Abstract
Nitrogen use in agriculture directly impacts food security, global warming, and environmental degradation. Forage grasses intercropped with maize produce feed for animals and or mulch for no-till systems. Forage grasses may exude nitrification inhibitors. It was hypothesized that brachiaria intercropping increases N recycling and maize grain yield and reduces nitrous oxide (N2O) emissions from soil under maize cropping. A field experiment was set up in December 2016 to test three cropping system (maize monocropped, maize intercropped with Brachiaria brizantha or with B. humidicola) and two N rates (0 or 150 kg ha-1). The grasses were sown with maize, but B. humidicola did not germinate well in the first year. B. brizantha developed slowly during the maize cycle because of shading but expanded after maize was harvested. The experiment was repeated in 2017/2018 when B. humidicola was replanted. N2O and carbon dioxide (CO2) emissions, maize grain yield and N content were measured during the two seasons. After the first maize harvest, the above- and below-ground biomass, C and N content of B. brizantha grown during fall-winter, and the biological nitrification inhibition potential of B. brizantha were evaluated. Maize yield responded to N fertilization (5.1 vs. 9.8 t ha-1) but not to brachiaria intercropping. B. brizantha recycled approximately 140 kg N ha-1 and left 12 t dry matter ha-1 for the second maize crop. However, the 2017/18 maize yields were not affected by the N recycled by B. brizantha, whereas N2O emissions were higher in the plots with brachiaria, suggesting that part of the recycled N was released too early after desiccation. Brachiarias showed no evidence of causing nitrification inhibition. The strategy of intercropping brachiarias did not increase maize yield, although it added C and recycled N in the system.
Keywords: Zea mays, Brachiaria spp, Nitrogen agronomic efficiency, Intercropping, Nitrous oxide emissions, Nutrient cycling
Highlights
-
•
N recycled by brachiaria mulch did not increase maize yield.
-
•
B. brizantha mulch increased N2O emissions with no effect on maize yield.
-
•
There was no evidence of nitrification inhibition by B. brizantha or B. humidicola.
1. Introduction
Maize is among the three major N-demanding crops on a global scale. In 2010, Zhang et al. (2015) estimated 28 Tg of reactive N input for global maize production, which resulted in 46% agronomic nitrogen use efficiency (NUE). Typically, maize plants take up less than 50% of the N fertilizer inputs in tropical soil (Rocha et al., 2019). A significant amount of applied N may be lost to the environment by leaching (Jankowski et al., 2018), NH3 volatilization (Chien et al., 2009), and N2O emissions (Meurer et al., 2016).
To reduce N losses, adequate management practices are necessary to supply the N demanded by plants in time, space, and chemically-available forms. Growing two species at the same time (intercropping) or in sequence (cover cropping) may aid in decreasing N losses (Brooker et al., 2015, Rosolem et al., 2017). A non-cash crop can capture the surplus N that the cash crop was unable to use, thereby increasing the NUE of the agroecosystem (Martinez-Feria et al., 2018) even if N recovery by the cash crop is not affected.
Forage grasses intercropped with maize that grow during the fall-winter-spring after maize harvest can result in high above- and below-ground yields of biomass. Such grasses have been used in Brazil to produce biomass to feed animals and or provide mulch for no-till crops, increasing land-use efficiency (Mateus et al., 2016) and improving soil fertility (Crusciol et al., 2015). The N applied to high demand crops, such as maize, that could not be absorbed, may move into deeper soil layers where it can be more easily accessed by the deep root system of forage grasses (Rosolem et al., 2017). N and other nutrients absorbed by the grasses may be released for the subsequent crop, improving soil fertility and soil organic carbon (SOC) in the long term (Carvalho et al., 2014, Cong et al., 2014, Crusciol et al., 2015). However, the N accumulated by the forage grasses during the fall-winter and used as mulch may not be effectively transferred to the following summer crop (Momesso et al., 2019). In the short-term, the cash crop use efficiency of N recycled by forage grasses is of interest because it may help define the crop need for mineral fertilizer. The scavenging capacity of intercropped grasses may also help to decrease the mineral N concentration in the soil and reduce N2O emissions (Rosolem et al., 2017).
Different species of Brachiaria and other grasses have been intercropped with maize and other grain crops at a large scale in Brazil (Momesso et al., 2019, Oliveira et al., 2019, Rocha et al., 2019). B. brizantha ‘Marandu’ is commonly used because of its high potential to produce biomass (Momesso et al., 2019, Pacheco et al., 2013). B. humidicola is less common in intercropping systems, but it has a high potential to release biological nitrification inhibitor (BNI) compounds in root exudates and may have an impact on grain yield of maize planted subsequently (Karwat et al., 2017, Subbarao et al., 2012, Subbarao et al., 2009).
Other species of Brachiaria are known to produce BNI but to a lesser extent than B. humidicola (Subbarao et al., 2012). However, the BNI activity of these grasses appears to depend not only on the species but also on soil conditions and management such as fertilization. The results reported in the literature have been varied. Byrnes et al. (2017) showed that soil under pastureland with B. humidicola presented lower nitrification and denitrification rates, N2O emissions, and an abundance of ammonium oxidizing archaea compared with that of other grasses. Karwat et al. (2017) also observed the effect of BNI when the pasture of B. humidicola was converted to maize production. When compared with areas under continuous maize growth, they observed improved performance in the first year of maize cultivation after B. humidicola pastureland conversion. However, no evidence of nitrification inhibition by B. humidicola, B. brizanta, B. decumbens, or B. ruzisiensis was detected in Brazilian soils under cultivation and fertilized with N in pot experiments (Castoldi et al., 2013, Castoldi et al., 2014).
Brachiaria intercropping has been extensively used by farmers, but, there is still controversy regarding whether the N recycled by brachiaria in tropical cropping systems has a significant role in the N nutrition of the subsequent crop. It is unclear whether BNI can be significant in systems with brachiaria and high N fertilization rates. Questions also remain regarding how changes in soil N caused by brachiaria intercropping affects N2O emissions associated with fertilizer application to maize. Given these unanswered questions, we conducted a study to determine the short-term effects of two brachiaria species with different biomass productions and BNI potentials intercropped with maize on N cycling, maize yield, and greenhouse gas (GHG) emissions.
2. Material and methods
A field experiment was conducted during two seasons (2016/17 and 2017/18) in Botucatu, State of São Paulo, Brazil, at 22º49’S, 48º25’W, and 740 m altitude. The climate is tropical of altitude (CWa) having dry winters and hot, wet summers according to the Köppen classification (Peel et al., 2007). The soil is a clay, kaolinitic, thermic Typic Haplorthox (USDA, 2014). Selected chemical and physical soil characteristics are shown in Table 1. Before our study, the experimental area was cropped with black oats (Avena strigosa), which were desiccated on October 24th, 2016, to supply the mulch for the experiment conducted under no-till.
Table 1.
Soil physical and chemical properties of the experimental area at 0–20 cm depth.
| pH | SOM | Resin P | S | Al+3 | H+Al+3 | K | Ca | Mg | CEC | BS | BD | Sand | Silt | Clay |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g dm-3 | --- mg dm-3 -- | ------------------ mmolc dm-3 --------------- | % | kg dm-3 | -------- g kg-1---------- | |||||||||
| 4.8 | 31.7 | 23 | 13 | 3.5 | 45 | 4.7 | 25 | 12 | 86 | 48 | 1.4 | 173 | 235 | 592 |
SOM: soil organic matter; CEC: cation exchange capacity; BS: base saturation; BD: bulk density.
The experimental design was a split-plot in complete randomized blocks with five replicates. The main plots (4.5 × 30 m) had three cropping system treatments: monocrop maize (M) hybrid 2B587 PW, maize intercropped with B. humidicola (MH) ‘Tully’ or with B. brizantha (MB) ‘Marandu’, combined with two N rates, 0 or 150 kg ha-1, placed in 4.5 × 15 m subplots. The subscripts 0 and 150 define the N rates in the abbreviations that represent the treatments. Each plot had six rows that were 15 m in length spaced 0.75 m in 2016/17; the interrow spacing was reduced to 0.45 m in 2017/2018, resulting in 10 rows per plot, but the plot area and the maize plant population remained the same. B. humidicola did not emerge in the first season (2016/17); thus, the treatments MH0 and MH150 were similar to the maize monocrop in that season. In the second season, both brachiaria species emerged such that the two intercropped treatments with maize were established.
Standard fertilization was performed at maize sowing in all plots in the first maize crop on December 13th, 2016, and again on November 24th, 2017, supplying 30, 70, and 50 kg ha-1 of N, P2O5, and K2O, respectively. For the N fertilizer treatments ammonium nitrate (150 kg ha-1 of N) was side-dressed on January 5th, 2017 (20 d after maize sowing; 20 DAS) and on January 4th, 2018 (41 DAS) for the first and second maize crops, respectively, i.e., the V4 to V6 stages. These were within the rates recommended by Cantarella et al. (1997). The N fertilizer was placed in narrow bands (~0.05 m) approximately 0.10 m to the side of the rows or maize plants.
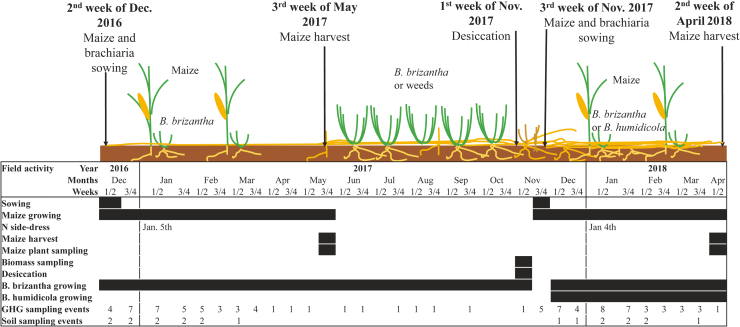
Maize was sown at 0.03 m depth to reach a plant population of circa 66,000 plants ha-1. Simultaneously, brachiaria seeds (1 kg ha-1 of pure live seeds) were mixed with fertilizer and band-incorporated 0.05 m deep and 0.05 m from the maize rows to attain between 80,000 and 100,000 plants ha-1. Maize was harvested at physiological maturity (R6) on May 11th, 2017 and April 10th, 2018, and the B. brizantha was left to grow unshaded during the dry season (fall-winter-spring) between maize crops because B humidicola did not emerge in the first year. B. brizantha desiccated in mid-spring served as mulch for the following no-till maize season (MB0 and MB150). In the second season, the mulch for monocrop maize (M0 and M150) and maize plus B. humidicola (MH0 and MH150) treatments were composed of weeds grown during the fall-winter and maize stover from the previous season. As in the first year, after the second maize crop was harvested, the intercropped grasses were left to grow unshaded during the fall-winter-spring period. The sequence of field operations and dates are shown in Fig. 1.
