0 Hz, 2H), 7.23 (t, J = 8.0 Hz, 2H), 7.18–7.04 (m, 3H), 2.46 (s, 6H), 2.45 (s, 3H); 13C NMR (100 MHz, CDCl3) δ165.27, 164.98, 151.77, 150.80, 139.72, 138.51, 138.46, 134.56, 134.44, 130.77, 130.72, 129.78, 129.41, 129.23, 128.55, 128.50, 127.75, 127.42, 127.37, 127.32, 122.08, 117.17, 114.80, 21.32; HRMS (ESI) (m/z): calcd for C38H30O6 [M + NH4]+ 600.2381, found 600.2379.
Data for (E)-5-(4-((2,4,6-trimethylbenzoyl)oxy)styryl)-1,3-phenylene bis(2,4,6-trimethylbenzoate) (I-9). White solid; yield 79%; mp 173–175 °C. 1H NMR (400 MHz, CDCl3) δ7.59 (d, J = 8.4 Hz, 2H), 7.33 (d, J = 1.6 Hz, 2H), 7.27–7.24 (m, 2H), 7.17–7.06 (m, 3H), 6.95–6.91 (m, 6H), 2.48 (s, 12H), 2.46 (s, 6H), 2.33 (s, 6H), 2.32 (s, 3H); 13C NMR (100 MHz, CDCl3) δ168.31, 167.88, 151.62, 150.56, 140.27, 140.09, 139.80, 135.93, 135.69, 134.62, 129.85, 129.51, 128.79, 128.70, 127.86, 127.33, 121.95, 117.04, 114.26, 21.23, 20.24, 20.07; HRMS (ESI) (m/z): calcd for C44H42O6 [M + NH4]+ 684.3320, found 684.3316.
Data for (E)-5-(4-((4-(tert-butyl)benzoyl)oxy)styryl)-1,3-phenylene bis(4-(tert-butyl)benzoate) (I-10). Pale yellow solid; yield 74%; mp 102–103 °C. 1H NMR (400 MHz, CDCl3) δ8.16–8.12 (m, 6H), 7.57–7.52 (m, 8H), 7.30 (d, J = 2.0 Hz, 2H), 7.21 (d, J = 8.6 Hz, 2H), 7.14–7.02 (m, 3H), 1.38 (s, 18H), 1.37 (s, 9H); 13C NMR (100 MHz, CDCl3) δ165.11, 164.86, 157.62, 157.49, 151.78, 150.80, 139.66, 134.51, 130.18, 130.14, 129.74, 127.74, 127.30, 126.66, 126.47, 125.67, 125.61, 122.12, 117.19, 114.87, 35.25, 31.14; HRMS (ESI) (m/z): calcd for C47H48O6 [M + NH4]+ 726.3789, found 726.4049.
Data for (E)-5-(4-((cyclopropanecarbonyl)oxy)styryl)-1,3-phenylene dicyclopropanecarboxylate (II-1). Yellow solid; yield 71%; mp 136–138 °C. 1H NMR (400 MHz, CDCl3) δ7.47 (d, J = 8.4 Hz, 2H), 7.13–6.91 (m, 6H), 6.83 (t, J = 2.0 Hz, 1H), 1.89–1.79 (m, 3H), 1.22–1.14 (m, 6H), 1.07–0.99 (m, 6H); 13C NMR (100 MHz, CDCl3) δ173.44, 173.11, 151.40, 150.52, 139.42, 134.36, 129.55, 127.59, 127.19, 121.92, 116.82, 114.50, 13.04, 9.40, 9.35; HRMS (ESI) (m/z): calcd for C26H24O6 [M + NH4]+ 450.1911, found 450.1914.
Data for (E)-5-(4-((cyclohexanecarbonyl)oxy)styryl)-1,3-phenylene dicyclohexanecarboxylate (II-2). White solid; yield 72%; mp 131–133 °C. 1H NMR (400 MHz, CDCl3) δ7.47 (d, J = 8.4 Hz, 2H), 7.08 (.m, J = 1.6 Hz, 3H), 7.04 (d, J = 5.12 Hz, 2H), 7.00–6.91 (m, 1H), 6.82–6.72 (m, 1H), 2.60–2.50 (m, 3H), 2.12–2.02 (m, 6H), 1.87–1.78 (m, 6H), 1.71–1.68 (m, 2H), 1.65–1.55 (m, 6H), 1.43–1.24 (m, 10H); 13C NMR (100 MHz, CDCl3) δ174.47, 174.11, 151.56, 150.65, 139.44, 134.34, 129.55, 127.58, 127.20, 121.88, 116.73, 114.42, 43.22, 28.94, 25.72, 25.36; HRMS (ESI) (m/z): calcd for C35H42O6 [M + NH4]+ 576.3320, found 576.3323.
