Significance
Nontypeable Haemophilus influenzae (NTHi) is a common cause of localized respiratory tract disease and results in significant morbidity in children and adults, prompting interest in developing a vaccine to prevent NTHi disease. It has been challenging to date to develop a vaccine that has broad activity against diverse NTHi strains. In this work, we found that immunization of mice with the NTHi HMW1 and HMW2 adhesive proteins stimulates protective immunity against nasopharyngeal colonization by genetically distinct NTHi strains. This immunity includes a strain-specific antibody response and a broadly protective cell-mediated response. Our results highlight the vaccine potential of the HMW1 and HMW2 proteins.
Keywords: Haemophilus influenzae, vaccine, intranasal immunization, colonization
Abstract
Nontypeable Haemophilus influenzae (NTHi) is a common cause of localized respiratory tract disease and results in significant morbidity. The pathogenesis of NTHi disease begins with nasopharyngeal colonization, and therefore, the prevention of colonization represents a strategy to prevent disease. The NTHi HMW1 and HMW2 proteins are a family of conserved adhesins that are present in 75 to 80% of strains and have been demonstrated to play a critical role in colonization of the upper respiratory tract in rhesus macaques. In this study, we examined the vaccine potential of HMW1 and HMW2 using a mouse model of nasopharyngeal colonization. Immunization with HMW1 and HMW2 by either the subcutaneous or the intranasal route resulted in a strain-specific antibody response associated with agglutination of bacteria and restriction of bacterial adherence. Despite the specificity of the antibody response, immunization resulted in protection against colonization by both the parent NTHi strain and heterologous strains expressing distinct HMW1 and HMW2 proteins. Pretreatment with antibody against IL-17A eliminated protection against heterologous strains, indicating that heterologous protection is IL-17A dependent. This work demonstrates the vaccine potential of the HMW1 and HMW2 proteins and highlights the importance of IL-17A in protection against diverse NTHi strains.
Nontypeable (nonencapsulated) Haemophilus influenzae (NTHi) is a common cause of acute otitis media (AOM), otitis media with effusion, sinusitis, and exacerbations of underlying lung disease in children (1). NTHi is also frequently associated with community-acquired pneumonia and exacerbations of chronic obstructive pulmonary disease in adults (2).
The pathogenesis of NTHi disease begins with colonization of the nasopharynx. Colonization occurs early in life and is common throughout childhood and into adulthood (3). NTHi spreads contiguously within the respiratory tract to cause localized disease, typically in the setting of a viral respiratory infection or allergic disease (4–6). Recent evidence indicates that colonization in early infancy may predispose to the development of neutrophilic asthma and allergic airway disease (7). Given the burden of NTHi disease and the association between early life NTHi colonization and asthma, there is interest in developing a vaccine against NTHi.
Currently, vaccines against H. influenzae are limited to polysaccharide conjugate vaccines targeting the capsule of type b strains (Hib). While these vaccines have been largely successful in eliminating Hib invasive disease, there has been no effect on strains of NTHi, which lack a capsule. Efforts to identify highly conserved antigens in NTHi and develop a vaccine against NTHi have been significantly more difficult. While Hib and other encapsulated strains of H. influenzae are clonal, NTHi strains exhibit much greater genetic diversity and heterogeneity (8). Across NTHi strains, common surface antigens exhibit high degrees of antigenic variation, resulting in variable and strain-specific antibody responses. In studies of mice, antibodies acquired during NTHi infection, including IgG in nasal washes directed against outer membrane proteins, are associated with reduced nasopharyngeal colonization density following subsequent challenge by NTHi (9). This suggests a protective role for antibodies at the mucosal surface. However, evidence in children indicates that despite the presence of antibody against an infecting strain, children remain susceptible to subsequent infections by new strains, suggesting that the antibody response may only protect against infection by the same strain.
In combination with antibody responses, cell-mediated immunity has been increasingly recognized as a key driver of protection against airway infection by respiratory pathogens. The production of IL-17A by host cells mediates many effector functions at mucosal surfaces, including the production of antimicrobial peptides, proinflammatory cytokines, chemotactic factors, and granulopoietic factors. These effector functions result in increased recruitment of macrophages and neutrophils and enhanced cytotoxicity and phagocytosis (10–12). There is also evidence that IL-17–producing cells drive humoral immunity, including the generation of antibody in mucosal secretions (13, 14). IL-17 production is known to be essential for defense against various mucosal pathogens, including Mycoplasma pneumoniae, Streptococcus pneumoniae, Klebsiella pneumoniae, and Bordetella pertussis (15–17). In studies of Staphylococcus aureus, clearance of bacteria colonizing the nasopharynx is driven by IL-17–mediated neutrophil influx and antimicrobial peptide production (16, 18). Thus far, it is unclear whether IL-17 production directly contributes to host defense against nasopharyngeal colonization by NTHi.
NTHi colonization of the nasopharynx begins with bacterial adherence to respiratory epithelial cells, which is dependent on NTHi adhesive proteins. The HMW1 and HMW2 high-molecular weight proteins are surface-exposed glycoproteins and are the predominant adhesins in ∼75 to 80% of NTHi strains (19–23). HMW1 and HMW2 also facilitate upper respiratory tract colonization in rhesus macaques as highlighted in studies comparing a wild-type strain and an isogenic mutant lacking both of these proteins (24). HMW1 and HMW2 are highly homologous to each other, sharing ∼70% identity and 80% similarity. HMW1 and HMW2 are also highly homologous among diverse NTHi strains (25, 26).
In children recovering from AOM, HMW1 and HMW2 are the major targets of the serum antibody response to infection. Similarly, rhesus macaques colonized with HMW1/HMW2-expressing strains develop serum antibody responses against HMW1 and HMW2, and serum antibodies against HMW1 and HMW2 are present in adults, suggesting that these adhesins are highly immunogenic. The development of HMW1/HMW2-specific antibodies in humans also coincides with increased serum bactericidal activity (27). However, antibody-mediated killing appears to be directed primarily against the homologous infecting strain rather than broadly acting against heterologous strains (28). The conservation of HMW1 and HMW2 among diverse strains, the immunogenicity of HMW1 and HMW2, the surface localization of HMW1 and HMW2, and the role of HMW1 and HMW2 in adherence to respiratory epithelial cells and in colonization make these adhesins promising antigens for a vaccine.
In this study, we evaluated whether immunization with HMW1 and HMW2 protects against nasopharyngeal colonization. Using a mouse model of immunization and nasopharyngeal challenge, we found that immunization with the HMW1 and HMW2 proteins results in protection against colonization by homologous and heterologous strains, despite a highly strain-specific antibody response. Protection against nasopharyngeal colonization is mediated by both antibody and T cell responses.
Results
Immunization with HMW1 and HMW2 Stimulates Serum Antibody.
