Abstract
Objective:
Gender differences in bladder cancer survival are well known. However, the impact of type of treatment, timing to surgery when rendered and survival outcomes according to gender has not been extensively examined. Given the relatively low incidence of bladder cancer in females, large multicenter and population-based studies are required to elucidate gender differences in survival. In the present study, we sought to characterize the impact of utilization and timing of radical cystectomy (RC) according to gender and survival outcomes.
Methods:
A total of 9,907 patients aged 66 years or older diagnosed with clinical stage II-IV N0M0 bladder cancer from January 1, 2001 to December 31, 2011 from SEER-Medicare data were analyzed. We used multivariable regression analyses to identify factors predicting the use and delay of RC. Cox proportional hazards models were used to analyze survival outcomes.
Results:
Of the 9,907 patients diagnosed with bladder cancer 3,256 (32.9%) were female. Women were significantly more likely to undergo RC across all stages compared to their male counterparts (Stage II: Relative Risk (RR) 1.48, 95% Confidence Interval (CI) = 1.33-1.65, P < 0.001; Stage III: RR 1.24, 95% CI = 1.13-1.37, p<0.001; Stage IV: RR 1.33, 95% CI = 1.19-1.49, p<0.001). Moreover, there was no significant difference in delay to RC according to gender across all clinical stages. Using propensity score matching, women had worse overall (HR 1.07, CI 1.01-1.14, p=0.024), and worse cancer-specific survival (HR 1.26, CI 1.17-1.36, p<0.001) than men, respectively.
Conclusion:
Gender differences persist with women significantly more likely to undergo RC independent of clinical stage. However, women have significantly worse survival than men. Delay from diagnosis to surgery did not account for this decreased survival among women.
Keywords: Bladder Cancer, Gender, Differences, Utilization, Radical Cystectomy
INTRODUCTION
There were an estimated 76,960 new cases and 16,390 deaths from bladder cancer in the United States in 2016, with men accounting for 76.5% of these new cases [1]. Although women are less likely to be diagnosed with bladder cancer, they present with more advanced disease and have worse survival outcomes compared to their male counterparts [2-6]. Moreover, prior studies have shown gender differences in survival following radical cystectomy [7-11]. The etiology of this gender discrepancy is still largely unknown, with prior studies suggesting inferior process of care measures such as delay to diagnosis among women leading to decreased chance for curative therapy and increased mortality [12]. This theory has been supported by studies attributing hematuria and voiding symptoms to be mistaken for infection, potentially leading to delayed referral to urology with delay in diagnosis of malignancy [13].
Current guidelines for patients with non-metastatic muscle-invasive bladder cancer recommend neoadjuvant chemotherapy followed by radical cystectomy (RC) with extended pelvic lymphadenectomy [14]. While underuse of neoadjuvant chemotherapy is well known, RC is significantly underutilized with use relatively unchanged over the past 3 decades which corroborate similar unchanged survival outcomes among patients with muscle-invasive disease [15]. Moreover, while underutilization of RC is paramount, timing to RC has been strongly associated with survival outcomes [16]. Prior work by Messer et al. identified the female gender as an adverse prognostic factor, independent of clinical and pathological features for patients undergoing RC [17, 18]. However, the impact of type of treatment, timing to surgery when rendered and survival outcomes according to gender has not been extensively examined [18, 19]. Therefore, we provide a population-based assessment in order to discern whether utilization of RC differs according to gender, specifically examining the receipt and timing of RC in relation to survival outcomes.
PATIENTS AND METHODS
Data Source
Our study utilized the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Medicare linked database. The dataset contains information on patients with newly diagnosed cancers in 18 US regions that are generalizable to the US population. Bladder cancer identified in the SEER database conformed to the standards of the North American Association of Central Cancer Registries, and case ascertainment in the SEER data was 98% complete [20]. The SEER database contains information on patient demographics, tumor characteristics (stage, grade, histology), and follow-up information. The Medicare database contains information on inpatient and outpatient claims. The study was deemed exempt by the Institutional Review Board at The University of Texas Medical Branch at Galveston and The University of Texas MD Anderson Cancer Center.
Ascertainment of Study Cohort
We restricted our analysis to patients with bladder cancer diagnosed as clinical stage II-IV N0M0 transitional cell or urothelial carcinoma from 2001 through 2011 with claims data available through December 31, 2013. Clinical stage is pathologically confirmed at radical cystectomy incorporating both clinical stage and pathological stage into a collaborative stage variable using the AJCC staging classification system in SEER. We restricted the study sample to subjects who had Medicare Fee-for-Service coverage and for whom Medicare Part A and Part B claims data were available 12 months before and 12 months after the date of diagnosis. The final cohort consisted of 9,907 patients (Supplementary Table 1).