Fig. 1.
Diagram with the sequence of field operations and respective dates for the two years of the maize-brachiaria intercropping study.
During the first maize crop, a herbicide (nicosulfuron [2-[(4,6-dimethoxypyrimidin-2-ylcarbamoyl) sulfamoyl]-N, N-dimethyl nicotinamide], 7 g ha-1) was sprayed 20 d after plant emergence (DAE) to delay the growth of brachiaria and prevent nutrient competition between brachiaria and maize. In the second crop, this was not necessary, because Brachiaria spp. growth was naturally delayed. Between the two maize crops, 2 t ha-1 of dolomitic lime increase the soil base saturation to 70% was surface-applied without incorporation because the area is managed under no-till.
2.1. Nitrous oxide and carbon dioxide emission
The efflux of nitrous oxide (N2O) and carbon dioxide (CO2) was monitored throughout the experiment using static chambers (Varner et al., 2003). Static chambers (0.3 m diameter and 0.2 m height) were placed (0.05 m depth) on the plant/fertilizer row of all plots. The chambers covered the maize row and the fertilizer band to capture the effect of treatments on N2O emissions. In the first season, one extra chamber was placed in the midrow of three replications of each treatment to measure the emission of the non-fertilized area and determine whether the proximity of the maize roots affected gas emissions. As those emissions were negligible, the midrow area was not sampled in the second year.
Intense gas sampling was performed at critical periods: daily for 6 d after maize sowing (December 13th, 2016), then three times per week until N application, which occurred 20 d DAE. The gas measurements were then recorded daily for 3 d, three times per week for 6 weeks, and finally twice a week until maize harvest (May 11th, 2017). During the winter, gases were sampled once a month, up to the second maize planting (November 24th, 2017). In the 2017/2018 crop cycle gases were sampled daily for 5 d after sowing, three times a week for 4 weeks, and once a week for 2 weeks until N application at 41 DAE. After fertilization gas sampling was performed daily for 4 days, three times per week for 4 weeks, twice a week for 4 weeks, and every 2 weeks until maize harvest (April 9th, 2018).
During sampling events, the static chamber was closed with PVC caps between 9:00 and 10:00 a.m. (Savage et al., 2014). Gas samples were collected (60 mL) at 0, 15, and 30 min after the chambers were closed and immediately transferred to glass vials with gas impermeable septa, following the methods described by Soares et al. (2016). Gas concentration was determined by gas chromatography (Shimadzu GC-2014®), where N2O and CO2 were measured using an electron capture detector (ECD).
Gas fluxes were calculated by linear interpolation of the concentrations of N2O and CO2 during the sampling as follows:
| (1) |
where F is the CO2-C (mg m-2 d-1) or N2O-N (µg m-2 d-1) efflux, is the variation (mol h-1) of gas concentration while the chamber is closed, V is the volume of the chamber headspace (m3), A is the soil area covered by the chamber (m2); m is the molar mass (g mol-1) of the gas, and Vm is the molar volume of the chamber (m3 mol-1).
The gas efflux between sampling dates was determined by linear interpolation, and the daily fluxes were summed to calculate the cumulative emissions. The cumulative N2O emissions of treatments with N fertilization (M150, MB150, and MH150) were subtracted from the respective controls (M0, MB0, and MH0) to calculate the emission factor (EF) (IPCC, 2007). The cumulative N2O emission of each treatment, expressed as CO2 equivalents, was divided by grain yield to calculate the emission intensity (EI).
2.2. Soil analyses
During the first season, soil samples were collected weekly for 11 weeks after maize sowing. In the second season, the soil was sampled 12, 19 and 41 d after sowing, and weekly during the 6 weeks following N application. Each sample was composed of eight subsamples of the 0–0.1 m soil layer. Soil moisture (gravimetric method), pH (CaCl2), and NH4+‒N and NO3-‒N were determined following the methods of Raij et al. (2001).
2.3. Maize grain yield and agronomic efficiency (AE) of applied N
Maize was harvested (10 m each of the two central rows per plot) on May 11th, 2017, and on April 10th, 2018, 149 and 137 DAS, respectively. Grain subsamples were dried at 105º C for 24 h, and maize grain yield was upscaled to 1 ha and 130 g kg-1 moisture content, which is the standard moisture for maize commercialization in Brazil. The N concentration in grains was determined with an elemental analyzer (LECO TruSpec ® CHNS). The agronomic efficiency (AE) of applied nitrogen was calculated as the increment of grain yield in fertilized plots compared to that of the control, following the equation suggested by Dobermann (2007):
| (2) |
where GYt is the grain yield observed for the fertilized treatment, GYc is the grain yield observed for the respective control treatment, and Nf is the N fertilizer added, in kg ha-1 so that AE is expressed as kg grain yield increase per kg of fertilizer N applied.
2.4. Brachiaria and fallow biomass production
The aboveground biomass of B. brizantha and weeds (fallow) grown during fall-spring in the maize plus brachiaria and maize treatments were sampled on November 6th, 2017, at the end of the dry season, using wooden frames (0.25 m2, five replications per treatment). The belowground biomass was sampled using monoliths of 0.2 m × 0.1 m up to 0.4 m. The soil attached to the roots was washed and the roots were dried at 45 ºC determine the dry mass. The N and C concentrations of the shoot and root tissues were determined using an elemental analyzer (LECO-TruSpec® CHNS).
B. brizantha and weeds (monocrop maize treatment) were desiccated on November 13th with glyphosate (6 L ha-1) to provide the mulch for the next maize crop. A summary of the main events and respective dates of the field experiment are presented in Table S1. In 2016/2017, we had data on the N export with grain and N accumulation in B. brizantha or weeds grown during the fall-spring. We calculated the total amount of N absorbed by both maize and forage during the season. Because we did not measure the N concentration in the maize stover, we estimated the total N in the plant using the N harvest index of 0.68 as suggested by Mueller et al. (2019).
2.5. BNI potential
Root samples of B. brizantha and weeds (fallow) were collected on November 15th, 2018, after the second maize crop, frozen at −80 ºC, freeze-dried, and ground to a fine powder. A sub-sample (100 mg) was placed in an Eppendorf tube. Then, three iron beads and 2 mL of 100% methanol were added. The tubes were shaken in a paint mixer machine (Harbil Paint Mixer, Harbil Manufacturing Co, Chicago, USA) for 3 min. The solution was filtered through a 0.22 µm filter membrane (Millex, Millipore, USA), vacuum evaporated and resuspended in 50 µL of dimethyl sulfoxide (DMSO) of which 2 µL was used for the BNI bioassay. A control without root tissue extract was prepared using the same procedure. The BNI bioassay was performed using recombinant Nitrosomonas europaea with a plasmid carrying the LuxAB (luciferase) gene (Iizumi et al., 1998) and used for the BNI potential calculation following the procedure described by Subbarao et al. (2006a) and Nuñez et al. (2018).
Nitrosomonas culture was grown for 7 d in 100 mL of growth media containing 100 µL kanamycin (50 mg mL-1) at 50 rpm and 28 ºC. The growth media had the following composition: KH2PO4 5.14 mM, Na2HPO4 95.1 mM, (NH4)2SO4 18.91 mM, NaHCO3 5.95 mM, CaCl2-2 H2O 0.034 mM, MgSO4-7H2O 0.041 mM, Fe (III) EDTA 0.0027 mM, and pH adjusted to 7.8. The bacterial pellet was collected by centrifugation at 4000 rpm for 20 min and suspended in 50 mL of fresh growth media.
For the bioassay, 2 µL of root extracts was incubated with 198 µL of distilled water and 250 µL of bacterial solution at 900 rpm and 15º C for 15 min (Fisher vortex Genie 2). A 100 µL sample was added to the luminometer Glomax 20/20 (Promega) and injected with 25 µL of decil-aldehyde (1%, resuspended in DMSO). The luminescence was determined for an integration time of 2 and 10 s. The inhibition potential of the root tissue was calculated as the percentage of light emitted by Nitrosomonas compared to the light emission of the control. The results were expressed in allylthiourea units (ATU), considering the inhibition of 80% of luminescence by 0.22 µM of allylthiourea, as described by Subbarao et al. (2006a).
2.6. Statistical analyses
Descriptive statistics were used to obtain the frequency and distribution of data. A two-way ANOVA and the LSD test (p < 0.10 and p < 0.05) were performed to compare the means of each treatment using SISVAR (Ferreira, 2014).
3. Results
3.1. C and N cycling by B. brizantha mulch
The above and below ground dry matter of the plant material of brachiaria and weeds that grew for 6 months in the fall-spring, after the first maize crop, and their C and N contents are shown in Table 2. These plant materials plus the remaining maize residues composed the mulch for the second maize crop.
Table 2.