Data for 5-((E)-4-(cinnamoyloxy)styryl)-1,3-phenylene (2E,2′E)-bis(3-phenylacrylate) (II-3). Yellow solid; yield 83%; mp 87–89 °C. 1H NMR (400 MHz, CDCl3) δ7.93–7.86 (m, 3H), 7.62–7.57 (m, 6H), 7.53 (d, J = 8.6 Hz, 2H), 7.46–7.38 (m, 10H), 7.25 (d, J = 2.3 Hz, 2H), 7.18 (d, J = 8.6 Hz, 2H), 7.15–6.98 (m, 3H), 6.67–6.61 (m, 3H); 13C NMR (100 MHz, CDCl3) δ165.36, 165.04, 151.52, 150.57, 147.07, 146.80, 139.62, 134.51, 134.16, 134.11, 130.86, 130.79, 129.70, 129.06, 128.98, 128.41, 128.36, 127.73, 127.30, 121.98, 117.19, 117.01, 114.58; HRMS (ESI) (m/z): calcd for C41H30O6 [M + NH4]+ 636.2381, found 636.2383.
Data for (E)-5-(4-(propionyloxy)styryl)-1,3-phenylene dipropionate (II-4). Brown solid; yield 89%; mp 56–58 °C. 1H NMR (400 MHz, CDCl3) δ7.48 (d, J = 8.4 Hz, 2H), 7.11 (d, J = 2.0 Hz, 2H), 7.10–7.07 (m, 2H), 7.05–6.92 (m, 2H), 6.82 (t, J = 2.0 Hz, 1H), 2.63–2.56 (m, 6H), 1.27 (t, J = 7.6 Hz, 9H); 13C NMR (100 MHz, CDCl3) δ172.92, 172.57, 151.41, 150.52, 139.50, 134.39, 129.60, 127.63, 127.20, 121.89, 116.82, 114.41, 27.78, 9.08, 9.05; HRMS (ESI) (m/z): calcd for C23H24O6 [M + NH4]+ 414.1911, found 414.1910.
Data for (E)-5-(4-acetoxystyryl)-1,3-phenylene diacetate (II-5). White solid; yield 65%; mp 186–188 °C. 1H NMR (400 MHz, CDCl3) δ7.48 (d, J = 8.5 Hz, 2H), 7.13–7.11 (m, 2H), 7.10–7.07 (m, 2H), 7.05–6.93 (m, 2H), 6.84–6.81 (m, 1H), 2.31 (s, 9H); 13C NMR (100 MHz, CDCl3) δ169.45, 169.04, 151.32, 150.44, 139.55, 134.48, 129.67, 127.68, 127.20, 121.91, 116.93, 114.42, 21.14; HRMS (ESI) (m/z): calcd for C20H18O6 [M + NH4]+ 372.1442, found 372.1443.
Data for (E)-5-(4-(nicotinoyloxy)styryl)-1,3-phenylene dinicotinate (III-1). Yellow solid; yield 71%; mp 135–137 °C. 1H NMR (400 MHz, CDCl3) δ9.45–9.39 (m, 3H), 8.92–8.85 (m, 3H), 8.51–8.44 (m, 3H), 7.58 (d, J = 8.4 Hz, 1H), 7.54–7.47 (m, 4H), 7.37 (d, J = 1.6 Hz, 1H), 7.25–7.20 (m, 2H), 7.17–7.14 (m, 1H), 7.12–6.92 (m, 3H); 13C NMR (100 MHz, CDCl3) δ163.79, 163.49, 154.18, 151.36, 151.26, 137.77, 134.74, 130.08, 127.91, 127.76, 127.19, 125.33, 123.65, 123.60, 121.96, 121.85, 117.37; HRMS (ESI) (m/z): calcd for C32H21N3O6 [M + H]+ 544.1503, found 544.1505.
Data for (E)-5-(4-((thiophene-2-carbonyl)oxy)styryl)-1,3-phenylene bis(thiophene-3-carboxylate) (III-2). Brown solid; yield 70%; mp 138–140 °C. 1H NMR (400 MHz, CDCl3) δ8.34–8.29 (m, 3H), 7.69–7.65 (m, 3H), 7.53 (d, J = 8.6 Hz, 2H), 7.40–7.36 (m, 3H), 7.29 (d, J = 2.0 Hz, 2H), 7.20 (d, J = 8.6 Hz, 2H), 7.16–7.01 (m, 3H); 13C NMR (100 MHz, CDCl3) δ160.96, 160.65, 151.42, 150.47, 139.70, 134.54, 134.35, 134.17, 132.78, 132.56, 129.77, 128.24, 127.76, 127.26, 126.55, 126.45, 122.06, 117.19, 114.74; HRMS (ESI) (m/z): calcd for C29H18O6S3 [M + NH4]+ 576.0604, found 576.0605.
Data for (E)-5-(4-((thiophene-2-carbonyl)oxy)styryl)-1,3-phenylene bis(thiophene-2-carboxylate) (III-3). Yellow solid; yield 78%; mp 113–115 °C. 1H NMR (400 MHz, CDCl3) δ8.03–7.96 (m, 3H), 7.71–7.65 (m, 3H), 7.54 (d, J = 8.3 Hz, 2H), 7.34–7.29 (m, 2H), 7.23 (d, J = 8.4 Hz, 2H), 7.20–7.16 (m, 3H), 7.13–7.01 (m, 3H); 13C NMR (100 MHz, CDCl3) δ160.50, 160.19, 151.27, 150.37, 139.70, 134.99, 134.81, 134.61, 133.88, 133.64, 132.78, 132.52, 129.84, 128.15, 128.09, 127.76, 127.25, 122.01, 117.22, 114.62; HRMS (ESI) (m/z): calcd for C29H18O6S3 [M + NH4]+ 576.0604, found 576.0605.