Identification of Bladder Cancer Treatments
Receipt of bladder cancer treatments was determined for one year after date of diagnosis. Subjects who underwent RC were identified based on International Classification of Diseases–Version 9 (ICD-9) and Common Procedural Terminology-4 (CPT-4) codes indicative for RC (Supplementary Table 2). RC utilized in this study included both open and robot-assisted laparoscopic surgery. Subjects who underwent surgery alone or in combination with radiation or chemotherapy are considered in the RC group. Subjects who received radiation were classified on the basis of diagnosis and procedure codes in Medicare claims that are consistent with ICD-9 and CPT codes specific for radiotherapeutic procedures used to treat bladder cancer (Supplementary Table 2). Among those without RC, we combined subjects who received chemotherapy alone, radiation alone, or combination chemotherapy and radiation into one treatment group because bladder-sparing therapeutic protocols for invasive bladder cancer typically combine radiation and chemotherapy [21]. We identified subjects who received chemotherapy based on ICD-9 and CPT-4 codes that are consistent with chemotherapeutic agents commonly used in the management bladder cancer in the absence of a simultaneous code for RC (Supplementary Table 2).
Study Covariates
Using the SEER database, we obtained age, sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and non-Hispanic other races), marital status (single, married, and unknown), and SEER region (Northeast, South, Midwest, and West). Urinary symptoms within one year before cancer diagnosis were collected and included the following ICD-9 codes grouped in 3 separate categories: irritative urinary symptoms, obstructive urinary symptoms, and hematuria as shown in Supplementary Table 2. We also obtained subject’s community socioeconomic characteristics. Census tract-level median household income was divided into quartiles. From the SEER database we determined cancer diagnosis year, grade, and stage. From claims database we identified presence of hydronephrosis, comorbidity score, and treatment method. Level of comorbidity was assessed using the Klabunde modification of the Charlson comorbidity index (CCI) during the year before diagnosis [22]. The Klabunde modification utilizes comorbid conditions identified by the CCI and incorporates the diagnostic and procedure data contained in Medicare physician Part B claims.
Statistical Analysis
Univariate analyses was performed to assess the association of RC with the list of covariates described above, using the Pearson chi-square test. We created a multivariable generalized linear model that incorporates stage, treatment method (RC, chemotherapy, and radiation therapy), and gender stratified by stage to evaluate the effect of gender associated with receipt of bladder cancer treatment, and to evaluate the association between gender and delayed RC from time of diagnosis. In the our multivariable analysis, we further classified the timing of RC into two groups, less or equal to 12 weeks and longer than 12 weeks, since previous studies have reported inferior overall survival and progression-free survival for patients who received RC more than 84–90 days after diagnosis. [23-26]. In the sensitivity analysis, we performed propensity score matching. Relative risks were reported from these models. Cox proportional hazards models were used to analyze overall and cancer specific survival outcomes. We used logistic regression analysis to generate probability to match male and female patients using the previously mentioned demographic and clinical covariates as predictors. We then conducted Cox proportional hazards models in the propensity score matched cohort were used to analyze overall and cancer specific survival outcomes. Proportional hazards assumption in Cox Model was tested using proportionality test. All statistical tests were 2-sided, and all analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC). Statistical significance was defined as p<0.05.
RESULTS
Patient demographics are summarized in Table 1. Of the 9,907 patients diagnosed with bladder cancer 3,256 (32.9%) were female. While there was no significant difference in bladder cancer diagnosis over the study period, women were older, non–Caucasian race/ethnicity, unmarried, had fewer comorbidities and presented with more advanced disease than men (all p<0.001). Men were more likely to present with hematuria and obstructive urinary symptoms, while women more were more likely to present with irritative urinary symptoms (all p<0.001). Overall 2,738 of the total patients in this study underwent RC, 1,038 (31.9%) of them were female (p<0.001) (Figure 1).
Table 1.
