Key Points
Question
What antiviral agents for treating seasonal influenza are associated with the most safety and best outcomes among healthy adults and children?
Findings
This network meta-analysis of 26 randomized clinical trials, including 11 897 patients, found that antiviral agents were associated with significantly greater efficacy than placebo in shortening the duration of influenza symptoms; zanamivir was associated with the shortest time to alleviation of influenza symptoms. Baloxavir was associated with a lower occurrence of influenza-related complications than other treatments.
Meaning
These findings suggest that zanamivir may be initiated as soon as possible for patients with influenza-like illness; in those who may be at high risk of developing influenza-related complications, baloxavir should be considered.
This systematic review and network meta-analysis assesses neuraminidase inhibitors and an endonuclease inhibitor for safety and efficacy against seasonal influenza in children and adults.
Abstract
Importance
Antiviral treatment of influenza is recommended for patients with influenza-like illness during periods of community cocirculation of influenza viruses and SARS-CoV-2; however, questions remain about which treatment is associated with the best outcomes and fewest adverse events.
Objective
To compare the efficacy and safety of neuraminidase inhibitors and the endonuclease inhibitor for the treatment of seasonal influenza among healthy adults and children.
Data Sources
Medline, Embase, and the Cochrane Register of Clinical Trials were searched from inception to January 2020 (the last search was updated in October 2020).
Study Selection
Included studies were randomized clinical trials conducted among patients of all ages with influenza treated with neuraminidase inhibitors (ie, oseltamivir, peramivir, zanamivir, or laninamivir) or an endonuclease inhibitor (ie, baloxavir) compared with other active agents or placebo.
Data Extraction and Synthesis
Two investigators identified studies and independently abstracted data. Frequentist network meta-analyses were performed; relative ranking of agents was conducted using P-score probabilities. Quality of evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluations criteria. Data were analyzed in October 2020.
Main Outcomes and Measures
The time to alleviation of influenza symptoms (TTAS), complications of influenza, and adverse events (total adverse events, nausea, and vomiting).
Results
A total of 26 trials were identified that investigated antiviral drugs at high or low doses; these trials included 11 897 participants, among whom 6294 (52.9%) were men and the mean (SD) age was 32.5 (16.9) years. Of all treatments comparing with placebo in efficacy outcomes, high-quality evidence indicated that zanamivir was associated with the shortest TTAS (hazard ratio, 0.67; 95% CI, 0.58-0.77), while baloxavir was associated with the lowest risk of influenza-related complications (risk ratio [RR], 0.51; 95% CI, 0.32-0.80) based on moderate-quality evidence. In safety outcomes, baloxavir was associated with the lowest risk of total adverse events (RR, 0.84; 95% CI, 0.74-0.96) compared with placebo based on moderate-quality evidence. There was no strong evidence of associations with risk of nausea or vomiting among all comparisons, except for 75 mg oseltamivir, which was associated with greater occurrence of nausea (RR, 1.82; 95% CI, 1.38-2.41) and vomiting (RR, 1.88; 95% CI, 1.47-2.41).
Conclusions and Relevance
In this systematic review and network meta-analysis, all 4 antiviral agents assessed were associated with shortening TTAS; zanamivir was associated with the shortest TTAS, and baloxavir was associated with reduced rate of influenza-related complications.
Introduction
Seasonal influenza is a serious global health threat of high prevalence that affects all countries. During the SARS-CoV-2 pandemic, coinfection with influenza A or B viruses and SARS-CoV-2 can occur and should be considered. Influenza and COVID-19 have overlapping signs and symptoms.1 Studies have found that patients with COVID-19 who were coinfected with influenza shed SARS-CoV-2 longer than other patients with only COVID-19.2 Therefore, empirical antiviral treatment is recommended as soon as possible, particularly for hospitalized patients with severe respiratory disease, outpatients with influenza-like illness, and patients with higher risk for influenza complications.1
Antiviral medications can help to shorten the duration of illness and prevent serious complications. Neuraminidase inhibitors selectively block an enzyme conserved within all influenza viruses to inhibit virus particles from exiting host cells and thus interrupt person-to-person viral spread. Neuraminidase inhibitors also shorten the duration of symptoms, likely by decreasing the viral load, preventing the spread and release of cytokines, and reducing the risk of complications.3 Another novel oral antiviral drug, baloxavir marboxil (hereafter, baloxavir), is a selective inhibitor of influenza cap-dependent endonuclease, and blocks proliferation of the influenza virus by inhibiting the initiation of mRNA synthesis.4
The Centers for Disease Control and Prevention has recommended 4 Food and Drug Administration–approved antiviral drugs for the treatment of influenza: oseltamivir, baloxavir (oral), zanamivir (inhaled), and peramivir (intravenous).5,6 When indicated, treatment should be initiated as soon as possible within the first 48 hours of symptoms developing. The adverse effects of each medication vary but generally are gastrointestinal. The most common adverse effects of oseltamivir are nausea and vomiting; zanamivir may cause bronchospasm; and peramivir may cause diarrhea.6 However, owing to a lack of high-quality evidence, questions about which treatment is associated with the best outcomes and fewest adverse events remain. In this study, we aimed to compare the efficacy and safety outcomes associated with different antiviral agents for adults and children with seasonal influenza.
Methods
This systematic review and network meta-analysis was performed in accordance with the PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions (PRISMA-NMA). The study protocol was published on the PROSPERO international prospective register of systematic reviews (CRD42020186433).
Eligibility Criteria
We included all published randomized clinical trials that compared the use of neuraminidase inhibitors (ie, oseltamivir, zanamivir, peramivir, and laninamivir) or endonuclease inhibitor (ie, baloxavir) with each other or with a placebo for the treatment of seasonal influenza. Studies assessing previously healthy people of all ages (children and adults) were included. Influenza-like illness was defined as a fever (≥38.0 °C), and at least 2 of the following 4 symptoms: headache, sore throat, myalgia, and cough, with a duration of symptoms of 48 hours or less.
Outcomes and Summary Measures
The primary efficacy outcome was time to symptom alleviation (TTAS), defined as time from the start of treatment to patient-reported improvement in all influenza-associated symptoms and measured using hazard ratios (HRs). When expressing the effect size of an intervention for time-to-event data, HRs were the most appropriate. Using median or mean time-to-event for continuous outcomes may introduce bias, given that censored participants must be excluded.7 Many clinical trials only report treatment effects as median or mean values, which were insufficient for our analysis. We used DigitizeIt software version 2.2 (DigitizeIt) to reconstruct the Kaplan-Meier curves and obtain the HRs and 95% CIs.8 The reliability and validity of the data obtained using this tool were confirmed by comparing the results with those of other data extraction programs.9 Most studies reported Kaplan-Meier curves in laboratory-confirmed population. We made the decision about using the intention-to-treat-influenza-infected (ITTI) population to enable a more comprehensive comparison of the effectiveness of all included antiviral agents.