Data for (E)-5-(4-((furan-2-carbonyl)oxy)styryl)-1,3-phenylene bis(furan-2-carboxylate) (III-4). Brown solid; yield 85%; mp 144–146 °C. 1H NMR (400 MHz, CDCl3) δ7.73–7.66 (m, 3H), 7.53 (d, J = 7.9 Hz, 2H), 7.43–7.37 (m, 3H), 7.32–7.28 (m, 2H), 7.22 (d, J = 8.1 Hz, 2H), 7.15–6.98 (m, 3H), 6.64–6.56 (m, 3H); 13C NMR (100 MHz, CDCl3) δ156.83, 156.46, 150.92, 149.99, 147.46, 147.27, 143.89, 143.68, 139.76, 134.65, 129.88, 127.81, 127.23, 121.95, 119.87, 119.63, 117.21, 114.48, 112.32, 112.26; HRMS (ESI) (m/z): calcd for C29H18O9 [M + NH4]+ 528.1289, found 528.1291.
Biological assay
Each bioassay was repeated three times at 25 ± 1 °C. The activity results were estimated according to a percentage scale of 0–100 (0 indicating no activity and 100 indicating total mortality). The bioassay procedures for the anti-TMV, fungicidal, and insecticidal activities of the synthesized compounds are described in detailed in our published literature and can also be found in the “Supporting Information S1”30.
Results and discussion
Synthesis
In order to investigate structure–activity relationships (SARs), resveratrol was chosen as a precursor according to the in vivo anti-TMV activity listed in Table 1. Several series of resveratrol derivatives I-1–I-10, II-1–II-5 and III-1–III-4 were synthesized according to procedures in Scheme 1. As shown in Scheme 1, commercially available E-resveratrol was reacted with corresponding aryl chloride, alkyl chloride and heterocyclic chloride to give resveratrol esters I-1–I-10, II-1–II-5 and III-1–III-4 in good yields. The detailed procedure is given in “Materials and methods”. It’s a simple route for the preparation of resveratrol derivatives with favorable yield.
Table 1.
The anti-TMV activity of I-1–I-10, II-1–II-5 and III-1–III-4.
| Compounds | Concentration (μg/mL) | Relative inhibition rate (%)a | ||
|---|---|---|---|---|
| Inactivation effect | Curative effect | Protection effect | ||
| I-1 | 500 | 32 ± 2 | ||
| I-2 | 500 | 36 ± 1 | ||
| I-3 | 500 | 34 ± 3 | ||
| I-4 | 500 | 35 ± 3 | ||
| I-5 | 500 | 42 ± 4 | ||
| I-6 | 500 | 38 ± 2 | ||
| I-7 | 500 | 37 ± 2 | ||
| I-8 | 500 | 38 ± 3 | ||
| I-9 | 500 | 54 ± 1 | 54 ± 4 | 55 ± 3 |
| 100 | 24 ± 1 | 15 ± 1 | 20 ± 1 | |
| I-10 | 500 | 51 ± 1 | 56 ± 2 | 47 ± 2 |
| 100 | 21 ± 1 | 18 ± 2 | 17 ± 2 | |
| II-1 | 500 | 23 ± 5 | ||
| II-2 | 500 | 43 ± 1 | 44 ± 4 | 40 ± 2 |
| 100 | 24 ± 2 | 15 ± 1 | 0 | |
| II-3 | 500 | 39 ± 3 | ||
| II-4 | 500 | 41 ± 5 | 43 ± 2 | 46 ± 2 |
| 100 | 26 ± 1 | 0 | 25 ± 1 | |
| II-5 | 500 | 54 ± 1 | 52 ± 2 | 51 ± 4 |
| 100 | 25 ± 2 | 28 ± 1 | 23 ± 1 | |
| III-1 | 500 | 30 ± 2 | ||
| III-2 | 500 | 50 ± 1 | 53 ± 1 | 59 ± 4 |
| 100 | 25 ± 2 | 20 ± 2 | 28 ± 1 | |
| III-3 | 500 | 38 ± 3 | ||
| III-4 | 500 | 57 ± 1 | 59 ± 3 | 51 ± 4 |
| 100 | 20 ± 2 | 26 ± 1 | 21 ± 1 | |
| Resveratrolb | 500 | 50 ± 1 | 43 ± 2 | 45 ± 2 |
| 100 | 13 ± 1 | 15 ± 1 | 18 ± 1 | |
| Ribavirinb | 500 | 38 ± 2 | 37 ± 1 | 40 ± 1 |
| 100 | 11 ± 2 | 13 ± 1 | 12 ± 1 | |
aAverage of three replicates. All results are expressed as the mean ± standard deviation (SD). Prominent activity data were presented in italics.
bPositive control.
