Abstract
Objective
Our scoping review aims to assess what legal, ethical, and socio-technical factors contribute to or inhibit the success of national eHealth system implementations. In addition, our review seeks to describe the characteristics and benefits of eHealth systems.
Materials and Methods
We conducted a scoping review of literature published in English between January 2000 and 2020 using a keyword search on 5 databases: PubMed, Scopus, Web of Science, IEEEXplore, and ProQuest. After removal of duplicates, abstract screening, and full-text filtering, 86 articles were included from 8276 search results.
Results
We identified 17 stakeholder groups, 6 eHealth Systems areas, and 15 types of legal regimes and standards. In-depth textual analysis revealed challenges mainly in implementation, followed by ethico-legal and data-related aspects. Key factors influencing success include promoting trust of the system, ensuring wider acceptance among users, reconciling the system with legal requirements, and ensuring an adaptable technical platform.
Discussion
Results revealed support for decentralized implementations because they carry less implementation and engagement challenges than centralized ones. Simultaneously, due to decentralized systems’ interoperability issues, federated implementations (with a set of national standards) might be preferable.
Conclusion
This study identifies the primary socio-technical, legal, and ethical factors that challenge and contribute to the success of eHealth system implementations. This study also describes the complexities and characteristics of existing eHealth implementation programs, and suggests guidance for resolving the identified challenges.
Keywords: eHealth, electronic health records, review
INTRODUCTION
eHealth refers to a growing variety of platforms, from complex national programs to mobile health applications.1,2 Just as we have seen the diversity of systems and technologies evolve in the last years, so too has the research sector increasingly framed eHealth systems as a means to promote accessibility, interoperability, security, and operationally efficiency. Increasingly, eHealth (defined as the use of information communication technologies for health3) is portrayed as a solution that can resolve many current problems with healthcare. These include the rising costs of treating aging populations and chronic diseases, inaccuracy of records and treatment due to human error, and fragmented delivery of healthcare. Accordingly, the problems with healthcare often get characterized as “wicked problems” or problems with fragmented and contradictory requirements that are difficult to solve.4
While the use of information communication technology in healthcare undeniably has benefits, attempts to implement these programs has led to significant problems. Rather than solving the issues they sought to address, in a cyclical fashion, these eHealth implementations often become “wicked problems.”5 A prominent example is the United Kingdom’s National Program for IT (NPfIT), designed to create a nationwide integrated electronic patient record system. The purported benefit of this program was to allow National Health Service (NHS) physicians to access patient records no matter which NHS practice or hospital the patient visited. However, NPfIT suffered severe delays due to a range of implementation, financial, and legal issues and was eventually dismantled in September 2011.6 The Dossier Médical Partagé in France encountered similar problems. However, the equivalent national electronic health record program in Estonia reports to have had higher rates of patient and physician uptake.7,8 With diverging outcomes of large-scale eHealth projects, an understanding of their benefits, challenges, and contributors to success would help for future planning of such a project.
Acknowledging that much can be learned from recent eHealth initiatives, the objective of this scoping review was to explore the relevant literature describing eHealth systems implementations. A scoping review can help to identify the main areas of a research field that has not been comprehensively studied both at a broad and in-depth level.9 Specifically, we seek to describe the characteristics of reported national health information systems and identify the socio-technical, legal, and ethical factors that challenged or led to the success of such programs. Although large scale projects are fraught with potential budget and time overruns,5 we seek to identify those specific to national eHealth systems. For this purpose, we used references to national or regional health information systems platforms in a federal system as inclusion criteria. Several scoping reviews have already examined monitoring and governance of national electronic health records and eHealth strategies.10–13 However, our review attempts to identify the specific factors that are reported as benefits, challenges, and factors in the implementation of these systems. Results from this study will help future implementation projects identify what might be the critical factors in success and failure.
MATERIALS AND METHODS
Our scoping review followed the PRISMA Extension for Scoping Reviews (PRISMA-ScR).14 As such, we report the method used according to the following steps: (1) identifying the research questions, (2) identifying the relevant literature, (3) selecting articles, (4) analysis of articles, (5) collating, summarizing, and reporting results.
Research questions
With this scoping review, we sought to answer 2 research questions:
What are the main characteristics of eHealth systems?
What are the benefits, challenges, and contributors to success for eHealth systems implementation on a national and regional level?
Identifying relevant literature
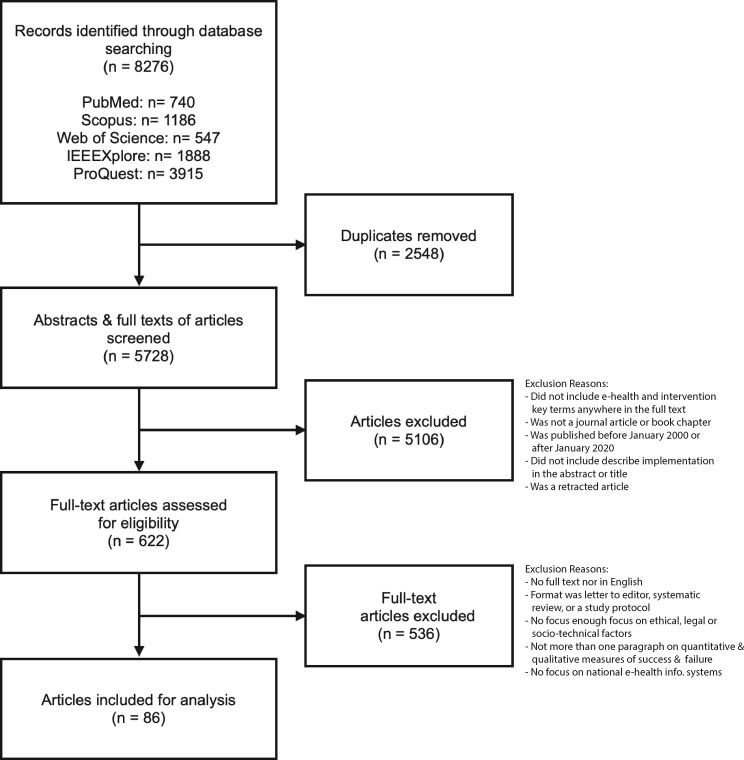
We developed the search strategy through discussions with the broader research team for the Data Protection and Personalized Health project, which included 2 senior academics in bioethics and a senior researcher in law. Table 1 displays the search strategy and Figure 1 provides an overview of the 6 stages of screening.