Patient demographic and clinical characteristics
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Total | No. | % | No. | % | p-value |
| Year of Diagnosis | 0.058 | |||||
| 2001 | 815 | 561 | 8.4 | 254 | 7.8 | |
| 2002 | 920 | 579 | 8.7 | 341 | 10.5 | |
| 2003 | 883 | 596 | 9.0 | 287 | 8.8 | |
| 2004 | 971 | 637 | 9.6 | 334 | 10.3 | |
| 2005 | 992 | 665 | 10.0 | 327 | 10.0 | |
| 2006 | 942 | 623 | 9.4 | 319 | 9.8 | |
| 2007 | 906 | 610 | 9.2 | 296 | 9.1 | |
| 2008 | 908 | 599 | 9.0 | 309 | 9.5 | |
| 2009 | 848 | 584 | 8.8 | 264 | 8.1 | |
| 2010 | 866 | 589 | 8.9 | 277 | 8.5 | |
| 2011 | 856 | 608 | 9.1 | 248 | 7.6 | |
| Age Group | <0.001 | |||||
| 66-69 | 1333 | 988 | 14.9 | 345 | 10.6 | |
| 70-74 | 1942 | 1364 | 20.5 | 578 | 17.8 | |
| 75-79 | 2405 | 1615 | 24.3 | 790 | 24.3 | |
| 80+ | 4227 | 2684 | 40.4 | 1543 | 47.4 | |
| Race/ethnicity | <0.001 | |||||
| White | 8564 | 5863 | 88.2 | 2701 | 83.0 | |
| Black | 604 | 292 | 4.4 | 312 | 9.6 | |
| Hispanics | 279 | 194 | 2.9 | 85 | 2.6 | |
| Other | 460 | 302 | 4.5 | 158 | 4.9 | |
| Marital Status | <0.001 | |||||
| Single | 1440 | 918 | 13.8 | 522 | 16.0 | |
| Married | 5420 | 4394 | 66.1 | 1026 | 31.5 | |
| Unknown | 3047 | 1339 | 20.1 | 1708 | 52.5 | |
| Rural area | 0.1088 | |||||
| No | 9691 | 6495 | 97.7 | 3196 | 98.2 | |
| Yes | 216 | 156 | 2.3 | 60 | 1.8 | |
| Census Region | 0.016 | |||||
| West | 3907 | 2694 | 40.5 | 1213 | 37.3 | |
| Northeast | 2354 | 1540 | 23.2 | 814 | 25.0 | |
| Midwest | 1139 | 761 | 11.4 | 378 | 11.6 | |
| South | 2507 | 1656 | 24.9 | 851 | 26.1 | |
| Median Household Income, $ | 0.511 | |||||
| ≤ 23,364 | 2808 | 1882 | 28.3 | 926 | 28.4 | |
| 23,365–31,906 | 2523 | 1701 | 25.6 | 822 | 25.2 | |
| 31,907–41,719 | 2344 | 1595 | 24.0 | 749 | 23.0 | |
| ≥41,720 | 2232 | 1473 | 22.1 | 759 | 23.3 | |
| Stage | 0.007 | |||||
| II | 5220 | 3578 | 53.8 | 1642 | 50.4 | |
| III | 1889 | 1241 | 18.7 | 648 | 19.9 | |
| IV | 2798 | 1832 | 27.5 | 966 | 29.7 | |
| Hydronephosis | <0.001 | |||||
| No | 8866 | 6018 | 90.5 | 2848 | 87.5 | |
| Yes | 1041 | 633 | 9.5 | 408 | 12.5 | |
| Grade | 0.182 | |||||
| Low | 625 | 439 | 6.6 | 186 | 5.7 | |
| High | 8754 | 5866 | 88.2 | 2888 | 88.7 | |
| Unknown | 528 | 346 | 5.2 | 182 | 5.6 | |
| Comorbidity Score | 0.001 | |||||
| 0 | 5361 | 3527 | 53.0 | 1834 | 56.3 | |
| 1 | 2415 | 1615 | 24.3 | 800 | 24.6 | |
| 2 | 1083 | 767 | 11.5 | 316 | 9.7 | |
| 3+ | 1048 | 742 | 11.2 | 306 | 9.4 | |
| Radical Cystectomy | <0.001 | |||||
| No | 7169 | 4951 | 74.4 | 2218 | 68.1 | |
| Yes | 2738 | 1700 | 25.6 | 1038 | 31.9 | |
| Treatment | <0.001 | |||||
| No curative treatment | 4405 | 3036 | 45.6 | 1369 | 42.0 | |
| Radical Cystectomy Alone | 365 | 224 | 3.4 | 141 | 4.3 | |
| Radical Cystectomy w/ LDS | 2373 | 1476 | 22.2 | 897 | 27.5 | |
| Radiation Therapy and/or Chemotherapy | 2764 | 1915 | 28.8 | 849 | 26.1 | |
| Neoadjuvant chemotherapy | 0.784 | |||||
| No | 9513 | 6389 | 96.1 | 3124 | 95.9 | |
| Yes | 394 | 262 | 3.9 | 132 | 4.1 | |
| Hematuria | <0.001 | |||||
| No | 3672 | 2385 | 35.9 | 1287 | 39.5 | |
| Yes | 6235 | 4266 | 64.1 | 1969 | 60.5 | |
| Irritative Symptoms | <0.001 | |||||
| No | 7726 | 5299 | 79.7 | 2427 | 74.5 | |
| Yes | 2181 | 1352 | 20.3 | 829 | 25.5 | |
| Obstructive Symptoms | <0.001 | |||||
| No | 8606 | 5587 | 84.0 | 3019 | 92.7 | |
| Yes | 1301 | 1064 | 16.0 | 237 | 7.3 | |
Figure 1.