The secondary efficacy outcome was complications of influenza, also based on the ITTI population. Complications of particular interest included pneumonia, bronchitis, otitis media, sinusitis, and other secondary illness, whether treated with antibiotics or not.
The safety outcomes were total adverse events, nausea, and vomiting targeting the safety population. We chose nausea and vomiting as our safety end points because they were common, especially in young adults, and studies also showed that nausea and vomiting were associated with discontinuation of study drugs.10,11 Both outcomes of complications and adverse events were binary, reported as risk ratios (RRs) and 95% CIs. These outcomes were defined according to the authors of each study and were not exactly the same in all trials (eTable 1 in the Supplement).10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42
Data Sources and Extraction
Comprehensive searches were conducted of Medline, Embase, and the Cochrane Register of Clinical Trials from inception until January 2020 (eAppendix 1 in the Supplement). We updated the last search in October 2020. The literature retrieval was independently carried out by 2 of us (J.-W.L. and S.-H.L.). There were no language restrictions. ClinicalTrials.gov was used to search clinical trial registers. We did not search for unpublished studies or contact experts in the field.
Two of us (J.-W.L. and S.-H.L.) independently extracted data from included trials, including published year, participants (mean age, inclusion criteria, influenza test results, and illness severity), interventions, results (outcome measures, TTAS, complications, total adverse events, nausea, and vomiting), and study design. Disagreements in extracted data were resolved by discussion.
Risk of Bias and Quality of Evidence Assessment
We summarized the potential biases for each individual study using the Cochrane risk of bias tool.43 We also presented information related to potential issues of clinical heterogeneity for each study. For the network meta-analysis, the quality of direct and network evidence was graded using Grading of Recommendations Assessment, Development, and Evaluation.44,45,46 For each comparison, 2 of us (J.-W.L. and S.-H.L.) independently rated the quality of direct and network evidence for each outcome; discrepancies were resolved by consensus, and if necessary, with arbitration by a third author (J.-A. L.).
Statistical Analysis
Network meta-analyses were fitted to a frequentist framework using a multivariate random-effects network meta-analyses model with the statistical package netmeta in R statistical software version 4.0.2 (R Project for Statistical Computing).47 For sparse binary outcome data (individual trials with zero events in either group), a continuity correction of 0.5 was added to each cell for each effect measure.7,48 We also pooled odds ratio outcomes using the Mantel-Haenszel method, which offers a reliable approach to assess binary outcomes, especially in the case of sparse data.49
We produced network plots for each outcome to visualize network geometry and node connectivity.50 We estimated the ranking probabilities of the different antiviral agents based on their P-scores. The P-score is measured on a scale from 0 (worst) to 1 (best), with a higher score indicating better overall performance of the competing treatment. The numerical P-score values are nearly identical to the surface under the cumulative ranking curve.51 It is also important to consider the relative risk and corresponding 95% CI for each comparison when interpreting the ranking results.52
We explored the transitivity assumption by assessing whether the distribution of patients and study characteristics, which could potentially modify the treatment effects across treatment comparisons, were significantly different.7 We assessed network heterogeneity across all treatment contrasts using τ2 and I2 statistics. We applied the Q statistic to test for global inconsistency using a design-by-treatment interaction random effects model.53 We evaluated local inconsistency via a node split method by splitting the network estimates into direct and indirect evidence using a back-calculation method.54,55,56 P values were 2-sided, and P values less than .05 were considered statistically significant.
We performed sensitivity analyses for the network meta-analysis outcomes of TTAS, nausea, and vomiting (self-report outcomes) to assess the robustness of the model by excluding trials with a high risk of bias in all domains overall and in the domain of blinding. We explored the potential for publication bias by visual inspection of the comparison-adjusted funnel plots and Egger test.7,46,57 We hypothesized that studies that find a new form of treatment is superior to an existing treatment may have a higher chance of getting published. Symmetry around the point estimate line in the scatter plot may indicate the absence of publication bias, or small study effects. Data were analyzed in October 2020.
Results
Study Selection and Characteristics
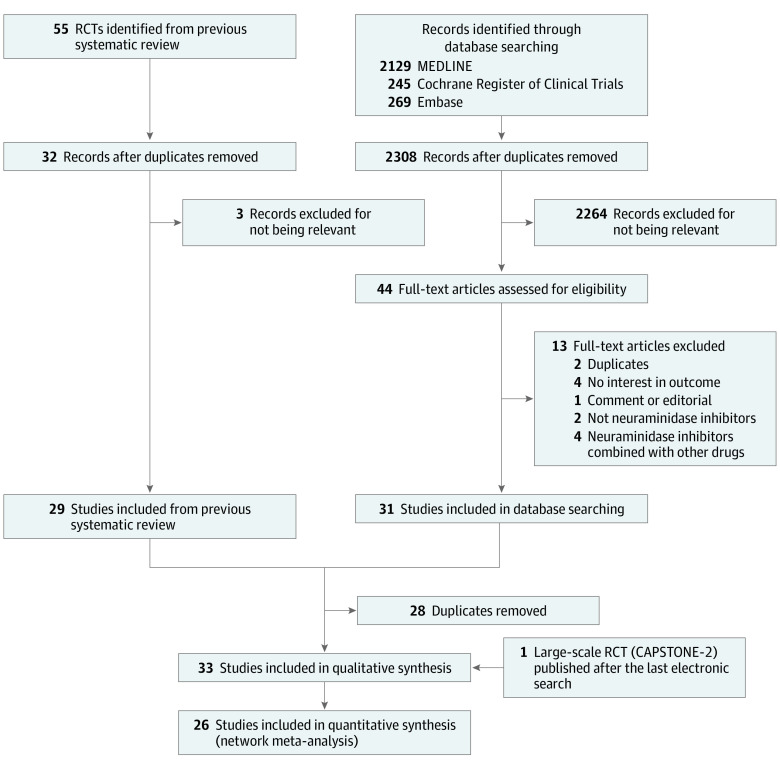
As shown in Figure 1, we identified 33 potentially eligible, completed randomized clinical trials,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42 after excluding 13 studies.58,59,60,61,62,63,64,65,66,67,68,69,70 Two studies (6%) published in Chinese12,13 and 1 study (3%) in Japanese20 were translated and included. A large-scale randomized clinical trial (CAPSTONE-2),11 published after our last electronic search, was also included.
Figure 1. Flowchart of Study Selection.
RCT indicates randomized clinical trial.
