Abstract
Recently, we identified seven novel hydroxy-carboxylic acids resulting from gas-phase reactions of isoprene in the presence of nitrogen oxides (NOx), ozone (O3), and/or hydroxyl radicals (OH). In the present study, we provide evidence that hydroxy-carboxylic acids, namely methyltartaric acids (MTA) are: (1) reliable isoprene tracers, (2) likely produced via rapid peroxy radical hydrogen atom (H) shift reactions (autoxidation mechanism) and analogous alkoxy radical H shifts in low and high NOx environments respectively and (3) representative of aged ambient aerosol in the low NOx regime. Firstly, MTA are reliable tracers of isoprene aerosol because they have been identified in numerous chamber experiments involving isoprene conducted under a wide range of conditions and are absent in the oxidation of mono- and sesquiterpenes. They are also present in numerous samples of ambient aerosol collected during the past 20 years at several locations in the U.S. and Europe. Furthermore, MTA concentrations measured during a year-long field study in Research Triangle Park (RTP), NC in 2003 show a seasonal trend consistent with isoprene emissions and photochemical activity. Secondly, an analysis of chemical ionization mass spectrometer (CIMS) data of several chamber experiments in low and high NOx environments show that highly oxidized molecules (HOMs) derived from isoprene that lead to MTAs may be produced rapidly and considered as early generation isoprene oxidation products in the gas phase. Density functional theory calculations show that rapid intramolecular H shifts involving peroxy and alkoxy radicals possess low barriers for methyl-hydroxy-butenals (MHBs) that may represent precursors for MTA. From these results, a viable rapid H shift mechanism is proposed to occur that produces isoprene derived HOMs like MTA. Finally, an analysis of the mechanism shows that autoxidation-like pathways in low and high NOx may produce HOMs in a few OH oxidation steps like commonly detected methyl tetrol (MT) isoprene tracers. The ratio of MTA/MT in isoprene aerosol is also shown to be significantly greater in field versus chamber samples indicating the importance of such pathways in the atmosphere even for smaller hydrocarbons like isoprene.
Keywords: isoprene, highly oxidized molecules, secondary organic aerosol, autoxidation, density functional theory, methyltartaric acid
1. Introduction
Organic aerosol (OA) makes up a significant fraction of PM2.5 and assessing contributions to OA from secondary processes remains a challenge (Hallquist et al., 2009; Noziere et al., 2015). Secondary organic aerosol (SOA) is formed by homogeneous reactions in the gas phase of volatile organic compounds emitted from biogenic and anthropogenic sources followed by gas-particle partitioning (Hallquist et al., 2009) and from the heterogeneous oxidation in the particle phase (Zheng et al., 2015). Oxidation products of isoprene represent a major input to the global SOA burden (Carlton et al., 2009; Henze and Seinfeld, 2006) and results from recent studies have been incorporated into atmospheric models, such as the Community Multiscale Air Quality (CMAQ) model (Carlton et al., 2010; Xie et al., 2013). Despite significant progress in our understanding of isoprene SOA, its role in ambient SOA formation has been reported to be a major cause of disagreement between different numerical models and observations. In fact, current models are still not able to predict ambient OA concentrations or the degree of oxidation accurately, as well as the magnitude and evolution of ambient OA. Several laboratory experiments and field studies showed the importance and role of the dynamic evolution of OA with aging in atmospheric oxidation processes (Jimenez et al., 2009).
High emissions of isoprene occur during the warmer months and follow a diurnal pattern with a midday maximum (Guenther et al., 1995). Atmospheric losses of isoprene are initiated by OH during the day and reaction with nitrate radicals at night (Wennberg et al., 2018), whereas, the atmospheric reaction of isoprene with ozone is a minor sink under most atmospheric conditions (Kleindienst et al., 2007b). For OH oxidation, the reaction of isoprene as with other alkenes is initiated by OH addition followed by molecular oxygen addition to the carbon-centered radical to produce an isoprene peroxy radical (ISOPOO·). Since OH can add at any of the four possible positions in the isoprene molecule and O2 may likewise add at multiple locations, eight different isomers of ISOPOO· result with the highest yields (ca. 88%) deriving from OH addition to the terminal carbon atoms of the double bonds. Further reactions of the ISOPOO· radicals may give rise to isoprene epoxides (IEPOX) (Paulot et al., 2009), 2-methyl- (or 3-methyl)-4-hydroxybut-2-enals (collectively MHB) (Zhao et al., 2004), isoprene dinitrates (Wennberg et al., 2018), isoprene hydroxyhydroperoxides (Riva et al., 2016), 2-methyltetrols (MT) (Claeys et al., 2004; Edney et al., 2005), 2-methylglyceric acid (2MGA) (Edney et al., 2005), and other related species. In many instances, these compounds or their precursors may be produced in less than three oxidation steps and therefore represent early generation isoprene oxidation compounds. For example, IEPOX is produced from two subsequent OH additions to isoprene and they serve as prominent precursors to aerosol formation (Paulot et al., 2009; Wennberg et al., 2018). Detailed mechanistic pathways leading to the early generation products under low and high NOx dominated regimes have been incorporated into the Master Chemical Mechanism (MCM) (Jenkin et al., 2015).
While the chemical pathways to produce these early generation products are varied, the complexity increases in subsequent generations because multiple oxidation sites appear in addition to new pathways. For example, recently Piletic et al. (2019) showed that hydrogen atom shift (H shift) reactions are kinetically favorable for second generation isoprene chemistry due to the increased propensity of hydrogen atom tunneling giving rise to highly oxidized molecules (HOMs). Here, we expand the definition provided by Bianchi et al. (2019) for HOMs to also include H transfers involving alkoxy radicals followed by O2 addition (Vereecken and Peeters, 2010). HOMs are therefore (1) formed from intramolecular H shift reactions involving peroxy radicals (autoxidation) or alkoxy radicals, (2) formed under atmospherically relevant conditions and (3) they contain six or more oxygen atoms. It is now thought that a significant fraction of ambient SOA results from organic compounds that have undergone several generations of atmospheric oxidation via successive OH oxidation steps or autoxidation in a process known as “oxidative aging” (Jimenez et al., 2009). Identifying highly oxidized species in aerosol particles therefore can report on the oxidative capacity of the atmosphere which may serve as an important constraint in atmospheric models.
Recently, we have identified a set of novel C4 and C5 hydroxy-substituted mono- and di-acids (referred to as Group 1 compounds) that were detected in laboratory oxidations of isoprene while field studies showed their prevalence in the atmosphere (Jaoui et al., 2019). 2MGA and MT are referred to as Group 2 compounds for consistency with this earlier work. Figure 1 displays the chemical structures of both Group 1 and Group 2 compounds. Analytical methods, including gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS), and high resolution-mass spectrometry (HR-MS) were employed to identify these compounds. Syntheses of reference compounds allowed for definitive structural identifications of the markers (Jaoui et al., 2019). The chemical structures of Groups 1 and 2 compounds having 1-4 OH groups and 1-2 COOH groups: 2-methyltartronic acid (MTtA); 2-methylthreonic acid (2MTrA); 2-methylerythronic acid (2MeTA); 3-methylthreonic acid (3MTrA); 3-methylerythronic acid (3MeTA); threo-methyltartaric acid (tMTA); erythro-methyltartaric acid (eMTA); 2-methylerythritol (2MeT); 2-methyltreitol (2MTr); 2-methylglyceric acid (2MGA) are summarized below. For ease of discussion, tMTA and eMT are collectively referred to as MTA.
Figure 1.
Chemical structures and acronyms of recently detected isoprene oxidation products including methyltartaric acids (tMTA and eMTA).