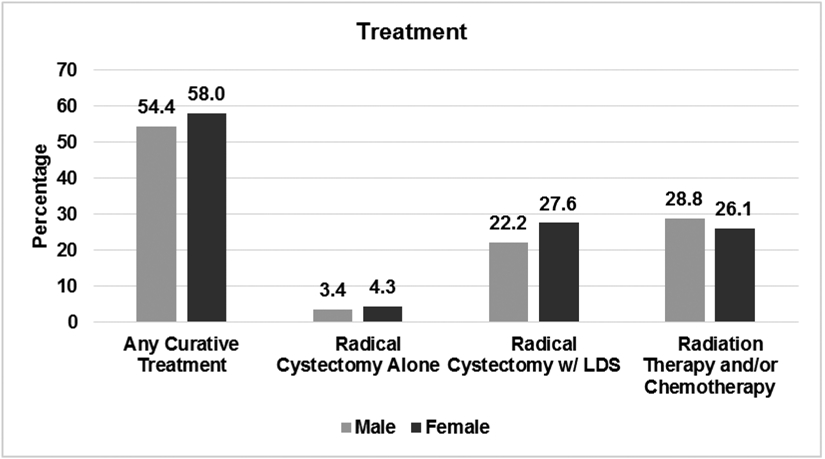
Treatments according to gender
LDS: Lymph node dissection
We analyzed predictors for receipt of radical cystectomy stratified by stage and gender as shown in Table 2. Patients who received neoadjuvant chemotherapy were more likely to have delayed RC across all stages (p<0.01). Women were significantly more likely to undergo RC across all stages compared to their male counterparts (Stage II: Relative Risk (RR) 1.48, 95% Confidence Interval (CI) = 1.33-1.65, P < 0.001; Stage III: RR 1.24, 95% CI = 1.13-1.37, p<0.001; Stage IV: RR 1.33, 95% CI = 1.19-1.49, p<0.001). Moreover, there was no significant difference in delay to RC according to gender across all clinical stages.
Table 2.
Multivariable model on predictors of receipt of radical cystectomy and receipt of delayed radical cystectomy stratified by stage.
| Receipt of Radical Cystectomy |
Receipt of Delayed Radical Cystectomy |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | No. (Percent) |
RR | 95% CI | P- value |
No. (Percent) |
RR | 95% CI | P-value | ||
|
|
|
|||||||||
| Stage II | ||||||||||
| Male | 643 (18.0) | 1.00 | 283 (7.9) | 1.00 | ||||||
| Female | 381 (23.2) | 1.48 | 1.33 | 1.65 | <.001 | 169 (10.3) | 0.99 | 0.94 | 1.05 | 0.744 |
| Stage III | 1.00 | |||||||||
| Male | 541 (43.6) | 180 (14.5) | 1.00 | |||||||
| Female | 330 (50.9) | 1.24 | 1.13 | 1.37 | <.001 | 105 (16.2) | 0.99 | 0.94 | 1.03 | 0.513 |
| Stage IV | ||||||||||
| Male | 516 (28.2) | 1.00 | 196 (10.7) | 1.00 | ||||||
| Female | 327 (33.9) | 1.33 | 1.19 | 1.49 | <.001 | 96 (9.9) | 0.96 | 0.90 | 1.02 | 0.181 |
Predictors in the model: year of diagnosis, age, race/ethnicity, marital status, rural area, census region, median income, tumor grade, stage, hydronephrosis, hematuria, irritative or obstructive symptom, and comorbidity score.
