Abstract
Background
Determining the trends and treatment outcomes of TB in health facilities is very important to inform better management of the disease and control efforts. Nevertheless, data from the rural, urban and suburban settings of Ethiopia show variability and inconsistency. This study was designed to evaluate trends and treatment outcomes of tuberculosis patients at Tepi Health Center and to identify the predictors of unsuccessful treatment outcome.
Method
Retrospective review of TB cases registered in Tepi health center between June 2011 and May 2018 was conducted using data extracted from medical records of TB patients. Structured data extraction form was prepared and used to extract socio-demographic, clinical and outcome data of study cases. Case definition and the treatment outcome of patients were ascertained and reported in accordance with World Health Organization guideline. Binary logistic regression model was fit to identify predictors of unsuccessful outcome.
Results
A total of 1651 TB patients registered at Tepi Public Health Center in between June 2011 and May 2018, were included in the study. Of all 924 (56%) were males and 1053 (63.8%) cases were in between the age range of 15 and 35 years. HIV-status of 1019 TB cases were unknown and 189 (11.4%) of participants were HIV-positive. Four hundred fifty seven (27.7%) cases were diagnosed with extra pulmonary TB (EPTB) and 1194 (72.3%) were pulmonary TB patients out of which, 376 (73.6%) were smear-positive pulmonary TB (PTB+). Overall treatment success rate (TSR) of patients was 80.4% (1327/1651), while it was 84.8% (134/158), 80.2% (410/511), and 78.3% (148/189) among the transfer-in, PTB+, and HIV + cases, respectively. Higher numbers of successful treatment outcomes were recorded among new patients (82.7%) and EPTB cases (84.7%). The cure rate were 73.6% (376/511) and 18% (34/189) among patients with PTB+ and HIV+, respectively. Multiple logistic regression analysis indicated that residence sites (OR 0.763 (0.584, 0.996) and TB/HIV co-infection (OR 0.661 (0.444, 0.985), were significantly associated with the treatment outcome. Rural residence was 27.1% less likely to have successful treatment. There was significant heterogeneity in the odds of having successful treatment outcomes across years of initiating treatment.
Conclusion
Treatment success rate among study cases was lower than the WHO’s target and further efforts like availability of TB clinics in nearby sites and reducing rate of HIV infection should be made to improve rate of successful treatment outcome.
Keywords: Treatment outcomes, Unsuccessful outcome, Sheka zone, Tepi health center, Ethiopia
1. Introduction
Tuberculosis (TB) remains among the major public health problem that accounted for one of the top 10 causes of death worldwide and the leading cause of death from a single infectious agent. Globally, an estimated ten million people fell ill with TB in 2018 with a global average of 130. The burden of the disease varies considerably among countries but the situation is worst in TB-HIV/AIDS-burdened countries of sub-Saharan Africa [1], [2].
TB is a curable and preventable disease, and about 85% of people who develop TB disease can be successfully treated with a 6-month drug regimen, and it has further benefit of curbing onward transmission of the infection. Globally, TB treatment has averted more than 60 million deaths since 2000 [2]. Trends in the number of patients enrolled on TB treatment have shown global improvement but in TB-HIV burdened countries of Africa due to persisting existence of gaps in detection and treatment [3].
In Ethiopia, TB is the second cause of death with an estimated incidence of 151 per 100,000 populations in 2018 [3], [4]. The country has been implementing the direct observed treatment short course (DOTS) strategy and it is currently provided in almost all of its health facilities since 1992 [5].
The WHO recommends analysis of TB treatment outcomes every year at both the national and health settings level. Determining the trends and treatment success rate of health facilities is very important to inform better management of the disease and control efforts [6]. In Ethiopia a number of studies have been conducted and reported TB treatment success rates from different parts of the country [7], [8]. Nevertheless, due to socioeconomic factors, access to health services, and/or availability of diagnostic facilities, data on the treatment outcomes of TB from rural, urban and suburban settings of Ethiopia show variability and inconsistency. Studies in health facilities of northeastern Ethiopia; Kobo and Raya Kobo [7], and Alamata Woreda [8] reported above 88% treatment success rate (TSR). In the northwestern parts in Metema, 65.3% [9] and in Enfranz, 94.8% [10] treatment success rates were recorded while it was 70.8% in western [11] and 79.4% in central Ethiopia [12]. In Afar and Gambella regional states 81.8% [13] and 70.76% [14] success rates were determined, respectively. Earlier, a more representative study reported an average of 80% TSR in Southern nations, nationalities and people’s regional state (SNNPRS) of the country [15]. In a study conducted by Wondale et al. at Jinka Hospital, 70.8% TSR was indicated [16].
Hence, determining the outcomes of TB treatment among all forms of TB and performing trend analysis of the outcome will in turn contribute to the improvement of TB control programs and to the decrease of TB related morbidity and mortality [3], [6], [17]. Though a number of studies have reported treatment success rates from different parts of the country [10], [13], [16], further information is required on the outcomes of TB treatment among all forms of TB and socio-demographic characteristics of patients with trend analysis of the outcome, as per the WHO recommendation.