This work presents the methyltartaric acids (MTAs) shown above as representative HOMs of aged isoprene aerosol because they have been quantified in many isoprene chamber reactions as well as field studies where they follow expected seasonal trends, are detected at comparable concentrations to established isoprene tracers and reaction mechanisms involving further OH oxidation and H shift reactions of precursor species may lead to their formation. The feasibility of H shift pathways is confirmed using quantum chemical calculations showing rapid H-shift and O2 addition steps. These HOMs may therefore be produced more promptly in this manner than via a series of successive OH oxidation steps. We also give qualitative evidence as to how these tracer compounds identified in ambient aerosol may indicate the importance of isoprene HOMs in the atmosphere. This approach will have the potential to determine the fine particle contribution of aged secondary aerosols from isoprene in urban and rural airsheds over a wide range of atmospheric conditions.
2. Materials and methods
All chemicals were purchased from Aldrich Chemical Co. (Milwaukee, WI) at the highest purity available and were used without further purification. All solvents (GC2 quality) were from Burdick and Jackson (Muskegon, MI).
Gas and particle samples for this study were generated in an indoor 14.5 m3 parallelepiped, Teflon-coated stainless-steel chamber. A description of the experiments and procedural details are described elsewhere (Jaoui et al., 2006; Jaoui et al., 2019). Table S1 (Supplementary Information: SI) summarizes a subset of isoprene experiments considered in this paper, representative of a large group conducted during the last 16 years in our laboratory. Six experiments (1-3, 7-9) were conducted in static mode to measure time profiles of primary and higher generation reaction products of isoprene, and four experiments (4-6, 10) in dynamic mode to produce a steady-state concentration of gas- and particle-phase reaction products. Two sets of irradiations were conducted: (1) in the presence of NOx (1, 4-6, 10), in which OH was produced by the conventional chain propagating mechanism where NO oxidizes ozone and peroxy radicals formed in the system; (2) in the absence of NOx (3, 7-9) in which OH was generated by photolyzing H2O2, where additional UV-sunlamps increased the wavelengths of radiation to 280-320 nm. Experiment 2 was conducted in the presence of ozone. Gas and particle phase isoprene products from experiment 1 were simultaneously collected with a sampling train that consisted of a 47 mm glass fiber filter (type T60A20, Pallflex Products Corp., Putnam, CT) followed by a downstream five-channel annular denuder (Jaoui and Kamens, 2001). The flow through the sampling system was 9.5 L/min. Five consecutive 20-, 40-, 60-, 90-, and 130-minute gas and particle samples were collected and analyzed using GC-MS at chromatographic conditions described by Jaoui et al. (2019). Sample components were identified by interpretation of mass spectra or by comparison to authentic standards. Experiments 2-6 are described in SI (Table S1).
Static (7-9) and dynamic (10) experiments were conducted in this study to measure time profiles of gas-phase oxygenated reaction products with a time-of-flight Chemical Ionization Mass Spectrometer (ToF-CIMS; Aerodyne Research Inc., Billerica, MA) operated in negative ion mode with iodide (I−) adduct reagent ion chemistry (Riedel et al., 2018). Samples from the chamber were drawn at 7 L/min through 10-m, 3.2-mm i.d. polytetrafluoroethylene tubing to the CIMS inlet where analyte species were ionized to I− molecular adducts. A peak fitting routine for the spectra enabled determination of the most probable molecular compositions. Since the analysis did not involve chemical separation, isomeric compounds having the same adduct molecular weight are not distinguished. Time-series plots for the molecular compositions of interest were then generated. Further details for the analytical approach have been reported (Riedel et al., 2018). For all experiments, NO and NOx were monitored with a TECO oxides of nitrogen analyzer (Model 42C, Franklin, MA). Ozone was measured with a Bendix ozone monitor (Model 8002, Lewisburg, WV). Chamber isoprene concentrations were measured by GC/FID (Hewlett Packard, Palo Alto, CA; Model 5890).
Field studies were conducted in several sites during the past 20 years in the United States and Europe. Detailed descriptions of the field sites and sampling procedures have been reported previously (Geron, 2011; Kleindienst et al., 2007a; Lewandowski et al., 2013; Nestorowicz et al., 2018) and are described in Section S1 for selected field studies. Although isoprene HOMs were observed in all field studies, we report data associated with a year-long study conducted at an EPA research field site in RTP, NC (UTM 35.894°N, 78.877°W) (Kleindienst et al., 2007a). PM2.5 field samples were collected onto quartz-fiber filters for 24 hours. The filters were Soxhlet extracted for 24-h in 50-ml dichloromethane/methanol mixture (1:1 v/v). Prior to extraction, known masses of ketopinic acid (KPA) and d50-tetracosane (TCS) were spiked on each filter as internal and recovery standard, respectively. The extraction efficiency of isoprene products varied from 95% to 100% (Jaoui et al., 2006; Jaoui et al., 2004). Each extract was evaporated to dryness under a gentle stream of N2 at room temperature, then derivatized for one hour with 200 μL of BSTFA and 100 μL of pyridine at 70°C. GC-MS analyses of derivatized extracts were conducted using a ThermoQuest (Austin, TX) GC coupled with an ion-trap mass spectrometer. The injector was operated in splitless mode at 270°C. Compounds were separated using the chromatographic conditions described by (Jaoui et al., 2006; Jaoui et al., 2019). Mass spectra were collected both in the electron and the methane-chemical ionization modes. SOA target compounds were not detected in the field and laboratory blanks nor in the background. Chromatographic considerations are also addressed by Jaoui et al. (2019).
3. Theory/calculations
In order to develop the chemical mechanisms described in the following section, electronic structure calculations were performed on reactive intermediates to determine reaction barriers. All electronic structure calculations were performed with Gaussian 16 (G16) software (Frisch et al., 2016). The M06-2X density functional was selected to compute H shift reaction barriers because it has been successfully applied to compute barrier heights on large datasets (Zhao and Truhlar, 2011). For all calculations, the M06-2X density functional was paired with the aug-cc-pVTZ basis set (Piletic et al., 2017; Piletic et al., 2019). Vibrational frequencies for all reactive intermediates and transition states were also computed to verify that they corresponded to stationary and transition states respectively. All stationary states were found to possess real vibrational frequencies while all transition states were found to have one imaginary vibrational frequency. The Gibbs free energies of activation were calculated within G16 by determining the electronic, vibrational, rotational and translational partition functions of all stationary and transition states within the rigid rotor and harmonic oscillator approximation to yield the enthalpies and entropies of activation (McQuarrie and Simon, 1999).
Rate constants were computed using a master equation approach capable of describing nonequilibrium kinetics of multiple well reaction pathways. The approach considers molecular collisions that dissipate energy in nonequilibrium systems. The Master Equation Solver for Multi-Energy well Reactions (MESMER v. 4.0) was used in this work to compute rate constants of either H shift reactions or fragmentation reactions of alkoxy radicals in high NOx environments (Glowacki et al., 2012). MESMER incorporates Eckart tunneling correction as presented by Miller (Miller, 1979) account for H atom tunneling which is prevalent in H shift reactions. Parameters used in these calculations were recently described and adopted in calculations of early generation isoprene H shift reactions (Piletic et al., 2019). An energy grain size of 20 cm−1 was used in all calculations and all final products were treated as infinite sinks. The errors in the rate constants are governed by the uncertainties in barrier heights computed using density functional methods described previously. Zhao and Truhlar determined that the average mean unsigned error of barrier heights computed using M06-2X over several data sets was 1.06 kcal/mol (Zhao and Truhlar, 2008). All barrier heights computed here are therefore assumed to have a +/−1 kcal/mol uncertainty which can give uncertainties in rate constants by an approximate factor of five. The results of these calculations in conjunction with experimental data were used to construct viable mechanisms that produce isoprene derived HOMs rapidly in the following section.
4. Results and discussion
This section is framed on demonstrating that MTAs: (1) are reliable atmospheric PM tracer compounds, (2) may be classified as HOMs and (3) are representative of aged aerosol. The subsections that follow are organized in this sequence and will commence with field study data from multiple sites where MTAs were detected. Subsequently, MTA will be classified as isoprene HOMs based on the newer definition described in the introduction using both time dependent data from chamber experiments and density functional theory calculations. In this section, the propensity of specific MTA precursors to autoxidize will be discussed. A mechanism will be developed and compared with time dependent data from GC-MS and CIMS instruments. The mechanism will show that two more OH oxidation steps are required to generate MTA in the particle phase relative to other established tracers like MT in low NOx environments confirming that the compounds represent aged aerosol. The section is concluded with a discussion of the use of MTA:MT ratio as a marker for the extent of aerosol aging as well as the possibility of interferences due to similar isomers being produced in toluene oxidation.