Dry mass, C and N content (mean ± standard deviation) of shoots and roots (0–40 cm) of B. brizantha and weeds grown during fall and winter after the first maize harvest (2016/17), as affected by a combination of N fertilization and intercrop with B. brizantha.
| Treatment | Above-ground |
Below-ground |
Above + below-ground |
||||||
|---|---|---|---|---|---|---|---|---|---|
| DM | N | C | DM | N | C | DM | N | C | |
| t ha-1 | kg ha-1 | t ha-1 | t ha-1 | kg ha-1 | t ha-1 | t ha-1 | kg ha-1 | t ha-1 | |
| M0 | 1.2b ± 0.4 | 23b ± 9.1 | 0.35b ± 0.12 | 0.4b ± 0.15 | 5b ± 1.2 | 0.16b ± 0.05 | 1.6b | 27b | 0.51b |
| M150 | 1.7b ± 0.6 | 33b ± 10.3 | 0.49b ± 0.18 | 0.6b ± 0.17 | 7b ± 2.7 | 0.21b ± 0.05 | 2.3b | 40b | 0.70b |
| MB0 | 8.1a ± 1.9 | 124a ± 23.6 | 2.56a ± 0.33 | 3.8a ± 1.55 | 21a ± 8.1 | 1.94a ± 0.64 | 11.9a | 146a | 4.50a |
| MB150 | 8.1a ± 2.6 | 110a ± 30.3 | 2.88a ± 0.65 | 3.6a ± 1.21 | 22a ± 7.4 | 1.41a ± 0.39 | 11.7a | 133a | 4.29a |
M0: maize monocrop with no N and only weeds grown after maize; M150: maize monocrop fertilized with 150 kg ha-1 of N and only weeds grown after maize; MB0: maize with no N and B. brizantha intercrop growing after maize; MB150: maize fertilized with 150 kg ha-1 of N and B. brizantha intercrop growing after maize. DM: Dry mass; N: N content; C: C content. Different letters denote significant differences between treatments in the columns for the LSD test (p ≤ 0.05).
Because B. humidicola failed to establish in the first year, only weeds grew after the first maize harvest in the plots of treatments M0, M150, MH0, and MH150, resulting in above-ground weed biomass production ranging from 1.2 to 1.7 t ha-1, whereas the below-ground biomass production ranged from 0.4 to 0.6 t ha-1 in the 0–40 cm soil layer (Table 2). The total (above and belowground) N and C cycled in those treatments varied from 27 to 40 kg ha-1 of N, and 0.51–0.70 t ha-1 of C. In the treatments with maize plus B. brizantha (MB0 and MB150), 8.1 t ha-1 of aboveground biomass and 3.6–3.8 t ha-1 of belowground biomass were produced, resulting in an accumulation of 133 and 146 kg ha-1 of N and 4.29 and 4.50 t ha-1 of C to be cycled in the subsequent cropping season. Thus, compared with weeds, the treatments with B. brizantha resulted in an increase of 106 kg ha-1 and 3.79 t ha-1 of N and C, respectively (Table 2).
3.2. Maize grain yield, N export and agronomic efficiency (AE) of applied N
Maize grain yields were affected by N fertilization, but not by B. brizantha intercropping in the first season (2016/2017) (Table 3). Grain yields ranged from 5.1 to 5.6 t ha-1 in treatments without N, and from 8.7 to 9.0 t ha-1 with 150 kg N ha-1, with AE between 22.6 and 23.3 kg kg-1 (Table 3). In the second season (2017/2018) there was a marked response to N. The highest grain yield was 11.5 t ha-1, observed in MH150 (p ≤ 0.1), followed by M150 and MB150, which produced 10.0 and 10.3 t ha-1, respectively. The higher grain yield of MH150 resulted in an AE of 48.1 kg kg-1, which was higher than the 34.4 and 33.2 kg kg-1 observed for M150 and MB150, respectively (Table 3). For treatments without N, MB0 exhibited higher yields than MH0, 5.3 and 4.3 t ha-1, respectively, however, both treatments did not differ statistically from M0, which produced 4.8 t ha-1 (Table 3).
Table 3.
Maize grain yield, N exported with grain and nitrogen agronomic efficiency (AE) of applied N (mean ± standard deviation) of two harvest seasons (2016/17 and 2017/18) as affected by a combination of N fertilization (0 or 150 kg ha-1) and intercrop with B. brizantha (B) or B. humidicola (H).
| Treatments | Grain Yield |
N exported by grain |
AE |
|||
|---|---|---|---|---|---|---|
| 2016/17 | 2017/18 | 2016/17 | 2017/18 | 2016/17 | 2017/18 | |
| --------- t ha-1 --------- | --------- kg ha-1 --------- | ------ kg kg-1 ---- | ||||
| M0 | 5.1b ± 0.8 | 4.8cd ± 1.1 | 54.9b ± 15.6 | 51.8c ± 14.9 | – | – |
| MB0 | 5.6b ± 0.9 | 5.3c ± 0.5 | 54.0b ± 3.9 | 55.9c ± 8.5 | – | – |
| MH0 | – | 4.3d ± 0.8 | – | 45.0c ± 14.5 | – | – |
| M150 | 8.7a ± 1.0 | 10.0b ± 1.6 | 118.0a ± 25.9 | 139.9b ± 27.9 | 23.3a ± 6.6 | 34.4b ± 10.9 |
| MB150 | 9.0a ± 0.9 | 10.3b ± 1.1 | 116.6a ± 8.9 | 141.7b ± 17.3 | 22.6a ± 5.4 | 33.2b ± 7.4 |
| MH150 | – | 11.5a ± 0.9 | – | 168.6a ± 18.6 | – | 48.1a ± 5.8 |
M: maize monocrop; MB and MH: maize intercropped with B. brizantha and B. humidicola, respectively; 0 and 150 subscripts indicate the 0 and 150 kg ha-1 of N applied to the maize crop. In 2016/17 B. humidicola germination did not germinate during the maize season: MH0 and MH150 plots were similar to the maize monocrop treatments. Different letters after means denote significant differences between treatments in the columns for the LSD test (p ≤ 0.1).
The amount of N accumulated in the maize plants plus that accumulated in B. brizantha in the 2016/2017 season varied from 225 kg N ha-1 in the plots with no N fertilizer to 305 kg N ha-1 in the MB150 plots. In the plots with maize plus weeds (in fall-spring), the corresponding figures were 108 and 213 kg N ha-1 (results not shown).
Yield, N accumulation, and AE of the two harvest seasons were not compared because they were probably affected by differences in climatic conditions. The second season had a better rainfall distribution. The first season (2016/2017) had three relatively dry periods longer than 10 d in duration, with no daily precipitation greater than 10 mm (Fig. 2); however, in the 2017/2018 season this happened only once (Fig. 3). This may explain the higher N response in the second season (Table 3).
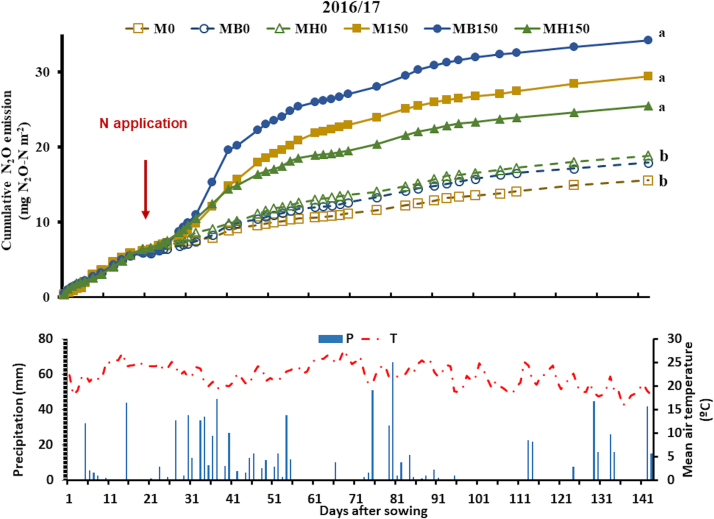
Fig. 2.
Cumulative nitrous oxide emission, precipitation and mean air temperature during maize growth in the 1st harvest season (2016/17), as affected by the combination of N fertilization (0 and 150 kg ha-1) and Brachiaria intercropping: B. brizantha (B) and B. humidicola (H). The arrow indicates when N fertilizer was applied to maize. Different letters indicate statistically significant differences among treatments (p ≤ 0.05). Legend abbreviations: M, MB, and MH denote monocrop maize, maize intercropped with B. brizantha, and maize intercropped with B. humidicola, respectively; 0 and 150 denote N rate applied to the maize crop.
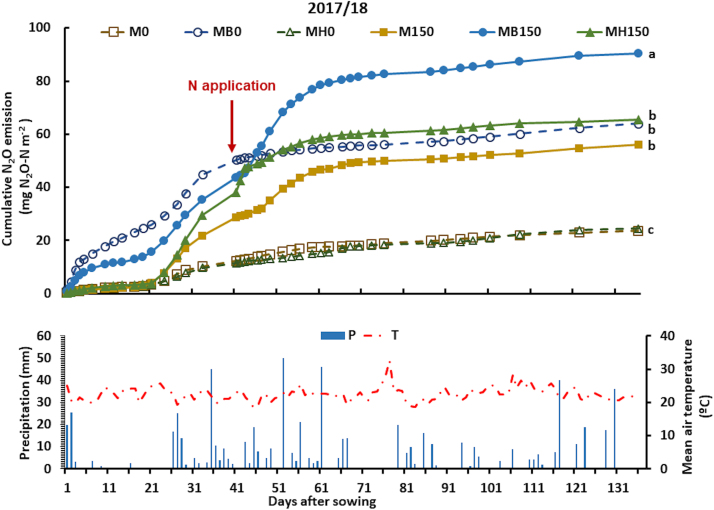
Fig. 3.
Cumulative nitrous oxide emission, precipitation and mean air temperature during maize growth in the 2nd harvest season (2017/18), as affected by the combination of N fertilization (0 and 150 kg ha-1) and Brachiaria intercropping: B. brizantha (B) and B. humidicola (H). The arrow indicates when N fertilizer was applied to maize. Different letters indicate statistically significant differences among treatments (p ≤ 0.05). Legend abbreviations: M, MB, and MH denote monocrop maize, maize intercropped with B. brizantha, and maize intercropped with B. humidicola, respectively; 0 and 150 denote N rate applied to the maize crop.