In SNNPRS the provision of DOTS services was introduced in 1996 and the program has now been introduced in all health facilities and, it has been active since 2001 in Tepi Health Center (THC) [16]. According to data from health officials of the center, TB is one of the major causes of mortality and morbidity in the region. Nevertheless, the treatment outcomes of TB patients and trend of the outcome in the Tepi district area have never been assessed so far. Therefore, this study was initiated to determine the treatment outcome, to perform trend analysis in all forms of TB patients treated and to identify factors associated with unsuccessful outcomes by reviewing eight years data of the patients in Tepi Health Center, southwestern Ethiopia.
2. Materials and methods
2.1. Study setting
This study was conducted using data extracted from medical records of TB patients registered in public health facility of Tepi town. Tepi town, which is situated in one of the three woredas of Sheka zone, is among the largest and oldest cities of SNNPRS of Ethiopia [18]. According to the projection of 2007 census report of Central Statistics Agency (CSA), the total population of Tepi town in 2018 was 24,169 of whom about 50% were men in [19]. Its climatic condition is characterized by higher temperature, higher moisture content and lower wind speed, and most inhabitants of the area are farmers while coffee plantation, crop and livestock productions are their common practices [18].
Tepi Health Center (THC) was established as a Clinic in 1966, and has been serving more than 100,000 inhabitants with an average annual outpatient visit of 40,000 peoples (information from Health Bureau of Sheka Zone). TB diagnosis and treatment is being provided as per the Ministry of Health guideline adapted from the WHO [5]. The DOTS clinic of THC remained the only TB clinic in the town until October 2018 (personal communication). In the Center, the diagnosis of TB relies on routine acid fast bacilli (AFB) microscopy and clinical examinations, based on the WHO recommended guideline [17].
2.2. Study design
A public health facility-based retrospective analysis was made to assess the treatment outcomes and determine associated factors of TB patients. TB treatment registry book obtained from the DOT’s clinic were reviewed for data extraction.
2.3. Source and study populations
All patients diagnosed for active TB disease and put on anti-TB treatment follow-up that were given under the DOTs Clinic of THC during the study period were considered. Those TB patients with complete and readable records were targeted and all eligible TB patients who took anti-TB treatment from June 2011 to May 2018 GC were included in the study. Eligibility was based on the enrollment period of study cases to DOTs service, and completeness and readability of each patients recorded data on the TB registration book. Patients who were diagnosed as non-TB after starting the DOTS service were excluded from the study.
2.4. Data collection method
Basic socio-demographic data (such as sex, age, and residence), category of TB patients (new, relapse, treatment after failure, return after default, transfer in), type of TB (PTB+, PTB-, and EPTB), HIV test result, year of enrolment, sputum smear result during follow up and treatment outcomes were collected from the registration book using a structured collecting format prepared in English.
2.5. Data quality assurance
To ensure the quality of collected data and entering into an Excel spreadsheet, the correctness of the data were cross-checked by two individuals independently, and meanwhile any doubts were requested to be clarified by data clerk of the clinic.
2.6. Data analysis
All the data entered on Microsoft Excel spreadsheets was transformed into IBM SPSS Statistics V.20, software package for analysis. The results were presented using descriptive statistics. The associations between TB treatment outcomes and independent variables were computed using binary logistic regression. Crude odds ratio (COR) and adjusted odds ratio (AOR) were used to present the results. Multi-variable analysis using logistic regression model were used to analyze the association between treatment outcomes and potential predictor variables. P-values of less than 0.05 were considered as statistically significant.
2.7. Operational definitions
Clinical definition and the treatment outcome of patients were reported in accordance with the guidelines of Ethiopian National TB and Leprosy Control Program (NTLCP), which was adopted from the guidelines of WHO [20]. Cured: case initially sputum smear-positive and completed with negative sputum smears at the end of treatment; Successfully treated: a patient who has been cured or completed the treatment; Failed: a case initially with smear positive TB result and remained smear-positive at the end of treatment; Treatment completed: a patient who completed treatment but without bacteriological result; Lost to follow-up : a patient who has taken anti-TB treatment for at least more than a month and interrupted for two or more consecutive months ; Died: who died during the course of TB treatment regardless of the causes of death; Transferred out: a patient with unknown treatment outcomes because of transfer to another TB clinic.
3. Results
3.1. Socio-demographic and clinical characteristics of TB patients
A total of 1651 TB patients registered at Tepi Public Health Center in between June 2011 and May 2018, were included in the study. Of these, 924(56%) were males and 727(44%) were females. The age range of the study participants were 0.33–90 years. Most of which (1053(63.8%)) were in between 15 and 35 years of age. The median age was 25 with an interquartile range of 14. The mean and median weights at initiation of treatment were 44.51 and 46.58Kgs, respectively.
Four hundred fifty seven (27.7%) cases were extra pulmonary TB (EPTB) and 1194(72.3%) were pulmonary TB of which, 683(41.4%) were smear negative PTB. The HIV-status of 1019 TB cases were unknown, 632 have known status (443(26.8%)–non-reactive) and 189(11.4%) of the study participants were HIV-positive (reactive). Majority of the cases resided in urban (1118(67.7)) (Table 1). About 90% (1469) of the cases were new TB cases while 158 (9.6%) were transferred in from other TB clinics, and only 23 (1.4%) were previously treated or relapsed cases (Table 2).