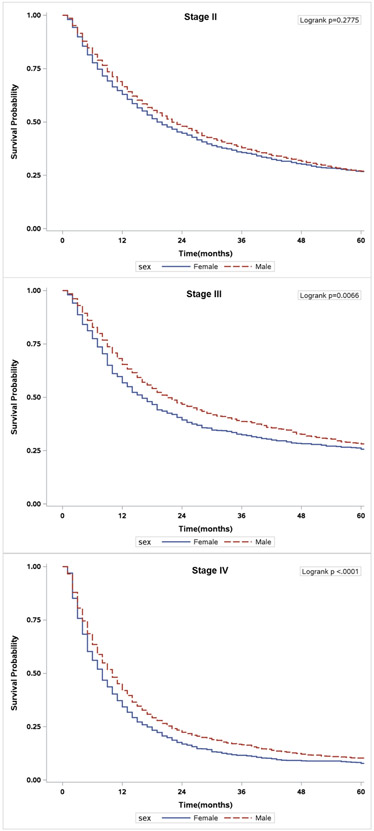
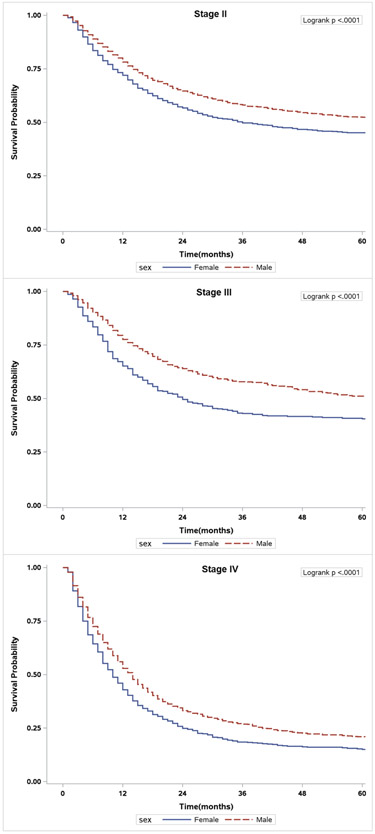
The overall and cancer-specific survival estimates for all bladder cancer patients according to stage and gender are presented in Table 3. Overall survival for all patients (Hazard Ratio [HR] 1.07, 95% CI = 1.02-1.12, p=0.010) and those with stage IV disease (HR 1.19, 95% CI 1.08-1.30, p<0.001) was significantly worse for women than men (Figure 2). Moreover, women had worse cancer-specific survival when compared to men for all stages (HR 1.27, 95% CI 1.19-1.35, p<0.001) and specifically among those diagnosed with stage II (HR 1.20, CI = 1.09–1.32, p<0.001), stage III (HR 1.45, CI 1.24–1.70, p<0.001), and stage IV (HR 1.29, CI 1.16–1.43, p<0.001) (Figure 3). Using propensity score matching (Supplementary Table 3), women had worse overall (HR 1.07, CI 1.01-1.14, p=0.024), and cancer-specific survival (HR 1.26, CI 1.17-1.36, p<0.001) than men, respectively.
Table 3.
Hazard ratios of overall survival and cancer-specific survival in patients diagnosed with bladder cancer
| Overall Survival | Cancer-Specific Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Gender | ||||||||
| Male | 1.00 | 1.00 | ||||||
| Female (All patients) | 1.07 | 1.02 | 1.12 | 0.010 | 1.27 | 1.19 | 1.35 | <.001 |
| Female (Stage II) | 0.98 | 0.91 | 1.05 | 0.531 | 1.20 | 1.09 | 1.32 | <.001 |
| Female (Stage III) | 1.12 | 0.99 | 1.26 | 0.071 | 1.45 | 1.24 | 1.70 | <.001 |
| Female (Stage IV) | 1.19 | 1.08 | 1.30 | <.001 | 1.29 | 1.16 | 1.43 | <.001 |
Predictors in the model: treatment, year of diagnosis, age, race/ethnicity, marital status, rural area, census region, median income, tumor grade, stage, neoadjuvant chemotherapy, hydronephrosis and comorbidity score.
Figure 2.
Unadjusted overall survival of patients stratified by sex. A) Stage II patients. B) Stage III patients. C) Stage IV patients.
Figure 3.
Unadjusted cancer-specific survival of patients stratified by sex. A) Stage II patients. B) Stage III patients. C) Stage IV patients.
Adjusted hazard ratios of delayed RC for overall survival and cancer-specific survival stratified by gender were performed (Supplementary Table 4). There was no significant difference in overall or cancer-specific survivals noted. As an attempt to control for further unknown confounders, we analyzed non-cancer survival by treatment (rather than all-cause survival). We noticed an effect of RC on the cancer-specific but not the non-cancer survival (p=0.207).
DISCUSSION
Men are more likely to be diagnosed with bladder cancer, however, women have increased bladder cancer-specific mortality [1-6, 27]. Our study utilized a large population-based cancer registry to critically examine gender differences in survival taking into account known predictors for survival as well as treatments including receipt and timing of RC. Our research confirms that although women have decreased incidence and increased bladder cancer-specific mortality, these differences in survival cannot be explained by RC use, delay to surgery and/or adverse clinical and pathological determinants.