3.3. Nitrous oxide emissions
In the 2016/2017 maize growing season, N2O emissions were higher with N fertilization than in unfertilized plots (Fig. 2). There was no effect of B. brizantha on N2O emissions. The treatments M150 and MB150 emitted 27.0 and 26.4 mg m-2 of N2O‒N, respectively during the 149 d between the maize sowing and harvesting. In this time, M0 and MB0 emitted 15.8 and 17.6 mg m-2 of N2O‒N, respectively (Fig. 2). During the fall-spring, 197 d between the two maize growing cycles, no difference was observed for the treatments, and the mean emission of N2O‒N was 0.06 mg m-2 d-1, which resulted in a cumulative emission of approximately 13.0 mg m-2 for all treatments (results not shown).
In contrast to the first season, in 2017/2018, besides the intercropping effect, some plots (MB0 and MB150) had a mulch of B. brizantha from the previous fall-winter period. The cumulative nitrous oxide emission was higher in treatments where the B. brizantha mulch was combined with N fertilization: MB150 emitted 90.3 mg N2O‒N m-2 during the 137 d between maize sowing and harvest, which was higher than the emission observed in M150 and MH150 (56.0 and 58.2 mg N2O‒N m-2, respectively). The cumulative emission of the unfertilized plots with B. brizantha mulch (MB0) was 64.0 mg N‒N2O m-2. These emissions were similar to those observed in maize monocrops or maize plus B. humidicola fertilized with 150 kg N ha-1 (M150 and MH150), but higher than that of other treatments without N: M0 and MH0, which emitted 23.7 and 24.4 mg N2O‒N m-2, respectively (Fig. 3).
Considering only the period between maize sowing and fertilization (41 DAS), MB0 resulted in an N2O‒N emission of 50.3 mg m-2, accounting for 79% of the total emissions in the maize cycle (137 d), higher than M0 and MH0 that emitted 12.4 and 11.4 mg m-2 of N2O‒N, accounting for 52% and 47% of the N2O emitted, respectively. For the period between fertilization and maize harvesting, MB150 resulted in higher N2O‒N emissions, up to 46.7 mg m-2, compared to M150 and MH150 which emitted 27.4 and 20.2 mg N2O‒N m-2, respectively. The emissions after fertilization accounted for 52%, 49%, and 35% of the total N2O emissions of MB150, M150, and MH150, respectively (Fig. 3).
The peak of N2O emission was observed in MB150 between 2 and 13 d after fertilization (DAF), ranging from 1.15 to 3.65 mg N2O‒N m-2 d-1. M150 presented two peaks 8 and 13 DAF when 2.38 and 1.35 mg N2O‒N m-2 d-1 were emitted, respectively (Fig. S1). After N fertilization, MH150 presented only one peak at 8 DAF, emitting 1.41 mg N2O‒N m-2 d-1. However, higher emissions were observed in MH150 before N fertilization, following 60 mm rainfall during 3 d after a relatively long dry period of 20 d (without precipitation greater than 4 mm). The daily N2O emissions ranged from 2.06 to 3.46 mg N2O‒N m-2 day-1 between 24 and 29 DAS (Fig. S1). For the same period, M150 and MB150 emissions ranged from 1.48 to 2.29 mg N2O‒N m-2 d-1. In treatments without N in the previous season, emissions ranged from 0.49 to 1.08, 1.38–2.75, and 0.44–0.84 mg N2O‒N m-2 d-1 for M0, MB0, and MH0, respectively.
3.4. Carbon dioxide emissions
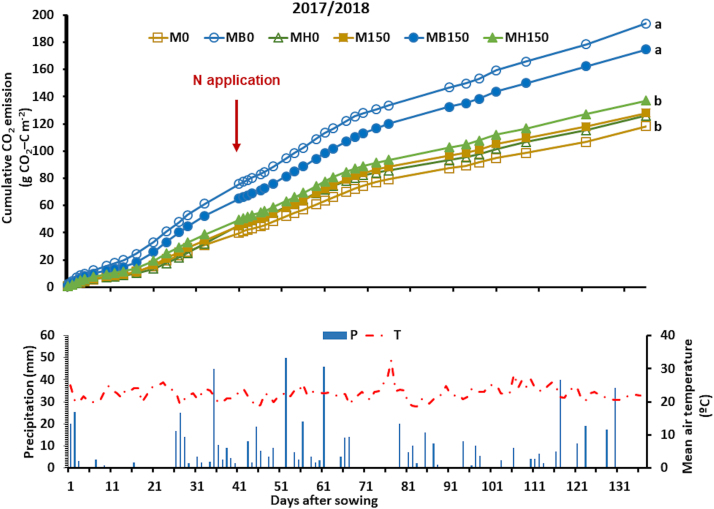
When maize was growing in the second season (2017/2018), CO2 emissions were higher in plots with B. brizantha intercropped with maize in the presence of the mulch from the previous cycle (Fig. 5). MB0 and MB150 emitted 193.6 and 174.6 g CO2‒C m-2, respectively. In plots without B. brizantha (M0, MH0, M150, and MH150), the cumulative emissions were 118, 126, 128, and 137 g CO2‒C m-2, respectively (Fig. 5). For all treatments, 41 days between sowing and N fertilization resulted in 33% and 39% of the CO2 emissions of the entire period.
Fig. 5.
Cumulative carbon dioxide emission, precipitation and mean air temperature during maize growth in the 2nd harvest season, as affected by the combination of N fertilization (0 and 150 kg ha-1) and Brachiaria intercropping: B. brizantha (B) and B. humidicola (H). The arrow indicates when N fertilizer was applied to maize. Different letters indicate statistically significant differences among treatments (p ≤ 0.05). Legend abbreviations: M, MB, and MH denote monocrop maize, maize intercropped with B. brizantha, and maize intercropped with B. humidicola, respectively; 0 and 150 denote N rate applied to the maize crop.
3.5. EF and EI of N fertilization
In the 2016/2017 season, the N2O EF was 0.06% and 0.07% of the fertilizer N applied for M150 and MB150, respectively. In the 2017/2018 season, the EFs were 0.22%, 0.18%, and 0.23% for M150, MB150, and MH150, respectively (Table 4).
Table 4.
Nitrous oxide emission factor (EF) and emission intensity (EI) (mean ± standard deviation) of the top-dressed N fertilization during two maize harvest seasons (2016/17 and 2017/18), as affected by the combination of N fertilization (0 or 150 kg ha-1) and intercrop with B. brizantha (B) or B. humidicola (H).
| Treatments | EF |
EI |
||
|---|---|---|---|---|
| 2016/17 | 2017/18 | 2016/17 | 2017/18 | |
| ------- % ------- | --- kg CO2 eq. t-1 of grain --- | |||
| M0 | – | – | 14.5a ± 2.49 | 21.0c ± 6.28 |
| MB0 | – | – | 11.9a ± 1.32 | 51.1a ± 18.1 |
| MH0 | – | – | – | 25.0c ± 8.22 |
| M150 | 0.06 | 0.22 | 13.11a ± 3.38 | 24.1c ± 13.9 |
| MB150 | 0.07 | 0.18 | 12.17a ± 2.60 | 36.6b ± 9.64 |
| MH150 | – | 0.23 | – | 21.0c ± 3. 43 |
M: maize monocrop; MB and MH: maize intercropped with B. brizantha and B. humidicola, respectively; 0 and 150 subscripts indicate the 0 and 150 kg ha-1 of N applied to the maize crop. Different letters after means denote significant differences between treatments in the columns for the LSD test (p ≤ 0.05).
Although the N2O emissions in 2016/2017 were higher in the fertilized plots than in the unfertilized plots (Fig. 1), no significant differences were observed between treatments when grain yield was used to compute the CO2eq EI. EI ranged from 11.9 to 14.5 kg CO2 eq t-1 of grain. In the 2017/2018 season, EI was higher in treatments with B. brizantha. The EI values for MB0 and MB150 were 51.1 and 36.6 kg t-1, respectively, whereas M0, M150, MH0, and MH150 resulted in EI values of 21.0, 24.1, 24.9, and 21.1 kg t-1, respectively (Table 4).
3.6. BNI potential of roots
The residual effect of the N fertilizer applied to maize did not affect the root BNI potential of weeds and brachiaria. The specific BNI potential of dry roots for the monocrop maize treatment, where weeds grew during the dry season, ranged from 127 to 130 ATU g-1 of dry roots (Table 5). The specific BNI potential of roots in monocrop maize was higher than that observed in B. brizantha roots (p < 0.05), which ranged from 113 to 119 ATU g-1 in the 0–10 cm soil layer (Table 5). However, taking into account the root biomass of each treatment and the specific BNI potential of the roots in the 0–10 cm soil layer (=total BNI capacity), MB0 and MB150 had a higher total BNI potentials of 23,641 and 24,927 ATU m-2, respectively, compared with 2958 and 3025 ATU m-2 observed on M0 and M150 (p < 0.05), respectively (Table 5).
Table 5.
Potential of biological nitrification inhibition of roots collected from the 0–10 cm soil layer of B. brizantha and weeds growing during fall and winter after the first maize harvest (2016/17). Data: mean ± standard deviation. ATU: allylthiourea unit.
| Treatments | BNI potential of roots |
|
|---|---|---|
| ATU g-1 | ATU m-2 | |
| Weeds (M0) | 127a ± 4.3 | 2957b ± 99 |
| Weeds (M150) | 130a ± 7.8 | 3025b ± 181 |
| B. brizantha (MB0) | 113b ± 5.7 | 23,641a ± 1183 |
| B. brizantha (MB150) | 119b ± 7.5 | 24,929a ± 1569 |
M: maize monocrop; MB: maize intercropped with B. brizantha; 0 and 150 subscripts indicate the 0 and 150 kg ha-1 of N applied to the maize crop. Brachiaria was sown with maize, grew slowly due to shading by the maize crop and developed in the fall-winter after maize was harvested. Weeds refer to plants that grew after the maize harvest in the treatments without brachiaria intercropping. Different letters after means denote significant differences between treatments in the columns for the LSD test (p ≤ 0.05).