4.1. MTA detected in multiple field studies
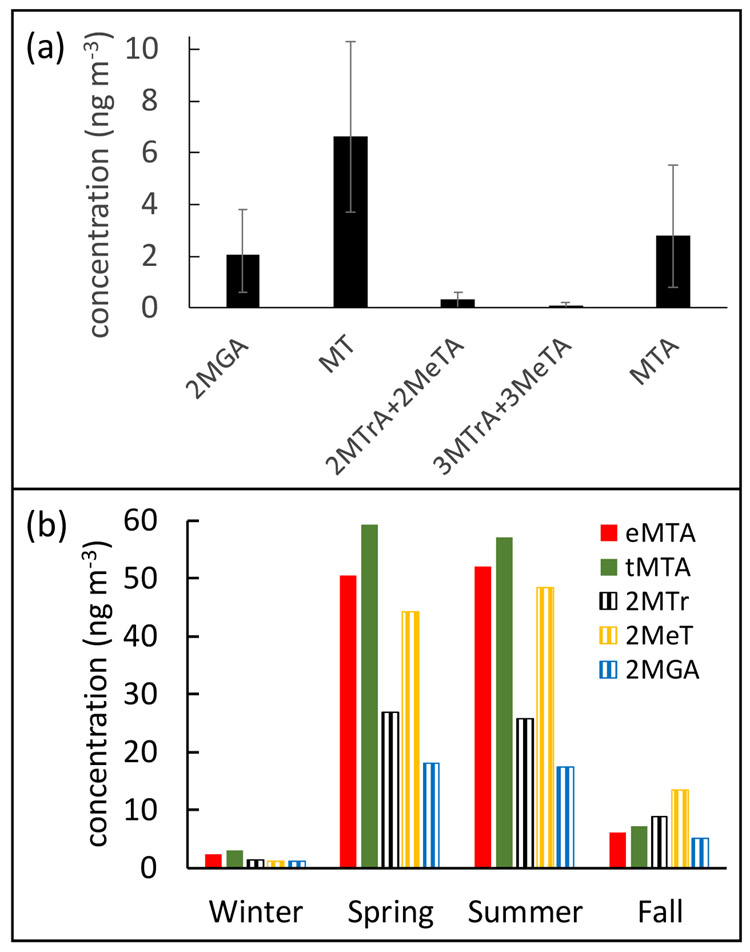
Oxidized compounds derived from isoprene (2MeTA, 3MeTA, 2MTrA, 3MTrA, tMTA, and eMTA) have been structurally characterized by interpreting chemical, chromatographic and mass spectral data, including comparison to authentic standards (Jaoui et al., 2019). These compounds were identified in urban, rural and regional field samples collected by our group during the past 20 years (Kleindienst et al., 2007a; Lewandowski et al., 2013). While both the methylthreonic and methyltartaric acids represent highly oxidized relatively unique species in atmospheric isoprene oxidation, only the MTAs are present in reasonably high abundance in field samples. To illustrate this, the results of samples collected during a field campaign at the Duke Forest (DF) site (Geron, 2011) in RTP, NC, USA during the calendar year 2003 are summarized in Figure 2a. The concentrations of the HOMs Group 1 tracers were estimated using BSTFA derivatization (Jaoui et al., 2019) described in Section S2 and shown in Figure 2a. Methyltartaric acids ambient concentrations ranged from 0.02 to 6.3 ng m−3, which are consistent with values typically obtained for atmospheric polar organic compounds (Jaoui et al., 2019; Kleindienst et al., 2007a; Lewandowski et al., 2013). The mean concentrations of MTAs are also comparable to two other isoprene organic aerosol tracers (2MGA and MT). As will be discussed further below, all tracers derive from disparate chemical mechanisms that depend differently on the concentrations of free radicals like HOx and NOx in the atmosphere and an assessment of their relative concentrations provides an indication of the oxidative capacity of the local atmosphere.
Figure 2.
(a) Mean concentrations of (2MTrA + 2MeTA), (3MTrA + 3MeTA), MTA, MT, and 2MGA in PM2.5 samples from the 2003 Duke Forest summer field study (variability marks indicate standard errors of the means). (b) The major Group 1 (eMTA and tMTA) and group 2 (2MTr, 2MeT, 2MGA) isoprene tracers detected in Research Triangle Park, NC during the 2003 calendar year and averaged by season (seasons are defined as in reference 22. The figure represents composites for the daily samples shown in Figure S1.
The ambient PM2.5 samples in Figure 2a show relatively low levels of 2-MTrA and 3-MTrA, typically less than 0.5 ng m−3 indicating that they either form in the atmosphere inefficiently, are converted to other compounds, or removed by other mechanisms. Considering their structural characteristics, it is possible that they are converted to stable γ-lactones through an intermolecular 5-exo-triggering closure under acidic conditions (Scheme 1). A similar process was proposed for potassium 2,3,4-trihydroxy-2-methylbutanoate (salt of 2-methylthreonic acid) under acidic conditions, resulting in leaf-closing in the Leucaena leucocephalam plant (Ueda et al., 2001). Consequently, 2-MTrA and 3-MTrA are considered unsuitable for use as isoprene HOM tracers. Henceforth, we focus our attention on MTA as the most viable atmospheric tracers for aged isoprene aerosol.
Scheme 1.

The γ-lactone formation from 2-methylthreonic acid occurs under acidic conditions.
During the Research Triangle Park campaign (Kleindienst et al., 2007a), field samples were taken over 33 single day periods (sampling for 24-h) during the calendar year 2003. The isoprene tracers 2MGA, 2MTr, and 2MeT, presented in that work are included here for comparison with Group 1 compounds. The MTA tracers were obtained by a chromatographic re-analysis of archived samples. Figure S1 presents the 24-h average concentrations of 2MGA, MT and MTA. MT and MTA concentrations range from 0 to 176, and from 0 to 146 ng m−3 respectively and are higher in the summer period. The MT and MTA tracers increase and decrease in unison over the whole year.
Figure 2b represents data from Figure S1 that are seasonally averaged. The observed concentrations show seasonal trends consistent with expected isoprene emission rates and photochemical activities. Thus, higher tracers’ contributions are measured during the warmer months when temperature dependent isoprene emissions rates are the greatest (Geron, 2011). Over the spring and summer the average ambient concentrations of MTA exceed the MT and 2MGA. The implications of this observation will be discussed further in Section 4.3 in the context of different formation mechanisms. MTA were readily seen in a number of field studies, including ambient PM2.5 samples collected at two European monitoring stations located in Zielonka and Godów (Poland) (Jaoui et al., 2019; Nestorowicz et al., 2018).
4.2. MTA as isoprene derived HOMs
According to the definition of HOMs described in the introduction, they are formed during oxidation under atmospherically relevant conditions. Section 4.1 has demonstrated that MTAs have been detected in numerous field studies in the ambient atmosphere. Another characteristic of HOMs is that they typically contain six or more oxygen atoms. This is certainly evident for MTA which contain six oxygen atoms analogous to some highly oxidized monoterpenes. The final criterion is that HOMs are formed either via H shift reactions involving peroxy radicals (autoxidation) or alkoxy radicals in high NOx environments. For MTA, it is difficult to decisively state whether they are produced via intramolecular H shift isomerization reactions or simply multiple oxidation steps involving OH radical. In this section, CIMS data and DFT calculations are used to argue that it is likely that MTA are produced via the former mechanism.