Participants included children and adults of all ages, with 12 trials15,21,22,23,24,28,29,31,33,35,37,42 (36%) assessing adult populations (age ≥18 years) and 5 trials17,18,34,38,41 (15%) including only children. Most trials recruited patients attending outpatient clinics (29 trials10,11,12,13,14,15,16,17,18,20,21,22,23,24,25,26,27,28,29,30,32,33,34,35,36,38,40,41,42 [88%]); 4 trials (12%) included hospitalized patients with rapid antigen test (RAT) results positive for influenza.19,31,37,39
We focused on 4 Food and Drug Administration–approved antiviral agents at high or low doses: 3 neuraminidase inhibitors (75 mg or 150 mg oseltamivir twice daily, 10 mg zanamivir twice daily, and 300 mg or 600 mg peramivir once daily or a single dose) and 1 endonuclease inhibitor (single 40 mg or 80 mg dose of baloxavir). Laninamivir was not included in this meta-analysis because no available primary efficacy outcome data were available, and the drug was only investigated in a few Japanese trials.38,41,42 Finally, 26 trials with 11 897 participants were included in the quantitative synthesis (network meta-analysis) (eTable 1 in the Supplement).10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42 Across the included studies, 6294 (52.9%) were men, and the mean (SD) age was 32.5 (16.9) years.
Risk of Bias of Included Studies
The risk of bias judgments for the studies contributing to the analysis of each outcome are presented in eFigure 1 in the Supplement. Open-labeled trials were determined to be at high risk of bias in the domain of blinding. Overall, 2 studies17,68 (6.1%) were judged to have a low risk of bias across all domains.
TTAS
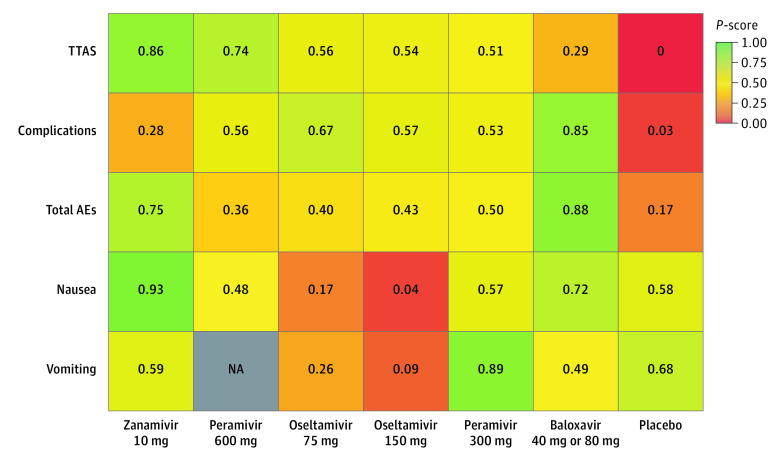
Among the ITTI population, the network of treatment comparisons for TTAS included 7 individual nodes among 15 trials (Figure 2A).10,11,15,17,18,20,21,23,24,25,29,32,33,34,35 No trial reported relevant TTAS data for laninamivir. Placebo was the most well-connected intervention and was directly linked to all other interventions. A total of 5 trials10,11,21,29,32 (33.3%) had 3 eligible groups, and the remaining 10 trials15,17,18,20,23,24,25,33,34,35 (66.7%) had 2 eligible groups. For the comparison of all treatments with placebo, 10 mg zanamivir was associated with the shortest TTAS (HR, 0.67; 95% CI, 0.58-0.77; P-score, 0.86), followed by 600 mg peramivir (HR, 0.69; 95% CI, 0.54-0.88; P-score. 0.74), 75 mg oseltamivir (HR, 0.74; 95% CI, 0.70-0.79; P-score, 0.56), 150 mg oseltamivir (HR, 0.75; 95% CI, 0.65-0.86; P-score, 0.54), 300 mg peramivir (HR, 0.75; 95% CI, 0.62-0.91; P-score, 0.51), and 40 mg or 80 mg baloxavir (HR, 0.79; 95% CI, 0.73-0.86; P-score, 0.29) (Figure 3 and Figure 4).
Figure 2. Network Graphs of Treatment Comparisons for Time to Alleviation of Influenza Symptoms and Complications.
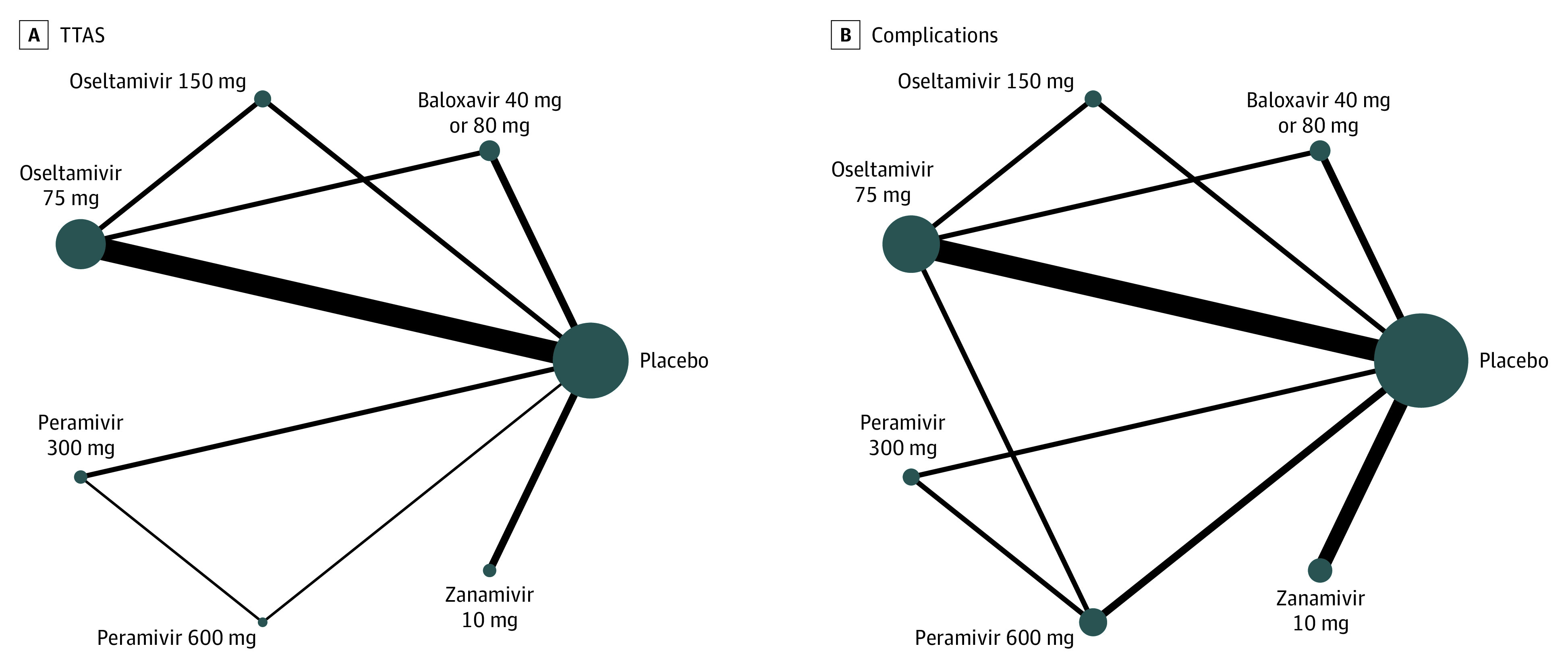
Nodes indicate different active interventions or placebo; size of nodes, number of studies; thickness of lines between nodes, number of randomized participants contributing to direct comparisons.