3.7. Soil characteristics
The only difference observed in soil inorganic N in the 2016/2017 maize growing season was caused by N fertilization, which was evident in soil NH4+‒N and NO3-‒N concentrations, which ranged from 28.0 to 66.8 mg kg-1 and from 51.1 to 63.8 mg kg-1, respectively, 1 week after fertilization. Unfertilized treatments presented soil NH4+‒N and NO3-‒N concentrations ranging from 12.2 to 14.2 mg kg-1 and 11.1–17.3 mg kg-1, respectively (results not shown).
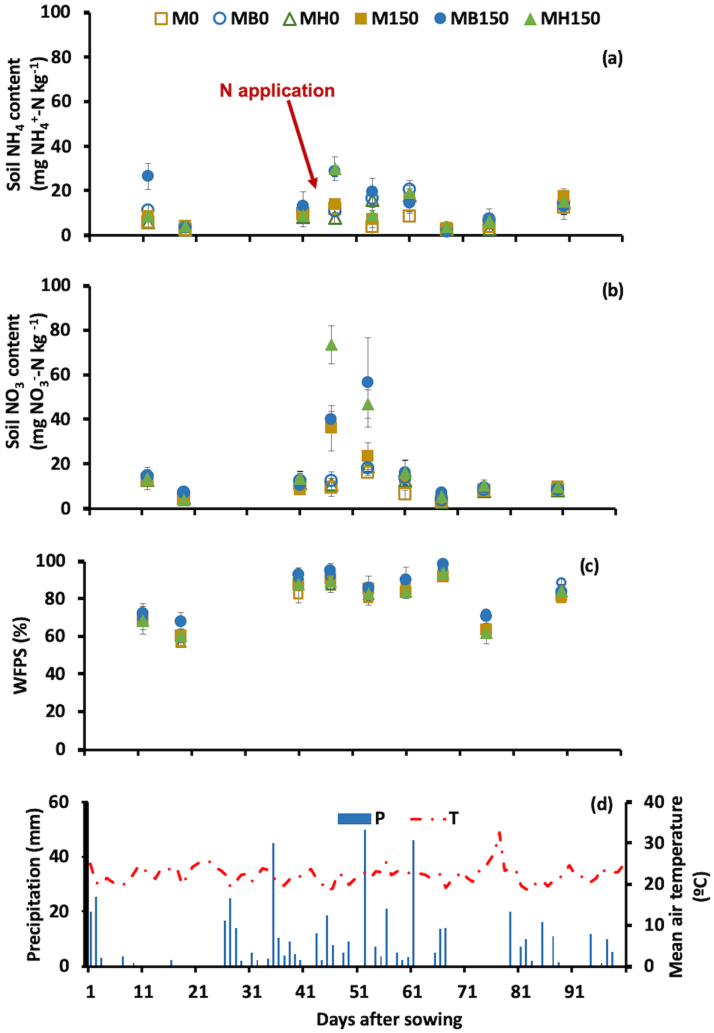
In the second season (2017/2018), soil samples taken 12 d after sowing (and before N fertilizer application) exhibited higher NH4+‒N (26.5 mg kg-1) in the MB150 treatment than in all other treatments, ranging from 6.0 to 11.5 mg kg-1 (Fig. 4). One week after maize fertilization with 150 kg N ha-1 as ammonium nitrate, all fertilized plots had higher soil NO3-‒N than did the unfertilized plots, suggesting the occurrence of rapid nitrification. The MH150 and MB150 treatments showed higher soil NH4+‒N than M0, M150, MB0, and MH0 (Fig. 4).
Fig. 4.
Soil ammonium (a) and nitrate (b) content, WFPS (c) and temperature and precipitation (d) during the maize growth the 2nd harvest season. Legend abbreviations: M: maize monocrop; MB and MH: maize intercropped with B. brizantha and B. humidicola, respectively; 0 and 150 subscripts indicate the 0 and 150 kg ha-1 of N applied to the maize crop. Bars over the symbols indicate the standard deviation and the arrow indicates the date of N fertilizer application.
The water-filled pore spaces (WFPS) were higher in treatments with B. brizantha mulch (MB0 and MB150) than in the other treatments during periods of lower rainfall (19 and 76 DAS).
4. Discussion
4.1. Effects of brachiaria intercropping and mulch on maize yield
Our data confirmed the findings of other studies that intercropping had no negative impacts on maize grain yield when appropriately fertilized (Borghi et al., 2014, Borghi et al., 2013, Momesso et al., 2019). This was further evidence that, if properly managed, the competition of forage grass with maize is negligible. In another study, growing B. humidicola and Panicum maximum intercropped with maize did not affect the N fertilizer utilization by maize because the forage grasses absorbed less than 5% of the 15N fertilizer applied (Coser et al., 2016).
Interestingly, there was no response of the biomass yield of B. brizantha growing unshaded after maize to the N fertilizer applied in the first maize crop, either because the residual N from fertilizer was small or because the grasses were effective in taking up N from the soil N pool that had not been affected by maize fertilization. However, the forage growing after maize in the plot with no N fertilizer (MB0) or fertilized (MB150) accumulated 119 and 93 kg ha-1 more N compared with weeds grown on plots with no fertilizer (M0) and fertilized (M150) maize, respectively (Table 2), indicating that the mixed maize-brachiaria system can improve N cycling. This N would be theoretically available in the system and could be taken up by the subsequent maize crop; however, there was no net increase in maize yields or N accumulation because of this extra nutrient recycled by B. brizantha (Table 3).
We expected that the N accumulated and recycled by B. brizantha would increase the yield and N uptake of maize grown subsequently, but this did not happen. Our data suggest that N may have been released slowly and not in synchrony with the maize demand and or may have been released earlier and part of it was lost.
Because N is bound to organic compounds in the plant, the residues must be mineralized to release the nutrients. It has been shown that residues with a high C:N ratio have a low decomposition rate (Boer et al., 2007). N from legume mulch is more easily released into the soil than that of grasses (Ranells and Wagger, 1997) and the release of N from the tissues of C4 plants is slower than from C3 plants (Rosolem et al., 2005). In our study, most of the brachiaria growth occurred in the spring after the rains started and leaves were young when desiccated. Thus, the C:N ratios of aboveground parts of B. brizantha were not high (C:N 19–24 for MB0 and 21–34 for MB150). However, the plant material remained on the soil surface, where mineralization is slow. Therefore, most of the N accumulated in the forage residue was probably not released in time for maize uptake.
Confirming the asynchrony between the N release by B. brizantha and maize uptake, other authors found that only small amounts of N from the brachiaria cover crop are transferred to the next crop. Borghi et al. (2014) observed that B. brizantha grown during the fall-winter season recovered between 71% and 82% of the 15N applied to the grass. However, the N accumulated by the forage was poorly used by maize sowed in the following summer over the mulch of B. brizantha; only 3–5% of the 15N was absorbed by the maize crop (Borghi et al., 2014). Similar results were reported by Momesso et al. (2019) and with other crops/mulch combinations by Boaretto et al. (2004), Silva et al., 2006a, Silva et al., 2006b, and Oliveira et al. (2018). Thus, the transfer of the N previously taken up by plants used as mulch for the following crop grown under no-till has been relatively small.
The high response of maize to N fertilizer in soil with and without mulch, regardless of more than 100 kg N ha-1 in brachiaria residue (Table 2, Table 3), suggests that the fertilizer must be supplied every year to support high maize yields under no-till when grasses are the cover crops, at least for the first few years after the system is established. Therefore, our hypothesis that forage grasses grown between maize seasons could cycle soil and fertilizer N and make most of it available to the following summer crop could not be proven. However, we only measured the AE of the cash crop and did not evaluate the effect of intercropping at the system level. Functional crop diversity, including intercropping and rotation, may lead to higher and more stable yields over time (Chimonyo et al., 2019). Although this study did not show short-term evidence, the benefits of cycling N and other nutrients and supplying organic C to the soil system will probably be noticed in the medium to long term (Qin et al., 2015, Crusciol et al., 2015), as most of the N from plant residues enriches the soil organic N pool (Fortes et al., 2013, Smith and Chalk, 2018, Rocha et al., 2019, Oliveira et al., 2019).
In addition to the lack of synchrony between the N released by B. brizantha and maize N uptake, part of the N accumulated in the grass may have been released early in the maize cycle. When the cover crop is herbicide-desiccated, ammonia volatilization is enhanced, and some N is released into the soil. Desiccation is usually conducted 20–30 d before sowing cash crops. The N from the cover crop released in the soil upon desiccation may not be efficiently taken up by maize because of the low demand in the early growth stages (first month) (Bender et al., 2013). After the end of the dry season, the temperature and precipitation were already high (Figs. 2 and 3) leading to N losses. Pacheco et al. (2013) evaluated the amount of N in the shoot residue of B. brizantha after desiccation and observed that the N content of B. brizantha residues was reduced from 135 kg ha-1 at desiccation to 82 kg ha-1 30 d later. Despite the difference of 53 kg N ha-1 in the shoot residue of B. brizantha during this period, these authors did not observe higher rice yield than fallows, where the amount of N in the desiccated mulch (weeds) decreased by only 17 kg ha-1. Castoldi et al. (2014) observed losses of 35% of the N from different forage grass species 28 d after desiccation with glyphosate. Similar losses were found by Damin et al. (2017) in black oats after desiccation with different herbicides. Thus, the desiccation of cover crops may be responsible for losses of at least part of the potentially cycled N through ammonia volatilization and rapid release in the soil (Damin et al., 2017, Damin et al., 2010, Pacheco et al., 2017). Denitrification losses cannot be ruled out although Oxisols usually have good drainage. However, the amount of N that was volatilized or released into the soil was probably not sufficient to justify the lack of response of the next crop.