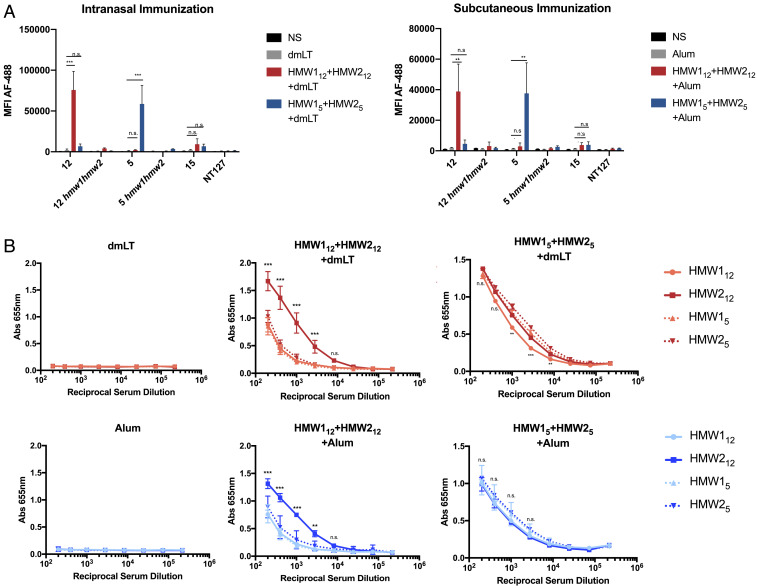
To evaluate the antibody response following immunization with HMW1 and HMW2, we immunized groups of mice by either the intranasal or subcutaneous route. Mice were immunized with HMW1 and HMW2 purified from NTHi strain 12 (HMW112 and HMW212) or NTHi strain 5 (HMW15 and HMW25). As shown in Fig. 1, animals immunized by either the subcutaneous or intranasal route developed a robust serum IgG response based on an assessment of pooled serum. A measurement of total IgG binding to whole bacteria by flow cytometry (Fig. 1A) revealed that immune serum was specific for NTHi expressing the HMW1 and HMW2 proteins. Antibodies generated by immunization with HMW112+HMW212 were specific for NTHi strain 12 and showed no significant binding to heterologous HMW1/HMW2-expressing strains (strain 5 and strain 15). Similarly, antibodies generated by immunization with HMW15+HMW25 were specific for strain 5. This specificity was present with both subcutaneous and intranasal immunization. There was no significant antibody reactivity with strains that do not possess the HMW1 and HMW2 proteins, including hmw1hmw2 mutants of strain 12 and strain 5 and strain NT127, which lacks the hmw1 and hmw2 loci. In enzyme-linked immunosorbent assays (ELISA) using purified HMW1 and HMW2, sera also exhibited reactivity with the HMW1 and HMW2 adhesins, including variable cross-reactivity with HMW1 and HMW2 from heterologous strains (Fig. 1B). There was no significant reactivity in serum from animals immunized with only adjuvant (alum or dmLT). These studies demonstrate that serum antibodies against HMW1 and HMW2 exhibited specificity for the parent strain from which HMW1 and HMW2 were purified in assays with whole bacteria and variable cross-reactivity with purified proteins from heterologous strains.
Fig. 1.
Immunization with HMW1+HMW2 and dmLT (intranasal) or HMW1+HMW2 and alum (subcutaneous) results in systemic IgG responses. (A) IgG binding to whole NTHi was evaluated by flow cytometry. IgG binding is expressed as mean antibody fluorescence intensity (MFI) based on 50,000 events per sample. (B) Binding to purified HMW1 and HMW2 by ELISA. Serum was pooled from five immunized animals per group. Control groups immunized with dmLT in PBS (intranasal) or alum in PBS (subcutaneous). Data are expressed as mean ± SD from three independent experiments. Statistical significance was determined using two-way ANOVA with Tukey’s correction for multiple comparisons. n.s., not significant. **P < 0.01, ***P < 0.001.
Immunization with HMW1 and HMW2 Stimulates Protection against Nasopharyngeal Colonization by Homologous and Heterologous Strains.
Following immunization with HMW112+HMW212, HMW15+HMW25, or adjuvant alone by either the subcutaneous or intranasal route, animals were challenged intranasally with either the parent strain, the heterologous HMW1/HMW2-expressing strain, the 12hmw1hmw2 mutant, or strain NT127. A total of 3 d following the challenge, the density of colonization in animals immunized with HMW112+HMW212 or HMW15+HMW25 was markedly reduced, based on colony-forming units (CFUs) recovered from homogenized nasal tissue (Fig. 2).
Fig. 2.
Immunization with HMW1+HMW2 reduces nasopharyngeal colonization density. Mice were immunized with purified HMW112+HMW212 (A) or HMW15+HMW25 (B) and subsequently challenged with NTHi strain 12, strain 5, strain 12hmw1hmw2, or strain NT127. Inoculums were ∼108 CFU. The density of colonization was represented as log(recovered CFU/g nasal tissue). Data represent means ± SD of five animals per group. Significance was determined using the Kruskall–Wallis test. If a significant variance was identified, the Mann–Whitney U test was used to compare individual groups to adjuvant controls. n.s., not significant. *P < 0.05.
Compared to animals immunized with dmLT in phosphate-buffered saline (PBS), the density of colonization of mice immunized intranasally with HMW112+HMW212 was reduced by ∼2 logs. The density of colonization of animals immunized subcutaneously with HMW112+HMW212 was reduced by 0.5 to 1 log compared to alum in PBS, though this change was not statistically significant (Fig. 2A). Animals immunized with HMW112+HMW212 were also protected against nasopharyngeal colonization with NTHi strain 5, with larger reductions in colonization density detected in the intranasally immunized group. Immunization with HMW15+HMW25 was also protective against both strain 5 and strain 12 (Fig. 2B). Intranasal immunization consistently resulted in greater reductions in colonization density compared to subcutaneous immunization, suggesting a role for local immune factors in the nasopharynx. Colonization density was not reduced in any animals challenged with strain 12hmw1hmw2 or strain NT127. These results suggest that mice were protected against nasopharyngeal colonization by heterologous strains, despite an antibody response that appears to be strain specific.
HMW1 and HMW2 Antibodies Promote NTHi Agglutination.
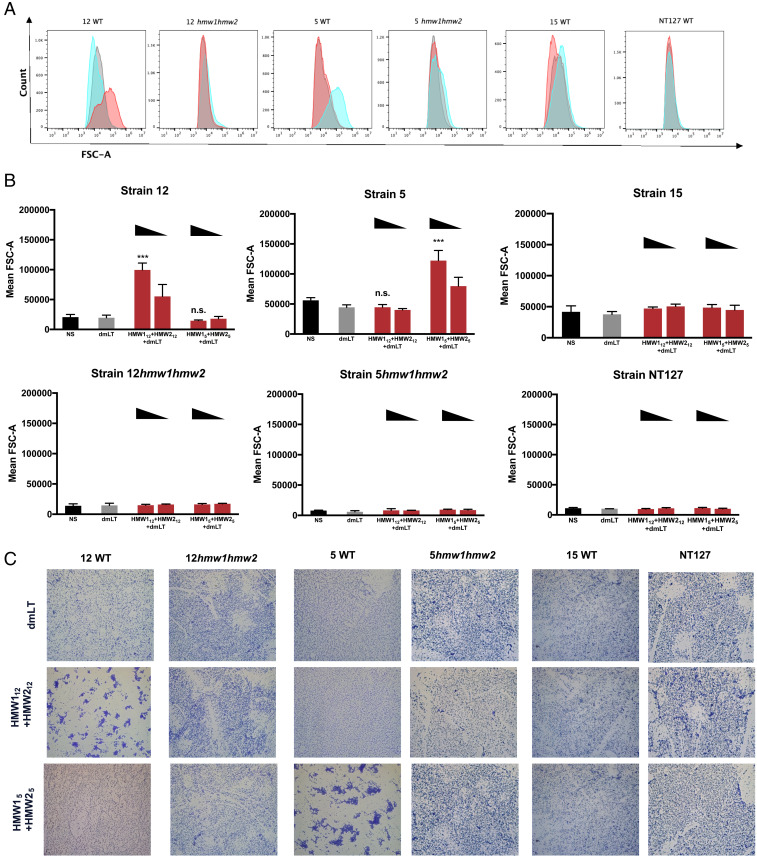
To study the specificity of antibody-driven antibacterial behaviors, we first measured agglutinating activity of HMW1 and HMW2 antibodies, employing a flow cytometry–based assay developed by Habets et al. (29). NTHi strains were exposed to immune serum or adjuvant-only serum. Following incubation, bacteria were fixed, stained, and evaluated using flow cytometry. Agglutination was detected based on increases in the forward scatter signal (FSC-A), which indicate increases in particle size. In the presence of HMW112+HMW212 immune serum, the mean FSC-A of strain 12 increased when compared to adjuvant-only serum (Fig. 3 A and B). The formation of aggregates was confirmed by staining bacteria with crystal violet and imaging with bright field microscopy (Fig. 3C). Serum generated against HMW112+HMW212 was associated with agglutination of NTHi strain 12 but not strain 5 or strain 15, again suggesting strain specificity of the antibody response. Similarly, immune serum generated against HMW15+HMW25 only agglutinated strain 5. Antibodies against strain 12 or strain 5 HMW1 and HMW2 proteins failed to agglutinate strain 12hmw1hmw2, strain 5hmw1hmw2, or strain NT127, indicating that agglutinating activity was due to HMW1- and HMW2-specific antibodies. Detectable agglutination was proportional to the concentration of immune serum, suggesting that the agglutination reaction is serum dependent (Fig. 3B). These results indicate that immunization with HMW1 and HMW2 results in serum antibodies that mediate strain-specific bacterial agglutination.
