Significance
The high-resolution cryogenic electron microscopy structure of the human parathyroid hormone receptor 2 (PTH2R), in complex with an endogenous tuberoinfundibular peptide (TIP39) and a heterotrimeric Gs protein, reveals that the unique loop conformation at the N terminus of TIP39 is indispensable for PTH2R activation, as the deletion of which results in a potent PTH2R antagonist TIP(7-39). The naturally occurring mutation G258D impairs the receptor signaling such as cAMP accumulation. This finding provides a potential molecular mechanism for syndromic short stature.
Keywords: parathyroid hormone receptor 2, cryo-electron microscopy, G protein–coupled receptor, ligand recognition, syndromic short stature
Abstract
The parathyroid hormone receptor 2 (PTH2R) is a class B1 G protein–coupled receptor (GPCR) involved in the regulation of calcium transport, nociception mediation, and wound healing. Naturally occurring mutations in PTH2R were reported to cause hereditary diseases, including syndromic short stature. Here, we report the cryogenic electron microscopy structure of PTH2R bound to its endogenous ligand, tuberoinfundibular peptide (TIP39), and a heterotrimeric Gs protein at a global resolution of 2.8 Å. The structure reveals that TIP39 adopts a unique loop conformation at the N terminus and deeply inserts into the orthosteric ligand-binding pocket in the transmembrane domain. Molecular dynamics simulation and site-directed mutagenesis studies uncover the basis of ligand specificity relative to three PTH2R agonists, TIP39, PTH, and PTH-related peptide. We also compare the action of TIP39 with an antagonist lacking six residues from the peptide N terminus, TIP(7-39), which underscores the indispensable role of the N terminus of TIP39 in PTH2R activation. Additionally, we unveil that a disease-associated mutation G258D significantly diminished cAMP accumulation induced by TIP39. Together, these results not only provide structural insights into ligand specificity and receptor activation of class B1 GPCRs but also offer a foundation to systematically rationalize the available pharmacological data to develop therapies for various disorders associated with PTH2R.
Class B1 G protein–coupled receptors (GPCRs) comprise 15 members involved in a wide spectrum of physiological functions (1, 2). A number of them are validated drug targets for different human diseases, such as osteoporosis, type 2 diabetes, obesity, psychiatric disorders, and migraine. Among them are two types of parathyroid hormone (PTH) receptors (PTH1R and PTH2R), whose actions are mediated by coupling primarily to the stimulatory G protein (Gs) (3, 4). Expressed in the central and peripheral nervous systems, PTH2R is a key mediator of nociception, wound healing, and maternal behavior (5–8). Furthermore, recent studies have shown that it regulates calcium transport and influences keratinocyte differentiation, pointing to its potential in the treatment of Darier disease or Hailey–Hailey disease (9). Furthermore, naturally occurring PTH2R mutations have been linked to familial early-onset generalized osteoarthritis, syndromic intellectual disability, and syndromic short stature (10, 11). The latter is presently being treated with recombinant human growth hormone (12).
PTH receptors have three endogenous ligands, namely, tuberoinfundibular peptide of 39 residues (TIP39), PTH, and PTH-related peptide (PTHrP). Unlike PTH and PTHrP that mainly expressed in peripheral systems, TIP39-containing neuronal cell bodies have been identified in the subparafascicular area and the medial paralemniscal nucleus (13). Both PTH and PTHrP are implicated in skeletal development, calcium homeostasis, and bone turnover (14). In fact, PTH (1-34) and abaloparatide, a variant of PTHrP (1-34) (15), are Food and Drug Administration–approved drugs for osteoporosis. Discovered in the bovine hypothalamus, TIP39 contains two identical and several similar residues common to PTH and PTHrP. However, there is no evidence to suggest that TIP39 plays a role in mineral or bone metabolism. In contrast to PTH that indistinguishably activates both receptors, TIP39 is selective for PTH2R (13, 16), while PTHrP only has a weak action on PTH2R (3, 4, 13). Deletion of six residues from the N terminus of TIP39 results in a PTH2R antagonist, TIP(7-39) (17). However, the underlying mechanism by which PTH2R selectively recognizes these related but distinct peptides is largely unknown. Although newly solved cryogenic electron microscopy (cryo-EM) structure of LA-PTH–PTH1R–Gs complex offers valuable insights into PTH recognition and receptor activation (18), questions remain relative to their applicability to PTH2R. Thus, we determined the single-particle cryo-EM structure of the human PTH2R in complex with TIP39 and a heterotrimeric Gs protein at a global resolution of 2.8 Å. Together with molecular dynamics (MD) simulation results, it provides an in-depth understanding of the structural basis of ligand specificity and PTH2R activation.
Results
Overall Structure.
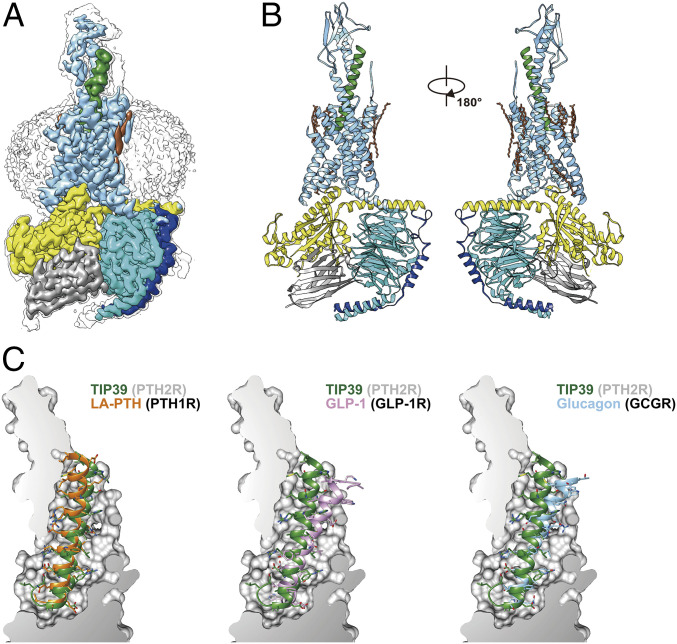
As shown in Fig. 1 and SI Appendix, Figs. S1 and S2, the final model of the PTH2R–Gs complex contains the first 34 amino acids of TIP39, the PTH2R (residues from Thr31ECD to Ser4348.64b) (class B1 GPCR numbering in superscript) (19), a dominant-negative human Gαs including eight mutations (S54N, G226A, E268A, N271K, K274D, R280K, T284D, and I285T), except for the α-helical domain (AHD), rat Gβ1, bovine Gγ2, and nanobody Nb35. Excluding the extracellular domain (ECD), the side chains of a majority of residues were well defined in the EM density maps (Fig. 1A and SI Appendix, Fig. S3 and Table S1). The overall structure of this complex is similar to that of other activated class B1 GPCRs such as LA-PTH–PTH1R–Gs (18), GLP-1–GLP-1R–Gs (20) and glucagon–GCGR–Gs (21) with Cα rmsd values of 0.98, 0.72, and 0.94 Å for the whole complex, respectively.
