Abstract
Increasing evidence supports a link between maternal prenatal cannabis use and altered neural and physiological development of the child. However, whether cannabis use relates to altered human brain development prior to birth, and specifically, whether maternal prenatal cannabis use relates to connectivity of fetal functional brain systems, remains an open question. The major objective of this study was to identify whether maternal prenatal cannabis exposure (PCE) is associated with variation in human brain hippocampal functional connectivity prior to birth. Prenatal drug toxicology and fetal fMRI data were available in a sample of 115 fetuses [43 % female; mean age 32.2 weeks (SD = 4.3)]. Voxelwise hippocampal connectivity analysis in a subset of age and sex-matched fetuses revealed that PCE was associated with alterations in fetal dorsolateral, medial and superior frontal, insula, anterior temporal, and posterior cingulate connectivity. Classification of group differences by age 5 outcomes suggest that compared to the non-PCE group, the PCE group is more likely to have increased connectivity to regions associated with less favorable outcomes and to have decreased connectivity to regions associated with more favorable outcomes. This is preliminary evidence that altered fetal neural connectome may contribute to neurobehavioral vulnerability observed in children exposed to cannabis in utero.
Keywords: Brain, Cannabis, Fetal, Hippocampus, Prenatal, Resting-state, THC
1. Introduction
Cannabis has become the most commonly used illicit drug during pregnancy. This is due in part to legalization of marijuana in many US states, and the softening position with regard to the negative impacts of the drug and recognition of its medical benefits (el Marroun et al., 2008; Chang et al., 2019; Brown et al., 2017). Preclinical studies of the most prevalent active ingredients of cannabis, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), demonstrate therapeutic benefits of the drug, particularly for anxiety disorders, epileptic encephalopathy, neurodegenerative and psychiatric disorders, pain and cancer (Devinsky et al., 2014; García-Gutiérrez et al., 2020; Velasco et al., 2016). However, much of this research has yet to advance to human clinical trials, though there are notable exceptions. (cf. Huntsman et al., 2019) Overall, clinical and observational cannabis studies in humans are complex; for example, mode/type of drug and co-occurrence of other health risk factors are consequential (Kafil et al., 2018; Day and Richardson, 1991).
Many women use cannabis prior to knowing they are pregnant, and many continue use as a natural remedy for morning sickness, depression, stress and anxiety (Roberson et al., 2014). Semi-structured interviews with obstetric providers suggest that providers perceive cannabis use to be less dangerous than other forms of perinatal substance use and many are not familiar with risks of cannabis use in pregnancy (Holland et al., 2016). Notably, cannabis-using pregnant women are more likely to meet criteria for cannabis use disorders relative to cannabis-using non-pregnant women of reproductive age (18.1 % as compared to 11.4 % in non-pregnant reproductive-age women) (Ko et al., 2015), suggesting usage patterns during pregnancy may be of greater concern than in the general population.
Prenatal cannabis exposure (PCE) is associated with impairments in child physical and neurological development. Systematic reviews of prenatal exposure to cannabis, such as that by Gunn and colleagues (2016) (Gunn et al., 2016), suggest reliable associations between maternal PCE and both decreased birthweight and increased neonatal intensive care unit stays. The impact of PCE on fetal growth has been shown to remain significant even when controlling for tobacco use (El Marroun et al., 2009). Studies have also shown links between PCE and diminished performance of higher-order functions, including visual-perception, language comprehension, impulse control and attention among school-age children (O’Connell and Fried, 1991). Studies in infants and toddlers have reported increased startle response and sleep disturbances, respectively (Dahl et al., 1995; Fried et al., 1987). Risks of PCE to the child are further compounded by increasing potency in today’s readily available cannabis products (Mehmedic et al., 2010).
Preclinical studies demonstrate that cannabinoids readily cross the placenta (Hurd et al., 2005; Hutchings et al., 1989) and act on fetal brain development by interfering with endogenous cannabinoid signaling (eCB), primarily through cannabinoid subtype 1 receptors (CB1-R). Interference with endogenous cannabinoid pathways dramatically influences cellular function and patterning of neural systems, including cellular differentiation, physiology, outgrowth, and migration (Galve-Roperh et al., 2013; Tortoriello et al., 2014; Mulder et al., 2008). In particular, CB1-R signaling is critical for proper axon pathfinding and connectivity of long-range glutamatergic projection neurons (de Salas-Quiroga et al., 2015). During embryonic development, CB1-R are also expressed in GABAergic interneurons that migrate from the caudal ganglionic eminence to the hippocampus (Keimpema et al., 2013). The long tangential migration course of hippocampal interneurons may be particularly vulnerable to PCE, given the known influence of eCB on growth cone dynamics and migration (Berghuis et al., 2005). Hippocampal interneurons co-express CHRNA7, the gene that forms the α7-nicotinic cholinergic receptor subunit (α7nACh) (Morales et al., 2008). Activation of α7-nicotinic receptors is necessary for conversion of fetal glutamate and GABA neurotransmission to their mature form (Liu et al., 2006). Modification of these processes following in utero exogenous cannabinoid exposure may give rise to lasting changes in neurotransmission and cellular physiology (Vargish et al., 2017). Frontal and striatal dopaminergic (DA) systems are amongst those impacted by these changes, as cannabinoid activation of hippocampal CB1-R increases ventral tegmental area (VTA) DA activity, which is causally associated with reward-seeking behavior and reduced sociability in animal models (Loureiro et al., 2015). Overall, disruptions in intrauterine cannabinoids impact brain development from the level of genes to systems, with widespread implications for the development of psychiatric disorders over the lifespan (Hurd et al., 2019).
Magnetic resonance imaging (MRI) studies have been informative in understanding the impacts of prenatal drug use on the human newborn brain. Very few, if any, of these studies have singularly focused on prenatal cannabis exposure effects. Instead, these studies have considered multiple illicit drugs and their interactions, which has the advantage of better reflecting frequent occurrence of polysubstance use. Structural brain findings in neonates show that prenatal drug exposure is associated with reduced cortical gray matter, most notably in prefrontal and frontal regions (Grewen et al., 2014), areas that support executive function and inhibitory control. Studies of the effects of prenatal illicit drug use on neonatal resting-state functional connectivity (RSFC) describe alterations in frontal connectivity to amygdala, insula and thalamic regions, and altered insula–sensorimotor connectivity. Specifically, exposure to cocaine and exposure to a mixture of non-cocaine drugs (THC, nicotine, antidepressants, alcohol) are both associated with newborn thalamo-frontal, amygdala–frontal, and insula–frontal hyperconnectivity, and insula–sensorimotor hypoconnectivity (Salzwedel et al., 2016; Salzwedel et al., 2015). A recent study that addressed prenatal drug exposure using multiple imaging modalities replicated and extended prior observations. Peterson et al. (2020) noted similar abnormalities across drug exposure types that included smaller volumes in the dorsal, medial, and ventral surfaces of the frontal lobe and dose-related increases in volumes in the lateral temporal lobe, dorsal parietal lobe, and superior frontal gyrus (Peterson et al., 2020). Because these studies were performed in the newborn brain, closely following birth, these observed effects are considered to reflect prenatal brain processes rather than attributes of postnatal maturation or variation in the postnatal environmental context.
An important consideration in human studies of the effects of PCE is that health and social inequities impact the likelihood of substance use during pregnancy. Socioeconomic disadvantage, lack of marital partnership, anxiety or depression diagnoses, and use of other substances are associated with increased cannabis use among pregnant women (el Marroun et al., 2008; Ko et al., 2015; Oh et al., 2017). This suggests that damaging effects of PCE may disproportionately impact children born to mothers with fewer resources and less support, contributing to continued inequities. It also undermines attributions of causality given that experiences stemming from health and social inequities are likely to additionally influence neural maturational processes in the womb. For example, prenatal stress and depression are related to altered limbic brain structure and function in the human fetus (Thomason et al., in press; De Asis-Cruz et al., 2020) and neonate (Graham et al., 2019; Karlsson et al., 2020; Lautarescu et al., 2020).
The present study sought to address the effect of isolated PCE on brain development during human gestation in a predominately low-resource community. Leveraging recent advances in fetal RSFC MRI, we examined functional connectivity across the hippocampal brain network in vivo. Recent fetal RSFC studies highlight existence of an elaborate and sophisticated neural connectomic structure prior to birth (Thomason et al., 2013; Turk et al., 2019; van den Heuvel et al., 2018) and confirm that the fetal connectome is sensitive to variation in maternal health and environmental exposures (Wheelock et al., 2019; Jakab et al., 2014; Norr et al., 2021; Thomason et al., 2019). We hypothesized that variation in the hippocampal functional connectivity network would be evident in PCE fetuses, especially in frontal, insula, and cingulate regions. We also investigated the relevance of observed patterns to school-aged outcomes. We recruited from a predominately low-resource, urban community, which reduced socioeconomic variation within the sample. PCE status was defined on the basis of prenatal urine toxicology analyses. Women positive for illicit drugs other than THC, n = 14, were excluded from analyses in an effort to focus analyses on a hippocampal network hypothesis of prenatal THC exposure.