Phytotoxic activity
Phytotoxic activity (according to the criterion of safety evaluation of pesticide to crops, NYT 1965.1-2010) of compounds I-1–I-10, II-1–II-5 and III-1–III-4 against tobacco was first tested. The data of phytotoxic activity at 500 μg/mL indicated that all of the compounds I-1–I-10, II-1–II-5 and III-1–III-4 showed no toxicity to the tested plant.
Antiviral activity
Compounds I-1–I-10, II-1–II-5 and III-1–III-4 were evaluated for their antiviral activity against TMV. The results of anti-TMV activities of these derivatives are listed in Table 1. To make a judgment on the antiviral potency of the synthesized compounds, the commercially available plant virucide ribavirin and lead compound resveratrol were used as the controls. All of the compounds were tested at both 500 and 100 μg/mL.
The synthesized compounds showed similar or higher in vivo activity against TMV than the commercial plant virucide ribavirin. At the concentration of 500 μg/mL, resveratrol ester derivative containing meta-thienyl moieties III-2 displayed the best inhibitory effect of inactivation activity, curative activity, and protection activity with values of 50, 53, and 59%, respectively. Ribavirin as a control was studied at the same conditions with values of 38, 37, and 40%, respectively. In contrast to the experimental data, the results indicated that compound III-2 was more efficient than ribavirin in vivo activity against TMV. At the concentration of 100 μg/mL, III-2 displayed inactivation activity, 25%; curative activity, 20%; and protection activity, 28%; which is higher than that of ribavirin (inactivation activity, 11%; curative activity, 13%; and protection activity, 12%). Resveratrol ester derivative III-4 bearing furan groups also gave relatively higher activity (57, 59, and 51% at 500 μg/mL) than ribavirin. Especially the curative activity at 500 μg/mL of III-4 was higher than ribavirin and resveratrol, which indicated that the introduction of heterocycle might lead to the increase of inhibitory effect. But introduction of pyridyl (III-1) and ortho-thienyl (III-4) resulted in a decline of anti-TMV activity27. Compound II-5 with alkyl groups such as methyl resulted in an obvious improvement of anti-TMV activity which exhibited better curative and protection activities in vivo against TMV than ribavirin. More importantly, the inactivation activity of compound II-5 was much higher than ribavirin. But other derivatives bearing alkyl groups II-2, II-4, II-1, II-3 in series II showed similar or lower in vivo anti-TMV activity than ribavirin31. The introduction of aromatic substituents resulted in a decline of anti-TMV activity, however aromatic substituents of resveratrol with electron-donating groups such as methyl (I-9) or t-butyl (I-10) showed more excellent in vivo anti-TMV activity than ribavirin27.
Fungicidal activity
The resveratrol and its derivatives were also evaluated for their fungicidal activities with the commercial fungicides chlorothalonil and carbendazim as the controls. Resveratrol and its derivatives all exhibited fungicidal activities to some extent against 14 kinds of plant pathogens (Fusarium oxysporum sp. cucumeris; Cercospora arachidicola Hori; Physalospora piricola; Rhizoctonia cerealis; Bipolaris maydis; Colletotrichum orbiculare; Fusarium moniliforme; Alternaria solani; Fusarium graminearum; Phytophthora infestans; Phytophthora capsici; Sclerotinia sclerotiorum; Botrytis cinerea; Rhizoctonia solani) by mycelial growth method (Table 2). As shown in Table 2, target compounds exhibited broad spectrum fungicidal activities against 14 kinds of phytopathogenic fungi at 50 μg/mL. However, compared with commercial fungicides carbendazim and chlorothalonil, these derivatives were less potent.
Table 2.
Fungicidal activity of the compounds I-1–I-10, II-1–II-5 and III-1–III-4 against 14 kinds of phytopathogens.