Fig. 3.
Agglutination of NTHi by serum from HMW1+HMW2-immunized animals. NTHi were incubated with pooled serum from groups of five mice immunized with HMW1+HMW2. (A) Agglutination was measured by flow cytometry and is represented as histograms of the FSC-A signal. NTHi were incubated with 2% serum from animals immunized with dmLT alone (gray), HMW112+HMW22+dmLT (red), or HMW15+HMW25+dmLT (blue). Mean FSC-A is quantified in (B) and is based on 50,000 events per sample. Error bars represent means ± SD from three independent experiments. Statistical significance was determined using two-way ANOVA with Tukey’s correction for multiple comparisons. n.s., not significant. ***P < 0.001. (C) Representative images of agglutination imaged by light microscopy following crystal violet staining. Images shown at 400× total magnification. NS, no serum.
HMW1 and HMW2 Antibodies Restrict HMW2-Mediated Adherence.
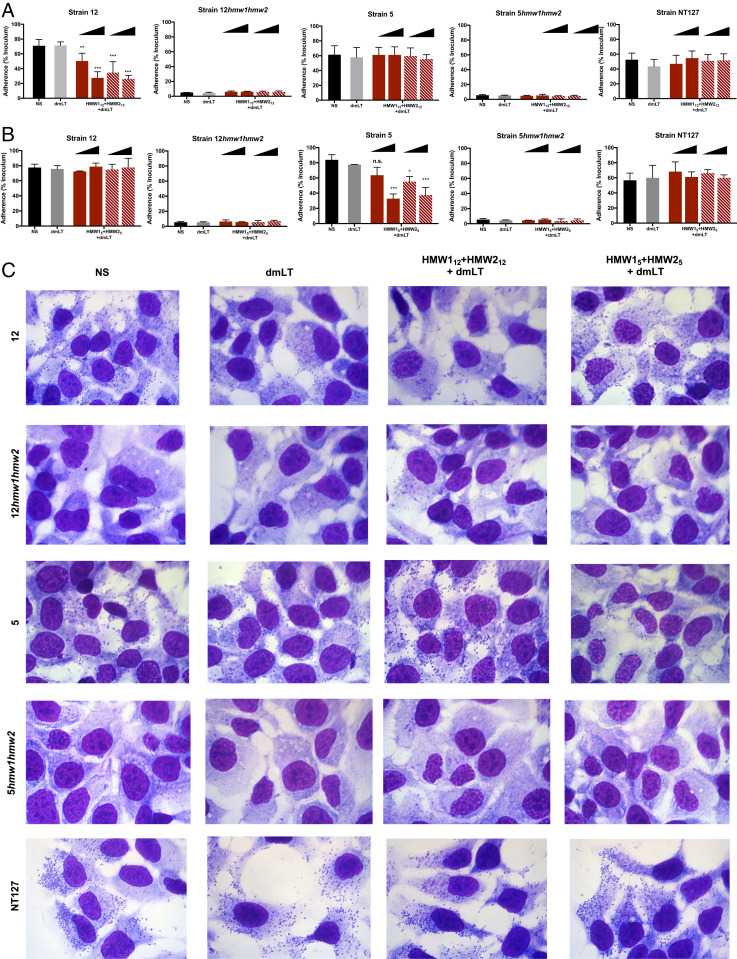
To further address the functional activity of the antibody response to the HMW1 and HMW2 proteins, we assayed the ability of immune sera to inhibit HMW1- and HMW2-mediated adherence to epithelial cells. NTHi strains were inoculated onto monolayers of HaCaT cells in the presence or absence of pooled immune serum. In the presence of 0.2% serum derived against HMW112+HMW212, adherence of strain 12 was reduced by ∼20%; when serum was increased to 2%, adherence was further reduced, demonstrating a dose-dependent effect (Fig. 4A, solid bars). Similarly, strain 5 adherence was reduced in the presence of serum generated against HMW15+ HMW25 (Fig. 4B, solid bars) and was proportional to the amount of serum present. Strain 12 and strain 5 bacterial adherence was not reduced in the presence of serum generated against proteins from the heterologous strain or in the presence of adjuvant-only serum. Immune serum derived against proteins from either parent strain had no impact on strain 12hmw1hmw2 or strain 5hmw1hmw2, which are unable to adhere to HaCaT cells. As shown in Fig. 4C, direct visualization of adherence by light microscopy after Giemsa staining was consistent with the results of quantitative adherence assays. Antibodies against adhesins can reduce bacterial adherence, and consequently colonization, due to either agglutination of bacteria or direct interference with the receptor–ligand interaction or both (30, 31). To evaluate whether agglutination was the sole mechanism of reduced adherence, we treated total serum with papain, digesting intact IgG in serum to produce nonagglutinating F(ab)2 fragments. Papain-digested serum retained the ability to inhibit adherence (Fig. 4 A and B, striped bars). Together, these results suggest that HMW1 and HMW2 antibodies are capable of promoting antibacterial function in a strain-specific manner, through both direct agglutination and blocking of adhesin–receptor interactions.
Fig. 4.
HMW1 and HMW2 antibodies reduce bacterial adherence. Bacteria were inoculated onto monolayers of HaCaT epithelial cells in the presence or absence of pooled immune serum derived against HMW112+HMW212 (A) or HMW15+HMW25 (B). Adherent bacteria represented as the recovered CFU percentage of the inoculum. For all strains, inoculums were ∼2 × 107 CFU/mL. Solid bars represent whole pooled serum. Striped bars represent pooled serum predigested with papain. Data expressed as means ± SD of three independent experiments. NS, no serum. Statistical significance was determined using two-way ANOVA with Tukey’s correction for multiple comparisons. Significance is determined relative to the dmLT serum condition. n.s., not significant. *P < 0.05, **P < 0.01, ***P < 0.001. (C) Representative images of bacterial adherence to HaCaT monolayers. Images shown at 400× total magnification. NS, no serum.
Passive Transfer of Antibodies Mediates Protection against Colonization by the Homologous Strain but Not a Heterologous NTHi Strain.
