Abstract
Objective
Guidelines for methotrexate (MTX) use in rheumatic disease may not be feasible for physicians practicing in the least developed countries. We aimed to understand the experiences of MTX prescribers relating to MTX use for rheumatic disease in African countries to inform the development of culturally and geographically appropriate recommendations.
Methods
African physicians who self‐identified as MTX prescribers from countries classified as having a low versus a medium or high Human Development Index (L‐HDI versus MH‐HDI) participated in semistructured interviews between August 2016 and September 2017. Interviews were transcribed verbatim, coded thematically, and stratified by HDI.
Results
Physicians (23 rheumatologists; six internists) from 29 African countries were interviewed (15 L‐HDI; 14 MH‐HDI). Identified barriers to MTX use included inconsistent MTX supply (reported by 87% L‐HDI versus 43% MH‐HDI), compounded by financial restrictions (reported by 93% L‐HDI versus 64% MH‐HDI), patient hesitancy based partly on cultural beliefs and societal roles (reported by 71%), few prescribers (reported by 33%), prevalent infections (especially viral hepatitis, tuberculosis, and human immunodeficiency virus), and both availability and cost of monitoring tests. MTX pretreatment evaluation and starting and maximal doses were similar between L‐HDI countries and MH‐HDI countries.
Conclusion
The challenges of treating rheumatic disease in African countries include unreliable drug availability and cost, limited subspecialists, and patient beliefs. Adapting recommendations for MTX use in the context of prevalent endemic infections; ensuring safe but feasible MTX monitoring strategies, enhanced access to stable drug supply, and specialized rheumatology care; and improving patient education are key to reducing the burden of rheumatic diseases in L‐HDI countries.
Significance & Innovations.
There are unique challenges in Africa regarding methotrexate (MTX) use, including limited prescribers, high prevalence of endemic infections, inconsistent drug supply, and low health literacy of patients.
Cultural beliefs and financial limitations influence MTX adherence.
Regional guidelines for MTX need to address screening for infections, strategies for affordable and feasible monitoring and follow‐up, consistent access to the medication, and patient education.
INTRODUCTION
Methotrexate (MTX) is considered standard of care for the treatment of rheumatoid arthritis (RA) and has an important role in the management of other rheumatic diseases (1). This relatively low‐cost disease‐modifying agent is effective when appropriately dosed, has versatile routes of administration, and has established safety profiles (2, 3, 4). However, MTX has potential for significant toxicity, including but not limited to hepatotoxicity, teratogenicity, and infection, especially with organisms targeted by cell‐mediated immunity (5). Clinical experience is required to use MTX safely. Existing guidelines vary regarding recommendations for MTX prescribing and monitoring (6). Moreover, it is unknown whether those guidelines are adequate to inform MTX use in resource‐poor regions (7).
The United Nations Human Development Program (UNDP) ranks countries in four tiers according to the Human Development Index (HDI; values between 0 and 1) (8). The HDI is a summary index assessing health, education, and standard of living using life expectancy at birth, expected years of schooling for children and mean years of schooling for adults, and gross national income per capita. Countries with very high development are those with an HDI of 0.8 or more, whereas those with low development have an HDI of less than 0.55. Countries with low socioeconomic development have high rates of poverty, experience greater economic instability, and have limited human resources for health and education (9). According to the 2019 UNDP report, more than half (58.5%) of all countries in Africa have low HDI (L‐HDI) (10). Clinicians caring for rheumatology patients in Africa face multiple challenges. These include malnutrition, a high burden of infections, lack of health personnel, and low health literacy (11, 12, 13, 14, 15). There are few published studies on the screening, safety, and efficacy of MTX in Africa to inform the development of guidelines for MTX use (16, 17, 18, 19, 20).
This study aimed to assess the experiences of MTX prescribers in African countries with different HDIs, to identify challenges related to the use of MTX for rheumatic disease, and to highlight priority management issues to consider when adapting existing disease management guidelines to their environment.
PATIENTS AND METHODS
Participants
Physicians were identified on the basis of membership in regional medical and rheumatology societies, physician registries, and contact tracing (Supplementary Table 1). Physicians (n = 80) were approached with an introductory email outlining the purpose of the study, followed by a reminder email if needed. Of the 50 respondents, all consenting physicians who self‐identified as MTX prescribers were interviewed. Countries were categorized based on the 2019 HDI, in which an HDI of less than 0.55 is categorized as low (L‐HDI), an HDI of 0.55 to 0.70 is categorized as medium, and an HDI of more than 0.7 is classified as high human development. We also compared results using the 2015 HDI categories, which more closely reflect the years interviews took place (21).
Interviews
Semistructured phone interviews based on a questionnaire (Supplementary Document) were conducted between August 2016 and September 2017 by a single interviewer (IC) in English or French (according to the preference of the interviewee) and audio‐recorded (22). Interviews lasted between 45 minutes and 60 minutes. Data collected included demographic information of the interviewee, type of medical practice, and estimated number of rheumatologists practicing in their country. Participants were asked to describe their experiences with the use of MTX specifically for the treatment of rheumatic diseases, including screening practices, dosage of MTX, and use of folic acid, and any barriers or challenges to prescribing MTX or to patient adherence. Finally, participants were asked to describe important considerations for future guideline development for MTX use in their region. The interview content was expanded as new concepts arose. Concept saturation (akin to study power) was achieved. The interviews were transcribed verbatim, translated to English if needed, checked for accuracy, and entered into DeDoose software (Sociocultural Research Consultants) for qualitative analysis.