Table 1.
Search strategy
| Process | Detail |
|---|---|
| Databases | PubMed, Scopus, Web of Science, IEEEXplore, and ProQuest |
| Search terms |
|
| Type of study | Journal article or book chapter |
| Time range | January 2000–January 2020 |
| Limits | English |
| Inclusion | Abstract describes implementing an eHealth system and challenges or success factors. |
| Exclusion | Articles that were retracted |
Figure 1.
PRISMA flowchart displaying the search protocol for articles.
We performed a search across 5 databases using various key terms relating to the review’s central concepts of “eHealth,” “intervention,” and “location” (Table 1.). There was no restriction on the type of publication, study design, or methods used. The search resulted in 8276 articles which, after duplicates were manually removed, left 5728. Next, we screened the full text of the resulting articles based on the search strategy presented in Table 1 and Figure 1. Articles were included if they had the relevant search terms in their full text, were published between January 2000 and 2020, were written in English, and were either a book chapter or a journal article. We excluded letters to the editor, correspondences, comments, and editorials. Articles were excluded if they did not include the “eHealth” and “Intervention” key terms or describe the implementation in their abstract or title. Retracted articles were also excluded.
Further for inclusion, articles had to either discuss and assess, retrospectively or prospectively, health information system implementations. Selecting this spectrum of different studies helped us adopt a narrative approach to assessing the success or failure of different programs.15 The challenges or factors of success described were not limited to either quantitative or qualitative measures. For example, levels of participation in a national health record system or a cost analysis showing an increase in healthcare efficiency could be considered quantitative measures of success. By contrast, availability, functionality, or perceptions about the trustworthiness of a national health record system could be considered a qualitative measure of success.16
Article selection
After the initial screening, a total of 622 remained for full-text review (Figure 1). For those articles identified, we used the Zotero reference management software to extract the year of publication, authors, title and abstract, full-body text (if available), and references. We then screened the full texts for the following inclusion and exclusion criteria.
Included were full-text articles in English that focused on national health information system platforms and implementation strategies. Formats included were implementation studies, empirical or comparative studies, or opinion, technical, survey-based, doctrinal, and legal articles. Further, articles needed at least 1 paragraph on 2 of the following factors: ethical, legal, or socio-technical. Articles also had to have at least 1 paragraph on potential quantitative and qualitative measures of success and failure, including ex-ante observations about the successes and failures/challenges of a health information system, or ex-ante comparative observations about the success and failure of a health system.
We excluded articles that did not have full texts in English or were letters to the editor, systematic reviews, and study protocols. We also excluded articles that only tangentially mentioned ethical, legal, or socio-technical factors (for example, mentioning ethical or legal requirements as part of an ethical review), or that focused on other factors such as only on economic or technical ones. Also excluded were articles that did not have at least 1 paragraph on potential quantitative and qualitative measures of success and failure. Finally, we excluded articles that focused only on mobile health applications or medical devices. Our justification for this was that these devices are often implemented on an institutional basis as opposed to software implementations that occur on a national or regional basis. Our overall approach was described in Figure 1.
Analysis of articles
The next stage of the project was to code each of the articles quantitatively. In this process, we identified the characteristics of the eHealth systems described and the factors leading to success or failure. We inductively identified these categories using the thematic model suggested by Braun and Clarke.17 Also, we conducted a thematic content analysis on specific categories identified from each of the articles. Table 2 provides details.
Table 2.
Inductive coding themes
| Category | Description |
|---|---|
| Article type | Refers to the type of study performed. After initial coding, an ex-ante approach conducted in concert between the 2 coders revealed the following categories and subcategories of study type:
|
| Taxonomy of system | This category was inductively coded and referred to the health information system as it was described within the document. For example, a distinction was drawn between electronic health systems (as a reference to a longitudinal record associated with a single individual) and electronic medical records (as a reference to a software system for storing electronic health records18). |
| Structure of system | As alluded to with respect to the level of health information systems, the authors identified 3 ways in which a health information system could be implemented. The first is a centralized or ‘top down’ model. Following this approach, the health information system is implemented by a government and rolled out to all hospitals and healthcare institutes. An example of this approach is the National Health Service Information for Health Strategy, a comprehensive rollout that was attempted in the United Kingdom following the electoral victory of Labour in 1997. This centralized approach has the advantage of increasing standardization and procurement processes and increasing the likelihood of health records being used for secondary purposes. However, the National Health IT System implementation process has also been plagued with ‘setbacks, misgivings, clinical unrest, delays, cost overruns and pairing back of promised functionality.’19 Further, placing all health records in 1 location creates a single point of failure, increasing the impact of a potential data breach. |
| Stakeholders | The coders agreed that different stakeholder categories would be coded inductively from each article. These included all actors that might be involved in the creation of health information systems. Once we had coded these categories, we then grouped them into subcategories. |
| Country/jurisdictions | The coders agreed to inductively code both the jurisdiction of the authors and the jurisdiction of study. |
| Legal regimes/regulations | This category referred to any international guidelines and national or supranational legislation mentioned within the article. These values were separated by commas. |
| Benefits, Challenges & Contributors to Success | An inductive coding mechanism was used to identify any factors which could support or undermine implementation. As discussed previously, these 3 code categories were subject to thematic content analysis.20 |
Coding was carried out in unison by 2 of the authors using the software MaxQDA. The authors coded phrases by sentence mentions (as opposed to full sentences). Except for legal regimes and regulations, the authors excluded footnotes, keywords, references, and titles from the scope of coding. To capture legislation, we included footnotes and references in our analysis. The same authors as above coded the first 10 documents separately to minimize subjective bias, then checked for congruence. Once these codes were reconciled, the authors split the remaining 77 documents and coded them independently. The authors then merged these documents together once coding was completed.