2. Materials and methods
2.1. Participants
This study included data from 115 pregnant, second-and third-trimester mothers from a larger ongoing project on fetal brain development who had available urine toxicology data (1–5 urine toxicology tests, mean = 1.83). Mothers were recruited during routine obstetrical appointments at Hutzel Women’s Hospital in Detroit, Michigan, into a protocol that included fetal MRI and child assessments at newborn, 7 months, 3 years and 5 years. Inclusionary criteria for the larger project included maternal age ≥18 years old, native English speaking, singleton pregnancy, and normal fetal brain anatomy as assessed by ultrasound and MRI examination. Forty-three percent of children in the N = 115 sample were female and MRI studies occurred when fetuses were between 22- and 39-weeks gestational age (GA). Additional exclusion criteria were applied to neuroimaging data, including exclusion of fetuses scanned before 27 weeks (n = 4); those with health concerns, such as delivery before 33 weeks, birth weight less than 2500 g (n = 18); lack of sufficient low-motion fMRI data (n = 11); or presence of multi-drug or other drugs in the drug toxicology screen (n = 14), resulting in a PCE/non-PCE neuroimaging sample of N = 68. In addition to the PCE/non-PCE neuroimaging sample, in the larger ongoing project on fetal brain development there were 67 children that had age 5 outcome data and quality neuroimaging data at the time of this analysis, with or without available urine toxicology. This group was used to isolate patterns of fetal connectivity that relate to age 5 outcomes in order to perform outcome-based region of interest (ROI) analyses. Rather than restricting age 5 outcome analyses to those also in the PCE analyses, the maximum available number of participants was used to define hippocampal network regions associated with behavioral outcomes. Participation was approved by Human Investigation Committee of Wayne State University. Detailed participant demographics and characteristics for the PCE and non-PCE neuroimaging groups are provided in Table 1.
Table 1.
Group descriptive variables for participant characteristics.
| Outcome | PCE group (N = 26) | Non-PCE group (N = 42) | p-value |
|---|---|---|---|
| Maternal age at scan years, M (SD) | 25.5 (3.7) | 25.0 (4.1) | 0.647 |
| Race, ethnicity n (%) | 0.517 | ||
| African-American | 21 (80.8) | 35 (83.3) | |
| Caucasian | 1 (3.8) | 3 (7.1) | |
| Latina | 0 (0.0) | 1 (2.4) | |
| Bi-racial | 1 (3.8) | 2 (4.8) | |
| Not disclosed | 3 (11.5) | 1 (2.4) | |
| Marital status, n (%) | 0.210 | ||
| Single | 14 (53.8) | 26 (61.9) | |
| Married/Partnered | 8 (30.8) | 14 (33.3) | |
| Divorced | 0 (0.0) | 1 (2.4) | |
| Not disclosed | 4 (15.4) | 1 (2.4) | |
| Annual household income, n (%) | 0.342 | ||
| <$10,000 | 8 (30.8) | 15 (35.7) | |
| $10,000−20,000 | 6 (23.1) | 6 (14.3) | |
| $20,000−30,000 | 3 (11.5) | 11 (26.2) | |
| >$30,000 | 4 (15.4) | 7 (16.7) | |
| Not disclosed | 5 (19.2) | 3 (7.1) | |
| Maternal education, n (%) | 0.260 | ||
| No diploma/No GED | 7 (26.9) | 6 (14.3) | |
| GED/high school diploma | 7 (26.9) | 19 (45.2) | |
| Some college | 8 (30.8) | 15 (35.7) | |
| > = 2yr college degree | 1 (3.8) | 1 (2.4) | |
| Not disclosed | 3 (11.5) | 1 (2.4) | |
| Child sex, n (%) | 0.813 | ||
| Female | 11 (42.3) | 19 (45.2) | |
| Male | 15 (57.7) | 23 (54.8) | |
| Child characteristics, M (SD) | |||
| Gestational weeks at birth | 39.0 (1.8) | 39.2 (1.4) | 0.496 |
| Birth weight (g) | 3173 (367) | 3352 (427) | 0.090 |
| Birth length (cm) | 50.2 (1.9) | 50.6 (2.2) | 0.442 |
| Head circumference (cm) | 33.6 (1.1) | 34.2 (1.7) | 0.067 |
| Number of drug tests, M (SD) | 2.6 (0.9) | 1.2 (0.4) | *< 0.001 |
| fMRI data characteristics, M (SD) | |||
| Fetal gestational weeks at scan | 33.8 (3.4) | 32.7 (3.7) | 0.198 |
| Number of fMRI frames analyzed | 175 (48.5) | 155 (43.6) | 0.088 |
| Translational mean movement (mm) | 0.21 (0.1) | 0.24 (0.1) | 0.218 |
| Rotational mean movement (degrees) | 0.35 (0.1) | 0.39 (0.2) | 0.280 |
| Translational drift (mm) | 0.87 (0.3) | 0.91 (0.3) | 0.651 |
| Rotational drift (degrees) | 1.18 (0.4) | 1.24 (0.4) | 0.502 |
Note: Chi-square tests compared race/ethnicity, marital status, income, education, and child sex between groups. Two-sample t tests compared all other variables.
2.2. Classification of maternal substance use, health behavior and mental health
Prenatal urine toxicology results were obtained via medical records. Urine toxicology examinations tested for use of amphetamines, barbiturates, benzodiazepines, methadone, opiates, and cannabis during pregnancy. The exact amount of time a drug stays in the human body is undetermined due to variation dependent on factors such as individuals’ age, sex, weight, and health behavior. The US Food and Drug Administration, however, states that THC is detectable in urine 1−3 h after use until up to 7 days after taking the drug. Participants also completed a 10-item version of the Wayne Indirect Drug Use Screener (WIDUS), a screener for identification of drug use during pregnancy that is not reliant on direct questioning about drug use behavior (Ondersma et al., 2012). Items 1–6 corresponded to the original screener developed and validated by Ondersma and colleagues (Ondersma et al., 2012, 2019). Items 7–10 were added based on subsequent research in pregnant women (Smith, 2011). These 4 additional true/false WIDUS questions were: I have seen or experienced worse things than most other people; When I was younger than 13-years-old I often stayed out past midnight; I am currently working twenty hours a week or more; I have had one or more abortions in my life. Assessment of the receiver operating characteristic (ROC) curve of the 10-item WIDUS is provided as Supplemental Material. Health behaviors were measured using an adapted version of the Health Practices Scale (HPS) (Thomason et al., in press; Jackson, 2006), a 53-item measure comprised of 5 subscales: diet, exercise, medical adherence, substance abuse (tobacco and alcohol), and sleep. Items were rated on a 6-point Likert Scale ranging from (1) Never to (6) Always; the substance use scale was reversed and renamed “substance avoidance”, thus for all scales, higher values correspond to better health behavior. Participants also completed the Revised Experiences with Close Relationships Scale (ECR-R) (Fraley et al., 2000), a 36-item measure of adult relationship style, comprised of 2 subscales: avoidance and anxiety; the Center for Epidemiological Studies Depression Scale (CES-D) (Radloff, 1977), the State Trait Anxiety Inventory (STAI) (Spielberger, 1984), the Penn State Worry Questionnaire (PSWQ) (Meyer et al., 1990), the Perceived Stress Scale (PSST) (Cohen et al., 1983), and the Satisfaction with Life Scale (SWLS) (Diener et al., 1985). We tested whether health behavior, WIDUS score, and self-reported mental health differed in participants with positive urine toxicology results.
2.3. Child age 5 neurobehavioral assessments
Children’s parent-reported inhibitory executive function was assessed using the Inhibitory Self-Control Index (ISCI) of the Behavior Rating Inventory of Executive Function Preschool Version (BRIEF-P) (Gioia et al., 2003). The BRIEF-P includes 63 items that assess multiple aspects of executive function (EF) such as working memory, planning, and inhibitory control. Children’s total problem behavior was assessed by parent report using the Child Behavior Checklist (CBCL) for ages 1.5–5 years (Achenbach and Rescorla, 2000). Children’s school readiness (SR) was assessed using the Bracken School Readiness Assessment Third Edition (BSRA-3), which has been shown to be predictive of child attainment in kindergarten and first grade (Panter and Bracken, 2009). The BSRA-3 Standard Score, also known as the Receptive School Readiness Composite (SRC), was reverse scored by subtracting the SRC from 160. This enabled visualization of all scales such that lower scores were associated with more favorable outcomes across the age 5 outcome measures. Individual scores on these measures were used to define hippocampal network regions associated with future behavior (visualized in Supplemental Material, Fig. 1).