| Compd | Fungicidal activity %/50 μg/mLa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F.Cb | C.H | P.P | R.C | B.M | W.A | F.M | A.S | F.G | P.I | P.C | S.S | R.S | B.C | |
| I-1 | 18 ± 1 | 33 ± 1 | 17 ± 1 | 26 ± 1 | 5 ± 1 | 18 ± 2 | 19 ± 2 | 43 ± 2 | 36 ± 2 | 5 ± 1 | 23 ± 2 | 40 ± 2 | 12 ± 1 | 13 ± 1 |
| I-2 | 14 ± 1 | 33 ± 1 | 33 ± 1 | 21 ± 1 | 5 ± 1 | 12 ± 2 | 6 ± 1 | 36 ± 1 | 36 ± 1 | 5 ± 1 | 26 ± 2 | 20 ± 1 | 16 ± 1 | 13 ± 1 |
| I-3 | 18 ± 1 | 25 ± 1 | 50 ± 1 | 33 ± 1 | 27 ± 1 | 35 ± 2 | 31 ± 1 | 43 ± 2 | 21 ± 1 | 41 ± 2 | 23 ± 1 | 20 ± 1 | 48 ± 2 | 7 ± 2 |
| I-4 | 5 ± 1 | 33 ± 1 | 33 ± 1 | 5 ± 1 | 14 ± 2 | 6 ± 1 | 13 ± 2 | 21 ± 1 | 14 ± 1 | 23 ± 2 | 29 ± 1 | 20 ± 1 | 15 ± 3 | 13 ± 1 |
| I-5 | 9 ± 1 | 33 ± 1 | 50 ± 1 | 37 ± 3 | 18 ± 1 | 6 ± 1 | 13 ± 1 | 21 ± 1 | 27 ± 2 | 27 ± 1 | 26 ± 2 | 20 ± 1 | 31 ± 1 | 27 ± 3 |
| I-6 | 5 ± 1 | 8 ± 1 | 17 ± 2 | 16 ± 3 | 9 ± 1 | 6 ± 1 | 19 ± 3 | 21 ± 1 | 36 ± 2 | 5 ± 1 | 16 ± 1 | 30 ± 1 | 16 ± 1 | 33 ± 1 |
| I-7 | 5 ± 1 | 33 ± 1 | 17 ± 2 | 21 ± 1 | 14 ± 2 | 12 ± 3 | 13 ± 2 | 14 ± 1 | 14 ± 1 | 9 ± 1 | 16 ± 1 | 10 ± 1 | 25 ± 2 | 13 ± 1 |
| I-8 | 14 ± 2 | 25 ± 1 | 8 ± 1 | 11 ± 2 | 14 ± 2 | 6 ± 1 | 13 ± 1 | 14 ± 1 | 36 ± 2 | 9 ± 1 | 16 ± 1 | 20 ± 1 | 20 ± 2 | 20 ± 1 |
| I-9 | 5 ± 1 | 17 ± 2 | 33 ± 1 | 16 ± 3 | 18 ± 1 | 12 ± 3 | 6 ± 1 | 7 ± 1 | 36 ± 2 | 14 ± 2 | 16 ± 1 | 30 ± 1 | 5 ± 2 | 13 ± 1 |
| I-10 | 5 ± 1 | 33 ± 1 | 25 ± 1 | 21 ± 1 | 14 ± 2 | 12 ± 3 | 13 ± 2 | 14 ± 1 | 21 ± 1 | 14 ± 2 | 16 ± 1 | 20 ± 1 | 15 ± 3 | 7 ± 2 |
| II-1 | 5 ± 1 | 33 ± 1 | 42 ± 3 | 37 ± 3 | 17 ± 2 | 6 ± 3 | 19 ± 2 | 7 ± 1 | 36 ± 2 | 14 ± 2 | 19 ± 1 | 30 ± 1 | 31 ± 1 | 33 ± 1 |
| II-2 | 14 ± 2 | 8 ± 1 | 17 ± 2 | 16 ± 3 | 5 ± 1 | 6 ± 3 | 13 ± 2 | 7 ± 1 | 21 ± 1 | 14 ± 2 | 19 ± 1 | 30 ± 1 | 20 ± 3 | 20 ± 1 |
| II-3 | 18 ± 1 | 8 ± 1 | 42 ± 3 | 21 ± 1 | 5 ± 1 | 6 ± 3 | 19 ± 3 | 7 ± 1 | 7 ± 1 | 5 ± 2 | 19 ± 1 | 20 ± 1 | 31 ± 1 | 33 ± 1 |
| II-4 | 18 ± 1 | 17 ± 2 | 33 ± 1 | 32 ± 2 | 18 ± 1 | 18 ± 2 | 25 ± 1 | 14 ± 1 | 7 ± 1 | 5 ± 2 | 26 ± 3 | 20 ± 1 | 21 ± 1 | 20 ± 1 |
| II-5 | 5 ± 2 | 33 ± 1 | 42 ± 2 | 42 ± 1 | 18 ± 1 | 47 ± 1 | 13 ± 1 | 36 ± 2 | 36 ± 2 | 18 ± 1 | 29 ± 1 | 10 ± 1 | 36 ± 1 | 13 ± 1 |
| III-1 | 14 ± 2 | 25 ± 1 | 8 ± 1 | 26 ± 3 | 14 ± 2 | 6 ± 3 | 25 ± 1 | 7 ± 1 | 7 ± 1 | 18 ± 1 | 13 ± 3 | 20 ± 1 | 31 ± 1 | 20 ± 1 |
| III-2 | 14 ± 2 | 8 ± 1 | 8 ± 1 | 21 ± 1 | 5 ± 1 | 6 ± 3 | 13 ± 2 | 14 ± 1 | 21 ± 1 | 14 ± 2 | 23 ± 2 | 10 ± 1 | 15 ± 3 | 7 ± 2 |
| III-3 | 5 ± 1 | 17 ± 2 | 8 ± 1 | 21 ± 1 | 14 ± 2 | 18 ± 2 | 6 ± 1 | 21 ± 1 | 36 ± 2 | 14 ± 2 | 23 ± 2 | 20 ± 1 | 16 ± 1 | 20 ± 1 |
| III-4 | 14 ± 2 | 8 ± 1 | 33 ± 1 | 11 ± 1 | 5 ± 1 | 17 ± 2 | 6 ± 1 | 36 ± 2 | 36 ± 2 | 9 ± 1 | 19 ± 1 | 20 ± 1 | 12 ± 1 | 20 ± 1 |
| Resveratr-old | 27 ± 1 | 42 ± 2 | 25 ± 1 | 42 ± 1 | 36 ± 1 | 35 ± 1 | 13 ± 1 | 43 ± 2 | 21 ± 1 | 36 ± 1 | 42 ± 2 | 40 ± 1 | 48 ± 1 | 40 ± 1 |
| Chlorothal-onilc | 100 | 70 ± 1 | 100 | 75 ± 1 | < 50 | 100 | < 50 | 100 | 100 | 92 ± 1 | 92 ± 1 | 87 ± 1 | 100 | 100 |
| Carbenda-zimc | < 50 | < 50 | < 50 | < 50 | 100 | < 50 | 100 | < 50 | 100 | 100 | 100 | 100 | 100 | < 50 |
aAverage of three replicates. All results are expressed as the mean ± SD.