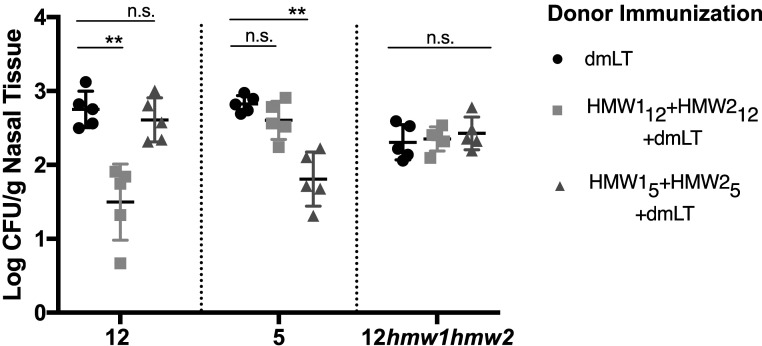
To evaluate the contribution of antibodies in the absence of other immune factors, we conducted passive transfer studies. Donor animals were immunized intranasally with purified HMW112+HMW212, purified HMW15+HMW25, or dmLT. Immune serum was administered to naïve animals, and recipient animals were then challenged with NTHi and assessed for colonization (Fig. 5). The density of colonization with strain 12 in animals receiving HMW112+HMW212-derived serum was reduced ∼1.5 logs compared to animals that received serum derived against dmLT alone. There was no significant reduction in density of colonization with strain 12 in animals that received HMW15+HMW25-derived serum. Similarly, the density of colonization with strain 5 in animals that received serum generated against HMW15+HMW25 was reduced ∼1.2 logs compared to control animals, but the density of colonization with strain 12 in these animals was unaffected. There were slight reductions (<0.5 log) in colonization density in animals that received serum derived against the heterologous strain, though these decreases were not statistically significant. Transfer of sera against purified HMW112+HMW212 or purified HMW15+HMW25 had no effect on colonization by an hmw1hmw2 mutant. Thus, passive receipt of antibodies granted protection against the homologous strain but was not sufficient to mediate protection against a heterologous strain, despite expression of HMW1 and HMW2.
Fig. 5.
Passive transfer of antibodies mediates homologous protection. Mice were passively immunized with immune serum from mice immunized with dmLT (circles), HMW112+HMW212 (squares), or HMW15+HMW25 (triangles) and subsequently challenged with NTHi strain 12, strain 5, and strain 12hmw1hmw2. Nasopharyngeal colonization densities were determined 3 d following challenge. Data represent means ± SD of n = 5 animals per group. Significance was determined using the Kruskall–Wallis test. If a significant variance was identified, the Mann–Whitney U test was used to compare individual groups to adjuvant controls. n.s., not significant. **P < 0.01.
IL-17A Is Required for Protection against Heterologous Strains.
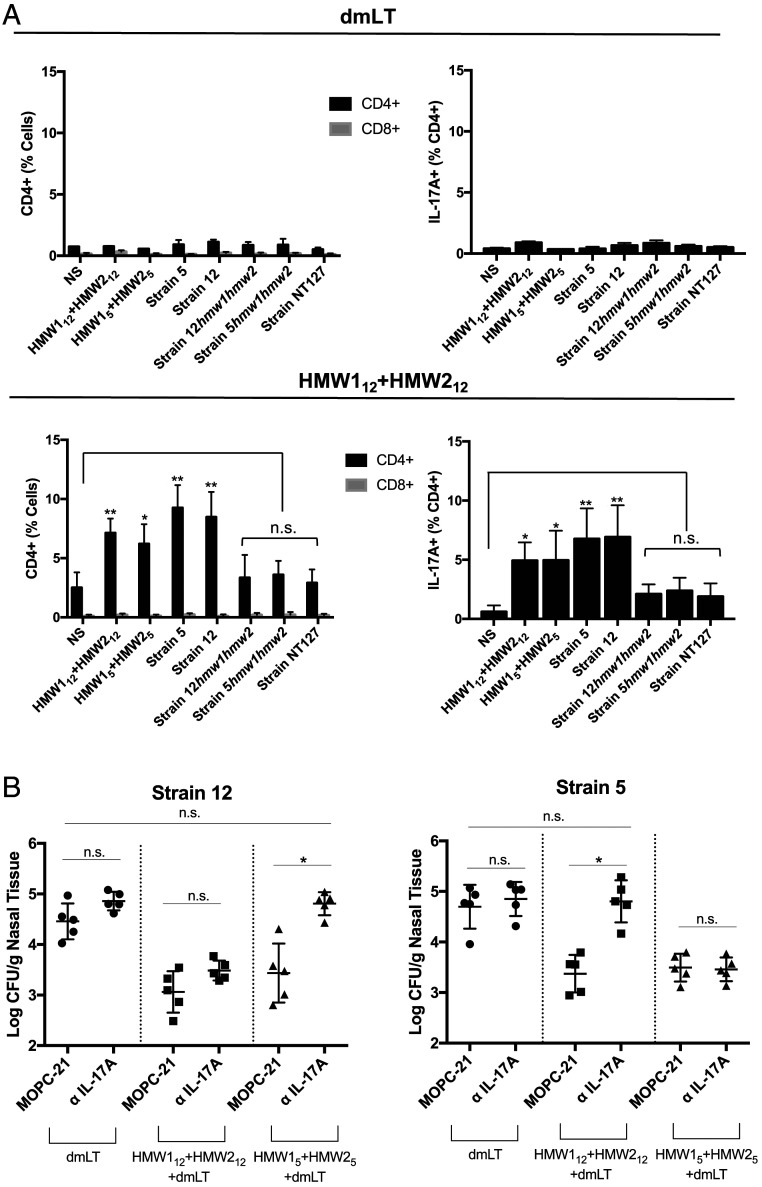
To evaluate whether cell-mediated IL-17 production was involved in the response to NTHi, we performed an in vitro stimulation of lymphocytes isolated from immunized mice. Groups of mice were immunized intranasally with purified HMW112+HMW212 or dmLT. Following immunization, lymphocytes were isolated from mouse nasal passages and stimulated in vitro with heat-killed NTHi strains or purified HMW1 and HMW2. Stimulated cells were stained for surface markers, and intracellular IL-17A was assessed by intracellular cytokine staining (Fig. 6A). In mice immunized with HMW112+HMW212, the stimulation of nasal lymphocytes resulted in an increase in CD4+ T cells but no significant increase in CD8+ T cells, suggesting a CD4-driven cell response. IL-17A production was detected in the CD4 cells following stimulation with purified proteins from either strain 12 or strain 5 as well as heterologous HMW1/2-expressing NTHi strains. Stimulation with HMW1/HMW2-deficient strains (strain NT127, strain 12hmw1hmw2, and strain 5hmw1hmw2) resulted in no significant IL-17A response compared with the no stimulation control.
Fig. 6.
IL-17A mediates heterologous protection. (A) Groups of five mice were immunized intranasally with dmLT (Top) or HMW112+HMW212 (Bottom). Following immunization, lymphocytes were isolated from mouse nasal passages and stimulated in vitro with heat-killed NTHi or purified protein as indicated. Following stimulation, cells were stained for the indicated surface markers, including CD4, CD8, and intracellular IL-17A, and evaluated by flow cytometry. Data represent means ± SD of pooled lymphocytes stimulated in triplicate. NS, no stimulation. Significance of each group was determined relative to the NS condition using a one-way ANOVA with Tukey’s correction for multiple comparisons. (B) Mice were immunized with dmLT, HMW112+HMW212, or HMW15+HMW25. Separate groups were administered anti–IL-17A antibody or IgG isotype control (MOPC-21) 1 d prior to challenge. Mice were then challenged intranasally with NTHi strain 12, NTHi strain 5, or NTHi strain 12hmw1hmw2. Nasopharyngeal colonization densities were determined 3 d following challenge. Data represent means ± SD of five animals per group. Significance was determined using the Kruskall–Wallis test. If a significant variance was identified, the Mann–Whitney U test was used to compare individual groups to adjuvant controls. n.s., not significant. *P < 0.05., **P < 0.01.