Analysis
The goals of the study analysis were to identify themes related to MTX use, to see how they differed by HDI, and to quantify different aspects of need to inform guideline development. Data from medium and high HDI (MH‐HDI) countries were combined because of the small number of interviews from high HDI countries and thematic commonalities. Descriptive statistics were used to compare demographics and medical practice patterns of participants from L‐HDI and MH‐HDI countries. Categorical variables are reported as number (percentage). Continuous variables are reported as median (25th percentile‐75th percentile). As this study was exploratory and only one physician per country was interviewed, we did not formally compare responses between L‐HDI and MH‐HDI groups.
Qualitative analysis
Thematic analysis of the transcripts was conducted separately by IC, CF, and CH in stages. First, general concepts identified during the conduct of interviews were explored. A pilot sample of four transcripts was independently reviewed by CF and CH to identify the main themes and subthemes, develop a coding strategy, and ensure consistency among reviewers. Themes were then applied to the complete set of interviews. Additional themes were assessed as they arose. Investigators reviewed and updated the thematic coding during the course of the analysis. Discrepancies were resolved by consensus. Narrative synthesis summarized the theme content. Representative quotes from participants in L‐HDI and MH‐HDI countries were highlighted.
Ethics
The study complies with the Declaration of Helsinki. It was approved by the institutional ethics boards of the University of Manitoba (H2016;020 and HS19277), McGill University (16‐037), and the University of KwaZulu‐Natal (BE376/16). Participants provided verbal informed consent.
RESULTS
Between 2016 and 2017, 30 physicians from 29 African countries were interviewed. In one L‐HDI country, two interviews were conducted, one with a trainee and one more informative interview with a practicing physician; we excluded the trainee interview. The countries represented were distributed across the African continent and included 15/31 (48%), 8/13 (62%), and 6/9 (67%) of all low, medium, and high HDI countries based on their 2019 HDI category (Figure 1). The demographics of the participants are shown in Table 1. Most participants were rheumatologists, were affiliated with a university or other academic institution, and worked in the country’s capital city. The estimated number of rheumatologists per country varied widely; however, MH‐HDI countries tended to have more rheumatologists than L‐HDI countries (0‐350 rheumatologists versus 0‐15 rheumatologists). Four L‐HDI countries and one MH‐HDI country had no practicing rheumatologist. In many countries, MTX was also prescribed by nonrheumatologists (82% of all countries; 93% L‐HDI versus 69% MH‐HDI). MTX prescribing practices were similar between L‐HDI and MH‐HDI countries, with comparable dosing targets and use of folic acid. Drug availability and cost (reported by 82% of all countries; 93% L‐HDI versus 64% MH‐HDI) and limited prescribers and monitoring capacity (reported by 33% of all countries; 28% L‐HDI versus 38% MH‐HDI) were identified as barriers to adherence (Table 2).
Figure 1.
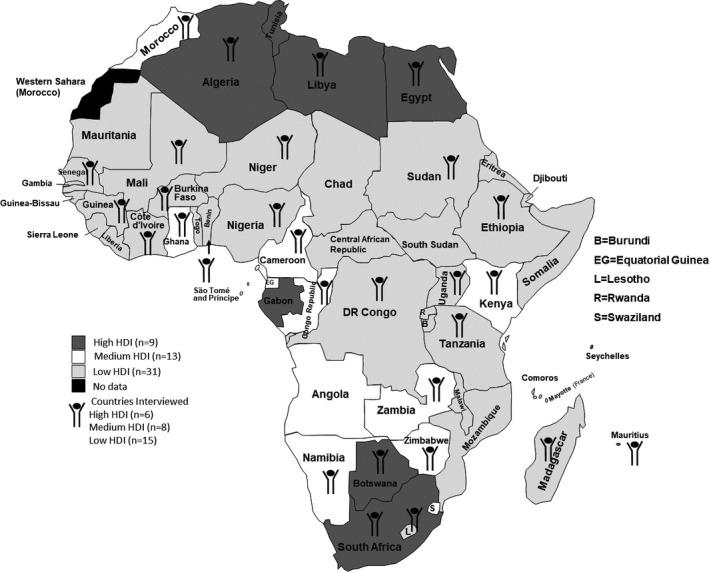
Countries participating in interviews. Shading indicates 2019 Human Development Index (HDI) category of country. Symbol identifies countries participating in interviews.
Table 1.