Figure 3. Network Meta-analysis Estimates of Time to Alleviation of Influenza Symptoms (TTAS) and Complications.
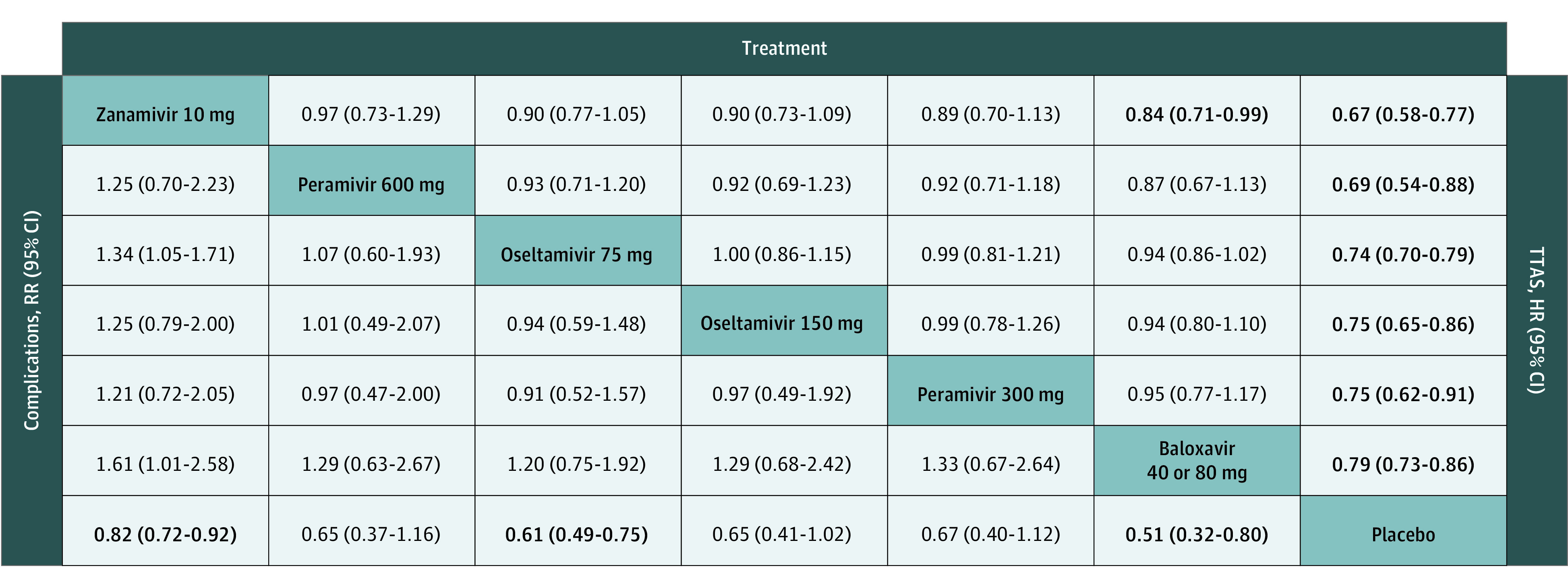
Effect sizes presented on the upper triangle correspond to hazard ratio (HR) (95% CI) of TTAS between the row and columns (eg, the HR of TTAS between zanamivir 10 mg and placebo is 0.97 [0.73-1.29]); On the lower triangle, effect sizes correspond to risk ratio (RR) (95% CI) of complications between the column and the row (eg, the RR [95% CI] of complications between zanamivir 10 mg and placebo is 0.82 [0.72-0.92]).
Figure 4. Heat Map of Antiviral Drugs Ranked According to Time to Alleviation of Influenza Symptoms (TTAS), Complications, Total Adverse Events (AEs), Nausea, and Vomiting.
Numbers reflect P-score, measured on a scale from 0 (worst) to 1 (best). A higher P-score indicates better effectiveness and fewer adverse events. Grey square indicates that data were not available (NA).
In the network meta-analysis, we found no evidence of heterogeneity (τ2 = 0 and I2 = 0%; 95% CI, 0%-50.2%). There was no measurable global inconsistency based on a random effects design-by-treatment model (χ24 = 2.37; P = .67) (eTable 2 in the Supplement) or local inconsistency within the network (eAppendix 2 in the Supplement).
Complications
Among the ITTI population, the network of treatment comparisons for complications reported from 21 trials10,11,12,13,15,17,19,21,22,23,24,25,26,27,28,29,30,32,33,34,35 was constituted of 7 individual nodes (Figure 2B). The placebo was the most well-connected group and was directly linked to all other interventions. In the comparison of all treatments with placebo, 40 mg or 80 mg baloxavir was associated with fewer influenza-related complications (RR, 0.51; 95% CI, 0.32-0.80; P-score, 0.85), followed by 75 mg oseltamivir (RR, 0.61; 95% CI, 0.49-0.75; P-score, 0.67), 150 mg oseltamivir (RR, 0.65; 95% CI, 0.41-1.02; P-score, 0.57), 600 mg peramivir (RR. 0.65; 95% CI, 0.37-1.16; P-score, 0.56), 300 mg peramivir (RR, 0.67; 95% CI, 0.40-1.12; P-score, 0.53), and 10 mg zanamivir (RR, 0.82; 95% CI, 0.72-0.92; P-score, 0.28) (Figure 3 and Figure 4).
No heterogeneity was observed (τ2 = 0 and I2 = 0%; 95% CI, 0%-41.6%). There was no measurable global inconsistency based on a random effects design-by-treatment model (χ29 = 5.05; P = .83) (eTable 2 in the Supplement) or local inconsistency within the network (eAppendix 2 in the Supplement).
Safety Outcomes
We established pooled estimates for total adverse events, nausea, and vomiting among the as-treated populations. The network graphs are presented in eFigure 2 in the Supplement, and the league tables are presented in eFigure 3 in the Supplement. The ranking of the treatment comparisons are shown in a heatmap in Figure 4.
For total adverse events, 21 trials10,11,12,13,14,15,16,17,19,20,21,22,23,24,25,26,28,30,31,33,35 compared 7 different interventions (eFigure 2 in the Supplement). There were almost no differences among the multiple treatment comparisons; only 40 mg or 80 mg baloxavir was associated with significantly fewer adverse events than the placebo (RR, 0.84; 95% CI, 0.74-0.96; P-score, 0.85) (eFigure 3 in the Supplement). Ranking of the risk of any adverse events identified 40 mg or 80 mg baloxavir as the best, indicating fewer adverse events, and peramivir 600 mg as the worst among all antiviral agents (Figure 4). No heterogeneity was observed (τ2 = 0 and I2 = 0%; 95% CI, 0%-42.7%). There was no measurable global inconsistency based on a random effects design-by-treatment model (χ28 = 6.42; P = .60) (eTable 2 in the Supplement) or local inconsistency within the network (eAppendix 2 in the Supplement).