bF.C, Fusarium oxysporium f. sp. cucumeris; C.H, Cercospora arachidicola Hori; P.P, Physalospora piricola; R.C, Rhizoctonia cerealis; B.M, Bipolaris maydis; W.A, Watermelon anthracnose; F.M, Fusarium moniliforme; A.S, Alternaria solani; F.G, Fusarium graminearum; P.I, Phytophthora infestans; P.C, Phytophthora capsici; S.S, Sclerotinia sclerotiorum; R.S, Rhizoctonia solani; B.C, Botrytis cinerea.
cThe commercial agricultural fungicides chlorothalonil and carbendazim were used for comparison of antifungal activities.
dPositive control.
Insecticidal activities
The insecticidal activities of target compounds and resveratrol against oriental armyworm (Mythimna separata), cotton bollworm (Helicoverpa armigera), corn borer (Ostrinia furnacalis), and Mosquito (Culex pipiens pallens) are listed in Table 3. The results indicated that many compounds exhibited higher activities than resveratrol. Especially compounds II-3, III-3, and III-4 exhibited obviously higher activities against oriental armyworm (70%, 70%, 60% at 600 μg/mL) than resveratrol.
Table 3.
Insecticidal activities of the target compounds I-1–I-10, II-1–II-5 and III-1–III-4 (mortalitya, percent).
| Compd | Cotton bollworm (600 μg/mL) | Corn borer (600 μg/mL) | Oriental armyworm (600 μg/mL) | Mosquito (10 μg/mL) |
|---|---|---|---|---|
| I-1 | 20 ± 2 | 5 ± 1 | 20 ± 2 | 5 ± 1 |
| I-2 | 0 | 0 | 10 ± 2 | 0 |
| I-3 | 5 ± 1 | 0 | 10 ± 2 | 0 |
| I-4 | 0 | 0 | 5 ± 1 | 20 ± 2 |
| I-5 | 0 | 0 | 0 | 0 |
| I-6 | 10 ± 2 | 10 ± 2 | 15 ± 2 | 5 ± 1 |
| I-7 | 0 | 5 ± 1 | 10 ± 2 | 0 |
| I-8 | 10 ± 2 | 15 ± 2 | 20 ± 2 | 0 |
| I-9 | 0 | 0 | 0 | 5 ± 1 |
| I-10 | 20 ± 2 | 10 ± 2 | 40 ± 2 | 0 |
| II-1 | 5 ± 1 | 20 ± 2 | 30 ± 2 | 10 ± 2 |
| II-2 | 0 | 15 ± 2 | 20 ± 2 | 0 |
| II-3 | 30 ± 2 | 35 ± 2 | 70 ± 3 | 5 ± 1 |
| II-4 | 10 ± 2 | 10 ± 2 | 30 ± 2 | 20 ± 2 |
| II-5 | 15 ± 2 | 20 ± 2 | 50 ± 2 | 10 ± 2 |
| III-1 | 5 ± 1 | 5 ± 1 | 10 ± 1 | 20 ± 2 |
| III-2 | 10 ± 2 | 15 ± 2 | 40 ± 2 | 0 |
| III-3 | 25 ± 2 | 35 ± 2 | 70 ± 2 | 30 ± 2 |
| III-4 | 30 ± 2 | 25 ± 2 | 60 ± 2 | 0 |
| Resveratrolb | 5 ± 1 | 0 | 10 ± 2 | 70 ± 2 |
aAverage of three replicates. All results are expressed as the mean ± standard deviation (SD).
bPositive control.
However, though some compounds exhibited insecticidal activity on some species to some extent, the potency of these compounds as insecticide was not comparable with that of commercial insecticides. More modification on the structure should be conducted.
Conclusion
In summary, the natural product resveratrol provides an unparalleled source of inspiration for the screening of antiviral drugs. With resveratrol as lead compound by chemical modification, a series of resveratrol ester derivatives were designed and synthesized. Their activities against TMV were evaluated. The optimum compounds III-2, III-4, and II-5, I-9, I-10 displayed higher activity than commercial plant virucide ribavirin. This paper also discussed the anti-TMV activities and the structure–activity relationships of resveratrol ester derivatives, providing a reference for the development of new drugs. Resveratrol as important phytoalexin, its structure is simple and is a kind of lead compound which is extremely potential in the field of pesticide development, so the structure modification of resveratrol is a very valuable and meaningful research work.
