Abstract
Salamanders exhibit the most extensive regenerative repertoire among vertebrates, being able to accomplish scar-free healing and faithful regeneration of significant parts of the eye, heart, brain, spinal cord, jaws and gills, as well as entire appendages throughout life. The cellular and molecular mechanisms underlying salamander regeneration are currently under extensive examination, with the hope of identifying the key drivers in each context, understanding interspecies differences in regenerative capacity, and harnessing this knowledge in therapeutic settings. The immune system has recently emerged as a potentially critical player in regenerative responses. Components of both innate and adaptive immunity have been found at critical stages of regeneration in a range of salamander tissues. Moreover, functional studies have identified a requirement for macrophages during heart and limb regeneration. However, our knowledge of salamander immunity remains scarce, and a thorough definition of the precise roles played by its members is lacking. Here, we examine the evidence supporting roles for immunity in various salamander regeneration models. We pinpoint observations that need revisiting through modern genetic approaches, uncover knowledge gaps, and highlight insights from various model organisms that could guide future explorations towards an understanding of the functions of immunity in regeneration.
Introduction
Regeneration is a remarkable biological process that reconstitutes damaged or missing structures yielding a functional replacement. In salamanders, after a limb has been severed, wound closure ensues through contraction and wound epithelium migration. This is followed by the induction of dedifferentiation of mature cell types in the stump, yielding a population of proliferating cells (termed ‘blastema’) that subsequently expands and re-differentiates into the tissues of origin, giving rise to the new structure 1–4. Similarly, during dorsal pallium regeneration in the brain, wound closure takes place by remodelling of pre-existing tissues, after which cell proliferation and differentiation of progenitor cells occurs 5. In contrast, during organ regeneration such as the heart after puncture or resection, cell proliferation of the epicardium takes place. Subsequently, a temporary extra-cellular matrix (ECM) is deposited and the tissue forms a blastema, which finally gives rise to the missing or damaged structure, replacing the temporary ECM 6,7. Importantly, the aforementioned salamander regeneration processes rely largely on cell proliferation for the replacement of missing structures, contrary to the hypertrophic enlargement that significantly contributes to many contexts of mammalian tissue repair, including liver8 and heart 9.
The regeneration of complex structures involves the coordinated response of a myriad of cell and tissue types, some of which constitute the raw material for regeneration, and others that provide critical cues for the process. The former includes specific cell types from and around the regenerating structure such as connective tissue, muscle, nervous system, vasculature and epidermis during limb regeneration 10–13, pigmented epithelial cells (PECs) during lens regeneration 14, or cardiomyocytes during heart regeneration 7,15. The latter involves the immune 16 and endocrine systems, whose impact on regenerative processes is not yet fully understood. Nevertheless, research in the past decade has highlighted that the immune system plays important roles in various regeneration and wound healing contexts, from salamanders and zebrafish through to mammals.
During axolotl (Ambystoma mexicanum) limb regeneration, macrophages, neutrophils, T and B cells are recruited to the regenerating stump 7,12,17–19. Similarly, during heart regeneration, recruitment of macrophages and upregulation of complement system components have been observed 7,20. In addition, during salamander limb and lens regeneration, complement components are differentially regulated upon tissue injury 21–23. Strikingly, upon pharmacological macrophage depletion, limb and heart regeneration are prevented following injury in the axolotl, suggesting a critical role for immunity in regeneration 7,17. However, the roles of immune system components in salamander regeneration are yet to be fully dissected.
Here, we examine what is currently known of the salamander immune system, with a critical focus on its possible roles in regeneration. We underline gaps in our current knowledge of salamander immunity, discuss insights derived from other highly regenerative species, highlight aspects that need revisiting taking advantage of recent technological developments, and suggest experimental approaches which may help refine our understanding of regenerative processes.
The salamander immune system
In vertebrates, the immune system is divided in two main branches: innate and adaptive immunity. Innate immunity is considered a non-specific response towards different pathogens that is executed through inflammation, the complement system, antimicrobial peptides (AMPs), and cell types such as macrophages, granulocytes (neutrophils, eosinophils, basophils and mast cells) and natural killer (NK) cells. In contrast, adaptive immunity is regarded as mediator of specific responses through T and B lymphocytes that can recognise specific antigens and generate memory against them. Components of both innate and adaptive immunity have been identified in salamanders through anatomical, histological and molecular approaches (Figure 1).
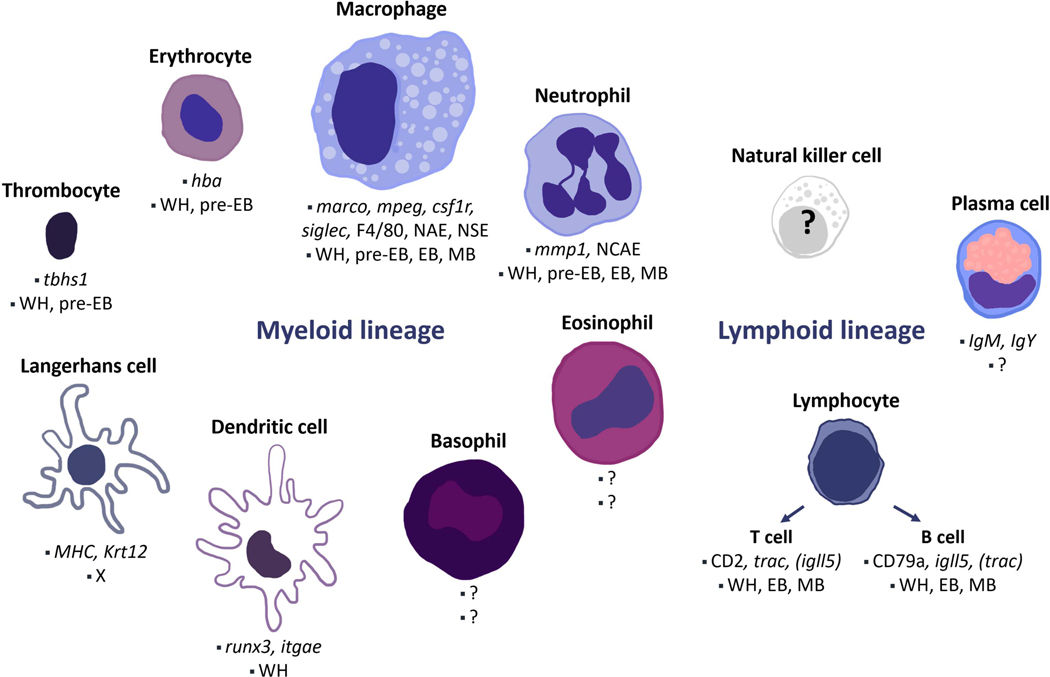
Figure 1. Salamander immune cell types.
Cell populations and cell-specific markers described in A. mexicanum are depicted, along with stages where the different populations have been described during axolotl limb regeneration. Note that the mobilisation dynamics of the cell types included here is not yet fully characterised for all regeneration stages. WH: wound healing, pre-EB: previous to blastema formation, EB: early blastema, MB: medium blastema, X: not found during regeneration. Dendritic and Langerhans cell populations have been identified by markers via scRNA-seq 18, without a histological description. Natural killer cells have not yet been observed in salamanders. Likewise, cell-specific markers have not been described for granulocytes besides neutrophils. Based on references 12,17–19,27,34,41.
Immune cells belong to either myeloid or lymphoid lineages: the myeloid lineage comprises monocytes, macrophages, granulocytes and dendritic cells, while the lymphoid lineage includes NK cells, T and B lymphocytes. In post-natal mammals, both lineages differentiate from haematopoietic stem cells residing in the bone marrow, which also contributes to the renewal of tissue-resident macrophages later in life. In contrast, it is unclear whether salamanders possess a classically-defined bone marrow. Bone marrow-dependent haematopoiesis has not been reported in urodeles with the exception of one species, Plethodon glutinosus, in which granulopoiesis and lymphopoiesis - but not erythropoiesis - occur in bone marrow-like tissue 24. Yet, salamanders exhibit other organs relevant for immune system development, maturation and function, including the spleen, liver, thymus, mucosa-associated lymphoid tissues and lymphatic vasculature.