2.4. MRI acquisition
Fetal MRI data were acquired on a Siemens Verio 70-cm open-bore 3 T MR system using a 550 g abdominal 4-Channel Siemens Flex Coil (Siemens, Munich, Germany). 12 min of fetal resting-state fMRI data were acquired using a gradient echo planar imaging sequence: TR/TE 2000/30 ms, flip angle 80 °, 360 frames, axial 4 mm slice thickness, voxel size 3.4 × 3.4 × 4 mm3. The sequence was repeated when time permitted. The average estimated specific absorption rate (SAR) was 0.24 W/kg, SD = 0.08.
2.5. Resting-state fMRI data preprocessing
Periods of fetal movement quiescence were identified using the Multi-image Analysis GUI (MANGO; Research Imaging Institute, UT Health Science Center at San Antonio, TX, USA; http://ric.uthscsa.edu/mango/) (FSL, 2021). Brainsuite (Shattuck and Leahy, 2002) was used to manually generate 3D masks for single reference images drawn for each of the identified time periods, or segments, of fetal quiescence. Masks were binarized and applied only to frames corresponding to their select segment, and only those data were retained for further analyses. Each segment was manually reoriented, realigned to the mean BOLD volume, resampled to 2 mm isotropic voxels, and normalized to a 32-week fetal brain template (Serag et al., 2012) using Statistical Parametric Mapping (SPM12) (Statistical Parametric Mapping 8 from the Wellcome Trust Centre for Neuroimaging, 2009) software implemented in MATLAB. Motion parameters for each low-motion segment were checked to ensure only segments that consisted of at least 20 s (10 frames) of low motion were retained in subsequent processing steps. This level of censoring has been reported previously (Wheelock et al., 2019; Thomason et al., 2014, 2017; Thomason et al., 2013; van den Heuvel et al., 2018). The average translational and rotational motion after censoring ranged from 0.09 mm to 0.49 mm and 0.1° to 1.1°, respectively and the mean number of frames was 163 (IQR = 125–192).
2.6. Functional connectivity processing
Subject-specific voxel maps of hippocampal connectivity were computed using the CONN toolbox (version 17f). Signal was extracted from a bilateral hippocampal mask defined manually on a 32-week fetal anatomical brain template (Serag et al., 2012). Following manual preprocessing methods, linear detrending, nuisance regression using aCompCor of five principal components extracted from a 32-week fetal atlas white matter and CSF masks, six head motion parameters, despiking, and band-pass filtering at 0.008 to 0.09 Hz were applied.
2.7. Statistical analyses
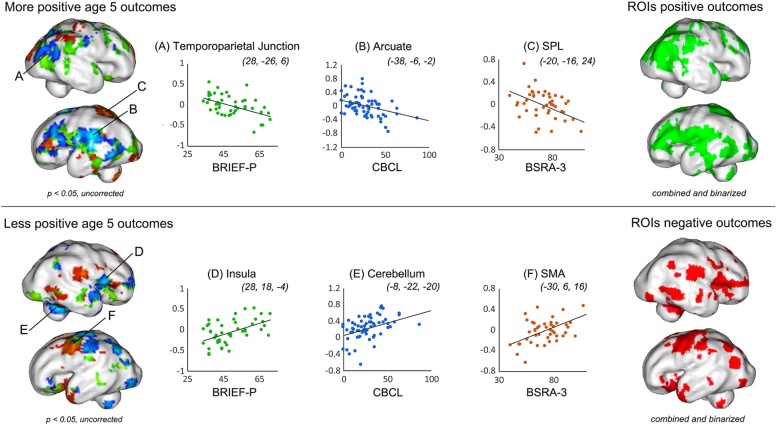
Demographic and questionnaire data were analyzed using standard Chi-squared test statistics or two-tailed independent samples t-tests. Hippocampal RSFC was compared between PCE and non-PCE groups using a two-sample t-test, controlling for gestational age at scan. Secondary analyses, using multiple linear regression, were performed to isolate differences in hippocampal connectivity related to age 5 behavioral outcomes. Nineteen cases in the behavioral outcome sample overlap with the cases included in the primary PCE/non-PCE investigation. Regions identified as having variation in hippocampal connectivity related to age 5 outcomes, (1) BRIEF-P ISCI norm score, (2) CBCL total problems score, and/or (3) BSRA-3 reversed standardized score, were defined as regions of interest for subsequent analyses. Specifically, age 5 regression results were viewed at a significance threshold of p < 0.05 (uncorrected), saved, binarized, and combined into 2 summary maps: (1) regions associated with more favorable outcomes (lower BRIEF-P, CBCL and reversed BSRA-3 scores) and (2) regions associated with less favorable outcomes (higher BRIEF-P, CBCL and reversed BSRA-3 scores).
2.8. Definition of regions of interest (ROIs)
Regions of interest based on age 5 outcomes as well as ROIs generated for bilateral dorsolateral prefrontal cortex (DLPFC), bilateral medial prefrontal cortex (MPFC), bilateral posterior cingulate cortex (PCC), bilateral anterior cingulate cortex (ACC), and bilateral anterior insula (aINS) were tested for THC-related hippocampal connectivity differences. Multiple testing correction was performed using the Analysis of Functional NeuroImages (AFNI) subroutines, 3dClustSim and 3dFWHMx, which used 10,000 Monte Carlo simulations to estimate the number of contiguous voxels one would expect to observe in a significant cluster given a p < 0.05 threshold, considerate of the number of comparisons made. This method addresses issues raised by Eklund et al. (2016) and Woo et al. (2014). Two ROIs reflecting more or less favorable age 5 outcomes were generated by performing 3 separate linear regression analyses, with fetal age at scan included as a covariate in each model. A p < 0.05 threshold was applied to resultant t-contrast images and these were combined and binarized for regions (a) positively and (b) negatively associated with favorable outcomes. Fig. 1 depicts each regression projected on an inflated surface model of a 32-week fetal template, and includes visualization of individual subject measures contributing to each of these analyses from representative regions. The resulting combined and binarized positive, negative outcome ROIs were used in subsequent analyses to test whether between group differences were co-localized with regions relevant to future developmental outcomes.
Fig. 1.
Regression models used to generate masks representing more or less favorable age 5 neurobehavioral outcomes. In addition to using a standard a priori approach for defining Regions of Interest (ROIs), two ROIs were constructed based on computed association with future neurobehavioral outcomes. Based on data from 67 children previously scanned in utero, we isolated and combined regions that showed significant association with age 5 outcome measures. Scatterplots depict observed values for representative regions in each regression analysis. The ROI depicted in green highlights areas in the fetal hippocampal network associated with more favorable future outcomes, whereas the ROI depicted in red highlights regions where higher (relative) connectivity was associated with worse performance scores at age 5. Abbreviations: BRIEF-P, Behavior Rating Inventory of Executive Function Preschool Version; BSRA-3, Bracken School Readiness Assessment Third Edition (reverse scored); CBCL, Child Behavior Checklist. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
2.9. Evaluation of influence of clinical and health variables on observed neural effects
As reported in Table 2, differences between the PCE and non-PCE groups included self-reported stress and anxiety, diet, and substance use (comprised of avoidance of tobacco and alcohol). In an effort to understand whether these clinical and health differences may have contributed to observed neural effects of PCE, we extracted functional connectivity values at the individual subject level for each brain region in which significant between group differences were observed. Starting with a reduced regression model with only the main effects for the group (PCE and non-PCE), variable selection for the clinical and health variables was performed through forward and backward stepwise regression, using Akaike information criterion (AIC) to evaluate model fit at each step. No interaction terms were considered in the stepwise regression process. The estimated coefficients from the regression models with selected clinical and health variables for each ROI were reported to assess the added contributions of these variables in explaining neural connectivity after accounting for group differences.
Table 2.
Group descriptive variables for prenatal measures.
| Outcome | PCE group (N = 26) | Non-PCE group (N = 42) | t-value | p-value |
|---|---|---|---|---|
| Health total | 184.49 (25.14) | 198.06 (24.42) | 2.22 | *0.03 |
| Diet | 59.4 (11.55) | 66.57 (11.92) | 2.46 | *0.017 |
| Exercise | 28.04 (6.42) | 28.95 (6.96) | 0.53 | 0.599 |
| Medical adherence | 27.94 (4.31) | 28.53 (5.32) | 0.48 | 0.634 |
| Substance avoidance | 47.08 (6.96) | 50.42 (5.22) | 2.22 | *0.03 |
| Sleep | 21.88 (5.74) | 23.72 (5.06) | 1.36 | 0.177 |
| Relationship (ECR-R) | ||||
| Anxiety | 2.37 (0.98) | 2.17 (1.16) | −0.71 | 0.48 |
| Avoidance | 2.61 (1.03) | 2.33 (1) | −1.12 | 0.266 |
| PSST Total | 16.12 (6.13) | 12.65 (6.34) | −2.23 | *0.029 |
| SWLS Total | 20.81 (7.24) | 23.69 (6.14) | 1.76 | 0.084 |
| STAI Total | 10.69 (2.6) | 9.37 (2.54) | −2.06 | *0.043 |
| PSWQ Total | 45.15 (9.98) | 41.24 (10.68) | −1.51 | 0.137 |
| CESD Total | 16.7 (8.65) | 13.35 (9.65) | −1.43 | 0.156 |
| WIDUS | 3.48 (1.22) | 2.51 (1.5) | −2.720 | *0.009 |
Note: Two-sample t tests compared all variables. Abbreviations: ECR-R, Revised Experiences in Close Relationships Scale; PSST, Perceived Stress Scale; SWLS, Satisfaction with Life Scale; STAI, State-Trait Anxiety Inventory; PSWQ, Penn State Worry Questionnaire; CESD, Center for Epidemiological Studies Depression; WIDUS, Wayne Indirect Drug Use Screener.