Supplementary Information
Acknowledgements
This work was supported by The National Natural Science Foundation of China (no. 22007043), A Project of Shandong Province Higher Educational Science and Technology Program (no. J18KA156), Doctoral Scientific Research Foundation of Linyi University (no. LYDX2016BS097).
Author contributions
Experiment, P.S.; structure–activity relationships, W.Y.; writing—original draft preparation, X.Y. and Q.W.; writing—review and editing, P.S., X.Y. and Q.W.; supervision, Q.W.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiuling Yu, Email: yxlwell@163.com.
Qingmin Wang, Email: wangqm@nankai.edu.cn.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-96069-1.
References
- 1.Song BA, Yang S, Jin LH, Bhadury PS. Environment-Friendly Anti-Plant Viral Agents. Chemical Industry Press (Beijing) & Springer Press; 2009. pp. 1–305. [Google Scholar]
- 2.Ritzenthaler C. Resistance to plant viruses: Old issue, news answers? Curr. Opin. Biotechnol. 2005;16:118–122. doi: 10.1016/j.copbio.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Wei, L. Z., Meng, F. S. Antiviral composition containing fucoidan and ribavirin for preventing and treating plant viral diseases. CN 101869111A, 2010-10-27.
- 4.Pervaiz S, Holme AL. Resveratrol: Its biologic targets and functional activity. Antioxid. Redox Signal. 2009;11:2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 5.Burns J, Yokota T, Ashihara H, Lean MEJ, Crozier A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002;50:3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 6.Ibern-Gómez M, Roig-Pérez S, Lamuela-Raventós RM, Carmen TBM. Resveratrol and piceid levels in natural and blended peanut butters. J. Agric. Food Chem. 2000;48:6352–6354. doi: 10.1021/jf000786k. [DOI] [PubMed] [Google Scholar]
- 7.Gerogiannaki-Christopoulou M, Athanasopoulos P, Kyriakidis N, Gerogiannaki IA, Spanos M. trans-Resveratrol in wines from the major Greek red and white grape varieties. Food Control. 2006;17:700–706. doi: 10.1016/j.foodcont.2005.04.006. [DOI] [Google Scholar]
- 8.Fremont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/S0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 9.Bavaresco L, Vezzulli S, Civardi S, Gatti M, Battilani P, Pietri A, Ferrari F. Effect of lime-induced leaf chlorosis on ochratoxin A, trans-resveratrol, and ε-viniferin production in grapevine (Vitis vinifera L.) berries infected by Aspergillus carbonarius. J. Agric. Food Chem. 2008;56:2085–2089. doi: 10.1021/jf073456+. [DOI] [PubMed] [Google Scholar]
- 10.Sobolev VS. Localized production of phytoalexins by peanut (Arachis hypogaea) kernels in response to invasion by Aspergillus species. J. Agric. Food Chem. 2008;56:1949–1954. doi: 10.1021/jf703595w. [DOI] [PubMed] [Google Scholar]
- 11.Soleas GJ, Diamandis EP, Goldberg DM. Resveratrol: A molecule whose time has come? And gone? Clin. Bio. Chem. 1997;30:91–113. doi: 10.1016/s0009-9120(96)00155-5. [DOI] [PubMed] [Google Scholar]
- 12.Jeandet P, Bessis R, Sbaghi M, Meunier P, Trollat P. Resveratrol content of wines of different ages: Relationship with fungal disease pressure in the vineyard. Am. J. Enol. Vitic. 1995;46:1–4. [Google Scholar]
- 13.Takaoka MJ. Of the phenolic substances of white hellebore (Veratrum grandiflorum Loes. Fil.) J. Fac. Sci. Hokkaido Imp. Univ. 1940;3:1–16. [Google Scholar]
- 14.Nonomura S, Kanagawa H, Makimoto A. Chemical constituents of polygonaceous plants. I. Studies on the components of Ko-jo-kon (Polygonum cuspidatum Sieb. et Zucc.) Yakugaku Zasshi. 1963;83:988–990. doi: 10.1248/yakushi1947.83.10_988. [DOI] [PubMed] [Google Scholar]
- 15.Wu CF, Yang JY, Wang F, Wang XX. Resveratrol, botanical origin, pharmacological activity and applications. Chin. J. Nat. Med. 2013;11:1–15. doi: 10.3724/SP.J.1009.2013.00001. [DOI] [Google Scholar]