If several OH oxidation steps give rise to oxidized species that lead to MTA, they will likely go through mechanistic pathways that involve some common first- or second-generation isoprene oxidation products or intermediates that retain the 5-carbon atom backbone. Every generation is defined here by the number of OH oxidation steps that are required to produce a particular species. Interestingly, a single OH oxidation step can lead to highly oxidized species if the reactive peroxy or alkoxy radicals continue to self-react via H-shift reactions followed by O2 additions as will be discussed further below. Examples of such compounds include isoprene epoxydiols (IEPOX), methyltetrols (MTs), isoprene dinitrates, and isoprene dihydroperoxydiols. All of these species contain oxidized functional groups at each carbon atom of isoprene except the methyl group that may be converted to the hydroxyl and carboxylic acid groups present in MTA by further reactions. The isoprene dinitrates and dihydroperoxydiols are typically present in lower yields in the gas phase because they are more likely to condense onto particles. Consequently, they are not considered further as significant gas phase precursors of isoprene HOMs. The MTs are found in the particle phase, where they form in part through the hydrolysis of IEPOX (Pye et al., 2013) or through non-IEPOX pathways (Liu et al., 2016). Other MT formation mechanisms include the degradation of particle phase oligomers (Lopez-Hilfiker et al., 2016) or MT-related organosulfates (Cui et al., 2018), and the hydrolysis of particle phase organic nitrates (Piletic et al., 2013) although all are particle phase mechanisms requiring MT to partition to the gas phase before oxidizing further. However, a gas phase mechanism of the RO2 + HO2 reaction that forms an unstable tetroxide intermediate and ultimately releases ozone and an alcohol makes it possible to produce MTs in the gas phase as shown in Figure S2, but may only provide low yields (Hasson et al., 2005). MTs are therefore possible gas phase precursors of MTA but likely give rise to low yields. IEPOX is another candidate MTA precursor and is a dominant early generation oxidation product of isoprene under low NOx conditions (Wennberg et al., 2018) and is even present in high NOx environments (D'Ambro et al., 2017). However, Bates et al. (2014) have shown that some of the major oxidation products of IEPOX are the result of fragmentation reactions. One lower yield C5 product however was an IEPOX aldehyde that may oxidize further to a carboxylic acid via OH/HO2 additions. The other hydroxyl functional group would also have to oxidize to the acid in two OH oxidation steps before the epoxide can hydrolyze in the particle phase to form MTA. As a result, IEPOX is also a potential precursor to MTA although it too may give lower yields because it requires several more OH oxidation steps under highly oxidizing conditions.
The MHBs represent volatile species that may also oxidize to MTA and not as hampered by fragmentation reactions or present in low concentrations in the gas phase. Structurally, all four carbon atoms (excluding the methyl group carbon) on MHBs are susceptible to further oxidation. They are produced via reactions involving δ-ISOPOO· isomers (Jenkin et al., 2015; Lopez-Hilfiker et al., 2016; St Clair et al., 2016). The NOx mediated formation of MHB has been shown experimentally by Zhao et al. (2004) and Baker et al. (2005) and is included in the MCM mechanism (Jenkin et al., 2015). Time profiles for the formation of these unsaturated hydroxy carbonyls associated with Experiment 1 (Table S1) are shown in Figure 3a. With NOx present, MHB builds up rapidly increasing in the first 30-min and decreasing rapidly afterwards due to subsequent reactions in the gas phase (see below). In the absence of NOx, MHB has been detected by GC-MS (not shown) and may be produced via ROOH + OH or RO2 + RO2 reactions. The hydrogen abstraction at a terminal hydroperoxide site releases H2O and OH while producing an aldehyde. The latter RO2 + RO2 reaction is a standard disproportionation reaction giving a carbonyl compound and an alcohol which strongly depends on the atmospheric concentration of peroxy radicals (St Clair et al., 2016). MHBs are therefore stable gas phase compounds sufficiently volatile to react rapidly with radical species.
Figure 3.

Time evolution of the early generation isoprene oxidation product (a) 4-hydroxy-2-methyl-but-2-enal and later generation isoprene HOM (b) methyltartaric acid in experiment 1 (Table S1). Dotted line: gas-phase; solid line: particle-phase.
The time profile for the formation of MTA is shown in Figure 3b. Here, a clear induction period is observed providing evidence that this compound is formed later in the reactive process involving further oxidation steps. MTAs also have a propensity to partition into the gas phase once it is present in the particle. This may not be too surprising given that its C* value is approximately ~3*103 (μg/m3) based on estimates using the EVAPORATION vapor pressure estimation method (Compernolle et al., 2011). This value is typically classified as an intermediate volatility organic compound (IVOC). However, diacids are known to have large Henry’s Law constants (Compernolle and Muller, 2014) and may feature prominently in an aqueous particle phase. The data in Figure 3 are consistent with the possibility that MHBs may serve as gas phase precursors to MTAs.
The time dependent chemistry of MHBs and other highly oxidized products was examined further in a series of isoprene chamber experiments that incorporated the I− CIMS. Figure 4 displays data for two experiments 8 and 10 (Table S1) in low and high NOx environments respectively where the ions representing IsopOOH/IEPOX and MHBs were tracked. Panel 4a represents a static run while in panel 4b the experiment was carried out in dynamic mode. In both cases, reaction dynamics occur once the UV lights are switched on at time zero with the only difference being that in dynamic mode the reactants are still being continuously injected into the chamber while the photochemical reactions are occuring and producing products that eventually reach a steady state as opposed to decaying to zero in a static experiment.
Figure 4.
Time dependent CIMS signals for experiments: (a) 8 (static) and (b) 10 (dynamic) – see Table S1. MHBs (C5H8O2), IsopOOH/IEPOX (C5H10O3), isoprene dihydroperoxydiols (C5H12O6), isoprene hydroxy-nitrates (C5H9NO4), isoprene dihydroxy-dinitrates (C5H10N2O8), structural isomers of MTA C5H8O6) and a subsequent oxidation product (C5H6O5) are displayed. The generation of appearance for known products with respect to OH reaction is also displayed.
The time dependence of the appearance of specific species may be used to develop a mechanism. Specifically, the rising edge of the signals may indicate when in a multi-step reaction sequence a product may appear. The peak of the signals may be misleading because it is affected not only by the rate of production of that species but also the rate at which it reacts to form other species later in the mechanism. However, an analysis of the rising edge on normalized CIMS signals is not definitive because there is a distribution of production rates for different products in every generation. In a chamber experiment with typically higher levels of oxidants, the appearance of products in different generations may even overlap considerably (see Figure S3c). Nonetheless, such an analysis may eliminate mechanistic pathways that would involve several additional oxidation steps if signals for a particular species appear early enough in the data (see Figure S5).
In the low and high NOx experiments, the rising edge of the MHB signal shows up early and is consistent with the Master Chemical Mechanism (Jenkin et al., 2015) because it involves one or two OH reactions with isoprene in high and low NOx environments, respectively (see Figure S4). In low NOx, MHBs may also be produced in one step via an RO2 + RO2 reaction involving δ-HO-Isop-OO·. However, in the atmosphere RO2 is generally present in lower concentrations than HO2 (Hornbrook et al., 2011) and the rate constants of RO2 + RO2 reactions are typically smaller than RO2 + HO2 (Atkinson, 1997)). Therefore, in low NOx ambient environments, it is assumed that MHBs are produced in two OH oxidation steps. Also displayed are known first- and second-generation oxidized species in low and high NOx with each generation defined here as a new reaction with OH. C5H10O3 and C5H12O6 signals are attributed to IsopOOH/IEPOX and isoprene dihydroperoxydiols while the C5H9NO4 and C5H10N2O8 signals are due to isoprene hydroxy-nitrates and dihydroxy-nitrates respectively.