Demographics of participants and rheumatology capacity
| Total (N = 29) | Low HDI (n = 15) | Medium/High HDI (n = 14) | |
|---|---|---|---|
| Rheumatologista | 23 (79) | 11 (73) | 12 (86) |
| Male sex | 20 (69) | 12 (80) | 8 (57) |
| Language of interview (English) | 17 (59) | 6 (40) | 11 (79) |
| Official language of country | |||
| English | 13 (45) | 4 (27) | 9 (64) |
| French | 10 (34) | 9 (60) | 1 (7) |
| Arabic | 5 (17) | 1 (7) | 4 (29) |
| Amharic | 1 (3) | 1 (7) | 0 (0) |
| Years in practicea | 8 (4‐12) | 8 (2.0‐12.0) | 6.5 (4.0‐16.8) |
| Practise located in capital city | 18 (62) | 11 (73) | 7 (50) |
| Main practice type | |||
| Hospital‐based | 3 (10) | 1 (7) | 2 (14) |
| University affiliate | 24 (83) | 14 (93) | 10 (71) |
| Private | 2 (7) | 0 (0) | 2 (14) |
| Treats adults and children | 23 (79) | 14 (93) | 9 (64) |
| MTX prescribed by nonrheumatologists in countryb | |||
| Only rheumatology | 5 (19) | 1 (7) | 4 (31) |
| Other prescriber | |||
| Internal medicine or medical subspecialties | 19 (70) | 11 (79) | 8 (62) |
| Orthopedics | 4 (15) | 1 (7) | 3 (23) |
| Generalists and medical officers | 2 (7) | 1 (7) | 1 (8) |
Abbreviation: HDI, human development index (2019); MTX, methotrexate.
Categorical data are n (%); continuous data are median (25th percentile‐75th percentile).
Years in practice were available for 11 low HDI countries and 12 medium/high HDI countries.
Prescriber status was missing for one low HDI country and one medium/high HDI country; total is greater than 27 as it includes countries with multiple prescriber categories (internal medicine + orthopedics [two medium/high HDI], orthopedics + generalists/house officers [one medium/high HDI], and other specialists + generalists/house officers [one low HDI]).
Table 2.
MTX prescribing patterns
| Totala (N = 29) | L‐HDI (n = 15) | MH‐HDI (n = 14) | |
|---|---|---|---|
| Rheumatology indication for prescribing | |||
| Main condition rheumatoid arthritis | 29 (100) | 15 (100) | 14 (100) |
| Secondary conditionsb | |||
| Connective tissue disease | 12 (43) | 7 (50) | 5 (36) |
| Psoriatic arthritis | 12 (43) | 5 (36) | 7 (50) |
| Juvenile idiopathic arthritis | 4 (14) | 2 (14) | 2 (14) |
| Pre‐MTX evaluation | |||
| Lung, liver, kidney, CBCb, c | 28 (97) | 14 (93) | 14 (100) |
| Radiographs done | 17 (59) | 9 (60) | 8 (57) |
| TB screen doned | 18 (62) | 10 (67) | 8 (57) |
| HIV serologies done or available | 16 (55) | 8 (53) | 8 (57) |
| Hepatitis B/C serologies done | 15 (52) | 7 (47) | 8 (57) |
| Pregnancy screen by historyb | 24/28 (85) | 12/14 (86) | 12/14 (86) |
| Alcohol counselling, no/NA/yes | 2/17/10 | 2/9/4 | 0/8/6 |
| MTX dose | |||
| Starting dose, mg/week | 10 (10‐14) | 10 (7.5‐15.0) | 10.0 (10.0‐14) |
| Maximum dose, mg/week | 25 (20‐25) | 20 (15‐25) | 25 (24‐25) |
| Folate prescribed | 24 (83) | 13 (87) | 11 (79) |
| Prescribing challenges | |||
| Cost per month, US dollarse | 17.5 (8.8‐23.5) | 17.0 (7.5‐25.0) | 20.0 (8.8‐223.8) |
| Injectable formulation available | 16 (55) | 8 (53) | 8 (57) |
| Inconsistent MTX supply | 19 (66) | 13 (87) | 6 (43) |
| MTX available in hospital pharmacy | 16 (55) | 4 (27) | 12 (86) |
| Adherence challengesb, f | |||
| Cost and drug availability | 23 (82) | 14 (93) | 9 (64) |
| Lack of prescribers/monitoring ability | 9 (33) | 4 (28) | 5 (38) |
| Patient belief/education/intolerance | 20 (71) | 10 (67) | 10 (77) |
| Patient stigma (hand deformities) | 14 (48) | 9 (60) | 5 (36) |
Abbreviation: CBC, complete blood count; HDI, human development index (2019); HIV, human immunodeficiency virus; L‐HDI, low HDI; MH‐HDI, medium or high HDI; MTX, methotrexate; NA, not applicable due to religion/restricted alcohol access; TB, tuberculosis; US, United States of America.
Connective tissue disease includes lupus vasculitis scleroderma.
Categorical data are reported as n (%); continuous data are reported as median (25th percentile‐75th percentile).
Available for 14 L‐HDI and 14 MH‐HDI countries only.
History, physical examination, and minimal basic laboratory studies done.
TB screen may include physical examination and chest radiograph (interferon γ release assay usually not available).
Estimated cost for MTX 10‐15 mg/week in US dollars.
Main categories reported.
Between 2015 and 2019, two countries (Cameroon and Zimbabwe) changed from an L‐HDI to an MH‐HDI category. Results were similar when the 2015 HDI definitions were applied (Supplementary Tables 2 and 3).
Thematic analysis of interviews
The experiences reported by the participants were grouped into the following three main categories: 1) barriers to the use of MTX, including drug availability, financial constraints, and patient hesitancy; 2) human resources; and 3) patterns of MTX use, including comorbidity screening, dosing and monitoring, and family planning. Representative quotes are presented below and in Supplementary Table 4.