For nausea, 15 trials10,11,14,17,19,20,21,23,24,25,26,28,29,32,35 compared 7 different interventions (eFigure 2 in the Supplement). Ranking identified 10 mg zanamivir as the best and 150 mg oseltamivir as the worst (Figure 4). No heterogeneity was observed (τ2 = 0 and I2 = 0%; 95% CI, 0%-44.6%). There was no measurable global inconsistency based on a random effects design-by-treatment model (χ25 = 7.89; P = .16) (eTable 2 in the Supplement). We only identified 1 contrast of inconsistency, indicating disagreement between the indirect and direct evidence for 150 mg oseltamivir vs placebo (eAppendix 2 in the Supplement). We found that 75 mg oseltamivir was associated with higher occurence of nausea vs placebo (RR, 1.82; 95% CI,1.38-2.41) (eTable 3 in the Supplement). Compared with 75 mg oseltamivir, zanamivir (RR, 0.30; 95% CI, 0.13-0.67) and baloxavir (risk ratio 0.47; 95% CI, 0.30-0.72) were associated with a lower occurrence of nausea. (eFigure 3 in the Supplement).
For vomiting, 9 trials10,14,17,18,20,23,29,32,35 compared 6 different interventions (eFigure 2 in the Supplement). No trial reported relevant data for 600 mg peramivir. In the ranking, 300 mg peramivir was identified as the best and 150 mg oseltamivir as the worst (Figure 4). No heterogeneity was observed (τ2 = 0 and I2 = 0%; 95% CI, 0%-66.5%). There was no measurable global inconsistency based on a random effects design-by-treatment model (χ22 = 5.76; P = .06) (eTable 2 in the Supplement). We only identified 2 contrasts of inconsistency, indicating disagreement between the indirect and direct evidence for 150 mg oseltamivir vs 75 mg oseltamivir and 150 mg oseltamivir vs placebo (eAppendix 2 in the Supplement). We found that 75 mg oseltamivir was associated with higher occurrence of vomiting compared with placebo (RR, 1.88; 95% CI, 1.47-2.41) ) (eTable 4 in the Supplement). Compared with 75 mg oseltamivir, 300 mg peramivir was associated with a lower frequency of vomiting (RR, 0.32; 95% CI, 0.11-0.93) (eFigure 3 in the Supplement).
Sensitivity Analysis
A sensitivity analysis was performed by excluding studies with a high risk of bias overall and in the domain of blinding. The overall estimates of TTAS and nausea did not change substantially compared with the base case analysis, indicating that the influence of trials with a high risk of bias was not significant (eTable 3 in the Supplement). The estimates (odds ratio) pooled with the Mantel-Haenszel method were similar to the original comparisons of the antiviral agents with placebo as binary outcomes (eTable 4 in the Supplement).
Publication Bias
We performed comparison-adjusted funnel plots and Egger test for the primary efficacy outcome, TTAS. Publication bias may present as funnel asymmetry and Egger test (P = .049) indicated trials of innovative treatments with favorable trial effects were more likely to get published (eFigure 4 in the Supplement).
Network Assumptions
We investigated the following factors: age, sex, underlying disease, disease severity, symptom duration until receiving treatment, intervention, treatment duration, concomitant medications, and outcome measures. The characteristics of the studies were mostly similar. The evidence showed there was no substantial heterogeneity or inconsistency in any analysis. However, the possibility of similarity and intransitivity could not be ruled out. Age and disease severity may be associated with modifying associations. Since the similarity of modification cannot be assured, we made the decision to downgrade the certainty of our conclusions owing to transitivity (eTable 6 in the Supplement).46
Discussion
The findings of this network meta-analysis suggest that 10 mg zanamivir twice daily was associated with shorter TTAS compared with placebo. The outcomes for all of the antiviral agents were similar for TTAS, except for 10 mg zanamivir compared with 40 mg or 80 mg baloxavir. Based on moderate-quality evidence, 40 mg or 80 mg baloxavir was associated with fewer complications of influenza compared with placebo.
With respect to safety outcomes, especially gastrointestinal adverse events, 75 mg oseltamivir twice daily, compared with placebo, was associated with more frequent occurrence of nausea based on moderate-quality evidence and of vomiting based on high-quality evidence. Furthermore, compared with oseltamivir, zanamivir and baloxavir were associated with a significantly lower occurrence of nausea, and 300 mg peramivir was associated with a lower frequency of vomiting.
To our knowledge, this is the first study to simultaneously compare the efficacy of baloxavir and 3 neuraminidase inhibitors, the 4 drugs most commonly used for patients with influenza in current clinical practice, using HRs. Compared with a 2019 network meta-analysis by Taieb et al,71 we included patients of all ages and a large-scale randomized clinical trial (CAPSTONE-2).11 Additionally, we assessed HRs as our treatment outcome in TTAS, while Taieb et al71 assessed median values. We also conducted a test of assumption and evaluation of the quality of evidence in this study.
We applied a frequentist framework to perform the analysis. In theory, similar results should be reported under a frequentist framework, as well as a Bayesian framework with noninformative priors.72 We also used the common-effect Mantel-Haenszel network meta-analysis, which is helpful to ensure the robustness of evidence when dealing with sparse data.49 We included participants of all ages with influenza-like illness; for the primary outcome, TTAS, we only focused on patients with laboratory-confirmed influenza based on a reverse transcription–polymerase chain reaction assay or viral culture with high specificity. The ITT population included patients without confirmed influenza and individuals who may have had poor medication adherence; thus, the analyses may underestimate the efficacy of the antiviral agents.73 Therefore, the efficacy analyses of the ITTI population may be closer to the truth than the analyses of the ITT population. Besides, most randomized clinical trials included in this study reported the Kaplan-Meier curves for their ITTI populations, which enabled a more comprehensive comparison of the effectiveness of the drugs.
Limitations
This network meta-analysis has several limitations. First, because HRs were not fully reported in most studies, our calculations based on reconstruction of the Kaplan-Meier curves may have subtle differences from the actual HRs. Second, there are a lack of studies on some antiviral agents, and the limited number of studies for some treatment comparisons may potentially dominate our network estimates. Third, the definition of complications varied among the studies, which could influence our interpretation of the effectiveness of the drugs for reducing the risk of complications. However, the network meta-analysis mostly identified influenza-related complications, which has a degree of credibility. Due to the lack of standardized definitions of influenza-related complications, meta‐analyses of these outcomes should be interpreted conservatively.74,75 Since this network meta-analysis was based on study-level data, the definition of influenza-related complications may be limited to original included trials. Additionally, we investigated all published reports of randomized clinical trials. Although nonrandomized clinical studies were not included, previous nonrandomized clinical studies reported minimal differences in the point estimates compared with our network meta-analysis. Given that nonrandomized clinical studies may be limited by the risk of confounding and selection, reporting, and publication bias, we have reservations about the credibility of their results.76,77 Since we did not include regulator reports, the possibility of publication bias cannot be completely ruled out.