The C5H8O6 ion corresponds to the formula consistent with MTA although it may represent other highly oxidized structural isomers. In both experiments, the ion appears extremely early even tracking the appearance of MHB in the high NOx experiment. The signal to noise ratio for C5H8O6 and other oxidized species like C5H6O5 in the low NOx experiment (panel 4a) is quite low likely because HO2 concentrations are high in the chamber which react with peroxy radicals to form hydroperoxides. Consequently, further averaging of the data was carried out consisting of a rolling average of five data points. Given the large number of data points tabulated every 30 seconds, a rolling average of five data points is not expected to affect the peak shape which changes substantially over a period of an hour. A fit to these data is included for visual acuity so that its early appearance can be easily discerned. The rapid appearance of this highly oxidized species was also observed in two other low NOx experiments with different initial isoprene and OH concentrations (see Figure S3). In Figure 4a, C5H8O6 is also observed to occur earlier than the second generation C5H12O6 species which is produced in only two OH/HO2 oxidation steps. Similarly, in the high NOx regime, the rising edge of C5H8O6 appears before C5H10N2O8 – a known second generation dinitrate species produced via two OH/NO oxidation steps. Interestingly, Figure 4b shows that C5H8O6 has two peaks with the early shoulder appearing at the same time as MHB – a first generation species. This raises the possibility that a potential HOM for isoprene may be produced with a single OH oxidation in high NOx environments. The early signal also appears before another oxidized species C5H6O5 that may be a precursor to MTAs as will be discussed further below. It is important to state that MTAs (diacids) may potentially adhere to chamber walls or sampling lines (Deming et al., 2019) and this may affect the time dependent peaks shown in Figure 4. This effect, however, would also suppress the rising edge of the CIMS signals, yet the C5H8O6 ion still appears very early in Figure 4. All these data suggest that C5H8O6 is produced by rapid mechanisms that involve one or two OH oxidation steps. One likely possibility is that it results from rapid intramolecular hydrogen atom shift reactions. Density functional theoretical calculations were performed to assess the viability of this hypothesis.
Table 1 displays the calculations of free energies of activation and first order rate constants for hydrogen shifts involving peroxy or alkoxy radicals that may be produced during the oxidation of isoprene. The first two entries represent calculations previously conducted on early generation isoprene reactions that are known to occur (D'Ambro et al., 2017; Piletic et al., 2019). Reactions 1 and 2 occur in low NOx environments while reaction 2 is more rapid despite a larger barrier because of hydrogen atom tunneling (Piletic et al., 2019) and has a comparable reaction rate (1.7E-1 s−1) to NO addition to peroxy radicals at 1 ppb and 298 K (2.2E−1 s−1) assuming its second order rate constant is 8.9 E-12 cm3 molec−1 s−1. Reactions 3 – 7 in Table 1 represent possible H-shift or fragmentation reactions pertaining to the oxidation of MHBs which as previously mentioned represent potential precursors for MTAs. The peroxy H shift reactions 3 and 4 are considerably faster than reactions 1 and 2 and can compete with NO reactions at higher concentrations. All subsequent reactions (5-7) involving alkoxy radicals have reaction rates that are orders of magnitude greater than the peroxy radical H shifts although fragmentation reactions are also incredibly fast and may be favored in certain instances, as shown in Table 1 (compare reactions 5a and b). However, the alkoxy H shift reactions are non-negligible within the computational uncertainty (see Section 3) and in some cases dominant (compare reactions 6a and b). Table S2 displays a more extensive set of reactions that include rate constants computed with and without H atom tunneling. It is readily evident that H tunneling plays a vital role in increasing reaction rates by an order of magnitude in many cases for H shift reactions. IEPOX-OO· may potentially autoxidize but this has a very small expected branching ratio because the peroxy radical is expected to abstract from the neighboring hydroxyl group to produce the IEPOX aldehyde preferentially (Peeters et al., 2009). MHBs on the other hand may experience rapid H shifts in low and high NOx environments involving peroxy and alkoxy radicals respectively that sustain autoxidation processes, many of which have favorable barrier heights. Some reactions in Table S2 may be used to infer mechanisms that produce isoprene HOMs like C5H8O6 in one OH oxidation step as will be discussed further in the following section. For example, reaction 8 in Table S2 may arise during the autoxidation of δ-HO-Isop-OO· isomers following the pathways described in the updated LIM1 mechanism (Peeters et al., 2014). This reaction represents the termination of the di-HPCARP peroxy radicals which give rise to C5H8O6 dihydroperoxy-dialdehydes. Peroxy radical precursors to this product have been detected recently by Berndt et al. (Berndt et al., 2019). In high NOx environments, reactions 11 and 19 in Table S2 illustrate that alkoxy radical H shifts are more rapid than the corresponding fragmentation reactions 12 and 20 respectively. These results, in conjunction with the CIMS data, illustrate the plausibility that MTAs are produced via isomerization reactions and may be consequently labeled as isoprene derived HOMs.
Table 1.
Computed free energies of activation and first order rate constants for hydrogen atom (H) shift reactions involving early generation peroxy and alkoxy radicals derived from isoprene oxidation. The first two entries labeled with * were computed by Piletic et al., (2019).
| Reaction | ΔG‡ (kcal/mol) | k (s−1) |
|---|---|---|

|
21.6* | 1.1E-03 |

|
22.5* | 1.7E-01 |

|
22.4 | 6.0E-01 |
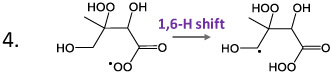
|
16.7 | 4.2E+01 |

|
7.4 | 1.7E+08 |
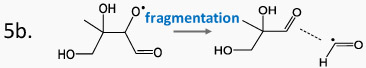
|
1.0 | 3.4E+09 |

|
8.7 | 1.7E+07 |

|
6.0 | 5.9E+06 |

|
16.5 | 1.8E+02 |
The computational results in Tables 1 and S2 were used to establish a mechanism for the formation of MTAs that may then be compared with traditional mechanisms producing other isoprene tracers like MT. A proposed mechanism invoking H shift reactions of MHB (as discussed in the previous section) is shown in Figure 5 for low and high NOx. It shows that a C5H8O6 species is produced in a single OH oxidation step from MHB in both low and high NOx environments consistent with CIMS data that indicate it should appear early in the mechanism. Once generated, a further OH oxidation step gives rise to cyclization reactions that produce lactones (Lin et al., 2013) or anhydrides that may subsequently hydrolyze in the particle phase to give MTA. This type of reaction is analogous to the production and fate of IEPOX.(Paulot et al., 2009) The C5H6O5 signal may represent these lactones and Figure 4b shows that it occurs after the first shoulder of the C5H8O6 signal which is consistent with the detailed mechanism shown in Figure S4 involving H shifts of the alkoxy radical. The first shoulder for the C5H8O6 ion also indicates that such HOMs may be produced in a single OH oxidation step. Given the uncertainty in definitively ascribing the generation in which a particular product appears because every generation produces a distribution of products at different rates, Figure S4 also illustrates pathways in low and high NOx that can lead to HOMs in a single OH oxidation step. The delayed onset of MTAs that appear after 2 hours of oxidation as shown in Figure 3b may be due to the adsorption and hydrolysis of lactones or anhydrides in the particle phase followed by a repartitioning to the gas phase. While such pathways have been shown in the laboratory and computationally predicted, in the following section the importance of these pathways are demonstrated in atmospheric samples.
Figure 5.
The oxidation of methyl-hydroxybutenals (MHBs) to methyltartaric acid (MTA) invoking H-shift reactions with peroxy radicals in low NOx environments (autoxidation) and alkoxy radicals in high NOx environments. The numbers in parentheses beside specific H shift reactions refer to the corresponding reactions listed in Table 1.