Table 1.
Socio-demographic and disease related characteristics of TB patients (n = 1651) registered in DOTs Clinic of Tepi Health Center (June 1, 2011 to May 30, 2018 GC).
| Variables | Categories | Types of TB (%) |
Total (%) | ||
|---|---|---|---|---|---|
| EPTB | PTB- | PTB+ | |||
| Sex | Male | 267(28.9) | 381(41.2) | 276(29.9) | 924(56) |
| Female | 190(26.1) | 302(41.5) | 235(32.3) | 727(44) | |
| Age in Years | 0–14 | 72(37.1) | 96(49.5) | 26(13.4) | 194(11.8) |
| 15–24 | 158(30.1) | 177(33.7) | 190(36.2) | 525(31.8) | |
| 25–34 | 149(28.2) | 209(39.6) | 170(32.2) | 528(32) | |
| 35–44 | 44(19.5) | 113(50.0) | 69(30.5) | 226(13.7) | |
| 45–54 | 21(19.3) | 53(48.6) | 35(32.1) | 109(6.6) | |
| 55–64 | 6(13.6) | 25(56.8) | 13(29.5) | 44(2.7) | |
| greater than 65 | 7(28.0) | 10(40.0) | 8(32.0) | 25(1.5) | |
| Residence | Urban | 304(27.2) | 491(43.9) | 323(28.9) | 1118(67.7) |
| Rural | 153(28.7) | 192(36.2) | 188(35.3) | 533(32.3) | |
| Category of TB patients | New TB Cases | 394(26.8) | 615(41.9) | 460(31.3) | 1469(89) |
| Retreatment TB Cases | 0 | 1(1 0 0) | 0 | 1(0.1) | |
| Relapse TB Cases | 2(8.7) | 4(17.4) | 17(73.9) | 23(1.4) | |
| TIN TB Cases | 61(38.6) | 63(39.9) | 34(21.50 | 158(9.6) | |
| HIV status | Reactive+ | 46(24.3) | 98(51.9) | 45(23.8) | 189(11.4) |
| Non-Reactive | 120(27.1) | 187(42.2) | 136(30.7) | 443(26.8) | |
| Unknown | 291(28.6) | 398(39.1) | 330(32.4) | 1019(61.7) | |
| Years of treatment | June 2010-May 2011 | 33(37.5) | 15(17.0) | 40(45.5) | 88(5.33) |
| June 2011-May 2012 | 65(34.8) | 29(15.5) | 93(49.7) | 187(11.33) | |
| June 2012-May 2013 | 71(29.6) | 97(40.4) | 72(30.0) | 240(14.54) | |
| June 2013-May 2014 | 63(21.3) | 178(60.1) | 55(18.6) | 296(17.93) | |
| June 2014-May 2015 | 64(27.8) | 94(39.1) | 72(31.3) | 230(13.92) | |
| June 2015- May 2016 | 60(29.7) | 90(44.6) | 52(25.7) | 202(12.23) | |
| June 2016- May 2017 | 53(24.5) | 94(43.5) | 69(31.9) | 216(13.10) | |
| June 2017- May 2018 | 48(25.0) | 86(44.8) | 58(30.2) | 192(11.62) | |
| Total | 457(27.7) | 683(41.4) | 511(31) | 1651 | |
Table 2.
Distribution of treatment outcomes of registered TB patients (n = 1651) by socio-demographic and disease characteristics in Tepi Health Center (June 1, 2011 to May 30, 2018 GC).