To determine whether the observed IL-17A responses facilitate protection against nasopharyngeal colonization in vivo, we performed an antibody-mediated in vivo depletion of IL-17A. Mice were immunized intranasally with HMW112+HMW212, HMW15+HMW25, or dmLT alone. A total of 1 d prior to intranasal inoculation (−1 dpi), mice were given an anti–IL-17A antibody (BioXCell; clone 1F17) or an IgG isotype control antibody (BioXCell; clone MOPC-21). Mice were then challenged with the parent strain or the heterologous HMW-expressing strain, and colonization density was determined 3 d later (Fig. 6B).
Among groups given the IgG isotype control antibody, mice immunized with purified protein were colonized at lower densities than mice immunized with dmLT alone, as expected. The administration of an anti–IL-17A antibody had no significant impact on nasopharyngeal colonization by the homologous strain in animals immunized with either HMW112+HMW212 or HMW15+HMW25. However, anti–IL-17A treatment reduced the degree of protection observed against heterologous strains. Compared to control animals immunized with dmLT, animals immunized with HMW15+HMW25 were colonized by strain 12 at ∼1.4 logs lower density when given the IgG isotype control. When given anti–IL-17A, animals immunized with HMW15+HMW25 were colonized by strain 12 at 0.05 logs lower density, a difference that was not statistically significant compared to the dmLT-immunized control. Likewise, animals that were given MOPC-21 and then immunized with HMW112+HMW212 were colonized by strain 5 at 1.3 logs lower density, but animals that were given anti–IL-17A and then immunized with HMW112+HMW212 had no significant reduction in strain 5 colonization density. These results suggest that IL-17A produced by CD4+ T cells is critical for protection against heterologous strains and that IL-17A may facilitate protection independent of antibody strain specificity.
Discussion
In this study, we have established that immunization with the HMW1 and HMW2 proteins stimulates protective immunity against nasopharyngeal colonization by NTHi. Importantly, the induced immunity protects against the parent strain as well as heterologous HMW1/HMW2-expressing strains, suggesting broad protection. To date, few studies have examined potential vaccine antigens that restrict NTHi nasopharyngeal colonization, an essential first step in the pathogenesis of all NTHi diseases.
Following immunization with purified HMW1 and HMW2, serum antibody responses were highly strain specific. In in vitro experiments, serum antibodies enhanced agglutination and inhibited adherence to epithelial cells by the homologous strain, with no significant effect on a heterologous HMW1/HMW2-expressing strain. In mice, passive transfer of immune sera resulted in reduced nasopharyngeal colonization by the homologous strain, presumably inhibiting bacterial adherence to host epithelium and enhancing opsonophagocytosis. In contrast, the passive transfer of antibodies had no effect on colonization by a heterologous HMW1/HMW2-expressing strain.
While the antibody response to HMW1 and HMW2 was strain specific and provided protection against only the homologous strain, immunization with HMW1 and HMW2 resulted in reduced nasopharyngeal colonization by heterologous strains as well, suggesting another mechanism of immunity. Immunization was associated with an increase in IL-17A production, and knockdown of IL-17A with a monoclonal antibody eliminated protection against colonization by a heterologous HMW1/HMW2-expressing strain, indicating IL-17A–mediated protection. In the upper airways, IL-17A is known to facilitate protection against colonization by bacterial pathogens by stimulating production of antimicrobial peptides, proinflammatory cytokines, chemotactic factors, and granulopoietic factors, resulting in increased recruitment of macrophages and neutrophils and enhanced cytotoxicity and phagocytosis (15, 32). Our findings demonstrate that immunization with HMW1 and HMW2 provides protection against nasopharyngeal colonization by heterologous NTHi strains by stimulating production of IL-17A.
Emerging evidence has begun to implicate IL-17 in the immune response to NTHi. Li et al. recently showed that Th17 cells drive antibody-independent protection against pulmonary infection by NTHi and that Th17 cells are capable of mediating protection against heterologous strains despite serotype-specific antibody responses (33). Similarly, intranasal immunization with NTHi outer membrane vesicles (OMVs) has been shown to stimulate Th17 memory and subsequent protection against heterologous strains (34, 35). While several immunogenic outer membrane proteins have been identified in OMVs, including the HMW1 and HMW2 adhesins, it has been unclear exactly which proteins directly contribute to IL-17 production and are capable of stimulating protective immunity in the nasopharynx. In this work, we identify the HMW1 and HMW2 adhesins as IL-17A–stimulating antigens that stimulate protective immunity against nasopharyngeal colonization.
While Th17 cells have been well described in immune responses to NTHi, innate CD4+ lymphoid cells (ILCs) have also been identified as a source of IL-17 at mucosal surfaces. Group 3 ILC (ILC3) cells have been shown to produce IL-17 in response to extracellular mucosal pathogens, including Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa (36–38). In our studies, protection against heterologous NTHi strains was limited to HMW1/2-expressing strains. Moreover, we were only able to observe significant increases in IL-17–producing CD4+ T cells in response to purified HMW1 and HMW2 or HMW1/2-expressing strains. These observations are indicative of an antigen-specific immune response, consistent with Th17-driven immunity. We were unable to identify CD4+ cells with an ILC3 profile in sufficient quantities to establish a role for these innate cells. However, given the relatively low lymphocyte counts isolated from nasal tissue and the established rarity of ILC3s at mucosal sites (39), we cannot exclude the possibility that ILC3s are involved in mediating broad host defense against nasal colonization by NTHi.
Animals immunized with HMW1 and HMW2 by the subcutaneous or intranasal route developed comparable amounts of NTHi-specific serum antibody. However, based on recovered CFU, our data suggest that intranasally immunized mice were protected to a greater degree, with greater reductions in colonization relative to adjuvant controls. This result suggests an advantage to intranasal immunization and a role for local immune factors in mediating protection against heterologous strains in the upper airways. This finding is consistent with other studies evaluating mucosal immunization, which have observed enhanced protection compared to immunization at distal sites (35, 40). The delivery of antigens to intranasal lymphoid tissue likely stimulates local immune cell development, facilitating cell-mediated protection in combination with antibody-mediated systemic immunity. In our studies, animals immunized subcutaneously with HMW1 and HMW2 were still protected against full levels of colonization by either the parent strain or the heterologous strain, suggesting development of IL-17A–producing CD4+ cells in the nasopharynx without direct antigen delivery in the nasal passage. Recent evidence suggests that parenteral immunization with bacterial antigens can stimulate the development of nasal memory cell populations, though the mechanism through which this occurs is unclear (40). These findings suggest that the route of antigen delivery is an important consideration in NTHi vaccine design and that intranasal immunization may be more effective.
Despite the high degrees of homology of the HMW1 and HMW2 proteins across strains, the antibody responses following immunization were remarkably strain specific. Based on amino acid sequences, HMW112 and HMW15 share 73.08% identity, and HMW212 and HMW25 share 69.66% identity (SI Appendix, Fig. S1). In our studies, while antibodies bound to whole bacteria in a strain-specific pattern, binding assays to purified proteins revealed that cross-reactive antibodies were present in immune sera, suggesting that strain specificity stems from factors beyond amino acid sequence, an ongoing area of investigation.