Fig. 1.
The overall cryo-EM structure of the TIP39–PTH2R–Gs complex. (A) Cut-through view of the cryo-EM density map that illustrates the TIP39–PTH2R–Gs complex and the disk-shaped micelle. The unsharpened cryo-EM density map at the 0.06 threshold shown as gray surface indicates a micelle diameter of 11 nm. The colored cryo-EM density map is shown at 0.12 threshold. (B) Model of the complex as a cartoon, with TIP39 as helix in green. The receptor is shown in blue, Gαs in yellow, Gβ subunit in cyan, Gγ subunit in navy blue, and Nb35 in gray. (C) The binding pocket of PTH2R accommodates peptide ligands of class B1 receptors. TIP39 is compared with LA-PTH (Left), GLP-1 (Middle), and glucagon (Right), respectively
A notable structural difference occurs in the transmembrane domain (TMD) ligand-binding pocket of these receptors. Fig. 1C and SI Appendix, Fig. S4 illustrate the shapes and the sizes of the TMD pockets and their cognate ligands. The interfacing structure of TIP39–PTH2R buried areas is 2,068 Å2, 65% of which was contributed by the N-terminal half of TIP39. Different from a typical peptide in the class B1 GPCR subfamily that adopts an extended helix with its N terminus inserted deeply into the TMD, TIP39 exhibits a single amphipathic α-helix from Leu4P (P indicates that the residue belongs to peptide) to Leu34P, with Leu4P being the deepest residue within the receptor core, and adopts a closed loop at the peptide N terminus (the first three residues) surrounded by TM5, TM6, extracellular loop 2 (ECL2), and ECL3 (Fig. 2A). In addition, unlike other class B1 GPCRs, PTH2R has an extended TM1 helix capable of interacting with a peptidic ligand. Diverse ECD positions in PTH2R and other class B1 GPCRs also presumably adjust individual peptide helix to respective TMD pocket in a manner that is specific for each receptor (Fig. 1C and SI Appendix, Fig. S4). In contrast to the ECL1 of growth hormone releasing hormone receptor (GHRHR) that stretches around GHRH to form broad interactions, no structural features in the ECL1 region of PTH2R were observed. This subtle difference supports our previous hypothesis that different activation requirement exists in class B1 GPCRs (22).
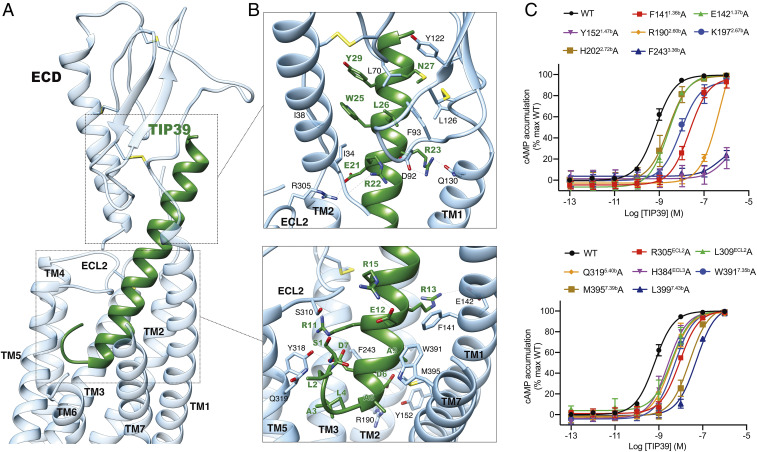
Fig. 2.
Molecular recognition and ligand specificity of PTH2R. (A) Overall contacts between PTH2R (blue) and TIP39 (green). (B) Detailed contacts between PTH2R (blue) and TIP39 (green) within the ECD or the TMD. Key residues are shown as sticks. (C) Effects of receptor mutations on TIP39-induced cAMP accumulation. Data shown are means ± SEM of at least three independent experiments.
With respect to the PTH2R–Gs interface, the outward movement of TM6 leads to a large opening of the cytoplasmic cavity for Gs coupling. The overall assembly of receptor–Gs complexes is very similar among class B1 GPCR structures solved to date (18, 23–25). In this study, the PTH2R–Gs complex is anchored with the α5-helix of Gαs, which fits snugly into the cytoplasmic cavity of the TMD. Additional contacts are observed between the extended helix 8 and the Gβ subunit (SI Appendix, Fig. S5). A number of detailed side chain interactions are visible in the receptor–Gs interface (SI Appendix, Fig. S5). The side chain of Glu392 and the last helical residue of the α5 helix in Gαs form a capping interaction with backbone amine of helix 8. Arg385 and Asp381 at the middle of the α5 helix in Gαs make charged interactions with Glu346ICL3 and Lys3435.64b of TM5, respectively. The carboxylate group at the C-terminal end of the α5 helix in Gαs forms a salt bridge with Lys3606.37b of TM6. Besides these polar and charged interactions, hydrophobic residues Leu388, Tyr391, Leu393, and Leu394 pack tightly against the hydrophobic surface comprised of residues of TM2, TM3, TM5, TM6, and TM7. Additionally, like other class B1 GPCRs, the α5 helix of Gαs also interacts with intracellular loop 2 (ICL2) and helix 8 of PTH2R (SI Appendix, Fig. S5).
Ligand Specificity.
An extensive network of complementary polar and nonpolar contacts between TIP39 and PTH2R was observed (Fig. 2 and SI Appendix, Table S2). Pointing to the receptor core, Ser1P forms one hydrogen bond with ECL2 (Ser310ECL2) via its side chain and has its amine terminus interacting with the α-helix part (Asp7P) of TIP39. Asp6, a highly conserved residue in glucagon-like peptides (26), makes one hydrogen bond and a salt bridge with Tyr1521.47b and Arg1902.60b, respectively, in line with abolished or decreased potencies for TIP39 observed in mutants Y152A and R190A (by 794-fold) (Fig. 2C and SI Appendix, Tables S3 and S4). Meanwhile, Glu21P forms a salt bridge with Arg305ECL2, consistent with a 16-fold reduction of TIP39 potency in mutant R305A (Fig. 2C and SI Appendix, Tables S3 and S4). Nonpolar interactions between TIP39 and PTH2R TMD are mainly contributed by the extracellular portions of TMs 1, 2, and 7, involving Phe1411.36b, Lys1972.67b, Phe2433.36b, Met3957.39b, and Leu3997.43b. Removal of the nonpolar contacts by alanine substitutions lowered the peptide potency by 12- to ∼80-fold (Fig. 2C and SI Appendix, Tables S2–S4). Of interest, the TM1 of PTH2R bends down toward TIP39, resulting in polar interactions between Arg23P and Gln1301.25b, and shifts the peptide C-terminal region toward ECL1, while the ECD clasps this region (residues 22 through 39) with massive hydrophobic contacts and several polar interactions (Fig. 2B and SI Appendix, Table S2).