In salamanders, the spleen and the liver cortex are widely populated by myeloid and lymphoid-lineage populations, and both have been histologically described as sites of haematopoiesis 25,26. In the axolotl, the spleen and liver have been functionally characterised as the main haematopoietic sites, as indicated by cell transplants into irradiated animals 27. In addition, the thymus has been described in a variety of species 25,28,29, where thymic nodules are found in different numbers near the base of the gills. For example, A. mexicanum presents three completely separate nodules in each side of the head 29, in Notophthalmus viridescens three nodules have been described although not fully separated by connective tissue 25, and in Pleurodeles waltl only one nodule per side is present 28. This variability is consistent with the plasticity of thymus ontogeny observed across phylogeny. In the species explored so far, a diversity of cell types has been morphologically described within the thymic tissue that resemble mammalian thymic epithelial cells, interdigitating cells and thymocytes, although a clear cortico-medullar segmentation of the organs is not evident from histological analysis. So far, lymph nodes have not been described in salamanders, though in spite of controversy over its presence in urodeles 30, gut-associated lymphoid tissue (GALT) has indeed been histologically described in P. waltl 31. In addition, lymphatic vasculature has been anatomically described in Salamandra salamandra 32, Salamandra maculosa and A. mexicanum 33.
In A. mexicanum, a variety of immune cell populations have been morphologically described 27,34. In addition, single-cell RNA sequencing (scRNA-seq) analysis of blood cells from sexually mature axolotls revealed the presence of seven principal cell population clusters, which include thrombocytes, erythrocytes, neutrophils, macrophages, T, B cells and a myeloid-like population 19. Furthermore, macrophages and neutrophils share common markers with their mammalian counterparts, such as F4/80 expression and α-naphthyl acetate esterase (NAE) enzymatic activity, and myeloperoxidase activity, respectively 27. In addition, the expression of genes relevant for the development of different lymphocyte populations has been described for A. mexicanum. Recombination activating gene 1 (Rag1), terminal deoxynucelotidyl transferase (Tdt), T cell receptor (TCR) α, β and δ chains, CD3 γ, δ and ε chains (CD3γ/δ, CD3ε), and immunoglobulins M and Y (IgM, IgY) expression has been reported during a variety of larval stages 35–41. Indeed, axolotl lymphocyte populations cross-react with the human T cell marker anti-CD2 or the B cell marker anti-CD79a antibodies 27. Likewise, in P. waltl the expression of Rag1, activation-induced cytidine deaminase (AID), CD3γ/δ, IgM and IgY has been described 42–45. The expression of classical immune cell markers suggests similarities between salamander macrophages, neutrophils, T and B lymphocytes and mammalian and zebrafish equivalents. However, whether this coincides with conservation of functions of T and B cells in salamanders has not yet been fully determined.
There remains a dearth of both in-depth characterisation and functional analysis of the different immune populations in the salamander, both during homeostasis and regeneration. Moreover, little is known about the development, maturation and ageing features of salamander immune cell types, even though these could be relevant to the exceptional regenerative abilities exhibited by these organisms. Tools such as scRNA-seq and sophisticated transgenic approaches recently available to salamander models will certainly contribute to filling the gaps in our understanding of immune cell ontogeny and function.
Roles of immune system components in regeneration
During regeneration, the release of cytokines and a variety of antigens to the extracellular space due to cell death, cell reprogramming and tissue remodelling, highlights the need for an immune system capable of recognising both decaying as well as newly-formed tissues in a way that allows for regeneration to occur. Of note, various studies in zebrafish have highlighted the importance of the immune system in regeneration of complex structures. Strikingly, roles of immune components during regeneration go beyond resolving potential infections or clearing damaged tissue. For example, acute inflammation provides essential signals for cell proliferation and neurogenesis during central nervous system regeneration 46. Further, during tail fin regeneration, macrophages promote an anti-inflammatory environment permissive for regeneration 47. In addition, during retina, heart and spinal cord regeneration, T regulatory cells deliver essential tissue-specific signals for progenitor cell development 48.
Several lines of evidence suggest that the immune system also contributes to regeneration in salamanders. Firstly, macrophages are dynamically recruited during limb and heart regeneration, whereby they produce both pro- and anti-inflammatory signals and contribute to ECM remodelling 7,17 as well as orchestrating clearance of cellular debris and senescent cells 49. Secondly, chemical depletion of macrophages impairs critical processes during limb regeneration, from initial regenerate formation to blastema growth 17 and senescent cell immunosurveillance 49. Thirdly, additional immune populations such as neutrophils, T and B cells have also been observed during limb regeneration, though their potential roles during the regeneration process have not been explored.
Innate immune cells in regeneration
Macrophages – an essential player in regeneration?
The circulating mononuclear phagocytic cells monocytes, and their differentiated tissue-resident counterpart macrophages comprise a major component of the innate immune system. While not yet fully characterised, recent single cell and bulk RNA sequencing data of leukocytes from axolotl blood and the intraperitoneal cavity has provided transcriptomic insights into these important immune populations 19. It is likely that, in concordance with other species, amphibian macrophages constitute a heterogeneous population, where circulating monocytes and tissue-resident macrophages from different locations and embryonic origins exhibit specialised phenotypes and functions, as well as context-dependent responses to activating stimuli in dynamic, pro- or anti-inflammatory manners (i.e. M1 or M2 respectively). While during infection, macrophage activity can be induced by pathogen-associated molecular patterns (PAMPs) from invading pathogens, in the context of injury, local occurrence of damage-associated molecular patterns (DAMPs) released by dead or dying cells can instead result in inflammation, recruitment and activation of macrophages. Further, macrophage function extends to the modulation of local inflammation 47,50, affecting other immune and likely non-immune cell populations, of relevance to wound healing and regenerative processes. Indeed, given the plethora of potential functions of macrophages, from defence against infection through phagocytosis of pathogens as well as cellular debris, to matrix remodelling, regulation of inflammation and promotion of cell proliferation, it is unsurprising that central roles for macrophages are beginning to be unveiled during regeneration of complex structures.
Macrophage recruitment during regeneration
Histological analysis of the axolotl blastema following limb amputation indicates that macrophages, positive for α-NAE activity as well as macrophage markers CD11b, cMAF, CD163, F4/80 and CSF1R, are recruited in high numbers during regeneration. Their infiltration into the regenerate peaks at around 6 days post amputation (dpa), following strong expression of macrophage chemoattractants in the stump upon amputation 17. This recruitment has since been confirmed by several single cell sequencing experiments, which indicate that an mpeg1+/marco+ cell population is present during the course of regeneration 18,19. Indeed, a substantial contribution to the blastemal macrophage population comprises recruited (trem2+) macrophages 18. While the signals that modulate macrophage recruitment and retention through the different stages of limb regeneration are ill-defined as yet, recent evidence suggests that blastema progenitors expressing the classical marker prrx1 51 contribute to myeloid cell recruitment to the blastema through IL-8 secretion 12. Knock-down of this interleukin leads to reduced myeloid cell retention and delayed limb regeneration 12. It is also possible that the induction of senescent cells which recurrently takes place during limb regeneration 49 also contributes to regulate macrophage recruitment dynamics -and perhaps macrophage functions-, as the latter are recruited to the former in salamander tissues, much like it happens in mammals 52.
Notably, a number of studies have revealed critical roles for macrophages in axolotl limb 17,49 and heart regeneration 7, and newt (N. viridescens and Cynops pyrroghaster) lens regeneration. In these contexts, macrophages have been hypothesised to regulate a number of processes, including inflammatory signalling, wound epithelialisation, vascularisation, local proliferation and matrix remodelling, for example through production of matrix metalloproteases such as MMP3 17. Further, macrophages also orchestrate local clearance of debris, including apoptotic neutrophils as well as senescent cells 49 through their phagocytic functions. Even within the immunoprivileged environment provided by the eye, macrophages have been observed to infiltrate the iris epithelium following lentectomy. There, they perform phagocytosis of melanosomes and fragments of iris epithelial cells 53. Moreover, following lens injury in the newt C. pyrroghaster, myeloid cells are rapidly recruited and have been proposed to be critical for the initiation of the regeneration process. In support of this hypothesis, myeloid cells isolated from the injury site and transplanted to uninjured eyes promote ectopic lens regeneration, resulting in eyes bearing two lenses 54.