First, women presented with more advanced disease which is consistent with prior studies. Moreover, there was no significant difference in diagnosis according to gender during the study period. While we did not account for delay to diagnosis of urinary symptoms (i.e. hematuria) to diagnosis of bladder cancer, we found women diagnosed to be older, non-Hispanic black race/ethnicity, and unmarried. These determinants, in addition to advanced stage of disease, have been previously associated with decreased survival among bladder cancer patients [21, 28]. Other studies have found that women present with more advanced stage for cystectomy and stage for stage, do worse. However, one study found that when matched 1:1 with males receiving cystectomy, taking in to account stage, grade, p53 status, chemotherapy use, hydronephrosis and time to cystectomy, there was no difference [29]. In the present study, using propensity score matching analyses to control for these and other determinants, we showed a persistent decreased overall and cancer specific-survival among women when compared to men. As an attempt to control for further unknown confounders, we analyzed non-cancer survival by treatment (rather than all-cause survival). We noticed an effect of treatment on the cancer-specific but not the non-cancer survival thus confirming the association between treatment and cancer-specific survival cannot be explained other unknown confounders [30]. While we certainly understand such analytic methods cannot control for all potential confounders, these data suggest advanced stage disease at presentation may not account for the survival difference according to gender [4]. Moreover, advanced stage disease such as stage IV is not necessarily the same for men and women. In the case of extension to the genital system in women, surgery can be easily proposed and performed, whereas it is more complicated in the case of rectal or parietal involvement in men. This is a major bias to discuss in order to consider when interpreting the survival difference according to gender in patients with stage IV cancer.
Second, when compared to men we observed women to have worse overall survival among all patients and in those with stage IV disease. In propensity score matching analyses, women had worse overall and cancer-specific survival when compared to men. Thus, while we cannot control for all potential confounders, when we attempted to do so using propensity score matching women have significantly worse survival than men. Age and comorbidity are likely determinants for overall survival, however, dietary intake and lifestyle are integral predictors for cancer-overall as well as cancer-specific survival [7, 31].
Third, women had increased use of treatments and specifically RC with no significant difference in delay to surgery. One plausible explanation for the increased use of treatments and more specifically RC, could be the fewer comorbidities observed (i.e. CCI ≥2) among women which could influence the decision to pursue surgery. In the present study, female patients with decreased comorbidities may need less preoperative evaluations, consultations and studies which may expedite timing to surgery. Moreover, advanced stage at presentation may prompt providers to act more aggressively. However, it should also be mentioned that use of RC was low regardless of gender. Despite these longstanding guidelines, radical cystectomy is markedly underused; only 19-21% of patients age 66 years of age and older with muscle-invasive disease are offered this potentially curative surgery. [15, 21] In the present study, despite the relative increased use of a potentially curable surgery with no increased delay to treatment (we even noted decrease delay to surgery among patients with stage IV disease), women have worse cancer-specific survival. Prior research concerning the biological aggressiveness of bladder cancer according to subtype of muscle-invasive disease may help elucidate the biological underpinnings of carcinogenesis according to gender [32]. Moreover, a plausible etiology for the known survival discrepancy pertains to prior the androgen receptor (AR) axis. The AR axis activates a number of known downstream oncogenes such as the epithelial growth factor receptor (EGFR/ERBB2) pathway and the increased β-catenin signaling [33, 34]. It is possible that increased circulating serum concentrations of androgen in male bladder cancer patients may result in the increased incidence among men. However, Daugherty et al. have shown that cumulative exposure to estrogen and progestin is protective against bladder cancer incidence [35]. The decreased production of estrogen and progesterone as observed in post-menopausal women and effects on biological aggressiveness of bladder cancer remains to be determined. From the present study we found process of care determinants (i.e. RC use and delay to treatment) were not associated with gender differences in survival which suggests further research discerning biological aggressiveness of bladder cancer according to gender is needed.
While our findings are clinically relevant, they must be interpreted within the context of the study design. First, patients utilized in this study are older and thus we cannot comment on our findings in relation to gender for younger patients. However, a majority of patients with bladder cancer are diagnosed in their sixth decade of life and therefore we provide a contemporary analysis of the gender differences in survival. Second, there is evidence supporting the use of neoadjuvant chemotherapy to significantly downstage and improved survival benefit at radical cystectomy [36]. In the present analysis, the use of perioperative chemotherapy was not accounted for due to the low utilization rates with no difference in use according to gender observed (data not shown) in the present cohort. Prior research by Booth et al. has shown that approximately 4% of patients with muscle-invasive bladder cancer receive neoadjuvant chemotherapy, thus potentially limiting this as a significant unmeasured confounding variable [37]. Third, we understand inherent limitations in using cancer registry data and in particular the inability to control for unknown confounders. We acknowledge the heterogeneity in staging according to gender as well as inherent staging limitations of using cancer registries. We attempted to control for potential confounders using propensity score matching. In addition, we determined an effect of treatment on the cancer-specific but not the non-cancer survival. Thus, the current data provide a robust, generalizable assessment of gender differences in survival at the population-based level.