Conclusions
The findings of this network meta-analysis suggest that the examined antiviral agents assessed were associated with shortening TTAS; zanamivir was associated with the lowest TTAS, and baloxavir was associated with the lowest rate of influenza-related complications. Moreover, baloxavir had the lowest risk of total adverse events. Compared with oseltamivir, zanamivir and baloxavir were associated with lower frequencies of nausea and 300 mg peramivir was associated with lower occurrences of vomiting. Network meta-analysis studies such as this may have utility in informing treatment guidelines for viral conditions, like influenza, when few direct comparisons between individual therapies are available.
eAppendix 1. Search Strategy
eTable 1. Characteristics of Included Studies
eFigure 1. Risk of Bias of Included Studies
eFigure 2. Network Graphs of Safety Outcomes
eFigure 3. League Tables
eTable 2. Global Inconsistency of All Outcomes
eAppendix 2. Netsplit Analysis of Inconsistency
eTable 3. Sensitivity Analysis
eTable 4. Mantel-Haenszel (MH) Method for Binary Outcomes
eFigure 4. Comparison-Adjusted Funnel Plot
eTable 5. Certainty of Direct Evidence Assessment
eTable 6. Certainty of Network Evidence Assessment
References
- 1.Centers for Disease Control and Prevention . Influenza antiviral medications: summary for clinicians. Accessed December 20, 2020. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm
- 2.Rubin R. What happens when COVID-19 collides with flu season? JAMA. 2020;324(10):923-925. doi: 10.1001/jama.2020.15260 [DOI] [PubMed] [Google Scholar]
- 3.Hayden FG, Treanor JJ, Fritz RS, et al. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282(13):1240-1246. doi: 10.1001/jama.282.13.1240 [DOI] [PubMed] [Google Scholar]
- 4.Heo YA. Baloxavir: First Global Approval. Drugs. 2018;78(6):693-697. doi: 10.1007/s40265-018-0899-1 [DOI] [PubMed] [Google Scholar]
- 5.Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68(6):e1-e47. doi: 10.1093/cid/ciy866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . What you should know about flu antiviral drugs. Accessed December 20, 2020. https://www.cdc.gov/flu/treatment/whatyoushould.htm
- 7.Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. John Wiley & Sons; 2020. [Google Scholar]
- 8.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16. doi: 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12(1):9. doi: 10.1186/1471-2288-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayden FG, Sugaya N, Hirotsu N, et al. ; Baloxavir Marboxil Investigators Group . Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379(10):913-923. doi: 10.1056/NEJMoa1716197 [DOI] [PubMed] [Google Scholar]
- 11.Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2020;20(10):1204-1214. doi: 10.1016/S1473-3099(20)30004-9 [DOI] [PubMed] [Google Scholar]
- 12.Deng WW, Li QY, Zhong NS; “Oseltamivir in the treatment of suspected influenza patients” Study Group . A multicenter study of efficacy and safety of oseltamivir in the treatment of suspected influenza patients. Article in Chinese. Zhonghua Yi Xue Za Zhi. 2004;84(24):2132-2136. [PubMed] [Google Scholar]
- 13.Fan HW, Han Y, Liu W, et al. A randomized controlled study of peramivir, oseltamivir and placebo in patients with mild influenza. Article in Chinese. Zhonghua Nei Ke Za Zhi. 2019;58(8):560-565. [DOI] [PubMed] [Google Scholar]
- 14.Fry AM, Goswami D, Nahar K, et al. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis. 2014;14(2):109-118. doi: 10.1016/S1473-3099(13)70267-6 [DOI] [PubMed] [Google Scholar]
- 15.Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections: GG167 Influenza Study Group. N Engl J Med. 1997;337(13):874-880. doi: 10.1056/NEJM199709253371302 [DOI] [PubMed] [Google Scholar]
- 16.Hayden FG, Gubareva LV, Monto AS, et al. ; Zanamivir Family Study Group . Inhaled zanamivir for the prevention of influenza in families. N Engl J Med. 2000;343(18):1282-1289. doi: 10.1056/NEJM200011023431801 [DOI] [PubMed] [Google Scholar]
- 17.Hedrick JA, Barzilai A, Behre U, et al. Zanamivir for treatment of symptomatic influenza A and B infection in children five to twelve years of age: a randomized controlled trial. Pediatr Infect Dis J. 2000;19(5):410-417. doi: 10.1097/00006454-200005000-00005 [DOI] [PubMed] [Google Scholar]
- 18.Heinonen S, Silvennoinen H, Lehtinen P, et al. Early oseltamivir treatment of influenza in children 1-3 years of age: a randomized controlled trial. Clin Infect Dis. 2010;51(8):887-894. doi: 10.1086/656408 [DOI] [PubMed] [Google Scholar]
- 19.de Jong MD, Ison MG, Monto AS, et al. Evaluation of intravenous peramivir for treatment of influenza in hospitalized patients. Clin Infect Dis. 2014;59(12):e172-e185. doi: 10.1093/cid/ciu632 [DOI] [PubMed] [Google Scholar]
- 20.Kashiwagi S, Kudoh S, Watanabe A, Yoshimura I. Clinical efficacy and safety of the selective oral neuraminidase inhibitor oseltamivir in treating acute influenza—placebo-controlled double-blind multicenter phase III trial. Article in Japanese. Kansenshogaku Zasshi. 2000;74(12):1044-1061. doi: 10.11150/kansenshogakuzasshi1970.74.1044 [DOI] [PubMed] [Google Scholar]
- 21.Kohno S, Kida H, Mizuguchi M, Shimada J; S-021812 Clinical Study Group . Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection. Antimicrob Agents Chemother. 2010;54(11):4568-4574. doi: 10.1128/AAC.00474-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohno S, Kida H, Mizuguchi M, et al. ; S-021812 Clinical Study Group . Intravenous peramivir for treatment of influenza A and B virus infection in high-risk patients. Antimicrob Agents Chemother. 2011;55(6):2803-2812. doi: 10.1128/AAC.01718-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Cai B, Wang M, Zhu Y. A double-blind, randomized, placebo-controlled multicenter study of oseltamivir phosphate for treatment of influenza infection in China. Chin Med J (Engl). 2003;116(1):44-48. doi: 10.3901/JME.2003.06.044 [DOI] [PubMed] [Google Scholar]
- 24.Lin JT, Yu XZ, Cui DJ, et al. A multicentre, randomized, controlled trial of oseltamivir in the treatment of influenza in a high-risk Chinese population. Curr Med Res Opin. 2006;22(1):75-82. doi: 10.1185/030079906X80297 [DOI] [PubMed] [Google Scholar]
