Abstract
Background
To compare the clinical outcomes of stereotactic body radiation therapy (SBRT) and fractionated radiation therapy (FRT) for primary liver cancer with portal vein tumor thrombus (PVTT).
Methods
This retrospective study included 36 patients who underwent SBRT and 36 patients who underwent FRT from August 2016 to June 2018. Patients were evaluated for short-term efficacy, long-term efficacy, AEs, and quality of life before and after treatment.
Results
With a median follow-up of 28.8 months (26–36 months), 27 patients survived in the SBRT group while 19 patients survived in the FRT group. The survival rate in the SBRT group was statistically higher than that of the FRT group after 6 months (80.56% vs. 58.33%; P = 0.041), 12 months (77.78% vs. 55.56%; P = 0.046) and 24 months 75.00% vs. 52.78%; P = 0.049). The median whole survival time of the SBRT group was 13.3 months (95% CI 12.83–13.97), which was statistically longer than 9.8 months in the FRT group (95% CI 8.83–10.97, P < 0.05) based on the Kaplan–Meier method. The SBRT group had better survival quality and fewer adverse events than the FRT group.
Conclusion
SBRT had better clinical outcomes than FRT for primary liver cancer with PVTT.
Keywords: Stereotactic body radiation therapy, Fractionated radiation therapy, Primary liver cancer, Portal vein tumor thrombus, Clinical outcomes
Background
Primary liver cancer (PLC) is one of the most common malignant tumors clinically. More than 500,000 new cases are reported all over the world each year, making it the third most common malignant cancer [1]. China is a high incidence area of PLC, and the mortality rate of PLC is the second highest among all cancer types in China [2]. Due to atypical manifestations and an aggressive course, most PLC patients were diagnosed at the mid or advanced stage and had tumor thrombus in the main trunk or branch of the portal vein at the time of diagnosis. It has been reported that 40–90% of PLC patients have portal vein tumor thrombus (PVTT) in China [3] while 62.2–90.2% in other countries [4]. The combination of PLC and PVTT can lead to high mortality. PLC is unresectable in most patients and has poor prognosis. Besides, PLC with PVTT can induce cancer cells to metastasize into the liver, contributing to esophageal variceal bleeding and portal hypertension, so that increasing the difficulty of treatment and improving the recurrence ratet [5]. Therefore, there is an urgent need for effective radiation therapy for PLC with PVTT in the clinic.
Stereotactic body radiation therapy (SBRT) is a promising radiotherapy technology which has been widely reported in the treatment of malignant tumors [6]. The efficacy of SBRT and fractionated radiation therapy (FRT) in PLC has also been reported and both modalities showed favorable outcomes [7]. However, only few studies have been done on comparison of SBRT and FRT for PLC with PVTT. And the outcomes were not consistent with each other.
This retrospective study compared the clinical outcomes of SBRT and FRT with short-term efficacy, long-term efficacy, adverse reactions (AEs), and quality of life before and after treatment as the primary outcomes. Based on these comparisons, we aimed to determine effective radiation therapy for treating PLC with PVTT.
Methods
This retrospective study reviewed the records of PLC patients with PVTT who received SBRT or FRT from August 2016 to June 2018 at The First Hospital of Hebei Medical University. The inclusion criteria were as follows: (1) the patients’ symptoms, imaging examination, histopathological examination, puncture cytology, and laboratory study met the diagnosis criteria of PLC with PVTT. Meanwhile, their diagnosis also met the criteria in the Diagnosis and Treatment of Primary Liver Cancer (2011, China) [8]. (2) The expected survival time was more than 6 months. (3) Normal coagulation function. (4) No contraindication for radiotherapy and chemotherapy. (5) Normal cardiac and renal function. (6) Good compliance with physician orders. The exclusion criteria were as follows: (1) patients with an infection or other malignant tumors. (2) Congenital malformation with hepatocellular carcinoma. (3) The tumor volume was more than 70% of the normal liver. (4) Metastases to other parts of the body. (5) Poor tolerance of radiation therapy. (6) Massive ascites. (7) Received prior radiotherapy.