Mean (Standard Deviation) provided.
p-values less than 0.05 appear in bold typeset.
3. Results
3.1. Demographic characteristics
The final neuroimaging sample consisted of 68 fetuses: 26 cannabis exposed and 42 controls. In the neuroimaging sample, mean fetal age at the time of MRI was 33.4 weeks GA (SD = 3.6), mean maternal age at time of MRI was 25.8 years (SD = 3.9, range 18–35), and mean fetal age at birth was 39.17 weeks GA (SD = 1.6). Those exposed to THC in utero did not differ from the comparison group in MRI variables (motion, frame count, age at scan), in birth outcomes, or in demographic factors. Group comparisons are detailed in Table 1 and represented visually in Supplementary Fig. 2.
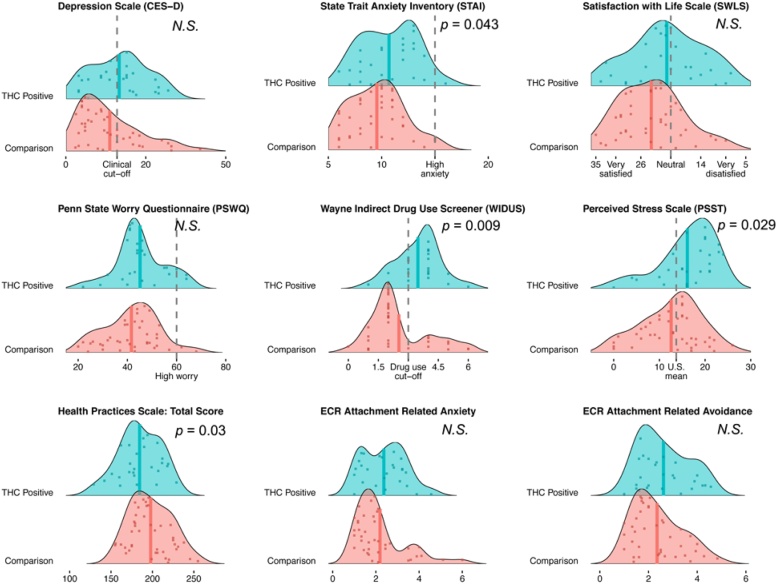
3.2. Maternal mental health, romantic support and physical health behavior
Mean self-reported ratings of depression and stress were high across both groups. Comparisons between women within the neuroimaging sample that did or did not test positive for prenatal THC yielded significant group differences in health behavior total score, t = 2.22, p = .03, anxiety (STAI), t = -2.06, p = .043, perceived stress (PSST), t = -2.23, p = .029, and in WIDUS total score, t = -2.72, p = .009. Post hoc analysis revealed that the health subscales contributing most to the aforementioned difference in overall health were diet, t = 2.46, p = .017 and avoidance of tobacco and alcohol use, t = 2.22, p = .03. In contrast, mothers that tested positive for THC did not differ from comparison mothers in self-reported sleep, medical adherence, exercise, romantic support (ECR-R), satisfaction with life (SWLS), perceived worry (PSWQ), or depression (CESD). Comparisons of these variables within the neuroimaging sample are provided in Table 2 and Fig. 2.
Fig. 2.
Distribution of maternal responses across multiple measures of mental health, physical health behavior and romantic support (N = 68). Overall, mean self-reported ratings of depression and stress are high across both subgroups. CESD and PSST were near or surpassed clinical cut-off and population mean, respectively. Between group comparisons are indicated with p-values where they are significant. Values correspond to data reported in Table 2.
3.3. Wayne Indirect Drug Use Screener (WIDUS)
Analysis of the classification accuracy of the adapted 10-item WIDUS in the full drug-screened sample of N = 115 demonstrated an optimal cut-off score of 3. Details of the WIDUS analysis along with receiver operating characteristic curve (ROC) are provided as Supplementary Material.
3.4. Intrinsic connectivity of hippocampal networks in THC and comparison fetuses
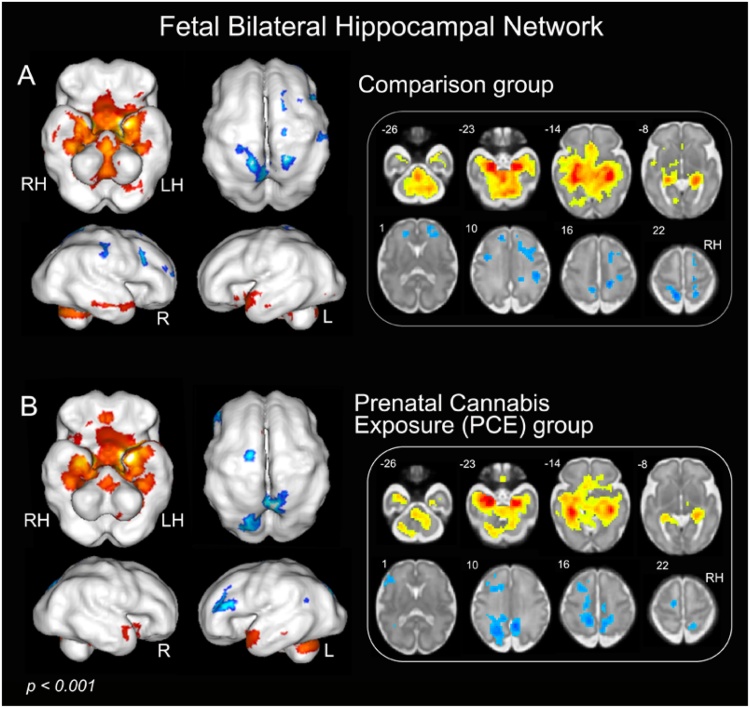
Fetal whole brain hippocampal connectivity results are displayed in Fig. 3 for fetuses of mothers that screened positive for THC and comparison fetuses. Connectivity patterns resemble those reported in children, adolescents and adults (Huijbers et al., 2014; Schleifer et al., 2019). For both groups, the hippocampus showed significant positive connectivity with parahippocampus (PHG), fusiform, superior and middle temporal gyrus, lingual gyrus, and cerebellum, while negative connectivity was observed with bilateral prefrontal cortex (PFC) and parietal lobe.
Fig. 3.