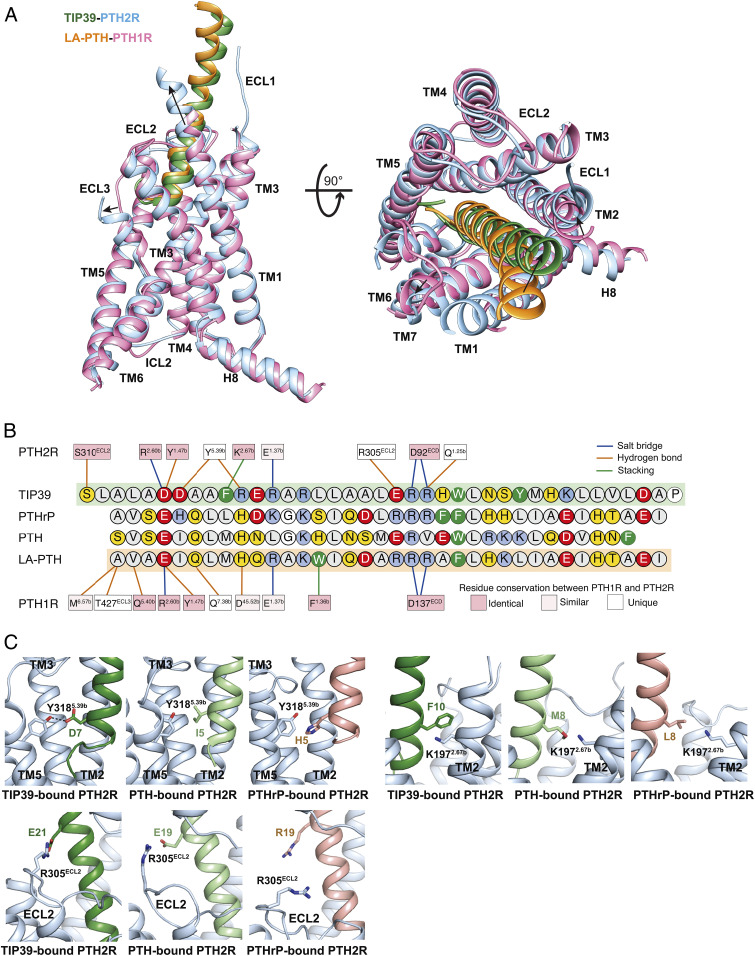
Structural comparison of TIP39–PTH2R–Gs and LA-PTH–PTH1R–Gs complexes reveals distinct features of the ligand recognition pattern between PTH1R and PTH2R. To specifically accommodate TIP39, PTH2R reforms its peptide-binding pocket by reorganizing the conformations of ECL3 and the extracellular parts of TM1 and TM7, as well as adopts receptor-specific amino acids at multiple positions that directly interact with the peptide. ECL3 is unstructured in PTH2R but is well solved in PTH1R that forms several additional direct contacts with the N-terminal portion of the bound LA-PTH (Figs. 2A and 3A). Such differences might contribute to the greater mobility of ECL3 in PTH2R that moves outward in response to the unique loop conformation at the N terminus of TIP39. Consequently, the extracellular tip of TM7 in PTH2R also shifts outward by 2 Å (measured at the Cα of Trp7.35b) thereby decreasing the contacts with the bound TIP39. Meanwhile, the extracellular tip of TM1 in PTH2R is extended by six residues, allowing the formation of a hydrogen bond between Gln1301.25b and Arg23P, which is not observed in the LA-PTH–PTH1R–Gs complex (18).
Fig. 3.
Ligand specificity between PTH1R and PTH2R. (A) Structural comparison of TIP39–PTH2R–Gs and LA-PTH–PTH1R–Gs complexes. Receptor ECD and G protein are omitted for clarity. (B) Schematic diagram of interactions between peptide and receptor. Conserved residues in PTH1R and PTH2R are highlighted in pink, while those similar are shown in light pink. Amino acid residues of peptides are colored red, negatively charged; blue, positively charged; yellow, hydrophilic; green, aromatic; gray, hydrophobic. Hydrophobic contacts are omitted for clarity. (C) Representative snapshots from MD simulations showing the key residues that determine the ligand specificity of PTH2R (blue). TIP39, PTH, and PTHrP are depicted in green, light green, and pink, respectively.
Besides the distinct conformations of TMs and ECLs, PTH1R and PTH2R use different amino acids (including Tyr3185.39b, Lys1972.67b, Arg305ECL2 in PTH2R) to recognize their peptides (Fig. 3B). PTH2R uses a polar residue Tyr3185.39b to form hydrogen bonds with Asp7P and Arg11P of TIP39, while PTH1R has a hydrophobic isoleucine (Ile3635.39b) at the corresponding site (Fig. 3B). Interestingly, Asp7P is one unique site of TIP39 that corresponds to Ile5P of PTH and His5P of PTHrP (Fig. 3B). In our MD simulations of PTH2R engaging different peptides, Asp7P of TIP39 stably formed hydrogen bonds with Tyr3185.39b, while Ile5P of PTH made hydrophobic interactions with Tyr3185.39b (Fig. 3C and SI Appendix, Fig. S6). In contrast, no hydrogen bond or hydrophobic interaction between His5P of PTHrP and Tyr3185.39b were observed in the PTHrP-bound PTH2R simulations. This observation is consistent with our mutagenesis studies, where Y318A mutation of PTH2R decreased TIP39 potency by 794-fold but increased PTH potency by fourfold (SI Appendix, Fig. S7). Lys1972.67b of PTH2R has stable hydrophobic interaction with the aromatic Phe10P of TIP39, which is stronger than the interactions with the smaller hydrophobic side chains of corresponding residues at PTH (Met8P) or PTHrP (Leu8P) (Fig. 3C). TIP39 and PTH share a conserved negatively charged residue (TIP39 Glu21P or PTH Glu19P), but PTHrP has a positively charged arginine (Arg19P) instead. In the simulations, either Glu21P (TIP39) or Glu19P (PTH) formed putative salt bridges with a positively charged ECL2 residue Arg305ECL2 (Fig. 3C), while PTHrP Arg19P repelled Arg305ECL2 and might impede the peptide binding. In addition to the residues crucial to ligand specificity, there are several conserved contacts shared by PTH1R and PTH2R. Either Glu4P of LA-PTH or Asp6P of TIP39 contributes salt bridges with Arg2.60b and hydrogen bonds with Tyr1.47b. At the middle region of these three peptides, two residues (Ala5P/Ala9P in TIP39, Ser3P/Leu7P in both PTH and PTHrP) hydrophobically interacted with Leu3997.43b in all simulations (SI Appendix, Fig. S6 B–D). At the C termini of peptides, a hydrophobic residue (Trp25P in TIP39, Trp23P in PTH and Phe23P in PTHrP) with a large side chain constantly interacts with two ECD residues Ile34ECD and Ile38ECD (SI Appendix, Fig. S6 H–J). I34A and I38A mutants significantly reduced the potencies of TIP39 and PTH (SI Appendix, Fig. S7 and Table S5), fully consistent with the simulation results.