RESULTS
Our search and selection resulted in 86 articles for full-text analysis. Of these, 53 were empirical studies, such as qualitative or quantitative stakeholder assessments, case studies, media analyses, or implementation reports. A breakdown of these stakeholders is contained in Figure 2. Sixteen articles were implementation studies describing a particular architecture or implementation strategy and the remaining 17 were ethico-legal articles.
Figure 2.

Distribution of stakeholders.
Description of eHealth systems
Taxonomy & structure of eHealth systems
The articles described a diversity of eHealth systems, surmised by 6 categories: (1) health data, such as patient data, (2) data collection, like medical records or registries (3) technologies, like meaning information communication technologies (4) systems, such as pharmacy information systems, (5) healthcare services, like e-prescription and telehealth, and (6) networks, such as clinical data research networks. Of these 6 areas, the majority of documents (n = 53/86, 61%) made references to types of data collections. This category also had the most subcategories (Supplementary Materials). The least reported category was networks, which had just 4 different subcategories and 49 instances across 5 documents. The Supplementary Materials details each category’s subcategory and the sum of their occurrences across documents. Centralized eHealth systems appeared most frequently (n = 38/86, 44%), followed by decentralized (n = 24/86, s = 58). Least reported was federated eHealth systems (n = 12/86, s = 21).
Stakeholders
We identified 17 different groups of e-Health system stakeholders. The majority of articles (n = 85/86) referenced the stakeholder groups of patients and the lay public, along with primary healthcare providers (n = 80/86) and healthcare organizations (n = 78/86). Government and academic stakeholders were also often mentioned (both n = 71/86), followed by information communication stakeholders (n = 37/86), legal (n = 37/86) and pharmaceutical stakeholders (n = 33/86). Less frequently mentioned across all documents were data protection stakeholders and insurance organizations (both n = 27/86).
Countries & jurisdictions
Analyses of the documents revealed 48 different countries and jurisdictions involved in the development or implementation of eHealth systems. As shown in Figure 3, the 3 most mentioned countries were the UK, USA, and Australia. Canada, the European Union, Denmark, Sweden and Singapore were also frequently mentioned.
Figure 3.
Jurisdictions included.
Legal regimes & regulations
We coded and clustered legal regimes into 17 categories (Supplementary Materials). The most frequently reported were national data and privacy protection laws and legislations (n = 29/86, s = 96). Following were national electronic health record and health funding legislations (n = 45/86, s = 17); these provide rules on processing health records and often supersede data protection laws. Likewise, health funding laws are crucial for guaranteeing the ongoing sustainability and support of eHealth systems. Although there was some mention of supranational data protection frameworks, these were often mentioned in the context of national data protection laws.21–33 National health service laws (n = 10/86, s = 20) also offered guidelines on using and implementing eHealth systems.34,35
Benefits, challenges, and contributors to success
Benefits of eHealth systems
The articles analyzed and reported a plethora of benefits to eHealth systems. Most frequently, they were said to improve the efficiency and effectiveness of healthcare coordination,36,37 processes, and delivery (n = 57, s = 156). For example, by “simplify[ing] administrative tasks,”38 helping with “optimizing physical resources,”39 and “reducing duplication of services, realizing operational efficiencies.”40 Many documents also described eHealth systems as a means to improve access and exchange of information and data (n = 57/86, s = 144).41–46 Over half of the documents also reported that eHealth would improve the quality of care (n = 48/86, s = 107),47 and support research and policy (n = 43/86, s = 92). Other perceived benefits included patient empowerment and engagement,46,48–51 improvements to patient safety and data security,53 reduction of costs, better service monitoring54 and generally increased ability to address challenges that emerge.55Supplementary Table 7 provides details.
Challenges of eHealth systems
More prevalent than the benefits, however, were challenges. Overall, we coded challenges within 5 categories; implementation, legal and ethical, data-related, engagement, and software-related challenges (Figure 4).
Figure 4.
Sunburst diagram of eHealth system challenges and subcategories according to number of segments.
Implementation challenges frequented most and included conflicting stakeholder requirements (n = 26/86, s = 48),56,57 difficulty demonstrating benefits (n = 32, s = 76/86),58 financial issues (n = 29/86, s = 104), government, policy, and political issues (n = 18/86, s = 28), as well as broader implementation challenges (n = 45/86, s = 238).59
Legal and ethical challenges also abounded. Most frequently reported were concerns about privacy (n = 54/86, s = 192)60 and research ethics (n = 15/86, s = 86). The latter referred to challenges with ethics committee approvals,61–64 ensuring representative samples, reporting results, protecting the rights of patients,38,61,65,66 and consent/reconsent processes.67–70 The articles we examined also identified patient autonomy, or the right of patients to make decisions about their medical care and overall health, as a significant challenge. In this context, patient autonomy specifically referred to patients controlling access to their electronic records and whether an opt-in or opt-out consent was preferable.59 Also, our sampled documents mentioned 3 categories of medical liability (n = 17/86, s = 42).70 These were the liability of physicians and healthcare organizations to provide adequate medical care, device or product liability for eHealth systems, and patient responsibility for their health records.21,71–73
Over two-thirds of the documents reported data challenges (n = 68/86, s = 318). First, this related to challenges of ensuring data availability (n = 50/86, s = 148), meaning the ability or willingness to share data with other hospitals, healthcare institutions, or research institutions.61,74–76 For example, on a jurisdictional level was the challenge of data transfer across national or international borders.62,68,77 Likewise, challenges arose regarding the availability of different data forms, such as metadata and data linkage.37,62,77,78 The second data challenge was information quality (n = 31/86, s = 53) referring to poor quality, missing, or lost records and information.19,79,80The third data challenge was interoperability (n = 43/86, s = 117), in that using heterogeneous standards would limit data sharing.42,44,81–83 Likewise, the use of “silo” or free text systems was said to undermine the free exchange of data.58
Stakeholder engagement was another major challenge. First, was the issue of lack of uptake. This involved a lack of patient uptake (n = 33/86, s = 108), which would undermine the use of patient data for secondary research purposes.52,84,85As well, physicians would refuse to use the systems because they did not trust or had had poor experiences,86–89 with software frequently being attributed as a cause in physician burnout (n = 41/86, s = 176). In turn, this would limit the benefits that might flow from these systems.90–93 Also impacting engagement was lack of access to benefits from using eHealth systems (n = 12/86, s = 23), such as from geographically isolated areas and developing countries,40,94 and communication issues (n = 8/86, s = 10).