Our data also suggest that the HMW1 and HMW2 proteins may have important immunological differences within a strain. Though animals were immunized with a 1:1 mixture of HMW112 and HMW212, the antibody response appeared to heavily favor HMW212. While HMW112 and HMW212 share ∼70% identity, they contain two regions of significant divergence, including an HMW112-specific region of 62 amino acids that is absent from HMW212 and an ∼360 amino acid region that harbors binding activity and accounts for the differences in HMW1 and HMW2 binding specificity. These regions could explain the observed differential immunogenicity between HMW112 and HMW212. Interestingly, immunization with HMW15 and HMW25 generated an antibody response that did not heavily favor either protein, suggesting that the differences are not solely due to the HMW1 and HMW2 binding domains.
Importantly, a number of other pathogens that colonize the upper respiratory tract express surface proteins that are orthologous to HMW1 and HMW2 and are involved in host cell adhesion and nasopharyngeal colonization. Examples include filamentous hemagglutinin in Bordetella pertussis (41), MchA in Moraxella catarrhalis (42), and HrpA in Neisseria meningitidis (43). With this information in mind, our observations regarding HMW1 and HMW2 as vaccine antigens may extend beyond NTHi to other respiratory pathogens and to other high-molecular weight adhesive proteins.
In summary, immunization with HMW1 and HMW2 stimulates an antibody response that protects against the homologous NTHi strain and an IL-17A response that protects against heterologous NTHi strains. Given the high prevalence of HMW1/HMW2-expressing strains among NTHi clinical isolates and the degree of protective immunity afforded by immunization, HMW1 and HMW2 may be valuable for inclusion in a vaccine to prevent nasopharyngeal colonization and provide protection against the full range of NTHi disease.
Materials and Methods
Bacterial Strains and Culture.
The bacterial strains used in the present study are described in Table 1. NTHi strain 12 is the prototype strain from which the hmw1 and hmw2 loci were first cloned and from which the HMW1 and HMW2 proteins were first characterized. NTHi strains were grown on chocolate agar (BD Biosciences) or brain heart infusion (BHI) agar supplemented with 0.1% (vol/vol) lysed horse blood as a source of hemin and 3.5 μg/mL NAD (BHIs), with 500 μg/mL streptomycin as appropriate. Agar plates were incubated overnight at 37 °C with 5% CO2. Spontaneous streptomycin-resistant derivatives of NTHi were generated by spreading a dense bacterial suspension onto agar plates containing 500 μg/mL streptomycin and then recovering survivors. For each set of isogenic strains, growth studies determined that the parent and derivative strain had similar growth rates. In preparation for intranasal inoculation, NTHi strains were resuspended in BHIs broth to an optical density at 600 nm (OD600) of 0.2. Cultures were grown shaking at 250 rpm to an OD600 of 0.8, pelleted, washed with PBS (Lonza Biologics), and resuspended in PBS.
Table 1.
Bacterial strains used in this study
| Strain | Description | Source or reference |
| 12 | Nontypeable Haemophilus influenzae, expresses HMW112 and HMW212 | Clinical isolate, middle ear fluid, AOM |
| 12hmw1hmw2 | 12 hmw1::kan hmw2::kan, expresses neither HMW112 nor HMW212 | (20) |
| 12hmw1 | 12 hmw1::kan, expresses only HMW212 | (20) |
| 12hmw2 | 12 hmw2::kan, expresses only HMW112 | (20) |
| 5 | Nontypeable Haemophilus influenzae, expresses HMW15 and HMW25 | Clinical isolate, middle ear fluid, AOM |
| 5hmw1hmw2 | 5 hmw1::kan hmw2::kan, expresses neither HMW15 nor HMW25 | (20) |
| 5hmw1 | 5 hmw1::kan, expresses only HMW25 | (20) |
| 5hmw2 | 5 hmw2::kan, expresses only HMW15 | (20) |
| 15 | Nontypeable Haemophilus influenzae, expresses HMW115 and HMW215 | Clinical isolate, middle ear fluid, AOM |
| NT127 | Nontypeable Haemophilus influenzae, expresses neither HMW1 or HMW2 and instead expresses the Hia adhesin | Clinical isolate, blood, meningitis (provided by Hao Shen, University of Pennsylvania) |
Cell Lines.
The cell lines used in this study were obtained from the American Tissue Culture Collection (ATCC). Cells were maintained at 37 °C with 7.5% CO2. Chang cells (human conjunctiva; ATCC CCL-20.2) were maintained in Eagle minimal essential media (MEM) (Sigma-Aldrich) supplemented with 1% nonessential amino acids (NEAA) and 10% fetal calf serum (FCS). HaCaT cells (derived from human keratinocytes) (44) were maintained in MEM + 0.1% NEAA + 10% FCS.
Purification of HMW1 and HMW2.
HMW1 and HMW2 were purified from the NTHi surface as described previously (24). NTHi mutants expressing HMW1 only or HMW2 only were inoculated into 10 L BHIs broth and grown 12 to 14 h shaking at 37 °C. The culture was then centrifuged for 20 min at 8,000 rpm, the supernatant was discarded, and the pellet was frozen at −80 °C overnight. To release HMW1 or HMW2 from the bacterial surface, the frozen pellet was thawed on ice and resuspended in 200 mL extraction buffer (0.5 M NaCl, 10 mM ethylenediaminetetraacetic acid (EDTA), 10 mM Tris pH 7.5, and 50 μM 1,10-phenanthroline) in the presence of protease inhibitors (Roche). The resuspended bacteria were incubated at 4 °C for 1 h and then centrifuged for 15 min at 8,500 rpm. The supernatant containing the released HMW1 or HMW2 was saved, and the pellet was discarded. The supernatants were dialyzed overnight into 20 mM 2-(N-morpholino)ethanesulfonic acid (MES), 75 mM NaCl pH 6.0 and then stepwise dialyzed in MES with 250 mM NaCl, in MES with 125 mM NaCl, and then in 80 mM NaCl. Dialyzed protein was loaded onto a Resource S cation exchange chromatography column (GE Life Sciences). The bound protein was eluted with 20 mM MES, 1 M NaCl pH 6.0. Fractions containing HMW1 or HMW2 were combined, concentrated, and then loaded onto a HiLoad SuperDex 16/60 200 pg size-exclusion column (GE Life Sciences) equilibrated with 20 mM MES, 150 mM NaCl pH 6.0, 5% glycerol. The fractions containing HMW1 or HMW2 were pooled, resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels, and stained with Coomassie blue to ensure purity.
Antibody Measurement by ELISA.
The purified protein (2.5 μg/mL in 0.1 M carbonate buffer, pH 9.6) was coated onto 96-well plates by incubation at 4 °C overnight. Plates were washed with PBS-0.1% Tween 20 and blocked with 2% nonfat milk in PBS. Serial dilutions of mouse sera were applied to wells in triplicate. The plates were incubated at 37 °C for 1 h. Mouse IgG was detected using a 1:2,000 dilution of rabbit anti-mouse IgG conjugated to horseradish peroxidase (Sigma). For detection, the wells were incubated with 100 μL 3,3′,5,5′-tetramethylbenzidine (TMB) ELISA substrate (Rockland), and absorbance was recorded at 655 nm.
Antibody Measurement by Flow.
NTHi strains were inoculated into 3 mL of BHIs broth at OD600 = 0.200 and grown shaking at 37 °C until OD600 = 0.800. Bacteria were then spun down and resuspended in 1× PBS to OD600 = 0.600. Bacteria were subsequently fixed in 1× tris-buffered saline + 1% formaldehyde for 1 h, and then incubated with the appropriate immune serum. Following incubation, bacteria were washed in 1× PBS and incubated with fluorescent anti-mouse IgG (Rockland) for 1 h, washed. Bacterial suspensions were brought up to 1 mL total volume and stained with propidium iodide (PI) to identify bacteria. Fluorescence was analyzed using a BD Accuri C6 flow cytometer and BD Accuri C6 plus software (BD Biosciences). A total of 50,000 PI+ events were recoded per sample.