| Variables | Categories | Successful treatment outcomes |
Not Successful treatment outcomes |
Total | ||||
|---|---|---|---|---|---|---|---|---|
| Cured | TC* | Died | DF** | Lost | TO*** | |||
| Sex | Male | 232 (25.1) | 530 (57.4) | 22(2.3) | 2(0.2) | 1(0.1) | 137(14.8) | 924(56.0) |
| Female | 183(25.2) | 422(58.0) | 17(2.3) | 1(0.14) | 3(0.4) | 101(13.9) | 727(44.0) | |
| Age in Years | 0–14 | 19(9.8) | 151(77.8) | 3(1.5) | 1(0.5) | 0 | 20(10.3) | 194(11.8) |
| 15–24 | 154(29.3) | 279(53.1) | 2(0.4) | 0 | 2(0.4) | 88(16.8) | 525(31.8) | |
| 25–34 | 135(25.6) | 303(57.4) | 12(2.3) | 1(0.19) | 2(0.38) | 75(14.2) | 528(32) | |
| 35–44 | 61(27.0) | 128(56.6) | 11(4.9) | 0 | 0 | 26(11.5) | 226(13.7) | |
| 45–54 | 27(24.8) | 57(52.3) | 8(7.3) | 1(0.9) | 0 | 16(14.7) | 109(6.6) | |
| 55–64 | 11(25) | 21(47.7) | 3(6.8) | 0 | 0 | 9(20.5) | 44(2.7) | |
| greater than65 | 8(32.0) | 13(52.0) | 0 | 0 | 0 | 4(16) | 25(1.5) | |
| Residence | Urban | 263(23.5) | 677(60.6) | 28(2.5) | 2(0.18) | 2(0.18) | 146(13.5) | 1118(67.7) |
| Rural | 152(28.5) | 275(51.60 | 11(2.1) | 1(0.2) | 2(0.4) | 92(17.3) | 533(32.3) | |
| Type of TB | PTB+ | 376(73.6) | 34(6.6) | 11(2.2) | 1(0.2) | 1(0.2) | 88(17.2) | 511(30.9) |
| PTB- | 19(2.8) | 551(80.7) | 17(2.5) | 1(0.1) | 0 | 95(13.9) | 683(41.4) | |
| EPTB | 20(4.4) | 367(80.3) | 11(2.4) | 1(0.2) | 3(0.7) | 55(12.0) | 457(27.7) | |
| Category of TB patients | New TB Cases | 375(25.5) | 840(57.2) | 30(2.0) | 3(0.2) | 4(0.3) | 217(14.8) | 1469(89) |
| Default | 0 | 0 | 0 | 0 | 0 | 1(1 0 0) | 1(0.1) | |
| Relapse Cases | 11(47.8) | 7(30.4) | 2(8.7) | 0 | 0 | 3(13.0) | 23(1.4) | |
| TIN TB Cases | 29(18.4) | 105(66.5) | 7(4.4) | 0 | 0 | 17(10.8) | 158(9.6) | |
| HIV-status | Reactive + | 34(18.0) | 114(60.3) | 7(3.7) | 0 | 0 | 34(18.0) | 189(11.4) |
| Non-Reactive | 104(23.4) | 261(58.9) | 1(0.2) | 0 | 0 | 77917.3) | 443(26.8) | |
| Unknown | 277(27.2) | 577(56.6) | 31(0.3) | 3(0.3) | 4(0.4) | 127(12.5) | 1019(61.7) | |
| Years of treatment | June 2010-May 2011 | 48(54.5) | 21(23.8) | 0 | 0 | 2 | 17(19.3) | 88(5.33) |
| June 2011-May 2012 | 71(37.9) | 62(33.2) | 4(2.1) | 3(1.6) | 2(1.1) | 45(24.1) | 187(11.33) | |
| June 2012-May 2013 | 55(22.9) | 157(65.4) | 14(5.8) | 0 | 0 | 14(5.8) | 240(14.54) | |
| June 2013-May 2014 | 47(15.9) | 200(67.6) | 7(2.4) | 0 | 0 | 42(14.2) | 296(17.93) | |
| June 2014-May 2015 | 59(25.7) | 139(60.4) | 8(3.4) | 0 | 0 | 24(10.4) | 230(13.92) | |
| June 2015- May 2016 | 31(15.3) | 138(68.3) | 4(1.9) | 0 | 0 | 29(14.4) | 202(12.23) | |
| June 2016- May 2017 | 57(26.4) | 117(54.20 | 2(0.9) | 0 | 0 | 40(18.5) | 216(13.10) | |
| June 2017- May 2018 | 47(24.5) | 118(61.5) | 0 | 0 | 0 | 27(14.1) | 192(11.62) | |
| Total | 415 | 952 | 39 | 3 | 4 | 238 | 1651 | |
*TC- treatment completed **DF - Defaulted ***TO- Transferred Out.
3.2. Treatment outcomes
Of all study targets, 952 (57.7%) completed the treatment, 415 (25.1%) cured and the rest 238(14.4%) were transferred out. A total of 39 (2.4%) were died during the follow-up (Table 2). The overall treatment success rate (TSR) of the study was 80.4% (1327/1651), while it was 84.8% (134/158), 80.2% (410/511), and 78.3% (148/189) among the transfer-in, smear-positive pulmonary TB (PTB + ), and HIV-positive cases, respectively. The cure rate was 73.6% (376/511) among patients with PTB+. Among all new cases registered the TSR was 82.7% in 2016 (Table 2). Higher rate of successful treatment outcomes were recorded in New and EPTB cases, 82.7% and 84.7% (387/457), respectively (Table 2).
3.3. Trends in treatment outcomes of cases across the years (June 2010-May 2018)
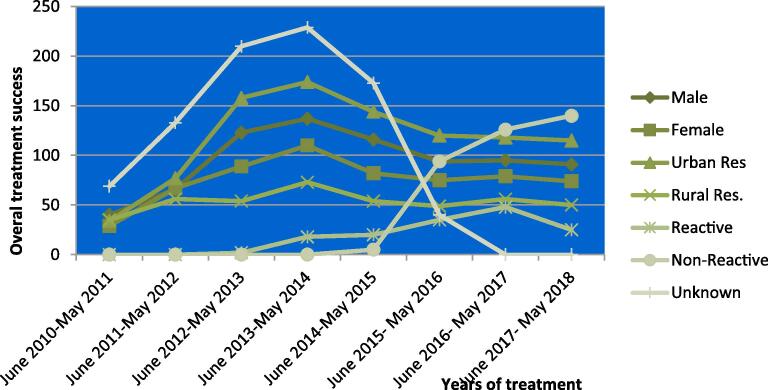
The overall treatment success rate of this study was 80.4% that had shown an increasing trend in the first four years (2010–2014) and decreasing in the following four consecutive years (2015–2018). This was also noticed among smear negative TB (PTB-) cases (Fig. 1). The number of TB cases registered across the study years had shown an increasing trend across the whole years with an exceptional decrease from June 2017- May 2018. Moreover, identifying serological status of TB patients also showed an increase but the treatment success rate had fallen among HIV-positive individuals over the period. As the overall success rate had decreased there was also a decreasing trend in successful treatment in both sexes and residence sites (Fig. 2).