Barriers to MTX use
Availability of MTX
The most significant barrier to the use of MTX for patients with rheumatic diseases was the lack of consistent access to the drug (for oral MTX, 66% of all countries; 87% L‐HDI versus 43% HM‐HDI) (Table 2). Country‐wide shortages occurred often, and, when supplies were limited, oncology patients received priority in some countries. In L‐HDI countries, the availability of oral MTX outside of large centers was particularly problematic and was believed to be related to limited prescribing; thus, pharmacies did not maintain stock. In MH‐HDI countries, supplies were generally more available when pharmacies were reminded to maintain stocks. Essentially, all countries reported limited availability of parenteral MTX for rheumatic disease, although this was primarily for rural locations in MH‐HDI countries. Some physicians raised concerns regarding drug quality, particularly during times of country‐wide stock limitations. These concerns primarily referred to the source and composition of the imported drug. As the use of MTX for rheumatic diseases increased, drug availability seemed to improve for some larger centers in MH‐HDI countries. An L‐HDI physician said “you feel like [methotrexate] can run out of the store at any point in time…[lack of drug] is true for any drug in this country…For rheumatic diseases…inconsistent availability of drugs is a big big problem.” One physician also said “methotrexate is one of the three main drugs used for cancer…So when the country goes through some financial hardship…the cancer patients will buy all what is available and the rheumatic patients will not have.”
Financial restrictions
Financial restrictions further compounded limitations associated with drug access and monitoring, sometimes affecting drug prescribing. One L‐HDI physician stated “sometimes we just give [patients] hydroxychloroquine and prednisone, which is cheaper.” Although many countries had infrastructure in place to provide partial coverage for drug costs, the costs were often prohibitive for those not covered. For some patients from rural areas who were eligible to receive drugs without payment, the cost of traveling to dispensing sites was unaffordable. Similarly, costs of laboratory and imaging tests for screening and monitoring, when the responsibility of the patient, were often not affordable. As a result, although all physicians monitored for potential MTX toxicity, many indicated that it was not feasible to follow existing recommendations for comorbidity screening and laboratory monitoring. The scope and frequency of tests were often reduced to decrease costs. Although many physicians raised these concerns, financial barriers appeared most significant in L‐HDI countries. The disparity between public and private health care systems was also raised with private medicine being “well equipped” and public medicine being in a “severe case of disrepair with unbelievable shortages [of drugs, laboratory resources, and staff].” An L‐HDI physician said “like all other chronic diseases we face, there are a lot of follow‐up problems, because as long as the population still remains poor…we have a good number of patients who fail to follow the treatment properly. Not because they do not want to, but maybe because they do not have the means.” An MH‐HDI physician said “[in my country about] 50% of people have health insurance but even when you have health insurance…you have to buy the medicine first and then you are reimbursed.”
Patient hesitancy
Identified patient factors impacting MTX use, particularly in L‐HDI countries, included misconceptions about the role of MTX for rheumatic disease, stigma relating to chronic disease and deforming arthritis, and cultural preferences for traditional healers and medicine (Table 2). Because MTX is primarily used (and prioritized) for oncology patients, dispensing pharmacists were often unfamiliar with the role of MTX in rheumatic disease, contributing to misinformation and medication nonadherence. Reinforcement of the rationale for MTX use was often required. In some regions, cultural influences contributing to stigma associated with chronic disease, in particular with deformities and resultant functional impairment, were thought to affect health‐seeking behavior. In some social groups, the concept of “chronic” disease was not culturally accepted, leading to inadequate patient follow‐up, treatment nonadherence, and alternative “immediate” solution seeking. Preferences for traditional healing and healers, combined with misattribution of chronic (deforming) disease to religious or mystic forces, were perceived to lead to delayed presentation to “Western medicine” clinics or nonadherence to prescribed medication (reported by 71%). Societal expectations regarding family roles also impacted the acceptance of MTX, particularly for young women reluctant to accept contraception while on MTX. According to an L‐DH physician,
“[the] number one challenge I have is trying to get the patient to understand the use of this drug…when the patients take their prescription to pharmacists outside the hospital, [the pharmacists tell them] ‘do you have cancer? Why the doctors prescribed this drug to you?’…Because of the information they get especially from pharmacists outside of hospital, a lot of them might not want to comply initially with the medication, because they may feel it is the wrong medication.”
Another L‐HDI physician said the following:
“…our African cultures, almost do not believe in chronic diseases. An illness must have treatment. When you tell the patients that their disease has no treatment, that they have to keep being followed…if the patient does not feel better, very soon he will look for other medicines, traditional medicines, or he will see the pastor … to find a solution quickly.”