Bacterial Adherence.
Quantitative adherence assays were performed as described previously (24). Approximately 1.8 × 105 cells were seeded into 24-well tissue culture plates and incubated overnight. Epithelial monolayers were inoculated with ∼2 × 107 CFU NTHi in the presence or absence of immune serum, and the plates were centrifuged at 165 × g for 5 min to facilitate contact between the bacteria and the epithelial cells. After incubation for 30 min at 37 °C in a 5% CO2 atmosphere, monolayers were rinsed with PBS to remove nonadherent bacteria. Trypsin-EDTA (0.25% trypsin, 0.5% EDTA) (Sigma) was added to the wells to release epithelial cells and adherent bacteria. Dilutions of adherent organisms were plated on BHIs agar and incubated overnight to determine the number of adherent bacteria per monolayer. To image epithelial cell monolayers, cells were seeded onto glass coverslips. Following inoculation and incubation with bacteria, nonadherent bacteria were washed away with PBS, and monolayers were stained with Giemsa.
Animal Immunizations.
All animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Children’s Hospital of Philadelphia. Groups of 6- to 8-wk-old female BALB/c mice were immunized intranasally or subcutaneously with purified HMW1 and HMW2. For subcutaneous immunizations, mice were given 10 μg purified protein diluted in PBS, conjugated to a suspension of Imject alum as an adjuvant (Thermo Scientific). Subcutaneous immunizations were given in a 100 μL volume (45). Intranasal immunizations were performed as described by Cutter et al., with purified protein diluted in PBS to a concentration of 15 μg/40 μL with 0.1 μg Escherichia coli labile toxin mutant LT(R192G/L211A) (dmLT) as a mucosal adjuvant (46). Purified dmLT was received as a gift from John Clements at Tulane University, New Orleans, LA (47). Prior to intranasal immunization, mice were anesthetized by intraperitoneal injection of ketamine/xylazine (75 mg/kg + 7.5 mg/kg body weight). Intranasal immunizations were delivered by pipetting 20 μL directly into each nostril. Following immunization, mice were placed in a supine position for 3 to 5 min. Mice were immunized on weeks 0, 1, 3, and 5. Blood was collected by terminal cardiac puncture 2 wk following the final immunization, and serum was isolated from whole blood.
Intranasal Challenge.
A total of 2 wk following the final immunization, mice were challenged intranasally with ∼1 × 108 CFU streptomycin-resistant NTHi in 10 μL. Mice were anesthetized as described for intranasal immunization, and 5 μL bacterial suspension was delivered into each nostril. Following inoculation, mice were placed in a supine position for 3 to 5 min. Animals were euthanized 3 d following challenge. Nasal washes were collected by inserting a catheter into the trachea and flushing PBS through the nose. Nasopharyngeal tissue was harvested, weighed, homogenized, diluted, and plated. Recovered NTHi was plated onto BHIs agar containing 500 μg/mL streptomycin and incubated at 37 °C for 48 h to determine colony counts.
Cytokine Staining and Flow Cytometry.
Lymphocytes were isolated from mouse nasal passages using enzymatic extraction with collagenase (48). Isolated lymphocytes were stimulated in vitro with preparations of heat-killed NTHi or purified HMW1 and HMW2 for 16 h at 37 °C, with protein transport inhibitor (BD GolgiStop, BD Biosciences) added for the final 5 h. Following stimulation, cells were stained for relevant surface markers, including CD4 and CD8 (Thermo Scientific) and intracellular IL-17A (BD Biosciences). Intracellular staining was performed using the BD Cytofix/Cytoperm Fixation/Permeabilization Kit (BD Biosciences). Stained lymphocytes were measured on a CytoFLEX LX flow cytometer and CytExpert software (Beckman Coulter).
Serum Transfer.
For the passive transfer of serum, naïve mice were injected intravenously with 200 μL pooled serum from immunized donor animals. Mice were challenged intranasally with NTHi 24 h following the transfer. A total of 3 d following challenge, mice were euthanized to determine nasopharyngeal colonization density.
Antibody-Mediated In Vivo Depletion of IL-17A.
Mice were immunized as described. A total of 2 wk following immunization, mice were given monoclonal anti–IL-17A antibody (clone 1F17) or the IgG isotype control antibody (clone MOPC-21) (BioXCell). Antibody was administered in a 1-mL intraperitoneal injection at a concentration of 0.500 mg/mL in sterile PBS. Mice were given antibody 1 d prior to intranasal challenge with NTHi (day −1). At 3 dpi, mice were euthanized to determine colonization density as described.
Agglutination.
Bacteria were resuspended from chocolate agar plates in 3 mL BHIs to OD600= 0.200, grown to OD600= 0.800, and then resuspended in 1× PBS. A 100-μL volume of the bacterial suspension was incubated with pooled, heat-inactivated serum for 1 hr at room temperature. Bacteria were then fixed by adding formaldehyde to a final concentration of 1% and incubating at room temperature for 1 hr. Following fixation, the samples were stained with crystal violet for imaging or diluted in PBS for analysis by flow cytometry.
Measurement of Agglutination by Flow Cytometry.
Bacteria were fixed and stained with PI to gate on bacterial cells. Bacterial populations were then evaluated in the FSC-A and side scatter dot plot to measure aggregate size and the FSC-A count plot to determine aggregate quantity (29). Samples were analyzed in duplicate, and 50,000 PI+ events were measured for each sample. Data were analyzed using FlowJo version 10 (BD).
Imaging.
Bacterial aggregates and epithelial monolayers were visualized by light microscopy. Images were acquired at a magnification of 400× using a Leica DM2500 microscope.
Statistical Analysis.