- 16.Pettit GR, Anderson CR, Herald DL, Jung MK, Lee DJ, Hamel E, Pettit RK. Antineoplastic agents. 487. Synthesis and biological evaluation of the antineoplastic agent 3,4-methylene dioxy-5,4′-dimethoxy-3′-amino-Z-stilbene and derived amino acid amides. J. Med. Chem. 2003;46:525–531. doi: 10.1021/jm020204l. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura H, Kuroda H, Saito H, Suzuki R, Yamori T, Maruyama K, Haga T. Synthesis and biological evaluation of boronic acid containing cis-stilbenes as apoptotic tubulin polymerization inhibitors. Chem. Med. Chem. 2006;1:729–740. doi: 10.1002/cmdc.200600068. [DOI] [PubMed] [Google Scholar]
- 18.Zheng X, Yu LY, Yao X, Lv B, Yang ZH, Zheng QT, Duan HY, Song C, Xie HL. Synthesis and anti-cancer activities of resveratrol derivatives. Open J. Med. Chem. 2016;6:51–57. [Google Scholar]
- 19.Li YQ, Li ZL, Zhao WJ, Wen RX, Meng QW, Zeng Y. Synthesis of stilbene derivatives with inhibition of SARS coronavirus replication. Eur. J. Med. Chem. 2006;41:1084–1089. doi: 10.1016/j.ejmech.2006.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merillon JM, Fauconneau B, Teguo PW, Barrier L, Vercauteren J, Huguet F. Antioxidant activity of the stilbene astringin, newly extracted from Vitis vinifera cell cultures. Clin. Chem. 1997;43:1092–1093. doi: 10.1093/clinchem/43.6.1092. [DOI] [PubMed] [Google Scholar]
- 21.Inamori Y, Kubo M, Ogawa M, Tsujibo H, Miki Y, Takemura S. Biological activities of 3,3′-dihydroxy-alpha, beta-diethylstilbene and its isomer, diethylstilbestrol. Chem. Pharm. Bull. 1987;35:3502–3506. doi: 10.1248/cpb.35.3502. [DOI] [PubMed] [Google Scholar]
- 22.Carella A, Castaldo A, Centore R, Fort A, Sirigu A, Tuzi A. Synthesis and second order nonlinear optical properties of new chromophores containing 1,3,4-oxadiazole and thiophene rings. J. Chem. Soc. Perkin Trans. 2002;2(11):1791–1795. doi: 10.1039/b207230k. [DOI] [Google Scholar]
- 23.Gester S, Wuest F, Pawelke B, Bergmann R, Pietzsch J. Synthesis and biodistribution of an 18F-labelled resveratrol derivative for small animal positron emission tomography. Amino Acids. 2005;29:415–428. doi: 10.1007/s00726-005-0205-x. [DOI] [PubMed] [Google Scholar]
- 24.Wang XM, Zhou YF, Yu WT, Wang C, Fang Q, Jiang MH, Lei H, Wang HZ. Two-photon pumped lasing stilbene-type chromophores containing various terminal donor groups: Relationship between lasing efficiency and intramolecular charge transfer. J. Mater. Chem. 2000;10:2698–2703. doi: 10.1039/b006764o. [DOI] [Google Scholar]
- 25.Christian E, Bernd M, Manfred N, Verena R, Barbara L, Peter B, Joachim S. Covalent binding of chloroacetamide herbicides to the active site cysteine of plant type III polyketide synthases. Phytochemistry. 2003;64:1045–1054. doi: 10.1016/S0031-9422(03)00516-8. [DOI] [PubMed] [Google Scholar]
- 26.Lu CJ, Guo YY, Li JH, Yao MC, Liao QF, Xie ZF, Li XF. Design, synthesis, and evaluation of resveratrol derivatives as Aß1–42 aggregation inhibitors, antioxidants, and neuroprotective agents. Bioorg. Med. Chem. Lett. 2012;22:7683–7687. doi: 10.1016/j.bmcl.2012.09.105. [DOI] [PubMed] [Google Scholar]
- 27.Huang XF, Ruan BF, Wang XT, Xu C, Ge HM, Zhu HL, Tan RX. Synthesis and cytotoxic evaluation of a series of resveratrol derivatives modified in C2 position. Eur. J. Med. Chem. 2007;42:263–267. doi: 10.1016/j.ejmech.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Hong T, Jiang W, Dong HM, Qiu SX, Lu Y. Synthesis and cytotoxic activities of E-resveratrol derivatives. Chin. J. Nat. Med. 2015;13:375–382. doi: 10.1016/S1875-5364(15)30029-7. [DOI] [PubMed] [Google Scholar]
- 29.Moon HI, Chung IM, Jung JC, Lim E, Lee Y, Oh S, Jung M. The convenient synthesis and evaluation of the anticancer activities of new resveratrol derivatives. J. Enzyme Inhib. Med. Chem. 2009;24:328–336. doi: 10.1080/14756360802185731. [DOI] [PubMed] [Google Scholar]
- 30.Kang J, Gao YY, Zhang MJ, Ding X, Wang ZW, Ma DJ, Wang QM. Streptindole and its derivatives as novel antiviral and anti-phytopathogenic fungus agents. J. Agric. Food Chem. 2020;68:7839–7849. doi: 10.1021/acs.jafc.0c03994. [DOI] [PubMed] [Google Scholar]
- 31.Acerson MJ, Andrus MB. Selective esterification of the polyphenol resveratrol at the 4′-position. Tetrahedron Lett. 2014;55:757–760. doi: 10.1016/j.tetlet.2013.12.019. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.