Finally, technical challenges associated with the systems themselves were mentioned (n = 42/86, s = 142). Out of these, security challenges were most frequent (n = 24/86, s = 51) and involved difficulties with security measures, including role-based access control and information security.55,95 Further, these challenges were directly related to broader ethico-legal concerns about patient autonomy and privacy, as well as impacting the engagement of physicians and patients.
Contributors to success
We identified 6 categories of factors contributing to successful eHealth systems and their implementation. As shown in Supplementary Table 9, the categories were socio-cultural, system-based, legal, implementation, and resources.
Most frequently highlighted as an underlying success factor was stakeholder engagement (n = 58/86, s = 218). Users’ trust and support were integral. One article describes “The adoption and diffusion of a large-scale IT programme necessitates the cultural and normative acceptance of a wide array of constituents, not merely within an organisation, but at the levels of the organisational field and wider society.”96,97 Many articles proposed methods of incentivization alongside skills training and the provision of guidance to foster support, trust, and shared ownership of the system. Multi-disciplinary collaborations too were advised, for example, by engaging with patient representatives, having public involvement at every stage, or by letting government policy makers set the agenda. Communicating about lessons learned and sharing past experiences was also suggested to help speed up implementation and reduce error.
The majority of articles also emphasized several system qualities (n = 64/86, s = 212). eHealth systems needed to be flexible, intuitive, accessible, transparent, reliable, interoperable, and safe. Having context-sensitive security processes and robust data governance structures would “demonstrate trustworthiness and sustain the social license.”97–99 Data accuracy and consent mechanisms were needed that served user needs by promoting informational autonomy.83,100,101 Recommended were also decentralized systems because “centralization causes paralysis.”102
Regarding legal contributors to success, over half of the documents reported the importance of abiding by and supporting the development of standards, protocols, guides, and recommendations (n = 45/86, s = 134).57 Also important were good implementation practices, such as defining goals, having a realistic timeline, and iterative evaluation processes (n = 40/86, s = 149).56 Interestingly, the least reported factor of success was resources (n = 32/86, s = 82).
DISCUSSION
This scoping review paints a comprehensive picture of eHealth system implementations, as documented in the current literature. To begin with, the results demonstrate that eHealth systems are characterized by complexity and diversity in their connection of divergent; data types, data collections, different technologies, systems and networks, are all made up of different actors. Beyond just referring to electronic health records, eHealth systems include patient-controlled records and eHealth cards, designed to make healthcare more accessible for patients. At the same time, eHealth refers to computerized physician order entry, decision support, and laboratory information systems, all of which help physicians with their work. Indeed, as the results show, eHealth systems promise benefits to all stakeholders, particularly medical practitioners and patients, but also policy makers, researchers, and the general public.
Interestingly, through this scoping review we see a decline in support for centralized eHealth implementations, and instead a growth in support for decentralized and federated platforms. As mentioned previously, the earliest implementations of national electronic health record systems were those implemented in the United Kingdom. However, these early implementations were plagued by numerous project management issues, and security concerns about storing data in 1 location. These shortcomings led to distrust and a lack of uptake from both physicians and patients.19,103 For this reason, the United Kingdom abandoned their centralized approach and have shifted to a decentralized one. Notably, decentralized systems have a higher degree of patient engagement.81 However, these systems also face challenges in terms of integration and interoperability. Therefore, federated systems may offer a useful compromise in guaranteeing the security of patient data while ensuring that healthcare systems remain interoperable.
Regarding challenges, our analysis demonstrated that technical shortcomings, such as system architecture, are not necessarily the greatest impediment to success. Nonetheless, technical issues have a flow-on effect, such as leading to low usability and security concerns. One means to avoid these 2 issues, as recommended by the articles analyzed, is to ensure from the onset that the eHealth system implemented is intuitive, interoperable, transparent, accessible, safe, and reliable. Ensuring these system qualities, along with robust data governance structures and context-sensitive security contributes to success.
Of greatest challenge to eHealth systems are ethico-legal factors, particularly privacy and research ethics concerns (such as informed and broad consent), secondary uses of data, and return of results. To address these, implementation projects need to actively foster support, trust, and ownership of the system among all actors, while also incentivizing engagement and providing training and guidance to the actors involved. The articles analyzed also stressed the importance of ensuring that eHealth systems comply with the laws of the jurisdiction in which they operate. Further, some jurisdictions had implemented national electronic health records legislation that determined the use of these systems (such as Australia and Germany). Ensuring that all systems are compliant with this legislation, which may supervene national privacy laws, and that there is no mismatch between technical and legislative solutions, however, can also be challenging. Finally, legislation on healthcare system funding is important to ensure the ongoing financial sustainability of eHealth systems.
Outside of implementation and legal challenges, the high rate at which data challenges were mentioned indicates the necessity for interoperable standards and vocabularies. In turn, this need may make federated systems that supply a set of national standards preferable to decentralized systems. Coiera (2011) notes that a “middle out” national strategy might be preferable for certain health data types, such as summary care records, which are more “liquid” than other records, such as prescription information.19 Likewise, the need for data availability and interoperability may inform the technical design for eHealth systems. For example, Doods et al (2014) and Hailemichael et al (2015) describe a distributed learning platform for conducting privacy-preserving computations on patient data. Both of these platforms crucially allow for these computations to be performed without the data leaving the facility where they are stored.75,104 These technical solutions may have an impact on another major challenge identified in this study, namely the lack of patient or physician uptake.