An L‐HDI physician also said that
“[there is stigma] when you have deformities like rheumatoid arthritis, there are people who have lost their jobs, there are women who have lost their marriages, there are those who have been dismissed from their society, they predict that maybe it is a witchcraft…he can no longer use his hands…”
An MH‐HDI physician reported that
“…a lot of patients consider it is a spiritual condition…and they need to seek spiritual interventions…”
Human resources
The shortage of rheumatologists and inadequate education and training of other medical practitioners on caring for patients with rheumatic diseases was a significant barrier in many L‐HDI countries. In these countries, patients with rheumatic diseases are managed by general internists, other specialists, or family practitioners. Lack of formal education and exposure to rheumatology during medical school were cited as factors leading to delayed recognition and referral of patients with rheumatic diseases by primary care physicians. In cases in which MTX treatment was initiated by primary care physicians, referring internists, or orthopedic surgeons, lack of confidence with prescribing and monitoring MTX were considered factors implicated in overconservative dosing, leading to inadequate disease control. As a result, when seen by rheumatology providers, most patients, particularly those from rural areas, had advanced deforming disease.
An L‐HDI physician said “the biggest problem is human capital. There are very, very few [physicians] who are comfortable prescribing or initiating methotrexate.” An MH‐HDI physician said “I have seen too many patients…who have been substandardly treated.”
Several interviewees from L‐HDI countries commented on the need to improve rheumatology education. Establishing rheumatology training during medical school, implementing a rheumatology curriculum for residents in training, and supporting physicians interested in obtaining formal rheumatology subspecialty training were considered ways to enhance rheumatology capacity and improve patient care. The need for improved rheumatology education for allied health care professionals, including nurses and pharmacists, was also identified. In several countries, physicians have identified an interest in pursuing rheumatology subspecialty training; such training is currently provided only in a few African countries. Participating in personal continuing medical education activities, educating local faculty, and maintaining networks with rheumatology colleagues across Africa were mentioned as strategies to increase rheumatology awareness.
Patterns of MTX use
Comorbidity screening
Interviewees were asked about the impact of infections on MTX prescribing. Tuberculosis (TB) was widely endemic. There was regional variation in the prevalence of hepatitis B and C, human immunodeficiency virus (HIV), and, in some countries, parasitic infections. TB screening was generally limited to clinical assessment; it was believed that high exposure rates made Mantoux testing unreliable, and serology testing was not widely available. TB reactivation was raised as a concern with MTX use. Practice patterns in the setting of TB varied from withholding MTX to starting MTX during or after completing TB treatment. In most settings where HIV was endemic or highly prevalent, patients were screened prior to referral or HIV testing was done as part of the rheumatology workup. MTX prescription was generally deferred until HIV treatment and viral suppression were achieved. Viral hepatitis prevalence was considered higher in most countries compared with Western or European countries, though the importance of hepatitis B versus hepatitis C varied and influenced screening practices. In general, MTX was held unless viral hepatitis was inactive or treated (Supplementary Table 5). Although common, malaria was not considered to be a barrier to MTX use, as it is easily treated. Practice patterns related to endemic infections were affected by regional infection prevalence more than HDI status. Similarly, the importance of alcohol use and related counselling varied depending on local cultural and religious factors.
One L‐HDI physician said “we are also overwhelmed by infectious conditions so there is a lot of emphasis on dealing with infectious conditions.” An MH‐HDI physician said “we have different diseases and different concerns compared to the West especially regarding infectious diseases…it is mainly chronic infectious diseases like hepatitis, because [in my country] there is much more hepatitis B and C than in the Western world…We also have tuberculosis which is very high compared to in Europe.”
MTX dosing and monitoring
Practice patterns of dosing MTX varied by prescribers but were generally similar between L‐HDI and MH‐HDI countries, as was the use of folate. Patient intolerance was cited as a common issue, and, at least according to some prescribers, acceptable disease control could be maintained with relatively low doses. Several doctors believed that an inability to obtain requested tests also contributed to lower MTX doses being prescribed, patient nonadherence, and patient noncompliance with follow‐up clinic visits.
An L‐HDI physician said “It is the minimal checkup that we ask for, but sometimes we are faced with difficulties where the patient may not be able to [pay for tests] …This is due to poverty and also to the fact that there is no system of health insurance, [patients] can no longer come back because of lack of means, lack of medicines, lack of money and everything.” An MH‐HDI physician said “I do not follow thoroughly the Western recommendations [for screening and monitoring]…I am adopting a somewhat lightened outline.”
Family planning
Pre‐MTX pregnancy screening was usually by clinical history alone in both L‐HDI and MH‐HDI countries. Most physicians counselled contraception; some also referred to family clinics or gynecologists. Cultural and religious considerations related to family planning complicated discussions and, in some regions, contraception was not formally prescribed, and women were told to stop MTX at the earliest sign of pregnancy. Approaches to family planning were similar for L‐HDI and MH‐HDI countries. An L‐HDI physician in a Muslim country said “this is a sociocultural problem…contraception is somewhat not well seen by women and men of childbearing age.”
Need for local adaptation of MTX prescribing recommendations
Finally, we asked about the perceived need for regional MTX prescribing recommendations and important issues those should address. There was a general consensus, particularly among physicians in L‐HDI countries, that regional recommendations for MTX use in rheumatic disease were needed. Recommendations need to address the use of MTX in the context of infections, including TB, hepatitis B and C, HIV, and other endemic infections. Recommendations about the scope and frequency of investigations for monitoring MTX safety are critical and need to incorporate local realities relating to restricted testing capacity and cost. Recommendations should also address differential diagnoses given the high prevalence in Africa of conditions not usually found in Western countries, such as infectious diseases with rheumatic manifestations and nutritional deficits. Although rheumatologists felt that MTX use is ideally directed by rheumatology, most interviewees felt that adapted recommendations should also target internists, who provide the majority of rheumatology care in some countries, and primary care providers and orthopedists to facilitate referral and follow‐up care.