Data were analyzed in GraphPad Prism (version 8.0) software (GraphPad Software, Inc.). Statistical significance was determined using ANOVA, the Tukey–Kramer nonparametric test, and Student’s t tests when appropriate. For nasopharyngeal colonization studies, the Kruskal–Wallis test was used to evaluate variance among all groups. If significant variance was found between groups, the Mann–Whitney U test was used to determine significant differences between individual groups.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases under the training grant in Microbial Pathogenesis and Genomics (1-T32-AI-141393-01). We thank Dr. John Clements for providing the purified dmLT used in these studies and Dr. Elizabeth Q. Littauer, Dr. Paul J. Planet, and Ying Yang for assistance with the animal work.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2019923118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Murphy T. F., et al., Nontypeable Haemophilus influenzae as a pathogen in children. Pediatr. Infect. Dis. J. 28, 43–48 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Sethi S., Evans N., Grant B. J. B., Murphy T. F., New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 347, 465–471 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Trottier S., Stenberg K., Svanborg-Edén C., Turnover of nontypable Haemophilus influenzae in the nasopharynges of healthy children. J. Clin. Microbiol. 27, 2175–2179 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao V. K., Krasan G. P., Hendrixson D. R., Dawid S., St Geme J. W. III, Molecular determinants of the pathogenesis of disease due to non-typable Haemophilus influenzae. FEMS Microbiol. Rev. 23, 99–129 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Smith-Vaughan H., et al., Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord. 6, 10 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leach A. J., Boswell J. B., Asche V., Nienhuys T. G., Mathews J. D., Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian aboriginal infants. Pediatr. Infect. Dis. J. 13, 983–989 (1994). [DOI] [PubMed] [Google Scholar]
- 7.McCann J. R., Mason S. N., Auten R. L., St Geme J. W. III, Seed P. C., Early-life intranasal colonization with nontypeable Haemophilus influenzae exacerbates juvenile airway disease in mice. Infect. Immun. 84, 2022–2030 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto M., et al., Insights into the population structure and pan-genome of Haemophilus influenzae. Infect. Genet. Evol. 67, 126–135 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Zola T. A., Lysenko E. S., Weiser J. N., Natural antibody to conserved targets of Haemophilus influenzae limits colonization of the murine nasopharynx. Infect. Immun. 77, 3458–3465 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kao C.-Y., et al., IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J. Immunol. 173, 3482–3491 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Hoshino H., et al., Increased elastase and myeloperoxidase activity associated with neutrophil recruitment by IL-17 in airways in vivo. J. Allergy Clin. Immunol. 105, 143–149 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Lu Y. J., et al., Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4, e1000159 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaffar Z., Ferrini M. E., Herritt L. A., Roberts K., Cutting edge: Lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J. Immunol. 182, 4507–4511 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao A. T., Yao S., Gong B., Elson C. O., Cong Y., Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J. Immunol. 189, 4666–4673 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Q., et al., IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 9, 78–86 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Archer N. K., et al., Interleukin-17A (IL-17A) and IL-17F are critical for antimicrobial peptide production and clearance of Staphylococcus aureus nasal colonization. Infect. Immun. 84, 3575–3583 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins S. C., Jarnicki A. G., Lavelle E. C., Mills K. H. G., TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: Role of IL-17-producing T cells. J. Immunol. 177, 7980–7989 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Archer N. K., Harro J. M., Shirtliff M. E., Clearance of Staphylococcus aureus nasal carriage is T cell dependent and mediated through interleukin-17A expression and neutrophil influx. Infect. Immun. 81, 2070–2075 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.St Geme J. W. III, Kumar V. V., Cutter D., Barenkamp S. J., Prevalence and distribution of the hmw and hia genes and the HMW and Hia adhesins among genetically diverse strains of nontypeable Haemophilus influenzae. Infect. Immun. 66, 364–368 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St Geme J. W. III, Falkow S., Barenkamp S. J., High-molecular-weight proteins of nontypable Haemophilus influenzae mediate attachment to human epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 90, 2875–2879 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buscher A. Z., Burmeister K., Barenkamp S. J., St Geme J. W. III, Evolutionary and functional relationships among the nontypeable Haemophilus influenzae HMW family of adhesins. J. Bacteriol. 186, 4209–4217 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grass S., et al., The Haemophilus influenzae HMW1 adhesin is glycosylated in a process that requires HMW1C and phosphoglucomutase, an enzyme involved in lipooligosaccharide biosynthesis. Mol. Microbiol. 48, 737–751 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Barenkamp S. J., Leininger E., Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect. Immun. 60, 1302–1313 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rempe K. A., Porsch E. A., Wilson J. M., St Geme J. W. III, The HMW1 and HMW2 adhesins enhance the ability of nontypeable Haemophilus influenzae to colonize the upper respiratory tract of rhesus macaques. Infect. Immun. 84, 2771–2778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis G. S., et al., Prevalence, distribution, and sequence diversity of hmwA among commensal and otitis media non-typeable Haemophilus influenzae. Infect. Genet. Evol. 28, 223–232 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahini Shams Abadi M., et al., Distribution and diversity of hmw1A among invasive nontypeable Haemophilus influenzae isolates in Iran. Avicenna J. Med. Biotechnol. 8, 99–102 (2016). [PMC free article] [PubMed] [Google Scholar]
- 27.Barenkamp S. J., Bodor F. F., Development of serum bactericidal activity following nontypable Haemophilus influenzae acute otitis media. Pediatr. Infect. Dis. J. 9, 333–339 (1990). [DOI] [PubMed] [Google Scholar]
- 28.Winter L. E., Barenkamp S. J., Naturally acquired HMW1-and HMW2-specific serum antibodies in adults and children mediate opsonophagocytic killing of nontypeable Haemophilus influenzae. Clin. Vaccine Immunol. 23, 37–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habets M. N., van Selm S., van der Gaast-de Jongh C. E., Diavatopoulos D. A., de Jonge M. I., A novel flow cytometry-based assay for the quantification of antibody-dependent pneumococcal agglutination. PLoS One 12, e0170884 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roche A. M., Richard A. L., Rahkola J. T., Janoff E. N., Weiser J. N., Antibody blocks acquisition of bacterial colonization through agglutination. Mucosal Immunol. 8, 176–185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitsi E., et al., Agglutination by anti-capsular polysaccharide antibody is associated with protection against experimental human pneumococcal carriage. Mucosal Immunol. 10, 385–394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z., Clarke T. B., Weiser J. N., Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Invest. 119, 1899–1909 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li W., et al., Recognition of conserved antigens by Th17 cells provides broad protection against pulmonary Haemophilus influenzae infection. Proc. Natl. Acad. Sci. U.S.A. 115, E7149–E7157 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noda K., et al., Th17 cells contribute to nontypeable Haemophilus influenzae-specific protective immunity induced by nasal vaccination with P6 outer membrane protein and α-galactosylceramide. Microbiol. Immunol. 55, 574–581 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Roier S., et al., Intranasal immunization with nontypeable Haemophilus influenzae outer membrane vesicles induces cross-protective immunity in mice. PLoS One 7, e42664 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beck K., Ohno H., Satoh-Takayama N., Innate lymphoid cells: Important regulators of host–bacteria interaction for border defense. Microorganisms 8, 1–22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broquet A., et al., Interleukin-22 level is negatively correlated with neutrophil recruitment in the lungs in a Pseudomonas aeruginosa pneumonia model. Sci. Rep. 7, 11010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Maele L., et al., Activation of Type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during Streptococcus pneumoniae infection. J. Infect. Dis. 210, 493–503 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Spits H., et al., Innate lymphoid cells – A proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013). [DOI] [PubMed] [Google Scholar]
- 40.O’Hara J. M., et al., Generation of protective pneumococcal-specific nasal resident memory CD4+ T cells via parenteral immunization. Mucosal Immunol. 13, 172–182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimura A., Mountzouros K. T., Relman D. A., Falkow S., Cowell J. L., Bordetella pertussis filamentous hemagglutinin: Evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect. Immun. 58, 7 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plamondon P., Luke N. R., Campagnari A. A., Identification of a novel two-partner secretion locus in Moraxella catarrhalis. Infect. Immun. 75, 2929–2936 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitt C., et al., A functional two-partner secretion system contributes to adhesion of Neisseria meningitidis to epithelial cells. J. Bacteriol. 189, 7968–7976 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boukamp P., et al., Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106, 761–771 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cutter D., et al., Immunization with Haemophilus influenzae Hap adhesin protects against nasopharyngeal colonization in experimental mice. J. Infect. Dis. 186, 1115–1121 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Leach S., Clements J. D., Kaim J., Lundgren A., The adjuvant double mutant Escherichia coli heat labile toxin enhances IL-17A production in human T cells specific for bacterial vaccine antigens. PLoS One 7, e51718 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clements J. D., Norton E. B., The mucosal vaccine adjuvant LT(R192G/L211A) or dmLT. MSphere 3, e00215–e00218 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asanuma H., Inaba Y., Aizawa C., Kurata T., Tamura S., Characterization of mouse nasal lymphocytes isolated by enzymatic extraction with collagenase. J. Immunol. Methods 187, 41–51 (1995). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.