CONCLUSIONS
Gender differences in survival persist despite women significantly more likely to undergo treatment including RC. These findings were independent of clinical stage. Delay from diagnosis to surgery did not account for the decreased cancer-specific survival among women, suggesting inherent characteristics of tumor biology likely impact gender differences in survival. These findings support further research to discern the biological underpinnings of bladder carcinogenesis according to gender.
Supplementary Material
Acknowledgement
This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a by a Clinical and Translational Science Award Mentored Career Development (KL2) Award (KL2TR001441) from the National Center for Advancing Translational Sciences, National Institutes of Health, Comparative Effectiveness Research on Cancer in Texas (CERCIT) (RP140020) and the National Cancer Institute (NCI) (K05 CA134923) (SBW) and in part by the fellowship from University of Texas MD Anderson Cancer Center's Halliburton Employees Foundation (JH).
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Fajkovic H, Halpern JA, Cha EK, Bahadori A, Chromecki TF, Karakiewicz PI, et al. Impact of gender on bladder cancer incidence, staging, and prognosis. World J Urol. 2011;29:457–63. [DOI] [PubMed] [Google Scholar]
- [3].Mungan NA, Kiemeney LA, van Dijck JA, van der Poel HG, Witjes JA. Gender differences in stage distribution of bladder cancer. Urology. 2000;55:368–71. [DOI] [PubMed] [Google Scholar]
- [4].Mungan NA, Aben KK, Schoenberg MP, Visser O, Coebergh JW, Witjes JA, et al. Gender differences in stage-adjusted bladder cancer survival. Urology. 2000;55:876–80. [DOI] [PubMed] [Google Scholar]
- [5].Najari BB, Rink M, Li PS, Karakiewicz PI, Scherr DS, Shabsigh R, et al. Sex disparities in cancer mortality: the risks of being a man in the United States. The Journal of urology. 2013;189:1470–4. [DOI] [PubMed] [Google Scholar]
- [6].Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68–74. [DOI] [PubMed] [Google Scholar]
- [7].Kluth LA, Rieken M, Xylinas E, Kent M, Rink M, Roupret M, et al. Gender-specific differences in clinicopathologic outcomes following radical cystectomy: an international multi-institutional study of more than 8000 patients. European urology. 2014;66:913–9. [DOI] [PubMed] [Google Scholar]
- [8].Otto W, May M, Fritsche HM, Dragun D, Aziz A, Gierth M, et al. Analysis of sex differences in cancer-specific survival and perioperative mortality following radical cystectomy: results of a large German multicenter study of nearly 2500 patients with urothelial carcinoma of the bladder. Gender medicine. 2012;9:481–9. [DOI] [PubMed] [Google Scholar]
- [9].May M, Bastian PJ, Brookman-May S, Fritsche HM, Tilki D, Otto W, et al. Gender-specific differences in cancer-specific survival after radical cystectomy for patients with urothelial carcinoma of the urinary bladder in pathologic tumor stage T4a. Urologic oncology. 2013;31:1141–7. [DOI] [PubMed] [Google Scholar]
- [10].May M, Stief C, Brookman-May S, Otto W, Gilfrich C, Roigas J, et al. Gender-dependent cancer-specific survival following radical cystectomy. World J Urol. 2012;30:707–13. [DOI] [PubMed] [Google Scholar]
- [11].Soave A, Dahlem R, Hansen J, Weisbach L, Minner S, Engel O, et al. Gender-specific outcomes of bladder cancer patients: a stage-specific analysis in a contemporary, homogenous radical cystectomy cohort. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2015;41:368–77. [DOI] [PubMed] [Google Scholar]
- [12].McGrath M, Michaud DS, De Vivo I. Hormonal and reproductive factors and the risk of bladder cancer in women. American journal of epidemiology. 2006;163:236–44. [DOI] [PubMed] [Google Scholar]
- [13].Mommsen S, Aagaard J, Sell A. Presenting symptoms, treatment delay and survival in bladder cancer. Scand J Urol Nephrol. 1983;17:163–7. [DOI] [PubMed] [Google Scholar]
- [14].Clark PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW, et al. Bladder cancer. J Natl Compr Canc Netw. 2013;11:446–75. [DOI] [PubMed] [Google Scholar]
- [15].Gore JL, Litwin MS, Lai J, Yano EM, Madison R, Setodji C, et al. Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst. 2010;102:802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gore JL, Lai J, Setodji CM, Litwin MS, Saigal CS. Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a Surveillance, Epidemiology, and End Results-Medicare analysis. Cancer. 2009;115:988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Messer JC, Shariat SF, Dinney CP, Novara G, Fradet Y, Kassouf W, et al. Female gender is associated with a worse survival after radical cystectomy for urothelial carcinoma of the bladder: a competing risk analysis. Urology. 2014;83:863–7. [DOI] [PubMed] [Google Scholar]