Strengths & limitations
One potential limitation of this study is that our search terms may have failed to capture all studies that would have met the inclusion criteria. Another limitation is that we focused only on articles published in the last 20 years. While this ensures that our review reflects current practice, given the rapidly changing nature of technology and cultural norms, some of the issues captured form 2 decades ago may no longer be relevant. Further, there may have been grey literature or articles in languages other than English relevant to our review; however, their inclusion was beyond the scope of this study. Despite these limitations, given the methodological approach taken and the inclusive search strategy, we believe this scoping review provides an overview of the characteristics, benefits, challenges, and factors contributing to the success of eHealth systems internationally. Finally, 1 of the difficulties in measuring the successes and challenges of eHealth implementations is that these projects are often multiyear initiatives. Our scoping review did not include longitudinal changes in benefits, challenges, and success factors. With respect to the different types of eHealth implementations, we note that different components of that system can be combined centralized/decentralized or federated systems, depending on their function. For example, the Australian centralized MyHealth Record system is limited to a summary care record, with decentralized records existing on a state level.37 Therefore, ongoing monitoring of eHealth implementations, particularly different aspects of these systems, is crucial for measuring any benefits and challenges.13
CONCLUSION
This review provides an overview of the benefits, challenges, and factors to success for respective implementations. The analysis of results brings us to the conclusion that eHealth systems provide a diversity of benefits. In particular, eHealth systems can contribute to improving access and exchange of information and data. Further, they can improve the quality of care, reduce costs, support research and policy, and safeguard patient empowerment and safety. However, for these benefits to actualize, it is critical to focus on their implementation which requires attention to more than just the technologies themselves.
FUNDING
This work was partially funded by the Personalized Health and Related Technologies Program (grant 2017-201; project: Data Protection and Personalized Health) supported by the Council of the Swiss Federal Institutes of Technology.
AUTHOR CONTRIBUTIONS
JScheibner, MI, JSleigh, and EV contributed to the conception of the study. JScheibner and MI tested the protocol. JScheibner and JSleigh performed the analysis and wrote the first draft of the manuscript. JSleigh designed the tables and diagrams. All authors contributed to the manuscript revision, read, and approved the submitted version.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES:
- 1.Gustafson DH, Wyatt JC.. Evaluation of ehealth systems and services. BMJ 2004; 328 (7449): 1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahern DK, Kreslake JM, Phalen JM.. What is eHealth (6): perspectives on the evolution of eHealth research. J Med Internet Res 2006; 8 (1): e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott R, Mars M.. Here we go again-‘digital health’. J Int Soc Telemed EHealth 2019; 7: e1–1. [Google Scholar]
- 4.Shaw SE, Rosen R.. Fragmentation: a wicked problem with an integrated solution? J Health Serv Res Policy 2013; 18 (1): 61–4. [DOI] [PubMed] [Google Scholar]
- 5.Garrety K, McLoughlin I, Dalley A, Wilson R, Yu P.. National electronic health record systems as ‘wicked projects’: the Australian experience. Inform Polity 2016; 21 (4): 367–81. [Google Scholar]
- 6.Justinia T.The UK’s National Programme for IT: why was it dismantled? Health Serv Manage Res 2017; 30 (1): 2–9. [DOI] [PubMed] [Google Scholar]
- 7.Séroussi B, Bouaud J.. The (re)-relaunching of the DMP, the French shared medical record: new features to improve uptake and use. Stud Health Technol Inform 2018; 247: 256–60. [PubMed] [Google Scholar]
- 8.de Lusignan S, Ross P, Shifrin M, Hercigonja-Szekeres M, Seroussi B.. A comparison of approaches to providing patients access to summary care records across old and new Europe: an exploration of facilitators and barriers to implementation. Stud Health Technol Inform 2013; 192: 397–401. [PubMed] [Google Scholar]
- 9.Arksey H, O'Malley L.. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005; 8 (1): 19–32. [Google Scholar]
- 10.Dixon BE, Pina J, Kharrazi H, Gharghabi F, Richards J.. What’s past is prologue: a scoping review of recent public health and global health informatics literature. Online J Public Health Inform 2015; 7 (2): e216. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4576440/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holeman I, Cookson TP, Pagliari C.. Digital technology for health sector governance in low and middle income countries: a scoping review. J Glob Health 2016; 6 (2): S41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Essén A, Scandurra I, Gerrits R, et al. Patient access to electronic health records: differences across ten countries. Health Policy Technol 2018; 7 (1): 44–56. [Google Scholar]
- 13.Villumsen S, Adler-Milstein J, Nøhr C.. National monitoring and evaluation of eHealth: a scoping review. JAMIA Open 2020; 3 (1): 132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169 (7): 467–73. [DOI] [PubMed] [Google Scholar]
- 15.Crampton NH, Reis S, Shachak A.. Computers in the clinical encounter: a scoping review and thematic analysis. J Am Med Inform Assoc 2016; 23 (3): 654–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang MZ, Gibson CJ, Terry AL.. Measuring electronic health record use in primary care: a scoping review. Appl Clin Inform 2018; 09 (01): 015–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun V, Clarke V.. Using thematic analysis in psychology. Qual Res Psychol 2006; 3 (2): 77–101. [Google Scholar]
- 18.Eden R, Burton-Jones A, Scott I, Staib A, Sullivan C.. Effects of eHealth on hospital practice: synthesis of the current literature. Aust Health Rev 2018; 42 (5): 568–78. [DOI] [PubMed] [Google Scholar]