DISCUSSION
African clinicians prescribing MTX for systemic rheumatic diseases were faced with a range of unique challenges. Critical barriers include limited numbers of trained rheumatologists, inconsistent access to oral or parenteral MTX for rheumatology patients, uncertainty regarding best approaches for assessing comorbidities and endemic infections, and patient‐related sociocultural influences, combined with low medical literacy. These barriers are compounded by severe financial limitations. Prescribers highlighted that existing international guidelines are often not applicable, as they do not address the unique concerns faced by African clinicians.
There remains a shortage of rheumatologists in Africa, especially in L‐HDI countries (11, 23). Some countries have few or no certified practicing rheumatologists. As a result, nonrheumatologists with variable expertise in rheumatology assume responsibility for rheumatology care in many countries. Strategies to increase rheumatology capacity are being explored; however, rheumatology training programs are limited to a few African countries, and thus potential trainees incur additional expenses that may be prohibitive. Enhanced rheumatology education during medical training, combined with innovative and regionally appropriate educational opportunities for practicing physicians, both nonrheumatology specialists and primary care providers, can bridge this rheumatology clinician shortage (23, 24, 25, 26, 27, 28).
Reliable access to MTX was identified as one of the biggest challenges to providing rheumatology care. Most countries did not have consistent access to MTX for patients with rheumatic diseases. In some regions, even other conventional disease‐modifying antirheumatic drugs were not widely available. Limited drug availability and poverty likely contribute to high rates of corticosteroid and nonsteroidal anti‐inflammatory use even though steroids pose a greater risk of side effects (17, 29, 30). Demand for MTX in Africa will likely increase with greater awareness of chronic rheumatic diseases, optimization of their treatment approaches, and growing number of specialty‐trained rheumatologists (31). Solutions to address MTX access will vary by region and will likely require multifaceted approaches engaging national and regional governments to stabilize supply pathways and enhance safe drug dispensing capacity. There is a global responsibility to advocate for the availability of key drugs used in noncommunicable diseases such as RA; such advocacy could be modeled on similar initiatives directed towards HIV or tuberculosis.
Additional challenges relate to the use of MTX in the setting of endemic infections and safety‐monitoring restrictions. Despite regional variation in the prevalence of those infections, there was a general consensus that existing guidelines on MTX prescribing do not adequately contemplate these issues. This uncertainty is compounded by a lack of local data on some of these aspects. Many guidelines recommend screening for HIV and avoiding MTX in the setting of active hepatitis B or C and untreated or latent TB (6, 32). However, there is no consensus regarding the duration of TB treatment needed prior to starting MTX, the risk of isoniazid hepatitis, or the use of MTX in the setting of treated HIV. Financial and logistical limitations affecting patients and health systems contributed to difficulty following existing recommendations for monitoring. Innovative screening and monitoring strategies, potentially incorporating point‐of‐care testing, may be one option if feasible and economically viable (33).
Patient adherence is critical for medication effectiveness (3). Many issues such as intolerance, though possibly compounded by limited access to parenteral MTX and monitoring challenges, were similar to those experienced by MTX users globally. Other sociocultural considerations, notably societal roles related to family planning and cultural beliefs regarding traditional healing, were also felt to impact patient health‐seeking behavior and acceptance of MTX. These concerns must be considered when developing and implementing regional recommendations for prescribing MTX. Patient education regarding the role of MTX in the management of rheumatic diseases is needed, and patient engagement during the development of recommendations is critical.
Strengths of this study include the broad regional representation of MTX prescribers based on geography and economic development. Participants were identified using multiple strategies, and our interview sample encompassed physicians from low, medium, and high HDI countries across the African continent; however, not all regions were represented, and only the viewpoints of a sample of physicians prescribing MTX were captured. For regions with many rheumatologists, the viewpoints expressed may not represent the breadth of local experiences. Having only one provider from each country limits our ability to understand variability by country; nevertheless, item saturation was reached when pooling providers according to HDI. Regions with severe rheumatology capacity limitations may not have been included; such regions may have unique regional infrastructure challenges or competing health priorities. Generalists, orthopedic surgeons, and primary care providers are critical for the identification, referral, and, in many cases, local follow‐up of rheumatology patients and likely have unique perceptions, educational needs, and comfort levels regarding managing patients receiving MTX. Similarly, allied health workers, including nursing and pharmacy workers, play key roles in patient education and MTX prescribing (15). Further work is required to determine the needs of these providers. Finally, we chose to stratify countries on the basis of the 2019 HDI, thereby providing a broader reflection of each country’s current development than economic indices alone with contemporary HDI classifications. However, the HDI does not incorporate the full breadth of factors affecting human development potential or disparities within a region, which may be extreme, and interviews were conducted in 2016 and 2017. Two L‐HDI countries converted to medium HDI between the 2015 and 2019 HDI reports, but this did not change the study findings.