4.3. Rapid oxidation mechanisms of isoprene in the ambient environment
Because MTAs likely represent isoprene HOMs produced by rapid H shift reactions, quantifying their concentrations in the atmosphere will determine to what extent these pathways play a role in the oxidation of isoprene. Table 2 compares the ratios of MTA:MT for the field samples from the calendar year 2003 RTP campaign (seasonally averaged) with those for the chamber measurements under conditions found in Table S1. The chamber ratios range from 0.008 for the isoprene/NOX irradiation with acidic aerosol to 0.040 for the same irradiation without acid. The difference is due almost exclusively to the increase in MT by a factor of five. From the ozonolysis and the isoprene/H2O2 irradiation, both without NOX, the ratios are intermediate compared to the experiments with NOX. For the field samples, Table 2 shows the MTA:MT ratios are one-to-two orders of magnitude greater than those observed in the laboratory. In many cases, MTA in the field is equal to or higher than MT. Thus, the seasonally averaged ratios are considerably enhanced to those detected in the laboratory. This finding may be due to several reasons. Firstly, enhanced MTs in chamber aerosol may be a consequence of large generated PM concentrations which will drive lower volatility compounds like IEPOX into the particle phase thereby limiting their exposure to gas-phase oxidants. Larger HO2 concentrations are also expected in the chamber because the initial burst of OH radicals produced from H2O2 photolysis may still react with the large concentrations of H2O2 to produce H2O and HO2 (Keyser, 1980). HO2 may limit autoxidation and therefore the production of MTAs because it may cap peroxy radicals to form hydroperoxides. The opposite effects are present in the field studies because both PM and HO2 concentrations are expected to be lower thereby driving more autoxidation in the gas phase with suppressed condensation to the particle phase. Other variables such as condensed phase chemistry may also affect the MTA:MT ratio. Because a greater extent of oxidation can occur in the actual atmosphere than in chamber experiments, a reliable HOM tracer must be representative of actual atmospheric conditions that cannot necessarily be reproduced in chamber experiments. A similar analysis is not possible using the LC data, since that method cannot detect the 2-methyltetrols.
Table 2.
Concentrations of 2-methyltetrols (MT) and methyltartaric acids (MTA) in chamber and field samples – the latter are season averages from the field campaign during the Calendar Year 2003 at RTP, NC (USA).
| Field samples, ng m−3 | Chamber samples, ng m−3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tracer compounds | Winter average |
Spring average |
Summer average |
Fall average |
Isoprene + O3 |
Isoprene + OH |
Isoprene + NOx/ non-acid |
Isoprene + NOx/acid |
|
| ∑ MTA | 5.43 | 110 | 109 | 13.3 | 4.30 | 70.2 | 23.7 | 19.1 | |
| ∑ MT | 2.48 | 71.2 | 74.3 | 22.4 | 277 | 2448 | 586 | 2456 | |
| MTA/MT ratio | 2.19 | 1.54 | 1.47 | 0.59 | 0.016 | 0.029 | 0.040 | 0.008 | |
| MTA/MT ratio (Mean) | 1.45 | 0.023 | |||||||
The MTA:MT ratio in the ambient atmosphere may be used as a gauge of the extent of autoxidation by peroxy radicals and oxidation by alkoxy radicals of organic PM derived from isoprene. This may then be used to improve atmospheric models that have not extensively incorporated such pathways. While relative comparisons of the ratio provide insights as to which atmospheres have a greater tendency to lead to autoxidation, the use of an absolute ratio to quantify the fractional mass of isoprene based HOMs requires a knowledge of which precursors are predominantly responsible from MTA production. An analysis of branching ratios and product yields in the MTA mechanism may then be used to quantify the fractional mass. An alternative method involves using an aerosol mass spectrometer (AMS) to measure the O:C ratios which may be correlated with MTA tracer concentrations in laboratory irradiations of isoprene under a wide range of conditions. In addition to constraining the extent of autoxidation in organic aerosols, the mechanisms proposed here may be used to modify existing explicit gas phase mechanisms of the oxidation chemistry of isoprene. The mechanisms are important because the diacids described here may play a role in nucleation events as has been discussed by Xu and Zhang (2012). In particular, the data presented in Figure 3 involved an experiment with no aerosol seed or acidity, yet measurable organic aerosol mass was detected. Further work (either laboratory or computational) is required to determine the role MTA play in nucleation and how they may be used to quantify the extent of oxidation in PM.
MTA and/or their structural isomers may not be completely unique to isoprene. Figure S6 in the supporting information displays that similar compounds may occur in the oxidation of toluene. The EI spectra in Figure S7 suggest that the major GC peaks from toluene oxidation experiments may be due to isomers of MTA because some MS fragments are present in toluene samples and not in isoprene samples and vice versa. Similar HOMs in isoprene and toluene oxidation may occur because similar oxidized precursor species are present in both mechanisms. For example, methylbutendials are produced in the atmospheric oxidation of toluene under high NOx conditions. Current research efforts are dedicated to determining the identities and relative yields of these HOMs from toluene oxidation. While toluene is typically associated with mobile sources present year-round, the seasonal trends shown in Figure 2b and S1 indicate that isoprene is the major precursor of the MTA measured in that atmosphere.
5. Conclusions
In summary, we provide three arguments for proposing MTA as reliable HOM tracers of aged isoprene aerosol. First of all, MTAs were consistently detected in locations in Europe and the United States and display the expected seasonal trend for biogenic SOA. Second, they represent HOMs because they are highly oxidized and are produced rapidly by autoxidation in low NOx environments and by alkoxy radical H shift reactions in the high NOx regime. Autoxidation has only relatively recently been investigated in atmospheric studies and has been shown to be important in producing HOMs. Alkoxy radical H shifts on the other hand have not been discussed extensively because many models predict that fragmentation reactions dominate. This study shows that both types of reactions may occur for isoprene neither of which have been incorporated in atmospheric models. HOMs are therefore an underrepresented component in models and this will affect predicted PM mass concentrations and particle nucleation mechanisms. Third, the increased ratio of MTA:MT in field versus laboratory studies indicate that chamber studies may potentially suppress autoxidation mechanisms. In the low NOx regime, this is likely because experimental chambers typically use elevated concentrations of oxidants that give rise to higher HO2 concentrations which may cap peroxy radicals to form hydroperoxides. Including these formation mechanisms in air quality models may therefore improve secondary organic aerosol predictions as well as help to constrain adjustable model parameters responsible for atmospheric aging.
Supplementary Material
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. The research at the Institute of Physical Chemistry of the Polish Academy of Sciences was partially supported by the funding from the Polish National Science Centre grant (Nr OPUS8-2014/15/B/ST10/04276). Mention of trade names does not constitute endorsement or recommendation of a commercial product by U.S. EPA.