Antagonism by TIP(7-39).
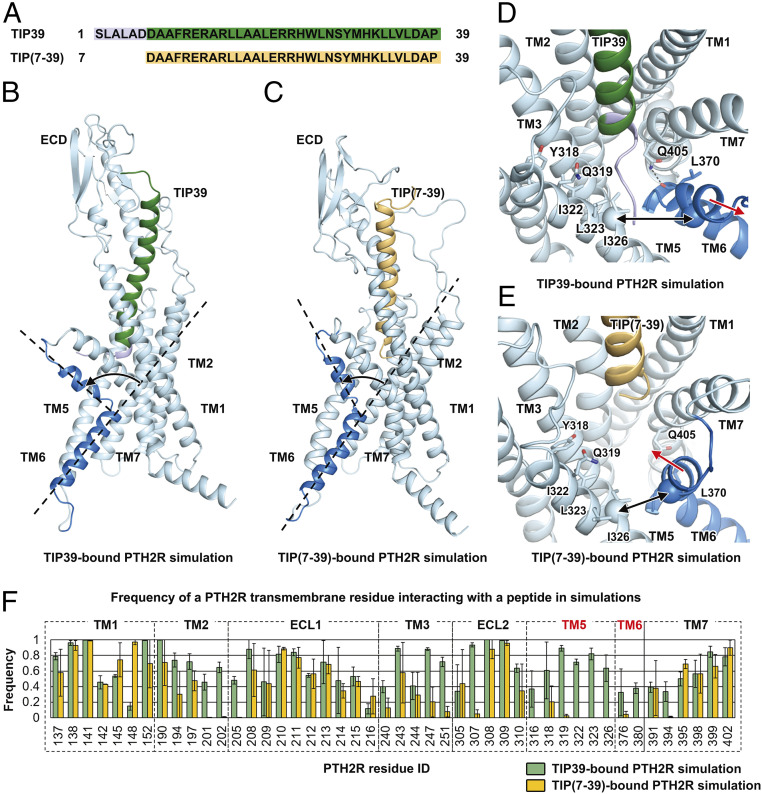
Deletion of six residues from the N terminus of TIP39 resulted in an antagonist, TIP(7-39) (Fig. 4A) (17). In the MD simulations of TIP(7-39)-bound PTH2R, the receptor spontaneously transitioned from the active conformation to an inactive-like one, displaying a smaller TM6 helix kinking angle (76.9° ± 9.1°) compared with that of TIP39-bound PTH2R (88.2° ± 8.3°) (Fig. 4 B and C). In the TIP39-bound PTH2R simulations, the N terminus of the peptide resided between TM5 and TM6 helices (Fig. 4 B and D). Particularly, the residues located at the N terminus of TIP39 interacted with TM5 residues (Tyr3185.39b, Gln3195.40b, Ile3225.43b, Leu3235.44b, and Ile3265.47b), close to the ligand-binding pocket (Fig. 4 D and F). Single-point mutations of these residues such as Y318A and Q319A showed significantly reduced cAMP accumulations induced by TIP39 (Fig. 2C), consistent with the MD observations. In the TIP(7-39)-bound PTH2R simulations, the interactions between the N terminus of the peptide and TM5 helix were missing (Fig. 4 E and F). Consequently, the average backbone distance between TIP(7-39) and TM5 helix was 12.9 ± 0.6 Å, ∼6 Å longer than that of TIP39-bound PTH2R (7.2 ± 0.7 Å). TIP(7-39) did not have stable interactions with TM6 helix (Fig. 4F). Without direct contacts with the peptide, the C terminus of TM6 helix moved upward to reduce the kinking (Fig. 4 C and E). In the TIP39-bound PTH2R simulations, stable insertion of the N terminus led to a large movement of 9.8 ± 0.8 Å between TM5 and TM6 helices on the extracellular side, which kept the large kinking angle of the TM6 helix (Fig. 4D). In addition, a conserved TM7 residue Gln4057.49b could form hydrogen bonds with the backbone atoms of the TM6 residue Leu3706.47b to further stabilize the kinking conformation of the TM6 helix during the simulations (Fig. 4D and SI Appendix, Fig. S8). In the TIP(7-39)-bound PTH2R simulations, however, the polar interactions between TM6 Leu3706.47b and TM7 Gln4057.49b were missing in the receptor core (Fig. 4E and SI Appendix, Fig. S8).
Fig. 4.
Molecular mechanism of the TIP(7-39) antagonism at PTH2R. (A) Sequence alignment between TIP39 and TIP(7-39). (B) A representative snapshot from the TIP39-bound PTH2R simulation system showing a TIP39-induced conformational change of the TM6 helix. (C) A representative snapshot from the TIP(7-39)-bound PTH2R simulation system showing a TIP(7-39)-induced conformational change of the TM6 helix. (D) A representative snapshot from the TIP39-bound PTH2R simulation system showing the N terminus of TIP39 insertion between TM5 and TM6 helices. Key residues are shown as sticks. Hydrogen bonds are shown as dashed lines. The Cα atoms of residues Ile3265.47b and Ile3776.54b are shown as spheres. (E) A representative snapshot from the TIP(7-39)-bound PTH2R simulation system showing a conformational change of the TM6 helix. (F) Frequency of a PTH2R residue interacting with TIP39 (green) or TIP(7-39) (yellow) in simulations. The frequency value suggests the stability of a particular residue–peptide interaction. A large interacting frequency suggests a stable interaction.
At the bottom of the ligand-binding pocket, the aspartic acid residue Asp6P of TIP39 was mainly responsible for interacting with Tyr1521.47b and Arg1902.60b (SI Appendix, Fig. S8), two residues that govern the functionality of PTH2R (Fig. 2). Multiple hydrogen bonds were formed between these residues. The average atom distances from the Asp6P in TIP39 to Tyr1521.47b and Arg1902.60b were 2.8 ± 0.3 Å and 2.8 ± 0.2 Å, respectively. Because TIP(7-39) does not have Asp6P, its Asp7P flipped into the receptor core to interact with Tyr1521.47b and Arg1902.60b instead of Asp6P seen with TIP39 (SI Appendix, Fig. S8). Through the C terminus, both of TIP39 and TIP(7-39) are capable of stably interacting with the ECD. Ligand binding patterns at the ECD were almost identical in TIP39-bound and TIP(7-39)-bound PTH2R simulations (SI Appendix, Fig. S8C). These findings demonstrate that the C terminus of TIP39 and TIP(7-39) contribute to ligand binding, while the N terminus determines receptor activation.