According to these criteria, 72 patients were included in this series, 36 patients were included in the SBRT group, and 36 patients were included in the FRT group. Data regarding age, sex, follow-up duration, alpha-fetoprotein, Child–Pugh grade, the number, location and stage of the tumor, location of tumor thrombus, and stage of tumor thrombus were collected. Informed consent was obtained from all study subjects.
This study was approved by the Clinical Academic Committee of The First Hospital of Hebei Medical University and was conducted in compliance with the Helsinki Declaration.
SBRT group
An OUR-QGD gamma-ray stereotactic body radiotherapy system was used in this study [9]. All patients were immobilized using a stereotactic body frame with a vacuum pillow to create reproducible immobilization. Then, the target areas were continuously scanned with a 3–5 mm CT-slide thickness. The obtained image data and related data were input into the planning system to construct a three-dimensional structure. Plan target volume (PTV) and clinical target volume (CTV) were delineated. According to the patient's condition, tumor location, CTV, and treatment purpose, the radiotherapy plan was customized, and dose distribution was adjusted. Thereafter, the patient was positioned and treated. In this group, the median CTV volume was 346.3 cm3 (range: 26–1012 cm3). The dose-volume histogram was used for quantitative evaluation. The median isodose curve was 55.6% (range: 50–70%). The total median dose of peripheral irradiation of PTV was 34.733 Gy (range: 3200–4800 cGy). The median fraction dose was 4.356 Gy (range: 3.800–5.200 cGy). The dose of the spinal cord and duodenum adjacent to the target area was 5–20% and 5–30%, respectively. Each patient received five sessions of treatment over 5–7 weeks.
FRT group
This group of patients received conventional FRT. Briefly, the GE Lightspeed VCT treatment system was used in this group (Eclipse10.0, American). Patients were placed in the supine position. Then, the target areas were continuously scanned with a 3–5 mm CT-slide thickness. In addition, CT images were processed by the VARIAN Eclipse TPS planning system (VARIAN, USA). Targets were delineated following the same procedure as for the SBRT group. In this group, a single radiation dose of 2 Gy was delivered 5 times a week for 5–7 weeks. Hepatoprotective drugs were given before and during the treatment.
Assessment of outcomes and follow-up
Both groups were evaluated for short-term efficacy, long-term efficacy, AEs, and quality of life before and after treatment as the primary outcomes.
The definition of short-term efficacy followed the RECISTI.1 criterion [10]. Short-term efficacy was defined at four levels: complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Each level was defined as follows: PD, the tumor volume increased by 25%, or new lesions appeared in the liver; CR, disappearance of all target lesions completely; PR, the maximum diameter of all tumors was reduced or necrotic > 50%; SD, the maximum diameter of all tumors was reduced or necrotic < 50%, or the tumor volume increase less than 25%. Response rate was the ratio of was the sum of CR and PR to whole patients. Disease control rate was the ratio of the sum of CR, PR, and SD to whole patients. The observation was started from the beginning of radiotherapy to the death or the last time follow-up.
Survival quality was evaluated using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) [11]. This questionnaire included 30 items. Except items 29 and 30, each item had a score of 1–4. A higher grade represented better survival quality. Items 29 and 30 had a score of 1–7. Toxicity was evaluated using Adiation Therapy Oncology Group (RTOG) [12]. The incidence of nausea, vomiting, fatigue, myelosuppression, liver pain, and other AEs was recorded and statistically analyzed. Besides, whole survival time was recorded. The whole survival time was analyzed by the Kaplan–Meier method. All patients were followed up by telephone once a week and once a month in outpatient service for 2 years.
Statistical analysis
All data were analyzed statistically using SPSS (version 21.0; IBM, Chicago, IL). Student’s t-test was performed to compare continuous variables. Chi-square test was performed to compare all categorical data. The survival curve was drawn by the Kaplan–Meier method. Statistical significance was set at P < 0.05.
Results
The series included a total of 72 patients; the SBRT group and the FRT group each had 36 patients. Patient data, including age, sex, follow-up duration, alpha-fetoprotein, Child–Pugh grade, the number, location and stage of the tumor, location of tumor thrombus, and stage of tumor thrombus side, smoking, and follow-up time, are summarized in Table 1. There was no significant difference between the two groups in all the parameters.