The vast range of functions attributed to macrophages in regenerative contexts is supported by the diverse phenotypic spectrum observed in these cells in other species, where macrophage function and polarization are highly plastic, and different macrophages subsets may exhibit complementary functions 55. Underlining this plasticity, co-expression of both pro-inflammatory ‘M1’ and anti-inflammatory ‘M2’ polarization markers have been observed, while further subsets of these polarization states have also been described 56. While a thorough dissection of macrophage sub-populations during regeneration in the salamander is currently lacking, evidence from zebrafish has revealed that accumulation of pro- and anti-inflammatory macrophages occurs rapidly following fin amputation, though only anti-inflammatory macrophages persist for the duration of regeneration 57. Indeed, M2-polarized macrophages appear particularly important in promoting regeneration in zebrafish, as depletion of heat shock protein HSP60 compromises both hair cell and fin regeneration, which is attributed to a novel extracellular function of HSP60 in triggering M2 polarization in monocytes 58.
However, macrophage dysregulation in the context of tissue damage may contribute to fibrosis and scarring, which can negatively impact tissue function. This is elegantly demonstrated by recent findings revealing that macrophages can directly contribute collagen to post-injury scars, in a cell-autonomous manner, in both zebrafish and mouse hearts 59. Strikingly, adoptive transfer of macrophages from GFP donors is actually detrimental to the repair process, enhancing scar formation 59. Further, evidence from Xenopus laevis, whose high regenerative ability is transiently lost during the ‘refractory’ period as the frog matures, indicates that a suppression of the immune response is beneficial for regeneration 60. Notably, treatment with immunosuppressants such as celastrol, FK506 and cyclosporin A, or knock-down of the transcription factor PU.1 -a transcription factor essential for the maturation and function of several haematopoietic lineages, including macrophages-, restores tail regeneration during the refractory period, underscoring that macrophages and other immune components can play anti-regenerative roles in certain contexts.
Macrophages in regeneration: insights from clodronate
Using liposomes loaded with the biphosphonate drug clodronate (Clo-Lipo), multiple studies have investigated the potential requirement for macrophages in axolotl regeneration. This treatment relies on the phagocytic capabilities of macrophages: following engulfment of liposomes into phagosomes and phagosome-lysosome fusion, the liposome phospholipid bilayer is disrupted through the action of lysosomal phospholipases, resulting in the intracellular release of clodronate. Here, clodronate is metabolized into an ATP analogue, which competitively inhibits the mitochondrial ADP/ATP translocase, resulting in the induction of apoptosis 61. As such, under this treatment, macrophage populations are substantially depleted, though there is little effect on neutrophils 17. Using Clo-Lipo, it has been observed that limb regeneration following amputation 17 and heart regeneration following cryoinjury 7 are severely impaired. In the context of limb regeneration, upon Clo-Lipo administration, an increase in collagen I and IV is observed, resulting in fibrotic scarring of the wound instead of successful regeneration 17. In fact, regeneration is completely prevented in an irreversible fashion, unless the treatment is stopped and the stump re-amputated. In addition, Clo-Lipo administration at later stages of regeneration has further negative effects, delaying the rate of regrowth and resulting in vasculature alterations [17], as well as preventing the elimination of senescent cells [49]. Similarly, cardiac cryoinjury upon Clo-Lipo treatment results in progressive scarring, with compromised ECM synthesis, remodelling and cross-linking, as well as impaired angiogenesis and failed regeneration, without affecting activation of cardiomyocytes. Such is the severity of impeding heart regeneration that the survival of clodronate-treated, cryo-injured animals is impaired both short-term due to infection post-injury, and long-term due to heart failure 7.
Clo-Lipo treatment has also been shown to prevent regeneration in a mammalian model of epimorphic regeneration, murine digit tip regrowth. In this context, macrophages are recruited during regeneration, peaking at 7 dpa and returning to baseline levels by 21 dpa. Clo-Lipo treatment prevents bone histolysis, inhibits re-epithelialization and blastema formation. Even following artificial epidermal wound closure, cell differentiation is impaired 62, suggesting a critical role for macrophages in digit tip regeneration. Further, in a mammalian model with enhanced regeneration capabilities, the African spiny mouse (Acomys cahirinus) 63, Clo-lipo treatment has been used to address the involvement of macrophages during ear pinna regeneration 64. Chemical depletion of macrophages results in impaired blastema formation and consequently impaired regeneration.
However, we advise a note of caution in interpreting these striking observations using clodronate. Indeed, while Clo-Lipo shows little toxicity to neutrophils, it remains possible that other cell populations may be affected. For example, fibroblasts have been shown to acquire phagocytic capacities during regeneration, which could result in targeting by Clo-Lipo 65. Further, osteoclasts (monocytic-derived cells responsible for bone resorption) are themselves depleted using Clo-Lipo; in the clinic, clodronate is used as a treatment for osteoporosis, and to relieve pain and strengthen bones in patients with secondary bone cancers 62. It is therefore possible that off-target effects of Clo-Lipo have been attributed to the absence of macrophages during axolotl regeneration. For example, targeting osteoclasts in murine digit tip regeneration more selectively (using free clodronate) results in delays to wound closure, blastema formation and regeneration 62. It is important to note that transgenic and CRISPR approaches have recently become available in the axolotl [4]. Therefore, whereas experiments using Clo-Lipo have been indispensable in highlighting macrophages as major players in regenerative processes, additional genetic analysis may be required to determine the exact roles of and requirement for this critical cell type.
Genetic insights from mice and zebrafish
While Clo-Lipo studies suggest an essential role for macrophages in axolotl regeneration, data from several knockout models in mice and zebrafish lacking macrophages has called this into question. Firstly, in PU.1 knock-out neonatal mice lacking both macrophages and functioning neutrophils, mice are able to repair skin wounds without delay, and without scar formation. While the growth factor and cytokine profile at the wound site is altered in comparison to wild-type controls, dying cells and cellular debris are instead engulfed by ‘stand-in phagocytic fibroblasts’ in the absence of neutrophil and macrophage populations 65. This indicates that these cell types are dispensable for scar-free healing in certain contexts, and that compensatory mechanisms exist for critical steps in this process.
In transgenic zebrafish, upon conditional depletion of mpeg1+ macrophages (specifically, a 80–90% reduction based on co-expression of E. coli nitroreductase, resulting in cell death upon administration of the pro-drug metronidazole), tail fin regeneration is impaired but not prevented. Here, macrophage depletion reduces tissue outgrowth and disrupts its patterning, phenomena attributed to a decrease in mesenchymal cell proliferation 66. Still, regeneration takes place. Additionally, in homozygous zebrafish CRISPR knockouts of the crucial macrophage-lineage transcription factor IRF8, where fish lack macrophages and microglial cells but show increased neutrophil populations, larval axonal regeneration efficiency is impaired but not prevented 50. The mild phenotype observed is attributed to an increased pro-inflammatory neutrophil population, with the absence of macrophages culminating in a failure to switch between pro- and anti-inflammatory phases of regeneration 50. Finally, in CSF1R−/− zebrafish, which show a ~60% reduction in tissue-resident macrophages, tail regeneration is again impaired yet not prevented. In the regenerate, an increase in the number of apoptotic cells present following injury is observed, coincident with a reduction in proliferation, as well as increased ROS and pro-inflammatory cytokines including IL1b 47.
Given the contrasting findings of clodronate and genetic experiments, it is clear that the roles of and potential requirement for macrophages in regeneration in salamanders need to be revisited using more precise tools for macrophage targeting. In particular, genetic macrophage depletion models will be critical in these studies, as they will facilitate analysis of the dynamics and functions of different macrophage populations during regeneration. This will include dissecting the contributions made by resident, blood-derived and peritoneal macrophages, as well as functionally differentiating M1 versus M2 activated macrophage states and possibly additional, as yet unidentified subpopulations. Indeed, recent research in mammals and zebrafish has highlighted the direct contribution of particular macrophage subpopulations to regenerative processes: following sterile liver injury in mice, not only are blood-derived monocytes and liver-resident Kupffer cells recruited, but also peritoneal macrophages which adopt an Arg1+ anti-inflammatory phenotype and contribute to regeneration 67. Further, peripheral macrophages have been recently proposed to fine-tune the inflammatory response during tail fin regeneration in zebrafish larvae 47, while an accurate M1 vs M2 macrophage subset balance has been implicated in priming this process 57. Lastly, a Wilms tumour 1b (wt1b)-expressing macrophage subpopulation with pro-regenerative functions in appendage and heart regeneration was recently identified in zebrafish 68. Adding to the complexity of macrophage subtypes, it is noteworthy that the embryonic origin can affect macrophage function, and that this particular cell type exhibits a high degree of plasticity, undergoing epigenetic reprogramming according to the local microenvironment 69,70. The use of multi-dimensional techniques such as single-cell RNA and ATAC sequencing will allow in-depth profiling of the various macrophage types that dynamically participate in regeneration, enabling genetic strategies to precisely address their functional relevance.