4.2. Effect of brachiaria intercropping on GHG emissions
The data on N2O emissions also appear to support the hypothesis that part of the brachiaria N was released before maize could use it. The large amounts of B. brizantha dry matter and the N recycled in the intercrop treatments, and the N released by desiccation probably caused increased N2O emissions in the early stages of the maize cycle in the second year. Our initial hypothesis that maize intercropping with forage grasses could reduce N2O emissions could not be proven (Table 4), at least for the first two years. During the first maize growing cycle significantly higher N2O emissions only occurred when N fertilizer was applied (Fig. 2), which was expected because of the higher concentration of mineral N, substrate for the processes that generate N2O (Bouwman et al., 2002, Lourenço et al., 2018, Soares et al., 2016). N fertilizer application also increased N2O emissions in the second growing season (2017/2018) (Fig. 3). However, in the second season, the emissions of N2O during the first 41 d in the maize cycle, before N fertilization, were higher in the plots where B. brizantha had been desiccated (MB0 and MB150) than in plots where there was no B. brizantha mulch (M0, MH0, M150, and MH150) (Fig. 3). N2O emissions of treatments MB0 and MB150 which occurred before maize fertilization with 150 kg N ha-1 accounted for 79% of the cumulative emission of the entire cycle (Fig. 3). This higher N2O emissions appeared to be caused by large amounts of brachiaria DM and N (Table 2). Nonetheless, the conservation of soil moisture by brachiaria mulch cannot be ruled out as a cause of increased N2O emissions.
The N release upon brachiaria desiccation as a cause of the high N2O emissions of treatments MB0 and MB150 is supported by indirect evidence. We did not measure the amount of N released from the 8.1 t ha-1 of forage dry matter when plants were desiccated. However, Castoldi et al. (2014) showed that the total N loss from 6.1 t ha-1 of brachiaria after desiccation ranged from 10.8 to 28.2 kg ha-1. The higher soil NH4+‒N content also supported the release of part of the N from B. brizantha soon after desiccation in our study in the MB150 plots in a concentration equivalent to that observed 1 week after N fertilization (Fig. 4).
The effect of mulch on N2O emissions may go beyond N released by desiccation. Chen et al. (2013) showed in their meta-analysis that the C and N input by crop residues might influence N2O emissions because of the supply of sufficient N for nitrification and denitrification (Guardia et al., 2016). This may explain why the cumulative N2O emissions of MB0 (which accumulated 124 kg ha-1 of N in the mulch and 2.56 t ha-1 of C) were higher than that of the non-fertilized treatments (M0 and MH0) and similar to the plots that received mineral N fertilizer.
In the second season (2017/18), CO2 emissions were higher in treatments with B. brizantha mulch (MB0 and MB150) than in other treatments, irrespective of N fertilization (Fig. 5). The higher maize yield (Table 3) because of N fertilization did not result in higher CO2 emissions. Thus, the higher CO2 emissions observed with B. brizantha were probably caused by its high C input in the soil, which was partially degraded by heterotrophic biota.
4.3. Fertilizer N2O EF and EI
The low fertilizer EF values observed in the first season (0.06–0.07% of the N applied, Table 4) may be attributed, at least partially, to the low soil pH (4.7–5.0) which may have limited nitrification and denitrification (Bremner and Shaw, 1958, Subbarao et al., 2006b). During the second season, the fertilizer EF was also low compared with the 1% default value suggested by the IPCC (IPCC, 2007). Another reason for the low EF values may be the rapid uptake of the fertilizer N, applied when maize was in a stage of active growth. Indeed, the soil of fertilized plots showed high inorganic N contents for just two weeks. These results agree with those of other studies under similar conditions (Hickman et al., 2015, Jankowski et al., 2018, Meurer et al., 2016).
The annual N2O emission in Brazilian croplands ranges from – 7 to 426 mg N2O‒N m-2, with an average of 80 mg N2O‒N m-2 (Meurer et al., 2016). In our study, the accumulated N2O emissions between maize cycles (346 d because of early sowing in 2017) varied from 28.8 to 40.0 mg N2O‒N m-2 and were lower than the average value for Brazilian croplands reported by Meurer et al. (2016).
We also calculated EI, the GHG emissions expressed in relation to maize production. In the first maize cycle, N application increased N2O emissions (Fig. 2) but did not increase the EI (Table 4) because grain yields increased by 67% (Table 3). In the second maize crop, the cumulative N2O emissions of the fertilized plots were higher than in the first season (Fig. 3), resulting in increased EI (Table 4). Likewise, in the first crop, the EI of both the fertilized and unfertilized monocrop maize plots were similar because the increased N2O emissions were compensated by higher grain yields (Table 3).
Other potential benefits of grass intercropping remain, such as the significant amounts of N cycled and C added to the soil, which may lead to improved soil fertility and long-term gains including high grain yields (Mateus et al., 2016, Crusciol et al., 2015).
Moreover, this study highlights that there was no negative effect of the intercropping system on maize grain yield, supporting the benefit of the interaction of two plant species growing at the same time and space (Ehrmann and Ritz, 2013, Rosolem et al., 2017).
4.4. Potential biological nitrification inhibition
The presence of B. humidicola intercropped with maize in the second season increased maize grain yield by more than 1.2 t ha-1 (p < 0.1) compared with that of the other fertilized treatments. The higher yield observed when N was applied to maize intercropped with B. humidicola may not be caused by the net mineralization of N from the biomass mulch because the treatments with B. humidicola had the same straw biomass input as monocrop maize.
There are reports of BNI from several tropical grass species, but especially B. humidicola (Subbarao et al., 2009), and evidence that these grasses could also decrease soil nitrification and N2O emissions (Byrnes et al., 2017, Karwat et al., 2018, Karwat et al., 2017, Moreta et al., 2014). The BNI potential of weed roots was slightly higher than that of B. brizantha when expressed as ATU g-1 roots, but the total BNI potential (ATU m-2) of the latter was higher because of the larger biomass yield. Despite the indications of high BNI potential of plots with B. brizantha (Table 5), our study did not reveal evidence of nitrification suppression of soil mineral N and N2O emissions. In agreement, Rocha et al. (2019), in a study conducted in the same region as our experiment, reported that different tropical grasses, including B. brizantha, failed to significantly decrease nitrification in the subsequent maize crop. It appears that the BNI effect requires up to 1–2 years after the grass is established (Nuñez et al., 2018, Subbarao et al., 2015), a condition that was not observed in our study.
We have no explanation for the positive effect of B. humidicola intercropped with maize. One possibility is that the slight soil disturbance caused by B. humidicola seeding before the second maize crop may have stimulated soil N mineralization. Another possibility is that B. humidicola is more active into producing BNI compounds than B. brizantha (Subbarao et al., 2008), and therefore may have released small amounts of exudate that somehow promoted physiological changes in maize, such as root branching or N immobilization by microbes, which could have increased maize yield. However, this hypothesis must be tested.
5. Conclusions
In this study, no negative effect of no-till intercropping was observed on maize grain yield. We observed the high potential of B. brizantha to cycle N and input C in the soil, which can result in long-term benefits. However, the hypothesis that intercropping results in higher grain yield and greater agronomic efficiency of the applied N in short-term for the cash crop was denied. B. brizantha intercropping increased N2O emissions. The absence of direct benefits of the N recycled by brachiarias to maize yields as well as the increase in N2O emissions may be caused by the relatively rapid N release of part of the B. brizantha N after desiccation and the desynchrony between the release of the remaining brachiaria N and the maize nutrient demand.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was undertaken as part of NUCLEUS: a virtual joint centre to deliver enhanced NUE via an integrated soil-plant systems approach for the United Kingdom and Brazil. Funded in Brazil by FAPESP—São Paulo Research Foundation [Grant 2015/50305–8], FAPEG—Goiás Research Foundation [Grant 2015–10267001479], and FAPEMA—Maranhão Research Foundation [Grant RCUK-02771/16]; and in the United Kingdom by the Biotechnology and Biological Sciences Research Council — BBSRC/Newton Fund [BB/N013201/1]. Additional grants from FAPESP [2018/20.2793–9] and CNPq—National Council for Technological and Scientific Development/Brazil [310.478/2017–0] funded this project. In Colombia, this study was undertaken by CIAT- International Center for Tropical Agriculture as part of the LivestockPlus project funded by the Consultative Group on International Agricultural Researh - CGIAR Research Program (CRP) on Climate Change, Agriculture, and Food Security (CCAFS). This work was also done as part of the Livestock CRP.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.agee.2021.107491.
Appendix A. Supplementary material
Supplementary material
.