- [18].Patafio FM, Robert Siemens D, Wei X, Booth CM. Is there a gender effect in bladder cancer? A population-based study of practice and outcomes. Canadian Urological Association journal = Journal de l'Association des urologues du Canada. 2015;9:269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Santos F, Dragomir A, Kassouf W, Franco E, Aprikian A. Urologist referral delay and its impact on survival after radical cystectomy for bladder cancer. Current oncology (Toronto, Ont). 2015;22:e20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Weir HK, Johnson CJ, Mariotto AB, Turner D, Wilson RJ, Nishri D, et al. Evaluation of North American Association of Central Cancer Registries' (NAACCR) data for use in population-based cancer survival studies. Journal of the National Cancer Institute Monographs. 2014;2014:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Williams SB, Huo J, Chamie K, Hu JC, Giordano SH, Hoffman KE, et al. Underutilization of Radical Cystectomy Among Patients Diagnosed with Clinical Stage T2 Muscle-invasive Bladder Cancer. European Urology Focus. 2016;online. [DOI] [PubMed] [Google Scholar]
- [22].Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of clinical epidemiology. 2000;53:1258–67. [DOI] [PubMed] [Google Scholar]
- [23].Lee CT, Madii R, Daignault S, Dunn RL, Zhang Y, Montie JE, et al. Cystectomy Delay More Than 3 Months From Initial Bladder Cancer Diagnosis Results in Decreased Disease Specific and Overall Survival. The Journal of Urology. 2006;175:1262–7. [DOI] [PubMed] [Google Scholar]
- [24].Gore JL, Lai J, Setodji CM, Litwin MS, Saigal CS. Mortality increases when radical cystectomy is delayed more than 12 weeks. Cancer. 2009;115:988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mahmud SM, Fong B, Fahmy N, Tanguay S, Aprikian AG. Effect of preoperative delay on survival in patients with bladder cancer undergoing cystectomy in quebec: A population based study. The Journal of Urology. 2006;175:78–83. [DOI] [PubMed] [Google Scholar]
- [26].SÁNchez-Ortiz RF, Huang WC, Mick R, Van Arsdalen KN, Wein AJ, Malkowicz SB. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. The Journal of Urology. 2003;169:110–5. [DOI] [PubMed] [Google Scholar]
- [27].M. Z, S. T, A. T, F. N, Takeuchi T, et al. Sex differences in bladder cancer pathology and survival: analysis of a population - based cancer registry. Cancer medicine. 2015;4:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sammon JD, Morgan M, Djahangirian O, Trinh QD, Sun M, Ghani KR, et al. Marital status: a gender-independent risk factor for poorer survival after radical cystectomy. BJU international. 2012;110:1301–9. [DOI] [PubMed] [Google Scholar]
- [29].Mitra AP, Skinner EC, Schuckman AK, Quinn DI, Dorff TB, Daneshmand S. Effect of gender on outcomes following radical cystectomy for urothelial carcinoma of the bladder: a critical analysis of 1,994 patients. Urologic oncology. 2014;32:52.e1–9. [DOI] [PubMed] [Google Scholar]
- [30].Giordano SH, Kuo YF, Duan Z, Hortobagyi GN, Freeman J, Goodwin JS. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112:2456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mai V, Kant AK, Flood A, Lacey JV Jr., Schairer C, Schatzkin A. Diet quality and subsequent cancer incidence and mortality in a prospective cohort of women. International journal of epidemiology. 2005;34:54–60. [DOI] [PubMed] [Google Scholar]
- [32].Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer cell. 2014;25:152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Izumi K, Zheng Y, Li Y, Zaengle J, Miyamoto H. Epidermal growth factor induces bladder cancer cell proliferation through activation of the androgen receptor. Int J Oncol. 2012;41:1587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li Y, Zheng Y, Izumi K, Ishiguro H, Ye B, Li F, et al. Androgen activates β-catenin signaling in bladder cancer cells. Endocr Relat Cancer. 2013;20:293–304. [DOI] [PubMed] [Google Scholar]
- [35].Daugherty SE, Lacey JV, Pfeiffer RM, Park Y, Hoover RN, Silverman DT. Reproductive factors and menopausal hormone therapy and bladder cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer. 2013;133:462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gakis G, Efstathiou J, Lerner SP, Cookson MS, Keegan KA, Guru KA, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63:45–57. [DOI] [PubMed] [Google Scholar]
- [37].Booth CM, Siemens DR, Li G, Peng Y, Tannock IF, Kong W, et al. Perioperative chemotherapy for muscle-invasive bladder cancer: A population-based outcomes study. Cancer. 2014;120:1630–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.