Fig. 1.
Trend analysis of treatment success rate in the peripheral settings of Ethiopia.
Fig. 2.
Trend analysis of treatment success with respect to HIV-status, residence and sex of TB cases of Tepi health Centre.
3.4. Treatment success and associated factors
Multiple logistic regression analysis indicated that residence sites (AOR = 0.699, 95% CI 0.535–0.914) and TB/HIV co-infection (AOR = 0.67, 95% CI 0.46–0.98) were significantly associated with the treatment outcome. Rural residence was less likely (27.1%) to have successful treatment outcome (OR 0.763(0.584,0.996), AOR, 95% CI 0.729(0.556,0.957)) as compared to urban residence (Table 3). There was significant heterogeneity in the odds of having successful treatment outcomes across years of initiating treatment had increased in from 2011-May 2015 and decreased in the following years.
Table 3.
Factors associated with treatment success rate of TB cases in Tepi Health Center (June 2011 to May 2018 GC).
| Variables | Categories | N (%) | Treatment success N (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | P-Value |
|---|---|---|---|---|---|---|
| Sex | Female | 727(44) | 605(36.6) | 1.054(.814,1.365) | 1.065(.817,1.387) | 0.643 |
| Male | 924(56) | 762(46.2) | 1 | 1 | ||
| Age in Years | 0-14 | 194(11.8) | 170 (10.3) | 1.349(.427,4.267) | 1.316(.412,4.204) | 0.643 |
| 15-24 | 525(31.8) | 433(26.2) | 0.896(.301,2.673) | 0.875(0.291,2.632) | 0.812 | |
| 25-34 | 528(32) | 438(26.5) | 0.927(.311,2.766) | 0.939(0.312,2.826) | 0.911 | |
| 35-44 | 226(13.7) | 189(11.4) | 0.973(.316,2.999) | 1.019(0.327,3.177) | 0.974 | |
| 45-54 | 109(6.6) | 84(5.1) | .640(.201,2.039) | 0.655(0.204,2.106) | 0.478 | |
| 55-64 | 44(2.7) | 32(1.9) | .0.508(.144,1.788) | 0.531(0.15,1.887) | 0.328 | |
| >65 | 25(1.5) | 21(1.3) | 1 | 1 | ||
| Residence | Rural | 533(32.3) | 427(25.9) | .763(.584,.996) | 0.729(0.556,0.957) | 0.023 |
| Urban | 1118(67.7) | 940(56.9) | 1 | 1 | ||
| Types of TB | EPTB | 457(27.7) | 387(23.4) | 1.362(.974, 1.904) | 1.277(0.907,1.799) | 0.161 |
| PTB- | 683(41.4) | 570(34.5) | 1.243(.923,1.672) | 1.217(0.895,1.653) | 0.21 | |
| PTB+ | 511(31) | 410(24.8) | 1 | 1 | ||
| Category of TB patients | Default | 1(0.1) | 0(0) | 0 | 0 | 1 |
| New | 1469(89) | 1215(73.6) | 0.857(.544, 1.35) | 0.851(0.534,1.357) | 0.499 | |
| Relapse | 23(1.4) | 18(1.1) | 0.645(.219,1.90) | 0.768(0.254,2.326) | 0.64 | |
| TIN | 158(9.6) | 134(8.1) | 1 | 1 | ||
| HIV status | Non-Reactive | 443(26.8) | 365(22.1) | .904(.673,1.215) | 0.888(0.657,1.20) | 0.0439 |
| Reactive | 189(11.4) | 148(9.0) | .697(.475,1.024) | 0.661(0.444, 0.985) | 0.042 | |
| Unknown | 1019(61.7) | 854(51.7) | 1 | 1 | ||
| Years of treatment | June 2010-May 2011 | 88(5.33) | 69(4.18) | 0.594(0.31,1.139) | 0.19(0.077,0.464) | 0.000 |
| June 2011-May 2012 | 187(11.33) | 133(8.06) | 0.403(0.241,0.675) | 0.133(.06,.295) | 0.000 | |
| June 2012-May 2013 | 240(14.54) | 212(12.84) | 1.239(0.703,2.183) | 0.407(.178, .929) | 0.033 | |
| June 2013-May 2014 | 296(17.93) | 247(14.96) | 0.825(0.496, 1.373) | .296(.14, .625) | 0.001 | |
| June 2014-May 2015 | 230(13.92) | 198(11.99) | 1.012(0.583, 1.759) | .415(.201,.856) | 0.017 | |
| June 2015- May 2016 | 202(12.23) | 169(10.24) | 0.838(0.483, 1.455) | .673(.382, 1.187) | .172 | |
| June 2016- May 2017 | 216(13.1) | 174(10.54) | 0.678(0.4, 1.15) | .702(.41, 1.20) | .195 | |
| June 2017- May 2018 | 192(11.62) | 165(9.99) | 1 | 1 | ||
| Total | 1651(100) | 1367(82.8) |
4. Discussion
In the study, the records of TB cases registered at THC between 2011 and 2018 were extracted and analyzed. The overall treatment success rate of tuberculosis patients of THC was 82.8% and this coincides with a similar study with an average of 80% in Southern Ethiopia [13], 79.4% in Debreberhan [12], 82.7% in Addis Ababa [21] and Eastern Ethiopia (Afar 81.8%) [13], and Iran 83.1% [22], Somalia (81.8%) [23]. But it was found to be higher than reports from Hospitals of Gambella 70.76% [14] and 70.8% of Jinka [16] and 67.8% of Bahawalpur, Pakistan [24].