- 19.Coiera E.Do we need a national electronic summary care record? Med J Aust 2011; 194 (2): 90–2. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh H-F, Shannon SE.. Three approaches to qualitative content analysis. Qual Health Res 2005; 15 (9): 1277–88. [DOI] [PubMed] [Google Scholar]
- 21.Guarda P, Ducato R.. From electronic health records to personal health records: emerging legal issues in the Italian regulation of e-health. Int Rev Law Comput Technol 2016; 30 (3): 271–85. [Google Scholar]
- 22.Herbek S, Eisl HA, Hurch M, et al. The electronic health record in Austria: a strong network between health care and patients. Eur Surg 2012; 44 (3): 155–63. [Google Scholar]
- 23.Asadi F, Moghaddasi H, Rabiei R, Rahimi F, Mirshekarlou SJ.. The evaluation of SEPAS national project based on Electronic Health Record System (EHRS) coordinates in Iran. Acta Inform Med 2015; 23 (6): 369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burton LC, Anderson GF, Kues IW.. Using electronic health records to help coordinate care. Milbank Q 2004; 82 (3): 457–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eigner I, Hamper A, Wickramasinghe N, Bodendorf F.. Success factors for national eHealth strategies: a comparative analysis of the Australian and German eHealth system. Int J Netw Virtual Organ 2019; 21 (4): 399–424. [Google Scholar]
- 26.Gummadi S, Housri N, Zimmers TA, Koniaris LG.. Electronic medical record: a balancing act of patient safety, privacy and health care delivery. Am J Med Sci 2014; 348 (3): 238–43. [DOI] [PubMed] [Google Scholar]
- 27.Gunter TD, Terry NP.. The emergence of national electronic health record architectures in the United States and Australia: models, costs, and questions. J Med Internet Res [Internet 2005; 7 (1): e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hägglund M, Scandurra I.. Patients’ online access to electronic health records: current status and experiences from the implementation in Sweden. Stud Health Technol Inform 2018; 245: 723–7. [PubMed] [Google Scholar]
- 29.Li Y-CJ, Yen J-C, Chiu W-T, Jian W-S, Syed-Abdul S, Hsu M-H.. Building a national electronic medical record exchange system - experiences in Taiwan. Comput Methods Programs Biomed 2015; 121 (1): 14–20. [DOI] [PubMed] [Google Scholar]
- 30.Mohd Nor NA, Taib NA, Saad M, et al. Development of electronic medical records for clinical and research purposes: the breast cancer module using an implementation framework in a middle income country—Malaysia. BMC Bioinformatics 2019; 19 (Suppl 13): 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scandurra I, Jansson A, Forsberg-Fransson M-L, Ålander T.. Patient accessible EHR is controversial: lack of knowledge and diverse perceptions among professions. In: Consumer-Driven Technologies in Healthcare: Breakthroughs in Research and Practice. Hershey: IGI Global; 2018: 77–96. [Google Scholar]
- 32.Vezyridis P, Timmons S.. Understanding the care.data conundrum: new information flows for economic growth. Big Data Soc 2017; 4 (1): 205395171668849. [Google Scholar]
- 33.Williams PAH, Hossack E.. It will never happen to us: the likelihood and impact of privacy breaches on health data in Australia. Stud Health Technol Inform 2013; 188: 155–61. [PubMed] [Google Scholar]
- 34.Heart T, O'Reilly P, Sammon D, O'Donoghue J.. Bottom-up or top-down? A comparative analysis of electronic health record diffusion in Ireland and Israel. J Syst Info Tech 2009; 11 (3): 244–68. [Google Scholar]
- 35.Metzger M-H, Durand T, Lallich S, Salamon R, Castets P.. The use of regional platforms for managing electronic health records for the production of regional public health indicators in France. BMC Med Inform Decis Mak 2012; 12 (1): 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georgiou A, Magrabi F, Hyppönen H, et al. The safe and effective use of shared data underpinned by stakeholder engagement and evaluation practice. Yearb Med Inform 2018; 27 (1): 25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearce C, Bainbridge M.. A personally controlled electronic health record for Australia. J Am Med Inform Assoc 2014. ; 21 (4): 707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.France FR.eHealth in Belgium, a new “secure” federal network: role of patients, health professions and social security services. Int J Med Inform 2011; 80 (2): e12–6. [DOI] [PubMed] [Google Scholar]
- 39.Abin J, Nemeth H, Friedmann I.. Systems architecture for a nationwide healthcare system. Stud Health Technol Inform 2015; 216: 12–6. [PubMed] [Google Scholar]
- 40.Alvarez RC.The promise of e-health–a Canadian perspective. World Hosp Health Serv 2004; 40 (4): 31–5. [PubMed] [Google Scholar]
- 41.Lupton D. “I’d like to think you could trust the government, but I don’t really think we can”: Australian women’s attitudes to and experiences of My Health Record. Digit Health 2019; 5: 205520761984701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGovern A, Hinton W, Correa A, Munro N, Whyte M, de Lusignan S.. Real-world evidence studies into treatment adherence, thresholds for intervention and disparities in treatment in people with type 2 diabetes in the UK. BMJ Open 2016; 6 (11): e012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scandurra I, Lyttkens L, Eklund B.. Implications of Swedish National Regulatory Framework of the patient accessible electronic health record. Stud Health Technol Inform 2016; 228: 695–9. [PubMed] [Google Scholar]
- 44.Schiza EC, Kyprianou T, Petkov N, Schizas CN.. Proposal for an eHealth based ecosystem serving national healthcare. IEEE J Biomed Health Inform 2018; 23 (3): 1. [DOI] [PubMed] [Google Scholar]
- 45.Stroetmann KA, Artmann J, Stroetmann V.. Developing national eHealth infrastructures – results and lessons from Europe. AMIA Annu Symp Proc 2011; 2011: 1347–54. [PMC free article] [PubMed] [Google Scholar]
- 46.Symons JD, Ashrafian H, Dunscombe R, Darzi A.. From EHR to PHR: let’s get the record straight. BMJ Open 2019; 9 (9): e029582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milani L, Leitsalu L, Metspalu A.. An epidemiological perspective of personalized medicine: the Estonian experience. J Intern Med 2015; 277 (2): 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Currie WL.Translating health IT policy into practice in the UK National Health Service. Scand J Inf Syst 2014; 26 (2): 3–26. [Google Scholar]