Resting-state functional connectivity pattern of bilateral hippocampal networks across participant groups (p < 0.001, k min = 20). Upper panel A depicts the hippocampal network for the comparison group, and lower panel B depicts the hippocampal network for the prenatal PCE group. The results illustrate variation in bilateral hippocampal networks for PCE and comparison fetuses derived using one sample t-tests. Warm colors (red) indicate positive connectivity and cool colors (blue) indicate negative connectivity. Abbreviations: PCE, prenatal cannabis exposure; LH, left hemisphere; RH, right hemisphere. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.5. Associations between THC status and hippocampal connectivity
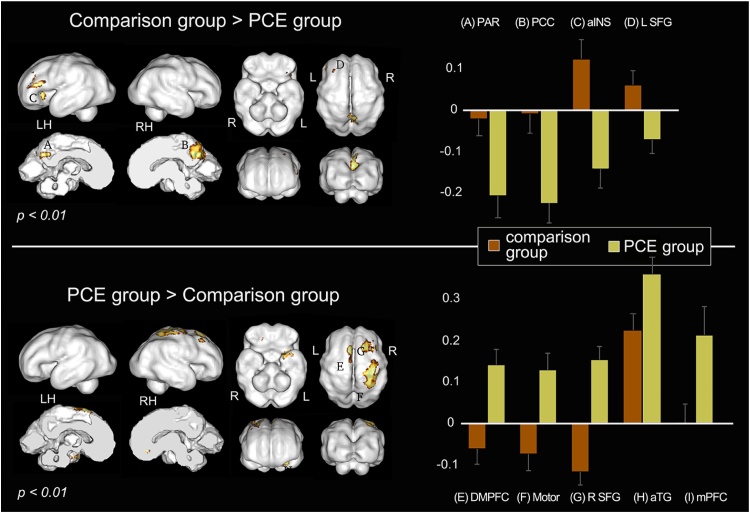
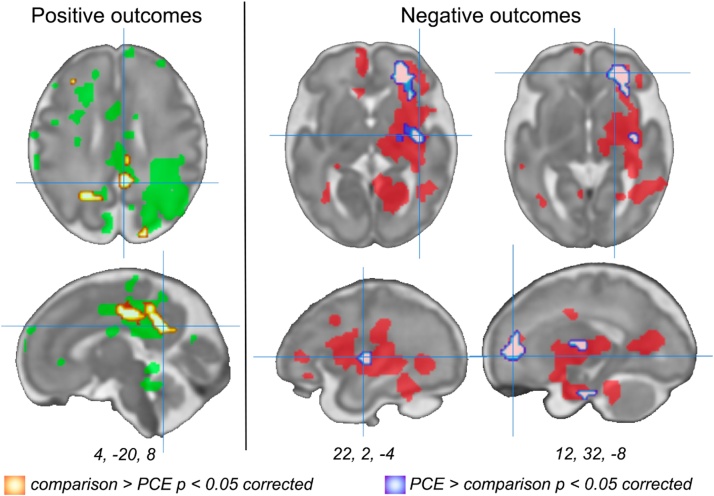
Nine regions showed significant group differences in hippocampal network connectivity in a whole brain two-tailed, two-sample t-test at a significance threshold of p < 0.01 [cluster (k) minimum = 20 voxels]. THC positive drug toxicology was associated with weaker hippocampal connectivity to parietal, posterior cingulate (PCC), anterior insula (aINS) and left superior frontal gyrus (SFG), and stronger hippocampal connectivity to medial and superior frontal regions, motor cortex, and anterior temporal gyrus (aTG), see Fig. 4 and Table 3. Three regions specified a priori, specifically the aINS, mPFC and PCC, remained significant after correcting for multiple comparisons. Examination of whether group differences fell within regions that predicted more or less favorable future child outcomes at age 5 revealed three significant effects after correcting for multiple comparisons. Specifically, a significant association with age 5 outcomes was observed in the posterior cingulate (4, -20, 8), where hippocampal connectivity was associated both with favorable outcomes and with higher RSFC in the non-PCE group. In addition, there were two regions, right prefrontal white matter (12, 32, -8) and right putamen (22, 2, -4), where hippocampal connectivity was associated with less favorable outcomes and with higher RSFC in the PCE group (Fig. 5). No regions showed the reverse effect. That is, there were no regions associated with favorable outcomes in which connectivity was higher in the PCE group, and no regions associated with less favorable outcomes in which connectivity was higher in the non-PCE comparison group.
Fig. 4.
Visualization of group differences in resting-state functional connectivity (RSFC). Top panel represents regions with significantly greater hippocampal connectivity in the comparison group, and bottom panel represents areas with significantly greater hippocampal connectivity in the PCE group, p value < 0.01, k min = 20. Column graphs depict group estimated means and standard error for significant clusters based on Fisher’s Z transformed values extracted from peaks summarized in Table 3. Data are visualized on an inflated surface model of a 32-week fetus. Abbreviations: L/LH, left hemisphere; R/RH, right hemisphere; PAR, parietal lobe; PCC, posterior cingulate cortex; aINS, anterior insula; SFG, superior frontal gyrus; DMPFC, dorsomedial prefrontal cortex; Motor, motor cortex; aTG, anterior temporal gyrus; mPFC, medial prefrontal cortex.
Table 3.
Group differences in resting-state functional connectivity.
|
Comparison group > PCE group | ||||||
|---|---|---|---|---|---|---|
| Area | x | y | z | Volume | Z score | Fig. 4 label |
| Parietal | −16 | −26 | 10 | 57 | 3.5728 | A |
| PCC | 4 | −20 | 10 | 130 | 4.8774 | B |
| aINS | −20 | 22 | −8 | 111 | 3.5888 | C |
| Left SFG | −20 | 28 | 14 | 29 | 2.9589 | D |
|
PCE group > Comparison group | ||||||
|---|---|---|---|---|---|---|
| Area | x | y | z | Volume | Z score | Fig. 4 label |
| DMPFC | −4 | 18 | 26 | 37 | 3.9424 | E |
| Motor | 20 | −2 | 26 | 110 | 3.9898 | F |
| Right SFG | 10 | 24 | 22 | 31 | 4.0287 | G |
| aTG | −20 | 8 | −24 | 31 | 3.0715 | H |
| mPFC | 8 | 28 | −16 | 21 | 3.0383 | I |
p < 0.01, k min = 20; N = 68; PCC, posterior cingulate cortex; aINS, anterior insula; SFG, Superior Frontal Gyrus; DMPFC, dorsomedial prefrontal cortex; Motor, motor cortex; aTG, anterior temporal gyrus; mPFC, medial prefrontal cortex.
Fig. 5.
Hippocampal network differences between study groups overlap regions associated with better or worse age 5 behavioral outcomes. Areas associated with more or less favorable future outcomes are depicted in green and red, respectively, corresponding to coloring in Fig. 1. After correction for multiple comparisons, a parietal region within the favorable outcome mask is more highly connected in non-PCE participants (orange), and two regions in the less favorable outcome mask (prefrontal white matter and right putamen) are more highly connected in PCE participants (blue). There were no significant regions showing the reverse association for either mask. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.6. Clinical and health variables account for a subset of observed RSFC differences
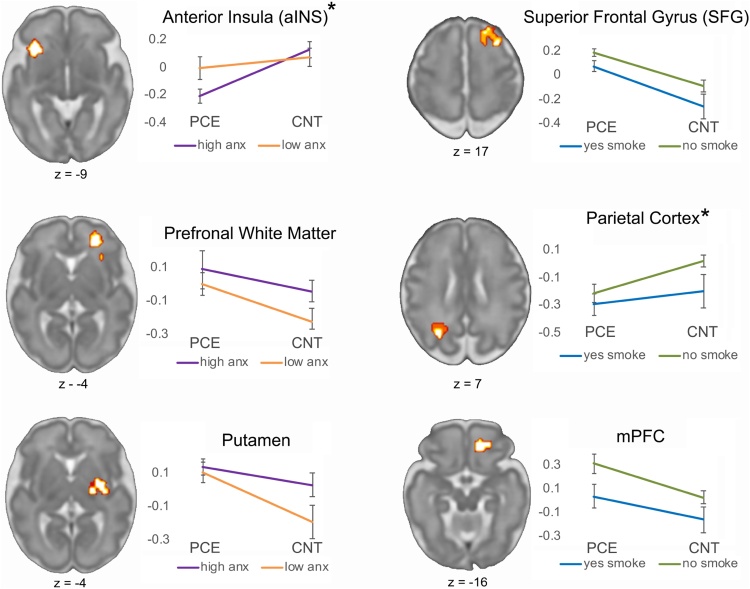
Stepwise regression models enabled testing the added contributions of clinical and health variables in explaining the variation in connectivity related to main effects of the group. We tested variables that were significantly different between groups, as reported in Table 2, diet, substance avoidance, perceived stress and anxiety. Omnibus F-tests confirmed that the addition of these variables improved model fit for four out of 12 regions, and in two regions resulted in trend-level improvements, p < 0.09 (Table 4). Notable contributions of anxiety symptoms and smoking to neural effects were observed. Anxiety was associated with PCE-related connectivity differences in the anterior insula (aINS), the right prefrontal white matter and right putamen (Fig. 6). Smoking was associated with PCE-related connectivity differences in the right superior frontal gyrus (SFG), the parietal cortex, the medial PFC and to lesser extent than anxiety, to the putamen (Table 4). In addition to these, diet and stress contributed to model fit of the aINS, and diet contributed to improved fit in the putamen. Thus, stress, anxiety, diet and smoking behavior were significantly different between PCE and control groups, and we find that all, but most notably, anxiety symptoms and smoking behavior, contribute to observed between-group hippocampal network effects.
Table 4.