Because of the unique barriers for patients and providers in African countries, there is a clear need for regional guidelines addressing safe prescribing of MTX for rheumatic diseases (34). Any adaptation of existing prescribing guidelines should include recommendations for MTX use in the context of common endemic infections and provide data‐driven but feasible, cost‐conscious recommendations for monitoring safety. Locally derived data are needed. Advocating for improved access to fundamental rheumatic disease medications, including MTX, should be both a regional and an international priority. Improved access to rheumatologists is critical and, given existing severe shortages, should be complimented by increasing educational training to generalists, orthopedic surgeons, and primary care and allied health providers and improving patient education. Organizations such as the International League of Associations for Rheumatology, which includes the American College of Rheumatology, have service to the global rheumatology community as one of their missions and could provide leadership for such initiatives (35, 36). Although this study focused on the experiences of African clinicians, these findings are applicable to resource‐limited regions worldwide, even to rural communities in better resourced countries (37, 38, 39), and are increasingly relevant to rheumatologists as international travel and globalization expand. Initiatives addressing identified concerns can contribute to reducing health disparities in rheumatology care all over the world.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Hitchon had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Hitchon, Mody, Feldman, Meltzer, Scuccimarri, Weinblatt, Colmegna.
Acquisition of data
Lau, Mody, Shi, Colmegna.
Analysis and interpretation of data
Hitchon, Feldman, Colmegna.
Supporting information
Table S1‐S5
ACKNOWLEDGMENTS
The authors thank the participants for providing their time and valuable insights into their experiences. They also thank Dr. Yewondwossen Tadese for critical review of the manuscript.
Supported by the International League of Associations for Rheumatology. Dr. Feldman research support from Pfizer and Office of Minority Health; NIH NIAMS K23‐AR‐071500.
Dr. Hitchon has received research funds from Health Sciences Centre Foundation, Research Manitoba, Pfizer Canada, and UCB Canada. Dr. Mody has received honoraria from Astra Zeneca (South Africa) and GlaxoSmithKline. Dr. Feldman receives research support from Pfizer and currently serves on the Board of Directors of the American College of Rheumatology and on the Medical‐Scientific Advisory Council of the Lupus Foundation of America. Dr. Weinblatt has received research funding from Amgen, Bristol Myers Squibb, Crescendo Bioscience, Eli Lilly, and Sanofi; he has consulted with Abbvie, Amgen, Arena, Bristol Myers Squibb, Corrona, Crescendo, Glaxo Smith Kline, Gilead, Horizon, Johnson and Johnson, Eli Lilly, Pfizer, Roche, Scipher, Set Point, Tremeau, and EqRx, has stock options in Canfite, Scipher, Inmedix, and Vorso, and received royalties as co‐editor of the textbook Rheumatology. No other disclosures relevant to this article were reported.
REFERENCES
- 1.Cronstein BN, Aune TM. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat Rev Rheumatol 2020;16:145–54. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem 2018;158:502–16. [DOI] [PubMed] [Google Scholar]
- 3.Curtis JR, Bykerk VP, Aassi M, Schiff M. Adherence and persistence with methotrexate in rheumatoid arthritis: a systematic review. J Rheumatol 2016;43:1997–2009. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi G, Caporali R, Todoerti M, Mattana P. Methotrexate and rheumatoid arthritis: current evidence regarding subcutaneous versus oral routes of administration. Adv Ther 2016;33:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedoui Y, Guillot X, Sélambarom J, Guiraud P, Giry C, Jaffar‐Bandjee MC, et al. Methotrexate an old drug with new tricks. Int J Mol Sci 2019;20:5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valerio V, Kwok M, Loewen H, Winkler J, Mody GM, Scuccimarri R, et al. Systematic review of recommendations on the use of methotrexate in rheumatoid arthritis. Clin Rheumatol 2021;40:1259–71. [DOI] [PubMed] [Google Scholar]
- 7.Misra DP, Sharma A, Agarwal V. Rheumatology science and practice in India. Rheumatol Int 2018;38:1587–600. [DOI] [PubMed] [Google Scholar]
- 8.United Nations Development Programme . Human Development Report 2019: beyond income, beyond averages, beyond today: inequalities in human development in the 21st century. URL: http://www.hdr.undp.org/en/2019‐report.
- 9.United Nations . LDC Identification Criteria & Indicators. URL: www.un.org/development/desa/dpad/least‐developed‐country‐category/ldc‐criteria.html.
- 10.United Nations Development Programme . Human development reports. URL: http://hdr.undp.org/en/humandev.