- 49.Hyppönen H, Doupi P, Hämäläinen P, Komulainen J, Nykänen P, Suomi R.. Towards a National Health Information System evaluation. Stud Health Technol Inform 2010; 160 (Pt 2): 1216–20. [PubMed] [Google Scholar]
- 50.Nøhr C, Parv L, Kink P, et al. Nationwide citizen access to their health data: analysing and comparing experiences in Denmark, Estonia and Australia. BMC Health Serv Res 2017; 17 (1): 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Protti D, Johansen I, Perez-Torres F.. Comparing the application of Health Information Technology in primary care in Denmark and Andalucía, Spain. Int J Med Inform 2009; 78 (4): 270–83. [DOI] [PubMed] [Google Scholar]
- 52.Williams H, Spencer K, Sanders C, et al. Dynamic consent: a possible solution to improve patient confidence and trust in how electronic patient records are used in medical research. JMIR Med Inform 2015; 3 (1): e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clarke A, Adamson J, Sheard L, Cairns P, Watt I, Wright J.. Implementing electronic patient record systems (EPRs) into England’s acute, mental health and community care trusts: a mixed methods study. BMC Med Inform Decis Mak 2015; 15 (1): 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plantier M, Havet N, Durand T, et al. Does adoption of electronic health records improve the quality of care management in France? Results from the French e-SI (PREPS-SIPS) study. Int J Med Inf 2017; 102: 156–65. [DOI] [PubMed] [Google Scholar]
- 55.Becker MY.Information governance in NHS’s NPfIT: A case for policy specification. Int J Med Inform 2007; 76 (5–6): 432–7. [DOI] [PubMed] [Google Scholar]
- 56.Klecun E, Zhou Y, Kankanhalli A, Wee YH, Hibberd R.. The dynamics of institutional pressures and stakeholder behavior in national electronic health record implementations: a tale of two countries. J Inform Technol 2019; 34 (4): 292–332. [Google Scholar]
- 57.Showell CM.Citizens, Patients and policy: a challenge for Australia’s national electronic health record. Health Inf Manag 2011; 40 (2): 39–43. [DOI] [PubMed] [Google Scholar]
- 58.Severinsen G-H, Silsand L, Ellingsen G, Pedersen R.. From free-text to structure in electronic patient records. Stud Health Technol Inform 2019; 265: 86–91. [DOI] [PubMed] [Google Scholar]
- 59.Moats D, McFall L.. In search of a problem: mapping controversies over NHS (England) patient data with digital tools. Sci Technol Hum Values 2019; 44 (3): 478–513. [Google Scholar]
- 60.Quantin C, Allaert F-A, Avillach P, et al. Building application-related patient identifiers: what solution for a European country? Int J Telemed Appl 2008; 678302: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ali J, Califf R, Sugarman J.. Anticipated ethics and regulatory challenges in PCORnet: the national patient-centered clinical research network. Account Res 2016; 23 (2): 79–96. [DOI] [PubMed] [Google Scholar]
- 62.Andrew NE, Sundararajan V, Thrift AG, et al. Addressing the challenges of cross-jurisdictional data linkage between a national clinical quality registry and government-held health data. Aust N Z J Public Health 2016; 40 (5): 436–42. [DOI] [PubMed] [Google Scholar]
- 63.Curtis LH, Brown J, Platt R.. Four health data networks illustrate the potential for a shared national multipurpose big-data network. Health Aff (Millwood) 2014; 33 (7): 1178–86. [DOI] [PubMed] [Google Scholar]
- 64.McGuire AL, Basford M, Dressler LG, et al. Ethical and practical challenges of sharing data from genome-wide association studies: the eMERGE Consortium experience. Genome Res 2011; 21 (7): 1001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chunyan D.Patient privacy protection in China in the age of electronic health records. Hong Kong Law J 2013; 43: 245–78. [Google Scholar]
- 66.McDonald L, Lambrelli D, Wasiak R, Ramagopalan SV.. Real-world data in the United Kingdom: opportunities and challenges. BMC Med 2016; 14 (1): 1–3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4921013/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riordan F, Papoutsi C, Reed JE, Marston C, Bell D, Majeed A.. Patient and public attitudes towards informed consent models and levels of awareness of electronic health records in the UK. Int J Med Inform 2015; 84 (4): 237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suominen H.Towards an international electronic repository and virtual laboratory of open data and open-source software for telehealth research: comparison of international, Australian and Finnish privacy policies. Stud Health Technol Inform 2012; 182: 153–60. [PubMed] [Google Scholar]
- 69.Teare HJA, de Masi F, Banasik K, et al. The governance structure for data access in the DIRECT consortium: an innovative medicines initiative (IMI) project. Life Sci Soc Policy 2018; 14 (1): 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valverde JL.The European electronic health record. Critics and future. Pharmceut Policy Law 2018; 19 (3-4): 247–57. [Google Scholar]
- 71.Mitchell C, Ploem C.. Legal challenges for the implementation of advanced clinical digital decision support systems in Europe. J Clin Transl Res 2018; 3: 424–30. [PMC free article] [PubMed] [Google Scholar]
- 72.Spriggs M, Arnold MV, Pearce CM, Fry C.. Ethical questions must be considered for electronic health records. J Med Ethics 2012; 38 (9): 535–9. Sep 1 [DOI] [PubMed] [Google Scholar]
- 73.Abd Ghani MK, Bali RK, Naguib RNG, Marshall IM, Wickramasinghe NS.. Electronic health records approaches and challenges: a comparison between Malaysia and four East Asian countries. Int J Electron Healthc 2008; 4 (1): 78–104. [DOI] [PubMed] [Google Scholar]
- 74.Kariotis TC, Harris KM.. Clinician perceptions of My Health Record in mental health care: medication management and sharing mental health information. Aust J Prim Health 2019; 25 (1): 66–71. [DOI] [PubMed] [Google Scholar]
- 75.Hailemichael MA, Marco-Ruiz L, Bellika JG.. Privacy-preserving Statistical Query and Processing on Distributed OpenEHR Data. Stud Health Technol Inform 2015; 210: 766–70. [PubMed] [Google Scholar]