Disease-Associated Mutation.
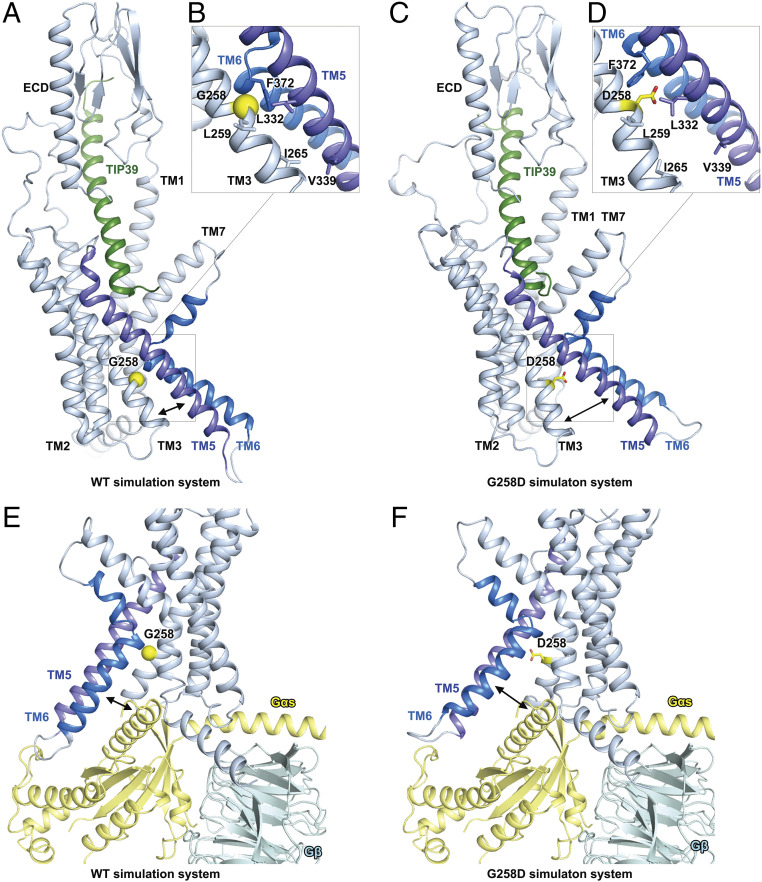
Several naturally occurring mutations in PTH2R have been reported to cause multiple hereditary human disorders (10, 11). Of them, two mutations (S158F and G258D) occur in regions that were well solved in our PTH2R structure, but only G258D significantly affected TIP39 elicited cAMP accumulation (SI Appendix, Fig. S9 and Table S6). Gly2583.51b is located at the intracellular side of TM3 helix (a part of the G protein-binding interface) and implicated in syndromic short stature (10). In the wild-type (WT) PTH2R MD simulations, Gly2583.51b was surrounded by several hydrophobic residues (Leu2593.52b, Leu3325.53b, and Phe3726.49b) of helices TM3, TM5, and TM6 (Fig. 5 A and B). Particularly, Gly2583.51b and Phe3726.49b are constantly interacting with an average distance of 3.3 ± 0.2 Å, forming the key helix–helix interface between helices TM3 and TM6. The hydrophobic interactions among Leu2593.52b, Leu3325.53b, and Phe3726.49b also stabilized the tight bundle of helices TM3, TM5, and TM6 at the G protein-binding interface. The interresidue distance between any two of these three residues was smaller than 4 Å in the WT simulations. However, in the G258D simulations, Asp2583.51b disrupted the hydrophobic interactions involving Leu2593.52b, Leu3325.53b, and Phe3726.49b (Fig. 5 C and D). The interresidue distance between any two of the four residues (Asp2583.51b, Leu2593.52b, Leu3325.53b, and Phe3726.49b) was larger than 5 Å in the G258D simulations. As a result, the conformations of helices TM3, TM5, and TM6 were interrupted at the intracellular side of the receptor. In the WT simulations, Ile2653.58b and Val3395.60b formed stable hydrophobic interactions to closely pack TM3 and TM5 helices at the intracellular side. However, in the G258D simulations, no direct interactions between these two residues were observed; therefore, the intracellular side of TM3, TM5, and TM6 were distorted and unfavorable to bind to a G protein (Fig. 5 E and F).
Fig. 5.
G258D mutation disrupts the G protein-binding interface of PTH2R in MD simulations. (A) A representative snapshot from the WT PTH2R simulations. (B) Key interactions stabilizing the helical bundle of TM3, TM5, and TM6 in the WT PTH2R simulations. Key residues are shown as sticks. Gly2583.51b is shown as a yellow sphere. (C) A representative snapshot from the G258D PTH2R simulations. (D) Asp2583.51b disrupts the hydrophobic interactions among helices TM3, TM5, and TM6 in the G258D PTH2R simulations. Key residues are shown as sticks. Asp2583.51b is highlighted in yellow. (E) A representative conformation of the G protein–binding interface of the WT PTH2R in simulations. The cryo-EM structure of TIP39–PTH2R–Gs complex was aligned to the simulation model to show the position of G protein with respect to the receptor. (F) A representative conformation of the G protein-binding interface of the G258D PTH2R in simulations.
Discussion
We used the single-particle cryo-EM approach to solve the high-resolution structure of the TIP39-bound PTH2R in complex with Gs. It provides essential structural information for understanding how PTH2R recognizes a peptide ligand and couples to Gs in the active state. Compared with other class B1 GPCRs (18, 20, 21), PTH2R shows a unique peptide-receptor binding interface, 65% of which is contributed by the N terminus of TIP39. Unlike typical peptides of class B1 GPCRs that adopt helix conformations at their N termini, TIP39 displays a closed loop at the N terminus (Fig. 2A and SI Appendix, Fig. S4). Both cryo-EM and MD simulation data indicate that the unique N terminus of TIP39 not only facilitates a deep insertion of the peptide into the receptor core (Figs. 2 and 3 and SI Appendix, Fig. S6A) but also participates in PTH2R activation via interactions with TM5 and TM6. These findings suggest a possible common mechanism of ligand-induced receptor activation by peptides with loop conformations at the N terminus (SI Appendix, Fig. S6A).