Neutrophils
While salamander neutrophils have not yet been fully characterised during homeostasis, rapid and dynamic neutrophil recruitment to the site of injury has been observed during axolotl limb regeneration. In particular, scRNA-seq has revealed neutrophil populations at the early and mid-bud stages 18,19. Additionally, granulocytes have been found prior to blastema formation by histology during axolotl limb regeneration via Naphthol AS-D Chloroacetate (NCAE) staining 12. Neutrophil numbers are found in higher proportions at 1dpa than 6dpa, prior to blastema formation, suggesting their recruitment as ‘first-wave’ incomers and potential roles during the early stages of regeneration, in contrast to macrophages, which are heavily mobilised later, showing higher proportions at 6dpa than 1dpa 19. However, whether neutrophils are required for successful salamander regeneration is as yet unknown.
Neutrophils are recognised as major players in tackling infection following injury. However, neutrophils may also perform pro-reparative and pro-regenerative roles in several contexts. In mammals, neutrophils provide the angiogenic signal Vascular Endothelial Growth Factor (VEGF) in a corneal injury model. In addition, neutrophils can release metalloproteinases that will further release and activate angiogenic factors from the ECM 71. Moreover, a role for neutrophils in the conversion of macrophages from a pro-inflammatory to an anti-inflammatory phenotype has been described in mice, via neutrophil phagocytosis by macrophages (termed efferocytosis) 72, and also during mice liver repair via ROS release 73. In zebrafish, neutrophils are recruited to the injury site both during neuromast regeneration and embryonic fin regeneration. As embryonic fin regeneration is considered a “sterile” wounding model, the recruitment of neutrophils suggests that they have roles beyond resolving infection 74. Further, live-imaging of neutrophils following zebrafish embryonic tailfin wounding revealed a directed migration process of these cells back into the vasculature after they had already infiltrated the tailfin tissue 75. This process of retrograde chemotaxis or reverse migration has also been observed during murine liver repair. In this sterile injury context, the infiltrating neutrophils contribute to vascular remodelling and afterwards up to 90% of them migrate back into circulation, passing through the lungs and arriving to the bone marrow, where they finally undergo apoptosis 76. These observations highlight the need for a deeper exploration of the influence of neutrophils over the inflammatory microenvironment and other potential roles during tissue repair and regeneration.
Adaptive immune cells
Despite the description of adaptive immunity components in urodeles such as A. mexicanum and P. waltl, functional analyses of their involvement in regenerative processes in salamanders have not been carried out to date. Hints of a possible requirement for T cells in salamander limb regeneration come from a study addressing the effect of immunosuppression on newts 77. While regeneration is impaired by treatment with the immunosuppressant Cyclosporin A in a dose-dependent manner, this phenotype is rescued by interleukin 2 (IL-2) treatment, suggesting that T-lymphocytes could contribute to this process - a hypothesis which remains to be tested. Notwithstanding this, evidence from additional model organisms suggests that adaptive immunity is indeed an important component of regeneration of complex structures. For example, histological approaches 78 show that, during tail regeneration in stage 50 X. laevis tadpoles, the thymus exhibits high expression of the inflammatory cytokine TNFα, which correlates with a significant infiltration of lymphocytes into the regenerating tail. In contrast, stage 55/56 tadpoles, incapable of regenerating the tail, exhibit thymus activation when the tail is severed, but thymic size irreversibly decreases compared to non-amputated controls. Together, these observations suggest that activation of adaptive immune components is required for tail regeneration, which is lost during X. laevis development. Moreover, in a recent landmark paper, T regulatory (Treg) cells have been shown to infiltrate the regenerating heart, spinal cord and retina following injury in zebrafish 48. Upon infiltration, Tregs stimulate the proliferation of precursor cells in each organ (cardiomyocytes, neural progenitor cells and Müller glia respectively), contributing to organ regeneration in each case. Interestingly, this stimulatory activity contrasts with classic Treg-mediated immunosuppression through IL-10 secretion, and constitutes a non-immunological role for Tregs, dependent on the transcription factor FoxP3a. In particular, FoxP3a induces the expression of the tissue-specific pro-regenerative factors neuregulin 1, neurotrophin 3 and insulin-like growth factor after heart, spinal cord and retina injury, respectively. Notably, this FoxP3 transcriptional activity is context-dependent, resulting in the provision of the right cue in each regenerating structure.
In salamanders, two independent scRNA-seq analysis of axolotl limb regeneration have described the presence of T and B cell markers at critical regeneration stages 18,19. In the first dataset, T cell receptor-alpha chain (trac)-positive T cells and immunoglobulin lambda-like polypeptide 5 (igll5)-positive B cell populations were identified during wound healing, early and mid-bud blastema formation 18. In the second dataset however, a single lymphoid population was defined by both trac and igll5 expression prior to blastema formation. Indeed, this single population differed from separate T and B cell populations defined using the same markers on cells from the blood stream 19. While the presence of trac+ cells in the early blastema has been validated using in situ hybridization, the potential existence and functional relevance of the trac and igll5 positive population(s) with classical T and B cell markers requires further exploration.
Our understanding of the role of adaptive immunity in regeneration of complex structures is in its infancy. However, the aforementioned considerations suggest this is an area of considerable interest. Characterising the potential roles of the adaptive immune system during regeneration in salamanders will require a thorough spatio-temporal characterisation of the different cell populations in terms of molecular features, heterogeneity of composition, cell behaviours and interactions, as well as functional probing through targeted cell depletion strategies, as have proven effective for analysis of innate immune components in other species.
Lymphatics in regeneration – new roads for new tissues
The lymphatic vasculature has been anatomically explored in salamanders 32,33, though molecular and functional analyses are still lacking. Notwithstanding this, unexpected roles for the lymphatic vasculature in tissue regeneration and repair have recently started to emerge. During zebrafish brain vasculature regeneration, newly generated lymph vessels infiltrate the brain where they participate in oedema resolution and, moreover, serve as “growing tracks” for the formation of new blood vessels. Notably, following blood vessel formation, the lymph vessels undergo apoptosis and restore a lymphatic-free brain tissue beyond the meninges 79. Further, during the mouse hair growth cycle, where hair follicle regeneration takes place, there is crosstalk between the hair follicle stem cell (HFSC) niche and the underlying lymph vessels. In this context, lymph vessels dissociate from the HFSC niche before proliferation takes place, suggesting potential roles for lymph vessels in drainage of stem cell proliferation stimuli. In addition, lymph vessels may provide a means for synchronised HFSC renewal across the skin 80. Thus, these studies clearly introduce the lymphatic system as a new, potentially important player in regenerative phenomena.
Other potential players – skin components and the complement system
Skin components: skin resident cells and antimicrobial peptides
Skin resident cells belonging to the immune system, such as dendritic cells (DCs), Langerhans cells (LCs) and a CD3+ dendritic epidermal T cell (DETC)-like population are found in anuran amphibians, similar to what is described in mammals 81. LCs are hypothesised to have deleterious effects during X. laevis and Rana skin regeneration during and after metamorphosis, when LCs become more abundant and skin injuries result in scar formation 82. This hypothesis is in agreement with the enhanced skin repair observed in PU.1 knock-out mice 65, where the myeloid lineage, which comprises DCs and LCs, is detrimentally affected. However, no functional role of DCs, LCs or DETCs during skin remodelling or regeneration has been explored in amphibians. Interestingly, in the axolotl a putative LC population defined by high Major Histocompatibility Complex (MHC) components and Krt12 expression is present during homeostasis but absent from the wound epithelium during limb regeneration 18. These findings suggest that LCs could have an overall negative impact on skin regeneration, and that organisms able to regenerate full-thickness wounds without scarring would be capable of LC clearance in the regenerating tissue. These interesting hypotheses are yet to be functionally approached.