Table 1.
Comparison of clinicopathologic characteristics between SBRT group and FRT group
| SBRT (36) | FRT (36) | t/χ2 | P | |
|---|---|---|---|---|
| Gender (n) | 0.058 | 0.81 | ||
| Male | 21 | 22 | ||
| Female | 15 | 14 | ||
| Age | 43.83 ± 6.21 | 43.67 ± 6.45 | 0.107 | 0.915 |
| Alpha fetoprotein (µg/L) | 0.056 | 0.813 | ||
| ≥ 400 | 16 | 17 | ||
| < 400 | 20 | 19 | ||
| Child–Pugh grade | 0.296 | 0.586 | ||
| A | 26 | 28 | ||
| B | 10 | 8 | ||
| Tumor number | 0.056 | 0.813 | ||
| ≤ 3 | 19 | 20 | ||
| > 3 | 17 | 16 | ||
| Location (n) | 0.230 | 0.891 | ||
| Left lobe | 7 | 6 | ||
| Right lobe | 19 | 21 | ||
| Both sides | 10 | 9 | ||
| Stage (n) | 0.239 | 0.887 | ||
| IIa | 20 | 22 | ||
| IIb | 10 | 9 | ||
| III | 6 | 5 | ||
| Location of tumor thrombus (n) | 0.397 | 0.982 | ||
| Left branch | 5 | 4 | ||
| Right branch | 4 | 5 | ||
| Main portal vein + left branch | 3 | 4 | ||
| Main portal vein + right branch | 8 | 8 | ||
| Main portal vein + left and right branch | 16 | 15 | ||
| Stage of tumor thrombus (n) | 1.368 | 0.713 | ||
| I | 3 | 6 | ||
| II | 11 | 11 | ||
| III | 15 | 14 | ||
| IV | 7 | 5 | ||
Comparison of short-term efficacy between the SBRT group and the FRT group
According to the analysis, the SBRT group had statistically better short-term efficacy than the FRT group. The response rate and disease control rate in the SBRT group were both higher than those in the FRT group (58.33% vs. 33.33%, P = 0.033; 88.89% vs. 69.44%, P = 0.042) (Table 2).
Table 2.
Comparison of short-term efficacy between SBRT group and FRT group
| Group | SBRT (n = 36) | FRT (n = 36) | χ2 | P |
|---|---|---|---|---|
| CR (n) | 5 | 2 | ||
| PR (n) | 16 | 10 | ||
| SD (n) | 12 | 12 | ||
| PD (n) | 3 | 12 | ||
| Response rate (%) | 58.33 (21/36) | 33.33 (12/36) | 4.531 | 0.033* |
| Disease control rate (%) | 91.67 (33/36) | 66.67 (24/36) | 6.821 | 0.009* |
CR complete response, PR partial response, SD stable disease, PD progressive disease. Response rate was the ratio of was the sum of CR and PR to whole patients. Disease control rate was the ratio of the sum of CR, PR, and SD to whole patients
*Have statistical difference
Comparison of long-term efficacy between SBRT group and FRT group
The follow-up of the two groups ended on August 30, 2019, and the median follow-up duration was 28.8 months (range: 26–36 months). No patients were lost to follow up in both groups. Twenty-seven patients survived in the SBRT group, and 19 patients survived in the FRT group. The 6, 12, and 24-month survival rates in the SBRT group were statistically higher than those in the FRT group (Table 3).
Table 3.