Pair-wise models evaluating the addition of clinical and health variables into group difference models of hippocampal functional connectivity.
| reduced model |
stepwise model |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Predictor | Estimate | SE | p-value | AIC | Estimate | SE | p-value | AIC | F stat | p-value |
| aINS | non-PCE > PCE group | −0.31 | 0.06 | 0.000 | −2.54 | −0.29 | 0.07 | 0.000 | −7.39 | 3.6333 | 0.019* |
| (-20, 22, -8) | anxiety (STAI) | 0.03 | 0.00 | 0.016 | |||||||
| stress (PSS) | −0.01 | 0.01 | 0.16 | ||||||||
| diet | 0.00 | 0.00 | 0.10 | ||||||||
| Parietal | non-PCE > PCE group | −0.18 | 0.07 | 0.008 | 0.2 | −0.15 | 0.07 | 0.035 | −0.9 | 3.0083 | 0.089 |
| (-16, -26, 10) | smoking | −0.14 | 0.08 | 0.09 | |||||||
| mPFC | PCE > non-PCE group | 0.22 | 0.08 | 0.008 | 22.66 | 0.28 | 0.08 | 0.001 | 17.86 | 6.849 | 0.012* |
| (8, 28, -16) | smoking | −0.25 | 0.09 | 0.012 | |||||||
| Right SFG | PCE > non-PCE group | 0.22 | 0.06 | 0.001 | −2.79 | 0.25 | 0.07 | 0 | −3.98 | 3.1059 | 0.084 |
| (10, 24, 22) | smoking | −0.13 | 0.08 | 0.08 | |||||||
| PFC white matter | PCE > non-PCE group | 0.18 | 0.09 | 0.050 | 34.75 | 0.11 | 0.09 | 0.22 | 30.42 | 6.3492 | 0.015* |
| (12, 32, -8) | anxiety (STAI) | 0.04 | 0.02 | 0.015 | |||||||
| Putamen | PCE > non-PCE group | 0.16 | 0.09 | 0.070 | 33.93 | 0.14 | 0.09 | 0.15 | 30.98 | 2.9425 | 0.042* |
| (22, 2, -4) | anxiety (STAI) | 0.03 | 0.02 | 0.080 | |||||||
| diet | 0.01 | 0 | 0.06 | ||||||||
| smoking | 0.18 | 0.11 | 0.13 | ||||||||
F-statistic compares the reduced model to the step-wise model. Trend level significance (p < 0.09) is observed in the parietal cortex and right superior frontal gyrus (SFG). Significant model improvement (p < 0.05) is observed with addition of clinical and health variables in four brain areas, including the anterior insula (aINS), medial prefrontal cortex (mPFC), prefrontal white matter and putamen. *p-values less than 0.05 appear in bold typeset.
Fig. 6.
Anxiety symptoms and smoking behavior improve model fit in 6 brain regions that differentiate study groups. Step-wise regression models improved model fit in six out of 12 regions that differed between groups (two at trend level; four at p < 0.05). Line plots depict functional connectivity for median split high/low anxiety groups (left) and smoke (self any/none) groups (right). “*” denotes regions of higher functional connectivity in the control group. Images are depicted in neurological convention (left is left). Prefrontal white matter and putamen are two of the regions isolated within the negative outcome mask, and these appear to be related to self-reported anxiety symptoms. Abbreviations: anxiety, anx; prefrontal cortex (PFC); prenatal cannabis exposure, PCE; control, CNT.
4. Discussion
This study investigated the effects of cannabis use during pregnancy on connectivity of the human fetal hippocampus during the third trimester. Preclinical research suggests that the effects of THC on neural processes modify animal behavior and underlie disposition to psychiatric illness (Hurd et al., 2019). We provide evidence in an in vivo human fetal fMRI study that altered fetal neural connectome may contribute to neurobehavioral vulnerability observed in children with PCE. Specifically, PCE fetuses demonstrated stronger hippocampal to frontocortical connectivity, particularly in DMPFC, right SFG and mPFC regions, as well as stronger connectivity to left anterior temporal gyrus and motor cortex. PCE fetuses showed reduced hippocampal connectivity to the PCC, right SFG, anterior insula and parietal lobe. Exposure-related differences in fetal frontal lobe development are in agreement with prior prenatal drug exposure studies in newborns. Salzwedel and colleagues identified significant group differences between regions of the amygdala, mPFC, insula and motor cortex (Salzwedel et al., 2015). Localization of their mPFC finding is closely aligned with the significant effect observed here in a 32-week fetal brain template space. Peterson and colleagues reported frontal and parietal differences in brain volume and mean diffusivity that were largely consistent across drug use groups. A comparison performed in a subset of their cases (n = 29) exposed to marijuana yielded volumetric differences in midline prefrontal regions, PCC, SFG and aTG (Peterson et al., 2020), areas observed as having differential functional connectivity in our fetal sample. Thus, significant effects observed in the present study are localized in regions reliably reported in whole-brain neonatal MRI studies of prenatal illicit drug use.
An important consideration in fetal connectomic research is that we possess limited understanding of associations between variation in prenatal brain network development and relevance of these networks to future neurobehavioral health. A small number of studies have linked intrauterine fetal brain measures to behavior and/or birth variables (Thomason et al., 2018, 2017). Here, we sought to identify regions associated with more or less favorable neurobehavioral outcomes by performing additional regression analyses in participants with available behavioral outcome data. This was done by combining 3 results from cognitive and socioemotional outcomes for the subset of participants that have quality neuroimaging data and have reached age 5. In the present sample, two significant clusters (p < .05, corrected) identified as having higher connectivity in the PCE group are located in regions associated with less favorable outcomes at age 5. In contrast, one significant cluster (p < .05, corrected) identified as having higher connectivity in the comparison group are located in regions associated with more favorable outcomes. This is preliminary evidence that altered fetal neural connectome may contribute to neurobehavioral vulnerability observed in children with PCE. However, it should be noted that PCE may alter developmental trajectories such that neural connectivity patterns associated with more or less favorable future behavioral outcomes may differ between groups, and the present study is not able to resolve this possibility within the available longitudinal data.
Prior neuroimaging studies of drug exposure during pregnancy and neonatal brain development demonstrate that the effects of different drugs are more overlapping than they are discrete (Salzwedel et al., 2015; Peterson et al., 2020). This is in contrast to animal studies that better differentiate the mechanisms underlying teratogenic exposure effects. Possible explanations are that invasive animal methodologies enable microscale analyses where mechanistic differences are easier to discern, or that animal experimentation enables better control over confounding variables. To the latter, data consistently relay that substance use during pregnancy is dangerous to the fetus, but human developmental neuroimaging studies have yet to rigorously control for the influence of genetic dispositional risk and additional clinical and environmental factors important for fetal development (e.g., maternal body mass, stress, anxiety, socioeconomic status, etc. (De Asis-Cruz et al., 2020; Karlsson et al., 2020; Lautarescu et al., 2020; Norr et al., 2021; Benavente-Fernández et al., 2019; Spann et al., 2020; Ramphal et al., 2020; Leijser et al., 2018)). As with the current study, this can be hard to do, especially in neuroimaging studies comprised of relatively small samples that would be underpowered to address this critical question. In the present study, imaging variables, fetal characteristics, and maternal age did not differ between groups. However, comparison of mental and physical health variables showed that our neuroimaging groups differed in dietary health behavior diet, p = .017, avoidance of tobacco and alcohol, p = .03, anxiety (STAI), p = .043, and perceived stress (PSST), p = .029. Groups also differed in WIDUS total score (p = .009), an indirect screener for drug use that asks questions including history of traumatic events, which are known to alter physiological processes and the epigenome in lasting ways (Buss et al., 2017; Gustafsson et al., 2017; McCormack et al., 2021; Scorza et al., 2020; Tottenham and Sheridan, 2009). Differences between comparison groups with potential to influence maternal biology and, in turn, fetal development are a notable limitation of this and prior neonatal neural studies.
To address this limitation, we performed step-wise regression, adding variables that differed between neuroimaging groups, and used F-tests to evaluate whether models improved with the addition of these variables. Each area associated with significant hippocampal connectivity differences between groups (12 total) was tested. Overall, we found that the addition of these variables improved model fit in 4 brain regions at p < 0.05, and 2 regions at p < 0.09, and we found that anxiety symptoms and smoking behavior were most informative across these models; each was influential in at least 3 of the models (Table 4). This confirms the need for control of confounds in imaging studies comparing drug exposure effects. We suggest that the lack of specificity in exposure to specific drug-types in prior human infant neuroimaging studies is likely the result of co-occurring confounds that frequently accompany illicit prenatal drug use.
The National Institutes of Health Healthy Brain and Child Development (HBCD) initiative seeks to address this limitation in human research by aggregating a nationally representative prospective, longitudinal study of early human development beginning in utero (Jordan et al., 2020). There will be opportunity to utilize this public resource data set to tackle questions about environmental programming of human development in ways that smaller scale studies are unable to do. Illicit substance use during pregnancy will be a major emphasis of this study, with potential to significantly advance understanding and inform public policy and health interventions. In addition, HBCD will provide a platform for evaluation of normal trajectories of early human brain development, an order of magnitude larger than priors. At the inception of developmental neuroimaging research, studies were comprised of 10–20 participants; the HBCD study will enroll as many as 7,500 families. Additional papers in this special issue address qualities and considerations of the HBCD study, including a commentary by Rajagopalan and colleagues relevant to data reported here on whether fetal imaging is a lost opportunity in the HBCD study design.