Antimicrobial peptides (AMPs) are multifunctional agents that perform a wide variety of functions in cell proliferation, cell death, chemoattraction and immunomodulation 83. Currently, a database listing AMPs with their described functions from different species is available 84. In amphibians, the majority of AMPs have been described for anurans, but urodele AMPs are now starting to be analysed 85–87. Among the few AMPs described so far in urodeles, tylotoin (isolated from the skin of Tylototriton verrucosus) was tested in mammalian models of wound healing 87. The purified 12-aminoacid peptide was shown to increase proliferation and cell motility in human keratinocytes (HaCaT cells), fibroblasts (HSFs) and vascular endothelial cells (HUVECs). Furthermore, tylotoin induced the secretion of TGF-β and IL-6 in the murine macrophage line RAW264.7. When purified tylotoin was topically applied to murine skin after skin full thickness wounding, wound healing was accelerated, along with an enhanced restoration of the dermis, epidermis and granulation tissue. Further, enhanced macrophage infiltration in the injury site was observed. While its role has not yet been explored during salamander wound healing or regeneration, the aforementioned observations suggest that tylotoin, and potentially other AMPS, could have pro-reparative features.
The complement system
The complement system is comprised of proteins that work in cascades of proteolytic reactions that promote opsonisation, chemotaxis and cell lysis. Complement system components C3 and C5 have been found to influence regenerative outcomes in a variety of models. In murine muscle regeneration, signalling of C3a through C3a receptor (C3aR- highly expressed in monocytes), is relevant for macrophage recruitment via CCL5 towards the regenerating tissue 88. Further, C5a receptor 1 enhances cardiomyocyte proliferation during mouse heart regeneration 20. Analysis of the zebrafish genome has shown high conservation of complement system components from mammals 89. However, the complement system has not been fully characterised or analysed at the genetic level in salamanders, though expression has been observed during limb and lens regeneration processes 20–23.
During limb regeneration, C3 is expressed in the axolotl and the newt N. viridescens 21, while C3 and C5 are upregulated both during limb and lens regeneration in N. viridescens 22. The upregulation of these components at the transcriptional level within regenerating tissue suggests potential regeneration-specific functions. Furthermore, using proteomics, the plasma protease C1 inhibitor (C1INH) and complement receptor 4 (CR4) were shown to be differentially expressed during blastema formation in neotenic animals 23. C1INH, which is upregulated at 7dpa, is regarded as an anti-inflammatory complement regulator which also interacts with ECM components 90. In contrast, CR4 is downregulated, while the rest of the complement system components remain unchanged. A decrease in complement system activity may influence the fibrinolytic system towards a higher degradation of fibrin products, thus allowing tissue remodelling. Interestingly, when complement system components were analysed in metamorphic animals, components C6 to C9 and fibrinogen were upregulated. This correlates with the lower regenerative capacities described for metamorphic axolotls, and raises the question as to whether more cell lysis is also induced due to increased complement system activity. However, whether inducing the activity of the complement system during limb or lens regeneration would induce changes in ECM remodelling, cell lysis, and other cellular processes relevant for regeneration is yet to be explored.
The complement system may also be important in heart regeneration in several species including neonatal mice, zebrafish and axolotls. For example, C5a receptor 1 (C5aR1) is upregulated at 24 hours after cardiac apical resection in neonatal mice, and after 48 hours in zebrafish and axolotls 20. Interestingly, when treating each species with the C5aR1 inhibitor PMX205, proliferation of cardiomyocytes was diminished, suggesting a conserved role for signalling via C5aR1 during heart regeneration. The differential regulation of members of the complement system during regenerative processes in salamanders strongly suggests that their expression is important for regeneration. Considering that C3 and C5-mediated signalling are broadly present in a variety of regeneration processes, it is currently unclear whether they belong to a common regeneration-specific program or a tissue-specific one. Nevertheless, further description of the specific cell types involved in this differential regulation, as well as the downstream effects of the different complement components is not yet available. Functional studies using existing pharmacological inhibitors as well as targeted genetic ablation systems will aid in establishing the importance and specific functions of the complement system in regeneration.
Role of immunity during wound healing - mapping the salamander path towards scar-free outcomes
Wound repair involves a series of organised and orderly dynamic stages including haemostasis, inflammation, re-epithelisation, and tissue remodelling 91. Dysregulation at any of these stages can result in delayed wound healing, chronic wounds, and/or fibrosis leading to scar formation 92. In contrast to humans, salamanders are capable of scar-free wound healing. Given that the immune system is engaged in every stage of the healing process, inter-species differences in immune components have been proposed to underlie the diverse outcomes.
After injury in mammals, haemostasis is the normal response of blood vessels, where coordinated efforts between platelets and numerous blood clotting proteins and factors result in the formation of a blood clot, thus limiting haemorrhage. Platelets then secrete the chemotactic molecules Platelet-Derived Growth Factor (PDGF), which attracts neutrophils and monocytes, and TGF-β, which attracts monocytes and T-lymphocytes 93. This amplified cell recruitment driven by chemoattractant secretion promotes a strong inflammatory response. Instead of platelets, amphibians contain thrombocytes, which are larger and nucleated, however, they appear to uphold the same characteristics as platelets during wound healing 34. The role and effect of thrombocyte degranulation in salamander wound healing nevertheless remains an opportunity for further research.
Showing strong parallels with regeneration, the stages of inflammation in wound healing are characterised by the influx of neutrophils, macrophages, and lymphocytes 94,95. Neutrophils help attract additional inflammatory cells, amplifying the inflammatory response. Macrophages aid in debris and neutrophil removal, which helps inflammation resolution. Neutrophils direct the production of ECM-degrading enzymes matrix metalloproteinase (MMPs) which degrade the collagen within the wound 96, further, neutrophils clean-up the wound site by phagocytosing bacteria and cellular and molecular debris 97. In comparison to mammalian wound healing, the axolotl shows a reduction in the processes of haemostasis, dampened inflammatory stages, lower infiltration and presence in circulating blood of monocytes and neutrophils, and a significant delay in the production of new ECM during scar-free healing 98. Cell count data from axolotl blood showed that they maintain around 21% of their circulating leukocyte population as neutrophils 98, in contrast to approximately 60% in mammals 99. The lower levels of circulating neutrophils could have an effect in the process of inflammation, leading to diminished scar formation. In mammalian contexts, depletion studies have shown that neutrophils are not required for wound healing 100,101, though our understanding of their potential roles during salamander scar-free wound healing is currently limited.
Previous research suggests that a prolonged or chronic inflammatory response results in disorganised ECM deposition – consisting mainly of collagen – which leads to scar formation 102. Studies of the initial wound healing stages in axolotls indicate the presence both pro-inflammatory (IFN- γ, IL-1β, and TNF-α) and anti-inflammatory cytokines (IL-4 and IL-10) concurrently 17. This is in stark contrast to mammalian wound healing, characterised by a wave of pro-inflammatory cytokines during haemostasis and inflammation, followed by anti-inflammatory signalling in the stages of re-epithelisation and tissue remodelling 103. Notably, studies in which mice and zebrafish where both treated with immunosuppressive agents (namely hydrocortisone or IL-10) at wound sites suggest that an attenuated inflammatory response at the time of injury leads to lower collagen deposition and decreased scar formation 104. These findings lead to the hypotheses that the salamander scar-free healing is connected to its dampened inflammatory response, or that a different molecular pathway may be activated. Evidence that a reduced inflammatory response promotes regeneration remains inconclusive yet suggestive that a more intricate and delicate interaction between the innate immune system and the inflammatory stage cascade of events is still to be discovered.
In order to understand the roles of inflammation in wound healing, it is critical to perform in depth characterisations of the immune populations, as well as to address the functional importance of the inflammatory response, particularly at the onset of injury. Recent studies demonstrate delayed and vastly reduced initial inflammatory response after injury in highly regenerative species. This is followed by a concomitant reduction in disorganised ECM deposition, along with a potential reactivation of developmental pathways that result in scar-free wounds 7,98. Thus, it would be of interest to study the effect of mimicking a mammalian-like inflammatory response during salamander wound healing, as well as performing comparative studies on the characteristics, heterogeneity and dynamics of the immune components throughout the repair process.