References
- Bender R.R., Haegele J.W., Ruffo M.L., Below F.E. Nutrient uptake, partitioning, and remobilization in modern, transgenic insect-protected maize hybrids. Agron. J. 2013;105:161–170. doi: 10.2134/agronj2012.0352. [DOI] [Google Scholar]
- Boaretto A.E., Spolidorio E.S., Aplicada D.D.E.N., Sucessão E.M. Fate of 15N-urea applied to wheat-soybean succession crop. Bragantia. 2004;63:265–274. doi: 10.1590/S0006-87052004000200011. [DOI] [Google Scholar]
- Boer C.A., Assis R.L.D., Silva G.P., Braz A.J.B.P., Barroso A.L.D.L., Cargnelutti Filho A., Pires F.R. Nutrient cycling in off-season cover crops on a Brazilian savanna soil. Pesqui. Agropecuária Bras. 2007;42:1269–1276. doi: 10.1590/S0100-204X2007000900008. [DOI] [Google Scholar]
- Borghi E., Crusciol C.A.C., Mateus G.P., Nascente A.S., Martins P.O. Intercropping time of corn and palisadegrass or guineagrass affecting grain yield and forage production. Crop Sci. 2013;53:629–636. doi: 10.2135/cropsci2012.08.0469. [DOI] [Google Scholar]
- Borghi E., Crusciol C.A.C., Trivelin P.C.O., Nascente A.S., Costa C., Mateus G.P. Nitrogen fertilization ((NH4NO3)-N15) of palisadegrass and residual effect on subsequent no-tillage corn. Rev. Bras. de Ciência do Solo. 2014;38:1457–1468. doi: 10.1590/S0100-06832014000500011. [DOI] [Google Scholar]
- Bouwman A.F., Boumans L.J.M., Batjes N.H. Emissions of N2O and NO from fertilized fields: summary of available measurement data. Glob. Biogeochem. Cycles. 2002;16:6–1–6–13. doi: 10.1029/2001GB001811. [DOI] [Google Scholar]
- Bremner J.M., Shaw K. Denitrification in soil. I. Methods of investigation. J. Agric. Sci. 1958;51:22–39. doi: 10.1017/S0021859600032767. [DOI] [Google Scholar]
- Brooker R.W., Bennett A.E., Cong W.-F., Daniell T.J., George T.S., Hallett P.D., Hawes C., Iannetta P.P.M., Jones H.G., Karley A.J., Li L., McKenzie B.M., Pakeman R.J., Paterson E., Schöb C., Shen J., Squire G., Watson C.A., Zhang C., Zhang F., Zhang J., White P.J. Improving intercropping: a synthesis of research in agronomy, plant physiology and ecology. N. Phytol. 2015;206:107–117. doi: 10.1111/nph.13132. [DOI] [PubMed] [Google Scholar]
- Byrnes R.C., Nùñez J., Arenas L., Rao I., Trujillo C., Alvarez C., Arango J., Rasche F., Chirinda N. Biological nitrification inhibition by Brachiaria grasses mitigates soil nitrous oxide emissions from bovine urine patches. Soil Biol. Biochem. 2017;107:156–163. doi: 10.1016/j.soilbio.2016.12.029. [DOI] [Google Scholar]
- Cantarella H., van Raij B., Camargo C.E.O. Cereals. In: van Raij B., Cantarella H., Quaggio J.A., Furlani A.C.M., editors. Lime and Fertilizer Recommendations for the State of Sao Paulo. (In Portuguese) 2nd ed., Tech. Bull. 100. Inst. Agronomico; Campinas, SP, Brazil: 1997. pp. 43–50. [Google Scholar]
- Carvalho J.L.N., Raucci G.S., Frazão L.A., Cerri C.E.P., Bernoux M., Cerri C.C. Crop-pasture rotation: a strategy to reduce soil greenhouse gas emissions in the Brazilian Cerrado. Agric. Ecosyst. Environ. 2014;183:167–175. doi: 10.1016/j.agee.2013.11.014. [DOI] [Google Scholar]
- Castoldi G., Reis J.G., Pivetta L.A., Rosolem C.A. Soil nitrogen dynamics after brachiaria desiccation. Rev. Bras. de Ciência do Solo. 2013;37:1620–1627. doi: 10.1590/S0100-06832013000600018. [DOI] [Google Scholar]
- Castoldi G., Pivetta L.A., Rosolem C.A. Nitrogen budget in a soil-plant system after brachiaria grass desiccation. Soil Sci. Plant Nutr. 2014;60:162–172. doi: 10.1080/00380768.2013.878641. [DOI] [Google Scholar]
- Chen H., Li X., Hu F., Shi W. Soil nitrous oxide emissions following crop residue addition: a meta-analysis. Glob. Change Biol. 2013;19:2956–2964. doi: 10.1111/gcb.12274. [DOI] [PubMed] [Google Scholar]
- Chien S.H., Prochnow L.I., Cantarella H. Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. Adv. Agron. 2009;102:267–322. doi: 10.1016/S0065-2113(09)01008-6. [DOI] [Google Scholar]
- Chimonyo V.G.P., Snapp S.S., Chikowo R. Grain legumes increase yield stability in maize based cropping systems. Crop Sci. 2019;59:1222–1235. doi: 10.2135/cropsci2018.09.0532. [DOI] [Google Scholar]
- Cong W.-F., Hoffland E., Li L., Six J., Sun J.-H., Bao X.-G., Zhang F.-S., Van Der Werf W. Intercropping enhances soil carbon and nitrogen. Glob. Change Biol. 2014;21:1715–1726. doi: 10.1111/gcb.12738. [DOI] [PubMed] [Google Scholar]
- Coser T.R., Ramos M.L.G., Figueiredo C.C., Urquiaga S., Carvalho A.M., Barros F.V., Mendonça M.T. Nitrogen uptake efficiency of maize in monoculture and intercropped with Brachiaria humidicola and Panicum maximum in a dystrophic Red-Yellow Latosol of the Brazilian Cerrado. Crop Pasture Sci. 2016;67:47–54. doi: 10.1071/CP15077. [DOI] [Google Scholar]
- Crusciol C.A.C., Nascente A.S., Borghi E., Soratto R.P., Martins P.O. Improving soil fertility and crop yield in a tropical region with palisadegrass cover crops. Agron. J. 2015;107:2271–2280. doi: 10.2134/agronj14.0603. [DOI] [Google Scholar]
- Damin V., Trivelin P.C.O., Carvalho S.J.P., Moraes M.F., Barbosa T.G. Herbicide application increases nitrogen (15N) exudation and root detachment of Brachiaria decumbens Stapf. Plant Soil. 2010;334:511–519. doi: 10.1007/s11104-010-0402-6. [DOI] [Google Scholar]
- Damin V., Trivelin P.C.O., Moraes L.A.C., Bruno I.P., Figueiredo L.A., Lima A.B., Genuário D.B. Losses of 15N-nitrogen by plant–soil system after herbicide application on black oat. Acta Agric. Scand. Sect. B: Soil Plant Sci. 2017;67:202–207. doi: 10.1080/09064710.2016.1245773. [DOI] [Google Scholar]
- Dobermann, A., 2007. Nutrient use efficiency - measurement and management. In: IFA International Workshop on Fertilizer Best Management Practices, Part 1. General principles of Fertilizer Best Management Practices. IFA - Intl. Fertilizer Industry Association, Brussels, pp. 1–30.
- Ehrmann J., Ritz K. Plant: soil interactions in temperate multi-cropping production systems. Plant Soil. 2013;376:1–29. doi: 10.1007/s11104-013-1921-8. [DOI] [Google Scholar]
- Ferreira, D.F., 2014. Análisesestatísticas por meio do Sisvar para Windows versão 4.0. 45a Reunião Anualda Região Brasileira da Sociedade internacional de Biometria.
- Fortes C., Vitti A.C., Otto R., Ferreira D.A., Franco H.C.J., Trivelin P.C.O. Contribution of nitrogen from sugarcane harvest residues and urea for crop nutrition. Sci. Agric. 2013;70:313–320. doi: 10.1590/S0103-90162013000500005. [DOI] [Google Scholar]
- Guardia G., Abalos D., García-Marco S., Quemada M., Alonso-Ayuso M., Cárdenas L.M., Dixon E.R., Vallejo A. Effect of cover crops on greenhouse gas emissions in an irrigated field under integrated soil fertility management. Biogeosciences. 2016;13:5245–5257. doi: 10.5194/bg-13-5245-2016. [DOI] [Google Scholar]
- Hickman J.E., Tully K.L., Groffman P.M., Diru W., Palm C.A. A potential tipping point in tropical agriculture: Avoiding rapid increases in nitrous oxide fluxes from agricultural intensification in Kenya. J. Geophys. Res.: Biogeosci. 2015;120:938–951. doi: 10.1002/2015JG002913. [DOI] [Google Scholar]
- Iizumi T., Mizumoto M., Nakamura K. A bioluminescence assay using Nitrosomonas europaea for rapid and sensitive detection of nitrification inhibitors. Appl. Environ. Microbiol. 1998;64:3656–3662. doi: 10.1128/aem.64.10.3656-3662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INTERGOVERNMENTAL PANEL ON CLIMATE CHANGE (IPCC), 2007. Fourth Assessment Report (AR4), Working Group I, UNEP. Geneva, Switzerland.