Limitations of the current study warrant discussion. An initial consideration is that drug screening results extracted from medical records are susceptible to biased sampling, due to non-uniform administration of discretionary urine toxicology in medical centers (FitzGerald and Hurst, 2017; Kunins et al., 2007). We specifically targeted a population with restricted range of sociodemographic characteristics, but even so, the implication remains that representativeness of this sample to a base population of predominately low-resource African American individuals is challenging to estimate due to medical bias in prenatal drug screen testing (FitzGerald and Hurst, 2017; Kunins et al., 2007). It also suggests that the observed high proportion of women with a positive THC screen in this population, 36.5 %, requires caution in interpretation. Another limitation is that THC is detectable in the urine for a limited amount of time. Alternatives that have been used in studies of prenatal drug use include hair and nail clippings, which are minimally invasive and minimally inconvenient, as these require fewer collections for reliable estimation (documenting up to 7 months of exposure history for specific substances) and are painless to collect. They are also less demanding in terms of storage and processing requirements. These approaches are likely to serve as a central strategy in the HBCD study. A drawback of hair and nail biospecimens for assessment of drug exposure is that these are costly materials to analyze, presently ∼$60 per sample to detect presence or absence of a single substance. The urine approach in the current study has potential to underestimate use, but remains preferable to self-report which can lead to underreporting due to social desirability and recall biases (Roberson et al., 2014; Gunn et al., 2016; Tourangeau and Yan, 2007) A final limitation of the current study is that the association between exposures, fetal connectivity and age 5 outcomes could not be assessed in a sufficiently powered mediation/moderation model, as the number of available participants with both outcome data and prenatal drug toxicology data were relatively few. Sample testing is ongoing as the sample increases in age; it is hoped that more comprehensive within subject modelling will be possible in the future.
Our data on the effects of PCE on the human fetal hippocampal network provides further support for a growing body of research conveying that cannabis use during pregnancy may increase adverse outcomes for women and their children. We add to prior studies that brain connectome changes associated with PCE begin before birth and can be studied in utero. We add study of this topic within a population at greater use for illicit substance use and subject to greater risk in terms of health inequities.
Data statement
The investigators contributing to this manuscript are committed to the principles of open and reproducible science. Data from the larger fetal neuroimaging study are available on OpenNeuro and on the NIMH NDAR Data Archive. Any additional queries can be directed to the corresponding author.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This project was supported by awards from the National Institutes of Health, MH110793, DA050287, MH122447 and ES032294. The authors thank Brendan Coyle, Jasmine Hect, Sophia Neuenfeldt, Rebekah Zielesch, and Pavan Jella for their assistance in data acquisition and thank Toni Lewis for assistance with data management and quality assurance. Importantly, the authors thank participant families who generously shared their time and expressed interest in helping future babies to achieve their best possible health outcomes.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.101000.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Achenbach T.M., Rescorla L.A. University of Vermont, Department of Psychiatry; Burlington, VT: 2000. Manual for the ASEBA Preschool Forms and Profiles. [Google Scholar]
- Benavente-Fernández I. Association of socioeconomic status and brain injury with neurodevelopmental outcomes of very preterm children. JAMA Netw. Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc. Natl. Acad. Sci. U. S. A. 2005;102:19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown Q.L. Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002–2014. JAMA. 2017;317(2):207–209. doi: 10.1001/jama.2016.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C. Intergenerational transmission of maternal childhood maltreatment exposure: implications for fetal brain development. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56:373–382. doi: 10.1016/j.jaac.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.C. Beliefs and attitudes regarding prenatal marijuana use: perspectives of pregnant women who report use. Drug Alcohol Depend. 2019;196:14–20. doi: 10.1016/j.drugalcdep.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Dahl R.E., Scher M.S., Williamson D.E., Robles N., Day N. A longitudinal study of prenatal marijuana use. Effects on sleep and arousal at age 3 years. Arch. Pediatr. Adolesc. Med. 1995;149:145–150. doi: 10.1001/archpedi.1995.02170140027004. [DOI] [PubMed] [Google Scholar]
- Day N.L., Richardson G.A. Prenatal marijuana use: epidemiology, methodologic issues, and infant outcome. Clin. Perinatol. 1991;18:77–91. [PubMed] [Google Scholar]
- De Asis-Cruz J. Association of prenatal maternal anxiety with fetal regional brain connectivity. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.22349. [DOI] [PubMed] [Google Scholar]
- de Salas-Quiroga A. Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB1 receptors on developing cortical neurons. Proc. Natl. Acad. Sci. U. S. A. 2015;112:13693–13698. doi: 10.1073/pnas.1514962112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55:791–802. doi: 10.1111/epi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E., Emmons R.A., Larsen R.J., Griffin S. The satisfaction with life scale. J. Pers. Assess. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Marroun H. Demographic, emotional and social determinants of cannabis use in early pregnancy: the Generation R study. Drug Alcohol Depend. 2008;98:218–226. doi: 10.1016/j.drugalcdep.2008.05.010. [DOI] [PubMed] [Google Scholar]
- El Marroun H. Intrauterine cannabis exposure affects fetal growth trajectories: the Generation R Study. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:1173–1181. doi: 10.1097/CHI.0b013e3181bfa8ee. [DOI] [PubMed] [Google Scholar]
- FitzGerald C., Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med. Ethics. 2017;18:19. doi: 10.1186/s12910-017-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley R.C., Waller N.G., Brennan K.A. An item response theory analysis of self-report measures of adult attachment. J. Pers. Soc. Psychol. 2000;78:350–365. doi: 10.1037//0022-3514.78.2.350. [DOI] [PubMed] [Google Scholar]
- Fried P.A., Watkinson B., Dillon R.F., Dulberg C.S. Neonatal neurological status in a low-risk population after prenatal exposure to cigarettes, marijuana, and alcohol. J. Dev. Behav. Pediatr. 1987;8:318–326. [PubMed] [Google Scholar]
- FSL, FMRIB Software Library. http://www.fmrib.ox.ac.uk/fsl/.
- Galve-Roperh I. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog. Lipid Res. 2013;52:633–650. doi: 10.1016/j.plipres.2013.05.004. [DOI] [PubMed] [Google Scholar]
- García-Gutiérrez M.S. Cannabidiol: a potential new alternative for the treatment of anxiety, depression, and psychotic disorders. Biomolecules. 2020;10 doi: 10.3390/biom10111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia G.A., Espy K.A., Isquith P.K. Psychological Assessment Resources, Inc.; 2003. The Behavior Rating Inventory of Executive Function-Preschool Version (BRIEF-P) [Google Scholar]
- Graham A.M. Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol. Psychiatry. 2019;85:172–181. doi: 10.1016/j.biopsych.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewen K. Prenatal cocaine effects on brain structure in early infancy. Neuroimage. 2014;101:114–123. doi: 10.1016/j.neuroimage.2014.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn J.K. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson H., Doyle C., Gilchrist M., Werner E., Monk C. Maternal abuse history and reduced fetal heart rate variability: abuse-related sleep disturbance is a mediator. Dev. Psychopathol. 2017;29:1023–1034. doi: 10.1017/S0954579416000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C.L. “Anything above marijuana takes priority”: obstetric providers’ attitudes and counseling strategies regarding perinatal marijuana use. Patient Educ. Couns. 2016;99:1446–1451. doi: 10.1016/j.pec.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W. Amyloid deposition is linked to aberrant entorhinal activity among cognitively normal older adults. J. Neurosci. 2014;34:5200–5210. doi: 10.1523/JNEUROSCI.3579-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntsman R.J. Dosage related efficacy and tolerability of cannabidiol in children with treatment-resistant epileptic encephalopathy: preliminary results of the CARE-E study. Front. Neurol. 2019;10:716. doi: 10.3389/fneur.2019.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd Y.L. Marijuana impairs growth in mid-gestation fetuses. Neurotoxicol. Teratol. 2005;27:221–229. doi: 10.1016/j.ntt.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Hurd Y.L. Cannabis and the developing brain: insights into its long-lasting effects. J. Neurosci. 2019;39:8250–8258. doi: 10.1523/JNEUROSCI.1165-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings D.E., Martin B.R., Gamagaris Z., Miller N., Fico T. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci. 1989;44:697–701. doi: 10.1016/0024-3205(89)90380-9. [DOI] [PubMed] [Google Scholar]
- Jackson T. Relationships between perceived close social support and health practices within community samples of American women and men. J. Psychol. 2006;140:229–246. doi: 10.3200/JRLP.140.3.229-246. [DOI] [PubMed] [Google Scholar]