Among all new cases registered for DOTs, the TSR was 82.7% (Table 2) and it is found lower than the TSR of the country in 2016 (90%). Moreover, the rate of successful treatment was also lower than the WHO set global target rate and 85.5 % of Addis Ababa [21], [25] and other parts of the country [26], [27], [28], [29], [30]. Altogether, this entailed the need for measures to improve on successful treatment outcomes [23], [30], [31], [32].
The rate of TB infection was found to be highest among study participants in between the age of 15 and 35 years (1053(63.8%)) which is in agreement with the reports of WHO [2] and with those of the previous studies in Ethiopia [12], [15], [21], [25], [26], [33] and in other countries [22], [24]. The rate of TB-HIV co-infection was 189(11.4%), and this was in contrast to other studies in western (17.1%), northeastern (Metema) (20.1%), and Northern (28.9%) Ethiopia [8], [11], [20], that reported higher percentages of TB/HIV co-infection. The TSR among HIV-TB co-infected study individuals were 78.3% which was higher than similar studies done in public hospitals of eastern and southern zone of Tigray region, Ethiopia [33], [34].
Multiple logistic regression analysis indicated that residence in rural areas (AOR = 0.699, 95% CI 0.535–0.914) and TB/HIV co-infection (AOR = 0.67, 95% CI 0.46–0.98) were significantly associated with reduced odds of successful treatment (Table 3). The low treatment success in TB/HIV co-infected patients could be due to co-administration of ART along with anti-TB therapy which can lead to drug–drug interactions, overlapping drug toxicities and immune reconstitution syndrome [23], [26].
New TB treatment cases were found to have a more likely successful treatment outcome (Table 3) and this was similar to studies in Ethiopia [25], [26], Iran [22] and Somalia [23]. Higher number of unsuccessful TB treatment outcome of the present study could be attributed to being HIV + and residing in rural areas, where there is less access to health facilities and poor socio-economic statuses that cut down adherence to the treatment regimens [9], [11], [23], [31], [32], [34].
In agreement with studies in Raya-Kobo [7], and Metema towns [8] the TSR was found to be highest among EPTB cases (84.7%) which is unlike other studies that reported higher TSR among PTB + cases [11], [12], [16].
The overall treatment success rate of this study had shown an increasing trend in the first four years and decreasing in the next four study years, and this was in contrast to other studies [10], [11], [14], [35]. Moreover, the TSR had also shown falling trend among HIV-positive individuals over the studied period, similar to studies conducted among HIV co-infected TB patients have reported lower successful treatment outcome [4], [9], [14].
5. Conclusions
Treatment success rate recorded in the present study was less than the target to be met by WHO and it necessitates an urgent management response to improve the treatment success rate of THC. The lower percentage of cure rate of THC also implies that Tepi is an area that requires a better intervention mechanism. The lower TSR of the study was associated with male gender and HIV positivity, indicating targeted control management of the disease in the area. As serological status (HIV/AIDS) of most of the cases is unknown and regular provision of test kits; improvement and strengthening of comprehensive and targeted TB–HIV control program are highly recommended to the study area. Moreover, further cooperative efforts should be done by governmental and non– governmental organizations in the area to identify and improve possible promoting hindering factors for successful tuberculosis treatment outcome.
Funding
The study was funded by Mizan-Tepi University based in Ethiopia
Ethical permission
The Institutional Ethical Clearance Committee of Mizan-Tepi University had ethically cleared the study while written permissions were obtained from the Health Bureaus of Sheka Zone, Yeki Woreda and Tepi Health Center.
Informed consent
Not applicable.
Limitations and strengths
This study had the following limitations, born from being secondary data: lack of HIV status for the significant number of cases that could affect treatment outcomes, the low percentage of PTB + cases, which could be attributed to negligence or poor skills of the technicians, inadequate sputum examination records for PTB + cases during treatment and this could be a reason for low cure rate. However, the study covered relatively longer time period and included a large number of patients’ data who attended the TB clinic situated in the remotest area of the country, and findings of the study could help as a basis for health officials better manage and control the disease.