- 25.Mäkelä MJ, Pauksens K, Rostila T, et al. Clinical efficacy and safety of the orally inhaled neuraminidase inhibitor zanamivir in the treatment of influenza: a randomized, double-blind, placebo-controlled European study. J Infect. 2000;40(1):42-48. doi: 10.1053/jinf.1999.0602 [DOI] [PubMed] [Google Scholar]
- 26.The MIST (Management of Influenza in the Southern Hemisphere Trialists) Study Group . Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet. 1998;352(9144):1877-1881. doi: 10.1016/S0140-6736(98)10190-3 [DOI] [PubMed] [Google Scholar]
- 27.Monto AS, Webster A, Keene O. Randomized, placebo-controlled studies of inhaled zanamivir in the treatment of influenza A and B: pooled efficacy analysis. J Antimicrob Chemother. 1999;44(suppl B):23-29. doi: 10.1093/jac/44.suppl_2.23 [DOI] [PubMed] [Google Scholar]
- 28.Nakamura S, Miyazaki T, Izumikawa K, et al. Efficacy and safety of intravenous peramivir compared with oseltamivir in high-risk patients infected with influenza a and b viruses: a multicenter randomized controlled study. Open Forum Infect Dis. 2017;4(3):ofx129. doi: 10.1093/ofid/ofx129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson KG, Aoki FY, Osterhaus AD, et al. ; Neuraminidase Inhibitor Flu Treatment Investigator Group . Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet. 2000;355(9218):1845-1850. doi: 10.1016/S0140-6736(00)02288-1 [DOI] [PubMed] [Google Scholar]
- 30.Puhakka T, Lehti H, Vainionpää R, et al. Zanamivir: a significant reduction in viral load during treatment in military conscripts with influenza. Scand J Infect Dis. 2003;35(1):52-58. doi: 10.1080/0036554021000026981 [DOI] [PubMed] [Google Scholar]
- 31.South East Asia Infectious Disease Clinical Research Network . Effect of double dose oseltamivir on clinical and virological outcomes in children and adults admitted to hospital with severe influenza: double blind randomised controlled trial. BMJ. 2013;346:f3039. doi: 10.1136/bmj.f3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treanor JJ, Hayden FG, Vrooman PS, et al. ; US Oral Neuraminidase Study Group . Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA. 2000;283(8):1016-1024. doi: 10.1001/jama.283.8.1016 [DOI] [PubMed] [Google Scholar]
- 33.Watanabe A, Ishida T, Hirotsu N, et al. Baloxavir marboxil in Japanese patients with seasonal influenza: Dose response and virus type/subtype outcomes from a randomized phase 2 study. Antiviral Res. 2019;163:75-81. doi: 10.1016/j.antiviral.2019.01.012 [DOI] [PubMed] [Google Scholar]
- 34.Whitley RJ, Hayden FG, Reisinger KS, et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J. 2001;20(2):127-133. doi: 10.1097/00006454-200102000-00002 [DOI] [PubMed] [Google Scholar]
- 35.Whitley R, Laughlin A, Carson S, et al. Single dose peramivir for the treatment of acute seasonal influenza: integrated analysis of efficacy and safety from two placebo-controlled trials. Antivir Ther. 2015;20(7):709-719. doi: 10.3851/IMP2874 [DOI] [PubMed] [Google Scholar]
- 36.Boivin G, Goyette N, Hardy I, Aoki F, Wagner A, Trottier S. Rapid antiviral effect of inhaled zanamivir in the treatment of naturally occurring influenza in otherwise healthy adults. J Infect Dis. 2000;181(4):1471-1474. doi: 10.1086/315392 [DOI] [PubMed] [Google Scholar]
- 37.Ison MG, Hui DS, Clezy K, et al. A clinical trial of intravenous peramivir compared with oral oseltamivir for the treatment of seasonal influenza in hospitalized adults. Antivir Ther. 2013;18(5):651-661. doi: 10.3851/IMP2442 [DOI] [PubMed] [Google Scholar]
- 38.Katsumi Y, Otabe O, Matsui F, et al. Effect of a single inhalation of laninamivir octanoate in children with influenza. Pediatrics. 2012;129(6):e1431-e1436. doi: 10.1542/peds.2011-2054 [DOI] [PubMed] [Google Scholar]
- 39.Ison MG, Fraiz J, Heller B, et al. Intravenous peramivir for treatment of influenza in hospitalized patients. Antivir Ther. 2014;19(4):349-361. doi: 10.3851/IMP2680 [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto K, Ogawa N, Nerome K, et al. Safety and efficacy of the neuraminidase inhibitor zanamivir in treating influenza virus infection in adults: results from Japan. GG167 Group. Antivir Ther. 1999;4(2):61-68. [PubMed] [Google Scholar]
- 41.Sugaya N, Ohashi Y. Long-acting neuraminidase inhibitor laninamivir octanoate (CS-8958) versus oseltamivir as treatment for children with influenza virus infection. Antimicrob Agents Chemother. 2010;54(6):2575-2582. doi: 10.1128/AAC.01755-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y; MARVEL Study Group . Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis. 2010;51(10):1167-1175. doi: 10.1086/656802 [DOI] [PubMed] [Google Scholar]
- 43.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puhan MA, Schünemann HJ, Murad MH, et al. ; GRADE Working Group . A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630 [DOI] [PubMed] [Google Scholar]
- 45.Brignardello-Petersen R, Bonner A, Alexander PE, et al. ; GRADE Working Group . Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36-44. doi: 10.1016/j.jclinepi.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 46.Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One. 2014;9(7):e99682. doi: 10.1371/journal.pone.0099682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rücker G, Krahn U, König J, Efthimiou O, Schwarzer G. Netmeta: network meta-analysis using frequentist methods. Version 1.3-0. CRAN.R Project. Accessed June 15, 2019. https://cran.r-project.org/web/packages/netmeta/index.html
- 48.Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007;7(1):5. doi: 10.1186/1471-2288-7-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Efthimiou O, Rücker G, Schwarzer G, Higgins JPT, Egger M, Salanti G. Network meta-analysis of rare events using the Mantel-Haenszel method. Stat Med. 2019;38(16):2992-3012. doi: 10.1002/sim.8158 [DOI] [PubMed] [Google Scholar]
- 50.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654. doi: 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15(1):58. doi: 10.1186/s12874-015-0060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eusebi LH, Black CJ, Howden CW, Ford AC. Effectiveness of management strategies for uninvestigated dyspepsia: systematic review and network meta-analysis. BMJ. 2019;367:l6483. doi: 10.1136/bmj.l6483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackson D, Barrett JK, Rice S, White IR, Higgins JP. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Stat Med. 2014;33(21):3639-3654. doi: 10.1002/sim.6188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98-110. doi: 10.1002/jrsm.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7-8):932-944. doi: 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- 56.Lu G, Ades A. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc. 2006;101(474):447-459. doi: 10.1198/016214505000001302 [DOI] [Google Scholar]