Comparison of survival rate between SBRT group and FRT group
| Group (n) | 6 months | 12 months | 24 months |
|---|---|---|---|
| SBRT (36) | 80.56% (29/36) | 77.78% (28/36) | 75.00% (27/36) |
| FRT (36) | 58.33% (21/36) | 55.56% (20/36) | 52.78% (19/36) |
| χ2 | 4.189 | 4.111 | 3.583 |
| P | 0.041 | 0.046 | 0.049 |
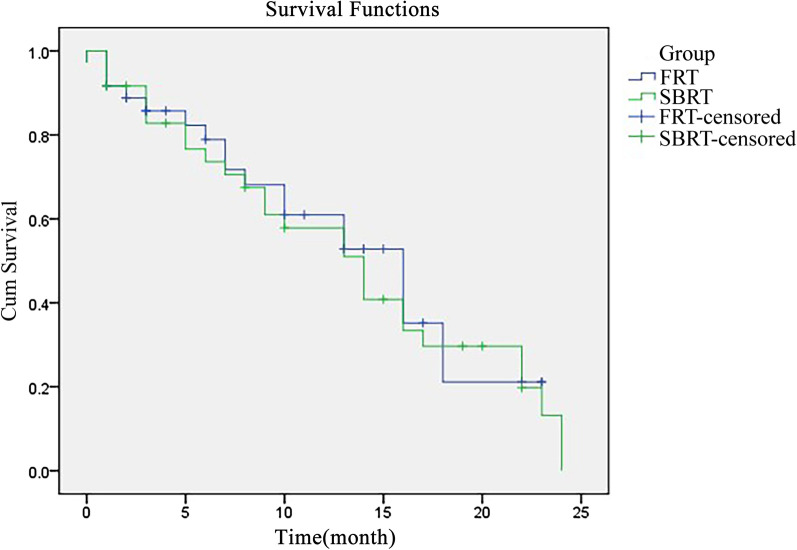
The median whole survival time of the SBRT group was 13.3 months (95% CI 12.83–13.97), which was statistically longer than 9.8 months in the FRT group (95% CI 8.83–10.97, P < 0.05) (Fig. 1).
Fig. 1.
Kaplan–Meier curve of survival for both groups. SBRT, stereotactic body radiation therapy. FRT fractionated radiation therapy
The quality of life of both groups showed no statistical difference before radiation therapy. However, both groups had better survival quality after receiving different treatments. In addition, the survival quality of the SBRT group was statistically better than that of the FRT group after therapy (Table 4).
Table 4.
Comparison of survival quality score of EORTC QLQ-C30 between SBRT group and FRT group
| Group (n) | Before treatment | After treatment | t | P |
|---|---|---|---|---|
| SBRT (36) | 41.03 ± 3.13 | 77.73 ± 3.29 | − 48.491 | < 0.001 |
| FRT (36) | 39.98 ± 3.09 | 63.29 ± 3.01 | 19.43 | < 0.001 |
| t | 1.432 | 19.430 | ||
| P | 0.157 | < 0.001 |
EORTC QLQ-C30 European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30. The student’s t-test was performed to compare the data, t t value
During the treatment, both groups developed AEs, including nausea, vomiting, fatigue, myelosuppression, and hepatic pain. According to the RTOG grade, all symptoms were graded I–II. The incidence of liver pain, fatigue, nausea, and vomiting in the SBRT group were lower than that of the FRT group. However, the incidence of myelosuppression showed no statistical difference between the two groups. After appropriate treatment, all AEs were ameliorated. Liver failure, gastrointestinal bleeding, perforation, and other severe AEs were not reported in both groups (Table 5).
Table 5.
Comparison of adverse reactions between SBRT group and FRT group
| Group (n) | Liver function damage | Myelosuppression | Nausea and vomiting | Fatigue and dizziness |
|---|---|---|---|---|
| SBRT (36) | 0 | 13.89% (5/36) | 8.33% (3/36) | 13.89% (5/36) |
| FRT (36) | 11.11% (4/36) | 25.00% (9/36) | 27.78% (10/36) | 52.78% (19/36) |
| χ2 | 4.235 | 1.419 | 4.603 | 12.25 |
| P | 0.039 | 0.234 | 0.032 | < 0.001 |
Discussion
Previous studies reported that hepatitis B virus is one of the main risk factors of liver cancer [13]. Researchers have been keeping to explore new treatment methods for the treatment of liver cancer; however, the survival time of patients has not increased significantly so far. Besides, patients with PVTT have an extremely poor prognosis, increasing the difficulty of treatment. PLC with PVTT is determined as a progressive stage according to the Barcelona Clinic Liver Cancer grade [14]. At present, sorafenib is the primary option for this disease, but it takes effect slowly and can not effectively relieve cancer cell metastasis to the liver induced by PVTT. Moreover, the therapeutic effect has certain limitations [15]. Kim et al. [16] reported that the median duration of the effects of sorafenib alone in the treatment of PLC with PVTT was less than 5 months. Therefore, it deserves to be explored whether radiotherapy combined with conventional treatment for PLC with PVTT could effectively control cancer cells, reduce the size of the lesions, maintain the normal flow of the portal vein, improve liver function and decrease the metastasis rate.