CRediT authorship contribution statement
Samuel Zewudie: Conceptualization, Methodology, Resources, Formal analysis, Investigation, Writing – original draft, Writing - review & editing. Abel Sirna: Conceptualization, Methodology, Resources, Formal analysis, Investigation, Writing – original draft, Writing - review & editing. Abiyot Terefe: Methodology, Formal analysis, Writing – original draft, Writing - review & editing. Abyot Asres: Conceptualization, Methodology, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.World Health Organization. (2019) Global tuberculosis report 2019. ISBN 978-92-4 156571-4.
- 2.World Health Organization Global tuberculosis report 2018. World Health Organization. 2018 https://apps.who.int/iris/handle/10665/274453.
- 3.WHO. Fact sheet on Top 10 causes of death. 2017. https://communitymedicine4asses.com/2017/02/01/who-updates-fact-sheet-on-top-10-cause s-of-death-27-January 2017/.
- 4.Teklu A.M., Nega A., Mamuye A.T., Sitotaw Y., Kassa D., Mesfin G. Factors associated with mortality of TB/HIV co-infected patients in Ethiopia. Ethiop J Health Sci. 2017;27(1):29. doi: 10.4314/ejhs.v27i1.4S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FMOH 2013 Federal Democratic Republic of Ethiopia Minster of Health. Guidelines for clinical and programmatic management of TB, TB/HIV and Leprosy in Ethiopia. Addis Ababa, Ethiopia.
- 6.Treatment of Tuberculosis: Guidelines. 2010 http://www.who.int/tb/publications/2010/9789241547833/en/. [PubMed]
- 7.Mekonnen D., Derbie A., Mekonnen H., Zenebe Y. Profile and treatment outcomes of patients with tuberculosis in Northeastern Ethiopia: a cross sectional study. Afri Health Sci. 2016;16(3):663–670. doi: 10.4314/ahs.v16i3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tesfahuneygn G., Medhin G., Legesse M. (2015) Adherence to Anti-tuberculosis treatment and treatment outcomes among tuberculosis patients in Alamata District, northeast Ethiopia. BMC Res Notes. 2015;8:503. doi: 10.1186/s13104-015-1452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jemal M., Tarekegne D., Atanaw T., Ebabu A., Endris M. Treatment outcomes of tuberculosis patients in metema hospital, northwest ethiopia: a four years retrospective study. Mycobact Dis. 2015;5:190. doi: 10.4172/2161-1068.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endris M., Moges F., Belyhun Y., Woldehana E., Esmael A., Unakal C. Treatment outcome of tuberculosis patients at enfraz health center, northwest Ethiopia: a five-year retrospective study. Tuberc. Res. Treat. 2014;2014(7):pages. doi: 10.1155/2014/726193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ejeta E., Chala M., Arega G., Ayalsew K., Tesfaye L., Birhanu T. Outcome of Tuberculosis patients under directly observed short course treatment in western Ethiopia. J Infect Dev Ctries. 2015;9(07):752–759. doi: 10.3855/jidc.5963. [DOI] [PubMed] [Google Scholar]
- 12.Firdie T., Tariku D., Tewelde T. Treatment outcomes of tuberculosis patients at debre berhan hospital, amhara region, northern ethiopia. Ethiop J Health Sci. 2016;26(1) doi: 10.4314/ejhs.v26i1.11. January 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zenebe T., Tefera E. Tuberculosis treatment outcome and associated factors among smear-positive pulmonary tuberculosis patients in Afar, Eastern Ethiopia: a retrospective study. Braz. J. Infect. Dis. 2016;20(6):635–636. doi: 10.1016/j.bjid.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asebe G., Dissasa H., Teklu T., Gebreegizeabher G., Tafese K. Treatment outcome of Tuberculosis Patients at Gambella Hospital, Southwest Ethiopia: Three-year Retrospective Study. J Infect Dis Ther. 2015;3:211. doi: 10.4172/2332-0877.1000211. [DOI] [Google Scholar]
- 15.Yassin MA, Datiko DG, Shargie EB. 92006) Ten-year experiences of the tuberculosis control program in the southern region of Ethiopia. Int J Tuberc Lung Dis. 10(10):1166-1171. [PubMed]
- 16.Wondale B , Medihn G, Teklu T, Mersha, W Tamirat M. and Ameni G. (2017) A retrospective study on tuberculosis treatment outcomes at Jinka General Hospital, southern Ethiopia. BMC Res Notes (2017) 10:680 https://doi.org/10.1186/s13104-017-3020-z. [DOI] [PMC free article] [PubMed]
- 17.Ahmed Yassin M., Takele L., Gebresenbet S., Girma E., Lera M., Lendebo E. HIV and tuberculosis co-infection in the southern region of Ethiopia: a prospective epidemiological study. Scand J Infect Dis. 2004;36(9):670–673. doi: 10.1080/00365540410020848. [DOI] [PubMed] [Google Scholar]
- 18.http//www falin grain.com/World /ET/54/Tpi,html.