Concluding remarks
The presence of immune system components in a broad range of regenerative contexts strongly suggests that immunity plays important roles during the regeneration of complex structures in salamanders. While functional experiments in urodeles are thus far limited to pharmacological approaches probing the roles of macrophages, observations using cell ablation strategies in other systems suggest that genetic approaches will be required to more accurately attribute cellular functions during salamander regeneration. In particular, as confounding findings often arise due to unexpected side-effects of pharmacological approaches, our knowledge of the involvement and relevance of immunity in salamander homeostatic and regenerative contexts needs to be critically revisited using accurate, cell population-specific genetic tools. For the purpose of investigating putative roles of molecular mediators, such as pro- or anti-inflammatory cytokines and complement system components, analysis of the signalling pathways and their conservation in salamanders awaits further characterisation. This will benefit from chemical as well as genetic approaches, but may also require further development of in vitro systems. Once we are able to extensively interrogate immune system-related signalling pathways, a more in-depth characterisation of the roles of inflammation and immune cells in regenerative contexts will be possible (Figure 2). Given the recent development of sophisticated bioinformatic and genetic tools for salamander models, advanced imaging techniques to visualise in vivo cell behaviours, as well as the wealth of information gained through single cell sequencing approaches, the ultimate goal is no longer out of reach. The coming years should bring a significant expansion and refinement of the current paradigm on the role of immunity in salamander regeneration, a critical step towards understanding the mechanisms underlying this fascinating process as well as interspecies differences in regenerative potential.
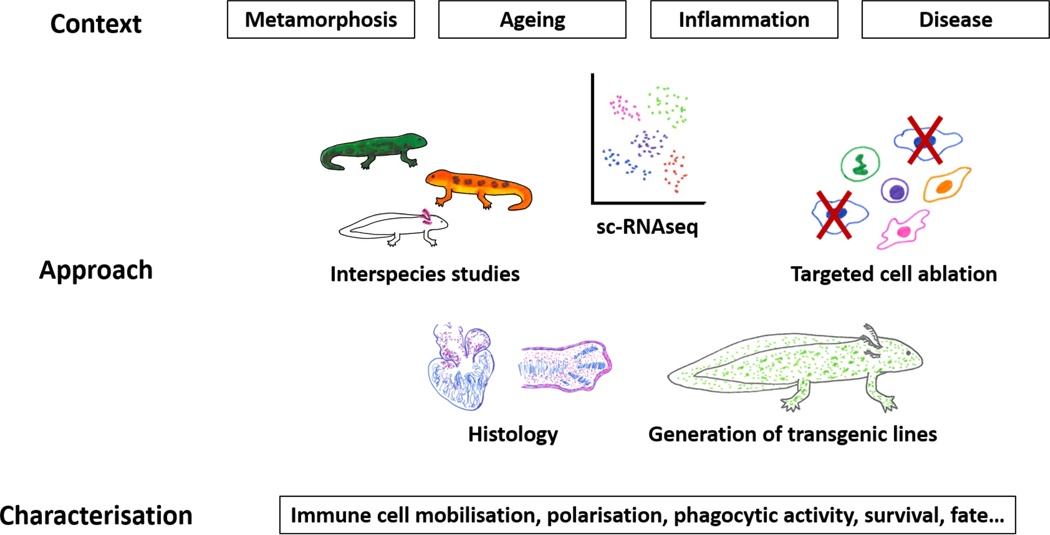
Figure 2.
Immunity in salamander regeneration: where are we headed? Little is known of how different physiological contexts affect the functions of immune system components in salamander regeneration. The combination of traditional and modern approaches such as scRNAseq, genetic tracing and conditional elimination, will spearhead the quest for answers. Further, findings from a variety of salamander species will provide valuable insights on the conservation or divergence of functions of immune players in regeneration, and on the relevance of the physiological context. Integrating observations derived from these diverse approaches will result in a deeper understanding of the role of immunity in regenerative processes.
Acknowledgements
The authors would like to thank Prof. Edward W. Scott and all members of the Yun Lab for useful comments.
Funding sources
L.A.B.C. is supported by a DAAD PhD Scholarship. H.E.W. is an Alexander von Humboldt postdoctoral fellow. R.O.G.V. was supported by a NIH T32 DK074367 training grant. M.H.Y. is supported by the DFG Research Center and Cluster of Excellence - Center for Regenerative Therapies TU Dresden (DFG FZ 111, DFG EXC 168).
References
- 1.Joven A, Elewa A, Simon A. Model systems for regeneration: salamanders. Development. July222019;146(14). 10.1242/dev.167700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brockes JP, Gates PB. Mechanisms underlying vertebrate limb regeneration: lessons from the salamander. Biochem Soc Trans. June2014;42(3):625–30. 10.1042/BST20140002. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka EM, Reddien PW. The cellular basis for animal regeneration. Dev Cell. July192011;21(1):172–85. 10.1016/j.devcel.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox BD, Yun MH, Poss KD. Can laboratory model systems instruct human limb regeneration? Development. October22019;146(20). 10.1242/dev.181016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amamoto R, Huerta VG, Takahashi E, et al. Adult axolotls can regenerate original neuronal diversity in response to brain injury. Elife. May92016;5. 10.7554/eLife.13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piatkowski T, Muhlfeld C, Borchardt T, Braun T. Reconstitution of the myocardium in regenerating newt hearts is preceded by transient deposition of extracellular matrix components. Stem Cells Dev. July12013;22(13):1921–31. 10.1089/scd.2012.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godwin JW, Debuque R, Salimova E, Rosenthal NA. Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape. NPJ Regen Med. 2017;2. 10.1038/s41536-017-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li N, Hua J. Immune cells in liver regeneration. Oncotarget. January102017;8(2):3628–3639. 10.18632/oncotarget.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foglia MJ, Poss KD. Building and re-building the heart by cardiomyocyte proliferation. Development. March12016;143(5):729–40. 10.1242/dev.132910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. November22007;318(5851):772–7. 10.1126/science.1147710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Currie JD, Kawaguchi A, Traspas RM, Schuez M, Chara O, Tanaka EM. Live Imaging of Axolotl Digit Regeneration Reveals Spatiotemporal Choreography of Diverse Connective Tissue Progenitor Pools. Dev Cell. November212016;39(4):411–423. 10.1016/j.devcel.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai SL, Baselga-Garriga C, Melton DA. Blastemal progenitors modulate immune signaling during early limb regeneration. Development. January22019;146(1). 10.1242/dev.169128. [DOI] [PubMed] [Google Scholar]
- 13.Tsai SL, Baselga-Garriga C, Melton DA. Midkine is a dual regulator of wound epidermis development and inflammation during the initiation of limb regeneration. Elife. January142020;9. 10.7554/eLife.50765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsonis PA, Madhavan M, Tancous EE, Del Rio-Tsonis K. A newt’s eye view of lens regeneration. Int J Dev Biol. 2004;48(8–9):975–80. 10.1387/ijdb.041867pt. [DOI] [PubMed] [Google Scholar]
- 15.Laube F, Heister M, Scholz C, Borchardt T, Braun T. Re-programming of newt cardiomyocytes is induced by tissue regeneration. J Cell Sci. November152006;119(Pt 22):4719–29. 10.1242/jcs.03252. [DOI] [PubMed] [Google Scholar]
- 16.Wagner I, Wang H, Weissert PM, et al. Serum Proteases Potentiate BMP-Induced Cell Cycle Re-entry of Dedifferentiating Muscle Cells during Newt Limb Regeneration. Dev Cell. Mar 27 2017;40(6):608–617 e6. 10.1016/j.devcel.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. June42013;110(23):9415–20. 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leigh ND, Dunlap GS, Johnson K, et al. Transcriptomic landscape of the blastema niche in regenerating adult axolotl limbs at single-cell resolution. Nat Commun. December42018;9(1):5153. 10.1038/s41467-018-07604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodgers AK, Smith JJ, Voss SR. Identification of immune and non-immune cells in regenerating axolotl limbs by single-cell sequencing. Exp Cell Res. June172020:112149. 10.1016/j.yexcr.2020.112149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Natarajan N, Abbas Y, Bryant DM, et al. Complement Receptor C5aR1 Plays an Evolutionarily Conserved Role in Successful Cardiac Regeneration. Circulation. May152018;137(20):2152–2165. 10.1161/CIRCULATIONAHA.117.030801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Rio-Tsonis K, Tsonis PA, Zarkadis IK, Tsagas AG, Lambris JD. Expression of the third component of complement, C3, in regenerating limb blastema cells of urodeles. J Immunol. December151998;161(12):6819–24. [PubMed] [Google Scholar]
- 22.Kimura Y, Madhavan M, Call MK, et al. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J Immunol. March12003;170(5):2331–9. 10.4049/jimmunol.170.5.2331. [DOI] [PubMed] [Google Scholar]
- 23.Sibai M, Altuntas E, Suzek BE, et al. Comparison of protein expression profile of limb regeneration between neotenic and metamorphic axolotl. Biochem Biophys Res Commun. February52020;522(2):428–434. 10.1016/j.bbrc.2019.11.118. [DOI] [PubMed] [Google Scholar]