Due to the relatively high-resolution (2.8 Å) of the structure, we were able to address the ligand specificity of PTH2R against three functionally important peptides (TIP39, PTH, and PTHrP). Their actions could be divided into three modes: potent (TIP39), mild (PTH), and weak (PTHrP), respectively (4, 13, 16). Integrating MD simulation with mutagenesis studies, we identified key residues responsible for ligand recognition and characterized important receptor–peptide interactions that govern ligand specificity. MD simulations showed that three residues in PTH2R (Lys1972.67b, Arg305ECL2, and Tyr3185.39b) are selective against different peptides. Lys1972.67b stably interacts with Phe10P of TIP39 (Fig. 3C). Arg305ECL2 forms putative salt bridges with Glu21P of TIP39 or Glu19P of PTH but repels Arg19P of PTHrP (Fig. 3C). Tyr3185.39b makes putative hydrogen bonds with Asp7P of TIP39 as well as hydrophobic interactions with Ile5P of PTH but fails to interact with His5P of PTHrP (Fig. 3C). Notably, Gardella and colleagues have reported that the substitution of Ile5P of PTH with a histidine decreases the peptide potency on PTH2R, but the substitution of His5P of PTHrP with an isoleucine significantly increases the potency (27), a phenomenon that is highly consistent with our MD simulations.
Activation of class B1 GPCRs is characterized by opening the transmembrane helix bundles at the extracellular side, while the intracellular side undergoes conformational changes to accommodate G protein. Such hourglass-like opening of both extracellular and intracellular portions of the TMD requires TM6 helix to be bent and tightly tethered to the other helices at the receptor core (18, 20, 21). In this work, we link the opposing activities of an agonist (TIP39) and an antagonist TIP(7-39) to the TM6 helix of PTH2R: both of them bind to the ECD, with TIP39 inserted into the base of the TMD orthosteric pocket. The N terminus of TIP39 inserts between TM5 and TM6 helices to enhance the kinking of TM6 helix, a step essential to class B1 GPCR activation. A conserved TM7 residue (Gln4057.49b) that forms hydrogen bonds with the backbone atoms of the TM6 (Leu3706.47b) might further stabilize the kinking conformation. A glutamine residue at the corresponding site of other class B1 GPCRs has been reported to act as a molecular switch between receptor activation states (18). Upon full activation of a receptor, this TM7 glutamine reorients downward to establish hydrogen bonds with the TM6 helix residues of a conserved LXXG motif (SI Appendix, Fig. S10). This conserved rearrangement of the TM7 residue close to the receptor core enables the stabilization of the distinct kink in TM6 helix to mediate the simultaneous opening of the intracellular and extracellular side. Compared with TIP39, TIP(7-39) has the same C terminus but misses six residues at the N terminus. MD simulations revealed that it binds to the ECD via the C terminus but fails to active the receptor due to the lack of stable interaction with TM6 helix. These findings disclose the structural basis of PTH2R antagonism and underscore an indispensable role of the N terminus of an agonist in activating PTH2R. This might extend to other class B GPCRs. In fact, N-terminal truncation of PTH, such as PTH (7-34), also results in antagonists for PTH1R (28–30).
Like some other class B1 GPCRs that are implicated in multiple hereditary human disorders (5–10, 12), PTH2R also has several disease-associated mutations, such as the naturally occurring mutation G258D that is associated with syndromic short stature (10). By means of MD simulations, we hypothesize that this mutation might disrupt the active conformation of PTH2R, leading to impaired receptor function (SI Appendix, Fig. S9). Surrounded by several hydrophobic residues (Leu2593.52b, Leu3325.53b, and Phe3726.49b) of helices TM3, TM5, and TM6, Gly2583.51b is located nearby the G protein-binding interface of PTH2R. In the simulations of the G258D mutant receptor, Asp2583.51b disturbs the hydrophobic interactions involving Leu2593.52b, Leu3325.53b, and Phe3726.49b to distort the helical bundle of TM3, TM5, and TM6 at the intracellular side (Fig. 5 A–D). Consequentially, the G protein-binding interface is disordered and unfavorable to bind to a heterotrimeric Gs protein (Fig. 5 E and F). While cAMP response was not affected in the S158F mutant (SI Appendix, Table S6), both S158F and G258D showed impaired Gq coupling (SI Appendix, Fig. S11). Based on the atomic-level structural information of PTH2R, we were able to quantitatively interpret the mutational data. This understanding provides valuable information to develop therapies for disorders associated with PTH2R.
Materials and Methods
The data that support the findings of this study are available in this paper and/or in SI Appendix. Atomic coordinates of the TIP39–PTH2R–Gs complex have been deposited in the Protein Data Bank (PDB) (https://www.rcsb.org/) under accession code 7F16.
Construct.
The human PTH2R (residues 25 through 442) was cloned into the pFastBac vector (Invitrogen) with its native signal peptide replaced by hemagglutinin (HA) signal peptide to enhance receptor expression. LgBiT subunit (Promega) was fused at the C terminus of PTH2R connected by a 20 amino acid linker. A TEV protease cleavage site and double maltose-binding protein (2MBP) tag were fused after LgBiT subunit. A dominant-negative human Gαs (S54N, G226A, E268A, N271K, K274D, R280K, T284D, and I285T) (31) was generated to stabilize the interaction with the βγ subunits. A 15 amino acid linker and SmBiT subunit (peptide 86, Promega) were attached to the C terminus of rat Gβ1. Human DNGαs, rat Gβ1, and bovine Gγ2 were cloned into pFastBac vector, respectively.
TIP39–PTH2R–Gs Complex Formation and Purification.
After dounce homogenization of High Five insect cell pellets in lysis buffer (20 mM Hepes [pH 7.4], 100 mM NaCl, 10% (vol/vol) glycerol supplemented with EDTA-free protease inhibitor mixture, Topscience), membrane was collected at 65,000 × g for 35 min and homogenized again in lysis buffer. The complex formation was initiated by addition of 20 μM TIP39 (GL Biochem), 15 μg/mL Nb35, 25 mU/mL apyrase (NEB), 5 mM CaCl2, 10 mM MgCl2, 1 mM MnCl2, and 100 μM TCEP for 1.5 h incubation at room temperature (RT). The membrane was solubilized by 0.5% (wt/vol) lauryl maltose neopentyl glycol (LMNG; Anatrace) and 0.1% (wt/vol) cholesterol hemisuccinate (CHS; Anatrace) for 2 h at 4 °C. After centrifugation at 65,000 × g for 35 min, the supernatant was separated and incubated with amylose resin (NEB) for 2 h at 4 °C. The resin was collected and packed into a gravity flow column and washed with 5 column volumes of 5 μM TIP39, 0.1% (wt/vol) LMNG, 0.02% (wt/vol) CHS, 20 mM Hepes (pH 7.4), 100 mM NaCl, 10% (vol/vol) glycerol, 5 mM MgCl2, 1 mM MnCl2, and 25 μM TCEP, followed by 20 column volumes of washing buffer with decreased concentrations of detergents 0.03% (wt/vol) LMNG, 0.01% (wt/vol) GDN, and 0.008% (wt/vol) CHS. 2MBP-tag was removed by His-tagged TEV protease (home-made) during overnight incubation. The complex was concentrated using an Amicon Ultra Centrifugal filter (MWCO, 100 kDa) and subjected to a Superose 6 Increase 10/300 GL column (GE Healthcare) that was pre-equilibrated with running buffer containing 20 mM Hepes (pH 7.4), 100 mM NaCl, 2 mM MgCl2, 100 μM TCEP, 5 μM TIP39, 0.00075% (wt/vol) LMNG, 0.00025% (wt/vol) GDN, and 0.0002% (wt/vol) CHS. Eluted fractions containing the TIP39–PTH2R–Gs complex were pooled and concentrated. All procedures mentioned above were performed at 4 °C.