- 57.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 58.Ramirez J, Peyrani P, Wiemken T, Chaves SS, Fry AM. A randomized study evaluating the effectiveness of oseltamivir initiated at the time of hospital admission in adults hospitalized with influenza-associated lower respiratory tract infections. Clin Infect Dis. 2018;67(5):736-742. doi: 10.1093/cid/ciy163 [DOI] [PubMed] [Google Scholar]
- 59.Hirotsu N, Saisho Y, Hasegawa T, Shishido T. Clinical and virologic effects of four neuraminidase inhibitors in influenza A virus-infected children (aged 4-12 years): an open-label, randomized study in Japan. Expert Rev Anti Infect Ther. 2018;16(2):173-182. doi: 10.1080/14787210.2018.1421945 [DOI] [PubMed] [Google Scholar]
- 60.Beigel JH, Bao Y, Beeler J, et al. IRC003 Study Team . Oseltamivir, amantadine, and ribavirin combination antiviral therapy versus oseltamivir monotherapy for the treatment of influenza: a multicentre, double-blind, randomised phase 2 trial. Lancet Infect Dis. 2017;17(12):1255-1265. doi: 10.1016/S1473-3099(17)30476-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He CH, Liu CY, Lin GY, et al. Efficacy and safety of oseltamivir in children with suspected influenza: a multicenter randomized open-label trial. Article in Chinese. Zhonghua Er Ke Za Zhi. 2017;55(6):462-467. [DOI] [PubMed] [Google Scholar]
- 62.Yoshino Y, Seo K, Koga I, Kitazawa T, Ota Y. Clinical efficacy of laninamivir and peramivir in patients with seasonal influenza: a randomized clinical trial. Infect Dis (Lond). 2017;49(5):417-419. doi: 10.1080/23744235.2016.1242773 [DOI] [PubMed] [Google Scholar]
- 63.Dawood FS, Jara J, Gonzalez R, et al. A randomized, double-blind, placebo-controlled trial evaluating the safety of early oseltamivir treatment among children 0-9 years of age hospitalized with influenza in El Salvador and Panama. Antiviral Res. 2016;133:85-94. doi: 10.1016/j.antiviral.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 64.Watanabe A. A randomized double-blind controlled study of laninamivir compared with oseltamivir for the treatment of influenza in patients with chronic respiratory diseases. J Infect Chemother. 2013;19(1):89-97. doi: 10.1007/s10156-012-0460-1 [DOI] [PubMed] [Google Scholar]
- 65.Kohno S, Yen MY, Cheong HJ, et al. ; S-021812 Clinical Study Group . Phase III randomized, double-blind study comparing single-dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection. Antimicrob Agents Chemother. 2011;55(11):5267-5276. doi: 10.1128/AAC.00360-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gravenstein S, Drinka P, Osterweil D, et al. Inhaled zanamivir versus rimantadine for the control of influenza in a highly vaccinated long-term care population. J Am Med Dir Assoc. 2005;6(6):359-366. doi: 10.1016/j.jamda.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 67.Li L, Cai B, Wang M, Zhu Y. A multicenter study of efficacy and safety of oseltamivir in treatment of naturally acquired influenza. Article in Chinese. Zhonghua Nei Ke Za Zhi. 2001;40(12):838-842. [PubMed] [Google Scholar]
- 68.Lin JT, Yu XZ, Cui DJ, et al. A multicenter randomized controlled study of the efficacy and safety of oseltamivir in the treatment of influenza in a high risk population. Article in Chinese. Zhonghua Jie He He Hu Xi Za Zhi. 2004;27(7):455-459. [PubMed] [Google Scholar]
- 69.Monto AS, Fleming DM, Henry D, et al. Efficacy and safety of the neuraminidase inhibitor zanamivirin the treatment of influenza A and B virus infections. J Infect Dis. 1999;180(2):254-261. doi: 10.1086/314904 [DOI] [PubMed] [Google Scholar]
- 70.Schilling M, Povinelli L, Krause P, et al. Efficacy of zanamivir for chemoprophylaxis of nursing home influenza outbreaks. Vaccine. 1998;16(18):1771-1774. doi: 10.1016/S0264-410X(98)00141-8 [DOI] [PubMed] [Google Scholar]
- 71.Taieb V, Ikeoka H, Ma F-F, et al. A network meta-analysis of the efficacy and safety of baloxavir marboxil versus neuraminidase inhibitors for the treatment of influenza in otherwise healthy patients. Curr Med Res Opin. 2019;35(8):1355-1364. doi: 10.1080/03007995.2019.1584505 [DOI] [PubMed] [Google Scholar]
- 72.Shim SR, Kim S-J, Lee J, Rücker G. Network meta-analysis: application and practice using R software. Epidemiol Health. 2019;41:e2019013. doi: 10.4178/epih.e2019013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doll MK, Winters N, Boikos C, Kraicer-Melamed H, Gore G, Quach C. Safety and effectiveness of neuraminidase inhibitors for influenza treatment, prophylaxis, and outbreak control: a systematic review of systematic reviews and/or meta-analyses. J Antimicrob Chemother. 2017;72(11):2990-3007. doi: 10.1093/jac/dkx271 [DOI] [PubMed] [Google Scholar]
- 74.Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014;2014(4):CD008965. doi: 10.1590/1516-3180.20141324T2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385(9979):1729-1737. doi: 10.1016/S0140-6736(14)62449-1 [DOI] [PubMed] [Google Scholar]
- 76.Santesso N, Hsu J, Mustafa R, et al. Antivirals for influenza: a summary of a systematic review and meta-analysis of observational studies. Influenza Other Respir Viruses. 2013;7(suppl 2):76-81. doi: 10.1111/irv.12085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsu J, Santesso N, Mustafa R, et al. Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Ann Intern Med. 2012;156(7):512-524. doi: 10.7326/0003-4819-156-7-201204030-00411 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Search Strategy
eTable 1. Characteristics of Included Studies
eFigure 1. Risk of Bias of Included Studies
eFigure 2. Network Graphs of Safety Outcomes
eFigure 3. League Tables
eTable 2. Global Inconsistency of All Outcomes
eAppendix 2. Netsplit Analysis of Inconsistency
eTable 3. Sensitivity Analysis
eTable 4. Mantel-Haenszel (MH) Method for Binary Outcomes
eFigure 4. Comparison-Adjusted Funnel Plot
eTable 5. Certainty of Direct Evidence Assessment
eTable 6. Certainty of Network Evidence Assessment