- 19.Central Statistical Agency of Ethiopia . Federal Democratic Republic of Ethiopia; Population Census Commission, with support from UNFPA. Addis Ababa, Ethiopia: 2008. Summary and statistical report of the 2007 population and housing census-population size by age and sex. [Google Scholar]
- 20.FMOH . Ministry of Health; Leprosy and TB/HIV Prevention and Control Program. Fourth Edition. Addis Ababa: 2008. Manual for Tuberculosis; p. 2008. [Google Scholar]
- 21.Tilahun G., Gebre-Selassie S. Treatment outcomes of childhood tuberculosis in Addis Ababa: a five-year retrospective analysis. BMC Public Health. 2016;16:612. doi: 10.1186/s12889-016-3193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khazaei S., Hassanzadeh J., Rezaeian S., Ghaderi E., Khazaei S., Mohammadian Hafshejani A. Treatment outcome of new smear positive pulmonary tuberculosis patients in Hamadan, Iran: A registry-based cross-sectional study. Egyptian Journal of Chest Diseases and Tuberculosis. 2016;65(4):825–830. [Google Scholar]
- 23.Ali M.K., Simon Karanja S., Karama M. Factors associated with tuberculosis treatment outcomes among tuberculosis patients attending tuberculosis treatment centers in 2016–2017 in Mogadishu, Somalia. Pan African Medical Journal. 2017;28:197. doi: 10.11604/pamj.2017.28.197.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atif, M, Anwar, Z, Fatima, RK, Malik, I, Asghar, S and Scahil S (2018) Analysis of tuberculosis treatment outcomes among pulmonary tuberculosis patients in Bahawalpur, Pakistan. BMC Res Notes. 11:370. doi.org/10.1186/s13104-018-3473-8. [DOI] [PMC free article] [PubMed]
- 25.Getahun B., Ameni G., Medhin G., Biadgilign S. Treatment outcome of tuberculosis patients under directly observed treatment in Addis Ababa, Ethiopia. Braz J Infect Dis. 2013;17(5):521–528. doi: 10.1016/j.bjid.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gebrezgabiher G., Romha G., Ejeta E., Asebe G., Zemene E., Ameni G. Treatment outcome of tuberculosis patients under direct observed treatment short course and factors affecting outcome in southern Ethiopia: a five year retrospective study. PLoS One. 2016 doi: 10.1371/journal.pone.0150560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sintayehu W., Abera A., Gebru T., Fiseha T. Trends of tuberculosis treatment outcomes at Mizan-Aman general hospital, southwest Ethiopia: a retrospective study. Int J Immunol. 2014;2(2):11–15. [Google Scholar]
- 28.Gebreegziabher S.B., Yimer S.A., Bjune G.A. Tuberculosis case notification and treatment outcomes in west Gojjam Zone, Northwest Ethiopia: a five-year retrospective study. J Tuberc Res. 2016;04(01):23–33. doi: 10.1155/2016/2036234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berhe G., Enquselasse F., Aseffa A. Treatment outcomes of smear-positive pulmonary tuberculosis patients in Tigray Region, northern Ethiopia. BMC Public Health. 2012;12:537. doi: 10.1186/1471-2458-12-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woldeyohannes D., Sisay S., Mengistu B., Kassa H. Direct observed treatment short-course (DOTS) for treatment of new tuberculosis cases in Somali Regional State, eastern Ethiopia: ten years retropspective study. BMC Res Notes. 2015;8:357. doi: 10.1186/s13104-015-1325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Federal Ministry of Health (FMOH) TB Research Advisory Committee (TRAC). Roadmap for Tuberculosis Operational Research in Ethiopia. March 2013, Addis Ababa, Ethiopia.
- 32.Yassin M.A., Datiko D.G., Tulloch O., Markos P., Aschalew M., Shargie E.B. Innovative community based approaches doubled tuberculosis case notification and improve treatment outcome in southern Ethiopia. PLoS ONE. 2013;8(5):e63174. doi: 10.1371/journal.pone.0063174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belayneh M., Giday K., Lemma H. Treatment outcome of human immunodeficiency virus and tuberculosis co-infected patients in public hospitals of eastern and southern zone of Tigray region. Ethiopia. Braz j infect dis. 2015;19(1):47–51. doi: 10.1016/j.bjid.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohammed T., Daniel K., Helamo D., Leta T. Treatment outcomes of tuberculosis patients in Nigist Eleni Mohammed General Hospital, Hosanna, southern nations, nationalities and peoples region, Ethiopia: a five year (June 2009 to August 2014) retrospective study. Arch Public Health. 2017;75(1) doi: 10.1186/s13690-017-0184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdulkader M, van Aken I, Niguse S, Hailekiros H, and Spigt M (2019) Treatment outcomes and their trend among tuberculosis patients treated at peripheral health settings of Northern Ethiopia between 2009 and 2014: a registry-based retrospective analysis. BMC Res Notes (2019) 12:786 https://doi.org/10.1186/s13104-019-4824-9. [DOI] [PMC free article] [PubMed]