In recent years, the continuous progress of RT technology has surely significantly improved the efficiency of its application to liver cancer patients. Some studies reported that radiotherapy can effectively control tumor progression, improve long-term survival, and have a low incidence of AEs [17]. Kwon et al. [18] reported that the 1-year and 3-year disease-free survival rates were 72% and 67.5%, respectively, after radiotherapy. Andolino et al. [19] proved that the 2-year local control rate was 90%, the disease-free survival rate was 48%, and the 2-year overall survival rate was 67% for radiotherapy. The above studies confirmed the important value of radiotherapy in the treatment of liver cancer. SBRT can accurately deliver high-doses towards the tumor target area, whilst the dose outside the target’s border decreases sharply, allowing for a very low radiation exposure of the surrounding normal liver. The application of SBRT in patients with advanced liver cancer has been gradually reported by several studies. Chan used SBRT for advanced liver cancer. The outcomes showed that the 1 survival rates was 62% [20]. Similar outcomes with fewer AEs were also reported in other studies, indicating the good prospect of this method in clinical application [21, 22]. In our study, the response rate and disease control rate in the SBRT group were both statistically better than those of the FRT group. Meanwhile, the 6, 12 and 24-month survival rate, and median survival time of the SBRT group were also higher than those of the FRT group. These results were similar to those of previous studies. It indicated that SBRT yielded better clinical outcomes than FRT for PLC with PVTT. This could be explained as follows. Firstly, SBRT could reduce the pressure of the portal vein, therefore, reduce the risk of refractory ascites and esophageal variceal bleeding. Secondly, SBRT could improve the resection rate of lesions and reduce intrahepatic metastasis caused by tumor thrombus. Thirdly, it may reduce tumor load and create conditions for transcatheter arterial chemoembolization. Moreover, this method may be beneficial to portal vein blood flow and improve liver function.
In clinical practice, it is necessary to pay attention to the influence of psychological and social factors on diseases [23]. How to effectively improve the quality of life of cancer patients and prolong their life span has become a common concern in society. Therefore, this study also focused on analyzing the survival quality of PLC patients with PVTT. The results indicated that both groups had improved quality after treatment. Besides, the SBRT group had better survival quality than the FRT group. All AEs were grade I–II according to the RTOG grade. No patients had severe AEs, and the moderate symptom was relieved after appropriate treatment. The better clinical outcomes of SBRT were expected to effectively alleviate the suffering of patients.
However, this study has some limitations. Firstly, we did not collect and analyze the data of distant metastasis in PLC patients with PVTT. Secondly, the sample size was small and the duration of follow-up was brief, and a multi-center and randomized controlled trial is necessary for future work. Besides, the application of SBRT for PLC patients with PVTT, needs further research and confirmation on how to better control the liver damage and how to tailor the appropriate dose escalation depending on the size of the tumor volume.
Conclusion
SBRT for PLC patients with PVTT has a certain curative effect and could improve the survival rate and median life cycle of patients, improving the quality of life with fewer AEs.
Acknowledgements
Not applicable.
Authors' contributions
SZ, LH, CB and ZZ designed the study. SZ, LH, CB, SY, YA, NL, YZ, LZ, WM and ZZ acquired and analyzed the data. YZ, LZ and ZZ performed the statistical analysis. SZ, LH and CB drafted the manuscript. YZ, LZ, WM and ZZ revised the manuscript; all authors approved the submitted version. All authors agreed both to be personally accountable or the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Funding
The authors don’t have received any financial support for this manuscript.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was performed following the rules of The First Hospital of Hebei Medical University, after review board consensus.
Consent for publication
Informed consent for publication was collected from all the patients.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sujing Zhang and Li He are contributed equally to this paper.