- Jankowski K., Neill C., Davidson E.A., Macedo M.N., Costa C., Galford G.L., Maracahipes Santos L., Lefebvre P., Nunes D., Cerri C.E.P., McHorney R., O’Connell C., Coe M.T. Deep soils modify environmental consequences of increased nitrogen fertilizer use in intensifying Amazon agriculture. Sci. Rep. 2018;8:13478. doi: 10.1038/s41598-018-31175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwat H., Moreta D., Arango J., Núñez J., Rao I., Rincón Á., Rasche F., Cadisch G. Residual effect of BNI by Brachiaria humidicola pasture on nitrogen recovery and grain yield of subsequent maize. Plant Soil. 2017;420:389–406. doi: 10.1007/s11104-017-3381-z. [DOI] [Google Scholar]
- Karwat H., Egenolf K., Nuñez J., Rao I., Rasche F., Arango J., Moreta D., Arevalo A., Cadisch G. Low 15N natural abundance in shoot tissue of Brachiaria humidicola is an indicator of reduced N losses due to biological nitrification inhibition (BNI) Front. Microbiol. 2018;9:2383. doi: 10.3389/fmicb.2018.02383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço K.S., Cassman N.A., Pijl A.S., van Veen J.A., Cantarella H., Kuramae E.E. Nitrosospira sp. govern nitrous oxide emissions in a tropical soil amended with residues of bioenergy crop. Front. Microbiol. 2018;9:1–11. doi: 10.3389/fmicb.2018.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Feria R.A., Castellano M.J., Dietzel R.N., Helmers M.J., Liebman M., Huber I., Archontoulis S.V. Linking crop- and soil-based approaches to evaluate system nitrogen-use efficiency and tradeoffs. Agric. Ecosyst. Environ. 2018;256:131–143. doi: 10.1016/j.agee.2018.01.002. [DOI] [Google Scholar]
- Mateus G.P., Crusciol C.A.C., Pariz C.M., Borghi E., Costa C., Martello J.M., Franzluebbers A.J., Castilhos A.M. Sidedress nitrogen application rates to sorghum intercropped with tropical perennial grasses. Agron. J. 2016;108:433–447. doi: 10.2134/agronj2015.0236. [DOI] [Google Scholar]
- Meurer K.H.E., Franko U., Stange C.F., Rosa J.D., Madari B.E., Jungkunst H.F. Direct nitrous oxide (N2O) fluxes from soils under different land use in Brazil—a critical review. Environ. Res. Lett. 2016;11 doi: 10.1088/1748-9326/11/2/023001. [DOI] [Google Scholar]
- Momesso L., Crusciol C.A.C., Soratto R.P., Vyn T.J., Tanaka K.S., Costa C.H.M., Neto J.F., Cantarella H. Impacts of nitrogen management on no-till maize production following forage cover crops. Agron. J. 2019;111:639–649. doi: 10.2134/agronj2018.03.0201. [DOI] [Google Scholar]
- Moreta D.E., Arango J., Sotelo M., Vergara D., Rincón A., Ishitani M., Castro A., Miles J., Peters M., Tohme J.O.E., Subbarao G.V., Rao I.M. Biological nitrification inhibition (BNI) in Brachiaria pastures: a novel strategy to improve eco-efficiency of crop-livestock systems and to mitigate climate change. Trop. Grassl. 2014;2:88–91. doi: 10.17138/TGFT(2)88-91. [DOI] [Google Scholar]
- Mueller S.M., Messina C.D., Vyn T.J. Simultaneous gains in grain yield and nitrogen efficiency over 70 years of maize genetic improvement. Sci. Rep. 2019;9:9095. doi: 10.1038/s41598-019-45485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez J., Arevalo A., Karwat H., Egenolf K., Miles J., Chirinda N., Cadisch G., Rasche F., Rao I., Subbarao G., Arango J. Biological nitrification inhibition activity in a soil-grown biparental population of the forage grass, Brachiaria humidicola. Plant Soil. 2018;426:401–411. doi: 10.1007/s11104-018-3626-5. [DOI] [Google Scholar]
- Oliveira S.M. d, Almeida R.E.M., Ciampitti I.A., Pierozan Junior C., Lago B.C., Trivelin P.C.O., Favarin J.L. Understanding N timing in corn yield and fertilizer N recovery: an insight from an isotopic labeled-N determination. PloS One. 2018;13 doi: 10.1371/journal.pone.0192776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira S.M.D., Almeida R.E.M.D., Pierozan Junior C., Reis A.F.D.B., Souza L.F.N., Favarin J.L. Contribution of corn intercropped with Brachiaria species to nutrient cycling. Pesqui. Agropecuária Trop. 2019;49 doi: 10.1590/1983-40632019v4955018. [DOI] [Google Scholar]
- Pacheco L.C.P.S., Damin V., Pelosi A.P., Ferreira K.R.S., Trivelin P.C.O. Herbicides increase emission of ammonia by pearl millet and congo grass. Agron. J. 2017;109:1232–1239. doi: 10.2134/agronj2016.04.0242. [DOI] [Google Scholar]
- Pacheco L.P., Barbosa J.M., Leandro W.M., Machado P.L.O.A., de Assis R.L., Madari B.E., Petter F.A. Ciclagem de nutrientes por plantas de cobertura e produtividade de soja e arroz em plantio direto. Pesqui. Agropecu. Bras. 2013;48:1228–1236. doi: 10.1590/S0100-204X2013000900006. [DOI] [Google Scholar]
- Peel M.C., Finlayson B.L., McMahon T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. Discuss. 2007;4:439–473. doi: 10.5194/hess-11-1633-2007. [DOI] [Google Scholar]
- Qin W., Hu C., Oenema O. Soil mulching significantly enhances yields and water and nitrogen use efficiencies of maize and wheat: a meta-analysis. Sci. Rep. 2015;5:16210. doi: 10.1038/srep16210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranells N.N., Wagger M.G. Nitrogen-15 recovery and release by rye and crimson clover cover crops. Soil Sci. Soc. Am. J. 1997;61:943–948. doi: 10.2136/sssaj1997.03615995006100030033x. [DOI] [Google Scholar]
- Rocha K.F., Mariano E., Grassmann C.S., Trivelin P.C.O., Rosolem C.A. Fate of 15N fertilizer applied to maize in rotation with tropical forage grasses. Field Crops Res. 2019;238:35–44. doi: 10.1016/j.fcr.2019.04.018. [DOI] [Google Scholar]
- Rosolem C.A., Calonego J.C., Foloni J.S.S. Leaching of nitrate and ammonium from cover crop straws as affected by rainfall. Commun. Soil Sci. Plant Anal. 2005;36:819–831. doi: 10.1081/CSS-200049458. [DOI] [Google Scholar]
- Rosolem C.A., Ritz K., Cantarella H., Galdos M.V., Hawkesford M.J., Whalley W.R., Mooney S.J. Enhanced plant rooting and crop system management for improved N use efficiency. Adv. Agron. 2017;146:205–239. doi: 10.1016/bs.agron.2017.07.002. [DOI] [Google Scholar]
- Savage K., Phillips R., Davidson E. High temporal frequency measurements of greenhouse gas emissions from soils. Biogeosciences. 2014;11:2709–2720. doi: 10.5194/bg-11-2709-2014. [DOI] [Google Scholar]
- Silva E.C., Muraoka T., Buzetti S., Ocheuze Trivelin P.C. Manejo de nitrogênio no milho sob plantio direto com diferentes plantas de cobertura, em Latossolo Vermelho. Pesqui. Agropecuária Bras. 2006;41:477–486. doi: 10.1590/S0100-204X2006000300015. [DOI] [Google Scholar]
- Silva E.C., Muraoka T., Buzetti S., Guimarães G.L., Trivelin P.C.O., Veloso M.E. da C. Utilização do nitrogênio (15N) residual de coberturas de solo e de uréia pela cultura do milho. Rev. Bras. de Cienc. do Solo. 2006;30:965–974. doi: 10.1590/S0100-06832006000600006. [DOI] [Google Scholar]
- Smith C.J., Chalk P.M. The residual value of fertiliser N in crop sequences: an appraisal of 60 years of research using 15N tracer. Field Crops Res. 2018;217:66–74. doi: 10.1016/j.fcr.2017.12.006. [DOI] [Google Scholar]
- Soares J.R., Cassman N.A., Kielak A.M., Pijl A., Carmo J.B., Lourenço K.S., Laanbroek H.J., Cantarella H., Kuramae E.E. Nitrous oxide emission related to ammonia-oxidizing bacteria and mitigation options from N fertilization in a tropical soil. Sci. Rep. 2016;6:30349. doi: 10.1038/srep30349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao G.V., Ishikawa T., Ito O., Nakahara K., Wang H.Y., Berry W.L. A bioluminescence assay to detect nitrification inhibitors released from plant roots: A case study with Brachiaria humidicola. Plant Soil. 2006;288:101–112. doi: 10.1007/s11104-006-9094-3. [DOI] [Google Scholar]
- Subbarao G.V., Ito O., Sahrawat K.L., Berry W.L., Nakahara K., Ishiwawa T., Watanabe T., Suenaga K., Rondon M., Rao I.M. Scope and strategies for regulation of nitrification in agricultural systems - challenges and opportunities. Crit. Rev. Plant Sci. 2006;25:303–335. doi: 10.1080/07352680600794232. [DOI] [Google Scholar]
- Subbarao G.V., Nakahara K., Ishikawa T., Yoshihashi T., Ito O., Ono H., Ohnishi-Kameyama M., Yoshida M., Kawano N., Berry W.L. Free fatty acids from the pasture grass Brachiaria humidicola and one of their methyl esters as inhibitors of nitrification. Plant Soil. 2008;313:89–99. doi: 10.1007/s11104-008-9682-5. [DOI] [Google Scholar]
- Subbarao G.V., Nakahara K., Hurtado M.P., Ono H., Moreta D.E., Salcedo A.F., Yoshihashi A.T., Ishikawa T., Ishitani M., Ohnishi-Kameyama M., Yoshida M., Rondon M., Rao I.M., Lascano C.E., Berry W.L., Ito O. Evidence for biological nitrification inhibition in Brachiaria pastures. Proc. Natl. Acad. Sci. USA. 2009;106:17302–17307. doi: 10.1073/pnas.0903694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao G.V., Sahrawat K.L., Nakahara K., Ishikawa T., Kishii M., Rao I.M., Hash C.T., George T.S., Srinivasa Rao P., Nardi P., Bonnett D., Berry W., Suenaga K., Lata J.C. Biological nitrification inhibition-a novel strategy to regulate nitrification in agricultural systems. Adv. Agron. 2012;114:249–302. doi: 10.1016/B978-0-12-394275-3.00001-8. [DOI] [Google Scholar]
- Subbarao G.V., Yoshihashi T., Worthington M., Nakahara K., Ando Y., Sahrawat K.L., Rao I.M., Lata J.-C., Kishii M., Braun H.-J. Suppression of soil nitrification by plants. Plant Sci. 2015;233:155–164. doi: 10.1016/j.plantsci.2015.01.012. [DOI] [PubMed] [Google Scholar]
- USDA . 12th. United States Department of Agriculture; Washington, DC: 2014. Keys to soil taxonomy; p. 372. [Google Scholar]
- van Raij B., Andrade J.C., Cantarella H., Quaggio J.A. Instituto Agronômico; Campinas: 2001. Análise química para avaliaçãoda fertilidade de solos tropicais; p. 284. [Google Scholar]
- Varner R.K., Keller M., Robertson J.R., Dias J.D., Silva H., Crill P.M., McGrody M., Silver W. Experimentally induced root mortality increased nitrous oxide emission from tropical forest soils. Geophys. Res. Lett. 2003;30:4–7. doi: 10.1029/2002GL016164. [DOI] [Google Scholar]
- Zhang X., Davidson E.A., Mauzerall D.L., Searchinger T.D., Dumas P., Shen Y. Managing nitrogen for sustainable development. Nature. 2015;528:51–59. doi: 10.1038/nature15743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material