References
- Atkinson R, 1997. Gas-phase tropospheric chemistry of volatile organic compounds .1. Alkanes and alkenes. J. Phys. Chem. Ref. Data 26, 215–290. 10.1063/1.556012. [DOI] [Google Scholar]
- Baker J, Arey J, Atkinson R, 2005. Formation and Reaction of Hydroxycarbonyls from the Reaction of OH Radicals with 1,3-Butadiene and Isoprene. Environ. Sci.Technol 39, 4091–4099. 10.1021/es047930t. [DOI] [PubMed] [Google Scholar]
- Bates KH, Crounse JD, St Clair JM, Bennett NB, Nguyen TB, Seinfeld JH, et al. , 2014. Gas Phase Production and Loss of Isoprene Epoxydiols. J. Phys. Chem. A 118, 1237–1246. 10.1021/jp4107958. [DOI] [PubMed] [Google Scholar]
- Berndt T, Hyttinen N, Herrmann H, Hansel A, 2019. First oxidation products from the reaction of hydroxyl radicals with isoprene for pristine environmental conditions. Communications Chemistry 2. 10.1038/s42004-019-0120-9. [DOI] [Google Scholar]
- Bianchi F, Kurten T, Riva M, Mohr C, Rissanen MP, Roldin P, et al. , 2019. Highly Oxygenated Organic Molecules (HOM) from Gas-Phase Autoxidation Involving Peroxy Radicals: A Key Contributor to Atmospheric Aerosol. Chem. Rev 119, 3472–3509. 10.1021/acs.chemrev.8b00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton AG, Bhave PV, Napelenok SL, Edney ED, Sarwar G, Pinder RW, et al. , 2010. Model Representation of Secondary Organic Aerosol in CMAQv4.7. Environ. Sci. Technol 44, 8553–8560. 10.1021/es100636q. [DOI] [PubMed] [Google Scholar]
- Carlton AG, Wiedinmyer C, Kroll JH, 2009. A review of secondary organic aerosol (SOA) formation from isoprene. Atmos. Chem. Phys 9, 4987–5005. 10.5194/acp-9-4987-2009. [DOI] [Google Scholar]
- Claeys M, Graham B, Vas G, Wang W, Vermeylen R, Pashynska V, et al. , 2004. Formation of secondary organic aerosols through photooxidation of isoprene. Science 303, 1173–1176. 10.1126/science.1092805. [DOI] [PubMed] [Google Scholar]
- Compernolle S, Ceulemans K, Muller JF, 2011. EVAPORATION: a new vapour pressure estimation methodfor organic molecules including non-additivity and intramolecular interactions. Atmos. Chem. Phys 11, 9431–9450. 10.5194/acp-11-9431-2011. [DOI] [Google Scholar]
- Compernolle S, Muller JF, 2014. Henry's law constants of diacids and hydroxy polyacids: recommended values. Atmos. Chem. Phys 14, 2699–2712. 10.5194/acp-14-2699-2014. [DOI] [Google Scholar]
- Cui TQ, Zeng ZX, dos Santos EO, Zhang ZF, Chen YZ, Zhang Y, et al. , 2018. Development of a hydrophilic interaction liquid chromatography (HILIC) method for the chemical characterization of water-soluble isoprene epoxydiol (IEPOX)-derived secondary organic aerosol. Environ. Sci.-Process Impacts 20, 1524–1536. 10.1039/c8em00308d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambro EL, Lee BH, Liu JM, Shilling JE, Gaston CJ, Lopez-Hilfiker FD, et al. , 2017. Molecular composition and volatility of isoprene photochemical oxidation secondary organic aerosol under low- and high-NOx conditions. Atmos. Chem. Phys 17, 159–174. 10.5194/acp-17-159-2017. [DOI] [Google Scholar]
- Deming BL, Pagonis D, Liu XX, Day DA, Talukdar R, Krechmer JE, et al. , 2019. Measurements of delays of gas-phase compounds in a wide variety of tubing materials due to gas-wall interactions. Atmos. Meas. Tech 12, 3453–3461. 10.5194/amt-12-3453-2019. [DOI] [Google Scholar]
- Edney EO, Kleindienst TE, Jaoui M, Lewandowski M, Offenberg JH, Wang W, et al. , 2005. Formation of 2-methyl tetrols and 2-methylglyceric acid in secondary organic aerosol from laboratory irradiated isoprene/NOX/SO2/air mixtures and their detection in ambient PM2.5 samples collected in the eastern United States. Atmos. Environ 39, 5281–5289. 10.1016/j.atmosenv.2005.05.031. [DOI] [Google Scholar]
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. Gaussian 16 Rev. C.01, Wallingford, CT, 2016. [Google Scholar]
- Geron C, 2011. Carbonaceous aerosol characteristics over a Pinus taeda plantation: Results from the CELTIC experiment. Atmos. Environ 45, 794–801. 10.1016/j.atmosenv.2010.07.015. [DOI] [Google Scholar]
- Glowacki DR, Liang CH, Morley C, Pilling MJ, Robertson SH, 2012. MESMER: An Open-Source Master Equation Solver for Multi-Energy Well Reactions. J. Phys. Chem. A 116, 9545–9560. 10.1021/jp3051033. [DOI] [PubMed] [Google Scholar]
- Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, et al. , 1995. A global model of natural volatile organic compound emissions. Journal of Geophysical Research-Atmospheres 100, 8873–8892. 10.1029/94jd02950. [DOI] [Google Scholar]
- Hallquist M, Wenger JC, Baltensperger U, Rudich Y, Simpson D, Claeys M, et al. , 2009. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys 9, 5155–5236. 10.5194/acp-9-5155-2009. [DOI] [Google Scholar]
- Hasson AS, Kuwata KT, Arroyo MC, Petersen EB, 2005. Theoretical studies of the reaction of hydroperoxy radicals (HO2 center dot) with ethyl peroxy (CH3CH2O2 center dot), acetyl peroxy (CH3C(O)O-2(center dot)) and acetonyl peroxy (CH3C(O)CH2O2 center dot) radicals. J. Photochem. Photobiol. A-Chem 176, 218–230. 10.1016/j.jphotochem.2005.08.012. [DOI] [Google Scholar]
- Henze DK, Seinfeld JH, 2006. Global secondary organic aerosol from isoprene oxidation. Geophys. Res. Lett 33, L09812. 10.1029/2006GL025976. [DOI] [Google Scholar]
- Hornbrook RS, Crawford JH, Edwards GD, Goyea O, Mauldin RL, Olson JS, et al. , 2011. Measurements of tropospheric HO2 and RO2 by oxygen dilution modulation and chemical ionization mass spectrometry. Atmos. Meas. Tech 4, 735–756. 10.5194/amt-4-735-2011. [DOI] [Google Scholar]
- Jaoui M, Corse E, Kleindienst TE, Offenberg JH, Lewandowski M, Edney EO, 2006. Analysis of secondary organic aerosol compounds from the photooxidation of d-limonene in the presence of NOX and their detection in ambient PM2.5. Environ. Sci. Technol 40, 3819–3828. 10.1021/es052566z. [DOI] [PubMed] [Google Scholar]
- Jaoui M, Kamens RM, 2001. Mass balance of gaseous and particulate products analysis from alpha-pinene/NOx/air in the presence of natural sunlight. Journal of Geophysical Research-Atmospheres 106, 12541–12558. 10.1029/2001jd900005. [DOI] [Google Scholar]
- Jaoui M, Kleindienst TE, Lewandowski M, Edney EO, 2004. Identification and quantification of aerosol polar oxygenated compounds bearing carboxylic or hydroxyl groups. 1. Method development. Anal. Chem 76, 4765–4778. 10.1021/ac049919h. [DOI] [PubMed] [Google Scholar]
- Jaoui M, Szmigielski R, Nestorowicz K, Kolodziejczyk A, Sarang K, Rudzinski KJ, et al. , 2019. Organic Hydroxy Acids as Highly Oxygenated Molecular (HOM) Tracers for Aged Isoprene Aerosol. Environ. Sci. Technol 53, 14516–14527. 10.1021/acs.est.9b05075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkin ME, Young JC, Rickard AR, 2015. The MCM v3.3.1 degradation scheme for isoprene. Atmos. Chem. Phys 15, 11433–11459. 10.5194/acp-15-11433-2015. [DOI] [Google Scholar]
- Jimenez JL, Canagaratna MR, Donahue NM, Prevot ASH, Zhang Q, Kroll JH, et al. , 2009. Evolution of Organic Aerosols in the Atmosphere. Science 326, 1525–1529. 10.1126/science.1180353. [DOI] [PubMed] [Google Scholar]
- Keyser LF, 1980. Absolute Rate-Constant of the Reaction OH + H2O2- HO2 + H2O from 245-K to 423-K. Journal of Physical Chemistry 84, 1659–1663. 10.1021/j100450a001. [DOI] [Google Scholar]