- 24.Curtis SK, Cowden RR, Nagel JW. Ultrastructure of the bone marrow of the salamander Plethodon glutinosus (Caudata: Plethodontidae). J Morphol. Feb 1979;159(2):151–183. 10.1002/jmor.1051590202. [DOI] [PubMed] [Google Scholar]
- 25.Hightower JA, Pierre RLS. Hemopoietic tissue in the adult newt, Notopthalmus viridescens. J Morphol. November1971;135(3):299–307. 10.1002/jmor.1051350304. [DOI] [PubMed] [Google Scholar]
- 26.Henry M, Charlemagne J. Plasmocytic series in the perihepatic layer of the urodele amphibian Pleurodeles waltlii Michah. (Salamandridae). Dev Comp Immunol. January1977;1(1):23–32. 10.1016/s0145-305x(77)80047-5. [DOI] [PubMed] [Google Scholar]
- 27.Lopez D, Lin L, Monaghan JR, et al. Mapping hematopoiesis in a fully regenerative vertebrate: the axolotl. Blood. August212014;124(8):1232–41. 10.1182/blood-2013-09-526970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry M, Charlemagne J. Development of amphibian thymus. I. Morphological differentiation, multiplication, migration and lysis of thymocytes in the urodele Pleurodeles waltlii. J Embryol Exp Morphol. June1980;57:219–32. [PubMed] [Google Scholar]
- 29.Tournefier A, Lesourd M, Gounon P. The axolotl thymus: cell types of the microenvironment - A scanning and transmission electron-microscopy study. Cell Tissue Res. 1990;262:387–396. [Google Scholar]
- 30.Goldstine SN, Manickavel V, N. C. Phylogeny of gut-associated lymphoid tissue. Integr Comp Biol. 1975;15:107–118. [Google Scholar]
- 31.Ardavin CF, Zapata A, Villena A, Solas MT. Gut-associated lymphoid tissue (GALT) in the amphibian urodele Pleurodeles waltl. J Morphol. Jul 1982;173(1):35–41. 10.1002/jmor.1051730105. [DOI] [PubMed] [Google Scholar]
- 32.Francis ETB. The anatomy of the salamander .: Oxford Univ. Press; 1934. [Google Scholar]
- 33.Isogai S, Hitomi J, Yaniv K, Weinstein BM. Zebrafish as a new animal model to study lymphangiogenesis. Anat Sci Int. September2009;84(3):102–11. 10.1007/s12565-009-0024-3. [DOI] [PubMed] [Google Scholar]
- 34.Charlemagne J [Morphological studies of blood cell differentiation in the axolotl, Ambystoma mexicanum Shaw]. Z Zellforsch Mikrosk Anat. 1972;123(2):224–39. [PubMed] [Google Scholar]
- 35.Durand C, Charlemagne J, Fellah JS. RAG expression is restricted to the first year of life in the Mexican axolotl. Immunogenetics. Jul 2000;51(8–9):681–7. 10.1007/s002510000191. [DOI] [PubMed] [Google Scholar]
- 36.Golub R, Andre S, Hassanin A, Affaticati P, Larijani M, Fellah JS. Early expression of two TdT isoforms in the hematopoietic system of the Mexican axolotl. Implications for the evolutionary origin of the N-nucleotide addition. Immunogenetics. June2004;56(3):204–13. 10.1007/s00251-004-0681-2. [DOI] [PubMed] [Google Scholar]
- 37.Fellah JS, Kerfourn F, Dumay AM, Aubet G, Charlemagne J. Structure and diversity of the T-cell receptor alpha chain in the Mexican axolotl. Immunogenetics. 1997;45(4):235–41. 10.1007/s002510050198. [DOI] [PubMed] [Google Scholar]
- 38.Fellah JS, Kerfourn F, Guillet F, Charlemagne J. Conserved structure of amphibian T-cell antigen receptor beta chain. Proc Natl Acad Sci U S A. July151993;90(14):6811–4. 10.1073/pnas.90.14.6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fellah JS, Andre S, Kerfourn F, et al. Structure, diversity and expression of the TCRdelta chains in the Mexican axolotl. Eur J Immunol. May2002;32(5):1349–58. . [DOI] [PubMed] [Google Scholar]
- 40.Andre S, Kerfourn F, Fellah JS. Molecular and biochemical characterization of the Mexican axolotl CD3 (CD3epsilon and CD3gamma/delta). Immunogenetics. Dec 2011;63(12):847–53. 10.1007/s00251-011-0560-6. [DOI] [PubMed] [Google Scholar]
- 41.Fellah JS, Vaulot D, Tournefier A, Charlemagne J. Ontogeny of immunoglobulin expression in the Mexican axolotl. Development. Oct 1989;107(2):253–63. [DOI] [PubMed] [Google Scholar]
- 42.Frippiat C, Kremarik P, Ropars A, Dournon C, Frippiat JP. The recombination-activating gene 1 of Pleurodeles waltl (urodele amphibian) is transcribed in lymphoid tissues and in the central nervous system. Immunogenetics. 2001;52(3–4):264–75. 10.1007/s002510000275. [DOI] [PubMed] [Google Scholar]
- 43.Bascove M, Frippiat JP. Molecular characterization of Pleurodeles waltl activation-induced cytidine deaminase. Mol Immunol. Apr 2010;47(7–8):1640–9. 10.1016/j.molimm.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Ropars A, Bautz AM, Dournon C. Sequencing and expression of the CD3 gamma/delta mRNA in Pleurodeles waltl (urodele amphibian). Immunogenetics. May 2002;54(2):130–8. 10.1007/s00251-002-0437-9. [DOI] [PubMed] [Google Scholar]
- 45.Schaerlinger B, Bascove M, Frippiat JP. A new isotype of immunoglobulin heavy chain in the urodele amphibian Pleurodeles waltl predominantly expressed in larvae. Mol Immunol. February2008;45(3):776–86. 10.1016/j.molimm.2007.06.356. [DOI] [PubMed] [Google Scholar]
- 46.Kyritsis N, Kizil C, Zocher S, et al. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science. December72012;338(6112):1353–6. 10.1126/science.1228773. [DOI] [PubMed] [Google Scholar]
- 47.Morales RA, Allende ML. Peripheral Macrophages Promote Tissue Regeneration in Zebrafish by Fine-Tuning the Inflammatory Response. Front Immunol. 2019;10:253. 10.3389/fimmu.2019.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hui SP, Sheng DZ, Sugimoto K, et al. Zebrafish Regulatory T Cells Mediate Organ-Specific Regenerative Programs. Dev Cell. December182017;43(6):659–672 e5. 10.1016/j.devcel.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Yun MH, Davaapil H, Brockes JP. Recurrent turnover of senescent cells during regeneration of a complex structure. Elife. May52015;4. 10.7554/eLife.05505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsarouchas TM, Wehner D, Cavone L, et al. Dynamic control of proinflammatory cytokines Il-1beta and Tnf-alpha by macrophages in zebrafish spinal cord regeneration. Nat Commun. November72018;9(1):4670. 10.1038/s41467-018-07036-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerber T, Murawala P, Knapp D, et al. Single-cell analysis uncovers convergence of cell identities during axolotl limb regeneration. Science. October262018;362(6413). 10.1126/science.aaq0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walters HE, Yun MH. Rising from the ashes: cellular senescence in regeneration. Current Opinion in Genetics & Development. 2020;64:1 – 7. [DOI] [PubMed] [Google Scholar]
- 53.Reyer RW. Macrophage invasion and phagocytic activity during lens regeneration from the iris epithelium in newts. Am J Anat. August1990;188(4):329–44. 10.1002/aja.1001880402. [DOI] [PubMed] [Google Scholar]
- 54.Kanao T, Miyachi Y. Lymphangiogenesis promotes lens destruction and subsequent lens regeneration in the newt eyeball, and both processes can be accelerated by transplantation of dendritic cells. Dev Biol. February12006;290(1):118–24. 10.1016/j.ydbio.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 55.Xue J, Schmidt SV, Sander J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. February202014;40(2):274–88. 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spiller KL, Koh TJ. Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Deliv Rev. December12017;122:74–83. 10.1016/j.addr.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen-Chi M, Laplace-Builhe B, Travnickova J, et al. TNF signaling and macrophages govern fin regeneration in zebrafish larvae. Cell Death Dis. August102017;8(8):e2979. 10.1038/cddis.2017.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pei W, Tanaka K, Huang SC, et al. Extracellular HSP60 triggers tissue regeneration and wound healing by regulating inflammation and cell proliferation. NPJ Regen Med. 2016;1. 10.1038/npjregenmed.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simoes FC, Cahill TJ, Kenyon A, et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat Commun. January302020;11(1):600. 10.1038/s41467-019-14263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukazawa T, Naora Y, Kunieda T, Kubo T. Suppression of the immune response potentiates tadpole tail regeneration during the refractory period. Development. Jul 2009;136(14):2323–7. 10.1242/dev.033985. [DOI] [PubMed] [Google Scholar]