References
- 1.Orcutt ST, Anaya DA. Liver resection and surgical strategies for management of primary liver cancer. Cancer Control. 2018;25:1073274817744621. doi: 10.1177/1073274817744621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xuefeng L, Lijun S. Advances in drug therapy for advanced hepatocellular carcinoma. Liver. 2019;24(08):955–958. [Google Scholar]
- 3.Yi Z, Hongying S, Xiangjun H. Efficacy and survival analysis of iodine-125 seed placement combined with transcatheter arterial chemoembolization for the treatment of hepatocellular cacinoma with portal vein tumor thrombus. Chin J Cancer Prevent Treat. 2018;25(23):1664–1669. [Google Scholar]
- 4.Shirabe K, Shimada M, Tanaka S, et al. Current therapeutic strategy of hepatocellular carcinoma with extensive portal thrombus. Fukuoka Igaku Zasshi. 2002;93(10):197–203. [PubMed] [Google Scholar]
- 5.Urahashi T, Yamamoto M, Ohtsubo T, Katsuragawa H, Katagiri S, Takasaki K. Liver metastases with massive portal venous tumor thrombi from colorectal cancer: can be treated by surgical resection? Hepatogastroenterology. 2007;54:210–213. [PubMed] [Google Scholar]
- 6.Haque W, Butler EB, Teh BS. Stereotactic body radiation therapy for prostate cancer—a review. Chin Clin Oncol. 2017;6:S10. doi: 10.21037/cco.2017.06.05. [DOI] [PubMed] [Google Scholar]
- 7.Schaub SK, Hartvigson PE, Lock MI, et al. Stereotactic body radiation therapy for hepatocellular carcinoma: current trends and controversies. Technol Cancer Res Treat. 2018;17:1533033818790217. doi: 10.1177/1533033818790217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministry of Health of the People’s Republic of China. Diagnosis, management, and treatment of hepatocellular carcinoma (V2011). J Clin Hepatol 2011.
- 9.Xie X, Yang G, Zhou H, et al. Structural shielding design for a gamma ray stereotactic body radiotherapy system. J Radiol Prot. 2006;26(3):301–308. doi: 10.1088/0952-4746/26/3/004. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Petersen MA, Aaronson NK, Arraras JI, et al. The EORTC CAT Core—the computer adaptive version of the EORTC QLQ-C30 questionnaire. Eur J Cancer. 2018;100:8–16. doi: 10.1016/j.ejca.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 13.Maucort-Boulch D, de Martel C. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142:2471–2477. doi: 10.1002/ijc.31280. [DOI] [PubMed] [Google Scholar]
- 14.EASL Clinical Practice Guidelines Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Fuqiang J, Jingbo K. Progress in treatment of hepatocellular carcinoma with portal vein tumor thrombus. J Clin Hepatol. 2013;16(03):286–288. [Google Scholar]
- 16.Kim GA, Shim JH, Yoon SM, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015;26:320–329.e326. doi: 10.1016/j.jvir.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Briens A, Castelli J, Barateau A, et al. [Adaptive radiotherapy: Strategies and benefits depending on tumor localization] Cancer Radiother. 2019;23:592–608. doi: 10.1016/j.canrad.2019.07.135. [DOI] [PubMed] [Google Scholar]
- 18.Kwon JH, Bae SH, Kim JY, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. doi: 10.1186/1471-2407-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447–453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Chan LC, Chiu SK, Chan SL. Chiu S K and Chan S L Stereotactic radiotherapy for hepatocellular carcinoma: report of a local single-centre experience. Hong Kong Med J. 2011;17:112–118. [PubMed] [Google Scholar]
- 21.Ibarra RA, Rojas D, Snyder L, et al. Multicenter results of stereotactic body radiotherapy (SBRT) for non-resectable primary liver tumors. Acta Oncol. 2012;51:575–583. doi: 10.3109/0284186X.2011.652736. [DOI] [PubMed] [Google Scholar]
- 22.Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 23.Wang P, Shen LQ, Zhang H, et al. Quality of life after I-125 seed implantation using computed tomography and three-dimensional-printed template guidance in patients with advanced malignant tumor. J Cancer Res Ther. 2018;14:1492–1496. doi: 10.4103/jcrt.JCRT_77_18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.