- Kleindienst TE, Jaoui M, Lewandowski M, Offenberg JH, Lewis CW, Bhave PV, et al. , 2007a. Estimates of the contributions of biogenic and anthropogenic hydrocarbons to secondary organic aerosol at a southeastern US location. Atmos. Environ 41, 8288–8300. 10.1016/j.atmosenv.2007.06.045. [DOI] [Google Scholar]
- Kleindienst TE, Lewandowski M, Offenberg JH, Jaoui M, Edney EO, 2007b. Ozone-isoprene reaction: Re-examination of the formation of secondary organic aerosol. Geophysical Research Letters 34. 10.1029/2006gl027485. [DOI] [Google Scholar]
- Lewandowski M, Piletic IR, Kleindienst TE, Offenberg JH, Beaver MR, Jaoui M, et al. , 2013. Secondary organic aerosol characterisation at field sites across the United States during the spring-summer period. Int. J. Environ. Anal. Chem 93, 1084–1103. 10.1080/03067319.2013.803545. [DOI] [Google Scholar]
- Lin YH, Zhang HF, Pye HOT, Zhang ZF, Marth WJ, Park S, et al. , 2013. Epoxide as a precursor to secondary organic aerosol formation from isoprene photooxidation in the presence of nitrogen oxides. Proc. Natl. Acad. Sci. U. S. A. 110, 6718–6723. 10.1073/pnas.1221150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JM, D'Ambro EL, Lee BH, Lopez-Hilfiker FD, Zaveri RA, Rivera-Rios JC, et al. , 2016. Efficient Isoprene Secondary Organic Aerosol Formation from a Non-IEPDX Pathway. Environ. Sci. Technol 50, 9872–9880. 10.1021/acs.est.6b01872. [DOI] [PubMed] [Google Scholar]
- Lopez-Hilfiker FD, Mohr C, D'Ambro EL, Lutz A, Riedel TP, Gaston CJ, et al. , 2016. Molecular Composition and Volatility of Organic Aerosol in the Southeastern US: Implications for IEPDX Derived SOA. Environ. Sci. Technol 50, 2200–2209. 10.1021/acs.est.5b04769. [DOI] [PubMed] [Google Scholar]
- McQuarrie DA, Simon JD Molecular Thermodynamics: University Science Books, 1999. [Google Scholar]
- Miller WH, 1979. Tunneling Corrections to Unimolecular Rate Constants, with Application to Formaldehyde. J. Am. Chem. Soc 101, 6810–6814. 10.1021/ja00517a004. [DOI] [Google Scholar]
- Nestorowicz K, Jaoui M, Rudzinski KJ, Lewandowski M, Kleindienst TE, Spolnik G, et al. , 2018. Chemical composition of isoprene SOA under acidic and non-acidic conditions: effect of relative humidity. Atmos. Chem. Phys 18, 18101–18121. 10.5194/acp-18-18101-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noziere B, Kaberer M, Claeys M, Allan J, D'Anna B, Decesari S, et al. , 2015. The Molecular Identification of Organic Compounds in the Atmosphere: State of the Art and Challenges. Chem. Rev 115, 3919–3983. 10.1021/cr5003485. [DOI] [PubMed] [Google Scholar]
- Paulot F, Crounse JD, Kjaergaard HG, Kurten A, St Clair JM, Seinfeld JH, et al. , 2009. Unexpected Epoxide Formation in the Gas-Phase Photooxidation of Isoprene. Science 325, 730–733. 10.1126/science.1172910. [DOI] [PubMed] [Google Scholar]
- Peeters J, Muller JF, Stavrakou T, Nguyen VS, 2014. Hydroxyl Radical Recycling in Isoprene Oxidation Driven by Hydrogen Bonding and Hydrogen Tunneling: The Upgraded LIM1 Mechanism. J. Phys. Chem. A 118, 8625–8643. 10.1021/jp5033146. [DOI] [PubMed] [Google Scholar]
- Peeters J, Nguyen TL, Vereecken L, 2009. HOx radical regeneration in the oxidation of isoprene. Phys. Chem. Chem. Phys 11, 5935–5939. 10.1039/b908511d. [DOI] [PubMed] [Google Scholar]
- Piletic IR, Edney EO, Bartolotti LJ, 2013. A computational study of acid catalyzed aerosol reactions of atmospherically relevant epoxides. Phys. Chem. Chem. Phys 15, 18065–18076. 10.1039/c3cp52851k. [DOI] [PubMed] [Google Scholar]
- Piletic IR, Edney EO, Bartolotti LJ, 2017. Barrierless Reactions with Loose Transition States Govern the Yields and Lifetimes of Organic Nitrates Derived from Isoprene. J. Phys. Chem. A 121, 8306–8321. 10.1021/acs.jpca.7b08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piletic IR, Howell R, Bartolotti LJ, Kleindienst TE, Kaushik SM, Edney EO, 2019. Multigenerational Theoretical Study of Isoprene Peroxy Radical 1-5-Hydrogen Shift Reactions that Regenerate HOx Radicals and Produce Highly Oxidized Molecules. J. Phys. Chem. A 123, 906–919. 10.1021/acs.jpca.8b09738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pye HOT, Pinder RW, Piletic IR, Xie Y, Capps SL, Lin YH, et al. , 2013. Epoxide Pathways Improve Model Predictions of Isoprene Markers and Reveal Key Role of Acidity in Aerosol Formation. Environ. Sci. Technol 47, 11056–11064. 10.1021/es402106h. [DOI] [PubMed] [Google Scholar]
- Riedel TP, DeMarini DM, Zavala J, Warren SH, Corse EW, Offenberg JH, et al. , 2018. Mutagenic atmospheres resulting from the photooxidation of aromatic hydrocarbon and NOx mixtures. Atmos. Environ 178, 164–172. 10.1016/j.atmosenv.2018.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva M, Budisulistiorini SH, Chen YZ, Zhang ZF, D'Ambro EL, Zhang X, et al. , 2016. Chemical Characterization of Secondary Organic Aerosol from Oxidation of Isoprene Hydroxyhydroperoxides. Environ. Sci. Technol 50, 9889–9899. 10.1021/acs.est.6b02511. [DOI] [PubMed] [Google Scholar]
- St Clair JM, Rivera-Rios JC, Crounse JD, Knap HC, Bates KH, Teng AP, et al. , 2016. Kinetics and Products of the Reaction of the First-Generation Isoprene Hydroxy Hydroperoxide (ISOPOOH) with OH. J. Phys. Chem. A 120, 1441–1451. 10.1021/acs.jpca.5b06532. [DOI] [PubMed] [Google Scholar]
- Ueda M, Sohtome Y, Ueda K, Yamamura S, 2001. Potassium 2,3,4-trihydroxy-2-methylbutanoate, a leaf-closing substance of Leucaena leucocephalam. Tetrahedron Lett. 42, 3109–3111. 10.1016/s0040-4039(01)00380-x. [DOI] [Google Scholar]
- Vereecken L, Peeters J, 2010. A structure-activity relationship for the rate coefficient of H-migration in substituted alkoxy radicals. Phys. Chem. Chem. Phys 12, 12608–12620. 10.1039/c0cp00387e. [DOI] [PubMed] [Google Scholar]
- Wennberg PO, Bates KH, Crounse JD, Dodson LG, McVay RC, Mertens LA, et al. , 2018. Gas-Phase Reactions of Isoprene and Its Major Oxidation Products. Chem. Rev 118, 3337–3390. 10.1021/acs.chemrev.7b00439. [DOI] [PubMed] [Google Scholar]
- Xie Y, Paulot F, Carter WPL, Nolte CG, Luecken DJ, Hutzell WT, et al. , 2013. Understanding the impact of recent advances in isoprene photooxidation on simulations of regional air quality. Atmos. Chem. Phys 13, 8439–8455. 10.5194/acp-13-8439-2013. [DOI] [Google Scholar]
- Xu W, Zhang RY, 2012. Theoretical Investigation of Interaction of Dicarboxylic Acids with Common Aerosol Nucleation Precursors. J. Phys. Chem. A 116, 4539–4550. 10.1021/jp301964u. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhang RY, Fortner EC, North SW, 2004. Quantification of hydroxycarbonyls from OH-isoprene reactions. J. Am. Chem. Soc 126, 2686–2687. 10.1021/ja0386391. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Truhlar DG, 2011. Applications and validations of the Minnesota density functionals. Chem. Phys. Lett. 502, 1–13. 10.1016/j.cplett.2010.11.060. [DOI] [Google Scholar]
- Zheng GJ, Duan FK, Su H, Ma YL, Cheng Y, Zheng B, et al. , 2015. Exploring the severe winter haze in Beijing: the impact of synoptic weather, regional transport and heterogeneous reactions. Atmos. Chem. Phys 15, 2969–2983. 10.5194/acp-15-2969-2015. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.