- 61.Lehenkari PP, Kellinsalmi M, Napankangas JP, et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol. May2002;61(5):1255–62. 10.1124/mol.61.5.1255. [DOI] [PubMed] [Google Scholar]
- 62.Simkin J, Sammarco MC, Marrero L, et al. Macrophages are required to coordinate mouse digit tip regeneration. Development. November12017;144(21):3907–3916. 10.1242/dev.150086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature. September272012;489(7417):561–5. 10.1038/nature11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simkin J, Gawriluk TR, Gensel JC, Seifert AW. Macrophages are necessary for epimorphic regeneration in African spiny mice. Elife. May162017;6. 10.7554/eLife.24623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin P, D’Souza D, Martin J, et al. Wound healing in the PU.1 null mouse--tissue repair is not dependent on inflammatory cells. Curr Biol. July12003;13(13):1122–8. 10.1016/s0960-9822(03)00396–8. [DOI] [PubMed] [Google Scholar]
- 66.Petrie TA, Strand NS, Yang CT, Rabinowitz JS, Moon RT. Macrophages modulate adult zebrafish tail fin regeneration. Development. July2014;141(13):2581–91. 10.1242/dev.098459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J, Kubes P. A Reservoir of Mature Cavity Macrophages that Can Rapidly Invade Visceral Organs to Affect Tissue Repair. Cell. April212016;165(3):668–78. 10.1016/j.cell.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 68.Sanz-Morejon A, Garcia-Redondo AB, Reuter H, et al. Wilms Tumor 1b Expression Defines a Pro-regenerative Macrophage Subtype and Is Required for Organ Regeneration in the Zebrafish. Cell Rep. July302019;28(5):1296–1306 e6. 10.1016/j.celrep.2019.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lavin Y, Winter D, Blecher-Gonen R, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. December42014;159(6):1312–26. 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gosselin D, Link VM, Romanoski CE, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. December42014;159(6):1327–40. 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J Neutrophils in tissue injury and repair. Cell Tissue Res. March2018;371(3):531–539. 10.1007/s00441-017-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fadok VA, McDonald PP, Bratton DL, Henson PM. Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem Soc Trans. November1998;26(4):653–6. 10.1042/bst0260653. [DOI] [PubMed] [Google Scholar]
- 73.Yang W, Tao Y, Wu Y, et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat Commun. March62019;10(1):1076. 10.1038/s41467-019-09046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keightley MC, Wang CH, Pazhakh V, Lieschke GJ. Delineating the roles of neutrophils and macrophages in zebrafish regeneration models. Int J Biochem Cell Biol. November2014;56:92–106. 10.1016/j.biocel.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 75.Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol. December2006;80(6):1281–8. 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science. October62017;358(6359):111–116. 10.1126/science.aam9690. [DOI] [PubMed] [Google Scholar]
- 77.Fahmy GH, Sicard RE. A role for effectors of cellular immunity in epimorphic regeneration of amphibian limbs. In Vivo. May-June2002;16(3):179–84. [PubMed] [Google Scholar]
- 78.Franchini A, Bertolotti E. The thymus and tail regenerative capacity in Xenopus laevis tadpoles. Acta Histochem. July2012;114(4):334–41. 10.1016/j.acthis.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 79.Chen J, He J, Ni R, Yang Q, Zhang Y, Luo L. Cerebrovascular Injuries Induce Lymphatic Invasion into Brain Parenchyma to Guide Vascular Regeneration in Zebrafish. Dev Cell. June32019;49(5):697–710 e5. 10.1016/j.devcel.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 80.Gur-Cohen S, Yang H, Baksh SC, et al. Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science. December62019;366(6470):1218–1225. 10.1126/science.aay4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mescher AL, Wolf WL, Moseman EA, et al. Cells of cutaneous immunity in Xenopus: studies during larval development and limb regeneration. Dev Comp Immunol. 2007;31(4):383–93. 10.1016/j.dci.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Mescher AL. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration (Oxf). April2017;4(2):39–53. 10.1002/reg2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mangoni ML, McDermott AM, Zasloff M. Antimicrobial peptides and wound healing: biological and therapeutic considerations. Exp Dermatol. March2016;25(3):167–73. 10.1111/exd.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang G, Li X, Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. January42016;44(D1):D1087–93. 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fredericks LP, Dankert JR. Antibacterial and hemolytic activity of the skin of the terrestrial salamander, Plethodon cinereus. J Exp Zool. October12000;287(5):340–5. [PubMed] [Google Scholar]
- 86.Meng P, Yang S, Shen C, Jiang K, Rong M, Lai R. The first salamander defensin antimicrobial peptide. PLoS One. 2013;8(12):e83044. 10.1371/journal.pone.0083044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mu L, Tang J, Liu H, et al. A potential wound-healing-promoting peptide from salamander skin. FASEB J. Sep 2014;28(9):3919–29. 10.1096/fj.13-248476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang C, Wang C, Li Y, et al. Complement C3a signaling facilitates skeletal muscle regeneration by regulating monocyte function and trafficking. Nat Commun. December122017;8(1):2078. 10.1038/s41467-017-01526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang S, Cui P. Complement system in zebrafish. Dev Comp Immunol. September2014;46(1):3–10. 10.1016/j.dci.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Davis AE, 3rd, Lu F, Mejia P. C1 inhibitor, a multi-functional serine protease inhibitor. Thromb Haemost. November2010;104(5):886–93. 10.1160/TH10-01-0073. [DOI] [PubMed] [Google Scholar]
- 91.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. May 15 2008;453(7193):314–21. 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 92.Landen NX, Li D, Stahle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. October2016;73(20):3861–85. 10.1007/s00018-016-2268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. July112011;13:e23. 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. December32014;6(265):265sr6. 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moro-Garcia MA, Mayo JC, Sainz RM, Alonso-Arias R. Influence of Inflammation in the Process of T Lymphocyte Differentiation: Proliferative, Metabolic, and Oxidative Changes. Front Immunol. 2018;9:339. 10.3389/fimmu.2018.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xue M, Jackson CJ. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv Wound Care (New Rochelle). March12015;4(3):119–136. 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Levin R, Grinstein S, Canton J. The life cycle of phagosomes: formation, maturation, and resolution. Immunol Rev. Sep 2016;273(1):156–79. 10.1111/imr.12439. [DOI] [PubMed] [Google Scholar]
- 98.Seifert AW, Monaghan JR, Voss SR, Maden M. Skin regeneration in adult axolotls: a blueprint for scar-free healing in vertebrates. PLoS One. 2012;7(4):e32875. 10.1371/journal.pone.0032875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davis AK, Maney DL, Maerz JC. The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Functional Ecology. 2008;22:760 – 772. [Google Scholar]
- 100.Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. April2003;73(4):448–55. 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 101.Simpson DM, Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest. August1972;51(8):2009–23. 10.1172/JCI107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Atala A, Irvine DJ, Moses M, Shaunak S. Wound Healing Versus Regeneration: Role of the Tissue Environment in Regenerative Medicine. MRS Bull. August12010;35(8). 10.1557/mrs2010.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology. July2011;216(7):753–62. 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 104.Gurevich DB, Severn CE, Twomey C, et al. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J. July22018;37(13). 10.15252/embj.201797786. [DOI] [PMC free article] [PubMed] [Google Scholar]