Cryo-EM Data Acquisition.
The purified TIP39–PTH2R–Gs complex (3 μL at 8.5 mg/mL) was applied to a glow-discharged holey carbon grid (Quantifoil R1.2/1.3). Vitrification was performed using a Vitrobot Mark IV (ThermoFisher Scientific) at 100% humidity and 4 °C. Cryo-EM imaging was processed on a Titan Krios (FEI) equipped with a Gatan K3 Summit direct electron detector in the Center of Cryo-Electron Microscopy, Shanghai Institute of Materia Medica, Chinese Academy of Science (China). The microscope was operated at 300 kV accelerating voltage, at a nominal magnification of 95,694× in counting mode, corresponding to a pixel size of 0.5225 Å. In total, 3,614 movies were obtained.
Model Building and Refinement.
Cryo-EM structure model of the PTH2R–Gs–Nb35 complex was built using the cryo-EM structure of PTH1R–Gs–Nb35 (PDB code: 6NBF) as initial model. The model was docked into the EM density map using Chimera (32), followed by iterative manual adjustment and rebuilding in COOT (33). Real-space refinement was performed using Phenix (34). The model statistics were validated using MolProbity (35). Structural figures were prepared in Chimera and PyMOL (https://pymol.org/2/). The final refinement statistics are provided in SI Appendix, Table S1.
cAMP Accumulation Assay.
The WT or mutant PTH2Rs were cloned into pcDNA3.1 vector (Invitrogen) for functional studies. cAMP signal was detected by LANCE cAMP kit (PerkinElmer) according to manufacturer’s instructions. Briefly, HEK-293T cells were seeded onto 6-well culture plates and transiently transfected with different PTH2R constructs using Lipofectamine 2000 transfection reagent (Invitrogen). After 24 h, cells were digested with 0.02% (wt/vol) EDTA and resuspended by HBSS supplemented with 5 mM Hepes, 0.5 mM IBMX, and 0.1% (wt/vol) BSA (pH 7.4) before seeding onto 384-well microtiter plates (3,000 cells per well). Increased concentrations of TIP39 or PTH (1-34) (1 pM to 1 µM) were used to stimulate transfected cells for 40 min at RT. Eu-tracer and ULight-anti-cAMP working solutions were added to the microtiter plates following a 1-h incubation at RT. Fluorescence signals were measured at 620 and 650 nm by an EnVision multilabel plate reader (PerkinElmer).
FITC-Labeled Ligand Binding Assay.
Competitive binding of TIP39-FITC (GL Biochem) to PTH2R was assessed as described previously (36). Briefly, 24 h after transfection with PTH2R (25-550) or PTH2R (25-442)-20AA-LgBiT, HEK-293T cells were harvested using 0.2% (wt/vol) EDTA. They (1 × 106 cells/mL) were then mixed with 0.2 μΜ TIP39-FITC on ice in the dark for 1 h. Seven decreasing concentrations of unlabeled peptide were added and competitively reacted with the cells in binding buffer (HBSS supplemented with 0.5% [wt/vol] BSA and 20 mM Hepes [pH 7.4]) on ice for 2 h. For each sample, 30,000 cells were analyzed for mean fluorescence intensity (using excitation and emission wavelengths of 488 and 518 nm) on a FACScan flow cytometer (ACEA Biosciences), with debris excluded by forward versus side scatter (FSC versus SSC) gating.
MD Simulation.
All peptide-bound PTH2R complex models were built based on the TIP39–PTH2R–Gs complex structure using Modeler (37). The default parameters were employed to construct the models. The missing backbone and side chains were added. The models with the lowest RMSDs from their template structures were selected. To build a simulation system, we placed the complex model into a 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine lipid bilayer. The lipid embedded complex model was solvated in a periodic boundary condition box (95 Å × 95 Å × 170 Å) filled with TI3P water molecules and 0.15 M KCl using CHARMM-GUI (38). Each system was replicated to perform two independent simulations. On the basis of the CHARMM36m all-atom force field (39–41), molecular dynamics simulations were conducted using GROMACS 5.1.4 (42, 43). Further details are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Chenyao Li and Wen Sun for technical assistance. This work was partially supported by National Natural Science Foundation of China 81872915 and 82073904 (M.-W.W.), 32071203 (L.Z), 81773792 (D.Y.), 81973373 (D.Y.), and 21704064 (Q.Z.); National Science and Technology Major Project of China–Key New Drug Creation and Manufacturing Program 2018ZX09735–001 (M.-W.W.), 2018ZX09711002–002–005 (D.Y.), and 2018ZX09711002–002–003 (X.C.); the National Key Basic Research Program of China 2018YFA0507000 (M.-W.W.); Ministry of Science and Technology of China 2018YFA0507002 (H.E.X.); Shanghai Municipal Science and Technology Major Project 2019SHZDZX02 (H.E.X.); the Strategic Priority Research Program of Chinese Academy of Sciences XDB37030103 (H.E.X.); Novo Nordisk-CAS Research Fund Grant NNCAS-2017–1-CC (D.Y.); Shanghai Science and Technology Development Fund 18ZR1447800 (L.Z.); The Young Innovator Association of Chinese Academy of Science 2018325 (L.Z.); SA-SIBS Scholarship Program (L.Z. and D.Y.); and The Youth Innovation Promotion Association of Chinese Academy of Science 2018319 (X.C). The cryo-EM data were collected at the Center of Cryo-Electron Microscopy, Shanghai Institute of Materia Medica.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2101279118/-/DCSupplemental.
Data Availability
Atomic coordinates and the cryo-EM density map have been deposited in PDB (ID code 7F16) and Electron Microscopy Data Bank (EMDB) (entry ID EMD-31405). All other study data are included in the article and/or SI Appendix.