- 11.Mody GM. Reflections on rheumatoid arthritis in selected sub‐Sahara African countries. East Afr Med J 2009;86:201–3. [PubMed] [Google Scholar]
- 12.Al Maini M, Adelowo F, al Saleh J, al Weshahi Y, Burmester GR, Cutolo M, et al. The global challenges and opportunities in the practice of rheumatology: white paper by the World Forum on Rheumatic and Musculoskeletal Diseases. Clin Rheumatol 2015;34:819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar B. Global health inequities in rheumatology. Rheumatology 2017;56:4–5. [DOI] [PubMed] [Google Scholar]
- 14.Adebajo A, Gabriel SE. Addressing musculoskeletal health inequity in Africa. Arthritis Care Res 2010;62:439–41. [DOI] [PubMed] [Google Scholar]
- 15.Hitchon CA, de Jong Y , Melkie A, Meltzer M, Scuccimarri R, Tadese Y, et al. Barriers to the use of methotrexate in Ethiopia for rheumatic disease: insights from pharmacy providers. African Journal of Rheumatology 2018;6:50–5. [Google Scholar]
- 16.Malemba JJ, Mbuyi Muamba JM, Mukaya J, Bossuyt X, Verschueren P, Westhovens R. Treatment of rheumatoid arthritis with methotrexate in Congolese patients. Clin Rheumatol 2013;32:1323–7. [DOI] [PubMed] [Google Scholar]
- 17.Elshafie AI, Elkhalifa AD, Elbagir S, Aledrissy MI, Elagib EM, Nur MA, et al. Active rheumatoid arthritis in central Africa: a comparative study between Sudan and Sweden. J Rheumatol 2016;43:1777–86. [DOI] [PubMed] [Google Scholar]
- 18.Ghazi MKA, Elrharras S, Niamane R. Procedures on rheumatoid arthritis management by rheumatologists in Morocco. Scholars Journal of Applied Medical Sciences 2018;6:1172–6. [Google Scholar]
- 19.Hodkinson B, Musenge E, Ally M, Meyer PW, Anderson R, Tikly M. Response to traditional disease‐modifying anti‐rheumatic drugs in indigent South Africans with early rheumatoid arthritis. Clin Rheumatol 2012;31:613–9. [DOI] [PubMed] [Google Scholar]
- 20.Hodkinson B, van Duuren E , Pettipher C, Kalla A. South African recommendations for the management of rheumatoid arthritis: an algorithm for the standard of care in 2013. S Afr Med J 2013;103:576–85. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . The Global Health Observatory. URL: www.who.int/data/gho/data/indicators/indicator‐details/GHO/population‐(in‐thousands).
- 22.Patton M. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage Publications; 2002. [Google Scholar]
- 23.Jawad AS. The role of the Royal College of Physicians in promoting rheumatology in low and middle‐income countries. Rheumatology 2020;59:3982–3. [DOI] [PubMed] [Google Scholar]
- 24.Hitchon C. Building rheumatology in East Africa. Canadian Rheumatology Association Journal 2018;28:9. [Google Scholar]
- 25.Sandhu VK, Hojjati M, Blanco I. Healthcare disparities in rheumatology: the role of education at a global level. Clin Rheumatol 2020;39:659–66. [DOI] [PubMed] [Google Scholar]
- 26.Erwin J, Woolf A, Oyoo O, Cederlund I, Mwaniki L, Etau P. The UWEZO project‐musculoskeletal health training in Kenya. Clin Rheumatol 2016;35:433–40. [DOI] [PubMed] [Google Scholar]
- 27.Oyoo GO, Espinoza LR. The practice of rheumatology in Kenya: challenges and opportunities. ILAR visiting professorship program report. Clin Rheumatol 2006;25:437–9. [DOI] [PubMed] [Google Scholar]
- 28.Colmegna I, Bartlett SJ, Oyoo OG. The ILAR‐East Africa initiative: current needs and progress in the globalization of rheumatology. Clin Rheumatol 2011;30:251–3. [DOI] [PubMed] [Google Scholar]
- 29.Lutf A, Poil AR, Hammoudeh M. Characteristics of patients with rheumatoid arthritis in Qatar: a cross‐sectional study. Int J Rheum Dis 2014;17:63–5. [DOI] [PubMed] [Google Scholar]
- 30.Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population‐based study. Arthritis Rheum 2002;46:2287–93. [DOI] [PubMed] [Google Scholar]
- 31.Adelowo O, Mody GM, Tikly M, Oyoo O, Slimani S. Rheumatic diseases in Africa. Nat Rev Rheumatol 2021;17:363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease‐modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59:762–84. [DOI] [PubMed] [Google Scholar]
- 33.Drain PK, Hyle EP, Noubary F, Freedberg KA, Wilson D, Bishai WR, et al. Diagnostic point‐of‐care tests in resource‐limited settings. Lancet Infect Dis 2014;14:239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elmamoun M, Eraso M, Anderson M, Maharaj A, Coates L, Chandran V, et al. International league of associations for rheumatology recommendations for the management of psoriatic arthritis in resource‐poor settings. Clin Rheumatol 2020;39:1839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American College of Rheumatology . URL: www.rheumatology.org/.
- 36.International League of Associations for Rheumatology . URL: http://www.ilar.org.
- 37.Deal CL. The regional distribution of rheumatologists: what can we do, what should we do? [Editorial]. Arthritis Rheum 2013;65:3011–3. [DOI] [PubMed] [Google Scholar]
- 38.Fernández‐Ávila DG, Patino‐Hernandez D, Kowalskii S, Vargas‐Caselles A, Sapag AM, Cachafeiro‐Vilar A, et al. Current status of the rheumatologists' workforce in Latin America: a PANLAR collaborative study. Clin Rheumatol 2021. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Aggarwal A, Haq SA. Rheumatology workforce issues in South Asia: challenges and solutions. Int J Rheum diseases 2020;23:443–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S5