- Jakab A. Fetal functional imaging portrays heterogeneous development of emerging human brain networks. Front. Hum. Neurosci. 2014;8:852. doi: 10.3389/fnhum.2014.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C.J., Weiss S.R.B., Howlett K.D., Freund M.P. Introduction to the special issue on "informing longitudinal studies on the effects of maternal stress and substance use on child development: planning for the Healthy Brain and Child Development (HBCD) study. Advers. Resil. Sci. 2020:1–5. doi: 10.1007/s42844-020-00022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafil T.S., Nguyen T.M., MacDonald J.K., Chande N. Cannabis for the treatment of Crohn’s disease. Cochrane Database Syst. Rev. 2018;11:CD012853. doi: 10.1002/14651858.CD012853.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson H. Association of cumulative paternal early life stress with white matter maturation in newborns. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.24832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keimpema E., Calvigioni D., Harkany T. Endocannabinoid signals in the developmental programming of delayed-onset neuropsychiatric and metabolic illnesses. Biochem. Soc. Trans. 2013;41:1569–1576. doi: 10.1042/BST20130117. [DOI] [PubMed] [Google Scholar]
- Ko J.Y., Farr S.L., Tong V.T., Creanga A.A., Callaghan W.M. Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am. J. Obstet. Gynecol. 2015;213:201.e201–201.e210. doi: 10.1016/j.ajog.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunins H.V., Bellin E., Chazotte C., Du E., Arnsten J.H. The effect of race on provider decisions to test for illicit drug use in the peripartum setting. J. Womens Health (Larchmt) 2007;16:245–255. doi: 10.1089/jwh.2006.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautarescu A. Maternal prenatal stress is associated with altered uncinate fasciculus microstructure in premature neonates. Biol. Psychiatry. 2020;87:559–569. doi: 10.1016/j.biopsych.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijser L.M., Siddiqi A., Miller S.P. Imaging evidence of the effect of socio-economic status on brain structure and development. Semin. Pediatr. Neurol. 2018;27:26–34. doi: 10.1016/j.spen.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Liu Z., Neff R.A., Berg D.K. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Loureiro M., Renard J., Zunder J., Laviolette S.R. Hippocampal cannabinoid transmission modulates dopamine neuron activity: impact on rewarding memory formation and social interaction. Neuropsychopharmacology. 2015;40:1436–1447. doi: 10.1038/npp.2014.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack C. Maternal childhood adversity and inflammation during pregnancy: interactions with diet quality and depressive symptoms. Brain Behav. Immun. 2021;91:172–180. doi: 10.1016/j.bbi.2020.09.023. [DOI] [PubMed] [Google Scholar]
- Mehmedic Z. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J. Forensic Sci. 2010;55:1209–1217. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- Meyer T.J., Miller M.L., Metzger R.L., Borkovec T.D. Development and validation of the Penn State Worry Questionnaire. Behav. Res. Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Morales M., Hein K., Vogel Z. Hippocampal interneurons co-express transcripts encoding the alpha7 nicotinic receptor subunit and the cannabinoid receptor 1. Neuroscience. 2008;152:70–81. doi: 10.1016/j.neuroscience.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder J. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc. Natl. Acad. Sci. U. S. A. 2008;105:8760–8765. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norr M.E., Hect J.L., Lenniger C.J., Van den Heuvel M., Thomason M.E. An examination of maternal prenatal BMI and human fetal brain development. J. Child Psychol. Psychiatry. 2021;62(4):458–469. doi: 10.1111/jcpp.13301. Epub 2020 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell C.M., Fried P.A. Prenatal exposure to cannabis: a preliminary report of postnatal consequences in school-age children. Neurotoxicol. Teratol. 1991;13:631–639. doi: 10.1016/0892-0362(91)90047-z. [DOI] [PubMed] [Google Scholar]
- Oh S., Salas-Wright C.P., Vaughn M.G., DiNitto D.M. Marijuana use during pregnancy: a comparison of trends and correlates among married and unmarried pregnant women. Drug Alcohol Depend. 2017;181:229–233. doi: 10.1016/j.drugalcdep.2017.09.036. [DOI] [PubMed] [Google Scholar]
- Ondersma S.J. Development and preliminary validation of an indirect screener for drug use in the perinatal period. Addiction. 2012;107(12):2099–2106. doi: 10.1111/j.1360-0443.2012.03982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma S.J. Accuracy of five self-report screening instruments for substance use in pregnancy. Addiction. 2019;114:1683–1693. doi: 10.1111/add.14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panter J.E., Bracken B.A. Validity of the Bracken School Readiness Assessment for predicting first grade readiness. Psychol. Sch. 2009;46:397–409. [Google Scholar]
- Peterson B.S. Associations of maternal prenatal drug abuse with measures of newborn brain structure, tissue organization, and metabolite concentrations. JAMA Pediatr. 2020;174:831–842. doi: 10.1001/jamapediatrics.2020.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Ramphal B. Brain connectivity and socioeconomic status at birth and externalizing symptoms at age 2 years. Dev. Cogn. Neurosci. 2020;45 doi: 10.1016/j.dcn.2020.100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson E.K., Patrick W.K., Hurwitz E.L. Marijuana use and maternal experiences of severe nausea during pregnancy in Hawai’i. Hawaii J. Med. Public Health. 2014;73:283–287. [PMC free article] [PubMed] [Google Scholar]
- Salzwedel A.P. Prenatal drug exposure affects neonatal brain functional connectivity. J. Neurosci. 2015;35:5860–5869. doi: 10.1523/JNEUROSCI.4333-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzwedel A.P., Grewen K.M., Goldman B.D., Gao W. Thalamocortical functional connectivity and behavioral disruptions in neonates with prenatal cocaine exposure. Neurotoxicol. Teratol. 2016;56:16–25. doi: 10.1016/j.ntt.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer C. Dissociable disruptions in thalamic and hippocampal resting-state functional connectivity in youth with 22q11.2 deletions. J. Neurosci. 2019;39:1301–1319. doi: 10.1523/JNEUROSCI.3470-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorza P. Epigenetic intergenerational transmission: mothers’ adverse childhood experiences and DNA methylation. J. Am. Acad. Child Adolesc. Psychiatry. 2020;59:900–901. doi: 10.1016/j.jaac.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serag A. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. Neuroimage. 2012;59:2255–2265. doi: 10.1016/j.neuroimage.2011.09.062. [DOI] [PubMed] [Google Scholar]
- Shattuck D.W., Leahy R.M. BrainSuite: an automated cortical surface identification tool. Med. Image Anal. 2002;6:129–142. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- Smith C. 2011. Virginia Commonwealth University. [Google Scholar]
- Spann M.N., Bansal R., Hao X., Rosen T.S., Peterson B.S. Prenatal socioeconomic status and social support are associated with neonatal brain morphology, toddler language and psychiatric symptoms. Child Neuropsychol. 2020;26:170–188. doi: 10.1080/09297049.2019.1648641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D. Consulting Psychologists Press; Palo Alto, CA: 1984. State-trait Anxiety Inventory: A Comprehensive Bibliography. [Google Scholar]
- Statistical Parametric Mapping 8 from the Wellcome Trust Centre for Neuroimaging . 2009. Statistical Parametric Mapping 8 From the Wellcome Trust Centre for Neuroimaging.http://www.fil.ion.ucl.ac.uk/spm/ [Google Scholar]
- Thomason M.E. Cross-hemispheric functional connectivity in the human fetal brain. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E. Intrinsic functional brain architecture derived from graph theoretical analysis in the human fetus. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E. Weak functional connectivity in the human fetal brain prior to preterm birth. Sci. Rep. 2017;7:39286. doi: 10.1038/srep39286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E. Prenatal neural origins of infant motor development: associations between fetal brain and infant motor development. Dev. Psychopathol. 2018;30:763–772. doi: 10.1017/S095457941800072X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E. Prenatal lead exposure impacts cross-hemispheric and long-range connectivity in the human fetal brain. Neuroimage. 2019;191:186–192. doi: 10.1016/j.neuroimage.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M., Hect J., Waller R., Curtin P. Interactive relations between maternal prenatal stress, fetal brain connectivity and gestational age at delivery. Neuropsychopharmacology. 2021 doi: 10.1038/s41386-021-01066-7. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortoriello G. Miswiring the brain: Δ9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J. 2014;33:668–685. doi: 10.1002/embj.201386035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Sheridan M.A. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourangeau R., Yan T. Sensitive questions in surveys. Psychol. Bull. 2007;133:859–883. doi: 10.1037/0033-2909.133.5.859. [DOI] [PubMed] [Google Scholar]
- Turk E. Functional connectome of the fetal brain. J. Neurosci. 2019;39(49):9716–9724. doi: 10.1523/JNEUROSCI.2891-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.I. Hubs in the human fetal brain network. Dev. Cogn. Neurosci. 2018;30:108–115. doi: 10.1016/j.dcn.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargish G.A. Persistent inhibitory circuit defects and disrupted social behaviour following in utero exogenous cannabinoid exposure. Mol. Psychiatry. 2017;22:56–67. doi: 10.1038/mp.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco G., Sánchez C., Guzmán M. Anticancer mechanisms of cannabinoids. Curr. Oncol. 2016;23:S23–32. doi: 10.3747/co.23.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock M.D. Sex differences in functional connectivity during fetal brain development. Dev. Cogn. Neurosci. 2019;36 doi: 10.1016/j.dcn.2019.100632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C.W., Krishnan A., Wager T.D. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.