References
- 1.Pal K., Melcher K., Xu H. E., Structure and mechanism for recognition of peptide hormones by Class B G-protein-coupled receptors. Acta Pharmacol. Sin. 33, 300–311 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wootten D., Miller L. J., Structural basis for allosteric modulation of class b g protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 60, 89–107 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jüppner H., et al., A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science 254, 1024–1026 (1991). [DOI] [PubMed] [Google Scholar]
- 4.Usdin T. B., Gruber C., Bonner T. I., Identification and functional expression of a receptor selectively recognizing parathyroid hormone, the PTH2 receptor. J. Biol. Chem. 270, 15455–15458 (1995). [DOI] [PubMed] [Google Scholar]
- 5.Dimitrov E. L., Kuo J., Kohno K., Usdin T. B., Neuropathic and inflammatory pain are modulated by tuberoinfundibular peptide of 39 residues. Proc. Natl. Acad. Sci. U.S.A. 110, 13156–13161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobolyi A., Ueda H., Uchida H., Palkovits M., Usdin T. B., Anatomical and physiological evidence for involvement of tuberoinfundibular peptide of 39 residues in nociception. Proc. Natl. Acad. Sci. U.S.A. 99, 1651–1656 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato E., et al., Activation of parathyroid hormone 2 receptor induces decorin expression and promotes wound repair. J. Invest. Dermatol. 137, 1774–1783 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varga T., et al., Paralemniscal TIP39 is induced in rat dams and may participate in maternal functions. Brain Struct. Funct. 217, 323–335 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato E., et al., The parathyroid hormone second receptor pth2r and its ligand tuberoinfundibular peptide of 39 residues tip39 regulate intracellular calcium and influence keratinocyte differentiation. J. Invest. Dermatol. 136, 1449–1459 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meulenbelt I., et al., Strong linkage on 2q33.3 to familial early-onset generalized osteoarthritis and a consideration of two positional candidate genes. Eur. J. Hum. Genet. 14, 1280–1287 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Tiosano D., et al., Mutations in PIK3C2A cause syndromic short stature, skeletal abnormalities, and cataracts associated with ciliary dysfunction. PLoS Genet. 15, e1008088 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuttler L., Safety and efficacy of growth hormone treatment for idiopathic short stature. J. Clin. Endocrinol. Metab. 90, 5502–5504 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Usdin T. B., Hoare S. R., Wang T., Mezey E., Kowalak J. A., TIP39: A new neuropeptide and PTH2-receptor agonist from hypothalamus. Nat. Neurosci. 2, 941–943 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Gardella T. J., Vilardaga J. P., International Union of Basic and Clinical Pharmacology. XCIII. The parathyroid hormone receptors—family B G protein-coupled receptors. Pharmacol. Rev. 67, 310–337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leder B. Z., et al., Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J. Clin. Endocrinol. Metab. 100, 697–706 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Usdin T. B., Dobolyi A., Ueda H., Palkovits M., Emerging functions for tuberoinfundibular peptide of 39 residues. Trends Endocrinol. Metab. 14, 14–19 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Hoare S. R. J., Usdin T. B., Tuberoinfundibular peptide (7-39) [TIP(7-39)], a novel, selective, high-affinity antagonist for the parathyroid hormone-1 receptor with no detectable agonist activity. J. Pharmacol. Exp. Ther. 295, 761–770 (2000). [PubMed] [Google Scholar]
- 18.Zhao L. H., et al., Structure and dynamics of the active human parathyroid hormone receptor-1. Science 364, 148–153 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wootten D., Simms J., Miller L. J., Christopoulos A., Sexton P. M., Polar transmembrane interactions drive formation of ligand-specific and signal pathway-biased family B G protein-coupled receptor conformations. Proc. Natl. Acad. Sci. U.S.A. 110, 5211–5216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., et al., Differential GLP-1R binding and activation by peptide and non-peptide agonists. Mol. Cell 80, 485–500.e7 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Qiao A., et al., Structural basis of Gs and Gi recognition by the human glucagon receptor. Science 367, 1346–1352 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Zhao L. H., et al., Differential requirement of the extracellular domain in activation of class B G protein-coupled receptors. J. Biol. Chem. 291, 15119–15130 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang Y. L., et al., Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature 546, 118–123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., et al., Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 546, 248–253 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang Y. L., et al., Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor-Gs complex. Nature 555, 121–125 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Ma S., et al., Molecular basis for hormone recognition and activation of corticotropin-releasing factor receptors. Mol. Cell 77, 669–680.e4 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Gardella T. J., Luck M. D., Jensen G. S., Usdin T. B., Jüppner H., Converting parathyroid hormone-related peptide (PTHrP) into a potent PTH-2 receptor agonist. J. Biol. Chem. 271, 19888–19893 (1996). [DOI] [PubMed] [Google Scholar]
- 28.Cheloha R. W., Watanabe T., Dean T., Gellman S. H., Gardella T. J., Backbone Modification of a parathyroid hormone receptor-1 antagonist/inverse agonist. ACS Chem. Biol. 11, 2752–2762 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doppelt S. H., et al., Inhibition of the in vivo parathyroid hormone-mediated calcemic response in rats by a synthetic hormone antagonist. Proc. Natl. Acad. Sci. U.S.A. 83, 7557–7560 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horiuchi N., Holick M. F., J. T.Potts, Jr, Rosenblatt M., A parathyroid hormone inhibitor in vivo: Design and biological evaluation of a hormone analog. Science 220, 1053–1055 (1983). [DOI] [PubMed] [Google Scholar]
- 31.Liang Y. L., et al., Dominant negative G proteins enhance formation and purification of agonist-GPCR-G protein complexes for structure determination. ACS Pharmacol. Transl. Sci. 1, 12–20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettersen E. F., et al., UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Emsley P., Cowtan K., Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Adams P. D., et al., PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen V. B., et al., MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan H., et al., The non-peptide GLP-1 receptor agonist WB4-24 blocks inflammatory nociception by stimulating β-endorphin release from spinal microglia. Br. J. Pharmacol. 172, 64–79 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sali A., Blundell T. L., Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993). [DOI] [PubMed] [Google Scholar]
- 38.Wu E. L., et al., CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 35, 1997–2004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guvench O., et al., CHARMM additive all-atom force field for carbohydrate derivatives and its utility in polysaccharide and carbohydrate-protein modeling. J. Chem. Theory Comput. 7, 3162–3180 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J., et al., CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacKerell A. D., et al., All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Hess B., Kutzner C., van der Spoel D., Lindahl E., GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Van Der Spoel D., et al., GROMACS: Fast, flexible, and free. J. Comput. Chem. 26, 1701–1718 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Atomic coordinates and the cryo-EM density map have been deposited in PDB (ID code 7F16) and Electron Microscopy Data Bank (EMDB) (entry ID EMD-31405). All other study data are included in the article and/or SI Appendix.