- 76.Levay C.Policies to foster quality improvement registries: lessons from the Swedish case. J Intern Med 2016; 279 (2): 160–72. Feb [DOI] [PubMed] [Google Scholar]
- 77.Boyd JH, Ferrante AM, O'Keefe CM, Bass AJ, Randall SM, Semmens JB.. Data linkage infrastructure for cross-jurisdictional health-related research in Australia. BMC Health Serv Res 2012; 12 (1): 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robertson ARR, Smith P, Sood HS, Cresswell K, Nurmatov UN, Sheikh A.. Tightrope walking towards maximising secondary uses of digitised health data: a qualitative study. J Innov Health Inform 2016; 23 (3): 847–9. [DOI] [PubMed] [Google Scholar]
- 79.Delvaux N, Aertgeerts B, Bussel J. V, Goderis G, Vaes B, Vermandere M.. Health data for research through a nationwide privacy-proof system in Belgium: design and implementation. JMIR Med Inform 2018; 6 (4): e11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gheorghiu B, Hagens S.. Measuring interoperable EHR adoption and maturity: a Canadian example. BMC Med Inform Decis Mak 2016; 16 (1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fragidis LL, Chatzoglou PD.. Implementation of a nationwide electronic health record (EHR): The international experience in 13 countries. Int J Health Care Qual Assur 2018; 31 (2): 116–30. [DOI] [PubMed] [Google Scholar]
- 82.Blobel B.Comparing approaches for advanced e-health security infrastructures. Int J Med Inform 2007; 76 (5-6): 454–9. [DOI] [PubMed] [Google Scholar]
- 83.Sweet LE, Moulaison HL.. Electronic health records data and metadata: challenges for big data in the United States. Big Data 2013; 1 (4): 245–51. [DOI] [PubMed] [Google Scholar]
- 84.Andrews L, Gajanayake R, Sahama T.. The Australian general public’s perceptions of having a personally controlled electronic health record (PCEHR). Int J Med Inform 2014; 83 (12): 889–900. [DOI] [PubMed] [Google Scholar]
- 85.Deutsch E, Duftschmid G, Dorda W.. Critical areas of national electronic health record programs—Is our focus correct? Int J Med Inform 2010; 79 (3): 211–22. [DOI] [PubMed] [Google Scholar]
- 86.Petrakaki D, Klecun E.. Hybridity as a process of technology’s ‘translation’: customizing a national electronic patient record. Soc Sci Med 2015; 124: 224–31. [DOI] [PubMed] [Google Scholar]
- 87.Bossen C.Accounting and co-constructing: the development of a standard for electronic health records. Comput Supported Coop Work 2011; 20 (6): 473–495. [Google Scholar]
- 88.Seroussi B, Bouaud J.. Use of a nationwide personally controlled electronic health record by healthcare professionals and patients: a case study with the French DMP. Stud Health Technol Inform 2017; 235: 333–7. [PubMed] [Google Scholar]
- 89.Zwaanswijk M, Ploem MC, Wiesman FJ, Verheij RA, Friele RD, Gevers JKM.. Understanding health care providers’ reluctance to adopt a national electronic patient record: an empirical and legal analysis. Med Law 2013; 32 (1): 13–31. Mar [PubMed] [Google Scholar]
- 90.Hackl WO, Hoerbst A, Ammenwerth E. “Why the hell do we need electronic health records?” EHR acceptance among physicians in private practice in Austria: a qualitative study. Methods Inf Med 2011; 50 (1): 53–61. [DOI] [PubMed] [Google Scholar]
- 91.Séroussi B, Bouaud J.. Adoption of a nationwide shared medical record in France: lessons learnt after 5 years of deployment. AMIA Annu Symp Proc 2016; 2016: 1100–9. [PMC free article] [PubMed] [Google Scholar]
- 92.Bosworth HB, Zullig LL, Mendys P, et al. Health information technology: meaningful use and next steps to improving electronic facilitation of medication adherence. JMIR Med Inform 2016; 4 (1): e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cho I, Kim J, Kim JH, Kim HY, Kim Y.. Design and implementation of a standards-based interoperable clinical decision support architecture in the context of the Korean EHR. Int J Med Inf 2010; 79 (9): 611–22. [DOI] [PubMed] [Google Scholar]
- 94.Were MC, Meslin EM.. Ethics of implementing electronic health records in developing countries: points to consider. AMIA Annu Symp Proc 2011; 2011: 1499–505. [PMC free article] [PubMed] [Google Scholar]
- 95.Mense A, Hoheiser-Pförtner F, Schmid M, Wahl H.. Concepts for a standard based cross-organisational information security management system in the context of a nationwide EHR. Stud Health Technol Inform 2013; 192: 548–52. [PubMed] [Google Scholar]
- 96.Currie WL, Finnegan DJ.. The policy-practice nexus of electronic health records adoption in the UK NHS: An institutional analysis. J Enterprise Inform Manag 2011; 24 (2): 146–70. [Google Scholar]
- 97.Wiljer D, Urowitz S, Apatu E, et al. ; Canadian Committee for Patient Accessible Health Records. Patient accessible electronic health records: exploring recommendations for successful implementation strategies. J Med Internet Res 2008; 10 (4): e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ballantyne A, Stewart C.. Big data and public-private partnerships in healthcare and research. Asian Bioeth Rev 2019; 11 (3): 315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tiik M, Ross P.. Patient opportunities in the Estonian electronic health record system. Stud Health Technol Inform 2010; 156: 171–7. [PubMed] [Google Scholar]
- 100.Grisot M, Vassilakopoulou P.. Re-infrastructuring for eHealth: dealing with turns in infrastructure development. Comput Supported Coop Work 2017; 26 (1-2): 7–31. [Google Scholar]
- 101.Kushniruk A, Kaipio J, Nieminen M, et al. Human factors in the large: experiences from Denmark, Finland and Canada in moving towards regional and national evaluations of health information system usability. Yearb Med Inform 2014; 23 (01): 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cockcroft S.A media analysis approach to evaluating national health information infrastructure development. J Syst Info Tech 2009; 11 (3): 208–29. [Google Scholar]
- 103.Wilson K, Khansa L.. Migrating to electronic health record systems: a comparative study between the United States and the United Kingdom. Health Policy 2018; 122 (11): 1232–9. [DOI] [PubMed] [Google Scholar]
- 104.Doods J, Bache R, McGilchrist M, Daniel C, Dugas M, Fritz F, Work Package 7. Piloting the EHR4CR feasibility platform across Europe. Methods Inf Med 2014; 53 (4): 264–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.